Abstract
BACKGROUND:
Theory of mind (ToM) has been shown to be impaired in Clinical High Risk (CHR) for psychosis populations and is linked to functional outcomes and symptom severity. Implicit versus explicit ToM has seldom been differentiated in this group, and underlying neural networks have also gone unexplored.
METHODS:
24 CHR and 26 healthy volunteers (HV) completed a behavioral ToM measure called the Short Story Task (SST), as well as a resting state functional MRI scan. SST performance was correlated to attenuated psychosis symptoms. Interactions between group and ToM variables (implicit, explicit, and comprehension) on global efficiency in the Mentalizing (MENT) and Mirror Neuron System (MNS) were also examined.
RESULTS:
CHR individuals made significantly fewer spontaneous mental state inferences. There were trend-level associations between ToM variables and symptoms, such that greater ToM performance predicted less severe symptoms. There was an interaction of group by spontaneous mental state inference within MENT bilateral dorsomedial prefrontal cortex (dmPFC), such that CHR individuals that made spontaneous mental state inferences showed greater global efficiency within the MENT network’s bilateral dmPFC.
DISCUSSION:
Findings suggest implicit ToM deficits are observable prior to psychotic disorder onset, and that these deficits implicate MENT network dmPFC efficiency. Explicit ToM performance was unaltered in the CHR group, and there were no interactions observed within MNS, suggesting specificity of implicit ToM associations with MENT network dmPFC global efficiency. Results identify potential treatment targets for the neural underpinnings of ToM, thus informing prevention and intervention efforts.
Keywords: dmPFC, graph theory, global efficiency, CHR, theory of mind, social cognition
Introduction
Theory of mind (ToM), constituting the ability to infer and understand others’ mental states, such as their beliefs and emotions, is critical for navigating the social world. This ability has been found to be impaired in psychotic disorders and is also strongly linked to symptom severity and prognosis (Fett et al., 2011). However, it is unclear to what extent ToM deficits emerge across different stages of psychotic illness progression. One contributing issue relates to extant methodology, as common ToM paradigms have faced issues surrounding ceiling effects in populations that do not exhibit significant global cognitive impairment (Dodell-Feder et al., 2013). This lack of variability in ToM performance may result in decreased sensitivity to the early emergence of ToM deficits that may occur prior to the onset of chronic illness. Further, previous ToM tasks have not often differentiated between implicit/spontaneous versus explicit/evoked ToM processes, decreasing specificity and sensitivity of measurement of this construct. Lastly, neural mechanisms underlying ToM ability in schizophrenia have been proposed and investigated; however these mechanisms have not often been examined early in disease course prior to neurotoxic effects of illness progression (Caspers et al., 2010; Schilbach et al., 2012a; Schurz et al., 2014; Kovacs et al., 2014; Schilbach et al., 2016; Kronbichler et al., 2017). Gaining a more thorough understanding of ToM deficits and possible neural underpinnings prior to psychosis onset would be instrumental in understanding the role that ToM plays in illness progression. These efforts would also aid in understanding shared etiological underpinnings of psychosis and ToM deficits as well as social cognition deficits more broadly.
ToM deficits have long been found in psychotic disorder patients (Fett et al., 2011; Bora and Pantelis, 2013). In these patients, the extent of social dysfunction has been closely tied to degree of ToM impairment (Fett et al., 2011; Kurtz and Richardson, 2012; Bora and Pantelis, 2013). ToM ability has also been shown to be impaired in those at Clinical High Risk (CHR) for developing psychosis (in addition to being linked to functional outcomes and symptom progression) (Stanford et al., 2011; Kim et al., 2011; Thompson et al., 2012; Bora and Pantelis, 2013; Healey et al., 2013). Stable deficits in ToM across psychotic stages of illness progression have also been reported (Green et al., 2012). Nonetheless, certain limiting methodological factors in assessing ToM have impacted the field’s understanding of this construct and its relationship to psychosis. In cases of less severe ToM impairment, established measures may lack sensitivity to both nuanced individual differences and subtler clinical impairment (Dodell-Feder et al., 2013). For example, ceiling effects are often observed among healthy volunteers (HV) (and sometimes among clinical groups) in conventional ToM measures (Corcoran et al., 1995; Corcoran and Frith, 2003; Craig et al., 2004; Marjoram et al., 2005; Martino et al., 2007; Zhu et al., 2007; Bertrand et al., 2007; Bora et al., 2008; Pijnenborg et al., 2009; de Achaval et al., 2010; Hooker et al., 2011). CHR individuals are a highly heterogeneous group, with functional and cognitive deficits that, despite having significant implications for psychopathology risk, can often be subtle in presentation and difficult to detect (Bora and Pantelis, 2013; Dodell-Feder et al., 2013). Thus, using ToM measures that are able to detect a full range of the construct, including mild or subtle impairment, is critical for both early identification and prevention.
The Short Story Task (SST) was chosen as a ToM task sensitive to individual differences and subtle variation. Importantly, the task provides separate measures of implicit versus explicit ToM ability, and, to our knowledge, these dissociable components of ToM have not been studied in CHR populations. Implicit ToM ability constitutes the ability to spontaneously attribute mental state to others, thus making “mental state inferences” (Dodell-Feder et al., 2013). In contrast, explicit ToM ability would include “mental state reasoning” whereby an individual is able to think and reason about others’ mental states when prompted (Dodell-Feder et al., 2013). This dissociation could be informative as, for example, research has shown that individuals with autism are able to make mental state inferences when prompted, though they are less likely to do so spontaneously (Senju et al., 2009; Moran et al., 2011). The SST has also proven promising in predicting individual differences in ToM correlates such as indirect request comprehension (Trott and Bergen, 2018).
Being able to engage in mental state reasoning when prompted is very different than spontaneously being prone to making these inferences. Understanding Implicit ToM ability thus could improve our ability to understand how likely CHR individuals are to spontaneously think about others’ mental states, which could significantly impact their day-to-day functioning and quality of life (Dodell-Feder et al., 2013; Trott and Bergen, 2018). There are studies that suggest deficits in implicit ToM as psychotic illness progresses, though this has not yet been explored prior to psychotic illness onset (Bliksted et al., 2016; Langdon et al., 2017). Thus, determining possible trait-level differences in likelihood to make implicit versus explicit mental state inferences could lend useful information with regards to illness progression, symptom severity and social functioning. Ultimately, it could be informative to prediction models for risk of conversion to a psychotic disorder.
The literature has consistently found abnormalities in neural ToM processing in both CHR (Takano et al., 2017) and psychotic phases of illness (Kronbichler et al., 2017). Two neural networks that have been consistently associated with ToM processing in both healthy and psychotic disorder patients (Caspers et al., 2010; Schilbach et al., 2012a; Schurz et al., 2014). These distinct networks are referred to as the “mentalizing network” (MENT) and the “mirror neuron system” (MNS). The MENT is activated by tasks that explicitly require or implicitly elicit individuals to think about the mental states of others (Schilbach et al., 2016). The MENT is also engaged during states of unconstrained cognition, which give rise to “social thoughts” (de Lange et al., 2008; Schilbach et al., 2008; Schilbach et al., 2010; Schilbach et al., 2012b). The MNS also plays a key role in social cognition through providing a pre-conscious sense of being and acting with others (Newman-Norlund et al., 2007; Gallese, 2009; Iacoboni, 2009; Rizzolatti and Sinigaglia, 2010; Shibata et al., 2011; Schilbach et al., 2016).
Recent investigations have discovered altered functional connectivity in schizophrenia patients in important network hubs of the MNS and MENT (Meda et al., 2012; Yu et al., 2012; Guo et al., 2014). Most recently, a study found decreased functional connectivity among the MENT and MNS networks in schizophrenia patients, which was linked to symptom severity (Schilbach et al., 2016). Exploring these resting state functional networks using brain graphs may be particularly important. Recent work strongly suggests that translating modality-specific connectivity statistics to topological measures on brain graphs may facilitate more reliable cognitive and clinical interpretations of neuroimaging systems (Bassett et al., 2009; Bullmore and Bassett, 2011). Brain graphs model the nervous system as nodes interconnected by a set of edges (Bullmore and Sporns, 2009). The edges can represent, for example, functional connections between cortical and subcortical regional nodes based on analysis of resting state data. Based on graph theory concepts, geometrical properties are estimated and related to network topology. Graph theory analyses allow us to compare across a variety of complex, information-processing systems (Bassett et al., 2009; Bullmore and Bassett, 2011). For example, it has been demonstrated across modalities (e.g. fMRI, EEG) that higher cognitive performanceis associated with brain graphs globally configured for greater efficiency of parallel information transfer (Bassett et al., 2009; Bullmore and Bassett, 2011). Given compelling and widely accepted evidence that the brain supports massively parallel information processing, adapting efficiency metrics of brain functional network topology has been argued to be conceptually preferable to other methods (Achard and Bullmore, 2007). Further, among schizophrenia populations, alterations in topological measures of local information processing, such as efficiency, have been found in fMRI studies (Zalesky et al., 2010b; Zalesky et al., 2010a; Alexander-Bloch et al., 2010; Lynall et al., 2010; Fornito et al., 2012). Despite evidence that connectivity in the MENT and MNS networks is impaired in psychotic disorders, no studies, to date, have examined these networks in CHR populations. Thus, it is unclear which stage of illness progression these abnormalities emerge and what the link is between risk for psychosis, efficiency in these networks, and ToM performance.
The present investigation hypothesized that ToM performance would relate to positive and negative symptoms (Stanford et al., 2011; Kim et al., 2011; Thompson et al., 2012; Bora and Pantelis, 2013; Healey et al., 2013). It was also hypothesized that topological properties of the MENT and MNS networks (assessed by resting state fMRI) would be altered in CHR, especially in those with lower SST ToM performance (Carrington and Bailey, 2009; Schilbach et al., 2016; Kronbichler et al., 2017). Thus, analyses relating symptoms to ToM performance were undertaken in CHR individuals. In addition, neural analyses examined between-group (CHR versus HV) differences in addition to interactions of group by ToM variables within each network.
Participants
Participants included 50 adolescents and young adults (24 CHR and 26 HVs) recruited to the Adolescent Development and Preventive Treatment (ADAPT) research program at the University of Colorado, Boulder (Institutional Review Board approved protocol 10–0398). Ages 12–24 were eligible for inclusion. Exclusion criteria included history of head injury, history of a neurological disorder, MRI contraindications, and having an Axis I psychotic disorder or substance dependence according to the Diagnostic and Statistical Manual of Mental Disorder 4th ed., text rev. (American Psychiatric Association, 2000). In addition, presence of a psychotic disorder in a first-degree relative or meeting for an Axis I disorder were exclusionary criteria for HVs. Participants 18 and older provided written informed consent. For participants under 18, parents or legal guardians additionally provided consent. After establishing eligibility, participants first completed clinical interviews. After, participants came back for another visit to complete the ToM task, and were additionally scheduled for an MRI session, which included a resting state scan (typically within a week of completing the clinical interviews and ToM task).
Measures and Procedure
Short Story Task (SST).
In the SST participants are asked to read “The End of Something,” a short story by Ernest Hemingway. After reading the story, participants are asked a series of 14 questions to assess comprehension, explicit mental state reasoning (EMSR), and spontaneous mental state inference (SMSI). Comprehension items assess the participant’s accurate understanding of non-mental state story content. Specifically, comprehension questions ask about factual information presented in the story, such as what object/item the character observed or what action they performed. Most importantly, accurate responses are based on available information and do not require inference or mental state understanding. As such, these questions assess the ability to comprehend concrete, factual information presented in narrative form. For purposes of task design, responses to comprehension questions help verify that the participant has sufficient cognitive capacity to complete the task and that they paid attention, understood instructions, and devoted adequate effort.
EMSR questions assess the accuracy of the mental state inference, number of character perspectives/emotions taken into account (i.e., second-order inferences generally received more points than first-order inferences), and understanding of non-verbal/indirect communications (e.g., sarcasm and body language). To assess SMSI, participants are asked a single question that simply asked them to summarize the story. Responses were coded for the presence or absence of a mental state inference (see Table 2 for sample questions). There were two raters, trained according to validated task guidelines (Dodell-Feder et al., 2013). Reliability was established for 5 subjects subsequently collected and coded. Kappas of at least .8 for SMSI and .9 for EMSR and Comprehension were obtained.
Table 2.
Sample prompt and scoring criteria from the Short Story Task (SST) for Spontaneous Mental State Inference (SMSI), Explicit Mental State Reasoning (EMSR), and Comprehension. After reading Ernest Hemingway’s short story, “The End of Something,” participants are asked a set of questions. Responses are scored from 0–2, with 2 being the most accurate response.
| Prompt | 2 score response | 1 score response | 0 score response | |
|---|---|---|---|---|
| Spontaneous Mental State Inference | In just a few sentences, how would you summarize the story? | N/A | During the response, the participant makes a mental state inference (i.e. about a belief, thought, desire, intention, goal, emotion) | Participant does not make a mental state inference. |
| Explicit Mental State Reasoning | Why is Nick afraid to look at Marjorie? | Response needs to reference Marjorie’s possible reaction to what he is saying; He knows she is hurt/upset by his comment, and he is afraid of her reaction/doesn’t want to see the hurt in her face; is afraid of her judgment of him. | Some response that conveys his feelings without referencing how Marjorie’s reactions affect his feelings; he is uncomfortable with the way the conversation is heading; he feels guilty/shameful/sad; he’s about to break up with her and it’s easier not to look at her; he’s afraid he’s making the wrong decision by breaking up with her. | He doesn’t want Marjorie to see his reaction; none of the responses in 2 or 1. |
| Comprehension | Nick and Marjorie have a pail of perch for what purpose? | Bait; catching fish | Any response that conveys understanding of its use for some aspect of fishing without being explicit about its function as bait for catching fish. | Any response that doesn’t convey understanding of its use for fishing. |
Structured Clinical Interviews and Assessment.
Participants were administered the Structured Clinical Interview for DSM-IV Axis I disorders (SCID) (First, 2012). The SCID was administered to rule out psychosis diagnosis in the CHR and HV groups, as well as to assess history of mood and anxiety disorders. The Structured Interview for Prodromal Syndromes (SIPS) was administered to CHR and HV participants in order to identify CHR subjects and rule out symptoms in HVs (Miller et al., 1999). CHR inclusion criteria included Attenuated Positive Symptom Syndrome (APSS), APSS and schizotypal personality disorder prior to 18 years old, and Genetic Risk and Deterioration Syndrome (GRDS; see table 1 for inclusion breakdown). All interviews were conducted by trained clinical doctoral students; Kappas of at least .8 for SIPS and .9 for prodromal (e.g. APSS, GRDS) and psychiatric diagnoses were obtained.
Table 1.
Demographic characteristics.
| Demographics | CHR1 (n = 24) | HV2 (n = 26) | CHR | HV | Group Diff. |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Range | |||
| Age | 19.83 (2.22) | 19.85 (2.72) | 15–23 | 13–24 | n.s |
| Sex | 41.67% female | 50 % female | -- | -- | n.s |
| Cognitive functiona | 57.48 (9.06) | 55 (8.98) | 28–70 | 33–69 | n.s |
| Parental educationb | 15.48 (2.37) | 16.30 (2.67) | 10–20 | 10–20 | n.s |
| Self-identified Race | |||||
| Black | 0% | 3.85% | -- | -- | -- |
| Central/South American | 16.67% | 3.85% | -- | -- | -- |
| East Asian | 4.17% | 7.69% | -- | -- | -- |
| First Nations | 8.33% | 0% | -- | -- | -- |
| Southeast Asian | 0% | 3.85% | -- | -- | -- |
| White | 70.83% | 80.77% | -- | -- | -- |
| Inclusion Criteria Met | |||||
| Attenuated Positive | 79.17% | -- | -- | -- | -- |
| Symptom Syndrome (APSS) | |||||
| APSS and Schizotypal | 4.17% | -- | -- | -- | -- |
| Personality Disorder prior to 18 years old | |||||
| Genetic Risk and | 16.67% | -- | -- | -- | -- |
| Deterioration Syndrome (GRDS) | |||||
| Symptomsc | Mean (SD) | Mean (SD) | |||
| Positive | 6.92 (5.08) | 0.12 (0.33) | 1–22 | 0–1 | -- |
| Negative | 5.54 (5.79) | 0.27 (0.53) | 0–19 | 0–2 | -- |
| Disorganized | 2.58 (2.24) | 0.42 (0.81) | 0–7 | 0–3 | -- |
| General | 5.63 (3.84) | 0.73 (1.40) | 1–13 | 0–6 | -- |
| SSTd Performance | Mean (SD) | Mean (SD) | |||
| SMSIe (Yes/No) | 29.17% (7/17) | 69.23% (18/8) | 0–1 | 0–1 | 1 < 2*g |
| EMSRf | 8.83 (2.24) | 8.54 (2.45) | 4–13 | 2–12 | n.s |
| Comprehension | 9.12 (1.23) | 8.54 (1.53) | 6–10 | 5–10 | n.s |
Note: Mean (SD),
= Clinical High Risk,
= Healthy Volunteer
MATRICS composite t score.
Averaged years of education of both parents.
Estimated by the SIPS interview.
Short Story Task.
Spontaneous Mental State Inference.
Explicit Mental State Reasoning.
Cramer’s V = 0.40
p < .05.
Imaging Acquisition and Processing.
Scans were acquired using a 3T Siemens Trio scanner. Resting-state blood oxygen level-dependent (BOLD) scans were acquired with a T2-weighted echo-planar functional protocol (number of volumes = 165; TR = 2,000 ms; TE = 29 ms; matrix size = 64 × 64 × 33; FA = 75°; 3.8 × 3.8 × 3.5 mm3 voxels; 33 slices; FOV = 240 mm). To investigate incidental pathology, a turbo spin echo proton density—T2 weighted acquisition (axial oblique aligned with anterior commissure-posterior commissure line; TR = 3,720 ms; TE = 89 ms; GRAPPA parallel imaging factor of 2; FOV = 240 mm; flip angle: 120°; 0.9 × 0.9 mm2 voxels; 77 interleaved 1.5-mm slices) was generated. Resting scans were 5 minutes and 34 seconds long. Participants were instructed to relax and keep their eyes closed. Previous studies have found that this functional connectivity MRI duration provides equal power to longer scan times (Van Dijk et al., 2010).
FSL Version 5 (Jenkinson et al., 2012) was used for data processing. This preprocessing involved motion correction, brain extraction, high-pass filtering (100s), and spatial smoothing (6 mm FWHM). Functional images were aligned to the Montreal Neurological Institute (MNI) 2-mm brain template. Motion correction (Power et al., 2012) was carried out with the artifact detection software (ART; https://www.nitrc.org/projects/artifact_detect), which created confound regressors for motion parameters (three translation and three rotation parameters) and additional confound regressors for specific image frames with outliers based on brain activation and head movement. Mean global brain activity was calculated to identify outliers. Outliers were defined as frames where the global mean signal exceeded 3 SDs. Framewise measures of motion were used to identify any motion outliers/motion spikes. Motion outliers were defined as frames where motion exceeded 1 mm.
Functional connectivity analyses were performed using the Conn Functional Connectivity Toolbox (CONN), Version 17.b (Whitfield-Gabrieli and Nieto-Castanon, 2012) in SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). SPM12 was used to segment anatomical images into gray matter, white matter, and cerebrospinal fluid (CSF) and create masks for signal extraction. The Conn Toolbox extracts five temporal components from the segmented CSF and white matter, which were then entered as confound regressors in the subject-level general linear model (GLM). Motion from the ART toolbox was additionally included as a confound regressor.
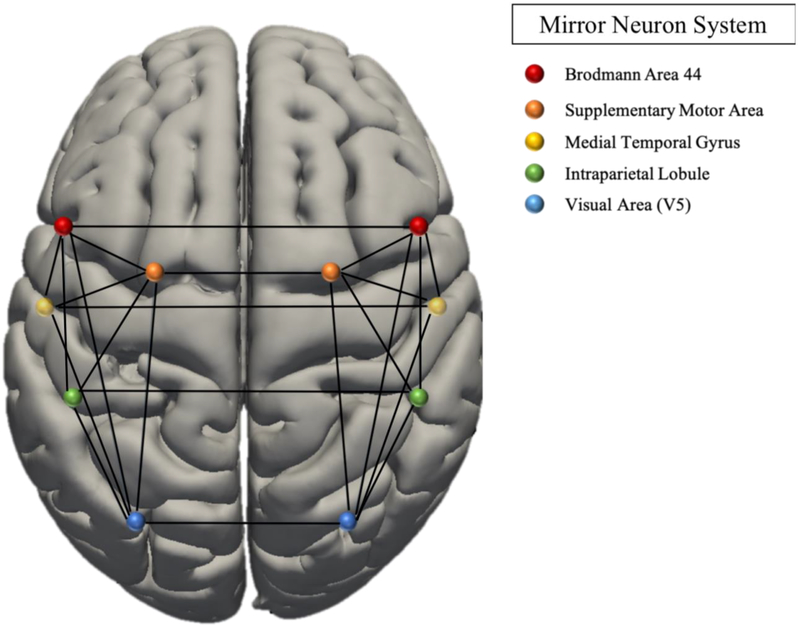
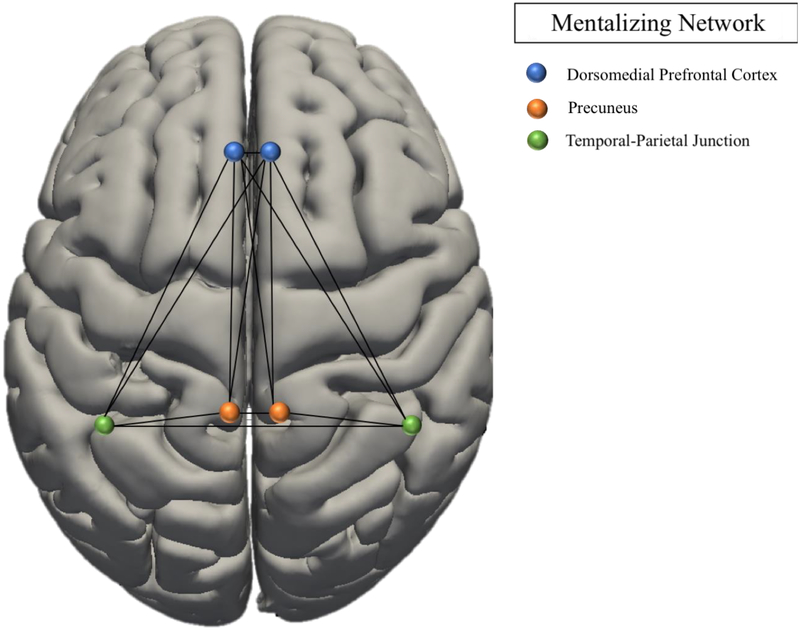
The MNS was derived from a meta-analysis implicating this network in activation observation and action imitation (see Figure 1, Table 3) (Caspers et al., 2010). The MENT network was derived from a meta-analysis of self-referential and social cognition, associating the network with introspective processes and socio-emotional function (see Figure 1b, Table 3) (Schilbach et al., 2012a). Regions of Interest (ROI) were extracted using SPM toolbox. Seed-to-seed functional connectivity analyses were computed for the MENT and MNS networks (see Table 3) using CONN (Whitfield-Gabrieli and Nieto-Castanon, 2012).
Figure 1(a).
Graph Network Models: Mirror Neuron System (Caspers et al., 2010).
Table 3.
MNI coordinates for networks of interest
| MNI coordinates | |||
|---|---|---|---|
| Region | x | y | z |
| MNS network | |||
| Right IFG (BA 44) | −56 | 8 | 28 |
| Left IFG (BA 44) | 58 | 16 | 10 |
| Right SMA | 10 | −2 | 45 |
| Left SMA | −1 | 16 | 52 |
| Right IPL | 51 | −36 | 50 |
| Left IPS/IPL | −38 | −40 | 50 |
| Right MTG | 53 | −45 | 5 |
| Left MTG | −54 | −50 | 10 |
| Right V5 | −52 | −70 | 6 |
| Left V5 | 54 | −64 | 4 |
| Right FG | 44 | −54 | −20 |
| Left FG | −28 | −9 | −38 |
| MENT network | |||
| Right PreC | 10 | −66 | 24 |
| Left PreC | −6 | −54 | 24 |
| Right dmPFC | 12 | 54 | 15 |
| Left dmPFC | −2 | 52 | 14 |
| Right TPJ | 52 | −62 | 16 |
| Left TPJ | −46 | −66 | 18 |
Note: cortex pMTG, posterior middle temporal gyrus; SMA, supplementary motor area (hidden within the interhemispheric fissure); IPS, intraparietal sulcus; MTG, middle temporal gyrus; FG, fusiform gyrus; PreC, Precuneus; dmPFC, dorsomedial prefrontal cortex; TPJ, temporo-parietal junction; MENT, mentalizing network; MNS, mirror neuron system; IPL, inferior parietal lobe; V5, extrastriate visual area; BA 44, Broca’s area; PCC, posterior cingulate cortex; mPFC, medial pre-frontal; MNI, Montreal Neurological Institute. All peaks are assigned to the most probable brain areas as revealed by the SPM Anatomy Toolbox.
Figure 1(b).
Graph Network Models: Mentalizing Network (Schilbach et al., 2012a).
Graph Theory Analysis.
Global efficiency was calculated for the two networks. Global efficiency constitutes the average of the inverse shortest path lengths for all ROI-ROI pairs in the network, providing a measure of the network’s overall connectedness, that is, how efficiently information is communicated between nodes (Bullmore and Bassett, 2011; Goparaju et al., 2014). Notably, global efficiency is a measure of the network’s capacity for parallel information transfer between nodes (via several series of edges) (Achard and Bullmore, 2007). Given strong evidence of widespread brain parallel information processing, efficiency metrics of brain functional network topology are hypothesized to be particularly suited for interpreting these processes (Achard and Bullmore, 2007). In order to reduce the number of total comparisons in the current study, global efficiency was focused on, as it accounts for multiple graph features (described above) in a single metric.
Statistical Analyses.
Chi-square tests and t-tests, when appropriate, were used to test for differences in demographic characteristics. Within the CHR group, due to evidence of skew in symptom data, relationships between positive, negative and disorganized symptoms and ToM variables were tested using Spearman correlations in the case of EMSR and Comprehension, and Independent Samples Mann-Whitney U test in the case of SMSI. Global efficiency was extracted for MENT and MNS networks, with network edges (adjacency matrix threshold) defined as z scores, using a two-sided FDR correction threshold of p < 0.05. For each network, group differences in global efficiency were tested. In addition, interactions of group by comprehension, EMSR, and SMSI were tested for each of the 2 networks.
Results
Demographics, Symptoms and ToM.
See Table 1 for demographic variables and group differences. A Pearson chi-square test of diagnosis by SMSI was significant [X2 = 8.01, p = 0.01], whereby significantly less CHR individuals made a SMSI in their story summary compared to HVs. There were no significant differences between groups with regards to both comprehension and EMSR (see Table 1). Relationships with symptoms are presented in Table 4. Trend-level associations were observed with symptoms, such that absence of SMSI was associated with greater positive and negative symptoms.
Table 4.
Relationships of SST variables with symptoms in CHR.
| Positive symptomsa | Negative symptomsa | Disorganized symptomsa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| statb | p | Effect sizec | 95% CI | statb | p | Effect sizec | 95% CI | statb | p | Effect sizec | 95% CI | |
| SMSId | −1.6 | 0.11 | 0.11 | −1.0–7.0 | −1.25 | 0.21 | 0.07 | −1.0–10.0 | −0.58 | 0.56 | 0.01 | −1.0–3.0 |
| EMSRe | −0.17 | 0.44 | 0.17 | −0.43–0.12 | −0.09 | 0.69 | 0.09 | −0.36–0.19 | −0.03 | 0.88 | 0.03 | −0.31–0.25 |
| Compf | 0.08 | 0.72 | 0.08 | −0.20–0.35 | −0.25 | 0.25 | 0.25 | −0.50–0.03 | −0.18 | 0.40 | 0.18 | −0.44–0.11 |
Estimated by the SIPS battery.
An Independent Samples Mann-Whitney U test was performed for SMSI, and Spearman correlations were performed for EMSR and Comp.
For SMSI, η2. For EMSR and Comp, |r|.
Spontaneous Mental State Inference.
Explicit Mental State Reasoning.
Comprehension
Graph Theory Analyses.
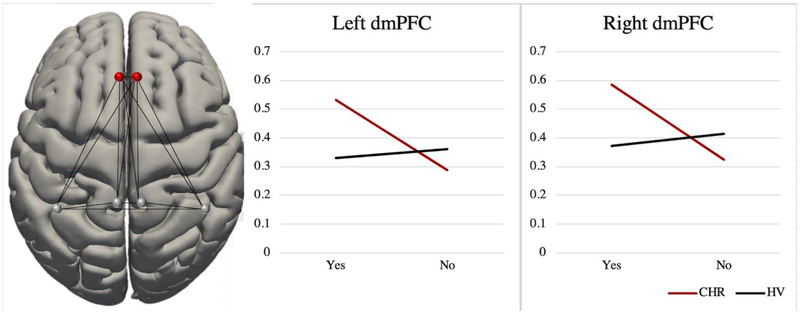
There were no differences in global efficiency between groups for networks of interest (Table 5). In addition, interactions of group by Comprehension, EMSR, and SMSI were not found for the MNS network. However, an interaction of group by SMSI was found within the MENT network, such that bilateral dorsomedial prefrontal cortex (dmPFC) showed greater global efficiency within the MENT network for those CHR individuals that made SMSIs, relative to those that did not (see Figure 2). This interaction was not observed in healthy individuals. Notably, interactions were not apparent within the MENT network for Comprehension or EMSR. The magnitude of the effects did not change when controlling for age and sex. Two CHR individuals eventually converted to a psychotic disorder. Running analyses without including these 2 individuals did not alter effect sizes.
Table 5.
Global efficiency statistics among networks of interest
| ROI | beta | T | puncorrected | p FDR | |
|---|---|---|---|---|---|
| Mentalizing (MENT) Network | |||||
| Group | Network | −0.01 | −0.16 | 0.87 | -- |
| Group by Comprehension | Network | −0.01 | −0.23 | 0.82 | -- |
| Group by EMSR | Network | −0.00 | −0.16 | 0.88 | -- |
| Group by SMSI | Network | 0.15 | 1.67 | 0.10 | -- |
| Right dmPFC | 0.30 | 2.59 | 0.01 | 0.039 | |
| Left dmPFC | 0.27 | 2.67 | 0.01 | 0.039 | |
| Mirror Neuron System (MNS) Network | |||||
| Group | Network | −0.03 | −0.96 | 0.34 | -- |
| Group by Comprehension | Network | 0.05 | 2.19 | 0.03 | -- |
| Group by EMSR | Network | 0.02 | 1.52 | 0.14 | -- |
| Group by SMSI | Network | −0.10 | −1.60 | 0.12 | -- |
Figure 2.
MENT network global efficiency interaction of SMSI by Group in bilateral dmPFC. On the x-axis, “Yes” refers to having made a SMSI, and “No” refers to not having made a SMSI. Bilateral dmPFC global efficiency within the MENT network was extracted as eigen values (y-axis).
Discussion
The present investigation used topographical functional resting state measures to explore the neural underpinnings of ToM in CHR individuals and HVs. First, behavioral differences between groups were examined. CHR individuals did not differ in explicit ToM ability, however they made significantly less SMSI, suggesting altered implicit and spontaneous ToM ability. Next, associations between ToM and symptoms in CHR were examined; there were trend-level relationships with positive and negative symptoms. Finally, topographical functional resting state measures were used to explore global efficiency (i.e. graph or network interconnectedness, or degree of global connectedness of a certain region within the network) in well-established ToM networks. Network global efficiency differences between groups were not observed. We aimed to see how resting state function in these networks correlated with ToM performance outside the scanner. A relation was observed whereby group interacted with SMSI such that CHR individuals that made SMSIs showed greater global efficiency in bilateral dmPFC within the MENT network. Findings offer nuance to the extant literature of ToM deficits in CHR populations. They suggest that CHR individuals exhibit impairment in implicit ToM (implying less spontaneous thinking about others’ mental states), while explicit ToM may be relatively more intact at this stage of illness progression (meaning CHR individuals are still able to exercise theory of mind and imagine others’ mental states when explicitly prompted). Further, CHR individuals with intact spontaneous mental reference abilities may be aided by increased efficiency of dmPFC within the MENT (see Figure 2). Taken together, results inform etiological models of ToM deficits and associated neural networks in psychotic disorders. In addition, findings offer specificity with regards to candidate neural mechanisms for treatment and intervention surrounding implicit ToM as well as with regards to what aspects of ToM may be more impaired prior to psychotic disorder onset.
The literature had yet to distinguish between implicit/spontaneous and explicit/evoked ToM ability in CHR populations, confounding understanding of specificity of neurocognitive function in this domain (Dodell-Feder et al., 2013). In this sample, there were no differences between CHR and HVs in story stimuli comprehension, nor were there differences in explicit/evoked mental state reasoning. However, compared to healthy individuals, CHR participants made significantly less SMSIs. This group difference suggests that implicit ToM may be particularly impacted in the CHR group, while explicit ToM remains relatively intact. This finding is consistent to what has been found in autism populations, who exhibit intact explicit ToM, but impaired implicit ToM function (Senju et al., 2009; Moran et al., 2011). However, given that previous meta analyses have found ToM deficits in CHR populations, future studies will be needed to replicate this finding using the SST (Bora et al., 2008). Further, there were trend-level associations between implicit ToM, positive and negative symptoms, such that those CHR participants that made SMSI had less severe symptoms. These symptom associations partially supports previous studies linking ToM to functional outcome and illness severity (Pijnenborg et al., 2009; Bae et al., 2010; Fett et al., 2011), though limited power may have precluded observation of a stronger relationship.
Topographical measures of MENT and MNS network efficiency did not yield group differences. This null network wide finding suggests that across groups the networks are not distinguishable in terms of globally integrated, parallel information-processing capacity (Achard and Bullmore, 2007). This finding is contrary to study hypotheses, though it adds to a wide body of literature examining global efficiency at the whole-brain level as well as in particular networks in schizophrenia, which has thus far yielded mixed findings (Liu et al., 2008; Alexander-Bloch et al., 2010; Lynall et al., 2010; Becerril et al., 2011; Fornito et al., 2012; Wang et al., 2012). It is necessary, however, to keep in mind that lack of differences in these activation patterns at rest does not preclude there being activation differences during performance of ToM tasks (Van Overwalle and Baetens, 2009; Schilbach et al., 2012a; Schilbach et al., 2016). It is possible that there are differences in efficiency that are only apparent during active ToM processing. The present study aimed to explore individual-level differences in “resting-state” neural efficiency and correlates to behavioral implicit and explicit ToM measures. This is valuable in investigating possible “trait-level” differences in ToM network efficiency that co-occur with ToM behavioral measures. Future studies will be needed using task-based fMRI paradigms of implicit and explicit ToM to further explore and expand this work.
In examining the convergence of ToM performance and global efficiency in MENT and MNS networks, however, an interaction was observed of group by SMSI within the MENT bilateral dmPFC region. Within the MENT, interactions of group by EMSR and group by comprehension were not detected, lending evidence to specificity of implicit/spontaneous ToM capacity (versus explicit ToM or attention to task stimuli). Likewise, for MNS there were no group interactions across any ToM variables. In all, results are consistent with previous research implicating the MENT in states of unconstrained cognition, which give rise to spontaneous “social thoughts” (de Lange et al., 2008; Schilbach et al., 2008; Schilbach et al., 2010; Schilbach et al., 2012b). Perhaps the MENT specifically serves a role in spontaneous ToM.
It is striking that rather than observe a global efficiency interaction in the MENT network as a whole, the interaction was localized to global efficiency in bilateral dmPFC within the MENT network. There are several plausible interpretations for this observation. It is a possibility that the dmPFC is responsible to a sizable degree when it comes to spontaneous mentalizing. There is a large body of evidence supporting this interpretation. Most recently, for example, a task-based fMRI study implicated the dmPFC in spontaneous mentalization specifically (Moessnang et al., 2017). Given the dmPFC has been found to be responsible for context-independent, flexible metacognitive representations of the social world, it may be particularly implicated in implicit ToM (Bzdok et al., 2013; Spunt and Adolphs, 2015).
CHR participants that made SMSI exhibited greater global efficiency in bilateral dmPFC (within the MENT network). This increased efficiency in dmPFC was not observed for HV individuals with SMSI. Again, there are several plausible interpretations for this finding. Previous investigations using brain graphs have found increased global efficiency in schizophrenia populations (Alexander-Bloch et al., 2010; Lynall et al., 2010). This increased global efficiency has been attributed to refined, subtle randomization of network topology (Fornito et al., 2012). It has also been hypothesized to contribute to robustness to random attacks of functional networks in schizophrenia, thus possibly providing a survival advantage (Lynall et al., 2010). That is, whole brain networks are less likely to break off into disconnected islands as regional nodes are removed at random (due to possible disease-caused multifocal brain lesions) (Lynall et al., 2010). This increased efficiency could be speculated to provide a survival advantage, lending greater resilience of global brain function in the face of possible multifocal brain lesions (Lynall et al., 2010). Thus, perhaps those CHR individuals that exhibit intact implicit ToM abilities, and that nonetheless experience clinical impairment and symptoms that put them at risk for psychosis, may develop compensatory neural mechanisms that serve as protective factors for conversion to a psychotic disorder. While this study is unique in linking global efficiency metrics within these networks to implicit ToM abilities, it will be essential for future studies to further delve into this question in order to determine whether global efficiency in these regions serves as a protective factor for at-risk individuals.
The current study elucidates multiple relevant etiological links that may serve to identify precursors of psychotic illness, as well as underlying biological factors. For example, research has shown that neural global efficiency is reduced in older individuals, and that these effects are largely localized to frontal cortical and subcortical regions (Achard and Bullmore, 2007). Perhaps the reduced global efficiency within dmPFC in the MENT network among those CHR individuals that exhibited impaired implicit ToM ability reflects deteriorating network function due to the same factors that put these individuals at risk (e.g. the latent or emerging pathophysiology of psychosis). Even more compelling, pharmacological blockade of dopamine transmission has also been shown to impair global and local efficiency of networks (Achard and Bullmore, 2007). Given widely observed cortical dopamine dysregulation in schizophrenia, this graph metric could serve as a candidate etiological factor informing risk for developing psychosis (Bauer et al., 2012; Bolton and Constantine-Paton, 2018; Gomes and Grace, 2018; Grace and Gomes, 2018).
There are several limitations that ought to be carefully considered when interpreting results. Most importantly, data is cross-sectional, thus causes of ToM deficits and neural underpinnings cannot be fully determined. Comparing CHR with psychotic disorder individuals in terms of implicit versus explicit ToM and involved networks would also be informative. Also, participants completed MRI scans in a separate visit than the SST task. We were interested in trait-level network features and relationships to ToM ability, which would, in theory, not be impacted by this delay in timing. It is also necessary to consider that there was high comorbidity of other Axis I disorders in our CHR sample, and it will be informative for future studies to compare to help-seeking controls with high incidence of Axis I disorders (in order to establish specificity to attenuated psychosis symptoms). Further, the study sample size was adequate but modest, and so lack of power may have precluded our finding of extant relationships with symptoms, for example. In addition, it would be beneficial for future explorations of this question to include multiple measures of social cognition, in order establish convergence and divergence of social cognitive functions. Relatedly to the above point, limited power prevented us from undertaking a whole brain approach, as well as from exploring other networks that may have been differentially involved in implicit and explicit ToM (in the interest of limiting the number of comparisons). Future studies would benefit from exploring other networks of interest, such as the fronto-parietal and salience networks.
Supplementary Material
Acknowledgments
We would like to thank our participants that kindly volunteered their time for this research.
Role of the Funding Source
This work was supported by a Northwestern University Society Biology and Health Cluster fellowship (T.V.), and by grants R01MH112545, R21/R33MH103231 and R21MH110374 (V.A.M).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
There are no conflicts of interest.
References
- Achard S, Bullmore E, 2007. Efficiency and cost of economical brain functional networks. PLoS Comput Biol 3(2), e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander-Bloch AF, Gogtay N, Meunier D, Birn R, Clasen L, Lalonde F, Lenroot R, Giedd J, Bullmore ET, 2010. Disrupted modularity and local connectivity of brain functional networks in childhood-onset schizophrenia. Front Syst Neurosci 4, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association A., 2000. Diagnostic and statistical manual of mental disorders (4th ed., text rev.), Washington, D.C. [Google Scholar]
- Bae SM, Lee SH, Park YM, Hyun MH, Yoon H, 2010. Predictive factors of social functioning in patients with schizophrenia: exploration for the best combination of variables using data mining. Psychiatry Investig 7(2), 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Bullmore ET, Meyer-Lindenberg A, Apud JA, Weinberger DR, Coppola R, 2009. Cognitive fitness of cost-efficient brain functional networks. Proc Natl Acad Sci U S A 106(28), 11747–11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Praschak-Rieder N, Kasper S, Willeit M, 2012. Is dopamine neurotransmission altered in prodromal schizophrenia? A review of the evidence. Curr Pharm Des 18(12), 1568–1579. [DOI] [PubMed] [Google Scholar]
- Becerril KE, Repovs G, Barch DM, 2011. Error processing network dynamics in schizophrenia. Neuroimage 54(2), 1495–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological) 57(1), 289–300. [Google Scholar]
- Bertrand MC, Sutton H, Achim AM, Malla AK, Lepage M, 2007. Social cognitive impairments in first episode psychosis. Schizophr Res 95(1–3), 124–133. [DOI] [PubMed] [Google Scholar]
- Bliksted V, Ubukata S, Koelkebeck K, 2016. Discriminating autism spectrum disorders from schizophrenia by investigation of mental state attribution on an on-line mentalizing task: a review and meta-analysis. Schizophrenia Research 171(1–3), 16–26. [DOI] [PubMed] [Google Scholar]
- Bolton AD, Constantine-Paton M, 2018. Synaptic Effects of Dopamine Breakdown and Their Relation to Schizophrenia-Linked Working Memory Deficits. Front Synaptic Neurosci 10, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Gokcen S, Kayahan B, Veznedaroglu B, 2008. Deficits of social-cognitive and social-perceptual aspects of theory of mind in remitted patients with schizophrenia: effect of residual symptoms. J Nerv Ment Dis 196(2), 95–99. [DOI] [PubMed] [Google Scholar]
- Bora E, Pantelis C, 2013. Theory of mind impairments in first-episode psychosis, individuals at ultra-high risk for psychosis and in first-degree relatives of schizophrenia: systematic review and meta-analysis. Schizophr Res 144(1–3), 31–36. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O, 2009. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10(3), 186–198. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Bassett DS, 2011. Brain graphs: graphical models of the human brain connectome. Annu Rev Clin Psychol 7, 113–140. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Langner R, Schilbach L, Engemann DA, Laird AR, Fox PT, Eickhoff SB, 2013. Segregation of the human medial prefrontal cortex in social cognition. Front Hum Neurosci 7, 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington SJ, Bailey AJ, 2009. Are there theory of mind regions in the brain? A review of the neuroimaging literature. Hum Brain Mapp 30(8), 2313–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB, 2010. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage 50(3), 1148–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran R, Frith CD, 2003. Autobiographical memory and theory of mind: evidence of a relationship in schizophrenia. Psychol Med 33(5), 897–905. [DOI] [PubMed] [Google Scholar]
- Corcoran R, Mercer G, Frith CD, 1995. Schizophrenia, symptomatology and social inference: investigating “theory of mind” in people with schizophrenia. Schizophr Res 17(1), 5–13. [DOI] [PubMed] [Google Scholar]
- Craig JS, Hatton C, Craig FB, Bentall RP, 2004. Persecutory beliefs, attributions and theory of mind: comparison of patients with paranoid delusions, Asperger’s syndrome and healthy controls. Schizophr Res 69(1), 29–33. [DOI] [PubMed] [Google Scholar]
- de Achaval D, Costanzo EY, Villarreal M, Jauregui IO, Chiodi A, Castro MN, Fahrer RD, Leiguarda RC, Chu EM, Guinjoan SM, 2010. Emotion processing and theory of mind in schizophrenia patients and their unaffected first-degree relatives. Neuropsychologia 48(5), 1209–1215. [DOI] [PubMed] [Google Scholar]
- de Lange FP, Spronk M, Willems RM, Toni I, Bekkering H, 2008. Complementary systems for understanding action intentions. Curr Biol 18(6), 454–457. [DOI] [PubMed] [Google Scholar]
- Dodell-Feder D, Lincoln SH, Coulson JP, Hooker CI, 2013. Using fiction to assess mental state understanding: a new task for assessing theory of mind in adults. PLoS One 8(11), e81279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L, 2011. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev 35(3), 573–588. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer RL, Gibbon M, Williams JBW, 2012. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I). New York State Psychiatric Institute, New York. [Google Scholar]
- Fornito A, Zalesky A, Pantelis C, Bullmore ET, 2012. Schizophrenia, neuroimaging and connectomics. Neuroimage 62(4), 2296–2314. [DOI] [PubMed] [Google Scholar]
- Gallese V, 2009. Motor abstraction: a neuroscientific account of how action goals and intentions are mapped and understood. Psychol Res 73(4), 486–498. [DOI] [PubMed] [Google Scholar]
- Gomes FV, Grace AA, 2018. Cortical dopamine dysregulation in schizophrenia and its link to stress. Brain 141(7), 1897–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goparaju B, Rana KD, Calabro FJ, Vaina LM, 2014. A computational study of whole-brain connectivity in resting state and task fMRI. Med Sci Monit 20, 1024–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Gomes FV, 2018. The Circuitry of Dopamine System Regulation and its Disruption in Schizophrenia: Insights Into Treatment and Prevention. Schizophr Bull. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Bearden CE, Cannon TD, Fiske AP, Hellemann GS, Horan WP, Kee K, Kern RS, Lee J, Sergi MJ, Subotnik KL, Sugar CA, Ventura J, Yee CM, Nuechterlein KH, 2012. Social cognition in schizophrenia, Part 1: performance across phase of illness. Schizophr Bull 38(4), 854–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Kendrick KM, Yu R, Wang HL, Feng J, 2014. Key functional circuitry altered in schizophrenia involves parietal regions associated with sense of self. Hum Brain Mapp 35(1), 123–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healey KM, Penn DL, Perkins D, Woods SW, Addington J, 2013. Theory of mind and social judgments in people at clinical high risk of psychosis. Schizophr Res 150(2–3), 498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker CI, Bruce L, Lincoln SH, Fisher M, Vinogradov S, 2011. Theory of mind skills are related to gray matter volume in the ventromedial prefrontal cortex in schizophrenia. Biol Psychiatry 70(12), 1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, 2009. Imitation, empathy, and mirror neurons. Annu Rev Psychol 60, 653–670. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM, 2012. Fsl. Neuroimage 62(2), 782–790. [DOI] [PubMed] [Google Scholar]
- Kim HS, Shin NY, Jang JH, Kim E, Shim G, Park HY, Hong KS, Kwon JS, 2011. Social cognition and neurocognition as predictors of conversion to psychosis in individuals at ultra-high risk. Schizophr Res 130(1–3), 170–175. [DOI] [PubMed] [Google Scholar]
- Kovacs AM, Kuhn S, Gergely G, Csibra G, Brass M, 2014. Are all beliefs equal? Implicit belief attributions recruiting core brain regions of theory of mind. PLoS One 9(9), e106558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler L, Tschernegg M, Martin AI, Schurz M, Kronbichler M, 2017. Abnormal Brain Activation During Theory of Mind Tasks in Schizophrenia: A Meta-Analysis. Schizophr Bull 43(6), 1240–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz MM, Richardson CL, 2012. Social cognitive training for schizophrenia: a meta-analytic investigation of controlled research. Schizophr Bull 38(5), 1092–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon R, Flynn M, Connaughton E, Brüne M, 2017. Impairments of spontaneous and deliberative mentalizing co- occur, yet dissociate, in schizophrenia. British Journal of Clinical Psychology 56(4), 372–387. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, Yu C, Liu H, Liu Z, Jiang T, 2008. Disrupted small-world networks in schizophrenia. Brain 131(Pt 4), 945–961. [DOI] [PubMed] [Google Scholar]
- Lynall ME, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, Bullmore E, 2010. Functional connectivity and brain networks in schizophrenia. J Neurosci 30(28), 9477–9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjoram D, Gardner C, Burns J, Miller P, Lawrie SM, Johnstone EC, 2005. Symptomatology and social inference: a theory of mind study of schizophrenia and psychotic affective disorder. Cogn Neuropsychiatry 10(5), 347–359. [DOI] [PubMed] [Google Scholar]
- Martino DJ, Bucay D, Butman JT, Allegri RF, 2007. Neuropsychological frontal impairments and negative symptoms in schizophrenia. Psychiatry Res 152(2–3), 121–128. [DOI] [PubMed] [Google Scholar]
- Meda SA, Gill A, Stevens MC, Lorenzoni RP, Glahn DC, Calhoun VD, Sweeney JA, Tamminga CA, Keshavan MS, Thaker G, Pearlson GD, 2012. Differences in resting-state functional magnetic resonance imaging functional network connectivity between schizophrenia and psychotic bipolar probands and their unaffected first-degree relatives. Biol Psychiatry 71(10), 881–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, Hoffman R, Davidson L, 1999. Symptom assessment in schizophrenic prodromal states. Psychiatr Q 70(4), 273–287. [DOI] [PubMed] [Google Scholar]
- Moessnang C, Otto K, Bilek E, Schafer A, Baumeister S, Hohmann S, Poustka L, Brandeis D, Banaschewski T, Tost H, Meyer-Lindenberg A, 2017. Differential responses of the dorsomedial prefrontal cortex and right posterior superior temporal sulcus to spontaneous mentalizing. Hum Brain Mapp 38(8), 3791–3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JM, Young LL, Saxe R, Lee SM, O’Young D, Mavros PL, Gabrieli JD, 2011. Impaired theory of mind for moral judgment in high-functioning autism. Proc Natl Acad Sci U S A 108(7), 2688–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman-Norlund RD, van Schie HT, van Zuijlen AM, Bekkering H, 2007. The mirror neuron system is more active during complementary compared with imitative action. Nat Neurosci 10(7), 817–818. [DOI] [PubMed] [Google Scholar]
- Pijnenborg GH, Withaar FK, Evans JJ, van den Bosch RJ, Timmerman ME, Brouwer WH, 2009. The predictive value of measures of social cognition for community functioning in schizophrenia: implications for neuropsychological assessment. J Int Neuropsychol Soc 15(2), 239–247. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE, 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59(3), 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Sinigaglia C, 2010. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nat Rev Neurosci 11(4), 264–274. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Bzdok D, Timmermans B, Fox PT, Laird AR, Vogeley K, Eickhoff SB, 2012a. Introspective minds: using ALE meta-analyses to study commonalities in the neural correlates of emotional processing, social & unconstrained cognition. PLoS One 7(2), e30920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L, Derntl B, Aleman A, Caspers S, Clos M, Diederen KM, Gruber O, Kogler L, Liemburg EJ, Sommer IE, Muller VI, Cieslik EC, Eickhoff SB, 2016. Differential Patterns of Dysconnectivity in Mirror Neuron and Mentalizing Networks in Schizophrenia. Schizophr Bull 42(5), 1135–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L, Eickhoff SB, Cieslik EC, Kuzmanovic B, Vogeley K, 2012b. Shall we do this together? Social gaze influences action control in a comparison group, but not in individuals with high-functioning autism. Autism 16(2), 151–162. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, Vogeley K, 2008. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious Cogn 17(2), 457–467. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Wilms M, Eickhoff SB, Romanzetti S, Tepest R, Bente G, Shah NJ, Fink GR, Vogeley K, 2010. Minds made for sharing: initiating joint attention recruits reward-related neurocircuitry. J Cogn Neurosci 22(12), 2702–2715. [DOI] [PubMed] [Google Scholar]
- Schurz M, Radua J, Aichhorn M, Richlan F, Perner J, 2014. Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neurosci Biobehav Rev 42, 9–34. [DOI] [PubMed] [Google Scholar]
- Senju A, Southgate V, White S, Frith U, 2009. Mindblind eyes: an absence of spontaneous theory of mind in Asperger syndrome. Science 325(5942), 883–885. [DOI] [PubMed] [Google Scholar]
- Shibata H, Inui T, Ogawa K, 2011. Understanding interpersonal action coordination: an fMRI study. Exp Brain Res 211(3–4), 569–579. [DOI] [PubMed] [Google Scholar]
- Spunt RP, Adolphs R, 2015. Folk explanations of behavior: a specialized use of a domain-general mechanism. Psychol Sci 26(6), 724–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford AD, Messinger J, Malaspina D, Corcoran CM, 2011. Theory of Mind in patients at clinical high risk for psychosis. Schizophr Res 131(1–3), 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano Y, Aoki Y, Yahata N, Kawakubo Y, Inoue H, Iwashiro N, Natsubori T, Koike S, Gonoi W, Sasaki H, 2017. Neural basis for inferring false beliefs and social emotions in others among individuals with schizophrenia and those at ultra-high risk for psychosis. Psychiatry Research: Neuroimaging 259, 34–41. [DOI] [PubMed] [Google Scholar]
- Thompson A, Papas A, Bartholomeusz C, Allott K, Amminger GP, Nelson B, Wood S, Yung A, 2012. Social cognition in clinical “at risk” for psychosis and first episode psychosis populations. Schizophr Res 141(2–3), 204–209. [DOI] [PubMed] [Google Scholar]
- Trott S, Bergen B, 2018. Individual Differences in Mentalizing Capacity Predict Indirect Request Comprehension. Discourse Processes, 1–33. [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL, 2010. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol 103(1), 297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K, 2009. Understanding others’ actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage 48(3), 564–584. [DOI] [PubMed] [Google Scholar]
- Wang Q, Su TP, Zhou Y, Chou KH, Chen IY, Jiang T, Lin CP, 2012. Anatomical insights into disrupted small-world networks in schizophrenia. Neuroimage 59(2), 1085–1093. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A, 2012. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2(3), 125–141. [DOI] [PubMed] [Google Scholar]
- Yu Q, Allen EA, Sui J, Arbabshirani MR, Pearlson G, Calhoun VD, 2012. Brain connectivity networks in schizophrenia underlying resting state functional magnetic resonance imaging. Curr Top Med Chem 12(21), 2415–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore ET, 2010a. Network-based statistic: identifying differences in brain networks. Neuroimage 53(4), 1197–1207. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Harding IH, Cocchi L, Yucel M, Pantelis C, Bullmore ET, 2010b. Whole-brain anatomical networks: does the choice of nodes matter? Neuroimage 50(3), 970–983. [DOI] [PubMed] [Google Scholar]
- Zhu CY, Lee TM, Li XS, Jing SC, Wang YG, Wang K, 2007. Impairments of social cues recognition and social functioning in Chinese people with schizophrenia. Psychiatry Clin Neurosci 61(2), 149–158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.