Abstract
Objective:
The role of heparin in acute ischemic stroke is controversial. We investigated the effect of heparin on ischemic lesion growth.
Methods:
Data were analyzed on non-thrombolysed ischemic stroke patients in whom diffusion/perfusion MRI (DWI and PWI) was performed <12 hours of last known well and showed a PWI-DWI lesion mismatch, and who underwent follow-up neuroimaging at least 4 days after admission. Lesion growth was assessed by (a) absolute lesion growth, and (b) percentage mismatch lost (PML). Univariate and multivariate regression analysis, and propensity score matching, were used to determine the effects of heparin on ischemic lesion growth.
Results:
Of the 113 patients meeting study criteria, 59 received heparin within 24 hours. Heparin use was associated with ~5-fold reductions in PML (3.5% vs. 19.2%, p=0.002) and absolute lesion growth (4.7 vs. 20.5 mL, p=0.009). In multivariate regression models, heparin independently predicted reduced PML (p=0.04) and absolute lesion growth (p=0.04) in the entire cohort, and in multiple subgroups (patients with and without proximal artery occlusion; DWI volume >5 mL; cardio-embolic mechanism; DEFUSE-3 target mismatch). In propensity score matching analysis where patients were matched by admission NIHSS, DWI volume and proximal artery occlusion, heparin remained an independent predictor of PML (p=0.048) and tended to predict absolute lesion growth (p=0.06). Heparin treatment did not predict functional outcome at discharge or 90 days.
Conclusion:
Early heparin treatment in acute ischemic stroke patients with PWI-DWI mismatch attenuates ischemic lesion growth. Clinical trials with careful patient selection are warranted to investigate the potential ischemic protective effects of heparin.
Keywords: Heparin, Anticoagulation, Acute Stroke, Diffusion Weighted MRI, Perfusion Weighted MRI, Ischemic Lesion Growth
Introduction
The role of heparin in ischemic stroke is controversial. Since the 1950s, anecdotal reports showed that stroke deficits improve with early heparin administration, especially in basilar artery thrombosis[1, 2]. While some clinical trials showed benefit[3–5], several large trials using intravenous or subcutaneous anticoagulation failed to show benefit in the broader stroke population[6–10]. A trend towards more favorable early outcome measures and reduced stroke recurrence was observed, but at a cost of increased hemorrhagic complications. As a result, the 2018 American Heart Association treatment guidelines do not recommend anticoagulation in the acute setting[11]. Some authors have attributed the negative trial results to suboptimal patient selection, delayed time to treatment, and inadequate heparin dosage. Stroke experts propose that heparin may still have utility in selected patient populations[12]. Indeed, subgroup analysis and subsequent studies suggest benefit in subpopulations such as symptomatic large artery atherosclerosis, intraluminal carotid or vertebro-basilar artery thrombus, and others[5, 13, 14]. Previous trials were based on heparin’s anticoagulant effects and focused on recurrent infarction rates and functional status as outcome measures. While heparin may facilitate early clot lysis, halt thrombus propagation, reduce blood viscosity and reduce recurrent thromboembolism, it also attenuates microvascular thrombosis and possibly has anti-inflammatory and neuroprotective effects [15–19]. The effect of heparin on tissue outcome has not been carefully investigated in humans. We investigated the effect of heparin administration on acute ischemic lesion growth.
Materials and Methods
The study was approved by the Partners Human Research Committee.
Study Population
We analyzed data from 274 ischemic stroke patients admitted to Massachusetts General Hospital between 1995 and 2009 who underwent diffusion-weighted (DWI) and perfusion-weighted MRI (PWI) within 12 hours of last known well, had a perfusion>diffusion lesion volume ‘mismatch’, and underwent a follow-up CT or MRI at least 4 days from presentation to assess final infarct volume. Of these, 170 were prospectively consented and enrolled into a NIH-funded study evaluating DWI-PWI in predicting tissue outcome after acute ischemic stroke. An additional 104 patients with admission DWI-PWI and follow-up imaging within the same timeframes were retrospectively included into the NIH study of serial imaging. Some patients have been included in previous publications[20–23]. For this analysis we excluded patients receiving intravenous/intra-arterial thrombolysis, experimental treatments, and in whom assessment of final infarct was not possible due to low-quality follow-up images, hemicraniectomy, extensive hemorrhagic conversion, or massive brain edema. CT or MRI were used for follow-up because they both conspicuously define infarct limits[24]. Because MRI volumetric determinations are unreliable for small lesions, we excluded patients with small PWI-DWI mismatch defined as “mean transit time (MTT) volume/DWI volume <1.2” and “MTT-DWI volume <10 mL”[25, 26].
Clinical Data
Demographics and clinical variables were obtained from chart abstraction. Stroke onset time was considered the time the patient was last known to be well. Stroke mechanism was ascribed using the Causative Classification of Stroke (CCS) system[27]. Clinical outcome was assessed prospectively using the modified Rankin scale (mRS) at discharge and 90 days for prospectively enrolled subjects and extrapolated from chart records for retrospectively enrolled subjects. All assessments were made blinded to mismatch findings. Heparin therapy was defined as the initiation of intravenous unfractionated heparin, or therapeutic doses of subcutaneous low-molecular weight heparin (i.e., not for prevention of deep vein thrombosis) within 24 hours of admission. At our hospital, unfractionated heparin is typically administered intravenously, with a variable bolus dose, with PTT monitoring every 4 hours until the PTT reaches goal of 50–70 seconds, and thereafter every 8–12 hours or more frequently based on clinical discretion. Time to heparin administration was the time from stroke onset to intravenous or low-molecular-weight heparin order. Time to therapeutic PTT (partial thromboplastin) was the time from stroke onset (or last seen well) to the time of first PTT >50 seconds.
Imaging Data
Imaging data were collected for the majority of patients using a 1.5 Tesla MRI (GE Medical Systems, Milwaukee, WI). DWI and apparent diffusion coefficient (ADC) maps were computed from raw diffusion data sets using methods described previously[28]. PWI was acquired using dynamic susceptibility contrast echo-planar imaging using techniques previously described[2023]. CT was performed using a helical scanner (High-Speed Advantage; GE Medical Systems). Acute lesions on admission DWI and PWI (mean transit time, MTT) and final infarcts on fluid-attenuated inversion recovery (FLAIR) or CT were manually outlined. Lesion volumes were manually outlined as previously described[20–23]. All MRI measurements were performed by investigators experienced in image analysis and processing, blinded to clinical data. Lesion growth was assessed by (a) Absolute Lesion Growth, i.e. final infarct volume – admission DWI lesion volume, and (b) Percentage mismatch lost (PML), i.e. (final infarct volume – admission DWI volume)/(mismatch volume) ×100%.
Proximal artery occlusion was defined as embolic cut-off or reduced/absent filling of the basilar artery, internal carotid artery (ICA), or M1 segment of the middle cerebral artery (MCA) before its bifurcation. Stroke arterial territory was classified as ICA, MCA, anterior cerebral artery (ACA), posterior cerebral artery (PCA) and vertebro-basilar (VB).
Statistical Analysis
Data are expressed as percentages, mean±SD or median (interquartile range [IQR]) as appropriate. The heparin-treated and heparin-untreated groups were compared using the Chi-Square test for categorical variables, Student’s t-test for normally distributed continuous variables, and Mann-Whitney U test for non-normally distributed continuous variables. Univariate analysis explored the relationship between clinical-imaging variables (including heparin treatment) and PML and absolute lesion growth using Mann-Whitney U and Kruskall-Wallis tests for categorical variables, and Spearman’s correlation (ρ) for continuous variables. Variables that were significantly different, and factors known to affect lesion growth, were included in linear and logistic regression models to identify independent predictors of PML and absolute lesion growth. For logistic regression, PML and absolute lesion growth were dichotomized by their mean values. The effect of heparin treatment was assessed in several subgroups (absence or presence of proximal artery occlusion, large artery atherosclerosis and cardioembolic stroke mechanisms, DWI lesions >5 mL, NIHSS≤5, prospective enrollment, DAWN criteria and DEFUSE-3 target mismatch criteria using MTT maps to define perfusion deficit[29]). We examined the effect of heparin on clinical outcome by constructing a logistic regression model where the dependent variable was mRS 0–2 (favorable outcome). Finally, to control for selection bias and confounding by unmeasured factors, we conducted a propensity score matched analysis to determine the effects of heparin on PML and absolute lesion growth. Pre-specified admission NIHSS values (≥ or <10), admission DWI volume (≥ or <30) and presence of large vessel occlusion were used in the propensity score matching. The linear assumptions such as linearity, normal distribution of residuals and homoscedasticity were met. A 2-tailed value of P<0.05 was considered significant. Analyses were performed using the RStudio statistical software (version 1.1.383, RStudio Inc., Boston, MA).
Results
A total of 274 patients with acute ischemic stroke had admission MRI and follow-up imaging available for analysis. Of these, 82 were excluded based on revascularization treatment, 22 for poor-quality MRI, and 57 for mismatch volume <10 mL or mismatch <20%, leaving 113 patients in the study cohort of whom 66 were prospectively enrolled.
Heparin was given to 59 patients within 24 hours of admission (median 5.0 (IQR, 3–8) hours), including 33 of 66 (50%) and 26 of 47 (55%) patients who were prospectively and retrospectively enrolled into the NIH-funded serial imaging study. Two of these patients received low-molecular-weight heparin (dalteparin) and the rest received unfractionated intravenous heparin. The median time to heparin was 9.0 (IQR, 6–11) hours, and median time to therapeutic PTT was 14 (IQR, 9–20) hours. Decisions concerning anticoagulation were made by the treating team; chart review suggested that indications for heparin were proximal artery occlusion in 32, cervical-artery dissection in 3, cardio-embolism in 18, hypercoagulable state in 2, and was unspecified in 4 patients.
Clinical-imaging features are shown in Table 1. There were no significant differences in age, gender, admission blood pressure and glucose levels between heparin-treated and non-treated groups. The groups were balanced with regards to CCS mechanism, arterial territory involved, proximal artery occlusion, and time from onset to initial MRI. Over half had proximal artery occlusions. Only 5 had infarcts in the VB territory. Patients receiving heparin had significantly lower admission NIHSS scores and DWI volumes, however the absolute mismatch volumes were similar between groups. Heparin-treated patients tended to have a larger percent mismatch volumes. Yet, the heparin-treated group had significantly smaller final infarcts, and correspondingly lower absolute lesion growth and PML. In the heparin treated group 5 patients had asymptomatic hemorrhagic transformation and 3 patients had mild hematuria. In the other group 6 patients had asymptomatic hemorrhagic transformation and 1 patient had mild hematuria. Functional outcome was significantly better in the heparin-treated group at discharge but not at 90 days.
Table 1.
Baseline Characteristics and Clinical and Imaging Features
| Variable | All (n=113) | Heparin (n=59) | Non-heparin (n=54) | P value |
|---|---|---|---|---|
| Age | 70 (53, 79) | 70 (51.5, 78.5) | 71 (54.5, 81.7) | 0.42 |
| Gender Male | 70 (61.9%) | 38 (64.4%) | 32 (59.3%) | 0.71 |
| Admission Glucose (mg/dL) | 118 (105, 137) | 117 (106, 128) | 122 (104, 147) | 0.36 |
| Admission SBP (mmHg) | 153 (25.2) | 154 (24) | 153 (27) | 0.90 |
| Admission NIHSS | 7.5 (4, 14) | 6 (3, 11.5) | 11 (6, 15.5) | 0.04 |
| CCS Subtypes | 0.29 | |||
| - Large artery | 25 (22%) | 16 (27%) | 9 (17%) | 0.25 |
| - Cardioembolic | 53 (47%) | 23 (39%) | 30 (55%) | 0.09 |
| - Other | 16 (14%) | 10 (17%) | 6 (11%) | 0.42 |
| - Cryptogenic | 19 (17%) | 10 (17%) | 9 (17%) | 1.0 |
| Arterial Territory Involved | 0.42 | |||
| - ICA | 7 (6%) | 3 (5%) | 4 (7%) | 0.70 |
| - ACA | 4 (3%) | 2 (3%) | 2 (4%) | 1.0 |
| - MCA | 92 (81%) | 49 (83%) | 43 (80%) | 0.8 |
| - PCA | 5 (5%) | 1 (2%) | 4 (7%) | 0.19 |
| - VB | 5 (5%) | 4 (7%) | 1 (2%) | 0.36 |
| Large Vessel Occlusion | 0.31 | |||
| - Complete | 50 (44%) | 25 (42%) | 25 (46%) | 0.45 |
| - Partial | 14 (12%) | 10 (17%) | 4 (7%) | 0.15 |
| Time to first MRI (h) | 6.0 (2.7) | 6.2 (2.5) | 5.7 (2.8) | 0.36 |
| Admission DWI vol. (mL) | 17.2 (5, 50) | 12.9 (4, 30) | 26.8 (8, 60) | 0.03 |
| Admission Mismatch vol. (mL) | 93.6 (46, 174) | 88.3 (40, 183) | 95.1 (51, 137) | 0.99 |
| Percent Mismatch (%) | 548 (169, 2053) | 770 (228, 2074) | 293 (120, 1675) | 0.069 |
| Time to follow-up exam (days) | 9.7 (5.9, 37.6) | 10.1 (6.1, 34) | 9.4 (5.5, 42.7) | 0.79 |
| Final infarct volume (mL) | 28.7 (9.6, 90.9) | 17.4 (7.6, 51.8) | 56.6 (15.1, 107) | 0.004 |
| Absolute Lesion Growth (mL) | 8.48 (0.1, 41.5) | 4.7 (−1.1, 15.2) | 20.5 (2.4, 54.2) | 0.009 |
| Percentage Mismatch Lost (%) | 7.4 (0.1, 31.2) | 3.5 (−0.9, 17.0) | 19.2 (2.6, 56.0) | 0.002 |
| Discharge mRS 0–2 | 37 (33%) | 25 (43%) | 12 (22%) | 0.03 |
| 90-day mRS 0–2 | 53 (47%) | 30 (73%) | 23 (55%) | 0.13 |
Abbreviations: SBP: Systolic blood pressure; NIHSS: National Institutes of Health Stroke Scale; CCS: Causative Classification System; ICA: Internal carotid artery; ACA: anterior cerebral artery; MCA: top cerebral artery; PCA: posterior cerebral artery; VB, vertebro-basilar arteries; Vol, volume.
Results of the univariate analysis are shown in Table 2. Both PML and absolute lesion growth correlated positively with admission glucose levels, admission DWI volume, and heparin use. The NIHSS score and presence of proximal artery occlusion correlated significantly with absolute lesion growth, and tended to correlate with PML.
Table 2.
Univariable Predictors of Infarct Growth
| Variable | PML (Median, IQR or Correlation Coefficient) | P value | Absolute Lesion Growth (mL, Median, IQR or Correlation Coefficient) | P value |
|---|---|---|---|---|
| Age | 0.12 | 0.19 | 0.14 | 0.15 |
| Gender: | 0.28 | 0.19 | ||
| - Male | 10.2% (1.6%, 37.0%) | 10.7 (1.0, 44.9) | ||
| - Female | 5.1% (−0.5%, 20.7%) | 6.5 (−0.4, 20.4) | ||
| Admission Glucose | 0.29 | 0.002 | 0.28 | 0.003 |
| Admission SBP | 0.008 | 0.94 | 0.05 | 0.59 |
| NIHSS score | 0.21 | 0.06 | 0.27 | 0.01 |
| CCS Subtypes | 0.20 | 0.01 | ||
| - Large-artery (n=25) | 9.7% (2.3%, 27.4%) | 14.3 (4.1, 56.9) | ||
| - Cardioembolic (n=53) | 7.8% (−0.9%, 30.7%) | 6.5 (−0.8, 22.9) | ||
| - Other (n=16) | 21.6% (4.3%, 50.4%) | 27.7 (9.2, 63.6) | ||
| - Cryptogenic (n=19) | 1.6% (−9.3%, 27.7%) | 0.5 (−5.9, 22.9) | ||
| Stroke territory | 0.20 | 0.36 | ||
| - ICA | 7.9% (4.4%, 57.8%) | 13.6 (8.3, 49.8) | ||
| - ACA | 1.2% (−2.1%, 25.3%) | 0.5 (−0.5, 17.2) | ||
| - MCA | 7.6% (0.4%, 29.6%) | 8.5 (0.2, 43.2) | ||
| - PCA | 72.1% (30.7%,136%) | 16.0 (8.0, 23.2) | ||
| - VB | 0.0% (−7.5%, 3.5%) | 0.0 (−6.6, 3.6) | ||
| Proximal-artery occlusion | 0.07 | <0.001 | ||
| - Yes | 9.5% (2.5%, 29.9%) | 14.1 (3.9, 53.4) | ||
| - No | 2.7% (−8.8%, 31.4%) | 2.1 (−4.1, 18.2) | ||
| Time to MRI | −0.11 | 0.24 | −0.09 | 0.36 |
| Admission DWI volume | 0.27 | 0.003 | 0.29 | 0.002 |
| Heparin | 0.002 | 0.009 | ||
| - Yes | 3.5% (−1.0%, 17.1%) | 4.7 (−1.2–15.3) | ||
| - No | 19.2% (2.6%, 56.1%) | 20.5 (2.4–54.2) | ||
Abbreviations: SBP: Systolic blood pressure; NIHSS: National Institutes of Health Stroke Scale; CCS: Causative Classification System; ICA: Internal carotid artery; ACA: anterior cerebral artery; MCA: middle cerebral artery; PCA: posterior cerebral artery; VB, vertebrobasilar arteries; PTT, partial thromboplastin time.
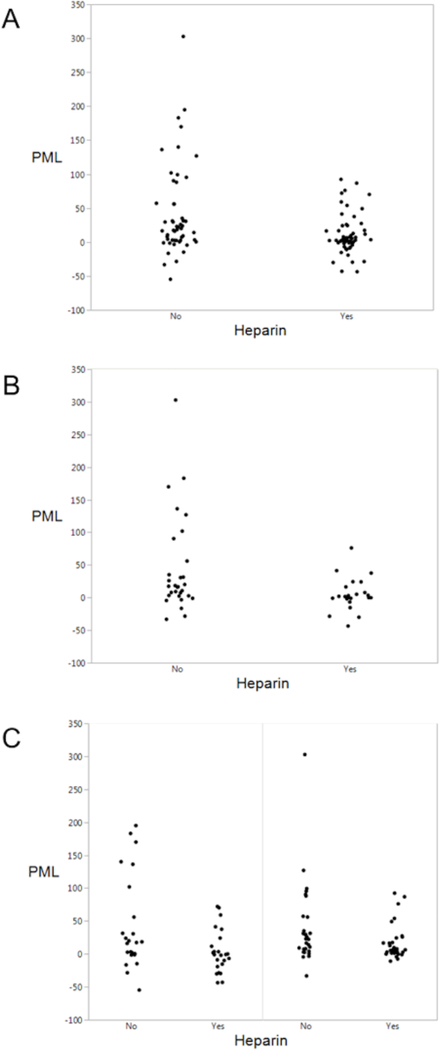
Heparin use was associated with approximately 5-fold reductions in both PML (3.5% vs. 19.2%, p=0.002) and absolute lesion growth (4.7 vs. 20.5 mL, p=0.009). The effects were even stronger in patients achieving therapeutic PTT within 18 hours (PML, 2.6 vs. 19.2, p<0.001; absolute lesion growth, 3.6 vs. 20.5 mL, p=0.008). As depicted in Figure 1, heparin use was associated with significantly reduced infarct growth in patients with proximal artery occlusion (PML, −0.9% vs. 17.4%, p=0.03; absolute lesion growth, 5.4 vs. 23.1 mL, p=0.01), and without proximal artery occlusion (PML, −0.7% vs. 8.0%, p=0.02; absolute lesion growth, 10.5 vs. 30.4 mL, p=0.048). Table 3 shows results of the linear regression model. After adjusting for variables shown in the table, heparin use proved to be the only independent predictor of PML and absolute lesion growth (both p=0.04). In logistic regression models, heparin use was again an independent predictor of absolute lesion growth (OR 0.17, 95% C.I. 0.03–0.64; p=0.01) and tended to predict PML (OR 0.35, 95% C.I. 0.11–1.1; p=0.07).
Figure 1. PML and Heparin Use.
The graphs depict the relationship between Percent Mismatch Lost (PML) and heparin treatment, in the entire cohort (A), and subgroups with cardioembolic stroke (B), without proximal artery occlusion (C, left) and with proximal artery occlusion (C, right).
Table 3.
Independent Predictors of PML and Absolute Lesion Growth
| Variable | PML | Absolute Lesion Growth | ||
|---|---|---|---|---|
| β value | P value | β value | P value | |
| Age | 0.01 | 0.91 | 0.03 | 0.80 |
| Male Gender | 0.07 | 0.55 | 0.11 | 0.31 |
| Admission Glucose | −0.006 | 0.96 | −0.05 | 0.66 |
| Admission NIHSS | 0.14 | 0.26 | 0.17 | 0.16 |
| Time to MRI | −0.18 | 0.12 | −0.12 | 0.28 |
| Admission DWI volume | −0.10 | 0.45 | 0.002 | 0.99 |
| Heparin use | −0.25 | 0.04 | −0.23 | 0.04 |
Abbreviations: DWI, diffusion-weighted MRI; NIHSS, NIH stroke scale score
For the propensity score analysis, patients were matched by admission NIHSS (≥ or <10), DWI volume (≥ or <30mL) and proximal artery occlusion (absent or present). Despite the small number of matched patients (n=29), heparin remained a predictor of PML (p=0.048) and tended to predict absolute lesion growth (p=0.059).
We performed sensitivity analyses in several subgroups by constructing regression models using the variables shown in Table 3. In the subgroup with DWI volume >5 mL (n=84), heparin was an independent predictor of reduced PML (p=0.046) and showed a trend for reduced absolute lesion growth (p=0.07). In cardio-embolic stroke (n=53), heparin use was not an independent predictor of reduced PML (p=0.11) but tended to predict reduced absolute lesion growth (p=0.08). There was no significant difference in patients with large-artery atherosclerosis subtype although the number of patients was small (n=25). In patients meeting the DEFUSE-3 criteria[29] for ‘target mismatch’ (n=109), heparin tended to independently predict reduced PML (p=0.06) and was an independent predictor of absolute lesion growth (p=0.04). In patients meeting the DAWN criteria (n=36), heparin did not predict reduced PML (p=0.23) or absolute lesion growth (p=0.11). In proximal artery occlusion (n=64), there was a trend for heparin to independently predict reduced PML (p=0.06) and reduced absolute lesion growth (p=0.05). In patients without proximal artery occlusion (n=49), heparin did not predict reduced PML (p=0.24) but showed a trend to independently predict reduced absolute lesion growth (p=0.06). In patients with NIHSS≤5 (n=31), heparin did not predict reduced PML (p=0.56) or absolute lesion growth (p=0.76). In prospectively enrolled patients (n=66), heparin did not predict reduced PML (p=0.13) but tended to reduce absolute lesion growth (p=0.09).
In logistic regression models (Table 4), lower age, admission NIHSS and DWI volumes correlated or tended to correlate with discharge and 90-day favorable outcomes, however heparin did not predict functional outcome.
Table 4.
Independent Predictors of Good Outcome (mRS 0–2) at Discharge and 90 days
| Variable | Discharge | 90 days | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age | 0.97 | 0.94, 1.00 | 0.09 | 0.93 | 0.88, 9.76 | 0.007 |
| Male Gender | 1.15 | 0.35, 3.80 | 0.80 | 1.31 | 0.34, 4.97 | 0.68 |
| Admission Glucose level | 0.98 | 0.96, 1.00 | 0.12 | 0.99 | 0.97, 1.00 | 0.23 |
| Admission NIHSS | 0.88 | 0.78, 0.97 | 0.02 | 0.90 | 0.79, 1.01 | 0.10 |
| Admission DWI volume | 0.96 | 0.92, 0.99 | 0.03 | 0.98 | 0.97, 1.00 | 0.21 |
| Heparin use | 1.84 | 0.59, 5.93 | 0.29 | 0.92 | 0.23, 3.40 | 0.91 |
Abbreviations: DWI, diffusion-weighted MRI; mRS, modified Rankin Scale score; NIHSS, National Institutes of Health Stroke Scale.
Discussion
Ischemic lesion growth is a frequent outcome measure used in neuroprotective and thrombolytic stroke trials[30–32]. In this study, we investigated the effects of early heparin use on ischemic lesion growth based on its antithrombotic, anti-inflammatory, and other tissue-level biologic ischemic protective effects[15–18]. Our results suggest that heparin is a powerful ischemic protectant, with a 5-fold reduction in PML and absolute lesion growth. These effects compare well against potent neuroprotectants such as citicoline (~2.5-fold reduction in lesion growth[32]), intravenous tissue plasminogen activator (~9-fold reduction in lesion growth[33]) and IA thrombectomy[29, 34, 35].
The validity of our results is supported by the consistent benefit across PML and absolute lesion growth, and observed association for known predictors of ischemic lesion growth such as admission glucose level, NIHSS score and DWI lesion volume[22, 36, 37]. Further, subgroup analysis showed or tended to show uniform benefit across subgroups with larger DWI volumes, cardio-embolic strokes, DEFUSE-3 ‘target’ mismatch, and with and without proximal artery occlusion, despite the relatively small sample sizes. Patients with DWI lesions >5 mL were specifically investigated since volume measurements are less reliable with small lesions[25]. The effect in patients with cardio-embolic strokes (which typically result in embolic occlusions), and in the subgroup with proximal artery occlusion, support our earlier results showing that prior anticoagulation is associated with 3.5-fold smaller admission lesion volumes[38]. The benefit of heparin appears to be similar in patients with and without proximal artery occlusion, suggesting tissue-level effects via prevention of microvascular thrombosis and ischemic protection in addition to its more widely recognized effects on clot lysis and thrombus propagation.
Time to imaging, rates of proximal artery occlusion, and stroke mechanism were balanced between groups. Heparin-treated patients had lower NIHSS and DWI volumes, suggesting that less severe strokes were offered heparin treatment, presumably to decrease the risk of post-ischemic brain hemorrhage. Similarly, heparin may have been avoided if ischemic lesions involved the deep-grey matter, which is supplied by perforating arteries and more susceptible to post-ischemic hemorrhage, although both groups had similar rates of proximal artery occlusion and arterial territory involvement. Other selection biases may have contributed to the positive results. We, therefore, performed a propensity score matching analysis, which allows one to more accurately estimate the effect of a treatment in an observational retrospective dataset accounting for the variables that determine receiving the treatment. Heparin remained beneficial in the propensity score matching analysis and in nearly all subgroups, suggesting that the observed effect is likely a true benefit. Of note, heparin was initiated after the baseline MRI in two-thirds of patients, suggesting a broad therapeutic time window[18]. However, heparin has been shown to have a time-dependent effect in animal models and in humans[5, 39], and we found a stronger effect with shorter time to therapeutic PTT.
Importantly, heparin reduced ischemic lesion growth, but did not improve functional outcome. Multiple trials have also failed to show improvement in functional outcomes. The discrepancy between reduced ischemic lesion growth and lack of improvement of functional outcome may explained by our small sample size. Demonstration of a clinically meaningful effect on functional outcome typically requires a much larger sample size[40]. Also, unlike lesion growth, final absolute lesion volume is more consistently associated with improved functional outcome, and a threshold volume is routinely used as a selection criterion for thrombectomy[41]. It is possible that far greater reductions in lesion growth are required to yield functional improvements.
The major strengths of our study include the recruitment of a large cohort of consecutive stroke patients with early DWI, PWI and follow-up imaging, and blinded analysis of lesion volumes by experienced reviewers. The propensity score matched analysis, and subgroup analyses, were attempts to bolster the validity and ensure consistency of our main results but these are not conclusive. Our study has several limitations. This was a single-center study using advanced imaging, and treatment was rendered by stroke neurologists based on their own preferences and following established anticoagulation-monitoring protocols, so our results are not generalizable and should be interpreted with caution. Unfortunately it was difficult to determine the duration of heparin from our retrospective chart review. We did note several elevated PTT values during the inpatient stay, suggesting heparin was continued for at least 2–5 days or more in most patients (this is consistent with our usual practice when heparin is administered for stroke). The retrospective analysis of the influence of heparin on lesion expansion might have led to our study being underpowered to detect an effect on functional outcomes. Since thresholded MTT maps or the RAPID software (used in the DEFUSE-3 trial) were not available at the time of the study, PWI volumes were assessed using non-thresholded MTT maps. This remains a limitation with regards to the DAWN and DEFUSE-3 subgroup analysis, and may have resulted in overestimation of the tissue-at-risk. Patients with extensive post-ischemic intracranial hemorrhage were excluded, and we did not collect data on systemic bleeding, so safety could not be fully assessed. Of note however the rates of asymptomatic post-ischemic hemorrhage and hematuria were comparable in this selected population. The strong influence of collateral circulation on lesion growth and outcome has been recognized only recently[42]; we did not collect data on collaterals although we did incorporate PWI data. We do not have data concerning reperfusion and arterial recanalization. It is conceivable that non-treated patients with more severe strokes also developed more complications such as infection, which may have contributed to infarct growth in controls; however, lesion growth observed in our study is within the range reported in the control population of large trials[33].
Thrombolysis and emerging acute stroke therapies are associated with a relatively high cost and require a robust 24/7 stroke team and infrastructure. In comparison, heparin is relatively inexpensive, globally available, and routinely used to treat deep vein thrombosis and pulmonary embolism. Our positive results are most likely explained by the presence of substantial DWI and PWI mismatch. Clinical trials using contemporary selection criteria, short therapeutic time windows and advanced neuroimaging, could better investigate the potential benefit of heparin.
Acknowledgements
This study was supported in parts by NIH grants (R01-NS038477, R01-NS051412, R01-NS059775, R01-NS063925, R21-NS077442, R21-NS085574, P50-NS051343, U01-NS086729, U01-NS095869, U24 NS107243) and the Capes Foundation, Ministry of Education, Brazil (grant no. 88881.133101/2016).
Grant Support: This study was supported in parts by NIH grants (R01-NS038477, R01-NS051412, R01-NS059775, R01-NS063925, R21-NS077442, R21-NS085574, P50-NS051343, U01-NS086729, U01-NS095869, U24 NS107243) and the Capes Foundation, Ministry of Education, Brazil (grant no. 88881.133101/2016).
Footnotes
Potential Conflicts of Interest
Ona Wu is coinventor of US Patent 7,512,435, March 31, 2009, licensed to General Electric, Siemens, Imaging Biometrics, and Olea Medical and consultant to Penumbra, Inc. The other authors report no relevant conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Fisher CM. The use of anticoagulants in cerebral thrombosis. Neurology. 1958;8:311–32. [DOI] [PubMed] [Google Scholar]
- [2].Fisher CM. Anticoagulant therapy in cerebral thrombosis and cerebral embolism. A national cooperative study, interim report. Neurology. 1961;11(4)Pt 2:119–31. [DOI] [PubMed] [Google Scholar]
- [3].Kay R, Wong KS, Yu YL, Chan YW, Tsoi TH, Ahuja AT, et al. Low-molecular-weight heparin for the treatment of acute ischemic stroke. N Engl J Med. 1995;333:1588–93. [DOI] [PubMed] [Google Scholar]
- [4].Wang Q, Chen C, Chen XY, Han JH, Soo Y, Leung TW, et al. Low-molecular-weight heparin and early neurologic deterioration in acute stroke caused by large artery occlusive disease. Arch Neurol. 2012;69:1454–60. [DOI] [PubMed] [Google Scholar]
- [5].Camerlingo M, Salvi P, Belloni G, Gamba T, Cesana BM, Mamoli A. Intravenous heparin started within the first 3 hours after onset of symptoms as a treatment for acute nonlacunar hemispheric cerebral infarctions. Stroke. 2005;36:2415–20. [DOI] [PubMed] [Google Scholar]
- [6].The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet. 1997;349:1569–81. [PubMed] [Google Scholar]
- [7].Low molecular weight heparinoid, ORG 10172 (danaparoid), and outcome after acute ischemic stroke: a randomized controlled trial. The Publications Committee for the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. JAMA. 1998;279:1265–72. [PubMed] [Google Scholar]
- [8].Chamorro A, Vila N, Ascaso C, Blanc R. Heparin in acute stroke with atrial fibrillation: clinical relevance of very early treatment. Arch Neurol. 1999;56:1098–102. [DOI] [PubMed] [Google Scholar]
- [9].Berge E, Abdelnoor M, Nakstad PH, Sandset PM. Low molecular-weight heparin versus aspirin in patients with acute ischaemic stroke and atrial fibrillation: a double-blind randomised study. HAEST Study Group. Heparin in Acute Embolic Stroke Trial. Lancet. 2000;355:1205–10. [DOI] [PubMed] [Google Scholar]
- [10].Paciaroni M, Agnelli G, Micheli S, Caso V. Efficacy and safety of anticoagulant treatment in acute cardioembolic stroke: a meta-analysis of randomized controlled trials. Stroke. 2007;38:423–30. [DOI] [PubMed] [Google Scholar]
- [11].Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018. [Google Scholar]
- [12].Caplan LR. Resolved: Heparin may be useful in selected patients with brain ischemia. Stroke. 2003;34:230–1. [DOI] [PubMed] [Google Scholar]
- [13].Wang QS, Chen C, Chen XY, Han JH, Soo Y, Leung TW, et al. Low-molecular-weight heparin versus aspirin for acute ischemic stroke with large artery occlusive disease: subgroup analyses from the Fraxiparin in Stroke Study for the treatment of ischemic stroke (FISS-tris) study. Stroke. 2012;43:346–9. [DOI] [PubMed] [Google Scholar]
- [14].Ruff IM, Jindal JA. Use of Heparin in Acute Ischemic Stroke: Is There Still a Role? Curr Atheroscler Rep. 2015;17:51. [DOI] [PubMed] [Google Scholar]
- [15].Mocco J, Shelton CE, Sergot P, Ducruet AF, Komotar RJ, Otten MLO, et al. -desulfated heparin improves outcome after rat cerebral ischemia/reperfusion injury. Neurosurgery. 2007;61:1297–303; discussion 303–4. [DOI] [PubMed] [Google Scholar]
- [16].Cassinelli G, Naggi A. Old and new applications of non-anticoagulant heparin. Int J Cardiol. 2016;212 Suppl 1:S14–21. [DOI] [PubMed] [Google Scholar]
- [17].Chamorro A, Obach V, Vila N, Revilla M, Cervera A, Ascaso C. Comparison of the acute-phase response in patients with ischemic stroke treated with high-dose heparin or aspirin. J Neurol Sci. 2000;178:17–22. [DOI] [PubMed] [Google Scholar]
- [18].Mary V, Wahl F, Uzan A, Stutzmann JM. Enoxaparin in experimental stroke: neuroprotection and therapeutic window of opportunity. Stroke. 2001;32:993–9. [DOI] [PubMed] [Google Scholar]
- [19].Erdi A, Thomas DP, Kakkar VV, Lane DA. Effect of low-dose subcutaneous heparin on whole-blood viscosity. Lancet. 1976;2:342–4. [DOI] [PubMed] [Google Scholar]
- [20].Kimberly WT, Wu O, Arsava EM, Garg P, Ji R, Vangel M, et al. Lower hemoglobin correlates with larger stroke volumes in acute ischemic stroke. Cerebrovasc Dis Extra. 2011;1:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ay H, Arsava EM, Rosand J, Furie KL, Singhal AB, Schaefer PW, et al. Severity of leukoaraiosis and susceptibility to infarct growth in acute stroke. Stroke. 2008;39:1409–13. [DOI] [PubMed] [Google Scholar]
- [22].Gokcay F, Arsava EM, Baykaner T, Vangel M, Garg P, Wu O, et al. Age-dependent susceptibility to infarct growth in women. Stroke. 2011;42:947–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wu O, Koroshetz WJ, Ostergaard L, Buonanno FS, Copen WA, Gonzalez RG, et al. Predicting tissue outcome in acute human cerebral ischemia using combined diffusion- and perfusion-weighted MR imaging. Stroke. 2001;32:933–42. [DOI] [PubMed] [Google Scholar]
- [24].Mohr JP, Biller J, Hilal SK, Yuh WT, Tatemichi TK, Hedges S, et al. Magnetic resonance versus computed tomographic imaging in acute stroke. Stroke. 1995;26:807–12. [DOI] [PubMed] [Google Scholar]
- [25].Ay H, Arsava EM, Vangel M, Oner B, Zhu M, Wu O, et al. Interexaminer difference in infarct volume measurements on MRI: a source of variance in stroke research. Stroke. 2008;39:1171–6. [DOI] [PubMed] [Google Scholar]
- [26].Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309. [DOI] [PubMed] [Google Scholar]
- [27].Ay H, Benner T, Arsava EM, Furie KL, Singhal AB, Jensen MB, et al. A computerized algorithm for etiologic classification of ischemic stroke: the Causative Classification of Stroke System. Stroke. 2007;38:2979–84. [DOI] [PubMed] [Google Scholar]
- [28].Sorensen AG, Wu O, Copen WA, Davis TL, Gonzalez RG, Koroshetz WJ, et al. Human acute cerebral ischemia: detection of changes in water diffusion anisotropy by using MR imaging. Radiology. 1999;212:785–92. [DOI] [PubMed] [Google Scholar]
- [29].Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med. 2018;378:708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Group MRSC, Phan TG, Donnan GA, Davis SM, Byrnes G. Proof-of-principle phase II MRI studies in stroke: sample size estimates from dichotomous and continuous data. Stroke. 2006;37:2521–5. [DOI] [PubMed] [Google Scholar]
- [31].Donnan GA, Baron JC, Ma H, Davis SM. Penumbral selection of patients for trials of acute stroke therapy. Lancet Neurol. 2009;8:261–9. [DOI] [PubMed] [Google Scholar]
- [32].Warach S, Pettigrew LC, Dashe JF, Pullicino P, Lefkowitz DM, Sabounjian L, et al. Effect of citicoline on ischemic lesions as measured by diffusion-weighted magnetic resonance imaging. Citicoline 010 Investigators. Ann Neurol. 2000;48:713–22. [PubMed] [Google Scholar]
- [33].Ogata T, Christensen S, Nagakane Y, Ma H, Campbell BC, Churilov L, et al. The effects of alteplase 3 to 6 hours after stroke in the EPITHET-DEFUSE combined dataset: post hoc case-control study. Stroke. 2013;44:87–93. [DOI] [PubMed] [Google Scholar]
- [34].Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. 2018;378:11–21. [DOI] [PubMed] [Google Scholar]
- [35].Chen CJ, Ding D, Starke RM, Mehndiratta P, Crowley RW, Liu KC, et al. Endovascular vs medical management of acute ischemic stroke. Neurology. 2015;85:1980–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Parsons MW, Barber PA, Desmond PM, Baird TA, Darby DG, Byrnes G, et al. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol. 2002;52:20–8. [DOI] [PubMed] [Google Scholar]
- [37].Desilles JP, Syvannarath V, Ollivier V, Journe C, Delbosc S, Ducroux C, et al. Exacerbation of Thromboinflammation by Hyperglycemia Precipitates Cerebral Infarct Growth and Hemorrhagic Transformation. Stroke. 2017;48:1932–40. [DOI] [PubMed] [Google Scholar]
- [38].Ay H, Arsava EM, Gungor L, Greer D, Singhal AB, Furie KL, et al. Admission international normalized ratio and acute infarct volume in ischemic stroke. Ann Neurol. 2008;64:499–506. [DOI] [PubMed] [Google Scholar]
- [39].Quartermain D, Li YS, Jonas S. The low molecular weight heparin enoxaparin reduces infarct size in a rat model of temporary focal ischemia. Cerebrovasc Dis. 2003;16:346–55. [DOI] [PubMed] [Google Scholar]
- [40].Saver JL. Clinical impact of NXY-059 demonstrated in the SAINT I trial: derivation of number needed to treat for benefit over entire range of functional disability. Stroke. 2007;38:1515–8. [DOI] [PubMed] [Google Scholar]
- [41].Leslie-Mazwi TM, Hirsch JA, Falcone GJ, Schaefer PW, Lev MH, Rabinov JD, et al. Endovascular Stroke Treatment Outcomes After Patient Selection Based on Magnetic Resonance Imaging and Clinical Criteria. JAMA Neurol. 2016;73:43–9. [DOI] [PubMed] [Google Scholar]
- [42].Shuaib A, Butcher K, Mohammad AA, Saqqur M, Liebeskind DS. Collateral blood vessels in acute ischaemic stroke: a potential therapeutic target. Lancet Neurol. 2011;10:909–21. [DOI] [PubMed] [Google Scholar]