Abstract
Objective
Approximately 55–76% of Service members use dietary supplements for various reasons, including pain and related outcomes. This work evaluates current research on dietary ingredients for chronic musculoskeletal pain to inform decisions for practice and self-care, specifically for Special Operations Forces personnel.
Methods
A steering committee convened to develop research questions and factors required for decision-making. Key databases were searched through August 2016. Eligible systematic reviews and randomized controlled trials were assessed for methodological quality. Meta-analysis was applied where feasible. GRADE was used to determine confidence in the effect estimates. The committee made evidence-informed judgments and recommendations for practice and self-care use.
Results
Nineteen eligible dietary ingredients were assessed for quality, efficacy, and safety. Avocado soybean unsaponifiables, capsaicin, curcuma, ginger (as a food source), glucosamine, melatonin, polyunsaturated fatty acids, and vitamin D were conditionally recommended as their benefits outweighed risks, but there was still some uncertainty about the trade-offs. No recommendations were made for boswellia, ginger (as a dietary supplement), rose hip, or s-adenosyl-L-methionine. Recommendations were made against the use of collagen, creatine, devil’s claw, l-carnitine, methylsulfonylmethane, pycnogenol, willow bark extract, and vitamin E. Research priorities were developed to address gaps precluding stronger recommendations.
Conclusions
Currently the scientific evidence is insufficiently robust to establish definitive clinical practice guidelines, but processes could be established to track the impact of these ingredients. Until then, providers have the evidence needed to make informed decisions about the safe use of these dietary ingredients, and future research can address existing gaps.
Keywords: Systematic Review, Meta-analysis, Dietary Ingredients, Dietary Supplements, Chronic Pain, Musculoskeletal Pain
Introduction
Pain is a major public health concern in the United States and worldwide [1,2]. A complex and multidimensional experience, pain affects individuals at physical, social, spiritual, behavioral, psychological, nutritional, financial, and emotional levels [3]. As pain persists and worsens, it can interfere with daily activities, sleep, and relationships; significantly impair quality of life, performance, and psychological health; and complicate concurrent medical conditions [2,4,5]. In the United States military, musculoskeletal (MSK) injuries are ubiquitous due to the demands of physical training for and executing combat missions, as well as carrying heavy loads; MSK injuries are one of the most burdensome conditions and a leading cause of pain, medical encounters, lost duty time, and disability [6–8].
Across the military, MSK pain management options are limited primarily to medications (e.g., nonsteroidal anti-inflammatories [NSAIDs]), injections, physical therapy, acupuncture, and other modalities. Alternative approaches are needed. In 2014, a Pain Medicine supplement [9] detailing the current evidence on what the authors termed active self-care complementary and integrative (ACT-CIM) modalities was published in response to the Army’s 2010 Pain Management Task Force Report recommendations [3] and the 2011 Institute of Medicine’s report “Relieving Pain in America” [2]. Additional recommendations for use of such therapies (e.g., yoga, tai chi, music therapy) and future research considerations were made for the military that are also generalizable to the general population. These suggestions, as well as other published work commenting on the ACT-CIM research [10], highlight the need for developing an evidence base for other potentially relevant self-care alternative approaches. Dietary ingredients are one area in need of an evidence base due to their popularity and widespread use.
In fact, although approximately 70–74% of the general adult population and 55–76% of Service members use dietary supplements [11,12], most individuals typically decide which supplements to use based on advice from both reliable and unreliable sources. The evidence supporting the outweighing of benefits over any potential harms across dietary supplements for pain management is unclear [1, 9,10,13]. Though supplements may be perceived to have fewer adverse events than conventional treatments, the safety of many products is unknown, and many have not been assessed for safety or efficacy, or prepared according to Good Manufacturing Processes [14]. A large number of dietary and herbal supplements may even contain various toxins because they do not undergo standard regulatory and quality control [15,16]. Because dietary ingredients could potentially be a promising self-care intervention [9,10], it is important to clearly determine their safety and efficacy for pain management and how ingredients might impact not only pain, but also other related (e.g., psychological health, quality of life) outcomes. If such products are going to be used for mitigating MSK pain, evidence-based research is required to inform appropriate and safe decisions about the available ingredients.
Purpose
As part of the US Special Operations Command’s Preservation of the Force and Family Behavioral Health Program, this project sought to determine whether current research on dietary ingredients for chronic MSK pain provided sufficient evidence to inform decisions for both clinical practice and self-care use. To achieve this, state-of-the-science evidence methodologies were applied to provide clear, comprehensive, and unbiased information to the Special Operations Forces personnel (SOF) and enable key stakeholders to make evidence-based recommendations regarding dietary ingredient use. Specifically, 1) key stakeholders and subject matter experts developed essential research questions and factors required for decision-making; 2) an independent series of systematic reviews was conducted to assess the current state of the evidence and to explore the safety and efficacy of various dietary ingredients for treating pain and related outcomes (Supplementary Data A: Summary Report); 3) the gathered evidence was integrated with the expertise of those subject matter experts; and 4) they were then convened for an expert panel meeting to develop evidence-based recommendations for the use of dietary ingredients and priority areas in need of future research.
The purpose of this paper is to describe these methodologies, resulting practice recommendations, and research priorities. This paper is the first in a series of articles that detail the relevance of this work to SOF, specific evidence-based recommendations, and implications for policy decisions [17–19].
Methods
Convening a Steering Committee
A steering committee was selected to provide high-level oversight and guidance to define the project’s scope and ensure meaningful impact for the intended audience; this group was completely independent of the research team that produced the systematic review evidence (Supplementary Data A: Summary Report). The committee, known as the Holistic Evidence Review Board (HERB), included a diverse group of stakeholders and subject matter experts, both within and outside the military, from research, clinical (patient and practitioner perspective), and policy realms. Committee members were well-rounded with expertise in human performance, dietary supplements, and nutrition, as well as pain and pain management. Although the intent was not to develop formal clinical practice guidelines, the authors followed the Institute of Medicine’s clinical practice recommendations [20] and used transparent processes to select a well-balanced group, free of potential conflicts of interest, and to ensure transparency throughout the process.
Scoping Review
A scoping review was performed to understand the extent of evidence that exists for dietary ingredients in pain populations and which dietary ingredients have been researched. The HERB devised a broad research question to first identify and map the various dietary ingredients and supplements reported in the pain management (across the spectrum of acute to chronic conditions) literature. The Population, Intervention, Comparison, Outcomes and Study Designs (PICOS) strategy [21] was used to scope the literature and represent the key concepts and eligibility criteria for initial inclusion (Table 1).
Table 1.
PICOS criteria used to define the research question “Are there dietary supplements/ingredients that can be safely used to mitigate or reduce pain?”
| Population | Individuals with pain, defined as an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage. Pain is always subjective. Pain can be acute or chronic [3]. |
| Intervention |
|
| Control/comparison | Sham, no treatment and/or active comparators. |
| Outcome(s) | Pain and pain-related outcomes to address the multidimensionality of pain, i.e., physical function, sleep, mood, stress, cognitive performance, global health, health-related quality of life, behavior, and resource use outcomes, as well as adverse events. |
| Study design | Peer-reviewed research presented in the English language. |
PICOS = Population, Intervention, Comparison, Outcomes and Study Designs strategy.
Search Strategy
Databases were searched from inception to August 2016 across PubMed, CINAHL, Embase, and PsycInfo for the purposes of scoping the literature. Searches were limited to peer-reviewed clinical trials, observational studies, systematic reviews, and meta-analyses including human populations and presented in the English language. The authors explored MeSH within MEDLINE and consulted with the HERB members to formulate a comprehensive search strategy (Supplementary Data A: Summary Report).
Study Selection
Using the predefined study eligibility criteria (Table 1) for scoping the literature, two investigators (LT, CL) independently screened the titles and abstracts of the citations retrieved from the broad literature search in duplicate. All disagreements were resolved through discussion and consensus with the lead authors (CC, CB). The activity was performed to identify the breadth of various pain populations [3], dietary ingredients [22, 23], controls/comparators, and outcomes studied and reported in the current peer-reviewed literature.
Development of Systematic Review Topic
The eligible peer-reviewed research was presented to the HERB after the scoping review. Given the vast extent of the literature, the HERB agreed to focus the systematic review on adults (i.e., at least 18 years old) with chronic MSK pain conditions [3, 24–26], as these were the types of conditions most commonly seen in SOF populations. Further, the HERB agreed to rely on systematic reviews, meta-analyses, and randomized controlled trial (RCT) study designs for the evidence. Following the scoping review, additional searches based on these narrowed criteria were executed to ensure comprehensiveness in the systematic review process. Specifically, each ingredient that emerged through the scoping review was searched by ingredient name and the MeSH term “pain” (e.g., melatonin and pain).
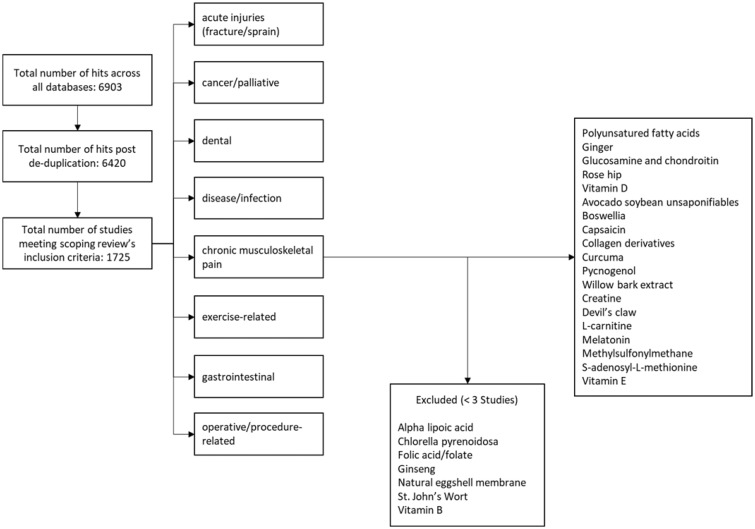
Two investigators (CB, CC), in duplicate, then screened the titles and abstracts of all citations yielded from the initial scope as well as the secondary searches, according to the more focused research question and eligibility criteria (Table 2). At this point, dietary ingredients without sufficient (i.e., at least three published trials meeting the review’s narrowed criteria) research to reach meaningful conclusions were excluded. Figure 1 displays the ingredients with sufficient evidence to evaluate and pain conditions excluded from full review.
Table 2.
Focused PICOS used to define the narrowed research question “Are there dietary supplements/ingredients that can safely mitigate chronic pain in adults (18+ years old) with musculoskeletal disorders?”
| Population | Adults (18+ years) with chronic pain due to musculoskeletal disorders. Chronic pain was defined as ongoing or recurrent pain, lasting beyond the usual course of acute illness or injury (i.e., more than 3 months and occurring at least half of the days over the past 6 months) and which adversely affects the individual’s well-being [3, 24]. Musculoskeletal pain was defined as pain affecting the bones, muscles, ligaments, or disorders of the muscles, nerves, tendons, joints, cartilage, and disorders of the nerves, tendons, muscles, and supporting structures of the upper and lower limbs, neck, and lower back that are caused, precipitated, or exacerbated by sudden exertion or prolonged exposure to physical factors such as repetition, force, vibration, or awkward posture [25, 26]. Note that headaches/migraines and MSK pain conditions resulting from another disease or injury (e.g., fracture, contusion, abrasion, laceration) were excluded. |
| Intervention | Any single or multiple (e.g., combination of ingredients) dietary ingredient(s). |
| Control/comparison | Sham, no treatment and/or active comparator. |
| Outcome(s) | Pain, physical function, sleep, mood (anxiety/depression), stress, cognitive performance, global health, health-related quality of life, behavior, resource use, adverse events. |
| Study design | Peer-reviewed systematic reviews/meta-analyses and/or randomized controlled trials presented in the English language. |
MSK = musculoskeletal; PICOS = Population, Intervention, Comparison, Outcomes and Study Designs strategy.
Figure 1.
Flowchart.
Data Extraction and Methodological Quality Assessment
Four reviewers (CB, CL, CP, LT) independently performed data extraction and methodological quality assessment for RCTs and integrated data with relevant existing systematic reviews present in the peer-reviewed literature. All reviewers followed a rulebook developed by the primary authors (CC, CB) and approved by the HERB.
Integration of Existing Systematic Reviews
Given the numerous amounts of dietary ingredients identified for full evaluation and the identification of high-quality systematic reviews/meta-analyses that mapped to the focused research question yielded from the search strategy (Table 2), the authors followed the guidance established by the Agency for Healthcare Research and Quality (AHRQ) Evidence Practice Centers Program Methods Guide [27] for integrating appropriate systematic reviews into new systematic reviews on related topics [28]. Using the Scottish Intercollegiate Guidelines Network (SIGN 50) Checklist [29], two investigators (CB, CC) first evaluated all systematic reviews/meta-analyses meeting the current review’s eligibility criteria for methodological quality in duplicate. Data from only high-quality systematic reviews/meta-analyses were considered for and integrated into the current analyses. All primary RCTs included in eligible reviews were then cross-checked against the primary studies captured from the literature search (as a secondary check to ensure completeness both from those reviews and for the current review). Included systematic reviews served as the sole, primary source of evidence only when no additional evidence was available within and across the primary trials independently examined under this scope.
Randomized Controlled Trials
Four reviewers (CB, CL, CP, LT) independently extracted data from all full-text articles of every RCT (including those primary RCTs also captured in the systematic review/meta-analyses above) meeting the focused eligibility criteria to describe PICOS characteristics. Any continuous or dichotomous pain outcomes with all associated time points were extracted in duplicate.
Additional outcomes related to physical function, sleep, mood (anxiety/depression), stress, cognitive performance, health-related quality of life, behavioral and resource use, and adverse events, as reported in the literature, were also extracted. All adverse events reported in primary studies were extracted and cross-checked with safety, toxicology, and pharmacokinetics profiles within the Natural Medicines Comprehensive Database [30].
Methodological quality and risk of bias were assessed in duplicate by using the SIGN 50 checklist [29]. All conflicts were tracked and resolved through consensus or by the primary authors (CC, CB). Once extraction was complete, the team re-examined the eligible systematic reviews/meta-analyses to determine whether the authors of those existing reviews included sufficient data in their analyses or whether additional data could be gleaned from the primary studies to contribute to the current analysis. For example, additional outcomes were considered for analysis if they were presented in the primary studies but not by the existing reviews.
In addition to methodological quality, the authors utilized the Standard for Reporting Interventions in Controlled Trials essential to Nutritional Elements (STRICT-NE) checklist to document the transparency of reporting. This allowed for a clearer understanding of results and provided the details necessary to replicate study findings for future research efforts. The proposed STRICT-NE checklist includes five criteria, adapted from and used in previous work [31, 32]: 1) preparation of dietary ingredients used in the intervention; 2) baseline/background diet; 3) control of diet during intervention; 4) nutritional ingredient analysis of intervention; and 5) absorption analysis (Table 3). Four reviewers (CB, CL, CP, LT) assessed, in duplicate, each RCT to determine whether these criteria were adequately reported on (Supplementary File A: Summary Report).
Table 3.
STRICT-NE criteria
| STRICT-NE Element | Description |
|---|---|
| 1. Preparation of dietary ingredient used in the intervention |
|
| 2. Baseline/background diet |
|
| 3. Control of diet during intervention |
|
| 4. Analysis of intervention conducted |
|
| 5. Analysis of absorption conducted |
|
STRICT-NE = Standard for Reporting Interventions in Controlled Trials essential to Nutritional Elements.
Data Synthesis and Analysis
The primary analysis across the included dietary ingredients, where feasible (i.e., sufficient data), was based on trials reporting a continuous outcome measure for pain reduction as compared with placebo. Meta-analyses compared with other active therapies were not attempted due to heterogeneity among various comparators. If more than one time point was provided for a single outcome, the authors chose the time point closest to three months for the main analysis. Secondary analyses were based on trials reporting: 1) a continuous measure for pain at any other time point (i.e., most commonly reported on the visual analog scale [VAS] or Western Ontario and McMaster Universities Osteoarthritis Index [WOMAC] pain subscale); 2) a dichotomous outcome measure at any time point (i.e., responder data for improvement marked as “global health”); 3) a continuous outcome measure for physical function or disability (i.e., commonly reported as physical function or stiffness WOMAC subscale) at any time point; 4) a continuous outcome measure for global function (i.e., often reported as a total WOMAC or total Lequesne’s Functional Index); 5) a dichotomous outcome measure of medication use (i.e., reported as the event to not require the use of rescue medication) at any time point; and 6) a dichotomous outcome measure of number of participants experiencing any adverse events. Psychological health (i.e., mood, anxiety, depression, stress), health-related quality of life (global, physical, and/or mental), sleep, and cognitive performance measures examined at any time point were also extracted when at least three studies for each ingredient measured such outcomes.
For continuous data, standardized mean differences (SMDs) were computed as the difference between groups in pre–post change scores using Comprehensive Meta-Analysis, version 3.3.070 (CMA; Biostat, Englewood, NJ, USA). When standard deviations for change scores were not reported, they were calculated from pre- and post-SDs [33], using r = 0.5 for the product–moment correlation. For studies with dichotomous outcomes, the risk difference (RD) between dietary ingredient and placebo groups (i.e., the percentage of responders with improvement, medication use, and adverse events) was calculated using Cochrane Collaboration’s Review Manager (RevMan) software (version 5.2.7). Meta-analyses of SMDs and RDs were performed with the generic inverse model of RevMan. Random-effects models were used. Statistical heterogeneity was examined by Cochrane’s Q test and I2, with low, moderate, and high I2 values of 25%, 50%, and 75%, respectively. A P value of less than 0.05 was set as the level of significance. SMDs of 0.2, 0.5, and 0.8 were considered small, medium, and large, respectively, according to Cohen’s d. Pooled effect sizes for the pain-related outcome of primary interest were translated into the VAS (0–100) for ease of clinical interpretation by using a standard deviation of 25 points [34]. Note that translations should be interpreted with caution as controversy remains on what is considered clinically “relevant” [34, 35].
The overall quality of the body of evidence and confidence in the effect estimates for each of the outcomes, as compared with placebo, was assessed using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach based on the following criteria: risk of bias, inconsistency, indirectness, imprecision, magnitude of the estimates of effects, and publication bias [36]. Two primary authors (CB, CC) independently performed this exercise before meeting with the HERB and coming to consensus. GRADE ratings of very low-, low-, moderate-, or high-quality evidence reflect the extent to which the authors were confident in the reported effect estimates. Additional studies (i.e., not compared to a placebo) comparing a dietary ingredient to an active comparator and/or combined dietary ingredients were evaluated separately; these functioned as an additional layer of evidence to consider beyond that of the primary question of efficacy for each ingredient, where data existed.
Moving Evidence to Recommendations
Integrating the Evidence with Clinical Acumen
Factors required for decision-making (adapted from the GRADE framework) that specifically addressed SOF personnel were mapped to the research evidence gleaned from the systematic reviews and presented using the GRADE Evidence to Decision (EtD) framework [36]. Factors included weighing the desirable/undesirable anticipated effects, overall certainty of the evidence, whether individuals might value the outcomes differently, resource requirements (i.e., cost and availability of the ingredient in the operational environment), feasibility/suitability (e.g., hospital vs theatre setting, shelf-life, speed of degradation in heat), and acceptability of the dietary ingredient. HERB members were educated on the findings from the systematic review and the process for making judgments across the factors and received the Summary Report (Supplementary Data A: Summary Report). The HERB members independently made judgments across each factor for each ingredient evaluated. A GRADE grid was constructed for each dietary ingredient to visually display the balance between the benefits and downsides of each ingredient for each factor on chronic MSK pain end points. A spread of their anonymous judgments was displayed on the grid for presentation (Supplementary Data B: GRADE Grid) [37].
Expert Panel Meeting
Eight HERB members convened for an in-person expert panel (KB, RC, SC, PD, TD, TH, JJ, NW) meeting to discuss the evidence, review the spread of judgments across each factor, and ultimately develop recommendations. A modified Delphi and multivoting approach was followed, whereby each member anonymously re-rated their judgments for each factor after discussing the evidence [38, 39]. The spread of votes and ratings was recorded and made instantly available using an online tool (Mentimeter, Stockholm, Sweden; www.mentimeter.com) before being placed on the grid. Convergence, not consensus, was examined across the ranges/distributions of judgments. Ultimately, the grid mapped the accumulated factors and provided a visual display of the direction, spread, and strength of each ingredient’s recommendation. Discussion was guided using predefined rules to explore areas of disagreement (wide spread) for or against current use as well as for the strength of a recommendation [37]. Ingredients were tiered into the following three recommendation groups based on GRADE’s EtD framework:
Conditional recommendations were made when desirable anticipated effects outweighed the undesirable effects, but there was uncertainty about the trade-offs: either because the key evidence was of low quality or because the benefits and downsides were closely balanced.
No recommendation was made for ingredients that required more and higher quality research due to overall low confidence in the effect estimate: either the recommendation was too speculative at the time or trade-offs were closely balanced. These issues made it difficult for the HERB to decide on the direction of a recommendation.
Recommendations against the current use of an ingredient, based on available evidence, were made when undesirable anticipated effects outweighed the desirable effects or the downsides clearly outweighed the benefits overall.
In addition to making practice recommendations, the HERB used a similar approach to identify research gaps and priorities for future research. Members were asked to anonymously vote on which dietary ingredients should be prioritized for future research, identify methodological gaps that needed to be addressed (identified specifically from the evidence evaluations), and identify additional priorities for research.
Results
The scoping review revealed >300 named dietary ingredients across >200 named pain conditions (Supplementary Data A: Summary Report). Twenty-six dietary ingredients meeting the focused research question remained after narrowing the focus to adults with chronic MSK pain for systematic review. Of those, seven dietary ingredients had fewer than three studies published to date and were therefore excluded from the current evaluation (Figure 1). Subsequently, only dietary ingredients with either a robust, high-quality systematic review with meta-analysis or a sufficient number of RCT studies were evaluated. This series of articles reports on the evidence-based recommendations yielded from the systematic review results; detailed systematic review results are reported elsewhere (Supplementary Data A: Summary Report).
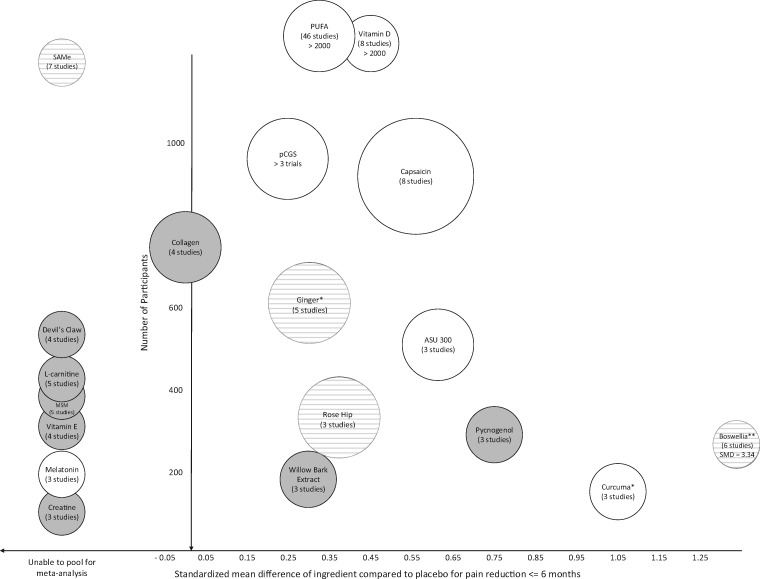
SMDs across the various outcomes assessed through the systematic review (Supplementary Data A: Summary Report) were relied upon to weigh undesirable/desirable anticipated effects derived from the meta-analysis. They were then translated for clinical interpretation. The HERB acknowledged, however, that statistically significant results did not necessarily translate into clinical significance; therefore, what is considered a clinically relevant point reduction for the population studied remains controversial [34, 35]. Figure 2 displays a comprehensive view of the SMD of each ingredient (x-axis) compared with placebo for pain reduction according to the number of patients pooled across studies at a time point closest to three months (y-axis) and the confidence in that effect (higher confidence associated with larger bubble sizes); the resulting evidence-based recommendations are indicated in white (conditional recommendations in favor), light gray (no recommendation), and dark gray (recommendations against current use).
Figure 2.
Evidence-based recommendations map.
Confidence in the effect = higher confidence associated with larger bubble sizes White = conditional recommendations in favor, striped pattern = no recommendation, dark gray = recommendations against current use.*Conditional recommendation for use as a food source, not as a dietary supplement at this time**Conditional recommendation for additional research, not use as a dietary supplement at this timeASU = avocado soybean unsaponifiables, MSM = methylsulfonylmethane, pCGS = patented crystalline glucosamine sulfate, PUFA = polyunsaturated fatty acids, SAMe = s-adenoysl-l-methionine
Evidence-Based Recommendations
Recommendations were based on current conditions and factors considered specific to SOF populations. The HERB only recommended ingredients that showed a benefit and did not negatively impact other areas (e.g., alleviating bodily pain but increasing financial stress due to the cost of an ingredient).
The HERB grouped recommendations for practice into three tiers based on their collective judgment. Conditional recommendations were made for the use of avocado soybean unsaponifiables (ASUs), capsaicin, curcuma, glucosamine prescription (Rx) and over-the-counter (OTC), melatonin, polyunsaturated fatty acids (PUFA), and vitamin D. Ginger was also conditionally recommended but only as a food source; no recommendation for use as a dietary supplement could be made at this time. No recommendations were made for boswellia, rose hip, and s-adenosyl-L-methionine (SAMe). Lastly, recommendations were made against the current use of collagen, creatine, devil’s claw, l-carnitine, methylsulfonylmethane, pycnogenol, willow bark extract, and vitamin E.
Specific details regarding the resulting evidence-based recommendations are provided in subsequent articles within this series [17–19].
Research Priorities
Prioritized Dietary Ingredients for Research
Based upon the evidence (Supplementary Data A: Summary Report), the HERB recommended additional research on the use of boswellia and only encouraged the use of curcuma in the daily diet at this time. Although both ingredients displayed large effect sizes (boswellia SMD = –3.34, curcuma SMD = –1.05), the published evidence was of low to very low quality. The HERB agreed that conducting a robust research study could either confirm or negate these effects. Subsequently, given their potential benefit for pain and related outcomes, these ingredients have been identified as the top two high-priority research areas, followed by ASU, ginger, SAMe, glucosamine Rx and OTC, vitamin D, PUFA, melatonin, rose hip, and capsaicin (Figure 3).
Figure 3.
Word cloud: Ingredients to prioritize for additional research.
Frequency of responses out of 8 total voters: boswellia (n = 8), curcuma (n = 7), ASU (n = 3), ginger (n = 3), s-adenoysl-L-methionine (SAMe, n = 3), glucosamine prescription (Rx)/over-the-counter (OTC, n = 3), vitamin D (n = 3), polyunsaturated fatty acids (PUFA, n = 2), melatonin (n = 2), rose hip (n = 1), capsaicin (n = 1)
Prioritized Research Gaps
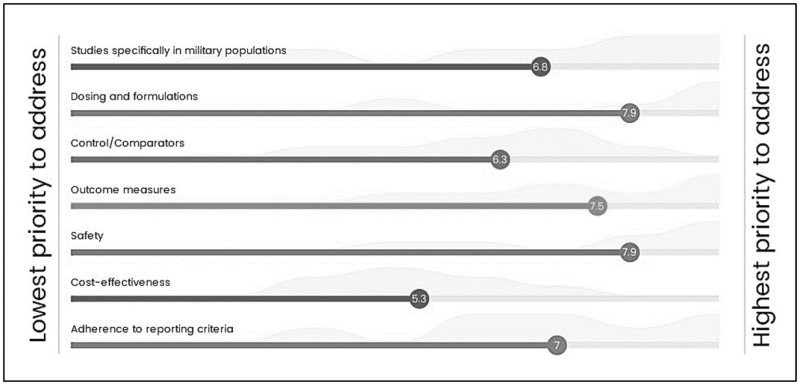
Several research gaps emerged from the evidence, including insufficient data specific to military populations, dosing/formulation variations, safety reporting of dietary ingredients, heterogeneity of controls/comparators used in studies, and outcome measures germane to military populations. Additionally, the authors were not able to identify any cost-effectiveness research studies, and methodological flaws existed across some reporting criteria within the trials that were included (Supplementary Data A: Summary Report). The HERB ranked research gaps and agreed safety was the highest priority to address in future research, followed by dosing/formulation of ingredients and data on additional outcome measures specific to the SOF population (Figure 4).
Figure 4.
Means and spread for prioritized research gaps.
Additional Priorities for Research
Several studies assessed interventions consisting of combinations of ingredients (e.g., MSM + boswellia). These combination studies were heterogeneous in terms of ingredients being combined, dosing, duration, and outcomes assessed, which made interpretation of their results challenging. The HERB agreed it would be worthwhile to explore what combination of selected ingredients would be most beneficial with minimal adverse events through this evaluation. Doing so would help determine if combining ingredients would yield greater pain reduction than single ingredients. The HERB also recommended additional research comparing interactions between dietary ingredients and prescription medications currently used to treat chronic MSK pain, as current research is sparse and too heterogeneous to draw conclusions. Stakeholders could rely on this evidence to make informed decisions about which ingredients and medications would be most appropriate for this research initiative. Additional research priorities that emerged included:
Validated methods: Overall, most evaluated studies either did not provide sufficient detail or failed to successfully carry out procedures relating to allocation concealment and intention-to-treat methods. STRICT-NE analyses also revealed inconsistencies in the details provided by the research studies regarding the dietary ingredient intervention/formulation. Future research should focus on adhering to standard reporting criteria to enhance transparency.
Delivery systems (Supplementary Data A: Summary Report): The HERB recommended investigating more fully the delivery methods, particularly when comparing dietary ingredients provided within the diet (e.g., food based) with those delivered as a pill, capsule, powder, gel, or tablet.
High-quality ingredients and purity of ingredients: Researchers need to be rigorous in their methods and reporting with regard to the source of the ingredient interventions by definitively quantifying their quality and purity.
Clinical significance, responder data, and pharmacogenetics: The HERB acknowledged that statistically significant results do not necessarily translate into clinical significance. Further, a study demonstrating non–statistically significant results for participants overall (mean) does not necessarily dismiss clinically relevant results, as many participants may have responded and others may not have. Therefore, the HERB believed researchers should report on individual responses to the intervention. Similarly, investigating genetic and epigenetic factors possibly associated with high and low responders to dietary ingredients would be important.
Dosing: The HERB recommended research and education on the most effective dosage of these dietary ingredients. Anecdotal reports suggest that the population of interest tends to consume more than the amounts recommended, as they believe this may confer an advantage. Clearly, this may not necessarily be the case. Considerations for dosing are discussed in subsequent articles within this series [18, 19].
Discussion
Evidence-based recommendations were made across various dietary ingredients for adults with chronic MSK pain with the hope of informing policy decisions regarding appropriate use and prioritizing future research directions. Although no strong recommendations were made for use among Special Operators, conditional recommendations were made when the desirable anticipated effects outweighed the undesirable. Emerging gaps can be prioritized for future research considerations, and education around the ingredients can be incorporated into the mission planning process, as many of the ingredients can be found in the diet or are already being used.
Strengths and Limitations
Some limitations are worth noting. First, because pain is complex and dietary ingredients are widely used across various pain conditions, the authors chose to focus on chronic MSK conditions. The majority of studies included within this effort involved patients with osteoarthritis, rheumatoid arthritis, low back pain, and fibromyalgia conditions; no studies involved military populations. Acknowledging that MSK conditions are highly prevalent among the military, the HERB agreed it was appropriate to generalize the current research to make evidence-based recommendations for SOF. Albeit, future research specific to military populations is still encouraged.
Dietary ingredients are marketed for a wide range of pain conditions, including joint pain, osteoarthritis, chronic gout, and gastrointestinal disorders [40, 41]. There is little knowledge, however, of what constitutes an effective and safe dose, formulary, and in some cases, delivery system, to relieve painful symptoms [15, 16, 42]. The STRICT-NE analyses revealed inconsistencies in the details of the dietary ingredient interventions reported in the research studies, which made it challenging to develop evidence-based decisions around the delivery methods, particularly when comparing food-based (i.e., available within the diet) ingredients with those delivered as a capsule, powder, gel, or patch. Researchers need to rigorously report their methods, specifically information pertaining to the source, quality, and purity of the ingredient, and conduct dose studies once efficacy of any ingredient is shown to be both effective and safe for the consumer. The authors cross-checked data with safety, toxicology, and pharmacokinetics profiles within the Natural Medicines Comprehensive Database. This was done to inform the HERB about these profiles as some of these data were not sufficiently detailed in the analyzed studies.
Among the studies evaluated, a wide variety of controls/comparators within various contexts specific to the populations were included. The authors attempted to pool data according to homogeneous subsets of studies, which precluded quantitative analyses for comparing ingredients with some active comparators (e.g., NSAIDs, opioids). Additionally, comparing the mean differences between groups on specific outcomes can, in some cases, fail to highlight either high or low responders to a particular therapy.
The original intent was not only to uncover the evidence for the efficacy of various dietary ingredients to alleviate pain, but also to examine the interaction on psychological health, quality of life, and other outcomes associated with chronic pain. For example, chronic MSK pain is often entangled with psychiatric comorbidities (e.g., depression, post-traumatic stress), especially among US military Service members who have served in the post-9/11 combat era [43]. Although there is agreement on the need to address pain/trauma psychiatric symptom comorbidities, limited data exist to guide practice. In fact, to date, outcomes surrounding these comorbidities have not been consistently and uniformly collected in research studies, which leaves interpretation of the evidence on other pain-related outcomes uncertain, as shown in this evidence-based evaluation. In addition, outcomes that do exist are primarily clinical outcomes, and what might be of greatest benefit is the introduction of outcomes used to pre-emptively mitigate pain and related symptoms from occurring for our Service members.
Conclusions
The methodological process and steps described in this article allowed for transparent and evidence-based recommendations to inform policy decisions regarding the use of dietary ingredients for chronic MSK pain and other related symptoms. Overall, the results demonstrate that some dietary ingredients may have the potential to be used adjunctively or even instead of certain NSAIDs and possibly other pain medications. Those working in health care should be prepared to help people make decisions consistent with their own values/lifestyles and participate in shared decision-making. Further, although SOF personnel may want to use dietary ingredients, without education, they may be unaware of these ingredients and their potential desirable and undesirable effects. Further, because no strong recommendations were made to endorse any ingredient for immediate use without caveats, there is a need to encourage discussion and debate among additional stakeholders before policy decisions are made. Research priorities include focusing efforts on understanding the true efficacy of boswellia and curcuma, as well as addressing safety concerns, dosing/formulation of all ingredients evaluated, and combining ingredients to test potential additive effects, if any, compared with other standards of care commonly being used and across outcomes specific to the SOF population.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the Holistic Evidence Review Board (HERB) for their commitment to the project: Dr. Kevin Berry, Vice President, TLI Foundation; Dr. Robert Bonakdar, Academy of Integrative Pain Management, and Director of Pain Management, Scripps Center for Integrative Medicine; SGM F. Bowling, Office of the Command Surgeon, US Special Operations Command, and US Army Special Forces Medic; CAPT Scott Cota, Command Surgeon, US Special Operations Command; Dr. Rebecca Costello, Scientific Consultant, Office of Dietary Supplements, National Institutes of Health; Dr. Tonya Dodge, Associate Professor of Psychology, George Washington University; Dr. C. Douglas Forcino, Director of the US Special Operations Command Programs at CHAMP/USUHS; Dr. Travis Harvey, Program Development Manager, US Special Operations Command, Preservation of the Force and Family, Human Performance; Col (ret.) Jeffery Johnson, 88th Pharmacy Flight Commander AFMC/TeamRx Consultant 88th DTS/88th MDG/88th, MDG/88th ABW/WPAFB, and member of the Air Force Nutrition and Supplements Subcommittee; COL (ret.) Steven Swann, Independent Military Medicine Consultant and former Command Surgeon, US Special Operations Command; CAPT (ret.) Necia Williams, Anesthesiologist and Command Surgeon, US Marine Corps Special Operations Command. None of the HERB members disclosed any conflicts of interest at the time of the project.
Supplementary Data
Supplementary data are available at Pain Medicine online.
Funding sources: Funding for this work was provided by the Preservation of the Force and Family Behavioral Health Program, Uniformed Services University (Award No. HU0001-15–2-0053).
Disclosure: The opinions or assertions contained herein are the collective views of the authors and the Holistic Evidence Review Board and are not to be construed as official or as reflecting the views of the Uniformed Services University, US Special Operations Command, or the Department of Defense.
Conflicts of interest: No conflicts of interest existed at the time of the project for any authors or steering committee members making evidence-based recommendations.
References
- 1. National Academies of Sciencies. Pain Management and Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Washington, DC: The National Academies Press; 2017. [PubMed] [Google Scholar]
- 2. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education and Research. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 3. Pain Management Task Force Report. Office of the Army Surgeon General: Pain Management Task Force. 2010. http://www.dvcipm.org/clinical-resources/pain-management-task-force/. Last accessed on March 7, 2019.
- 4. Dahan A, van Velzen M, Niesters M.. Comorbidities and the complexities of chronic pain. Anesthesiology 2014;121(4):675–7. [DOI] [PubMed] [Google Scholar]
- 5. Fine P. Long-term consequences of chronic pain: Mounting evidence for pain as a neurological disease and parallels with other chronic disease states. Pain Med 2011;12(7):996–1004. [DOI] [PubMed] [Google Scholar]
- 6. Armed Forces Health Surveillance Center. Absolute and relative morbidity burdens attributable to various illnesses and injuries, U.S. Armed Forces, 2013. MSMR 2014;21(4):2–7. [PubMed] [Google Scholar]
- 7. Bullock S, Jones B, Gilchrist J, Marshall S.. Prevention of physical training-related injuries recommendations for the military and other active populations based on expedited systematic reviews. Am J Prev Med 2010;38(Suppl 1):S156–81. [DOI] [PubMed] [Google Scholar]
- 8. Hauret K, Jones B, Bullock S, Canham-Chervak M, Canada S.. Musculoskeletal injuries description of an under-recognized injury problem among military personnel. Am J Prev Med 2010;38(Suppl 1):S61–70. [DOI] [PubMed] [Google Scholar]
- 9. Buckenmaier C, Crawford C, Lee C, Schoomaker E.. Are active self-care complementary and integrative therapies effective for management of chronic pain? A rapid evidence assessment of the literature and recommendations for the field. Pain Med 2014;15(1):1–113.24433467 [Google Scholar]
- 10. Complementary Medicine Research. Journal club. Complement Med Res 2017;24:207–13. [Google Scholar]
- 11. Knapik J, Steelman R, Hoedebecke S, et al. A systematic review and meta-analysis on the prevalence of dietary supplement use by military personnel. BMC Complement Altern Med 2014;14:143.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Council for Responsible Nutrition. CRN 2016 Annual Survey on Dietary Supplements. Available at: https://www.crnusa.org/resources/crn-2016-annual-survey-dietary-supplements. Last accessed on March 7, 2019.
- 13. De Silva V, El-Metwally A, Ernst E, Lewith G, Macfarlane GJ.. Evidence for the efficacy of complementary and alternative medicines in the management of osteoarthritis: A systematic review. Rheumatology 2011;50(5):911–20. [DOI] [PubMed] [Google Scholar]
- 14. Jones D, Kasper K, Deuster P.. Third-party evaluation: A review of dietary supplements dispensed by military treatment facilities from 2007-2011. Mil Med 2015;180(7):737–41. [DOI] [PubMed] [Google Scholar]
- 15. Van der Voet G, Sarafanov A, Todorov T, et al. Clinical and analytical toxicology of dietary supplements: A case study and a review of the literature. Biol Trace Elem Res 2008;125(1):1–12. [DOI] [PubMed] [Google Scholar]
- 16. Saper R, Phillips R, Sehgal A, et al. Lead, mercury, and arsenic in US- and Indian-manufactured Ayurvedic medicines sold via the Internet. JAMA 2008;300(8):915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cota S, Williams N, Neff R, Deuster P.. How Evidence-Based Recommendations May Direct Policy Decisions Regarding Appropriate Selection and Use of Dietary Ingredients for Improving Pain. Pain Med 2019;20(6):1063–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boyd C, Crawford C, Berry K, Deuster P; HERB Working Group. Conditional Recommendations for Specific Dietary Ingredients as an Approach to Chronic Musculoskeletal Pain: Evidence-Based Decision Aid for Healthcare Providers, Participants and Policy Makers. Pain Med 2019; (doi: 10.1093/pm/pnz051). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crawford C, Boyd C, Berry K, Deuster P; HERB Working Group. Dietary Ingredients Requiring Further Research before Evidence-Based Recommendations Can be Made for their Use as an Approach to Mitigating Pain. Pain Med 2019; (doi: 10.1093/pm/pnz050). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Institute of Medicine. Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press; 2011. [Google Scholar]
- 21. Richardson W, Wilson M, Nishikawa J, Hayward R.. The well-built clinical question: A key to evidence-based decisions. ACP J Club 1995;123(3):A12–3. [PubMed] [Google Scholar]
- 22. United States Special Operations Command. Policy Memorandum 16-29: Policy on Performance Enhancing Substance Use for Special Operations Forces. 2016.
- 23. Office of Dietary Supplements. Available at: www.ods.od.nih.gov. Last accessed on March 7, 2019.
- 24. Deyo R, Dworkin S, Amtmann D, et al. Report of the Task Force on Research Standards for Chronic Low-Back Pain. Phys Ther 2015;95(2):e1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Centers for Disease Control and Prevention. The National Institute for Occupational Safety and Health (NIOSH) Musculoskeletal Health Program. Available at: https://www.cdc.gov/niosh/programs/msd/default.html. Last accessed on March 7, 2019.
- 26. Cleveland Clinic. Musculoskeletal Pain. Available at: http://my.clevelandclinic.org/health/articles/musculoskeletal-pain. Last accessed on March 7, 2019.
- 27. White C, Ip S, McPheeters M, Carey T, Chou R, Lohr K.. Using Existing Systematic Reviews to Replace de Novo Processes in Conducting Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality; 2009. [PubMed] [Google Scholar]
- 28. Robinson K, Chou R, Berkman N, et al. Twelve recommendations for integrating existing systematic reviews into new reviews: EPC guidance. J Clin Epidemiol 2016;70:38–44. [DOI] [PubMed] [Google Scholar]
- 29. Scottish Intercollegiate Guidelines Network (SIGN). A Guideline Developer’s Handbook. Available at: https://www.sign.ac.uk/checklists-and-notes.html. Last accessed on March 7, 2019.
- 30. Natural Medicines. Food, Herbs and Supplements Database. Available at: https://naturalmedicines.therapeuticresearch.com/databases/food,-herbs-supplements.aspx. Last accessed on March 7, 2019.
- 31. Costello RB, Lentino CV, Boyd CC, et al. The effectiveness of melatonin for promoting healthy sleep: A rapid evidence assessment of the literature. Nutr J 2014;13:106.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Attipoe S, Zeno S, Lee C, et al. Tyrosine for mitigating stress and enhancing performance in healthy adult humans, a rapid evidence assessment of the literature. Mil Med 2015;180(7):754–65. [DOI] [PubMed] [Google Scholar]
- 33. Follmann D, Elliott P, Suh I, Cutler J.. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol 1992;45(7):769–73. [DOI] [PubMed] [Google Scholar]
- 34. Farrar J, Portenoy R, Berlin J, Kinman J, Strom B.. Defining the clinically important difference in pain outcome measures. Pain 2000;88(3):287–94. [DOI] [PubMed] [Google Scholar]
- 35. Emshoff R, Bertram S, Emshoff I.. Clinically important difference thresholds of the visual analog scale: A conceptual model for identifying meaningful intraindividual changes for pain intensity. Pain 2011;152(10):2277–82. [DOI] [PubMed] [Google Scholar]
- 36. GRADE Working Group. Grading of Recommendations Assessment, Development and Evaluation (GRADE). Available at: http://www.gradeworkinggroup.org/. Last accessed on March 7, 2019.
- 37. Jaeschke R, Guyatt G, Dellinger P, et al. Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. BMJ 2008;337:a744.. [DOI] [PubMed] [Google Scholar]
- 38. Coulter I, Adams A.. Consensus methods, clinical guidelines, and the RAND study of chiropractic. ACA J Chiropr 1992;50–61. https://www.rand.org/pubs/external_publications/EP19920003.html. Last accessed on March 7, 2019. [Google Scholar]
- 39. Coulter I, Shekelle P, Mootz R, Hansen D.. The Use of Expert Panel Results: The RAND Panel for Appropriateness of Manipulation and Mobilization of the Cervical Spine. Santa Monica, CA: RAND; 1997. [Google Scholar]
- 40. Andres M, Sivera F, Falzon L, Buchbinder R, Carmona L.. Dietary supplements for chronic gout. Cochrane Database Syst Rev 2014;7(10):CD010156.. [DOI] [PubMed] [Google Scholar]
- 41. Siah K, Wong R, Ho K.. Melatonin for the treatment of irritable bowel syndrome. World J Gastroenterol 2014;20(10):2492–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Crawford C, Saldanha L, Costello RB, Deuster P.. Dietary supplements for musculoskeletal pain: Science versus claims. An ongoing series for human performance optimization. J Spec Oper Med 2018;18:110–4. [DOI] [PubMed] [Google Scholar]
- 43. McGeary C, McGeary D, Moreno J, Gatchel R.. Military chronic musculoskeletal pain and psychiatric comorbidity: Is better pain management the answer? Healthcare 2016;4(3):38.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.