Abstract
Interictal spike is a biomarker of epilepsy that can occur frequently between seizures. Its potential effects on brain oscillations, especially on theta rhythm (4–8 Hz) that is related to a variety of cognitive processes, remain controversial. Using local field potentials recorded from patients with temporal lobe epilepsy (TLE), we investigated here the impact of spikes on theta rhythm immediately after spikes and during the prolonged periods (lasting 4–36 s) between adjacent spikes. Local field potentials (LFPs) were recorded in different epileptogenic areas including the anterior hippocampus (aH) and the entorhinal cortex (EC) as well as in the extended propagation pathway. We found that interictal spikes had a significant inhibitory effect on theta rhythm. Power of theta rhythm was reduced immediately after spikes, and the inhibitory effect on theta rhythm might sustain during the prolonged between-spike periods. The inhibitory effect was more severe when the epileptogenic areas involved both the aH and EC compared to that involved only a single structure. These observations suggest that interictal spikes have a significant negative impact on theta rhythm and may thus play a role in theta-related cognition changes in patients with TLE.
Keywords: Temporal lobe epilepsy, Local field potential, Theta rhythm, Interictal spike
1. Introduction
The most common form of interictal-like activity (ILA) is high-amplitude, sharp spike that can occur frequently and in distributed brain areas [1–10]. The physiological underpinning of ILA remains only partially understood. Traditionally, ILA has been related to glutamatergic networks, γ-aminobutyric acid (GABA)ergic networks, and the unbalance between the two types of networks [3–5,7]. Studies with animal models and human subjects have provided ample evidence that ILA can affect ongoing brain oscillations, especially theta rhythm in the medial temporal lobe (MTL) that may be related to various cognitive processes [11–19]. In epilepsy, theta power is related to many factors including long-term plasticity, age, age of first seizure, and medication [11,15,20,21]. Changes in theta rhythm may be associated with cognitive deficits in temporal lobe epilepsy (TLE) [11,15]. For example, it was found that loss of hippocampal theta rhythm in epileptic rat models could result in spatial memory deficit [15]. Using deep brain electroencephalography (EEG) recorded from pilocarpine rats, we recently found that theta power was significantly reduced after ILA, i.e., LFP theta rhythm was at high level during exploration before injections but were disrupted by ILA after injections [16,17]. Moreover, LFP theta power around ILA reduced more in the early stage than in the later stage of epileptogenesis, implying that early ILA had more severe effects on theta oscillations than later ILA [16,17].
How ILA affects theta rhythm in human subjects, especially in patients with TLE, remains only partially understood. Theta rhythm in the human brain is prominent during voluntary movements, attention, rapid eye movement (REM) sleep, arousals from sleep, and calm wakefulness [20,22], particularly in the entorhinal-hippocampal system including the anterior hippocampus (aH) and the entorhinal cortex (EC) [23–30]. The aH plays an important role in theta-dependent memory, and the EC has intrinsically oscillatory membranes with theta frequencies independent of the hippocampus and drives the hippocampal circuits [24,31,32]. To date, there are only few invasive studies on the relation between LFP theta rhythm and ILA in human patients [12]. There is in vitro evidence to suggest that ILA and theta rhythm both involve GABAergic circuits [27,33,34]. As the propagation of ILA varies with GABA-mediated inhibition and theta rhythm depends on the recruitment of GABAergic circuits [20,26,27,33], it is possible that ILA affects theta rhythm through GABAergic circuits.
To gain a deeper understanding of the impact of ILA on theta rhythm, we recorded here LFPs using intracerebral electrodes implanted in the aH and EC of four patients with TLE during quiet wakefulness. In these patients, the epileptogenic areas involved the aH, EC, or both. Theta rhythm was investigated around spikes as well as in the prolonged between-spike periods that lasted up to 36 s.
2. Materials and methods
2.1. Participants and intracerebral EEG recordings
Intracerebral EEG data and related clinical information were acquired from 4 patients with refractory TLE. Participants provided written-informed consent in accordance with guidelines set by the institutional review boards of La Timone Hospital, Marseille, France. The patients included 3 males (Patient1, 33 yrs; Patient3, 19 yrs; Patient4, 16 yrs) and 1 female (Patient2, 40 yrs). The inclusion criteria satisfied the following: (1) epileptic foci involved the mesial temporal lobe; (2) structure Magnetic resonance imaging (MRI) was either normal or showed patterns suggestive of hippocampal atrophy and hyperintensity in Fluid attenuated inversion recovery (FLAIR) images. The epileptogenic areas involved the aH, EC, or both (Patient1: epileptogenic area involved only the aH, with the EC in the propagation pathway; Patient2: epileptogenic area involved only the EC, with the aH in the propagation pathway; Patient3 and Patient4: epileptogenic area involved both the aH and EC).
Each patient was implanted with 5–8 intracerebral electrodes in the temporal region based on the clinical needs. Each intracerebral electrode had 10–15 contacts (length: 2 mm, diameter: 0.8 mm; 1.5 mm apart). Electroencephalography data were recorded using a 128channel long-term video-EEG monitoring system (Deltamed™). The placements of electrodes were first trained on the patients’ 3-D T1 MRI using a virtual machine with the BrainVISA software. The implantation accuracy was then calculated using telemetric X-ray imaging, postoperative computerized tomography (CT) scan, and MRI. Finally, the placements were determined by Talairach’s reference frame under stereotactic conditions [8]. All patients had electrodes that spatially sampled mesial/limbic regions including the amygdala, the EC, the internal part of the temporal pole, the anterior part of the hippocampus, the posterior part of the hippocampus, and the lateral/neocortical regions of the temporal lobe. Please see the materials and methods in the work of Bartolomei and Wendling for more details [8,35].
During the experiments, the patients were awake and resting. No obvious limb movements were observed in the video recordings. The EEG signals were recorded at a sampling rate of 256 Hz. The recording lasted 1 h long for each patient. Electrical power-line noise was notch filtered, and nonphysiological slow variations were high-pass filtered using analog filters embedded in the hardware.
2.2. Data processing
Exemplar EEG recordings of these patients are shown in Fig. S1. Using a spike-sorting technique (epoch sorting toolbox of EEGLAB software package [36]), the spikes were aligned at the peak (time 0). The onset and offset of a spike were determined by an experienced neurologist based on visual inspection. An epoch was defined for each spike, which started from 350 ms before the spike onset and ended 350 ms after the spike offset. A total of 168 spikes were identified from the recordings (Patient1: 40 spikes; Patient2: 40 spikes; Patient3: 40 spikes; Patient4: 48 spikes).
Data between spikes were also extracted for analyses. During the prolonged between-spike period, epochs were extracted using a 350ms time window without overlap. Each 350-ms epoch was defined as a between-spike epoch. Between-spike epochs with connotative tiny spikes (see Fig. S2 for an example) in the epileptogenic areas and propagation pathway were excluded from further analyses. A total of 300 between-spike epochs were randomly extracted for each patient.
Power of theta rhythm was derived from Gabor wavelet transform:
| (1) |
where Wx(t0f) is the complex form of signal x(t) at time (t0) and frequency (f). η is the center frequency (5 cycles here). The sum of squares of real part and imaginary part corresponding to Wx(t0f) is regarded as Gabor power at frequency (f) and time (t0). To avoid power estimation errors around the edge of the short-time window, we used here an extended time window of 4 s centered at each epoch for power estimation, and then the theta power within the 350-ms epoch was extracted. Theta power was computed as the power values in the 4- to 8-Hz frequency band averaged over time and frequency per epoch and then averaged over epochs.
The transient inhibitory effect of spikes was evaluated by the percent change of theta power after spike compared with prespike theta power. During the between-spike period, Gabor wavelet transform was carried out in a 4-s long data frame centered at the middle of each betweenspike epoch. Paired t-test was employed to test the significance of inhibitory effect.
2.3. Loss of theta rhythm during the between-spike period
To quantify the disruption of theta rhythm during between-spike periods, we evaluated theta power in each between-spike epoch and identified epochs that demonstrated disrupted theta power. Theta power was considered disrupted in an epoch if it fell below the mean theta power within the 4-s long time window centered at the epoch, and the period with low theta power lasted longer than 50 ms (see Fig. S3 for an example). The loss of theta rhythm was then quantified as the percent of epochs demonstrating disrupted theta rhythm among all between-spike epochs.
3. Results
3.1. Transient reduction of theta power after spikes
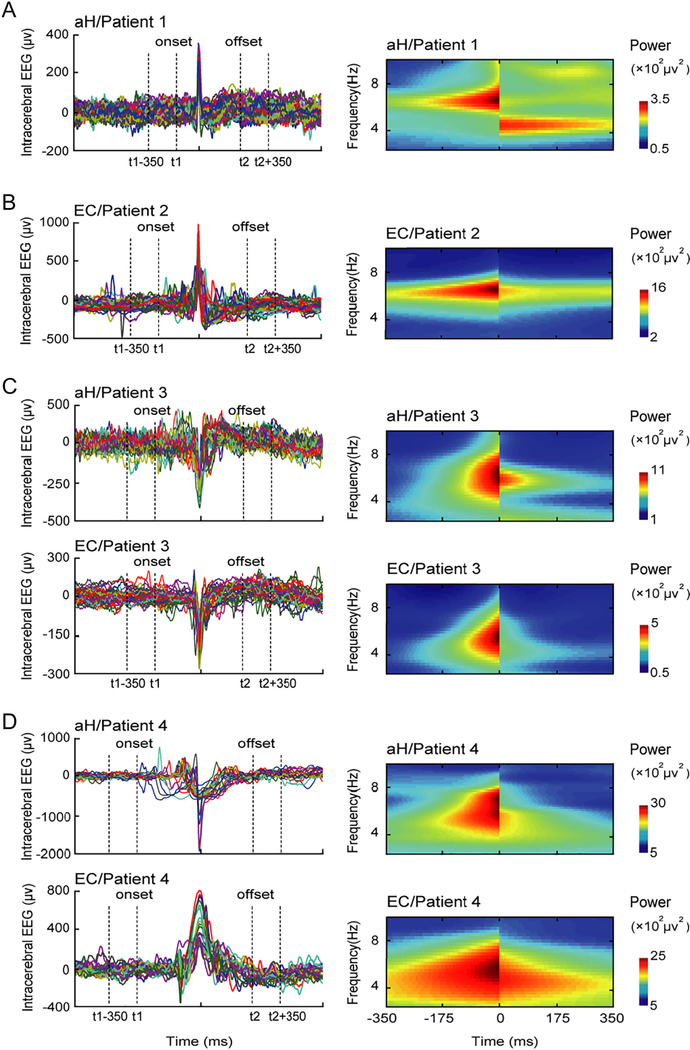
Theta power dropped immediately after spikes in epileptogenic regions (Fig. 1). However, theta power did not show a transient reduction in the propagation pathway (see Fig. S4). The transient inhibitory effect of spikes on theta rhythm was summarized in Table 1. Theta power in the aH was reduced by 55.29% and 53.79% in Patient3 (p = 0.0191) and Patient4 (p = 0.0325), whose epileptogenic areas involved both the aH and EC, whereas theta power was only reduced by 41.25% in Patient1 whose epileptogenic area only involved the aH. Similarly, theta power in the EC was reduced by 60.22% and 68.30% in Patient3 (p = 0.0039) and Patient4 (p = 0.0414), whereas theta power was only reduced by 21.23% in the EC of Patient2 who had a single epileptogenic area in the EC. The impairment of theta rhythm was more severe when epileptogenic areas involved both the aH and EC than when cases involved only a single epileptic area. See supplemental materials for additional analyses on single spikes and spike bursts. Finally, we found that gamma power also showed a transient reduction in epileptogenic regions (see Fig. S5 and Table S1).
Fig. 1.
Transient reduction of theta power after spikes in 4 patients. Peak-sorting results of the spikes are shown in the left panel. Time-frequency power is shown in the right panel. (A) Patient1; (B) Patient2; (C) Patient3; (D) Patient4. The negative effect of spikes on theta rhythm is more severe in epileptogenic areas involving both the aH and EC (Patient3 and Patient4) than that involving only one structure (aH in Patient1; EC in Patient2).
Table 1.
Theta power level around spikes and the inhibitory effect of spikes.a
| Brain area | Spikes in aH (Patient1) |
Spikes in EC (Patient2) |
Spikes in both aH and EC (P3/P4) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prespike (μv2) |
Postspike (μv2) |
Decrease percentage (%) |
p-Value | Prespike (μv2) |
Postspike (μv2) |
Decrease percentage (%) |
p-Value | Prespike (μv2) |
Postspike (μv2) |
Decrease percentage (%) |
p-Value | |
| aH | 577.30 ±313.08 | 339.16 ± 210.51 | 41.25 | 0.0004 | - | - | - | - | 1137.10 ± 458.83/2783.40 ± 1428.30 | 508.45 ± 284.04/1286.30 ± 816.00 | 55.29/53.79 | 0.0191/0.0033 |
| EC | - | - | - | - | 487.97 ± 291.98 | 384.88 ± 196.23 | 21.13 | 0.0172 | 543.59 ± 246.60/2596.00 ± 1504.50 | 216.23 ± 102.75/822.96 ± 486.57 | 60.22/68.30 | 0.0039/0.0414 |
P3/P4 represented for Patient3 and Patient4.
3.2. Disruption of LFP theta rhythm during the between-spike period
We then examined theta power in the between-spike epochs. The loss of LFP theta rhythm was evaluated as the percent of epochs demonstrating theta power disruption (see Materials and methods). The results are summarized in Table 2. We found that disruption of LFP theta power in epileptogenic area was more than that in the propagation pathway, and the disruption was more severe when epileptogenic areas involved both the aH and EC than cases with a single epileptogenic area. Disruption of LFP theta rhythm during the between-spike period may indicate that the negative effect of spikes was sustained for a prolonged period.
Table 2.
Disruption of theta rhythm in between-spike epochs.
| Brain area | Spikes in aH (Patient1) |
Spikes in EC (Patient2) |
Spikes in both aH and EC (P3/P4) |
|||
|---|---|---|---|---|---|---|
| Number of epochs with theta disruption | Percentage (%) | Number of epochs with theta disruption | Percentage (%) | Number of epochs with theta disruption | Percentage (%) | |
| aH | 135 | 45.00 | 55 | 18.33 | 229/195 | 76.33/65.00 |
| EC | 50 | 16.67 | 75 | 25.00 | 219/234 | 73.00/78.00 |
3.3. Control analyses
To investigate whether our results were dependent on the precise determination of spike onset and offset, we repeated the analyses by shifting the onset to 350 ms earlier (50 ms) and the offset to 350 ms later (50 ms) (see Fig. S6). Our conclusions were unchanged. Therefore, the difference between prespike and postspike is insensitive to the small variation in the determination of spike onset/offset. Additionally, to investigate the effect of window length (350 ms), we reanalyzed all data using a time window of 700 ms (Table S2). The conclusions also remained unchanged.
4. Discussion
In the present study, we found that interictal spikes had a significant inhibitory effect on theta rhythm. Power of theta rhythm was reduced immediately after spikes, and the inhibitory effect on theta rhythm may be sustained during the prolonged between-spike periods. The inhibitory effect was more severe when the epileptogenic areas involved both the aH and EC compared to when the epileptogenic areas involved only a single structure. These observations suggest that interictal spikes have a significant negative impact on theta rhythm and may thus play an important role in theta-related cognition changes in patients with TLE.
4.1. Transient negative effect of interictal spikes on theta rhythm
Theta rhythm in the hippocampus has been related to a variety of cognitive functions. For example, using simultaneous recordings from rat prefrontal cortex and hippocampus during spatial working memory, Jones and Wilson found rapid configuration of functional connectivity through the theta-frequency entrainment of oscillatory networks across these two brain regions [37]. Using Magnetoencephalography (MEG) recording in human subjects, Cornwell et al. observed greater theta activity in the hippocampus and parahippocampal cortices during goal-directed navigation relative to aimless movements, suggesting that human spatial learning is dependent on hippocampal and parahippocampal theta oscillations [38]. Furthermore, mounting evidence indicated that theta rhythm may play a role in attention and sensorimotor integration [39], episodic memory [40], and other voluntary behaviors (see [41,42] for reviews). Theta oscillation has been hypothesized as the navigation rhythm through both physical and mnemonic spaces, facilitating the formation of maps and episodic/semantic memories [43]. Revealing how ILA affects theta rhythm in the MTL is thus crucial for a better understanding of cognitive deficits related to epilepsy.
In the present study, while there are individual differences in theta power among the 4 patients, the reduced theta power could be observable around spikes (see Fig. 1 and Table 1) in all patients. In particular, theta power decreased immediately after interictal spikes in the epileptogenic area. However, theta power did not reduce in the propagation pathway (see Fig. S4), such as in the EC in Patient1 and in the aH in Patient2. These observations suggest that the interictal spikes could impair theta rhythm transiently. These findings are in line with the previous animal work that reported reduced theta power after spikes [16,17]. Interestingly, we also found that gamma power dropped immediately after spikes in epileptogenic regions (see Fig. S5 and Table S1). When the coupling between theta phase and gamma power [44] was examined, we further observed that the phase-amplitude coupling (PAC) in the aH showed a decrease after spikes (see Fig. S7), suggesting that epileptic spikes might have a negative impact not only on theta power, but also on the coupling between theta phase and gamma power in the aH.
The occurrence of spikes in multiple structures may indicate more severe network impairment than cases with spikes limited to one structure. Here, we observed that loss of theta rhythm was more prominent after spikes when epileptogenic areas involved both the aH and EC compared to that when epileptogenic areas involved either the aH or EC. These observations suggest that impairment of theta rhythm after spikes is likely to be the result of abnormal functional network rather than the result of abnormality in local regions.
4.2. Disruption of LFP theta rhythm during the between-spike period
Loss of hippocampal theta rhythm may be related to loss of principal neurons and interneurons in the hippocampus [2,11,15] or to selective loss of excitability and action potential firing in inhibitory GABAergic neurons in various types of epilepsy [45–47]. Impaired theta rhythm can lead to deficits in the spatial memory in the rat model [11,15]. In addition, the degree of hippocampal theta power impairment was correlated with the degree of spatial memory deficit during epileptogenesis [11]. Our observation that theta was disrupted during the prolonged between-spike periods near the epileptogenic area may be in line with these previous reports. In the present study, we observed that the more severe theta power was reduced after spikes (e.g., last columns in Table 1), the more theta disruption occurred during the betweenspike period (e.g., last columns in Table 2). This suggested that the interictal spike might be the main cause of theta rhythm disruptions in the ongoing oscillations that can in turn influence theta-related cognitive processes.
4.3. Caveats
There are several limitations of the present study that should be mentioned. First, the study was based on a small number of subjects (n = 4) who met the criteria that the electrodes were implanted in the hippocampal area, and multiple interictal spikes were recorded during wakefulness. Second, there are substantial individual differences between patients, and the analyses were carried out at the level of individuals. However, the observations reported here were consistent across all subjects. A follow-up study with larger sample size and more rigorous statistical analyses is warranted.
Supplementary Material
Acknowledgment
The authors thank Fabrice Bartolomei, Christophe Bernar, Christian Bénar, Laëtitia Chauvel, and Julien Krieg for the valuable data and computational scripts. The project is supported by the Colleges and Universities of Hebei Province Science and Technology Research Projects (grant no. ZD2014026) and University Innovation Team Leader Program of Hebei Province (grant no. LJRC003). H.L was supported by NIH (grant nos. 1R01NS091604 and P50MH106435), Beijing Municipal Science and Technology Commission (no. Z161100002616009), and National Natural Science Foundation of China (grant no. 81790652).
Footnotes
Conflict of interest
None of the authors have any conflict of interest.
Ethical publication statement
We confirm that we have read the journal’s position on issues involved in ethical publication and affirm that this paper is consistent with those guidelines.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.yebeh.2018.07.014.
References
- [1].Buzsáki G, Hsu M, Slamka C, Gage FH, Horváth Z. Emergence and propagation of interictal spikes in the subcortically denervated hippocampus. Hippocampus 1991; 1(2):163–80. [DOI] [PubMed] [Google Scholar]
- [2].Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents EEG, ECoG, LFP and spikes. Nat Rev Neurosci 2012;3:407–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cossart R, Dinocourt C, Hirsch JC, Merchan Perez A, De Felipe J, Ben Ari Y, et al. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat Neurosci 2001;4(1):52–62. [DOI] [PubMed] [Google Scholar]
- [4].Demont-Guignard S, Benquet P, Gerber U, Wendling F. Analysis of intracerebral EEG recordings of epileptic spikes: insights from a neural network model. IEEE Trans Biomed Eng 2009;56(12):2782–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].El-Hassar L, Milh M, Wendling F, Ferrand N, Esclapez M, Bernard C. Cell domain-dependent changes in the glutamatergic and GABAergic drives during epileptogenesis in the rat CA1 region. J Physiol 2007;578(Pt.1):193–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Searle AG, Ford CE, Beechey CV. Origin and propagation of interictal discharges in the acute electrocorticogram. Implications for pathophysiology and surgical treatment of temporal lobe epilepsy. Brain 1997;28(5):446–52. [DOI] [PubMed] [Google Scholar]
- [7].Shah MM, Anderson AE, Leung V, Lin X, Johnston D. Seizure-induced plasticity of h channels in entorhinal cortical layer III pyramidal neurons. Neuron 2004;44(3): 495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bartolomei F, Chauvel P, Wendling F. Epileptogenicity of brain structures in human temporal lobe epilepsy: a quantified study from intracerebral EEG. Brain 2008;131 (Pt. 7):1818–30. [DOI] [PubMed] [Google Scholar]
- [9].King D, Spencer SS, Mccarthy G, Spencer DD. Surface and depth EEG findings in patients with hippocampal atrophy. Neurology 1997;48(5):1363–7. [DOI] [PubMed] [Google Scholar]
- [10].Staley K, Hellier JL, Dudek FE. Do interictal spikes drive epileptogenesis? Neuroscientist 2005;11(4):272–6. [DOI] [PubMed] [Google Scholar]
- [11].Chauvière L, Rafrafi N, Thinus-Blanc C, Bartolomei F, Esclapez M, Bernard C. Early deficits in spatial memory and theta rhythm in experimental temporal lobe epilepsy. J Neurosci 2009;29(17):5402–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kraskov A, Quiroga R, Reddy L, Fried I, Koch C. Local field potentials and spikes in the human medial temporal image lobe are selective to image category. J Cogn Neurosci 2007;19(3):479–92. [DOI] [PubMed] [Google Scholar]
- [13].Trenité DGAK-N, Bakker DJ, Binnie CD, Buerman A, Raaij MV. Psychological effects of subclinical epileptiform EEG discharges. I. Scholastic skills. Epilepsy Res 1988;2(2): 111–6. [DOI] [PubMed] [Google Scholar]
- [14].Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron 2006;52(52):155–68. [DOI] [PubMed] [Google Scholar]
- [15].Winson J Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science 1978;201(4351):160–3. [DOI] [PubMed] [Google Scholar]
- [16].Ge ML, Guo BQ, Chen X, Sun Y, Chen SH, Zheng Y, et al. Inhibitory effects of epileptic spikes on theta rhythm in rat pilocarpine model of temporal lobe epilepsy. Acta Phys Sin 2014;66(2):118–28. [PubMed] [Google Scholar]
- [17].Ge M, Wang D, Dong G, Guo B, Gao R, Sun W, et al. Transient impact of spike on theta rhythm in temporal lobe epilepsy. Exp Neurol 2013;250(4):136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ertl M, Hildebrandt M, Ourina K, Leicht G, Mulert C. Emotion regulation by cognitive reappraisal—the role of frontal theta oscillations. NeuroImage 2013;81(11):412–21. [DOI] [PubMed] [Google Scholar]
- [19].Vijver IVD, Cohen MX, Ridderinkhof KR. Aging affects medial but not anterior frontal learning-related theta oscillations. Neurobiol Aging 2014;35(3):692–704. [DOI] [PubMed] [Google Scholar]
- [20].Cantero JL, Atienza M, Stickgold R, Kahana MJ, Madsen JR, Kocsis B. Sleep-dependent θ oscillations in the human hippocampus and neocortex. J Neurosci 2003;23(34): 10897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lindén H, Pettersen KH, Einevoll GT. Intrinsic dendritic filtering gives low-pass power spectra of local field potentials. J Comput Neurosci 2010;29(3):423–44. [DOI] [PubMed] [Google Scholar]
- [22].Montgomery SM, Betancur MI, Buzsáki G. Behavior-dependent coordination of multiple theta dipoles in the hippocampus. J Neurosci 2009;29(5):1381–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Basu J, Zaremba JD, Cheung SK, Hitti FL, Zemelman BV, Losonczy A, et al. Gating of hippocampal activity, plasticity, and memory by entorhinal cortex long-range inhibition. Science 2016;351(6269):138–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Buzsáki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci 2013;16(2):130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schlesiger MI, Cannova CC, Boublil BL, Hales JB, Mankin EA, Brandon MP, et al. The medial entorhinal cortex is necessary for temporal organization of hippocampal neural activity. Nat Neurosci 2015;18(8):217–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lubenov EV, Siapas AG. Hippocampal theta oscillations are travelling waves. Nature 2009;459(7246):534–9. [DOI] [PubMed] [Google Scholar]
- [27].Handelmann GE, Olton DS. Spatial memory following damage to hippocampal pyramidal cells with kainic acid: impairment and recovery with preoperative training. Brain Res 1981;217(1):41–58. [DOI] [PubMed] [Google Scholar]
- [28].Lee I, Jerman TS, Kesner RP. Disruption of delayed memory for a sequence of spatial locations following CA1 lesions of the dorsal hippocampus. Neurobiology 2005;84 (2):138–47. [DOI] [PubMed] [Google Scholar]
- [29].Morris AM, Churchwell JC, Kesner RP, Gilbert PE. Selective lesions of the dentate gyrus produce disruptions in place learning for adjacent spatial locations. Neurobiology 2012;97(3):326–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Buzsáki G Theta oscillations in the hippocampus. Neuron 2002;33(3):325–40. [DOI] [PubMed] [Google Scholar]
- [31].Patel J, Fujisawa S, Berényi A, Royer S, Buzsáki G. Traveling theta waves along the entire septotemporal axis of the hippocampus. Neuron 2012;75(3):410–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ogawa S, Koga M, Osanai S. Theta oscillations provide temporal windows for local circuit computation in the entorhinal-hippocampal loop. Neuron 2009;64(2): 267–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cappaert NLM, Lopes da Silva FH, Wadman WJ. Spatio-temporal dynamics of theta oscillations in hippocampal-entorhinal slices. Hippocampus 2009;19 (11):1065–77. [DOI] [PubMed] [Google Scholar]
- [34].Sabolek HR, Swiercz WB, Lillis KP, Cash SS, Huberfeld G, Zhao G, et al. A candidate mechanism underlying the variance of interictal spike propagation. J Neurosci 2012;32(9):3009–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wendling F, Bartolomei F, Bellanger JJ, Bourien J, Chauvel P. Epileptic fast intracerebral EEG activity: evidence for spatial decorrelation at seizure onset. Brain 2003;126 (6):1449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 2004;134 (1):9–21. [DOI] [PubMed] [Google Scholar]
- [37].Jones MW, Wilson MA. Theta rhythms coordinate hippocampal–prefrontal interactions in a spatial memory task. PLoS Biol 2005;3(12):2187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cornwell BR, Johnson LL, Holroyd T, Carver FW, Grillon C. Human hippocampal and parahippocampal theta during goal-directed spatial navigation predicts performance on a virtual Morris water maze. J Neurosci 2008;28(23):5983–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ekstrom AD, Caplan JB, Ho E, Shattuck K, Fried I, Kahana MJ. Human hippocampal theta activity during virtual navigation. Hippocampus 2005;15(7):881–9. [DOI] [PubMed] [Google Scholar]
- [40].Lega BC, Jacobs J, Kahana M. Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus 2012;22(4):748–61. [DOI] [PubMed] [Google Scholar]
- [41].Kahana MJ. The cognitive correlates of human brain oscillations. J Neurosci 2006;26 (6):1669–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jacobs J, Kahana MJ. Direct brain recording fuel advances in cognitive electrophysiology. Trends Cogn Sci 2001;4(14):162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Buzsáki G Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus 2005;15(7): 827–40. [DOI] [PubMed] [Google Scholar]
- [44].Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, et al. High gamma power is phase-locked to theta oscillations in human neocortex. Science 2006;313(5793):1626–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].De Lanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res 1989;495(495): 387–95. [DOI] [PubMed] [Google Scholar]
- [46].Sanabria ERG, Castañeda MT, Banuelos C, Perez-Cordova MG, Hernandez S,Colom LV. Septal GABAergic neurons are selectively vulnerable to pilocarpine-induced status epilepticus and chronic spontaneous seizures. Neuroscience 2006;142(3):871–83. [DOI] [PubMed] [Google Scholar]
- [47].Kalume F, Oakley JC, Westenbroek RE, Gile J, de la Iglesia HO, Scheuer T, et al. Sleep impairment and reduced interneuron excitability in a mouse model of Dravet syndrome. Neurobiol Dis 2015;77:141–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.