Abstract
Objectives:
HIV-infected persons with chronic herpesvirus infections may experience paradoxical worsening after initiation of antiretroviral therapy (ART), but the impact of longer term ART is unclear. We evaluated the relationships between genital herpes simplex virus (HSV) shedding and ART initiation and time on therapy in HIV and HSV-2 infected persons.
Design:
Prospective observational study.
Methods:
Rates of HSV shedding in 45 HIV and HSV-2 infected persons on or off ART were prospectively followed over up to three, non-contiguous, 60-day periods, during which participants performed daily genital swabs for HSV detection by real-time HSV DNA PCR and reported symptoms. Initiation or discontinuation of ART was at the discretion of participants’ healthcare providers.
Results:
6,425 daily genital swabs were obtained from 45 persons (38 men and 7 women) during 105 swabbing sessions. During the three sessions, 67%, 74%, and 92% of persons were on ART. HSV was detected on 26.5% of days in men and 22.3% of days in women. The overall rates of genital HSV shedding were 19.4% of days in persons not on ART, 30.2% in persons within 90 days of ART initiation, and 23.3% in persons on ART for longer than 90 days. After initiation of ART, HSV shedding decreased by 2% per month, or 23% per year (RR 0.98 /month on ART, p=0.0003 in adjusted analysis). This finding was consistent after including consideration of HIV viral load and CD4 count.
Conclusions:
HSV shedding increased significantly shortly after ART initiation but decreased with time on prolonged ART.
Keywords [MESH]: HIV, Immune reconstitution, Opportunistic infection, Antiretroviral therapy, Herpesvirus, Sexually transmitted diseases
Summary:
In a prospective study of genital HSV shedding in HIV and HSV-2 infected persons, the rate of HSV-2 shedding was highest within 90 days of initiation of ART, then decreased by 2% for every month on ART. Shedding rate was higher in untreated persons with higher plasma HIV RNA.
INTRODUCTION
Antiretroviral therapy (ART) leads to effective immune recovery with dramatic improvement in overall life expectancy of patients with HIV/AIDS [1–3]. As plasma HIV RNA drops at initiation of ART [4], immune reconstitution inflammatory syndrome (IRIS) may arise in response to subclinical or occult infections [5,6] causing substantial morbidity [7–9]. Herpesviruses, including HSV as well as CMV, VZV, and KSV, are well-recognized causes of IRIS [10–13]. Clinical manifestations of IRIS associated with herpesviruses can include flares of CMV retinitis or enterocolitis [14–16], varicella zoster [11], or unmasking or paradoxical worsening of Kaposi’s sarcoma [17]. The new appearance or increased frequency of herpetic genital ulcers are among the most common syndromes reported during immune reconstitution [18–22]; most of these are caused by HSV-2.
While the mechanism underlying herpesvirus-related IRIS is unknown [23,24], it may involve an increase in viral reactivation and shedding, implying a loss of control of viral replication. To define these complex host-pathogen interactions more precisely in newly-treated HIV-infected patients, we evaluated the virologic and clinical aspects of genital HSV in the setting of ART initiation.
METHODS
Study Participants and Procedures
From 1998 to 2003 several research studies were conducted at the University of Washington Virology Research Clinic in Seattle to define genital HSV reactivation in HIV-infected and uninfected men and women. For this analysis, we selected all participants who met the following entry criteria: 1. HIV-1 seropositive on or off of ART, 2. HSV-2 seropositive, 3. participated 1 or more times in a genital HSV shedding study between September 1998 and June 2003. ART was prescribed by primary care providers and was defined to be at least 3 drugs in 2 of the following categories: protease inhibitor, nucleoside analogue, and nonnucleoside analogue. Standardized demographic and medical history information was obtained at the initial visit, and medical history was updated at each visit. The protocol was approved by the Institutional Review Board of the University of Washington and written informed consent was obtained from all participants.
To evaluate the frequency of HSV reactivation, participants used Dacron swabs to obtain daily samples of genital secretions for at least 45 consecutive days. Up to three separate sessions of intensive daily home collection were separated by intervals of approximately one year [19,25,26]. Women collected a swab specimen from the cervicovaginal, vulvar, and perianal areas. Men collected a swab specimen from the penile skin and perianal area. All participants kept symptom diaries during periods of swab collection. Episodic treatment for symptomatic herpes recurrences was provided but suppressive therapy was not permitted during the study.
Laboratory Methods
HSV-1 and HSV-2 serostatus were assessed by University of Washington HSV Western blot [27]. Detection of HSV in specimens from mucosal sites was performed by quantitative, real-time, fluorescence-based PCR, as described previously [25,26]. HIV-1 infection was confirmed by commercial EIA and Western blot. Plasma HIV RNA was measured by use of the Roche Amplicor assay (detection level, 400 HIV-1 RNA copies/mL) and for those samples which were negative, the ultrasensitive assay (detection level, 50 HIV-1 RNA copies/mL; Roche Diagnostic Systems). CD4+ cell count (cells/mL) was assessed by flow cytometry. The laboratories performing these assays were certified by the College of American Pathologists and the National Institute of Allergy and Infectious Diseases (NIAID) AIDS Clinical Trial Group (ACTG).
Statistical Analysis
Shedding rate for each person and swabbing session was calculated as the number of days on which HSV was detected from a genital site in relation to the total number of days on which a swab was obtained. Swabs were considered to be positive if they contained ≥3 copies HSV DNA/PCR reaction (20 μL), or 150 copies HSV DNA/mL [26]. Samples obtained on days when anti-HSV therapy was used for symptomatic recurrences of HSV were not excluded from these calculations, as HSV was often still detectable in these samples [28]. Shedding episodes were defined as any contiguous period of shedding beginning and ending with two consecutive negative swabs; those deemed to be of uncertain duration were those that were ongoing at the start or end of an observation period. ART use was categorized as 1) no ART, 2) ART initiated within fewer than 90 days, and 3) ART use for more than 90 days at the start of the swabbing period. CD4+ cell count and plasma HIV RNA within 60 days of the start of each period of genital swab collection were included in analyses.
The potential predictors assessed for association with genital HSV shedding included age, gender, race, HSV-1 coinfection status, time since HSV acquisition, whether HSV was acquired symptomatically, plasma HIV RNA, use of ART, and CD4+ cell count. Generalized estimating equations (GEE) models with a Poisson link were used to assess rate ratios for HSV shedding; this model accounts for the correlation between multiple sampling sessions on the same participants and makes no model-based assumptions about variance. We simultaneously included current ART with time on ART, setting time on ART to zero for untreated persons. Plasma HIV RNA was log10 transformed and centered at the mean value of 1000, or 3 logs. Plausible interactions were examined: between ART use and each of plasma HIV RNA and CD4+ cell count. A final model was selected using backward elimination. Starting variables included those significant in univariate analysis at p<0.10 and biologically plausible 2-way interactions.
Differences in shedding episode duration and in HSV DNA copies/mL detected on positive days were examined by ART history using GEE with a linear link, including up to 5 episodes per session. All analyses were performed using SAS 9.4 (SAS institute) and statistical significance was defined as a two-sided p value ≤0.05.
RESULTS
We studied 45 HIV and HSV-2 infected persons (38 men and 7 women); 15 with HSV-2 antibodies only and 30 with HSV-1 and HSV-2 antibodies (Table 1). The median age was 41 (range, 20 to 64 years); 35 were white, 4 were African-American and 6 were of other racial background. At study entry, the median plasma HIV RNA was 994 copies/mL (range, <50 to 4,087,019 copies/mL), and median CD4+ cell count was 301 cells/mL (range, 1 to 1,064 cells/mL). Thirty persons (67%) were taking ART at the onset of the study. The median plasma HIV RNA among these 30 treated persons was 201 copies/mL, and among the 15 untreated persons the median plasma HIV RNA was 21,312 copies/mL. All 45 persons completed one sampling session, 34 (76%) completed two sessions, and 26 (58%) completed three sessions. The median interval between the first two sessions was 321 days (range, 66–857 days); and between the second and third sessions, 310 days (range, 230–553 days). Ten persons initiated ART during the course of the study, including six who initiated ART within 90 days of an HSV swabbing session. Sixty-seven percent of individuals were on ART during the first session, 74% during the second, and 92% during the third.
Table 1.
Demographic and clinical characteristics of 45 HIV-1 & HSV-2-seropositive persons.
| Session 1 N=45 (%) |
Session 2 N=34 (%) |
Session 3 N=26 (%) |
|
|---|---|---|---|
| Age, median (range) | 41 (20–64) | 41 (29–64) | 42 (29–64) |
| White Race | 35 (78) | 25 (74) | 20 (77) |
| Men | 38 (84) | 29 (85) | 23 (88) |
| Men who have sex with men | 37 (82) | 28 (82) | 22 (85) |
| Prior history of genital herpesa | 38 (84) | 30 (88) | 22 (85) |
| Serostatus | |||
| HSV-1 & HSV-2 | 30 (67) | 26 (76) | 21 (81) |
| HSV-2 only | 15 (33) | 8 (23) | 5 (19) |
| Days with swabs, median (range) | 62 (34–176) | 62.5 (36–69) | 63 (30–79) |
| Days with valid diary, median (range)b | 63 (0–176) | 66 (0–93) | 66 (0–84) |
| Persons on ART | 30 (67) | 26 (76) | 24 (92) |
| CD4+ count (cells/mm3)c | |||
| ≤200 | 12 (27) | 13 (38) | 6 (23) |
| 201 – 350 | 16 (36) | 7 (21) | 8 (31) |
| >350 | 17 (38) | 14 (41) | 12 (46) |
| HIV RNA (copies/mL)c | |||
| undetectabled | 11 (24) | 8 (23) | 7 (27) |
| 51 – 1,000 | 12 (27) | 8 (23) | 5 (19) |
| 1,001 – 10,000 | 7 (16) | 9 (26) | 8 (31) |
| >10,000 | 15 (33) | 9 (26) | 6 (23) |
as determined by self-report
one person in each session did not provide diary information
at time closest to onset of observation session (within 60 days)
<50 copies/mL
Rates of genital HSV shedding measured by PCR
HSV DNA was detected at least once in genital mucosa in 88.9% of persons in the first session, 88.2% in the second, and 77.9% in the third session. In the first session, overall genital shedding rate was 22.2%, (per person range, 0 to 84.7% of days); in the second, 13.9% (person range, 0 to 95.5% of days); and in the third session, 14.3% (per person range, 0 to 77.7% of days).
The total number of genital samples collected was 6,425 (5,531 from men and 894 from women). The total number of days with available diary information was 7,164 (6,114 from men and 1,050 from women). HSV was detected in the genital tract on 1,235 days (22.3%) in men and on 237 days (26.5%) in women. Of 1,376 days with genital HSV detection and a valid diary, only 19.5% were associated with symptomatic lesions.
Factors associated with HSV shedding
We examined the genital HSV shedding rates by severity of HIV disease. Among 18 participants with CD4+ cell count <200 cells/mL, HSV was detected on 35.3% of days, versus 17.2% of days among 39 participants with CD4+ cell count >200 cells/mL. Seventeen participants with plasma HIV RNA less than 50 copies/mL had HSV DNA detected on 20.0% of days, versus 39 participants with detectable plasma HIV RNA had HSV DNA detected on 23.8% of days.
To analyze the relationship between ART and HSV shedding, we compared HSV shedding rates in untreated persons to persons starting ART within 90 days of the swabbing session (recent ART), and persons on ART for more than 90 days (established ART) (Table 2). HSV shedding occurred on 19.4% of days in untreated persons, 30.2% of days in persons with recent ART, and 23.3% of days in persons on established ART. We also examined frequency of days with genital lesions with longer term ART use. Genital lesions were reported on 11.0% of days for untreated persons, 11.3% of days in persons with recent ART and 5.5% of days in persons with established ART.
Table 2.
Genital HSV total and subclinical shedding rates, median HSV DNA detected, and days with lesions by ART use.
| ART status | N (# sessions) | Total (%) | Median HSV DNA (log10 copies/mL) | Subclinical (%) | Days with reported lesions (%) |
|---|---|---|---|---|---|
|
Not on
ART |
25 | 298/1538 (19.4%) | 5.02 | 208/1360 (15.3%) | 193/1749 (11.0%) |
|
ART initiated
<90 days |
6 | 155/512 (30.2%) | 5.84 | 115/454 (25.3%) | 61/540 (11.3%) |
| ART >3 months | 74 | 1019/4375 (23.3%) | 4.81 | 880/4149 (21.2%) | 256/4679 (5.5%) |
The median HSV DNA copy number detected (irrespective of symptoms) was highest in samples from persons on recent ART (5.84 log10 copies/mL) compared to persons on established ART (4.81 log10 copies/mL) or those off ART (5.02 log10 copies/mL). In regression analysis, the quantity of HSV DNA detected (log10 copies/mL) was 0.95 logs higher for those within 3 months of initiating HAART versus those not on HAART (p=0.018) but not significantly different for those on established HAART versus untreated (difference 0.27 logs higher, p=0.52).
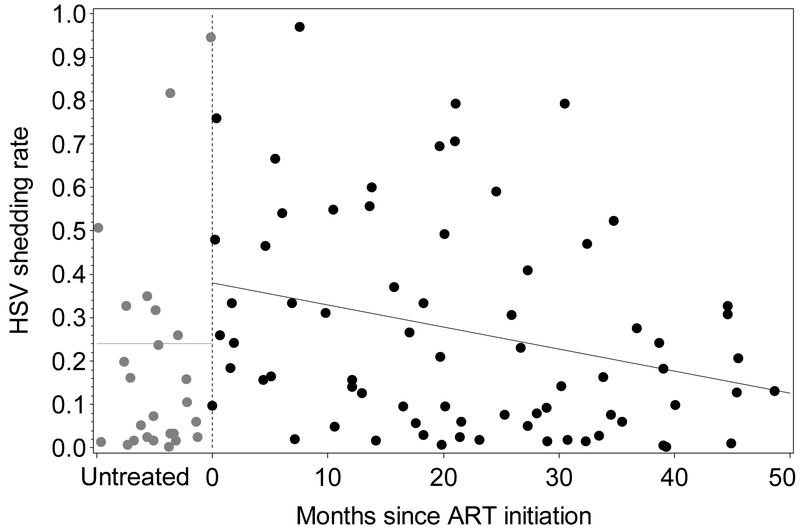
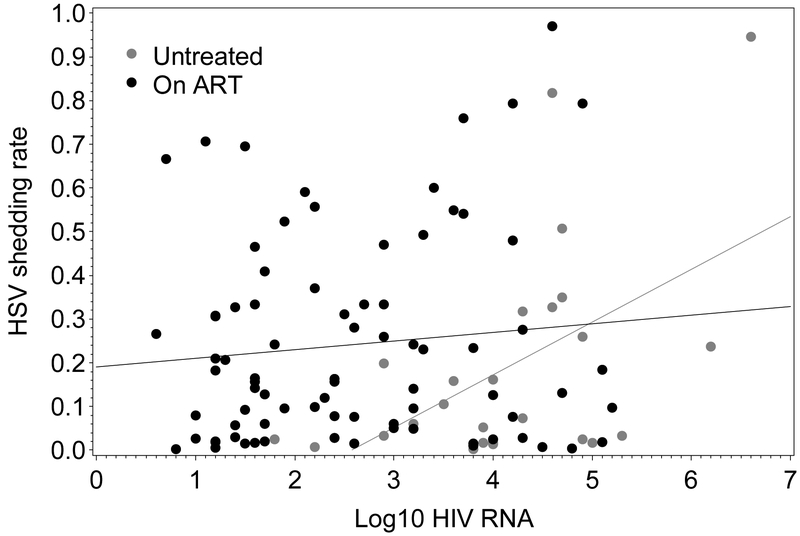
In univariate analyses, HSV shedding frequency was higher among those who had CD4+ cell count <200 cells/mL in the previous 6 months (RR=1.91, p<0.0001). HSV shedding frequency was higher in persons with recent ART (RR=1.59, p=0.050), then lower by 2% on a relative scale, for each subsequent month on treatment (RR=0.98, p<0.0001) (Table 3). Similarly, in multivariate analysis, HSV shedding was 2.87 times higher (for those with average plasma HIV RNA copies/mL, p<0.0001) immediately following ART initiation and decreasing thereafter (RR=0.98, p=0.0003) (Figure 1). In untreated persons, log10 plasma HIV RNA was directly associated with higher HSV shedding frequencies (RR=1.59 for each 1 log10 increase in RNA, p<0.0001) but no such relationship was observed for treated persons (Figure 2).
Table 3.
Univariate and multivariate analyses of factors associated with frequency of HSV DNA detection by PCR.
| Factor | Observed HSV shedding rate (N) |
Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
| Risk ratio (95% CI) | p-value | Risk ratio (95% CI) | p-value | |||
| Sex | 26.5% (894) 22.3% (5531) |
(ref) 0.86 (0.49, 1.51) |
0.60 | - | ||
| Race | 23.2% (4795) 22.0% (1630) |
(ref) 0.86 (0.38, 1.93) |
0.71 | - | ||
| Age (/year) | - | 0.98 (0.95, 1.01) | 0.22 | - | ||
| HSV2 acquisition | 15.7% (1041) 24.3% (5384) |
(ref) 1.99 (0.75, 5.24) |
0.17 | - | ||
| Time since acquisitiona (/5 years) | - | 0.92 (0.72, 1.19) | 0.54 | - | ||
| Serostatus | 24.4% (1616) 22.4% (4809) |
(ref) 0.75 (0.41, 1.37) |
0.35 | - | ||
| CD4 cell nadir in past 6 months | 16.2% (3866) 33.1% (2559) |
(ref) 1.91 (1.41, 2.58) |
<0.0001 | (ref) 1.52 (1.15, 2.01) |
0.0033 | |
| ART useb | 19.4% (1538) 24.0% (4887) |
(ref) 1.59 (1.00, 2.52) |
0.050 | (ref) 2.87 (1.79, 4.62) |
<0.0001 | |
| Time since ART initiationb (/month) | - | 0.98 (0.97, 0.99) | <0.0001 | 0.98 (0.97, 0.99) | 0.0003 | |
| Log10 HIV RNAc | - | 1.06 (0.93, 1.20) | 0.37 | 1.59 (1.36, 1.85) | <0.0001 | |
| Log10 HIV RNA*ART used | - | - | 0.58 (0.47, 0.72) | <0.0001 | ||
Risk ratio for each additional 5 years since time of acquisition, if known
These two terms were evaluated jointly in a single univariate model, in which months since ART initiation was set to zero for those not on ART. As such, the interpretation of the risk ratio for ‘on ART’ is the increase in HSV detection rate between those not on ART and those who have just initiated it (months = 0)
Risk ratio for each log10 increase in HIV RNA
The significant interaction term for log10 HIV RNA
ART use means simultaneously that a) HIV RNA is not predictive of HSV detection frequency in those on ART and b) that the difference in HSV detection frequency between those off versus on ART is larger for those at lower levels of HIV RNA.
Figure 1.
Genital HSV shedding rate and ART status by months on therapy. Those not on ART are positioned arbitrarily to the left of zero (grey circles). From a univariate regression model, the predicted shedding rate for those off ART was 24% (95% CI 15–39%), for those just initiating ART was 38% (95% CI 28–53%), and for those on ART 50 months was 13% (95% CI 8–19%). The rate of HSV shedding among persons on established ART decreased, in a relative sense, by 2% for each subsequent month on treatment (or 23% over each year of use).
Figure 2.
The association between HSV shedding rate and HIV viral load (log10 copies/mL) depends on the presence of ART.
Duration of HSV shedding episodes
A total of 308 viral shedding episodes occurred among 42 of 45 study participants, with 67% of them having certain duration (starting and ending with two negative daily swabs) and the remaining lasting potentially longer than observed; the first 291 were retained to allow no more than 5 episodes per session. Fifteen persons had 1 shedding episode, 18 had 2, 16 had 3, 13 had 4, and 28 had 5 or more. Fifty episodes occurring while persons were not on ART lasted an average of 6.1 days. The duration of shedding episodes was associated with ART use: within 90 days of initiating ART, episodes lasted an average of 7.1 days (n=23), versus 4.8 days (n=218) for those with longer use of ART (p=0.0053). Duration of episodes during established ART did not differ from untreated (p=0.30). The peak HSV copy number detected per episode was also highest in those with recent ART initiation: peak HSV DNA was 0.82 logs higher for those with recent ART versus those on established ART (p=0.002) but was not different between untreated versus those on established ART (difference 0.58 logs, p=0.13).
Factors associated with genital lesions
In univariate analysis, lesion frequency was not found to change immediately after ARV initiation (RR=0.79, 95% CI 0.39 to 1.60, p=0.51). However, consistent with patterns observed with HSV shedding frequency, genital lesions were predicted to occur only half as often after 50 months on ARVs (4.2%) versus at initiation (9.1%): the frequency was lower by 2% on a relative scale for each month on treatment (RR=0.98, p=0.044). After adjustment for plasma HIV viral load, lesion frequency was 2.53 times higher (95% CI 0.99 to 6.48; p=0.053) immediately following ART initiation and was marginally associated with subsequent monthly decrease (RR=0.98, p=0.064).
DISCUSSION
Our data show the following: 1) HSV genital shedding rates are highest, 2) quantities of detected HSV DNA are highest, and 3) HSV shedding episodes are longer in persons within the first 90 days of ART initiation relative to persons who have not initiated therapy and persons on established therapy. This provides additional support to previous clinical observations of herpetic genital ulcers as a manifestation of HIV-related immune reconstitution inflammatory syndrome [19,22,29] and suggests that the mechanism of reactivation is loss of immune control and increased epithelial viral replication. This is consistent with most studies of HSV-2 shedding in the post-ART period [19,21], as well as one study of vaginal CMV shedding and another of oral herpesvirus shedding [30,31]. Of note, no lesions were reported on most (80.5%) of the days with shedding; this as well as a transient increase in shedding rate and copy number indicates a potential increase in the risk of HSV-2 transmission to sexual partners [32,33], especially soon after initiation of ART.
In our study, among persons on established ART the rates of HSV shedding and lesions declined by about 2% a month. In previous investigations of the impact of long-term ART on HSV shedding, many studies have not shown a decrease in HSV-2 shedding or disease with ART [21,22,34], though in two there was a non-significant decrease in shedding or genital ulcers [19,29]. Many of these studies were limited by a short duration of follow up after ART initiation. Furthermore, as HSV shedding is highly variable between individuals, protocols that include prolonged periods of daily swabs may be more informative [35–37]. Tan et al. [34] sought to answer whether ART decreased genital HSV shedding, employed daily sampling, and focused on individuals who have received prolonged ART (median 2.8 years, all virologically suppressed), but found no difference in HSV shedding rates between ART-treated and -untreated persons. However, study eligibility was limited to persons with infrequent HSV symptoms (no symptoms in the last 4 months and no more than 2 outbreaks a year), so it is possible that the impact of ART on HSV shedding rate may be different in this population.
The important strengths of this study are the longitudinal sampling of genital secretions over a broad range of ART experience and plasma HIV RNA, and the use of daily genital swabs to minimize the inherent stochasticity of HSV shedding. We focused on HSV shedding as the primary outcome because it is a sensitive indicator of HSV activity and an effective surrogate marker of HSV disease severity [38,39]. The conclusion that HSV shedding is increased following ARV initiation is further strengthened by the parallel finding of the same phenomenon in lesion frequency.
The mechanism leading to loss of control of HSV reactivation during immune reconstitution deserves further study. An “imbalance” of CD4+ and CD8+ T cells has been described in VZV-related IRIS; a higher number of circulating CD8+ cells is associated with an increased likelihood of VZV reactivation [10]. Why initiation of ART would lead to a loss of function of these CD8+ T cells is unclear, though a higher circulating CD8+ cell count may be a marker of other underlying pathology. Another possible mechanism by which immune reconstitution leads to loss of control of viral replication could be via regulatory CD4+ T cells (Tregs). Recently, Phetsouphan et al. showed by single cell RNA sequencing that the relative proportion of antigen-specific Tregs increased substantially during immune reconstitution [40]. Importantly, Tregs are known to be present in the genital skin response to HSV-2 reactivation, and a higher density in tissue has been correlated with increased HSV-2 DNA detection [41]. An alteration in the balance of T-cell subsets in tissue could lead to an increase in HSV-2 reactivation during immune reconstitution.
Should these findings change clinical management of HSV infections in HIV-infected persons? Current guidelines (aidsinfo.nih.gov) recommend anti-HSV therapy as determined by symptoms, with consideration of chronic suppressive therapy at ART initiation for those with CD4+ cell count <250 cells/mL. While daily suppressive therapy with acyclovir did not reduce the risk of HSV-2 transmission from HIV-infected persons [42], provision of acyclovir at the time of ART initiation does reduce the risk of genital ulcer disease that occurs following ART initiation in HSV-2 infected persons [22]. Our findings also support the utility of long-term ART in reducing the frequency of HSV shedding and symptomatic lesions in HIV-infected persons.
ACKNOWLEDGMENTS:
Funding sources: T32 AI007140, U19 AI11317 (ESF), P01 AI030731 (SS, SK, ASM)
Sources of Support: U19 AI11317 (ESF), P01 AI030731 (SS, SK, ASM, LC, AW)
Disclosures: EF, SS, SK: None
ASM is a consultant for AiCuris and for Immune Design.
CS: Consulting fees: Astellas, Viracor, Gilead, Research grants: ViiV, Pfizer, Gilead
Speaking fees: Astellas, Allergan, Merck, The Medicines Company, Pfizer, Cubist
LC has received research support from Sanofi and Immune Design and is a co-inventor on several patents associated with the development of an HSV-2 vaccine.
AW is a consultant for AiCuris and has received research funding from Genocea and Vical.
REFERENCES:
- 1.Smith CJ, Ryom L, Weber R, Morlat P, Pradier C, Reiss P, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): A multicohort collaboration. Lancet 2014; 384:241–248. [DOI] [PubMed] [Google Scholar]
- 2.Wandeler G, Johnson LF, Egger M. Trends in life expectancy of HIV-positive adults on antiretroviral therapy across the globe: Comparisons with general population. Curr Opin HIV AIDS 2016; 11:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teeraananchai S, Kerr S, Amin J, Ruxrungtham K, Law M. Life expectancy of HIV-positive people after starting combination antiretroviral therapy: A meta-analysis. HIV Med 2017; 18:256–266. [DOI] [PubMed] [Google Scholar]
- 4.Finzi D, Siliciano RF. Viral dynamics in HIV-1 infection. Cell 1998; 93:665–671. [DOI] [PubMed] [Google Scholar]
- 5.Stoll M, Schmidt RE. Immune restoration inflammatory syndromes: The dark side of successful antiretroviral treatment. Curr Infect Dis Rep 2003; 5:266–276. [DOI] [PubMed] [Google Scholar]
- 6.Shelburne SA, Hamill RJ. The immune reconstitution inflammatory syndrome. AIDS Rev 2003; 5:67–79. [PubMed] [Google Scholar]
- 7.Race EM, Adelson-Mitty J, Kriegel GR, Barlam TF, Reimann KA, Letvin NL, et al. Focal mycobacterial lymphadenitis following initiation of protease-inhibitor therapy in patients with advanced HIV-1 disease. Lancet 1998; 351:252–255. [DOI] [PubMed] [Google Scholar]
- 8.Narita M, Ashkin D, Hollender ES, Pitchenek AE. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. Am J Respir Crit Care Med 1998; 158:157–161. [DOI] [PubMed] [Google Scholar]
- 9.Foudraine N, Hovenkamp E, Notermans DW, Meenhorst PL, Klein MR, Lange JM, et al. Immunopathology as a result of highly active antiretroviral therapy in HIV-1-infected patients. AIDS 1999; 13:177–184. [DOI] [PubMed] [Google Scholar]
- 10.Domingo P, Torres OH, Ris J, Vazquez G. Herpes zoster as an immune reconstitution disease after initiation of combination antiretroviral therapy in patients with human immunodeficiency virus type-1 infection. Am J Med 2001; 110:605–9. [DOI] [PubMed] [Google Scholar]
- 11.Dunić I, Djurković-Djaković O, Vesić S, Zerjav S, Jevtović D. Herpes zoster as an immune restoration disease in AIDS patients during therapy including protease inhibitors. Int J STD AIDS 2005; 16:475–8. [DOI] [PubMed] [Google Scholar]
- 12.Schrier RD, Song MK, Smith IL, Karavellas MP, Bartsch DU, Torriani FJ, et al. Intraocular viral and immune pathogenesis of immune recovery uveitis in patients with healed cytomegalovirus retinitis. Retina 2006; 26:165–169. [DOI] [PubMed] [Google Scholar]
- 13.Ratnam I, Chiu C, Kandala NB, Easterbrook PJ, I. R CC, et al. Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1-infected cohort. Clin Infect Dis 2006; 42:418–427. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson MA, Michael Z, Pavan PR, Donnell JJO, Sattler F, Rao N, et al. Cytomegalovirus retinitis after initiation of highly active antiretroviral therapy. Lancet 1997; 349:1443–1445. [DOI] [PubMed] [Google Scholar]
- 15.Faldetta KF, Kattakuzhy S, Wang H-W, Sereti I, Sheikh V. Cytomegalovirus immune reconstitution inflammatory syndrome manifesting as acute appendicitis in an HIV-infected patient. BMC Infect Dis 2014; 14:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutiérrez-Delgado EM, Villanueva-Lozano H, García Rojas-Acosta MJ, Miranda-Maldonado IC, Ramos-Jiménez J. A case report of small bowel perforation secondary to cytomegalovirus-related immune reconstitution inflammatory syndrome in an AIDS patient. Ann Med Surg 2017; 13:20–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Letang E, Almeida JM, Miró JM, Ayala E, White IE, Carrilho C, et al. Predictors of immune reconstitution inflammatory syndrome associated with Kaposi sarcoma in Mozambique: A prospective study. J Acquir Immune Defic Syndr 2010; 53:589–97. [DOI] [PubMed] [Google Scholar]
- 18.Fox PA, Barton SE, Francis N, Youle M, Henderson DC, Pillay D, et al. Chronic erosive herpes simplex virus infection of the penis, a possible immune reconstitution disease. HIV Med 1999; 1:10–8. [DOI] [PubMed] [Google Scholar]
- 19.Posavad CM, Wald A, Kuntz S, Huang M-L, Selke S, Krantz E, et al. Frequent reactivation of herpes simplex virus among HIV-1-infected patients treated with highly active antiretroviral therapy. J Infect Dis 2004; 190:693–696. [DOI] [PubMed] [Google Scholar]
- 20.Couppié P, Sarazin F, Clyti E, Guedj M El, Vaz T, Sainte-Marie D, et al. Increased incidence of genital herpes after HAART initiation: A frequent presentation of immune reconstitution inflammatory syndrome (IRIS) in HIV-infected patients. AIDS Patient Care STDS 2006; 20:143–145. [DOI] [PubMed] [Google Scholar]
- 21.Tobian AAR, Grabowski MK, Serwadda D, Newell K, Ssebbowa P, Franco V, et al. Reactivation of herpes simplex virus type 2 after initiation of antiretroviral therapy. J Infect Dis 2013; 208:839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fife KH, Mugwanya K, Thomas KK, Baeten JM, Celum C, Bukusi E, et al. Transient increase in HSV-2-associated genital ulcers following initiation of antiretroviral therapy in HIV-1/HSV-2 co-infected individuals. J Infect Dis 2016; 213:1573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Espinosa E, Ormsby CE, Vega-Barrientos RS, Ruiz-Cruz M, Moreno-Coutiño G, Peña-Jiménez Á, et al. Risk factors for immune reconstitution inflammatory syndrome under combination antiretroviral therapy can be aetiology-specific. Int J STD AIDS 2010; 21:573–579. [DOI] [PubMed] [Google Scholar]
- 24.Mutnal MB, Schachtele SJ, Hu S, Lokensgard JR. T-cell reconstitution during murine acquired immunodeficiency syndrome (MAIDS) produces neuroinflammation and mortality in animals harboring opportunistic viral brain infection. J Neuroinflammation 2013; 10:863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wald A, Huang M-L, Carrell D, Selke S, Corey L. Polymerase chain reaction for detection of herpes simplex virus (HSV) DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis 2003; 188:1345–51. [DOI] [PubMed] [Google Scholar]
- 26.Magaret AS, Wald A, Huang M-L, Selke S, Corey L. Optimizing PCR positivity criterion for detection of herpes simplex virus DNA on skin and mucosa. J Clin Microbiol 2007; 45:1618–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashley RL, Militoni J, Lee F, Nahmias A, Corey L. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol 1988; 26:662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta R, Wald A, Krantz E, Selke S, Warren T, Vargas-Cortes M, et al. Valacyclovir and acyclovir for suppression of shedding of herpes simplex virus in the genital tract. J Infect Dis 2004; 190:1374–81. [DOI] [PubMed] [Google Scholar]
- 29.Graham SM, Masese L, Gitau R, Mwakangalu D, Jaoko W, Ndinya-Achola J, et al. Increased risk of genital ulcer disease in women during the first month after initiating antiretroviral therapy. J Acquir Immune Defic Syndr 2009; 52:600–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gianella S, Redd AD, Grabowski MK, Tobian AAR, Serwadda D, Newell K, et al. Vaginal cytomegalovirus shedding before and after initiation of antiretroviral therapy in Rakai, Uganda. J Infect Dis 2015; 212:899–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dittmer DP, Tamburro K, Chen H, Lee A, Sanders MK, Wade TA, et al. Oral shedding of herpesviruses in HIV-infected patients with varying degrees of immune status. AIDS 2017; 31:2077–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mertz GJ, Schmidt O, Jourden JL, Guinan ME, Remington ML, Fahnlander A, et al. Frequency of acquisition of first-episode genital infection with herpes simplex virus from symptomatic and asymptomatic source contacts. Sex Transm Dis 1985; 12:33–9. [DOI] [PubMed] [Google Scholar]
- 33.Schiffer JT, Mayer BT, Fong Y, Swan DA, Wald A. Herpes simplex virus-2 transmission probability estimates based on quantity of viral shedding. J R Soc Interface 2014; 11. doi: 10.1098/rsif.2014.0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan DHS, Raboud JM, Kaul R, Walmsley SL. Antiretroviral therapy is not associated with reduced herpes simplex virus shedding in HIV coinfected adults: An observational cohort study. BMJ Open 2014; 4:e004210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schiffer JT, Swan D, Al R, Magaret A, Johnston C, Mark KE, et al. Rapid localized spread and immunologic containment define herpes simplex virus-2 reactivation in the human genital tract. eLIFE 2013; 2:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnston C, Zhu J, Jing L, Laing KJ, McClurkan CM, Klock A, et al. Virologic and immunologic evidence of multifocal genital herpes simplex virus 2 infection. J Virol 2014; 88:4921–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schiffer JT, Wald A, Selke S, Corey L, Magaret A. The kinetics of mucosal herpes simplex virus-2 infection in humans: Evidence for rapid viral-host interactions. J Infect Dis 2011; 204:554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agyemang E, Magaret AS, Selke S, Johnston C, Corey L, Wald A. Herpes simplex virus shedding rate: Surrogate outcome for genital herpes recurrence frequency and lesion rates, and phase 2 clinical trials end point for evaluating efficacy of antivirals. J Infect Dis 2018; :1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agyemang E, Magaret A, Selke S, Johnston C, Corey L, Wald A. Herpes simplex virus shedding: Biomarker for disease severity and response to antivirals. Open Forum Infect Dis 2015; 22:1–66. [Google Scholar]
- 40.Phetsouphanh C, Xu Y, Munier ML, Zaunders JJ, Kelleher AD. Single-cell profiling of lineage determining transcription factors in antigen-specific CD4+ T cells reveals unexpected complexity in recall responses during immune reconstitution. Immunol Cell Biol 2017; 95:640–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milman N, Zhu J, Johnston C, Cheng A, Magaret A, Koelle DM, et al. In situ detection of regulatory T cells in human genital herpes simplex virus type 2 (HSV-2) reactivation and their influence on spontaneous HSV-2 reactivation. J Infect Dis 2016; 214:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mujugira A, Magaret AS, Celum C, Baeten JM, Lingappa JR, Morrow RA, et al. Daily acyclovir to decrease herpes simplex virus type 2 (HSV-2) transmission from HSV-2/HIV-1 coinfected persons: A randomized controlled trial. J Infect Dis 2013; 208:1366–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]