Abstract
Recent research suggests that language processing (LP) may rely heavily on sequential processing (SP), a cognitive ability that allows people to process the patterns of environmental stimuli that unfold in time, such as spoken language or music. Indeed, spoken language corresponds to a set of linguistic units (e.g., phonemes, syllables, words) that are organized in time in a non-random way, according to phonotactic and syntactic rules. In this review, we discuss recent research highlighting the importance of SP for learning and processing such linguistic regularities and argue that interventions focused on improving SP may provide a potentially effective way to rehabilitate language impairments. The first part of this review presents a series of findings showing that LP is intimately related to SP. We review the literature on populations with normal LP performance suggesting that LP relies upon SP. We then report two recent studies from our lab that demonstrated a direct link between LP and SP: (1) a behavioral study showing that variations on a non-linguistic SP task are significantly associated with LP, and (2) an event-related potential study showing that the neural correlates of SP interact with LP abilities in healthy adults. The second part of this review summarizes the literature suggesting that populations with LP impairments (such as language delays due to hearing loss, dyslexia, specific language impairment, and aphasia) also display SP impairments. Thus, disturbances to SP appear to be a commonality among what appears to be very different types of LP impairments, suggesting that impaired SP causes or exacerbates LP impairment. This leads to the third part of this review, where we first summarize recent findings from brain plasticity showing that: (1) cognitive training can improve cognitive processing, and that (2) increasing cognitive processing performance through training can result in a cognitive “transfer” by also increasing performance on other related cognitive skills. We then present a potentially new method for LP remediation that is based on the idea that some LP impairments might stem directly from SP disturbances and that improving SP processing will, via transfer, result in increased LP performance. This method was applied by our research team to conduct a study aimed at improving SP and LP mechanisms. To our knowledge, the SP training study presented here shows the first evidence that SP performance can be improved and therefore has strong clinical implications as a potentially effective and novel intervention to treat LP impairments.
Keywords: Language, Rehabilitation, Training, Sequential Processing, Language Impairment
INTRODUCTION
The longstanding approach to language deficit rehabilitation is to apply traditional communication programs that focus on speech perception, hearing, and auditory skill development (Balkany et al., 2002; Geers, 2006; Hodges et al., 1999). However, the problem is that these research and therapeutic approaches are predicated on the implicit assumption that populations with language deficits suffer solely from speech-related, hearing, or auditory skill development problems and that successful rehabilitation will occur if these skills are improved. Such a narrow focus can have misleading and potentially detrimental consequences by ignoring the larger cognitive and neural context in which language acquisition is embedded.
As an alternative to these standard language rehabilitation techniques, there has been a surge of interest in using computerized training techniques to improve aspects of neurocognitive function. Whereas traditionally the brain was thought to be relatively immutable past a certain age, there is a growing body of evidence suggesting that neural connections remain modifiable even late into adulthood (Kleim & Jones, 2008; van Praag, Kempermann, & Gage, 2000). Recent research has demonstrated that even relatively short-term training can lead to improvements to certain neurocognitive abilities such as working memory (WM) capacity (Klingberg, 2010), with improvements transferring to a host of non-trained tasks of memory and cognition.
Might such computerized training be beneficial for treating language disorders, and if so, which aspect(s) of cognition ought to be targeted? One aspect of neurocognition that is important for language processing (LP) is sequential processing (SP). SP provides sensitivity to patterns in the environment that unfold in time. SP is assumed to occur without attention and hence is often thought to reflect an instance of implicit learning (Perruchet & Pacton, 2006). Performance on SP has been shown to be related to LP performance (Conway & Pisoni, 2008; Gervain & Mehler, 2010; Gogate & Hollich, 2010; Gupta & Dell, 1999; Kuhl, 2004; Reber, 1967; Saffran, 2003; Ullman, 2004). In addition, a number of language and communication disorders, including dyslexia (Howard, Howard, Japikse, & Eden, 2006), specific language impairment (Evans, Saffran, & Robe-Torres, 2009), and language delays caused by a period of deafness early in development (Conway, Pisoni, Anaya, Karpicke, & Henning, 2011), may be due, at least in part, to disturbances to cognitive abilities such as SP and procedural memory (Nicolson & Fawcett, 2007; Ullman & Pierpont, 2005).
Given that SP is important for LP, then we should expect that if SP can be enhanced through some type of training regimen, LP performance would be enhanced too. In this review, we first present recent evidence highlighting the importance of SP for LP in populations both with and without a language or communication disorder. We then describe a study that used a computerized training technique to improve SP with the ultimate goal to provide a new type of language rehabilitation.
1. SEQUENTIAL PROCESSING AND LANGUAGE PROCESSING
Much recent research has suggested that SP is important for LP. For instance, SP is thought to be important for word segmentation (Saffran, Aslin, & Newport, 1996), word learning (Graf Estes, Evans, Alibali, & Saffran, 2007; Mirman, Magnuson, Graf Estes, & Dixon, 2008), and syntax processing (Gomez & Gerkin, 1999; Ullman, 2004).
Surprisingly, despite the voluminous work on SP and the suggestions of its importance for LP, up until recently, no study has shown a direct relation between individual performance on SP and individual performance on LP. Recently, we investigated whether SP would be associated with one particular measure of everyday LP: how well one uses the context of a sentence to process the next upcoming word of this sentence (Conway et al., 2010). The rationale is that the context of a sentence is a sequence of words that are not randomly organized but instead follow a pattern defined by the syntax. It is this syntax together with the meaning of individual words presented within the sentence context that allows the listener to build the global meaning of the sentence. Thus, SP of the syntax in addition to the words of the sentence helps to provide meaning of what is being said, and hence helps to process the upcoming words of the sentence.
For example, consider the following two sentences:
Her entry should win first prize.
The arm is riding on the beach.
The final word in sentence (1) is highly predictable given the global meaning of the preceding context (i.e., the context can help to process the final word of the sentence) while the final word in sentence (2) is not predictable (i.e., the global meaning of the preceding context cannot help to process the final word). Therefore, when these two sentences are presented to participants under degraded listening (i.e., noisy) conditions, long-term knowledge of language syntax (and semantics) can improve the perception of the final word in sentence (1) more so than in (2). We argue then, that performance on the first type of sentence ought to be more closely associated with fundamental SP abilities because it relies on one’s knowledge of syntax that accrued implicitly over many years of exposure to language. On the other hand, performance on the second type of sentence simply relates to how well one perceives speech in noise, where knowledge of syntax, and hence SP, is less useful.
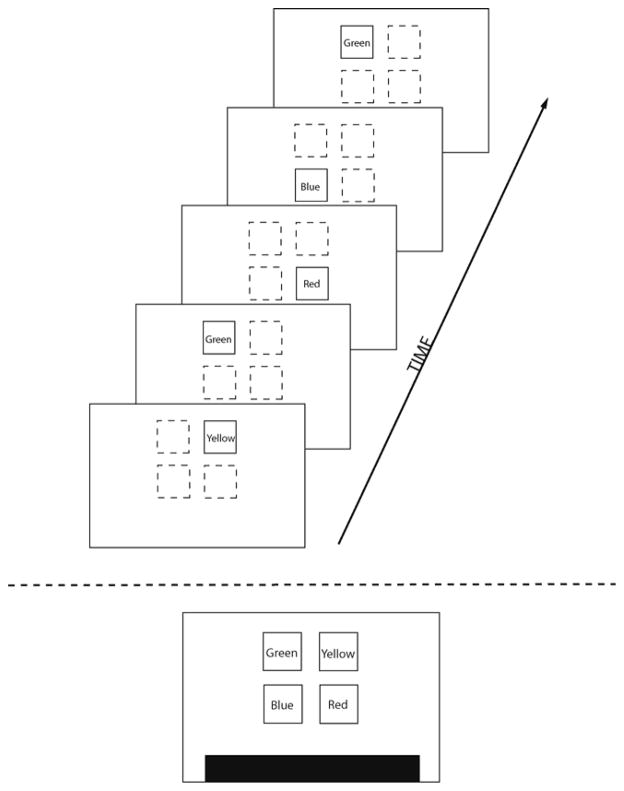
We directly tested this hypothesis by assessing healthy adult participants on both an SP task and this “speech perception task” (i.e., an assessment of LP performance). In the SP task, participants observed and then immediately reproduced visual color sequences that followed an artificial syntax (or set of rules) on a touch-screen monitor (Figure 1). The visual color sequences followed a syntax in which only certain colors (e.g., blue) would ever occur following certain others (e.g., green). SP performance is assessed by improvement at recalling (reproducing) these sequences. The test is separated into two parts: a “learning phase”, where the participant learns the syntax of the sequences, and a “test phase”, where the participant’s recall performance is compared between new sequences that follow the same syntax and new sequences that do not follow a syntax, i.e., are completely random, with any color followed any other random color. SP was assessed by observing improvements to immediate reproduction of the sequences (Botvinick, 2005; Conway et al., 2007; Hebb, 1961; Jamieson & Mewhort, 2005; Karpicke & Pisoni, 2004; Miller & Selfridge, 1950). That is, as participants were exposed to the sequence, if any SP improvement occurred, their immediate reproduction of the sequence (or immediate “serial recall”) should improve for sequences that followed the learned syntax compared to random sequences.
Figure 1.
Depiction of the SP task used in Conway et al., 2010. Participants viewed a sequence of colored squares (each colored square is displayed for 700-msec followed 500-msec later by the presentation of the next colored square) appearing on the computer screen (top). Then, 2000-msec after sequence presentation, participants must attempt to reproduce the sequence by pressing the touch-panels in correct order (bottom). The next sequence occurs 2000-msec following their response.
We measured LP with the above-mentioned speech perception in noise task. In this task, participants had to identify sentences spoken under degraded listening conditions in which half of the sentences ended on a highly predictable word (sentences of type 1) and half ended on a low predictable word (sentences of type 2) (Elliott, 1995; Kalikow et al., 1977). To assess performance, we used the difference score suggested by Bilger and Rabinowitz (1979). This score was calculated by taking the difference between how well one perceives the final word in high-predictability sentences and how well they perceive the final word in low-predictability sentences. This difference score provides a means of assessing how well an individual is able to use sentence context to guide spoken LP.
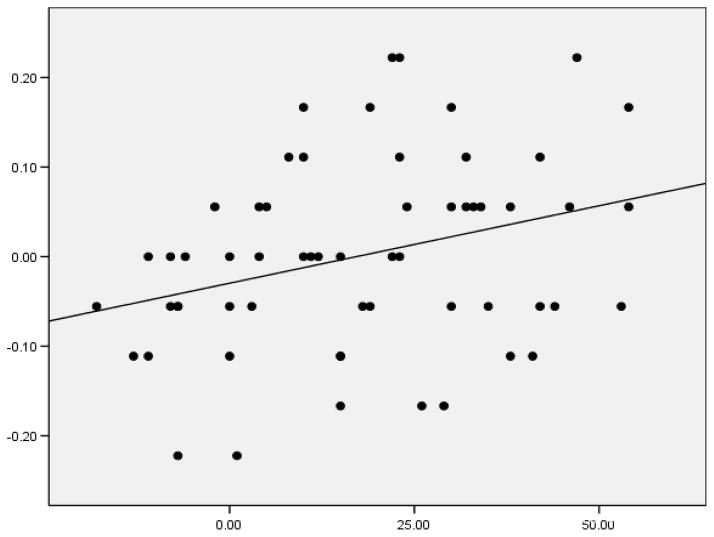
Across three experiments, we found that individual differences in SP were significantly correlated with LP, according to the sentence perception difference score [Pearson r = .308, p < .05, n=59, 2-tailed] (Figure 2). Importantly, the correlation remained even after controlling for sources of variance associated with non-verbal intelligence, verbal short-term memory and WM, attention and inhibition, and knowledge of vocabulary and syntax. We concluded that there exists a direct relation between SP and LP (Conway et al., 2010). Indeed, superior SP would increase the ability to use the syntax of spoken sentences and hence improve LP performance.
Figure 2.
Scatterplot of data from Experiment 3 (n=59) of Conway et al. (2010). The x-axis displays the SP scores; the y-axis displays the LP according to the spoken sentence perception difference scores.
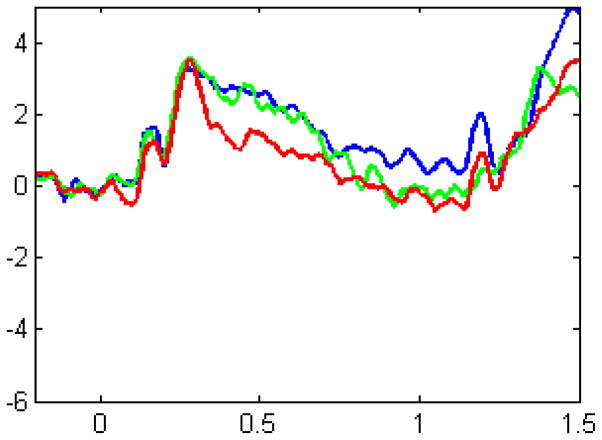
These behavioral data suggest a link between SP and LP, but in order to even more precisely identify whether there are shared neurocognitive mechanisms in SP and LP, neural measures are needed. To this aim, we tested whether a neural correlate of SP, a late latency event-related potential similar to the P300 and P600 components (Jost et al., 2011; also see Christiansen, Conway, & Onnis, 2012) was modulated by several measures of LP, namely: (1) a measure of vocabulary skill, the Peabody Picture Vocabulary Test (PPVT-IV), (2) a measure of sentence comprehension, the Sentence Completion subtest of the Comprehensive Assessment of Spoken Language (CASL), (3) and a measure of syntactic processing skill, the Grammaticality Judgment subtest of the CASL (for a preliminary version of this study, based on the analysis of the first 10 participants, see Daltrozzo et al., 2013). Seventeen adult participants without any LP or other cognitive impairment were presented visual sequences. Instead of colored squared as in the SP task of Figure 1, white abstract shapes were displayed in the center of the screen on a dark background. The presentation of different shapes unfolded in time according to an artificial syntax. The sequences included two types of stimuli: “predictors” and “targets”. A predictor preceded each target with varying probability. These probabilities defined the artificial syntax of the non-random sequences. We replicated the results of Jost et al. (2011), showing a P600 component that was larger as the probability increased, thus indicating that this event-related component is a neuronal correlate of SP (Figure 3).
Figure 3.
Grand average of ERP responses to the SL task in the left parietal region of interest with high probability predictor (blue), low probability predictor (green), and zero probability predictor (red). The y-axis represents ERPs in microvolts (positivity is upwards) and the x-axis represents time in seconds. Responses are recorded from the onset of the predictor to 1.5 seconds following this onset.
Most importantly, we found that this neuronal effect of SP interacted with our three measures of LP: PPVT [750–850ms post-predictor onset: F(4,28)=3.57, p=.018], Sentence Completion [150–250ms post-target onset: F(8,56)=2.94, p=.015], and Grammaticality [400–500ms post-target onset: F(16,112)=2.92, p=.012]. These results confirm that SP and LP are highly related, not only with behavioral measures of SP, but also, at a neurophysiological level.
2. SEQUENTIAL PROCESSING AND LANGUAGE PROCESSING DISORDERS
If SP is important for LP, then we might expect that what initially appear to be language-specific disorders may be due in part to disturbances with SP. There is in fact, a growing body of evidence suggesting that this is indeed the case. Here, we review research examining SP in specific language impairment, aphasia, and dyslexia. Then, we present the results of a study with deaf children with cochlear implants, who also have LP impairment, that supports the theory that SP is a crucial part of typical language acquisition, and if disturbed or developmentally delayed, can impair successful language development (Conway, Pisoni, Anaya, Karpicke, & Henning, 2011).
Recent accumulating evidence has established SP impairments in individuals with various types of language disorders. For example, Plante, Gomez, and Gerken (2002) showed that a group of adults with language and reading impairments had more difficulty with SP tasks (as reflected by reduced improvement of SP to the presentation of sequences following an artificial syntax) than adults without a diagnosed language disorder. As concerns specific language impairment (SLI), recent research indicates that SP may be intact but significantly slower than in normal controls. For example, one study showed that in a standard SP task referred to as “serial reaction time task”, adolescents with and without SLI showed evidence for SP (i.e., reaction times improved over trials), but improvement rates were slower for the SLI group compared to controls (Tomblin, Mainela-Arnold, & Zhang, 2007). Similarly, children diagnosed with SLI showed sufficient learning of an SP task after 42 minutes, whereas controls learned it after only 21 minutes (Evans, Saffran, & Robe-Torres, 2009). Likewise, evidence suggests that aphasia is associated with disturbances to SP (Christiansen, Kelly, Shillcock, & Greenfield, 2010; Goschke et al., 2001).
Regarding reading disorders such as dyslexia, the evidence of SP impairment is mixed and seems to depend on the type of SP task. Studies using the visual serial reaction time task appear to show an absence of SP (Menghini, Hagberg, Caltagirone, Petrosini, & Vicari, 2006; Vicari, Marotta, Menghini, Molinari, & Petrosini, 2003). However, studies using other tasks to assess SP, such as “cued reaction time” (Roodenrys & Dunn, 2008) and other paradigms including artificial syntax (Russeler, Gerth, & Munte, 2006) showed unimpaired SP. Between these two extremes, other studies such as Howard et al. (2006) found impaired SP in dyslexics. Overall, SP might be impaired in dyslexics although the task used to measure SP might be critical in assessing this ability.
A final population that offers an interesting test of the role of SP in LP is deaf children who have received a cochlear implant (CI). A CI is a medical prosthesis surgically implanted into the inner ear of a deaf child in order to provide sound by directly stimulating the auditory nerve. Although a CI provides the potential to develop age-appropriate speech and language abilities, it is well known that some children obtain little language benefit other than the awareness of sound from their implant (American Speech-Language-Hearing Association, 2004). Some of this variation in outcome has been shown to be due to demographic factors, such as age at implantation and length of deafness (Kirk et al., 2002; Tomblin, Barker, & Hubbs, 2007). However, these demographic variables leave a large amount of unexplained variance. It is likely that intrinsic cognitive factors, especially fundamental learning and memory abilities, contribute to language outcomes following implantation (Pisoni, 2000). Disturbances to SP specifically may hold the key to understanding the enormous range of variation in LP performance in this population.
Deaf children with CIs also provide a unique opportunity to study neurocognitive plasticity and neural reorganization following the introduction of sound and spoken language after a period of auditory deprivation. Whereas most previous work with this clinical population has investigated the development of auditory perception, speech perception, and spoken language development following cochlear implantation, relatively few studies have examined other cognitive abilities.
Recently we assessed SP using sequences of visual items (similar to the items of the task described in Figure 1) in a group of deaf children with CIs (Conway et al., 2011). Our aims were twofold: to assess the effects that a period of auditory deprivation (including speech deprivation) and language delay may have on SP; and to investigate the role that SP plays in LP performance following cochlear implantation. Our hypothesis was that deaf children with CIs would show disturbances in SP as a result of their relative lack of experience with (auditory) environmental sequential patterns (including speech, music, and other environmental sounds that are non-randomly organized in time) early on in development. Furthermore, we expected that SP performance would be associated with measures of LP, with post-implanted children showing the best performance on SP, showing also the best LP performance.
A group of deaf children with CIs engaged in a visual SP task similar to the sequence reproduction task used on adult participants (see Figure 1). The results revealed that the CI children, on average, showed no evidence of SP following exposure to (non-random, syntactic) sequences [t(22) = −.77, p = .50, two-tailed], and were significantly worse than an age-matched group of hearing children [t(47) = −2.01, p < .05, two-tailed] (Conway et al., 2011). Furthermore, performance on the SP task was found to be significantly correlated with a standardized measure of language outcome, the Clinical Evaluation of Language Fundamentals, 4th Ed. (CELF-4; Semel, Wiig, & Secord, 2003), which has a particular emphasis on syntax-related language functions [r = .571, p < .05, two-tailed, n=23]. That is, those children who showed the highest levels of SP also showed the best LP performance as measured by the CELF-4. For the most part, these correlations remained significant even after controlling for the shared variance associated with duration of implant use, age at which the device was implanted, forward and backward digit span, and vocabulary scores. In addition, performance on the SP task was associated with LP performance as measured by a spoken sentence comprehension task (Pisoni, Conway, Kronenberger, Henning, & Anaya, 2010) similar to that described in section 1, and hence is consistent with the adult findings (Conway et al., 2010).
Why did these children show a disturbance to SP? There is some indication that a period of auditory deprivation occurring early in development may have secondary cognitive and neural ramifications in addition to the obvious hearing-related effects (Conway, Pisoni, & Kronenberger, 2009). Specifically, because sound is a temporally-organized signal, a lack of experience with sound may affect how well one is able to process patterns of stimuli unfolding non-randomly in time, that is (non-random, syntactic) sequences (Marschark, 2006; Rileigh & Odom, 1972; Todman & Seedhouse, 1994). Exposure to sound may provide a kind of “auditory scaffolding” in which a child gains vital experience and practice of SP from the environment (Conway et al., 2009). We suggest that a lack of experience with sound may delay or alter the development of cognitive processing skills such as SP. Poor SP skills therefore might help explain why this particular population may have impaired LP even after hearing is restored through a CI.
In sum, across a variety of populations having a language or communication disorder, we find that LP impairment is associated with SP impairment. Therefore, if an intervention is conducted to improve SP performance, it might be that the associated LP performance is also improved. Such transfer of cognitive ability from SP to LP may thus provide a key to alleviate LP in populations with language and communication disorders. The purpose of the next section is to present an attempt to develop such a rehabilitation strategy.
3. IMPROVING SEQUENTIAL PROCESSING IN HEALTHY ADULTS
The relationship between SP and LP in both healthy individuals and those with language disorders makes it important to ask whether it is possible to improve LP by enhancing SP. A number of studies have demonstrated the efficacy of using different kinds of cognitive training paradigms to improve aspects of perception, attention, and cognition (Dye, Green, & Bavelier, 2009; Klingberg, 2010; Rueda et al., 2005; Shalev, Tsal, & Mevorach, 2007; Tallal & Gaab, 2006).
To our knowledge, there have been no published attempts to improve SP. However, one cognitive domain that has received much interest in the cognitive training literature is WM. While the training tasks and populations have varied, there is a growing body of evidence suggesting that computerized training tasks can improve WM capacity, and importantly, result in transfer to non-trained tasks of spatial and verbal WM, attention, and other cognitive functions (Curtis and D’Esposito, 2003, Olesen, Westerberg & Klingberg 2004, Holmes, Gathercole, & Dunning 2009, Thorell, Lindqvist, Nutley, Bohlin, & Klingberg 2009, Westerberg, Jacobaeus, Hirvikoski, Clevberger, Ostensson, Bartfai, & Klingberg 2007, Klingberg, Fernell, Olesen, Johnson, Gustafsson, Dahlstrom, Gillgberg, Forssberg, & Westerberg 2005; Verhaeghen et al., 2004).
The findings from these studies suggest that improving WM performance appears to transfer to performance to other non-trained tasks of WM and other cognitive functions. For example, visuospatial WM training transfers to the processing of inhibition (Klingberg et al. 2002; Klingberg et al. 2005, Olesen et al. 2004), to the level of attention (Westerberg et al. 2007), and to verbal WM performance (Holmes et al. 2009; Thorell et al. 2009).
These studies demonstrate the rational of improving cognitive function through computerized training techniques, leaving open the possibility that like WM, SP might also be amenable to training that would also result in a transfer to performance on other cognitive abilities such as LP. As Klingberg (2010) rightfully pointed out, the synaptic mechanisms underlying WM capacity are governed by the same principles of neural plasticity as the rest of the brain. Thus, we might expect that SP can also be enhanced using a similar training and that a similar transfer to other cognitive abilities such as LP can occur.
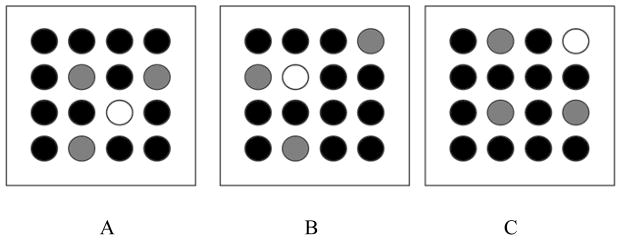
To test this hypothesis, we created a novel computerized visual SP training task and tested it with healthy adults (Bauernschmidt, Conway, & Pisoni, 2009; Smith, Conway, Bauernschmidt, & Pisoni. Improving the ability to learn structure in time: Adaptive training of structured sequence processing and language. Submitted). The training task is a visuo-spatial training procedure that is conceptually similar to other WM training tasks designed to improve WM abilities in adults and children (e.g., Holmes et al., 2009; Thorell et al., 2009). However, in addition to training WM using random sequences, the present training task also trains SP, using non-random sequences that follow an artificial syntax. Thus, the novel facet of our task is that it trains both SP and WM, when non-random (syntactic) sequences are used. Similarly to the SP task described in section 1 (Figure 1), participants are asked to reproduce (i.e., to recall) sequences of visual stimuli that conform to a syntax (i.e., the non-random, syntactic sequences) requiring both SP and WM or to random sequences, requiring only WM (see Figure 4).
Figure 4.
Computerized training task. Participants view a 4 × 4 matrix of circles. A sequence of circles light up, one at a time (the white circle depicted in each of the three scenes A, B, and C). Participants must reproduce (i.e., recall) each sequence in its entirety. When these sequences are random, the task require only WM. When these sequences are non-random (i.e., conform to an artificial syntax), the task require both WM and SP. The syntax is defined as follows: each circle can be followed by only 1 of 3 possible circles (shaded light grey). If the sequence is random, each circle can be followed by any other pseudo-randomly selected circle. Note that in the actual task, all circles are colored the same, except for the one that is currently lit.
In the training task, participants view a sequence of colored lights, occurring one at a time (see Figure 4) and then are required to reproduce what they saw by pressing the circles in correct order on a touch-sensitive monitor. Improvement on SP is evidenced as participants begin to perform better on non-random compared to random sequences, that is, when their performance is facilitated by SP in addition to WM.
Participants engaged in this SP training task for four days (Days 2–5), with each training session lasting about 45 minutes. Crucially, the syntax of the non-random (syntactic) sequences is changed for each participant on each subsequent training day. Because each of the four days of training incorporated a new set of syntactic rules, our intention was that participants would gradually improve their general SP abilities independently of any specific set of syntactic rules.
The key test is whether any such improvements to SP would result in a transfer to LP. To test this transfer, all participants were given a set of pre-training measures on Day 1 that included a measure of initial LP using the speech perception task used in Conway et al. (2010) and described in section 1, and a measure of initial SP using the same SP task also used in Conway et al. (2010) and described in section 1 (Figure 1). These same measures were given again after the SP training was completed, on Day 6, in order to ascertain improvements to LP, and general improvement to SP (i.e., using a non-trained SP task containing a different set of syntactic rules).
Finally, in order to ensure that any observed gains on non-trained tasks were not merely a result of a test-retest effect, participants were randomly assigned to one of two different training conditions. Group 1 engaged in the training of SP plus WM using non-random sequences, while Group 2 engaged in the training of WM only, using random sequences. In sum, any training effects observed in Group 1 but not in Group 2 can be safely regarded as being due to the effect of including non-random sequences in the task, and hence must relate to SP rather than to a test-retest or a WM effect.
Initial results are presented below for 43 adult participants (ages 18–30), with 22 participants in Group 1 and 21 participants in Group 2 (a complete analysis of the results is presented in Smith, Conway, Bauernschmidt, & Pisoni. Improving the ability to learn structure in time: Adaptive training of structured sequence processing and language, submitted). As predicted, the effect of training on SP (according to the difference of performance between pre- and post-training) was larger in Group 1 [M=4.27, SEM=5.38] compared to Group 2 [M=−1.67, SEM=5.32], although this difference did not reach significance [t(41)=.784, p=.438, two-tailed]. Also, according to our prediction, the effect of training on LP (according to the difference of performance between pre- and post-training) was larger in Group 1 [M=4.38, SEM=0.68] compared to Group 2 [M=4.20, SEM=0.82], although this difference did not reach significance [t(39)=.170, p=.866, two-tailed].
These trends suggest that SP and LP may be enhanced by SP training. Although the effect size of the training on SP appears rather large, the effect did not reach significance due to large between-participants variability. In comparison, the effect size of the training on LP is rather small. This result suggests that the expected cognitive transfer from SP to LP may require extended training or changes to the way in which LP gains are measured. For instance, it may be beneficial to measure LP after more than one night of sleep memory consolidation (Peigneux et al., 2001). Indeed, sleep consolidation research indicates that memories, such as those encoded by SP training, are further consolidated during the nights of the week following learning (Peigneux et al., 2003; Rasch & Born, 2013; Stickgold et al., 2000). Hence, measuring the effect of this transfer the day immediately following the last day of training may have underestimated the amount of LP gains. Thus, this study could be fruitfully replicated using a post-training assessment performed one week after the last day of training.
Another possible improvement to this paradigm would be to test whether SP and LP improvement through training have long lasting effects. Indeed, it is important to determine whether these training effects have the potential to alleviate the long-term quality of life of LP impaired populations.
4. CONCLUSION
In agreement with the literature on populations with and without LP impairment, the recent behavioral and neurophysiological findings reported here suggest that LP is intimately related to SP. The behavioral study reports a correlation between the performance on a non-linguistic SP task and LP performance, as measured by a speech perception in noise task. The neurophysiological study found significant interactions between event-related potential effects of SP and three measures of LP performance. Based on the recent literature on brain plasticity showing that cognitive processing can be improved by training and that increasing cognitive processing performance through training can result in a cognitive “transfer” by also increasing performance on other related cognitive skills, we tested whether SP could be amenable to improvement through training and whether the expected increased SP performance would result in a cognitive transfer by also increasing LP performance. We believe that this study was important to attempt to alleviate LP impairments in populations with language and communication disorders.
The computerized training task that we have developed was based conceptually on recent WM training task designs. Our training task is relatively easy to implement, short in duration (45 minutes per day over 4–10 days), and crucially incorporates non-random sequences (i.e. organized according to an artificial syntax). The results with adults showed that training resulted in gains to a non-trained task of SP, indicating a general improvement on SP independently of the specific set of syntactic rules used in the non-random sequences. The SP improvement also transferred to LP, although only moderately, as reflected by a small increased performance on the spoken sentence comprehension task.
Although the findings are encouraging, they need to be replicated by assessing the extent of the transfer in a more optimal way (see section 3). This training task shows promise as a novel intervention for treating various disorders of language and learning, in particular if the transfer effect can be improved and shown to have a long-lasting effect.
In addition to treating LP disorders, it may be possible to use this approach to help improve LP acquisition for individuals learning a second language. As recent research aptly indicates, we are beginning to realize the importance of SP for LP. But we ought not to stop there. Our cognitive and neural systems are far more plastic and modifiable by experience than initially believed. By capitalizing on these theoretical and empirical developments, it may be possible to improve LP by using novel computerized training techniques that specifically target SP, offering great promise for alleviating disorders of language and communication.
Acknowledgments
Preparation of this manuscript was supported by the following grant from the National Institutes of Health: (NIH 1R01DC012037-01A1). An earlier version of this review appeared in: Conway, Gremp, Walk, Bauernschmidt, & Pisoni (2012).
References
- American Speech-Language-Hearing Association. Cochlear implants [Technical report] 2004 Retrieved on October 24, 2013 from www.asha.org/policy.
- Balkany TJ, Hodges AV, Eshraghi AA, Butts S, Bricker K, Lingvai J, Polak M, King J. Cochlear implants in children—A review. Acta Oto-Laryngologica. 2002;122(4):356–362. doi: 10.1080/00016480260000012. [DOI] [PubMed] [Google Scholar]
- Bar M. The proactive brain: Using analogies and associations to generate predictions. Trends in Cognitive Sciences. 2007;11(7):280–289. doi: 10.1016/j.tics.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Bauernschmidt A, Conway CM, Pisoni DB. Research on Spoken Language Processing Progress Report No. 29. Indiana University; 2009. Working memory training and implicit learning. [Google Scholar]
- Bilger RC, Rabinowitz WM. Relationships between high- and low-probability SPIN scores. The Journal of the Acoustical Society of America. 1979;65(S1):S99. [Google Scholar]
- Botvinick MM. Effects of domain-specific knowledge on memory for serial order. Cognition. 2005;97:135–151. doi: 10.1016/j.cognition.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Christiansen MH, Conway CM, Onnis L. Similar neural correlates for language and sequential learning: Evidence from event-related brain potentials. Language and Cognitive Processes. 2012;27:231–256. doi: 10.1080/01690965.2011.606666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen MH, Kelly ML, Shillcock RC, Greenfield K. Impaired artificial grammar learning in agrammatism. Cognition. 2010;116:382–393. doi: 10.1016/j.cognition.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Conway CM, Bauernschmidt A, Huang SS, Pisoni DB. Implicit statistical learning in language processing: Word predictability is the key. Cognition. 2010;114:356–371. doi: 10.1016/j.cognition.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway CM, Gremp MA, Walk AD, Bauernschmidt A, Pisoni DB. Can we enhance domain-general learning abilities to improve language function? In: Rebuschat P, Williams J, editors. Statistical Learning and Language Acquisition. Walter de Gruyter; Boston: 2012. pp. 305–336. [Google Scholar]
- Conway CM, Karpicke J, Pisoni DB. Contribution of implicit sequence learning to spoken language processing: Some preliminary findings with hearing adults. Journal of Deaf Studies and Deaf Education. 2007;12:317–334. doi: 10.1093/deafed/enm019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway CM, Pisoni DB. Neurocognitive basis of implicit learning of sequential structure and its relation to language processing. Annals of New York Academy of Sciences. 2008;1145:113–131. doi: 10.1196/annals.1416.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway CM, Pisoni DB, Anaya EM, Karpicke J, Henning SC. Implicit sequence learning in deaf children with cochlear implants. Developmental Science. 2011;14:69–82. doi: 10.1111/j.1467-7687.2010.00960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway CM, Pisoni DB, Kronenberger WG. The importance of sound for cognitive sequencing abilities. Current Directions in Psychological Science. 2009;18:275–279. doi: 10.1111/j.1467-8721.2009.01651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends in Cognitive Sciences. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Daltrozzo J, Deocampo J, Trapani J, Sims S, Conway CM. Statistical learning is correlated with language performance: An event-related potential study. Annual Meeting of the Psychonomic Society; Toronto, Ontario, Canada. November 14–17; 2013. Abstract. [Google Scholar]
- Dye MWG, Green CS, Bavelier D. Increasing speed of processing with action video games. Current Directions in Psychological Science. 2009;18:321–326. doi: 10.1111/j.1467-8721.2009.01660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott LL. Verbal auditory closure and the speech perception in noise (SPIN) test. Journal of Speech, Language, and Hearing Research. 1995;38(6):1363–1376. doi: 10.1044/jshr.3806.1363. [DOI] [PubMed] [Google Scholar]
- Evans JL, Saffran JR, Robe-Torres K. Statistical learning in children with specific lanugage impairment. Journal of Speech, Language, and Hearing Research. 2009;52:321–335. doi: 10.1044/1092-4388(2009/07-0189). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster J. The prefrontal cortex—An update: Time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Geers AE. Factors influencing spoken language outcomes in children following early cochlear implantation. Advanced Otolaryngology. 2006;64:50–65. doi: 10.1159/000094644. [DOI] [PubMed] [Google Scholar]
- Gervain J, Mehler J. Speech perception and language acquisition in the first year of life. Annual Review of Psychology. 2010;61:191–218. doi: 10.1146/annurev.psych.093008.100408. [DOI] [PubMed] [Google Scholar]
- Gogate LJ, Hollich G. Invariance detection within an interactive system: A perceptual gateway to language development. Psychological Review. 2010;117:496–516. doi: 10.1037/a0019049. [DOI] [PubMed] [Google Scholar]
- Gomez RL, Gerken L. Artificial grammar learning by 1-year-olds leads to specific and abstract knowledge. Cognition. 1999;70:109–135. doi: 10.1016/s0010-0277(99)00003-7. [DOI] [PubMed] [Google Scholar]
- Goschke T, Friederici AD, Kotz SA, van Kampen A. Procedural learning in Broca’s aphasia: Dissociation between the implicit acquisition of spatio-motor and phoneme sequences. Journal of Cognitive Neuroscience. 2001;13(3):370–388. doi: 10.1162/08989290151137412. [DOI] [PubMed] [Google Scholar]
- Graf Estes K, Evans JL, Alibali MW, Saffran JR. Can infants map meaning to newly segmented words? Psychological Science. 2007;18:254–260. doi: 10.1111/j.1467-9280.2007.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P, Dell GS. The emergence of language from serial order and procedural memory. In: MacWhinney B, editor. The emergence of language. Lawrence Erlbaum Associates; Hillsdale, NJ: 1999. pp. 447–481. [Google Scholar]
- Hebb DO. Distinctive features of learning in the higher animal. In: Delafresnaye JF, editor. Brain mechanisms and learning. Blackwell Scientific Publications; Oxford: 1961. pp. 37–51. [Google Scholar]
- Hodges AV, Ash MD, Balkany TJ, Schloffman JJ, Butts SL. Speech perception results in children with cochlear implants: Contributing factors. Otolaryngology—Head and Neck Surgery. 1999;121(1):31–34. doi: 10.1016/S0194-5998(99)70119-1. [DOI] [PubMed] [Google Scholar]
- Holmes J, Gathercole SE, Dunning DL. Adaptive training leads to sustained enhancement of poor working memory in children. Developmental Science. 2009;12(4):F9–15. doi: 10.1111/j.1467-7687.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Howard JH, Jr, Howard DV, Japikse KC, Eden GF. Dyslexics are impaired on implicit higher-order sequence learning, but not on implicit spatial context learning. Neuropsychologia. 2006;44:1131–1144. doi: 10.1016/j.neuropsychologia.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Jamieson RK, Mewhort DJK. The influence of grammatical, local, and organizational redundancy on implicit learning: An analysis using information theory. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2005;31(1):9–23. doi: 10.1037/0278-7393.31.1.9. [DOI] [PubMed] [Google Scholar]
- Jost E, Conway CM, Purdy JD, Hendricks MA. Neurophysiological correlates of visual statistical learning in adults and children. 33rd Annual meeting of the Cognitive Science Society; July 2011.2011. [Google Scholar]
- Kalikow DN, Stevens KN, Elliott LL. Development of a test of speech intelligibility in noise using materials with controlled word predictability. Journal of the Acoustical Society of America. 1977;61(5):1377–1351. doi: 10.1121/1.381436. [DOI] [PubMed] [Google Scholar]
- Karpicke JD, Pisoni DB. Using immediate memory span to measure implicit learning. Memory & Cognition. 2004;32(6):956–964. doi: 10.3758/bf03196873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk KI, Miyamoto RT, Lento CL, Ying E, O’Neil T, Fears B. Effects of age at implantation in young children. Annals of Otology, Rhinology, & Laryngology. 2002;189:69–73. doi: 10.1177/00034894021110s515. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. Journal of Speech, Language, and Hearing Research. 2008;51:225–239. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- Klingberg T. Training and plasticity of working memory. Trends in Cognitive Sciences. 2010;14(7):317–324. doi: 10.1016/j.tics.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlstrom K, Gillberg CG, Forssberg H, Westerberg H. Computerized training of working memory in children with ADHD—A randomized, controlled trial. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44:177–186. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Training of working memory in children with ADHD. Journal of Clinical and Experimental Neuropsychology. 2002;24:781–791. doi: 10.1076/jcen.24.6.781.8395. [DOI] [PubMed] [Google Scholar]
- Kuhl PK. Early language acquisition: Cracking the speech code. Nature Reviews Neuroscience. 2004;5:831–843. doi: 10.1038/nrn1533. [DOI] [PubMed] [Google Scholar]
- Marschark M. Intellectual functioning of deaf adults and children: Answers and question. European Journal of Cognitive Psychology. 2006;18:70–89. [Google Scholar]
- Menghini D, Hagberg GE, Caltagirone C, Petrosini L, Vicari S. Implicit learning deficits in dyslexic adults: and fMRI study. NeuroImage. 2006;33:1218–1226. doi: 10.1016/j.neuroimage.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Miller GA, Cohen J. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miller GA, Heise GA, Lichten W. The intelligibility of speech as a function of the context of the test materials. Journal of Experimental Psychology. 1951;41:329–335. doi: 10.1037/h0062491. [DOI] [PubMed] [Google Scholar]
- Miller GA, Selfridge JA. Verbal context and the recall of meaningful material. American Journal of Psychology. 1950;63:176–185. [PubMed] [Google Scholar]
- Mirman D, Magnuson JS, Estes KG, Dixon JA. The link between statistical segmentation and word learning in adults. Cognition. 2008;108:271–280. doi: 10.1016/j.cognition.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ. Procedural learning difficulties: Reuniting the developmental disorders? Trends in Neuroscience. 2007;30(4):135–141. doi: 10.1016/j.tins.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nature Neuroscience. 2004;7:75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- Peigneux P, Laureys S, Delbeuck X, Maquet P. Sleeping brain, learning brain. The role of sleep for memory systems. Neuroreport. 2001;12(18):A111–24. doi: 10.1097/00001756-200112210-00001. [DOI] [PubMed] [Google Scholar]
- Peigneux P, Laureys S, Fuchs S, Destrebecqz A, Collette F, Delbeuck X, Phillips C, Aerts J, Del Fiore G, Degueldre C, Luxen A, Cleeremans A, Maquet P. Learned material content and acquisition level modulate cerebral reactivation during posttraining rapid-eye-movements sleep. Neuroimage. 2003;20(1):125–134. doi: 10.1016/s1053-8119(03)00278-7. [DOI] [PubMed] [Google Scholar]
- Perruchet P, Pacton S. Implicit learning and statistical learning: One phenomenon, two approaches. Trends in Cognitive Sciences. 2006;10(5):233–238. doi: 10.1016/j.tics.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Pisoni DB. Word identification in noise. Language and Cognitive Processes. 1996;11(6):681–687. doi: 10.1080/016909696387097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni DB. Cognitive factors and cochlear implants: Some thoughts on perception, learning, and memory in speech perception. Ear & Hearing. 2000;21:70–78. doi: 10.1097/00003446-200002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni DB, Conway CM, Kronenberger W, Henning S, Anaya E. Executive function, cognitive control, and sequence learning in deaf children with cochlear implants. In: Marschark M, Spencer P, editors. Oxford Handbook of Deaf Studies, Language, and Education. Oxford University Press; New York, NY: 2010. pp. 439–457. [Google Scholar]
- Plante E, Gomez R, Gerken L. Sensitivity to word order cues by normal and language/learning disabled adults. Journal of Communication Disorders. 2002;35:453–462. doi: 10.1016/s0021-9924(02)00094-1. [DOI] [PubMed] [Google Scholar]
- Rasch B, Born J. About sleep’s role in memory. Physiological reviews. 2013;93(2):681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber AS. Implicit learning of artificial grammars. Journal of Verbal Learning and Verbal Behavior. 1967;6:855–863. [Google Scholar]
- Rileigh KK, Odom PB. Perception of rhythm by subjects with normal and deficient hearing. Developmental Psychology. 1972;7:54–61. [Google Scholar]
- Roodenrys S, Dunn N. Unimpaired implicit learning in children with development dyslexia. Dyslexia. 2008;14:1–15. doi: 10.1002/dys.340. [DOI] [PubMed] [Google Scholar]
- Rueda MR, Rothbart MK, McCandliss BD, Saccomanno L, Posner MI. Training, maturation, and genetic influences on the development of executive attention. Proceedings of the National Academy of Sciences. 2005;102:14931–14936. doi: 10.1073/pnas.0506897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russeler J, Gerth I, Munte TF. Implicit learning is intact in adult developmental dyslexic readers: Evidence from serial reaction time task and artificial grammar learning. Journal of Clinical and Experimental Neuropsychology. 2006;28:808–827. doi: 10.1080/13803390591001007. [DOI] [PubMed] [Google Scholar]
- Saffran JR. Statistical learning learning: Mechanisms and constraints. Current Directions in Psychological Science. 2003;12(4):110–114. [Google Scholar]
- Saffran JR, Aslin RN, Newport EL. Statistical learning by 8-month-old infants. Science. 1996;274:1926–1928. doi: 10.1126/science.274.5294.1926. [DOI] [PubMed] [Google Scholar]
- Semel E, Wiig EH, Secord WA. Clinical Evaluation of Language Fundamentals (CELF-4) Harcourt Assessment. Inc; San Antonio, TX: 2003. [Google Scholar]
- Shalev L, Tsal Y, Mevorach C. Computerized progressive attentional training (CPAT) program: Effective direct intervention for children with ADHD. Child Neuropsychology. 2007;13:382–388. doi: 10.1080/09297040600770787. [DOI] [PubMed] [Google Scholar]
- Stickgold R, James L, Hobson JA. Visual discrimination learning requires sleep after training. Nature neuroscience. 2000;3(12):1237–1238. doi: 10.1038/81756. [DOI] [PubMed] [Google Scholar]
- Tallal P, Gaab N. Dynamic auditory processing, musical experience, and language development. Trends in Neuroscience. 2006;29:382–390. doi: 10.1016/j.tins.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Thorell L, Lindqvist S, Nutley S, Bohlin G, Klingberg T. Training and transfer effects of executive functions in preschool children. Developmental Science. 2009;12(1):106–113. doi: 10.1111/j.1467-7687.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- Todman J, Seedhouse E. Visual-action code processing by deaf and hearing children. Language and Cognitive Processes. 1994;9:129–141. [Google Scholar]
- Tomblin JB, Barker BA, Hubbs S. Developmental constraints on language development in children with cochlear implants. International Journal of Audiology. 2007;46:512–523. doi: 10.1080/14992020701383043. [DOI] [PubMed] [Google Scholar]
- Tomblin JB, Mainela-Arnold E, Zhang X. Procedural learning in adolescents with and without specific language impairments. Language Learning and Development. 2007;3:269–293. [Google Scholar]
- Ullman MT. Contributions of memory circuits to language: The declarative/procedural model. Cognition. 2004;92:231–270. doi: 10.1016/j.cognition.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Ullman MT, Pierpont EI. Specific language impairment is not specific to language: The procedural deficit hypothesis. Cortex. 2005;41:399–433. doi: 10.1016/s0010-9452(08)70276-4. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nature Reviews: Neuroscience. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Carella J, Basak C. A working memory workout: How to expand the focus of serial attention from one to four items in 10 hours or less. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2004;30:1322–1337. doi: 10.1037/0278-7393.30.6.1322. [DOI] [PubMed] [Google Scholar]
- Vicari S, Marotta L, Menghini D, Molinari M, Petrosini L. Implicit learning deficit in children with developmental dyslexia. Neuropsychologia. 2003;41:108–114. doi: 10.1016/s0028-3932(02)00082-9. [DOI] [PubMed] [Google Scholar]
- Westerberg H, Jacobaeus H, Hirvikoski T, Clevberger P, Ostensson ML, Bartfai A, Klingberg T. Computerized working memory training after stroke—A pilot study. Brain Injury. 2007;21(1):21–29. doi: 10.1080/02699050601148726. [DOI] [PubMed] [Google Scholar]