Abstract
We addressed the relationship between white matter architecture, represented by MRI fractional anisotropy (FA), and cognition in individuals with first-episode psychosis (FEP) by applying for a new methodology that allows whole brain parcellation of core and peripheral white matter in a biologically meaningful fashion. Regionally specific correlations were found in FEP between three specific domains of cognition (processing speed, attention/working memory, and executive functioning) and FA at the deep (cerebral peduncles, sagittal striatum, uncinate, internal/external capsule, cingulum) and peripheral white matter (adjacent to inferior temporal, angular, supramarginal, insula, occipital, rectus gyrus).
Keywords: DTI, first-episode psychosis, schizophrenia, cognition, MRI
1. Introduction
Abnormalities in diffusion tensor images (DTI) have been reported in patients with psychotic disorders, such as Schizophrenia (SZ) (Cheung et al., 2008; Mitelman et al., 2007; Perez-Iglesias et al., 2010a; Price et al., 2007; Schmidt et al., 2015; Wang et al., 2011; Whitford et al., 2010). Decreases in fractional anisotropy (FA) have been described in major tracts and widespread areas (Kelly et al., 2017; Oestreich et al., 2017). These changes are observed in patients with psychosis in early disease stages (Lee et al., 2012) and non-medicated patients (Cheung et al., 2008; Lei et al., 2015). Furthermore, many studies have reported associations between the white matter microstructure and cognition in psychotic patients (Alloza et al., 2016; Karbasforoushan et al., 2015; Nazeri et al., 2013; Perez-Iglesias et al., 2010b).
Nevertheless, there were methodological limitations in studying specific white matter regions and structures. Studies focusing on tracts of interest (Alloza et al., 2016; Karbasforoushan et al., 2015; Nazeri et al., 2013; Perez-Iglesias et al., 2010b) suffer from the limitations of tract-tracing and population variability. Voxel-based hypothesis-free studies suffer from poor signal-to-noise ratio and imperfections in spatial normalization, particularly in the peripheral white matter (Karlsgodt et al., 2009; Kochunov et al., 2017; Kuswanto et al., 2012).
To address these limitations, we recently developed a novel method in automated brain segmentation and quantification for biologically meaningful regions of interest (Miller and Qiu, 2009; Mori et al., 2009; Tang et al., 2014). This method can be applied for the whole white matter, including the, usually neglected, peripheral association areas. This initial reduction in the dimensions of the (voxel-based) neuroimaging data increases the signal-to-noise ratio and the statistical power (Faria, 2017; Miller et al., 1997; Miller et al., 2013).
In this study, we examined white matter anisotropy of patients with first episode of psychosis (FEP) using this novel automated atlas-based segmentation method. Furthermore, we assessed the association of white matter anisotropy with cognitive changes.
2. Materials and Methods
2.1. Cohort
Individuals with FEP, as well as neurologically and psychologically healthy participants, were recruited by the Johns Hopkins Schizophrenia Center. Details about the recruitment, inclusion and exclusion criteria, demographics, and clinical features can be found elsewhere (Kamath, 2018; Kamath et al., 2018). In this study, we included individuals with FEP (n=82) [SZ (n=45), schizoaffective disorder (n=13), bipolar disorder with psychotic features (n=19), major depressive disorder with psychotic features (n=5)] and 93 healthy controls.
2.2. Neuropsychological Evaluation
A complete clinical and neuropsychological evaluation was performed. The cognitive scores were scaled in normally distributed standardized units, and grouped by “factor scores” into: 1) processing speed (calculated from the combined scores of the Grooved Pegboard test and the Salthouse test); 2) attention / working memory (Digit Span and Brief Attention Memory test); 3) verbal learning and memory (Hopkins Verbal Learning test); 4) visual learning and memory (Brief Visuospatial Memory test); 5) ideational fluency (Ideational Fluency assessment for Word Fluency and Acceptable Designs); and 6) executive functioning (Modified Wisconsin Card Sorting test). “Adjusted” scores were calculated after adjusting for age, gender, and race.
2.3. MRI and imaging processing
The MRI was obtained in the same day as the neuropsychological evaluation, on a Phillips 3T scanner. The diffusion tensor imaging (DTI) parameters were: axial orientation; TR/TE=2000/30 ms; 32 gradients; b factor=1000; voxel size=0.8281×0.8281×2.2 mm; 70 slices. The DTI was automatically processed in MRICloud (www.MRICloud.org), a public web-based service for multi-contrast, multi-atlas imaging segmentation and quantification (Mori et al., 2016). Each individual was represented by a vector of FA values in 96 brain regions, as defined by (Mori et al., 2008; Oishi et al., 2009; Oishi et al., 2011) (see supplemental material).
2.4. Statistical Analysis
After confirming the normal distribution of FA values with Shapiro-Wilk test and Q-Q plots, we used t-test to compare the global and regional FA between groups matched by age, gender, and race. Groups were defined as healthy controls, FEP, and two FEP subgroups: individuals with schizophrenia and schizoaffective disorders (S-FEP) and those with major depressive disorder and bipolar disorder with psychotic features (M-FEP). This was based on previous studies and two recent meta-analyses (Grossman et al., 1991; Maj et al., 1991; Pagel et al., 2013; Pini et al., 2001; Radomsky et al., 1999; Rink et al., 2016; Tsuang and Coryell, 1993) that found patients with schizoaffective disorders have illness characteristics similar to patients with schizophrenia, in comparison with patients with bipolar disorder or major depressive disorder with psychotic features (M-FEP).
Using linear models, we evaluated the relationship between white matter FA and the six cognitive factors in FEP group and subgroups, and controls. Significance was considered when the p-value corrected for multiple comparisons (FDR), as well as a permutation test (1000-folds), was lower than 0.1 (0.05 at one-tail regression). We chose a one-tail regression based on the previously reported positive correlation between FA and cognition (Kochunov et al., 2017). Correlations were declared significant only if they met the criteria above when using BOTH the non-adjusted and the age-, gender-, and race-adjusted cognitive scores.
For the significant relationships, we tested whether the partial correlation between FA and cognition remained significant after adjusting age, gender, race, and antipsychotic medication. Finally, we conducted interaction analysis to investigate the difference in slopes between groups (controls vs. FEP group and subgroups).
3. Results
3.1. Cohort
Controls and S-FEP differed in gender, reflecting the prevalence of the diseases (table 1). SFEP and M-FEP differed in gender and race, but not in antipsychotic medication dosages, converted to chlorpromazine equivalents using published reference tables (Woods, 2003). Information about education level, handiness, disease stage, and non-antipsychotic medications was not fully quantitatively available; therefore these factors were not included in our analysis, which is a limitation of this study.
Table 1:
Demographic and neuropsychological summary
| HC (n=93) |
FEP (n=82) |
S-FEP (n=58) |
M-FEP (n=24) |
HC x FEP | HC x S-FEP | HC x M-FEP | S-FEP x M-FEP | ||
|---|---|---|---|---|---|---|---|---|---|
| Antipsychotic dose* | 356.9±285.9 | 368.7±303.7 | 332.2±248.3 | 0.6 | |||||
| Processing speed | |||||||||
| adjusted | 108.8±15.1 | 87.9±19.0 | 90.9±18.4 | 108.5±4.8 | <0.0001 | <0.0001 | <0.0001 | 0.223 | |
| Attention / working memory | |||||||||
| adjusted | 104.1±14.5 | 87.4±18.2 | 94.4±15.7 | 102.5±12.6 | <0.0001 | <0.0001 | 0.007 | 0.01 | |
| Verbal learning memory | |||||||||
| adjusted | 103.8±14.3 | 87.5±16.4 | 92.5±15.4 | 89.2±18.9 | <0.0001 | <0.0001 | 0.003 | 0.049 | |
| Visual learning memory | |||||||||
| adjusted | 103.5±14.1 | 88.6±17.4 | 92.3±18.7 | 92.5±13.6 | <0.0001 | <0.0001 | 0.026 | 0.226 | |
| Ideational fluency | |||||||||
| adjusted | 111.9±12.0 | 95.4±16.8 | 101.6±16.9 | 93.7±15.2 | <0.0001 | <0.0001 | 0.008 | 0.045 | |
| Executive functioning | |||||||||
| adjusted | 101.8±13.6 | 88.5±17.5 | 94.8±13.3 | 91.7±24.4 | <0.0001 | <0.0001 | 0.08 | 0.017 | |
Race codes: aa: african american, as: asian, c: caucasian, h: hispanic, o: others
S-FEP: schizophrenia and schizoaffective disorders; M-FEP: major depression and bipolar disorder with psychiatric features. HC: healthy controls. Adjusted / no adj. refers to adjustment of cognitive scores for age, gender, and race
Antipsychotic medication dosage information was unavailable for six patients.
3.2. Neuropsychological Evaluation
FEP patients scored lower than controls in all neurocognitive domains with the exception of executive functioning in which M-FEP patients did not score significantly different from controls. S-FEP scored lower than M-FEP in all cognitive scores, except for visual learning and memory, and processing speed (Table1).
3.3. Group differences in FA between FEP and controls
Compared with controls, FEP patients had significantly lower FA in the global white matter. In particular, the FEP group and the S-FEP subgroup showed lower FA than controls in the subsegments of the projection fibers (at the pons level, cerebral peduncle, internal capsule), main commissural fibers (corpus callosum), association pathways (anterior corona radiata and inferior occipital-frontal fasciculus); and higher FA in the caudate, a deep gray matter nucleus (Table 2). The M-FEP group showed similar trends, although some areas were not significantly different from controls (table 2).
Table 2:
Differences in fractional anisotropy (FA) between FEP (and subgroups) and controls (HC), paired by age, gender, and race.
| mean FA | p-value (p-multiple comparisons corrected) | ||||||
|---|---|---|---|---|---|---|---|
| HC | FEP | S-FEP | M-FEP | HC x FEP | HC x S-FEP | HC x M-FEP | |
| Cerebral peduncles | 0.670 | 0.653 | 0.654 | 0.649 | <0.0001(<0.0001) | <0.0001(<0.0001) | <0.0001(<0.0001) |
| Corpus callosum | 0.610 | 0.593 | 0.597 | 0.585 | <0.0001(<0.0001) | <0.0001 (0.002) | <0.0001(<0.0001) |
| Projection fibers at pons level | 0.552 | 0.539 | 0.536 | 0.545 | <0.0001(<0.0001) | <0.0001(<0.0001) | 0.084 (0.3) |
| Caudate | 0.220 | 0.233 | 0.236 | 0.223 | <0.0001 (0.003) | <0.0001 (0.002) | 0.412(0.6) |
| Internal capsule | 0.636 | 0.626 | 0.626 | 0.628 | <0.0001 (0.003) | 0.001 (0.007) | 0.003 (0.045) |
| Inferior occipital-frontal fasciculus | 0.445 | 0.435 | 0.438 | 0.430 | 0.003 (0.022) | 0.042 (0.2) | 0.006 (0.05) |
| Anterior corona radiata | 0.433 | 0.427 | 0.428 | 0.425 | 0.02 (0.048) | 0.045 (0.3) | 0.029(0.16) |
| Total white matter | 0.456 | 0.452 | 0.453 | 0.450 | 0.005 (0.025) | 0.047 (0.2) | 0.005 (0.05) |
3.4. Correlations between FA and cognition in FEP
Next, we studied the relationship between changes in white matter and cognitive manifestations. In FEP patients, the global white matter FA, measured by averaging all segmented areas, was positively correlated with the scores for specific domains of cognitive function, such as those of processing speed (p-value=0.005, non-adjusted score; p=0.009, adjusted score) and attention / working memory (p-value=0.029, non-adjusted score; p=0.028, adjusted score). These correlations were not observed in healthy controls.
The FEP group showed regionally-specific correlations between processing speed and white matter FA in the cerebral peduncles, the inferior temporal, the angular, and the supramarginal gyrus. Furthermore, the FEP group showed correlations between attention / working memory and the white matter FA in the occipital, the sagittal striatum, the uncinate fasciculus, and the external capsule / insula (Table 3).
Table 3:
Summary of correlations between cognitive tests and fractional anisotropy (FA) in FEP, S-FEP, and healthy controls (HC). Correlations were accessed using the cognitive test scores, (“cog. score”), as well as the scores adjusted for age, gender, and race (“adj. cog. score”). “R2adj” and “P” are the r squared adjusted and p-value for the linear model fitting the correlation between regional FA and cognition; “P-mv” is the p-value for the partial correlation between regional FA and cognition, including age, race, gender, and antipsychotic dosage as covariates; “P-gr.sl.” is the p-value for the difference between the group slopes (S-FEP vs. controls, or FEP vs. controls).
| Processing Speed | Attention / Working Memory | Executive Functioning | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cerebral peduncle | Inferior temporal | Angular left | Supramarginal Left | Superior occipital | Inferior occipital | Sagittal striatum | External capsule / Insula | Uncinate right | Cingulum left | Rectus left | Retrolenticular int. capsule right | Sagittal striatum right | |||
| S-FEP | cog. score | R2adj | 0.164 | 0.121 | 0.155 | 0.153 | 0.174 | 0.135 | 0.14 | 0.124 | 0.093 | 0.146 | 0.075 | 0.118 | 0.159 |
| P | 0.001 | 0.004 | 0.001 | 0.001 | 0.001 | 0.003 | 0.002 | 0.004 | 0.012 | 0.002 | 0.021 | 0.005 | 0.001 | ||
| P-mv | 0.001 | 0.01 | 0.0002 | 0.002 | 0.001 | 0.004 | 0.0004 | 0.0009 | 0.021 | 0.0003 | 0.002 | 0.0009 | 0.001 | ||
| P-gr.sl. | 0.003 | 0.005 | 0.012 | 0.016 | 0.003 | 0.004 | 0.005 | 0.006 | 0.43 | 0.006 | 0.089 | 0.18 | 0.051 | ||
| adj. cog. score | R2adj | 0.216 | 0.146 | 0.185 | 0.121 | 0.148 | 0.092 | 0.134 | 0.147 | 0.103 | 0.122 | 0.095 | 0.101 | 0.132 | |
| P | 0.0001 | 0.002 | 0.0005 | 0.004 | 0.002 | 0.012 | 0.003 | 0.002 | 0.008 | 0.004 | 0.011 | 0.009 | 0.003 | ||
| P-mv | 0.0007 | 0.008 | 0.0001 | 0.005 | 0.003 | 0.005 | 0.0004 | 0.0005 | 0.012 | 0.0004 | 0.004 | 0.0005 | 0.0008 | ||
| P-gr.sl. | 0.003 | 0.002 | 0.002 | 0.004 | 0.029 | 0.002 | 0.025 | 0.005 | 0.09 | 0.018 | 0.042 | 0.06 | 0.064 | ||
| FEP | cog. score | R2adj | 0.129 | 0.085 | 0.088 | 0.111 | 0.133 | 0.066 | 0.049 | 0.117 | 0.094 | 0.107 | 0.039 | 0.07 | 0.049 |
| P | 0.0002 | 0.004 | 0.003 | 0.001 | 0.0007 | 0.01 | 0.023 | 0.001 | 0.002 | 0.001 | 0.038 | 0.008 | 0.023 | ||
| P-mv | 0.0002 | 0.004 | 0.001 | 0.0009 | 0.0006 | 0.006 | 0.004 | 0.0007 | 0.0146 | 0.0005 | 0.02 | 0.003 | 0.048 | ||
| P-gr.sl. | 0.003 | 0.015 | 0.04 | 0.049 | 0.011 | 0.026 | 0.04 | 0.008 | 0.43 | 0.01 | 0.222 | 0.286 | 0.334 | ||
| adj. cog. score | R2adj | 0.145 | 0.085 | 0.091 | 0.072 | 0.11 | 0.039 | 0.044 | 0.122 | 0.073 | 0.109 | 0.06 | 0.068 | 0.032 | |
| P | 0.0001 | 0.004 | 0.003 | 0.007 | 0.001 | 0.039 | 0.029 | 0.001 | 0.007 | 0.001 | 0.013 | 0.009 | 0.038 | ||
| P-mv | 0.0002 | 0.003 | 0.001 | 0.003 | 0.001 | 0.007 | 0.003 | 0.005 | 0.009 | 0.0005 | 0.022 | 0.003 | 0.048 | ||
| P-gr.sl. | 0.008 | 0.009 | 0.01 | 0.022 | 0.016 | 0.009 | 0.034 | 0.0004 | 0.137 | 0.027 | 0.181 | 0.127 | 0.377 | ||
| HC | cog. score | R2adj | −0.011 | −0.01 | −0.004 | −0.011 | −0.01 | −0.005 | −0.01 | −0.009 | 0.021 | −0.005 | −0.006 | 0.028 | 0.001 |
| P | 0.93 | 0.764 | 0.446 | 0.955 | 0.74 | 0.458 | 0.835 | 0.686 | 0.089 | 0.815 | 0.486 | 0.058 | 0.31 | ||
| P-mv | 0.72 | 0.651 | 0.465 | 0.557 | 0.588 | 0.539 | 0.848 | 0.204 | 0.512 | 0.822 | 0.408 | 0.125 | 0.474 | ||
| adj. cog. score | R2adj | −0.01 | −0.011 | −0.005 | −0.004 | −0.01 | 0.01 | −0.009 | 0.017 | −0.007 | −0.001 | −0.001 | 0.005 | −0.003 | |
| P | 0.754 | 0.862 | 0.477 | 0.436 | 0.818 | 0.163 | 0.719 | 0.108 | 0.54 | 0.629 | 0.344 | 0.233 | 0.395 | ||
| P-mv | 0.746 | 0.607 | 0.556 | 0.43 | 0.523 | 0.769 | 0.95 | 0.154 | 0.582 | 0.838 | 0.447 | 0.163 | 0.514 | ||
The partial correlations between regional FA and function remained significant after inclusion of age, race, gender, and antipsychotic dosage in the models (Table 3, “P-mv”). Generally, the slopes of the linear models were significantly different in FEP and controls (Table 3, “P-gr.sl.”).
3.5. Correlations between FA and cognition in FEP subgroups
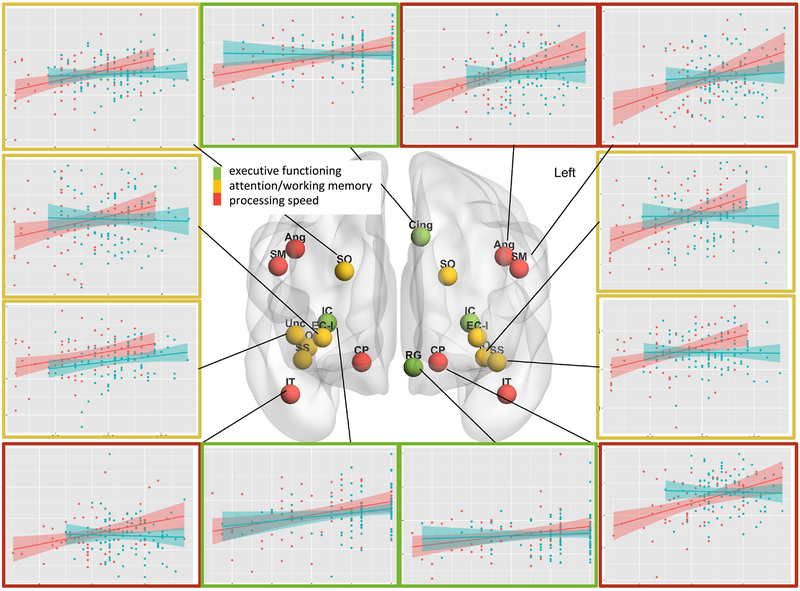
All significant correlations were stronger in the S-FEP sub-group than the whole FEP group (Table 3 and Figure 1). The S-FEP, but not the whole FEP group, displayed regional correlations between executive memory and white matter FA in the left cingullum, the rectus gyrus, the internal capsule, and the sagittal striatum (Table 3 and Figure 1).
Figure 1:
Correlations between regional fractional anisotropy and cognitive scores. The type of cognitive test is color-coded on the spheres overlaid in the anatomical areas on the glass brain, and in the scatterplot frames (red: processing speed, yellow: attention / working memory, green: executive functioning). In the scatterplots, red are S-FEP participants and blue are controls; the shadow represents the 95% interval for the linear fitting (line); y-axis is FA [0.35–0.5]; x-axis is cognitive score [60–130]. Significant correlations between fractional anisotropy and cognition were found in patient’s group, but not in controls, in the white matter adjacent to the following gyrus: angular (Ang), supramarginal (SM), superior and inferior occipital (SO, IO), fusiform (Fu), middle and inferior temporal (MT, IT), rectus (RG), superior frontal (SF), and insula, which was considered in combination with external capsula (EC-I), as well as in the internal capsule (IC), sagittal striatum (SS), and cerebral peduncle (CP). The brain is visualized with the BrainNet Viewer (http://www.nitrc.org/projects/bnv/).
The partial correlations between regional FA and function remained significant after inclusion of age, race, gender, and antipsychotic dosage in the models (Table 3, “P-mv”). In general, the slopes of the linear models were significantly different in S-FEP and controls (Table 3, “P-gr.sl.”).
There was no significant correlation between FA and cognition in the M-FEP group. Note, the M-FEP sample size limited the power of the correlational analysis.
4. Discussion
As in previous studies (Kuswanto et al., 2012) and a meta-analysis of patients with chronic SZ, we found widespread FA decrease in FEP, and a strong correlation between processing speed and executive / working memory in the core white matter and deep white matter tracts (Kochunov et al., 2017). The corroboration of these findings attests to the high power of our approach, as we used a sample size much smaller than the previous meta-analysis.
Unlike previous studies, this novel methodology enabled extension the analysis from the core to the peripheral white matter association areas, in a data-driven approach, and to report associations not previously described in FEP patients. We found that processing speed tests, driven by both motor coordination (speed of navigating the board) and semantic performance (reading and understanding the task), correlated with FA in motor (cerebral peduncles) and language processing areas (inferior temporal, angular, and supramarginal gyrus). Attention / working memory correlated with FA in the core white matter (sagittal striatum, external capsule / insula), the uncinate fasciculus (considered a locus of episodic memory and a zone of DTI abnormalities in patients with schizophrenia (Burns et al., 2003; Kitis et al., 2012; Kubicki et al., 2005; Marin et al., 2017; McIntosh et al., 2008; Price et al., 2008; Voineskos et al., 2010; Von Der Heide et al., 2013; Wilmsmeier et al., 2010), and the occipital area. Executive function correlated with FA in the white matter adjacent to frontal, rectus gyrus, and cingullum, which were previously identified as neural correlates of executive function by fMRI (Wilmsmeier et al., 2010).
The correlations between regional FA and cognition were stronger in S-FEP than in the whole FEP group, which highlights the importance of population stratification. While we did not observe such relationships in controls, previous studies using other methodologies, elderly populations, and diseases with higher effect size demonstrated similar function-anatomical relations (Aukema et al., 2009; Cacciaglia et al., 2018; Gu et al., 2013; Jirsaraie et al., 2018; Jung et al., 2012; Sasson et al., 2012, 2013; Tartaglia et al., 2012; Turken et al., 2008; Williams et al., 2017). Therefore, although such relations may not be FEP-specific, FEP patients (in particular S-FEP) present a large range of FA and cognition scores that increases the power to detect anatomic-functional links, indirectly pointing to FA as a candidate functional biomarker.
Supplementary Material
Acknowledgements
We thank study participants and the recruitment team staff members led by Ms. Yukiko Lema.
Funding
This work was supported by NIH (MH-094268, MH-092443, MH-105660, and MH-107730), as well as foundation grants of Stanley, RUSK/S-R, NARSAD/BBRF to AS. A part of the recruitment cost was also supported by Mitsubishi Tanabe Pharm. Co. Ltd. This work represents the author’s view, independently of the funding sources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest.
References
- Alloza C, Cox SR, Duff B, Semple SI, Bastin ME, Whalley HC, Lawrie SM, 2016. Information processing speed mediates the relationship between white matter and general intelligence in schizophrenia. Psychiatry research. Neuroimaging 254, 26–33. [DOI] [PubMed] [Google Scholar]
- Aukema EJ, Caan MW, Oudhuis N, Majoie CB, Vos FM, Reneman L, Last BF, Grootenhuis MA, Schouten-van Meeteren AY, 2009. White matter fractional anisotropy correlates with speed of processing and motor speed in young childhood cancer survivors. International journal of radiation oncology, biology, physics 74(3), 837–843. [DOI] [PubMed] [Google Scholar]
- Burns J, Job D, Bastin ME, Whalley H, Macgillivray T, Johnstone EC, Lawrie SM, 2003. Structural disconnectivity in schizophrenia: a diffusion tensor magnetic resonance imaging study. The British journal of psychiatry : the journal of mental science 182, 439–443. [PubMed] [Google Scholar]
- Cacciaglia R, Molinuevo JL, Sanchez-Benavides G, Falcon C, Gramunt N, Brugulat-Serrat A, Grau O, Gispert JD, 2018. Episodic memory and executive functions in cognitively healthy individuals display distinct neuroanatomical correlates which are differentially modulated by aging. Human brain mapping 39(11), 4565–4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung V, Cheung C, McAlonan GM, Deng Y, Wong JG, Yip L, Tai KS, Khong PL, Sham P, Chua SE, 2008. A diffusion tensor imaging study of structural dysconnectivity in never-medicated, first-episode schizophrenia. Psychological medicine 38(6), 877–885. [DOI] [PubMed] [Google Scholar]
- Faria AV, Liang Z, Miller MI, Mori S, 2017. Brain MRI Pattern Recognition Translated to Clinical Scenarios. Frontiers in neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman LS, Harrow M, Goldberg JF, Fichtner CG, 1991. Outcome of schizoaffective disorder at two long-term follow-ups: comparisons with outcome of schizophrenia and affective disorders. The American journal of psychiatry 148(10), 1359–1365. [DOI] [PubMed] [Google Scholar]
- Gu L, Li J, Feng DF, Cheng ET, Li DC, Yang XQ, Wang BC, 2013. Detection of white matter lesions in the acute stage of diffuse axonal injury predicts long-term cognitive impairments: a clinical diffusion tensor imaging study. The journal of trauma and acute care surgery 74(1), 242–247. [DOI] [PubMed] [Google Scholar]
- Jirsaraie RJ, Sheffield JM, Barch DM, 2018. Neural correlates of global and specific cognitive deficits in schizophrenia. Schizophrenia research 201, 237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Chavez RS, Flores RA, Qualls C, Sibbitt WL Jr., Roldan CA, 2012. White matter correlates of neuropsychological dysfunction in systemic lupus erythematosus. PloS one 7(1), e28373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath V, Crawford J, DuBois S, Nucifora FC Jr., Nestadt G, Sawa A, Schretlen DJ, 2018. Contributions of olfactory and neuropsychological assessment to the diagnosis of first-episode schizophrenia. Neuropsychology in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath V, Lasutschinkow P, Ishizuka K, Sawa A, 2018. Olfactory Functioning in First-Episode Psychosis. Schizophrenia bulletin 44(3), 672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbasforoushan H, Duffy B, Blackford JU, Woodward ND, 2015. Processing speed impairment in schizophrenia is mediated by white matter integrity. Psychological medicine 45(1), 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, Niendam TA, Bearden CE, Cannon TD, 2009. White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biological psychiatry 66(6), 562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S, Jahanshad N, Zalesky A, Kochunov P, Agartz I, Alloza C, Andreassen OA, Arango C, Banaj N, Bouix S, Bousman CA, Brouwer RM, Bruggemann J, Bustillo J, Cahn W, Calhoun V, Cannon D, Carr V, Catts S, Chen J, Chen JX, Chen X, Chiapponi C, Cho KK, Ciullo V, Corvin AS, Crespo-Facorro B, Cropley V, De Rossi P, Diaz-Caneja CM, Dickie EW, Ehrlich S, Fan FM, Faskowitz J, Fatouros-Bergman H, Flyckt L, Ford JM, Fouche JP, Fukunaga M, Gill M, Glahn DC, Gollub R, Goudzwaard ED, Guo H, Gur RE, Gur RC, Gurholt TP, Hashimoto R, Hatton SN, Henskens FA, Hibar DP, Hickie IB, Hong LE, Horacek J, Howells FM, Hulshoff Pol HE, Hyde CL, Isaev D, Jablensky A, Jansen PR, Janssen J, Jonsson EG, Jung LA, Kahn RS, Kikinis Z, Liu K, Klauser P, Knochel C, Kubicki M, Lagopoulos J, Langen C, Lawrie S, Lenroot RK, Lim KO, Lopez-Jaramillo C, Lyall A, Magnotta V, Mandl RCW, Mathalon DH, McCarley RW, McCarthy-Jones S, McDonald C, McEwen S, McIntosh A, Melicher T, Mesholam-Gately RI, Michie PT, Mowry B, Mueller BA, Newell DT, O’Donnell P, Oertel-Knochel V, Oestreich L, Paciga SA, Pantelis C, Pasternak O, Pearlson G, Pellicano GR, Pereira A, Pineda Zapata J, Piras F, Potkin SG, Preda A, Rasser PE, Roalf DR, Roiz R, Roos A, Rotenberg D, Satterthwaite TD, Savadjiev P, Schall U, Scott RJ, Seal ML, Seidman LJ, Shannon Weickert C, Whelan CD, Shenton ME, Kwon JS, Spalletta G, Spaniel F, Sprooten E, Stablein M, Stein DJ, Sundram S, Tan Y, Tan S, Tang S, Temmingh HS, Westlye LT, Tonnesen S, Tordesillas-Gutierrez D, Doan NT, Vaidya J, van Haren NEM, Vargas CD, Vecchio D, Velakoulis D, Voineskos A, Voyvodic JQ, Wang Z, Wan P, Wei D, Weickert TW, Whalley H, White T, Whitford TJ, Wojcik JD, Xiang H, Xie Z, Yamamori H, Yang F, Yao N, Zhang G, Zhao J, van Erp TGM, Turner J, Thompson PM, Donohoe G, 2017. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Molecular psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitis O, Ozalay O, Zengin EB, Haznedaroglu D, Eker MC, Yalvac D, Oguz K, Coburn K, Gonul AS, 2012. Reduced left uncinate fasciculus fractional anisotropy in deficit schizophrenia but not in non-deficit schizophrenia. Psychiatry and clinical neurosciences 66(1), 34–43. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Coyle TR, Rowland LM, Jahanshad N, Thompson PM, Kelly S, Du X, Sampath H, Bruce H, Chiappelli J, Ryan M, Fisseha F, Savransky A, Adhikari B, Chen S, Paciga SA, Whelan CD, Xie Z, Hyde CL, Chen X, Schubert CR, O’Donnell P, Hong LE, 2017. Association of White Matter With Core Cognitive Deficits in Patients With Schizophrenia. JAMA psychiatry 74(9), 958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Park H, Westin CF, Nestor PG, Mulkern RV, Maier SE, Niznikiewicz M, Connor EE, Levitt JJ, Frumin M, Kikinis R, Jolesz FA, McCarley RW, Shenton ME, 2005. DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. NeuroImage 26(4), 1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuswanto CN, Teh I, Lee TS, Sim K, 2012. Diffusion tensor imaging findings of white matter changes in first episode schizophrenia: a systematic review. Clin Psychopharmacol Neurosci 10(1), 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY, Smith GN, Su W, Honer WG, Macewan GW, Lapointe JS, Vertinsky AT, Vila-Rodriguez F, Kopala LC, Lang DJ, 2012. White matter tract abnormalities in first-episode psychosis. Schizophrenia research 141(1), 29–34. [DOI] [PubMed] [Google Scholar]
- Lei W, Li N, Deng W, Li M, Huang C, Ma X, Wang Q, Guo W, Li Y, Jiang L, Zhou Y, Hu X, McAlonan GM, Li T, 2015. White matter alterations in first episode treatment-naive patients with deficit schizophrenia: a combined VBM and DTI study. Scientific reports 5, 12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maj M, Starace F, Pirozzi R, 1991. A family study of DSM-III-R schizoaffective disorder, depressive type, compared with schizophrenia and psychotic and nonpsychotic major depression. The American journal of psychiatry 148(5), 612–616. [DOI] [PubMed] [Google Scholar]
- Marin D, Madotto E, Fabbro F, Skrap M, Tomasino B, 2017. Design fluency and neuroanatomical correlates in 54 neurosurgical patients with lesions to the right hemisphere. Journal of neuro-oncology 135(1), 141–150. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Munoz Maniega S, Lymer GK, McKirdy J, Hall J, Sussmann JE, Bastin ME, Clayden JD, Johnstone EC, Lawrie SM, 2008. White matter tractography in bipolar disorder and schizophrenia. Biological psychiatry 64(12), 1088–1092. [DOI] [PubMed] [Google Scholar]
- Miller M, Banerjee A, Christensen G, Joshi S, Khaneja N, Grenander U, Matejic L, 1997. Statistical methods in computational anatomy. Statistical methods in medical research 6(3), 267–299. [DOI] [PubMed] [Google Scholar]
- Miller MI, Faria AV, Oishi K, Mori S, 2013. High-throughput neuro-imaging informatics. Frontiers in neuroinformatics 7, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MI, Qiu A, 2009. The emerging discipline of Computational Functional Anatomy. NeuroImage 45(1 Suppl), S16–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitelman SA, Torosjan Y, Newmark RE, Schneiderman JS, Chu KW, Brickman AM, Haznedar MM, Hazlett EA, Tang CY, Shihabuddin L, Buchsbaum MS, 2007. Internal capsule, corpus callosum and long associative fibers in good and poor outcome schizophrenia: a diffusion tensor imaging survey. Schizophrenia research 92(1–3), 211–224. [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Faria AV, 2009. White matter atlases based on diffusion tensor imaging. Curr Opin Neurol 22(4), 362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J, 2008. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage 40(2), 570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Wu D, Ceritoglu C, Li Y, Kolasny A, Valliant MA, faria AV, Oishi K, Miller MI, 2016. MRICloud: Delivering High-Throughput MRI Neuroinformatics as Cloud-Based Software as a Service. Comput. Sci. Eng 18(21), 15. [Google Scholar]
- Nazeri A, Chakravarty MM, Felsky D, Lobaugh NJ, Rajji TK, Mulsant BH, Voineskos AN, 2013. Alterations of superficial white matter in schizophrenia and relationship to cognitive performance. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 38(10), 1954–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreich LK, Lyall AE, Pasternak O, Kikinis Z, Newell DT, Savadjiev P, Bouix S, Shenton ME, Kubicki M, Whitford TJ, McCarthy-Jones S, 2017. Characterizing white matter changes in chronic schizophrenia: A free-water imaging multi-site study. Schizophrenia research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Faria A, Jiang H, Li X, Akhter K, Zhang J, Hsu JT, Miller MI, van Zijl PC, Albert M, Lyketsos CG, Woods R, Toga AW, Pike GB, Rosa-Neto P, Evans A, Mazziotta J, Mori S, 2009. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer’s disease participants. NeuroImage 46(2), 486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Faria AV, van Ziji PCM, Mori S, 2011. MRI atlas of human white matter, Second ed. Elsevier. [Google Scholar]
- Pagel T, Baldessarini RJ, Franklin J, Baethge C, 2013. Characteristics of patients diagnosed with schizoaffective disorder compared with schizophrenia and bipolar disorder. Bipolar Disord 15(3), 229–239. [DOI] [PubMed] [Google Scholar]
- Perez-Iglesias R, Tordesillas-Gutierrez D, Barker GJ, McGuire PK, Roiz-Santianez R, Mata I, de Lucas EM, Quintana F, Vazquez-Barquero JL, Crespo-Facorro B, 2010a. White matter defects in first episode psychosis patients: a voxelwise analysis of diffusion tensor imaging. NeuroImage 49(1), 199–204. [DOI] [PubMed] [Google Scholar]
- Perez-Iglesias R, Tordesillas-Gutierrez D, McGuire PK, Barker GJ, Roiz-Santianez R, Mata I, de Lucas EM, Rodriguez-Sanchez JM, Ayesa-Arriola R, Vazquez-Barquero JL, Crespo-Facorro B, 2010b. White matter integrity and cognitive impairment in first-episode psychosis. The American journal of psychiatry 167(4), 451–458. [DOI] [PubMed] [Google Scholar]
- Pini S, Cassano GB, Dell’Osso L, Amador XF, 2001. Insight into illness in schizophrenia, schizoaffective disorder, and mood disorders with psychotic features. The American journal of psychiatry 158(1), 122–125. [DOI] [PubMed] [Google Scholar]
- Price G, Cercignani M, Parker GJ, Altmann DR, Barnes TR, Barker GJ, Joyce EM, Ron MA, 2007. Abnormal brain connectivity in first-episode psychosis: a diffusion MRI tractography study of the corpus callosum. NeuroImage 35(2), 458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G, Cercignani M, Parker GJ, Altmann DR, Barnes TR, Barker GJ, Joyce EM, Ron MA, 2008. White matter tracts in first-episode psychosis: a DTI tractography study of the uncinate fasciculus. NeuroImage 39(3), 949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomsky ED, Haas GL, Mann JJ, Sweeney JA, 1999. Suicidal behavior in patients with schizophrenia and other psychotic disorders. The American journal of psychiatry 156(10), 1590–1595. [DOI] [PubMed] [Google Scholar]
- Rink L, Pagel T, Franklin J, Baethge C, 2016. Characteristics and heterogeneity of schizoaffective disorder compared with unipolar depression and schizophrenia - a systematic literature review and meta-analysis. Journal of affective disorders 191, 8–14. [DOI] [PubMed] [Google Scholar]
- Sasson E, Doniger GM, Pasternak O, Tarrasch R, Assaf Y, 2012. Structural correlates of cognitive domains in normal aging with diffusion tensor imaging. Brain structure & function 217(2), 503–515. [DOI] [PubMed] [Google Scholar]
- Sasson E, Doniger GM, Pasternak O, Tarrasch R, Assaf Y, 2013. White matter correlates of cognitive domains in normal aging with diffusion tensor imaging. Frontiers in neuroscience 7, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Lenz C, Smieskova R, Harrisberger F, Walter A, Riecher-Rossler A, Simon A, Lang UE, McGuire P, Fusar-Poli P, Borgwardt SJ, 2015. Brain Diffusion Changes in Emerging Psychosis and the Impact of State-Dependent Psychopathology. Neuro-Signals 23(1), 71–83. [DOI] [PubMed] [Google Scholar]
- Tang X, Yoshida S, Hsu J, Huisman TA, Faria AV, Oishi K, Kutten K, Poretti A, Li Y, Miller MI, Mori S, 2014. Multi-contrast multi-atlas parcellation of diffusion tensor imaging of the human brain. PloS one 9(5), e96985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia MC, Zhang Y, Racine C, Laluz V, Neuhaus J, Chao L, Kramer J, Rosen H, Miller B, Weiner M, 2012. Executive dysfunction in frontotemporal dementia is related to abnormalities in frontal white matter tracts. Journal of neurology 259(6), 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang D, Coryell W, 1993. An 8-year follow-up of patients with DSM-III-R psychotic depression, schizoaffective disorder, and schizophrenia. The American journal of psychiatry 150(8), 1182–1188. [DOI] [PubMed] [Google Scholar]
- Turken A, Whitfield-Gabrieli S, Bammer R, Baldo JV, Dronkers NF, Gabrieli JD, 2008. Cognitive processing speed and the structure of white matter pathways: convergent evidence from normal variation and lesion studies. NeuroImage 42(2), 1032–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos AN, Lobaugh NJ, Bouix S, Rajji TK, Miranda D, Kennedy JL, Mulsant BH, Pollock BG, Shenton ME, 2010. Diffusion tensor tractography findings in schizophrenia across the adult lifespan. Brain : a journal of neurology 133(Pt 5), 1494–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR, 2013. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain : a journal of neurology 136(Pt 6), 1692–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Deng W, Huang C, Li M, Ma X, Wang Y, Jiang L, Lui S, Huang X, Chua SE, Cheung C, McAlonan GM, Sham PC, Murray RM, Collier DA, Gong Q, Li T, 2011. Abnormalities in connectivity of white-matter tracts in patients with familial and non-familial schizophrenia. Psychological medicine 41(8), 1691–1700. [DOI] [PubMed] [Google Scholar]
- Whitford TJ, Kubicki M, Schneiderman JS, O’Donnell LJ, King R, Alvarado JL, Khan U, Markant D, Nestor PG, Niznikiewicz M, McCarley RW, Westin CF, Shenton ME, 2010. Corpus callosum abnormalities and their association with psychotic symptoms in patients with schizophrenia. Biological psychiatry 68(1), 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams OA, Zeestraten EA, Benjamin P, Lambert C, Lawrence AJ, Mackinnon AD, Morris RG, Markus HS, Charlton RA, Barrick TR, 2017. Diffusion tensor image segmentation of the cerebrum provides a single measure of cerebral small vessel disease severity related to cognitive change. NeuroImage. Clinical 16, 330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmsmeier A, Ohrmann P, Suslow T, Siegmund A, Koelkebeck K, Rothermundt M, Kugel H, Arolt V, Bauer J, Pedersen A, 2010. Neural correlates of set-shifting: decomposing executive functions in schizophrenia. Journal of psychiatry & neuroscience : JPN 35(5), 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SW, 2003. Chlorpromazine equivalent doses for the newer atypical antipsychotics. The Journal of clinical psychiatry 64(6), 663–667. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.