Abstract
Objective
This review provides a comprehensive assessment of the effectiveness of burst spinal cord stimulation (SCS). Ratings of pain intensity (visual analog scale or numeric rating scale) and patient-reported outcomes (PROs) on functional/psychometric domains such as depression (Beck Depression Index), catastrophizing (Pain Catastrophizing Scale), surveillance (Pain Vigilance and Attention Questionnaire), and others are addressed.
Design
Articles were identified and selected from the literature according to prospective, replicable methods. Effectiveness data—pain scores and PRO ratings—were weighted by study sample sizes and pooled. The effects of burst SCS were compared against values at baseline and with tonic SCS. For PROs, published population norms were used for comparison.
Results
Fifteen articles, with a combined sample size of 427, were included. Follow-up ranged from a few hours to two years. A variety of prospective designs were employed, including crossover studies, single-arm cohorts, and a randomized controlled trial, as well as retrospective case reports. The weighted pooled mean pain rating across articles at baseline was 76.7 (±27.4). With tonic SCS, this was reduced to 49.2 (±12.9), and with burst SCS it was further reduced to 36.7 (±11.6), a 12.5-point difference between tonic and burst values. Psychometric analyses of PROs noted preferential improvement with burst SCS. In addition, 65% of subjects stated a preference for burst SCS.
Conclusions
In pooled analyses that incorporated all available published evidence, the improvement over baseline for burst SCS was shown to have a clinically important incremental benefit over tonic SCS. In addition, burst SCS may support resolution of the emotional or cognitive aspects of pain that are mediated by medial thalamo-cortical pathways. This study highlights the value in considering the entire knowledge base in therapeutic assessments as well as adopting a consistent set of outcome variables within neuromodulation. Burst SCS is a valuable intervention, providing both analgesia and psychometric benefits that warrant further thoughtful applications.
Keywords: Burst Stimulation, Spinal Cord Stimulation, Chronic Pain, Neuromodulation, Depression, Pooled Analysis, Affective and Medial Pathway
Introduction
Since 1967, spinal cord stimulation (SCS) has been providing relief for neuropathic pain that is otherwise refractory to conventional treatments [1]. Conventional SCS delivers short pulses (approximately 200 µsec) of electricity to the dorsal surface of the spinal cord in a tonic—constant, unchanging—fashion, typically at approximately 40 Hz. Considerable work has demonstrated the value of this intervention [1].
After decades with tonic stimulation as the only SCS option, albeit with dramatic advances in technology, implantation procedures, and programming options, in 2010 a new SCS waveform emerged [2]. Termed “burst” SCS, it delivers packets of five longer (1,000 µsec) pulses at 500 Hz, with the bursts repeated at 40 Hz. Monophasic charge accumulation occurs during the burst packet; this accumulation passively discharges during the interburst quiescent period. Despite requiring amplitudes much lower than for tonic SCS, burst SCS delivers more electrical current per second than tonic SCS [2,3]. Burst SCS is ideally paresthesia-free when properly administered [2].
Burst SCS has been positively received by the neuromodulation community due to the growing consensus that it relieves pain while also improving functional and psychological outcomes. It has shown utility for back and limb pain due to etiologies commonly treated with SCS, such as failed back surgery syndrome (FBSS), complex regional pain syndrome (CRPS), and diabetic peripheral neuropathy (DPN) [4–7]. Burst SCS has been identified as providing better pain suppression than tonic SCS [5,8]. The value of burst SCS as a salvage therapy has also been noted [5]. In addition to clinical outcomes, burst SCS has shown higher degrees of hyperalgesia resolution than tonic SCS in animal models [9,10].
The largest randomized controlled trial (RCT) of burst SCS to date, the SUNBURST trial, compared burst SCS with tonic SCS in a crossover design [11]. This trial led to FDA approval for burst SCS therapy in October 2016 [11]. Although the statistical superiority of burst SCS relative to tonic was confirmed, the magnitude of the difference between the two groups at the primary end point (43.5 mm [on a standard 100-mm visual analog scale [VAS] with burst SCS vs 48.7 mm with tonic SCS) was modest. Interestingly, this was in contrast to the strongly endorsed preference data: 70.8% of SUNBURST subjects preferred burst SCS, compared with 15.8% who preferred tonic SCS (10.4% had no preference) [11]. The modest difference between the SUNBURST waveforms’ pain scores was unexpected because it followed nearly a decade of smaller publications that consistently presented burst SCS as a considerably more robust intervention compared with tonic SCS. Thus, this report was completed with the objective of comprehensively reviewing the burst SCS literature—real-world observations as well as more stringently controlled designs—and pooling the data in analyses that may provide a more realistic reflection of outcomes with burst SCS and avoid potential unforeseen biases in smaller sample sizes. The objective is also to reframe future burst SCS studies with emphasis on collecting a greater number of objective measures reflective of the burst SCS waveform’s mechanism of action.
Methods
Databases queried included MEDLINE 1946 to present, MEDLINE InProcess and other nonindexed citations, the Cochrane Methodology Register, Health Technology Assessment, NHS Economic Evaluation Database, and Cochrane Clinical Answers, all via Ovid. The search strategy was (burst OR BurstDR) AND (spinal cord stimulat* OR SCS OR dorsal column stimulat*), with a publication date range of 2010–2019. Additionally, key word searches for “burst spinal cord stimulation” and the above terms were completed using Google Scholar and the journal Neuromodulation. Finally, the citation lists of recent systematic reviews were checked for additional citations.
Articles were included on the basis of reporting prospective or retrospective data on the clinical effectiveness (pain ratings and/or associated domains such as function or quality of life) of burst SCS. The following categories of articles were excluded: reviews, protocol-only publications, non-SCS treatment, indication outside of trunk/limb pain, use of a nonhuman model, technical data (e.g., electroencephalography [EEG]) only, non-peer-reviewed communications (e.g., letters to the editor), and conference proceedings. In instances in which multiple reports were made on the same cohort of patients, only the most recent and/or most complete publication was summarized to ensure that data were not duplicated in the systematic review. All authors collaborated on the selection process; any disagreements were resolved by consensus.
Data Analysis
From the selected articles, abstracted data included study design, sample size, subject demographics, indication being treated with SCS, pain scores (either VAS or numeric rating scale [NRS]), and patient-reported outcomes (PROs; e.g., questionnaires about mood, disability, or quality of life). Each article’s level of evidence was rated according to a standard methodology (Level 1 evidence: high-quality RCT; Level 2: lesser-quality RCT or prospective comparative study; Level 3: case–control study or retrospective comparative study; Level 4: case series; Level 5: expert opinion) [12,13]. Additionally, the quality of recommendations based on the selected literature as a whole was evaluated using the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) methodology, in which interventions are evaluated based on the whole of the evidence in a step-wise fashion. Initially, randomized controlled trials are given a default “high” ranking, and observational studies are ranked “low.” The quality of the evidence is then considered against consistent criteria and can be downgraded due to limitations in study quality, inconsistencies in results, uncertainty about the directness of the intervention on outcome, imprecise or sparse data, or high probability of reporting bias. Conversely, the quality of the evidence can be upgraded due to strong effect sizes, evidence of a dose–response gradient, or favorable interpretation of any confounders. A final grade is then assigned to the evidence: “High = Further research is unlikely to change our confidence in the estimate of effect; Moderate = Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; Low = Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; Very low = Any estimate of effect is very uncertain” [14,15]. GRADE recommendations were made for controlled trials as a group and for observational studies as a group.
For a pooled analysis of pain scores, VAS and NRS were considered equivalent and were analyzed together. If multiple pain scores were reported in an article (e.g., overall pain, back pain, and leg pain), the most comprehensive option (overall pain) was used. Scores were transformed, if necessary, from a 0–10 scale to a 0–100 scale for consistency. Mean pain scores in each article for baseline, burst SCS treatment, and tonic SCS treatment conditions were identified and weighted by the study N. Then, a single pooled mean and standard error of the mean (SEM) were calculated for all studies.
For preference scores (for burst SCS vs tonic) and the most common PROs (that is, those reported in at least three articles), similar methods were used to calculate pooled means. As comparisons, population norms, based on nonpain respondents, were identified from the literature (Beck Depression Inventory [BDI] [16], Pain Catastrophizing Scale [PCS] [17], Pain Vigilance and Awareness Questionnaire [PVAQ] [18]).
Because the same PROs were not used consistently across the 15 articles and therefore could not be analyzed individually, all reported PRO outcomes were normalized and pooled. The mean PRO scores reported in each study for baseline and during burst SCS treatment were transformed to the proportion of the highest (worst) possible score for the relevant instrument. As above, these proportions were then weighted by each study N, and a single pooled mean and SEM were calculated. For comparison, population norms, based on nonpain respondents, were identified from the literature (as above, and the Pittsburgh Sleep Quality Index [PSQI] [19], McGill Pain Questionnaire number of words chosen [MPQ NWC] [20], McGill Pain Questionnaire pain rating index [MPQ PRI] [20], McGill Pain Questionnaire, Short Form, total and sensory and affective domains [MPQSF T/S/A] [21], Oswestry Disability Index [ODI] [22]). To convert the population norms to a similar metric, they were transformed (as above) to proportions of the worst possible score for that instrument and weighted according to the sample size of each burst SCS article employing that PRO. The converted population norms were then expressed as a single pooled weighted mean and SEM, for a comparison.
Safety data (adverse events [AEs] and complications) were collated across the articles and presented in a narrative format.
Results
Summary of Patient Demographics
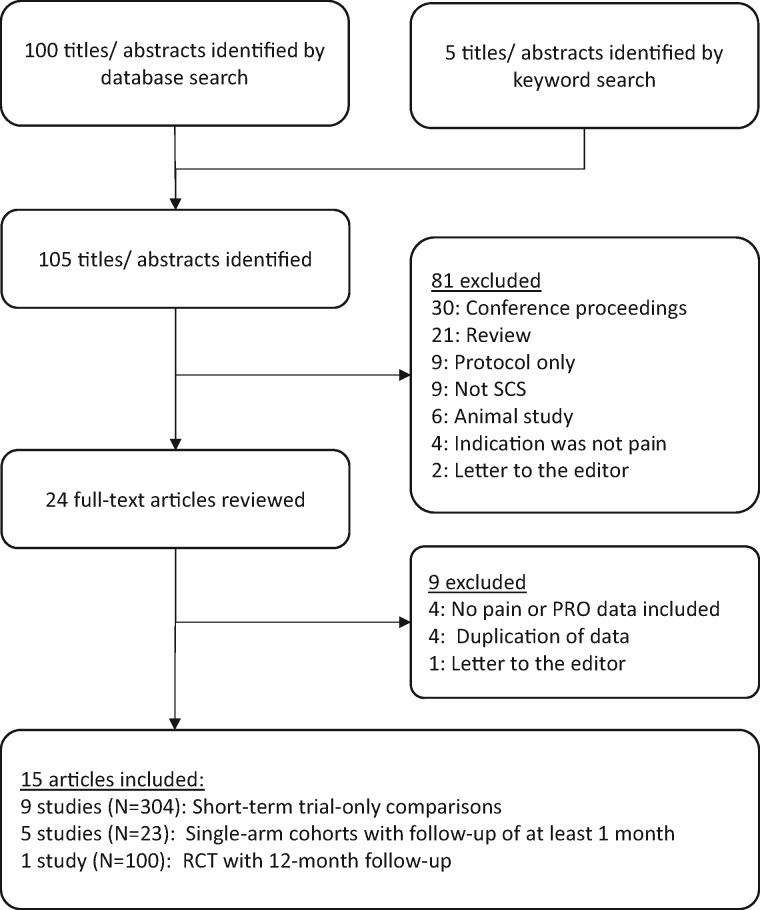
After removal of duplicate titles and assurance of date range restriction, there were 105 titles/abstracts identified. Of these, 81 were excluded on the basis of the criteria above. The remaining 24 full-text titles were reviewed, and nine were excluded. Of these were four articles [23–26] that would have otherwise been eligible for inclusion but were excluded due to being comprised of subject samples that were the same as, or overlapping with, selected articles. Thus, 15 articles were reviewed for this report (Figure 1).
Figure 1.
Summary of article selection.
All included studies used technology manufactured by St. Jude/Abbott, delivering the BurstDR waveform. Follow-up duration varied from one week to two years. Nine studies were short-term, applying stimulation for hours to weeks. Five studies had longer follow-up, although three of these were single case reports. One study was a randomized controlled trial with its primary end point at three months. Apart from the case reports, all were prospective, and a crossover design was used in a majority (nine studies). Nine studies compared outcomes with burst vs tonic SCS, whereas five studies compared burst SCS against baseline, and a single study compared outcomes under burst SCS programming options. One study was identified as Level 1 evidence and six as Level 2, with the remainder at Level 3 or Level 4. Despite the inclusion of articles with high levels of evidence, the conservative GRADE rating system set the level of evidence for controlled studies at “low” and “very low” for observational studies. In both categories, when taken together (not as individual articles), the quality of evidence was downgraded due to limitations/inconsistencies in study quality and imprecise/sparse data. Study design elements and level of evidence for each included article are presented in Table 1.
Table 1.
Designs and levels of evidence of included studies
| First Author, Year | No. (with Burst) | Prospective vs Retrospective | Single Arm vs Multiple Arms | Design | Comparison Being Made | Study Control via | Blinding Used? | Description of Follow-up | Follow-up Duration | Pain Rating Used | PROs Used | NASS Level of Evidence | GRADE Level of Evidence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deer 2018 [11] | 100* | Prospective | Single | Crossover | Tonic vs burst | Randomized order of presentation | No | Longer-term follow-up | 12 wk each* | VAS | PCS, BDI, ODI | 1 | Low† |
| De Ridder 2010 [2] | 12 | Prospective | Single | Crossover | Tonic vs burst | Randomized order of presentation | Yes | Trial only | 1 h–1 mo each | VAS | MPQSF sensory, affective | 2 | |
| De Ridder 2013 [27] | 15 | Prospective | Single | Crossover | Tonic vs burst | Randomized order of presentation and placebo | Yes | Trial only | 1 wk each | VAS | PVAQ | 2 | |
| Kriek 2017 [28] | 29 | Prospective | Single | Crossover | Tonic (40, 500, 1,200 Hz) vs placebo vs burst | Randomized order of presentation and placebo | Yes | Trial only | 2 wk each | VAS | MPQ NWC, MPQ PRI | 2 | |
| Schu 2014 [29] | 20 | Prospective | Single | Crossover | Tonic vs burst | Randomized order of presentation and placebo | Yes | Trial only | 1 wk each | NRS | PCS, MPQSF, PVAQ, ODI | 2 | |
| Tjepkema-Cloostermans 2016 [30] | 40 | Prospective | Single | Crossover | Tonic vs burst (high and low amplitude) | Randomized order of presentation | Yes | Trial only | 2 wk each | VAS | MPQ NWC, MPQ PRI | 2 | |
| Van Haverbergh 2015 [31] | 15 | Prospective | Single | Crossover | Burst 500 Hz vs burst 1,000 Hz) | Randomized order of presentation | Yes | Trial only | 2 wk each | VAS | PCS, PVAQ | 2 | |
| De Ridder 2015 [32] | 102 | Prospective | Single | Pre–post | Tonic vs burst | – | No | Trial only | 2 wk | NRS | 3 | Very low‡ | |
| De Ridder 2015 [33] | 49 | Prospective | Single | Pre–post | Tonic vs burst | – | No | Trial only | 2 wk | NRS | 3 | ||
| Courtney 2015 [34] | 22 | Prospective | Single | Pre–post | Tonic at baseline vs burst | – | No | Trial only | 2 wk | VAS | PCS | 3 | |
| Kinfe 2017 [35] | 12 | Prospective | Single | Pre–post | No-stimulation baseline vs burst | – | No | Longer-term follow-up | 3 mo | VAS | BDI, PSQI | 3 | |
| Muhammad 2017 [36] | 8 | Prospective | Single | Pre–post | No-stimulation baseline vs burst | – | No | Longer-term follow-up | 15 mo (average 12–19 mo) | VAS | BDI, PSQI | 3 | |
| Kriek 2015 [37] | 1 | Retrospective | – | Case report | No-stimulation baseline vs burst | – | No | Longer-term follow-up | 2 y | NRS | 4 | ||
| Rasekhi 2018 [38] | 1 | Retrospective | – | Case report | No-stimulation baseline vs burst | – | No | Longer-term follow-up | 1 mo | VAS | 4 | ||
| Reck 2018 [39] | 1 | Retrospective | – | Case report | No-stimulation baseline vs burst | – | No | Longer-term follow-up | 3 mo | NRS | 4 |
BDI = Beck Depression Index; GRADE = Grades of Recommendation, Assessment, Development, and Evaluation; MPQ = McGill Pain Questionnaire; MPQ NWC = McGill Pain Questionnaire number of words chosen; MPQ PRI = McGill Pain Questionnaire pain rating index; MPQSF = McGill Pain Questionnaire, Short Form; NASS = North American Spine Society; NRS = numeric rating scale; ODI = Oswestry Disability Index; PCS = Pain Catastrophizing Scale; PROs = patient-reported outcomes; PSQI = Pittsburgh Sleep Quality Index; PVAQ = Pain Vigilance and Attention Questionnaire; VAS = visual analog scale.
The intent-to-treat analysis, after 12 weeks (each) of treatment with tonic and burst SCS, included N = 100. Additionally, the study continued open-label through 12 months, with N = 88 contributing pain ratings for any treatment (that is, tonic or burst outcomes, combined).
Due to limitations of study quality (various combinations of small sample size, lack of placebo control, and lack of blinding could reduce statistical power and obscure therapeutic effect against unclear placebo effect) and imprecise or sparse data (majority of studies are trial-only, with one including three months of follow-up; this is an incomplete assessment of a chronic intervention).
Due to limitations of study quality (various combinations of small sample size, lack of control/placebo/blinding and variability in the comparisons being made) and imprecise or sparse data (trial-only data, N = 1 data).
The 15 articles included 427 subjects (1–102 per article). Across the 13 studies that reported gender, approximately 40% of subjects were female and 60% were male. Outside of case reports, articles’ mean reported ages ranged from 42 to 62 years, with a grand mean age of 55.2 years. The most prevalent diagnosis was FBSS, followed by CRPS and DPN. Subject demographics for each article are presented in Table 2.
Table 2.
Summary of patient demographics
| First Author, Year | No. (with Burst) | Average Age, y | Gender, % | Etiology of Pain, % |
||||
|---|---|---|---|---|---|---|---|---|
| FBSS | CRPS | Radiculopathy | DPN | Other | ||||
| Deer 2018 [11] | 100* | 59.1 | 60 female | 42 | 1 | 37 | 20 | |
| 40 male | ||||||||
| De Ridder 2010 [2] | 12 | 52.3 | 33.3 female | 92 | 8 | |||
| 66.6 male | ||||||||
| De Ridder 2013 [27] | 15 | 54.1 | 73.3 female | 87 | 13 | |||
| 26.7 male | ||||||||
| Kriek 2017 [28] | 29 | 42.6 | 14 female | 100 | ||||
| 86 male | ||||||||
| Schu 2014 [29] | 20 | 58.6 | 65 female | 100 | ||||
| 35 male | ||||||||
| Tjepkema-Cloostermans 2016 [30] | 40 | 58 | 40 female | 80 | 2.5 | 7.5 | 10 | |
| 60 male | ||||||||
| Van Haverbergh 2015 [31] | 15 | 52 | 46.7 female | 100 | ||||
| 53 male | ||||||||
| De Ridder 2015 [32] | 102 | † | ‡ | § | § | |||
| De Ridder 2015 [33] | 49 | 56.2 | ‡ | 47 | 25 | 29 | ||
| Courtney 2015 [34] | 22 | 58 | 60 female | 32 | 5 | 36 | 27 | |
| 40 male | ||||||||
| Kinfe 2017 [35] | 12 | 54.3 | 58.3 female | 100 | ||||
| 41.6 male | ||||||||
| Muhammad 2017 [36] | 8 | 62.1 | ¶ | 100 | ||||
| Kriek 2015 [37] | 1 | 65 | 100 female | 100 | ||||
| Rasekhi 2018 [38] | 1 | 72 | 100 male | 100 | ||||
| Reck 2018 [39] | 1 | 53 | 100 male | 100 | ||||
CRPS = complex regional pain syndrome; DPN = diabetic peripheral neuropathy; FBSS = failed back surgery syndrome.
The intent-to-treat analysis, after 12 weeks (each) of treatment with tonic and burst SCS, included N = 100. Additionally, the study continued open-label through 12 months, with N = 88 contributing pain ratings for any treatment (that is, tonic or burst outcomes, combined).
Ages were reported as the mean ages of subjects recruited at hospital 1 (56, N = 57) and at hospital 2 (53, N = 45).
Not reported.
Text indicated that the 102 neuropathic pain diagnoses were “mostly related” to FBSS or DPN, but precise numbers were not provided.
Text reported genders pooled across all enrolled subjects (N = 16), which included eight subjects treated with high-frequency SCS.
Pain, Preference, and PRO Scores
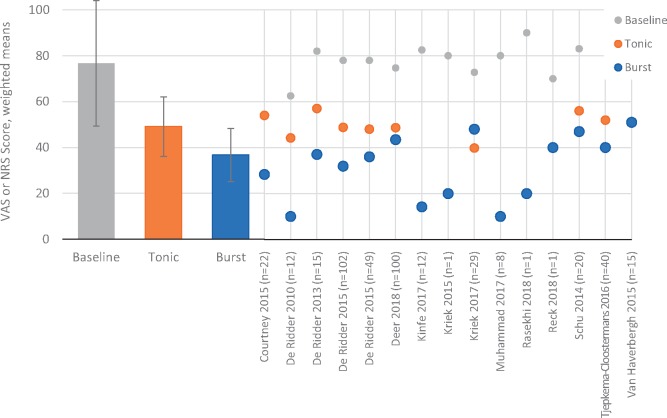
Pain scores were compared across baseline, tonic SCS, and burst SCS conditions. At baseline, the weighted pooled mean was 76.7 (±27.4). With tonic SCS, this was reduced to 49.2 (±12.9), and with burst SCS it was further reduced to 36.7 (±11.6) (Figure 2).
Figure 2.
Pain scores (visual analog scale or numeric rating scale) at baseline or with active spinal cord stimulation (tonic vs burst) are compared. Left side: Bar heights represent pooled weighted means across studies for each of the stimulation conditions. Error bars represent standard error of the mean. Right side: Points represent means for each of the studies that contributed to the pooled means for each of the stimulation conditions.
When weighted and pooled across all studies that reported preference, 65% of subjects stated a preference for burst SCS, whereas 20% preferred tonic SCS and 16% had no preference or preferred some other SCS waveform (Figure 3).
Figure 3.
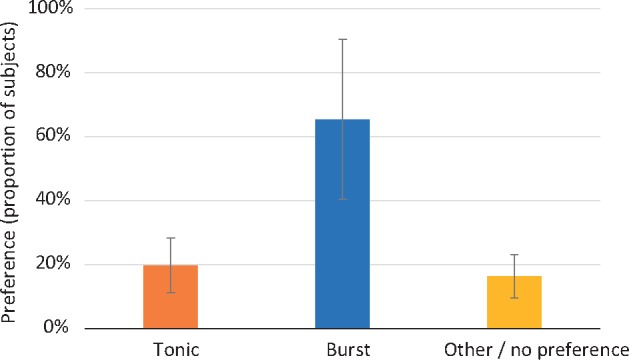
Across studies, a higher proportion of subjects preferred burst spinal cord stimulation (SCS) than preferred tonic SCS or another SCS/had no preference. Bar heights represent pooled means across studies for each of the stimulation conditions. Error bars represent standard error of the mean.
Weighted pooled BDI scores were 12.1 and improved to 9.2 with burst SCS treatment. For comparison, the nonpain population norm is 9.1. Weighted pooled PCS scores were 18.2 at baseline and improved to 6.3 with burst SCS treatment. For comparison, the nonpain population norm is 13.9. Weighted pooled PVAQ scores were 35.0 at baseline and improved to 17.1 with burst SCS treatment. For comparison, the nonpain population norm is 33.5 (Figure 4).
Figure 4.
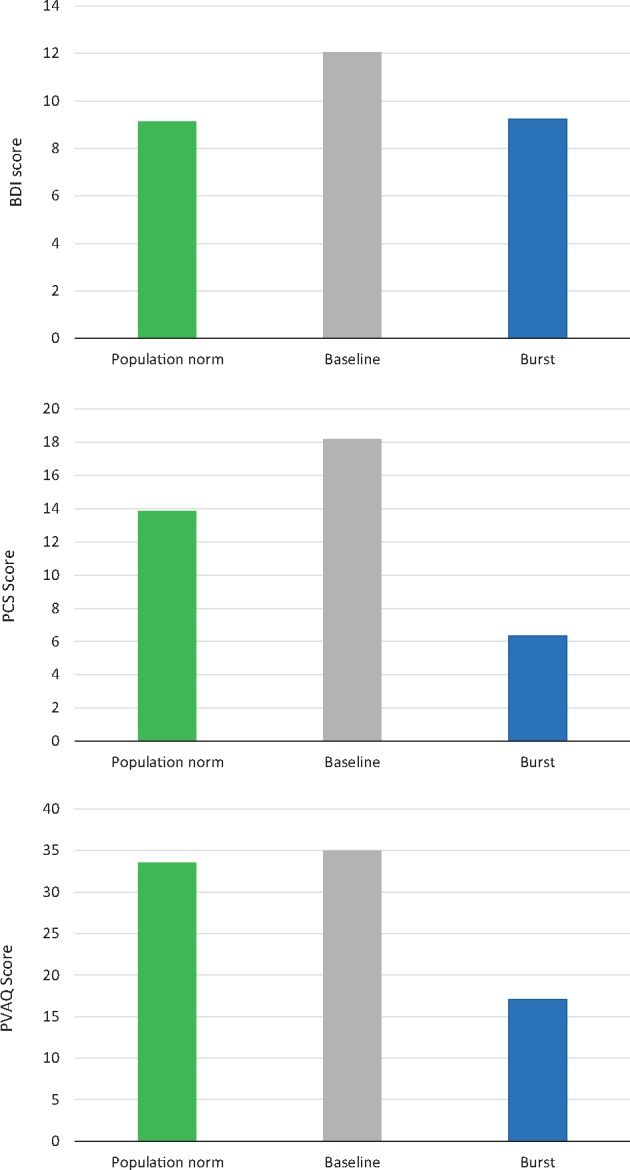
Depression (Beck Depression Inventory, top), pain catastrophizing (Pain Catastrophizing Scale, middle), and pain vigilance and awareness (Pain Vigilance and Attention Questionnaire, bottom) scores were highest at baseline and were reduced after treatment with burst spinal cord stimulation. Bar heights represent pooled means across studies. Population norms from the literature are included for comparisons (green bars).
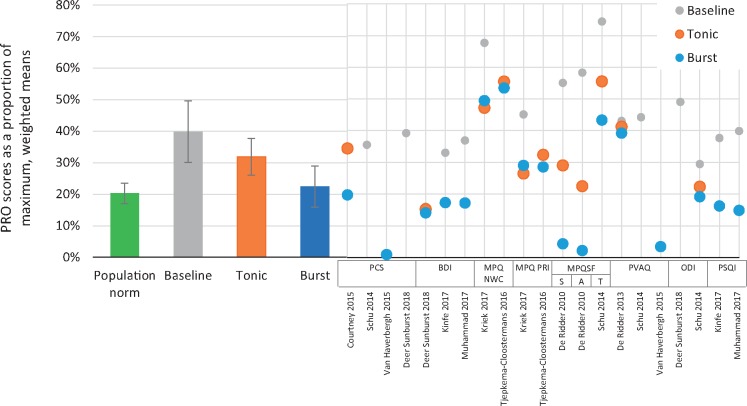
The normalized weighted pooled scores for all PROs, combined, were compared for baseline, tonic SCS, and burst SCS conditions, and with the normalized population norms. At baseline, PRO scores were 37.8% (±9.8%) of their maximum (worst) score. With tonic SCS, this was reduced to 31.9% (±5.9%), and with burst SCS it was further reduced to 25.8% (±6.6%). For comparison, the normalized nonpain population norm was 19.8% (±3.2%) (Figure 5).
Figure 5.
Patient-reported outcome (PRO) scores were normalized as proportions of the maximum/worst possible score for each instrument and weighted by sample size. Combined pooled means were then calculated for all PROs across studies at baseline or with active spinal cord stimulation (SCS; tonic vs burst). Left side: Bar heights represent pooled means across SCS studies at baseline, with tonic SCS, and with burst SCS. Error bars represent standard error of the mean. Pooled population norms from the literature (also normalized and weighted by the same method; green bar) are included as a comparison. Right side: Points represent normalized scores for each of the studies that contributed to the pooled means for each of the stimulation conditions.
Safety Data
Eight of the 15 articles reported on AEs and complications. Safety events were largely mild and readily resolved. In all, two study-related serious AEs were reported, both in the SUNBURST study (Table 3).
Table 3.
Listing of AEs and complications reported in the included articles
| Study | No. (with Burst) | Safety Language from Article |
|---|---|---|
| Deer 2018 [11] | 100* | 2: Study-related SAEs (persistent pain and/or numbness; unsuccessful lead placement) |
| 62: Study-related AEs | ||
| 19: SAEs unrelated to study (including two deaths) | ||
| 75: AEs unrelated to study | ||
| De Ridder 2010 [2] | 12 | Not reported |
| De Ridder 2013 [27] | 15 | Not reported |
| Kriek 2017 [28] | 29 | 0: SAEs |
| 3: Lead dislocation/migration | ||
| 22: Long and frequent charging times | ||
| 1: Stimulation stopped involuntarily | ||
| 1: Stimulation switches off | ||
| 8: Electrode reconfiguration required | ||
| 27: Pulse width adjusted | ||
| 1: Comfortable paresthesia not reached | ||
| 8: Pmax too high | ||
| 2: Itching or rash | ||
| 3: Stimulation could not be set high enough | ||
| 1: Standard stimulation set to 60 Hz instead of 40 Hz | ||
| 1: Axial paresthesia, uncomfortable | ||
| 4: Headache | ||
| 3: Converted to standard stimulation | ||
| 1: Stimulation discontinued | ||
| Schu 2014 [29] | 20 | 0 AEs |
| Tjepkema-Cloostermans 2016 [30] | 40 | 3: Heavy feeling or pressure in legs or feet |
| 1: Increased sensation of local stimulation around IPG | ||
| 3: Perception of soft paresthesia at least once | ||
| Van Haverbergh 2015 [31] | 15 | Not reported |
| De Ridder 2015 [32] | 102 | Not reported |
| De Ridder 2015 [33] | 49 | Not reported |
| Courtney 2015 [34] | 22 | 1: Dizziness and sensation of warm feet |
| 1: Warm sensation in foot with moderate discomfort | ||
| 2: AEs unrelated to study procedures or device | ||
| Kinfe 2017 [35] | 12 | 0: SAEs |
| 3: Temporary skin irritation at IPG site | ||
| Muhammad 2017 [36] | 8 | 0: SAEs |
| 4: Mild AEs along the extension and IPG, resolving without therapy within one to two weeks | ||
| Kriek 2015 [37] | 1 | 1: Tingling sensation in left arm; resolved with lower amplitude |
| 1: Increased pain score due to increase in CRPS activity; resolved with reprogramming | ||
| Rasekhi 2018 [38] | 1 | Not reported |
| Reck 2018 [39] | 1 | Not reported |
AE = adverse event; CRPS = complex regional pain syndrome; IPG = Implanted pulse generator; SAE = serious adverse event.
Safety data were reported for all subjects through the 12-month end point.
Discussion
This systematic review of burst SCS included 15 peer-reviewed articles with a combined total of 427 subjects. A pooled analysis of pain intensity ratings, preference for burst SCS, and PROs was completed. Although the data were collected from heterogenous patient populations suffering from various pain diagnoses and with varying follow-up durations, the pooled analysis revealed consistent and clear effect in the incremental benefit of burst SCS above that of tonic SCS. The pooled analysis showed that the average pooled pain score with tonic SCS was 49.2, whereas with burst SCS it was 36.7, a 12.5-point difference. This is likely to be experienced by the subjects as a clinically important difference, given that the minimal clinically important difference (MCID) for multidisciplinary pain treatment for low back pain was 1 point on a 0–10 NRS [40] and the MCID for SCS treatment of postlaminectomy syndrome was 1–1.2 cm on a 10-cm VAS [41]. The clinical importance of the incremental benefit of burst was supported by the large majority of subjects who preferred burst SCS over tonic. Likewise, a common pooled analysis of all reported PROs showed that burst SCS achieved better outcomes than tonic. Pooled analyses of several relevant PROs also showed that burst SCS improved outcomes relative to baseline values and achieved scores that were similar to published reference values for nonpain populations. Complications appeared generally mild and consistent with those of other waveforms.
A notable finding in the pooled analysis of pain intensities was that the burst vs tonic difference (12.5 points) was more pronounced than the difference reported in the SUNBURST RCT (5.2 points) [11]. This report’s pooled analysis included SUNBURST data as well as prospective and observational data from the other 14 available studies. One possible explanation is that the SUNBURST study enrolled only those who responded to tonic SCS during the pre-implant trial period, which enriched the population for tonic responders and may have led some of that group to favor tonic SCS. Moreover, during the crossover phase of the trial, burst SCS stimulation used high amplitude (average of 1.73 mA, which is higher than that reported in other trials [2]) and may have been influenced by the higher stimulation amplitudes used for tonic SCS. This may have resulted in suboptimal outcome. On the other hand, another explanation is that the tightly controlled inclusion and exclusion criteria of the SUNBURST study may have made the study population less representative of the typical SCS population and therefore contributed to a less observable difference between the studied interventions. Order effects were ruled out statistically. Regardless, 70.8% of SUNBURST subjects preferred burst SCS, which is very similar to the 65% pooled preference proportion in this report. This suggests the utility of burst SCS for patients who may have lost efficacy with tonic SCS or are ready for a modification of their therapy due to the implant’s battery being at the end of its life. There are many factors that may contribute to the preference for one waveform over another, such as the lack of perceptible paresthesia, improved emotional status, better pain relief, or a combination of factors that may form novel questions for future research into SCS waveforms.
In addition to the preferential effect of burst SCS on pain intensity, PRO outcomes were consistently improved with burst SCS. This was demonstrated in pooled analyses that showed that burst SCS achieved a global PRO improvement (across multiple domains such as function, quality of life, pain interference, and mood) and individual PRO improvements to similar levels as nonpain population norms for the BDI, PCS, and PVAQ. These improvements in PROs indicate that burst SCS may have benefits for the holistic pain experience, not only pain intensity. In addition, the BDI, PCS, and PVAQ are all tied to mood, emotion, and attention regarding pain. This effect was very pronounced, especially with the PCS and PVAQ, which actually improved to better than population norms. There is considerable evidence that different brain pathways may mediate the sensory and affective components of pain [42,43].
Burst stimulation SCS is based on the observation that fibers originating from thalamus, when exposed to burst stimulation, are more likely to activate cortical areas [44]. Central processing of pain stimuli are believed to be processed in parallel in two signal pathways: a medial affective (attention-controlled) pathway and a lateral discriminatory signal pathway [45]. It is postulated that the medial (affective) pain system is triggered by nociceptive neurons in lamina I of the dorsal horn and that this is achieved by the burst, not tonic, SCS waveform. The charge accumulation and passive discharge are key factors in the burst waveform that mimic natural neuronal burst firing in order to modulate the medial pathway. Tonic stimulation, on the other hand, primarily triggers wide–dynamic range neurons and thus is thought to activate only the lateral (discriminative) pain pathway [23,46]. Compelling data regarding the effect of burst SCS on pooled outcomes for PROs that may be related to emotional functioning support the hypothesis that burst SCS is mediated by the medial supraspinal pathway. These findings align with a previous report that showed that subjects’ attention to pain, as measured by the PVAQ, was significantly improved with burst SCS, compared with both sham and tonic stimulation [27]. Source-localized EEG data also showed significantly higher alpha activity in the dorsal thalamus in burst stimulation compared with other stimulation modes in a subset of patients in the same trial [27]. Additionally, objective measures of the cortical response to pain in both the medial and lateral pathways have been shown to be decreased selectively by burst SCS [47]. This suggests that, for future studies, affective measures such as the BDI, PVAQ, and PCS should be recorded in conjunction with VAS and functional improvement. This is in accordance with recommendations of the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials group (IMMPACT) [48,49]. It also opens some consideration for the validity of pre-SCS screening for comorbid depression and mood if burst SCS may have a beneficial effect on those aspects as well as pain intensity.
Potential Limitations
This summary of published evidence about burst SCS has several limitations. One is that studies of different designs and follow-up durations were combined. For example, the SUNBURST study was set alongside short-term studies in which burst and tonic SCS were compared with trial-only reports (five studies) and against N-of-one case reports (three studies). Additionally, the follow-up time in nine studies was two weeks or less, and only five trials followed subjects for three months or more. Furthermore, there were differences in comparators (nine were tonic vs burst, five were baseline vs burst, and one was burst vs burst). This heterogeneity in the selected literature is reflective of the currently limited state of the knowledge base for this emerging technology. To attempt to restrict our review to one or another category of article would have reduced the number of available studies to the point of precluding meaningful conclusions. Thus, the quantitative analysis—essentially a grand mean weighted by sample size in order to avoid unfairly emphasizing the outcomes of small studies and case reports—does not approach meta-analysis methodology, which is not yet possible due to the underlying literature. The primary strength with this approach, however, is its simplicity in that it forms a representative “snapshot” of all existing information in a raw data format. It should be noted that care was taken not to double-count data (as can be a concern in some meta-analyses including multiple reports from the same research groups).
However promising and beneficial to selected patients, future clinical trials on burst SCS could benefit from implementing established concepts of study design aimed at minimizing bias and raising the level of evidence produced. RCTs should have proper treatment allocation, blinded outcome assessment, and longer duration of follow-up. Crossover trials should consider wash-in and washout periods, as well as treatment order effects. More trials without industry sponsorship would be beneficial. Additionally, instead of simple documentation of treatment in a case report style, N-of-one studies could more valuably implement prospective randomization to treatment and control periods, and thus be of great potential value in exploring new indications and new treatment algorithms [50].
The IMMPACT group has produced a number of consensus papers with recommendation for design and interpretation of clinical research in chronic pain. Recommended core outcome domains and corresponding specific measurement for each domain have been proposed [48]. Adherence to these recommendations in future clinical trials, when suitable, would facilitate cross-study comparisons of trial data, pooled data analysis, and meta-analyses in this field. Highlighted in other IMMPACT publications, a critical consideration in study design is to incorporate the concept of clinical importance. What change in a measured outcome measurement represents a meaningful, clear change for individual patients should be established in order for the trial to be of value to patients, clinicians, and payers. Clear and concise recommendations for methodology exist [49].
Moreover, it is well established that the clinical importance of individual patient improvements and the clinical importance of mean group differences must be interpreted differently [49]. These are two distinct aspects of the result of a clinical trial, and both are important when evaluating the result. Differences in the clinical changes (e.g., mean reductions in pain intensity) observed between two interventions may not adequately describe the overall potential benefit of an investigated treatment for an individual and may obscure clinically important aspects. A comprehensive reporting on percentage of responders with meaningful improvement, evaluation of secondary outcomes, and safety and tolerability must all be weighed to understand the full benefit of the investigated treatment.
Apart from high-quality efficacy studies, the field in general would benefit from systematic aggregation of real-world outcome data collected in a standardized way, preferably in multinational registries, to investigate long-term clinical effectiveness and monitor safety. This would again serve as a useful foundation for exploring the cost-effectiveness of different neuromodulation treatments.
A systematic review of burst SCS clinical outcomes was published in 2016 and concluded that there was not sufficient evidence to recommend for or against the use of burst SCS and that there was “very low” confidence in recommending burst SCS on the strength of the evidence [6]. That finding was, however, based on the five studies that were available at the time. This report provides a much more complete picture of the burst SCS literature base, including a newly published large RCT. The GRADE rating of controlled studies here, although low overall, indicates that the balance of evidence regarding burst SCS as an intervention has improved since the 2016 systematic review. However, overall interpretation may benefit from the development of a rating system that is specific to the treatment patterns and study design details found in neuromodulation, as has been suggested previously (51). Importantly, this review provides, for the first time, quantitative evidence for the overall efficacy of burst SCS in the pooled analyses with specific objective data on medial and lateral pathway parameters.
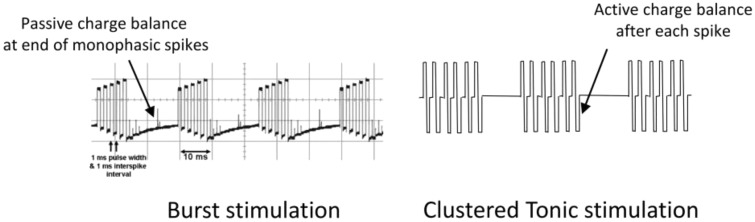
All of the articles included in this systematic review used the BurstDR waveform. It should be noted, however, that there are other SCS waveforms that are also termed “burst,” most notably that delivered by the Boston Scientific Spectra system. Considerable differences exist, however, in their production, functional consequences, and clinical outcomes. The BurstDR waveform functions by passive recharge, in which charge is built up sequentially during the five-spike train and discharges only after the train ends; in contrast, Boston Scientific’s burst waveform uses active recharge to repeatedly balance the charge after every spike [52]. It has been argued that because the latter does not exhibit a charge summation, it should more accurately be termed “clustered tonic stimulation” (Figure 6) [53]. A study of conventional tonic vs active recharge burst SCS in an animal model of chronic pain showed that conventional SCS provided more effective pain relief with equivalent charge densities [54]. A response suggested that there may have been a different outcome if passive recharge BurstDR stimulation had been used [55]. Indeed, behavioral animal testing has shown that the recharge phase is a relevant factor in pain responses [56]. A recent study of electrophysiological monitoring during intraoperative SCS activation indicated that passive recharge burst SCS required lower thresholds to generate an EMG response, activated distal musculature (indicating charge penetration deep into the dorsal columns of the spinal cord), and creation of a hyperexcitable state. Active recharge burst SCS, on the other hand, although also exhibiting some degree of threshold-lowering, had a stimulation artifact pattern that was similar to that of tonic SCS. This neurological difference was theorized to be due to the BurstDR waveform creating a “primed” state for energy-efficient ongoing stimulation [57]. Active recharge SCS was selected by patients as the best option from among a number of available waveforms only 8.4% of the time [58]. As has been pointed out in the literature, however, the ideal set of parameters for clinically useful burst SCS has not been definitively identified [59].
Figure 6.
Passive-recharge BurstDR waveform (left) and active recharge burst waveform (right), termed “clustered tonic stimulation” by one author. Reprinted with permission from De Ridder [53].
Here, a novel approach was used to address the apparent dichotomy of findings between SUNBURST vs other reports and provides a method to integrate real-world evidence from various peer-reviewed sources on burst SCS. Burst SCS is likely to be a valuable addition to the resources with which the neuromodulator can employ ingenuity toward the otherwise intractable problem of chronic pain.
Acknowledgments
The authors thank Allison Foster, PhD, an independent medical writer, for her assistance in preparing the manuscript.
Funding sources: This manuscript was prepared with financial support from Abbott.
Conflicts of interest: Dr. Chakravarthy is a consultant to Abbott, MedinCell, and Bioness Inc. and founder of Douleur Therapeutics, NanoAxis, and Newrom Biomedical. Dr. Malayil is a consultant to Depomed and Abbott. Dr. Kirketeig has received lecturing fees and Uppsala University Hospital research grants from Abbott/St. Jude Medical. Dr. Deer is a consultant for Abbott, Axonics, Bioness, Nalu, Saluda, Vertos, Vertiflex, Spinethera, Flowonix, and Cornorloc.
Supplement sponsorship: This article appears as part of the supplement “Neuromodulation of the Spine and Nervous System” sponsored by Abbott.
References
- 1. Verrills P, Sinclair C, Barnard A.. A review of spinal cord stimulation systems for chronic pain. J Pain Res 2016;9:481–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Ridder D, Vanneste S, Plazier M, Van Der Loo E, Menovsky T.. Burst spinal cord stimulation: Toward paresthesia-free pain suppression. Neurosurgery 2010;665:986–90. [DOI] [PubMed] [Google Scholar]
- 3. Miller JP, Eldabe S, Buchser E, et al. Parameters of spinal cord stimulation and their role in electrical charge delivery: A review. Neuromodulation 2016;194:373–84. [DOI] [PubMed] [Google Scholar]
- 4. Chakravarthy K, Kent AR, Raza A, Xing F, Kinfe TM.. Burst spinal cord stimulation: Review of preclinical studies and comments on clinical outcomes. Neuromodulation 2018;215:431–9. [DOI] [PubMed] [Google Scholar]
- 5. Deer TR, Campos LW, Pope JE.. Evaluation of Abbott’s BurstDR stimulation device for the treatment of chronic pain. Exp Rev Med Dev 2017;146:417–22. [DOI] [PubMed] [Google Scholar]
- 6. Hou S, Kemp K, Grabois M.. A systematic evaluation of burst spinal cord stimulation for chronic back and limb pain. Neuromodulation 2016;194:398–405. [DOI] [PubMed] [Google Scholar]
- 7. Pope JE, Falowski S, Deer TR.. Advanced waveforms and frequency with spinal cord stimulation: Burst and high-frequency energy delivery. Expert Rev Med Devices 2015;124:431–7. [DOI] [PubMed] [Google Scholar]
- 8. De Ridder D, Perera S, Vanneste S.. Are 10 kHz stimulation and burst stimulation fundamentally the same? Neuromodulation 2017;207:650–3. [DOI] [PubMed] [Google Scholar]
- 9. Tang R, Martinez M, Goodman-Keiser M, et al. Comparison of burst and tonic spinal cord stimulation on spinal neural processing in an animal model. Neuromodulation 2014;172:143–51. [DOI] [PubMed] [Google Scholar]
- 10. Gong WY, Johanek LM, Sluka KA.. A comparison of the effects of burst and tonic spinal cord stimulation on hyperalgesia and physcial activity in an animal model of neuropathic pain. Anesth Analg 2016;1224:1178–85. [DOI] [PubMed] [Google Scholar]
- 11. Deer T, Slavin KV, Amirdelfan K.. Success Using Neuromodulation with BURST (SUNBURST) study: Results from a prospective, randomized controlled trial using a novel burst waveform. Neuromodulation 2018;211:56–66. [DOI] [PubMed] [Google Scholar]
- 12. North American Spine Society. Levels of evidence for primary research question. Available at: https://www.spine.org/Documents/ResearchClinicalCare/LevelsOfEvidence.pdf (accessed September 2018).
- 13. North American Spine Society. Clinical Guidelines for Multidisciplinary Spine Care: Diagnosis and Treatment of Degenerative Lumbar Spinal Stenosis. Burr Ridge, IL: North American Spine Society; 2007. [Google Scholar]
- 14. Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Balshem H, Helfand M, Schunemann HJ.. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;644:401–6. [DOI] [PubMed] [Google Scholar]
- 16. Whisman MA, Richardson ED.. Normative data on the Beck Depression Inventory—second edition (BDI-II) in college students. J Clin Psychol 2015;719:898–907. [DOI] [PubMed] [Google Scholar]
- 17. Osman A, Barrios FX, Gutierrez PM, et al. The Pain Catastrophizing Scale: Further psychometric evaluation with adult samples. J Behav Med 2000;234:351–65. [DOI] [PubMed] [Google Scholar]
- 18. McWilliams LA, Asmundson GJG.. Assessing individual differences in attention to pain: Psychometric properties of the Pain Vigilance and Awareness Questionnaire modified for a non-clinical pain sample. Pers Individ Dif 2001;312:239–46. [Google Scholar]
- 19. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ.. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res 1989;282:193–213. [DOI] [PubMed] [Google Scholar]
- 20. Melzack R. The McGill Pain Questionnaire: Major properties and scoring methods. Pain 1975;13:277–99. [DOI] [PubMed] [Google Scholar]
- 21. Melzack R. The short-form McGill Pain Questionnaire. Pain 1987;302:191–7. [DOI] [PubMed] [Google Scholar]
- 22. Fairbank JCT, Pynsent PB.. The Oswestry Disability Index. Spine 2000;2522:2940–53. [DOI] [PubMed] [Google Scholar]
- 23. De Ridder D, Vanneste S.. Burst and tonic spinal cord stimulation: Different and common brain mechanisms. Neuromodulation 2016;191:47–59. [DOI] [PubMed] [Google Scholar]
- 24. de Vos CC, Bom MJ, Vanneste S, Lenders MW, de Ridder D.. Burst spinal cord stimulation evaluated in patients with failed back surgery syndrome and painful diabetic neuropathy. Neuromodulation 2014;172:152–9. [DOI] [PubMed] [Google Scholar]
- 25. Kinfe TM, Pintea B, Link C, et al. High frequency (10 kHz) or burst spinal cord stimulation in failed back surgery syndrome patients with predominant back pain: Preliminary data from a prospective observational study. Neuromodulation 2016;193:268–75. [DOI] [PubMed] [Google Scholar]
- 26. Muhammad S, Chaudhry SR, Yearwood TL, Krauss JK, Kinfe TM.. Changes in metabolic disorders associated peripheral cytokine/adipokine traffic in non-obese chronic back patients responsive to burst spinal cord stimulation. Neuromodulation 2018;211:31–7. [DOI] [PubMed] [Google Scholar]
- 27. De Ridder D, Plazier M, Kamerling N, Menovsky T, Vanneste S.. Burst spinal cord stimulation for limb and back pain. World Neurosurg 2013;805:642–9. [DOI] [PubMed] [Google Scholar]
- 28. Kriek N, Groeneweg JG, Stronks DL, De Ridder D, Huygen FJ.. Preferred frequencies and waveforms for spinal cord stimulation in patients with complex regional pain syndrome: A multicentre, double-blind, randomized and placebo-controlled crossover trial. Eur J Pain 2017;213:507–19. [DOI] [PubMed] [Google Scholar]
- 29. Schu S, Slotty PJ, Bara G, et al. A prospective, randomised, double-blind, placebo-controlled study to examine the effectiveness of burst spinal cord stimulation pattersn for the treatment of failed back surgery syndrome. Neuromodulation 2014;175:443–50. [DOI] [PubMed] [Google Scholar]
- 30. Tjepkema-Cloostermans MC, de Vos CC, Wolters R, Dijkstra-Scholten C, Lenders MW.. Effect of burst stimulation evaluated in patients familiar with spinal cord stimulation. Neuromodulation 2016;195:492–7. [DOI] [PubMed] [Google Scholar]
- 31. Van Havenbergh T, Vancamp T, Van Looy P, Vanneste S, De Ridder D.. Spinal cord stimulation for the treatment of chronic back pain patients: 500-Hz vs 1000-Hz burst stimulation. Neuromodulation 2015;181:9–12. [DOI] [PubMed] [Google Scholar]
- 32. De Ridder D, Lenders MW, De Vos CC, et al. A 2-center comparative study on tonic versus burst spinal cord stimulation. Amount of responders and amount of pain suppression. Clin J Pain 2015;315:433–7. [DOI] [PubMed] [Google Scholar]
- 33. De Ridder D, Vancamp T, Lenders MW, De Vos CC, Vanneste S.. Is preoperative pain duration important in spinal cord stimulation? A comparison between tonic and burst stimulation. Neuromodulation 2015;181:13–7. [DOI] [PubMed] [Google Scholar]
- 34. Courtney P, Espinet A, Mitchell B, et al. Improved pain relief with burst spinal cord stimulation for two weeks in patients using tonic stimulation: Results from a small clinical study. Neuromodulation 2015;185:361–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kinfe TM, Muhammad S, Link C, et al. Burst spinal cord stimulation increases peripheral antineuroinflammatory interleukin 10 levels in failed back surgery syndrome patients with predominant back pain. Neuromodulation 2017;204:322–30. [DOI] [PubMed] [Google Scholar]
- 36. Muhammad S, Roeske S, Chaudhry SR, Kinfe TM.. Burst or high-frequency (10 kHz) spinal cord stimulation in failed back surgery syndrome patients with predominant back pain: One year comparative data. Neuromodulation 2017;207:661–7. [DOI] [PubMed] [Google Scholar]
- 37. Kriek N, Groeneweg G, Huygen FJ.. Burst spinal cord stimulation in a patient with complex regional pain syndrome: A 2-year follow-up. Pain Pract 2015;156:E59–64. [DOI] [PubMed] [Google Scholar]
- 38. Rasekhi R, Babb D, Price C.. Neuromodulatory burst therapy for Agent Orange-induced peripheral neuropathy: A case report. A A Pract 2018;107:165–7. [DOI] [PubMed] [Google Scholar]
- 39. Reck TA, Landmann G.. Successful spinal cord stimulation for neuropathic below-level spinal cord injury pain following complete paraplegia: A case report. Spinal Cord Ser Cases 2017;3:17049.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lauridsen HH, Hartvigsen J, Manniche C, Korsholm L, Grunnet-Nilsson N.. Responsiveness and minimal clinically important difference for pain and disability instruments in low back pain patients. BMC Musculoskelet Disord 2006;7:82–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Paul AR, Kumar V, Roth S, Gooch MR, Pilitsis JG.. Establishing minimal clinically important difference of spinal cord stimulation therapy in post-laminectomy syndrome. Neurosurgery 2017;816:1011–5. [DOI] [PubMed] [Google Scholar]
- 42. Xiao X, Zhang YQ.. A new perspective on the anterior cingulate cortex and affective pain. Neurosci Biobehav Rev 2018;90:200–11. [DOI] [PubMed] [Google Scholar]
- 43. Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science 2000;2885472:1769–72. [DOI] [PubMed] [Google Scholar]
- 44. Swadlow HA, Gusev AG.. The impact of ‘bursting’ thalamic impulses at a neocortical synapse. Nature Neuroscience 2001;44:402–8. [DOI] [PubMed] [Google Scholar]
- 45. Kulkarni B, Bentley DE, Elliott R, et al. Attention to pain localization and unpleasantness discriminates the functions of the medial and lateral pain systems. Eur J Neurosci 2005;2111:3133–42. [DOI] [PubMed] [Google Scholar]
- 46. De Ridder D, Vancamp T, Vanneste S.. Fundamentals of burst stimulation of the spinal cord and brain In: Krames ES, Peckham PH, Rezai AR, eds. Neuromodulation: Comprehensive Textbook of Principles, Technologies, and Therapies. London, UK: Elsevier Academic Press. 2nd ed.2018: pp 147–160. [Google Scholar]
- 47. Bocci T, De Carolis G, Paroli M, et al. Neurophysiological comparison among tonic, high frequency, and burst spinal cord stimulation: Novel insights into spinal and brain mechanisms of action. Neuromodulation 2018;215:480–8. [DOI] [PubMed] [Google Scholar]
- 48. Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;1131:9–19. [DOI] [PubMed] [Google Scholar]
- 49. Dworkin RH, Turk DC, McDermott MP, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain 2009;1463:238–44. [DOI] [PubMed] [Google Scholar]
- 50. Shamseer L, Sampson M, Bukutu C, et al. CONSORT extension for reporting N-of-1 trials (CENT) 2015: Explanation and elaboration. BMJ 2015;350:h1793.. [DOI] [PubMed] [Google Scholar]
- 51. De Ridder D, Vanneste S.. Response: A systematic evaluation of burst spinal cord stimulation for chronic back and limb pain. Neuromodulation 2016;197:785–6. [DOI] [PubMed] [Google Scholar]
- 52. Ahmed S, Yearwood T, De Ridder D, Vanneste S.. Burst and high frequency stimulation: Underlying mechanism of action. Expert Rev Med Devices 2018;151:61–70. [DOI] [PubMed] [Google Scholar]
- 53. De Ridder D. Comment in Meuwissen, et al. 2017, ‘Conventional-SCS vs burst-SCS and the behavioral effect on mechanical hypersensitivity in a rat model of chronic neuropathic pain: Effect of amplitude.’ Neuromodulation 2018;21:26–8. [DOI] [PubMed] [Google Scholar]
- 54. Meuwissen KPV, Gu JW, Zhang TC, Joosten EAJ.. Conventional-SCS vs burst-SCS and the behavioral effect on mechanical hypersensitivity in a rat model of chronic neuropathic pain: Effect of amplitude. Neuromodulation 2018;211:19–30. [DOI] [PubMed] [Google Scholar]
- 55. Falowski SM. Fundamental differences in burst stimulation waveform design: Eliminating confusion in the marketplace. Neuromodulation 2018;213:320.. [DOI] [PubMed] [Google Scholar]
- 56. Weisshaar CL, Kent AR, Venkatesan L, Winkelstein BA. Comparison of burst SCS paradigms on acute spinal neuronal activity in a rat model of painful radiculopathy. Paper presented at: American Society of Regional Anesthesia and Pain Medicine (ASRA); Nov 17–19, 2016; San Diego, CA.
- 57. Falowski SM. An observational case series of spinal cord stimulation waveforms visualized on intraoperative neuromonitoring. Neuromodulation 2019; 222:219–28. [DOI] [PubMed] [Google Scholar]
- 58. Berg AP, Mekel-Bobrov N, Goldberg E, Huynh D, Jain R.. Utilization of multiple spinal cord stimulation (SCS) waveforms in chronic pain patients. Expert Rev Med Devices 2017;148:663–8. [DOI] [PubMed] [Google Scholar]
- 59. Meuwissen KPV, Gu JW, Zhang TC, Joosten EAJ.. Response to: Fundamental differences in burst stimulation waveform design: Eliminating confusion in the marketplace. Neuromodulation 2018;217:721–2. [DOI] [PubMed] [Google Scholar]