Abstract
Background:
In recent years, psychiatry research has increasingly focused on understanding mental illnesses from a cross-diagnostic, dimensional perspective in order to better align their neurocognitive features with underlying neurobiological mechanisms. In this multi-site study, we examined two measures of cognitive control (d-prime context and lapsing rate) during the Dot Probe Expectancy (DPX) version of the AX-Continuous Performance Task in patients with either schizophrenia (SZ), schizoaffective disorder (SZ-A), or Type I bipolar disorder (BD) as well as healthy control (HC) subjects. We hypothesized significantly lower d-prime context and higher lapsing rate in SZ and SZ-A patients and intermediate levels in BD patients relative to HC.
Methods:
72 HC, 84 SZ, 77 SZ-A, and 58 BD patients (ages 18–56) were included in the final study sample.
Results:
Significant main effects of diagnosis were observed on d-prime context (F(3,279) = 9.59, p < 0.001) and lapsing (F(3,279) = 8.08, p < 0.001). A priori linear contrasts suggesting intermediate dysfunction in BD patients were significant (p < 0.001), although post-hoc tests showed the BD group was only significantly different from HC on d-prime context. Group results for d-prime context remained significant after covarying for lapsing rate. Primary behavioral measures were associated with mania and disorganization symptoms as well as everyday functioning.
Conclusions:
These findings suggest a continuum of dysfunction in cognitive control (particularly d-prime context) across diagnostic categories in psychiatric illness. These results further suggest that lapsing and d-prime context, while related, make unique contributions towards explaining deficits in cognitive control in these disorders.
Keywords: ax-cpt, bipolar disorder, cognitive control, d-prime context, schizoaffective disorder, schizophrenia
Introduction
Cognitive control, defined as the neurocognitive processes that permit flexible adaptation of stimulus processing and behavior as a function of task goals, is not only one of the most robust, widely replicated cognitive deficits in schizophrenia (SZ) but also predicts functional and clinical outcome (reviewed by Lesh et al., 2011). Indeed, dysfunction in the neuronal mechanisms that underlie cognitive control may influence a myriad of other cognitive processes leading to an overall poor level of functioning (Niendam et al., 2012). Interestingly, more recent work has suggested that patients with bipolar disorder (BD) are also behaviorally and functionally impaired in cognitive control albeit to a lesser extent that in SZ (Brambilla et al., 2007; Fortgang et al., 2016; Smucny et al., 2018a; Smucny et al., 2018b; Thakkar et al., 2015). Previous findings of intermediate deficits in BD (relative to SZ) have thus fueled speculation that deficits in control in BD and SZ patients may share a common mechanism, with BD patients presenting with a less-severe phenotype (Kuswanto et al., 2016; Smucny et al., 2018b). Related to this point, identification of neuronal mechanisms that underlie cognitive dysfunction agnostic to DSM-diagnosis is in accordance with the National Institutes of Mental Health Research Domain Criteria (RDoC) framework (Insel et al., 2010).
Although previous studies have suggested intermediate impairment in cognitive control in BD, this finding has not been extensively studied using large datasets or across multiple sites. The present study, therefore, sought to addresses the replicability of this pattern and provide additional insights by examining other aspects of cognitive control. Specifically, the goal of this study was to test the hypothesis that cognitive control deficits in mental illness represent a continuum of dysfunction with BD patients falling in-between HC and patients with SZ. Based on previous work (Owoso et al., 2013) we also hypothesized that patients with schizoaffective disorder (SZ-A) would show deficits comparable to individuals with SZ. Amongst the many facets of cognitive control, we focused particularly on goal maintenance, or the processes involved in activating and maintaining goals and rules proactively in order to bias attention and responses (Braver et al., 2009). Using the dot-probe expectancy (DPX) version of the AX-CPT (which substitutes letters of the AX-CPT with dot patterns), our primary measure of goal maintenance was d-prime context, an index of the ability to utilize cue information to guide probe responses. We also examined attentional control (i.e. attention lapses, indexed by errors during catch trials). This measure of cognitive control may reflect a complete disruption in goal maintenance leading to transient task disengagement (Smallwood et al., 2007b); to our knowledge, this is the first study to examine this aspect of performance on this task across SZ, SZ-A, and BD. Of clinical and cognitive significance, loss of attentional control may account for performance deficits in psychiatric populations (Barch et al., 2012; Boudewyn et al., 2017) and individual differences in healthy adults (Boudewyn et al., 2015; Engle and Kane, 2004; Smallwood et al., 2007a; Smallwood et al., 2008). As d-prime context and lapsing reflect overlapping yet potentially distinct neuronal processes (Lesh et al., 2011; Weissman et al., 2006) we further hypothesized 1) significant associations between the two measures, and 2) separate contributions of the two measures towards explaining group effects. Associations between symptoms/functioning and behavior were also transdiagnostically examined to probe the dimensionality of these relationships. The current study was conducted across multiple sites in order to ascertain whether behavioral effects were sufficiently robust to survive increased variability associated with different settings.
Materials and Methods
Participants
Participants were recruited as part of the CNTRACS Consortium (http://cntracs.ucdavis.edu). The consortium consists of five sites: The University of California—Davis, The Maryland Psychiatric Research Center at the University of Maryland, Rutgers University –Robert Wood Johnson Medical School, The University of Minnesota—Twin Cities, and Washington University. The respective institutional review boards for each site approved study procedures, and informed consent was acquired for all subjects. Additional details regarding the consortium are provided in Henderson et al. (2012).
303 participants (ages 18–56) participated in this study — 90 patients with SZ, 83 patients with SZ-A, 58 patients with BD Type I with a history of psychotic features, and 72 HC subjects. Exclusion criteria included head trauma, diagnosis of mental retardation or a pervasive developmental disorder, substance dependence in the past 6 months or substance abuse in the past month, and failed drug and alcohol urine screen on the day of testing. All patients were medication stable in the month leading up to test day and were either stable outpatients or had partial hospital status. All HCs had no history of SZ, SZ-A, BD, depression, or any other psychotic disorder and were not taking psychotropic or cognitive-enhancing drugs. Clinical assessments were performed or supervised by Master’s level clinicians and all raters achieved agreement with “gold” standard ratings (see Henderson et al. (2012) for details). Clinical ratings scales included the Young Mania Rating Scale (YMRS) (Young et al., 1978), the 24-item Brief Psychiatric Rating Scale (BPRS) (which includes subscales for negative, positive, disorganization, mania, and depressed mood symptoms (Overall and Gorham, 1962)) and the SZ-BD scale (used to determine if patient symptoms are primarily mood (BD-like) or psychotic/delusional (SZ-like)) (Keshavan et al., 2011). Everyday functioning was measured using the University of California San Diego Performance Skills Assessment-B (UPSA-B), which includes Financial Skills and Communications Skills subscales (Mausbach et al., 2007). Symptom data was not collected for HC individuals.
DPX Task Description
The DPX is a more challenging (less susceptible to ceiling effects) variant of the AX-CPT, in which the letter (A, B, X, Y) cues found in the AX-CPT are replaced by dot patterns (Henderson et al., 2012; Jones et al., 2010). Specifically, subjects were asked to respond (button press) to a target probe (“A”) after it follows a target cue (“X”) (i.e. an “AX” trial), but not otherwise (“AY”, “BX”, or “BY” trials) (Supplementary Figure 1). All cues and nontarget probes required nontarget responses (pressing a button with the middle finger). Target sequence (AX) trials were frequent (68.75% occurrence) and set up a prepotent tendency to make a target response when the probe letter X occurs. As a result, a nontarget sequence trial in which any non-A cue (collectively called “B” cues) was presented and followed by the probe letter X (i.e. BX trials) required the most cognitive control. The task was presented using EPrime2 software (Psychology Software Tools, Inc.). Task parameters were taken from short form #1 in Henderson et al. (2012) (ITI = 1200 ms, ISI = 2000 ms, cue length 1000 ms, probe length 500 ms, 128 total trials, %AX = 68.75, %AY = 12.5, %BX = 12.5, %BY = 6.25). Consistent with Henderson et al. (2012), subjects with greater than 56% incorrect AX or 50% incorrect BY trials were excluded from analysis.
The primary DPX outcome measure of interest in this study was d-prime context. D-prime context is an index of goal maintenance of cognitive control during the task and is calculated as a function of AX hits minus BX false alarms (Cohen et al., 1999). A secondary behavioral measure of interest was attentional control (the ability to stay on task), another key aspect of cognitive control. In this study, attentional control was indexed by “lapsing” rate, defined as the percentage of missed “catch” trials (i.e. average error rate of AX or BY trials, calculated as (1 – (AX accuracy + BY accuracy)) ÷ 2 * 100). To understand why this equation can index attention lapses, consider that DPX accuracy is usually highest on AX and BY trials. For AX trials, nothing is presented to counteract the tendency to respond to the X target; for BY trials, neither cue nor probe should prompt a response of “target.” Errors during these trials were therefore more likely to be due to an attention lapse (Phillips et al., 2015). Notably, a similar approach has been previously utilized to measure attention lapsing and its functional correlates during sustained attention tasks (Li et al., 2007; Phillips et al., 2015). Auxiliary measures included accuracy and reaction time for each trial type.
Data Analysis
All analyses were performed using SPSS25 (IBM, Armonk NY). D-prime context and lapsing rate were analyzed by ANOVA with gender, site, and diagnosis as between-subjects factors. Age was not included in the model as it did not significantly differ between groups. Significance of an a priori contrast of a linear trend for each measure ((HC > BD > (SZ = SZ-A)) for d-prime context and the reverse for lapsing rate) was also ascertained. Significant group effects and/or interactions were followed up by post-hoc tests with significance set to p < 0.05 (Sidak-corrected) to further probe the nature of these effects.
Accuracy and reaction time were analyzed by ANOVA with gender, site, and diagnosis as between-subjects factors and trial type as a within-subjects factor. Significant group effects and/or interactions were followed up by exploratory post-hoc tests with significance set to p < 0.05 (uncorrected) to probe the nature of these effects.
Exploratory analyses of associations between d-prime context and lapsing were analyzed using non-parametric, Spearman’s ρ coefficients. Correlation strengths were compared using Fisher’s z-transformation. Significance was set to p < 0.05 (uncorrected). To determine if d-prime context results persisted after removing lapsing-associated variance, 1) standardized residuals were calculated from the regression of d-prime context by lapsing across all subjects, 2) these residuals were entered as dependent variables in an ANOVA analysis with the factors specified above.
Clinical and Functional Correlates
Relationships between symptoms/functioning and the primary (d-prime context) and secondary (lapsing rate) measures of interest were analyzed using Spearman’s ρ coefficients. Correlations were also examined after controlling for diagnostic group differences as we were interested in examining dimensional relationships between symptoms and behavior independent of diagnosis. As these analyses were exploratory, significance was set to p < 0.05 (uncorrected).
Antipsychotic Medication Effects
Medication effects were examined by comparing d-prime context and lapsing rate between unmedicated and medicated patients using independent samples t-tests as well as analyzing relationships between these measures and antipsychotic dose (Chlorpromazine equivalents) using Spearman’s ρ coefficients. Significance was set to p < 0.05 (uncorrected). CPZ equivalent antipsychotic doses were calculated using published guidelines for conventional (American Psychiatric Association, 1997) and atypical (Woods, 2003) antipsychotics.
Results
Demographic and Clinical
Six SZ patients and 6 SZ-A patients did not meet AX performance criteria (see Methods). 72 controls, 84 SZ patients, 77 SZ-A patients, and 58 BD patients were therefore included in the final sample.
Demographic and clinical information for participants included in the final sample are shown in Table 1. Clinical information segregated by gender is shown in Supplementary Table 2. Groups did not differ by age but did differ by gender and site distribution. Groups also differed by education but not parental education (Table 1). Group differences were also observed on YMRS/BPRS symptom severity and everyday functioning (UPSA-B scores) (Table 1).
Table 1.
Demographic and clinical information for participants, excluding subjects who did not meet task performance criteria (see Methods). Numbers in parentheses represent the standard error.
| HC | SZ | SZ-A | BD | F or X2 (p) | |
|---|---|---|---|---|---|
| N | 72 | 84 | 77 | 58 | – |
| N BD with Current (< 1 Month) Psychotic Features | – | – | – | 28/12 (18 missing or inadequate info) | – |
| N SZ-A Depressive/Bipolar Type | – | – | 32/44 (1 missing) | – | – |
| Age | 37.46 (1.35) | 35.84 (1.18) | 36.57 (1.26) | 38.66 (1.39) | 0.83 (0.48) |
| N per Gender (M/F) | 39/33 | 53/31 | 42/35 | 19/39 | 13.06 (0.005) |
| Education Level (Years) | 14.66 (0.27) | 12.99 (0.23) | 13.42 (0.28) | 14.84 (0.34) | 10.99 (<0.001) |
| Parental Education Level (Years) | 13.86 (0.34) | 13.51 (0.40) | 14.11 (0.34) | 14.34 (0.35) | 0.96 (0.41) |
| N per Site (1/2/3/4/5) | 10/10/12/18/22 | 20/16/10/14/24 | 18/9/17/6/27 | 17/15/18/3/5 | 36.68 (<0.001) |
| YMRS Total | – | 10.19 (0.69) | 13.30 (0.80) | 9.50 (1.00) | 6.12 (0.003) |
| BPRS Total | 43.46 (1.36) | 49.68 (1.53) | 40.04 (1.17) | 11.44 (<0.001) | |
| Negative | 7.61 (0.28) | 7.52 (0.31) | 5.84 (0.25) | 10.25 (<0.001) | |
| Positive | 8.02 (0.49) | 9.25 (0.52) | 4.64 (0.27) | 22.90 (<0.001) | |
| Disorganization | 6.10 (0.32) | 5.70 (0.25) | 4.76 (0.15) | 5.90 (0.003) | |
| Mania | 6.75 (0.32) | 8.17 (0.42) | 7.38 (0.38) | 3.96 (0.020) | |
| Depressed Mood | 7.94 (0.34) | 10.80 (0.50) | 9.72 (0.52) | 11.49 (<0.001) | |
| UPSA-B Total | 94.63 (1.36) | 75.12 (1.56) | 77.55 (1.57) | 85.08 (1.19) | 11.65 (<0.001) |
| Financial Skills | 44.15 (0.72) | 39.11 (0.92) | 41.04 (0.97) | 43.06 (0.74) | 7.23 (<0.001) |
| Communication Skills | 40.49 (0.92) | 36.01 (0.92) | 36.51 (0.98) | 42.02 (0.85) | 10.10 (<0.001) |
| N per Antipsychotic Type (Typ/Atyp/Both/No ne) | – | 8/50/5/21 | 3/43/8/23 | 2/25/1/30 | 16.49 (0.011) |
| Antipsychotic Dose (mg CPZ equivalent) | – | 377.49 (39.94) | 560.07 (110.64) | 415.71 (102.96) | 1.45 (0.24) |
| N Taking Lithium (Yes/No) | – | 5/79 | 8/69 | 12/46 | 5.03 (0.081) |
| N Taking Antidepressant(s) (Yes/No) | – | 20/64 | 39/38 | 26/32 | 13.90 (0.001) |
Abbreviations: Atyp = Atypical, BD = Bipolar Disorder, BPRS = Brief Psychiatric Rating Scale, CPZ = Chlorpromazine, HC = Healthy Control, SZ = Schizophrenia, SZ-A = Schizoaffective, SZ-BD = Schizo-Bipolar, Typ = Typical, YMRS = Young Mania Rating Scale.
D-Prime Context
Behavioral data and results are presented in Figure 1 (ANOVA d-prime context ANOVA beta estimates), Figure 2 (lapsing rate ANOVA beta estimates) and Table 2 (raw data). Raw behavioral data segregated by site and gender and are provided in Supplementary Tables 1 and 2, respectively. For ANOVA analyses, group, site, gender, and the group X gender interaction were included as between-subjects factors. Analyses of accuracy and reaction time included trial type as a within-subjects factor.
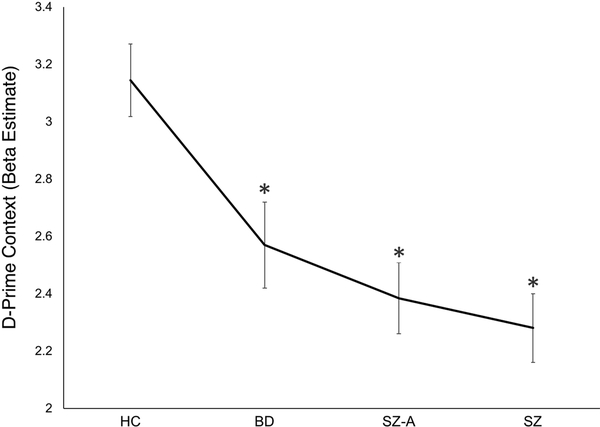
Figure 1.
D-prime context beta estimates for each diagnostic group taken from ANOVA analysis. Error bars represent the standard error. *p < 0.05 (vs. healthy control (HC)). Abbreviations: BD = Bipolar Disorder, SZ-A = Schizoaffective Disorder, SZ = Schizophrenia.
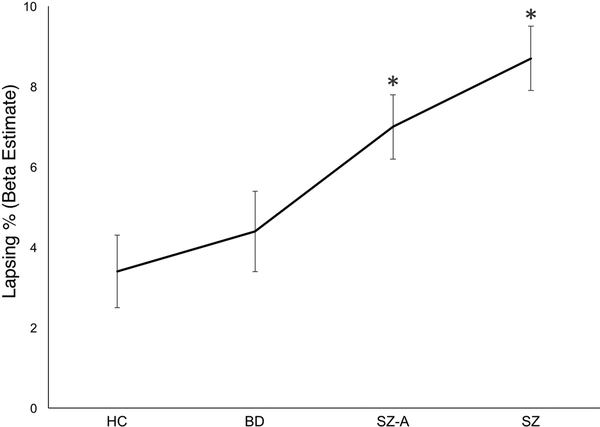
Figure 2.
Lapsing rate (%) beta estimates for each diagnostic group taken from ANOVA analysis. Error bars represent the standard error. *p < 0.05 (vs. healthy control (HC)). Abbreviations: BD = Bipolar Disorder, SZ-A = Schizoaffective Disorder, SZ = Schizophrenia.
Table 2.
AX-CPT performance comparison (raw data). Numbers in parentheses represent the standard error.
| Measure | HC | SZ | SZ-A | BP | ANOVA Effect of Group F(p) |
|---|---|---|---|---|---|
| D-Prime Context | 3.15 (0.11) | 2.38 (0.13) | 2.39 (0.14) | 2.65 (0.11) | 9.59 (<0.001) |
| Lapsing (%) | 3.29 (0.63) | 8.06 (1.03) | 7.03 (0.85) | 4.52 (0.61) | 8.08 (<0.001) |
| Accuracy (%) | |||||
| AX | 96.03 (0.61) | 91.91 (0.96) | 92.28 (0.98) | 95.06 (0.51) | 7.23 (<0.001) |
| AY | 89.93 (1.44) | 78.79 (2.40) | 82.47 (2.06) | 86.42 (1.77) | 6.50 (<0.001) |
| BX | 87.24 (1.94) | 73.96 (2.64) | 73.46 (2.67) | 77.80 (2.39) | 7.53 (<0.001) |
| BY | 97.40 (0.82) | 91.96 (1.38) | 93.67 (1.07) | 95.91 (0.99) | 5.21 (0.002) |
| Reaction Time (ms) | |||||
| AX | 433.79 (7.67) | 483.45 (10.50) | 489.88 (9.58) | 472.28 (10.79) | 8.04 (<0.001) |
| AY | 569.26 (10.70) | 645.85 (13.56) | 643.57 (13.92) | 631.49 (14.57) | 9.36 (<0.001) |
| BX | 413.08 (14.21) | 522.76 (18.34) | 535.68 (18.76) | 501.16 (18.87) | 9.92 (<0.001) |
| BY | 426.97 (15.72) | 545.92 (17.91) | 542.42 (14.96) | 523.85 (17.40) | 11.85 (<0.001) |
Abbreviations: BD = Bipolar Disorder, HC = HealthyControl, SZ = Schizophrenia, SZ-A = Schizoaffective.
For d-prime context, a significant main effect of group was observed (F(3,279) = 9.59, p < 0.001) as well as a significant group X gender interaction (F(3,279) = 5.03, p = 0.002). No main effects of site or gender were observed. Post-hoc tests showed the group effect was driven by significant deficits (vs. HC) in SZ (p < 0.001), SZ-A (p < 0.001), and BD (p = 0.025) patients. The group X gender interaction was driven by deficits in d-prime context in women (relative to men) with SZ (p = 0.009) and men (relative to women) with BD (p = 0.023) (Supplementary Table 2). The a priori contrast hypothesizing a linear trend (HC > BD > (SZ = SZ-A)) was also significant (contrast estimate = 0.65, p < 0.001).
Lapsing
Behaviorally, “lapsing” rate was indexed by the equation 1 – ((AX accuracy + BY accuracy) ÷ 2). For this measure, a significant main effect of group was observed (F(3,279) = 8.08, p < 0.001). No main effects of nor interaction effects with gender or site were observed. Post-hoc tests showed the group effect was driven by significant deficits (vs. HC) in SZ (p < 0.001) and SZ-A (p = 0.016) patients. BD patients also showed significantly less lapsing than SZ (p = 0.007) patients. The a priori contrast hypothesizing a linear trend ((SZ = SZ-A) > BD > HC) was also significant (contrast estimate = 0.039, p < 0.001).
Relationships between D-Prime Context and Lapsing
Across all subjects, a significant negative correlation was observed between d-prime context and lapsing rate (ρ = −0.80, p < 0.001). Within diagnostic groups, significant negative correlations were observed between these measures in HC (ρ = −0.80, p < 0.001), SZ (ρ = −0.82, p < 0.001), SZ-A (ρ = −0.82, p < 0.001), and BD patients (ρ = −0.55, p < 0.001). Although significant, the correlation in BD patients was significantly weaker than in HC (Fisher’s z = −2.66, p = 0.008), SZ (Fisher’s z = −3.08, p = 0.002), and SZ-A (Fisher’s z = −3.02, p = 0.003) patients.
As the same behavioral metric (AX accuracy) was used as part of the calculation for both lapsing and d-prime context, we also analyzed correlations between d-prime context and BY accuracy (as BY accuracy was used as part of the lapsing rate calculation but not d-prime context). Across all subjects, a significant association was observed between d-prime context and BY accuracy (ρ = 0.49, p < 0.001). Within diagnostic groups, significant associations were observed between these measures in HC (ρ = 0.33, p = 0.005), SZ (ρ = 0.55, p < 0.001) and SZ-A patients (ρ = 0.58, p < 0.001), but not BD patients (ρ = 0.19, p < 0.16). The correlation in BD patients was significantly weaker than in SZ (Fisher’s z = 2.44, p = 0.015) and SZ-A (Fisher’s z = 2.64, p = 0.008) patients.
After removing lapsing-associated variance (see Methods), a main effect of group was still observed for d-prime context (F(3,287) = 3.59, p = 0.014).
Auxiliary Behavioral Results
Results for auxiliary behavioral measures (accuracy, reaction time) are provided in Table 2, Supplementary Figures 2a-b, and Supplementary Results.
Clinical Correlates
Across all subjects, a negative association was observed between d-prime context and total YMRS score (ρ = −0.21, p = 0.002, Supplementary Figure 3a) as well as total BPRS score (ρ = −0.19, p = 0.005, Supplementary Figure 3b). The BPRS association was driven by negative associations with disorganization (ρ = −0.22, p = 0.001) and mania (ρ = −0.24, p < 0.001). These correlations persisted after controlling for diagnostic group (all p < 0.005).
Across all subjects, significant associations were observed between lapsing and BPRS disorganization (ρ = 0.21, p = 0.001) and BPRS mania (ρ = 0.15, p = 0.023). After controlling for diagnostic group, associations remained significant with BPRS disorganization (ρ = 0.25, p < 0.001) but not mania (ρ = 0.13, p = 0.052).
Within-group correlations primarily revealed associations between performance and symptoms in SZ but not SZ-A or BD. Additional details are provided in Supplementary Results.
Functional Correlates
Across all subjects, a positive association was observed between d-prime context and total UPSA-B score (ρ = 0.31, p < 0.001, Supplementary Figure 3c). Both the Financial Skills (ρ = 0.30, p < 0.001) and Communication Skills (ρ = 0.23, p < 0.001) subscales contributed to the association. These relationships persisted after controlling for effects of group (all p < 0.001).
Across all subjects, a negative association was observed between lapsing and total UPSA-B score (ρ = −0.36, p < 0.001, Supplementary Figure 3d). Both the Financial Skills (ρ = −0.33, p < 0.001) and Communication Skills (ρ = −0.25, p < 0.001) subscales contributed to the association. These relationships persisted after controlling for effects of group (all p < 0.001).
Medication Effects
No differences were observed in d-prime context between patients treated with antipsychotics and unmedicated patients across all subjects or within any diagnostic group. No relationships were observed between d-prime context and antipsychotic dose across all patients or within any diagnostic group.
Discussion
In agreement with our hypothesis, significant group differences were observed on d-prime context as well as attentional control (lapsing rate). Consistent with previous studies (Brambilla et al., 2007; Smucny et al., 2018a; Smucny et al., 2018b) and as demonstrated by significant linear contrasts, a continuum of dysfunction was observed in which the BD group showed intermediate levels of control between HC and patients with either SZ or SZ-A (with SZ and SZ-A patients showing the poorest control). Post-hoc tests further showed that SZ, SZ-A and BD patients significantly differed from HC on d-prime context and BD patients significantly differed from SZ patients on lapsing. HC and BD patients did not significantly differ on lapsing, although it should be mentioned that the low frequency of BY trials (half as many as BX trials) was expected to reduce the discriminating power of lapsing compared to d-prime context. Although subjects with higher d-prime context showed lower lapsing rates, group d-prime context effects remained significant after controlling for lapsing, consistent with the view that these metrics index overlapping yet distinct neurocognitive processes. Associations were also observed between the behavioral measures of interest (d-prime context, lapsing) and clinical symptoms (particularly mania and disorganization) as well as everyday functioning (financial and communication skills), suggesting these metrics of performance reflect cognitive impairments that have clinical relevance. These associations, furthermore, persisted after controlling for group differences, suggesting these relationships are dimensional and cross-diagnostic.
The observation of intermediate d-prime context in BD patients relative to HC and patients with SZ and SZ-A is consistent with recent findings on the standard letter version of the AX-CPT (Smucny et al., 2018b), suggesting that the previously observed pattern of dysfunction may generalize to other task versions. Also consistent with previous work (Barch et al., 2003; Smucny et al., 2018b; Yoon et al., 2008), significant associations were observed between deficits in d-prime context and symptom severity across patient groups as well as within the SZ group. Novel associations were also observed between d-prime context/lapsing and financial/communication skills. These results support the continued use of cognitive control processes as clinically relevant candidate biomarkers for mental illness (Carter et al., 2012). These associations provide further validation for the DPX task as an instrument to measure top down control, given that disorganization and mania (impulsivity) are thought to arise in part due to failures in control (Dalley et al., 2011; Lesh et al., 2011). Why might deficits in control be related to these clusters of symptoms? One possibility is that these symptoms are a consequence of the relative inability of the cognitive control system to direct and maintain coordinated behavior (Cole et al., 2014). The reverse relationship may also be true in that poor performance on cognitive control tasks may result from resources already being taxed by attempts to manage symptoms (Cole et al., 2014). Importantly, in this framework deficits in control are predicted to exist in a continuum dependent on symptom severity (as observed with disorganization in this study and others (Barch et al., 2003; Smucny et al., 2018b; Yoon et al., 2008)) and otherwise agnostic to DSM diagnosis, consistent with alternative hypothetical constructs for understanding psychiatric disease (e.g. RDoC (Insel et al., 2010)). In alignment with this view, the correlations observed here remained significant after controlling for group effects. Although within-group analysis suggested symptom correlations were only significant within the SZ group, lack of relationships in the other groups may have been driven by decreased variance in lapsing (variances = 0.2 for BD, 0.6 for SZ-A, and 0.9 for SZ) and d-prime context (variances = 0.74 for BD, 1.40 for SZ-A, and 1.47 for SZ) relative to the SZ group.
A significant association was observed between d-prime context and lapsing (as well as for BY accuracy, which is fully d-prime context-independent), consistent with the view that these metrics both provide an index of goal maintenance. How might we further understand the role of attentional control in explaining cognitive deficits in mental illness? Previous work has found that lapsing during a perceptual task may explain variance in episodic memory and visual perception (Barch et al., 2012). Combined with the present findings, these results suggest that the abnormal processes that increase lapsing rate may have effects that cut across traditional cognitive domains. Traditionally, psychiatry has treated the fact that patients often display deficits in multiple cognitive domains as an undesirable confound (the so-called “generalized deficit” – a catch-all for lack of motivation, poor test-taking skills, etc.). As noted by Green et al. (2013), however, shared variance among different domains may be suggestive of a common neuronal substrate. We suggest that lapsing rate may be an index of the integrity of this substrate. Related to this point, a 2012 functional neuroimaging meta-analysis of executive function studies extracted a core “cognitive control network” of frontoparietal brain areas that were consistently recruited across tasks (Niendam et al., 2012). Dysfunction in this network might therefore be predicted to broadly affect various cognitive domains. Tying the network into attentional control processes, an fMRI study in SZ patients by Phillips et al. (2015) found that lapsing (indexed by AX errors) was associated with reductions in frontoparietal activation (relative to HC). Following the recent work of Smucny et al. (2018b) showing deficits in d-prime context and frontoparietal activation in psychotic disorders, future studies investigating the neural correlates of attention lapsing and their relationship(s) with other goal maintenance measures in these populations could shed further light on the generalizability of these deficits across traditional cognitive domains.
Interestingly, group differences in d-prime context remained significant after controlling for lapsing, suggesting that although these metrics are associated with one another, deficits in the former measure may not be fully explained by variations in attentional control. Although both d-prime context and lapsing are measures of goal maintenance and cognitive control, the neuronal processes that are involved in each process may be at least partially differentiated. D-prime context is most frequently associated with activation of frontoparietal regions (e.g. Lesh et al. (2013)), whereas studies of lapsing/attentional control have observed involvement of a number of brain regions including the default mode network, prefrontal cortex, and anterior cingulate (e.g. Phillips et al. (2015); see Fox et al. (2015) for meta-analysis). Region-specific contributions to lapsing may also occur depending on disease status (Phillips et al., 2015). Related to this point, we observed an interesting pattern of results in the BD group, with significant deficits in d-prime context but not lapsing (relative to HC). Reduced discriminating power of the lapsing measure due to low numbers of trials together with the overall milder level of d-prime context impairments in BD may have contributed to the negative group comparison. Future studies examining the relationship between cognitive control deficits and lapsing will be needed using lapsing measures with comparable discrimination power in order to definitively address this question. How specific brain regions are differentially recruited during lapsing and other aspects of cognitive control in SZ, SZ-A, BD, and other mental illnesses may be investigated in future studies using neuroimaging.
In this study, attentional control was indexed by accuracy on “easy,” “catch” trials (AX and BY). AX trials primarily index pre-potent responses; BY trials do not prompt a target response after either cue or probe. Errors during these trials are therefore more likely to be due to an attention lapse (Phillips et al., 2015). A limitation of this approach, however, is that this metric is only an indirect measure of top-down attentional control. It is possible that other dysfunctional processes (e.g. visuospatial processing of the DPX dot patterns or increased “noise” driving uncertainty in sensory, motor, or other systems (Nguyen et al., 2016)) contributed to accuracy during these trials. More direct methods of probing lapses have recently been developed, the majority of which involve asking participants where their attention is directed at various points during a task (Weinstein et al., 2017). Lapsing may also be examined physiologically by tracking alpha oscillatory activity, as previously conducted in HC and SZ patients (Boudewyn and Carter, 2017). An important area for future study is to examine attentional control in mental illness using these more direct measures to more precisely determine the extent to which this process is impaired.
A significant diagnosis X gender interaction was observed on d-prime context, driven by higher d-prime context in men with SZ (compared to women) and lower d-prime context in men with BD (compared to women). This pattern of gender effects in SZ is generally inconsistent with the literature. The majority of studies have found cognitive deficits in men relative to women in SZ, although there are exceptions (Mendrek and Mancini-Marie, 2016). Possible reasons for the discrepancy are site effects (the two sites with the lowest d-prime context scores had the lowest male to female ratios (Supplementary Table 1)), differences in onset of illness (increased impairment has been observed in early onset men compared to late onset men with SZ, while the opposite pattern has been observed in women with SZ (Lewine et al., 1997), and differences in symptom severity (female patients with SZ were more symptomatic than male patients with SZ in this study (Supplementary Table 2)). It should also be noted that this study was relatively underpowered to examine group X gender interactions (e.g. the number of men with BD was relatively small (n = 19)) and these findings require replication in a larger sample.
As with any study involving medicated psychiatric populations, the potential confounding effects of antipsychotics should be considered. No relationships between antipsychotic type or dose and any measures of interest were observed in the present study, suggesting antipsychotics did not significantly affect the results. In contrast to these findings, a 2015 cross-sectional study by Lesh et al. (2015) reported higher d-prime context in medicated patients with SZ relative to unmedicated patients on the letter version of the AX-CPT. Several differences between our study and the previous work may explain this discrepancy, including age disparity (Lesh et al. (2015) studied young, recent onset patients), duration of illness (the present study examined a more chronic sample), and task design (lower d-prime context scores were observed on the DPX relative to the AX task used in the prior study, highlighting the increased difficulty of the DPX). Given the potential therapeutic implications, future longitudinal or case-control studies may more closely examine the effects of antipsychotics on cognitive control.
In conclusion, in the present, multi-site study, patients with BD showed intermediate goal maintenance of cognitive control (particularly d-prime context) relative to HC and patients with SZ or SZ-A. The fact that significant results were observed across multiple sites further suggests these findings were quite robust and replicable. A strong relationship was also observed between d-prime context and lapsing, consistent with predictions that these measures reflect shared deficits in goal maintenance processes in these disorders. These abnormalities were also associated with mania and disorganization symptom severity across patient groups, illustrating the clinical relevance of dysfunctional cognitive control processes across mental illnesses and the need to develop new treatments to target this essential aspect of cognitive functioning.
Supplementary Material
Acknowledgements
The authors thank the study participants and their families.
Role of the Funding Source
This study was supported by a research grant from the NIMH (5R01MH059883).
Footnotes
Conflicts of Interest.
None.
Ethics Statement
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association, 1997. Practice Guideline for the Treatment of Patients with Schizophrenia. American Journal of Psychiatry 154(4 Suppl), 1–63. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Dakin SC, Gold J, Luck SJ, Macdonald A 3rd, Ragland JD, Silverstein S, Strauss ME, 2012. The clinical translation of a measure of gain control: the contrast-contrast effect task. Schizophr Bull 38(1), 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Carter CS, MacDonald AW 3rd, Braver TS, Cohen JD, 2003. Context-processing deficits in schizophrenia: diagnostic specificity, 4-week course, and relationships to clinical symptoms. J Abnorm Psychol 112(1), 132–143. [PubMed] [Google Scholar]
- Boudewyn MA, Carter CS, 2017. Electrophysiological correlates of adaptive control and attentional engagement in patients with first episode schizophrenia and healthy young adults. Psychophysiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudewyn MA, Carter CS, Long DL, Traxler MJ, Lesh TA, Mangun GR, Swaab TY, 2017. Language context processing deficits in schizophrenia: The role of attentional engagement. Neuropsychologia 96, 262–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudewyn MA, Long DL, Traxler MJ, Lesh TA, Dave S, Mangun GR, Carter CS, Swaab TY, 2015. Sensitivity to Referential Ambiguity in Discourse: The Role of Attention, Working Memory, and Verbal Ability. J Cogn Neurosci 27(12), 2309–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla P, Macdonald AW 3rd, Sassi RB, Johnson MK, Mallinger AG, Carter CS, Soares JC, 2007. Context processing performance in bipolar disorder patients. Bipolar Disord 9(3), 230–237. [DOI] [PubMed] [Google Scholar]
- Braver TS, Paxton JL, Locke HS, Barch DM, 2009. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proc Natl Acad Sci U S A 106(18), 7351–7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Minzenberg M, West R, Macdonald A 3rd, 2012. CNTRICS imaging biomarker selections: Executive control paradigms. Schizophr Bull 38(1), 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Barch DM, Carter C, Servan-Schreiber D, 1999. Context-processing deficits in schizophrenia: converging evidence from three theoretically motivated cognitive tasks. J Abnorm Psychol 108(1), 120–133. [DOI] [PubMed] [Google Scholar]
- Cole MW, Repovs G, Anticevic A, 2014. The frontoparietal control system: a central role in mental health. Neuroscientist 20(6), 652–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW, 2011. Impulsivity, compulsivity, and top-down cognitive control. Neuron 69(4), 680–694. [DOI] [PubMed] [Google Scholar]
- Engle RW, Kane MJ, 2004. Executive attention, working memory capacity, and a two-factor theory of cognitive control, in: Ross B (Ed.), The psychology of learning and motivation. Academic Press, New York, NY, pp. 145–199. [Google Scholar]
- Fortgang RG, Hultman CM, van Erp TG, Cannon TD, 2016. Multidimensional assessment of impulsivity in schizophrenia, bipolar disorder, and major depressive disorder: testing for shared endophenotypes. Psychol Med 46(7), 1497–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox KC, Spreng RN, Ellamil M, Andrews-Hanna JR, Christoff K, 2015. The wandering brain: meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage 111, 611–621. [DOI] [PubMed] [Google Scholar]
- Green MF, Horan WP, Sugar CA, 2013. Has the generalized deficit become the generalized criticism? Schizophr Bull 39(2), 257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson D, Poppe AB, Barch DM, Carter CS, Gold JM, Ragland JD, Silverstein SM, Strauss ME, MacDonald AW 3rd, 2012. Optimization of a goal maintenance task for use in clinical applications. Schizophr Bull 38(1), 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P, 2010. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 167(7), 748–751. [DOI] [PubMed] [Google Scholar]
- Jones JA, Sponheim SR, MacDonald AW 3rd, 2010. The dot pattern expectancy task: reliability and replication of deficits in schizophrenia. Psychol Assess 22(1), 131–141. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Morris DW, Sweeney JA, Pearlson G, Thaker G, Seidman LJ, Eack SM, Tamminga C, 2011. A dimensional approach to the psychosis spectrum between bipolar disorder and schizophrenia: the Schizo-Bipolar Scale. Schizophr Res 133(1–3), 250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuswanto C, Chin R, Sum MY, Sengupta S, Fagiolini A, McIntyre RS, Vieta E, Sim K, 2016. Shared and divergent neurocognitive impairments in adult patients with schizophrenia and bipolar disorder: Whither the evidence? Neurosci Biobehav Rev 61, 66–89. [DOI] [PubMed] [Google Scholar]
- Lesh TA, Niendam TA, Minzenberg MJ, Carter CS, 2011. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology 36(1), 316–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesh TA, Tanase C, Geib BR, Niendam TA, Yoon JH, Minzenberg MJ, Ragland JD, Solomon M, Carter CS, 2015. A multimodal analysis of antipsychotic effects on brain structure and function in first-episode schizophrenia. JAMA Psychiatry 72(3), 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesh TA, Westphal AJ, Niendam TA, Yoon JH, Minzenberg MJ, Ragland JD, Solomon M, Carter CS, 2013. Proactive and reactive cognitive control and dorsolateral prefrontal cortex dysfunction in first episode schizophrenia. Neuroimage Clin 2, 590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewine R, Haden C, Caudle J, Shurett R, 1997. Sex-onset effects on neuropsychological function in schizophrenia. Schizophr Bull 23(1), 51–61. [DOI] [PubMed] [Google Scholar]
- Li CS, Yan P, Bergquist KL, Sinha R, 2007. Greater activation of the “default” brain regions predicts stop signal errors. Neuroimage 38(3), 640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mausbach BT, Harvey PD, Goldman SR, Jeste DV, Patterson TL, 2007. Development of a brief scale of everyday functioning in persons with serious mental illness. Schizophr Bull 33(6), 1364–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrek A, Mancini-Marie A, 2016. Sex/gender differences in the brain and cognition in schizophrenia. Neurosci Biobehav Rev 67, 57–78. [DOI] [PubMed] [Google Scholar]
- Nguyen J, Majmudar U, Papathomas TV, Silverstein SM, Torres EB, 2016. Schizophrenia: The micro-movements perspective. Neuropsychologia 85, 310–326. [DOI] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS, 2012. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci 12(2), 241–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE, Gorham DR, 1962. The Brief Psychiatric Rating Scale. Psychol Rep 10, 799–812. [Google Scholar]
- Owoso A, Carter CS, Gold JM, MacDonald AW 3rd, Ragland JD, Silverstein SM, Strauss ME, Barch DM, 2013. Cognition in schizophrenia and schizo-affective disorder: impairments that are more similar than different. Psychol Med 43(12), 2535–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RC, Salo T, Carter CS, 2015. Distinct neural correlates for attention lapses in patients with schizophrenia and healthy participants. Front Hum Neurosci 9, 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J, Fishman DJ, Schooler JW, 2007a. Counting the cost of an absent mind: mind wandering as an underrecognized influence on educational performance. Psychon Bull Rev 14(2), 230–236. [DOI] [PubMed] [Google Scholar]
- Smallwood J, McSpadden M, Schooler JW, 2007b. The lights are on but no one’s home: meta-awareness and the decoupling of attention when the mind wanders. Psychon Bull Rev 14(3), 527–533. [DOI] [PubMed] [Google Scholar]
- Smallwood J, McSpadden M, Schooler JW, 2008. When attention matters: the curious incident of the wandering mind. Mem Cognit 36(6), 1144–1150. [DOI] [PubMed] [Google Scholar]
- Smucny J, Lesh TA, Iosif AM, Niendam TA, Tully LM, Carter CS, 2018a. Longitudinal stability of cognitive control in early psychosis: Nondegenerative deficits across diagnoses. J Abnorm Psychol 127(8), 781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucny J, Lesh TA, Newton K, Niendam TA, Ragland JD, Carter CS, 2018b. Levels of Cognitive Control: A Functional Magnetic Resonance Imaging-Based Test of an RDoC Domain Across Bipolar Disorder and Schizophrenia. Neuropsychopharmacology 43(3), 598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar KN, Schall JD, Logan GD, Park S, 2015. Cognitive control of gaze in bipolar disorder and schizophrenia. Psychiatry Res 225(3), 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein Y, De Lima HJ, van der Zee T, 2017. Are you mind-wandering, or is your mind on task? The effect of probe framing on mind-wandering reports. Psychon Bull Rev. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG, 2006. The neural bases of momentary lapses in attention. Nat Neurosci 9(7), 971–978. [DOI] [PubMed] [Google Scholar]
- Woods SW, 2003. Chlorpromazine equivalent doses for the newer atypical antipsychotics. Journal of Clinical Psychiatry 64, 663–667. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Minzenberg MJ, Ursu S, Ryan Walter BS, Wendelken C, Ragland JD, Carter CS, 2008. Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: relationship with impaired cognition, behavioral disorganization, and global function. Am J Psychiatry 165(8), 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA, 1978. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 133, 429–435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.