Abstract
Leptospirosis, an emerging infectious disease caused by bacteria of the genus Leptospira, is thought to be the most widespread zoonotic disease in the world. A first step in preventing the spread of Leptospira is delineating the animal reservoirs that maintain and disperse the bacteria. Quantitative PCR (qPCR) methods targeting the LipL32 gene were used to analyze kidney samples from 124 House mice (Mus musculus), 94 Black rats (Rattus rattus), 5 Norway rats (R. norvegicus), and 89 small Indian mongooses (Herpestes auropunctatus) from five cattle farms in Puerto Rico. Renal carriage of Leptospira was found in 38% of the sampled individuals, with 59% of the sampled mice, 34% of Black rats, 20% of Norway rats, and 13% of the mongooses. A heterogeneous distribution of prevalence was also found among sites, with the highest prevalence of Leptospira-positive samples at 52% and the lowest at 30%. Comparative sequence analysis of the LipL32 gene from positive samples revealed the presence of two species of Leptospira, L. borgpetersenii and L. interrogans in mice, detected in similar percentages in samples from four farms, while samples from the fifth farm almost exclusively harbored L. interrogans. In rats, both Leptospira species were found, while mongooses only harbored L. interrogans. Numbers tested for both animals, however, were too small (n = 7 each) to relate prevalence of Leptospira species to location. Significant associations of Leptospira prevalence with anthropogenic landscape features were observed at farms in Naguabo and Sabana Grande, where infected individuals were closer to human dwellings, milking barns, and ponds than were uninfected individuals. These results show that rural areas of Puerto Rico are in need of management and longitudinal surveillance of Leptospira in order to prevent continued infection of focal susceptible species (i.e. humans and cattle).
Author summary
Leptospirosis is a global zoonotic disease that recently has shown an increase of human cases in many regions, Puerto Rico being one of them. To decrease human Leptospira infections it is necessary to ascertain the role of animal reservoirs to maintain the pathogen in the environment. However, no studies of wild mammal reservoirs in Puerto Rico have been done since 1963. We addressed the prevalence of Leptospira in four species of introduced wild mammals, which included three commensal rodent species (Mus and Rattus spp.) and the small Indian mongoose (Herpestes auropunctatus) on dairy farms in Puerto Rico. Pathogen prevalence ranged from high to moderate in mice and mongooses, respectively. We also found that the pathogen is not distributed homogeneously among the sites we sampled and that landscape features, both natural and manmade, can play a role in the distribution of Leptospira. Our study provides an initial exploration of wild mammal reservoirs for Leptospira in Puerto Rico, and our data highlight the need for management of these species to potentially decrease pathogen transmission rates in both humans and cattle.
Introduction
Along with increasing globalization, climate change, and urban expansion, the rising number of emerging infectious diseases is a major concern. Among emerging infectious diseases, over 60% are multi-host zoonoses many of which are classified as “neglected” owing to a general lack of knowledge about their epidemiology, more so in the tropics [1]. Perhaps the most widespread neglected zoonotic disease in the world is leptospirosis, which has an estimated annual global incidence of 1.03 million human cases with a projected number of 60,000 as fatal cases [2, 3]. Leptospirosis is caused by spirochetes of the genus Leptospira [4], with at least 15 known pathogenic species possessing over 250 serovars [5–7]. In known natural reservoirs, such as dogs, rodents, and cattle, Leptospira persists and multiplies within the renal tubules from which they are dispersed by urination of the moving hosts throughout the local landscape [4, 6]. Once in soil and water, these bacteria can remain viable for several months and can infect susceptible species through open-skin wounds and mucus membranes [5, 8]. Humans are incidentally infected with Leptospira following exposure to soils or water that is contaminated with animal urine [6].
Leptospirosis is an endemic disease in South Pacific island countries [9–11]. It is also widespread in the Caribbean islands including Haiti, Jamaica, Martinique, and Trinidad and Tobago [12–14]. However, incidence and prevalence of leptospirosis are largely underestimated throughout tropical environments especially since clinical signs associated to other febrile diseases, such as with dengue, malaria, and Zika, are strikingly similar; therefore, diagnosis and treatment is problematic [15–19]. In Puerto Rico, leptospirosis was first suspected in 1918 and confirmed in 1939 [20]. Current data for this island are limited, but reported incidence has increased over the past decade [16, 21]. Leptospirosis has been known to cause abortions, birth complications, and reduced milk production in cattle [22–24]. Due to the shared environment and level of contact with the animals, livestock workers are at risk of contracting leptospirosis areas where the pathogen is present [21, 25, 26]. The dairy industry in Puerto Rico comprises up to 25% of agriculture-related income and is historically the most important agricultural commodity on the island [27, 28]. Employing leptospirosis prevention regimens for cattle does not completely eliminate the threat of contraction if wildlife reservoirs are maintaining this pathogen in the farm environment. Therefore, assessing the risk associated with potential wildlife vectors in rural farm areas of Puerto Rico will inform future plans that aim to reduce transmission rates. The first step to this approach is identifying the wildlife species that are potentially acting as reservoirs on and around farms.
One of the most effective methods for managing zoonotic disease outbreaks is managing the wildlife reservoirs responsible for spreading the disease [21, 29, 30]. Invasive and pest (i.e. commensal rodents) species are of particular concern, because they readily adapt to human activity and urban settings, which places them in closer proximity to humans [29, 31]. This can be particularly true in farm settings where rodents and other pests can have direct access to animal feed and bedding areas. The objective of this study was to provide data on the prevalence of Leptospira in four invasive and pest species on rural farms of Puerto Rico; namely, House mice (Mus musculus), two rat species (Rattus rattus and R. norvegicus), and small Indian mongooses (Herpestes auropunctatus), which are now ubiquitous throughout the island and known reservoirs for Leptospira [1, 12]. We sampled at five rural locations in different parts of Puerto Rico, analyzed kidney tissue for renal carriage of Leptospira, and correlated Leptospira presence and absence data to individual distances from signature features within each location.
Methods
Sampling sites and sample collection
Rodents and mongooses were trapped on cattle farms from five municipalities in Puerto Rico during the summers of 2014 and 2015 (Fig 1). Municipalities sampled for 2014 included dairy cow farms in Lajas (18.041189, -67.042908), Isabela (18.46116, -67.05652), San Sebastián (18.378265, -67.022423), and Naguabo (18.238525, -65.719208). During 2015, the same municipalities were sampled along with the addition of a beef cattle farm in Sabana Grande (18.036125, -66.931173). Lajas and Sabana Grande are both located in the southwestern Caribbean Sea side island and have a tropical savannah climate. Sampling sites San Sebastián and Isabela are on the northeastern Atlantic Ocean side of the island and they are characterized by a tropical rainforest climate. Naguabo represents the coastal east side of the island and receives the most amount of rain of the five farms. These sites formed part of a larger project focused on livestock health, with an emphasis on the impacts of Cattle Fever Ticks (CFTs, Rhipicephalus spp.) as pathogen vectors. Sampling for small mammals to ascertain their potential role as tick hosts opened the opportunity to collect samples for the present project. Selection of the farm sites was driven by the original goal of studying CFTs. Criteria for including farm in the study required the presence of the CFT as well as the willingness of farm owners to volunteer to participate in the study. An additional consideration was the distribution of these farms along different ecological zones from the island. Thus, it should be emphasized that farm selection was not driven by any previous information associated to presence of Leptospira in humans or cattle. More precisely the present project is ancillary to a larger one and thus of an exploratory nature.
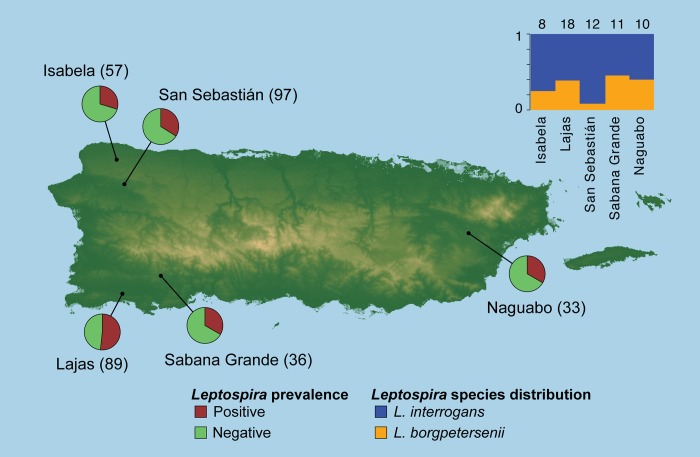
Fig 1. Leptospira prevalence for small invasive mammals (Mus, Rattus, and Herpestes) collected from five farms in Puerto Rico.
Pie charts represent the total ratio of positive and negative individuals captured at each farm location. The bar chart (top right) represents the ratio of L. interrogans and L. borgpertersenii detected in the animal reservoirs at each site. Sample sizes for each farm location are indicated with parentheses. Prevalence per site is overlaid on an elevation map based on the Puerto Rico, PR 1 arc-second MHW DEM [68]. Open source raster accessed from: https://www.ngdc.noaa.gov/dem/squareCellGrid/download/1561.
Sherman live traps (3"x 3.5"x 9") (H.B. Sherman Traps, Inc., Tallahassee, FL, USA) baited with rolled oats, and Tomahawk Live Traps (20"x7"x7") (Tomahawk Live Traps, Hazelhurst, WI, USA) baited with tuna fish were placed in transects of either one line of 40 or two lines of 20 traps. Tomahawk traps were placed approximately 15–20 meters apart and Sherman traps were placed approximately 2–5 meters apart depending on the habitat being sampled, which included ecotones, grasslands, cattle pastures, riparian zones, and around human dwellings. The positions of captured animals were recorded with a GPS unit (Garmin Montana 650, Garmin Corp., Kansas City, KS, USA). Tomahawk traps were checked for captures throughout diurnal hours, three times a day (early morning, midday, and evening) to target mongooses. Tomahawk and Sherman traps were left overnight to target rodents.
Immediately after capture, animals were euthanized by cervical dislocation after first being rendered unconscious with isoflurane. Weight and size measurements of individuals were taken along with tissue samples that included kidneys, a liver fragment, the GI tract, heart, and lungs obtained using sterilized equipment. During summer 2014, tissue samples were stored in 70% ethanol (EtOH) and transferred to 95% EtOH at the end of the field season. During the summer of 2015, kidney samples were stored in 95% EtOH and kept cool at approximately 4°C throughout the field season. Samples were stored at different EtOH concentrations between years due to a lack of resources during 2014.
Molecular analyses
DNA was extracted from kidneys using the Qiagen DNeasy Blood and Tissue Extraction Kit following the manufacturer’s instructions (Qiagen Inc., Valencia, CA, USA). As per these instructions, we extracted DNA from approximately 20mg of tissue, however this measurement was not systematically standardized. DNA extracts were stored frozen at -18°C. A qPCR TaqMan assay with primers designed to target the LipL32 gene present in pathogenic Leptospira sp. was used to test for the presence of Leptospira [32, 33]. For the purpose of this exploratory study, qPCR was used for the detection of Leptospira but was not used for quantification. TaqMan based analyses were carried out on an Applied Biosystems StepOne Plus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) in duplicate in a volume of 25 μl containing 12.5 μl of TaqMan Fast Advanced Master Mix (Applied Biosystem, Foster City, CA, USA) 1 μl of each forward primer LipL32-45F (700 nM, 5’AAG CAT TAC CGC TTG TGG TG) and reverse primer LipL32-286R (700 nM, 5’GAA CTC CCA TTT CAG CGA TT), 1 μl of probe LipL32-189P (150 nM, 5’[6-FAM]- AA AGC CAG GAC AAG CGC CG -[BHQ1]), 1 μl of DNA template and 8.5 μl of water. An initial denaturation at 95°C for 5 minutes was followed by 40 cycles of 95°C for 15 seconds and 60°C for 30 seconds [32, 33]. LipL32 gene amplicons obtained by end-point PCR with primers LipL32-45F and LipL32-286R from DNA of Leptospira interrogans serovar Copenhageni strain Fiocruz L1-130, kindly provided by Dr. Albert I. Ko (Yale University Schools of Public Health and Medicine, New Haven, CT), served as a positive control. Samples that amplified at ≤ 40 were considered positive. All samples were run in duplicate along with two negative controls. Assays were only considered valid if the negative control did not show an amplification signal.
Partial LipL32 genes from a subset of positive samples were re-amplified by end-point PCR with primers LipL32-45F and LipL32-286R. Amplicons (242 bp) were sequenced on an Applied Biosystems Genetic Analyzer 3500xL (Life Technologies, Carlsbad, CA), and sequences deposited at Genbank. Sequences were assembled in Geneious 8.1.7 (Biomatters Ltd, Auckland, New Zealand), and checked in GenBank/EMBL databases using the BLAST algorithm [34]. This subset was selected to include representative groups of samples from all species and farm locations included in this study and represents approximately 50% of the identified positive samples.
Landscape analyses
Landscape features included natural ponds, slurry ponds, milking areas within farms, and human buildings for which GPS positions were recorded. GPS positions of animals collected in the field were used to calculate the distance of each individual to landscape features. Average distances between these landscape features and positive or negative samples were then used in Welch’s t-test (unequal variances) to assess the relationships between prevalence of Leptospira in animals and landscape features on the farm. Leptospira presence/absence data were analyzed with SaTScan v9.4.2 software to test for significant clusters of positive cases on the landscape [35, 36]. Given that prevalence is binomial (infected or not) and presented as a proportion, we used Jeffrey’s confidence intervals for our estimates of prevalence among and within species and farms. We used the “prevalence” R package v.0.4.0 and an alpha value of 0.05 for significance assessments. A chi-squared test was conducted to determine if there was a heterogeneous distribution of Leptospira among the five farms.
Ethics statement
Collection and handling of wild rodents followed the Guidelines of the American Society of Mammalogists for the use of wild mammals in research [37]. Our protocol was reviewed and approved by the Texas State University Institutional Animal Care and Use Committee (protocol #0514_0303_07) and scientific collecting permits for wild mammal collection were provided by Departamento de Recursos Naturales y Ambientales from Puerto Rico (2014-IC-063 to Ivan Castro-Arellano). Access to private property was granted by landowners.
Results
Prevalence of Leptospira
Over two trapping seasons, we sampled 312 mammals comprising of 124 house mice (Mus musculus), 94 black rats (Rattus rattus), 5 Norway rats (R. norvegicus), and 89 Small Indian mongooses (Herpestes auropunctatus), sampling and prevalence data are summarized in Table 1. Sample sizes per site ranged from 33 (Naguabo) to 97 (San Sebastián) with an overall Leptospira prevalence of 0.38 (0.33–0.44) across all species. Although sample sizes among species were not uniform the overall prevalence values among and within sites it is of epidemiological significance to understand the level at which Leptospira is present among all animal reservoirs for both the island and each farm. Lajas had significantly higher prevalence compared to the other sites (x2 = 9.97, df = 4, p < 0.04). San Sebastián, Naguabo, Sabana Grande, and Isabela, had a similar prevalence values (0.30–0.34) regardless of varying sample sizes (n = 33–97). Mice generally showed higher prevalence of Leptospira (0.42–0.70), followed by Black rats (0.00 to 0.61), and mongooses (0.00–0.21). Norway rats were caught at two sites and in low numbers (n = 5), only one individual was positive for Leptospira. Overall, detections of Leptospira in mice were significantly higher than in rats and mongooses (x2 = 42.347, df = 2, p < 0.0001).
Table 1. Leptospira prevalence, with Jefferys confidence intervals, among invasive small mammals sampled from cattle farms in Puerto Rico during summer 2014 and 2015.
Positives columns represent numbers of infected individuals (outside parenthesis) and total sample size (n, in parenthesis). Jeff. C.I. = Jefferys Confidence Interval.
| Overall | Mus musculus | Rattus rattus | Rattus norvegicus | Herpestes auropunctatus | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Locality | positives (n) |
Prevalence (Jeff. C.I.) |
positives (n) |
Prevalence (Jeff. C.I.) |
positives (n) |
Prevalence (Jeff. C.I.) |
positives (n) |
Prevalence (Jeff. C.I.) |
Positives (n) |
Prevalence (Jeff. C.I.) |
| Lajas | 46 (89) | 0.52 (0.41–0.62) | 26 (37) | 0.70 (0.54–0.83) | 14 (23) | 0.61 (0.41–0.79) | – | – | 6 (29) | 0.21 (0.09–0.38) |
| San Sebastián | 33 (97) | 0.34 (0.25–0.44) | 20 (34) | 0.59 (0.42–0.74) | 10 (39) | 0.26 (0.14–0.41) | 0 (3) | 0.00 (0.00–0.44) | 3 (21) | 0.14 (0.04–0.33) |
| Naguabo | 11 (33) | 0.33 (0.19–0.50) | 10 (18) | 0.56 (0.33–0.76) | 0 (7) | 0.00 (0.00–0.23) | – | – | 1 (8) | 0.12 (0.01–0.45) |
| Sabana Grande | 12 (36) | 0.33 (0.20–0.50) | 10 (24) | 0.42 (0.24–0.61) | 2 (5) | 0.40 (0.09–0.79) | – | – | 0 (7) | 0.00 (0.00–0.23) |
| Isabela | 17 (57) | 0.30 (0.19–0.42) | 7 (11) | 0.64 (0.35–0.86) | 7 (20) | 0.36 (0.17–0.57) | 1 (2) | 0.50 (0.06–0.94) | 2 (24) | 0.08 (0.02–0.24) |
| Overall | 119 (312) | 0.38 (0.21–0.49) | 73 (124) | 0.59 (0.50–0.67) | 33 (94) | 0.34 (0.25–0.45) | 1 (5) | 0.20 (0.02–0.63) | 12 (89) | 0.13 (0.08–0.22) |
Leptospira diversity
Endpoint PCRs of Leptospira positive samples for LipL32 gene fragments (242 bp) from 45 mice, 7 rats, and 7 mongooses generated a sufficient number of amplicons to allow automated Sanger sequencing (MK328816–MK328874). Sequences showed either 100% identity to GenBank reference sequences for L. borgpetersenii (KF928037) or L. interrogans (U89708, DQ149595). Overall L. interrogans (68%) was detected more among small mammals compared to L. borgpetersenii (32%) (t = -2.58, df = 7.9, p = 0.03). Both Leptospira species were detected in similar proportions in Lajas, Naguabo, Sabana Grande, and Isabela, while samples from the fifth farm San Sebastián harbored mainly L. borgpetersenii (Fig 1, Table 2). In rats, both Leptospira species were found, while mongooses only harbored L. interrogans (Table 2).
Table 2. Leptospira species infecting invasive small mammals captured on Puerto Rican cattle farms during summer 2014 and 2015.
Assignments were inferred using LipL32 sequence data from renal tissue.
| Locality | n | L. borgpeterseniia | L. interrogansa |
|---|---|---|---|
| Lajas | 18 | 7 (5, 2, 0) | 11 (6, 1, 4) |
| San Sebastián | 12 | 1 (0, 1, 0) | 11 (10, 0, 1) |
| Naguabo | 10 | 4 (4, 0, 0) | 6 (5, 0, 1) |
| Sabana Grande | 11 | 5 (5, 0, 0) | 6 (5, 1, 0) |
| Isabela | 8 | 2 (2, 0, 0) | 6 (3, 2, 1) |
| Overall | 59 | 19 (16, 3, 0) | 40 (29, 4, 7) |
a Total (Mus, Rattus, Herpestes)
Association of Leptospira with landscape features
Association of infection with landscape features was significant in Naguabo, where infected individuals tended to be closer to chosen landscape features (including a human dwelling, dairy cow milking area, and a pond) than were uninfected individuals (Table 3). At Sabana Grande, infected individuals were closer to the human dwelling than were uninfected individuals (Table 3). At the other locations, i.e. Lajas, San Sebastián and Isabela, infected and uninfected individuals did not differ in distance to the anthropogenic landscape features.
Table 3. Correlation of landscape features present on individual farms in Puerto Rico to Leptospira prevalence among host species collected from five farms in Puerto Rico during summers 2014 and 2015.
Host species included Rattus rattus, R. norvegicus, Mus musculus, and Herpestes auropunctatus. Distances between host species and selected landscape features were calculated from GPS coordinates using the haversine equation. Welch’s t-tests were used to determine if the average distance of positive samples to the landscape features were closer than the average distance of negative samples using an aloha value of 0.05. Significant results are denoted in bold.
| Location | Feature | Distance (m) to negatives1 | Distance (m) to positives2 | Df | T | P |
|---|---|---|---|---|---|---|
| Lajas | ||||||
| Human dwelling | 2043.12 | 2237.99 | 80.95 | 0.70 | 0.48 | |
| Human dwelling | 1339.47 | 1038.90 | 80.81 | -0.97 | 0.34 | |
| Milking area | 1390.05 | 1076.59 | 80.84 | -0.97 | 0.34 | |
| Pond | 1646.12 | 1466.84 | 79.87 | -0.68 | 0.50 | |
| Wetland | 1452.45 | 1174.42 | 80.48 | -0.85 | 0.39 | |
| San Sebastián | ||||||
| Milking area | 392.33 | 346.09 | 61.70 | -0.85 | 0.40 | |
| Wetland Pond | 454.29 | 537.13 | 69.53 | 1.10 | 0.27 | |
| Slurry Pond | 414.85 | 380.96 | 60.44 | -0.67 | 0.50 | |
| Naguabo | ||||||
| Human dwelling | 278.93 | 126.35 | 23.94 | 2.60 | <0.02 | |
| Milking area | 234.42 | 100.71 | 24.00 | 2.53 | <0.02 | |
| Slurry Pond | 254.38 | 128.19 | 23.95 | 2.44 | <0.05 | |
| Sabana Grande | ||||||
| Human dwelling | 337.88 | 133.45 | 28.98 | -2.28 | <0.05 | |
| Stock Pond | 450.78 | 314.50 | 28.90 | -1.37 | 0.18 | |
| Isabela | ||||||
| Horse Stables | 565.04 | 571.06 | 29.23 | -0.05 | 0.96 | |
| Milking area | 520.26 | 534.54 | 29.45 | -0.13 | 0.90 | |
| Slurry Pond | 524.93 | 559.57 | 29.94 | -0.33 | 0.74 | |
| Stock Pond | 601.45 | 620.19 | 29.23 | -0.13 | 0.89 | |
1Average distance of features to captured animals testing negative for Leptospira
2Average distance of features to captured animals testing positive for Leptospira
Spatial analyses with SaTScan using a Bernoulli model identified four clusters within the studied farms but none were statistically significant. However, a cluster identified at the Lajas farm, although not significant at the level (p < 0.05) we had chosen, had a substantially different p-value than the other clusters (Fig 2 and Table 4). This cluster was in a field in close proximity to the milking area (0.14 km SE) and a building (0.15 km SW).
Fig 2. Potential cluster of reservoir animals (Herpestes auropunctatus, Rattus spp., Mus musculus) that tested positive for Leptospira at the farm location in the municipality of Lajas.
Each point indicates the location of one individual. Map image includes the main milking and processing area for the farm. Structures in view include the milking area and a human dwelling. Base map for creating this figure was taken from an open source (https://datagateway.nrcs.usda.gov/GDGOrder.aspx).
Table 4. Spatial clustering of samples positive for Leptospira at four locations, analyzed in SaTScan using a Bernoulli model.
Analyses included Herpestes auropunctatus, Rattus spp. and Mus musculus collected from cattle farms in Puerto Rico. All positive samples throughout sampling sites were analyzed to determine if more positive samples that expected were detected in any given area using ci-square analyses.
| Site | Radius | Expected | Observed | p | |
|---|---|---|---|---|---|
| Cluster 1 | Lajas | 0.029 | 5.49 | 12 | 0.07 |
| Cluster 2 | Sabana Grande | 0.024 | 1.57 | 4 | 0.95 |
| Cluster 3 | Naguabo | 0.086 | 1.57 | 4 | 0.95 |
| Cluster 4 | San Sebastián | 0.029 | 2.75 | 6 | 0.97 |
Discussion
Although all four species of mammals analyzed in our study tested positive for Leptospira, prevalence of Leptospira was much higher in mice than in mongooses or both Black or Norway rats (Tables 1 and 2). Results from this study suggest that mice potentially play a more important role as reservoir for Leptospira in rural parts of Puerto Rico than rats. However, since mice excrete less urine than rats they are likely also shedding fewer Leptospira into the environment. A major limitation to this study was not quantifying Leptospira load among individuals. Since this was an exploratory study that aimed to identify a potential health risk to cattle and farmworkers on Puerto Rican dairy farms, the main goal was to verify the presence of the pathogenic Leptospira spp. among potential reservoirs present at each farm. Now that the presence has been verified, future studies on farms should concentrate on accurately quantifying the load of reservoir animals. Quantifying pathogen load will provide a better idea of the extent to which each species contributes to Leptospira maintenance in the environment.
In Puerto Rico, all four animal species we sampled had been identified as reservoirs for Leptospira before, with high prevalence, i.e. 48% in House mice, 37% in Black rats, 40% in Norway rats, and 20% in small Indian mongooses [20]. In a previous study, both rat species were found to carry Leptospira, at a prevalence of 39% [38]. Although both of these studies were limited to the urban area of San Juan, our data confirm that House mice, black rats, and Norway rats are also important reservoirs in rural areas of Puerto Rico with high Leptospira prevalence in all animal species.
It should be noted that few Norway rats were caught in sampled farms. Previous studies have found large numbers and with high prevalence of Leptospira in urban areas [39, 40]. In both tropical and temperate urban areas, Norway rats are frequently encountered and infected with Leptospira, with prevalence values often around 40% [41–43], but up to 89% as well [44, 45]. In our study, Norway rats were rarely encountered and caught (n = 5), which was likely a consequence of this species preferring urban areas over rural areas [46]. Thus, our prevalence values of 20% that reflected detection of Leptospira in one individual only, are without statistical significance though still similar to other published data [47, 48].
High prevalence of Leptospira has been shown for different species of mongooses in previous studies [49–51]. Our study resulted in few detections of Leptospira in Indian mongooses at any site on Puerto Rico corresponding to an overall prevalence rate of 13%. Results on other Caribbean islands like Barbados, however, show much higher prevalence, with prevalence values close to 41% in mongooses, while mice were infected at much lower prevalence (28%) [51]. Even though similar numbers of mongooses and mice were tested in this study, the results do not match our data with 13% mongooses harboring Leptospira, and mice being the most infected with 59% prevalence. The result trends, however, are similar to numbers ascertained from a previous survey conducted in San Juan, Puerto Rico [20].
Two species of Leptospira, i.e. L. interrogans and L. borgpetersenii were detected in mice and rats, while mongooses only harbored L. interrogans. The lack of detection of L. borgpetersenii in mongooses, however, is potentially a function of the small sampling size used for species analyses of Leptospira, i.e. seven individuals from 4 locations. Since a higher prevalence of L. borgpetersenii is seen in both rats and mice, it could also be that mongooses are not regularly coming into direct contact with this pathogen due to mongooses living in lower densities than mice or rats and the short environmental persistence of L. borgpetersenii outside of the host. L. borgpetersenii is thought to survive poorly in the environment due to point mutations in environmental sensing and metabolite transport and utilization genes, and thus is transmitted most frequently through direct contact [52]. In contrast, L. interrogans survives for extended times in the environments [53] and is transmitted readily through contact with surface waters [54, 55]. L. borgpetersenii (i.e. serovar Hardjo) has been reported as most prominent Leptospira sp. in cattle in Chile [56], and cattle were proposed as maintenance host [24]. L. borgpetersenii has been detected in cattle from other countries, however, equally often in other animals, including many different rodent species [57]. Thus, while the tropical conditions on Puerto Rico generally favor environmental survival and transmission of Leptospira [53], animal host preferences of different Leptospira species could not be established in our study.
Both Leptospira species identified in this study are known to persist in urban rat populations, as demonstrated for samples from Malaysia [58]. Both species were detected in mice, in similar percentages in four of the five sampling locations in Puerto Rico, while samples from the fifth location, San Sebastián, almost exclusively harbored L. interrogans. Since there were no apparent landscape features ecologically isolating this location from the other study locations, it is interesting that there was an absence of L. borgpetersenii in mice. Low abundance and small sampling size affecting detection of L. borgpetersenii is not a likely explanation, since sampling size was similar to those of the other locations and L. borgpetersenii was identified in the only rat sample analyzed from this location.
In some farms, such as those is Barbados, agricultural workers were identified as having a high risk of contracting leptospirosis due to their proximity to contaminated water and soil [59], and in an urban slum in Brazil lower elevations were related to higher Leptospira concentrations [55]. Furthermore, previous studies have associated the persistence of Leptospira with moist environments [55, 60]. Naguabo was the only location in this study for which several landscape feature-Leptospira prevalence relationships were found. These relationships are, in part, possibly due to the location of the farm, i.e. a valley in a mountainous area in close proximity to the El Yunque National Rainforest which experiences an average rainfall of approx. 2,134 mm which is much higher than in all of the other sampling sites. Heavy rainfall results in runoff that might carry bacteria to and concentrate them at structures located in areas with the lowest elevation of this farm and in a nearby stream. Here, the moist environments would provide suitable conditions for some species of Leptospira to persist for longer lengths of time; therefore, rodents would have a greater opportunity to come into contact with the pathogens. Ponds, milking areas, and human dwellings are also desirable rodent habitats due to providing easier access to resources, so rodents are more likely to persist in higher abundances in close proximity to these areas.
The relationship between landscape features and the distance of positive and negative samples were inconsistent between the five farms included in this study. While it is generally thought that Leptospira is associated with the presence of environmental features such water bodies, data presented in this study do not support this hypothesis. This inconsistency could be due to interspecific interactions among animal reservoirs having a greater effect on Leptospira prevalence than the environmental features themselves. Ansersen-Ranberg et al. (2016) found that although some groups of animal reservoirs had similar prevalence values, these were inconsistently correlated to environmental factors [61]. Additionally, there is some indication that sociality in reservoir species or human effects on landscape has the potential to create hot spots for Leptospira presence. While not statistically significant, the spatial cluster of positive samples detected in the Lajas farm (Fig 2) held some ecological and epidemiological significance because it coincided spatially with an area where discarded farm materials (cut tree branches, tires, metal pieces, etc.) had been deposited as a pile in the middle of an open field. This created a habitat suitable for mice where individuals, both positive and negative, congregated (Fig 2) likely raising pathogen transmission among those individuals. As a precaution, farmers were informed that waste management around farm buildings to prevent rodent infestations should be a high priority to avoid concentration of Leptospira positive individuals. Since it is likely that the small sample size and uneven coverage of the farm influenced the significance of the cluster analysis, future research should aim to increase samples size and sample coverage at the individual farms to better identify potential disease hotspots.
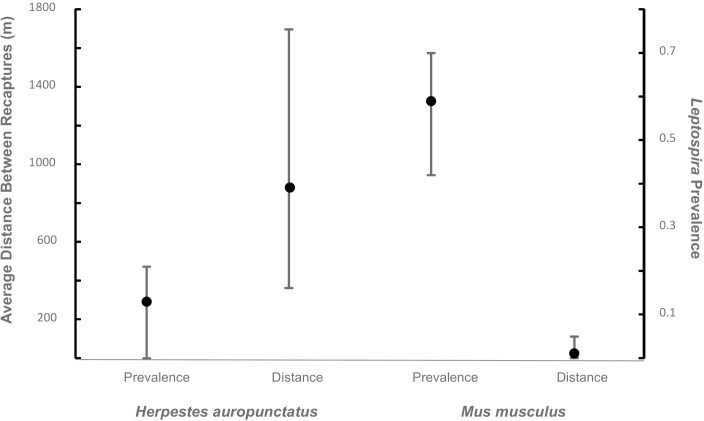
Another issue that needs to be addressed is the dynamics of interspecific transmission and pathogen maintenance at each farm and among farms in a landscape. The contrast between usual spatial movements and Leptospira prevalence between mongooses and mice shows a clear opposite pattern (Fig 3) in which mice usually travel short distances but show greater pathogen prevalence whereas the opposite is true for mongooses. Our study found a higher prevalence of Leptospira in mice thus pointing out a potential important role for this species to maintain the pathogen at a given site. However, this rodent species is usually found in close association to human dwellings and likely will not be a relevant factor to spread the pathogen to other sites. In contrast, mongooses readily travel longer distances and would be capable of spreading Leptospira among adjoining farms and further into areas of high human use, a behavior reported for other Herpestidae species that are also reservoirs for this pathogen [50]. Since small Indian mongooses readily prey on commensal rodents a high opportunity of contact between these species exist so the need to evaluate this as a potential route for interspecies transmission and its role in maintaining the pathogen in Puerto Rico deserves further evaluation. Future studies that investigate this possible relationship should also consider other factors, such as host carriage rate, host urine extraction rates, and pathogen life history traits to gain a clear picture of how much host behaviors potentially affect prevalence between farms.
Fig 3. Comparison of Leptospira prevalence and movement distances of Herpestes auropunctatus and Mus musculus.
Spatial movement data of H. auropunctatus was collected via a parallel telemetry sampling on four of the dairy farms in Puerto Rico that formed part of present study. Detailed results from this telemetry study forms part of a larger project about zoonotic diseases in cattle farms in the island and will be published elsewhere. The average distance between telemetry fixes among mongoose individuals equaled 362.61 meters, ranging from 362.61 meters– 1696.5 meters (extreme values represented with bars around point that represents average). Spatial movements of M. musculus were retrieved from a published review [69] that included capture-recapture studies for M. musculus in predominantly rural settings and included both feral and commensal house mice. The average distance between recaptures in this review equaled 25.27 meters, and the range was 0.9 meters to 112 meters (extreme values represented with bars around point that represents average). Prevalence estimates for both species represented in this graph are from the current study (extreme values represented by bars with averages as points).
According to news sources, after hurricane Maria landed in September of 2017 this event increased the number of leptospirosis infections in humans across Puerto Rico. This was likely because people were obligated to drink contaminated water as a result of failed infrastructure. As many as 76 individuals were likely infected and a small handful of these were fatal cases [62]. As climatic events such as this increase in intensity and frequency as a result of climate change, it is becoming increasingly important to monitor the epidemiological consequences on pathogenic agents of neglected infectious diseases. This is especially true in tropical areas such as Puerto Rico because these are the most severely affected by intense climatic events such as hurricanes and monsoons, as was illustrated with Hurricane Maria. This study demonstrates the need for leptospirosis monitoring programs to be implemented in rural areas of Puerto Rico along with urban areas.
In conclusion, this study established baseline data on the prevalence of Leptospira species in four animal species in five rural areas, at both the east and west coasts of Puerto Rico. The capture of large numbers of rodents and mongooses with high prevalence of Leptospira in animals from all locations supports suggestions for the implementation of management plans for rodent and mongoose control to reduce the risk of susceptible focal species (i.e. humans and cattle) to contract Leptospira. These management plans could focus on all or selected animal species depending on their abundance at the respective location, and should include monitoring prevalence of Leptospira in cattle and adjacent soils and waters to assess environmental risks of infection in rural areas of Puerto Rico [54, 55, 63–67].
Acknowledgments
We are grateful to Dr. Albert I. Ko (Yale University Schools of Public Health and Medicine, New Haven, CT) for providing DNA of Leptospira interrogans serovar Copenhageni strain Fiocruz L1-130 that was used as reference material. We also thank farm owners that allowed access to their property.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Support for graduate studies of KMB was provided by the United States Department of Agriculture (USDA NIFA 201 3-70001-20524) and funding for fieldwork activities was provided by the USDA Agricultural Research Service made possible by the Puerto Rico Department of Agriculture (USDA MTRA 58-6205-4-004 grant to RJM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Webster JP, Gower CM, Knowles SCL, Molyneux DH, Fenton A. One health—an ecological and evolutionary framework for tackling Neglected Zoonotic Diseases. Evol Appl. 2016;9(2):313–33. 10.1111/eva.12341 WOS:000368841800001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guerra MA. Leptospirosis: Public health perspectives. Biologicals. 2013;41(5):295–7. 10.1016/j.biologicals.2013.06.010 WOS:000325447300002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jancloes M, Bertherat E, Schneider C, Belmain S, Munoz-Zanzi C, Hartskeerl R, et al. Towards a “One Health” strategy against Leptospirosis. Planet@Risk. 2014;2:204–6. [Google Scholar]
- 4.Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3(12):757–71. 10.1016/S1473-3099(03)00830-2 WOS:000186910800018. [DOI] [PubMed] [Google Scholar]
- 5.Ko AI, Goarant C, Picardeau M. Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol. 2009;7(10):736–47. 10.1038/nrmicro2208 WOS:000269831000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plank R, Dean D. Overview of the epidemiology, microbiology, and pathogenesis of Leptospira spp. in humans. Microbes Infect. 2000;2(10):1265–76. 10.1016/S1286-4579(00)01280-6 WOS:000089488900014. [DOI] [PubMed] [Google Scholar]
- 7.Thibeaux R, Girault D, Bierque E, Soupé-Gilbert M-E, Rettinger A, Douyère A, et al. Biodiversity of environmental Leptospira: improving identification and revisiting the diagnosis. Frontiers in microbiology. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casanovas-Massana A, Pedra GG, Wunder EA, Diggle PJ, Begon M, Ko AI. Quantitative survival of Leptospira interrogans in soil and water microcosms. Applied and environmental microbiology. 2018:AEM. 00507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Victoriano AFB, Smythe LD, Gloriani-Barzaga N, Cavinta LL, Kasai T, Limpakarnjanarat K, et al. Leptospirosis in the Asia Pacific region. Bmc Infect Dis. 2009;9 Artn 147 10.1186/1471-2334-9-147 WOS:000270413400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez-Silveira MS, et al. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. Plos Neglect Trop D. 2015;9(9). ARTN e0003898 10.1371/journal.pntd.0003898 WOS:000363031200005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hotez PJ, Bottazzi ME, Strych U, Chang LY, Lim YAL, Goodenow MM, et al. Neglected Tropical Diseases among the Association of Southeast Asian Nations (ASEAN): Overview and Update. Plos Neglect Trop D. 2015;9(4). ARTN e0003575 10.1371/journal.pntd.0003575 WOS:000354972200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desvars A, Cardinale E, Michault A. Animal leptospirosis in small tropical areas. Epidemiol Infect. 2011;139(2):167–88. 10.1017/S0950268810002074 WOS:000286012800001. [DOI] [PubMed] [Google Scholar]
- 13.Petrakovsky J, Bianchi A, Fisun H, Najera-Aguilar P, Pereira MM. Animal Leptospirosis in Latin America and the Caribbean Countries: Reported Outbreaks and Literature Review (2002–2014). Int J Env Res Pub He. 2014;11(10):10770–89. 10.3390/ijerph111010770 WOS:000344358700047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotez PJ. Neglected Tropical Diseases—Latin America and the Caribbean Foreword. Neglect Trop Dis. 2015:V-Vi Book_Doi 10.1007/978-3-7091-1422-3 WOS:000374638500001. [DOI] [Google Scholar]
- 15.Rodriguez NMP, Galloway R, Blau DM, Traxler R, Bhatnagar J, Zaki SR, et al. Case Report: Case Series of Fatal Leptospira spp./Dengue Virus Co-Infections-Puerto Rico, 2010–2012. Am J Trop Med Hyg. 2014;91(4):760–5. 10.4269/ajtmh.14-0220 WOS:000342957600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharp TM, Garcia BR, Perez-Padilla J, Galloway RL, Guerra M, Ryff KR, et al. Early Indicators of Fatal Leptospirosis during the 2010 Epidemic in Puerto Rico. Plos Neglect Trop D. 2016;10(2). ARTN e0004482 10.1371/journal.pntd.0004482 WOS:000372567300077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanders EJ, Rigau-Perez JG, Smits HL, Deseda CC, Vorndam VA, Aye T, et al. Increase of leptospirosis in dengue-negative patients after a hurricane in Puerto Rico in 1966. Am J Trop Med Hyg. 1999;61(3):399–404. WOS:000082697800011. 10.4269/ajtmh.1999.61.399 [DOI] [PubMed] [Google Scholar]
- 18.Bruce MG, Sanders EJ, Leake JAD, Zaidel O, Bragg SL, Aye T, et al. Leptospirosis among patients presenting with dengue-like illness in Puerto Rico. Acta Trop. 2005;96(1):36–46. 10.1016/j.actatropica.2005.07.001 WOS:000231886400006. [DOI] [PubMed] [Google Scholar]
- 19.Neaterour P, Rivera A, Galloway RL, Negrón MG, Rivera-Garcia B, Sharp TM. Fatal Leptospira spp./Zika Virus Coinfection—Puerto Rico, 2016. The American journal of tropical medicine and hygiene. 2017;97(4):1085–7. 10.4269/ajtmh.17-0250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexander AD, Benenson AS, Byrne RJ, Díaz-Rivera RS, Evans LB, Gochenour WS, et al. Leptospirosis in Puerto Rico. Zoonoses Research 1963;2:152–227. [PubMed] [Google Scholar]
- 21.CDC. Notes from the field: investigation of leptospirosis underreporting–Puerto Rico, 2010. Morbidity and Mortality Weekly Report 2012;61:421 [PubMed] [Google Scholar]
- 22.Brioudes A, Warner J, Hedlefs R, Gummow B. Diseases of livestock in the Pacific Islands region: Setting priorities for food animal biosecurity. Acta Trop. 2015;143:66–76. 10.1016/j.actatropica.2014.12.012 WOS:000350526800010. [DOI] [PubMed] [Google Scholar]
- 23.Lilenbaum W, Martins G. Leptospirosis in Cattle: A Challenging Scenario for the Understanding of the Epidemiology. Transbound Emerg Dis. 2014;61:63–8. 10.1111/tbed.12233 WOS:000340537900008. [DOI] [PubMed] [Google Scholar]
- 24.Alonso-Andicoberry C, Garcia-Pena FJ, Pereira-Bueno J, Costas E, Ortega-Mora LM. Herd-level risk factors associated with Leptospira spp. seroprevalence in dairy and beef cattle in Spain. Prev Vet Med. 2001;52(2):109–17. Pii S0167-5877(01)00249-5 10.1016/S0167-5877(01)00249-5 WOS:000176454000002. [DOI] [PubMed] [Google Scholar]
- 25.Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296–+. 10.1128/CMR.14.2.296-326.2001 WOS:000168047600004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cestero-Rivera R, Ramirez-Rivera J, Jaume-Anselmi F, Guerrero-Dujarric O. Acute renal failure in a man with a small farm. Boletin de la Asociacion Medica de Puerto Rico. 2006;98(2):114–6. [PubMed] [Google Scholar]
- 27.Gruebele JW, Barahona LFC. Growth of the dairy industry in Puerto Rico. Illinois Agriculture Economics 1974;14:32–8. [Google Scholar]
- 28.USDA. Puerto Rico Island and Municipio Data. 2012 Census of Agriculture, Washington, D.C., USA2014.
- 29.Reperant LA. Applying the Theory of Island Biogeography to Emerging Pathogens: Toward Predicting the Sources of Future Emerging Zoonotic and Vector-Borne Diseases. Vector-Borne Zoonot. 2010;10(2):105–10. 10.1089/vbz.2008.0208 WOS:000276080100001. [DOI] [PubMed] [Google Scholar]
- 30.Mendes MS, de Moraes J. Legal aspects of public health: Difficulties in controlling vector-borne and zoonotic diseases in Brazil. Acta Trop. 2014;139:84–7. 10.1016/j.actatropica.2014.07.008 WOS:000343352300012. [DOI] [PubMed] [Google Scholar]
- 31.McKinney ML. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosystems 2008;11:161–76. [Google Scholar]
- 32.Stoddard RA, Gee JE, Wilkins PP, McCaustland K, Hoffmaster AR. Detection of pathogenic Leptospira spp. through TaqMan polymerase chain reaction targeting the LipL32 gene. Diagn Micr Infec Dis. 2009;64(3):247–55. 10.1016/j.diagmicrobio.2009.03.014 WOS:000267277300002. [DOI] [PubMed] [Google Scholar]
- 33.Bourhy P, Bremont S, Zinini F, Giry C, Picardeau M. Comparison of Real-Time PCR Assays for Detection of Pathogenic Leptospira spp. in Blood and Identification of Variations in Target Sequences. J Clin Microbiol. 2011;49(6):2154–60. 10.1128/JCM.02452-10 WOS:000291024500014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pearson WR, Lipman DJ. Improved tools for biological sequence comparison. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(8):2444–8. WOS:A1988N023400007. 10.1073/pnas.85.8.2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kulldorff M. A spatial scan statistic. Communications in Statistics-Theory and methods. 1997;26(6):1481–96. [Google Scholar]
- 36.Kulldorff M. Information Management Services, Inc. SaTScan version 4.0: software for the spatial and space-time scan statistics, 2004. 2009.
- 37.Sikes RS, Gannon WL, Mammalogists AS. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J Mammal. 2011;92(1):235–53. 10.1644/10-Mamm-F-355.1 WOS:000287898700025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rust JH. Leptospirosis in Puerto Rican wild rats. Puerto Rico Journal of Public Health and Tropical Medicine 1948;24:105–11. [PubMed] [Google Scholar]
- 39.Desvars A, Michault A, Bourhy P. Leptospirosis in the western Indian Ocean islands: what is known so far? Vet Res. 2013;44 Artn 80 10.1186/1297-9716-44-80 WOS:000326184000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Costa F, Richardson JL, Dion K, Mariani C, Pertile AC, Burak MK, et al. Multiple Paternity in the Norway Rat, Rattus norvegicus, from Urban Slums in Salvador, Brazil. J Hered. 2016;107(2):181–6. 10.1093/jhered/esv098 WOS:000370063900010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santos AAN, Figueira CP, dos Reis MG, Costa F, Ristow P. Heterogenic colonization patterns by Leptospira interrogans in Rattus norvegicus from urban slums. Braz J Microbiol. 2015;46(4):1161–4. 10.1590/S1517-838246420140873 WOS:000367387800026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Theuerkauf J, Perez J, Taugamoa A, Niutoua I, Labrousse D, Gula R, et al. Leptospirosis risk increases with changes in species composition of rat populations. Naturwissenschaften. 2013;100(4):385–8. 10.1007/s00114-013-1033-6 WOS:000317348600011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aviat F, Blanchard B, Michel V, Blanchet B, Branger C, Hars J, et al. Leptospira exposure in the human environment in France: A survey in feral rodents and in fresh water. Comp Immunol Microb. 2009;32(6):463–76. 10.1016/j.cimid.2008.05.004 WOS:000269599300001. [DOI] [PubMed] [Google Scholar]
- 44.Costa F, Porter FH, Rodrigues G, Farias H, de Faria MT, Wunder EA, et al. Infections by Leptospira interrogans, Seoul Virus, and Bartonella spp. Among Norway Rats (Rattus norvegicus) from the Urban Slum Environment in Brazil. Vector-Borne Zoonot. 2014;14(1):33–40. 10.1089/vbz.2013.1378 WOS:000329360400004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krojgaard LH, Villumsen S, Markussen MDK, Jensen JS, Leirs H, Heiberg AC. High prevalence of Leptospira spp. in sewer rats (Rattus norvegicus). Epidemiol Infect. 2009;137(11):1586–92. 10.1017/S0950268809002647 WOS:000270661800009. [DOI] [PubMed] [Google Scholar]
- 46.Feng AYT, Himsworth CG. The secret life of the city rat: a review of the ecology of urban Norway and black rats (Rattus norvegicus and Rattus rattus). Urban Ecosystems. 2014;17(1):149–62. 10.1007/s11252-013-0305-4 WOS:000332698500010. [DOI] [Google Scholar]
- 47.Agudelo-Florez P, Londono AF, Quiroz VH, Angel JC, Moreno N, Loaiza ET, et al. Prevalence of Leptospira spp. in Urban Rodents from a Groceries Trade Center of Medellin, Colombia. Am J Trop Med Hyg. 2009;81(5):906–10. 10.4269/ajtmh.2009.09-0195 WOS:000271956500030. [DOI] [PubMed] [Google Scholar]
- 48.Loan HK, Cuong NV, Takhampunya R, Kiet BT, Campbell J, Them LN, et al. How Important Are Rats As Vectors of Leptospirosis in the Mekong Delta of Vietnam? Vector-Borne Zoonot. 2015;15(1):56–64. 10.1089/vbz.2014.1613 WOS:000348570600008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Millan J, Candela MG, Lopez-Bao JV, Pereira M, Jimenez MA, Leon-Vizcaino L. Leptospirosis in Wild and Domestic Carnivores in Natural Areas in Andalusia, Spain. Vector-Borne Zoonot. 2009;9(5):549–54. 10.1089/vbz.2008.0081 WOS:000270864200014. [DOI] [PubMed] [Google Scholar]
- 50.Jobbins SE, Sanderson CE, Alexander KA. Leptospira interrogans at the Human-Wildlife Interface in Northern Botswana: A Newly Identified Public Health Threat. Zoonoses Public Hlth. 2014;61(2):113–23. 10.1111/zph.12052 WOS:000331184900004. [DOI] [PubMed] [Google Scholar]
- 51.Matthias MA, Levett PN. Leptospiral carriage by mice and mongooses on the island of Barbados. W Indian Med J. 2002;51(1):10–3. WOS:000176078000003. [PubMed] [Google Scholar]
- 52.Cosson JF, Picardeau M, Mielcarek M, Tatard C, Chaval Y, Suputtamongkol Y, et al. Epidemiology of Leptospira Transmitted by Rodents in Southeast Asia. Plos Neglect Trop D. 2014;8(6). ARTN e2902 10.1371/journal.pntd.0002902 WOS:000338846100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adler B, Moctezuma AD. Leprospira and leptospirosis. Vet Microbiol. 2010;140(3–4):287–96. 10.1016/j.vetmic.2009.03.012 WOS:000274986400012. [DOI] [PubMed] [Google Scholar]
- 54.Ganoza CA, Matthias MA, Collins-Richards D, Brouwer KC, Cunningham CB, Segura ER, et al. Determining risk for severe leptospirosis by molecular analysis of environmental surface waters for pathogenic Leptospira. PLoS medicine. 2006;3(8):e308 10.1371/journal.pmed.0030308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Casanovas-Massana A, Costa F, Riediger IN, Cunha M, de Oliveira D, Mota DC, et al. Spatial and temporal dynamics of pathogenic Leptospira in surface waters from the urban slum environment. Water research. 2018;130:176–84. 10.1016/j.watres.2017.11.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salgado M, Otto B, Sandoval E, Reinhardt G, Boqvist S. A cross sectional observational study to estimate herd level risk factors for Leptospira spp. serovars in small holder dairy cattle farms in southern Chile. Bmc Vet Res. 2014;10 Artn 126 10.1186/1746-6148-10-126 WOS:000338359500001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Allan KJ, Biggs HM, Halliday JEB, Kazwala RR, Maro VP, Cleaveland S, et al. Epidemiology of Leptospirosis in Africa: A Systematic Review of a Neglected Zoonosis and a Paradigm for 'One Health' in Africa. Plos Neglect Trop D. 2015;9(9). ARTN e0003899 10.1371/journal.pntd.0003899 WOS:000363031200006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benacer D, Zain SNM, Sim SZ, Khalid MKNM, Galloway RL, Souris M, et al. Determination of Leptospira borgpetersenii serovar Javanica and Leptospira interrogans serovar Bataviae as the persistent Leptospira serovars circulating in the urban rat populations in Peninsular Malaysia. Parasite Vector. 2016;9 ARTN 117 10.1186/s13071-016-1400-1 WOS:000371454900001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Everard C, Edwards C, Everard J, Carrington D. A twelve-year study of leptospirosis on Barbados. European journal of epidemiology. 1995;11(3):311–20. [DOI] [PubMed] [Google Scholar]
- 60.Saito M, Villanueva SY, Chakraborty A, Miyahara S, Segawa T, Asoh T, et al. Comparative analysis of Leptospira strains isolated from environmental soil and water in the Philippines and Japan. Appl Environ Microbiol. 2013;79(2):601–9. 10.1128/AEM.02728-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andersen-Ranberg EU, Pipper C, Jensen PM. GLOBAL PATTERNS OF LEPTOSPIRA PREVALENCE IN VERTEBRATE RESERVOIR HOSTS. Journal of wildlife diseases. 2016;52(3):468–77. 10.7589/2014-10-245 [DOI] [PubMed] [Google Scholar]
- 62.Wire C. Suspected leptospirosis cases increasing in Puerto Rico after Hurricane Maria.: Oklahoma News 4; [updated 24 October 2017January 4 2018]. Available from: https://kfor.com/2017/10/24/suspected-leptospirosis-cases-increasing-in-puerto-rico-after-hurricane-maria/.
- 63.Andre-Fontaine G, Aviat F, Thorin C. Waterborne Leptospirosis: Survival and Preservation of the Virulence of Pathogenic Leptospira spp. in Fresh Water. Curr Microbiol. 2015;71(1):136–42. 10.1007/s00284-015-0836-4 WOS:000355749400018. [DOI] [PubMed] [Google Scholar]
- 64.Benacer D, Woh PY, Zain SNM, Amran F, Thong KL. Pathogenic and Saprophytic Leptospira Species in Water and Soils from Selected Urban Sites in Peninsular Malaysia. Microbes Environ. 2013;28(1):135–40. 10.1264/jsme2.ME12154 WOS:000316005100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wojcik-Fatla A, Zajac V, Wasinski B, Sroka J, Cisak E, Sawczyn A, et al. Occurrence of Leptospira DNA in water and soil samples collected in eastern Poland. Ann Agr Env Med. 2014;21(4):730–2. 10.5604/12321966.1129924 WOS:000346625800013. [DOI] [PubMed] [Google Scholar]
- 66.Saito M, Villanueva SYAM, Chakraborty A, Miyahara S, Segawa T, Asoh T, et al. Comparative Analysis of Leptospira Strains Isolated from Environmental Soil and Water in the Philippines and Japan. Applied and Environmental Microbiology. 2013;79(2):601–9. 10.1128/AEM.02728-12 WOS:000312941800020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schneider AG, Casanovas-Massana A, Hacker KP, Wunder EA Jr, Begon M, Reis MG, et al. Quantification of pathogenic Leptospira in the soils of a Brazilian urban slum. PLoS neglected tropical diseases. 2018;12(4):e0006415 10.1371/journal.pntd.0006415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taylor LA, Eakins BW, Carignan KS, Warnken RR, Sazonova TS, Schoolcraft DC. Digital elevation models of Puerto Rico: procedures, data sources and analysis. 2008.
- 69.POCOCK MJ, HAUFFE HC, SEARLE JB. Dispersal in house mice. Biological Journal of the Linnean Society. 2005;84(3):565–83. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.