Abstract
Background
Cardiac remodelling, following a myocardial insult, often causes progression to heart failure. The relationship between alterations in left ventricular (LV) blood flow, including kinetic energy, and remodelling is uncertain. We hypothesised that increasing derangements in LV blood flow would relate to: 1)conventional cardiac remodelling markers 2)increased levels of biochemical remodelling markers 3)altered cardiac energetics and 4)worsening patient symptoms and functional capacity.
Methods
34 dilated cardiomyopathy (DCM), 30 ischaemic cardiomyopathy (IHD) patients and 36 controls underwent magnetic resonance including 4D flow, Brain-type natriuretic peptide measurement, functional capacity assessment (6-minute walk test) and symptom quantification. A subgroup of DCM and control subjects underwent cardiac energetic assessment. LV flow was separated into four components: direct flow, retained inflow, delayed ejection flow and residual volume. Average kinetic energy throughout the cardiac cycle was calculated.
Results
Patients had reduced direct flow proportion and direct flow average kinetic energy compared to controls (P<0.0001). The residual volume proportion and residual volume average kinetic energy were increased in patients (P<0.0001). Importantly, in a multiple linear regression model to predict the patient’s 6-minute walk test, the independent predictors were age (β=-0.3015, P=0.019) and direct flow average kinetic energy (β=0.280, P=0.035, R2 model=0.466, P=0.002). In contrast, neither ejection fraction nor LV volumes were independently predictive.
Conclusions
This study demonstrates an independent predictive relationship between the direct flow average kinetic energy and a prognostic measure of functional capacity. Intra-cardiac 4D flow parameters are novel biomarkers in heart failure and may provide additive value in monitoring new therapies and predicting prognosis.
Subject terms and Keywords: Magnetic Resonance Imaging, Failure chronic, cardiac remodelling, 4D flow
Introduction
Heart failure (HF) is a global health burden with significant morbidity and mortality.1 It is a complex multifactorial syndrome that is initiated by a myocardial insult which activates cardiac remodelling; a process encompassing numerous transcriptional, cellular and architectural changes within both cardiac myocytes and surrounding extracellular structures.2 The ability of the heart to remodel in response to stimuli is important for cardiovascular adaptation in altered physiological conditions, such as pregnancy.3 However, in pathological remodelling this initially beneficial plasticity response becomes maladaptive with a propensity toward hypertrophy, ventricular dilatation, systolic dysfunction and electrophysiological changes resulting in ventricular arrhythmias and HF.2,3
Fluid dynamic studies indicate that the morphological structure of a compliant vessel is inextricably linked to the flow within it.4 Hence, as ventricular flow is altered in the early stages of remodelling5, it is probable that the flow itself can influence disease progression.4 Insights into and quantification of left ventricular (LV) blood flow and kinetic energy (KE) are now afforded by three-dimensional, time-resolved magnetic resonance imaging (“4D flow”).6 Previous studies have demonstrated altered LV flow patterns in seemingly compensated dilated cardiomyopathy (DCM) patients5, as well as higher KE in severe HF.7 However, no studies have found relationships between intra-cardiac blood flow parameters and the functional ability of HF patients.
Brain type natriuretic peptide (BNP) produced by cardiac myocytes in response to volume expansion and pressure overload is a powerful prognostic HF marker.8 Functional capacity in HF, as represented by the distance covered during a 6-minute walk test, is also a predictor of mortality and morbidity9, as is the presence of symptoms as assessed with a standardised questionnaire (Minnesota HF questionnaire).10
Cardiac phosphorus magnetic resonance spectroscopy (31P-MRS) allows non-invasive measurement of the phosphocreatine to adenosine triphosphate concentration ratio (PCr/ATP), which is a sensitive marker of myocardial energetics. Impaired myocardial energetics (decreased PCr/ATP) in DCM patients are predictive of mortality.11 However, the relationship between derangements in myocardial energetics and LV blood flow is unknown.
Much remains to be understood about cardiac remodelling2; the aim of this study was to investigate the relationship between ventricular morphology, function and blood flow during cardiac remodelling. In this study, patients were included with two of the commonest causes of HF- ischaemic heart disease (IHD) and dilated cardiomyopathy (DCM).1 We hypothesised that increasing derangements in LV blood flow would relate to: 1) conventional cardiac remodelling markers 2) increased levels of biochemical remodelling markers 3) altered cardiac energetics and 4) worsening patient symptoms and functional capacity.
Further, we hypothesised these changes to be independent of the aetiology of the myocardial damage, instead reflecting the self-propagating nature of cardiac remodelling and that 4D flow parameters would be more powerful predictors of the functional consequences of cardiac remodelling than conventional imaging parameters.
Methods
The data, analytic methods and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure, due to a lack of ethical approval to share datasets beyond the host institution’s research team.
Study population
This study was approved by the National Research Ethics Committee. Each participant gave written informed consent. 100 participants were recruited; 34 dilated cardiomyopathy (DCM), 30 ischaemic cardiomyopathy (IHD) and 36 healthy controls. See supplementary methods for inclusion/exclusion criteria.
Cardiac magnetic resonance protocol
Imaging was performed at 3.0-Tesla (Trio, Siemens Healthcare Erlangen, Germany) using a 32-channel cardiac coil. Standard cine and strain imaging were performed (see supplementary methods).12,13
4D flow acquisitions were free breathing, using a retrospectively ECG triggered, respiratory navigator gated three-dimensional, three-directional, time-resolved phase contrast MRI sequence with a 52ms measurement temporal resolution and 3x3x3mm3 voxel size, with velocity encoding 100cm/s.
CMR data analysis
LV volumes were analysed using cmr42© (Circle Cardiovascular Imaging Inc, Calgary, Canada) as previously described.12 LV sphericity index was calculated by division of the horizontal long axis (HLA) length by the maximum diameter at end-diastole.
Tagged images were analysed for mid ventricular peak systolic circumferential strain and diastolic strain rate using Cardiac Image Modeller software (CIMTag2D v7 Auckland, New Zealand).13
4D flow data analysis
LV blood flow was analysed using methodology described by Eriksson et al14; consisting of endocardial segmentation at end diastole (ED) and end systole (ES), with pathline generation from each segmented voxel. The position of pathlines at ES divides them into four functional flow components as described previously5,14; 1)direct flow: blood that enters and exits the LV in the analysed cardiac cycle; 2)retained inflow: enters the LV but does not exit during the analysed cycle; 3)delayed ejection flow: starts within the LV and exits during the analysed cycle and 4)residual volume: blood that remains in the LV for at least two cardiac cycles. Each components volume was calculated as a proportion of the total end diastolic volume. LV segmentation was performed in Segment (version 1.9R2842) and flow visualisation in EnSight (CEI Inc., NC, USA).
Each components KE was calculated throughout the cardiac cycle using KE=½▪ρblood▪Vpathline▪v2pathline where ρblood is blood density, Vpathline the blood volume represented by one pathline and vpathline the pathline velocity. The KE for each component is the sum of KE for each of its pathlines. Two different measurements of KE are reported within this study 1) KE at end diastole and 2) average KE. 1) KE at end diastole: as in previous studies, KE for each component was recorded at end-diastole, as these reflect the preservation of the inflowing KE prior to the rapid systolic ejection of blood.5 2) Average KE: this was calculated for each flow component to assess whether the inclusion of all time frames provided additional information. The average KE was calculated by adding the KE values for the entire flow component’s pathlines throughout the cardiac cycle. This summed value was then divided by thirty to reflect the average KE for that flow component per time frame. Using the average KE values, the proportion of the direct flow average KE was derived by dividing the direct flow average KE by the total average KE for all components. The same calculation was performed with the residual volume average KE to derive the proportion of the residual volume average KE. Both measures of KE (KE at ED and average KE) were additionally normalised to the end diastolic volume.
31P-MR spectroscopy
25 DCM patients and 10 controls underwent 31P MR spectroscopy at 7T (Magnetom, Siemens, Germany), as previously described by our group in controls and patients (see supplementary methods).15,16 IHD patients were not included in this sub-study due to the regionality of the LV dysfunction.
Statistical analysis
Statistics were analysed using SPSS 22 (Chicago, IL). Normality testing utilised the D’Agostino and Pearson omnibus normality test; data are presented as mean±standard deviations, unless otherwise specified. One-way ANOVA with post hoc Tukeys’s or Kruskal-Wallis H with post hoc Dunn’s multiple comparison tests were performed as appropriate. Correlation was assessed using the Pearson or Spearman method. P values <0.05 were considered significant. Multiple linear regression models were created, using stepwise entry and the dependent variable as the patient 6MWT result. Variables with P<0.05 that had the strongest relationship with 6MWT were included in the model. Linear model fit was assessed by visually checking the linearity assumption. Residuals were normally distributed. Standardised (β) values are reported.
Results
Participant characteristics
Demographic and clinical data are shown in Table 1. There were no significant differences in age or heart rate between groups. Blood pressure tended to be lower in the patients, likely reflecting HF and pharmacotherapy (supplementary Table 1). As expected, IHD and DCM patients had higher BNP levels compared to controls (P<0.0001). Mean distance walked was 20% less in DCM and 25% less in IHD patients compared to controls (P<0.0001).
Table 1. Baseline characteristics.
| Controls (n=36) |
DCM (n=34) |
IHD (n=30) |
P value DCM vs Controls | P value IHD vs Controls | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, yrs | 57±12 | 57±14 | 63±12 | 1.0 | 0.125 |
| Male, n(%) | 25(70) | 22(65) | 28(93) | 0.89 | 0.062 |
| Body mass index, kg/m2 | 25±4 | 28±4 | 28±4 | 0.04 | 0.007 |
| Systolic BP, mmHg | 134±20 | 128±18 | 120±15 | 0.375 | 0.007 |
| Diastolic BP, mmHg | 78±10 | 72±12 | 69±9 | 0.044 | 0.003 |
| Heart rate, bpm | 64±14 | 65±14 | 65±14 | 0.988 | 0.967 |
| Prognostic markers | |||||
| BNP, pmol/L | 7±5 | 51±105 | 77±108 | <0.0001 | <0.0001 |
| 6 minute walk test, m | 624±77 | 500±84 | 470±101 | <0.0001 | <0.0001 |
| Minnesota heart failure questionnaire | - | 18±19 | 22±22 | - | - |
Values are mean ± standard deviations or percentages. BNP indicates brain natriuretic peptide; BP, blood pressure; bpm, beats per minute; DCM, dilated cardiomyopathy; IHD, ischaemic heart disease; yrs, years.
Myocardial structure and function
Results for LV volumes and function are summarised in Table 2. The two patient groups, as expected, had significantly increased LV volumes and decreased systolic function compared to controls (P<0.0001). Both patient groups had a more spherical ventricle with impaired systolic strain compared to controls (P<0.0001). There were no significant differences between the patient groups.
Table 2. CMR results in controls, IHD patients and DCM patients.
| Variable | Controls (n=36) |
DCM (n=34) |
IHD (n=30) |
P value DCM vs Controls | P value IHD vs Controls |
|---|---|---|---|---|---|
| LV end diastolic volume, ml | 159±31 | 273±118 | 231±68 | <0.0001 | <0.0001 |
| LV EDV indexed BSA, ml/m2 | 82±14 | 135±52 | 116±33 | <0.0001 | <0.0001 |
| LV end systolic volume, ml | 53±13 | 182±108 | 146±65 | <0.0001 | <0.0001 |
| LV stroke volume, ml | 106±20 | 90±25 | 85±22 | 0.012 | 0.001 |
| LV ejection fraction, % | 67±4 | 36±11 | 39±12 | <0.0001 | <0.0001 |
| Cardiac output, L/min | 6.7 | 5.6 | 5.2 | 0.003 | <0.0001 |
| LV mass, g | 113±35 | 137±46 | 137±30 | 0.010 | 0.081 |
| LV mass index, g/m2 | 58±15 | 69±20 | 68±13 | <0.0001 | 0.038 |
| LV sphericity index | 1.7±0.2 | 1.4±0.2 | 1.4±0.2 | <0.0001 | <0.0001 |
| Mid-ventricular circumferential systolic strain, | 19±3 | 10±4 | 12±4 | <0.0001 | <0.0001 |
| %(negative) Mid-ventricular diastolic strain rate, s-1 | 83±19 | 48±21 | 53±18 | <0.0001 | <0.0001 |
Values are mean ± standard deviations or percentages. BSA indicates body surface area; DCM, dilated cardiomyopathy; EDV, end diastolic volume; IHD, ischaemic heart disease; LV, left ventricle.
Changes in flow components
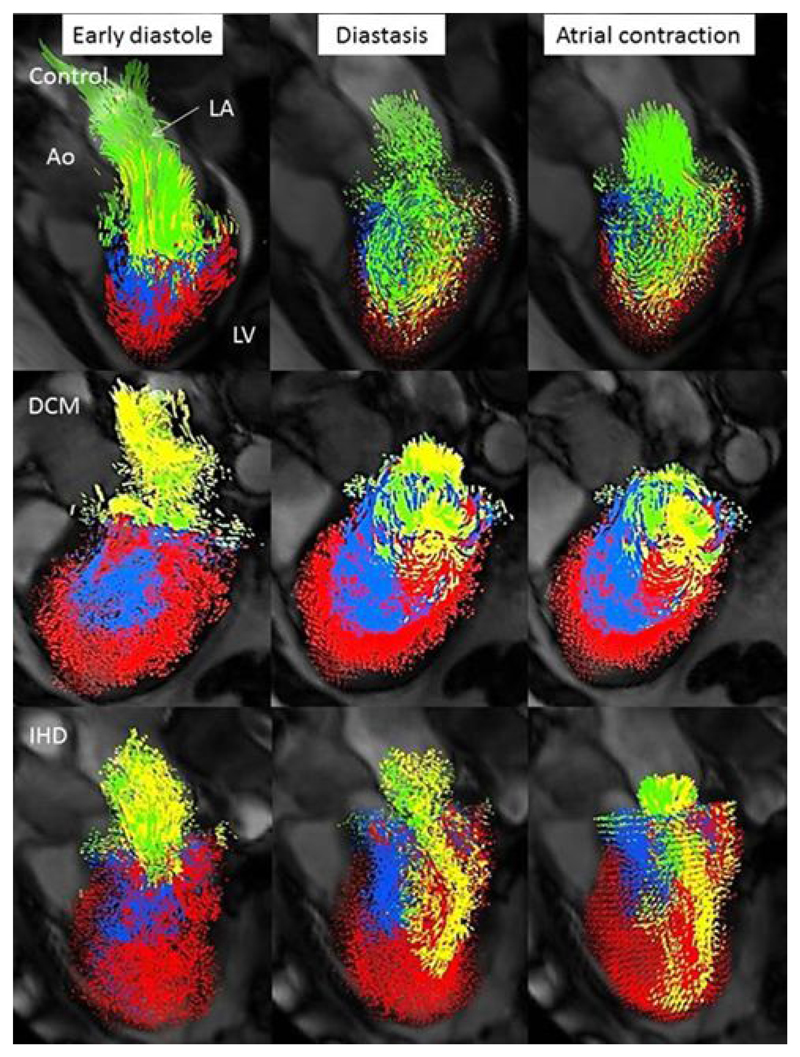
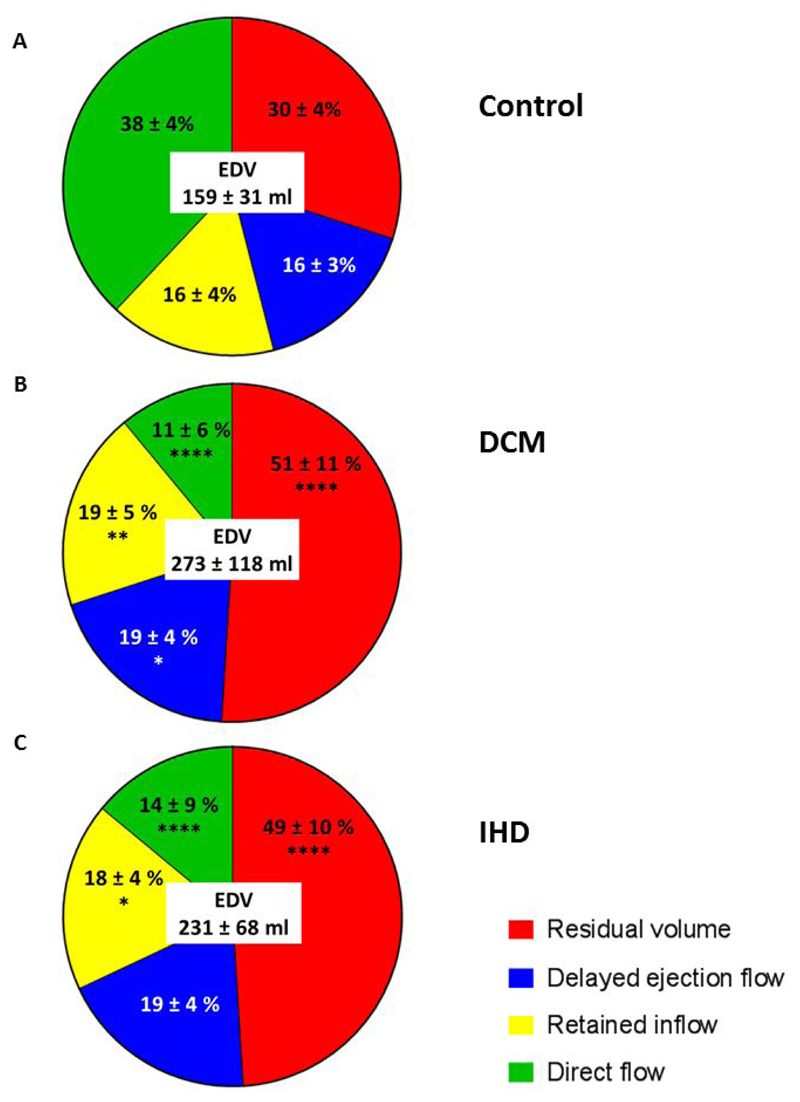
Flow visualisations are shown in Figure 1 and videos available in supplemental material. The changes in proportion of the flow components, compared to controls, were similar between the DCM and IHD groups, Figure 2. DCM was associated with a 71% and IHD a 63% decrease in the direct flow component proportion compared to controls (P<0.0001). This decrease in direct flow corresponded to a similar increase in the residual volume component in both DCM (63% increase) and IHD patients (70% increase) compared to controls (P<0.0001).
Figure 1. Representative diastolic LV visualisations in a control (direct flow 35%, residual volume 29%), DCM (direct flow 10%, residual volume 55%) and IHD patient with an antero-apical infarct (direct flow 8%, residual volume 56%).
Despite similar proportions of residual volume between the IHD and DCM patient the distribution differs; with a global distribution in the DCM patient and a more localised distribution in the IHD patient, corresponding to the area of infarction. Direct flow, green; retained inflow, yellow; delayed ejection flow, blue and residual volume, red. Ao, aorta; DCM, dilated cardiomyopathy; IHD, ischaemic cardiomyopathy; LA, left atrium; LV left ventricle.
Figure 2. Flow components by percentage of the end-diastolic volume for (A) Control, (B) DCM and (C) IHD.
Data are mean ± standard deviation. ****P<0.0001, **P<0.01, *P<0.05 compared to corresponding component in controls. All comparisons between DCM and IHD patients were non-significant. DCM, dilated cardiomyopathy; EDV, end diastolic volume; IHD ischaemic cardiomyopathy.
Changes in kinetic energy profiles
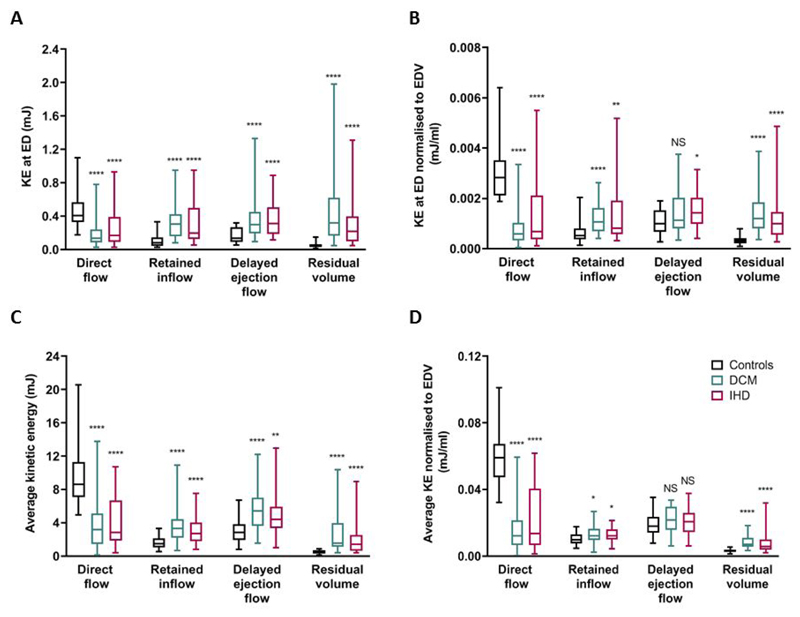
KE values for all four flow components differed significantly for the DCM and IHD groups compared to controls, but not between the patient groups, Figure 3. In controls the efficient direct flow component possessed the greatest KE, in DCM and IHD patients this was decreased (DCM average KE 60% decrease and IHD 56% decrease versus controls, P<0.0001, Figure 3B) and the KE of the other 3 flow components increased compared to controls. These KE changes were seen for both KE at end-diastole and the average kinetic energy, but the magnitude of change differed depending upon which KE measure was assessed (Figure 3A and B).
Figure 3. Kinetic energy profiles. (A)Kinetic energy at end diastole; (B)Kinetic energy at end diastole normalised to EDV; (C)Average kinetic energy throughout the cardiac cycle and (D)Average kinetic energy normalised to EDV.
Bars show minimum and maximum values.. ****P<0.0001, ***P<0.001 compared to corresponding component value in controls. All comparisons between DCM and IHD patients were non-significant. DCM, dilated cardiomyopathy; KE ED, kinetic energy end diastole; IHD ischaemic cardiomyopathy.
The proportion of the direct flow average KE compared to the total average KE of all flow components was highest for the control group (64±8%), compared to DCM 23±14% and IHD 29±19%(P<0.0001). The residual volume average KE proportion was significantly higher in the two patient groups (DCM 17±11%, IHD 15±12%) compared to controls (4±2%, P<0.0001).
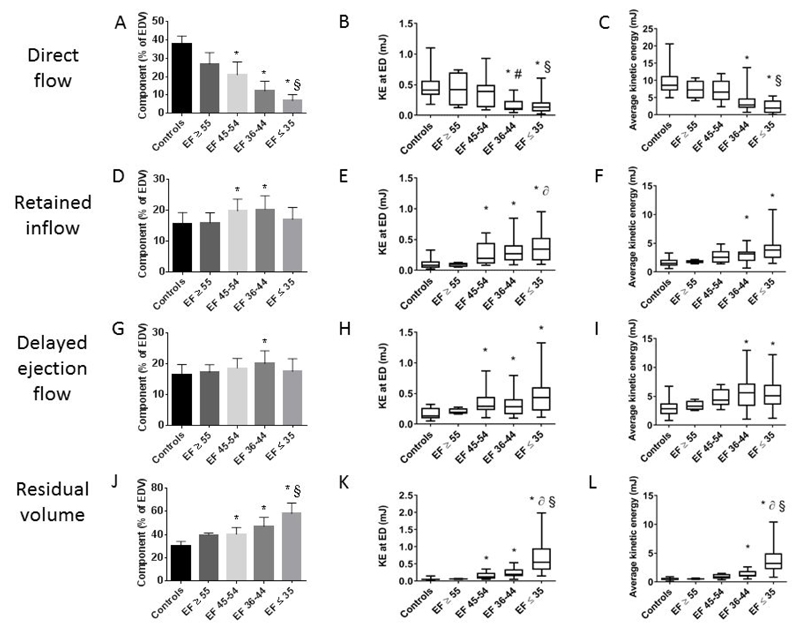
The derangement in the proportion and KE values of the flow components progressed as the LVEF decreased, as illustrated in Figure 4. The proportion of the direct flow decreased in line with the EF. However, the decrease in direct flow KE occurred only with the development of more advanced HF, with an EF ≤44% (Figure 4B and C). The proportion and KE of the residual volume component increased steadily as LV impairment worsened (Figure 4J-L). As with the direct flow the change in the KE of the residual volume, in this case an increase, occurred with more advanced HF, with EF ≤44%.
Figure 4. Differences in flow component percentage, kinetic energy at end diastole and average kinetic energy according to left ventricular ejection fraction (LVEF).
LVEF>55%, n=4; EF 45-54% n=11; EF 36-44% n=21; EF≤35%, n=28. Panels A, D, G and J bars show mean value and error bars indicate standard deviation. Other panels bars show minimum and maximum values. *P<0.05 compared to controls, §P<0.05 LVEF≤35% compared to 45-54%, ∂P<0.05 LVEF≤35% compared to ≥55%, #P<0.05 LVEF 36-44% compared to 45-54%. EDV, end diastolic volume; EF, ejection fraction; KE ED, kinetic energy end diastole.
Association of novel 4D flow parameters with classical remodelling and prognostic markers
The correlation coefficients for the direct flow and residual volume KE across all participants are shown in Table 3. Direct flow KE correlated negatively with the conventional remodelling parameters of LVEDV, LVESV and positively with LVEF(P<0.0001). Residual volume KE correlated negatively with the LVEF and positively with the LVEDV and LVESV(P<0.0001). Both the direct flow and residual volume KE correlated, but in opposite directions, with the patients’ symptoms (MHFQ), their functional capacity (6MWT) and biochemical evidence of cardiac remodelling (BNP).
Table 3. Correlations between ventricular remodelling parameters, prognostic markers, kinetic energy at both end-diastole and average kinetic energy for direct flow and residual volume.
| Variable | Direct flow kinetic energy at ED | Direct flow average kinetic energy | Residual volume kinetic energy at ED | Residual volume average kinetic energy | ||||
|---|---|---|---|---|---|---|---|---|
| r | P value (95% CI) | r | P value (95% CI) | r | P value (95% CI) | r | P value (95% CI) | |
| LV EDV | -0.35 | <0.0001 (-0.50 to -0.14) |
-0.41 | <0.0001 (-0.52 to -0.18) |
0.78 | <0.0001 (0.64 to 0.85) |
0.88 | <0.0001 (0.79 to 0.92) |
| LV ESV | -0.55 | <0.0001 (-0.65 to -0.37) |
-0.64 | <0.0001 (-0.70 to -0.46) |
0.89 | <0.0001 (0.81 to 0.91) |
0.93 | <0.0001 (0.86 to 0.95) |
| LV EF | 0.66 | <0.0001 (0.50 to 0.75) |
0.79 | <0.0001 (0.65 to 0.84) |
-0.88 | <0.0001 (-0.90 to -0.79) |
-0.86 | <0.0001 (-0.90 to -0.76) |
| MHFQ | -0.56 | <0.0001 (-0.70 to -0.40) |
-0.63 | <0.0001 (-0.73 to -0.50) |
0.60 | <0.0001 (0.43 to 0.73) |
0.58 | <0.0001 (0.42 to 0.71) |
| 6MWT | 0.46 | <0.0001 (0.28 to 0.62) |
0.60 | <0.0001 (0.45 to 0.72) |
-0.50 | <0.0001 (-0.63 to -0.33) |
-0.43 | <0.0001 (-0.57 to -0.23) |
| Circumferential systolic strain | -0.56 | <0.0001 (-0.69 to -0.39) |
-0.73 | <0.0001 (-0.80 to -0.59) |
0.78 | <0.0001 (0.69 to 0.86) |
0.77 | <0.0001 (0.66 to 0.84) |
| BNP | -0.45 | <0.0001 (-0.57 to -0.20) |
-0.58 | <0.0001 (-0.64 to -0.34) |
0.53 | <0.0001 (0.29 to 0.62) |
0.51 | <0.0001 (0.22 to 0.60) |
Correlations are performed with Pearson or Spearman’s correlation as appropriate. 6MWT indicates 6 minute walk test; BNP, Brain-type natriuretic peptide; ED, end diastole; EDV, end diastolic volume; EF, ejection fraction; ESV, end systolic volume; LV indicates, left ventricular; MHFQ, Minnesota heart failure questionnaire.
Kinetic energy values for direct flow and residual volume according to distance covered during the six minute walk test are shown in Table 4. The direct flow average kinetic energy was found to be different depending upon the distance walked (P=0.008).
Table 4. Results for direct flow percentage and kinetic energy values and residual volume percentage and kinetic energy values according to distance covered during six minute walk test.
| 6MWT <450m (n= 17) |
6MWT 451-550m (n= 31) |
6MWT >551m (n=16) |
P value | |
|---|---|---|---|---|
| Direct flow (% of EDV) | 9.8±4.4 | 13.6±8.7 | 13.7±8.9 | 0.264 |
| Direct flow KE at ED (mJ) | 0.17±0.12 | 0.24±0.25 | 0.24±0.15 | 0.456 |
| Direct flow average KE (mJ) | 2.41±1.32 | 3.93±2.80 | 5.71±3.95 | 0.008 |
| Residual volume (% of EDV) | 53.7±9.3 | 49.0±11.6 | 48.0±9.4 | 0.261 |
| Residual volume KE at ED (mJ) | 0.40±0.29 | 0.35±0.41 | 0.44±0.38 | 0.745 |
| Residual volume average KE (mJ) | 2.23±1.51 | 2.23±2.43 | 2.27±1.59 | 0.989 |
Values are mean ± standard deviations. 6MWT indicates six minute walk test; EDV, end diastolic volume; KE, kinetic energy
In order to assess whether remodelling parameters were predictive of the patient’s functional capacity, as represented by the 6MWT, a multiple linear regression model was created. The independent variables entered into the model were age, height, LVEF, BNP, direct flow average kinetic energy and peak systolic circumferential strain. Importantly, the independent predictors of the 6MWT were found to be age (β=-0.315, P=0.019) and direct flow average kinetic energy (β=0.280, P=0.035, overall R2 of the model =0.466, supplemental table 2). In order to avoid co-linearity of predictors the other prognostic remodelling parameters of ESV and EDV were substituted into the model above instead of LVEF, but in these subsequent models age and direct flow average kinetic energy remained the only independent predictors. Thus, direct flow average kinetic energy was, but traditional remodelling parameters were not, independent predictors of functional capacity in these heart failure patients.
Associations between myocardial energetics and 4D flow parameters in DCM
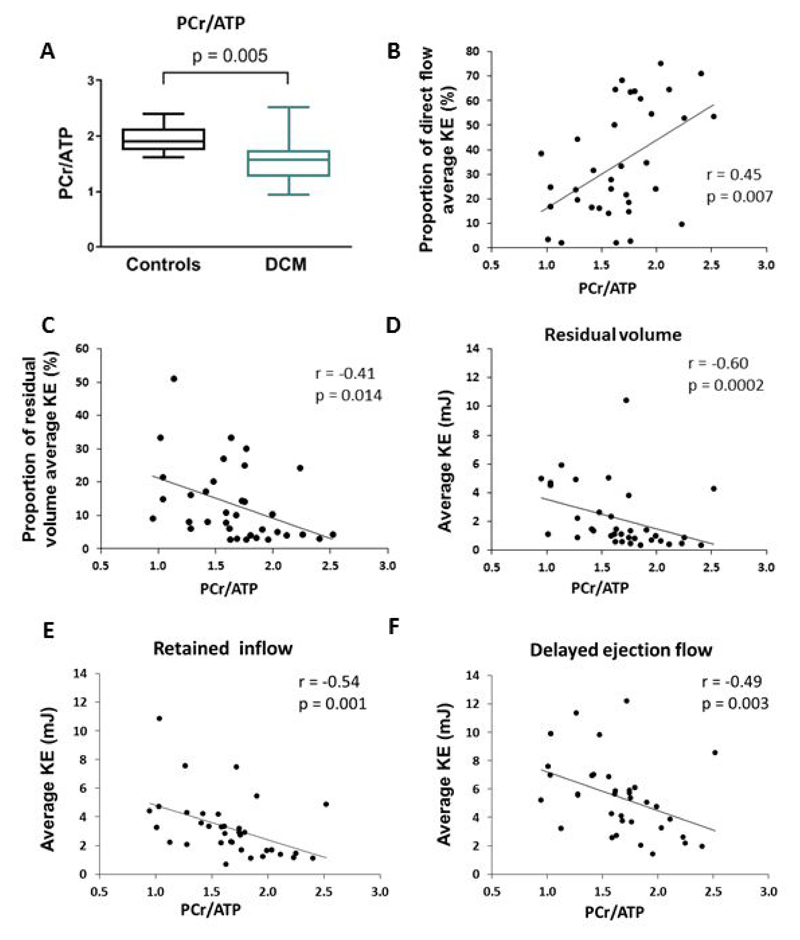
In keeping with previous studies11 we found a reduced PCr/ATP ratio in DCM compared to controls (PCr/ATP 1.54±0.39 vs 1.95±0.25, P=0.005,Figure 5A). The PCr/ATP ratio correlated with the classical remodelling parameters of LVEF(r=0.527, P =0.01, 95%CI 0.11 to 0.72), LVEDV(r=- 0.587, P=0.0002, 95%CI -0.79 to -0.15) and LVESV(r=-0.601, P=0.0001, 95%CI -0.80 to -0.21) as well as the peak systolic circumferential strain(r=0.507, P=0.003, 95%CI 0.19 to 0.74). Additionally the PCr/ATP ratio correlated with 4D flow parameters (Figure 5B-F) including the proportion of the direct flow average KE(r=0.45, P=0.007, 95%CI 0.05 to 0.73) and proportion of the residual volume average KE(r=-0.41, P=0.014, 95%CI -0.67 to -0.03).
Figure 5. Myocardial energetics results and correlations in DCM patients. (A)PCr/ATP ratio in controls compared to DCM. Correlations between PCr/ATP ratio and (B)proportion of direct flow average KE; (C)proportion of residual volume average KE; (D)residual volume average KE; (E)retained inflow average KE and (F)delayed ejection flow average KE.
PCr/ATP, phosphocreatine to adenosine triphosphate concentration ratio; KE kinetic energy.
Discussion
In this work, the relationships between ventricular morphology, prognostic markers, and novel 4D flow parameters during cardiac remodelling due to dilated and ischaemic cardiomyopathy were assessed using CMR. We demonstrate that the average KE of the direct flow and residual volume correlate with conventional remodelling parameters and prognostic markers, suggesting a role as novel cardiac remodelling imaging biomarkers. Importantly, we show that the direct flow average KE is predictive of the patient’s functional capacity, whereas the LVEF and LV volumes were not. We demonstrate that changes in flow components and KE, as seen previously in DCM patients5, are similar in ischaemic cardiomyopathy, despite a different myocardial insult aetiology. Finally, we demonstrate that in DCM there is a relationship between the impaired myocardial energetics and the KE of the LV flow components.
Consequences of alterations in LV flow components and kinetic energy
In health, most inflow volume and hence KE of blood from the left atrium (direct flow and retained inflow) is due to direct flow, which preserves its KE as it transits the LV.17 We identified that in DCM and IHD the majority of the inflowing volume and KE is due to the retained inflow component. Hence, instead of immediate ejection as part of the direct flow, the KE possessed by the retained inflow resides within the LV for at least one cardiac cycle prior to ejection. The KE of this blood has several possible fates in the receiving ventricle, it may: 1)be transferred as KE to the blood already residing in the LV (delayed ejection flow and residual volume); 2)be converted into potential energy that is either a)stored within the elastic recoil of the myocardium or b)causes an elevation in ventricular pressure; or 3)be dissipated in the form of friction/heat.5 With any of these fates, energy is dissipated or converted into less efficient configurations within the ventricle, and the KE of the LV residing components is increased compared to the situation in health.
Many processes that occur as a consequence of cardiac remodelling have initial advantageous effects that become deleterious over time; it may be that increasing the residual volume in ventricular dysfunction initially confers an advantage such as acting as a buffer to redistribute KE, so as to reduce transfer of KE to potential energy that would result in elevated ventricular pressure. However, when either the myocardium remodels becoming less compliant or the LV pressure exceeds a certain level, the conversion of KE to potential energy declines and may explain why we see the sharp rise in the residual volume average KE once end-stage remodelling is reached, suggesting failure of any compensatory mechanisms. The KE of the residual volume may also have a role in prevention of blood stasis and thrombus formation, as suggested by a Doppler study that found lower apical blood velocities in patients with thrombus compared to those without.18
Relation to earlier studies
Previous work by Eriksson et al 5and Kanski et al 7found that patients with HF have higher KE values compared to controls. Eriksson et al 5studied patients with clinically compensated DCM and found similar but less pronounced alterations in the flow components volume’s with reduced direct flow, and increases in the other flow components. This study looked at KE values at end diastole and found, as we did, an increase in KE of the retained inflow, delayed ejection flow and residual volume. Unlike our results, they did not see a difference in the KE of the direct flow between the DCM patients and controls, but their patients had better systolic function (mean LVEF 42% and preserved stroke volume versus our values of LVEF 36% DCM and 39% IHD, with reduced stroke volume), which may explain this difference.
Kanski et al7 evaluated the average KE of the total ventricular blood volume in HF patients. They found no difference in diastolic average KE between patients and controls, but higher average systolic KE. They did not find any relationship between the patients’ symptoms or functional capacity and the total KE. This lack of association is likely due to consideration of the blood volume as a whole rather than as flow components as in our study. Interestingly in Fontan patients, Sjöberg et al19 found that the peak diastolic but not systolic KE indexed to stroke volume was lower in Fontan patients than controls. These varying results depending upon the aetiology of myocardial injury suggest that there is still much to be understood about intra-cardiac KE.
Potential clinical utility of intra-cardiac kinetic energy assessment
To our knowledge our work is the first to demonstrate that the kinetic energy of both the direct flow and the residual volume flow components correlated with conventional, established prognostic markers for heart failure, including BNP levels, heart failure symptoms and functional capacity. These results support a clinical utility for kinetic energy evaluation in the management and follow up of patients with heart failure. Current volumetric based cardiac imaging techniques have limited ability to provide prognostic information for this patient cohort. In the future it is hoped that by incorporating more advanced imaging techniques, such as 4D flow kinetic energy assessments, the predictive ability of cardiac imaging to provide prognostication in heart failure patients can be further refined, and aid appropriate targeting of new therapies to those patients most at risk of complications. However, before generalised use of 4D flow for clinical assessments occurs our results require further validation in a larger multi-centre study to fully establish the reproducibility between centres and the clinical potential of this technique.
Candidate pathophysiological mechanisms for transduction of blood flow abnormalities to cardiac remodelling
Blood flow within the left ventricle is subject to the laws of mechanics including that of Laplace; ventricular wall stress is proportional to ventricular pressure and cavity radius and inversely proportional to wall thickness.2 If KE conversion to potential energy causes increased LV pressure, especially diastolic pressure, this would result in increased ventricular wall stress/stretch, which may be important in the activation of cardiac remodelling pathways.3 Translating stretch-stimuli to downstream signalling requires numerous complex pathways including transient receptor potential channels, integrins, as well as the sarcomere spanning protein titin.20 Once cardiac myocytes have sensed mechanical stretch they convert this into intracellular growth signals and changes in gene expression.21
A common early feature of cardiac remodelling is an increase in wall thickness in order to reduce wall stress and decrease oxygen demand, however when the wall stress is sustained the myocardium slowly transitions to a state of decompensation and subsequent HF. Part of the cardiac myocyte response to mechanical stress is to reactivate a pattern of gene expression similar to that required during fetal growth which includes BNP.2 Re-expression of fetal genes during remodelling provides further evidence for the potential influence of cardiac blood flow upon morphological changes; in fetal cardiac development mechanical signals from blood flow, via induction of gene expression, promote ventricular cell enlargement and contractility.22
Additional support for the importance of intra-cardiac blood flow upon myocardial cellular processes is provided by tissue samples obtained before and after implantation of a left ventricular assist device in HF patients, which demonstrated reverse remodelling changes including regression of cell thickening/elongation and reversion of gene expression controlling calcium cycling.23
Myocardial energetics and intra-ventricular blood flow
Myocardial energetics were associated with the proportion of the direct flow average KE. This suggests that, as well as the direct flow KE, the KE of the components that remain within the LV for at least one cycle are also important. One explanation for this may relate to altered cardiac substrate metabolism as a consequence of reactivation of the fetal gene program by abnormal LV stretch (caused by the KE of the LV residing components). Hence, it may be that the activation of this gene program shifts myocardial metabolism from dominant fat to dominant glucose metabolism.24 Metabolising glucose requires less oxygen per unit of ATP generated than metabolising fat, but, a mole of glucose has significantly lower chemical potential energy than a mole of fat. This metabolic shift might impair ATP generation in advanced HF.25 In support of this, mechanical unloading of failing hearts with left ventricular assist devices is associated with at least partial normalisation of cardiac metabolism.26
Study limitations
4D flow acquisitions were at rest and, although associations were found with the patients’ functional capacity, these relationships and understanding of blood flow changes in HF may elucidate additional mechanisms if assessed during pharmacological or exercise stress.
An alternative selection method for the variables to include in the multiple linear regression model could have been utilised, such as setting the significance level higher (e.g. p<0.2) or using index criteria. However these methods were not utilised as this would have resulted in more eligible variables, which with the limited sample size available, may have resulted in overfitting of the data. However, we acknowledge that the selection method utilised means other relevant variables may have been excluded from the current model which should be investigated further in future studies with larger sample sizes. Additionally the results are primarily unadjusted and the sample size has limited ability to fully adjust for covariates. The lack of significance of covariates in the analysis cannot exclude these as parameters of importance and may instead be due to a small effect that the limited study size sample was unable to detect. This will require future larger sample size studies to investigate further.
Potential selection bias based on recruitment from patients under tertiary level care, as well as for controls who volunteered for the study may also confound the applicability of these results beyond the present study cohort. Additionally the patients enrolled for this study were recruited on the basis of systolic heart failure and were mostly well compensated patients. Therefore, as such it was perhaps not surprising that the results for IHD and DCM patients were similar, despite the differing original myocardial insult. No patients with heart failure preserved ejection fraction (HFpEF) were recruited to this study. Further studies enrolling a cohort of HFpEF patients would be of benefit to understand if novel 4D flow parameters may be of clinical utility in this patient population.
This study has highlighted important relationships between classical remodelling parameters and novel 4D flow markers, but, in line with its proof of principle concept, cross sectional and observational nature, it cannot assess the causality of these relationships. Additionally, the exploratory nature of this study means that multiple parameters have been assessed at once and therefore mass significance is a potential limitation. Although we found with statistical modelling the direct flow average KE to be a superior predictor of patients’ functional capacity compared to volumetric parameters, assessment of the applicability of this result to all patients with different aetiologies of HF is beyond the scope of this study design.
Clinical implications
Therapies for HF, including angiotensin converting enzyme inhibitors and β-blockers, have significantly reduced morbidity and mortality.1 However, the incidence of HF and burden of disease continues to increase and the need for new therapies remains.2 Despite numerous phase I-II studies describing potential novel therapies, very few of these compounds have been successfully translated in clinical trials. The reasons for this failure are multifactorial including: the difficulty of achieving adequate power to demonstrate a mortality benefit and the inability to identify effective therapies in phase II trials, which may be compounded by the use of surrogate endpoints that are a consequence of remodelling rather than an active part of the process.27 Therefore, the identification in this study of novel 4D flow imaging biomarkers that may be mechanistic in the cardiac remodelling process, rather than surrogate markers, warrants further investigation with longitudinal therapeutic intervention studies, potentially providing an early efficacy signal indicating prognostic benefit more strongly than traditional remodelling markers.
Conclusions
In heart failure patients, the direct flow average kinetic energy was the only imaging based independent predictor of functional capacity. 4D flow parameters are novel imaging biomarkers that provide additional information about disease severity and cardiac remodelling over conventional imaging parameters. We speculate that 4D flow parameters may become a powerful surrogate for clinical endpoints in future heart failure studies.
Supplementary Material
Clinical Perspective.
Cardiac magnetic resonance imaging plays a central role in the diagnosis and prognostication of heart failure patients. In this current study we investigated the clinical utility of 4D flow imaging, compared to conventional imaging and clinical prognostic markers. The left ventricular flow was divided into four functional components and the kinetic energy of each flow component calculated throughout the cardiac cycle. We found that in patients with either ischaemic or dilated cardiomyopathy there was a decrease in the volume and kinetic energy of the direct flow component compared to healthy controls. The degree of derangement in the direct flow parameters worsened as the left ventricular ejection fraction declined. The direct flow average kinetic energy correlated negatively with the conventional remodelling parameters of left ventricular end diastolic and end systolic volumes, patients symptoms (as measured by a validated questionnaire) and B-type natriuretic peptide levels (P<0.0001). This is the first study that found in a multiple linear regression model that the direct flow average kinetic energy was predictive of the patient’s functional capacity, as measured by the distance covered during a six minute walk test. Conventional imaging parameters including left ventricular ejection fraction were not predictive of the patient’s functional capacity. These results suggest that intra-cardiac 4D flow parameters are novel biomarkers in heart failure and warrant further investigation in longitudinal studies as a marker of prognosis in heart failure patients.
Acknowledgements
The authors gratefully acknowledge Hayley Harvey, Judith Del Santos and Joanne Sellwood for their help and support with patient care.
Sources of Funding
This study was supported by the British Heart Foundation [grant number FS/12/14/29354 to VMS]; Medical Research Council (ATH); Oxford British Heart Foundation Centre of Research Excellence (ATH and SN); Sir Henry Dale Fellowship from the Wellcome Trust and the Royal Society (098436/Z/12/B to CTR); National Institute for Health Research Oxford Biomedical Research Centre Programme (SN and SGM); Swedish Research Council (PD and TE); the Swedish Heart and Lung Foundation [grant number 20140398 to CJC]. The research leading to these results has received funding from the European Union's Seventh Framework Programme (FP7/2007-2013) under grant agreement 310612 to TE.
Footnotes
Disclosures
All authors have none to declare.
Disclaimer: The manuscript and its contents are confidential, intended for journal review purposes only, and not to be further disclosed.
References
- 1.Nichols M, Townsend N, Scarborough P, Rayner M. Cardiovascular disease in Europe 2014: epidemiological update. Eur Heart J. 2014;35:2950–2959. doi: 10.1093/eurheartj/ehu299. [DOI] [PubMed] [Google Scholar]
- 2.Burchfield JS, Xie M, Hill JA. Pathological Ventricular Remodeling: Mechanisms: Part 1 of 2. Circulation. 2013;128:388–400. doi: 10.1161/CIRCULATIONAHA.113.001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill JA, Olson EN. Mechanisms of disease: Cardiac plasticity. New Engl J Med. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 4.Yang GZ, Merrifield R, Masood S, Kilner PJ. Flow and myocardial interaction: an imaging perspective. Philos T R Soc B. 2007;362:1329–1341. doi: 10.1098/rstb.2007.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eriksson J, Bolger AF, Ebbers T, Carlhall CJ. Four-dimensional blood flow-specific markers of LV dysfunction in dilated cardiomyopathy. Eur Heart J-Card Img. 2013;14:417–424. doi: 10.1093/ehjci/jes159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dyverfeldt P, Bissell M, Barker AJ, Bolger AF, Carlhall CJ, Ebbers T, Francios CJ, Frydrychowicz A, Geiger J, Giese D, Hope MD, et al. 4D flow cardiovascular magnetic resonance consensus statement. J Cardiovasc Magn R. 2015;17:72. doi: 10.1186/s12968-015-0174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanski M, Arvidsson PM, Toger J, Borgquist R, Heiberg E, Carlsson M, Arheden H. Left ventricular fluid kinetic energy time curves in heart failure from cardiovascular magnetic resonance 4D flow data. J Cardiovasc Magn R. 2015;17:111. doi: 10.1186/s12968-015-0211-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger R, Huelsman M, Strecker K, Bojic A, Moser P, Stanek B, Pacher R. B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation. 2002;105:2392–2397. doi: 10.1161/01.cir.0000016642.15031.34. [DOI] [PubMed] [Google Scholar]
- 9.B V, Weiner DH, Yusuf S, Rogers WJ, Mcintyre KM, Bangdiwala SI, Kronenberg MW, Kostis JB, Kohn RM, Guillotte M, Greenberg B, et al. Prediction of Mortality and Morbidity with a 6-Minute Walk Test in Patients with Left-Ventricular Dysfunction. Jama-J Am Med Assoc. 1993;270:1702–1707. [PubMed] [Google Scholar]
- 10.Alla F, Briancon S, Guillemin F, Juilliere Y, Mertes PM, Villemot JP, Zannad F. Self-rating of quality of life provides additional prognostic information in heart failure. Insights into the EPICAL study. Eur J Heart Fail. 2002;4:337–343. doi: 10.1016/s1388-9842(02)00006-5. [DOI] [PubMed] [Google Scholar]
- 11.Neubauer S, Horn M, Cramer M, Harre K, Newell JB, Peters W, Pabst T, Ertl G, Hahn D, Ingwall JS, Kochsiek K. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation. 1997;96:2190–6. doi: 10.1161/01.cir.96.7.2190. [DOI] [PubMed] [Google Scholar]
- 12.Rider OJ, Lewandowski A, Nethononda R, Petersen SE, Francis JM, Pitcher A, Holloway CJ, Dass S, Banerjee R, Byrne JP, Leeson P, et al. Gender-specific differences in left ventricular remodelling in obesity: insights from cardiovascular magnetic resonance imaging. Eur Heart J. 2013;34:292–9. doi: 10.1093/eurheartj/ehs341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stuber M, Spiegel MA, Fischer SE, Scheidegger MB, Danias PG, Pedersen EM, Boesiger P. Single breath-hold slice-following CSPAMM myocardial tagging. MAGMA. 1999;9:85–91. doi: 10.1007/BF02634597. [DOI] [PubMed] [Google Scholar]
- 14.Eriksson J, Carlhall CJ, Dyverfeldt P, Engvall J, Bolger AF, Ebbers T. Semi-automatic quantification of 4D left ventricular blood flow. J Cardiovasc Magn R. 2010;12:9. doi: 10.1186/1532-429X-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodgers CT, Clarke WT, Snyder C, Vaughan JT, Neubauer S, Robson MD. Human cardiac 31P magnetic resonance spectroscopy at 7 Tesla. Magnetic resonance in medicine. 2014;72:304–15. doi: 10.1002/mrm.24922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoll VM, Clarke WT, Levelt E, Liu A, Myerson SG, Robson MD, Neubauer S, Rodgers CT. Dilated Cardiomyopathy: Phosphorus 31 MR Spectroscopy at 7 T. Radiology. 2016;281:409–417. doi: 10.1148/radiol.2016152629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlhall CJ, Bolger A. Passing Strange Flow in the Failing Ventricle. Circ-Heart Fail. 2010;3:326–331. doi: 10.1161/CIRCHEARTFAILURE.109.911867. [DOI] [PubMed] [Google Scholar]
- 18.Maze SS, Kotler MN, Parry WR. Flow Characteristics in the Dilated Left-Ventricle with Thrombus - Qualitative and Quantitative Doppler Analysis. J Am Coll Cardiol. 1989;13:873–881. doi: 10.1016/0735-1097(89)90230-1. [DOI] [PubMed] [Google Scholar]
- 19.Sjoberg P, Heiberg E, Wingren P, Ramgren Johansson J, Malm T, Arheden H, Liuba P, Carlsson M. Decreased Diastolic Ventricular Kinetic Energy in Young Patients with Fontan Circulation Demonstrated by Four-Dimensional Cardiac Magnetic Resonance Imaging. Pediatr Cardiol. 2017;38:669–680. doi: 10.1007/s00246-016-1565-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spaich S, Katus HA, Backs J. Ongoing controversies surrounding cardiac remodeling: is it black and white-or rather fifty shades of gray? Frontiers in physiology. 2015;6:202. doi: 10.3389/fphys.2015.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadoshima J, Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu Rev Physiol. 1997;59:551–571. doi: 10.1146/annurev.physiol.59.1.551. [DOI] [PubMed] [Google Scholar]
- 22.Lindsey SE, Butcher JT, Yalcin HC. Mechanical regulation of cardiac development. Frontiers in physiology. 2014;5:318. doi: 10.3389/fphys.2014.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heerdt PM, Holmes JW, Cai B, Barbone A, Madigan JD, Reiken S, Lee DL, Oz MC, Marks AR, Burkhoff D. Chronic unloading by left ventricular assist device reverses contractile dysfunction and alters gene expression in end-stage heart failure. Circulation. 2000;102:2713–9. doi: 10.1161/01.cir.102.22.2713. [DOI] [PubMed] [Google Scholar]
- 24.Taegtmeyer H, Sen S, Vela D. Return to the fetal gene program: a suggested metabolic link to gene expression in the heart. Annals of the New York Academy of Sciences. 2010;1188:191–8. doi: 10.1111/j.1749-6632.2009.05100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neubauer S. The failing heart--an engine out of fuel. New Engl J Med. 2007;356:1140–51. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 26.Chokshi A, Drosatos K, Cheema FH, Ji R, Khawaja T, Yu S, Kato T, Khan R, Takayama H, Knoll R, Milting H, et al. Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circulation. 2012;125:2844–53. doi: 10.1161/CIRCULATIONAHA.111.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kramer DG, Trikalinos TA, Kent DM, Antonopoulos GV, Konstam MA, Udelson JE. Quantitative Evaluation of Drug or Device Effects on Ventricular Remodeling as Predictors of Therapeutic Effects on Mortality in Patients With Heart Failure and Reduced Ejection Fraction A Meta-Analytic Approach. J Am Coll Cardiol. 2010;56:392–406. doi: 10.1016/j.jacc.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.