Abstract
Habitual short sleep duration (<7 hours/night) is associated with increased morbidity and mortality due, in large part, to increased inflammatory burden and endothelial dysfunction. microRNAs (miRNAs) play a key role in regulating vascular health and circulating levels are now recognized to be sensitive and specific biomarkers of cardiovascular function, inflammation and disease. The aim of this study was to determine whether the circulating expression of: miR-34a; miR-92a; miR-125a; miR-126; miR-145; miR-146a; and miR-150; are disrupted in adults who habitually sleep <7 h/night (short sleep). These were chosen based upon their well-established links with vascular inflammation, function and, in-turn, cardiovascular risk. Twenty-four adults were studied: 12 with normal nightly sleep duration (6M/6F; age: 55±3 y; sleep duration: ≥7.0 h/night) and 12 with short nightly sleep duration (7M/5F; 55±2 y; sleep duration: <7.0 h/night) and circulating miRNA expression was assayed by RT-PCR. All subjects were non-smokers, normolipidemic, non-medicated and free of overt CVD. Circulating levels of miR-125a (3.07±1.98 vs 7.34±5.34 AU), miR-126 (1.28 (0.42 to 2.51) vs 1.78 (1.29 to 4.80) AU) and miR-146a (2.55 (1.00 to 4.80) vs 6.46 (1.50 to 11.44) AU) were significantly lower (~60%, 40% and 60%, respectively) in the short compared with the normal sleep group. However, there were no significant group differences in circulating levels of miR-34a, miR-92a, miR-145, and miR-150. In summary, chronic short sleep is associated with marked reduction in circulating levels of miR-125a, miR-126 and miR-146a. Dysregulation of these miRNAs may contribute to the increased inflammatory burden and endothelial dysfunction associated with habitual insufficient sleep.
INTRODUCTION
Habitual insufficient nightly sleep, defined as <7 h/night, is associated with increased cardiovascular disease (CVD) risk, events and mortality (Cappuccio et al., 2011; Liu, 2016; Yin et al., 2017). The mechanisms underlying the detrimental cardiovascular effects of habitual short sleep duration are not fully understood. Heightened inflammation and impaired endothelial function are recognized as contributing factors to sleep-related CVD risk. We (Weil et al., 2010; Bain et al., 2017) and others (Calvin et al., 2014; Akinseye et al., 2015) have demonstrated profound endothelial vasomotor dysfunction and heightened systemic inflammation in adults who habitually sleep <7 h/night. The factors leading to this proinflammatory, endothelial dysfunction state are not well defined.
microRNA (miRNAs) are short non-coding RNAs which regulate gene expression on the post-transcriptional level by targeting mRNA and inhibiting translation. A specific subset of miRNA have been identified as critical regulators of vascular inflammation (i.e. miR-92a, miR-145, miR-146a, miR-150, miR-181b and miR-Let-7a), endothelial cell dysfunction (i.e. miR-126 and miR-34a) and vasoconstrictor tone (miR-125a)(Empel et al., 2012; Hao et al., 2014; Ma et al., 2016). For example, inhibition of miR-92a and over-expression of miR-146a, miR-181b, and miR-Let-7a is associated with suppressed endothelial inflammation and atherogenesis (Loyer et al., 2014; Bao et al., 2014; Ma et al., 2016). Both miR-145 and miR-150 limit immune cell activation, cytokine production, and vascular inflammation (Lovren & Verma, 2013; Sang et al., 2016). miR-34a has been identified as a key driver of endothelial senescence and apoptosis through the inhibition of the sirtuin system (Yamakuchi et al., 2008; Ito et al., 2010; Han et al., 2015). Conversely, miR-126 and miR-125a have been shown to be key, pleiotropic promotors of endothelial health and vasomotor function. Furthermore, altered circulating levels of these vascular-related miRNAs have been shown to be indicative of elevated vascular inflammation and endothelial dysfunction as well as predictive of cardiovascular morbidity and mortality (Empel et al., 2012; Sayed et al., 2014; Wronska et al., 2015). Currently, it is unknown if habitual short sleep is associated with altered circulating miRNA expression. Circulating miRNA desynchrony may contribute to the increased cardiovascular risk associated with short sleep.
Accordingly, the aim of the present study was to determine the influence of habitual short sleep on a subset of specific vascular-related miRNAs. Specifically, we tested the hypothesis that circulating miR-34a and miR-92a would be higher and miR-125a, miR-126, miR-145, miR-146a, and miR-150 would be lower in middle-aged adults who habitually sleep <7 h/night compared with adults who sleep 7–9 h/night. The rational for focusing on these specific circulating miRNAs is based on their established regulatory links with endothelial cell function and inflammatory pathways and association with CVD risk.
METHODS
Ethical Approval
All subjects had the research study and its potential risks and benefits explained before providing written informed consent according to the guidelines of the University of Colorado Boulder. All aspects of this research study complied with the Declaration of Helsinki, except for registration in a public database (clause 35) (World Medical Association, 2013). This study was approved by the University of Colorado Institutional Review Board (approval # B5079).
Subjects
Twenty-four sedentary middle-aged adults (age range: 44–62 years) were studied: 12 normal sleep duration (6M/6F; range: 7.0–8.5 h/night) and 12 short sleepers (7M/5F; range: 5.0–6.8 h/night). All subjects were sedentary, non-smokers, normolipidemic, non-medicated and free of overt cardiovascular, metabolic, renal, and hematologic disease, as assessed by medical history, resting and exercise electrocardiograms, and fasting blood chemistries. Female subjects were at least 1 year postmenopausal and had never taken or had discontinued use of hormone replacement therapy at least 1 year before the start of the study.
Sleep Duration
Sleep duration was self-reported as a component of the Stanford Physical Activity Questionnaire as previously described (Weil et al., 2010). Nightly mean reported sleep duration was calculated as the weighted mean of weeknight and weekend values as follows: (5 X weekday sleep duration) + (2 X weekend sleep duration)/7. Subjects were divided into 2 groups based upon their reported sleep duration: 7–9 h/night = “normal sleep” and <7 h/night = “insufficient or short sleep” (Bain et al., 2017). These criteria were chosen based on reports that indicate that habitual sleep duration shorter than 7 h/night is associated with increased health risks (Cappuccio et al., 2011; Yin et al., 2017).
Body Composition and Metabolic Measures
Body mass was measured to the nearest 0.1 kg using a medical beam balance. Percent body fat was determined by dual energy X-ray absorptiometry (Lunar Corp., Madison, WI, USA). Body mass index (BMI), fasting plasma lipid, lipoprotein, glucose, and insulin concentrations were determined using standard techniques.
MicroRNA isolation and Reverse Transcription Quantitative Polymerase Chain Reaction Analysis (RT-qPCR)
Blood samples were collected from the antecubital vein between 8:00 and 10:00am following an overnight fast. Blood was centrifuged at 600 x g for 20 minutes and the supernatant was centrifuged 1500 x g for 15 minutes at 4°C to remove any additional cellular debris.
Total RNA was isolated from platelet poor plasma using the miRNeasy Serum/Plasma Kit (Qiagen, Hilden, Germany) (Hijmans et al., 2018). Briefly, RNA was isolated from 100μL of plasma using the QIAsol lysis reagent, washed and eluted in RNAse free water. To normalize between samples 3.5μL (1.6×108 copies/μL) Canorhabditis elegans miR-39 (cel-miR-39) was added to each sample. Immediately after RNA isolation, 12μL of RNA was reverse transcribed using the miScript Reverse Transcription Kit (Qiagen, Hilden, German). cDNA was PCR-amplified (BioRad CFX96 Touch Real Time System) using the miScript SYBR green PCR kit (Qiagen, Hilden, Germany) and miRNA specific primers for miR-34a, miR-92a, miR-125a, miR-126, miR-145, miR-146a and miR-150 (Qiagen, Hilden, Germany). All samples were assayed in duplicate. Relative expression level for a given miR was normalized to cel-miR-39, calculated as ΔCt =2-(Ct[miR]-Ct[cel-miR−39]) and expressed as arbitrary units (AU) (Hijmans et al., 2018).
Statistical Analysis
The distribution of the data was assessed by the Shapiro-Wilk test and the homogeneity of variances by the Levene test. Group differences in subject characteristics, circulating microparticles concentrations, cellular protein expression, miRNA expression, oxidative stress, and senescence were determined by independent Student t-test or Mann-Whitney U test. Data were presented as mean ± standard deviation (SD) or normally distributed variables, and as the median (interquartile range [IQR]) for non-normally distributed variables. Pearson correlations were determined between variables of interest. Statistical significance was set a priori at P<0.05.
RESULTS
Selected subject characteristics are presented in the Table. There were no significant differences in any anthropometric, hemodynamic or metabolic variables between the groups, however, by design, nightly sleep was significantly lower (~20%) in the short vs normal sleep group. Circulating levels of miR-125a ([short vs. normal sleep] 3.07±1.98 vs 7.34±5.34 arbitrary units [AU]), (1.28 (0.42 to 2.51) vs 1.78 (1.29 to 4.80) AU) and miR-146a (2.55 (1.00 to 4.80) vs 6.46 (1.50 to 11.44) AU) were significantly lower (~60%, ~40%, and ~60% respectively) in the short sleep compared with normal sleep group (Figure 1). There were no significant group differences in circulating miR-34a ([short vs. normal sleep] 1.63±1.00 vs 1.70±1.23 AU), miR-92a (6.86 (1.95 to 9.51) AU), miR-145 (0.74 (0.10 to 2.02) vs 1.08 (0.21 to 2.17) AU) and miR-150 ([short vs. normal sleep] 0.91±0.51 vs 1.35±1.20 AU) (Figure 2).
Table.
Selected subject characteristics
| Variable | Normal Sleep (n=12) |
Short Sleep (n=12) |
|---|---|---|
| Sleep Duration (h/night) | 7.6±0.3 | 6.0±0.7* |
| Age (yr) | 58±6 | 55±5 |
| Body Mass (kg) | 80.2±21.1 | 86.6±12.4 |
| BMI (kg m−2) | 27.3±6.5 | 27.6±3.4 |
| Body Fat (%) | 34.8±10.1 | 30.5±7.9 |
| Relative VO2 max (mL/kg/min) | 35.6±8.9 | 33.0±6.5 |
| Systolic Blood Pressure, (mmHg) | 122±9 | 118±9 |
| Diastolic Blood Pressure (mmHg) | 75±8 | 75±7 |
| Total Cholesterol (mg/dL) | 198±36 | 198±34 |
| HDL-C (mg/dL) | 60±18 | 57±4=15 |
| LDL-C (mg/dL) | 116±21 | 120±30 |
| Triglycerides (mg/dL) | 110±40 | 104±36 |
| Glucose (mg/dL) | 91±6 | 93±7 |
| Insulin (μU.ml−1) | 7.6±3.2 | 7.2±2.4 |
| HOMA-IR | 1.7±1.0 | 1.6±0.7 |
Values expressed as Mean±SD. BMI: body mass index. HDL-C: high-density lipoprotein. LDL-C: low-density lipoprotein. HOMA-IR: homeostasis model of insulin resistance.
P<0.05
Figure 1.
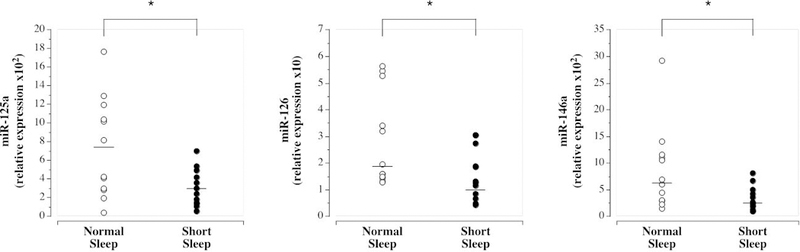
Circulating miR-125a, miR-126, and miR-146a in the normal sleep and short sleep duration groups. Mean circulating level is denoted for miR-125a; median for miR-126 and miR-146a. *P<0.05
Figure 2.
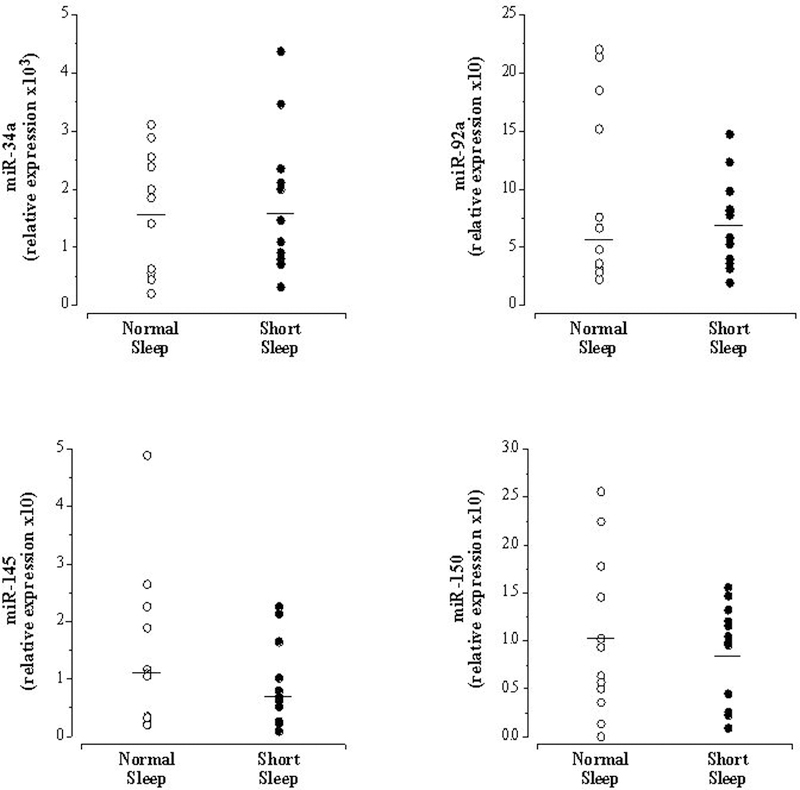
Circulating miR-34a, miR-92a, miR-145 and miR-150 in the normal sleep and short sleep duration groups. Mean circulating level is denoted for miR-34a and miR-150; median for miR-92a and miR-150.
In the overall study population, miR-125a (r=0.59; P<0.05), miR-126 (r=0.43; P<0.05) and miR-146a levels (r=0.41; P<0.05) were each significantly related to average nightly sleep duration (Figure 3). No other miRNAs were associated with nightly sleep duration.
Figure 3.
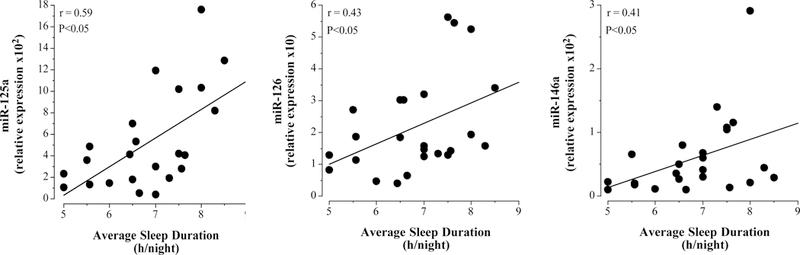
Relation between circulating miR-125a, miR-126 and miR-146a and nightly sleep duration.
DISCUSSION
Interest in circulating miRNA profiles has intensified as their role as biomarkers and mediators of cardiovascular dysfunction and potential therapeutic targets has become increasingly established (Wronska et al., 2015). The key finding of the present study is that habitual insufficient sleep (<7 h/night) is associated with disruption in circulating levels of miR-125a, miR-126 and miR-146a. Altered circulating profiles of these vascular-related miRNAs have been linked to vascular dysfunction and increased CVD risk and events (Zampetaki et al., 2010; Empel et al., 2012; Hao et al., 2014; Bao et al., 2015). To our knowledge this the first study to determine the influence of short sleep duration on circulating miRNA signatures.
A primary factor underlying the elevated incidence of myocardial infarction and stroke associated with habitual short sleep is endothelial dysfunction, specifically impaired vasomotor function and reduced fibrinolytic capacity (Weil et al., 2010, 2013; Levy et al., 2012; Bain et al., 2017). For example, we have demonstrated that short sleep is associated with reduced nitric oxide-mediated endothelium-dependent vasodilation (Bain et al., 2017). In a similar population to the present study, forearm blood flow responses to the endothelial agonist acetylcholine was significantly lower in adults who habitually slept less than 6.5 h/night compared with their cohorts of similar age who slept >7 h/night. The co-administration of the endothelial nitric oxide synthase inhibitor, L-NG-monomethyl arginine, with acetylcholine demonstrated that the insufficient sleep-related loss in vasodilator function was due, in part, to reduced nitric oxide bioavailability (Bain et al., 2017). In addition, enhanced endothelin(ET)-1-mediated vasoconstrictor tone has been shown to be elevated with insufficient sleep, further compounding the vasomotor dysfunction and increasing CVD risk (Weil et al., 2010). In the present study, circulating concentrations of both miR-125a and miR-126 were significantly lower in the short sleep group. This finding is congruent with previous studies demonstrating impaired vasomotor function with insufficient sleep (Weil et al., 2010; Calvin et al., 2014; Bain et al., 2017). The endo-miR, miR-126, is critical for proper endothelial function and vascular homeostasis (Chistiakov et al., 2016). miR-126 promotes eNOS activation and endothelial cell survival by targeting the PI3K/AKT/eNOS pathway regulator PI3KRA as well as suppressing the proatherogenic proteins SPRED1 and CXCL12 (Jansen et al., 2013; Chen et al., 2016). Clinically, circulating miR-126 expression has been associated with endothelial vasodilatory capacity and function (Widmer et al., 2014; Park et al., 2015; Chistiakov et al., 2016). Along with vasomotor function, miR-126 also regulates endothelial fibrinolytic function and diminished miR-126 is thought to result in a prothrombotic state (Gao et al., 2017). Contrastingly, miR-125a directly targets the 3’UTR of ET-1 mRNA inhibiting translation and, in turn, ET-1 system activity. Thus, reduced expression of miR-125a is associated with greater ET-1 production and release (Li et al., 2010; Hao et al., 2014). Lower circulating miR-125a in the short vs normal sleepers observed herein is consistent with previous studies reporting increased ET-1 system activity with insufficient sleep (Palma et al., 2002; Weil et al., 2010). Collectively, reduced levels of miR-125a and miR-126 may be etiologically involved in the increased incidence of endothelial dysfunction and CVD with insufficient sleep.
A proinflammatory vascular environment is a common consequence of insufficient sleep and is thought to be a major contributor to insufficient sleep-related CVD risk (Hansson, 2005). It is well established that short sleep is associated with elevated levels of pro-inflammatory cytokines, such as IL-6 and IL-8, and markers of vascular inflammation, such as C-reactive protein (Grandner et al., 2013). In a seminal study, Aho and colleagues (2013) demonstrated that short sleep is associated with increased activation of the proinflammatory transcription factor, nuclear factor-κB (NF-κB) resulting in increased inflammatory gene expression and cytokine production (Aho et al., 2013). Dysregulation of miR-146a has been directly linked with increased NF-κB activation. Reduced miR-146a expression limits TRAF-6 and IRAK-1 suppression allowing unregulated NF-κB activation. Elevated NF-κB activation promotes increased cytokine production, endothelial dysfunction and atherogeneis (Ma et al., 2016; Paterson & Kriegel, 2017). Ma and colleagues (2016) demonstrated that low circulating levels of miR-146a is associated with increased vascular inflammation and atherosclerosis (Ma et al., 2016); whereas, exogenous restoration of circulating miR-146a blunted vascular inflammation in mice (Ma et al., 2016). Thus, it is plausible that lower miR-146a levels may contribute to the increased inflammatory burden associated with habitual short nightly sleep (Aronica et al., 2010). Moreover, considering lower circulating miR-146a levels have been shown to be predictive of atherosclerosis and coronary events (Ramkaran et al., 2014; Bao et al., 2015), the negative influence of insufficient sleep on miR-146a may have yet untold cardiovascular consequences.
In contrast to miR-125a, miR-126 and miR-146, there was no significant impact of nightly sleep duration on circulating miR-34a, miR-92a, miR-145 and miR-150 levels. Each of these miRs contribute to the regulation of vascular health and are associated with CVD risk (Zeller et al., 2014; Satoh et al., 2015). miR-34a promotes cellular senescence and dysfunction through the regulation of sirtuin-1 and the apoptotic protein BCL-2 (Boon et al., 2013). Circulating miR-34a has been reported to be two-fold higher in adults with coronary artery disease compared with healthy controls (Han et al., 2015) and to play contributing role in the development of heart failure and cardiac death (Boon et al., 2013; Han et al., 2015). miR-92a negatively regulates KLF-2 and KLF-4 proteins resulting in diminished endothelial repair capacity, eNOS expression and endothelial function (Daniel et al., 2014; Shang et al., 2017). Increased expression of miR-92a is associated with the progression of atherosclerotic lesions (Daniel et al., 2014). miR-145, on the other hand, interacts with vascular smooth muscle cells to drive differentiation from a proliferative state to a contractile phenotype and miR-145 mediated reduction in vascular smooth muscle cell proliferation has been shown to blunt atherosclerotic lesion progression (Lovren et al., 2012). miR-150 limits atherogenesis by moderating immune cell activation and secretion of cytokines and enhances vascular health by promoting endothelial and endothelial progenitor cell function (Desjarlais et al., 2017). Lower circulating levels of miR-145 and miR-150 have been linked with increased CVD risk and events (Rhodes et al., 2013; Dong et al., 2015). Lack of alteration in these miRNAs with insufficient sleep suggest a differential effect of sleep on circulating miRNA and demonstrate the complexity of the interaction of sleep and circadian physiology with miRNA regulation and, in turn, cellular function. In fact, it is possible that other sleep disorders such as insomnia or sleep apnea may influence the circulating profile of these miRNAs.
There are a few experimental considerations regarding the present study that deserve mention. Firstly, inherent with all cross-sectional studies involving humans it is possible that genetic, dietary or lifestyle factors may have influenced our results. However, to minimize the potential confounding effects of other lifestyle behaviors, besides habitual sleep duration, all subjects were similar in age, body composition and cardiorespiratory fitness; additionally they were sedentary, non-smokers, free of overt cardiometabolic diseases and not taking vitamins or medications that could influence circulating miRNAs. Secondly, we assessed habitual nightly sleep duration by self-report opening the possibility for bias and experimental error. Although we did not utilize more objective measures of sleep duration through actigraphic monitoring, previous studies have reported a strong correlation between self-report sleep duration and actigraphic data (Hauri & Wisbey, 1992; Lockley et al., 1999). Thirdly, we did not screen for sleep disorders such as sleep apnea. Considering that the study population was non-obese and cardiometabolically healthy, the risk of sleep apnea would be small. Finally, although we are ascribing the disruption in circulating miR-125a, miR-126 and miR-146a to insufficient sleep, we are unable to identify which cells or tissue are involved in the production, release or clearance of these miRNAs in the circulation. Thus, the mechanisms by which sleep may affect their circulating signature is outside the scope of this study. Nevertheless, circulating levels of miR-125a, miR-126 and miR-146a, regardless of cell of origin or mechanism of release, are correlated with disease and provide mechanistic insight into disease development and progression (Empel et al., 2012; Sayed et al., 2014).
In conclusion, the results of the present study suggest that habitual insufficient nightly sleep adversely affects the circulating profile of miR-125a, miR-126 and miR-146a. Sleep related changes in these miRNA may play a role in the aberrant vascular physiology and increased vascular risk associated with short sleep (Empel et al., 2012; Wronska et al., 2015). Indeed, lower circulating levels of miR-125a, miR-126 and miR-146a are consistent with, and can be linked to, the reduction in nitric oxide-mediated vasodilation, increased ET-1 vasoconstrictor tone, diminished fibrinolytic function and increased vascular inflammation associated with insufficient sleep (Weil et al., 2010, 2013; Hao et al., 2014; Chistiakov et al., 2016; Paterson & Kriegel, 2017; Bain et al., 2017). Future studies are needed to establish whether circulating miRNAs may be used as biomarkers of sleep-related vascular risk.
What is the central question of the study
Is habitual short sleep associated with altered circulating levels of specific inflammation and vascular-related miRNAs?
What is the main finding and its importance?
Circulating levels of miR-125a, miR-126 and miR-146a were significantly lower in the short compared with normal sleep group. Altered circulating profiles of these vascular-related miRNAs have been linked to vascular inflammation, dysfunction and increased CVD events. Sleep related changes in these miRNAs are consistent with, and may play a role in, the aberrant vascular physiology and increased vascular risk associated with short sleep
ACKNOWLDEGEMNTS
The authors would like to thank all subjects who participated in this study and the University of Colorado Boulder, Clinical and Translational Research Center clinical staff for their assistance. This study was supported by National Institutes of Health awards HL131458, HL135598 and NIH/NCATS UL1 TR001082.
Footnotes
COMPETING INTERESTS
The authors have no conflicts of interest to disclose.
Conflicts of Interest: The authors have no conflicts of interest to disclose.
REFERENCES
- Aho V, Ollila HM, Rantanen V, Kronholm E, Surakka I, Leeuwen van WMA, Lehto M, Matikainen S, Ripatti S, Härmä M, Sallinen M, Salomaa, Jauhiainen M, Alenius H, Paunio T & Porkka-Heiskanen T (2013). Partial Sleep Restriction Activates Immune Response-Related Gene Expression Pathways: Experimental and Epidemiological Studies in Humans. PLOS ONE 8, e77184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinseye OA, Williams SK, Seixas A, Pandi-Perumal SR, Vallon J, Zizi F & Jean-Louis G (2015). Sleep as a Mediator in the Pathway Linking Environmental Factors to Hypertension: A Review of the Literature. Int J Hypertens; DOI: 10.1155/2015/926414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica E, Fluiter K, Iyer A, Zurolo E, Vreijling J, Van Vliet EA, Baayen JC & Gorter JA (2010). Expression pattern of miR-146a, an inflammation-associated microRNA, in experimental and human temporal lobe epilepsy. Eur J Neurosci 31, 1100–1107. [DOI] [PubMed] [Google Scholar]
- Bain AR, Weil BR, Diehl KJ, Greiner JJ, Stauffer BL & DeSouza CA (2017). Insufficient sleep is associated with impaired nitric oxide-mediated endothelium-dependent vasodilation. Atherosclerosis 265, 41–46. [DOI] [PubMed] [Google Scholar]
- Bao M, Zhang Y, Lou X, Cheng Y & Zhou H (2014). Protective Effects of Let-7a and Let-7b on Oxidized Low-Density Lipoprotein Induced Endothelial Cell Injuries. PLoS ONE; DOI: 10.1371/journal.pone.0106540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao M-H, Xiao Y, Zhang Q-S, Luo H-Q, Luo J, Zhao J, Li G-Y, Zeng J & Li J-M (2015). Meta-Analysis of miR-146a Polymorphisms Association with Coronary Artery Diseases and Ischemic Stroke. Int J Mol Sci 16, 14305–14317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon RA et al. (2013). MicroRNA-34a regulates cardiac ageing and function. Nature 495, 107–110. [DOI] [PubMed] [Google Scholar]
- Calvin AD, Covassin N, Kremers WK, Adachi T, Macedo P, Albuquerque FN, Bukartyk J, Davison DE, Levine JA, Singh P, Wang S & Somers VK (2014). Experimental Sleep Restriction Causes Endothelial Dysfunction in Healthy Humans. J Am Heart Assoc Cardiovasc Cerebrovasc Dis; DOI: 10.1161/JAHA.114.001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccio FP, Cooper D, D’Elia L, Strazzullo P & Miller MA (2011). Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J 32, 1484–1492. [DOI] [PubMed] [Google Scholar]
- Chen L, Wang J, Wang B, Yang J, Gong Z, Zhao X, Zhang C & Du K (2016). MiR-126 inhibits vascular endothelial cell apoptosis through targeting PI3K/Akt signaling. Ann Hematol 95, 365–374. [DOI] [PubMed] [Google Scholar]
- Chistiakov DA, Orekhov AN & Bobryshev YV (2016). The role of miR-126 in embryonic angiogenesis, adult vascular homeostasis, and vascular repair and its alterations in atherosclerotic disease. J Mol Cell Cardiol 97, 47–55. [DOI] [PubMed] [Google Scholar]
- Daniel J-M, Penzkofer D, Teske R, Dutzmann J, Koch A, Bielenberg W, Bonauer A, Boon RA, Fischer A, Bauersachs J, van Rooij E, Dimmeler S & Sedding DG (2014). Inhibition of miR-92a improves re-endothelialization and prevents neointima formation following vascular injury. Cardiovasc Res 103, 564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjarlais M, Dussault S, Dhahri W, Mathieu R & Rivard A (2017). MicroRNA-150 Modulates Ischemia-Induced Neovascularization in Atherosclerotic Conditions. Arterioscler Thromb Vasc Biol 37, 900–908. [DOI] [PubMed] [Google Scholar]
- Dong Y-M, Liu X-X, Wei G-Q, Da Y-N, Cha L & Ma C-S (2015). Prediction of long-term outcome after acute myocardial infarction using circulating miR-145. Scand J Clin Lab Invest 75, 85–91. [DOI] [PubMed] [Google Scholar]
- Empel VPM van Windt LJD & Martins PA da C(2012). Circulating miRNAs: Reflecting or Affecting Cardiovascular Disease? Curr Hypertens Rep 14, 498–509. [DOI] [PubMed] [Google Scholar]
- Gao J, Ma X, Zhang Y, Guo M & Shi D (2017). The role of microRNAs in prethrombotic status associated with coronary artery disease. Thromb Haemost 117, 429–436. [DOI] [PubMed] [Google Scholar]
- Grandner MA, Sands-Lincoln MR, Pak VM & Garland SN (2013). Sleep duration, cardiovascular disease, and proinflammatory biomarkers. Nat Sci Sleep; DOI: 10.2147/NSS.S31063 Available at: https://www.dovepress.com/sleep-duration-cardiovascular-disease-and-proinflammatory-biomarkers-peer-reviewed-article-NSS [Accessed June 19, 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H, Qu G, Han C, Wang Y, Sun T, Li F, Wang J & Luo S (2015). MiR-34a, miR-21 and miR-23a as potential biomarkers for coronary artery disease: a pilot microarray study and confirmation in a 32 patient cohort. Exp Mol Med 47, e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson GK (2005). Inflammation, Atherosclerosis, and Coronary Artery Disease. N Engl J Med 352, 1685–1695. [DOI] [PubMed] [Google Scholar]
- Hao L, Wang X, Cheng J, You S, Ma S, Zhong X, Quan L & Luo B (2014). The up-regulation of endothelin-1 and down-regulation of miRNA-125a-5p, −155, and −199a/b-3p in human atherosclerotic coronary artery. Cardiovasc Pathol 23, 217–223. [DOI] [PubMed] [Google Scholar]
- Hauri PJ & Wisbey J (1992). Wrist Actigraphy in Insomnia. Sleep 15, 293–301. [DOI] [PubMed] [Google Scholar]
- Hijmans JG, Diehl KJ, Bammert TD, Kavlich PJ, Lincenberg GM, Greiner JJ, Stauffer BL & DeSouza CA (2018). Association between hypertension and circulating vascular-related microRNAs. J Hum Hypertens; DOI: 10.1038/s41371-018-0061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Yagi S & Yamakuchi M (2010). MicroRNA-34a regulation of endothelial senescence. Biochem Biophys Res Commun 398, 735–740. [DOI] [PubMed] [Google Scholar]
- Jansen F, Yang X, Hoelscher M, Cattelan A, Schmitz T, Proebsting S, Wenzel D, Vosen S, Franklin BS, Fleischmann BK, Nickenig G & Werner N (2013). Endothelial Microparticle-Mediated Transfer of MicroRNA-126 Promotes Vascular Endothelial Cell Repair via SPRED1 and is Abrogated in Glucose-Damaged Endothelial Microparticles. CirculationCIRCULATIONAHA.113.001720. [DOI] [PubMed] [Google Scholar]
- Levy P, Tamisier R, Arnaud C, Monneret D, Baguet JP, Stanke-Labesque F, Dematteis M, Godin-Ribuot D, Ribuot C & Pepin J-L (2012). Sleep deprivation, sleep apnea and cardiovascular diseases. Front Biosci Elite Ed 4, 2007–2021. [DOI] [PubMed] [Google Scholar]
- Li D, Yang P, Xiong Q, Song X, Yang X, Liu L, Yuan W & Rui Y-C (2010). MicroRNA-125a/b-5p inhibits endothelin-1 expression in vascular endothelial cells. J Hypertens 28, 1646. [DOI] [PubMed] [Google Scholar]
- Liu Y (2016). Prevalence of Healthy Sleep Duration among Adults — United States, 2014. MMWR Morb Mortal Wkly Rep; DOI: 10.15585/mmwr.mm6506a1. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Skene DJ & Arendt J (1999). Comparison between subjective and actigraphic measurement of sleep and sleep rhythms. J Sleep Res 8, 175–183. [DOI] [PubMed] [Google Scholar]
- Lovren F, Pan Y, Quan A, Singh KK, Shukla PC, Gupta N, Steer BM, Ingram AJ, Gupta M, Al-Omran M, Teoh H, Marsden PA & Verma S (2012). MicroRNA-145 Targeted Therapy Reduces Atherosclerosis. Circulation 126, S81–S90. [DOI] [PubMed] [Google Scholar]
- Lovren F & Verma S (2013). Evolving Role of Microparticles in the Pathophysiology of Endothelial Dysfunction. Clin Chem 59, 1166–1174. [DOI] [PubMed] [Google Scholar]
- Loyer X, Potteaux S, Vion A-C, Guérin CL, Boulkroun S, Rautou P-E, Ramkhelawon B, Esposito B, Dalloz M, Paul J-L, Julia P, Maccario J, Boulanger CM, Mallat Z & Tedgui A (2014). Inhibition of MicroRNA-92a Prevents Endothelial Dysfunction and Atherosclerosis in Mice. Circ Res 114, 434–443. [DOI] [PubMed] [Google Scholar]
- Ma S, Tian XY, Zhang Y, Mu C, Shen H, Bismuth J, Pownall HJ, Huang Y & Wong WT (2016). E-selectin-targeting delivery of microRNAs by microparticles ameliorates endothelial inflammation and atherosclerosis. Sci Rep; DOI: 10.1038/srep22910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma BD, Gabriel Jr A, Bignotto M & Tufik S (2002). Paradoxical sleep deprivation increases plasma endothelin levels. Braz J Med Biol Res 35, 75–79. [DOI] [PubMed] [Google Scholar]
- Park MY, Herrmann SM, Saad A, Widmer RJ, Tang H, Zhu X-Y, Lerman A, Textor SC & Lerman LO (2015). Circulating and renal vein levels of microRNAs in patients with renal artery stenosis. Nephrol Dial Transplant 30, 480–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson MR & Kriegel AJ (2017). MiR-146a/b: a family with shared seeds and different roots. Physiol Genomics 49, 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramkaran P, Khan S, Phulukdaree A, Moodley D & Chuturgoon AA (2014). miR-146a polymorphism influences levels of miR-146a, IRAK-1, and TRAF-6 in young patients with coronary artery disease. Cell Biochem Biophys 68, 259–266. [DOI] [PubMed] [Google Scholar]
- Rhodes CJ et al. (2013). Reduced MicroRNA-150 Is Associated with Poor Survival in Pulmonary Arterial Hypertension. Am J Respir Crit Care Med 187, 294–302. [DOI] [PubMed] [Google Scholar]
- Sang W, Wang Y, Zhang C, Zhang D, Sun C, Niu M, Zhang Z, Wei X, Pan B, Chen W, Yan D, Zeng L, Loughran Jr TP & Xu K (2016). MiR-150 impairs inflammatory cytokine production by targeting ARRB-2 after blocking CD28/B7 costimulatory pathway. Immunol Lett 172, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh M, Takahashi Y, Tabuchi T, Tamada M, Takahashi K, Itoh T, Morino Y & Nakamura M (2015). Circulating Toll-like receptor 4-responsive microRNA panel in patients with coronary artery disease: results from prospective and randomized study of treatment with renin-angiotensin system blockade. Clin Sci Lond Engl 1979 128, 483–491. [DOI] [PubMed] [Google Scholar]
- Sayed ASM, Xia K, Salma U, Yang T & Peng J (2014). Diagnosis, Prognosis and Therapeutic Role of Circulating miRNAs in Cardiovascular Diseases. Heart Lung Circ 23, 503–510. [DOI] [PubMed] [Google Scholar]
- Shang F, Wang S-C, Hsu C-Y, Miao Y, Martin M, Yin Y, Wu C-C, Wang Y-T, Wu G, Chien S, Huang H-D, Tarng D-C, Shiu Y-T, Cheung AK, Huang P-H, Chen Z & Shyy JY-J (2017). MicroRNA-92a Mediates Endothelial Dysfunction in CKD. J Am Soc NephrolASN.2016111215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil BR, Greiner JJ, Stauffer BL & DeSouza CA (2013). Self-Reported Habitual Short Sleep Duration Is Associated with Endothelial Fibrinolytic Dysfunction in Men: A Preliminary Report. Sleep 36, 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil BR, Mestek ML, Westby CM, Van Guilder GP, Greiner JJ, Stauffer BL & DeSouza CA (2010). Short sleep duration is associated with enhanced endothelin-1 vasoconstrictor toneThis article is one of a selection of papers published in the two-part special issue entitled 20 Years of Endothelin Research. Can J Physiol Pharmacol 88, 777–781. [DOI] [PubMed] [Google Scholar]
- Widmer RJ, Chung W-Y, Herrmann J, Jordan KL, Lerman LO & Lerman A (2014). The Association between Circulating MicroRNA Levels and Coronary Endothelial Function. PLOS ONE 9, e109650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Medical Association (2013). World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310, 2191–2194. [DOI] [PubMed] [Google Scholar]
- Wronska A, Kurkowska-Jastrzebska I & Santulli G (2015). Application of microRNAs in diagnosis and treatment of cardiovascular disease. Acta Physiol 213, 60–83. [DOI] [PubMed] [Google Scholar]
- Yamakuchi M, Ferlito M & Lowenstein CJ (2008). miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci U S A 105, 13421–13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Jin X, Shan Z, Li S, Huang H, Li P, Peng X, Peng Z, Yu K, Bao W, Yang W, Chen X & Liu L (2017). Relationship of Sleep Duration With All-Cause Mortality and Cardiovascular Events: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. J Am Heart Assoc; DOI: 10.1161/JAHA.117.005947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E, Shah A, Willeit J & Mayr M (2010). Plasma MicroRNA Profiling Reveals Loss of Endothelial MiR-126 and Other MicroRNAs in Type 2 DiabetesNovelty and Significance. Circ Res 107, 810–817. [DOI] [PubMed] [Google Scholar]
- Zeller T, Keller T, Ojeda F, Reichlin T, Twerenbold R, Tzikas S, Wild PS, Reiter M, Czyz E, Lackner KJ, Munzel T, Mueller C & Blankenberg S (2014). Assessment of microRNAs in patients with unstable angina pectoris. Eur Heart J 35, 2106–2114. [DOI] [PubMed] [Google Scholar]


