Dear Editor,
Introduction:
We examined the relationship between face emotion recognition (FER) and experiences of self-stigma related feelings of shame in regards to symptoms in young adults at clinical high risk (CHR) for psychosis. FER deficits and stigma are both characteristic of schizophrenia and exist prior to onset of index psychosis (Corcoran et al., 2015; Yang et al., 2015). Further, transition to psychosis in CHR individuals has been predicted by both FER deficits, particularly for “threat” emotions like fear (Corcoran et al., 2015), and by stigma stress (i.e., when one’s appraisal of stigma as harmful exceeds appraisal of one’s perceived resources to cope with this threat), independent of symptom severity (Rusch et al., 2015). Misperception of fear may be related to experience of stigma, which relies on the perception of affective appraisal by others. To our knowledge, this is the first study of FER and stigma in a CHR cohort, albeit in a small sample.
Experimental:
The cohort was comprised of 28 CHR participants (18 males/10 females), ages 18–27 (mean(SD) = 22.2(3.0) years), ascertained using the Structured Interview for Psychosis-Risk Syndromes/Scale of Psychosis-Risk Symptoms (SIPS/SOPS) (Miller et al., 1999). FER was assessed using the Penn Emotion Recognition Task (ER-40) (Gur et al., 2002). Self-stigma related feelings of shame (herein referred to as stigma shame) was assessed using the index of ‘negative emotions related to symptoms’ scale from Yang’s CHR stigma measure. Comprising this scale were items assessing shame, embarrassment, and/or feeling different from others on the basis of their symptoms and experiences that led them to come to the CHR clinic (Yang et al., 2015). Spearman correlations were done to investigate the relationship between stigma shame and fear emotion processing, with regression analyses done to determine any confounding by clinical or demographic features.
Results:
We found that fear accuracy was negatively correlated with stigma shame (r = −0.41; p = .029). Further, stratifying the cohort using a median split, the “low shame” subgroup showed better fear accuracy than the “high shame” group (mean (SD): 7.5 (0.7) vs. 6.7 (0.9); t1,26 = −2.6, p = .01) (Figure 1a). The association of fear accuracy and stigma shame survived adjustment for prodromal symptom severity and could not be accounted for by age or sex. Of note, the association of FER accuracy and stigma shame was specific to fear, as no significant associations were found with stigma shame for happy, angry, sad, or neutral faces (all p’s > .05). Associations of fear processing deficits with stigma shame extended beyond accurate identification of fear, as there was also a significant association between misattribution of fear to non-fearful faces and stigma shame (r = .37, p = .05) (Figure 1b), which also was unrelated to symptoms, age or sex.
Figure 1.
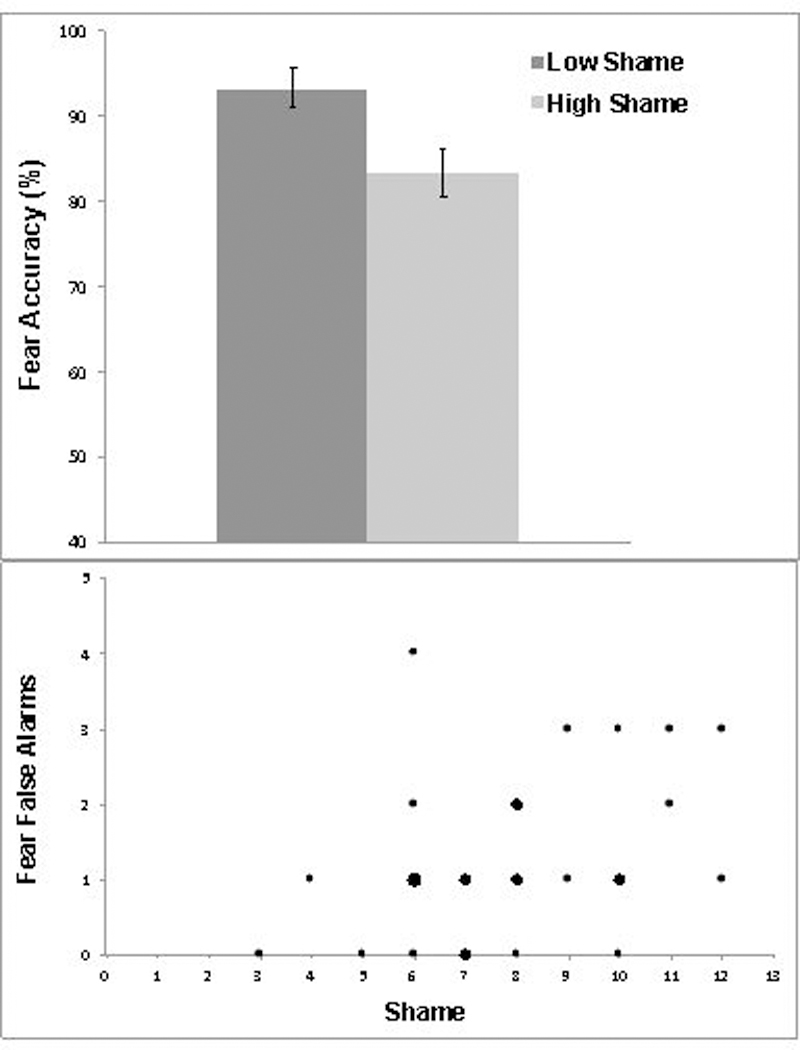
a. Fear Accuracy and Stigma Shame in CHR Individuals b. Fear Misperception and Stigma Shame in CHR Individuals. Note: Larger dots represent more than one participant.
Discussion:
Overall, we showed that among CHR individuals, greater stigma shame was associated with worse accuracy in identification of fear in faces, and greater misattribution of fear to non-fearful face emotion stimuli. Of course, in this cross-sectional study, causal direction can only be inferred. It may be that FER deficits lead to stigma, and there is evidence that FER deficits emerge quite early in the course of illness. A population based study of individuals with psychotic symptoms demonstrated that FER deficits are present as early as eight years old (Gur et al., 2014). It is plausible that such early FER deficits, especially in respect to threat and fear, could lead to misattribution of others’ emotions and intentions, and subsequent suspiciousness, stigma stress and stigma shame.
However, there is also a body of literature that shows that anxiety (trait and induced state) can modify FER, including both impairment in FER accuracy and increase in misattribution, specifically of fear (Attwood et al., 2017). We have previously shown that stigma shame related to symptoms, using this same scale, is associated with self-reported depression in CHR patients (Yang et al., 2015). Therefore, it is plausible that the depression common to the psychosis risk syndrome, and the related shame associated with symptoms, can also lead to impairment in FER.
It may also be that the association of fear processing deficits and stigma in CHR individuals is iterative and bidirectional in causation, which can be tested in longitudinal studies. The neural mechanisms underlying their association likely entail changes in amygdala function and activation. CHR youth show increased amygdala activity in response to threating facial expressions (Wolf et al., 2015), and amygdala functioning has been associated in schizophrenia with both FER deficits, especially fear (Taylor et al., 2012), and stigma (Raij et al., 2014). Longitudinal studies therefore might include both resting and task-related neuroimaging paradigms for FER and stigma in CHR.
As FER deficits and stigma are interrelated, and both are associated with psychosis risk and functional impairment, trials of interventions are warranted. In schizophrenia, FER deficits have been variably improved in schizophrenia with oxytocin administration(Averbeck et al., 2012), and interventions targeting social cognition (Grant et al., 2017), while anti-stigma interventions related to psychosis have proved promising (Yang et al., 2015). It would be useful to implement these trials in CHR cohorts, and to determine if treatments targeting FER deficits also reduce stigma shame, and if efforts to reduce stigma might also improve FER, especially fear processing; these interventions hold promise for preventive intervention and improvement of concurrent functional impairments.
Footnotes
Data statement All de-identified study data are available in Excel format upon request.
References
- Attwood AS, Easey KE, Dalili MN, Skinner AL, Woods A, Crick L, Ilett E, Penton-Voak IS, Munafo MR, 2017. State anxiety and emotional face recognition in healthy volunteers. R Soc Open Sci 4(5), 160855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbeck BB, Bobin T, Evans S, Shergill SS, 2012. Emotion recognition and oxytocin in patients with schizophrenia. Psychol Med 42(2), 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran CM, Keilp JG, Kayser J, Klim C, Butler PD, Bruder GE, Gur RC, Javitt DC, 2015. Emotion recognition deficits as predictors of transition in individuals at clinical high risk for schizophrenia: a neurodevelopmental perspective. Psychol Med 45(14), 2959–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant N, Lawrence M, Preti A, Wykes T, Cella M, 2017. Social cognition interventions for people with schizophrenia: a systematic review focussing on methodological quality and intervention modality. Clin. Psychol. Rev. 56, 55–64. [DOI] [PubMed] [Google Scholar]
- Gur RC, Calkins ME, Satterthwaite TD, Ruparel K, Bilker WB, Moore TM, Savitt AP, Hakonarson H, Gur RE, 2014. Neurocognitive growth charting in psychosis spectrum youths. JAMA Psychiatry 71(4), 366–374. [DOI] [PubMed] [Google Scholar]
- Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, Turner T, Bajcsy R, Posner A, Gur RE, 2002. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J. Neurosci. Methods 115(2), 137–143. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, Hoffman R, Davidson L, 1999. Symptom assessment in schizophrenic prodromal states. Psychiatr. Q. 70(4), 273–287. [DOI] [PubMed] [Google Scholar]
- Raij TT, Korkeila J, Joutsenniemi K, Saarni SI, Riekki TJ, 2014. Association of stigma resistance with emotion regulation - functional magnetic resonance imaging and neuropsychological findings. Compr Psychiatry 55(3), 727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch N, Heekeren K, Theodoridou A, Muller M, Corrigan PW, Mayer B, Metzler S, Dvorsky D, Walitza S, Rossler W, 2015. Stigma as a stressor and transition to schizophrenia after one year among young people at risk of psychosis. Schizophr Res 166(1–3), 43–48. [DOI] [PubMed] [Google Scholar]
- Taylor SF, MacDonald AW 3rd, Cognitive Neuroscience Treatment Research to Improve Cognition in, S., 2012. Brain mapping biomarkers of socio-emotional processing in schizophrenia. Schizophr Bull 38(1), 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf DH, Satterthwaite TD, Calkins ME, Ruparel K, Elliott MA, Hopson RD, Jackson CT, Prabhakaran K, Bilker WB, Hakonarson H, Gur RC, Gur RE, 2015. Functional neuroimaging abnormalities in youth with psychosis spectrum symptoms. JAMA Psychiatry 72(5), 456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LH, Link BG, Ben-David S, Gill KE, Girgis RR, Brucato G, Wonpat-Borja AJ, Corcoran CM, 2015. Stigma related to labels and symptoms in individuals at clinical high-risk for psychosis. Schizophr. Res. 168(1–2), 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]


