Abstract
PURPOSE
Lorlatinib is a potent, brain-penetrant, third-generation anaplastic lymphoma kinase (ALK)/ROS1 tyrosine kinase inhibitor (TKI) with robust clinical activity in advanced ALK-positive non–small-cell lung cancer, including in patients who have failed prior ALK TKIs. Molecular determinants of response to lorlatinib have not been established, but preclinical data suggest that ALK resistance mutations may represent a biomarker of response in previously treated patients.
PATIENTS AND METHODS
Baseline plasma and tumor tissue samples were collected from 198 patients with ALK-positive non–small-cell lung cancer from the registrational phase II study of lorlatinib. We analyzed plasma DNA for ALK mutations using Guardant360. Tumor tissue DNA was analyzed using an ALK mutation–focused next-generation sequencing assay. Objective response rate, duration of response, and progression-free survival were evaluated according to ALK mutation status.
RESULTS
Approximately one quarter of patients had ALK mutations detected by plasma or tissue genotyping. In patients with crizotinib-resistant disease, the efficacy of lorlatinib was comparable among patients with and without ALK mutations using plasma or tissue genotyping. In contrast, in patients who had failed 1 or more second-generation ALK TKIs, objective response rate was higher among patients with ALK mutations (62% v 32% [plasma]; 69% v 27% [tissue]). Progression-free survival was similar in patients with and without ALK mutations on the basis of plasma genotyping (median, 7.3 months v 5.5 months; hazard ratio, 0.81) but significantly longer in patients with ALK mutations identified by tissue genotyping (median, 11.0 months v 5.4 months; hazard ratio, 0.47).
CONCLUSION
In patients who have failed 1 or more second-generation ALK TKIs, lorlatinib shows greater efficacy in patients with ALK mutations compared with patients without ALK mutations. Tumor genotyping for ALK mutations after failure of a second-generation TKI may identify patients who are more likely to derive clinical benefit from lorlatinib.
INTRODUCTION
Lung cancers that harbor chromosomal rearrangements of anaplastic lymphoma kinase (ALK) are highly responsive to small-molecule tyrosine kinase inhibitors (TKIs) that target ALK.1-3 Standard treatment of patients with advanced ALK-positive non–small-cell lung cancer (NSCLC) has recently shifted from sequential crizotinib followed by more potent second-generation ALK TKIs4-6 to front-line second-generation TKIs.7-10 Whereas most patients derive clinical benefit from second-generation ALK TKIs, acquired resistance invariably develops and leads to clinical relapse.
Lorlatinib is a third-generation oral, reversible, ATP-competitive, macrocyclic TKI of ALK and ROS1.11 Compared with second-generation inhibitors, lorlatinib was specifically designed to penetrate the CNS and to overcome known secondary resistance mutations in the ALK tyrosine kinase domain. Preclinical studies demonstrate that lorlatinib is more potent than earlier-generation TKIs against nonmutant ALK and retains potency against most known single ALK resistance mutations, including the highly refractory ALK G1202R solvent front mutation.12 Whereas ALK mutations can be observed after the failure of crizotinib,13,14 they are more commonly detected after failure of second-generation ALK TKIs in which ALK mutations account for approximately 50% of acquired resistance cases.15
In the clinic, the safety and efficacy of lorlatinib were evaluated in a phase I and II trial in patients with advanced ALK- or ROS1-positive NSCLC.16 In the phase I portion, 54 patients were treated with escalating doses of lorlatinib, and the recommended phase II dose was established to be 100 mg per day. The phase II portion of the study enrolled 228 ALK-positive patients to one of multiple different expansion cohorts defined by prior treatments.17 Among patients who were treated with prior crizotinib, objective response rate (ORR) with lorlatinib was 69% and median progression-free survival (PFS) was not reached (NR). Among patients who had failed two or more ALK TKIs, ORR was 39% and median PFS was 6.9 months. On the basis of these results, lorlatinib was recently approved in the United States and Japan for previously treated, advanced ALK-positive NSCLC.
Compared with patients with crizotinib-resistant disease, most of whom are responsive to more potent second-generation ALK TKIs, a smaller proportion of patients who have failed a second-generation ALK TKI respond to lorlatinib.17 Preclinical studies of patient-derived cell lines suggest that the presence of ALK resistance mutations may identify cancers with continued ALK dependency and sensitivity to lorlatinib.15 To determine whether ALK resistance mutations may serve as biomarkers of lorlatinib response, we performed a planned molecular analysis of tumor tissue and cell-free DNA (cfDNA)—or circulating tumor DNA—from patients enrolled in the phase II study of lorlatinib. Here, we report on the correlation between ALK kinase domain mutations and the efficacy of lorlatinib in previously treated ALK-positive patients.
PATIENTS AND METHODS
Study design, objectives, and eligibility criteria of the phase II trial have been recently published.17 All patients received the standard dose of lorlatinib 100 mg per day. The protocol was approved by the institutional review board or independent ethics committee at each site and complied with the International Ethical Guidelines for Biomedical Research Involving Human Subjects, Good Clinical Practice guidelines, the Declaration of Helsinki, and local laws. All patients provided written informed consent.
The primary objective of the phase II trial was to evaluate the overall and intracranial efficacy of lorlatinib. A secondary objective was to evaluate tumor- and blood-based molecular markers of response and resistance to lorlatinib. All patients were required to have a mandatory de novo biopsy obtained within 28 days of starting lorlatinib; however, if a biopsy was not possible because of a safety risk, then archival tissue was accepted. All patients also had peripheral blood collected for cfDNA analysis before initiating lorlatinib.
Molecular profiling was performed on both tumor tissue and cfDNA. Formalin-fixed, paraffin-embedded tumor tissue was profiled using a central, customized next-generation sequencing (NGS) assay on the Ion Torrent PGM platform at MolecularMD (Portland, OR). We isolated tissue DNA using the AllPrep DNA/RNA extraction kit (Qiagen, Germantown, MD). NGS assay was validated in accordance with the Clinical Laboratory Improvement Amendments for the detection of ALK kinase domain (exons 20 to 25) mutations in formalin-fixed, paraffin-embedded tumor tissue NSCLC samples, with a 2% to 5% allele frequency limit of detection.
We performed extraction and analysis of cfDNA using a validated, commercially available 73-gene cfDNA NGS assay (Guardant360, panel version 2.10, bioinformatics pipeline version 3.0; Guardant Health, Redwood City, CA), as previously described.18-20 Results of the cfDNA analysis are reported here for ALK kinase domain mutations.
Clinical efficacy and statistical parameters were described previously17 and detailed in the Data Supplement. Data cutoff date was February 2, 2018.
RESULTS
Patient Population
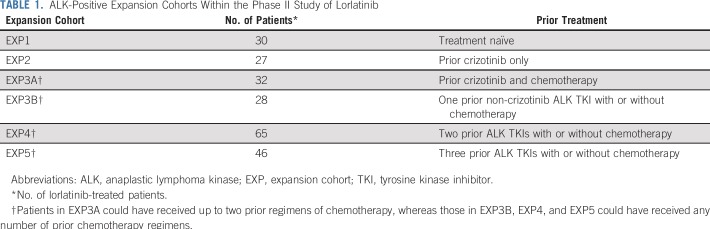
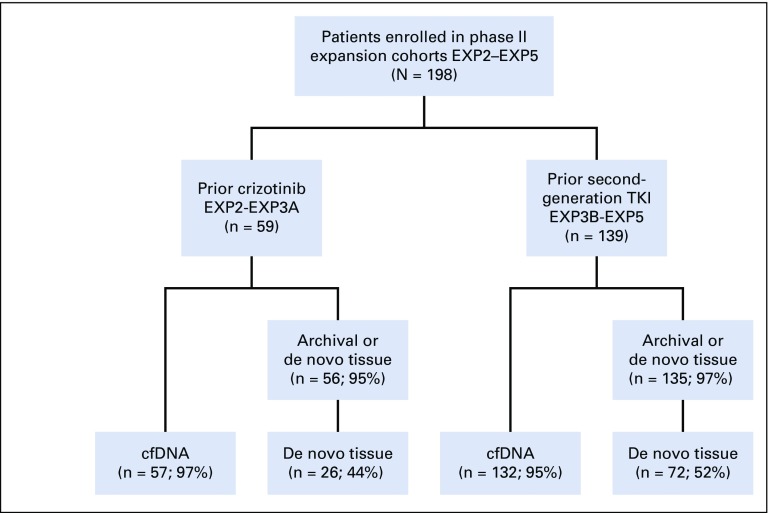
A total of 228 patients with ALK-positive NSCLC were enrolled in the phase II study. Thirty ALK-positive patients who were treatment naïve (expansion cohort 1 [EXP1]) were excluded from this analysis. The remaining 198 patients had received one or more prior ALK TKIs and were enrolled in expansion cohorts EXP2 to EXP5, depending on previous treatment (Table 1). Baseline clinical characteristics of these patients were consistent with an ALK-positive population, as previously reported.17 Fifty-nine patients had received prior crizotinib (EXP2 to EXP3A), whereas 139 patients had received one or more second-generation ALK TKIs (EXP3B to EXP5), often with crizotinib preceding the second-generation inhibitor(s). For both groups (EXP2 to EXP3A and EXP3B to EXP5), 95% of patients or greater had baseline cfDNA testing and either archival or de novo tumor biopsies (Fig 1). Clinical characteristics of the larger group of EXP3B to EXP5 patients according to ALK mutation status are shown in the Data Supplement.
TABLE 1.
ALK-Positive Expansion Cohorts Within the Phase II Study of Lorlatinib
FIG 1.
CONSORT diagram. A total of 198 anaplastic lymphoma kinase (ALK)-positive patients in expansion cohorts EXP2 to EXP5 were treated. Of these, 59 had received prior crizotinib and 139 had received one or more second-generation inhibitors, often in addition to crizotinib. The numbers of patients with cell-free DNA (cfDNA) and tissue samples are shown. De novo tissue refers to a biopsy obtained within 28 days of starting lorlatinib. EXP2, prior crizotinib only; EXP3A, prior crizotinib and chemotherapy; EXP3B, one prior non-crizotinib ALK tyrosine kinase inhibitor (TKI) with or without chemotherapy; EXP4, two prior ALK TKIs with or without chemotherapy; EXP5, three prior ALK TKIs with or without chemotherapy.
Molecular Profiling
Among 189 ALK-positive patients in EXP2 to EXP5 with baseline plasma genotyping, 45 (24%) had one or more ALK mutations detectable in cfDNA (Data Supplement). Forty patients (21%) in EXP2 to EXP5 had no detectable cfDNA. Among 191 tumor tissue samples—both archival and de novo specimens—164 (86%) were adequate for NGS analysis, of which 40 (24%) harbored one or more ALK mutations. Of the 98 de novo specimens, 76 (78%) were adequate for NGS analysis, of which 36 (47%) were found to harbor one or more ALK mutations.
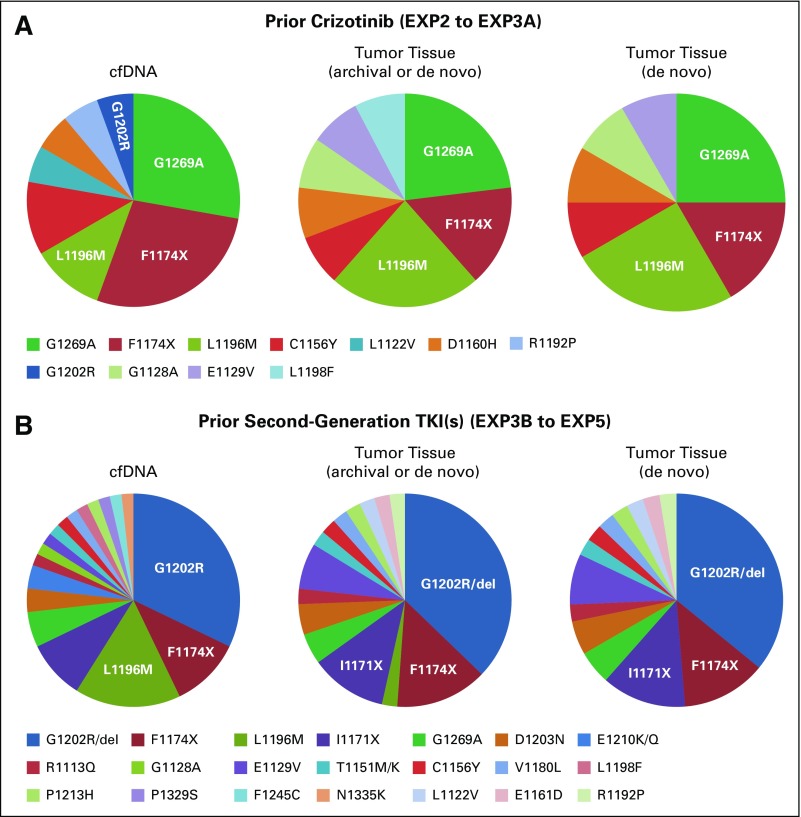
Numerous different ALK mutations were detected using plasma and tissue genotyping of patients in EXP2 to EXP5 (Fig 2 and Data Supplement). On the basis of plasma genotyping, the most common ALK mutations were G1202R/del (42%), L1196M (24%), F1174X (24%), G1269A (18%), and I1171X (11%; Data Supplement). On the basis of tumor genotyping, the most common ALK mutations were G1202R/del (40%), F1174X (20%), I1171X (13%), and G1269A (13%; Data Supplement). All of these single ALK resistance mutations have been shown to be sensitive to lorlatinib in preclinical models.12,15 A similar spectrum and frequency of ALK mutations was observed in de novo tissue specimens. Of note, both the spectrum and frequency of ALK mutations differed depending on prior ALK TKI therapy. For the subset of patients who received prior crizotinib (EXP2 to EXP3A), the most common ALK mutations were G1269A, F1174X, and L1196M (Fig 2A). In contrast, for the subset of patients who previously received one or more second-generation TKIs (EXP3B to EXP5), the predominant ALK mutation was G1202R/del, which was detected in 53% and 55% of cfDNA and tumor tissue cases, respectively (Fig 2B).
FIG 2.
Summary of anaplastic lymphoma kinase (ALK) mutations identified by plasma and tumor genotyping. (A) Post-crizotinib patients (expansion cohorts EXP2 to EXP3A). The most common ALK mutation observed in cell-free DNA (cfDNA) and tumor tissue was G1269A. ALK G1202R was detected in one cfDNA sample. (B) Patients who have failed 1 or more second-generation ALK TKIs (EXP3B–5). The most common ALK mutation observed in cfDNA and tumor tissue was G1202R/del. (Note: only one G1202del mutation was detected.) Pie charts display the frequency of indicated ALK mutations as a percentage of the total number of patients with ALK mutations.
Using ALK mutation status in de novo tumor tissue as reference, the sensitivity of plasma genotyping for any ALK mutations in EXP2 to EXP5 was 61% (19 of 31; 95% CI, 44% to 76%), which is consistent with a false-negative rate of 39%. For the most common ALK mutations detected in de novo tumor tissue, sensitivity of plasma genotyping for each mutation ranged from 50% (for I1171X) to 80% (for G1269A; Data Supplement). The specificity of plasma genotyping for any ALK mutation was 82% (31 of 38; 95% CI, 67% to 91%). The overall agreement or accuracy of plasma genotyping and de novo tumor tissue genotyping was 73% (50 of 69; 95% CI, 61% to 82%) with κ-statistics of 0.44 (95% CI, 0.22 to 0.65).
Clinical Outcomes Analysis
We previously reported efficacy results from this phase 2 study after an estimated median follow up of approximately 7 months.17 Here, we present updated efficacy data (median follow up, 16.6 months [95% CI, 15.2 to 17.0 months]) for EXP2–5, according to prior therapy and presence/absence of ALK mutations.
Post-crizotinib.
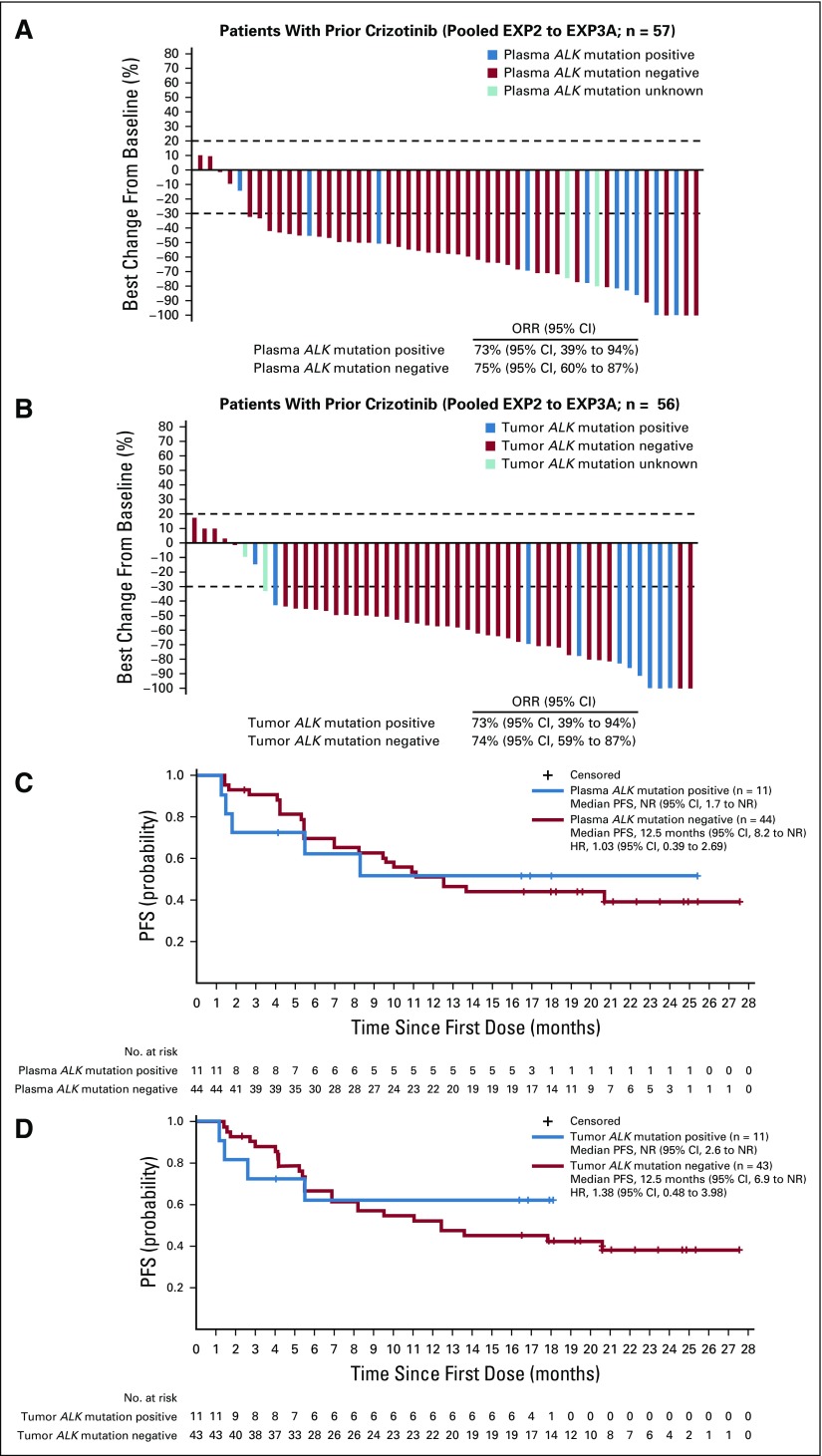
Among 59 patients who were previously treated with crizotinib (EXP2 to EXP3A), ORR was 73% (95% CI, 60% to 84%). Median duration of response (DOR) was NR (95% CI, 8.4 months to NR) and median PFS was 11.1 months (95% CI, 8.2 months to NR). On the basis of plasma genotyping, 11 patients (19%) had detectable ALK mutations and 44 patients (77%) did not. ORR was 73% among mutation-positive patients and 75% among mutation-negative patients (Fig 3A). Similar results were observed when ALK mutation status was assessed in tumor tissue. In mutation-positive and mutation-negative patients, ORRs with lorlatinib were 73% and 74%, respectively (Fig 3B and Data Supplement).
FIG 3.
Efficacy of lorlatinib in crizotinib-resistant patients (expansion cohorts EXP2 to EXP3A), according to anaplastic lymphoma kinase (ALK) mutation status. Shown are waterfall plots summarizing the best percentage change in target lesions, with ALK mutation status determined by (A) plasma genotyping or (B) tissue genotyping. Blue bars indicate patients with one or more ALK mutations, red bars indicate patients without detectable ALK mutations, and aqua bars indicate two samples that failed analysis. Two patients in EXP2 to EXP3A did not have plasma samples for cell-free DNA analysis. Patients with at least one on-study target lesion assessment were included. If any assessment procedures differed from or were not interchangeable with the procedure at screening, the change from baseline could not be calculated and is not displayed. Kaplan-Meier curves of progression-free survival (PFS) are also shown according to ALK mutation status, as determined by (C) plasma genotyping or (D) tissue genotyping. Vertical lines on the curves indicate censoring of data. HR, hazard ratio; NR, not reached; ORR, objective response rate.
Among patients who were treated with prior crizotinib, median PFS was NR (95% CI, 1.7 months to NR) and 12.5 months (95% CI, 8.2 months to NR) in patients with and without ALK mutations detected by plasma genotyping, respectively (Fig 3C). Nearly identical results were observed with tumor tissue (Fig 3D). In addition, DOR was also similar regardless of ALK mutation status (Data Supplement). Thus, in patients who have received prior crizotinib as their only ALK TKI, lorlatinib is highly effective regardless of the presence or absence of detectable ALK mutations.
Post–Second-Generation ALK TKI.
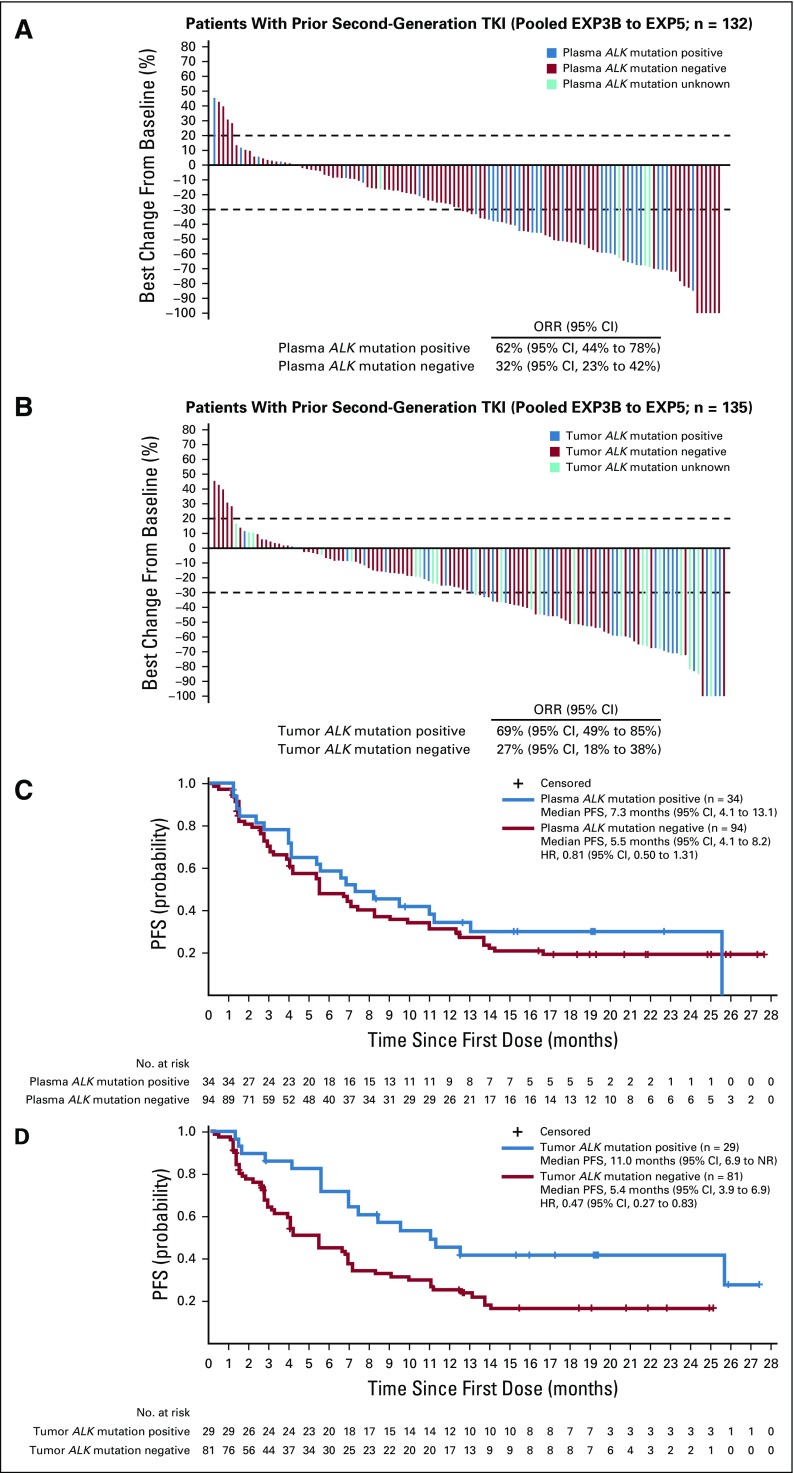
Among 139 patients who were previously treated with one or more second-generation ALK TKIs (EXP3B to EXP5), ORR was 40% (95% CI, 32% to 49%). Median DOR was 7.1 months (95% CI, 5.6 months to 24.4 months) and median PFS was 6.9 months (95% CI, 5.4 months to 8.2 months). On the basis of plasma genotyping, 34 patients (26%) had detectable ALK mutations, whereas 94 patients (71%) did not. In contrast to the post-crizotinib setting, response rates to lorlatinib after a prior second-generation ALK TKI differed based on ALK mutation status. ORR was 62% among mutation-positive patients and 32% among mutation-negative patients (Fig 4A). Tumor tissue testing yielded similar results, with ORRs of 69% and 27% in patients with and without an ALK mutation, respectively (Fig 4B). Similarly, on the basis of testing of de novo tissue samples only, ORRs were 69% and 31% in patients with and without an ALK mutation, respectively (Data Supplement).
FIG 4.
Efficacy of lorlatinib in patients who have failed 1 or more second-generation ALK inhibitors (EXP3B–5), according to ALK mutation status. Shown are waterfall plots summarizing the best percentage change in target lesions, with ALK mutation status determined by (A) plasma genotyping or (B) tissue genotyping. Blue bars indicate patients with one or more ALK mutations, red bars indicate patients without detectable ALK mutations, and aqua bars indicate four samples that failed analysis. Seven patients in EXP3B to EXP5 did not have plasma samples for cell-free DNA analysis. Patients with at least one on-study target lesion assessment were included. If any assessment procedures differed from or were not interchangeable with the procedure at screening, the change from baseline could not be calculated and is not displayed. Kaplan-Meier curves of progression-free survival (PFS) are also shown according to ALK mutation status, as determined by (C) plasma genotyping or (D) tissue genotyping. Vertical lines on the curves indicate censoring of data. HR, hazard ratio; NR, not reached; ORR, objective response rate; TKI, tyrosine kinase inhibitor.
As shown in Figure 4C, PFS did not differ significantly according to ALK mutation status as determined by plasma genotyping. Median PFS was 7.3 months (95% CI, 4.1 months to 13.1 months) and 5.5 months (95% CI, 4.1 months to 8.2 months) in patients with and without ALK mutations detected in cfDNA, respectively (hazard ratio [HR], 0.81; 95% CI, 0.50 to 1.31). DOR was also similar in patients with and without ALK mutations (Data Supplement). Conversely, both PFS and DOR were significantly longer in mutation-positive patients compared with mutation-negative patients when tumor tissue was genotyped. Median PFS was 11.0 months (95% CI, 6.9 months to NR) in patients with ALK mutations compared with 5.4 months (95% CI, 3.9 months to 6.9 months) in patients without ALK mutations (HR, 0.47; 95% CI, 0.27 to 0.83; Fig 4D). Median DOR was 24.4 months in mutation-positive patients compared with 4.3 months in mutation-negative patients (Data Supplement). These differences were more pronounced when tumor tissue genotyping was limited to de novo biopsies. For example, median PFS was 11.0 months (95% CI, 6.9 months to 25.6 months) versus 4.0 months (95% CI, 2.6 months to 5.5 months) in patients with and without ALK mutations, respectively (HR, 0.20; 95% CI, 0.10 to 0.40; Data Supplement). Taken together, these findings suggest that in patients who have previously received one or more second-generation ALK inhibitors, the presence of an ALK mutation based on tissue genotyping may identify a subgroup of patients more likely to derive durable benefit from lorlatinib.
Clinical Efficacy According to Type and Number of ALK Mutations
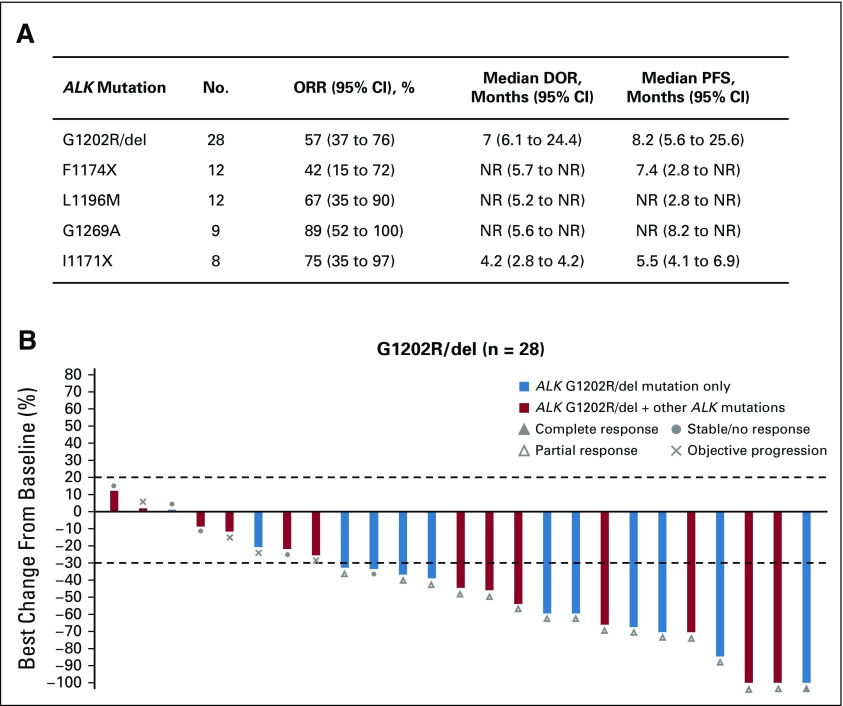
We next examined the efficacy of lorlatinib according to the exact ALK mutation identified by plasma or tissue genotyping. Lorlatinib demonstrated antitumor activity against the five most common ALK mutations observed in EXP2 to EXP5, with ORRs ranging from 42% to 89% (Fig 5 and Data Supplement). Of the five ALK mutations, G1202R/del was the most common mutation detected and has been shown to confer resistance to both first- and second-generation ALK inhibitors.15 As shown in Figure 5B, lorlatinib was highly effective against ALK G1202R/del, with an ORR of 57%, median DOR of 7.0 months, and median PFS of 8.2 months.
FIG 5.
Clinical activity of lorlatinib against common anaplastic lymphoma kinase (ALK) resistance mutations. (A) Efficacy of lorlatinib in patients in expansion cohorts EXP2 to EXP5 harboring the indicated ALK mutations, as detected by plasma or tissue genotyping. (B) Waterfall plot showing the best percentage change in target lesions in patients harboring G1202R/del. Blue bars indicate patients with G1202R/del only and red bars indicate patients with G1202R/del and one or more other ALK mutations. Patients with at least one on-study target lesion assessment were included. If any assessment procedures differed from or were not interchangeable with the procedure at screening, the change from baseline could not be calculated and is not displayed. ALK, anaplastic lymphoma kinase; cfDNA, cell-free plasma DNA; CI, confidence interval; DOR, duration of response; EXP, expansion cohort; HR, hazard ratio; NR, not reached; ORR, objective response rate; PFS, progression-free survival.
Among patients with detectable ALK mutations, approximately one third harbored more than one ALK mutation (Data Supplement). The presence of more than one ALK mutation could indicate compound ALK mutations—that is, mutations located on the same allele—or heterogeneous resistance mechanisms, both of which have been described in heavily pretreated patients15,21 and could affect the efficacy of lorlatinib. We compared the efficacy of lorlatinib among patients in EXP3B to EXP5 who harbored either one ALK mutation or more than one ALK mutation. AS the numbers were small based on plasma genotyping, we limited this analysis to patients with tumor genotyping. ORR trended higher among patients with only one ALK mutation compared with those with more than one ALK mutation (75% v 56%, respectively). Median DOR was also longer in patients with only one ALK mutation (24.4 months v 6.1 months with more than one ALK mutation). These results suggest that in patients who have failed 1 or more second-generation inhibitors, the number of ALK resistance mutations may affect the efficacy of lorlatinib, but larger studies are required to validate this finding.
DISCUSSION
The therapeutic landscape in advanced ALK-positive NSCLC is rapidly evolving. Second-generation ALK TKIs are widely used in the crizotinib-resistant setting4-6,22,23 and have become the preferred first-line therapy for patients with advanced disease.7-10 Recently, the third-generation TKI lorlatinib has demonstrated clinical activity in previously treated patients, including those who have failed 1 or more second-generation TKIs,16,17 leading to the regulatory approval of lorlatinib in the United States and Japan.
Here, we evaluated the efficacy of lorlatinib according to prior TKI therapy and ALK mutation status. For patients who failed crizotinib only, lorlatinib was highly active, with efficacy parameters comparable to those of second-generation TKIs in the post-crizotinib setting.4-6,22,23 Of importance, on the basis of either plasma or tissue genotyping, ALK mutation status did not correlate with response to lorlatinib, with both mutation-positive and mutation-negative patients responding equally well to lorlatinib (Figs 3A and 3B). Similar findings were observed in a small cohort of crizotinib-resistant patients in the phase I ceritinib study in which responses were noted in patients with and without ALK mutations.4 These results demonstrate that most crizotinib-resistant tumors, including those without a detectable ALK mutation, are still driven by ALK and remain responsive to more potent ALK inhibitors.
Lorlatinib was also active in patients who failed 1 or more second-generation ALK inhibitors; however, overall efficacy in these patients was less robust than in patients who had failed crizotinib only. Unlike in the post-crizotinib setting, ALK mutation status seems to be an important predictor of lorlatinib response in patients who have failed a second-generation TKI. On the basis of plasma or tissue genotyping, mutation-positive patients had significantly higher response rates to lorlatinib compared with mutation-negative patients (Figs 4A and 4B). In addition, patients with ALK mutations that were detected by tumor (but not plasma) genotyping had longer PFS and DOR compared with those without mutations (median PFS, 11.0 months v 5.4 months; median DOR, 24.4 months v 4.3 months, respectively). These data suggest that, in patients who have failed a second-generation ALK TKI, ALK mutations may identify tumors with continued ALK dependency, making them more likely to respond to lorlatinib. The absence of an ALK mutation suggests that tumors may have developed ALK-independent mechanisms of resistance, making them less likely to respond to ALK inhibition.
The association of ALK mutations with clinical response to lorlatinib after failure of a second-generation ALK TKI is reminiscent of epidermal growth factor receptor (EGFR) T790M serving as a predictive biomarker for the third-generation EGFR inhibitor osimertinib after failure of earlier-generation TKIs.24-26 On the basis of tumor genotyping, T790M-positive cases are associated with an ORR of 62% and median PFS of 9.7 months, whereas T790M-negative cases are associated with an ORR of 26% and median PFS of 3.4 months. Similarly, among the ALK-positive patients who failed one or more second-generation TKIs and had a de novo biopsy, ORR and median PFS were 69% and 11.0 months in ALK mutation-positive cases, respectively, compared with 31% and 4.0 months in mutation-negative cases. Thus, in patients who have failed a second-generation ALK TKI, ALK mutations as a group may be analogous to EGFR T790M in identifying patients who are more responsive to a highly potent third-generation TKI.
Plasma genotyping for resistance mutations adds another layer of complexity, and again there are parallels between ALK mutations and EGFR T790M. For example, the PFS of patients with T790M-negative plasma genotyping was comparable to that of patients with T790M-positive plasma (median, 8.2 months v 9.7 months, respectively).25 Similarly, in the case of lorlatinib and plasma genotyping, PFS did not differ significantly in patients with and without ALK mutations (median, 7.3 months v 5.5 months, respectively). These results may reflect the current limitations of plasma genotyping and the fact that plasma-negative cases include both true negatives and false negatives, potentially compromising the ability of plasma to identify two distinct subgroups with different PFS.25,27 For patients without a sensitizing mutation identified in plasma, additional investigation with tumor biopsy and genotyping may be considered; however, it should be noted that after failure of a second-generation ALK TKI, even ALK mutation–negative patients by tumor genotyping can respond to lorlatinib (ORR, 31%). In addition, for patients with CNS-predominant relapse, many of whom may not have detectable cfDNA, lorlatinib may be highly effective given its potent intracranial activity.17 Thus, the absence of ALK mutations, in some cases, may be helpful in estimating the likelihood of response but should not be used to exclude patients from treatment with lorlatinib.
This study has several important limitations. First, although we enrolled almost 200 previously treated ALK-positive patients,17 the multiplicity of different ALK mutations and the various lines of therapy resulted in relatively small subgroups of patients. Second, this study mandated de novo tumor biopsies at screening unless it was not technically feasible or safe to perform. Consequently, 51% of patients underwent de novo tumor biopsies, whereas the remaining 49% of patients had only archival tumor specimens that could have been collected at any time during the course of disease and, therefore, may have been less informative in terms of relevant biomarkers. The inclusion of archival specimens may have potentially weakened the correlation between ALK resistance mutations and the efficacy of lorlatinib. Finally, both the plasma and tumor genotyping studies focused solely on ALK mutations. Expanding the evaluation to include other cancer-associated genes may uncover novel biomarkers of response and resistance to lorlatinib.
In summary, in patients who experience relapse on a second-generation ALK inhibitor, ALK mutations may serve as a biomarker to identify those who are more likely to respond to lorlatinib. Additional investigations into plasma versus tissue genotyping of ALK mutations are needed.28 The National Cancer Institute’s ALK Master Protocol will incorporate both plasma and tissue genotyping after failure of a second-generation ALK TKI to establish concordance and to prospectively match patients to appropriate ALK TKIs on the basis of the underlying ALK resistance mutation. These and other studies should help refine the optimal sequencing of next-generation ALK inhibitors in patients with advanced ALK-positive NSCLC.
ACKNOWLEDGMENT
The authors thank the participating patients and their families, as well as the investigators, subinvestigators, research nurses, study coordinators, and operations staff. The authors also thank Deborah Shepard for support with the biomarker analyses for this study. Vasupradha Vethantham of inScience Communications, Springer Healthcare (Philadelphia, PA), provided editorial support funded by Pfizer.
Footnotes
Presented in part at the American Association for Cancer Research 2018 Annual Meeting, Chicago, IL, April 14-18, 2018.
Supported by Pfizer and National Cancer Institute Grant No. R01-CA164273 (to A.T.S.).
Processed as a Rapid Communication manuscript.
Preprint version available on bioRxiv.
Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the United States and/or European Union or (2) in programs that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The deidentified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
AUTHOR CONTRIBUTIONS
Conception and design: Alice T. Shaw, Benjamin J. Solomon, Sai-Hong Ignatius Ou, Jill S. Clancy, Antonello Abbattista, Holger Thurm, D. Ross Camidge, Jean-François Martini
Administrative support: Jill S. Clancy
Provision of study material or patients: Alice T. Shaw, Benjamin J. Solomon, Todd M. Bauer, Chia-Chi Lin, Gregory J. Riely, Sai-Hong Ignatius Ou, Miyako Satouchi, D. Ross Camidge, Steven Kao, Rita Chiari, Shirish M. Gadgeel, Enriqueta Felip
Collection and assembly of data: Alice T. Shaw, Benjamin J. Solomon, Benjamin Besse, Todd M. Bauer, Chia-Chi Lin, Ross A. Soo, Gregory J. Riely, Sai-Hong Ignatius Ou, Antonello Abbattista, Miyako Satouchi, D. Ross Camidge, Steven Kao, Rita Chiari, Shirish M. Gadgeel, Enriqueta Felip, Jean-François Martini
Data analysis and interpretation: Alice T. Shaw, Benjamin J. Solomon, Benjamin Besse, Todd M. Bauer, Chia-Chi Lin, Ross A. Soo, Jill S. Clancy, Sherry Li, Antonello Abbattista, Holger Thurm, Miyako Satouchi, D. Ross Camidge, Steven Kao, Rita Chiari, Shirish M. Gadgeel, Enriqueta Felip, Jean-François Martini
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
ALK Resistance Mutations and Efficacy of Lorlatinib in Advanced Anaplastic Lymphoma Kinase-Positive Non–Small-Cell Lung Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Alice T. Shaw
Honoraria: Pfizer, Novartis, Genentech, Foundation medicine, Guardant Health
Consulting or Advisory Role: Pfizer, Novartis, Genentech, Roche, ARIAD Pharmaceuticals, Ignyta, Blueprint Medicines, Daiichi Sankyo, EMD Serono, Taiho Pharmaceutical, KSQ Therapeutics, Natera, Loxo Pharmaceuticals, Takeda, Bayer, Chugai Pharma
Research Funding: Pfizer (Inst), Novartis (Inst), Genentech (Inst), TP Therapeutics (Inst), Ignyta (Inst), ARIAD Pharmaceuticals (Inst), Daiichi Sankyo (Inst)
Benjamin J. Solomon
Honoraria: Bristol-Myers Squibb, AstraZeneca (Inst)
Consulting or Advisory Role: Bristol-Myers Squibb, Merck Sharp & Dohme (Inst), AstraZeneca, Pfizer (Inst), Genentech (Inst), Eisai
Research Funding: Pfizer (Inst)
Patents, Royalties, Other Intellectual Property: Royalties from Veristrat (Biodesix), UpToDate
Travel, Accommodations, Expenses: AstraZeneca, Roche, Merck, Bristol-Myers Squibb, Novartis
Benjamin Besse
Research Funding: AstraZeneca (Inst), Pfizer (Inst), Eli Lilly (Inst), Onxeo (Inst), Bristol-Myers Squibb (Inst), Inivata (Inst), AbbVie (Inst), Amgen (Inst), Biogen (Inst), Blueprint Medicines (Inst), Celgene (Inst), GlaxoSmithKline (Inst), Ignyta (Inst), Ipsen (Inst), Merck (Inst), MSD Oncology (Inst), Nektar (Inst), PharmaMar (Inst), Sanofi (Inst), Spectrum Pharmaceuticals (Inst), Takeda (Inst), Tiziana Therapeutics (Inst)
Todd M. Bauer
Employment: Tennessee Oncology, Sarah Cannon Research Institute
Consulting or Advisory Role: Ignyta (Inst), Guardant Health, Loxo Pharmaceuticals, Pfizer, Moderna Therapeutics (Inst)
Speakers' Bureau: Bayer
Research Funding: Daiichi Sankyo (Inst), Medpacto (Inst), Incyte (Inst), Mirati Therapeutics (Inst), MedImmune (Inst), AbbVie (Inst), AstraZeneca (Inst), Leap Therapeutics (Inst), MabVax (Inst), Stemline Therapeutics (Inst), Merck (Inst), Eli Lilly (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Pfizer (Inst), Genentech (Inst), Deciphera (Inst), Merrimack Pharmaceuticals (Inst), Immunogen (Inst), Millennium Pharmaceuticals (Inst), Ignyta (Inst), Calithera Biosciences (Inst), Kolltan Pharmaceuticals (Inst), Principa Biopharma (Inst), Peleton (Inst), Immunocore (Inst), Roche (Inst), Aileron Therapeutics (Inst), Bristol-Myers Squibb (Inst), Amgen (Inst), Moderna Therapeutics (Inst), Sanofi (Inst), Boehringer Ingelheim (Inst), Astellas Pharma (Inst), Five Prime Therapeutics (Inst), Jacobio (Inst), Top Alliance BioScience (Inst), Loxo Pharmaceuticals (Inst), Janssen Pharmaceuticals (Inst), Clovis Oncology (Inst), Takeda (Inst), Karyopharm Therapeutics (Inst), Onyx Pharmaceuticals (Inst), Phosplatin Therapeutics (Inst), Foundation Medicine (Inst)
Chia-Chi Lin
Honoraria: Novartis, Roche, Daiichi Sankyo
Consulting or Advisory Role: Novartis, Boehringer Ingelheim, Blueprint Medicines
Travel, Accommodations, Expenses: Eli Lilly, Daiichi Sankyo, BeiGene, Novartis
Ross A. Soo
Honoraria: AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Genentech, Taiho Pharmaceutical, Takeda, Yuhan, Celgene
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Merck Sharp & Dohme, Novartis, Pfizer, Genentech, Taiho Pharmaceutical, Celgene, Yuhan, Takeda
Research Funding: AstraZeneca
Gregory J. Riely
Research Funding: Novartis (Inst), Genentech (Inst), Millennium Pharmaceuticals (Inst), GlaxoSmithKline (Inst), Pfizer (Inst), Infinity Pharmaceuticals (Inst), ARIAD Pharmaceuticals (Inst), Mirati Therapeutics (Inst), Merck (Inst)
Patents, Royalties, Other Intellectual Property: Patent application submitted covering pulsatile use of erlotinib to treat or prevent brain metastases (Inst)
Travel, Accommodations, Expenses: Merck Sharp & Dohme
Sai-Hong Ignatius Ou
Stock and Other Ownership Interests: TP Therapeutics
Honoraria: Pfizer, Roche, Genentech, ARIAD Pharmaceuticals, Takeda, AstraZeneca, Foundation Medicine, Merck
Consulting or Advisory Role: Pfizer, Genentech, AstraZeneca, Takeda, Foundation Medicine, TP Therapeutics, Ignyta
Speakers' Bureau: Genentech, AstraZeneca, Takeda
Research Funding: Pfizer (Inst), Roche (Inst), AstraZeneca (Inst), MedImmune (Inst), ARIAD Pharmaceuticals (Inst), Ignyta (Inst), Astellas Pharma (Inst), Chugai Pharma (Inst), Revolution Medicines (Inst)
Jill S. Clancy
Employment: Pfizer
Research Funding: Pfizer
Sherry Li
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Antonello Abbattista
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Holger Thurm
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Miyako Satouchi
Honoraria: Chugai Pharma, Taiho Pharmaceutical, Pfizer, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Ono Pharmaceutical, Novartis, MSD Oncology, Eli Lilly
Research Funding: Chugai Pharma (Inst), Pfizer (Inst), Eli Lilly (Inst), AstraZeneca (Inst), Bristol-Myers Squibb (Inst), Ono Pharmaceutical (Inst), Novartis (Inst), MSD Oncology (Inst), Astellas Pharma (Inst), AbbVie (Inst), Ignyta (Inst), Takeda (Inst), Boehringer Ingelheim (Inst)
D. Ross Camidge
Honoraria: Roche, G1 Therapeutics, Mersana, Takeda, AstraZeneca, Genoptix, Ignyta, Daiichi Sankyo, Hansoh, Lycera, Biothera, Revolution Medicines
Research Funding: Takeda
Steven Kao
Honoraria: Pfizer, AstraZeneca, Roche, Bristol-Myers Squibb, MSD Oncology
Travel, Accommodations, Expenses: Boehringer Ingelheim, Roche, AstraZeneca, Bristol-Myers Squibb
Rita Chiari
Speakers' Bureau: Takeda
Shirish M. Gadgeel
Consulting or Advisory Role: Pfizer, Genentech, ARIAD Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, AbbVie
Speakers' Bureau: AstraZeneca
Research Funding: Pfizer (Inst), Merck, Genentech (Inst), Merck (Inst), Blueprint Medicines (Inst), ARIAD Pharmaceuticals (Inst), Takeda (Inst)
Travel, Accommodations, Expenses: ARIAD Pharmaceuticals, Takeda
Enriqueta Felip
Consulting or Advisory Role: Pfizer, Roche, Boehringer Ingelheim, AstraZeneca, Bristol-Myers Squibb, Celgene, Guardant Health, Novartis, Takeda, AbbVie, Blueprint Medicines, Eli Lilly, Merck, Merck Sharp & Dohme
Speakers' Bureau: AstraZeneca, Bristol-Myers Squibb, Novartis, Boehringer Ingelheim, Merck Sharp & Dohme, Roche, Pfizer, AbbVie, Eli Lilly, Merck, Takeda
Research Funding: Fundación Merck Salud (Inst), EMD Serono (Inst)
Jean-François Martini
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
REFERENCES
- 1.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non–small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin JJ, Riely GJ, Shaw AT. Targeting ALK: Precision medicine takes on drug resistance. Cancer Discov. 2017;7:137–155. doi: 10.1158/2159-8290.CD-16-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non–small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 4.Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non–small-cell lung cancer. N Engl J Med. 2014;370:1189–1197. doi: 10.1056/NEJMoa1311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ou SH, Ahn JS, De Petris L, et al. Alectinib in crizotinib-refractory ALK-rearranged non–small-cell lung cancer: A phase II global study. J Clin Oncol. 2016;34:661–668. doi: 10.1200/jco.2015.63.9443. [DOI] [PubMed] [Google Scholar]
- 6.Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non–small-cell lung cancer: A randomized, multicenter phase II trial. J Clin Oncol. 2017;35:2490–2498. doi: 10.1200/JCO.2016.71.5904. [DOI] [PubMed] [Google Scholar]
- 7.Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non–small-cell lung cancer (ASCEND-4): A randomised, open-label, phase 3 study. Lancet. 2017;389:917–929. doi: 10.1016/S0140-6736(17)30123-X. [DOI] [PubMed] [Google Scholar]
- 8.Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non–small-cell lung cancer (J-ALEX): An open-label, randomised phase 3 trial. Lancet. 2017;390:29–39. doi: 10.1016/S0140-6736(17)30565-2. [DOI] [PubMed] [Google Scholar]
- 9.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non–small-cell lung cancer. N Engl J Med. 2017;377:829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 10.Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in ALK-positive non–small-cell lung cancer. N Engl J Med. 2018;379:2027–2039. doi: 10.1056/NEJMoa1810171. [DOI] [PubMed] [Google Scholar]
- 11.Johnson TW, Richardson PF, Bailey S, et al. Discovery of (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(metheno)pyrazolo[4,3-h][2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem. 2014;57:4720–4744. doi: 10.1021/jm500261q. [DOI] [PubMed] [Google Scholar]
- 12.Zou HY, Friboulet L, Kodack DP, et al. PF-06463922, an ALK/ROS1 inhibitor, overcomes resistance to first and second generation ALK inhibitors in preclinical models. Cancer Cell. 2015;28:70–81. doi: 10.1016/j.ccell.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katayama R, Khan TM, Benes C, et al. Therapeutic strategies to overcome crizotinib resistance in non–small-cell lung cancers harboring the fusion oncogene EML4-ALK. Proc Natl Acad Sci USA. 2011;108:7535–7540. doi: 10.1073/pnas.1019559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non–small-cell lung cancer. Clin Cancer Res. 2012;18:1472–1482. doi: 10.1158/1078-0432.CCR-11-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6:1118–1133. doi: 10.1158/2159-8290.CD-16-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw AT, Felip E, Bauer TM, et al. Lorlatinib in non–small-cell lung cancer with ALK or ROS1 rearrangement: An international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017;18:1590–1599. doi: 10.1016/S1470-2045(17)30680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non–small-cell lung cancer: Results from a global phase 2 study. Lancet Oncol. 2018;19:1654–1667. doi: 10.1016/S1470-2045(18)30649-1. [DOI] [PubMed] [Google Scholar]
- 18.Cristescu R, Lee J, Nebozhyn M, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449–456. doi: 10.1038/nm.3850. [DOI] [PubMed] [Google Scholar]
- 19.Lanman RB, Mortimer SA, Zill OA, et al. Analytical and clinical validation of a digital sequencing panel for quantitative, highly accurate evaluation of cell-free circulating tumor DNA. PLoS One. 2015;10:e0140712. doi: 10.1371/journal.pone.0140712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Odegaard JI, Vincent JJ, Mortimer S, et al. Validation of a plasma-based comprehensive cancer genotyping assay utilizing orthogonal tissue- and plasma-based methodologies. Clin Cancer Res. 2018;24:3539–3549. doi: 10.1158/1078-0432.CCR-17-3831. [DOI] [PubMed] [Google Scholar]
- 21.Yoda S, Lin JJ, Lawrence MS, et al. Sequential ALK inhibitors can select for lorlatinib-resistant compound ALK mutations in ALK-positive lung cancer. Cancer Discov. 2018;8:714–729. doi: 10.1158/2159-8290.CD-17-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non–small-cell lung cancer: A single-group, multicentre, phase 2 trial. Lancet Oncol. 2016;17:234–242. doi: 10.1016/S1470-2045(15)00488-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horn L, Infante JR, Reckamp KL, et al. Ensartinib (X-396) in ALK-positive non–small-cell lung cancer: Results from a first-in-human phase I/II, multicenter study. Clin Cancer Res. 2018;24:2771–2779. doi: 10.1158/1078-0432.CCR-17-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non–small-cell lung cancer. N Engl J Med. 2015;372:1689–1699. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]
- 25.Oxnard GR, Thress KS, Alden RS, et al. Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non–small-cell lung cancer. J Clin Oncol. 2016;34:3375–3382. doi: 10.1200/JCO.2016.66.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oxnard GR, Paweletz CP, Sholl LM. Genomic analysis of plasma cell-free DNA in patients with cancer. JAMA Oncol. 2017;3:740–741. doi: 10.1001/jamaoncol.2016.2835. [DOI] [PubMed] [Google Scholar]
- 28.Dagogo-Jack I, Brannon AR, Ferris LA, et al. Tracking the evolution of resistance to ALK tyrosine kinase inhibitors through longitudinal analysis of circulating tumor DNA. JCO Precis Oncol. doi: 10.1200/PO.17.00160. 10.1200/PO.17.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]