Abstract
Administration of the influenza vaccine has been associated with development of several autoimmune phenomena. We describe the case of a 72-year-old male who developed double seropositive vasculitis following seasonal influenza vaccination. On presentation, he was positive for both myeloperoxidase anti-neutrophil cytoplasmic antibody and anti-glomerular basement membrane antibody. He had stage three acute kidney injury requiring dialysis and was treated with methylprednisolone, intravenous cyclophosphamide and plasma exchange. He was also noted to have an incidental seven centimetre abdominal aortic aneurysm. He achieved remission with recovery in renal function and became haemodialysis independent. We hypothesize that the temporal relationship between influenza vaccination and double seropositive vasculitis directly triggered a systemic immune response in a susceptible patient, although a causal relationship cannot be proved. To the best of our knowledge, this is the first case of double seropositive vasculitis occurring in close temporal association with seasonal influenza vaccination.
INTRODUCTION
The seasonal influenza vaccine is widely used within the United Kingdom to provide protection against the influenza virus. Several rare autoimmune phenomena, including vasculitis, have been widely reported following its administration, and to date, a causal relationship between influenza vaccine and vasculitis has not been established [1]. Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is one of several vasculitides that has occurred in temporal relationship with the influenza vaccine [2, 3]. AAV is a rare, small-vessel vasculitis that refers to three syndromes: granulomatosis with polyangiitis (GPA), microscopic polyangiitis and eosinophilic GPA [4]. In Europe, the annual incidence is 13–20 cases per million individuals and it can lead to a rapidly progressive necrotizing glomerulonephritis [5]. Several reports have documented development or relapse of AAV following administration of the influenza vaccine [2, 3].
Anti-glomerular basement membrane (Anti-GBM) disease is another rare small-vessel vasculitis that occurs at an annual incidence of one case per million population [6]. Co-presentation with ANCA and anti-GBM antibodies represents double seropositive vasculitis, which accounted for up to 50% of patients presenting with anti-GBM in one cohort [7]. Outcomes in double seropositive vasculitis are variable and represent a mixture between the clinical courses of both vasculitides. Here, we report a novel case of double seropositive vasculitis following seasonal influenza vaccination, which to the best of our knowledge, has never been reported.
CASE REPORT
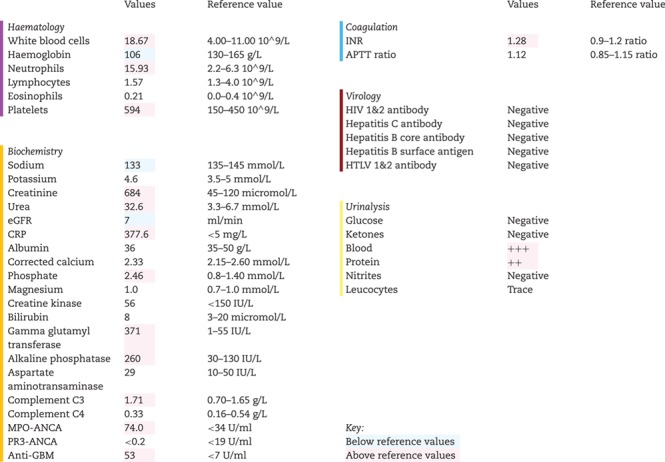
A 72-year-old Caucasian male retired engineer, with no significant medical or surgical history, presented with a 4-week history of general malaise, myalgia and fevers. Four weeks before presentation, he received the inactivated influenza vaccination in primary care. Four days following vaccination, he became generally unwell and visited his general practitioner on two occasions over 14 days. He was initially prescribed a co-amoxiclav course for a presumed lower respiratory tract infection, followed by a course of doxycycline. There was no history of haemoptysis but he did develop visible haematuria. He had no regular medications, no family history and was an otherwise well ex-smoker (50 pack/years) who played golf regularly with no recent foreign travel. Due to persistent symptoms, he underwent blood tests that revealed a creatinine of 684 micromol/L, which prompted referral to secondary care.
On presentation to hospital, his physical examination was normal and his initial investigations are shown in Table 1. Briefly, these showed raised inflammatory markers, a positive urine dip (Blood 3+, protein 2+) and stage three acute kidney injury (AKI) with a Birmingham Vasculitis Activity Score of 14. An urgent ultrasound revealed normal sized kidneys without evidence of obstruction. However, it did show a large abdominal aortic aneurysm (AAA). He subsequently underwent computed tomography aortogram showing a seven-centimetre infra-renal AAA with no evidence of leak. He was admitted to the renal ward where his renal function continued to deteriorate with a peak creatinine of 768 micromol/L. Urgent immunological tests showed ANCA positive with MPO antibodies of 74 U/ml and anti-GBM positive with antibodies of 53 U/mL. He was initiated on haemodialysis via temporary vascath and was given high-dose intravenous methylprednisolone (500 mg) for two consecutive days. Concurrently, he underwent an ultrasound-guided renal biopsy consistent with double seropositive vasculitis (Fig. 1). Three days following presentation, he started seven rounds of plasma exchange over a 10-day period and was inducted with oral cyclophosphamide (100 mg) before starting ‘CYCLOPS’, which is the induction/consolidation regimen using pulsed cyclophosphamide for AAV.
Table 1.
Results summary of the investigations performed on the patient in secondary care
|
|
Figure 1.
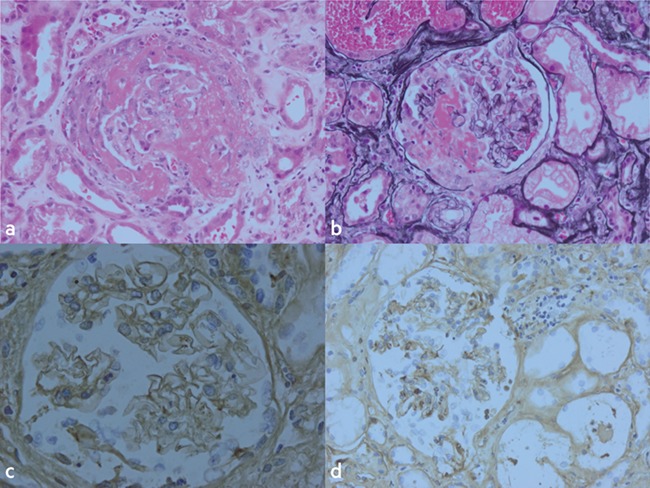
Histopathology images of the renal biopsy specimen. (a) Haemotoxylin and Eosin. (b) Periodic acid silver methenamine. (c and d) Immunperoxidase. All images show a glomerular cellular crescent with fibrinoid necrosis. Acute tubular injury best evidenced in (a) with red cell casts seen in background tubules of (b). Glomerular capillary linear IgG in (c) showing evidence of anti-GBM disease and glomerular capillary linear C3 shown in (d).
He remained dialysis-dependent during admission and received a tunnelled line to continue intermittent dialysis on discharge. An early multidisciplinary decision was made to hold off intervention for the AAA until completion of treatment for vasculitis. He was successfully discharged and continued to receive cyclophosphamide as an outpatient, showing good renal recovery following treatment (creatinine 321 micromol/L). Nine months following the acute episode, he successfully underwent an elective three-vessel fenestrated endovascular aortic repair without need for renal replacement therapy.
DISCUSSION
In this report, we described a patient who developed double seropositive vasculitis within four weeks of influenza vaccine administration. Previous reports describe development of AAV in association with the influenza vaccine, but to the best of our knowledge, this is the first reported case associated with double seropositive vasculitis.
Multiple reports document the development of vasculitis following administration of the influenza vaccine but a causal relationship has yet to be concluded [1–3]. The aetiology of post-influenza vaccination vasculitis is currently unknown, although several hypotheses have been postulated. The influenza vaccine contains inactivated preparations of immunogenic antigens representative of influenza viruses. Therefore, it has been suggested that molecular mimicry, whereby a foreign antigen shares structural similarities with self-antigens, could be implicated in development of this autoimmune phenomena [8]. Other hypotheses focus on vaccine adjuvants and how they may lead to post-vaccination adverse reactions known as ‘autoimmune syndrome induced by adjuvants’ [9]. Like post-influenza vaccination vasculitis, the aetiology of AAV remains unclear with many postulating roles for infectious, genetic and/or environmental factors [10].
In double seropositive vasculitis, the clinical course is variable and it has been suggested outcomes reflect the severity of the presentation rather than actual diagnosis. It has been postulated that the predominant older age of onset, longer prodromal illness and high propensity to renal recovery suggests ANCA-mediated glomerular inflammation may precede, and be the driver for, development of anti-GBM disease [7]. Therefore, we hypothesize that administration of the influenza vaccine in our case may have caused an immunological response that stimulated an inflammatory process leading to double seropositive vasculitis and AKI. Alternatively, the older age of onset and more prolonged prodromal illness could represent prior ANCA-mediated glomerular inflammation with subsequent superimposed anti-GBM disease with vaccination a triggering event. Regarding the AAA, the most likely explanation is a pre-existing aneurysm in a patient with cardiovascular risk factors. However, we cannot exclude accelerated aneurysmal growth as a sequela of the pro-inflammatory response following vaccination.
Ultimately, the temporal association of influenza vaccination with double seropositive vasculitis may suggest a trigger for development of vasculitis in susceptible individuals. Further studies are needed to characterize and confirm the mechanism of post-influenza vasculitis. Clinicians should be drawn to the rare possibility of vasculitis as a cause of unexplained symptom complexes following influenza vaccination.
ACKNOWLEDGEMENTS
None to declare.
CONFLICT OF INTEREST STATEMENT
No conflicts of interest.
FUNDING
No sources of funding.
ETHICAL APPROVAL
No ethical approval required.
CONSENT
Full informed written consent was gained from the patient in this case using the Oxford University Press Patient Consent Form provided.
GUARANTOR
Benjamin Norton is nominated as the guarantor of the manuscript.
REFERENCES
- 1. Bonetto C, Trotta F, Felicetti P, Alarcon GS, Santuccio C, Bachtiar NS, et al. Vasculitis as an adverse event following immunization—systematic literature review. Vaccine 2016;34:6641–6651. [DOI] [PubMed] [Google Scholar]
- 2. Duggal T, Segal P, Shah M, Carter-Monroe N, Manoharan P, Geetha D. Antineutrophil cytoplasmic antibody vasculitis associated with influenza vaccination. Am J Nephrol 2013;38:174–178. [DOI] [PubMed] [Google Scholar]
- 3. Eindhoven S, Levels J, Huisman M, Ruizeveld de Winter K, Dalm V, Alwani R. MPO-ANCA associated vasculitis with mononeuritis multiplex following influenza vaccination. Allergy Asthma Clin Immunol 2017;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill consensus conference Nomenclature of Vasculitides. Arthritis Rheum 2013;65:1–11. [DOI] [PubMed] [Google Scholar]
- 5. Watts RA, Mahr A, Mohammad AJ, Gatenby P, Basu N, Flores-Suarez LF. Classification, epidemiology and clinical subgrouping of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Nephrol Dial Transplant 2015;30:i14–i22. [DOI] [PubMed] [Google Scholar]
- 6. McAdoo SP, Pusey CD. Anti-glomerular basement membrane disease. Clin J Am Soc Nephrol 2017;12:1162–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McAdoo S, Tanna A, Hrušková Z, Holm L, Weiner M, Arulkumaran N, et al. Patients double-seropositive for ANCA and anti-GBM antibodies have varied renal survival, frequency of relapse, and outcomes compared to single-seropositive patients. Kidney Int 2017;92:693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salemi S, D’Amelio R. Could autoimmunity be induced by vaccination? Int Rev Immunol 2010;29:247–269. [DOI] [PubMed] [Google Scholar]
- 9. Pellegrino P, Clementi E, Radice S. On vaccine’s adjuvants and autoimmunity: current evidence and future perspectives. Autoimmun Rev 2015;14:880–888. [DOI] [PubMed] [Google Scholar]
- 10. de Lind van Wijngaarden RA, van Rijn L, Hagen EC, Watts RA, Gregorini G, Tervaert JW, et al. Hypotheses on the etiology of antineutrophil cytoplasmic autoantibody associated vasculitis: the cause is hidden, but the result is known. Clin J Am Soc Nephrol 2008;3:237–252. [DOI] [PubMed] [Google Scholar]