Abstract
Background:
Patients with psychotic spectrum disorders share overlapping clinical/biological features, making it often difficult to separate them into a discrete nosology (i.e., Diagnostic and Statistical Manual of Mental Disorders [DSM]).
Methods:
The current study investigated whether a continuum classification scheme based on symptom burden would improve conceptualizations for cognitive and real-world dysfunction relative to traditional DSM nosology. Two independent samples (New Mexico [NM] and Bipolar and Schizophrenia Network on Intermediate Phenotypes [B-SNIP]) of patients with schizophrenia (NM: N=93; B-SNIP: N=236), bipolar disorder Type I (NM: N=42; B-SNIP: N=195) or schizoaffective disorder (NM: N=15; B-SNIP: N=148) and matched healthy controls (NM: N=64; B-SNIP: N=717) were examined. Linear regressions examined how variance differed as a function of classification scheme (DSM diagnosis, negative and positive symptom burden, or a three-cluster solution based on symptom burden).
Results:
Symptom-based classification schemes (continuous and clustered) accounted for a significantly larger portion of captured variance of real-world functioning relative to DSM diagnoses across both samples. The symptom-based classification schemes accounted for large percentages of variance for general cognitive ability and cognitive domains in the NM sample. However, in the B-SNIP sample, symptom-based classification schemes accounted for roughly equivalent variance as DSM diagnoses. A potential mediating variable across samples was the strength of the relationship between negative symptoms and impaired cognition.
Conclusions:
Current results support suggestions that a continuum perspective of psychopathology may be more powerful for explaining real-world functioning than the DSM diagnostic nosology, whereas results for cognitive dysfunction were sample dependent.
Keywords: Research Domain Criteria (RDoC), Hierarchical Taxonomy of Psychopathology (HiTOP), psychotic spectrum disorder, cluster, classification, symptoms
1. Introduction
Psychotic spectrum disorders are difficult to differentially diagnose and treat, often leaving their victims with lifetime disability (van Praag, 2000). It is increasingly recognized that traditionally distinct disorders such as schizophrenia, schizoaffective disorder and bipolar disorder with psychotic features share common genetic risk factors, neurobiological features, clinical presentations (i.e., positive and negative symptoms), neuroimaging findings, and treatment regimens (Kuswanto et al., 2016; Pearlson, 2015). These commonalities suggest that patients with psychotic spectrum disorders (PSD) may not fall neatly into a discrete nosology as currently conceptualized with the Diagnostic and Statistical Manual of Mental Disorders (DSM; Aukes et al., 2012; Keshavan et al., 2011). Rather, they may fall along a continuum of psychoses and other neuropsychiatric symptoms (Kuswanto et al., 2016; Pearlson, 2015). Although this perspective of a continuum is not new (Coryell et al., 1984; Crow, 1986), it has been revitalized by the Research Domain Criteria (RDoC; Cuthbert, 2015) and Hierarchical Taxonomy of Psychopathology (HiTOP; Kotov et al., 2017) frameworks.
The primary differentiating factor between RDoC and HiTOP frameworks are the respective emphasis on more basic biological constructs (e.g., neural circuits) relative to more clinical phenomenon (Kotov et al., 2017). Potential current sources of methodological discrepancies for studying PSD within these frameworks include differences in inclusion criteria for psychosis characteristics (bipolar disorder Type I [BP-I] vs. Type II [BP-II]; Martinez-Aran et al., 2008) as well as how the onset and progression of cognitive deficits are defined (Bora, 2015; Lewandowski et al., 2011). Additional methodological and statistical differences include whether PSD are defined by a discreet classification system (i.e., DSM), through continuous variables (a continuum with increased statistical range), or by other objective clustering methods that do not involve expert diagnosis (Pearlson, 2015). Critically, depending on the methods/statistics employed, the same data set can yield different conclusions.
In a review, Kuswanto et al. (2016) found support for a continuum of neurocognitive impairments in which patients with schizophrenia (SP) performed the worst, followed by patients with BP-I, with BP-II performing the best across multiple cognitive domains. The Bipolar and Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study (Hill et al., 2013) also reported cognitive differences using a discreet classification (healthy control [HC]>BPI>patients with schizoaffective disorder [SA]>SP). In addition, they noted a relationship between psychosis, measured by the Schizo-Bipolar Scale (SBS), and impaired cognition. These studies suggest that cognitive deficits fall along a continuum of psychosis severity. However, it is not evident if the data lie on a unimodal (i.e., actual continuum) or multimodal (i.e., discrete disorders) distribution (Pearlson, 2015). The latter suggests that PSD share the same type of deficits but are quantitatively different, with SP more impaired than BP-I (Vohringer et al., 2013). Other literature suggests a more generalized cognitive impairment in SP, with specific cognitive domains (e.g., memory and executive function) also impaired in BP (Altshuler et al., 2004; Seidman et al., 2002; Sperry et al., 2015).
Others have adopted bottom-up clustering approaches based on cognitive performance to examine whether PSD group into traditional diagnostic or trans-diagnostic groups (Bora et al., 2016: euthymic BP and SP; Lewandowski et al., 2014: BP-I, SP, and SA). Both studies reported a four-cluster solution (one cognitively normal, one substantially impaired, and two with mixed neurocognitive profiles) that spanned traditional diagnostic nosology. However, SP were more likely to be included in the substantially impaired cluster than BP (Bora et al., 2016: 27.8% vs. 9.3%; Lewandowski et al., 2014: 41.5% vs. 15.1%), whereas BP were more likely to fall in the cognitively normal cluster than SP (Bora et al., 2016: 25.6% vs. 9.3%; Lewandowski et al., 2014: 39.7% vs. 12.2%). Very recently, in an independent sample of PSD, Lewandowski et al. (2018) reported a similar four cognitive subgroup solution.
Importantly, Lewandowski et al. (2014; 2018) also consistently found that the substantially cognitively impaired PSD cluster exhibited a higher symptom burden (positive and negative) and medication dose, whereas cognitively normal PSD exhibited the best community functioning. Others have also reported a strong relationship between symptoms, poor cognition, and functional outcome, with negative symptoms posited to be the mediator of these relationships (Lin et al., 2013; Ventura et al., 2009). Thus, after stratifying PSD based on cognition, it is still not clear which PSD clinical characteristics (e.g., diagnosis vs. psychotic features vs. negative symptoms) predict cognitive abilities and how all three of these clinical characteristics relate to real-word functioning.
The current study therefore stratified PSD based on traditional DSM diagnoses or symptom burden (a continuum based on positive and/or negative symptoms) to determine which classification system would provide improved explanatory power for cognitive and real-world dysfunction. In addition, patients were stratified with clusters based on symptom burden in order to mimic the range of variance available for DSM diagnoses, eliminating statistical differences associated with comparing a continuous (i.e., symptom burden) versus categorical (i.e., DSM diagnosis) variable. It also allows for comparisons to previous literature based on cognitive clustering (Bora et al., 2016; Lewandowski et al., 2014; 2018). We hypothesized that both the symptom-based continuum and cluster approaches would account for significantly more unique variance relative to the DSM nosology. This hypothesis was purposely tested across four separate statistical models in an attempt to bridge current findings with existent literature and to directly compare different methods for examining spectrum (i.e., RDoC or HiTOP) based hypotheses. We next tested the reproducibility of our findings (New Mexico [NM] sample) with a larger cohort of PSD from the B-SNIP study (Hill et al., 2013).
2. Methods and materials
2.1. NM Sample
2.1.1. Participants
One-hundred sixty-two PSD and 64 HC (18–50 years old) were consented to participate. Patients were recruited from local psychiatric centers and newspaper advertisements. Exclusion criteria included 1) contraindications for MRI, 2) developmental disorders (e.g., autism spectrum disorder or intellectual disability), 3) history of neurological diagnoses, 4) history of moderate or severe head injury (loss of consciousness >30 minutes), 5) current pregnancy, 6) electroconvulsive therapy (scheduled or within the previous month) and 7) recent history of substance abuse disorders (excluding marijuana or nicotine use). Additional exclusion criteria for HC were 1) history of an Axis 1 disorder, 2) history of substance abuse, 3) first-degree relative with a psychotic spectrum disorder, and 4) Beck Depression Inventory-II >29 (BDI; Beck et al., 1996).
All participants completed urine-based drug screens and were subsequently excluded if positive (except for marijuana for PSD). Twelve PSD did not complete all assessments, leaving a final cohort of 150 PSD (94 males; mean age=32.31±9.07 years) and 64 HC (40 males; mean age=32.67±7.96 years; see Supplemental Table S1 for sample race information). All participants provided informed consent according to institutional guidelines at the University of NM School of Medicine and were paid for participating. Psychiatric diagnoses were verified by a board-certified psychiatrist based on the Structured Clinical Interview for DSM-IV-TR (SCID-II; First et al., 2012) and included SP (N=93), BP-I (N=42), and SA (N=15).
2.1.2. Clinical Assessments
Clinical symptoms were assessed with the Positive and Negative Syndrome Scale (PANSS; Kay et al., 1987), Schizo-Bipolar Scale (SBS; Keshavan et al., 2011), Clinical Global Impressions Scale (CGI; Guy, 1976), Montgomery-Asberg Depression Rating Scale (MADRS; Montgomery and Asberg, 1979), Young Mania Rating Scale (YMRS; Young et al., 1978) and a medical history form. Data on extrapyramidal symptoms, smoking history, antipsychotic exposures (olanzapine equivalence; Gardner et al., 2010), as well as other psychotropic exposures (i.e., mood stabilizers, antidepressants), were also collected (see Supplemental Materials).
2.1.3. Neuropsychological and Functioning Assessments
All participants completed the Wechsler Test of Adult Reading (WTAR) to estimate pre-morbid intelligence (Wechsler, 2001) and the Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery (MCCB; Kern et al., 2004) to determine current cognitive functioning. Primary analyses focused on RDoC’s committee recommended domains (attention, working memory, and declarative memory) and processing speed. A general cognitive ability (GCA) score was calculated via principle component analysis using HC and PSD patients’ data (see Supplemental Methods).
Real-world functioning was assessed with the UCSD Performance-Based Skills Assessment Brief Version (UPSA-B; Patterson et al., 2001) and the Quality of Life Questionnaire in Schizophrenia 18 (S-QoL 18; Boyer et al., 2010). A family member of the PSD (N=110) also completed the Specific Levels of Functioning Informant Scale (SLOF-I; Schneider and Struening, 1983) for an independent assessment of functioning.
2.1.4. Statistical Analyses
Group comparisons for demographic, clinical, cognitive and functioning domains were conducted using one-way ANOVAs with either two (PSD, HC) or three (SP, BP-I, SA) factor levels. These analyses were performed to characterize the sample and therefore were not corrected for multiple comparisons. A series of linear regressions tested a priori hypotheses for cognitive performance (four domains), GCA, and real-world functioning (three domains). Analyses were Bonferonni corrected separately for the four cognitive (corrected p<0.013) and three real-world functioning (corrected p<0.017) domains, with primary analyses focusing on significant R2 change (ΔR2) from a base model that controlled for demographic and general illness variables (sex, age, and illness duration). The second step investigated the amount of unique variance captured by DSM-IV-TR diagnosis (SP, BP-I, and SA) and continuous representations of symptom burden in terms of cognitive and real-world functioning domains. Symptom burden was assessed with the PANSS positive and negative symptom scores and the SBS score, as these scores have been previously associated with cognitive function across PSD (Hill et al., 2013; Lewandowski et al., 2014). The primary model compared the DSM and symptom burden scores simultaneously to parse out unique variance (Model 1), whereas subsequent models examined for unique and shared variance (DSM=Model 2; Sx Burden=Model 3).
Finally, a limited range in the independent variable represents a critical but understudied confound for spectrum analyses. Specifically, a continuous independent variable (i.e., symptom burden) has a higher probability of capturing additional variance in a continuous dependent variable (e.g., real-world functioning) relative to a discrete variable (i.e., DSM diagnosis) due to increased range. Therefore, supplemental regressions (Model 4) were conducted using an empirically (k-means) determined three-cluster solution based on symptom burden to mimic the range of variance available for DSM diagnoses and compare to previous literature based on cognitive clustering (Bora et al., 2016; Lewandowski et al., 2014; 2018).
2.2. B-SNIP Replication Sample
B-SNIP data from the NIMH Data Archive were utilized as an independent replication sample (for study details see Hill et al., 2013; Tamminga et al., 2013). The B-SNIP study was a five-site consortium that collected clinical and intermediate phenotypes on PSD (Tamminga et al., 2013). B-SNIP participants were included if they were 1) 18–50 years old to mirror the NM sample, 2) had valid cognitive data (Brief Assessment of Cognition in Schizophrenia; BACS) and 3) PSD had symptom ratings (PANSS). This resulted in a total replication sample of 717 HC (281 males; mean age=33.67±10.48 years), 236 SP (166 males; mean age=31.99±9.45 years), 148 SA (60 males; mean age=33.60±9.14 years), and 195 BP-I (76 males; mean age=32.51±9.67 years; see Supplemental Table S1 for sample race information). Groups did not differ in age (p=0.104). GCA and the Social Functioning Scale (SFS; Birchwood et al., 1990) served as primary replication variables.
3. Results
3.1. NM Sample: Demographic, Clinical and Neuropsychological Data
Results for demographic, clinical, neuropsychological and functioning data are summarized in Tables 1 and 2. Age and sex distribution (63% male in both groups) was similar both between PSD and HC as well as within disorder sub-groups. As expected, relative to HC, PSD had lower years of education, lower pre-morbid intelligence (WTAR), worse functioning (UPSA-B), poorer quality of life (S-QoL 18), greater cognitive deficits (attention, processing speed, working memory and declarative memory) and were more addicted to nicotine (FTND). Education also varied among PSD sub-groups.
Table 1:
Summary of demographic, cognitive and functioning assessments across all groups for the New Mexico sample.
| HC (N = 64) | PSD (N = 150) | SP (N = 93) | SA (N = 15) | BP-I (N = 42) | |||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | p value | Mean (SD) | Mean (SD) | Mean (SD) | p value | |
| Demographics | |||||||
| Sex (Females/Males) | 24/40 | 56/94 | p = 0.982 | 32/61 | 5/10 | 19/23 | p = 0.457 |
| Age (years) | 32.67 (7.96) | 32.31 (9.07) | p = 0.784 | 31.51 (8.54) | 32.53 (9.65) | 34.02 (9.96) | p = 0.329 |
| Education (years) | 14.98 (2.20) | 13.34 (2.39) | p < 0.001 | 13.01 (2.48) | 12.93 (1.94) | 14.21 (2.14) | p = 0.019 |
| FTND | 0.50 (1.41) | 1.51 (2.43) | p < 0.001 | 1.72 (2.53) | 1.50 (2.35) | 1.05 (2.22) | p = 0.340 |
| Cognition | |||||||
| WTAR | 56.40 (6.86) | 51.24 (10.57) | p < 0.001 | 49.11 (11.25) | 53.69 (8.98) | 55.06 (8.20) | p = 0.006 |
| Attention | 46.19 (9.68) | 36.53 (12.90) | p < 0.001 | 35.60 (12.60) | 35.73 (9.51) | 38.86 (14.49) | p = 0.404* |
| Processing speed | 50.05 (10.07) | 39.25 (11.49) | p < 0.001 | 38.22 (11.93) | 38.07 (10.57) | 41.95 (10.58) | p = 0.211* |
| Working memory | 47.28 (9.43) | 39.63 (11.58) | p < 0.001 | 38.06 (11.96) | 39.00 (12.02) | 43.31 (9.88) | p = 0.040* |
| Declarative memory | 48.35 (8.59) | 37.57 (10.41) | p < 0.001 | 35.43 (10.32) | 40.02 (10.18) | 41.44 (9.56) | p = 0.002* |
| General Cognitive Ability | 0.68 (0.72) | −0.29 (0.96) | p < 0.001 | −0.43 (0.98) | −0.22 (0.91) | 0.00 (0.91) | p = 0.041* |
| Functioning | |||||||
| UPSA-B total | 77.51 (11.84) | 68.73 (15.78) | p < 0.001 | 65.25 (15.97) | 70.77 (14.30) | 75.70 (13.56) | p = 0.002* |
| S-QoL 18 | 66.49 (9.59) | 56.69 (14.41) | p < 0.001 | 57.13 (13.97) | 57.55 (14.05) | 55.41 (15.70) | p = 0.631* |
| SLOF-I total | 87.43 (17.60) N=110 |
85.14 (17.50) N=68 |
73.00 (19.36) N=9 | 96.09 (13.15) N=33 |
p = 0.045* |
Notes: HC = Healthy controls; PSD = Psychotic Spectrum Disorders; SP = Patients with schizophrenia; SA = Patients with schizoaffective disorder; BP-I = Patients with bipolar disorder, Type I; FTND = Fagerstrom Test for Nicotine Dependence; WTAR = Wechsler Test of Adult Reading; UPSA-B = UCSD Performance Based Skills Assessment Brief Version; S-QoL 18 = Quality of Life Questionnaire in Schizophrenia 18; SLOF-I = Specific Levels of Functioning Informant Scale;
Table 2:
Summary of clinical assessments across all groups for the New Mexico sample.
| PSD (N = 150) | SP (N = 93) | SA (N = 15) | BP-I (N = 42) | ||
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | p value | |
| Clinical Measures | |||||
| Age of onset (years) | 20.59 (6.01) | 21.31 (5.81) | 20.07 (8.00) | 19.19 (5.51) | p = 0.155 |
| Illness duration (years) | 11.72 (9.17) | 10.19 (8.35) | 12.47 (9.30) | 14.83 (10.22) | p = 0.022 |
| PANSS positive | 14.03 (5.67) | 15.02 (5.86) | 15.20 (5.29) | 11.43 (4.55) | p = 0.002 |
| PANSS negative | 13.78 (5.36) | 15.22 (5.71) | 11.67 (3.74) | 11.36 (3.75) | p < 0.001 |
| PANSS total | 54.43 (15.49) | 58.37 (16.24) | 53.47 (12.98) | 46.07 (10.77) | p < 0.001 |
| CGI | 3.52 (0.91) | 3.74 (0.90) | 3.53 (0.52) | 3.02 (0.87) | p < 0.001 |
| MADRS | 8.44 (7.09) | 9.48 (7.08) | 9.53 (8.80) | 5.74 (5.78) | p = 0.014 |
| YMRS | 4.31 (6.36) | 3.67 (6.07) | 5.80 (5.06) | 5.19 (7.27) | p = 0.276 |
| SBS | 5.80 (2.89) | 7.58 (1.51) | 5.47 (1.13) | 1.98 (1.59) | p < 0.001 |
| Olanzapine equivalent | 12.49 (10.29) | 14.40 (9.67) | 14.54 (16.28) | 7.54 (7.03) | p = 0.001 |
| Extrapyramidal Symptoms | |||||
| AIMS | 0.72 (1.34) | 0.64 (1.07) | 1.40 (1.99) | 0.64 (1.53) | p = 0.112 |
| BAS | 0.53 (1.19) | 0.46 (1.04) | 1.07 (1.75) | 0.48 (1.23) | p = 0.178 |
| SAS | 0.99 (1.83) | 1.07 (1.90) | 1.13 (1.60) | 0.76 (1.75) | p = 0.639 |
Notes: PSD = Psychotic Spectrum Disorders; SP = Patients with schizophrenia; SA = Patients with schizoaffective disorder; BP-I = Patients with bipolar disorder, Type I; AIMS = Abnormal Involuntary Movement Scale (average total for the first 7 variables); BAS = Barnes Akathisia Scale; SAS = Simpson Angus Scale; PANSS = Positive and Negative Syndrome Scale; CGI = Clinical Global Impressions Scale; MADRS = Montgomery and Asberg Depression Rating Scale; YMRS = Young Mania Rating Scale; SBS = Schizo-Bipolar Scale. All p-values derived from sub-group comparisons.
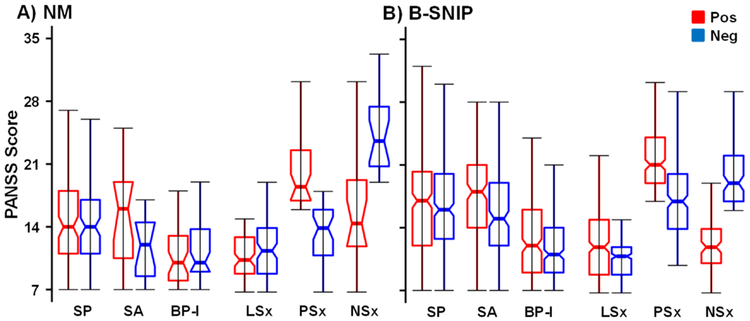
From a clinical perspective (Table 2), PSD subgroups did not statistically differ in manic (YMRS) or extrapyramidal symptoms (AIMS, BAS and SAS). The SBS was significantly stratified according to DSM-IV-TR diagnosis (SP>SA>BP-I), and the PANSS positive (SP≈SA>BP-I) and negative (SP>SA≈BP-I) scales also significantly differed across subgroup membership (Figure 1A). SP and SA were more clinically severe (CGI) and took higher antipsychotic medication dosages (olanzapine equivalent) relative to BP-I (SP≈SA>BP-I). Finally, illness duration (BP-I>SP) and depression scores (MADRS: SP>BP-I) significantly differed between the sub-groups.
Figure 1:
Box plots are used to illustrate Positive and Negative Syndrome Scale (PANSS) negative (Neg; blue color) and PANSS positive (Pos: red color) symptom scores for both the New Mexico (NM; Panel A) and the Bipolar and Schizophrenia Network on Intermediate Phenotypes (B-SNIP; Panel B) samples. Data are plotted for patients with psychotic spectrum disorders (PSD; notched boxes), as well as for individual patient subgroups diagnosed with schizophrenia (SP), schizoaffective disorder (SA) or bipolar disorder Type I (BP-I). Data are also plotted for symptom clusters of patients exhibiting low positive and negative symptoms (LSx), high positive and lower negative symptoms (PSx) and high negative and lower positive symptoms (NSx). Asterisks were not used to denote significance due to the between subject nature of analyses.
3.2. Cognitive Domains
3.2.1. NM Sample
For the regressions conducted using cognitive domain scores and GCA as the dependent variables (Table 3), the base model (i.e., sex, age, and illness duration) was only significant for the attention domain (p=0.041), accounting for a minimal amount of variance (ΔR2=5.5%). A direct comparison of DSM diagnosis and continuous symptom burden (Model 1) indicated that cognitive variance was mainly accounted for by negative (p range<0.001) and positive (p range=0.001–0.022) symptom burden rather than DSM diagnosis (p range=0.371–0.998) or SBS (p range=0.675–0.900).
Table 3:
Summary of results from regression analyses using cognitive domains and GCA as dependent variables.
| Cognitive Domains | DSM and Sx Burden (Model 1) | DSM (Model 2) | Sx Burden (Model 3) | Sx Burden Clust (Model 4) |
|---|---|---|---|---|
| Attention | ||||
| ΔR2 (p value) | 0.228 (<0.001) | 0.005 (0.404) | 0.226 (<0.001) | 0.179 (<0.001) |
| IV: Beta (p value) | DSM = −0.086 (0.541) | DSM = 0.070 (0.404) | DSM = NA | Sx Clust = −0.437 (<0.001) |
| Positive = −0.255 (0.001) | Positive = NA | Positive = −0.252 (0.001) | ||
| Negative = −0.417 (<0.001) | Negative = NA | Negative = −0.415 (<0.001) | ||
| SBS = 0.026 (0.849) | SBS = NA | SBS = 0.097 (0.213) | ||
| Processing Speed | ||||
| ΔR2 (p value) | 0.239 (< 0.001) | 0.011 (0.211) | 0.239 (< 0.001) | 0.202 (<0.001) |
| IV: Beta (p value) | DSM = −0.056 (0.694) | DSM = 0.106 (0.211) | DSM = NA | Sx Clust= −0.464 (<0.001) |
| Positive = −0.192 (0.013) | Positive = NA | Positive = −0.190 (0.014) | ||
| Negative = −0.465 (<0.001) | Negative = NA | Negative = −0.464 (< 0.001) | ||
| SBS = 0.018 (0.900) | SBS = NA | SBS = 0.063 (0.419) | ||
| Working Memory | ||||
| ΔR2 (p value) | 0.205 (< 0.001) | 0.028 (0.040) | 0.205 (<0.001) | 0.136 (<0.001) |
| IV: Beta (p value) | DSM = 0.000 (0.998) | DSM = 0.173 (0.040) | DSM = NA | Sx Clust = −0.381 (< 0.001) |
| Positive = −0.227 (0.004) | Positive = NA | Positive = −0.227 (0.004) | ||
| Negative = −0.368 (<0.001) | Negative = NA | Negative = −0.368 (<0.001) | ||
| SBS = −0.019 (0.897) | SBS = NA | SBS = −0.018 (0.820) | ||
| Declarative Memory/ | ||||
| ΔR2 (p value) | 0.325 (< 0.001) | 0.063 (0.002) | 0.321 (< 0.001) | 0.186 (<0.001) |
| IV: Beta (p value) | DSM = 0.121 (0.371) | DSM = 0.259 (0.002) | DSM = NA | Sx Clust = −0.446 (<0.001) |
| Positive = −0.169 (0.022) | Positive = NA | Positive = −0.174 (0.018) | ||
| Negative = −0.519 (< 0.001) | Negative = NA | Negative = −0.522 (< 0.001) | ||
| SBS = 0.056 (0.675) | SBS = NA | SBS = −0.043 (0.565) | ||
| GCA | ||||
| ΔR2 (p value) | 0.310 (< 0.001) | 0.028 (0.041) | 0.310 (< 0.001) | 0.209 (< 0.001) |
| IV: Beta (p value) | DSM = 0.009 (0.947) | DSM = 0.173 (0.041) | DSM = NA | Sx Clust = −0.472 (< 0.001) |
| Positive = −0.195 (0.008) | Positive = NA | Positive = −0.196 (0.008) | ||
| Negative = −0.528 (< 0.001) | Negative = NA | Negative = −0.528 (< 0.001) | ||
| SBS = 0.037 (0.782) | SBS = NA | SBS = 0.030 (0.690) | ||
| B-SNIP GCA | ||||
| ΔR2 (p value) | 0.123 (< 0.001) | 0.083 (<0.001) | 0.089 (<0.001) | 0.058 (<0.001) |
| IV: Beta (p value) | DSM = 0.209 (< 0.001) | DSM = 0.299 (<0.001) | DSM = NA | Sx Clust = 0.243 (<0.001) |
| Positive = −0.076 (0.073) | Positive = NA | Positive = −0.115 | ||
| Negative = −0.177 (< 0.001) | Negative = NA | Negative = −0.234 (<0.001) | ||
Notes: DSM = Diagnostic and Statistical Manual of Mental Disorders Diagnoses (i.e. schizophrenia, schizoaffective disorder, or bipolar disorder, Type I); Sx Burden = symptom burden based on the Positive and Negative Syndrome Scale (PANSS) and the Schizo-Bipolar Scale (SBS) scores; Sx Burden Clust = three clusters of PANSS positive and negative symptom scores; IV = independent variables; Positive = PANSS positive symptom score; Negative = PANSS negative symptom score; SBS = Schizo-Bipolar Scale; GCA = general cognitive ability. B-SNIP = Bipolar and Schizophrenia Network on Intermediate Phenotypes; Beta values are standardized.
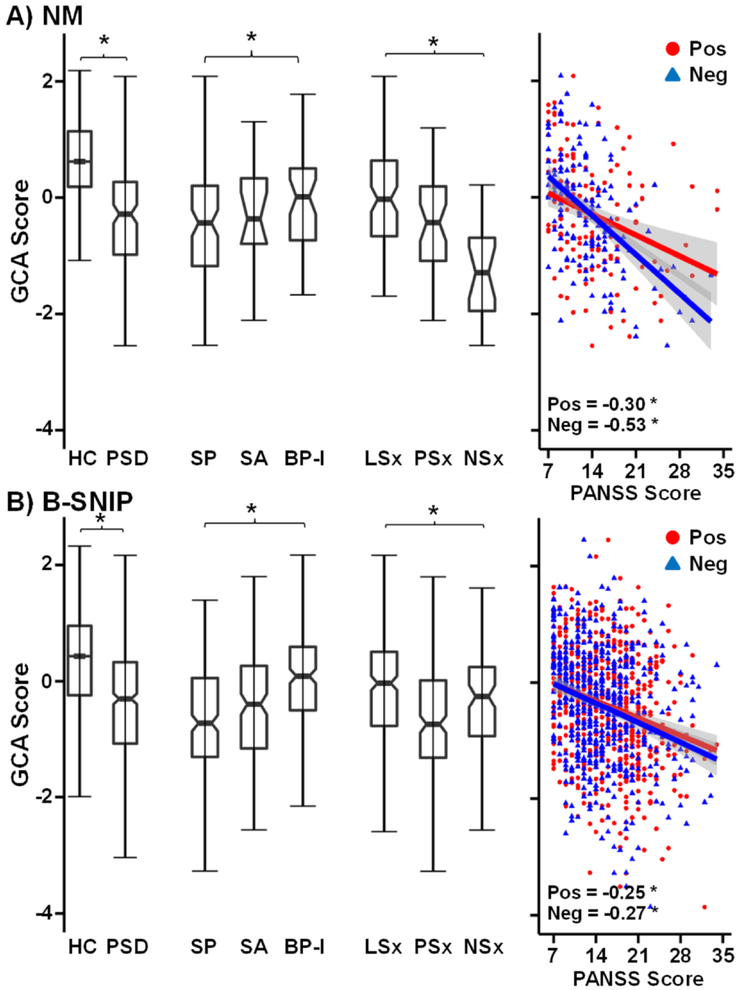
When examined alone, DSM diagnosis (Model 2) accounted for significant variance only for declarative memory performance (ΔR2=6.3%) following correction for multiple comparisons. Follow-up analyses indicated that performance differences on declarative memory resulted from subgroup differences between SP and BP-I (p=0.003; Supplemental Figure S1B) rather than between SP and SA or BP-I and SA. DSM diagnosis alone also accounted for a significant percentage of GCA (ΔR2=2.8%; Figure 2A), with SP performing worse than BP-I (p=0.017).
Figure 2:
Box plots on the left depict general cognitive ability (GCA) score for both the New Mexico (NM; Panel A) and the Bipolar and Schizophrenia Network on Intermediate Phenotypes (B-SNIP; Panel B) samples. Data are separately plotted for healthy controls (HC; unnotched boxes) and patients with psychotic spectrum disorders (PSD; notched boxes), as well as for patient subgroups diagnosed with schizophrenia (SP), schizoaffective disorder (SA) or bipolar disorder Type I (BP-I). Data are also plotted for symptom clusters (PSD only) of patients exhibiting low positive and negative symptoms (LSx), high positive and lower negative symptoms (PSx) and high negative and lower positive symptoms (NSx). Scatterplots are included on the right for GCA against Positive and Negative Syndrome Scale (PANSS) negative (Neg; blue triangles) and PANSS positive (Pos: red circles) symptom scores. Correlation coefficients are provided for each sample with model significance denoted by an asterisk (p < 0.05).
Regression analyses for symptom burden alone (PANSS positive and negative symptom scores and SBS; Model 3) were statistically significant (Table 3; Supplemental Figure S1) and accounted for large percentages of variance in all cognitive domains (ΔR2 range=20.5%–32.1%) and GCA (ΔR2=31.0%), similar to Model 1. Both positive and negative symptoms accounted for unique variance, with negative symptoms exhibiting the strongest correlation (Supplemental Figure S1 and Figure 2A). The SBS did not account for unique variance for any cognitive measure, with supplementary analyses confirming that the same was true for depression symptoms (MADRS; see Supplemental Results/Supplemental Table S2).
Model 4 imitated the categorical nature of the three DSM diagnoses using an empirically (k-means) determined three-cluster solution of the PANSS positive and negative symptom scores (see Supplemental Material). PSD were clustered into groups with low positive and negative symptoms (LSx), high positive and lower negative symptoms (PSx) and high negative and lower positive symptoms (NSx; see Supplementary Table S3).
In contrast to DSM results (Model 2), the nominal symptom clustered solution (Model 4) explained significant and substantial variance for all cognitive domains and GCA (see Supplemental Results).
3.2.2. B-SNIP Replication Sample
Clinically, PANSS positive (SP≈SA>BP-I; F2,576=44.92, p<0.001) and negative (SP≈SA>BP-I; F2,576=48.24, p<0.001) scales significantly differed as a function of DSM subgroup membership (Figure 1B). A k-means clustering approach (3 clusters based on PANSS positive and negative symptom scores) again generated groups of PSD clustered by low positive and negative symptoms (LSx; N=278), high positive symptoms (PSx; N=207) and high negative symptoms (NSx; N=94). Supplementary Table S3 displays the percentage of PSD from each DSM diagnostic category represented in each cluster.
Results from the B-SNIP sample indicated that the base model (i.e., sex and age) was significant for GCA (p<0.001). DSM diagnosis and continuous symptoms burden (Model 1) collectively accounted for a significant percentage of the variance in GCA (ΔR2=12.3%; Table 3), with unique variance accounted for by DSM diagnosis (p<0.001) and negative symptoms (p<0.001). Positive symptoms were not significant (p=0.073). DSM diagnosis (Model 2) alone continued to account for a significant amount of the variance (ΔR2=8.3%; Figure 2B). Follow-up analyses indicated lower GCA in SP than SA (p=0.009), who had worse ability than BP-I (p<0.001). The PANSS continuous measure of symptom burden (Model 3) was statistically significant (ΔR2=8.9%), with both positive and negative (strongest correlation) symptoms accounting for unique variance (Table 3; Figure 2B). The symptom cluster solution (Model 4) also explained significant variance with a pattern of decreasing GCA as a function of symptom burden (see Supplemental Results).
3.3. Functioning Domains
3.3.1. NM Sample
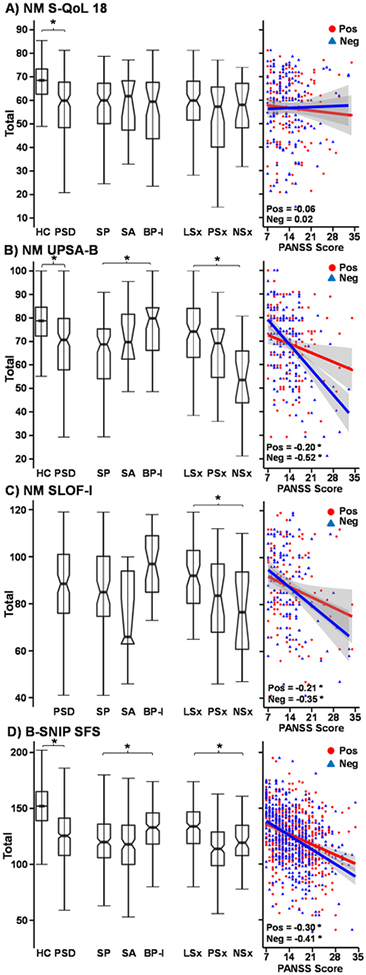
Regressions performed on real-world functioning (Table 4) were separated into performance-based (UPSA-B), self-report (S-QoL 18) and collateral report (SLOF-I) measures. Results indicated that the base model (i.e., sex, age, and illness duration) was significant for both the UPSA-B (ΔR2=9.2%; p=0.003) and the SLOF-I (ΔR2=12.1%; p=0.003). None of the demographic/illness variables exhibited a significant and unique relationship with the UPSA-B, whereas patient’s sex was significant for the SLOF-I (Beta=−0.247; p=0.010). DSM diagnosis and continuous symptom burden (Model 1) accounted for significant overall variance in UPSAB (ΔR2=22.2%), with the SLOF-I (ΔR2=9.6%) p-value just above the multiple comparisons threshold. However, inspection of the individual variables showed negative symptoms to be the only significant (p<0.001) predictor of unique UPSA-B variance. When considered alone (Model 2), DSM diagnosis was associated with significant variance in the UPSA-B (ΔR2=5.6%), with differences between SP and BP-I only (p=0.003) rather than between SP and SA or BP-I and SA (Figure 3).
Table 4:
Summary of results from regression analyses using measures of function as dependent variables.
| Function | DSM and Sx Burden (Model 1) | DSM (Model 2) | Sx Burden (Model 3) | Sx Burden Clust (Model 4) |
|---|---|---|---|---|
| UPSA-B | ||||
| ΔR2 (p value) | 0.222 (< 0.001) | 0.056 (0.002) | 0.221 (<0.001) | 0.133 (<0.001) |
| IV: Beta (p value) | DSM = 0.065 (0.634) | DSM = 0.244 (0.002) | DSM = NA | Sx Clust = −0.376 (<0.001) |
| Positive = −0.048 (0.516) | Positive = NA | Positive = −0.051 (0.492) | ||
| Negative = −0.439 (< 0.001) | Negative = NA | Negative = −0.441 (<0.001) | ||
| SBS = −0.050 (0.711) | SBS = NA | SBS = −0.104 (0.172) | ||
| S-QoL 18 | ||||
| ΔR2 (p value) | 0.010 (0.849) | 0.002 (0.631) | 0.010 (0.711) | 0.008 (0.292) |
| IV: Beta (p value) | DSM = 0.000 (0.998) | DSM = −0.041 (0.631) | DSM = NA | Sx Clust = −0.090 (0.292) |
| Positive = −0.087 (0.330) | Positive = NA | Positive = −0.087 (0.326) | ||
| Negative = −0.002 (0.983) | Negative = NA | Negative = −0.002 (0.983) | ||
| SBS = 0.073 (0.657) | SBS = NA | SBS = 0.072 (0.426) | ||
| SLOF-I | ||||
| ΔR2 (p value) | 0.096 (0.018) | 0.033 (0.045) | 0.095 (0.008) | 0.063 (0.005) |
| IV: Beta (p value) | DSM = −0.049 (0.777) | DSM = 0.191 (0.045) | DSM = NA | Sx Clust = −0.257 (0.005) |
| Positive = −0.095 (0.320) | Positive = NA | Positive = −0.092 (0.331) | ||
| Negative = −0.214 (0.034) | Negative = NA | Negative = −0.213 (0.034) | ||
| SBS = −0.170 (0.330) | SBS = NA | SBS = −0.130 (0.195) | ||
| B-SNIP SFS | ||||
| ΔR2 (p value) | 0.185 (< 0.001) | 0.048 (<0.001) | 0.182 (<0.001) | 0.130 (<0.001) |
| IV: Beta (p value) | DSM = 0.069 (0.143) | DSM = 0.229 (<0.001) | DSM = NA | Sx Clust = 0.363 (<0.001) |
| Positive = −0.141 (0.003) | Positive = NA | Positive = −0.151 (0.001) | ||
| Negative = −0.323 (< 0.001) | Negative = NA | Negative = −0.342 (<0.001) | ||
Notes: DSM = Diagnostic and Statistical Manual of Mental Disorders Diagnoses (i.e. schizophrenia, schizoaffective disorder, or bipolar disorder, Type I); Sx Burden = symptom burden based on the Positive and Negative Syndrome Scale (PANSS) and the Schizo-Bipolar Scale (SBS) scores; Sx Burden Clust = three clusters of PANSS positive and negative symptom scores; UPSA-B = UCSD Performance Based Skills Assessment Brief Version; S-QoL 18 = Quality of Life Questionnaire in Schizophrenia 18; SLOF-I = Specific Levels of Functioning Informant Scale; IV = independent variables; Positive = PANSS positive symptom score; Negative = PANSS negative symptom score; SBS = Schizo-Bipolar Scale; B-SNIP = Bipolar and Schizophrenia Network on Intermediate Phenotypes; SFS = Social Functioning Scale. Beta values are standardized.
Figure 3:
On the left are box plots depicting the total score for each functioning measure examined. For the New Mexico (NM) sample, this included the Quality of Life Questionnaire in Schizophrenia 18 (S-QoL 18; Panel A), UCSD Performance Based Skills Assessment Brief Version (UPSA-B; Panel B), and the Specific Level of Functioning Informant Scale (SLOF-I; Panel C). For the Bipolar and Schizophrenia Network on Intermediate Phenotypes (B-SNIP) sample, this included the Social Functioning Scale (SFS; Panel D). Results are displayed for healthy controls (HC; unnotched boxes) and patients with psychotic spectrum disorders (PSD; notched boxes), as well as for individual patient subgroups diagnosed with schizophrenia (SP), schizoaffective disorder (SA) or bipolar disorder Type I (BP-I). Data are also plotted for symptom clusters (PSD only) of patients exhibiting low positive and negative symptoms (LSx), high positive and lower negative symptoms (PSx) and high negative and lower positive symptoms (NSx). Scatterplots for each functioning measure (S-QoL 18, UPSA-B, SLOF-I, and SFS) against Positive and Negative Syndrome Scale (PANSS) negative (Neg; blue triangles) and PANSS positive (Pos: red circles) symptom scores are included on the right. Correlation coefficients are provided for each sample, and model significance is denoted with an asterisk (NM: corrected p < 0.017; B-SNIP: p < 0.05).
Symptom burden alone (PANSS positive/negative and SBS; Model 3) was statistically significant for UPSA-B (ΔR2=22.1%) and SLOF-I (ΔR2=9.5%; Table 4), with the only significant relationships observed with negative symptoms (Figure 3). Similarly, the three-cluster solution (Model 4; Figure 3) accounted for significant UPSA-B and SLOF-I variance (see Supplemental Results).
3.3.2. B-SNIP Replication Sample
Regression results from the B-SNIP sample indicated that the base model (i.e. sex and age) was not significant for social functioning (SFS; p=0.130). Collectively DSM diagnosis and continuous symptom burden (Model 1; Table 4) accounted for overall variance in SFS (ΔR2=18.5%), with unique variance captured by both positive (p=0.003) and negative (p<0.001) symptom burden rather than DSM diagnosis. DSM diagnosis accounted for significant SFS variance (ΔR2=4.8%; SP≈SA<BP-I; both p’s<0.001) when it was the sole predictor in Model 2. The regression including PANSS symptoms alone (Model 3) accounted for almost four times the amount of variance relative to DSM symptoms (ΔR2=18.2%), with positive and negative symptoms (strongest) each accounting for unique variance (Table 4; Figure 3D). The clustered symptom solution (Model 4) explained twice as much variance for social functioning as the DSM (see Supplemental Results).
4. Discussion
The current study investigated whether dimensional, spectrum-based classification schemes using positive and/or negative symptom burden would improve conceptualizations for cognitive and real-world dysfunction relative to traditional DSM nosology. Consistent with previous results (Mausbach et al., 2010), findings from two independent, large samples overwhelmingly supported a symptom-burden perspective for explaining real-world functioning. The amount of unique variance was much larger for symptom burden relative to DSM nosologies and remained relatively consistent when symptom burden was discretized into three categories, eliminating confounds associated with the narrower DSM statistical range (Model 4). However, in the NM sample only negative symptoms accounted for unique real-world functioning variance, where both positive and negative symptoms were significant in the B-SNIP sample. Collectively, current and previous results support HiTOP suggestions that clinical dimensions (e.g., symptoms, personality traits, syndromes) may more accurately account for the functional impairment associated with psychopathology (Kotov et al., 2017).
In contrast, results for cognitive dysfunction were more variable. DSM nosology alone (Model 2) accounted for a significant, albeit modest, amount of variance in terms of GCA deficits across two independent PSD samples, with worse impairment observed in SP (SP<SA<BP-I). These results are generally consistent with previous work (Hill et al., 2013; Kuswanto et al., 2016), and reinforce the notion of increased general cognitive impairment in SP relative to other PSD. However, metrics of symptom burden accounted for substantially more variance than DSM diagnoses in the NM sample regardless if modeled simultaneously (Model 1), or considered as a continuous (Model 3) or a categorical (Model 4) variable. In contrast, DSM diagnoses and symptom burden accounted for approximately equivalent and unique amounts of variance in the B-SNIP sample across all models (Hill et al., 2013; Tamminga et al., 2013).
There are several potential explanations for the discrepancies observed across our two samples. Previous work suggests that negative symptoms represent a mediating variable between poor cognition and functional outcome (Lin et al., 2013; Ventura et al., 2009) and share a similar clinical course/onset time with cognitive deficits in SP (Cullen et al., 2011). Negative symptoms, lower cognitive performance and real-world functioning are also largely unaffected by current antipsychotic medications (Buchanan et al., 2007; Harvey et al., 2004; Strassnig and Harvey, 2014), suggesting that all three deficits may rely on a similar neural network or genetic disposition. Although positive and negative symptoms accounted for unique cognitive variance in both samples, the relationships between cognitive ability and negative symptoms was much stronger (i.e., almost double) than it was with positive symptoms in the NM sample. Moreover, the B-SNIP (high positive-lower negative symptom cluster=worst cognitive impairment/real-world functioning) and NM (high negative-lower positive symptom cluster=worst cognitive impairment/real-world functioning) samples differed in terms of how cognition related to symptoms.
Second, there were several differences between the NM and B-SNIP samples including single versus multisite recruitment, sex and race distribution among DSM diagnoses, cognitive test battery (screen vs. extensive battery), sample size and the percentage of SA included. The nosological status of SA has been controversial for some time (Madre et al., 2016; Maier, 2006), with poor diagnostic reliability, stability, and validity, resulting in excessive misdiagnosis for an intended uncommon disorder (Malaspina et al., 2013). Third, the relationship between cognitive deficits and symptom burden may be state dependent, whereas DSM diagnosis is determined from past symptoms/traits. Thus, it is challenging to disambiguate whether the relationship represents current disease state, which could have varied based on sample, versus a phenotype/biomarker/trait that differed based on the previously noted sample differences.
Evidence for specific cognitive impairment in SP was present only for declarative memory in the NM sample (data not available for B-SNIP). Deficits in verbal learning/memory are one of the most commonly reported findings in SP (Bowie and Harvey, 2005; Hanlon et al., 2011; Heinrichs and Vaz, 2004; Saykin et al., 1991), even when controlling for symptom-based scores (Sperry et al., 2015). However, the significant relationship between DSM diagnosis and memory deficits was no longer present when symptom burden was considered (Model 1), which was similar to previous results with null findings in relation to memory (Ivleva et al., 2012; Kuswanto et al., 2013). Thus, the concept of “domain-specific” cognitive impairment across psychotic spectrum disorders remains actively debated (Altshuler et al., 2004; Bora and Ozerdem, 2017; Mausbach et al., 2010; Seidman et al., 2002; Sperry et al., 2015; Woodward, 2016), with results complicated by sampling differences, inclusion/exclusion criteria and inconsistencies in the testing measures utilized (Kuswanto et al., 2016).
Finally, a myriad of analyses, measures and outcomes exist when researching RDoC/HiTOP frameworks, with each choice having ramifications on the types of conclusions that can potentially be drawn from each study (Kozak and Cuthbert, 2016). Importantly, these different approaches should generally converge, as previous PSD studies clustering based on cognition (Bora et al., 2016; Lewandowski et al., 2014; 2018) and symptoms (current study) have done (i.e., higher percentage of SP in most impaired clusters vs. higher percentage of BP in the cognitively unimpaired cluster). Conceptually, both appear to be a different methodological approach for arriving at the similar conclusion of generally greater general cognitive impairment in SP. However, as exemplified by the “different” results obtained from the models in the current study, the “significance” of the DSM nosology partially depends on statistical methodology.
The current study had several limitations. First, traditional neuropsychological batteries have been questioned by the RDoC Workgroup (2016) for their specificity. However, neuropsychological batteries (e.g., MCCB) are extensively standardized (August et al., 2012; Kern et al., 2008), whereas certain recommended RDoC tasks (e.g., AX-CPT) have been questioned on basic psychometric properties (Cooper et al., 2017). Second, SP and SA were more clinically severe/medicated relative to BP-I. Although expected given typical disease course, future work with non-medicated or early-onset PSD would be informative. Finally, although commonly used for comparative and therapeutic purposes in the literature, it is important to note that DSM nosology was originally designed to increase diagnostic reliability (Pearlson, 2015).
In summary, the overarching purpose of RDoC/HiTOP frameworks is not to replace current diagnostic systems (DSM-V or International Classification of Diseases), but to potentially inform the field of more optimal classifications for mental illness based on dimensional clinical symptoms and biomarkers (Clark et al., 2017). To this end, a continuum perspective based on symptom burden accounted for greater variance in real-world functioning than traditional DSM diagnostic nosology, whereas results for cognitive dysfunction were sample dependent. Continuum based perspectives may potentially serve as a powerful mediating or moderating variable for determining outcomes during clinical trials.
Supplementary Material
Acknowledgements
We would like to thank Diana South and Catherine Smith for their assistance with data collection.
Funding
This work was supported by the National Institutes of Health (grant number 1R01MH101512–03 to A.R.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- Altshuler LL, Ventura J, van Gorp WG, Green MF, Theberge DC, Mintz J, 2004. Neurocognitive function in clinically stable men with bipolar I disorder or schizophrenia and normal control subjects. Biol. Psychiatry 56 (8) 560–569. [DOI] [PubMed] [Google Scholar]
- August SM, Kiwanuka JN, McMahon RP, Gold JM, 2012. The MATRICS Consensus Cognitive Battery (MCCB): clinical and cognitive correlates. Schizophr. Res 134 (1) 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aukes MF, Laan W, Termorshuizen F, Buizer-Voskamp JE, Hennekam EA, Smeets HM, Ophoff RA, Boks MP, Kahn RS, 2012. Familial clustering of schizophrenia, bipolar disorder, and major depressive disorder. Genet. Med 14 (3) 338–341. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK, 1996. Manual for the Beck depression inventory-II San Antonio. [Google Scholar]
- Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S, 1990. The Social Functioning Scale. The development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. Br. J. Psychiatry 157 853–859. [DOI] [PubMed] [Google Scholar]
- Bora E, 2015. Developmental trajectory of cognitive impairment in bipolar disorder: comparison with schizophrenia. Eur. Neuropsychopharmacol 25 (2) 158–168. [DOI] [PubMed] [Google Scholar]
- Bora E, Ozerdem A, 2017. Meta-analysis of longitudinal studies of cognition in bipolar disorder: comparison with healthy controls and schizophrenia. Psychol. Med 47 (16) 2753–2766. [DOI] [PubMed] [Google Scholar]
- Bora E, Veznedaroglu B, Vahip S, 2016. Theory of mind and executive functions in schizophrenia and bipolar disorder: A cross-diagnostic latent class analysis for identification of neuropsychological subtypes. Schizophr. Res 176 (2–3) 500–505. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Harvey PD, 2005. Cognition in schizophrenia: impairments, determinants, and functional importance. Psychiatr. Clin. North Am 28 (3) 613–33, 626. [DOI] [PubMed] [Google Scholar]
- Boyer L, Simeoni MC, Loundou A, D’Amato T, Reine G, Lancon C, Auquier P, 2010. The development of the S-QoL 18: a shortened quality of life questionnaire for patients with schizophrenia. Schizophr. Res 121 (1–3) 241–250. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Javitt DC, Marder SR, Schooler NR, Gold JM, McMahon RP, Heresco-Levy U, Carpenter WT, 2007. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am. J. Psychiatry 164 (10) 1593–1602. [DOI] [PubMed] [Google Scholar]
- Clark LA, Cuthbert B, Lewis-Fernandez R, Narrow WE, Reed GM, 2017. Three Approaches to Understanding and Classifying Mental Disorder: ICD-11, DSM-5, and the National Institute of Mental Health’s Research Domain Criteria (RDoC). Psychol. Sci. Public Interest 18 (2) 72–145. [DOI] [PubMed] [Google Scholar]
- Cooper SR, Gonthier C, Barch DM, Braver TS, 2017. The Role of Psychometrics in Individual Differences Research in Cognition: A Case Study of the AX-CPT. Front. Psychol 8 1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coryell W, Lavori P, Endicott J, Keller M, VanEerdewegh M, 1984. Outcome in schizoaffective, psychotic, and nonpsychotic depression. Course during a six- to 24-month follow-up. Arch. Gen. Psychiatry 41 (8) 787–791. [DOI] [PubMed] [Google Scholar]
- Crow TJ, 1986. The continuum of psychosis and its implication for the structure of the gene. Br. J. Psychiatry 149 419–429. [DOI] [PubMed] [Google Scholar]
- Cullen K, Guimaraes A, Wozniak J, Anjum A, Schulz SC, White T, 2011. Trajectories of social withdrawal and cognitive decline in the schizophrenia prodrome. Clin. Schizophr. Relat. Psychoses 4 (4) 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, 2015. Research Domain Criteria: toward future psychiatric nosologies. Dialogues. Clin. Neurosci 17 (1) 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB, 2012. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), Clinician Version, Administration Booklet American Psychiatric Pub, Arlington, VA. [Google Scholar]
- Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ, 2010. International consensus study of antipsychotic dosing. Am. J. Psychiatry 167 (6) 686–693. [DOI] [PubMed] [Google Scholar]
- Guy W, 1976. Clinical global impression scale. ECDEU assessment manual for psychopharmacology 338 218–222. [Google Scholar]
- Hanlon FM, Houck JM, Pyeatt CJ, Lundy SL, Euler MJ, Weisend MP, Thoma RJ, Bustillo JR, Miller GA, Tesche CD, 2011. Bilateral hippocampal dysfunction in schizophrenia. Neuroimage 58 (4) 1158–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Green MF, Keefe RS, Velligan DI, 2004. Cognitive functioning in schizophrenia: a consensus statement on its role in the definition and evaluation of effective treatments for the illness. J. Clin. Psychiatry 65 (3) 361–372. [PubMed] [Google Scholar]
- Heinrichs RW, Vaz SM, 2004. Verbal memory errors and symptoms in schizophrenia. Cogn. Behav. Neurol 17 (2) 98–101. [DOI] [PubMed] [Google Scholar]
- Hill SK, Reilly JL, Keefe RS, Gold JM, Bishop JR, Gershon ES, Tamminga CA, Pearlson GD, Keshavan MS, Sweeney JA, 2013. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Am. J. Psychiatry 170 (11) 1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivleva EI, Morris DW, Osuji J, Moates AF, Carmody TJ, Thaker GK, Cullum M, Tamminga CA, 2012. Cognitive endophenotypes of psychosis within dimension and diagnosis. Psychiatry Res 196 (1) 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA, 1987. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bull 13 (2) 261–276. [DOI] [PubMed] [Google Scholar]
- Kern RS, Green MF, Nuechterlein KH, Deng BH, 2004. NIMH-MATRICS survey on assessment of neurocognition in schizophrenia. Schizophr. Res 72 (1) 11–19. [DOI] [PubMed] [Google Scholar]
- Kern RS, Nuechterlein KH, Green MF, Baade LE, Fenton WS, Gold JM, Keefe RS, Mesholam-Gately R, Mintz J, Seidman LJ, Stover E, Marder SR, 2008. The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. Am. J. Psychiatry 165 (2) 214–220. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Morris DW, Sweeney JA, Pearlson G, Thaker G, Seidman LJ, Eack SM, Tamminga C, 2011. A dimensional approach to the psychosis spectrum between bipolar disorder and schizophrenia: the Schizo-Bipolar Scale. Schizophr. Res 133 (1–3) 250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Krueger RF, Watson D, Achenbach TM, Althoff RR, Bagby RM, Brown TA, Carpenter WT, Caspi A, Clark LA, Eaton NR, Forbes MK, Forbush KT, Goldberg D, Hasin D, Hyman SE, Ivanova MY, Lynam DR, Markon K, Miller JD, Moffitt TE, Morey LC, Mullins-Sweatt SN, Ormel J, Patrick CJ, Regier DA, Rescorla L, Ruggero CJ, Samuel DB, Sellbom M, Simms LJ, Skodol AE, Slade T, South SC, Tackett JL, Waldman ID, Waszczuk MA, Widiger TA, Wright AGC, Zimmerman M, 2017. The Hierarchical Taxonomy of Psychopathology (HiTOP): A dimensional alternative to traditional nosologies. J. Abnorm. Psychol 126 (4) 454–477. [DOI] [PubMed] [Google Scholar]
- Kozak MJ, Cuthbert BN, 2016. The NIMH Research Domain Criteria Initiative: Background, Issues, and Pragmatics. Psychophysiology 53 (3) 286–297. [DOI] [PubMed] [Google Scholar]
- Kuswanto C, Chin R, Sum MY, Sengupta S, Fagiolini A, McIntyre RS, Vieta E, Sim K, 2016. Shared and divergent neurocognitive impairments in adult patients with schizophrenia and bipolar disorder: Whither the evidence? Neurosci. Biobehav. Rev 61 66–89. [DOI] [PubMed] [Google Scholar]
- Kuswanto CN, Sum MY, Sim K, 2013. Neurocognitive functioning in schizophrenia and bipolar disorder: clarifying concepts of diagnostic dichotomy vs. continuum. Front. Psychiatry 4 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski KE, Baker JT, McCarthy JM, Norris LA, Ongur D, 2018. Reproducibility of cognitive profiles in psychosis using cluster analysis. J. Int. Neuropsychol. Soc 24 (4) 382–390. [DOI] [PubMed] [Google Scholar]
- Lewandowski KE, Cohen BM, Ongur D, 2011. Evolution of neuropsychological dysfunction during the course of schizophrenia and bipolar disorder. Psychol. Med 41 (2) 225–241. [DOI] [PubMed] [Google Scholar]
- Lewandowski KE, Sperry SH, Cohen BM, Ongur D, 2014. Cognitive variability in psychotic disorders: a cross-diagnostic cluster analysis. Psychol. Med 44 (15) 3239–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Huang CL, Chang YC, Chen PW, Lin CY, Tsai GE, Lane HY, 2013. Clinical symptoms, mainly negative symptoms, mediate the influence of neurocognition and social cognition on functional outcome of schizophrenia. Schizophr. Res 146 (1–3) 231–237. [DOI] [PubMed] [Google Scholar]
- Madre M, Canales-Rodriguez EJ, Ortiz-Gil J, Murru A, Torrent C, Bramon E, Perez V, Orth M, Brambilla P, Vieta E, Amann BL, 2016. Neuropsychological and neuroimaging underpinnings of schizoaffective disorder: a systematic review. Acta Psychiatr. Scand 134 (1) 16–30. [DOI] [PubMed] [Google Scholar]
- Maier W, 2006. Do schizoaffective disorders exist at all? Acta Psychiatr. Scand 113 (5) 369–371. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Owen MJ, Heckers S, Tandon R, Bustillo J, Schultz S, Barch DM, Gaebel W, Gur RE, Tsuang M, van OJ, Carpenter W, 2013. Schizoaffective Disorder in the DSM-5. Schizophr. Res 150 (1) 21–25. [DOI] [PubMed] [Google Scholar]
- Martinez-Aran A, Torrent C, Tabares-Seisdedos R, Salamero M, Daban C, Balanza-Martinez V, Sanchez-Moreno J, Manuel GJ, Benabarre A, Colom F, Vieta E, 2008. Neurocognitive impairment in bipolar patients with and without history of psychosis. J. Clin. Psychiatry 69 (2) 233–239. [DOI] [PubMed] [Google Scholar]
- Mausbach BT, Harvey PD, Pulver AE, Depp CA, Wolyniec PS, Thornquist MH, Luke JR, McGrath JA, Bowie CR, Patterson TL, 2010. Relationship of the Brief UCSD Performance-based Skills Assessment (UPSA-B) to multiple indicators of functioning in people with schizophrenia and bipolar disorder. Bipolar. Disord 12 (1) 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M, 1979. A new depression scale designed to be sensitive to change. Br. J. Psychiatry 134 382–389. [DOI] [PubMed] [Google Scholar]
- National Advisory Mental Health Council Workgroup on Tasks and Measures for Research Domain Criteria Behavioral Assessment Methods for RDoC Constructs https://www.nimh.nih.gov/about/advisory-boards-and-groups/namhc/reports/rdoc_council_workgroup_report_153440.pdf, 1–167. 2016. Bethesda, MD. [Google Scholar]
- Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste DV, 2001. UCSD Performance-Based Skills Assessment: development of a new measure of everyday functioning for severely mentally ill adults. Schizophr. Bull 27 (2) 235–245. [DOI] [PubMed] [Google Scholar]
- Pearlson GD, 2015. Etiologic, phenomenologic, and endophenotypic overlap of schizophrenia and bipolar disorder. Annu. Rev. Clin. Psychol 11 251–281. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, Kester DB, Stafiniak P, 1991. Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Arch. Gen. Psychiatry 48 (7) 618–624. [DOI] [PubMed] [Google Scholar]
- Schneider LC, Struening EL, 1983. SLOF: a behavioral rating scale for assessing the mentally ill. Soc. Work Res. Abstr 19 (3) 9–21. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Kremen WS, Koren D, Faraone SV, Goldstein JM, Tsuang MT, 2002. A comparative profile analysis of neuropsychological functioning in patients with schizophrenia and bipolar psychoses. Schizophr. Res 53 (1–2) 31–44. [DOI] [PubMed] [Google Scholar]
- Sperry SH, O’Connor LK, Ongur D, Cohen BM, Keshavan MS, Lewandowski KE, 2015. Measuring cognition in bipolar disorder with psychosis using the MATRICS Consensus Cognitive Battery. J. Int. Neuropsychol. Soc 21 (6) 468–472. [DOI] [PubMed] [Google Scholar]
- Strassnig MT, Harvey PD, 2014. Treatment resistance and other complicating factors in the management of schizophrenia. CNS. Spectr 19 Suppl 1 16–23. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, Morris DW, Bishop J, Thaker GK, Sweeney JA, 2013. Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am. J. Psychiatry 170 (11) 1263–1274. [DOI] [PubMed] [Google Scholar]
- van Praag HM, 2000. Nosologomania: a disorder of psychiatry. World J. Biol Psychiatry 1 (3) 151–158. [DOI] [PubMed] [Google Scholar]
- Ventura J, Hellemann GS, Thames AD, Koellner V, Nuechterlein KH, 2009. Symptoms as mediators of the relationship between neurocognition and functional outcome in schizophrenia: a meta-analysis. Schizophr. Res 113 (2–3) 189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohringer PA, Barroilhet SA, Amerio A, Reale ML, Alvear K, Vergne D, Ghaemi SN, 2013. Cognitive impairment in bipolar disorder and schizophrenia: a systematic review. Front. Psychiatry 4 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, 2001. Wechsler Test of Adult Reading: WTAR Psychological Corporation, New York, NY. [Google Scholar]
- Woodward ND, 2016. The course of neuropsychological impairment and brain structure abnormalities in psychotic disorders. Neurosci. Res 102 39–46. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA, 1978. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry 133 429–435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.