Abstract
Background
Bipolar disorder is a common condition associated with high morbidity; developing efficacious, safe treatments is therefore essential. Lithium is an effective maintenance treatment for bipolar disorder. It acts as mood stabiliser and reduces the risk of suicide. However, evidence assessing the efficacy of lithium in the treatment of acute mania is less robust. Current evidence‐based guidelines cite multiple anti‐dopaminergic and mood‐stabilising agents as initial treatments: more definite evidence is needed to decide if lithium should be the first‐line therapy.
Objectives
1. To assess the effects of lithium in comparison with placebo or other active treatment in alleviating the acute symptoms of a manic or mixed episode in people with bipolar disorder. 2. To review the acceptability and tolerability of treatment with lithium in comparison with placebo or other active treatments in alleviating the acute symptoms of a manic or mixed episode in people with bipolar disorder.
Search methods
We searched the Cochrane Common Mental Disorders Controlled Trials Register, CENTRAL, MEDLINE, Embase, and PsycINFO. We also searched the World Health Organization trials portal (ICTRP) and ClinicalTrials.gov. We checked the reference lists of all included studies and relevant systematic reviews. We have incorporated studies from searches to 18 May 2018 into the current analyses.
Selection criteria
Prospective randomised controlled studies comparing lithium with placebo or alternative drug treatment in treatment of acute mania. We included anyone with bipolar disorder, male and female, of any age.
Data collection and analysis
At least two review authors independently extracted data and assessed methodological quality. We used odds ratios (ORs) to analyse binary efficacy outcomes, and mean differences (MDs) or standardised mean differences (SMDs) for continuously distributed outcomes. We used a fixed‐effect model unless heterogeneity was moderate or substantial, in which case we used a random‐effects model. We used Review Manager 5 to analyse data. We assessed the certainty of evidence for individual outcomes using the GRADE approach.
Main results
We found 36 randomised controlled studies comparing lithium with placebo, one of 12 drugs, or electroconvulsive therapy for treatment of acute mania. Studies included male and female participants (n = 4220), of all ages, who all fitted criteria for a manic episode within the context of a diagnosis of bipolar disorder.
Risk of bias was variable; 12 studies had a high risk of bias in one domain and 27 gave inadequate information on randomisation leading to an 'unclear' rating for selection bias.
Lithium versus placebo
High‐certainty evidence found that lithium was an effective treatment for acute mania and was more effective than placebo at inducing a response (OR 2.13, 95% confidence interval (CI) 1.73 to 2.63; participants = 1707; studies = 6; I2 = 16%; high‐certainty evidence), or remission (OR 2.16, 95% CI 1.73 to 2.69; participants = 1597; studies = 5; I2 = 21%; high‐certainty evidence).
Lithium was more likely than placebo to cause tremor (OR 3.25, 95% CI 2.10 to 5.04; participants = 1241; studies = 6; I2 = 0%; high‐certainty evidence), and somnolence (OR 2.28, 95% CI 1.46 to 3.58; participants = 1351; studies = 7; I2 = 0%; high‐certainty evidence).
There was insufficient evidence to determine the effect of lithium for all‐cause dropouts (OR 0.76; 95% CI 0.46 to 1.25; participants = 1353; studies = 7; I2 = 75%; moderate‐certainty evidence), and weight gain (OR 1.48, 95% CI 0.56 to 3.92; participants = 735, studies = 3; I2= 51%; moderate‐certainty evidence).
Lithium versus antipsychotics or mood stabilisers
For the outcome of inducing a response, there was only very low‐certainty evidence regarding lithium compared to haloperidol (MD −2.40, 95% CI −6.31 to 1.50; participants = 80; studies = 3; I2 = 95%), quetiapine (OR 0.66, 95% CI 0.28 to 1.55; participants = 335; studies = 2; I2 = 71%), and carbamazepine (SMD 0.21, 95% CI −0.18 to 0.60; participants = 102; studies = 3; I2 = 0%).
Lithium was probably less likely to induce a response than olanzapine (OR 0.44, 95% CI 0.20 to 0.94; participants = 180; studies = 2; I2 = 0%; moderate‐certainty evidence).
Lithium may be less likely to induce a response than risperidone (MD 7.28, 95% CI 5.22 to 9.34; participants = 241; studies = 3; I2 = 49%; low‐certainty evidence).
There was no evidence of a difference between lithium and valproate (OR 1.22, 95% CI 0.87 to 1.70; participants = 607; studies = 5; I2 = 22%; moderate‐certainty evidence).
There was moderate‐certainty evidence that lithium was more effective than topiramate at treating acute mania (OR 2.28, 95% CI 1.63 to 3.20; participants = 660; studies = 1).
Data on adverse events for these comparisons contained too few studies to provide high‐certainty evidence.
Authors' conclusions
This systematic review indicates that lithium is more effective than placebo as a treatment for acute mania but increases the risk for somnolence and tremor. Limited evidence suggests little or no difference between lithium and other mood stabilisers (valproate, carbamazepine) or antipsychotics (risperidone, quetiapine, haloperidol). Olanzapine may be an exception, as it is probably slightly more effective than lithium. There is uncertain evidence that risperidone may also be more effective than lithium. Lithium is probably more effective at treating acute mania than topiramate. When compared to placebo, lithium was more likely to cause adverse events. However, when compared to other drugs, too few studies provided data on adverse effects to provide high‐certainty evidence. More, rigorously designed, large‐scale studies are needed to definitively conclude if lithium is superior to other interventions in treating acute mania.
Plain language summary
Lithium for the treatment of acute mania
Review question
Is lithium (a mood‐stabilising medication) as effective at treating an episode of mania (high mood) as other available drug treatments or electroconvulsive therapy (ECT)?
Background
Bipolar disorder is a common condition in which people experience episodes of low mood (depression) and high mood (mania). The symptoms of bipolar disorder may lower quality of life. Traditionally a range of medications have been used to treat mania, including medications that try to lessen changes in mood (e.g. lithium, valproate, lamotrigine, carbamazepine, divalproex, topiramate), and those that reduce distressing experiences, such as hearing voices or having unusual ideas (e.g. olanzapine, risperidone, quetiapine, aripiprazole, haloperidol, chlorpromazine). ECT (delivering an electric shock to the brain whilst the patient is under a general anaesthetic) is also a treatment for mania. We already know that lithium is the most effective of all these treatments for keeping people with bipolar disorder well in the long term, but we do not know if it is as effective for treating mania.
Method
The review authors searched for studies comparing lithium to other treatments for mania published up to May 2018. We identified 36 randomised studies, including 4220 participants who attended hospitals in at least 30 different countries. Randomisation means that each participant has the same chance of being assigned to each of the study groups, and reduces the chance that unknown but important factors could influence the study accidentally. Three studies included children and adolescents aged under 18 years. The studies compared lithium to placebo (inactive substance), ECT and 12 other medications for between three and 12 weeks.
Results
Lithium is an effective treatment for acute mania. Lithium was more effective than a placebo or the anti‐epileptic drug topiramate. There was some evidence that lithium may be less effective than the antipsychotic drug olanzapine, but this needs further investigation. There was no evidence that lithium was better or worse at treating mania than any of the other drugs, and not enough evidence to draw a conclusion for ECT.
There was not enough evidence to provide a definite answer as to which treatment for mania has the fewest side effects. It is probable that more people will develop a mild tremor when treated with lithium than other treatments. Participants were not more likely to withdraw from a study if they were treated with lithium compared to another treatment.
Unanswered questions remain, and these would be best resolved by further large, well‐designed studies comparing lithium to other treatments for acute mania.
Summary of findings
Background
Description of the condition
Bipolar disorder is a chronic, severe mental disorder characterised by episodes of elevated mood (mania), depression or mixed states. The estimated prevalence worldwide is 1%, but there is a wider group (1.5% to 2% total), who have clinically relevant milder symptoms that do not quite meet diagnostic criteria (Montgomery 2000; Philips 2013). Both genders and all nationalities, ethnicities and cultures appear to be equally affected (Philips 2013). Average age of onset is 15 to 19 years, although the mean delay in diagnosis between onset of symptoms and formal diagnosis is seven years (Berk 2007). The impact of bipolar disorder is considerable: globally it accounts for 0.3% of disability‐adjusted life years, impacts upon the sufferer's ability to carry out normal daily activities and is associated with a high suicide rate (Alsonso 2011; Chen 1996). Bipolar disorder also reduces life expectancy ‐ this is due to a combination of a greater risk of physical health conditions and the high suicide rate (Goldstein 2015; Laursen 2011).
A diagnosis of bipolar disorder is usually made using one of the two major diagnostic classification systems, the Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV; APA 2013) or the International Classification of Diseases 10th revision (ICD‐10; WHO 1992). Two main subtypes are recognised: bipolar 1, which requires at least one manic or mixed episode with or without a history of depressive episode(s) and bipolar 2, which requires at least one hypomanic episode and a depressive episode. People who present with bipolar spectrum symptoms but do not fit criteria for bipolar 1 or 2 may be diagnosed with bipolar disorder not‐otherwise‐specified (bipolar‐NOS). Bipolar disorder is a chronic condition, at least 80% of people who have an episode of mania will have recurrent episodes (NIMH‐NIH 1985).
A manic episode is characterised by elevated or irritable mood, excess energy, racing thoughts, pressured speech, grandiosity, decreased need for sleep, poor attention and an increase in goal‐directed activities. Symptoms must last at least seven days (unless hospitalisation occurs before that time). It is often associated with an increase in risk‐taking behaviours (e.g. over‐spending, promiscuity, dangerous driving), which may be the precipitant for hospitalisation. Many people with bipolar disorder develop mood‐congruent psychotic symptoms, usually along grandiose or paranoid themes, and may show pronounced psychomotor agitation or aggression. Hypomania differs from mania in the degree of severity: mood must be elevated for four days and four additional typical manic symptoms must be present (Samara 2017). A mixed episode is diagnosed when people with bipolar disorder experience manic and depressive symptoms (low mood, loss of energy, lack of interest in life) at the same time. Manic episodes may also occur in people who have symptoms of both schizophrenia and mood disorder (schizoaffective disorder).
The costs of bipolar disorder are high for both the patient and health services. Admissions to hospital for a manic disorder typically last at least several weeks, and as treatment within a psychiatric intensive care unit is often necessary it can be very costly (de Zelicourt 2003). During a manic episode, patients are typically at high risk of accidental injury due to reckless behaviour, of not eating and drinking sufficiently or of interfering with family or members of the general public and putting themselves at risk. There is a particularly high risk of harm to self during mixed episodes (Balazs 2006). For the individual, as well as the period of acute illness, manic episodes often leave an aftermath of psychological, social and financial problems. Overall, 1/3 of people with bipolar disorder attempt suicide during their lifetime, with 10% to 12% eventually completing suicide (Pallaskorpi 2017).
Management of bipolar disorder has two main aspects: treatment of an acute mood episode and maintenance treatment. The latter is designed to prevent or reduce the either the frequency or the intensity of episodes of illness, or both. The pharmacological agent with the strongest evidence base for maintenance treatment in bipolar disorder is lithium (Burgess 2001; Hayes 2016; Severus 2014). Systematic review evidence has consistently shown that lithium reduces the risk of a mood episode by about one‐third (Severus 2014). In addition lithium independently reduces the risk of completed suicide in bipolar disorder and unipolar depression (Cipriani 2013; Riblet 2017; Smith 2017). Other options for long‐term mood stabilisation include anticonvulsive agents (e.g. sodium valproate, lamotrigine, carbamazepine), or atypical antipsychotics, such as quetiapine or olanzapine (Hayes 2016).
Options for treatment of acute episodes of mood disorder depend upon the pole of illness; depressive, mania or a mixed state. The evidence base for treatment of bipolar depression is growing, but still in its infancy. Present guidelines recommend either fluoxetine combined with olanzapine/quetiapine, or quetiapine/ olanzapine alone as the first‐line option (Goodwin 2016; NICE 2014). Other options include lithium plus an antidepressant (first line for those in whom lithium has been previously effective), lamotrigine, an atypical antipsychotic alone, sodium valproate or antidepressants alone (Taylor 2014). The latter is frequently avoided due to the small risk of precipitating a manic episode. Mixed states are typically treated along the same guidelines as a manic episode. Treatment of acute mania has traditionally been with antipsychotics or mood stabilisers, with the addition of sedatives or anxiolytic drugs used as needed. There has been randomised controlled trial (RCT)‐level evidence that typical and atypical antipsychotics are effective in treating mania; meta‐analysis has suggested that olanzapine, risperidone, quetiapine and haloperidol are the most efficacious (Cipriani 2011; Smith 2007; Yildiz 2015).
For severe mania, or if drug treatments fail, electroconvulsive therapy (ECT) is an effective alternative. ECT has been used since the 1950s and involves passing an electric current through the brain to intentionally trigger a brief seizure. ECT is done under a general anaesthetic and the patient is given muscle relaxant, so the majority only experience mild twitching of their limbs during the few minutes of the procedure. It is not understood how ECT works, but it is known to cause sudden release of neurotransmitters and neurohormones, and effectively relieves symptoms of depression, mania and psychosis more quickly than other interventions. ECT is usually given twice weekly, and patients usually need between six to 12 sessions in total. The main side effects are those related to the general anaesthetic and short‐term memory loss, which for most patients does not persist long term.
Description of the intervention
Lithium (Li, from the Greek 'lithos', meaning stone), is a chemical element with the atomic number 3. It is a member of the alkali family that also includes sodium and potassium. These latter elements, in ionic form, are essential for physiological functioning in humans. The uses of lithium are numerous; it is widely used across the manufacturing and energy sectors, as well as in medicine for the treatment of mood disorders. Medical uses of lithium account for about 2% of global consumption per year, with the majority being used within the energy industry to produce lithium‐ion batteries (Malhi 2017).
Lithium was first used therapeutically by John Cade in 1949, to treat what he termed 'psychotic excitement'. Over the intervening 60 plus years, lithium has been widely shown to be an effective mood stabiliser and protect against completed suicide (Burgess 2001; Cipriani 2013; Geddes 2010). It is now the first‐line drug for maintenance treatment of bipolar disorder, and an adjunctive treatment for unipolar depression.
Lithium is prescribed as one of its salts ‐ citrate or carbonate ‐ and due to being a simple natural element is an inexpensive drug. Lithium is an oral medication and is well absorbed in the small intestine, with a bioavailability of 80% to 100% (Malhi 2017). It distributes equally across intracellular and extracellular spaces. Lithium does not undergo any form of metabolism. Lithium is handled very similarly to sodium by the kidney. It is freely filtered by the glomerulus and reabsorbed (˜80%) in the proximal tubule. Lithium renal excretion is in proportion to its plasma level; half‐life is 16 to 30 hours (Bauer 2006). Lithium is excreted as a free ion. Clearance is influenced by intrinsic renal disease, age, body weight, low sodium intake, dehydration, cardiac failure and drugs that affect renal function (e.g. diuretics, non‐steroidal anti‐inflammatory drugs, angiotensin‐converting enzyme (ACE)‐inhibitors). Care is therefore needed if these situations should arise whilst a patient is taking lithium.
Lithium therapy requires regular monitoring of plasma levels. This is because lithium has a narrow therapeutic index, meaning that the dose range that is therapeutic is very close to levels that can become toxic. Lithium is started at a low dose and gradually titrated over a few weeks, taking weekly blood levels 12 hours after a dose until the plasma level is within the therapeutic range, typically 0.5 to 0.8 mmol/L (BNF 2017; NICE 2014). The usual maintenance dose varies from 400 mg to 1500 mg daily. Once the dose is stable, blood samples need only be taken every three months. Lithium toxicity is dangerous; coarse tremor, diarrhoea and nausea, muscle weakness, confusion and eventually seizures may occur. The main risk factors for toxicity are changes in sodium levels, for example, due to the drugs previously mentioned, dehydration or a low‐salt diet.
Most side effects of lithium are dose‐related. Common minor symptoms include mild gastrointestinal upset (this usually resolves), fine tremor, polyuria and polydipsia. Longer term, there is a risk of thyroid dysfunction, especially hypothyroidism in women, and hyperparathyroidism (Shine 2015). Thyroid function and calcium levels should be regularly monitored. Lithium is strongly associated with reduced urinary concentrating ability; this is due to a (mostly) reversible nephrogenic diabetes insipidus. In the great majority of patients, the risk of a clinically significant decline in renal function is very low, even in the long term (McKnight 2012; Shine 2015). The risk of developing end‐stage renal function is extremely low, but renal function should be monitored in all people taking lithium (McKnight 2012). Lithium is associated with a congenital cardiac malformation called Ebstein's anomaly, but the risk to the foetus if exposed to lithium is low, approximately 1:1000. Women of childbearing age can take lithium during pregnancy but the risks to the mothers' mood destabilising need to be carefully balanced against potential risks to the developing foetus (McKnight 2012).
How the intervention might work
Lithium has been the mainstay of treatment of manic episodes since John Cade's serendipitous discovery of the antimanic effects of lithium and has repeatedly been shown to be effective (Burgess 2001; Cipriani 2013; Geddes 2010). Lithium is handled by the body in a very similar way to sodium, which is essential for physiological homeostasis. Sodium (and therefore lithium) is present in all parts of the body and is involved in virtually all biological processes. Narrowing down the process by which lithium exerts its mood stabilising effect has therefore proved extremely challenging.
Current evidence points towards lithium acting as a neuroprotective agent in the brain: reducing cell death (apoptosis) and enhancing new neuronal growth (neuroproliferation). On a macroscopic level, functional imaging has shown that people treated with lithium have a global increase in grey matter across the cerebrum, but especially concentrated in the prefrontal cortex, amygdala and hippocampus (Malhi 2013). Compared to controls or non‐treated bipolar patients, lithium‐treated patients have greater grey matter volume. This is important because evidence has shown that bipolar disorder may well be a neurodegenerative condition (Berk 2009). How these changes relate to mood stabilisation is not understood.
At a neuronal level, lithium acts to modulate neurotransmission, probably by 'dampening down' the system (Malhi 2013). Lithium appears to have an effect on both excitatory (glutamate/dopamine) and inhibitory (gamma‐aminobutyric acid (GABA)) transmission. There is strong evidence that in mania there is an excess of dopamine, with dopamine agonists inducing mania in healthy people, and elevated dopamine levels found in manic people (Post 1980). It appears that lithium reduces dopamine‐induced excitatory neurotransmission by interacting with the G‐protein‐coupled post‐synaptic dopamine receptors (Manji 2000). It is not clear how this is mediated at present. Similarly, increased levels of glutamate are seen during mania. Glutamate acts via the N‐methyl‐D‐aspartate (NMDA)‐receptor which, when glutamate and its cofactor glycine are absent, has magnesium bound to it. Lithium competes with magnesium to bind, and when bound, unlike magnesium, stimulates the receptor. Chronic lithium stimulation (such as during regular therapy) leads to downregulation of the NMDA‐receptors, and an overall reduction in glutamate transmission (Tsapakis 2002). People with bipolar disorder are known to have lower levels of GABA‐neurotransmission than controls: this reduction in inhibition leads to excess excitation via glutamate/dopamine and eventually apoptosis and cell loss (Ng 2009). Lithium counteracts this by facilitating inhibition via GABA. Lithium directly enhances GABA release and increases upregulation of the GABA‐B receptor (Ahluwalia 1982). Lithium also increases brain‐derived neurotrophic factor (BDNF) and B‐cell lymphoma 2 (Bcl‐2), which are neuroprotective factors, via activation of Gs‐protein‐coupled receptors (Quiroz 2010). It appears that these complex actions of lithium occur through multiple second messenger signalling cascades (cyclic adenosine monophosphate (cAMP), inositol phosphate (IP3), protein kinase C (PKC), myristoylated alanine‐rich C‐kinase substrate (MARCKS) and glycogen synthase kinase 3 (GSK‐3)). In some way, not clear as yet, lithium moderates the actions of these cascades, which leads to changes in gene transcription and, ultimately, mood stabilisation.
Why it is important to do this review
Bipolar disorder is a common, chronic condition that represents a high burden of disability for the individual and society. Effective treatments are needed, both for acute mood episodes and maintenance. Unlike maintenance therapy, for which the evidence strongly supports the use of lithium as a first‐line treatment, it remains unclear which psychotropic drugs are the most effective for the treatment of mania (Burgess 2001; Cipriani 2013). Systematic reviews and network analysis have suggested that multiple antipsychotics (especially haloperidol, olanzapine, risperidone and quetiapine) and mood stabilisers (lithium, valproate, carbamazepine) can all treat mania (Cipriani 2011; Yildiz 2015). Current clinical guidelines recommend that if a person with bipolar disorder is not on medication, starting one of the antidopamine agents above should be first line. Other options include valproate, lithium, aripiprazole or carbamazepine (Goodwin 2016; NICE 2014). As lithium is the most efficacious treatment for maintenance, and there is evidence that it is effective in mania, it is a strong contender for being a first‐line agent (Cipriani 2011; Yildiz 2015). Lithium has the advantage that it can be used for both acute treatment and maintenance, which is attractive to many patients. Similarly, whilst lithium carries its own set of potential adverse events, it can be used in patients who have not tolerated anti‐dopaminergic agents (e.g. had extra‐pyramidal symptoms or raised prolactin) and has much less risk of teratogenicity than valproate. The previous network meta‐analyses that have been done using studies dating up to January 2014, had fairly narrow criteria (no children/adolescents, one main outcome measure), and therefore the knowledge base remains incomplete (Cipriani 2011; Yildiz 2015). This review aims to assess the available evidence to date (up to January 2017) comparing the effectiveness of lithium to other antimanic agents in treating acute mania in all ages and settings.
Objectives
To assess the effects of lithium in comparison with placebo or other active treatment in alleviating the acute symptoms of a manic or mixed episode in people with bipolar affective disorder.
To review the acceptability and tolerability of treatment with lithium in comparison with placebo or other active treatments in alleviating the acute symptoms of a manic or mixed episode in people with bipolar affective disorder.
Methods
Criteria for considering studies for this review
Types of studies
We included prospective double‐ or single‐blinded randomised controlled trials (RCTs) where lithium was used in the treatment of acute manic episodes in comparison with other antimanic treatments or placebo. For the comparison between lithium and ECT we also considered single‐blind studies, due to the nature of the comparator (see also Differences between protocol and review). For studies that had a cross‐over design, we only considered results from the first period prior to the cross‐over. We included cluster‐randomised studies, if the effect of clustering could be accounted for in the statistical analysis.
Types of participants
Subset data
We considered for inclusion, people of both sexes of any age with a primary diagnosis of bipolar affective disorder and experiencing a manic episode, according to any of the following standard operational criteria: Feighner criteria, Research Diagnostic Criteria, DSM‐III , DSM‐III‐R , DSM‐IV, DSM‐IV‐TR, DSM‐5 (APA 2013), or ICD‐10 (WHO 1992). We included studies using operational diagnostic criteria essentially similar to the above. We excluded studies using ICD‐9, as it has only disease names and no diagnostic criteria. We also excluded studies that defined mania as scoring above a certain cut‐off on a screening questionnaire. Finally, we included studies recruiting participants with treatment‐resistant mania and, if any, we planned to examine these in a sensitivity analysis. We did not include studies of acute treatment with lithium, which recruited people with diagnoses other than bipolar disorder or schizoaffective disorder and did not stratify randomisation according to diagnosis in this review.
Comorbidities
We did not consider concurrent secondary diagnosis of another psychiatric disorder an exclusion criterion. However, we excluded studies in which all participants had a concurrent primary diagnosis of another Axis I or II disorder. We also excluded participants with a serious concomitant medical illness or with postpartum depression.
Setting
We did not apply restrictions on setting.
Types of interventions
Experimental intervention
Lithium: at any dose within the therapeutic range and pattern of administration.
Comparator interventions
Placebo
All other antimanic drugs (mood stabilisers, antipsychotics, anticonvulsants or sedatives)
ECT
All comparator interventions were used either as monotherapy or combined with other treatments.
We included studies that allowed rescue medications (as required, short‐term, infrequent use of medications aimed at emergent symptom relief only, for example, short‐term use of hypnotics) as long as these medications were equally distributed among the randomised arms.
Types of outcome measures
We included studies that met the above inclusion criteria regardless of whether they reported on the following outcomes.
Primary outcomes
Efficacy outcomes: response (categorical). Number of participants who responded to treatment, where treatment response was defined as a decrease in score on the Young Mania Rating Scale (YMRS), or any other equivalent standardised rating scale, of at least 50% from baseline to the end of the study.
Efficacy outcomes: response (continuous). Mean endpoint scores or mean change scores in manic symptoms (YMRS or other equivalent standardised rating scale) from baseline to the end of the study.
Efficacy outcomes: remission (categorical). Number of participants who achieved remission by the end of the study out of the total number of randomised participants. We defined remission as a YMRS score of 12 or less (or equivalent on other validated mania rating scales).
Acceptability (categorical): overall number of participants who dropped out during the study as a proportion of the total number of randomised participants.
-
Acceptability: adverse events (categorical). We evaluated adverse events using the following outcome measures.
Total number of participants who experienced at least one side effect between first treatment dose and end of study.
-
Total number of participants who experienced the following specific side effects between the first treatment dose and the end of the study (BNF 2016).
Depression
Mania
Weight gain
Akathisia
Headache
Somnolence
Dizziness
Insomnia
Diarrhoea
Nausea
Vomiting
Dry mouth
Pain
Extra‐pyramidal side effects
Tremor
Constipation
Fever
Rash
Attempted suicide
Anorexia
Infection
Weight loss
Agitation
Convulsions or seizures
Dyspepsia
Psychosis
Suicidal ideation
Blood disorders
Hyperprolactinaemia
Thyroid disorders
Arthralgia
Rhinitis
Pruritis
Renal impairment
Sexual dysfunction
In order to avoid missing any relatively rare or unexpected, yet important side effects in the data extraction phase we collected information on all side effects data reported in the studies and discussed ways to summarise them post hoc. We extracted descriptive data regarding adverse effect profiles from all available studies. In a specific number of cases, we combined terms describing similar side effects: for example, we combined ’dry mouth’ and ’reduced salivation’ into ’dry mouth’.
We included a higher number of primary outcomes than is standard in a Cochrane Review in order to capture the full breadth of the available evidence. Included studies were published from 1970 onwards, and earlier studies did not necessarily use the standardised outcome measures of recent times. Excluding those data would reduce the value of the analysis results.
Secondary outcomes
Efficacy outcome (continuous): mean endpoint scores or mean change scores in depressive symptoms (Montgomery and Asberg Depression Rating Scale (MADRS), Hamilton Rating Scale for Depression (HAMD) or other equivalent standardised rating scale) from baseline to the end of the study
Efficacy outcome (continuous): mean endpoint scores or mean change scores in psychotic symptoms (Positive and Negative Syndrome Scale (PANSS) or other equivalent standardised rating scale) from baseline to the end of the study
Efficacy outcome (continuous): mean endpoint scores or mean change scores in general wellness and social functioning (Clinical Global Impressions‐Bipolar (CGI‐BP), Goal Attainment Scale (GAS) or other equivalent standardised rating scale) from baseline to the end of the study
Efficacy outcome (categorical): response. Number of participants who responded to treatment, where treatment response was defined as a decrease in score between baseline and end of study as defined by the study authors on standardised rating scales not within the primary outcomes (e.g. CGI‐BP).
Use of rescue medications (categorical or continuous): either number of participants who required treatment with rescue medications as a proportion of the total number of randomised participants or mean/total dosage use of rescue medications from baseline to the end of the study.
-
Acceptability (categorical), evaluated using the following outcome measures.
Number of participants who dropped out due to lack of efficacy during the study as a proportion of the total number of randomised participants
Number of participants who dropped out due to side effects during the study as a proportion of the total number of randomised participants
Timing of outcome assessment
Outcomes were measured at three weeks (21 days) and 12 weeks (84 days).
Search methods for identification of studies
Cochrane Common Mental Disorders' Controlled Trials Register (CCMD‐CTR)
Cochrane Common Mental Disorders maintains a specialised register of RCTs, the CCMD‐CTR. This register contains over 40,000 reference records (reports of RCTs) for anxiety disorders, depression, bipolar disorder, eating disorders, self‐harm and other mental disorders within the scope of this Group. The CCMD‐CTR is a partially studies‐based register with more than 50% of reference records tagged to around 12,500 individually PICO‐coded study records. Reports of studies for inclusion in the register are collated from (weekly) generic searches of MEDLINE (1950 onwards), Embase (1974 onwards) and PsycINFO (1967 onwards), quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) and review‐specific searches of additional databases. Reports of studies are also sourced from international trials registries, drug companies, the handsearching of key journals, conference proceedings and other (non‐Cochrane) systematic reviews and meta‐analyses. Details of CCMD's core search strategies (used to identify RCTs) can be found on the Group's website, with an example of the core MEDLINE search displayed in Appendix 1.
In 2016 the Group’s Specialised Register (CCMD‐CTR) became out of date with the Editorial Group’s move from Bristol to York.
Electronic searches
Cochrane Common Mental Disorders' Specialised Register (CCMD‐CTR)
The Cochrane Common Mental Disorders' Information Specialist (IS) searched the CCMD‐CTR using the following terms.
CCMD‐CTR Studies Register: condition = (mania or hypomania) and Intervention = lithium
CCMD‐CTR‐References Register: (lithium and (mania* or manic* or hypomani* or ((bipolar or schizoaffective) NEAR (acute or psychos* or psychotic or "mixed episode*" or “mixed state*” or "rapid cycl*"))))
The IS applied no date, language or publication restrictions to the searches. The CCMD‐CTR was up to date as of June 2016 (Appendix 1).
Additional bibliographic database searches
The IS performed additional searches on the following databases, in February 2017 and April/May 2018. The search strategies are displayed in Appendix 2:
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 4) in the Cochrane Library;
Ovid MEDLINE (2014 to 17 May 2018);
Ovid Embase (2014 to 17 May 2018);
Ovid PsycINFO (2014 to 17 May 2018);
World Health Organization (WHO) trials portal (ICTRP) (17 May 2018);
ClinicalTrials.gov (17 May 2018).
We applied no restriction on date, language or publication status to the searches.
Searching other resources
Reference checking
We handsearched any major textbooks of affective disorder, journals or conference proceedings specifically relating to lithium therapy in mania (up to May 2018).
Personal communication
We identified the authors of significant papers over the last five years from authorship lists. We contacted them and other experts in the field to ask if they knew of any other published or unpublished studies relevant to the review article. We requested relevant published and unpublished data from pharmaceutical companies marketing lithium.
Grey literature
We searched the following drug regulatory authorities for additional unpublished data: the US Food and Drug Administration, the Medicines and Healthcare products Regulatory Agency in the UK, the European Medicines Agency in the EU, the Pharmaceuticals and Medical Devices Agency in Japan, and the Therapeutic Goods Administration in Australia.
Handsearching
We handsearched and incorporated into the CCMD‐CTR appropriate journals and conference proceedings relating to the treatment of mania with lithium (up to May 2018).
Reference lists
We checked the reference lists of all included studies and relevant systematic reviews to identify additional studies missed from the original electronic searches (for example unpublished or in‐press citations). We also conducted a cited reference search on Web of Science (up to May 2018).
Data collection and analysis
Selection of studies
Two out of three review authors (SdLM, BA, RMK) independently screened titles and abstracts for inclusion of all the references retrieved by the search strategy. Subsequently, we retrieved full‐text study reports/publications, which two out of three review authors (SdLM, BA, RMK) independently screened for inclusion. At this stage, we recorded the reasons for excluding the ineligible studies.
We resolved any disagreement through discussion or, if required, by consulting a third review author (AC). We identified and removed duplicate records and collated multiple reports that related to the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA (Moher 2009), flow diagram and the characteristics of excluded studies table.
Data extraction and management
We used a data collection form to extract study characteristics and outcome data that we piloted on at least one study in the review. Three review authors (SdLM, EC and RMK) independently extracted study characteristics and outcome data from each included study and compared their results. We resolved any disagreement through discussion with a third member of the team (AC). We contacted the study authors when necessary, to obtain supplemental information. We extracted the following study characteristics.
Participant characteristics (age, sex, depression diagnosis, comorbidity, depression severity, antidepressant treatment history for the index episode, study setting)
Intervention details (intended dosage range, mean daily dosage actually prescribed, co‐intervention if any, lithium as investigational drug or as comparator drug, sponsorship)
Outcome measures of interest from the included studies. We noted in the Characteristics of included studies if outcome data were not reported in a usable way. We resolved disagreements by consensus or by involving a third person (AC). Two review authors (SdLM and RMK) entered data into the Review Manager 5 (RevMan 5) software (Review Manager 2014).
We double‐checked that we had entered data correctly by comparing the data presented in the systematic review with the study reports.
Main comparisons
Lithium versus placebo
Lithium versus all other antimanic drugs (mood stabilisers, antipsychotics, anticonvulsants or sedatives)
Lithium versus ECT
Assessment of risk of bias in included studies
Two out of four review authors (SdLM, RMK, EC and BA) independently assessed the risks of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We resolved any disagreements by discussion or by involving another review author (AC). We assessed the risk of bias according to the following domains.
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective outcome reporting
Other bias
We judged each potential source of bias as high, low, or unclear and provided a supporting quotation from the study report together with a justification for our judgement in the ’Risk of bias’ table. We summarised the risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary. Where information on risk of bias relates to unpublished data or correspondence with a study author, we noted this in the ’Risk of bias’ table. When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Measures of treatment effect
Categorical data
We calculated the odds ratio (OR) with corresponding 95% confidence interval (CI) for categorical or event‐like outcomes. We calculated response rates out of the total number of randomised participants.
Continuous data
We calculated the mean difference (MD) or standardised mean difference (SMD) along with corresponding 95% CI for continuous outcomes. We used the MD where studies used the same scale to measure an outcome. We employed the SMD where studies used different scales to measure the same underlying construct. For both continuous and categorical data, we undertook meta‐analyses only where this was meaningful, that is if the treatments, participants, and the underlying clinical question were similar enough for pooling to make sense. We narratively described skewed data reported as medians and interquartile ranges. Where a single study reported multiple study arms, we planned to include only the relevant arms.
Unit of analysis issues
Cluster‐randomised studies
We included cluster‐randomised studies if either of the two methods below were possible.
When the original report had correctly analysed the cluster‐randomised study, we entered the effect estimate and standard error using the generic inverse variance method in RevMan 5 (Review Manager 2014).
-
If the original report failed to adjust for cluster effects, we included such a study in the meta‐analysis if we could extract the following information.
Number of clusters randomised to each intervention or the average size of each cluster.
Outcome data ignoring the cluster design for the total number of participants.
Estimate of the intra‐cluster correlation coefficient (ICC). The ICC was borrowed from similarly designed studies when such were available. We then conducted the approximately correct analysis following the procedures described in section 16.3.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Cross‐over trials
A major concern of cross‐over studies was the potential of carry‐over effects, which occur if an effect (for example, pharmacological, physiological, or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase, the participants could differ systematically from their initial state, despite a washout phase. For the same reason, cross‐over studies are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in bipolar affective disorder, we only used data from the first phase of cross‐over studies. However, we are aware that cross‐over studies for which only first period data are available, should be considered to be at risk of bias (Higgins 2017).
Studies with multiple treatment groups
Where a study involved more than two treatment arms, we included all relevant treatment arms in comparisons. If data were binary, we combined them into one group or divided the comparison arm into two (or more) groups as appropriate. If data were continuous, we combined data following the formula in section 7.7.3.8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
Dealing with missing data
Categorical data
We calculated responders to treatment and remitters on a strict intention‐to‐treat (ITT) basis: we included dropouts in this analysis. Where participants had been excluded from a study before the endpoint, we assumed that they had experienced a negative outcome by the end of the study (e.g. failure to respond to treatment). We examined the validity of this decision in sensitivity analyses by applying worst‐ and best‐case scenarios (that is, we assumed missing participants to be either a responder or non‐responder in the corresponding sensitivity analysis).
Continuous data
When there were missing data and the study had used the 'last observation carried forward' (LOCF) method to perform an ITT analysis, we used the LOCF data. We contacted the original study authors for missing data.
When only the standard error (SE) or t‐test or P values were reported, we calculated SDs according to Altman 1996. Where studies did not report SDs, we contacted study authors and asked them to supply the data but, in the absence of data from the study authors, we borrowed SDs from other studies in the review (Furukawa 2006). We examined the validity of this imputation in the sensitivity analyses.
Missing data
We contacted the original study authors for missing data.
Missing statistics
When only the SE or t‐test or P values were reported, we calculated SDs as suggested by Altman 1996. Where studies did not report SDs, we contacted study authors and asked them to supply the data. In the absence of a response from the study authors, we borrowed SDs from other studies in the review (Furukawa 2006). We examined the validity of this imputation in sensitivity analyses.
Assessment of heterogeneity
We first investigated heterogeneity between studies by visual inspection of the forest plots. If the 95% CIs of the ORs for each study in the pooled analysis did not include means of other studies, we investigated potential sources of heterogeneity. We also calculated the I2 statistic (Higgins 2003). The I2 statistic describes approximately the proportion of variation in point estimates due to heterogeneity rather than sampling error. We used the Cochrane Handbook for Systematic Reviews of Interventions’ rough guide to its interpretation as follows: 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; and 75% to 100% considerable heterogeneity (Deeks 2017). We also kept in mind that the importance of the observed value of the I2 statistic depends on the magnitude and direction of effects and the strength of evidence for heterogeneity (for example P value from the Chi2 test, or a CI for I2). If the I2 value was below 50% but the direction and magnitude of treatment effects was suggestive of important heterogeneity, we investigated the potential sources of heterogeneity. Finally, we performed subgroup analyses to investigate heterogeneity. We used random‐effects models to investigate the sensitivity of results to the choice of statistical method.
Assessment of reporting biases
We entered data from included studies into a funnel plot (study effect against study variance) to investigate small‐study effects. We used the test for funnel plot asymmetry when we included at least 10 studies in the meta‐analysis. When using a funnel plot, we interpreted results cautiously, with visual inspection of the funnel plots (Sterne 2017). If we identified evidence of small‐study effects, we investigated possible reasons for funnel plot asymmetry, including publication bias (Egger 1997).
Data synthesis
For the primary analysis, we calculated the pooled OR with corresponding 95% CI for categorical outcomes. We calculated the pooled MD or SMD as appropriate with corresponding 95% CI for continuous outcomes. We presented any skewed data and non‐quantitative data descriptively. An outcome that has a minimum score of zero could be considered skewed when the mean is smaller than twice the SD. However, the skewness of change score is difficult to depict as the possibility of negative values exists. We, therefore, used change scores for meta‐analysis of MDs (Deeks 2017).
We considered a P value of less than 0.05 and a 95% CI that does not cross the line of no effect to be statistically significant. In forest plots with two or more studies, we used a random‐effects model for both categorical and continuous variables. We adopted the random‐effects model under these circumstances because it has the highest generalisability for empirical examination of summary effect measures in meta‐analyses (Furukawa 2002). However, as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (10.4.4.1; Sterne 2017), when concerned about the influence of small‐study effects on the results of a meta‐analysis with between‐study heterogeneity, we examined the robustness by comparing the fixed‐effect model and the random‐effects model. We reported any material differences between the models.
Subgroup analysis and investigation of heterogeneity
As multiple analyses can lead to false‐positive and false‐negative conclusions, subgroup analyses should be performed and interpreted with caution (Brookes 2004). We planned the following subgroup analysis for primary outcomes.
Lithium alone and studies using lithium with a mood stabiliser or antipsychotic
If data were available, analysis by length of treatment would be performed to ascertain whether any treatment differences detected varied with time.
Sensitivity analysis
We used random‐effects models to investigate the sensitivity of results to the choice of statistical method.
We conducted the following sensitivity analyses for primary outcomes:
excluding studies that recruited participants with treatment‐resistant mania;
excluding studies with a dropout rate greater than 20%;
excluding studies for which the SD had to be borrowed from other studies (Furukawa 2006).
'Summary of findings' table
We constructed a ’Summary of findings’ table for each comparison, with regard to the following four outcomes.
Response
Remission
Main adverse events
Total withdrawal from the study
We used we used GRADE proGDT software (GRADEproGDT 2015) to produce the ’Summary of findings’ tables, and followed the principles of the GRADE approach (Atkins 2004), which assess the certainty of a body of evidence based on the extent to which there can be confidence that the obtained effect estimate reflects the true underlying effect. The certainty of a body of evidence is judged on the basis of the included studies’ risks of bias, the directness of the evidence, unexplained heterogeneity, imprecision, and the risk of publication bias.
Results
Description of studies
See Characteristics of included studies.
Results of the search
The process of the search is shown in Figure 1. The initial database searching (updated to include searches up to May 2018) identified 1276 records and 254 were identified from other sources (primarily 'grey literature' described above). After removing duplicates 1530 remained. We then excluded 1361 records as they did not meet our inclusion criteria. We attempted to contact the primary investigators and drug companies for six studies (all listed on ClinicalTrials.gov), but had no reply to any of our emails or telephone messages. Two of these studies are currently awaiting classification (NCT00183443; NCT00893581). We were able to match up the other three protocols to reports of included studies (with the help of a review by Yildiz 2011): NCT00448578 is Li 2008; NCT00485680 Niufan 2008; and NCT00035230 is Kushner 2006 PDMD‐008)
1.
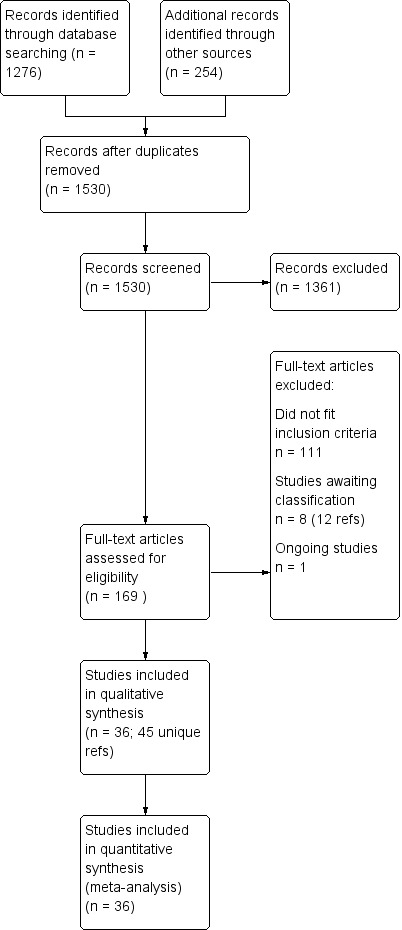
Study flow diagram
We assessed 169 full‐text records for eligibility; 111 did not fit our inclusion criteria and there are eight studies awaiting classification (12 references) plus one ongoing study. One of the studies awaiting classification only presented the results graphically as a regression. We contacted the study authors for numerical results but had no reply (Young 2017 (NCT0025448); results posted on ClinicalTrials.gov 14 August 2018). We identified 36 studies that met our inclusion criteria to May 2018 (within 35 publications). Kushner 2006 reported combined adverse event data from two studies but did not report these data separately (attempts to contact authors for these data separately by study were unsuccessful). We judged it appropriate to include these combined adverse event data, due to similarities in trial methods used by the company, rather than exclude them from the analyses. Although Kushner 2006 reported the effectiveness results separately, to be consistent with the adverse event data, we combined the data from these two studies (Kushner 2006 PDMD‐004 NCT00037674 and Kushner 2006 PDMD‐008 NCT00035230).
We included all 36 studies (represented by a total of 45 references) in the qualitative and quantitative analysis. Out of the 36 studies, only three were unpublished data (Astra Zeneca 2009; GlaxoSmithKline 2005; GlaxoSmithKline 2008) and the remainder were published.
Studies from searches to 18 May 2018 have been incorporated into the current analyses.
Included studies
The characteristics of the 36 included studies are shown in Characteristics of included studies.
Study design and setting
All of the studies used parallel‐group design. Kushner 2006 PDMD‐004 and Kushner 2006 PDMD‐008 used a complex cross‐over design; we included only the first randomisation, as outlined in the methods section. Banga 2003 has only been published as a conference abstract; we could not find any further information, despite attempts to contact the study authors. There is therefore a lack of data about the detailed methodology or setting of the study. Where the information was available, we have listed the country in which the study was set in the Characteristics of included studies; at least 25 countries were represented.
Length of the studies
The range of study length was between two weeks and 12 weeks (Table 16). Due to the variability in study length and time points at which the studies provided data, the initial plan to analyse data at the time points of three weeks (21 days) and twelve weeks (84 days) was not possible. We therefore analysed data from baseline to end of study.
1. Length of study.
Participants
In total, the 36 studies included 4220 individual participants. Only three studies included children and adolescents (Findling 2015; Geller 2012; Kowatch 2000). Studies diagnosed acute mania according to DSM‐IV criteria in 22 of the 36 studies (APA 2013). The other studies used the DSM‐III (6 studies) or the Chinese Classification of Mental Disorders (Li 2008). Six studies published before 1990 all used clinical interviews by psychiatrists (Chouinard 1983; Garfinkel 1980; Lusznat 1988; Platman 1970; Prien 1972; Spring 1970), and Shopsin 1975 did not describe the method of diagnosis.
Interventions and comparisons
All of the studies compared lithium with one of the comparators (placebo, antimanic drugs or ECT). Lithium treatment was with either lithium carbonate or lithium citrate, with dosing aimed to reach a plasma level of 0.6 to 1.4 mmol/L.
Lithium versus placebo
Eight studies compared lithium with placebo (Astra Zeneca 2009; Bowden 1994; Bowden 2005; Findling 2015; GlaxoSmithKline 2005; GlaxoSmithKline 2008; Keck 2009; Kushner 2006). All of these studies looked at the efficacy and tolerability of using lithium to treat acute mania. Six of the studies included a third comparator (valproate, quetiapine, lamotrigine, aripiprazole or topiramate respectively for Bowden 1994; Bowden 2005; GlaxoSmithKline 2005; GlaxoSmithKline 2008; Keck 2009; Kushner 2006), whilst Astra Zeneca 2009 had quetiapine in addition to either lithium or placebo. Findling 2015 was the only study conducted in children and adolescents, the others were all in adults.
Lithium versus valproate
Four studies reported studies comparing the efficacy of lithium to sodium valproate (Banga 2003; Bowden 2010; Freeman 1992; Shafti 2008). None of these studies included a third comparator. Four studies compared divalproex to lithium (Bowden 1994; Geller 2012; Hirschfeld 1999; Kowatch 2000). Kowatch 2000 and Geller 2012 enrolled children and adolescents, whereas the other studies involved adults. Dosing varied across the studies; all the studies used divided doses (typically twice or three times daily), with three studies starting a titration at 20 mg/kg/day (Banga 2003; Bowden 2010; Shafti 2008), whilst Freeman 1992 titrated up to 1500 mg to 3000 mg daily. Hirschfeld had two subgroups within the divalproex arm; they gave one group a loading dose of divalproex (30 mg/kg/day for two days reducing to 20 mg/kg/day) but not the other group (starting at 20 mg/kg/day). Both of these subgroups are included in the analysis. The other studies all started a titration at divalproex 20 mg/kg/day and did not use a loading dose. As the active ingredient of both sodium valproate and divalproex is valproic acid, we combined these in the analysis.
Lithium versus quetiapine
We found only two studies comparing quetiapine with lithium that fitted our study criteria (Bowden 2005; Li 2008). Bowden 2005 had a third placebo arm, whereas Li 2008 did not. The main difference between these studies was that Bowden 2005 ran for 12 weeks, whereas Li 2008 reported their outcomes at four weeks. Both studies titrated quetiapine up to 800 mg daily.
Lithium versus clonazepam
Two studies compared clonazepam with lithium (Chouinard 1983; Clark 1996). Clark 1996 was a single‐blinded study whereas Chouinard 1983 was double‐blinded. Chouinard 1983 reports using a dose range of 9 mg to 21 mg daily, Clark 1996, 2 mg to 16 mg daily.
Lithium versus lamotrigine
Three studies, including data from two unpublished studies, compared lithium to lamotrigine for treatment of acute mania. Studies from Glaxosmithkline (GlaxoSmithKline 2005; GlaxoSmithKline 2008), were conducted for six and three weeks respectively, whilst Ichim 2000 reported outcomes at the interim period of four weeks. All studies reported starting lamotrigine at 25 mg daily and titrating up to at least 100 mg over three to four weeks. GlaxoSmithKline 2005 and GlaxoSmithKline 2008 titrated to 100 mg over four weeks, then up to 200 mg over the following two weeks.
Lithium versus carbamazepine
We identified data from five studies comparing carbamazepine to lithium. Four of these contributed efficacy data (Kowatch 2000; Lerer 1987; Lusznat 1988; Small 1991), whilst one only examined and reported adverse events (Trivedi 1996). This adverse‐events study had three arms, with haloperidol given to the third group. Kowatch 2000 was a study of children and adolescents, and included a third arm given divalproex, whereas the other studies were all on adults and only had two arms. The studies described a range of dosing strategies. Three studies aimed for a carbamazepine plasma level within the range of six to 12 μ/mL (Kowatch 2000; Lerer 1987; Lusznat 1988), whilst Small 1991 aimed for a higher range of 25 to 50 μg/mL. Trivedi 1996 gave a set dose of 800 mg of carbamazepine daily.
Lithium versus chlorpromazine
Four studies provided data comparing chlorpromazine to lithium (Platman 1970; Prien 1972; Shopsin 1975; Spring 1970). All these studies included adults and lasted for three weeks. The mean dose of chlorpromazine was 800 mg to 900 mg in all studies, although we are unsure about Shopsin 1975, which gave inadequate information on dosing.
Lithium versus haloperidol
Four studies compared haloperidol to lithium in four studies, although only three of these reported efficacy outcomes (Garfinkel 1980; Segal 1998; Shopsin 1975). These studies gave a dose range of 10 mg to 30 mg of haloperidol daily. Trivedi 1996 only reported adverse events from a set dose of 15 mg of haloperidol daily.
Lithium versus olanzapine
Three studies compared olanzapine to lithium (Berk 1999; Niufan 2008; Shafti 2010), which all reported using a standard titration from 5 mg up to maximum 20 mg.
Lithium versus risperidone
Three studies compared risperidone to lithium (Barekatain 2005; Geller 2012; Segal 1998). Geller 2012 had a third comparator arm treating with divalproex and Segal 1998 had an arm using haloperidol. All the studies reported titrating risperidone to 4 mg to 6 mg/day.
Lithium versus aripiprazole
Only one study comparing aripiprazole to lithium in acute mania fitted our inclusion criteria. Keck 2009 compared lithium (titrated to mean plasma level 0.76 mmol/L) to 30 mg of aripiprazole daily. This could be given as a single or divided doses. The study lasted three weeks.
Lithium versus topiramate
Two studies from the same research group compared topiramate to lithium (Kushner 2006 PDMD‐004; Kushner 2006 PDMD‐008). These studies had a complex design of three weeks of core treatment of either placebo + topiramate or placebo + lithium and then a cross‐over in which those treated with placebo were either treated with topiramate or lithium. We have only included the first randomisation and first drug treatment in the analysis. The studies gave topiramate at 400 mg/day and lithium at 1500 mg/day.
Lithium versus zuclopenthixol
Gouliaev 1996 was the only study comparing zuclopenthixol to lithium. They gave zuclopenthixol at 20 mg/day and titrated lithium to plasma level 0.9 mmol/L to 1.0 mmol/L. The study lasted 28 days.
Lithium versus ECT
Small 1988 was the only study fitting the inclusion criteria that compared ECT to lithium treatment. This study had 17 participants in each arm. The lithium group had a mean plasma level of 0.69 mmol/L and the ECT arm received an average of nine bilateral ECT treatments over three to five weeks.
Lithium versus all antimanic agents
We were able to combine data from 16 studies comparing lithium to all the other antimanic agents for a categorical outcome of response (n = 3856), and 20 studies for a continuous outcome (n = 2410). We also combined withdrawals for any cause, including 4211 participants.
Outcomes
The outcome measures used by studies varied considerably. All studies bar one either reported a continuous measure of manic symptoms as their primary outcome or a categorical outcome, whereby an arbitrary relative improvement of a reduction of at least 50% from baseline in the scale rating mania was taken to signify a response, or both. It should be noted that whilst this is a commonly used method of defining 'response', a 50% improvement in a scale may not necessarily indicate a clinically relevant improvement. The one study that did not use this method of reporting was Trivedi 1996, whose study was aimed at reporting adverse events only.
Mental state
The most common scales used to assess mental state were as follows.
Young Mania Rating Scale (YMRS; Young 1978). This scale, used by Astra Zeneca 2009; Banga 2003; Barekatain 2005; Berk 1999; Bowden 1994; Bowden 2005; Bowden 2010; Findling 2015; Geller 2012; Hirschfeld 1999; Keck 2009; Kowatch 2000; Kushner 2006; Li 2008; Lusznat 1988; Niufan 2008; Segal 1998; Shafti 2008; Shafti 2010; Small 1988; and Small 1991, has 11 items that are rated after a clinical interview. Irritability, speech rate and amount, content of thought and disruptive behaviour items are given extra weight in the total by being scored from 0 to 8, whereas the remaining items are scored from 0 to 4. Higher scores indicate more symptoms.
SADS‐C Manic Syndrome Subscale (Endicott 1978). The SADS scale was developed with the primary aim of differentiating between schizophrenia and mood disorders. The scale makes use of collateral information and past history. The SADS‐C scale is adapted to measure change over time. The manic syndrome subscale score examines elevated mood, sleep, energy activity and grandiosity. Higher scores indicate more symptoms.
Brief Psychiatric Rating Scale (BPRS) (Overall 1962). This scale rates 24 symptoms from 1 to 7 and gives a generalised view of how abnormal the mental state is. Higher scores indicate more symptoms.
Montgomery Asberg Depression Rating scale (MADRS) (Montgomery 1979). This is a 10‐item diagnostic questionnaire used to measure the severity of depression. Higher scores indicate more severe depression.
Hamilton Rating Scale for Depression (HAMD) (Hamilton 1960). This is a questionnaire that can be used to diagnose depression. It is validated for adults and can rate the severity of depression. Higher scores indicate more severe depression.
Positive and Negative symptom scale (PANSS) (Kay 1987). This is a scale used to measure the severity of symptoms of psychosis, originally validated in schizophrenia. It is a standardised diagnostic interview.
For all of the above scales, a reduction of at least 50% in score from baseline during a study (definition of a 'response') represents a substantial decrease in symptom severity.
Global state of health
Some studies reported an outcome assessing global state of health or recent change in health.
Clinical Global Impression (CGI) (Guy 1976). The majority of studies used this scale, which assesses both severity of illness and clinical improvement by comparing the condition of the patient standardised against others with the same diagnosis. The 7‐point scoring system is usually employed, with low scores showing decreased severity and overall improvement. Some studies used a modification of the Clinical Global Impressions Scale for use in bipolar illness: the CGI‐BP. Modifications include the correction of perceived inconsistencies in scaling, detailed definitions of illness severity and change, the inclusion of time frames and the separation of the assessment of improvement in illness from the assessment of the adverse events of treatment. Previous phases of illness are also used as comparators for the assessed period. A CGI score of greater than 3 is often taken to indicate a response.
Global Assessment Scale (Spitzer 1970) (GAS). The Global Assessment Scale evaluates the overall functioning of a patient. Their general functioning is given a score following consideration of any behavioural disturbance, levels of distress, social functioning, self‐care, impulsivity and reality testing. Higher scores indicate a higher level of functioning.
A few studies used different outcomes:
Inpatient Multidimensional Psychiatric Scale (IMPS) (Lorr 1966) was used by Chouinard 1983 and Prien 1972. It includes a manic subscale that scores 5 items (overactivity, elevated mood, pressure of speech, logorrhea and insight) each out of 7. This is a clinician‐rated scale. Higher scores reflect more severe symptoms.
Bech‐Rafachen Mania Rating Scale (Bech 1979) is a clinician‐rated scale in which 11 manic symptoms are each rated on a scale of 0 to 4. Higher scores reflect more severe symptoms (Gouliaev 1996; Lusznat 1988).
Trivedi 1996 investigated the side effect profiles of lithium, carbamazepine and haloperidol for acute mania. They used a side‐effect checklist published by the World Health Organization (WHO 1986).
Spring 1970 did not use a scale to measure response to treatment. They had a team of three psychiatrists who independently rated the participants on target symptoms: euphoria, expansiveness, grandiosity, flight of ideas, distractibility, pressured speech, motor activity and sleep disturbance. It is worth noting that this study occurred before the majority of the validated scales above were published.
Assessment of adverse events
In general, adverse events experienced by participants were either listed in tables with statements of frequency, or were described in the text. A few studies (in addition) used specific scales:
Simpson‐Angus Scale: Clark 1996; Keck 2009 and Segal 1998 used this scale, which assesses extra‐pyramidal symptoms (signs relating to the abnormality of gait, muscle rigidity and resistance to movement, the glabellar tap, tremor and salivation). These items are scored from 0 to 4. The score increases with the severity of symptoms.
Abnormal Involuntary Movement Scale (Guy 1976): Geller 2012 used this scale. It scores facial and oral movements (four items), movements of the limbs and the trunk (three items), dental problems (two items) and global judgements, such as the severity of abnormal movements, their resultant incapacitation and patient's awareness of abnormal movements (three items). Each item, with the exception of dental problems, is scored on a 5‐point scale, from normal to severe.
Excluded studies
There are 21 studies formally excluded from this review, please see Characteristics of excluded studies for details.
Ongoing studies
There is one ongoing study (NCT01893229), which is a study of adults with a manic or mixed episode being randomised to one of lithium, valproate, oxcarbazepine, quetiapine, olanzapine or ziprasidone for 14 days. Please see Characteristics of ongoing studies for details.
Studies awaiting classification
There are eight studies currently awaiting classification:
Grunze 2006 is a conference abstract for a study examining the effects of valproate and lithium in acute and continuation treatment of bipolar mania. The abstract doesn't include any results data, randomisation or blinding strategies and we were unable to get any further details of this study.
Itoh 1974 is a double‐blind comparison of lithium carbonate and chlorpromazine in mania but we were unable to retrieve the full‐text.
Kumar 2009 is a conference abstract for a study comparing the efficacy and side effects of lamotrigine compared with lithium in acute mania. The methodology is unclear and there is no efficacy data reported, only side effects. We were unable to get further information.
Maggs 1963 is a comparative study of lithium carbonate in the treatment of manic illness but the full‐text report is ambiguous. Unclear number of participants, unclear methodology and poor reporting of the findings.
NCT00183443 is a published conference abstract of a randomised double‐blind study of open‐label divalproex plus adjunctive lithium, quetiapine or placebo in manic patients with bipolar disorder. A full methodology is needed to determine if the study fits inclusion criteria. We emailed the Principal Investigator via the address on clinicaltrials.gov to request results but the email bounced back as an invalid address. (Results posted on ClinicalTrials.gov 17 July 2018.)
NCT00893581 is a neuroimaging study examining the effects of quetiapine and lithium on neural function in adolescents with a first episode of mania. Only a conference abstract is available: a full methodology is required to determine if this study fits inclusion criteria. We contacted the study authors via clinicaltrials.gov but we had no reply to our email.
Penick 1971 is another conference abstract for a study comparing lithium carbonate and chlorpromazine in the treatment of manic states, but we were unable to contact the authors or to get further details of this study.
Young 2017 is a randomised double‐blind study of lithium and divalproex to treat mania in older patients with bipolar disorder (GERI‐bd). The results for this study were presented graphically & as a logistical regression; we contacted the study authors to ask for the numerical results but received no answer to our emails. If this information were received the study would otherwise fit criteria for inclusion. (Results posted on ClinicalTrials.gov 14 August 2018.)
Risk of bias in included studies
For details of the 'Risk of bias' judgements for each study, see Characteristics of included studies. We have presented a graphical representation of the overall risk of bias in included studies in Table 17, Figure 2 and Figure 3.
2. Risk of bias summary table.
| Area of potential bias | Number of studies (N = 35) | ||
| High risk | Unclear risk | Low risk | |
| Randomisation | 1 | 27 | 7 |
| Allocation bias | 0 | 34 | 1 |
| Blinding | 3 | 9 | 23 |
| Incomplete outcomes | 3 | 10 | 22 |
| Selective reporting | 6 | 5 | 24 |
| Other bias | 0 | 9 | 26 |
2.
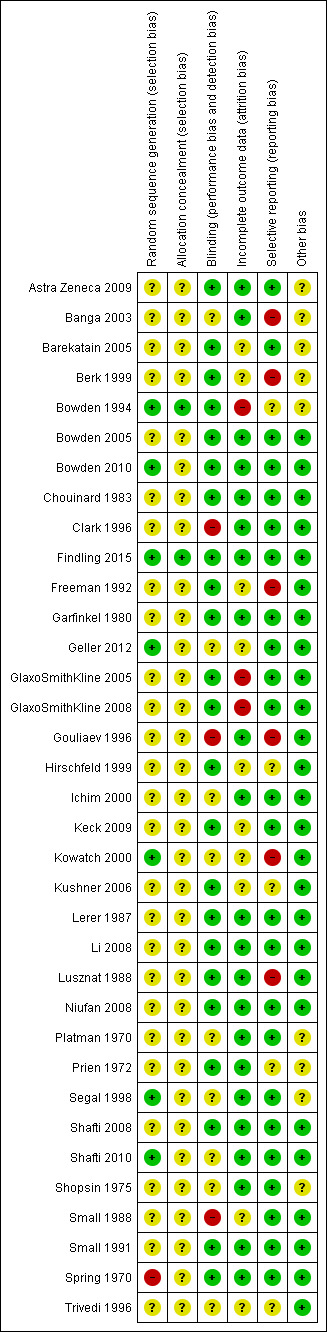
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
3.
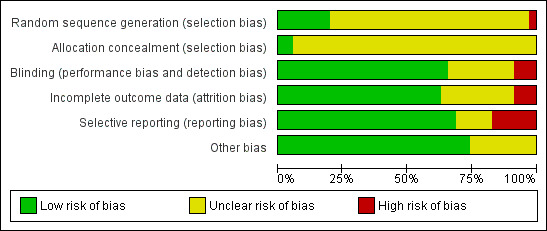
'Risk of bias graph': review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
Randomisation and concealment of allocation
All included studies described themselves as 'randomised'. Using the Cochrane 'Risk of bias' criteria, which rate the adequacy and concealment of random allocation, we rated 28 studies as unclear, seven as low and one as high risk of bias (Spring 1970). The studies rated unclear all described 'randomisation' without further explanation. Spring 1970 probably used a quasi‐randomisation method, but this was unclear.
Blinding
Thirty‐four studies described themselves as double‐blinded, but unfortunately all 34 of these studies gave no further explanation as to what they meant by this term. We therefore deemed these at unclear risk of bias. This included Gouliaev 1996, which was singled‐blinded, with only the rater being blind to the allocation. As in any study, it is always possible that both the assessors and participants previously in receipt of the study medications may have been unblinded by the distinctive adverse effect profile of the study drugs. Clark 1996 and Small 1988 gave no description of their blinding techniques and we therefore deemed them at high risk. Regarding performance bias, no specific differences in the care provided to different groups were identified in any study, however, the number of studies published before detailed methodologies were required is high. This therefore puts a element of uncertainty into the picture. Similarly, for detection bias, there were no reported differences in the outcome determination methods between groups in any study. Kowatch 2000 reported that the participants and treating clinician were blinded during the study, but the rater of outcomes at the end of the study was not. This appears to have been for both arms.
Incomplete outcome data
In general the studies were comprehensive in their reporting of the flow of participants and the outcomes. We deemed 22 studies at low risk, with all participants accounted for and all outcomes well reported. Ten studies had a high (more than 20%) dropout rate from the study, which we deemed high risk (Barekatain 2005; Bowden 1994; Bowden 2010; Garfinkel 1980; GlaxoSmithKline 2005; GlaxoSmithKline 2008; Hirschfeld 1999; Keck 2009; Kowatch 2000, Prien 1972) For four studies it was unclear if the reporting was comprehensive, as the process was poorly described (Banga 2003; Berk 1999; Kushner 2006 ‐ both studies).
Selective reporting
On the whole, the study authors comprehensively reported the studies. Five studies were poorly reported, with either outcome data missing or adverse effect data not described, so we deemed these at high risk of bias (Banga 2003; Berk 1999; Freeman 1992; Gouliaev 1996; Lusznat 1988;) In addition, in Prien 1972, it was unclear what the time point of the last data collection was.
Other potential sources of bias
Handling of withdrawals: intention‐to‐treat analyses and use of last observation carried forward
The studies included in this review date from a 45‐year time period. Many were published well before the ITT and LOCF methods were introduced. Given this, 21 papers (19 published prior to the year 2000) did not use either ITT or LOCF. Four studies described using LOCF, all of which only included participants who had had at least one dose of the randomised treatment (Astra Zeneca 2009; Bowden 2005; Ichim 2000; Keck 2009). The more recent publications (nine studies) all reported using ITT and LOCF, where the participant needed to have had at least one dose of the treatment they were randomised to, and had at least one outcome measure (e.g. a YMRS) done post‐baseline (Geller 2012; GlaxoSmithKline 2005; GlaxoSmithKline 2008; Kowatch 2000; Kushner 2006; Li 2008; Niufan 2008; Shafti 2008; Shafti 2010). For, Banga 2003 it is unclear which, if any, of these techniques were employed. The LOCF approach is usually thought to give a conservative estimate of the effectiveness of a treatment in an acute illness, but when withdrawal is non‐random (i.e. associated with one of the treatments, perhaps through failure of blinding), it can give a biased estimate of that treatment effect. The withdrawal rates from the studies are variable (more than 50% of placebo‐treated participants withdrew in Bowden 1994 and Keck 2009 but there were no withdrawals in Clark 1996). The use of LOCF introduces more uncertainty and potential for bias the higher the withdrawal rate and this should be considered when interpreting the findings of this review.
Numbers of participants
Many of the included studies, especially the older ones, were too small to reliably detect moderate but clinically important treatment effects. Their small size limits their ability to detect small differences in acceptability or differences in the rates of rare outcomes and rendered them prone to potential confounding by baseline differences between groups. Several of the most recent papers reported power calculations (Astra Zeneca 2009; Bowden 2010; Findling 2015; GlaxoSmithKline 2005; GlaxoSmithKline 2008).
Selection of participants
The participants of all the studies except two were adults (either inpatients or outpatients), who met operationally defined diagnostic criteria for acute mania. Kowatch 2000 and Findling 2015 studied children and adolescents.
Inevitably, the participants of all the studies were selected in one way or another. Only some of the most recent studies provided a clear indication of the manner in which they had selected participants for the study, as recommended by the CONSORT statement (Schulz 2010). One identified problem was the tendency to exclude severely affected participants. The majority of the studies mentioned that participants were able to give informed consent to participate, potentially excluding those with severe mania. Similarly, the majority excluded participants who had axis‐I comorbidities or comorbid substance misuse difficulties. Hirschfeld 1999 and Kowatch 2000 defined a manic episode on the basis of a low threshold YMRS Score of 14 or more, whereas the standard cut‐off is 20. Another difficulty was a tendency to select on the grounds of previous experience of study medications. Geller 2012 divided participants into known and unknown lithium responders; however, these participants were all included in the analysis. Bowden 1994 and Freeman 1992 excluded those with previous experience of valproate. Bowden 1994 and Hirschfeld 1999 excluded those who had previously experienced severe adverse events on lithium, and those who had shown an intolerance to valproate or lithium respectively. Helpfully, in their study of lithium versus ECT, Small 1988 clearly state that they did not exclude participants with previous non‐response to lithium.
Discontinuation effects
Although some study authors dispute its existence (Schou 1993), it is widely accepted that 'rebound mania' occurs in some people with bipolar disorder on discontinuation of lithium (Goodwin 1994). In acute treatment studies, discontinuation of lithium prior to the experimental phase might lead to exacerbation of mania, possibly affecting the observed response to the study drug. It is not clear whether a similar effect exists on anticonvulsant withdrawal.
The majority of studies report that they used a washout period prior to the start of the study, typically three to five days. Few of the studies appeared to have given specific consideration to the discontinuation effects. The exception to this was Bowden 2010, who clearly state that the washout period was reduced if there were signs of discontinuation effects. However, most studies reported the withdrawal of psychotropic medication prior to randomisation. This occurred three to 14 days previously. It was unclear in almost all studies how many participants had been on lithium (or any other given psychotropic) prior to randomisation. Bowden 1994 specified that plasma lithium was undetectable prior to randomisation.
Use of rescue medication and adjustment of dose of investigational drug
The doses of medication used were well described by all 36 studies. These are described under 'Description of studies' above. In general, similar dose regimes were used across comparator groups. In all studies where an explanation of how dose changes were made, lithium was titrated according to lithium plasma levels and not clinical symptoms. The target range for lithium was very similar between studies. This was similar for carbamazepine and divalproex; drugs were titrated to predetermined serum ranges. Valproate was the comparator with the most variation of dose. Half of the studies used a titration starting at 20 mg/kg/day whilst the others used a fixed‐dose titration up to 1500 to 3000 mg daily. Banga 2003 did not give details of the doses used. It should be noted that Bowden 1994 had one characteristic different to the other studies: it allowed the dosage of the study drug to be altered in response to clinical deterioration.
All the studies used other psychotropic agents as rescue medication. The agents used depended upon the publication date of the study. The older studies tended to use anticholinergics (e.g. orphenadrine) or benzodiazepines. One study used chloral hydrate as a sleep aid (Freeman 1992). The use of these was typically poorly reported. The majority of more modern studies clearly reported the use of benzodiazepines (usually lorazepam) in terms of either a categorical use/did not use or the mean dose used over the study. Chouinard 1983 used haloperidol as a rescue medication, as the active comparator to lithium was the benzodiazepine clonazepam.
Publication bias
We did not examine the presence of publication bias in this systematic review because there were insufficient studies in each intervention category to allow meaningful formal assessment using funnel plots.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15
Summary of findings for the main comparison. Lithium compared to placebo for acute mania.
| Lithium compared to placebo for acute mania | ||||||
| Patient or population: acute mania Setting: inpatients and a specialist paediatric psychiatry outpatient clinic Intervention: lithium Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with lithium | |||||
|
Efficacy: response (categorical) YMRS/MRS decrease by ≥ 50% at end of study |
Study population | OR 2.13 (1.73 to 2.63) | 1707 (6 RCTs) | ⊕⊕⊕⊕ High | ||
| 347 per 1000 | 531 per 1000 (479 to 583) | |||||
|
Efficacy: response (continuous) YMRS change from baseline at end of study |
The mean efficacy: response (continuous) measured as YMRS change from baseline to end of study in the placebo group ranged between −20.1 and −6.71 | The mean efficacy: response (continuous) measured as YMRS change from baseline to end of study in the lithium group ranged between −22.8 and −12 | ‐ | 935 (4 RCTs) | ⊕⊕⊕⊕ High | |
|
Efficacy: remission (categorical) YMRS < 12 at end of study |
Study population | OR 2.16 (1.73 to 2.69) | 1597 (5 RCTs) | ⊕⊕⊕⊕ High | ||
| 301 per 1000 | 482 per 1000 (427 to 536) | |||||
| Acceptability: total withdrawals | Study population | OR 0.76 (0.46 to 1.25) | 1353 (7 RCTs) | ⊕⊕⊕⊝ Moderate1 | ||
| 427 per 1000 | 362 per 1000 (255 to 482) | |||||
| Adverse event: tremor | Study population | OR 3.25 (2.10 to 5.04) | 1241 (6 RCTs) | ⊕⊕⊕⊕ High | ||
| 48 per 1000 | 141 per 1000 (96 to 203) | |||||
| Adverse event: somnolence | Study population | OR 2.28 (1.46 to 3.58) | 1351 (7 RCTs) | ⊕⊕⊕⊕ High2 | ||
| 46 per 1000 | 99 per 1000 (66 to 147) | |||||
| Adverse event: weight gain | Study population | OR 1.48 (0.56 to 3.92) | 735 (3 RCTs) | ⊕⊕⊕⊝ Moderate3 | ||
| 19 per 1000 | 28 per 1000 (11 to 70) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MRS: Mania Rating Scale; OR: odds ratio; RCT: randomised controlled trial; YMRS: Young Mania Rating Scale | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level due to imprecision: wide confidence interval for most of the studies. 2Seven studies of variable size, all of which give imprecise results individually, and six cross line of no difference. Overall result is precise but likely over‐exaggeration of true effect. 3Downgraded as all three studies cross line of no difference with wide confidence interval.
Summary of findings 2. Lithium compared to valproate for acute mania.
| Lithium compared to valproate for acute mania | ||||||
| Patient or population: acute mania Setting: inpatients and outpatients attending mood disorders clinics Intervention: lithium Comparison: valproate | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with valproate | Risk with lithium | |||||
|
Efficacy: response (categorical) YMRS/SADS‐C decrease ≥ 50% by end of study |
Study population | OR 1.22 (0.87 to 1.70) | 607 (5 RCTs) | ⊕⊕⊕⊝ Moderate1 | ||
| 438 per 1000 | 487 per 1000 (404 to 569) | |||||
|
Efficacy: response (continuous) Change in YMRS (ITT‐LOCF) from baseline to end of study |
The mean efficacy: response (continuous) change in YMRS (ITT‐LOCF) from baseline to end of study in the valproate group ranged from −23.8 to −7.4 | The mean efficacy: response (continuous) change in YMRS (ITT‐LOCF) from baseline to end of study in the lithium group ranged from −23.55 to −6.1 | ‐ | 398 (5 RCTs) | ⊕⊝⊝⊝ Very low2,3 | |
|
Efficacy: remission (categorical) YMRS ≤ 12 and no increase in MADRS at end of study |
Study population | OR 0.78 (0.46 to 1.32) | 257 (1 RCT) | ⊕⊕⊕⊝ Moderate4 | ||
| 713 per 1000 | 660 per 1000 (533 to 766) | |||||
| Acceptability: total withdrawals | Study population | OR 1.20 (0.86 to 1.69) | 629 (5 RCTs) | ⊕⊕⊕⊕ High | ||
| 330 per 1000 | 372 per 1000 (298 to 455) | |||||
| Adverse event: tremor | Study population | OR 10.51 (1.96 to 56.48) | 449 (2 RCTs) | ⊕⊕⊕⊕ High5,6 | ||
| 4 per 1000 | 44 per 1000 (9 to 200) | |||||
| Adverse event: somnolence | Study population | OR 0.47 (0.29 to 0.76) | 575 (4 RCTs) | ⊕⊕⊕⊕ High | ||
| 221 per 1000 | 118 per 1000 (76 to 177) | |||||
| Adverse event: nausea | Study population | OR 1.53 (0.97 to 2.40) | 583 (4 RCTs) | ⊕⊕⊕⊝ Moderate7 | ||
| 158 per 1000 | 222 per 1000 (154 to 310) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ITT: intention‐to‐treat; LOCF: last observation carried forward; MADRS: Montgomery‐Åsberg Depression Rating Scale; OR: odds ratio; RCT: randomised controlled trial; SADS‐C: Schedule for Affective Disorders and Schizophrenia‐change; YMRS: Young Mania Rating Scale | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level due to imprecision: two small studies with very wide confidence intervals; overall result is precise which is probably an overestimate of true precision. 2Downgarded two levels due to imprecision and inconsistency. Six studies, three missing standard deviation, which could not be imputed. Three studies giving highly variable results. Overall estimate is therefore much more consistent than heterogeneity of studies should suggest. 3Downgraded for imprecision. Three studies all with differing, imprecise results. 4Downgraded one level due to suspicion of reporting bias. Single study reported this outcome when multiple other larger studies will have had this data available; strongly suggests few participants met remission criteria by end of study. 5Both studies have very wide confidence intervals. 6Likely publication bias, but not downgraded as the included studies are of high quality. Four large studies examined this question but did not report this common adverse event with lithium. 7Downgraded one level due to imprecision. All values fairly imprecise, especially Kowatch 2000, may be overestimating true effect.
Summary of findings 3. Lithium compared to lamotrigine for acute mania.
| Lithium compared to lamotrigine for acute mania | ||||||
| Patient or population: acute mania Setting: inpatients (GlaxoSmithKline studies did not state if the participants were inpatients or outpatients) Intervention: lithium Comparison: lamotrigine | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with lamotrigine | Risk with lithium | |||||
|
Efficacy: response (categorical) YMRS/SADS‐C decrease ≥ 50% by end of study |
‐ | ‐ | ‐ | ‐ | ‐ | No study measured this outcome |
|
Efficacy: response (continuous) Change in BPRS from baseline to end of study |
The mean efficacy: response (continuous) change in BPRS from baseline to end of study in lamotrigine group ranged between −3.6 and −2.9 | The mean efficacy: response (continuous) change in BPRS from baseline to end of study in lithium group ranged between −5.1 and −4.9 | ‐ | 301 (3 RCTs) | ⊕⊕⊕⊝ Moderate1 | |
|
Efficacy: remission (categorical) YMRS ≤ 12 and no increase in MADRS at end of study |
‐ | ‐ | ‐ | ‐ | ‐ | No study measured this outcome |
| Acceptability: total withdrawals | Study population | OR 0.80 (0.50 to 1.29) | 303 (3 RCTs) | ⊕⊝⊝⊝ Very low2,3 | ||
| 408 per 1000 | 355 per 1000 (256 to 471) | |||||
| Adverse event: tremor | Study population | OR 1.28 (0.48 to 3.41) | 272 (2 RCTs) | ⊕⊕⊕⊝ Moderate4 | ||
| 57 per 1000 | 72 per 1000 (28 to 171) | |||||
| Adverse event: somnolence | Study population | OR 1.14 (0.34 to 3.85) | 272 (2 RCTs) | ⊕⊕⊕⊝ Moderate3,5 | ||
| 44 per 1000 | 50 per 1000 (16 to 151) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MADRS: Montgomery‐Åsberg Depression Rating Scale; OR: odds ratio; RCT: randomised controlled trial; SADS‐C: Schedule for Affective Disorders and Schizophrenia‐change; YMRS: Young Mania Rating Scale | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level for imprecision. 2Downgraded two levels for inconsistency. Heterogeneity was 82%. Two studies by GlaxoSmithKline have similar methodology and inclusion criteria but had very different dropout rates. The study authors do not provide any insight into the cause of this. 3Downgraded one level for imprecision: wide confidence interval. 4Downgraded one level for imprecision. 5Ichim 2000 would have been expected to have reported somnolence as it is a common adverse effect, however, as we have no objective measure of reporting bias we decided not to downgrade on the basis of a suspicion only.
Summary of findings 4. Lithium compared to carbamazepine for acute mania.
| Lithium compared to carbamazepine for acute mania | ||||||
| Patient or population: acute mania Setting: inpatients Intervention: lithium Comparison: carbamazepine | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with carbamazepine | Risk with lithium | |||||
|
Efficacy: response (categorical) YMRS/SADS‐C decrease ≥ 50% by end of study |
‐ | ‐ | ‐ | ‐ | ‐ | No study measured this outcome |
|
Efficacy: response (continuous) YMRS/BPRS change from baseline to end of study |
The mean efficacy: response (continuous) YMRS/BPRS endpoint score in the carbamazepine group ranged between 9 and 24 | The mean efficacy: response (continuous) YMRS/BPRS endpoint score in the lithium group ranged between 9.46 and 30.9 | ‐ | 102 (3 RCTs) | ⊕⊝⊝⊝ Very low1 | |
|
Efficacy: response (continuous) CGI change from baseline to end of study |
The mean efficacy: response (continuous) CGI endpoint score ranged between 4.1 and 5.6 | The mean efficacy: response (continuous) CGI endpoint score ranged between 5.3 and 5.7 | ‐ | 76 (2 RCTs) | ⊕⊕⊕⊝ Moderate2 | |
|
Efficacy: remission (categorical) YMRS ≤ 12 and no increase in MADRS at end of study |
‐ | ‐ | ‐ | ‐ | ‐ | No study measured this outcome |
| Acceptability: total withdrawals | Study population | OR 0.20 (0.02 to 1.94) | 34 (1 RCT) | ⊕⊕⊝⊝ Low3 | ||
| 263 per 1000 | 67 per 1000 (7 to 409) | |||||
| Adverse event: sedation | Study population | OR 0.16 (0.01 to 3.64) | 27 (1 RCT) | ⊕⊕⊝⊝ Low4 | ||
| 154 per 1000 | 28 per 1000 (2 to 398) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CGI: Clinical Global Impression; CI: confidence interval; MADRS: Montgomery‐Åsberg Depression Rating Scale; OR: odds ratio; RCT: randomised controlled trial; SADS‐C: Schedule for Affective Disorders and Schizophrenia‐change; YMRS: Young Mania Rating Scale | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded for risk of bias, and two levels for imprecision. 2Downgraded for risk of bias. Kowatch 2000 blinding strategy unclear, very poor completion rate to the study and not all outcomes reported. Lusznat 1988 selective reporting. Other studies low risk of bias. 3Pooled estimate has wide confidence interval, reflective of imprecision of all studies. Sample size is very small, would not meet optimal information size (OIS). Downgraded two levels for this. 4Downgraded two levels for risk of bias. Studies included participants aged older than 25 years; methodology poorly reported compared to modern studies. 5Downgraded for risk of bias (methodology) and imprecision. Single study with wide confidence intervals.
Summary of findings 5. Lithium compared to quetiapine for acute mania.
| Lithium compared to quetiapine for acute mania | ||||||
| Patient or population: acute mania Setting: inpatients Intervention: lithium Comparison: quetiapine | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with quetiapine | Risk with lithium | |||||
|
Efficacy: response (categorical) YMRS decrease by ≥ 50% by end of study |
Study population | OR 0.66 (0.28 to 1.55) | 335 (2 RCTs) | ⊕⊝⊝⊝ Very low1,2,3 | ||
| 644 per 1000 | 544 per 1000 (336 to 737) | |||||
|
Efficacy: response (continuous) YMRS change from baseline to end of study |
The mean efficacy: response (continuous) YMRS change from baseline to end of study in the quetiapine group ranged from −20.28 to −18.2 | The mean efficacy: response (continuous) YMRS change from baseline to end of study in the lithium group ranged from −20.76 to −15.9 | ‐ | 359 (2 RCTs) | ⊕⊕⊝⊝ Low1,3 | |
|
Efficacy: remission (categorical) Decrease in YMRS ≤ 12 by end of study |
Study population | OR 0.64 (0.26 to 1.57) | 359 (2 RCTs) | ⊕⊕⊝⊝ Low1,3 | ||
| 538 per 1000 | 427 per 1000 (232 to 646) | |||||
| Acceptability: total withdrawals | Study population | OR 1.38 (0.83 to 2.28) | 359 (2 RCTs) | ⊕⊝⊝⊝ Very low1,3,4 | ||
| 212 per 1000 | 271 per 1000 (182 to 380) | |||||
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | No study measured this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial; YMRS: Young Mania Rating Scale | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level for inconsistency. High heterogeneity from two similar studies. 2Downgraded one level for imprecision: wide confidence intervals. 3Outcome 1 downgraded for publication bias. Only two studies found for this outcome, which makes it likely that there are unpublished data not found by our search. 4Downgraded one level for imprecision. Large study with tight confidence interval, small study with wide confidence interval. This is to be expected, but it means the precision is highly dependent upon one study.
Summary of findings 6. Lithium compared to olanzapine for acute mania.
| Lithium compared to olanzapine for acute mania | ||||||
| Patient or population: acute mania Setting: inpatients Intervention: lithium Comparison: olanzapine | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with olanzapine | Risk with lithium | |||||
|
Efficacy: response (categorical) MSRS/YMRS ≥ 50% decrease in score by end of study |
Study population | OR 0.44 (0.20 to 0.94) | 180 (2 RCTs) | ⊕⊕⊕⊝ Moderate1 | ||
| 730 per 1000 | 544 per 1000 (351 to 718) | |||||
|
Efficacy: response (continuous) CGI severity score at end of study |
The mean efficacy: response (continuous) CGI severity endpoint score in the olanzapine group ranged between 2.26 and 3.69 | The mean efficacy: response (continuous) CGI severity endpoint score in the lithium group ranged between 2.83 and 3.41 | ‐ | 210 (3 RCTs) | ⊕⊕⊝⊝ Low2 | |
|
Efficacy: remission (categorical) YMRS ≤ 12 at end of study |
Study population | OR 2.00 (0.89 to 4.46) | 140 (1 RCT) | ⊕⊕⊝⊝ Low3 | ||
| 826 per 1000 | 905 per 1000 (809 to 955) | |||||
| Acceptability: total withdrawals | Study population | OR 2.60 (1.13 to 5.99) | 210 (3 RCTs) | ⊕⊕⊝⊝ Low4 | ||
| 87 per 1000 | 198 per 1000 (97 to 362) | |||||
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | No study measured this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CGI: Clinical Global Impression; CI: confidence interval; MSRS: Manic‐State Rating Scale; OR: odds ratio; RCT: randomised controlled trial; YMRS: Young Mania Rating Scale | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level for publication bias. Olanzapine is used widely to treat acute mania; why so few studies? The lack of studies precluded the use of funnel plots. 2Berk 1999 rated as high risk of bias for selective reporting. Downgraded one level for risk of bias. Downgraded one level for suspected publication bias as described above. 3Downgraded one level for imprecision. Wide confidence interval on single small study. Downgraded one level for suspected publication bias as described above. 4All studies have wide confidence intervals; strongly suspect result to be an overestimate of favouring olanzapine. Downgraded one level for imprecision. Downgraded one level for suspected publication bias as described above.
Summary of findings 7. Lithium compared to chlorpromazine for acute mania.
| Lithium compared to chlorpromazine for acute mania | ||||||
| Patient or population: acute mania Setting: inpatients Intervention: lithium Comparison: chlorpromazine | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with chlorpromazine | Risk with lithium | |||||
|
Efficacy: response (categorical) MSRS/YMRS ≥ 50% decrease in score by end of study |
‐ | ‐ | ‐ | ‐ | ‐ | No study measured this outcome |
|
Efficacy: response (continuous) BPRS score change from baseline to end of study |
The mean efficacy: response (continuous) BPRS score change from baseline to end of study in the chlorpromazine group ranged from 1.21 to 2.23 | The mean efficacy: response (continuous) BPRS score change from baseline to end of study in the lithium group ranged from 0.99 to 1.16 | ‐ | 284 (2 RCTs) | ⊕⊝⊝⊝ Very low1,2,3 | |
|
Efficacy: remission (categorical) YMRS ≤ 12 at end of study |
‐ | ‐ | ‐ | ‐ | ‐ | No study measured this outcome |
| Acceptability: total withdrawals | Study population | OR 1.75 (0.92 to 3.31) | 262 (2 RCTs) | ⊕⊕⊝⊝ Low3,4 | ||
| 141 per 1000 | 223 per 1000 (131 to 351) | |||||
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | No study measured this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BPRS: Brief Psychiatric Rating Scale; CI: confidence interval; MSRS: Manic‐State Rating Scale; OR: odds ratio; RCT: randomised controlled trial; YMRS: Young Mania Rating Scale | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded for risk of bias and publication bias. We identified only two studies comparing lithium and chlorpromazine that we could pool (a third, Spring 1970 did not provide any outcomes that were comparable): these were both conducted > 30 years ago. It is possible that the trend to using atypical antipsychotics means research using chlorpromazine has reduced, but we would have expected more publications. Also note that the methodology used in the 1970s/80s was not as rigorous as today, but these studies do give relatively clear accounts of systematic study procedures. 2Downgraded for inconsistency. Heterogeneity 90%. Methodology of studies might well be the explanation but this is hard to investigate with minimal information. 3Downgraded for publication bias. Chlorpromazine was a mainstay of psychiatry in mid 20th century. We would have expected more publications on this topic. Part of the issue is the lack of combinable outcomes. 4Downgraded for risk of bias as Spring 1970 was quasi‐randomised. Removing Spring 1970 does not change the result. Prien 1972 does not give a detailed enough methodology to feel confident about risk of bias.
Summary of findings 8. Lithium compared to haloperidol for acute mania.
| Lithium compared to haloperidol for acute mania | ||||||
| Patient or population: acute mania Setting: inpatients (one study did not specify a setting) Intervention: lithium Comparison: haloperidol | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with haloperidol | Risk with lithium | |||||
|
Efficacy: response (categorical) MSRS/YMRS ≥ 50% decrease in score by end of study |
‐ | ‐ | ‐ | ‐ | ‐ | No study measured this outcome |
|
Efficacy: response (continuous) change in BPRS (total) from baseline to end of study ‐all data |
The mean efficacy: response (continuous) change in BPRS (total) from baseline to end of study in the haloperidol group ranged from 1.24 to 12 | The mean efficacy: response (continuous) change in BPRS (total) from baseline to end of study in the lithium group ranged from 0.99 to 1 | ‐ | 80 (3 RCTs) | ⊕⊝⊝⊝ Very low1 | |
|
Efficacy: remission (categorical) YMRS ≤ 12 at end of study |
‐ | ‐ | ‐ | ‐ | ‐ | No study measured this outcome |
| Acceptability: total withdrawals | Study population | OR 0.29 (0.03 to 3.12) | 30 (1 RCT) | ⊕⊝⊝⊝ Very low2 | ||
| 200 per 1000 | 68 per 1000 (7 to 438) | |||||
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | No study measured this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BPRS: Brief Psychiatric Rating Scale; CI: confidence interval; MSRS: Manic‐State Rating Scale; OR: odds ratio; RCT: randomised controlled trial; YMRS: Young Mania Rating Scale | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded two levels for potential risk of bias. Includes studies from 1980 and 1975 with sparse published methodology. Downgraded for inconsistency. Heterogeneity very high (88%); even with removal of outlier in Analysis 8.1 (95%). 2Downgraded for imprecision. Single study with wide confidence interval. Downgraded for publication bias. Similar to other categories, haloperidol versus lithium is a question we would have expected to see more literature on. It is not possible to do funnel plots with such a limited number of studies.
Summary of findings 9. Lithium compared to zuclopenthixol for acute mania.
| Lithium compared to zuclopenthixol for acute mania | ||||||
| Patient or population: acute mania Setting: inpatients Intervention: lithium Comparison: zuclopenthixol | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with zuclopenthixol | Risk with lithium | |||||
|
Efficacy: response (categorical) BMRS change ≥ 50% from baseline to end of study |
Study population | OR 1.33 (0.30 to 5.91) | 28 (1 RCT) | ⊕⊝⊝⊝ Very low1,2,3 | ||
| 462 per 1000 | 533 per 1000 (205 to 835) | |||||
|
Efficacy: response (continuous) change in BPRS (total) from baseline to end of study ‐all data |
‐ | ‐ | ‐ | ‐ | ‐ | No study measured this outcome |
|
Efficacy: remission (categorical) YMRS ≤ 12 at end of study |
‐ | ‐ | ‐ | ‐ | ‐ | |
| Acceptability: total withdrawals | Study population | OR 0.78 (0.17 to 3.49) | 28 (1 RCT) | ⊕⊝⊝⊝ Very low1,2,3 | ||
| 462 per 1000 | 401 per 1000 (127 to 749) | |||||
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | No study measured this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BPRS: Brief Psychiatric Rating Scale; BRMS: Bech‐Rafaelsen Mania Scale; CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level: single study with high risk of bias for blinding and selective reporting. 2Downgraded one level for imprecision. Single small study with wide confidence interval. 3Downgraded one level for publication bias. Only a single study found in the literature fitting our inclusion criteria.
Summary of findings 10. Lithium compared to risperidone for acute mania.
| Lithium compared to risperidone for acute mania | ||||||
| Patient or population: acute mania Setting: inpatients and specialist mood disorders clinic (one study did not describe the setting) Intervention: lithium Comparison: risperidone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with risperidone | Risk with lithium | |||||
|
Efficacy: response (categorical) BMRS change ≥ 50% from baseline to end of study |
‐ | ‐ | ‐ | ‐ | ‐ | No study measured this outcome |
|
Efficacy: response (continuous) YMRS/MRS change at the end of the study |
The mean efficacy: response (continuous): YMRS/MRS change at the end of the study was 0 | MD 7.28 more (5.22 to 9.34) | ‐ | 241 (3 RCTs) | ⊕⊕⊝⊝ Low1,2 | |
|
Efficacy: remission (categorical) YMRS ≤ 12/absence of DSM‐IV mania by end of study |
Study population | OR 1.30 (0.11 to 14.95) | 211 (2 RCTs) | ⊕⊕⊝⊝ Low3,4 | ||
| 590 per 1000 | 652 per 1000 (137 to 956) | |||||
| Acceptability: total withdrawals | Study population | OR 1.85 (1.02 to 3.34) | 255 (3 RCTs) | ⊕⊕⊕⊝ Moderate2 | ||
| 181 per 1000 | 290 per 1000 (184 to 425) | |||||
| Adverse events: drowsiness/ somnolence | Study population | OR 0.43 (0.24 to 0.75) | 219 (2 RCTs) | ⊕⊕⊕⊝ Moderate2 | ||
| 455 per 1000 | 264 per 1000 (167 to 385) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BRMS: Bech‐Rafaelsen Mania Scale; CI: confidence interval; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, fourth edition; OR: odds ratio; MRS: Mania Rating Scale; YMRS: Young Mania Rating Scale | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level for imprecision. All studies have wide confidence intervals. 2Downgraded one level for publication bias. Very few studies found for this important clinical question using widely used medications. 3Downgraded one level for imprecision. Two studies each with wide confidence interval. 4Downgraded one level for high probability of publication bias. This is an important clinical question using widely used medications. Seems probable few studies were long enough to show remission and so this outcome has not been reported.
Summary of findings 11. Lithium compared to aripiprazole for acute mania.
| Lithium compared to aripiprazole for acute mania | ||||||
| Patient or population: acute mania Setting: inpatients Intervention: lithium Comparison: aripiprazole | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with aripiprazole | Risk with lithium | |||||
|
Efficacy: response (categorical) BMRS change ≥ 50% from baseline to end of study |
‐ | ‐ | ‐ | ‐ | ‐ | No study measured this outcome |
|
Efficacy: response (continuous) YMRS change by ≥ 50% at end of study |
Study population | OR 0.96 (0.62 to 1.51) | 309 (1 RCT) | ⊕⊕⊕⊝ Moderate1 | ||
| 468 per 1000 | 457 per 1000 (352 to 570) | |||||
|
Efficacy: remission (categorical) YMRS ≤ 12 at end of study |
Study population | OR 0.99 (0.63 to 1.56) | 309 (1 RCT) | ⊕⊕⊕⊝ Moderate1 | ||
| 403 per 1000 | 400 per 1000 (298 to 513) | |||||
| Acceptability: total withdrawals | Study population | OR 0.94 (0.60 to 1.46) | 315 (1 RCT) | ⊕⊕⊕⊝ Moderate1 | ||
| 529 per 1000 | 514 per 1000 (403 to 621) | |||||
| Adverse events: tremor | Study population | OR 1.45 (0.65 to 3.24) | 313 (1 RCT) | ⊕⊕⊕⊝ Moderate1 | ||
| 71 per 1000 | 100 per 1000 (48 to 200) | |||||
| Adverse events: somnolence/sedation | Study population | OR 0.56 (0.26 to 1.23) | 313 (1 RCT) | ⊕⊕⊕⊝ Moderate1 | ||
| 117 per 1000 | 69 per 1000 (33 to 140) | |||||
| Adverse events: clinically relevant (> 7%) weight gain | Study population | OR 2.07 (0.18 to 23.21) | 184 (1 RCT) | ⊕⊕⊝⊝ Low1,2 | ||
| 11 per 1000 | 22 per 1000 (2 to 201) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BRMS: Bech‐Rafaelsen Mania Scale; CI: confidence interval; OR: odds ratio; YMRS: Young Mania Rating Scale | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level for publication bias. Despite a full repeated database search and handsearching of grey literature, we found only Keck 2009 lithium verus aripiprazole. We found no ongoing studies for this category. This strongly suggests that data are missing. 2Downgraded one level for imprecision. Single study with wide confidence interval.
Summary of findings 12. Lithium compared to topiramate for acute mania.
| Lithium compared to topiramate for acute mania | ||||||
| Patient or population: acute mania Setting: inpatients Intervention: lithium Comparison: topiramate | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with topiramate | Risk with lithium | |||||
|
Efficacy: response (categorical) YMRS change ≥ 50% from baseline to end of study |
Study population | OR 2.28 (1.63 to 3.20) | 660 (1 RCT) | ⊕⊕⊕⊕ High | ||
| 270 per 1000 | 458 per 1000 (376 to 542) | |||||
|
Efficacy: response (continuous) YMRS change by ≥ 50% at end of study |
‐ | ‐ | ‐ | ‐ | ‐ | No study measured this outcome |
|
Efficacy: remission (categorical) YMRS ≤ 12 at end of study |
Study population | OR 2.24 (1.58 to 3.15) | 660 (1 RCT) | ⊕⊕⊕⊕ High | ||
| 240 per 1000 | 415 per 1000 (333 to 499) | |||||
| Acceptability: total withdrawals | Study population | OR 1.28 (0.66 to 2.48) | 1352 (2 RCTs) | ⊕⊕⊕⊕ High | ||
| 74 per 1000 | 93 per 1000 (50 to 166) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; YMRS: Young Mania Rating Scale | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
Summary of findings 13. Lithium compared to clonazepam for acute mania.
| Lithium compared to clonazepam for acute mania | ||||||
| Patient or population: acute mania Setting: inpatients Intervention: lithium Comparison: clonazepam | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with clonazepam | Risk with lithium | |||||
|
Efficacy: response (categorical) YMRS change ≥ 50% from baseline to end of study |
‐ | ‐ | ‐ | ‐ | ‐ | No study measured this outcome |
|
Efficacy: response (continuous) change in CGI score from baseline to end of study |
The mean efficacy: response (continuous) mean CGI endpoint score in the clonazepam group ranged from 2.71 to 4 | The mean efficacy: response (continuous) mean CGI endpoint score in the lithium group ranged from 2.07 to 4.4 | ‐ | 41 (2 RCTs) | ⊕⊕⊕⊝ Moderate1 | |
|
Efficacy : remission (categorical) YMRS ≤ 12 at end of study |
‐ | ‐ | ‐ | ‐ | ‐ | No study measured this outcome |
| Acceptability: total withdrawals | ‐ | ‐ | ‐ | ‐ | ‐ | No study measured this outcome |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | No study measured this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CGI: Clinical Global Impression; CI: confidence interval; OR: odds ratio; YMRS: Young Mania Rating Scale | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level for risk of bias; includes a single blinded study.
Summary of findings 14. Lithium compared to electroconvulsive therapy (ECT) for acute mania.
| Lithium compared to electroconvulsive therapy (ECT) for acute mania | ||||||
| Patient or population: acute mania Setting: inpatients Intervention: lithium Comparison: ECT | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with ECT | Risk with lithium | |||||
|
Efficacy: response (categorical) YMRS change ≥ 50% from baseline to end of study |
‐ | ‐ | ‐ | ‐ | ‐ | No study measured this outcome |
|
Efficacy: response (continuous) MRS mean change from baseline to end of study |
The mean efficacy: response (continuous) MRS mean change from baseline to end of study in the ECT group was −18.5 | The mean efficacy: response (continuous) MRS mean change from baseline to end of study in the ECT group was −16.4 | ‐ | 34 (1 RCT) | ⊕⊕⊝⊝ Low1 | |
|
Efficacy: remission (categorical) YMRS ≤ 12 at end of study |
‐ | ‐ | ‐ | ‐ | ‐ | No study measured this outcome |
| Acceptability: total withdrawals | ‐ | ‐ | ‐ | ‐ | ‐ | No study measured this outcome |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | No study measured this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ECT: electroconvulsive therapy; MRS: Mania Rating Scale; OR: odds ratio; YMRS: Young Mania Rating Scale | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level for imprecision. Downgraded one level for suspected publication bias. We only found this one study looking at electroconvulsive therapy in mania. It is not possible to do a funnel plot in this situation.
Summary of findings 15. Lithium compared to all antimanic agents for acute mania.
| Lithium compared to all antimanic agents for acute mania | ||||||
| Patient or population: acute mania Setting: inpatients and specialised outpatient clinics Intervention: lithium Comparison: all antimanic agents | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with all antimanic agents | Risk with lithium | |||||
|
Efficacy: response (categorical) YMRS/MRS/BPRS change by ≥ 50% at end of study |
Study population | OR 1.36 (1.01 to 1.83) | 3666 (14 RCTs) | ⊕⊕⊕⊕ High | ||
| 395 per 1000 | 476 per 1000 (407 to 545) | |||||
|
Efficacy: response (continuous) YMRS/BPRS: change from baseline to end of study |
The mean efficacy: response (continuous) YMRS/BPRS change from baseline to end of study was 0 | MD 0.30 lower (−1.45 to lower 0.85) | ‐ | 2410 (19 RCTs) | ⊕⊕⊝⊝ Low1 | |
|
Efficacy: remission (categorical) YMRS ≤ 12 at end of study |
‐ | ‐ | ‐ | ‐ | ‐ | No study measured this outcome |
| Acceptability: total withdrawals | Study population | OR 1.16 (0.89 to 1.52) | 4201 (24 RCTs) | ⊕⊕⊕⊕ High | ||
| 308 per 1000 | 341 per 1000 (284 to 404) | |||||
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | No study measured this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BPRS: Brief Psychiatric Rating Scale; CI: confidence interval; MD: mean difference; MRS: Mania Rating Scale; OR: odds ratio; RCT: randomised controlled trial; YMRS: Young Mania Rating Scale | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded two levels for inconsistency. The overall result of this meta‐analysis is precise, but the individual results are highly heterogeneous. This is probably explainable by small sample sizes in early studies and missing standard deviations that could not be imputed, however, with such a high I2 value (99%) the results could not be said to be consistent.
Lithium versus placebo
Comparison 1: lithium versus placebo
Eight studies (including 1991 participants) contributed data to this comparison. See also: Table 1
We found eight RCTs, which are included in the outcomes, although only a maximum of six studies provided efficacy data that fitted any of our primary outcomes. We identified seven studies via the database search, and we found one (Astra Zeneca 2009), in the grey literature (ClinicalTrials.gov).
Primary outcomes
Efficacy: response (categorical). At least 50% reduction on YMRS or Mania Rating Scale (MRS) by end of the study
Six studies contributed to the comparison (Analysis 1.1). Lithium was twice as effective as placebo at achieving a response (OR 2.13, 95% CI 1.73 to 2.63; participants = 1707; studies = 6; I2 = 16%; high‐certainty evidence).
1.1. Analysis.
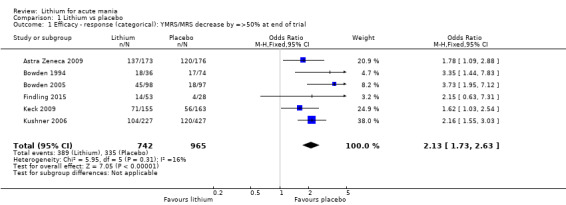
Comparison 1 Lithium vs placebo, Outcome 1 Efficacy ‐ response (categorical): YMRS/MRS decrease by =>50% at end of trial.
Efficacy: response (continuous). Mean change in YMRS from baseline to end of the study
Participants treated with lithium had a greater mean reduction in YMRS by day 21 of treatment than those on placebo (MD −2.85, 95% CI −3.14 to −2.55; participants = 935; studies = 4; I2 = 84%; high‐certainty evidence). Six studies reported this outcome (Analysis 1.2), but we were unable to include two studies (n = 276 participants), in the meta‐analysis as we could not obtain or calculate SDs (Bowden 2005; Findling 2015). These studies both reported a statistically greater reduction in YMRS in the lithium group compared to placebo.
1.2. Analysis.
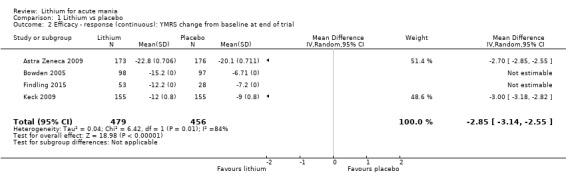
Comparison 1 Lithium vs placebo, Outcome 2 Efficacy ‐ response (continuous): YMRS change from baseline at end of trial.
Efficacy (categorical): remission. YMRS less than 12 at end of study
Five studies reported the outcome of remission measured by YMRS less than 12 at end of study when comparing lithium with placebo (Analysis 1.4). Lithium was twice as effective in achieving remission as placebo (OR 2.16, 95% CI 1.73 to 2.69; participants = 1597; studies = 5; I2 = 21%; high‐certainty evidence).
1.4. Analysis.
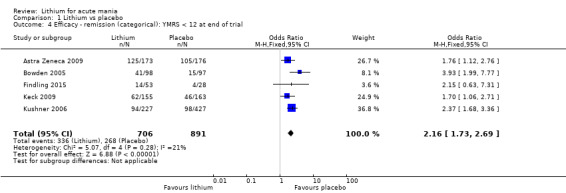
Comparison 1 Lithium vs placebo, Outcome 4 Efficacy ‐ remission (categorical): YMRS < 12 at end of trial.
Acceptability: adverse events
Depression (categorical): four studies measured the emergence of depression during treatment (Analysis 1.6). The lithium group were half as likely to develop depression as those treated with placebo (OR 0.57, 95% CI 0.33 to 0.98; participants = 1360; studies = 4; I2 = 0%).
Mania (categorical): four studies reported on the proportion of participants who developed further manic symptoms during the study (Analysis 1.7). The lithium group were less likely to experience worsened symptoms than those on placebo (OR 0.59, 95% CI 0.38 to 0.93; participants = 1296; studies = 4; I2 = 0%).
Somnolence (categorical): we included seven studies in the analysis; lithium was twice as likely to cause somnolence as placebo (OR 2.28, 95% CI 1.46 to 3.58; participants = 1351; studies = 7; I2 = 0%; high‐certainty evidence Analysis 1.12).
Dizziness (categorical): five studies were included in the analysis for dizziness during the study; lithium was more likely to cause dizziness than placebo (OR 2.12, 95% CI 1.21 to 3.74; participants = 873; studies = 5; I2 = 0%; Analysis 1.13).
Nausea (categorical): participants treated with lithium were more likely to report nausea that those on placebo (OR 2.32, 95% CI 1.54 to 3.50; participants = 1220; studies = 6; I2 = 0%; Analysis 1.16).
Vomiting (categorical): participants treated with lithium were more likely to report vomiting that those on placebo (OR 6.06, 95% CI 3.21 to 11.45; participants = 1028; studies = 6; I2 = 0%; Analysis 1.17).
Pain (categorical): three studies (n = 396) reported that the lithium group had significantly fewer participants presenting with pain compared to placebo (OR 0.23, 95% CI 0.07 to 0.79; participants = 396; studies = 3; I2 = 0%; Analysis 1.19).
Tremor (categorical): six studies contributed to the analysis. Lithium was three times more likely to cause a tremor than placebo (OR 3.25, 95% CI 2.10 to 5.04; participants = 1241; studies = 6; I2 = 0%; high‐certainty evidence; Analysis 1.21).
Attempted suicide (categorical): two studies reported the incidence of attempted suicide during the study. We could not use these data for meta‐analysis due to the low incidence (only one attempted suicide out of 811 participants). GlaxoSmithKline 2005 reported one attempted suicide from the placebo group and zero in the lithium group; Kushner 2006 reported no suicide attempts across the study.
-
Other adverse events: meta‐analysis showed no significant difference between lithium and placebo for the following adverse events.
Weight gain ‐ categorical (OR 1.48, 95% CI 0.56 to 3.92; participants = 735, I2= 51%; high‐certainty evidence; Analysis 1.8)
Weight gain ‐ continuous (MD 0.16, 95% CI −0.50 to 0.82; participants = 599; I2 = 90%; Analysis 1.9)
Akathisia (OR 0.86, 95% CI 0.39 to 1.91; participants = 673; I2 = 32%; Analysis 1.10)
Headache (OR 1.06, 95% CI 0.76 to 1.48; participants = 1270; I2 = 0%; Analysis 1.11)
Insomnia (OR 0.76, 95% CI 0.44 to 1.29; participants = 706; I2 = 0%; Analysis 1.14)
Diarrhoea (OR 1.51, 95% CI 0.90 to 2.54; participants = 1028; I2 = 16%; Analysis 1.15)
Dry mouth (OR 1.10, 95% CI 0.58 to 2.09; participants = 682; I2 = 33%; Analysis 1.18)
Extrapyramidal side effects (EPSEs) (OR 1.23, 95% CI 0.68 to 2.19; participants = 478; I2 = 0%; Analysis 1.20)
Constipation (OR 1.28, 95% CI 0.81 to 2.01; participants = 1075; I2 = 20%; Analysis 1.22)
Fever (OR 1.63, 95% CI 0.75 to 3.55; participants = 466; I2 = 41%; Analysis 1.23)
Rash (OR 0.91, 95% CI 0.37 to 2.29; participants = 367; I2 = 48%; Analysis 1.24)
1.6. Analysis.
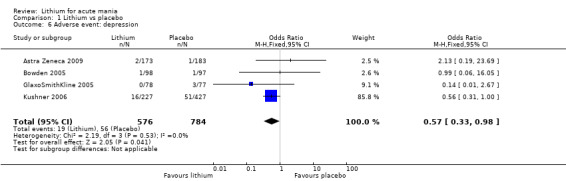
Comparison 1 Lithium vs placebo, Outcome 6 Adverse event: depression.
1.7. Analysis.
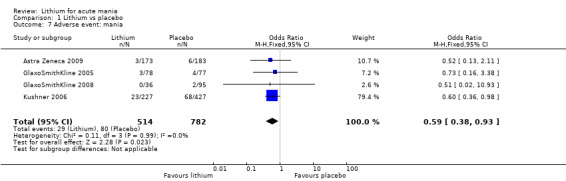
Comparison 1 Lithium vs placebo, Outcome 7 Adverse event: mania.
1.12. Analysis.
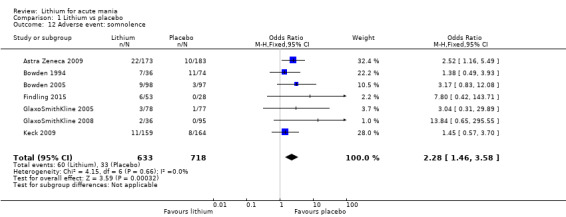
Comparison 1 Lithium vs placebo, Outcome 12 Adverse event: somnolence.
1.13. Analysis.
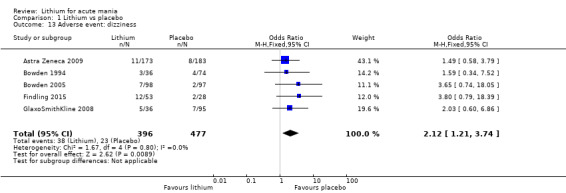
Comparison 1 Lithium vs placebo, Outcome 13 Adverse event: dizziness.
1.16. Analysis.
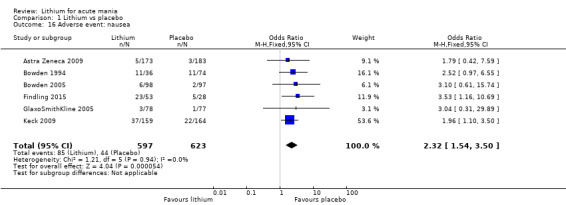
Comparison 1 Lithium vs placebo, Outcome 16 Adverse event: nausea.
1.17. Analysis.
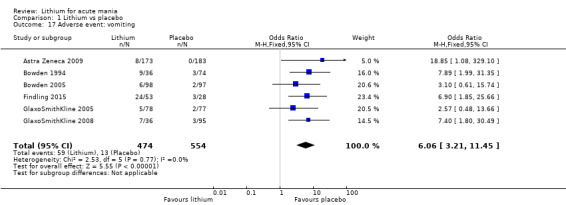
Comparison 1 Lithium vs placebo, Outcome 17 Adverse event: vomiting.
1.19. Analysis.
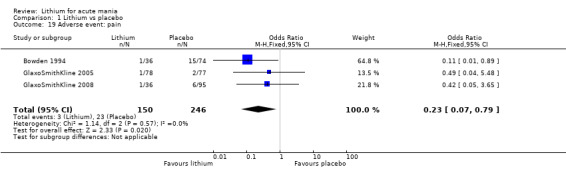
Comparison 1 Lithium vs placebo, Outcome 19 Adverse event: pain.
1.21. Analysis.
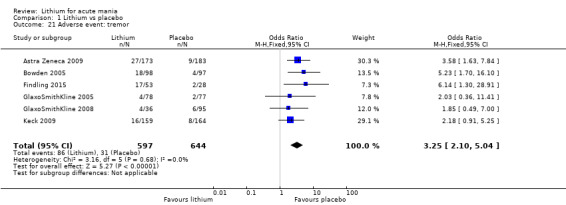
Comparison 1 Lithium vs placebo, Outcome 21 Adverse event: tremor.
1.8. Analysis.
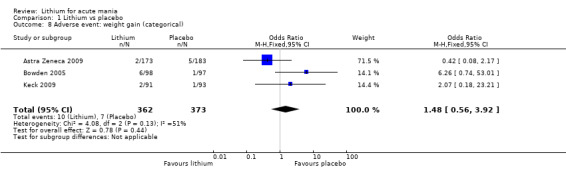
Comparison 1 Lithium vs placebo, Outcome 8 Adverse event: weight gain (categorical).
1.9. Analysis.
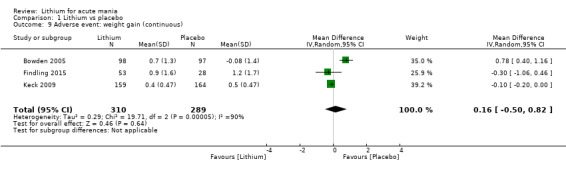
Comparison 1 Lithium vs placebo, Outcome 9 Adverse event: weight gain (continuous).
1.10. Analysis.
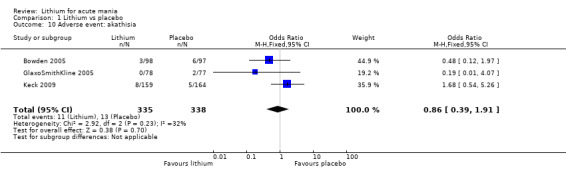
Comparison 1 Lithium vs placebo, Outcome 10 Adverse event: akathisia.
1.11. Analysis.
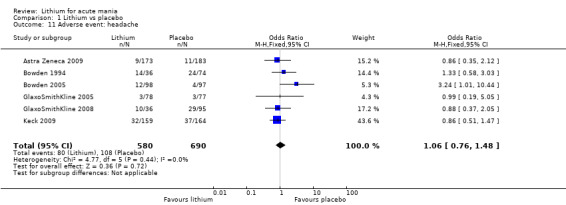
Comparison 1 Lithium vs placebo, Outcome 11 Adverse event: headache.
1.14. Analysis.
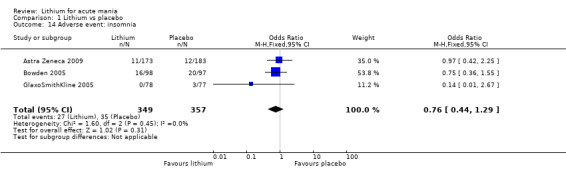
Comparison 1 Lithium vs placebo, Outcome 14 Adverse event: insomnia.
1.15. Analysis.
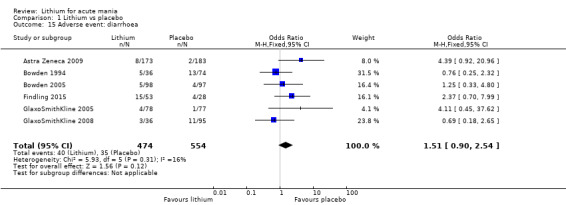
Comparison 1 Lithium vs placebo, Outcome 15 Adverse event: diarrhoea.
1.18. Analysis.
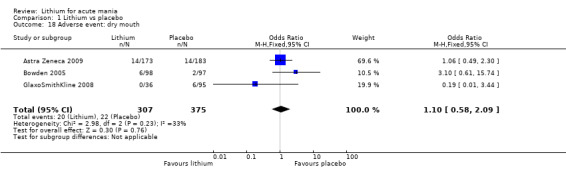
Comparison 1 Lithium vs placebo, Outcome 18 Adverse event: dry mouth.
1.20. Analysis.
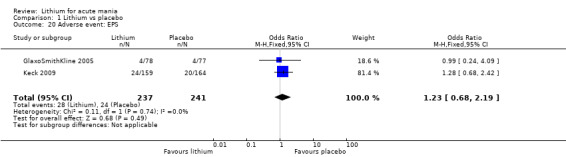
Comparison 1 Lithium vs placebo, Outcome 20 Adverse event: EPS.
1.22. Analysis.
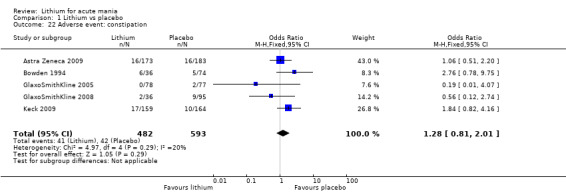
Comparison 1 Lithium vs placebo, Outcome 22 Adverse event: constipation.
1.23. Analysis.
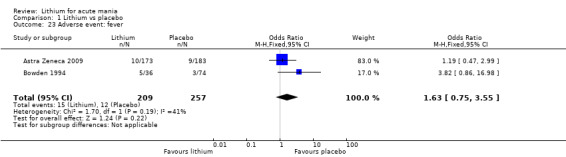
Comparison 1 Lithium vs placebo, Outcome 23 Adverse event: fever.
1.24. Analysis.
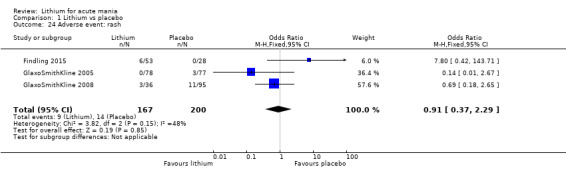
Comparison 1 Lithium vs placebo, Outcome 24 Adverse event: rash.
Only one study reported data for each of the other adverse events. The data are shown in Analysis 16.1 to Analysis 16.32.
16.1. Analysis.
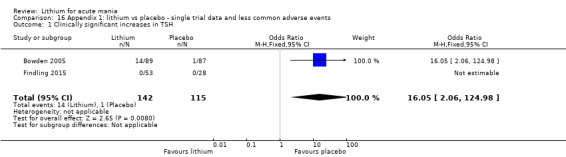
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 1 Clinically significant increases in TSH.
16.32. Analysis.
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 32 Pruritus.
Acceptability: all‐cause dropouts from the study
All eight publications contributed data to this analysis. Participants treated with lithium were less likely to withdraw from the study than those taking a placebo. However, there was considerable heterogeneity between the results (OR 0.76; 95% CI 0.46 to 1.25; participants = 1353; I2 = 75%; moderate‐certainty evidence; Analysis 1.5).
1.5. Analysis.
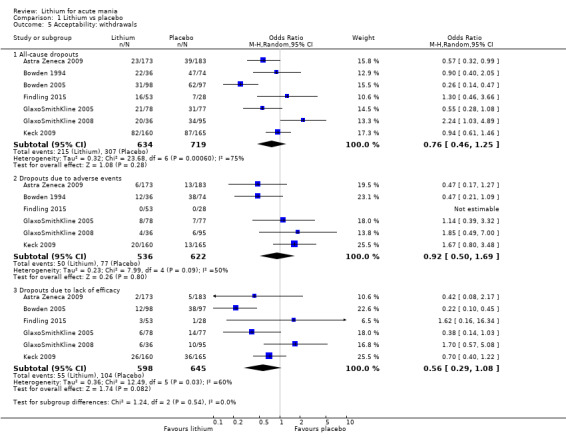
Comparison 1 Lithium vs placebo, Outcome 5 Acceptability: withdrawals.
Secondary outcomes
Efficacy: response (continuous). CGI change from baseline to end of study
Four studies demonstrated a greater decrease in mean CGI score in lithium‐treated participants compared to placebo (MD −0.25, 95% CI −0.35 to −0.16; participants = 1147; studies = 4; I2 = 95%; Analysis 1.3). A fifth study (Bowden 2005; n=195), provided CGI data but we could not use it in the meta‐analysis due to a lack of standard deviations.
1.3. Analysis.
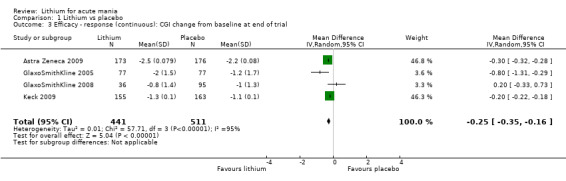
Comparison 1 Lithium vs placebo, Outcome 3 Efficacy ‐ response (continuous): CGI change from baseline at end of trial.
Efficacy: response (continuous). Mean change in MADRS from baseline to the end of the study
Two studies contributed to the analysis; lithium was superior in reducing mean MADRS scores compared to placebo (MD −0.58, 95% CI −0.78 to −0.37; participants = 862; studies = 2; I2 = 87%; Analysis 1.28). We could not combine one study (n = 195) due to lack of SD (Bowden 2005).
1.28. Analysis.
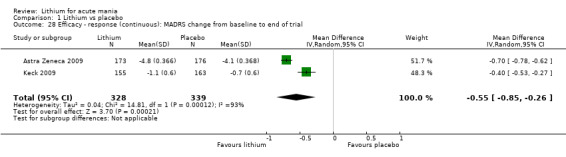
Comparison 1 Lithium vs placebo, Outcome 28 Efficacy ‐ response (continuous): MADRS change from baseline to end of trial.
Efficacy: response (continuous). Mean change in MRS from baseline to end of the study
There was no significant difference in the mean change in MRS scores between the lithium and placebo groups (MD −1.19, 95% CI −2.78 to 0.39; participants = 285; studies = 2; I2 = 0%; Analysis 1.29).
1.29. Analysis.
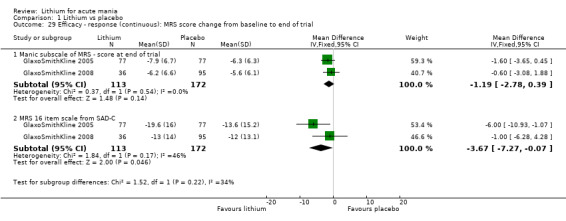
Comparison 1 Lithium vs placebo, Outcome 29 Efficacy ‐ response (continuous): MRS score change from baseline to end of trial.
Efficacy (continuous). Mean change in MRS (16‐item scale from SAD‐C) from baseline to end of study
Using the 16‐item scale from SAD‐C, lithium‐treated participants showed a greater reduction in mean scores across the length of the study (MD −3.67, 95% CI −7.27 to −0.07; participants = 285; studies = 2; I2 = 46%; Analysis 1.29).
Efficacy (continuous). Manic subscale of MRS, mean change from baseline to end of study
There was no significant difference in the mean change in MRS ‐manic subscale scores between the lithium and placebo groups (MD −1.19, 95% CI −2.78 to 0.39; participants = 285; studies = 2; I2 = 0%; Analysis 1.29).
Efficacy (continuous). Mean change in the HAMD‐31 scale from baseline to end of study
There was no significant difference in the mean change in HAMD scores between the lithium and placebo groups (MD −1.12, 95% CI −7.69 to 5.44; participants = 285; studies = 2; I2 = 91%; Analysis 1.31).
1.31. Analysis.
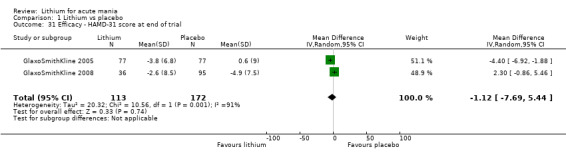
Comparison 1 Lithium vs placebo, Outcome 31 Efficacy ‐ HAMD‐31 score at end of trial.
Efficacy (continuous). Mean change in the BPRS from baseline to end of study
Two studies contributed to the analysis; lithium was no better than placebo in reducing mean BPRS scores (MD −1.74, 95% CI −3.70 to 0.23; participants = 285; studies = 2; I2 = 33%; Analysis 1.32).
1.32. Analysis.
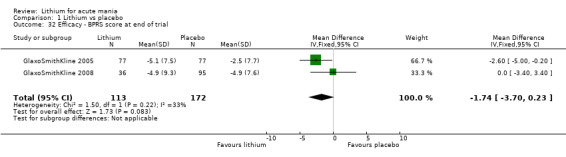
Comparison 1 Lithium vs placebo, Outcome 32 Efficacy ‐ BPRS score at end of trial.
Efficacy (continuous). Mean change in the PANSS cognitive subscale from baseline to end of study
Two studies contributed to this analysis; the lithium group showed greater improvement than placebo (MD −2.86, 95% CI −4.33 to −1.39; participants = 629; studies = 2; I2 = 98%; Analysis 1.33).
1.33. Analysis.
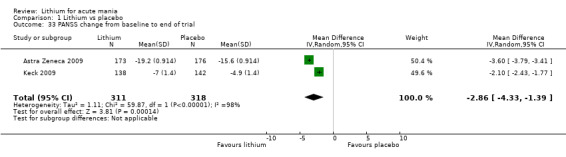
Comparison 1 Lithium vs placebo, Outcome 33 PANSS change from baseline to end of trial.
Other efficacy results
Several of our secondary outcomes were only reported by one study. Many of these did not provide statistical analysis or the means to do this ourselves. The following outcome was reported by Keck 2009 as showing a greater effect in the lithium group compared to placebo:
Change in CGI‐BP severity of illness score (MD −2.00, 95% CI −2.02 to −1.98; participants = 316; studies = 1; Analysis 1.34).
1.34. Analysis.
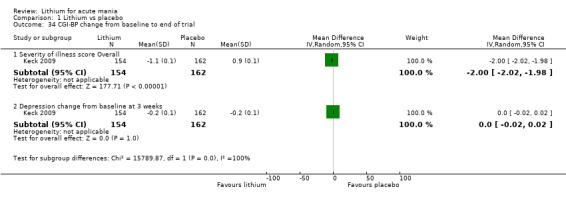
Comparison 1 Lithium vs placebo, Outcome 34 CGI‐BP change from baseline to end of trial.
The following outcome did not find a significant difference between lithium and placebo:
Change in CGI‐BP depression score (MD 0.00, 95% CI −0.02 to 0.02; participants = 316; studies = 1; Analysis 1.34).
Acceptability: withdrawal from the study due to lack of efficacy
Participants treated with lithium were less likely to withdraw from the study due to lack of efficacy than those taking a placebo (OR 0.56, 95% CI 0.29 to 1.08; participants = 1243; studies = 6; I2 = 60%; Analysis 1.5). It should be noted that the withdrawal rates between studies were quite variable.
Acceptability: withdrawal from the study due to adverse events
There was no significant difference between the number of participants who withdrew for adverse events on lithium compared to placebo (OR 0.92, 95% CI 0.50 to 1.69; participants = 1158; studies = 6; I2 = 50%; Analysis 1.5).
Use of rescue medications
Three studies looked at these outcomes; there was no significant difference between lithium and placebo for the use of:
any allowed concomitant medication, including anxiolytics, benzodiazepines and z‐drugs (OR 0.90, 95% CI 0.52 to 1.57; participants = 479; studies = 2; I2 = 40%; Analysis 1.35);
use of sleep medications (OR 0.90, 95% CI 0.51 to 1.58; participants = 195; studies = 1; Analysis 1.36);
use of anticholinergic medications (OR 1.39, 95% CI 0.73 to 2.62; participants = 520; studies = 2; I2 = 0%; Analysis 1.37).
1.35. Analysis.
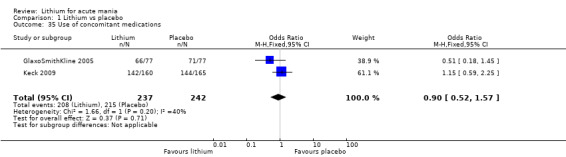
Comparison 1 Lithium vs placebo, Outcome 35 Use of concomitant medications.
1.36. Analysis.
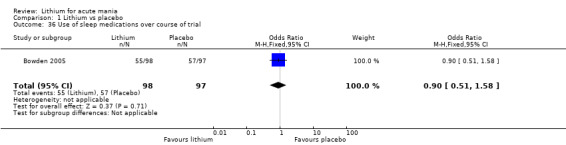
Comparison 1 Lithium vs placebo, Outcome 36 Use of sleep medications over course of trial.
1.37. Analysis.
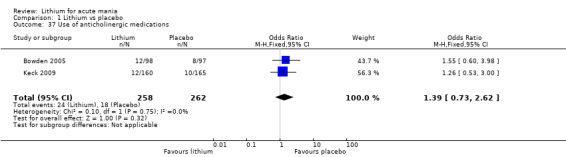
Comparison 1 Lithium vs placebo, Outcome 37 Use of anticholinergic medications.
Lithium versus mood stabilisers
Comparison 2: lithium versus valproate
Eight studies including 801 participants contributed data to this comparison (Banga 2003; Bowden 1994; Bowden 2010; Freeman 1992; Geller 2012; Hirschfeld 1999; Kowatch 2000; Shafti 2008). See also Table 2.
Primary outcomes
Efficacy: response (categorical). YMRS/SADS‐C decrease by at least 50% by end of study
Five studies (n = 607) contributed to the analysis: there was no significant difference between lithium and valproate/divalproex in terms of efficacy using this outcome measure (OR 1.22, 95% CI 0.87 to 1.70; participants = 607; studies = 5; I2 = 22%; moderate‐certainty evidence; Analysis 2.1). When we considered only adults (by removing Kowatch 2000), there was no significant difference in the results (OR 1.30, 95% CI 0.84 to 1.99; participants = 579; studies = 4; I2 = 25%; Analysis 2.2).
2.1. Analysis.
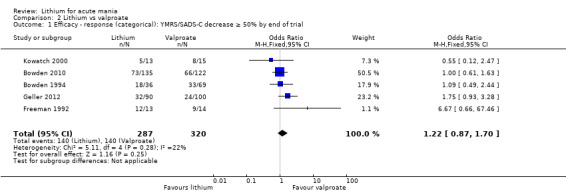
Comparison 2 Lithium vs valproate, Outcome 1 Efficacy ‐ response (categorical): YMRS/SADS‐C decrease ≥ 50% by end of trial.
2.2. Analysis.
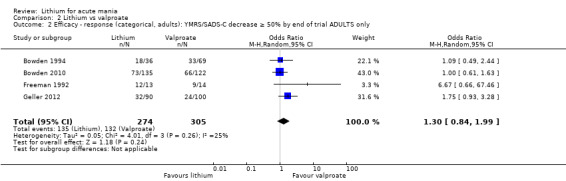
Comparison 2 Lithium vs valproate, Outcome 2 Efficacy ‐ response (categorical, adults): YMRS/SADS‐C decrease ≥ 50% by end of trial ADULTS only.
Efficacy: response (continuous). Change in YMRS (ITT‐LOCF) from baseline to end of study (12 weeks)
There was no significant difference in the mean change in YMRS scores between the lithium and valproate/divalproex groups by the end of the study (MD 0.43, 95% CI −0.36 to 1.23; participants = 398; studies = 5; I2 = 26%; low‐certainty evidence; Analysis 2.3).
2.3. Analysis.
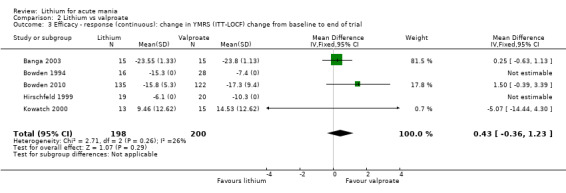
Comparison 2 Lithium vs valproate, Outcome 3 Efficacy ‐ response (continuous): change in YMRS (ITT‐LOCF) change from baseline to end of trial.
Efficacy: response (continuous). Change in CGI‐BP from baseline to the end of the study (3 weeks)
Two studies (n = 287) contributed to the comparison; there was no significant difference in change in CGI scores between the groups (MD −0.02, 95% CI −0.29 to 0.25; participants = 287; studies = 2; I2 = 0%; Analysis 2.4).
2.4. Analysis.
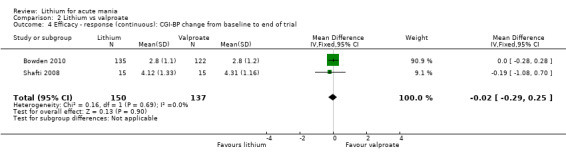
Comparison 2 Lithium vs valproate, Outcome 4 Efficacy ‐ response (continuous): CGI‐BP change from baseline to end of trial.
Efficacy: remission (categorical). YMRS 12 or less and a reduction of at least 2 points on the CGI‐BP at 12 weeks
This outcome is a more restrictive definition of remission than outlined in our predetermined outcomes; it was the definition of remission used by the study authors. Only one study reported this outcome (Analysis 2.7). Under this definition, fewer lithium‐treated participants achieved remission compared to valproate/ divalproex (OR 0.57, 95% CI 0.34 to 0.96; participants = 257; studies = 1, moderate‐certainty evidence).
2.7. Analysis.
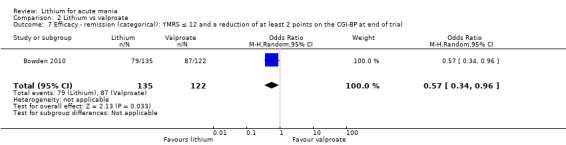
Comparison 2 Lithium vs valproate, Outcome 7 Efficacy ‐ remission (categorical): YMRS ≤ 12 and a reduction of at least 2 points on the CGI‐BP at end of trial.
Acceptability: adverse events
Tremor: participants taking lithium were more likely to experience a tremor than those on valproate or divalproex (OR 10.51, 95% CI 1.96 to 56.48; participants = 449; studies = 2; I2 = 0%; high‐certainty evidence). It should be noted that the absolute number of events was fairly small, so the confidence intervals on both studies contributing data were very wide (Analysis 2.10).
Headache: headache was more commonly reported by the participants in the lithium group compared to valproate or divalproex (OR 2.64, 95% CI 1.52 to 4.59; participants = 286; studies = 2; I2 = 0%; Analysis 2.8).
Somnolence: participants taking sodium valproate/divalproex were more likely than those on lithium to report somnolence (OR 0.47, 95% CI 0.29 to 0.76; participants = 575; studies = 4; I2 = 27%; high‐certainty evidence; Analysis 2.9).
-
Other adverse events: we found no significant difference between lithium and valproate/ divalproex for the following adverse events (P > 0.05 for each):
any adverse effect (OR 0.99, 95% CI 0.62 to 1.57; participants = 298; studies = 2; I2 = 0%; Analysis 2.19);
diarrhoea (OR 1.09, 95% CI 0.59 to 2.01; participants = 583; studies = 4; I2 = 46%; Analysis 2.21);
nausea (OR 1.53, 95% CI 0.97 to 2.40; participants = 583; studies = 4; I2 = 0%; high‐certainty evidence; Analysis 2.22).
There was no significant difference between lithium and valproate for a series of less common or important adverse events, or both; data shown in Analysis 17.1 onwards.
2.10. Analysis.
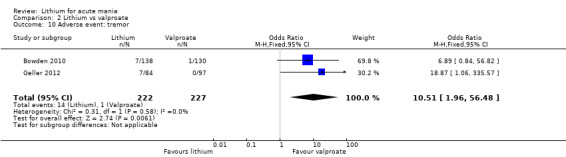
Comparison 2 Lithium vs valproate, Outcome 10 Adverse event: tremor.
2.8. Analysis.
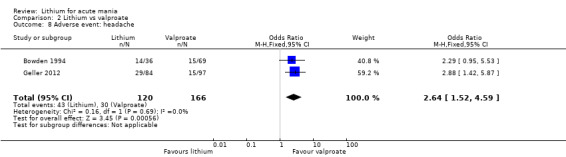
Comparison 2 Lithium vs valproate, Outcome 8 Adverse event: headache.
2.9. Analysis.
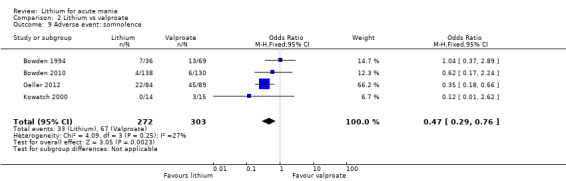
Comparison 2 Lithium vs valproate, Outcome 9 Adverse event: somnolence.
2.19. Analysis.
Comparison 2 Lithium vs valproate, Outcome 19 Any adverse events.
2.21. Analysis.
Comparison 2 Lithium vs valproate, Outcome 21 Adverse event: diarrhoea.
2.22. Analysis.
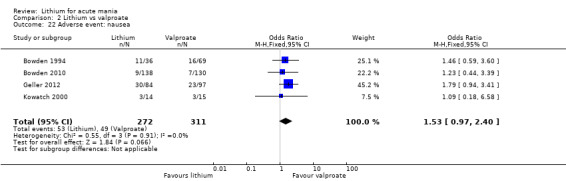
Comparison 2 Lithium vs valproate, Outcome 22 Adverse event: nausea.
17.1. Analysis.
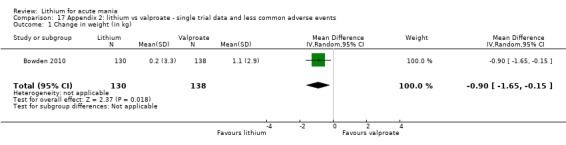
Comparison 17 Appendix 2: lithium vs valproate ‐ single trial data and less common adverse events, Outcome 1 Change in weight (in kg).
Acceptability: withdrawals
There was no significant difference in withdrawal rates between those participants taking valproate and those on lithium (OR 1.20, 95% CI 0.86 to 1.69; participants = 629; studies = 5; I2 = 0%; Analysis 2.11).
2.11. Analysis.
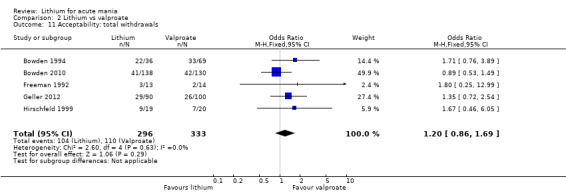
Comparison 2 Lithium vs valproate, Outcome 11 Acceptability: total withdrawals.
Secondary outcomes
Outcomes for which a single study reported data
Only the first of these single studies reported a significant difference between lithium and valproate/divalproex.
SADS‐C mania score change from baseline (MD −16.90, 95% CI −28.85 to −4.95; participants = 27; studies = 1; Analysis 2.13).
Change in MADRS (ITT‐LOCF) from baseline to end of study (12 weeks) (MD 0.40, 95% CI −0.73 to 1.53; participants = 257; studies = 1; Analysis 2.5).
SADS‐C depression score (MD −3.40, 95% CI −9.62 to 2.82; participants = 27; studies = 1; Analysis 2.14).
Mean change in MADRS (MD 0.20, 95% CI −0.83 to 1.23; participants = 257; studies = 1; Analysis 2.12).
GAS post‐treatment score (MD 9.30, 95% CI −4.18 to 22.78; participants = 27; studies = 1; Analysis 2.15).
Mean CGI‐BP score at end of study (MD 0.20, 95% CI −0.13 to 0.53; participants = 257; studies = 1; Analysis 2.16).
2.13. Analysis.
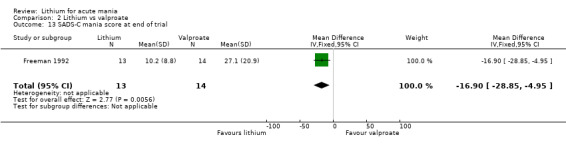
Comparison 2 Lithium vs valproate, Outcome 13 SADS‐C mania score at end of trial.
2.5. Analysis.
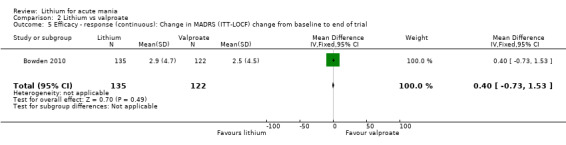
Comparison 2 Lithium vs valproate, Outcome 5 Efficacy ‐ response (continuous): Change in MADRS (ITT‐LOCF) change from baseline to end of trial.
2.14. Analysis.
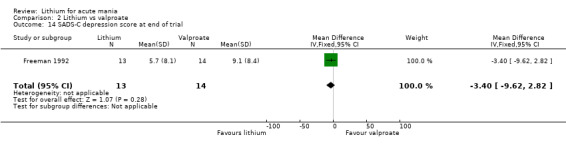
Comparison 2 Lithium vs valproate, Outcome 14 SADS‐C depression score at end of trial.
2.12. Analysis.
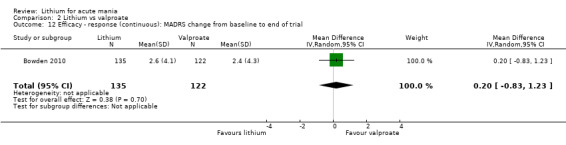
Comparison 2 Lithium vs valproate, Outcome 12 Efficacy ‐ response (continuous): MADRS change from baseline to end of trial.
2.15. Analysis.
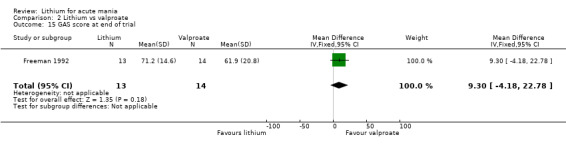
Comparison 2 Lithium vs valproate, Outcome 15 GAS score at end of trial.
2.16. Analysis.
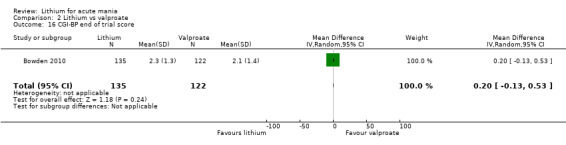
Comparison 2 Lithium vs valproate, Outcome 16 CGI‐BP end of trial score.
Use of rescue medications
Bowden 2010 and Freeman 1992 compared the use of various other psychotropic medications, unfortunately we could not combine any of these for meta‐analysis due to Bowden 2010 providing only categorical data and Freeman 1992 providing only continuous data.
Hirschfeld 1999 found that there was no significant difference in the amount of either lorazepam (MD 1.25, 95% CI 0.28 to 5.59; participants = 39; studies = 1; Analysis 2.23) or chloral hydrate (MD 1.50, 95% CI −2.76 to 5.76; participants = 27; studies = 1; Analysis 2.24) used by either lithium or valproate/divalproex; Freeman 1992 also found no significant difference in use of lorazepam between the two groups (MD −0.70, 95% CI −5.04 to 3.64; participants = 27; studies = 1; Analysis 2.25).
2.23. Analysis.
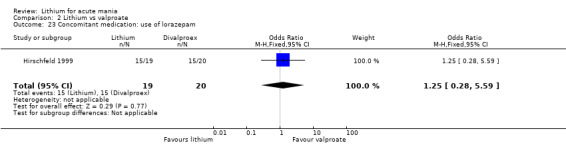
Comparison 2 Lithium vs valproate, Outcome 23 Concomitant medication: use of lorazepam.
2.24. Analysis.
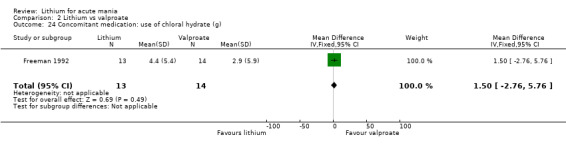
Comparison 2 Lithium vs valproate, Outcome 24 Concomitant medication: use of chloral hydrate (g).
2.25. Analysis.
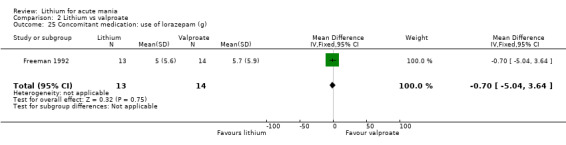
Comparison 2 Lithium vs valproate, Outcome 25 Concomitant medication: use of lorazepam (g).
Similarly, Bowden 2010 also found no significant difference in the categorical use of anxiolytics (OR 1.24, 95% CI 0.75 to 2.05; participants = 257; studies = 1; Analysis 2.26), or antidepressants (OR 2.17, 95% CI 0.55 to 8.58; participants = 257; studies = 1; Analysis 2.27), between lithium and valproate/divalproex treated participants).
2.26. Analysis.
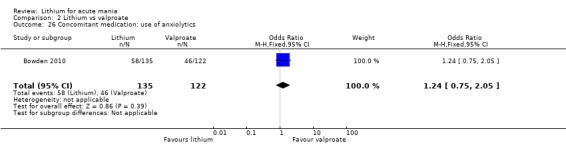
Comparison 2 Lithium vs valproate, Outcome 26 Concomitant medication: use of anxiolytics.
2.27. Analysis.
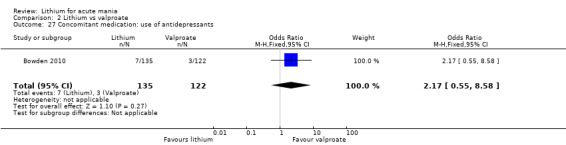
Comparison 2 Lithium vs valproate, Outcome 27 Concomitant medication: use of antidepressants.
Comparison 3: lithium versus lamotrigine
We found three studies, with 304 participants.
Primary outcomes
Efficacy (continuous): change in BPRS from baseline to end of study
There was no significant difference in the change in BPRS score in the lithium compared to the lamotrigine group (MD −1.82, 95% CI −3.78 to 0.14; participants = 301; studies = 2; I2 = 0%; moderate‐certainty evidence; Analysis 3.1). Ichim 2000 did not report SDs, but we used the method recommended by Furukawa 2006 to impute them. Inclusion or exclusion of the data from Ichim 2000 had minimal effects on the result estimate.
3.1. Analysis.
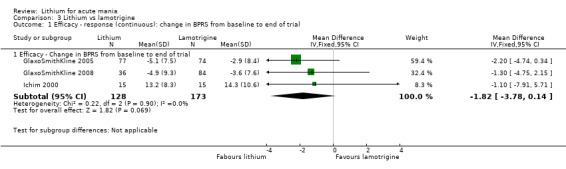
Comparison 3 Lithium vs lamotrigine, Outcome 1 Efficacy ‐ response (continuous): change in BPRS from baseline to end of trial.
Efficacy (continuous): change in MRS‐16 item from SAD‐C from baseline to end of study
There was a trend towards lithium being more efficacious than lamotrigine (MD −3.74, 95% CI −7.55 to 0.08; participants = 271; studies = 2; I2 = 0%; Analysis 3.2). It may be, however, that the change in scores is too small to make a clinically relevant difference
3.2. Analysis.
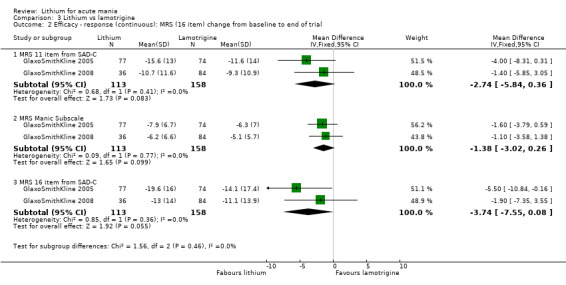
Comparison 3 Lithium vs lamotrigine, Outcome 2 Efficacy ‐ response (continuous): MRS (16 item) change from baseline to end of trial.
Acceptability: adverse events
There was no significant difference in the frequency of the following adverse events between the lithium and lamotrigine groups.
Vomiting (OR 1.77, 95% CI 0.75 to 4.18; participants = 272; studies = 2; I2 = 46%; Analysis 3.10)
Worsening of manic symptoms (OR 0.63, 95% CI 0.16 to 2.46; participants = 272; studies = 2; I2 = 0%; Analysis 3.11)
Diarrhoea (OR 3.83, 95% CI 0.92 to 15.92; participants = 272; studies = 2; I2 = 0%; Analysis 3.12)
Headache (OR 0.96, 95% CI 0.45 to 2.02; participants = 272; studies = 2; I2 = 35%; Analysis 3.13)
Tremor (OR 1.28, 95% CI 0.48 to 3.41; participants = 272; studies = 2; I2 = 30%; moderate‐certainty evidence; Analysis 3.14)
Rash (OR 0.58, 95% CI 0.17 to 1.97; participants = 272; studies = 2; I2 = 35%; Analysis 3.15)
Somnolence (OR 1.14, 95% CI 0.34 to 3.85; participants = 272; studies = 2; I2 = 0%; moderate‐certainty evidence; Analysis 3.16)
Any side effect (OR 0.89, 95% CI 0.47 to 1.70; participants = 272; studies = 2; I2 = 31%; Analysis 3.17)
Constipation (OR 0.78, 95% CI 0.18 to 3.40; participants = 272; studies = 2; I2 = 72%; Analysis 3.19)
Accidental injury (OR 1.32, 95% CI 0.43 to 4.08; participants = 272; studies = 2; I2 = 0%; Analysis 3.20)
Pain (OR 0.34, 95% CI 0.07 to 1.65; participants = 272; studies = 2; I2 = 0%; Analysis 3.21)
3.10. Analysis.
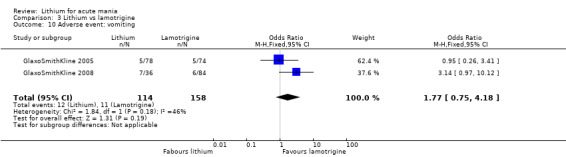
Comparison 3 Lithium vs lamotrigine, Outcome 10 Adverse event: vomiting.
3.11. Analysis.
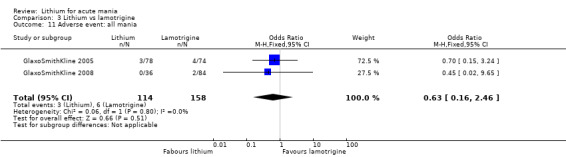
Comparison 3 Lithium vs lamotrigine, Outcome 11 Adverse event: all mania.
3.12. Analysis.
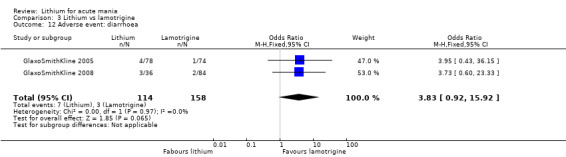
Comparison 3 Lithium vs lamotrigine, Outcome 12 Adverse event: diarrhoea.
3.13. Analysis.
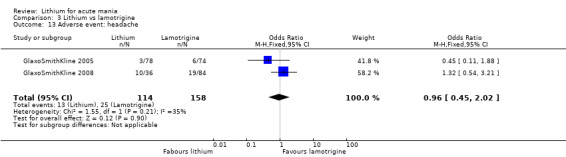
Comparison 3 Lithium vs lamotrigine, Outcome 13 Adverse event: headache.
3.14. Analysis.
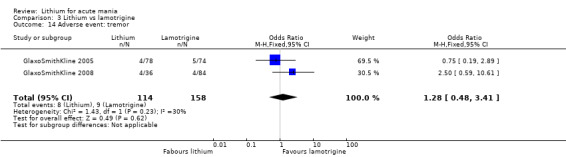
Comparison 3 Lithium vs lamotrigine, Outcome 14 Adverse event: tremor.
3.15. Analysis.
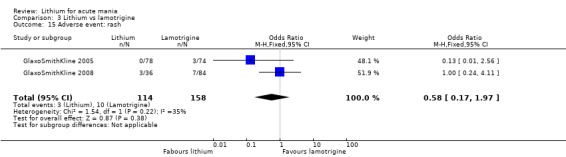
Comparison 3 Lithium vs lamotrigine, Outcome 15 Adverse event: rash.
3.16. Analysis.
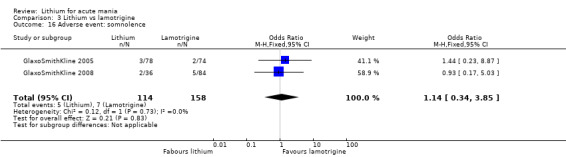
Comparison 3 Lithium vs lamotrigine, Outcome 16 Adverse event: somnolence.
3.17. Analysis.
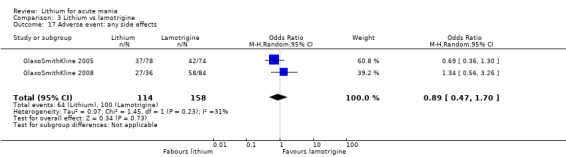
Comparison 3 Lithium vs lamotrigine, Outcome 17 Adverse event: any side effects.
3.19. Analysis.
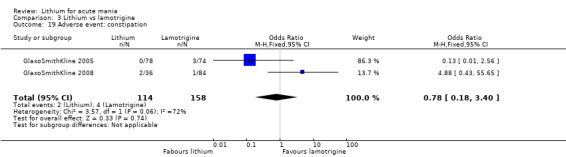
Comparison 3 Lithium vs lamotrigine, Outcome 19 Adverse event: constipation.
3.20. Analysis.
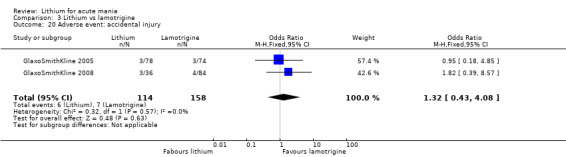
Comparison 3 Lithium vs lamotrigine, Outcome 20 Adverse event: accidental injury.
3.21. Analysis.
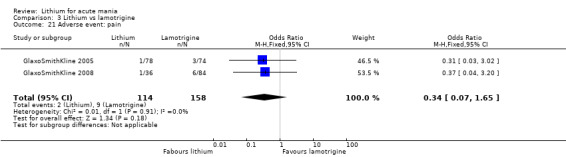
Comparison 3 Lithium vs lamotrigine, Outcome 21 Adverse event: pain.
A large number of adverse events were only reported by one study; these are shown in data analysis Analysis 19.1 onwards.
19.1. Analysis.
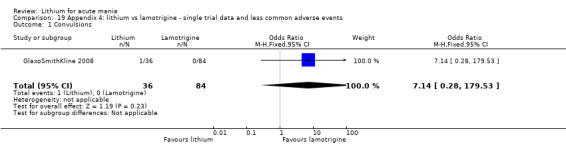
Comparison 19 Appendix 4: lithium vs lamotrigine ‐ single trial data and less common adverse events, Outcome 1 Convulsions.
Acceptability: total withdrawal from the study
Three studies reported total withdrawal data. They did not find any significant difference between lithium and lamotrigine, but there was high heterogeneity in the data (OR 0.80, 95% CI 0.50 to 1.29; participants = 303; studies = 3; I2 = 82%; low‐certainty evidence; Analysis 3.5).
3.5. Analysis.
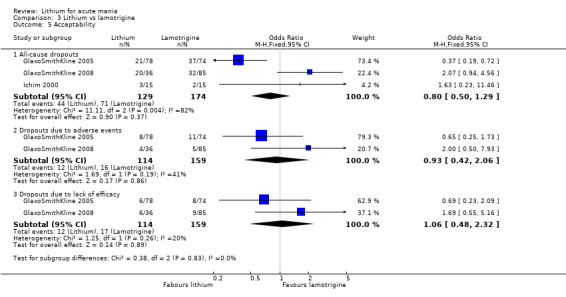
Comparison 3 Lithium vs lamotrigine, Outcome 5 Acceptability.
Secondary outcomes
Efficacy (continuous): change in CGI from baseline to tend of study
There was no significant difference in the change in CGI severity score in the lithium compared to the lamotrigine group (MD −0.35, 95% CI −1.24 to 0.53; participants = 304; studies = 2; I2 = 83%; Analysis 3.3). There was considerable heterogeneity between these two studies. Ichim 2000 also reported this outcome, but could not be included in the meta‐analysis as it was not possible to impute SDs. They reported no significant difference between the groups.
3.3. Analysis.
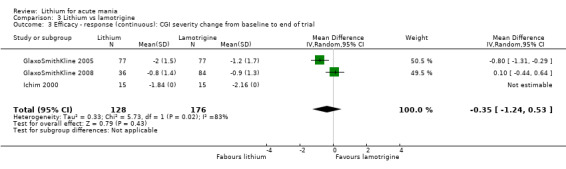
Comparison 3 Lithium vs lamotrigine, Outcome 3 Efficacy ‐ response (continuous): CGI severity change from baseline to end of trial.
Efficacy (continuous): change in GAS from baseline to end of study
Two studies contributed to the analysis. There was no significant difference in the change in GAS scores between groups (MD 4.36, 95% CI −0.65 to 9.37; participants = 270; studies = 2; I2 = 47%; Analysis 3.4).
3.4. Analysis.
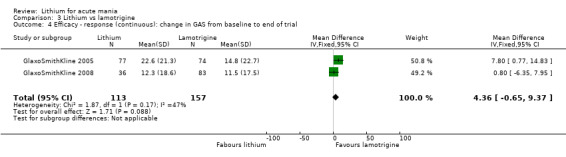
Comparison 3 Lithium vs lamotrigine, Outcome 4 Efficacy ‐ response (continuous): change in GAS from baseline to end of trial.
Efficacy (continuous): mean change in HAMD‐31 score from baseline to end of study
There was no evidence that lithium caused the emergence of more depressive symptoms, as scored by the HAMD‐31, compared to lamotrigine (MD −1.74, 95% CI −3.72 to 0.24; participants = 271; studies = 2; I2 = 26%). In both groups, and across the two contributing studies, treatment actually reduced depressive symptoms by a small amount (Analysis 3.6).
3.6. Analysis.
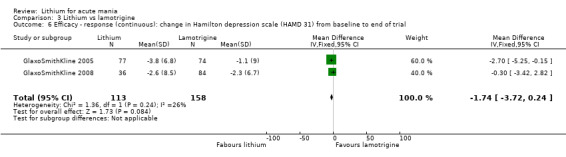
Comparison 3 Lithium vs lamotrigine, Outcome 6 Efficacy ‐ response (continuous): change in Hamilton depression scale (HAMD 31) from baseline to end of trial.
Efficacy results reported by only one study
Efficacy results reported by only one study showed no significant differences:
MRS reduction by at least 50% at end of study (OR 1.31, 95% CI 0.31 to 5.58; participants = 30; studies = 1; Analysis 3.8);
BPRS reduction by at least 50% at end of study (OR 0.42, 95% CI 0.09 to 1.92; participants = 30; studies = 1; Analysis 3.7).
3.8. Analysis.
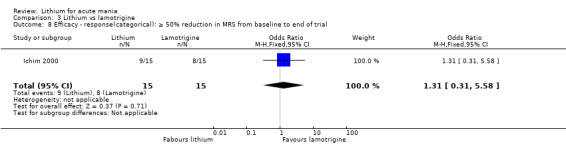
Comparison 3 Lithium vs lamotrigine, Outcome 8 Efficacy ‐ response(categorical): ≥ 50% reduction in MRS from baseline to end of trial.
3.7. Analysis.
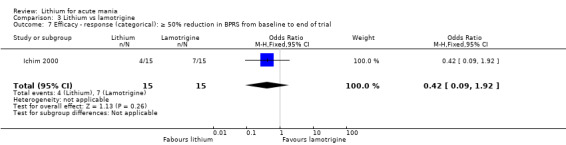
Comparison 3 Lithium vs lamotrigine, Outcome 7 Efficacy ‐ response (categorical): ≥ 50% reduction in BPRS from baseline to end of trial.
Acceptability: withdrawal due to lack of efficacy/adverse events
There was no significant difference in withdrawal due to lack of efficacy (OR 1.06, 95% CI 0.48 to 2.32; participants = 273; studies = 2; I2 = 20%) or adverse events (OR 0.93, 95% CI 0.42 to 2.06; participants = 273; studies = 2; I2 = 41%) between the lithium and lamotrigine treatment groups (Analysis 3.5).
Use of rescue medications
There were no combinable outcomes for this data. GlaxoSmithKline 2005 found that the lithium group used significantly lower doses of concomitant psychotropic medications (these were not further identified) than the lamotrigine group (OR 0.25, 95% CI 0.07 to 0.95; participants = 151; studies = 1; Analysis 3.22). Ichim 2000 reported no significant difference between the mean dose of lorazepam used by their lithium versus lamotrigine participants, but they did not provide SDs for this data (Analysis 3.23).
3.22. Analysis.
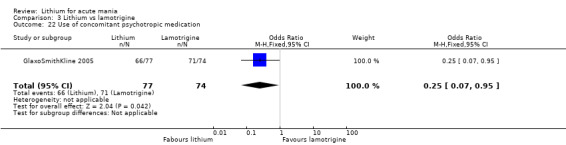
Comparison 3 Lithium vs lamotrigine, Outcome 22 Use of concomitant psychotropic medication.
3.23. Analysis.
Comparison 3 Lithium vs lamotrigine, Outcome 23 Mean total dose of lorazepam (g).
Comparison 4: lithium versus carbamazepine
Five studies included 123 participants in this comparison.
Primary outcomes
Five studies compared carbamazepine to lithium, of which one (Trivedi 1996), only reported data on adverse events. The studies did not report the majority of primary outcomes. There were no adverse events reported by more than one study. Kowatch 2000 reported that significantly more participants experienced nausea, sedation, a rash or dizziness with carbamazepine treatment than with lithium. They did not give any statistics in their publication (Analysis 4.12; Analysis 4.13; Analysis 4.14; Analysis 4.15).
4.12. Analysis.
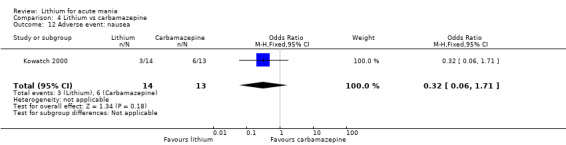
Comparison 4 Lithium vs carbamazepine, Outcome 12 Adverse event: nausea.
4.13. Analysis.
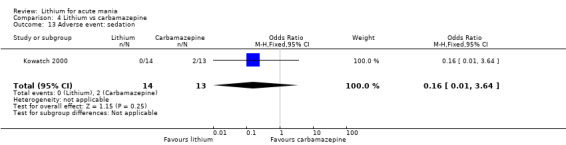
Comparison 4 Lithium vs carbamazepine, Outcome 13 Adverse event: sedation.
4.14. Analysis.
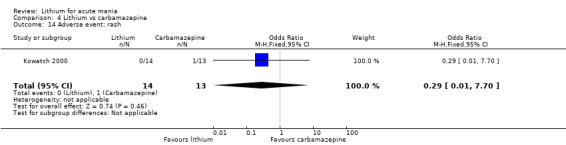
Comparison 4 Lithium vs carbamazepine, Outcome 14 Adverse event: rash.
4.15. Analysis.
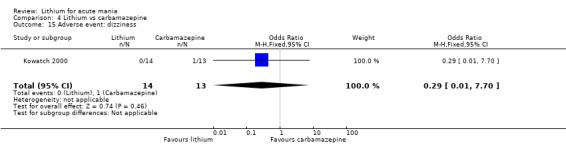
Comparison 4 Lithium vs carbamazepine, Outcome 15 Adverse event: dizziness.
Efficacy: response (continuous). Change in YMRS/BPRS from baseline to end of study
Three studies contributed to this outcome. We found no significant difference in the improvement in scale scores between the lithium and carbamazepine groups (SMD 0.21, 95% CI −0.18 to 0.60; participants = 102; studies = 3; I2 = 0%; very low‐certainty evidence; Analysis 4.1). The fourth study, Lusznat 1988, reported data from the Bech‐Rafaelsen mania scale, but only in graph format, and with no raw data available, so we did not combine this within the meta‐analysis. They reported that there was no significant difference in the reduction in scores between their two groups.
4.1. Analysis.
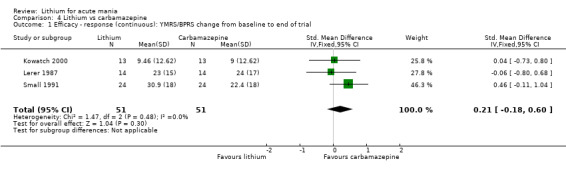
Comparison 4 Lithium vs carbamazepine, Outcome 1 Efficacy ‐ response (continuous): YMRS/BPRS change from baseline to end of trial.
Acceptability: withdrawals
Lerer 1987 reported that there was no significant difference in the number of withdrawals between the lithium and carbamazepine groups (OR 0.20, 95% CI 0.02 to 1.94; participants = 34; studies = 1, low‐certainty evidence; Analysis 4.4).
4.4. Analysis.
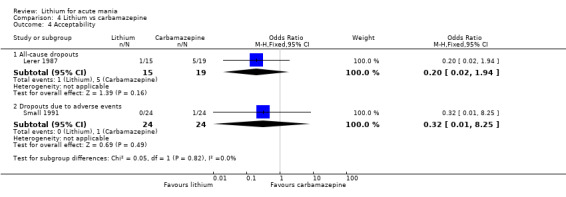
Comparison 4 Lithium vs carbamazepine, Outcome 4 Acceptability.
Secondary outcomes
Efficacy: response (continuous). CGI change from baseline to end of study
There was no significant difference in the change in CGI score in the lithium compared to the carbamazepine group (MD 0.68, 95% CI −0.40 to 1.76; participants = 76; studies = 2; I2 = 88%; low‐certainty evidence; Analysis 4.2).
4.2. Analysis.
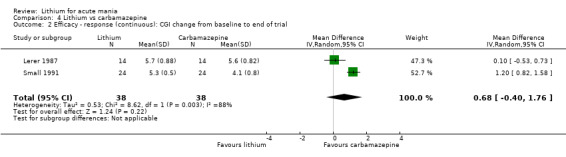
Comparison 4 Lithium vs carbamazepine, Outcome 2 Efficacy ‐ response (continuous): CGI change from baseline to end of trial.
Proxy measure of efficacy: mean length of treatment in weeks
Although not a pre‐stated outcome, three studies reported the mean length of treatment in weeks (Kowatch 2000; Lerer 1987; Lusznat 1988). In each of these studies the participants were admitted to hospital and treated as inpatients. Unfortunately it was not possible to meta‐analyse these data due to lack of SDs. All three studies reported no significant difference in the treatment length between participants on lithium versus carbamazepine (Analysis 4.3).
4.3. Analysis.
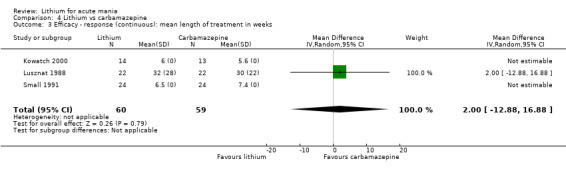
Comparison 4 Lithium vs carbamazepine, Outcome 3 Efficacy ‐ response (continuous): mean length of treatment in weeks.
Acceptability: change in adverse events scores from baseline to end of study (day 28)
Trivedi 1996 used a side‐effect score to explore the relative adverse events of carbamazepine and lithium. They reported that there was no significant difference between treatments in the overall side‐effect score changes across the study (MD 0.90, 95% CI −0.80 to 2.60; participants = 27; studies = 1) They did, however, note that for all participants who experienced any side effects, they tended to decrease in the second week of treatment and had resolved by the end of the fourth week (Analysis 4.5).
4.5. Analysis.
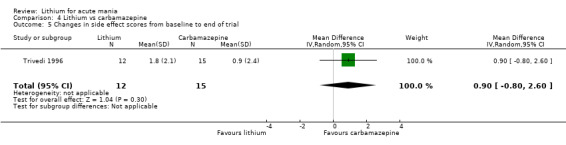
Comparison 4 Lithium vs carbamazepine, Outcome 5 Changes in side effect scores from baseline to end of trial.
Acceptability: withdrawals due to adverse events
Rash: Small 1991 did not find a difference in the numbers of participants who experienced a rash in the lithium compared to carbamazepine arms (OR 0.29, 95% CI 0.01 to 7.70; participants = 27; studies = 1; Analysis 4.14).
Comparison 5: lithium versus quetiapine
We found two studies with 359 participants for this comparison.
Primary outcomes
Efficacy: response (categorical). YMRS decrease by at least 50% from baseline to end of study (day 21)
There was no significant difference in the number of participants who responded to treatment between the lithium and quetiapine groups (OR 0.66, 95% CI 0.28 to 1.55; participants = 335; studies = 2; I2 = 71%; low‐certainty evidence; Analysis 5.1).
5.1. Analysis.
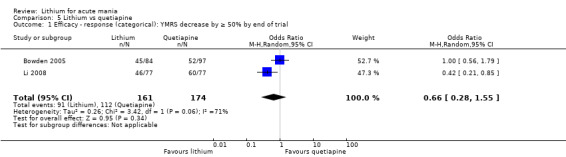
Comparison 5 Lithium vs quetiapine, Outcome 1 Efficacy ‐ response (categorical): YMRS decrease by ≥ 50% by end of trial.
Efficacy (continuous): mean change in YMRS score from baseline to the end of the study (day 21)
No meta‐analysis was possible for this outcome. Two studies reported a mean change in YMRS, but only one provided SDs (Analysis 5.2). We were unable to impute the SDs for Bowden 2005. Both studies reported that there was no significant difference between the change in YMRS scores between groups. Both studies showed a considerable improvement in manic symptoms (about 20 points on YMRS) with both lithium or quetiapine therapy.
5.2. Analysis.
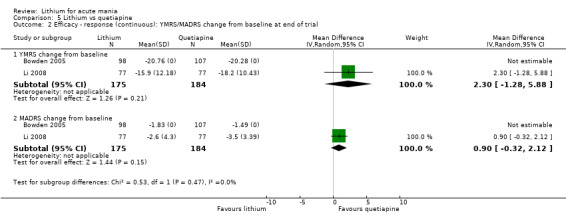
Comparison 5 Lithium vs quetiapine, Outcome 2 Efficacy ‐ response (continuous): YMRS/MADRS change from baseline at end of trial.
Efficacy (categorical): remission. Number of participants who achieved YMRS 12 or less by end of study
Neither lithium nor quetiapine was shown to be more effective in achieving remission than the other treatment (OR 0.64, 95% CI 0.26 to 1.57; participants = 359; studies = 2; I2 = 77%; low‐certainty evidence; Analysis 5.3).
5.3. Analysis.
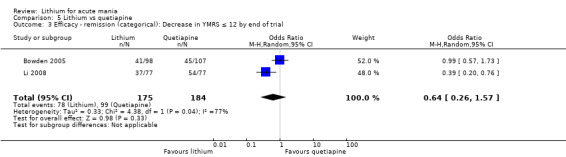
Comparison 5 Lithium vs quetiapine, Outcome 3 Efficacy ‐ remission (categorical): Decrease in YMRS ≤ 12 by end of trial.
Acceptability: adverse events
Dizziness: participants treated with lithium were less likely to report dizziness than those treated with quetiapine (OR 0.47, 95% CI 0.23 to 0.97; participants = 360; studies = 2; I2 = 0%; Analysis 5.5).
Diarrhoea: there was no significant difference in the numbers of participants reporting diarrhoea in the lithium compared to the quetiapine group (OR 0.79, 95% CI 0.34 to 1.86; participants = 360; studies = 2; I2 = 0%; Analysis 5.6).
Weight gain: both Bowden 2005 and Li 2008 reported the mean change in weight (kg) across the study. Neither study provided SDs. Bowden 2005 reported a greater weight gain in the quetiapine group compared to lithium (2.6 kg versus 0.7 kg), but there was no statistical analysis. Li 2008 reported a significantly greater increase in weight in the quetiapine group compared to lithium (1.45 kg versus 0.25 kg); again, this was not accompanied by a P value (Analysis 5.7).
Other adverse events: the majority of adverse events showed no significant difference between those treated with lithium compared to quetiapine. These are shown in Analysis 18.1 onwards.
5.5. Analysis.
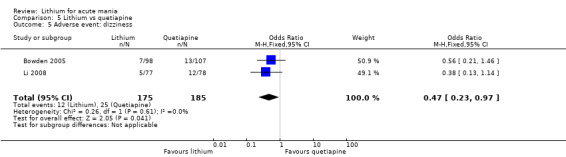
Comparison 5 Lithium vs quetiapine, Outcome 5 Adverse event: dizziness.
5.6. Analysis.
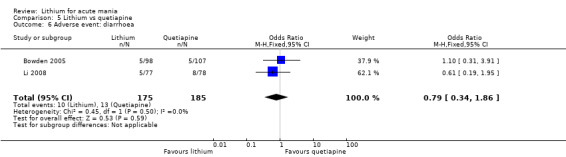
Comparison 5 Lithium vs quetiapine, Outcome 6 Adverse event: diarrhoea.
5.7. Analysis.
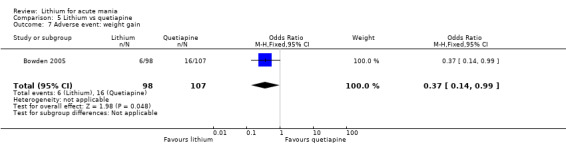
Comparison 5 Lithium vs quetiapine, Outcome 7 Adverse event: weight gain.
18.1. Analysis.
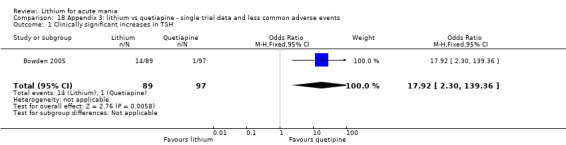
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 1 Clinically significant increases in TSH.
Acceptability: total withdrawal from the study
There was no significant difference in the number of withdrawals in the lithium compared to the quetiapine group (OR 1.38, 95% CI 0.83 to 2.28; participants = 359; studies = 2; I2 = 82%; very low‐certainty evidence; Analysis 5.8).
5.8. Analysis.
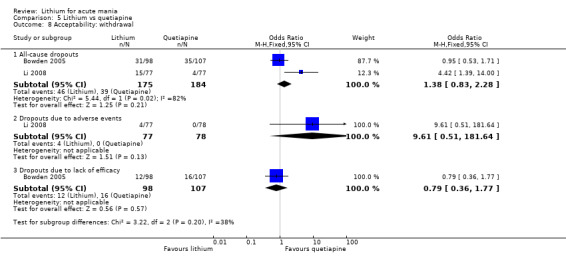
Comparison 5 Lithium vs quetiapine, Outcome 8 Acceptability: withdrawal.
Secondary outcomes
Efficacy (continuous): MADRS mean change from baseline to end of study (day 21)
Two studies reported this outcome; no meta‐analysis was possible due to a lack of SDs, which we could not impute (Bowden 2005; Li 2008). Both studies reported no significant differences between the reduction in depression scores on the MADRS between lithium and quetiapine groups (Analysis 5.4).
5.4. Analysis.
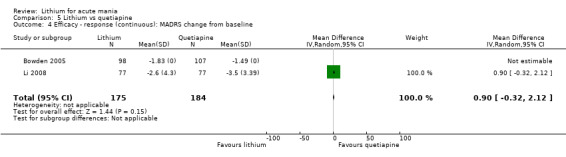
Comparison 5 Lithium vs quetiapine, Outcome 4 Efficacy ‐ response (continuous): MADRS change from baseline.
Efficacy (continuous): PANSS score change from baseline to end of study
Only Li 2008 reported this outcome; there was no significant difference between lithium and quetiapine (MD −3.20, 95% CI −6.71 to 0.31; participants = 154; studies = 1; Analysis 5.9).
5.9. Analysis.
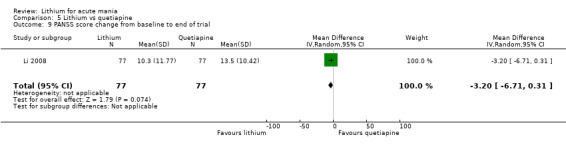
Comparison 5 Lithium vs quetiapine, Outcome 9 PANSS score change from baseline to end of trial.
Acceptability: withdrawal due to lack of efficacy/withdrawal due to adverse events
Only one study reported each of these outcomes (Analysis 5.8): there was no significant difference between lithium and quetiapine (lack of efficacy: Bowden 2005; adverse events: Li 2008).
Use of rescue medications
Two studies compared the use of rescue medications. Meta‐analysis showed no difference between sleep medications (benzodiazepines or z‐drugs, (OR 1.21, 95% CI 0.78 to 1.87; participants = 359; studies = 2; I2 = 0%; Analysis 5.11), nor anticholinergics (OR 1.19, 95% CI 0.61 to 2.30; participants = 359; studies = 2; I2 = 0%; Analysis 5.13), between lithium and quetiapine groups.
5.11. Analysis.
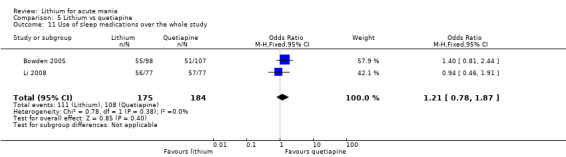
Comparison 5 Lithium vs quetiapine, Outcome 11 Use of sleep medications over the whole study.
5.13. Analysis.
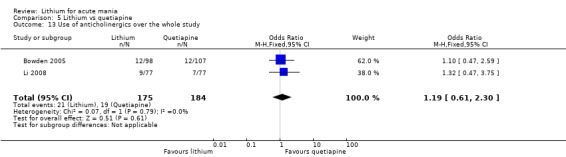
Comparison 5 Lithium vs quetiapine, Outcome 13 Use of anticholinergics over the whole study.
Comparison 6: lithium versus olanzapine
We found three studies with 210 participants for this comparison.
Primary outcomes
Efficacy: response (categorical). MSRS/YMRS at least 50% reduction from baseline to end of study
Lithium was less likely to improve manic symptoms than olanzapine (OR 0.44, 95% CI 0.20 to 0.94; participants = 180; studies = 2; I2 = 0%; moderate‐certainty evidence; Analysis 6.1).
6.1. Analysis.
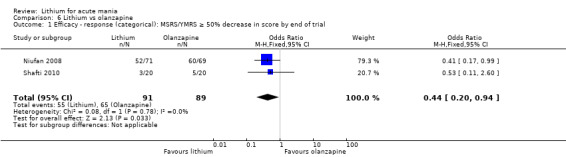
Comparison 6 Lithium vs olanzapine, Outcome 1 Efficacy ‐ response (categorical): MSRS/YMRS ≥ 50% decrease in score by end of trial.
Efficacy (categorical): remission. YMRS 12 or less at the end of the study
Only Niufan 2008 reported this outcome. They did not find a difference between lithium and olanzapine at inducing remission (OR (non‐event) 2.00, 95% CI 0.89 to 4.46; participants = 140; studies = 1; Analysis 6.4).
6.4. Analysis.
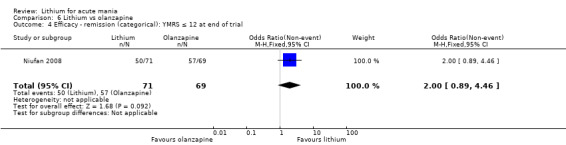
Comparison 6 Lithium vs olanzapine, Outcome 4 Efficacy ‐ remission (categorical): YMRS ≤ 12 at end of trial.
Acceptability: adverse events
Tremor: significantly more participants in the lithium arm reported a tremor than those taking olanzapine (OR 0.34, 95% CI 0.06 to 1.99; participants = 46; studies = 1).
Other adverse events: only Niufan 2008 reported data on other adverse events (from Analysis 20.1 onwards). They reported no significant difference between olanzapine and lithium for nausea, EPSEs, constipation, somnolence, gastrointestinal disorders, dizziness, cough, tachycardia, fatigue, headache, tonsillitis, upper respiratory tract infection, dry mouth, haemorrhoids, metabolic disorders, hepatic disorders, high cholesterol or high glucose. They did find a significantly greater weight gain in the participants treated with olanzapine: weight gain more than 7% of baseline at end of study (Analysis 20.28).
20.1. Analysis.
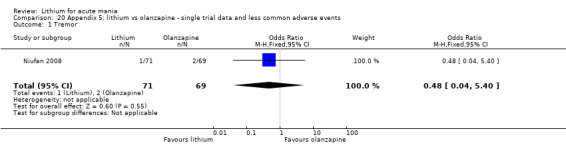
Comparison 20 Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events, Outcome 1 Tremor.
20.28. Analysis.
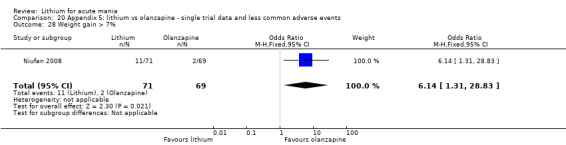
Comparison 20 Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events, Outcome 28 Weight gain > 7%.
Acceptability: total withdrawal
Participants treated with lithium were more likely to withdraw from the study than those on olanzapine (OR 2.60, 95% CI 1.13 to 5.99; participants = 210; studies = 3; I2 = 0%; low‐certainty evidence; Analysis 6.5).
6.5. Analysis.
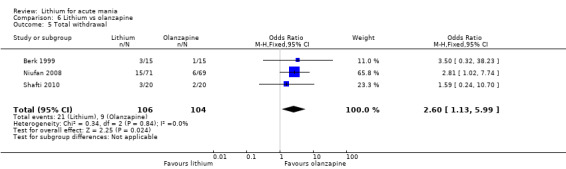
Comparison 6 Lithium vs olanzapine, Outcome 5 Total withdrawal.
Secondary outcomes
Efficacy: response (continuous). CGI severity score at the end of the study
Three studies contributed data to this analysis (Analysis 6.3); all reported that there were no significant differences in CGI score between groups by the end of the study (MD 0.35, 95% CI −0.04 to 0.74; participants = 210; studies = 3; I2 = 87%; low‐certainty evidence).
6.3. Analysis.
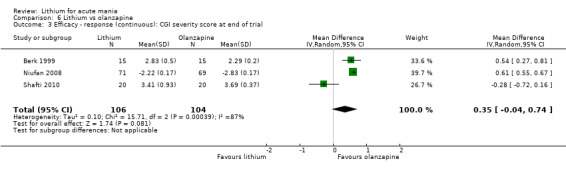
Comparison 6 Lithium vs olanzapine, Outcome 3 Efficacy ‐ response (continuous): CGI severity score at end of trial.
Efficacy (continuous): change in CGI score from baseline to end of study
Lithium caused a greater reduction in CGI score than olanzapine but this was objectively a small effect (MD 0.58, 95% CI 0.52 to 0.64; participants = 170; studies = 2; I2 = 0%; Analysis 6.2).
6.2. Analysis.
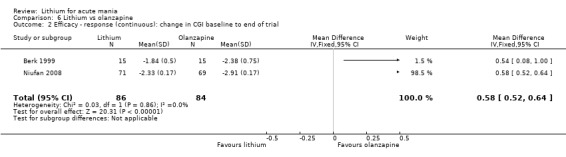
Comparison 6 Lithium vs olanzapine, Outcome 2 Efficacy ‐ response (continuous): change in CGI baseline to end of trial.
Only Niufan 2008 (n = 140) reported any other secondary outcomes; they found olanzapine to be favourable (by a small amount in each case) for the following outcomes:
change in BPRS from baseline to end of study (MD 2.12, 95% CI 1.87 to 2.37: Analysis 6.8);
change in CGI‐BP depression score (MD 0.03, 95% CI 0.01 to 0.05; Analysis 6.9);
change in MADRS from baseline to end of study (MD 0.75, 95% CI 0.60 to 0.90; Analysis 6.10).
6.8. Analysis.
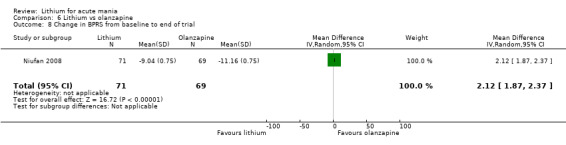
Comparison 6 Lithium vs olanzapine, Outcome 8 Change in BPRS from baseline to end of trial.
6.9. Analysis.
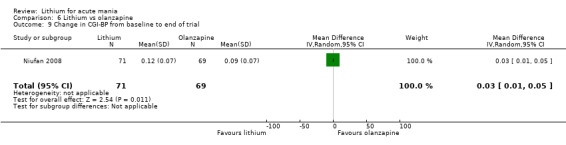
Comparison 6 Lithium vs olanzapine, Outcome 9 Change in CGI‐BP from baseline to end of trial.
6.10. Analysis.
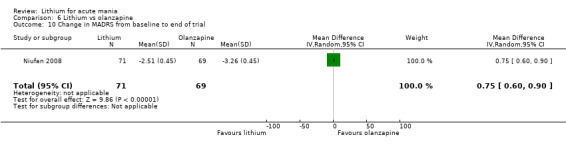
Comparison 6 Lithium vs olanzapine, Outcome 10 Change in MADRS from baseline to end of trial.
Acceptability: withdrawals due to lack of efficacy/adverse events
Only Niufan 2008 reported these withdrawal figures; they found no significant difference between lithium and olanzapine for lack of efficacy (OR 1.67, 95% CI 0.38 to 7.26; participants = 140; studies = 1; Analysis 6.6), and adverse events (OR 2.96, 95% CI 0.12 to 73.85; participants = 140; studies = 1; Analysis 6.7).
6.6. Analysis.
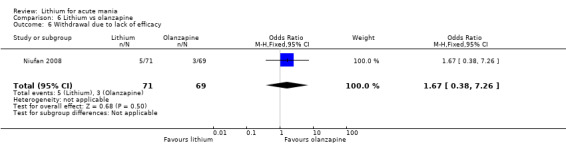
Comparison 6 Lithium vs olanzapine, Outcome 6 Withdrawal due to lack of efficacy.
6.7. Analysis.
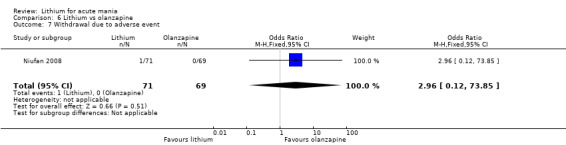
Comparison 6 Lithium vs olanzapine, Outcome 7 Withdrawal due to adverse event.
Use of rescue medications
Regarding rescue medications (benzodiazepines), there was no significant difference in the use between participants taking lithium or olanzapine (OR 1.66, 95% CI 0.63 to 4.35; participants = 140; studies = 1; Analysis 6.11).
6.11. Analysis.
Comparison 6 Lithium vs olanzapine, Outcome 11 Concomitant medication: benzodiazepine use.
Lithium versus antipsychotics
Comparison 7: lithium versus chlorpromazine
Four studies with 313 participants met the inclusion criteria in this category (Platman 1970; Prien 1972; Shopsin 1975; Spring 1970). They were all published in the 1970s and used variable outcome measures; this limited the amount of meta‐analysis possible.
Primary outcomes
Efficacy (continuous): change in BPRS score from baseline to end of study
Two studies reported this outcome (Prien 1972; Shopsin 1975). There was no significant difference in efficacy between lithium and chlorpromazine (MD −0.59, 95% CI −1.75 to 0.57; participants = 284; studies = 2; I2 = 90%; low‐certainty evidence; Analysis 7.1).
7.1. Analysis.
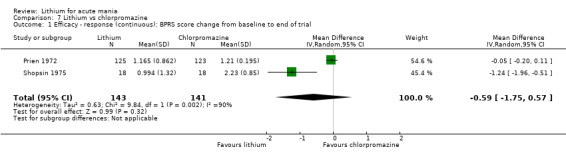
Comparison 7 Lithium vs chlorpromazine, Outcome 1 Efficacy ‐ response (continuous): BPRS score change from baseline to end of trial.
Efficacy (continuous): Psychiatric Evaluation Scale at end of study
Platman 1970 reported the Psychiatric Evaluation Scale as their only efficacy outcome and did not provide SDs or any statistical analysis. They found a score of 2/6 in the lithium group and 2.7/6 in the chlorpromazine group (n = 23). The small size and lack of analysis makes this result hard to place in context (Analysis 7.2).
7.2. Analysis.
Comparison 7 Lithium vs chlorpromazine, Outcome 2 Efficacy ‐ response (continuous): PES change from baseline to end of trial.
Efficacy: response (categorical): defined by the study authors at 21 days
Spring 1970 used a categorical outcome measure of efficacy, with a clinician scoring presence (3 degrees) or absence of euphoria, expansiveness, flight or ideas, pressured speech, activity and sleep disturbance. They found that chlorpromazine was more effective at treating manic symptoms than lithium (OR 4.00, 95% CI 0.25 to 63.95; participants = 12; studies = 1). However, this was an extremely small study (Analysis 7.3). Note: this was a quasi‐randomised study.
7.3. Analysis.
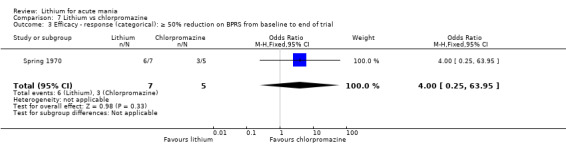
Comparison 7 Lithium vs chlorpromazine, Outcome 3 Efficacy ‐ response (categorical): ≥ 50% reduction on BPRS from baseline to end of trial.
Acceptability: adverse events
There were no outcomes for which it was possible to perform meta‐analysis. Prien 1972 reported an array of common adverse events, but only provided comparative analysis for the overall number of participants who experienced any "serious side effect". They reported no significant difference in the numbers of participants on lithium or chlorpromazine: 31% versus 18%; n = 255, P = 0.1 (Chi2 analysis). Platman 1970, Shopsin 1975 and Spring 1970 provided only narrative comments about common side effects experienced by participants.
Acceptability: total withdrawals
There were no more total withdrawals from the study in the lithium group compared to the chlorpromazine group (OR 1.75, 95% CI 0.92 to 3.31; participants = 262; studies = 2; I2 = 0%; very ow certainty evidence; Analysis 7.4). There was no change to the result removing Spring 1970, which was quasi‐randomised.
7.4. Analysis.
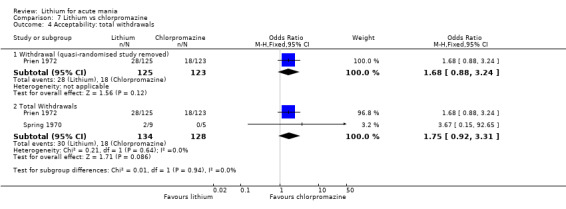
Comparison 7 Lithium vs chlorpromazine, Outcome 4 Acceptability: total withdrawals.
Comparison 8: lithium versus haloperidol
Four studies investigated treatment of acute mania with lithium or haloperidol; three of these examined efficacy (Garfinkel 1980; Segal 1998; Shopsin 1975; n = 80) and one examined adverse events (Trivedi 1996).
Primary outcomes
Efficacy (continuous): change in BPRS score from baseline to end of study
Three studies reported efficacy data (Analysis 8.1); there was no significant difference found between lithium or haloperidol (MD −2.40, 95% CI −6.31 to 1.50; participants = 80; studies = 3; I2 = 95%; low‐certainty evidence). We investigated heterogeneity by removing Garfinkel 1980, a result outlier, but the heterogeneity remained high and the result did not change. The long timescale over which these three studies were published (1975 to 1998) may be a cause of unknown methodological differences.
8.1. Analysis.
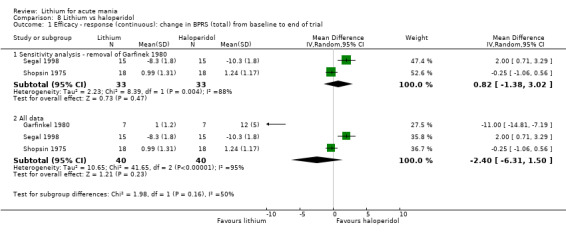
Comparison 8 Lithium vs haloperidol, Outcome 1 Efficacy ‐ response (continuous): change in BPRS (total) from baseline to end of trial.
Acceptability: adverse events. Changes in side‐effect scores from baseline to day 28
Trivedi 1996 used a side‐effect score to explore the relative adverse events of haloperidol and lithium. They reported that there was no significant difference between treatments in the overall side‐effect score changes across the study (MD −0.20, 95% CI −2.05 to 1.65; participants = 28; studies = 1; Analysis 8.3).
8.3. Analysis.
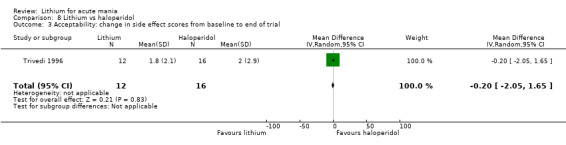
Comparison 8 Lithium vs haloperidol, Outcome 3 Acceptability: change in side effect scores from baseline to end of trial.
Acceptability: total withdrawal
Only Segal 1998 reported withdrawal data; they reported no significant difference between the lithium and haloperidol groups, but this was a very small study (OR 0.29, 95% CI 0.03 to 3.12; participants = 30; very low‐certainty evidence; Analysis 8.4).
8.4. Analysis.
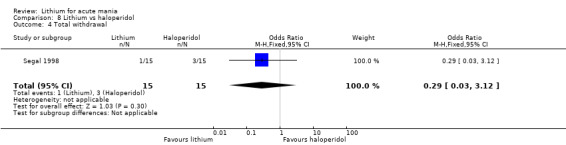
Comparison 8 Lithium vs haloperidol, Outcome 4 Total withdrawal.
Secondary outcomes
Efficacy (continuous): change in CGI score from baseline to end of study
Two studies reported this outcome (Segal 1998; Shopsin 1975), but we could not carry out meta‐analysis as neither study provided SDs or the data to calculate them. Both studies reported a reduction in scores for both lithium and haloperidol but that this was not significantly different between groups (Analysis 8.2).
8.2. Analysis.
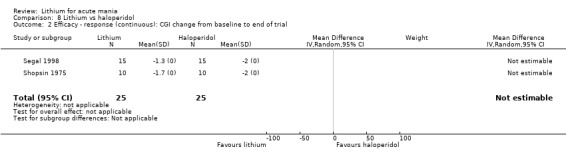
Comparison 8 Lithium vs haloperidol, Outcome 2 Efficacy ‐ response (continuous): CGI change from baseline to end of trial.
Use of rescue medications
Only Segal 1998 reported the use of rescue medications. They did not find any significant difference between the use of either orphenadrine (OR 0.05, 95% CI 0.00 to 0.94; participants = 30; Analysis 8.5), or lorazepam between the lithium and haloperidol groups. They did not provide any SDs for the lorazepam data (Analysis 8.6).
8.5. Analysis.
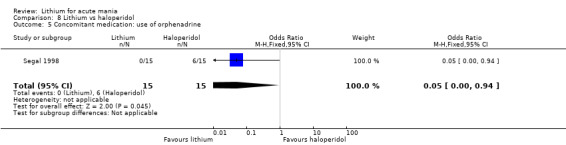
Comparison 8 Lithium vs haloperidol, Outcome 5 Concomitant medication: use of orphenadrine.
8.6. Analysis.
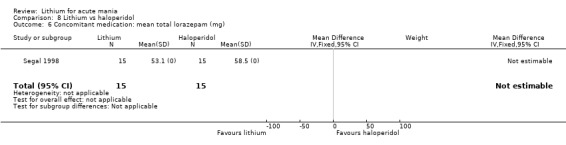
Comparison 8 Lithium vs haloperidol, Outcome 6 Concomitant medication: mean total lorazepam (mg).
Comparison 9: lithium versus zuclopenthixol
Only Gouliaev 1996 fitted the inclusion criteria; this was a small study with only 28 participants.
Primary outcomes
Efficacy: response (categorical). Reduction of at least 50% in Bech‐Rafaelsen Mania Scale by end of study
Gouliaev 1996 found no significant difference between lithium and zuclopenthixol (OR 1.33, 95% CI 0.30 to 5.91; participants = 28; studies = 1, very‐low‐certainty evidence; Analysis 9.1).
9.1. Analysis.
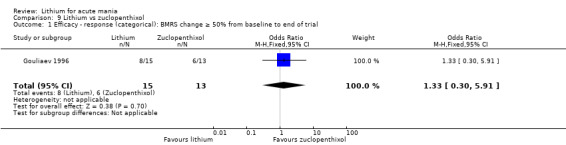
Comparison 9 Lithium vs zuclopenthixol, Outcome 1 Efficacy ‐ response (categorical): BMRS change ≥ 50% from baseline to end of trial.
Acceptability: adverse events
Gouliaev 1996 divided adverse events into 'neurological', 'psychological' and 'autonomic'. They found no significant difference between lithium and zuclopenthixol for the psychological or autonomic categories. Zuclopenthixol was associated with more frequent reporting of neurological symptoms, including EPSEs (mean side‐effect score lithium 0.3, zuclopenthixol 1.8, no SD/CI provided, n = 28, P value reported as < 0.05; Analysis 9.4; Analysis 9.5; Analysis 9.6; Analysis 9.7).
9.4. Analysis.
Comparison 9 Lithium vs zuclopenthixol, Outcome 4 Adverse event rating scale psychic.
9.5. Analysis.
Comparison 9 Lithium vs zuclopenthixol, Outcome 5 Adverse event rating scale neurological.
9.6. Analysis.
Comparison 9 Lithium vs zuclopenthixol, Outcome 6 Adverse event rating scale autonomic.
9.7. Analysis.
Comparison 9 Lithium vs zuclopenthixol, Outcome 7 Adverse event rating scale other.
Acceptability: total withdrawal
There was no significant difference in the number of withdrawals from the lithium and zuclopenthixol arms of the study (OR 0.78, 95% CI 0.17 to 3.49; participants = 28; studies = 1, very low‐certainty evidence; Analysis 9.2).
9.2. Analysis.
Comparison 9 Lithium vs zuclopenthixol, Outcome 2 Total withdrawal.
Secondary outcomes
Use of rescue medications: mean dose of clonazepam
There was no significant difference in the amount of clonazepam used by the lithium or zuclopenthixol groups (2.8 mg versus 4 mg, no SD or statistics provided; Analysis 9.3).
9.3. Analysis.
Comparison 9 Lithium vs zuclopenthixol, Outcome 3 Mean dose (mg) of extra clonazepam.
Comparison 10: lithium versus risperidone
We found three studies with 255 participants for this comparison.
Primary outcomes
Efficacy: response (continuous). Change in YMRS score from baseline to end of study
Meta‐analysis found that risperidone was more effective at reducing manic symptoms than lithium (MD 7.28, 95% CI 5.22 to 9.34; participants = 241; studies = 3; I2 = 49%; low‐certainty evidence; Analysis 10.1).
10.1. Analysis.
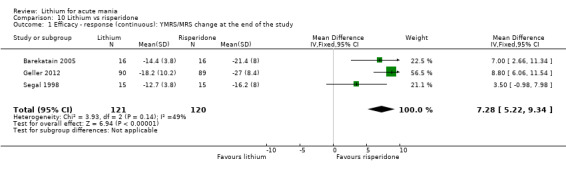
Comparison 10 Lithium vs risperidone, Outcome 1 Efficacy ‐ response (continuous): YMRS/MRS change at the end of the study.
Efficacy: remission (categorical). YMRS less than 12 or absence of DSM‐IV mania by the end of the study
There was considerable heterogeneity between the studies, and no significant difference between lithium or risperidone (OR 1.30, 95% CI 0.11 to 14.95; participants = 211; studies = 2; I2 = 89%; low‐certainty evidence; Analysis 10.2).
10.2. Analysis.
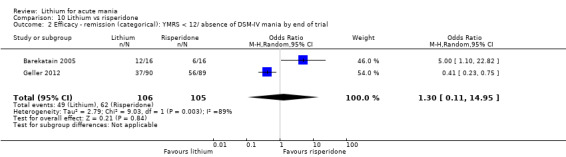
Comparison 10 Lithium vs risperidone, Outcome 2 Efficacy ‐ remission (categorical): YMRS < 12/ absence of DSM‐IV mania by end of trial.
Acceptability: adverse events
Just one study reported most of the common adverse events. Geller 2012 reported no significant difference between frequency in participants treated with lithium or risperidone for the majority of adverse events. The exceptions included appetite increase (OR 0.31, 95% CI 0.16 to 0.59; participants = 173; Analysis 10.12), weight gain (OR 0.10, 95% CI 0.03 to 0.32; participants = 173; Analysis 10.15), dry mouth (OR 2.42, 95% CI 1.02 to 5.75; participants = 173; Analysis 10.16), and abdominal pain (OR 3.98, 95% CI 1.91 to 8.27; participants = 173; Analysis 10.17), which were all more common in lithium‐treated participants.
10.12. Analysis.
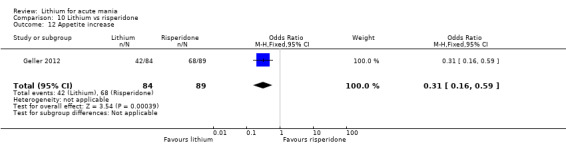
Comparison 10 Lithium vs risperidone, Outcome 12 Appetite increase.
10.15. Analysis.
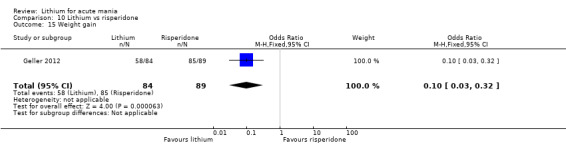
Comparison 10 Lithium vs risperidone, Outcome 15 Weight gain.
10.16. Analysis.
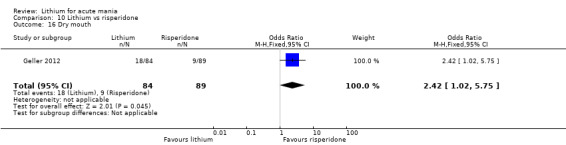
Comparison 10 Lithium vs risperidone, Outcome 16 Dry mouth.
10.17. Analysis.
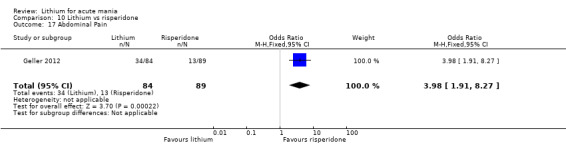
Comparison 10 Lithium vs risperidone, Outcome 17 Abdominal Pain.
More participants treated with risperidone than lithium reported somnolence or drowsiness (OR 0.43, 95% CI 0.24 to 0.75; participants = 219; studies = 2; I2 = 50%; Analysis 10.4).
10.4. Analysis.
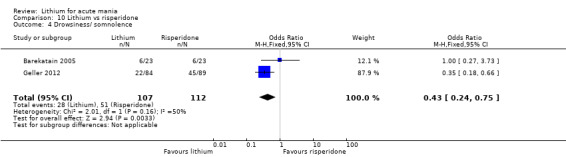
Comparison 10 Lithium vs risperidone, Outcome 4 Drowsiness/ somnolence.
Data from two studies (Barekatain 2005; Geller 2012), found that lithium was more likely than risperidone to cause the following adverse events:
diarrhoea (OR 4.14, 95% CI 1.12 to 15.26; participants = 219; studies = 2; I2 = 0%; Analysis 10.5);
nausea (OR 2.49, 95% CI 1.32 to 4.69; participants = 219; studies = 2; I2 = 0%; Analysis 10.6);
vomiting (OR 2.80, 95% CI 1.22 to 6.42; participants = 219; studies = 2; I2 = 0%; Analysis 10.9);
frequent urination (OR 5.29, 95% CI 2.12 to 13.21; participants = 219; studies = 2; I2 = 0%; Analysis 10.14).
10.5. Analysis.
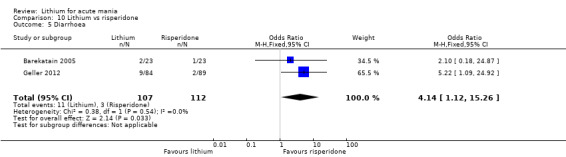
Comparison 10 Lithium vs risperidone, Outcome 5 Diarrhoea.
10.6. Analysis.
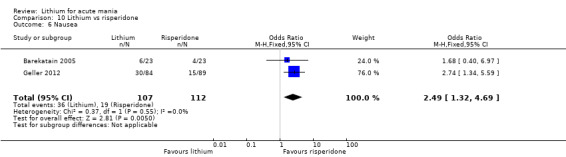
Comparison 10 Lithium vs risperidone, Outcome 6 Nausea.
10.9. Analysis.
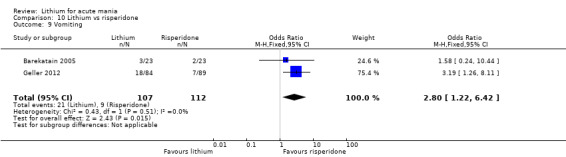
Comparison 10 Lithium vs risperidone, Outcome 9 Vomiting.
10.14. Analysis.
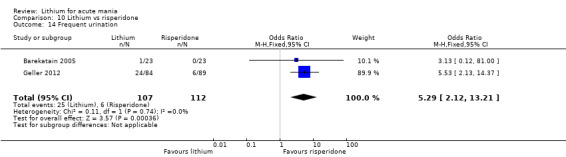
Comparison 10 Lithium vs risperidone, Outcome 14 Frequent urination.
Acceptability: total withdrawal
Three studies reported total withdrawal data. The participants in the lithium group were more likely to withdraw than those treated with risperidone (OR 1.85, 95% CI 1.02 to 3.34; participants = 255; studies = 3; I2 = 29%; moderate‐certainty evidence; Analysis 10.8).
10.8. Analysis.
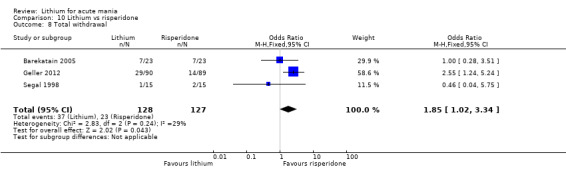
Comparison 10 Lithium vs risperidone, Outcome 8 Total withdrawal.
Secondary outcomes
Efficacy: response (continuous). CGI change from baseline to end of study
The meta‐analysis found a small but significantly greater reduction in CGI score in those treated with lithium compared to risperidone (MD 0.90, 95% CI 0.39 to 1.41; participants = 62; studies = 2; I2 = 0%; Analysis 10.3).
10.3. Analysis.
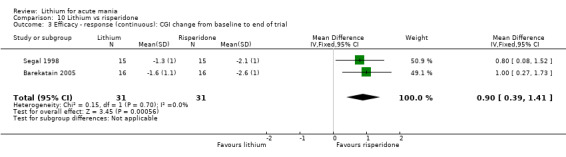
Comparison 10 Lithium vs risperidone, Outcome 3 Efficacy ‐ response (continuous): CGI change from baseline to end of trial.
Acceptability: withdrawal due to adverse events
Geller 2012 reported no significant difference in the number of withdrawals between the lithium and risperidone groups (OR 2.80, 95% CI 0.72 to 10.91; participants = 179; Analysis 10.10).
10.10. Analysis.
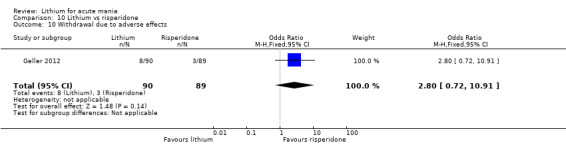
Comparison 10 Lithium vs risperidone, Outcome 10 Withdrawal due to adverse effects.
Use of rescue medications
Segal 1998 was the only study to report use of lorazepam or orphenadrine in addition to the randomised medication. They did not provide SDs or any means to calculate these, but reported in the paper that there was no significant difference between the use of these between the lithium and risperidone groups (Analysis 10.18; Analysis 10.19).
10.18. Analysis.
Comparison 10 Lithium vs risperidone, Outcome 18 Concomitant medication: mean total lorazepam (mg).
10.19. Analysis.
Comparison 10 Lithium vs risperidone, Outcome 19 Concomitant medication: use of orphenadrine.
Comparison 11: lithium versus aripiprazole
Only one study with 309 participants met the inclusion criteria for lithium compared to aripiprazole (Keck 2009).
Primary outcomes
Efficacy: response (categorical). YMRS reduction by at least 50% from baseline
There was no significant difference between lithium and aripiprazole (OR 0.96, 95% CI 0.62 to 1.51; participants = 309; moderate‐certainty evidence; Analysis 11.1).
11.1. Analysis.
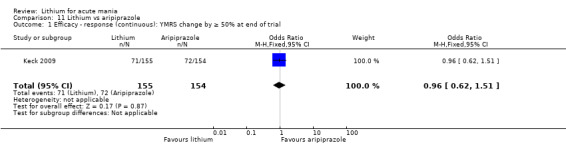
Comparison 11 Lithium vs aripiprazole, Outcome 1 Efficacy ‐ response (continuous): YMRS change by ≥ 50% at end of trial.
Efficacy: remission (categorical). YMRS 12 or less at end of study
Neither lithium or aripiprazole were better at inducing remission from mania (OR 0.99, 95% CI 0.63 to 1.56; participants = 309; moderate‐certainty evidence; Analysis 11.3).
11.3. Analysis.
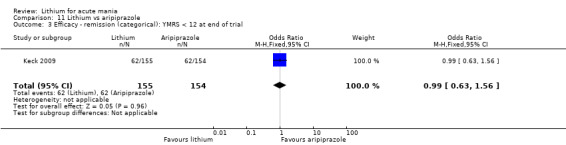
Comparison 11 Lithium vs aripiprazole, Outcome 3 Efficacy ‐ remission (categorical): YMRS < 12 at end of trial.
Acceptability: adverse events
Keck 2009 did not find any significant difference in the frequency of akathisia, constipation, headache, nausea, somnolence, tremor or EPSEs between lithium and aripiprazole (Analysis 11.10; Analysis 11.11; Analysis 11.12; Analysis 11.13; Analysis 11.14; Analysis 11.15; Analysis 11.16). There was a trend towards more participants in the lithium group gaining weight than in the aripiprazole group but this was not significant (OR 2.07, 95% CI 0.18 to 23.21; n = 184, P = 0.56, low‐certainty evidence; Analysis 11.17).
11.10. Analysis.
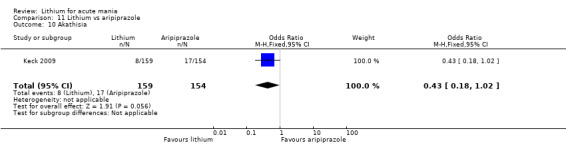
Comparison 11 Lithium vs aripiprazole, Outcome 10 Akathisia.
11.11. Analysis.
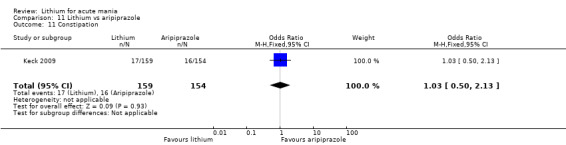
Comparison 11 Lithium vs aripiprazole, Outcome 11 Constipation.
11.12. Analysis.
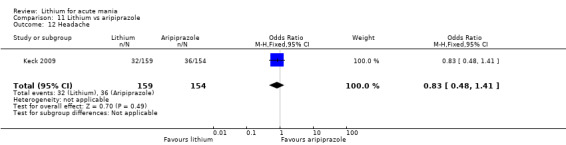
Comparison 11 Lithium vs aripiprazole, Outcome 12 Headache.
11.13. Analysis.
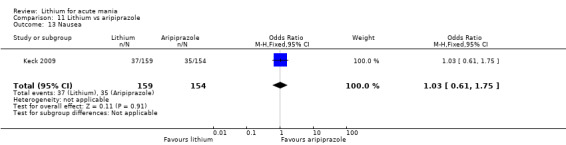
Comparison 11 Lithium vs aripiprazole, Outcome 13 Nausea.
11.14. Analysis.
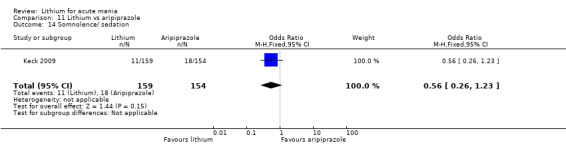
Comparison 11 Lithium vs aripiprazole, Outcome 14 Somnolence/ sedation.
11.15. Analysis.
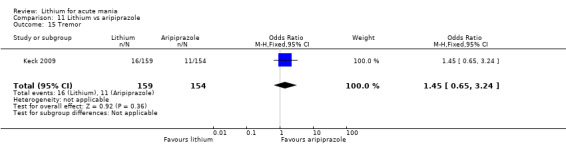
Comparison 11 Lithium vs aripiprazole, Outcome 15 Tremor.
11.16. Analysis.
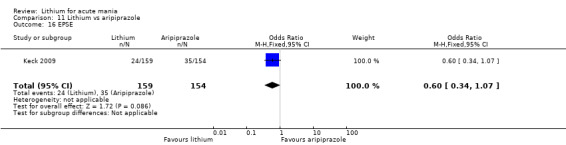
Comparison 11 Lithium vs aripiprazole, Outcome 16 EPSE.
11.17. Analysis.
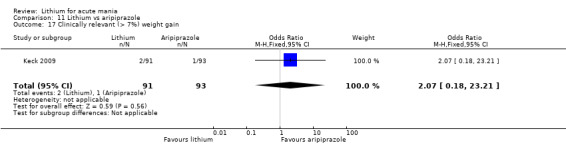
Comparison 11 Lithium vs aripiprazole, Outcome 17 Clinically relevant (> 7%) weight gain.
Acceptability: withdrawals
There was no significant difference between total withdrawals in the lithium compared to aripiprazole groups (Analysis 11.5), however, Keck 2009 did find that significantly more participants withdrew from the lithium group than from the aripiprazole group when just withdrawals due to lack of efficacy were considered (OR 3.15, 95% CI 1.42 to 6.96; participants = 315, moderate‐certainty evidence; Analysis 11.20).
11.5. Analysis.
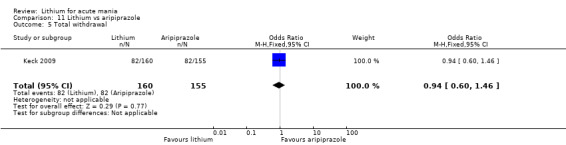
Comparison 11 Lithium vs aripiprazole, Outcome 5 Total withdrawal.
11.20. Analysis.
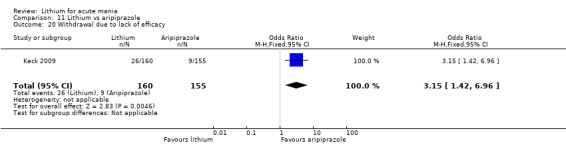
Comparison 11 Lithium vs aripiprazole, Outcome 20 Withdrawal due to lack of efficacy.
Secondary outcomes
Efficacy: response (continuous). Change CGI‐BP severity score from baseline to end of study
There was a greater reduction in CGI scores in the aripiprazole group compared to lithium (MD 0.20, 95% CI 0.18 to 0.22; participants = 309; studies = 1; Analysis 11.2).
11.2. Analysis.
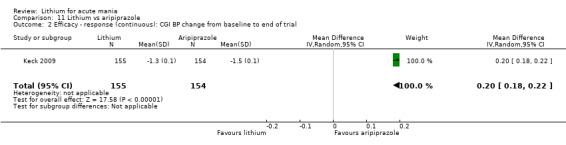
Comparison 11 Lithium vs aripiprazole, Outcome 2 Efficacy ‐ response (continuous): CGI BP change from baseline to end of trial.
Efficacy (continuous): change in PANSS scores from baseline
Aripiprazole treatment led to a greater reduction in PANSS scores than lithium (MD 2.50, 95% CI 2.16 to 2.84; participants = 268; studies = 1; Analysis 11.4).
11.4. Analysis.
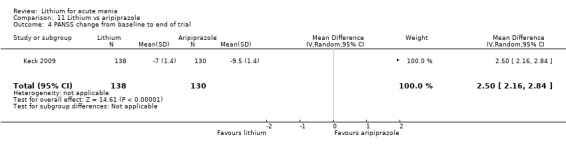
Comparison 11 Lithium vs aripiprazole, Outcome 4 PANSS change from baseline to end of trial.
Use of rescue medications
The use of anxiolytics between lithium‐ and aripiprazole‐treated participants did not differ (Analysis 11.22), but the lithium group was significantly less likely to use anticholinergics (OR 0.34, 95% CI 0.17 to 0.69; participants = 315; studies = 1; Analysis 11.24).
11.22. Analysis.
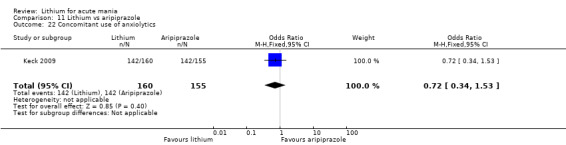
Comparison 11 Lithium vs aripiprazole, Outcome 22 Concomitant use of anxiolytics.
11.24. Analysis.
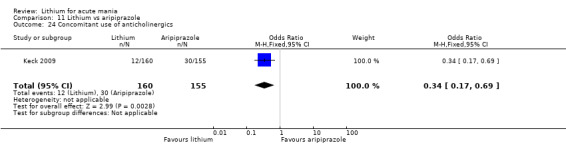
Comparison 11 Lithium vs aripiprazole, Outcome 24 Concomitant use of anticholinergics.
Lithium versus anti‐epileptic mood stabiliser
Comparison 12: lithium versus topiramate
Two studies with 660 participants, from the same research group, investigated the comparative efficacy of lithium and topiramate for acute mania (Kushner 2006 PDMD‐004; Kushner 2006 PDMD‐008). These were published in one publication and the methodology for both studies was identical, except for the experimental dose of topiramate, so the results have been combined.
Primary outcomes
Lithium was more effective than topiramate at reducing the symptoms of mania for the following outcomes.
Efficacy: response (categorical). YMRS reduction by at least 50% by the end of the study (OR 2.28 (95% CI 1.63 to 3.20; participants = 660; studies = 1, high‐certainty evidence; Analysis 12.1).
Efficacy (categorical): remission. YMRS 12 or less at the end of the study (OR 2.24 (95% CI 1.58 to 3.15; participants = 660; studies = 1, high‐certainty evidence; Analysis 12.2).
12.1. Analysis.
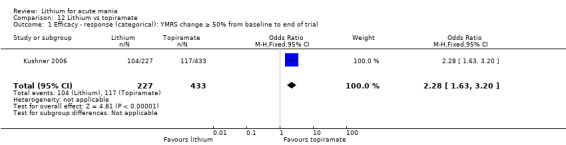
Comparison 12 Lithium vs topiramate, Outcome 1 Efficacy ‐ response (categorical): YMRS change ≥ 50% from baseline to end of trial.
12.2. Analysis.
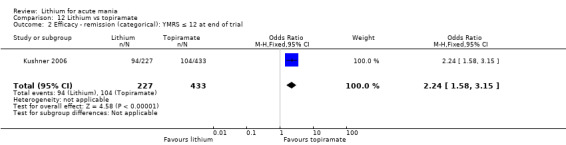
Comparison 12 Lithium vs topiramate, Outcome 2 Efficacy ‐ remission (categorical): YMRS ≤ 12 at end of trial.
Acceptability: adverse events
There were no adverse events found to be significantly different between the groups at the end of the studies.
Acceptability: withdrawals due to lack of efficacy/adverse events
Kushner 2006 did not find a significant difference between withdrawals for any reason between lithium and topiramate (OR 1.28, 95% CI 0.66 to 2.48; participants = 1352; studies = 1; I2 = 53%; high‐certainty evidence; Analysis 12.8).
Lithium versus benzodiazepines
Comparison 13: lithium versus clonazepam
Two studies with only 41 participants met the inclusion criteria.
Primary outcomes
None of the included studies reported any of our primary outcomes.
Secondary outcomes
Efficacy: response (continuous). Change in CGI scores from baseline to end of the study
Two studies contributed to this analysis; there was no significant difference between participants treated with lithium versus clonazepam (MD −0.41, 95% CI −1.46 to 0.65; participants = 41; studies = 2; I2 = 0%; moderate‐certainty evidence; Analysis 13.1). A sensitivity analysis removing Clark 1996, the only single‐blinded study, left only one set of results, which show no significant difference between lithium and clonazepam (Chouinard 1983; Analysis 13.2).
13.1. Analysis.
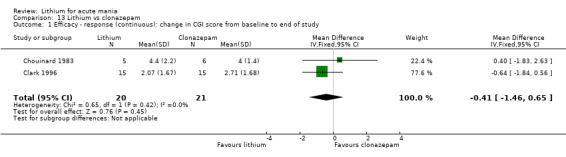
Comparison 13 Lithium vs clonazepam, Outcome 1 Efficacy ‐ response (continuous): change in CGI score from baseline to end of study.
13.2. Analysis.
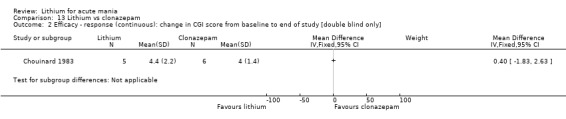
Comparison 13 Lithium vs clonazepam, Outcome 2 Efficacy ‐ response (continuous): change in CGI score from baseline to end of study [double blind only].
Clark 1996 also reported that there was no significant difference in change in MRS or IMPS scores between groups from baseline to end of the study (Analysis 13.3; Analysis 13.5).
13.3. Analysis.
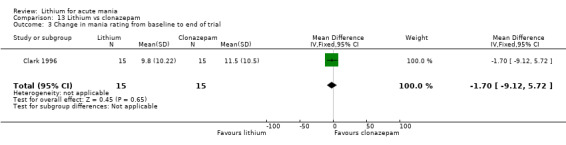
Comparison 13 Lithium vs clonazepam, Outcome 3 Change in mania rating from baseline to end of trial.
13.5. Analysis.
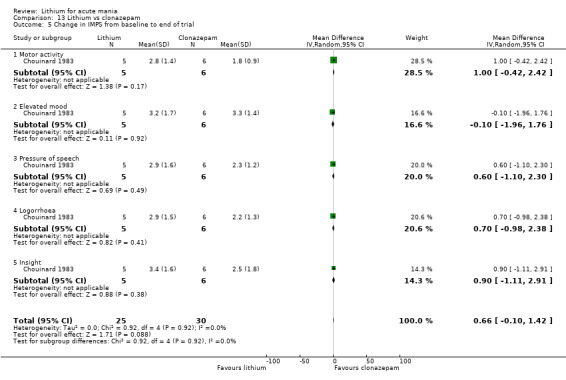
Comparison 13 Lithium vs clonazepam, Outcome 5 Change in IMPS from baseline to end of trial.
Acceptability: adverse events
Clark 1996 reported the Angus‐Simpson scale of adverse events. They found no significant difference in the side effects reported by those in the lithium arm compared to those treated with clonazepam (MD −0.17, 95% CI −0.83 to 0.49; participants = 30; studies = 1; Analysis 13.7).
13.7. Analysis.
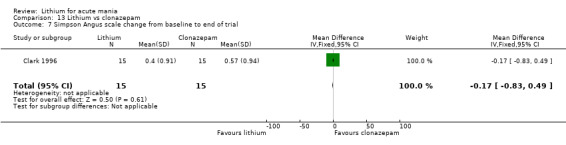
Comparison 13 Lithium vs clonazepam, Outcome 7 Simpson Angus scale change from baseline to end of trial.
Use of rescue medications
Chouinard 1983 reported the use of their stipulated rescue medication (haloperidol), to be no greater in the lithium compared to haloperidol group. However, this study was extremely small, leading to very wide confidence intervals and limiting the application of this finding (OR 4.00, 95% CI 0.27 to 60.32; participants = 11; studies = 1; Analysis 13.8).
13.8. Analysis.
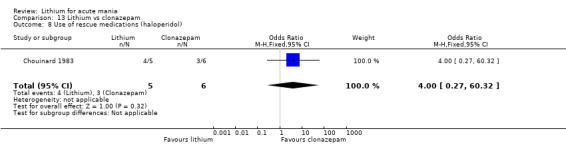
Comparison 13 Lithium vs clonazepam, Outcome 8 Use of rescue medications (haloperidol).
Lithium versus electroconvulsive therapy (ECT)
Comparison 14: lithium versus ECT
There was only one study with 38 participants that fitted the inclusion criteria (Small 1988). This publication did not provide SEs or SDs but did report P values relating to t‐tests.
Primary outcomes
ECT was more effective at reducing manic symptoms than lithium (P < 0.05) on the following outcomes: mean change in MRS (Analysis 14.1, low‐certainty evidence), and in BPRS (Analysis 14.2, low‐certainty evidence), from baseline to end of week 8.
14.1. Analysis.
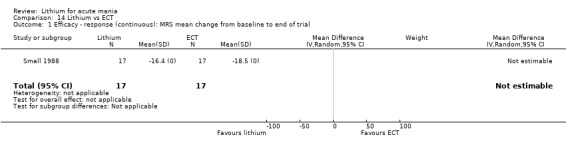
Comparison 14 Lithium vs ECT, Outcome 1 Efficacy ‐ response (continuous): MRS mean change from baseline to end of trial.
14.2. Analysis.
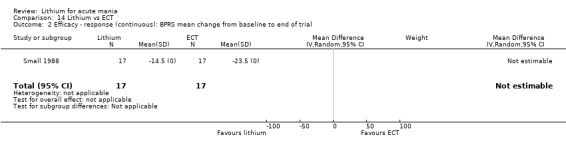
Comparison 14 Lithium vs ECT, Outcome 2 Efficacy ‐ response (continuous): BPRS mean change from baseline to end of trial.
Small 1988 did not report data about acceptability or adverse events.
Secondary outcomes
ECT was more effective at reducing scores on the following overall ratings of severity than lithium (P < 0.05): change in CGI severity (Analysis 14.3), GAS (Analysis 14.4), and Hamilton Depression Scale ratings (Analysis 14.5), from baseline to end of week 8.
14.3. Analysis.
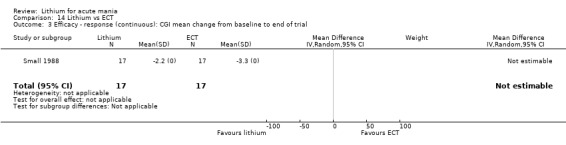
Comparison 14 Lithium vs ECT, Outcome 3 Efficacy ‐ response (continuous): CGI mean change from baseline to end of trial.
14.4. Analysis.
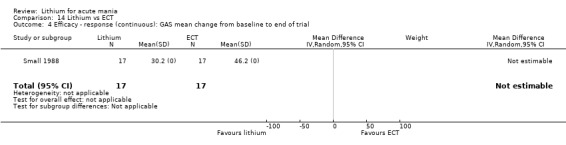
Comparison 14 Lithium vs ECT, Outcome 4 Efficacy ‐ response (continuous): GAS mean change from baseline to end of trial.
14.5. Analysis.
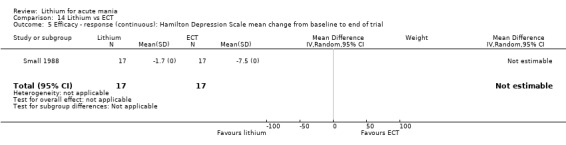
Comparison 14 Lithium vs ECT, Outcome 5 Efficacy ‐ response (continuous): Hamilton Depression Scale mean change from baseline to end of trial.
Comparison 15: lithium versus all antimanic agents
We compared data from all antimanic agents that had combinable primary outcome measures to lithium.
Primary outcomes
Efficacy: response (categorical). At least 50% reduction on YMRS/MRS/BPRS by end of the study
Fourteen studies (including 18 datasets, as four studies had two arms), contributed to the analysis. Lithium was more effective at inducing a response in acute mania than placebo (OR 1.36, 95% CI 1.01 to 1.83; participants = 3666; studies = 14; I2 = 70%; high‐certainty evidence; Analysis 15.1).
15.1. Analysis.
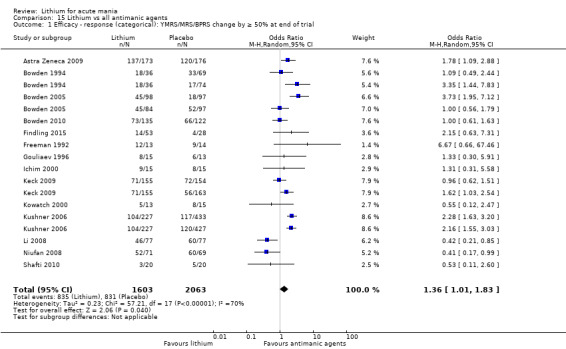
Comparison 15 Lithium vs all antimanic agents, Outcome 1 Efficacy ‐ response (categorical): YMRS/MRS/BPRS change by ≥ 50% at end of trial.
Efficacy: response (continuous). Change from baseline YMRS/BPRS score to end of study
There was no significant difference between the efficacy of lithium and the other antimanic agents (MD −0.30, 95% CI −1.45 to 0.85; participants = 2231; studies = 18; I2 = 99%; moderate‐certainty evidence; Analysis 15.2).
15.2. Analysis.
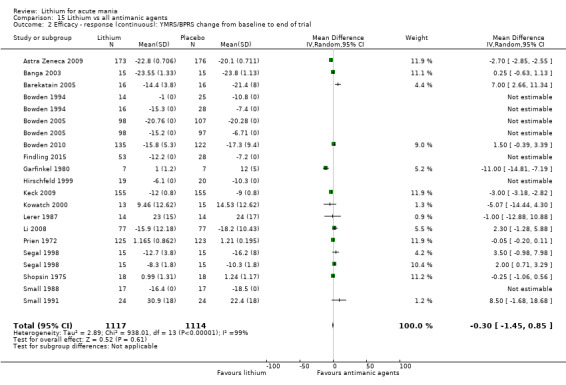
Comparison 15 Lithium vs all antimanic agents, Outcome 2 Efficacy ‐ response (continuous): YMRS/BPRS change from baseline to end of trial.
Acceptability: total withdrawals
We compared lithium to all other antimanic agents (whose studies provided data, studies = 23, datasets =28) in terms of withdrawals for any reason; there was no significant difference between groups (OR 1.11, 95% CI 0.84 to 1.47; participants = 3832; studies = 23; I2 = 62%; high‐certainty evidence; Analysis 15.3).
15.3. Analysis.
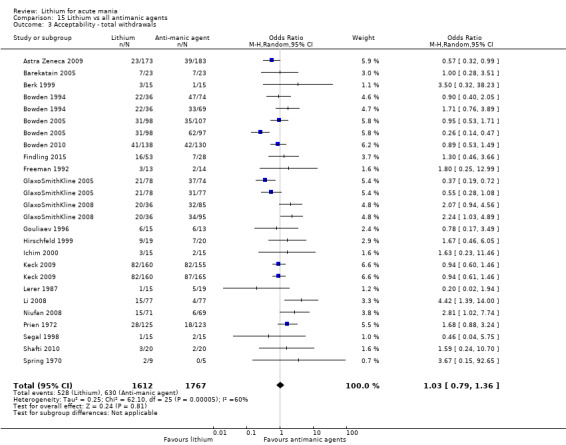
Comparison 15 Lithium vs all antimanic agents, Outcome 3 Acceptability ‐ total withdrawals.
Subgroup analyses
Due to the limited number of studies per comparison, we could not perform any of the originally planned subgroup analyses.
Sensitivity analyses
We could not perform a sensitivity analysis excluding studies that recruited participants with treatment‐resistant mania, because none of the included studies recruited such participants. Almost all the studies reported an all‐cause dropout rate greater than 20%, so it was not possible to carry out this sensitivity analysis. However, our routine comparisons of random‐effects and fixed‐effect models, as well as our secondary outcomes of remission rates and continuous severity measures, may be considered additional forms of sensitivity analyses.
Discussion
Summary of main results
We aimed to identify, synthesise and assess the available evidence from double‐ and single‐blinded randomised controlled studies to answer the following questions.
How does the efficacy of lithium compare with placebo or other active treatment in alleviating the acute symptoms of a manic or mixed episode in people with bipolar disorder?
How does the acceptability and tolerability of lithium compare with placebo or other active treatments in alleviating the acute symptoms of a manic or mixed episode in people with bipolar disorder?
We identified 36 studies that fitted our inclusion criteria. The studies were published over a wide time scale (1970‐2015) and were mostly small in size. The earlier studies tended to have fewer participants. All the studies included men and women, but three studies only enrolled participants under 18 years. The duration of follow‐up varied across studies, within the range of three to 12 weeks and studies included a variety of measures of efficacy from our primary and secondary outcomes. Overall, meta‐analysis for adverse events was limited by only one or two studies tending to report any given outcome.
Lithium versus placebo
We included eight studies that compared lithium treatment with placebo for acute mania (1991 participants). Lithium was found to be superior to placebo in achieving response or remission; this was high‐certainty evidence. Greater decreases in continuous outcome scales (YMRS, CGI, MADRS) were also seen in the lithium groups compared to placebo, but objectively these were small reductions and imprecision led to a grading of only moderate‐certainty evidence. There was insufficient evidence to analyse speed of response to lithium, or the optimal dosing strategies. The short time scale (e.g. three to four weeks) of many of the studies may well fail to capture the full effect of lithium on the treatment of acute mania compared to fast‐acting antipsychotics. Most other mood stabilisers (lamotrigine, carbamazepine, valproate) also take time to act ‐ but even if longer head‐to‐head studies of mood stabilisers in mania were conducted, there is often a substantial clinical benefit to a rapid treatment effect.
The data on adverse events are less robust, as only a few studies reported outcomes that could be combined. Moderate‐certainty evidence showed that participants treated with lithium were less likely to develop an episode of depression or a worsening of their mania. Tremor, dizziness, somnolence and gastrointestinal symptoms were more common in the lithium group than placebo (moderate‐ or low‐certainty evidence). It is notable that for the majority of the adverse events, these were reported equally by those on active treatment and placebo. No significant difference was found for weight gain, but as the mean length of follow‐up was only five weeks, this is unsurprising. Participants on lithium were less likely to withdraw from a study for any reason or for lack of efficacy than those on placebo.
Lithium versus anticonvulsants/mood stabilisers
We found no evidence of a difference in the efficacy of valproate/divalproex and lithium in treating mania in either categorical or continuous outcomes. The heterogeneity of results was high, leading to moderate‐certainty evidence. Both drugs appeared to be tolerated equally; the only exceptions were that participants on lithium were more likely to experience a tremor, whilst those on valproate/divalproex were more sedated. Withdrawals from the study for any reason were not significantly different between the two groups (high‐certainty evidence).
Moderate‐certainty evidence compared the efficacy of lithium to lamotrigine (three studies): we found no significant difference for any measure of efficacy in treating acute mania or in total study withdrawals. We found no significant differences between lithium and lamotrigine in terms of tolerability (low‐certainty evidence). It is notable that lamotrigine needs to be slowly titrated to therapeutic level over several weeks: the length of these studies was three to six weeks, which may not have been long enough to see the full effects of lamotrigine.
There was high‐certainty evidence that lithium was superior to topiramate in causing a response or remission from mania. Weight loss was seen in those treated with topiramate but not in those on lithium. There was no significant difference in total withdrawals between lithium and topiramate (moderate‐certainty evidence).
Lithium versus antipsychotics
Lithium was less likely to induce a response than olanzapine, but this was low‐certainty evidence from only two studies. Continuous outcomes of efficacy showed a very small, but significant improvement in manic symptoms in those on olanzapine (moderate‐certainty evidence). More participants treated with lithium reported a tremor (moderate‐certainty evidence). Unfortunately, there was inadequate data to comment on the relative effects of these interventions on weight gain. By contrast, there was no evidence that risperidone was more effective than lithium in terms of reducing manic symptoms (moderate‐certainty evidence) or inducing remission (low‐certainty evidence). Evidence covering adverse events was all low in certainty, and suggested that risperidone was more sedating but gastrointestinal symptoms and polyuria were more common with lithium.
There was no evidence that quetiapine was more effective than lithium in terms of response or remission (low‐certainty evidence). However, as the analysis only contained two studies with heterogeneous results, we deemed all efficacy outcomes to be of low certainty. Total withdrawals from the study were not significantly different between the two groups (low‐certainty evidence). We found only one study comparing lithium with aripiprazole, and there was no evidence that either drug was superior in inducing a response.
Chlorpromazine was not found to be more effective in reducing manic symptoms compared to lithium (moderate‐ or low‐certainty evidence). The evidence available for meta‐analysis was limited and several of the studies were very small; the four studies were all published in the 1970s. No side effects were reported by more than one study. Efficacy was not significantly different between the two groups (moderate‐certainty evidence) and neither were total withdrawals. Three studies looking at efficacy compared haloperidol to lithium, but these included only 80 participants. We found no significant difference in efficacy between groups (moderate‐certainty evidence). Adverse events outcomes only had single studies or could not be meta‐analysed.
Lithium versus benzodiazepines
We found only two studies comparing lithium to clonazepam. There was no significant difference in efficacy found between the two comparators, but the number of participants was low and results heterogeneous.
Lithium versus ECT
We found one small, single‐blinded study from 1988, which compared lithium with ECT: this reported narratively that ECT was more effective than lithium at treating acute mania but did not provide adequate data for analysis.
Lithium versus all antimanic comparators
When compared to all the antimanic comparators, lithium was more effective at inducing a categorical response. This combining of data led to high heterogeneity, but this is primarily explainable by the small sample sizes in many studies and variable methodology used over the period 1970 to 2017. The meta‐analysis size led to a precise estimate and therefore high‐certainty evidence. Similarly, there was high‐certainty evidence that participants randomised to lithium were not more likely to drop out of a study than those randomised to another antimanic agent or placebo.
Overall completeness and applicability of evidence
We completed a comprehensive search and reviewed a large quantity of potential published and unpublished studies. We found 36 studies that directly addressed our review questions. Thirty‐five of these investigated the efficacy of lithium versus comparator(s), plus or minus adverse events, whilst one reported adverse events only. Overall the reporting of the studies was very good, only a few reported adverse events poorly. As we looked at 14 different comparisons, the overall amount of evidence for each comparison (except lithium versus placebo) was smaller than expected. The maximum number of studies within any meta‐analysis for our 14 key comparisons was nine, which clearly demonstrates that there are limitations in the available data. Overall the studies reported appropriate efficacy outcomes, but the range of categorical/continuous outcomes used made it hard to combine data. We were able address the question of efficacy in lithium versus placebo well, but for all other outcomes the evidence is less robust due to insufficient data.
In terms of adverse events, the study authors reported information about many adverse events but available data were sparse and we did not manage to carry out a meta‐analysis from a large number of included studies. Some of the most problematic side effects (for instance, renal dysfunction) are uncommon but not rare consequences of long‐term treatment with lithium, however special attention should also be devoted to elderly patients during the acute treatment, as they carry a particular risk for chronic kidney disease, given the age‐related decline in estimate glomerular filtration rate (eGFR), age‐related comorbidities and polypharmacy (Bocchetta 2016).
The studies all included people formally diagnosed with bipolar disorder, and 33 out of 36 were in adults.There is therefore a lack of evidence for people with bipolar disorder under 18 years of age. The studies were mainly in inpatient settings, but some outpatients, and were conducted across high‐ and middle‐income countries. There is no particular reason why the findings should not be applicable to any clinical setting and all countries.
Given that lithium has been in continuous use since 1949, and over that period the number of potential interventions for mania has gradually increased, we would have expected to find considerably more studies. In order to try and maintain the highest quality of findings, we excluded studies that were open‐label or did not use a standardised diagnostic process; this did reduce the amount of data considerably.
Quality of the evidence
We assessed the certainty of the evidence collected using the GRADE system, which takes into consideration the risk of bias, consistency of effect, indirectness, publication bias and imprecision. Individual GRADE assessments can be seen within the 'Summary of findings' tables. In view of the large number of outcomes retrieved, this was only done for the most important outcomes.
Overall GRADE assessments
Overall, two comparisons (lithium versus placebo and lithium versus topiramate) had high‐certainty evidence for our main efficacy outcomes. This means that we are confident in our result and feel further research is unlikely to change the direction of the effect. This is especially so for lithium versus placebo, which had significantly more studies included. The evidence for adverse events was more heterogeneous, with somnolence/tremor in lithium versus placebo being downgraded to moderate certainty due to imprecision.
All the other comparisons had moderate‐, low‐ or very low‐certainty evidence. We are therefore less confident in our estimate of the effect. We downgraded the majority of outcomes due to small quantities of data, studies of extremely variable size, and imprecision in single study estimates. This can be summarised as follows.
Lithium versus valproate/divalproex: efficacy outcomes ‐ moderate certainty. The majority of the studies that looked at divalproex had wide confidence intervals, high dropout rates and there was a suspicion of publication bias.
Lithium versus quetiapine: low‐certainty evidence for all outcomes due to high heterogeneity and likely publication bias (only two studies found)
Lithium versus clonazepam: moderate‐certainty evidence from two studies, likely publication bias
Lithium versus lamotrigine: moderate‐certainty evidence for efficacy results (downgraded due to likely publication bias), low‐certainty evidence for adverse events (downgraded for potential publication bias and imprecision)
Lithium versus carbamazepine: low‐ to moderate‐certainty evidence. Downgraded as two studies had very poor reporting (selective reporting bias) and likely publication bias
Lithium versus chlorpromazine: moderate‐certainty evidence ‐ we could include only two studies published a long time ago, these did not give as robust an account of study protocols as would be expected in modern studies and we suspected publication bias
Lithium versus haloperidol: moderate‐certainty evidence due to lack of SDs meaning pooled analysis was very limited
Lithium versus olanzapine: moderate‐ to low‐certainty evidence ‐ downgrading was due to the limited number of studies available, wide confidence intervals in the data and minimal reporting of certain important side effects such as weight gain
Lithium versus risperidone: low‐certainty evidence due to the limited number and variable size of studies with imprecise estimates
Lithium versus aripiprazole: moderate‐certainty evidence but from a single study; publication bias seems very likely
Lithium versus zuclopenthixol: the evidence was found to be of very low certainty, due to the single study, small sample size and wide confidence intervals
Lithium versus ECT: low‐certainty evidence from one small, single‐blinded study that did not report adequate statistical analysis
Lithium versus valproate or divalproex: moderate‐certainty evidence ‐ downgrading was due to imprecision from variable size studies and wide confidence intervals.
Risk of bias
The primary bias we identified in multiple comparisons was the likelihood of publication bias. We would have expected considerably more research over the 60 years since lithium has been in regular usage. We attempted to contact study authors to get access to unpublished data or to try and get raw data missing from publications to facilitate meta‐analysis. Unfortunately we were not fruitful in this approach. We did not assess publication bias through a funnel plot as none of the outcomes examined yielded more than 10 studies.
Inconsistency
We found significant heterogeneity across the studies, which limited the reliability of the evidence. We believed that this was largely down to the effect of small studies. The amount of data available meant that it was difficult to do subanalyses to investigate this further.
Indirectness
Overall, the available evidence matched our objective questions very well. The included studies treated most participants in inpatient settings, which would be expected given the pathology studied. All ages and both sexes were included from a wide range of countries. Furthermore, studies used rescue medications (usually benzodiazepines) for both lithium and all comparators, which further added to the generalisability of this data. Some studies allowed for the use of stimulants (Findling 2015), which may have had an impact on the results. Generally the outcomes measured were directly related to symptoms of mania. One potential limitation is that due to all studies requiring the participants to be able to give informed consent, many participants who were severely unwell (e.g. psychotic, highly behaviourally disturbed or with co‐morbidities affecting their cognition) may well have been excluded. Another is that whilst the outcomes of response/remission are very concrete, those of reduction on a scale may be statistically significant but were often too small to be likely to be clinically significant. Most studies followed up for about four weeks (range 3 to 12); a longer length of study would improve generalisability and reliability of results.
Imprecision
The precision of our outcome estimates was significantly hampered by several factors.
Studies tending to report one or two results from a large range of different outcomes (e.g. YMRS, BPRS, CGI, GAS, BMRS, MADRS, HAMD etc). This limited our ability to combine data and yield more precise estimates of effect size.
Missing SDs or data to impute these. This further limited our ability to meta‐analyse.
Variable size of studies. The number of participants in the studies seems to have gradually increased as time has gone by; those in the 1970s especially included very few participants.
Wide confidence intervals. This is in part relating to the small size of some of the studies.
Potential biases in the review process
In order to capture as much data as possible, we made a comprehensive search of the literature, including multiple sources of published and unpublished data. Two literature searches were conducted, firstly in 2014 and then when the complexity of the review meant considerable time had passed, we repeated the searches in 2017 and 2018 to make sure the review was up to date. As one of our review authors (BA) was unavailable in 2017 and 2018, another review author (SD) carried out the second screening. RM was involved in both searches. A third review author (AC) settled any disputes about whether studies should be included. Two review authors independently checked all data entered to avoid errors.
Unfortunately, our requests to study authors/drug companies about studies registered on clinicaltrials.gov that had not been published or reported online all went unanswered so there is a gap surrounding several modern studies. It is unclear if any of these studies have actually occurred (NCT00183443; NCT00448578; NCT00485680). We also attempted to contact study authors regarding NCT00893581; NCT00183443 and Young 2017, which are studies published as conference abstracts or that did not publish data in the paper such that it could be numerically extracted. Disappointingly, we did not get any replies to our emails.
Given that significant amounts of research occurred on lithium in the period 1950 to 1980, we would have expected more studies to be found between these dates. However, this was an era when registering studies pre‐emptively was not the norm, and many studies were observational in design so did not fit our inclusion criteria. Early randomised studies frequently did not meet inclusion criteria (e.g. early studies were often not blinded or used non‐standard diagnostic criteria) or may not have been published in journals linked to modern databases. This reduces the comprehensive nature of the review but does increase the homogeneity and quality of included data.
We did not examine the doses or regime of any drugs given during the study. We included lithium carbonate and lithium citrate, although most studies clearly stated that they used lithium carbonate. There are some differences in pharmacokinetics between preparations, but no evidence for a difference in clinical efficacy (Guelen 1992; Shelley 1986). The target range of serum levels of lithium was variable: this represents differing practice internationally, but also changes in prescribing patterns over time. These differences are unlikely to have changed the outcome of the meta‐analyses, but could have impacted upon the strength of associations.
We did not further analyse the reasons for withdrawal from each study. The reason for this was the heterogeneity in the way studies reported withdrawals, meaning that the data were impossible to combine. Early studies typically gave no explanation for withdrawals.
Given the nature of mania, if severely unwell participants did not respond to medication and deteriorated, there is a high risk they would have been withdrawn from the study. Similarly, the most unwell patients would not have had capacity to consent for research and would not have been included in the studies.
The outcomes reported by studies were numerous, including both categorical and quantitative measures. We tried to capture as much data as possible by including a wide range of primary and secondary outcomes. Inevitably, we could not include all data from studies or combined them with other studies for analysis; this was mostly from older papers using non‐standard non‐validated questionnaires or qualitative measures.
As many of the included studies were published before it became routine to use/report using ITT or LOCF analysis, there was inadequate quantity of data to allow subgroup analyses dividing the studies between those that did and did not use these strategies. As LOCF introduces more uncertainty and potential for bias than only using collected end‐of‐study data, this may have inadvertently introduced bias.
Agreements and disagreements with other studies or reviews
To our knowledge this is the first comprehensive systematic review and meta‐analysis focusing on the effects of lithium in acute mania and comparing it to a wide range of other interventions.
We found four primary studies that we did not include in this review due to being open‐label but that otherwise fitted our inclusion criteria (Bowden 2008; Calabrese 2005; Christie 1989; Pavuluri 2004). Three of these studies reported no significant difference in efficacy or withdrawal outcomes between lithium and the comparator (valproate or sulpiride). This is similar to our findings for valproate. Christie 1989 was primarily a maintenance study with an open, randomised, acute stabilisation: they did not report efficacy data for the acute phase.
Two high‐quality network meta‐analyses (NMA) have been published examining the comparative efficacy of psychotropics in treating acute mania (Cipriani 2011; Yildiz 2015). Both of these studies used inclusion criteria that were very similar to ours. One important difference was that Cipriani 2011 included combination or augmentation studies (e.g. both arms given lithium plus drug X in one arm or both arms treated with an antipsychotic whilst valproate was added to one and lithium to the other), whereas neither Yildiz 2015 nor this review did. The reason for excluding these reviews was that we wanted to be able to isolate the effects of individual comparators from that of lithium. This meant that only 59% of data included in Yildiz 2015 was present in the first as well. Similarly to this review, the quality of evidence was variable in both NMAs, mostly due to high dropout rates or data heterogeneity. Neither of these reviews included ECT or clonazepam.
Cipriani 2011 reported that lithium was more effective than placebo, finding an OR 2.33 (95% CI 1.39 to 3.85), for a response. Yildiz 2015 also found lithium to be more effective than placebo by approximately two‐fold. These results are very similar to our findings.
Analysis between active comparators by Cipriani 2011 found that with the exception of topiramate, all the antimanic drugs included were more efficacious than placebo. They did not find any significant difference in efficacy between lithium and risperidone, olanzapine, quetiapine, aripiprazole, carbamazepine, valproate, ziprasidone or lamotrigine. Lithium was more efficacious than topiramate. Except for olanzapine, which we found to be more effective than lithium (but this was low‐certainty evidence), these findings are in line with our own. Yildiz 2015 also reported that they found no significant difference in efficacy between lithium and risperidone, olanzapine, carbamazepine, aripiprazole, quetiapine, zuclopenthixol, valproate and lamotrigine. As previously, lithium was found to be more efficacious than topiramate. Taken together, this evidence strongly suggests that lithium is effective in treating acute mania, and is superior to topiramate, but there is no high‐certainty evidence that it is better than other antimanic treatments. Further investigation of the relative efficacies of lithium and olanzapine would be worthwhile.
This review found that participants on lithium were less likely to withdraw from the study than those on placebo. Neither of the NMAs found a significant difference between lithium and placebo withdrawals. Interpretation of our data for withdrawal from studies was limited by low‐certainty evidence in many comparisons. Excluding placebo, we did not find any evidence for a difference in withdrawals between lithium and any comparator. Cipriani 2011 found that participants on risperidone or olanzapine were less likely to withdraw than those on lithium. Yildiz 2015 reported no significant difference for risperidone, but found results favouring olanzapine in terms of withdrawals compared to lithium. Whilst the results are all broadly similar, these variations in findings could be due to differences in our inclusion criteria, or perhaps due to data quality.
A recently published systematic review (De Fazio 2017), examined the efficacy of lithium in treating mania in "late‐life" (this was defined as patients aged over 50 years). De Fazio 2017's inclusion criteria were the same as in this review, except that there was no definition of what was meant by 'a diagnosis of bipolar disorder'. De Fazio 2017 gave a narrative results section as they did not find data compatible with meta‐analysis. The authors reported that the evidence suggests that lithium is more effective than placebo in older patients, but there appears to be no difference in efficacy between lithium and valproate or lamotrigine. It is difficult to compare our findings to this narrative approach, but the results are broadly similar.
There is clear evidence that lithium is effective in treating acute mania. Evidence is also emerging that strongly suggests that mood in bipolar disorder is much more complex than discrete episodes of illness with a return to baseline euthymia in between (McKnight 2017). Mood instability is a strong component of bipolar disorder, and the effect of lithium on this instability is yet to be characterised. The OxLith Trial is the first RCT to examine the potential role of lithium in early mood stabilisation (Geddes 2015). It is using portable technology to capture continuous physiological responses to lithium in the first six weeks of treatment and record weekly mood ratings. This will be linked to longer‐term data. If a manic individual's physiological response to lithium in the very early stages of treatment can be used to predict longer‐term efficacy, then this could be used to determine if lithium is an appropriate treatment for mania or if they should be switched to another proven antimanic agent.
Authors' conclusions
Implications for practice.
Within the limitations of this review, there is consistent evidence from randomised studies that lithium is an effective treatment for acute mania. There is clear and at least moderate‐certainty evidence that lithium is more effective than a placebo or topiramate at treating acute mania and is at least as well tolerated. It may be that olanzapine is slightly more effective than lithium, but the certainty of evidence is not high. The wide range of adverse events experienced by the placebo group compared to those on lithium could provide a useful perspective for counselling patients regarding the risks and benefits of taking lithium. There is not enough evidence at present to clearly guide clinicians on the best dosing strategy for lithium ‐ the recommendation would be as per the National Institute for Health and Care Excellence (NICE) or British Association of Pharmacology Guidelines of aiming for plasma level 0.6 mmol/L to 0.8 mmol/L (Goodwin 2016; NICE 2014), or on the timescale over which lithium could be expected to work compared to other drugs.
The results are consistent with current guidelines for treatment mania, that antipsychotics such as olanzapine, quetiapine, risperidone or haloperidol are reasonable first‐line choices for patients not already on a long‐term antimanic (Goodwin 2016; NICE 2014). This study suggests that lithium is not inferior to the antipsychotics (perhaps to olanzapine, but the evidence is of low certainty), but is a second‐line choice in published guidelines. The probable reason for that is the clinical experience that lithium is slower to act than antipsychotics, and as mania is associated with high clinical risk, a faster working medication ‐ and one that does not required blood level monitoring in agitated patients‐ may be preferred. This is a reasonable strategy given the current evidence, especially for olanzapine. If patients are already taking an antipsychotic, the addition of lithium does seem a logical next step, especially given the recent guidance issued regarding valproate use in women of reproductive age (MHRA 2018).
We found no evidence that lamotrigine is less effective than lithium, as would be suggested by the current NICE or Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines, but the evidence was not of high certainty (CANMAT 2018; NICE 2014). The time (and therefore cost) needed to safely titrate lamotrigine make other antimanic agents a more practical choice. There is insufficient high‐certainty evidence to guide clinical practice on the use of carbamazepine or benzodiazepines in acute mania compared to lithium. Similarly, the relative efficacy and tolerability of lithium to ECT in acute mania remains unclear.
It should be noted that the paucity of evidence relating to children and adolescents mean that the results here apply only to adults.
Implications for research.
More well‐designed, randomised controlled studies investigating the relative efficacy and acceptability of lithium in the treatment of acute mania are required. This is especially the case in children and adolescents. Future researchers should learn from existing studies and aim to include a power calculation to ensure detecting moderate but worthwhile benefits and include as wide a clinical range of people with bipolar disorder as practicable. Outcome measures of relevance to both patients and clinicians, such as length of hospital stay, occupational and social assessments and reports of patient satisfaction should be included. In view of the practical and ethical difficulties of the inclusion of a placebo group, especially for severely ill patients, future studies should focus on the comparison of lithium with other medications. Considering the cost implications of large studies, and the multiple available treatments for acute mania, network meta‐analysis may prove a useful tool to investigate direct and indirect treatments in future.
Acknowledgements
The review authors are grateful to Cochrane Common Mental Disorders for help in constructing and implementing the search strategy.
CRG Funding Acknowledgement: the NIHR is the largest single funder of Cochrane Common Mental Disorders.
Disclaimer: the views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the NIHR, UK NHS or the Department of Health and Social Care.
Appendices
Appendix 1. Specialised Register: CCMD's core MEDLINE search strategy
The search strategy listed below is the weekly OVID MEDLINE search used to inform the Group’s specialised register. It is based on a list of terms for all conditions within the scope of the Cochrane Common Mental Disorders Group plus a sensitive RCT filter. 1. [MeSH Headings]: eating disorders/ or anorexia nervosa/ or binge‐eating disorder/ or bulimia nervosa/ or female athlete triad syndrome/ or pica/ or hyperphagia/ or bulimia/ or self‐injurious behavior/ or self mutilation/ or suicide/ or suicidal ideation/ or suicide, attempted/ or mood disorders/ or affective disorders, psychotic/ or bipolar disorder/ or cyclothymic disorder/ or depressive disorder/ or depression, postpartum/ or depressive disorder, major/ or depressive disorder, treatment‐resistant/ or dysthymic disorder/ or seasonal affective disorder/ or neurotic disorders/ or depression/ or adjustment disorders/ or exp antidepressive agents/ or anxiety disorders/ or agoraphobia/ or neurocirculatory asthenia/ or obsessive‐compulsive disorder/ or obsessive hoarding/ or panic disorder/ or phobic disorders/ or stress disorders, traumatic/ or combat disorders/ or stress disorders, post‐traumatic/ or stress disorders, traumatic, acute/ or anxiety/ or anxiety, castration/ or koro/ or anxiety, separation/ or panic/ or exp anti‐anxiety agents/ or somatoform disorders/ or body dysmorphic disorders/ or conversion disorder/ or hypochondriasis/ or neurasthenia/ or hysteria/ or munchausen syndrome by proxy/ or munchausen syndrome/ or fatigue syndrome, chronic/ or obsessive behavior/ or compulsive behavior/ or behavior, addictive/ or impulse control disorders/ or firesetting behavior/ or gambling/ or trichotillomania/ or stress, psychological/ or burnout, professional/ or sexual dysfunctions, psychological/ or vaginismus/ or Anhedonia/ or Affective Symptoms/ or *Mental Disorders/
2. [Title/ Author Keywords]: (eating disorder* or anorexia nervosa or bulimi* or binge eat* or (self adj (injur* or mutilat*)) or suicide* or suicidal or parasuicid* or mood disorder* or affective disorder* or bipolar i or bipolar ii or (bipolar and (affective or disorder*)) or mania or manic or cyclothymic* or depression or depressive or dysthymi* or neurotic or neurosis or adjustment disorder* or antidepress* or anxiety disorder* or agoraphobia or obsess* or compulsi* or panic or phobi* or ptsd or posttrauma* or post trauma* or combat or somatoform or somati#ation or medical* unexplained or body dysmorphi* or conversion disorder or hypochondria* or neurastheni* or hysteria or munchausen or chronic fatigue* or gambling or trichotillomania or vaginismus or anhedoni* or affective symptoms or mental disorder* or mental health).ti,kf.
3. [RCT filter]: (controlled clinical trial.pt. or randomised controlled trial.pt. or (randomi#ed or randomi#ation).ab,ti. or randomly.ab. or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*)).ab. or placebo*.ab,ti. or drug therapy.fs. or trial.ab,ti. or groups.ab. or (control* adj3 (trial* or study or studies)).ab,ti. or ((singl* or doubl* or tripl* or trebl*) adj3 (blind* or mask* or dummy*)).mp. or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or randomised controlled trial/ or pragmatic clinical trial/ or (quasi adj (experimental or random*)).ti,ab. or ((waitlist* or wait* list* or treatment as usual or TAU) adj3 (control or group)).ab.)
4. (1 and 2 and 3)
Records are screened for reports of RCTs within the scope of the Cochrane Common Mental Disorders Group. Secondary reports of RCTs are tagged to the appropriate study record.
Appendix 2. Database searches 2017/2018
Update search‐1, February 2017
Cochrane Common Mental Disorders Group Specialised Register (CCMDCTR) (1 February 2014 to 1 February 2017) 1. The CCDAN Studies Register was searched using the following terms: Condition = (mania or hypomania) and Intervention = lithium 2. The CCDANCTR‐References Register was searched using a more sensitive set of free‐text terms to find additional, untagged, uncoded trial reports: (lithium and (mania* or manic* or hypomani* or ((bipolar or schizoaffective) NEAR (acute or psychos* or psychotic or "mixed episode*" or “mixed state*” or "rapid cycl*")))) 3. (1 or 2) (n = 103) Cochrane Central Register of Controlled Trials (CENTRAL), 2017, Issue 1 (all years) #1 (lithium and (mania* or manic* or hypomani* or ((bipolar or schizoaffective) near (acute or psychos* or psychotic or "mixed episode*" or "mixed state*" or "rapid cycl*")))) #2 SR‐DEPRESSN or HS‐DEPRESSN #3 #1 not #2 (n = 231) Ovid MEDLINE (databases) (2014 to 1 February 2017) Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1. lithium.ti,ab,kf,hw. 2. ((bipolar or schizoaffective) and (acute or psychos* or psychotic or mixed episode* or mixed state* or rapid cycl*)).ti,ab,kf,hw. 3. 1 and 2 4. randomized controlled trial.pt. 5. randomi*.ti,ab,kf. 6. (RCT or at random or (random* adj (assign* or allocat* or divid* or division or number))).ti,ab,kf. 7. trial.ti. 8. clinical trials as topic.sh. 9. placebo.ti,ab,kf. 10. ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask* or dummy)).ti,ab,kf. 11. or/5‐10 12. exp animals/ not humans.sh. 13. 11 not 12 14. 13 and 3 15. ..dedup 14 16. (2014* or 2015* or 2016* or 2017*).yr,ed. 17. 15 and 16 (n = 64) Ovid PsycINFO (2014 to 1 February 2017) 1. treatment effectiveness evaluation.sh. 2. clinical trials.sh. 3. mental health program evaluation.sh. 4. placebo.sh. 5. placebo.ti,ab,id. 6. randomly.ab. 7. randomi*.ti,ab,id. 8. trial.ti,id. 9. ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask* or dummy)).ti,ab,id. 10. (control* adj3 (trial or study or group*)).ti,ab,id. 11. factorial*.ti,ab,id. 12. allocat*.ti,ab,id. 13. assign*.ti,ab,id. 14. ((waitlist* or wait* list* or treatment as usual or TAU) adj3 (control or group)).ab. 15. (random* adj3 (administ* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*)).ab. 16. or/1‐15 17. lithium.ti,ab,id,hw. 18. ((bipolar or schizoaffective) and (acute or psychos* or psychotic or mixed episode* or mixed state* or rapid cycl*)).ti,ab,id,hw. 19. mania/ or hypomania/ 20. 17 and (18 or 19) 21. 16 and 20 22. (2014* or 2015* or 2016* or 2017*).yr,an,up. 23. 21 and 22 (n = 46) Ovid Embase (2014 to 1 February 2017) 1. randomized controlled trial.sh. 2. randomization.sh. 3. placebo.sh. 4. placebo.ti,ab,kw. 5. randomi#ed.ti,ab,kw. 6. (RCT or at random or (random* adj3 (administ* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*))).ti,ab,kw. 7. ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask* or dummy)).ti,ab,kw,hw. 8. crossover procedure.sh. 9. (crossover* or cross over*).ti,ab,kw. 10. (control* adj3 (trial or study or group*)).ti,ab,kw. 11. or/1‐10 12. ((animal or nonhuman) not (human and (animal or nonhuman))).sh. 13. 11 not 12 14. ((bipolar or schizoaffective) and (acute or psychos* or psychotic or mixed episode* or mixed state* or rapid cycl*)).ti,ab,kw,hw. 15. mania/ or hypomania/ or manic psychosis/ 16. bipolar mania/ 17. manic depressive psychosis.mp. 18. mania assessment/ or bech‐rafaelsen mania scale/ or hypomania checklist 32/ or young mania rating scale/ 19. "mixed mania and depression"/ 20. or/14‐19 21. lithium.af. 22. 13 and 20 and 21 23. (2014* or 2015* or 2016* or 2017*).yr,dd. 24. 22 and 23 25. ..dedup 24 (n = 318) 2017 Search Total = 762 After de‐duplication = 544
Update search‐2, April/May 2018
Cochrane Library Trials database (Issue 4 of 12, April 2018) #1 (lithium and (mania* or manic* or hypomani* or ((bipolar or schizoaffective) near (acute or psychos* or psychotic or "mixed episode*" or "mixed state*" or "rapid cycl*")))) #2 SR‐DEPRESSN or HS‐DEPRESSN #3 #1 not #2 (n = 46)
Ovid MEDLINE Databases (1946 to 17 May 2018) date limited 2017 onwards 1 lithium.ti,ab,kf,hw. (50923) 2 ((bipolar or schizoaffective) and (acute or psychos* or psychotic or mixed episode* or mixed state* or rapid cycl*)).ti,ab,kf,hw. (18953) 3 (1 and 2) (2632) 4 randomized controlled trial.pt.461940 5 randomi*.ti,ab,kf.543100 6 (RCT or at random or (random* adj (assign* or allocat* or divid* or division or number))).ti,ab,kf.188041 7 trial.ti.182905 8 clinical trials as topic.sh.183803 9 placebo.ti,ab,kf.194598 10 ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask* or dummy)).ti,ab,kf.158432 11 or/4‐10 (1052295) 12 (3 and 11) (652) 13 exp animals/ not humans.sh.4461712 14 (12 not 13) (650) 15 (2017* or 2018*).yr,ed. (2393762) 16 (14 and 15) (44)
Ovid PsycINFO (1806 to May Week 2 2018), date limited 2017 onwards 1 treatment effectiveness evaluation.sh. (22049) 2 clinical trials.sh. (10902) 3 mental health program evaluation.sh. (2033) 4 placebo.sh. (5096) 5 placebo.ti,ab,id. (37454) 6 randomly.ab. (65746) 7 randomi*.ti,ab,id. (73778) 8 trial.ti,id. (30055) 9 ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask* or dummy)).ti,ab,id. (24361) 10 (control* adj3 (trial or study or group*)).ti,ab,id.127299 11 factorial*.ti,ab,id. (17973) 12 allocat*.ti,ab,id. (27496) 13 assign*.ti,ab,id. (88272) 14 ((waitlist* or wait* list* or treatment as usual or TAU) adj3 (control or group)).ab. (4726) 15 (random* adj3 (administ* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*)).ab. (47831) 16 or/1‐15 (346634) 17 lithium.ti,ab,id,hw. (10538) 18 ((bipolar or schizoaffective) and (acute or psychos* or psychotic or mixed episode* or mixed state* or rapid cycl*)).ti,ab,id,hw. (13086) 19 mania/ or hypomania/ (5891) 20 17 and (18 or 19) (2189) 21 (16 and 20) (496) 22 (2017* or 2018*).yr,an,up. (290086) 23 (21 and 22) (33)
Ovid Embase (1974 to 2018 May 17), date limited 2017 onwards 1 randomized controlled trial.sh. (502772) 2 randomization.sh. (78117) 3 placebo.sh. (325165) 4 placebo.ti,ab,kw. (272805) 5 randomi#ed.ti,ab,kw. (753158) 6 (RCT or at random or (random* adj3 (administ* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*))).ti,ab,kw. (495563) 7 ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask* or dummy)).ti,ab,kw,hw. (275282) 8 crossover procedure.sh. (55512) 9 (crossover* or cross over*).ti,ab,kw. (94338) 10 (control* adj3 (trial or study or group*)).ti,ab,kw. (987574) 11 or/1‐10 (1972516) 12 ((animal or nonhuman) not (human and (animal or nonhuman))).sh.5545720 13 (11 not 12) (1717138) 14 ((bipolar or schizoaffective) and (acute or psychos* or psychotic or mixed episode* or mixed state* or rapid cycl*)).ti,ab,kw,hw. (34878) 15 mania/ or hypomania/ or manic psychosis/ (19370) 16 bipolar mania/ (1670) 17 manic depressive psychosis.mp. (9079) 18 mania assessment/ or bech‐rafaelsen mania scale/ or hypomania checklist 32/ or young mania rating scale/ (2666) 19 "mixed mania and depression"/ (1027) 20 or/14‐19 (54267) 21 lithium.af. (81055) 22 (13 and 20 and 21) (2717) 23 (2017* or 2018*).yr,dd. (2409033) 24 (22 and 23) (125)
2018 Search total = 248 After de‐duplication, n = 161
Data and analyses
Comparison 1. Lithium vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Efficacy ‐ response (categorical): YMRS/MRS decrease by =>50% at end of trial | 6 | 1707 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.73, 2.63] |
| 2 Efficacy ‐ response (continuous): YMRS change from baseline at end of trial | 4 | 935 | Mean Difference (IV, Random, 95% CI) | ‐2.85 [‐3.14, ‐2.55] |
| 3 Efficacy ‐ response (continuous): CGI change from baseline at end of trial | 4 | 952 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.35, ‐0.16] |
| 4 Efficacy ‐ remission (categorical): YMRS < 12 at end of trial | 5 | 1597 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.16 [1.73, 2.69] |
| 5 Acceptability: withdrawals | 7 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 All‐cause dropouts | 7 | 1353 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.46, 1.25] |
| 5.2 Dropouts due to adverse events | 6 | 1158 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.50, 1.69] |
| 5.3 Dropouts due to lack of efficacy | 6 | 1243 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.29, 1.08] |
| 6 Adverse event: depression | 4 | 1360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.33, 0.98] |
| 7 Adverse event: mania | 4 | 1296 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.38, 0.93] |
| 8 Adverse event: weight gain (categorical) | 3 | 735 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.56, 3.92] |
| 9 Adverse event: weight gain (continuous) | 3 | 599 | Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.50, 0.82] |
| 10 Adverse event: akathisia | 3 | 673 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.39, 1.91] |
| 11 Adverse event: headache | 6 | 1270 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.76, 1.48] |
| 12 Adverse event: somnolence | 7 | 1351 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.28 [1.46, 3.58] |
| 13 Adverse event: dizziness | 5 | 873 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.12 [1.21, 3.74] |
| 14 Adverse event: insomnia | 3 | 706 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.44, 1.29] |
| 15 Adverse event: diarrhoea | 6 | 1028 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.90, 2.54] |
| 16 Adverse event: nausea | 6 | 1220 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.32 [1.54, 3.50] |
| 17 Adverse event: vomiting | 6 | 1028 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.06 [3.21, 11.45] |
| 18 Adverse event: dry mouth | 3 | 682 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.58, 2.09] |
| 19 Adverse event: pain | 3 | 396 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.07, 0.79] |
| 20 Adverse event: EPS | 2 | 478 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.68, 2.19] |
| 21 Adverse event: tremor | 6 | 1241 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.25 [2.10, 5.04] |
| 22 Adverse event: constipation | 5 | 1075 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.81, 2.01] |
| 23 Adverse event: fever | 2 | 466 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.75, 3.55] |
| 24 Adverse event: rash | 3 | 367 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.37, 2.29] |
| 25 Efficacy ‐ response (continuous): CGI change from baseline to end of trial | 2 | 513 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐1.00, 0.07] |
| 26 Response: YMRS decrease by ≥ 50% end of the trial | 1 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.12, 0.42] |
| 27 Response: remission YMRS < 12 at end of trial | 1 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.11, 0.36] |
| 28 Efficacy ‐ response (continuous): MADRS change from baseline to end of trial | 2 | 667 | Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐0.85, ‐0.26] |
| 29 Efficacy ‐ response (continuous): MRS score change from baseline to end of trial | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 29.1 Manic subscale of MRS ‐ score at end of trial | 2 | 285 | Mean Difference (IV, Fixed, 95% CI) | ‐1.19 [‐2.78, 0.39] |
| 29.2 MRS 16 item scale from SAD‐C | 2 | 285 | Mean Difference (IV, Fixed, 95% CI) | ‐3.67 [‐7.27, ‐0.07] |
| 30 Efficacy ‐ (continuous): GAS score at end of trial | 2 | 284 | Mean Difference (IV, Random, 95% CI) | 3.17 [‐7.02, 13.37] |
| 31 Efficacy ‐ HAMD‐31 score at end of trial | 2 | 285 | Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐7.69, 5.44] |
| 32 Efficacy ‐ BPRS score at end of trial | 2 | 285 | Mean Difference (IV, Fixed, 95% CI) | ‐1.74 [‐3.70, 0.23] |
| 33 PANSS change from baseline to end of trial | 2 | 629 | Mean Difference (IV, Random, 95% CI) | ‐2.86 [‐4.33, ‐1.39] |
| 34 CGI‐BP change from baseline to end of trial | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 34.1 Severity of illness score Overall | 1 | 316 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐2.02, ‐1.98] |
| 34.2 Depression change from baseline at 3 weeks | 1 | 316 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 35 Use of concomitant medications | 2 | 479 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.52, 1.57] |
| 36 Use of sleep medications over course of trial | 1 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.51, 1.58] |
| 37 Use of anticholinergic medications | 2 | 520 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.73, 2.62] |
| 38 Concomitant use of analgesics/antipyretics | 1 | 325 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.58, 1.48] |
| 39 Acceptability: withdrawal due to adverse events | 6 | 1158 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.61, 1.37] |
1.25. Analysis.
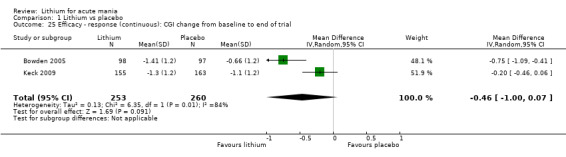
Comparison 1 Lithium vs placebo, Outcome 25 Efficacy ‐ response (continuous): CGI change from baseline to end of trial.
1.26. Analysis.
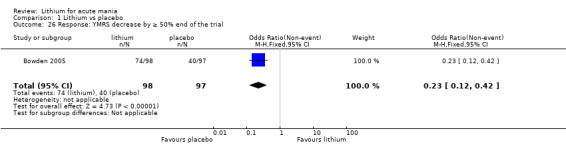
Comparison 1 Lithium vs placebo, Outcome 26 Response: YMRS decrease by ≥ 50% end of the trial.
1.27. Analysis.
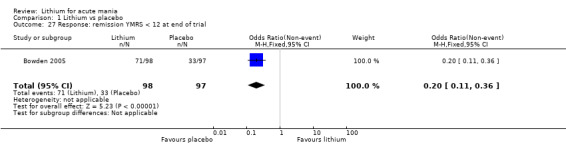
Comparison 1 Lithium vs placebo, Outcome 27 Response: remission YMRS < 12 at end of trial.
1.30. Analysis.
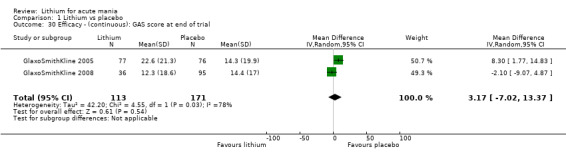
Comparison 1 Lithium vs placebo, Outcome 30 Efficacy ‐ (continuous): GAS score at end of trial.
1.38. Analysis.
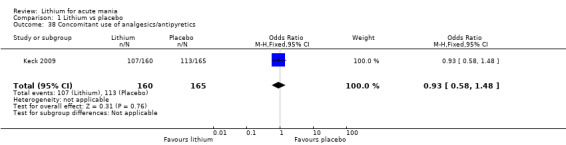
Comparison 1 Lithium vs placebo, Outcome 38 Concomitant use of analgesics/antipyretics.
1.39. Analysis.
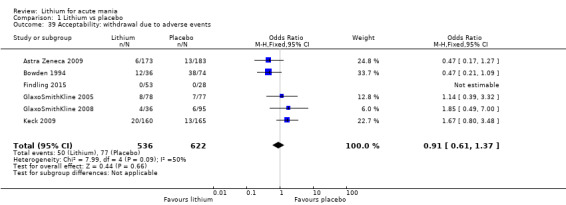
Comparison 1 Lithium vs placebo, Outcome 39 Acceptability: withdrawal due to adverse events.
Comparison 2. Lithium vs valproate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Efficacy ‐ response (categorical): YMRS/SADS‐C decrease ≥ 50% by end of trial | 5 | 607 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.87, 1.70] |
| 2 Efficacy ‐ response (categorical, adults): YMRS/SADS‐C decrease ≥ 50% by end of trial ADULTS only | 4 | 579 | Odds Ratio (M‐H, Random, 95% CI) | 1.30 [0.84, 1.99] |
| 3 Efficacy ‐ response (continuous): change in YMRS (ITT‐LOCF) change from baseline to end of trial | 5 | 398 | Mean Difference (IV, Fixed, 95% CI) | 0.43 [‐0.36, 1.23] |
| 4 Efficacy ‐ response (continuous): CGI‐BP change from baseline to end of trial | 2 | 287 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.29, 0.25] |
| 5 Efficacy ‐ response (continuous): Change in MADRS (ITT‐LOCF) change from baseline to end of trial | 1 | 257 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.73, 1.53] |
| 6 Efficacy ‐ remission (categorical): YMRS ≤ 12 and no increase in MADRS at end of trial | 1 | 257 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.46, 1.32] |
| 7 Efficacy ‐ remission (categorical): YMRS ≤ 12 and a reduction of at least 2 points on the CGI‐BP at end of trial | 1 | 257 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.34, 0.96] |
| 8 Adverse event: headache | 2 | 286 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.64 [1.52, 4.59] |
| 9 Adverse event: somnolence | 4 | 575 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.29, 0.76] |
| 10 Adverse event: tremor | 2 | 449 | Odds Ratio (M‐H, Fixed, 95% CI) | 10.51 [1.96, 56.48] |
| 11 Acceptability: total withdrawals | 5 | 629 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.86, 1.69] |
| 12 Efficacy ‐ response (continuous): MADRS change from baseline to end of trial | 1 | 257 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.83, 1.23] |
| 13 SADS‐C mania score at end of trial | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐16.90 [‐28.85, ‐4.95] |
| 14 SADS‐C depression score at end of trial | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐9.62, 2.82] |
| 15 GAS score at end of trial | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 9.30 [‐4.18, 22.78] |
| 16 CGI‐BP end of trial score | 1 | 257 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.13, 0.53] |
| 17 YMRS insight score at end of trial | 1 | 257 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [0.02, 0.58] |
| 18 BPRS score at end of trial | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐6.3 [‐11.99, ‐0.61] |
| 19 Any adverse events | 2 | 298 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.62, 1.57] |
| 20 Serious adverse events | 1 | 268 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.15, 1.36] |
| 21 Adverse event: diarrhoea | 4 | 583 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.59, 2.01] |
| 22 Adverse event: nausea | 4 | 583 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.97, 2.40] |
| 23 Concomitant medication: use of lorazepam | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.28, 5.59] |
| 24 Concomitant medication: use of chloral hydrate (g) | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 1.50 [‐2.76, 5.76] |
| 25 Concomitant medication: use of lorazepam (g) | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐5.04, 3.64] |
| 26 Concomitant medication: use of anxiolytics | 1 | 257 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.75, 2.05] |
| 27 Concomitant medication: use of antidepressants | 1 | 257 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.55, 8.58] |
2.6. Analysis.
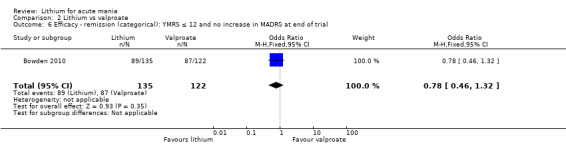
Comparison 2 Lithium vs valproate, Outcome 6 Efficacy ‐ remission (categorical): YMRS ≤ 12 and no increase in MADRS at end of trial.
2.17. Analysis.
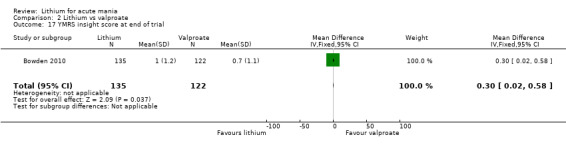
Comparison 2 Lithium vs valproate, Outcome 17 YMRS insight score at end of trial.
2.18. Analysis.
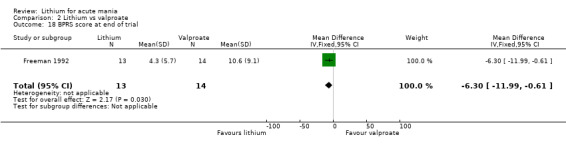
Comparison 2 Lithium vs valproate, Outcome 18 BPRS score at end of trial.
2.20. Analysis.
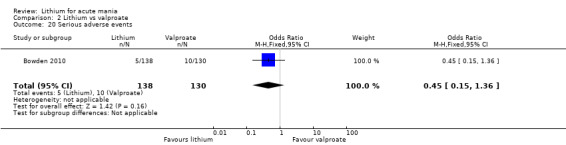
Comparison 2 Lithium vs valproate, Outcome 20 Serious adverse events.
Comparison 3. Lithium vs lamotrigine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Efficacy ‐ response (continuous): change in BPRS from baseline to end of trial | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Efficacy ‐ Change in BPRS from baseline to end of trial | 3 | 301 | Mean Difference (IV, Fixed, 95% CI) | ‐1.82 [‐3.78, 0.14] |
| 2 Efficacy ‐ response (continuous): MRS (16 item) change from baseline to end of trial | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 MRS 11 item from SAD‐C | 2 | 271 | Mean Difference (IV, Fixed, 95% CI) | ‐2.74 [‐5.84, 0.36] |
| 2.2 MRS Manic Subscale | 2 | 271 | Mean Difference (IV, Fixed, 95% CI) | ‐1.38 [‐3.02, 0.26] |
| 2.3 MRS 16 item from SAD‐C | 2 | 271 | Mean Difference (IV, Fixed, 95% CI) | ‐3.74 [‐7.55, 0.08] |
| 3 Efficacy ‐ response (continuous): CGI severity change from baseline to end of trial | 3 | 304 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐1.24, 0.53] |
| 4 Efficacy ‐ response (continuous): change in GAS from baseline to end of trial | 2 | 270 | Mean Difference (IV, Fixed, 95% CI) | 4.36 [‐0.65, 9.37] |
| 5 Acceptability | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 All‐cause dropouts | 3 | 303 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.50, 1.29] |
| 5.2 Dropouts due to adverse events | 2 | 273 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.42, 2.06] |
| 5.3 Dropouts due to lack of efficacy | 2 | 273 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.48, 2.32] |
| 6 Efficacy ‐ response (continuous): change in Hamilton depression scale (HAMD 31) from baseline to end of trial | 2 | 271 | Mean Difference (IV, Fixed, 95% CI) | ‐1.74 [‐3.72, 0.24] |
| 7 Efficacy ‐ response (categorical): ≥ 50% reduction in BPRS from baseline to end of trial | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.09, 1.92] |
| 8 Efficacy ‐ response(categorical): ≥ 50% reduction in MRS from baseline to end of trial | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.31, 5.58] |
| 9 Efficacy ‐ response (categorical): CGI scores of 1 or 2 at the end of the trial | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.09, 1.92] |
| 10 Adverse event: vomiting | 2 | 272 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.75, 4.18] |
| 11 Adverse event: all mania | 2 | 272 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.16, 2.46] |
| 12 Adverse event: diarrhoea | 2 | 272 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.83 [0.92, 15.92] |
| 13 Adverse event: headache | 2 | 272 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.45, 2.02] |
| 14 Adverse event: tremor | 2 | 272 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.48, 3.41] |
| 15 Adverse event: rash | 2 | 272 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.17, 1.97] |
| 16 Adverse event: somnolence | 2 | 272 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.34, 3.85] |
| 17 Adverse event: any side effects | 2 | 272 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.47, 1.70] |
| 18 Adverse event: any serious event | 1 | 152 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.26, 3.41] |
| 19 Adverse event: constipation | 2 | 272 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.18, 3.40] |
| 20 Adverse event: accidental injury | 2 | 272 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.43, 4.08] |
| 21 Adverse event: pain | 2 | 272 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.07, 1.65] |
| 22 Use of concomitant psychotropic medication | 1 | 151 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.07, 0.95] |
| 23 Mean total dose of lorazepam (g) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.9. Analysis.
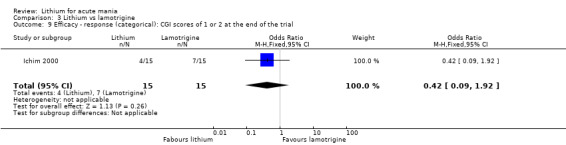
Comparison 3 Lithium vs lamotrigine, Outcome 9 Efficacy ‐ response (categorical): CGI scores of 1 or 2 at the end of the trial.
3.18. Analysis.
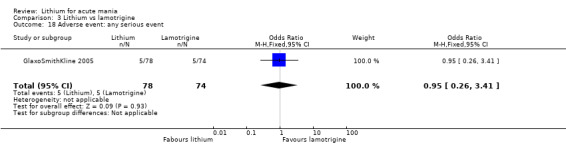
Comparison 3 Lithium vs lamotrigine, Outcome 18 Adverse event: any serious event.
Comparison 4. Lithium vs carbamazepine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Efficacy ‐ response (continuous): YMRS/BPRS change from baseline to end of trial | 3 | 102 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.18, 0.60] |
| 2 Efficacy ‐ response (continuous): CGI change from baseline to end of trial | 2 | 76 | Mean Difference (IV, Random, 95% CI) | 0.68 [‐0.40, 1.76] |
| 3 Efficacy ‐ response (continuous): mean length of treatment in weeks | 3 | 119 | Mean Difference (IV, Random, 95% CI) | 2.0 [‐12.88, 16.88] |
| 4 Acceptability | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 All‐cause dropouts | 1 | 34 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.02, 1.94] |
| 4.2 Dropouts due to adverse events | 1 | 48 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.25] |
| 5 Changes in side effect scores from baseline to end of trial | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 0.9 [‐0.80, 2.60] |
| 6 Response rate ≥ 50% change in YMRS from baseline to end of trial | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.21, 4.86] |
| 7 Score of CGI‐BP of 1 or 2 at end of trial | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.39, 9.60] |
| 8 Response: CGI change of 2 or more from baseline to end of trial | 1 | 28 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.17 [1.63, 51.43] |
| 9 HDRS 6 weeks | 1 | 54 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐2.21, 0.61] |
| 10 HDRS >11 | 1 | 54 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.03, 3.16] |
| 11 Adverse event: serious adverse events | 1 | 34 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.15, 3.59] |
| 12 Adverse event: nausea | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.06, 1.71] |
| 13 Adverse event: sedation | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.64] |
| 14 Adverse event: rash | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 7.70] |
| 15 Adverse event: dizziness | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 7.70] |
| 16 Adverse event: increased appetite | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.4 [0.24, 123.81] |
| 17 Adverse event: polyuria | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.11, 80.39] |
| 18 Adverse event: diarrhoea | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.11, 80.39] |
4.6. Analysis.
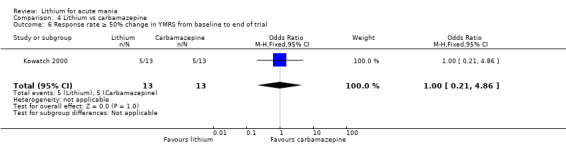
Comparison 4 Lithium vs carbamazepine, Outcome 6 Response rate ≥ 50% change in YMRS from baseline to end of trial.
4.7. Analysis.
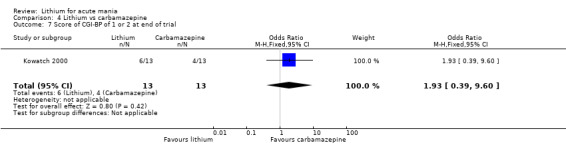
Comparison 4 Lithium vs carbamazepine, Outcome 7 Score of CGI‐BP of 1 or 2 at end of trial.
4.8. Analysis.
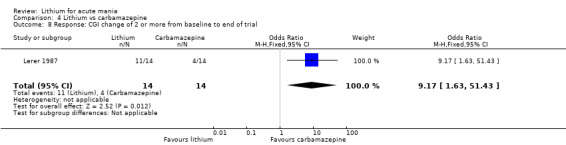
Comparison 4 Lithium vs carbamazepine, Outcome 8 Response: CGI change of 2 or more from baseline to end of trial.
4.9. Analysis.
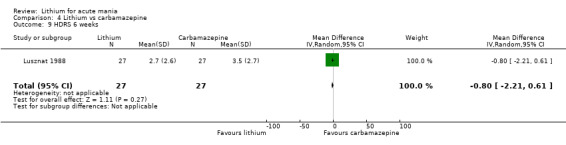
Comparison 4 Lithium vs carbamazepine, Outcome 9 HDRS 6 weeks.
4.10. Analysis.
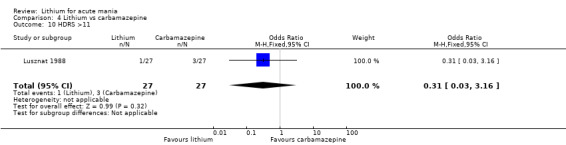
Comparison 4 Lithium vs carbamazepine, Outcome 10 HDRS >11.
4.11. Analysis.
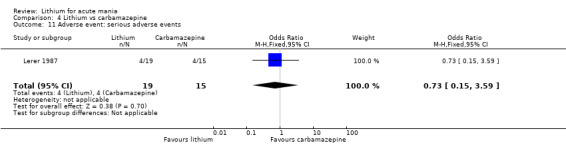
Comparison 4 Lithium vs carbamazepine, Outcome 11 Adverse event: serious adverse events.
4.16. Analysis.
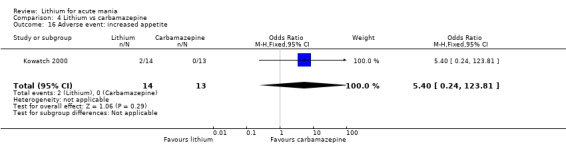
Comparison 4 Lithium vs carbamazepine, Outcome 16 Adverse event: increased appetite.
4.17. Analysis.
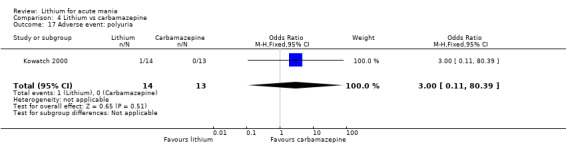
Comparison 4 Lithium vs carbamazepine, Outcome 17 Adverse event: polyuria.
4.18. Analysis.
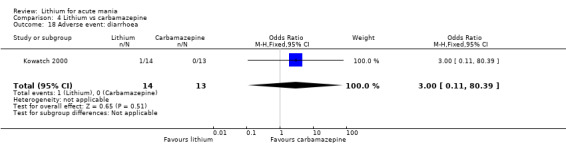
Comparison 4 Lithium vs carbamazepine, Outcome 18 Adverse event: diarrhoea.
Comparison 5. Lithium vs quetiapine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Efficacy ‐ response (categorical): YMRS decrease by ≥ 50% by end of trial | 2 | 335 | Odds Ratio (M‐H, Random, 95% CI) | 0.66 [0.28, 1.55] |
| 2 Efficacy ‐ response (continuous): YMRS/MADRS change from baseline at end of trial | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 YMRS change from baseline | 2 | 359 | Mean Difference (IV, Random, 95% CI) | 2.30 [‐1.28, 5.88] |
| 2.2 MADRS change from baseline | 2 | 359 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐0.32, 2.12] |
| 3 Efficacy ‐ remission (categorical): Decrease in YMRS ≤ 12 by end of trial | 2 | 359 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.26, 1.57] |
| 4 Efficacy ‐ response (continuous): MADRS change from baseline | 2 | 359 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐0.32, 2.12] |
| 5 Adverse event: dizziness | 2 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.23, 0.97] |
| 6 Adverse event: diarrhoea | 2 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.34, 1.86] |
| 7 Adverse event: weight gain | 1 | 205 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.14, 0.99] |
| 8 Acceptability: withdrawal | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 All‐cause dropouts | 2 | 359 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.83, 2.28] |
| 8.2 Dropouts due to adverse events | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.61 [0.51, 181.64] |
| 8.3 Dropouts due to lack of efficacy | 1 | 205 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.36, 1.77] |
| 9 PANSS score change from baseline to end of trial | 1 | 154 | Mean Difference (IV, Random, 95% CI) | ‐3.20 [‐6.71, 0.31] |
| 10 Change in YMRS from baseline to end of trial | 1 | 462 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.28, 0.62] |
| 10.1 YMRS > or = 50% by day 28 | 1 | 154 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.21, 0.85] |
| 10.2 YMRS < or = 12 AND MADRS < or = 8 at day 28 | 1 | 154 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.20, 0.76] |
| 10.3 YMRS < or = 8 at day 28 | 1 | 154 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.23, 0.86] |
| 11 Use of sleep medications over the whole study | 2 | 359 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.78, 1.87] |
| 12 Use of lorazepam over the whole study | 1 | 154 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.27, 2.64] |
| 13 Use of anticholinergics over the whole study | 2 | 359 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.61, 2.30] |
5.10. Analysis.
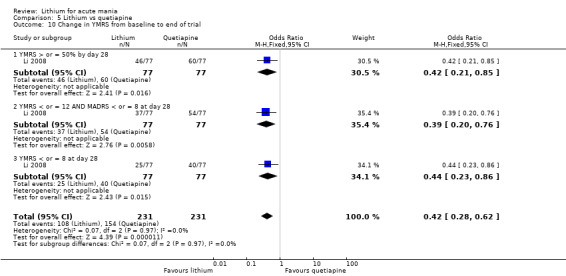
Comparison 5 Lithium vs quetiapine, Outcome 10 Change in YMRS from baseline to end of trial.
5.12. Analysis.
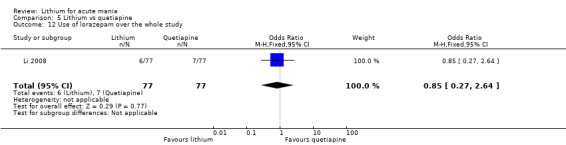
Comparison 5 Lithium vs quetiapine, Outcome 12 Use of lorazepam over the whole study.
Comparison 6. Lithium vs olanzapine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Efficacy ‐ response (categorical): MSRS/YMRS ≥ 50% decrease in score by end of trial | 2 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.20, 0.94] |
| 2 Efficacy ‐ response (continuous): change in CGI baseline to end of trial | 2 | 170 | Mean Difference (IV, Fixed, 95% CI) | 0.58 [0.52, 0.64] |
| 3 Efficacy ‐ response (continuous): CGI severity score at end of trial | 3 | 210 | Mean Difference (IV, Random, 95% CI) | 0.35 [‐0.04, 0.74] |
| 4 Efficacy ‐ remission (categorical): YMRS ≤ 12 at end of trial | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.89, 4.46] |
| 5 Total withdrawal | 3 | 210 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.60 [1.13, 5.99] |
| 6 Withdrawal due to lack of efficacy | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.38, 7.26] |
| 7 Withdrawal due to adverse event | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.96 [0.12, 73.85] |
| 8 Change in BPRS from baseline to end of trial | 1 | 140 | Mean Difference (IV, Random, 95% CI) | 2.12 [1.87, 2.37] |
| 9 Change in CGI‐BP from baseline to end of trial | 1 | 140 | Mean Difference (IV, Random, 95% CI) | 0.03 [0.01, 0.05] |
| 10 Change in MADRS from baseline to end of trial | 1 | 140 | Mean Difference (IV, Random, 95% CI) | 0.75 [0.60, 0.90] |
| 11 Concomitant medication: benzodiazepine use | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.63, 4.35] |
| 12 Concomitant medication: mean daily dose of lorazepam | 1 | 140 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.06, 0.30] |
| 13 Concomitant medication: anticholinergic use | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.46, 3.38] |
| 14 Adverse event: tremor | 1 | 46 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.06, 1.99] |
6.12. Analysis.
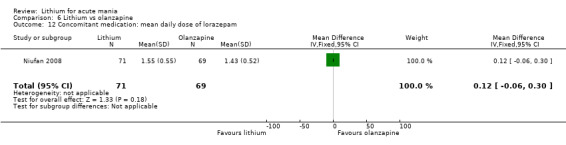
Comparison 6 Lithium vs olanzapine, Outcome 12 Concomitant medication: mean daily dose of lorazepam.
6.13. Analysis.
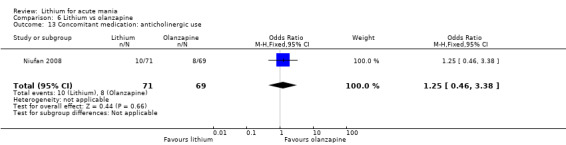
Comparison 6 Lithium vs olanzapine, Outcome 13 Concomitant medication: anticholinergic use.
6.14. Analysis.
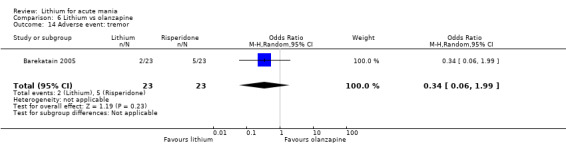
Comparison 6 Lithium vs olanzapine, Outcome 14 Adverse event: tremor.
Comparison 7. Lithium vs chlorpromazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Efficacy ‐ response (continuous): BPRS score change from baseline to end of trial | 2 | 284 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.75, 0.57] |
| 2 Efficacy ‐ response (continuous): PES change from baseline to end of trial | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Efficacy ‐ response (categorical): ≥ 50% reduction on BPRS from baseline to end of trial | 1 | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.25, 63.95] |
| 4 Acceptability: total withdrawals | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Withdrawal (quasi‐randomised study removed) | 1 | 248 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.88, 3.24] |
| 4.2 Total Withdrawals | 2 | 262 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.92, 3.31] |
Comparison 8. Lithium vs haloperidol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Efficacy ‐ response (continuous): change in BPRS (total) from baseline to end of trial | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Sensitivity analysis ‐ removal of Garfinek 1980 | 2 | 66 | Mean Difference (IV, Random, 95% CI) | 0.82 [‐1.38, 3.02] |
| 1.2 All data | 3 | 80 | Mean Difference (IV, Random, 95% CI) | ‐2.40 [‐6.31, 1.50] |
| 2 Efficacy ‐ response (continuous): CGI change from baseline to end of trial | 2 | 50 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Acceptability: change in side effect scores from baseline to end of trial | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐2.05, 1.65] |
| 4 Total withdrawal | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.03, 3.12] |
| 5 Concomitant medication: use of orphenadrine | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.94] |
| 6 Concomitant medication: mean total lorazepam (mg) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. Lithium vs zuclopenthixol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Efficacy ‐ response (categorical): BMRS change ≥ 50% from baseline to end of trial | 1 | 28 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.30, 5.91] |
| 2 Total withdrawal | 1 | 28 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.17, 3.49] |
| 3 Mean dose (mg) of extra clonazepam | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Adverse event rating scale psychic | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Adverse event rating scale neurological | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Adverse event rating scale autonomic | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Adverse event rating scale other | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 10. Lithium vs risperidone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Efficacy ‐ response (continuous): YMRS/MRS change at the end of the study | 3 | 241 | Mean Difference (IV, Fixed, 95% CI) | 7.28 [5.22, 9.34] |
| 2 Efficacy ‐ remission (categorical): YMRS < 12/ absence of DSM‐IV mania by end of trial | 2 | 211 | Odds Ratio (M‐H, Random, 95% CI) | 1.30 [0.11, 14.95] |
| 3 Efficacy ‐ response (continuous): CGI change from baseline to end of trial | 2 | 62 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [0.39, 1.41] |
| 4 Drowsiness/ somnolence | 2 | 219 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.24, 0.75] |
| 5 Diarrhoea | 2 | 219 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.14 [1.12, 15.26] |
| 6 Nausea | 2 | 219 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.49 [1.32, 4.69] |
| 7 Drowsiness/ somnolence | 1 | 46 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.27, 3.73] |
| 8 Total withdrawal | 3 | 255 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.02, 3.34] |
| 9 Vomiting | 2 | 219 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.80 [1.22, 6.42] |
| 10 Withdrawal due to adverse effects | 1 | 179 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.80 [0.72, 10.91] |
| 11 CGI‐BP‐IM response | 1 | 179 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.14, 0.47] |
| 12 Appetite increase | 1 | 173 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.16, 0.59] |
| 13 Constipation | 1 | 173 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.11, 1.74] |
| 14 Frequent urination | 2 | 219 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.29 [2.12, 13.21] |
| 15 Weight gain | 1 | 173 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.03, 0.32] |
| 16 Dry mouth | 1 | 173 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.42 [1.02, 5.75] |
| 17 Abdominal Pain | 1 | 173 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.98 [1.91, 8.27] |
| 18 Concomitant medication: mean total lorazepam (mg) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Concomitant medication: use of orphenadrine | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 3.96] |
10.7. Analysis.
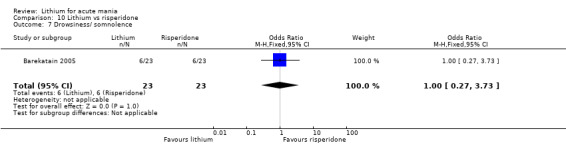
Comparison 10 Lithium vs risperidone, Outcome 7 Drowsiness/ somnolence.
10.11. Analysis.
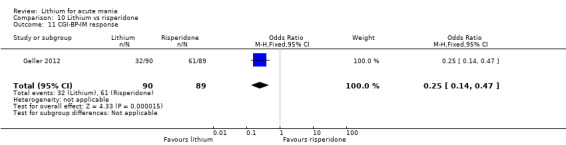
Comparison 10 Lithium vs risperidone, Outcome 11 CGI‐BP‐IM response.
10.13. Analysis.
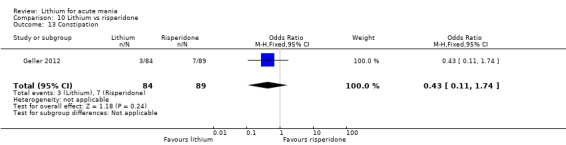
Comparison 10 Lithium vs risperidone, Outcome 13 Constipation.
Comparison 11. Lithium vs aripiprazole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Efficacy ‐ response (continuous): YMRS change by ≥ 50% at end of trial | 1 | 309 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.62, 1.51] |
| 2 Efficacy ‐ response (continuous): CGI BP change from baseline to end of trial | 1 | 309 | Mean Difference (IV, Random, 95% CI) | 0.20 [0.18, 0.22] |
| 3 Efficacy ‐ remission (categorical): YMRS < 12 at end of trial | 1 | 309 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.63, 1.56] |
| 4 PANSS change from baseline to end of trial | 1 | 268 | Mean Difference (IV, Random, 95% CI) | 2.5 [2.16, 2.84] |
| 5 Total withdrawal | 1 | 315 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.60, 1.46] |
| 6 CGI‐BP severity of illness score change from baseline to end of trial | 1 | 307 | Mean Difference (IV, Random, 95% CI) | 0.30 [0.28, 0.32] |
| 7 MADRS change from baseline to end of trial | 1 | 309 | Mean Difference (IV, Random, 95% CI) | 1.0 [0.87, 1.13] |
| 8 CGI BP depression change from baseline to end of trial | 1 | 307 | Mean Difference (IV, Random, 95% CI) | 0.10 [0.08, 0.12] |
| 9 Weight | 1 | 313 | Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐0.89, ‐0.67] |
| 10 Akathisia | 1 | 313 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.18, 1.02] |
| 11 Constipation | 1 | 313 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.50, 2.13] |
| 12 Headache | 1 | 313 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.48, 1.41] |
| 13 Nausea | 1 | 313 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.61, 1.75] |
| 14 Somnolence/ sedation | 1 | 313 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.26, 1.23] |
| 15 Tremor | 1 | 313 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.65, 3.24] |
| 16 EPSE | 1 | 313 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.34, 1.07] |
| 17 Clinically relevant (> 7%) weight gain | 1 | 184 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.18, 23.21] |
| 18 Simpson Angus scale EPS LOCF | 1 | 313 | Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.54, ‐0.48] |
| 19 BARS score | 1 | 313 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.18, ‐0.16] |
| 20 Withdrawal due to lack of efficacy | 1 | 315 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.15 [1.42, 6.96] |
| 21 Withdrawal due to adverse events | 1 | 315 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.56] |
| 22 Concomitant use of anxiolytics | 1 | 315 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.34, 1.53] |
| 23 Concomitant use of analgesics/antipyretics | 1 | 315 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.66, 1.67] |
| 24 Concomitant use of anticholinergics | 1 | 315 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.17, 0.69] |
11.6. Analysis.
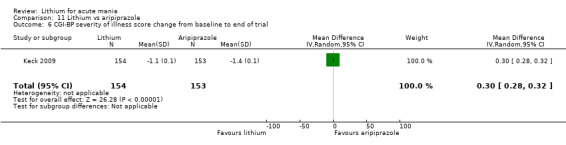
Comparison 11 Lithium vs aripiprazole, Outcome 6 CGI‐BP severity of illness score change from baseline to end of trial.
11.7. Analysis.
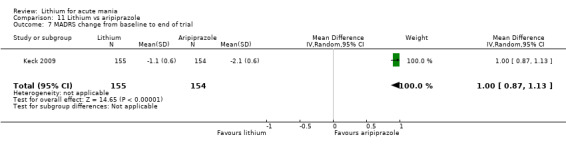
Comparison 11 Lithium vs aripiprazole, Outcome 7 MADRS change from baseline to end of trial.
11.8. Analysis.
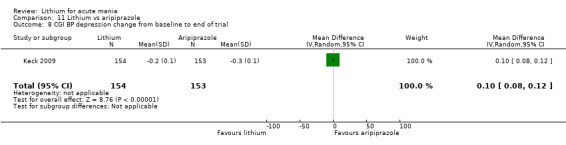
Comparison 11 Lithium vs aripiprazole, Outcome 8 CGI BP depression change from baseline to end of trial.
11.9. Analysis.
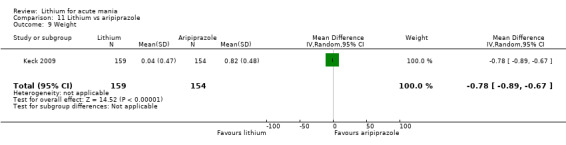
Comparison 11 Lithium vs aripiprazole, Outcome 9 Weight.
11.18. Analysis.
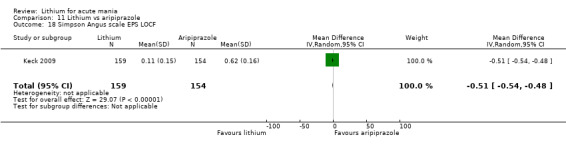
Comparison 11 Lithium vs aripiprazole, Outcome 18 Simpson Angus scale EPS LOCF.
11.19. Analysis.
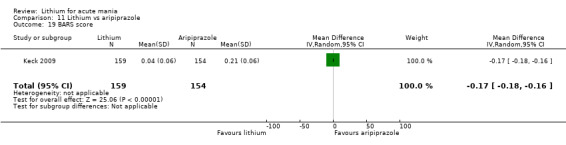
Comparison 11 Lithium vs aripiprazole, Outcome 19 BARS score.
11.21. Analysis.
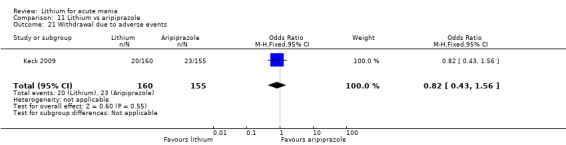
Comparison 11 Lithium vs aripiprazole, Outcome 21 Withdrawal due to adverse events.
11.23. Analysis.
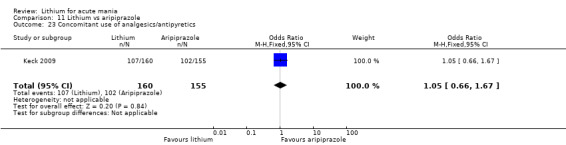
Comparison 11 Lithium vs aripiprazole, Outcome 23 Concomitant use of analgesics/antipyretics.
Comparison 12. Lithium vs topiramate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Efficacy ‐ response (categorical): YMRS change ≥ 50% from baseline to end of trial | 1 | 660 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.28 [1.63, 3.20] |
| 2 Efficacy ‐ remission (categorical): YMRS ≤ 12 at end of trial | 1 | 660 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.24 [1.58, 3.15] |
| 3 Treatment emergent depression | 1 | 883 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.42, 1.31] |
| 4 DSM‐IV responders at day 21 | 1 | 660 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.28, 2.56] |
| 5 Mania exacerbation ≥ YMRS 10% | 1 | 660 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.31, 0.84] |
| 6 Suicidal ideation | 1 | 883 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.02, 5.94] |
| 7 Suicide attempt | 1 | 883 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.03, 12.03] |
12.3. Analysis.
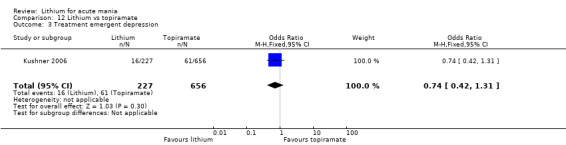
Comparison 12 Lithium vs topiramate, Outcome 3 Treatment emergent depression.
12.4. Analysis.
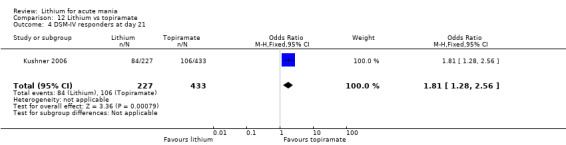
Comparison 12 Lithium vs topiramate, Outcome 4 DSM‐IV responders at day 21.
12.5. Analysis.
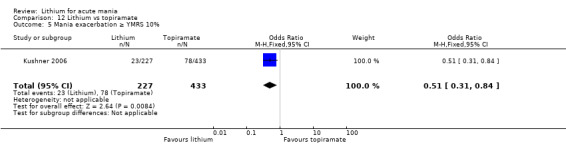
Comparison 12 Lithium vs topiramate, Outcome 5 Mania exacerbation ≥ YMRS 10%.
12.6. Analysis.
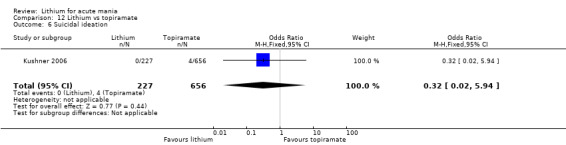
Comparison 12 Lithium vs topiramate, Outcome 6 Suicidal ideation.
12.7. Analysis.
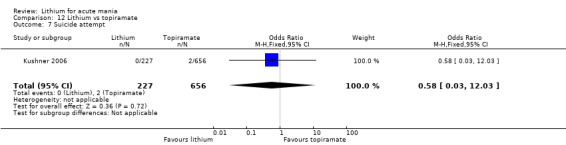
Comparison 12 Lithium vs topiramate, Outcome 7 Suicide attempt.
Comparison 13. Lithium vs clonazepam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Efficacy ‐ response (continuous): change in CGI score from baseline to end of study | 2 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐1.46, 0.65] |
| 2 Efficacy ‐ response (continuous): change in CGI score from baseline to end of study [double blind only] | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 Change in mania rating from baseline to end of trial | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐9.12, 5.72] |
| 4 Change in BPRS from baseline to end of trial | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Day 3 | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐4.4 [‐10.10, 1.30] |
| 4.2 Day 28 | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.52 [‐6.68, 3.64] |
| 5 Change in IMPS from baseline to end of trial | 1 | 55 | Mean Difference (IV, Random, 95% CI) | 0.66 [‐0.10, 1.42] |
| 5.1 Motor activity | 1 | 11 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐0.42, 2.42] |
| 5.2 Elevated mood | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.96, 1.76] |
| 5.3 Pressure of speech | 1 | 11 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐1.10, 2.30] |
| 5.4 Logorrhoea | 1 | 11 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐0.98, 2.38] |
| 5.5 Insight | 1 | 11 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐1.11, 2.91] |
| 6 Change in GAF from baseline to end of trial | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐10.29 [‐26.28, 5.70] |
| 7 Simpson Angus scale change from baseline to end of trial | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.83, 0.49] |
| 8 Use of rescue medications (haloperidol) | 1 | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.27, 60.32] |
13.4. Analysis.
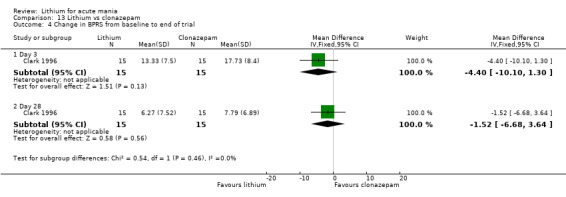
Comparison 13 Lithium vs clonazepam, Outcome 4 Change in BPRS from baseline to end of trial.
13.6. Analysis.
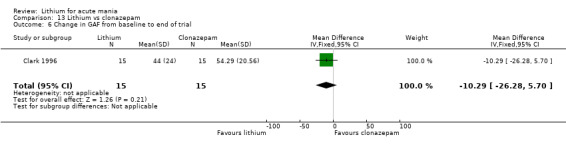
Comparison 13 Lithium vs clonazepam, Outcome 6 Change in GAF from baseline to end of trial.
Comparison 14. Lithium vs ECT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Efficacy ‐ response (continuous): MRS mean change from baseline to end of trial | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Efficacy ‐ response (continuous): BPRS mean change from baseline to end of trial | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Efficacy ‐ response (continuous): CGI mean change from baseline to end of trial | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Efficacy ‐ response (continuous): GAS mean change from baseline to end of trial | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Efficacy ‐ response (continuous): Hamilton Depression Scale mean change from baseline to end of trial | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 15. Lithium vs all antimanic agents.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Efficacy ‐ response (categorical): YMRS/MRS/BPRS change by ≥ 50% at end of trial | 14 | 3666 | Odds Ratio (M‐H, Random, 95% CI) | 1.36 [1.01, 1.83] |
| 2 Efficacy ‐ response (continuous): YMRS/BPRS change from baseline to end of trial | 18 | 2231 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.45, 0.85] |
| 3 Acceptability ‐ total withdrawals | 21 | 3379 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.79, 1.36] |
Comparison 16. Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinically significant increases in TSH | 2 | 257 | Odds Ratio (M‐H, Fixed, 95% CI) | 16.05 [2.06, 124.98] |
| 2 Any serious adverse event | 4 | 723 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.39, 1.67] |
| 3 Attempted suicide | 2 | 811 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.10] |
| 4 Accidental injury | 2 | 286 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.39, 3.42] |
| 5 Any adverse event | 3 | 396 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.59, 2.21] |
| 6 Anorexia | 2 | 276 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.85, 7.12] |
| 7 Infection | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.19, 5.05] |
| 8 Weight loss | 1 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.26 [0.74, 53.01] |
| 9 Agitation | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 1.31] |
| 10 Back pain | 1 | 131 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.01, 4.18] |
| 11 Convulsions | 1 | 131 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.07 [0.32, 202.74] |
| 12 Dyspepsia | 1 | 131 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.49, 7.00] |
| 13 AIMS score | 1 | 323 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.14, ‐0.10] |
| 14 Emotional lability | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.10] |
| 15 Influenza | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.19, 5.05] |
| 16 Psychotic disorder | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.24, 9.24] |
| 17 Suicidal ideation | 1 | 656 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.01, 5.21] |
| 18 Simpson Angus scale EPS LOCF | 1 | 323 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.07, ‐0.01] |
| 19 Motor dysfunction | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.03, 3.15] |
| 20 Leukopenia | 1 | 131 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.03, 21.67] |
| 21 Aggression/ hostility | 1 | 356 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.67] |
| 22 BARS score | 1 | 323 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 23 Gastroenteritis | 1 | 356 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.19 [0.13, 78.87] |
| 24 Increased appetite | 1 | 356 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.31 [0.48, 38.93] |
| 25 Hyperprolactinaemia > 20 mcg for men and > 30 mcg in women | 1 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.31, 3.20] |
| 26 Change in thyrotropin in mIU/L | 1 | 81 | Mean Difference (IV, Random, 95% CI) | 3.1 [2.05, 4.15] |
| 27 Arthralgia | 1 | 131 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.12, 15.12] |
| 28 Dysarthria | 1 | 356 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.14 [0.39, 11.85] |
| 29 Twitching | 1 | 105 | Odds Ratio (M‐H, Fixed, 95% CI) | 14.52 [0.73, 289.29] |
| 30 Exacerbation of cough | 1 | 131 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.07 [0.32, 202.74] |
| 31 Rhinitis | 1 | 131 | Odds Ratio (M‐H, Fixed, 95% CI) | 33.35 [1.79, 620.15] |
| 32 Pruritus | 1 | 131 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.15, 3.74] |
16.2. Analysis.
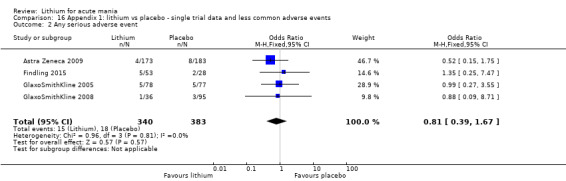
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 2 Any serious adverse event.
16.3. Analysis.
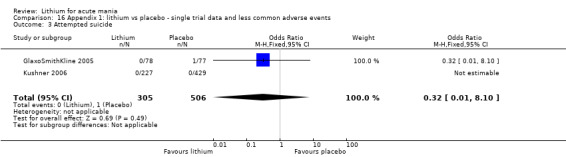
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 3 Attempted suicide.
16.4. Analysis.
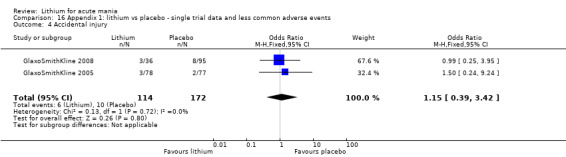
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 4 Accidental injury.
16.5. Analysis.
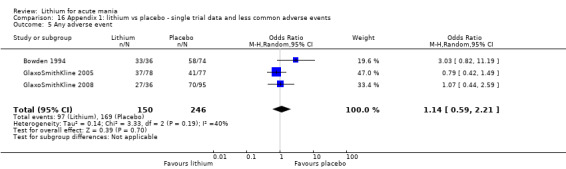
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 5 Any adverse event.
16.6. Analysis.
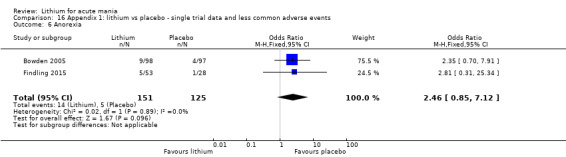
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 6 Anorexia.
16.7. Analysis.
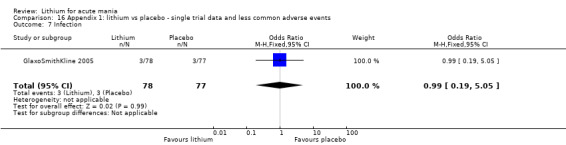
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 7 Infection.
16.8. Analysis.
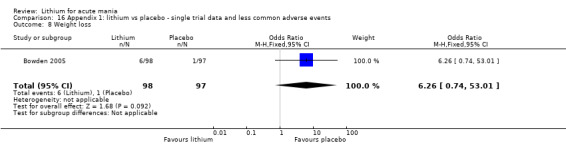
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 8 Weight loss.
16.9. Analysis.
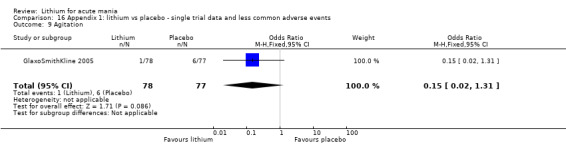
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 9 Agitation.
16.10. Analysis.
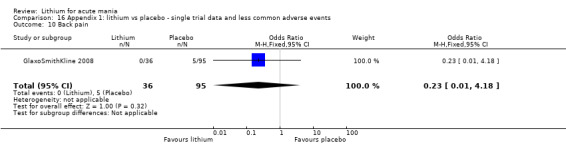
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 10 Back pain.
16.11. Analysis.
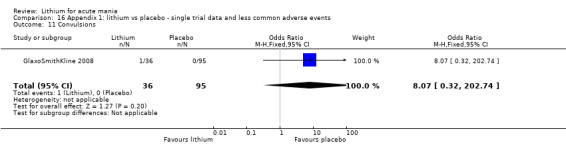
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 11 Convulsions.
16.12. Analysis.
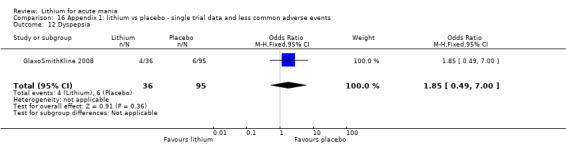
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 12 Dyspepsia.
16.13. Analysis.
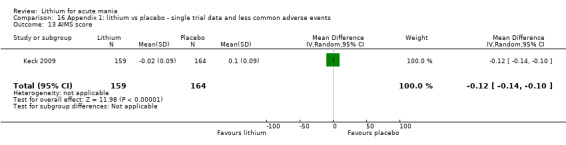
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 13 AIMS score.
16.14. Analysis.
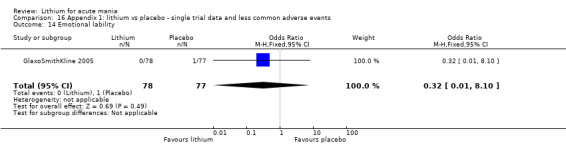
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 14 Emotional lability.
16.15. Analysis.
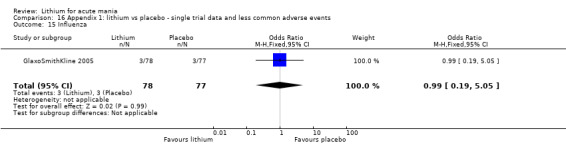
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 15 Influenza.
16.16. Analysis.
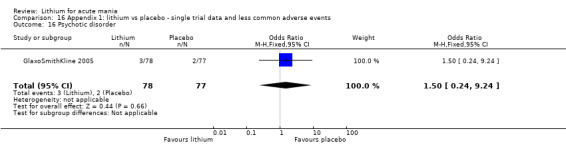
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 16 Psychotic disorder.
16.17. Analysis.
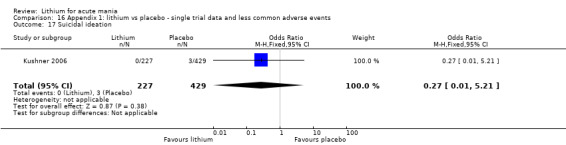
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 17 Suicidal ideation.
16.18. Analysis.
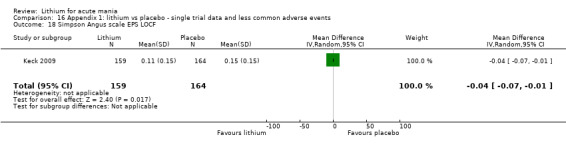
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 18 Simpson Angus scale EPS LOCF.
16.19. Analysis.
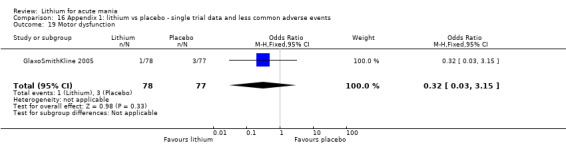
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 19 Motor dysfunction.
16.20. Analysis.
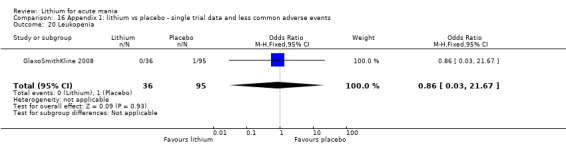
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 20 Leukopenia.
16.21. Analysis.
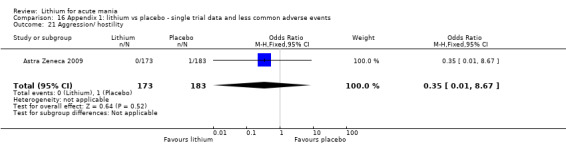
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 21 Aggression/ hostility.
16.22. Analysis.
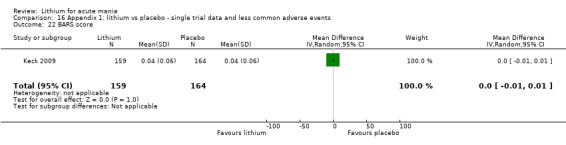
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 22 BARS score.
16.23. Analysis.
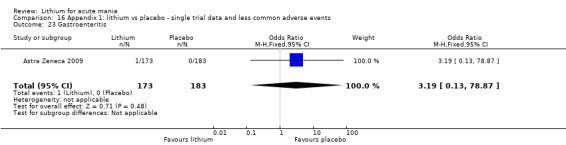
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 23 Gastroenteritis.
16.24. Analysis.
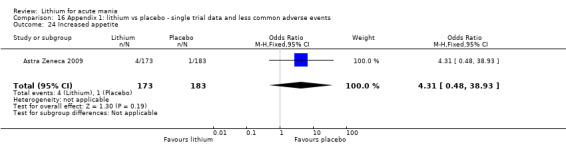
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 24 Increased appetite.
16.25. Analysis.
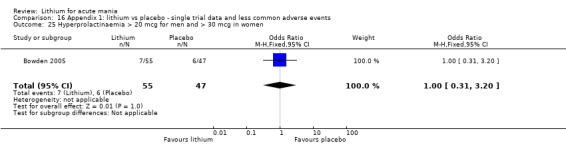
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 25 Hyperprolactinaemia > 20 mcg for men and > 30 mcg in women.
16.26. Analysis.
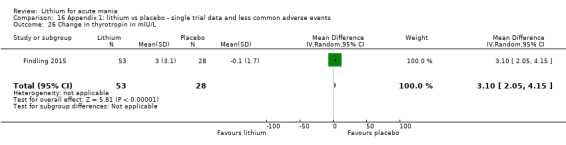
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 26 Change in thyrotropin in mIU/L.
16.27. Analysis.
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 27 Arthralgia.
16.28. Analysis.
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 28 Dysarthria.
16.29. Analysis.
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 29 Twitching.
16.30. Analysis.
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 30 Exacerbation of cough.
16.31. Analysis.
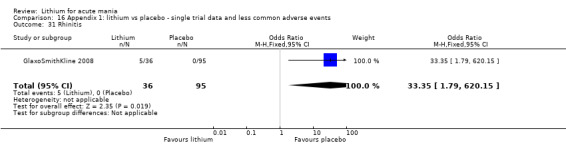
Comparison 16 Appendix 1: lithium vs placebo ‐ single trial data and less common adverse events, Outcome 31 Rhinitis.
Comparison 17. Appendix 2: lithium vs valproate ‐ single trial data and less common adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in weight (in kg) | 1 | 268 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.65, ‐0.15] |
| 2 Weight gain greater than 7% | 1 | 181 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.56] |
| 3 Change in aspartate aminotransferase IU/L | 1 | 268 | Mean Difference (IV, Random, 95% CI) | ‐5.1 [‐9.97, ‐0.23] |
| 4 Change in alanine aminotransferase IU/L | 1 | 268 | Mean Difference (IV, Random, 95% CI) | ‐2.90 [‐8.69, 2.89] |
| 5 Change in platelet count (x10^9/L) | 1 | 268 | Mean Difference (IV, Random, 95% CI) | 48.00 [31.20, 64.80] |
| 6 Fatigue | 1 | 181 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.58, 2.09] |
| 7 Constipation | 1 | 268 | Odds Ratio (M‐H, Fixed, 95% CI) | 14.89 [0.84, 263.32] |
| 8 Change in creatinine mcmol/L | 1 | 268 | Mean Difference (IV, Random, 95% CI) | 3.60 [0.57, 6.63] |
| 9 Thyroid function | 1 | 804 | Mean Difference (IV, Random, 95% CI) | 0.48 [0.11, 0.86] |
| 9.1 Change in TSH in mUI/L | 1 | 268 | Mean Difference (IV, Random, 95% CI) | 0.48 [0.09, 0.87] |
| 9.2 Change in T4 in pmol/L | 1 | 268 | Mean Difference (IV, Random, 95% CI) | ‐1.9 [‐6.88, 3.08] |
| 9.3 Change in T3 in pmol/L | 1 | 268 | Mean Difference (IV, Random, 95% CI) | 0.77 [‐0.79, 2.33] |
| 10 Change in glucose in mmol/L | 1 | 268 | Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.13, 0.65] |
| 11 Change in cholesterol in mmol/L | 1 | 268 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.20, 0.30] |
| 12 Change in triglycerides in mmol/L | 1 | 268 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.17, 0.35] |
| 13 Somnolence | 3 | 402 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.33, 1.50] |
| 14 Constipation | 1 | 268 | Odds Ratio (M‐H, Fixed, 95% CI) | 14.89 [0.84, 263.32] |
17.2. Analysis.
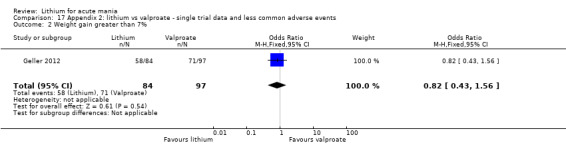
Comparison 17 Appendix 2: lithium vs valproate ‐ single trial data and less common adverse events, Outcome 2 Weight gain greater than 7%.
17.3. Analysis.
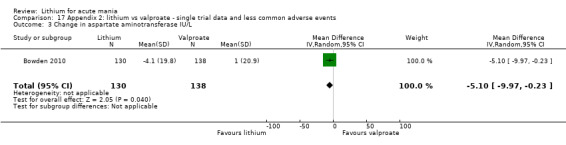
Comparison 17 Appendix 2: lithium vs valproate ‐ single trial data and less common adverse events, Outcome 3 Change in aspartate aminotransferase IU/L.
17.4. Analysis.
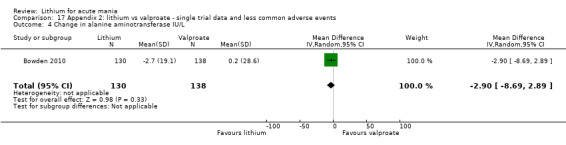
Comparison 17 Appendix 2: lithium vs valproate ‐ single trial data and less common adverse events, Outcome 4 Change in alanine aminotransferase IU/L.
17.5. Analysis.
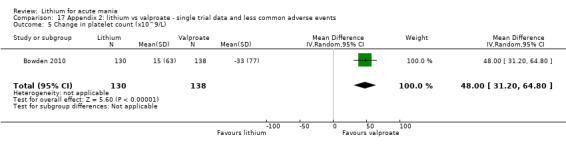
Comparison 17 Appendix 2: lithium vs valproate ‐ single trial data and less common adverse events, Outcome 5 Change in platelet count (x10^9/L).
17.6. Analysis.
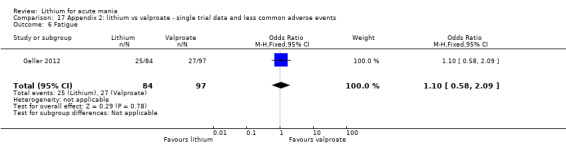
Comparison 17 Appendix 2: lithium vs valproate ‐ single trial data and less common adverse events, Outcome 6 Fatigue.
17.7. Analysis.
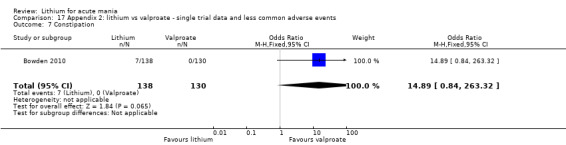
Comparison 17 Appendix 2: lithium vs valproate ‐ single trial data and less common adverse events, Outcome 7 Constipation.
17.8. Analysis.
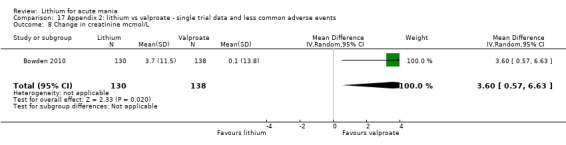
Comparison 17 Appendix 2: lithium vs valproate ‐ single trial data and less common adverse events, Outcome 8 Change in creatinine mcmol/L.
17.9. Analysis.
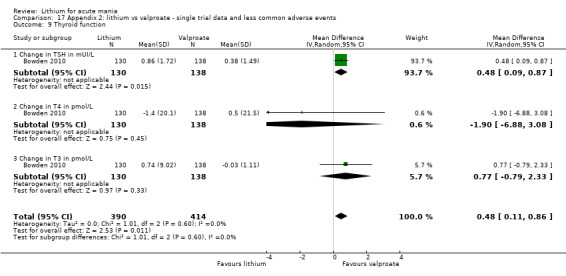
Comparison 17 Appendix 2: lithium vs valproate ‐ single trial data and less common adverse events, Outcome 9 Thyroid function.
17.10. Analysis.
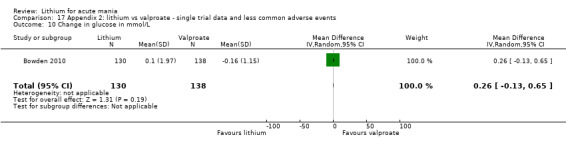
Comparison 17 Appendix 2: lithium vs valproate ‐ single trial data and less common adverse events, Outcome 10 Change in glucose in mmol/L.
17.11. Analysis.
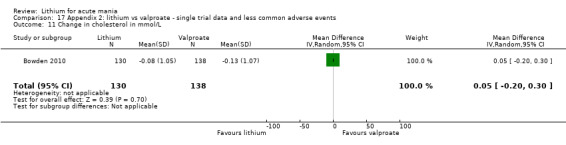
Comparison 17 Appendix 2: lithium vs valproate ‐ single trial data and less common adverse events, Outcome 11 Change in cholesterol in mmol/L.
17.12. Analysis.
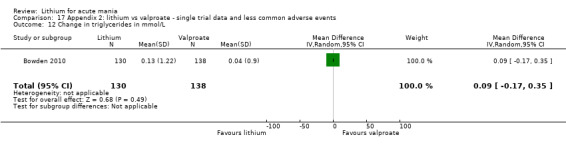
Comparison 17 Appendix 2: lithium vs valproate ‐ single trial data and less common adverse events, Outcome 12 Change in triglycerides in mmol/L.
17.13. Analysis.
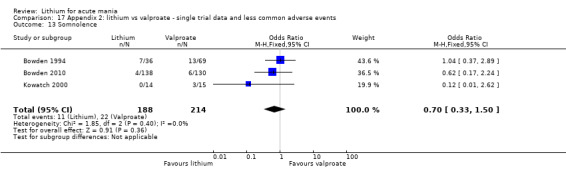
Comparison 17 Appendix 2: lithium vs valproate ‐ single trial data and less common adverse events, Outcome 13 Somnolence.
17.14. Analysis.
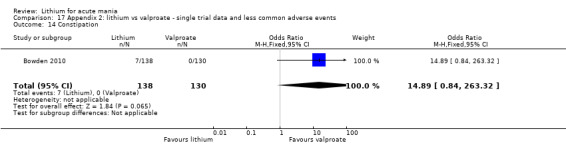
Comparison 17 Appendix 2: lithium vs valproate ‐ single trial data and less common adverse events, Outcome 14 Constipation.
Comparison 18. Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinically significant increases in TSH | 1 | 186 | Odds Ratio (M‐H, Fixed, 95% CI) | 17.92 [2.30, 139.36] |
| 2 Dry mouth | 1 | 205 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.08, 0.52] |
| 3 Somnolence | 1 | 205 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.18, 0.95] |
| 4 Akathisia | 1 | 205 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.35 [0.34, 32.73] |
| 5 Insomnia | 1 | 205 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.81, 4.40] |
| 6 URTI | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.28, 3.65] |
| 7 Headache | 1 | 205 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.67, 4.42] |
| 8 EPS | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.33, 4.98] |
| 9 ECG changes | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.50, 3.28] |
| 10 Asthenia | 1 | 205 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.17, 2.14] |
| 11 Constipation | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.13, 0.63] |
| 12 Depression | 2 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.09, 1.76] |
| 13 Cardiac disorders | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.07, 0.92] |
| 14 Weight loss | 1 | 205 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.42 [0.67, 17.38] |
| 15 Anorexia | 1 | 205 | Odds Ratio (M‐H, Fixed, 95% CI) | 10.72 [1.33, 86.24] |
| 16 Bone marrow depression | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.12, 76.74] |
| 17 Nausea | 1 | 205 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.91 [0.82, 58.48] |
| 18 Raised neutrophils > 10x109 /L | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.61 [0.51, 181.64] |
| 19 Deranged LFTs > 3x normal range | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.07, 2.07] |
| 20 Vomiting | 1 | 205 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.91 [0.82, 58.48] |
| 21 High blood glucose | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.20, 5.18] |
| 22 Hyperprolactinaemia > 20mcg for men and > 30mcg in women | 1 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.36, 3.65] |
| 23 GI disorder | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.37, 1.34] |
| 24 Nervous system disorders | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.39, 1.91] |
| 25 Infections and infestations | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.63, 3.29] |
| 26 Tremor | 1 | 205 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.79 [1.44, 9.99] |
| 27 Respiratory, thoracic and mediastinal disorders | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.02, 1.68] |
| 28 Skin and subcutaneous tissue disorders | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.28, 3.65] |
| 29 Eye disorders | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.23] |
| 30 Psychiatric disorders | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.40, 7.53] |
| 31 Metabolism and nutritional disorders | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.25, 9.49] |
| 32 MSK and connective tissue disorders | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.14, 7.38] |
| 33 Renal and urinary disorders | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.18] |
| 34 Reproductive system and breast disorders | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.18] |
| 35 Vascular disorders | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.04, 5.63] |
| 36 Blood and lymphatic disorders | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.20 [0.25, 110.07] |
| 37 Hepatobiliary disorders | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.12, 76.74] |
| 38 Injury, poisoning and procedural complications | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.12, 76.74] |
| 39 Weight gain | 2 | 360 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
18.2. Analysis.
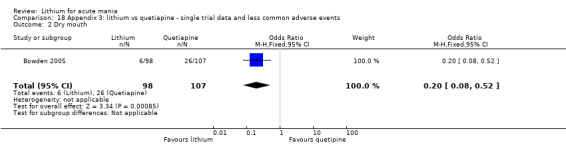
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 2 Dry mouth.
18.3. Analysis.
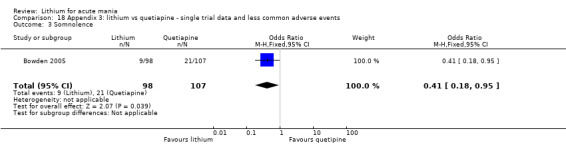
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 3 Somnolence.
18.4. Analysis.
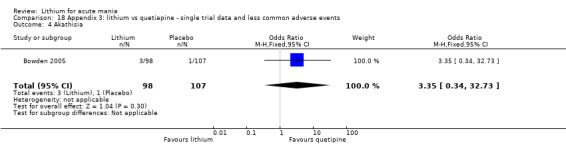
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 4 Akathisia.
18.5. Analysis.
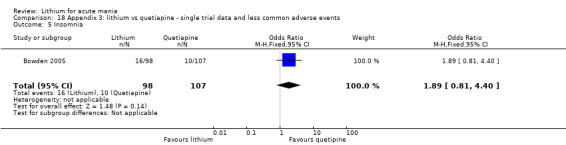
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 5 Insomnia.
18.6. Analysis.
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 6 URTI.
18.7. Analysis.
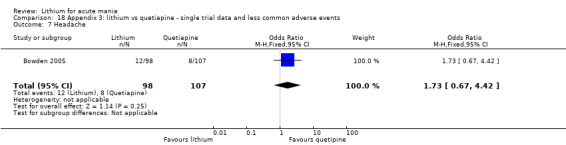
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 7 Headache.
18.8. Analysis.
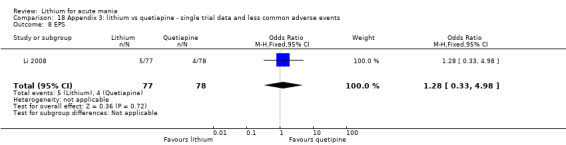
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 8 EPS.
18.9. Analysis.
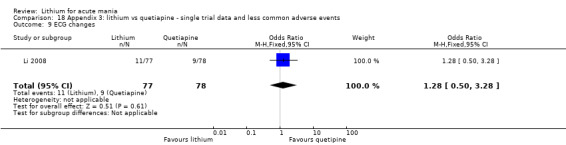
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 9 ECG changes.
18.10. Analysis.
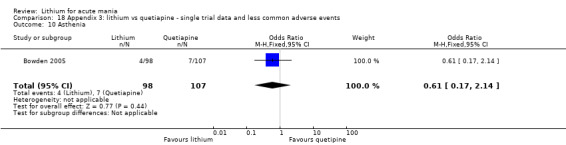
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 10 Asthenia.
18.11. Analysis.
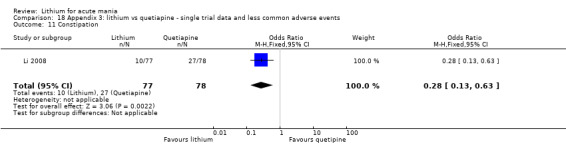
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 11 Constipation.
18.12. Analysis.
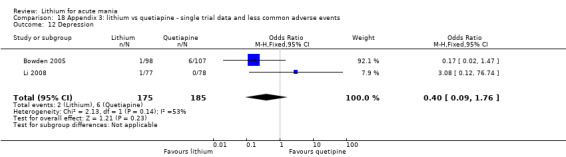
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 12 Depression.
18.13. Analysis.
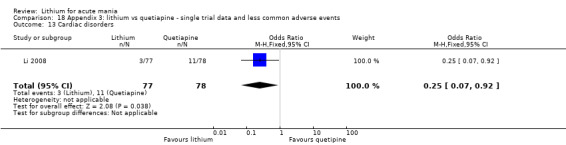
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 13 Cardiac disorders.
18.14. Analysis.
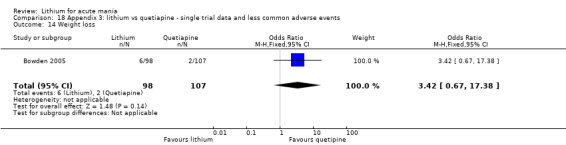
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 14 Weight loss.
18.15. Analysis.
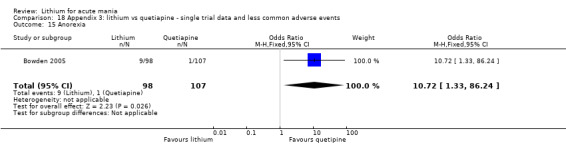
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 15 Anorexia.
18.16. Analysis.
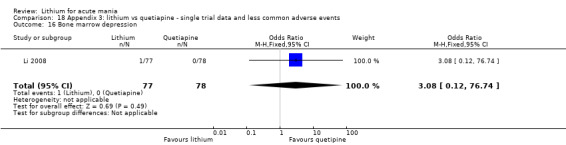
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 16 Bone marrow depression.
18.17. Analysis.
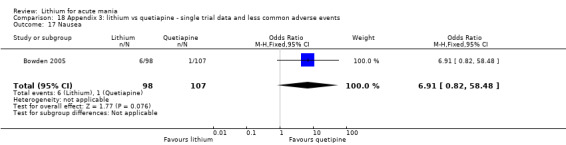
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 17 Nausea.
18.18. Analysis.
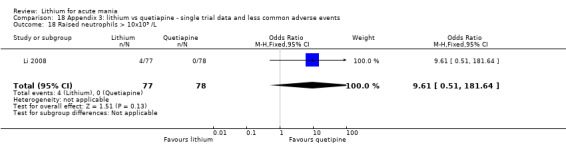
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 18 Raised neutrophils > 10x109 /L.
18.19. Analysis.
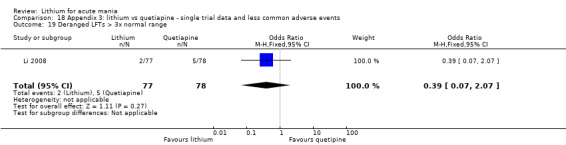
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 19 Deranged LFTs > 3x normal range.
18.20. Analysis.
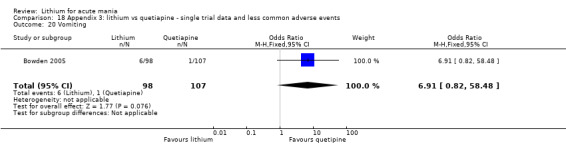
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 20 Vomiting.
18.21. Analysis.
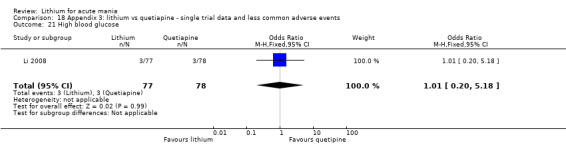
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 21 High blood glucose.
18.22. Analysis.
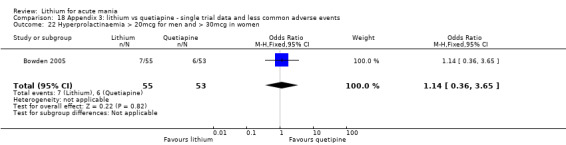
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 22 Hyperprolactinaemia > 20mcg for men and > 30mcg in women.
18.23. Analysis.
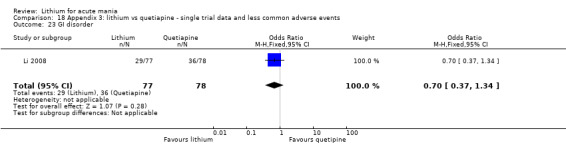
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 23 GI disorder.
18.24. Analysis.
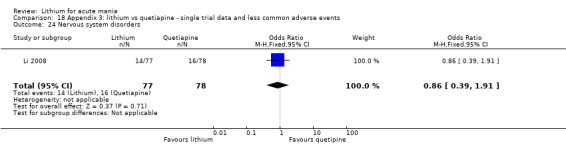
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 24 Nervous system disorders.
18.25. Analysis.
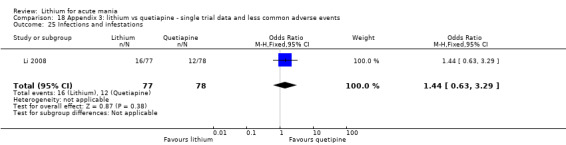
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 25 Infections and infestations.
18.26. Analysis.
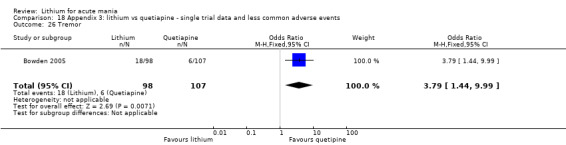
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 26 Tremor.
18.27. Analysis.
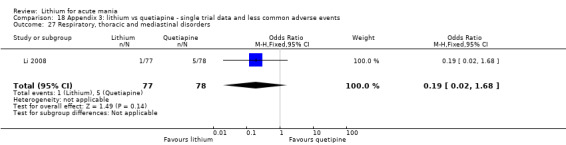
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 27 Respiratory, thoracic and mediastinal disorders.
18.28. Analysis.
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 28 Skin and subcutaneous tissue disorders.
18.29. Analysis.
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 29 Eye disorders.
18.30. Analysis.
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 30 Psychiatric disorders.
18.31. Analysis.
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 31 Metabolism and nutritional disorders.
18.32. Analysis.
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 32 MSK and connective tissue disorders.
18.33. Analysis.
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 33 Renal and urinary disorders.
18.34. Analysis.
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 34 Reproductive system and breast disorders.
18.35. Analysis.
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 35 Vascular disorders.
18.36. Analysis.
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 36 Blood and lymphatic disorders.
18.37. Analysis.
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 37 Hepatobiliary disorders.
18.38. Analysis.
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 38 Injury, poisoning and procedural complications.
18.39. Analysis.
Comparison 18 Appendix 3: lithium vs quetiapine ‐ single trial data and less common adverse events, Outcome 39 Weight gain.
Comparison 19. Appendix 4: lithium vs lamotrigine ‐ single trial data and less common adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Convulsions | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.14 [0.28, 179.53] |
| 2 Insomnia | 1 | 152 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.88] |
| 3 EPS | 1 | 152 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.17, 2.26] |
| 4 Dry Mouth | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.03, 19.16] |
| 5 Infection | 1 | 152 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.15, 3.24] |
| 6 Nausea | 1 | 152 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.92 [0.30, 28.72] |
| 7 Akathisia | 1 | 152 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.56] |
| 8 Dyspepsia | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.38, 5.02] |
| 9 Agitation | 1 | 152 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 1.25] |
| 10 Emotional lability | 1 | 152 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.56] |
| 11 Influenza | 1 | 152 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.92 [0.30, 28.72] |
| 12 Psychotic disorder | 1 | 152 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.23, 8.87] |
| 13 Motor dysfunction | 1 | 152 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.06, 15.44] |
| 14 Dizziness | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.55 [0.69, 9.42] |
| 15 Syncope | 1 | 152 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.78] |
| 16 Depression | 1 | 152 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.91] |
| 17 Arthralgia | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.05, 4.01] |
| 18 Back pain | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 3.68] |
| 19 Exacerbation of cough | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.05, 4.01] |
| 20 Rhinitis | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.23 [0.81, 12.80] |
| 21 Pruritus | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 12.25 [0.57, 261.73] |
| 22 Non fatal serious adverse events | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.08, 7.68] |
19.2. Analysis.
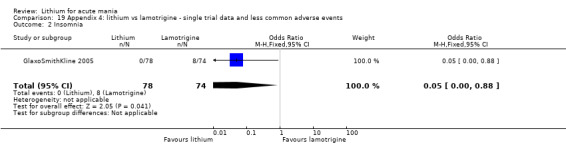
Comparison 19 Appendix 4: lithium vs lamotrigine ‐ single trial data and less common adverse events, Outcome 2 Insomnia.
19.3. Analysis.
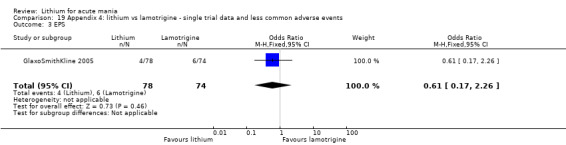
Comparison 19 Appendix 4: lithium vs lamotrigine ‐ single trial data and less common adverse events, Outcome 3 EPS.
19.4. Analysis.
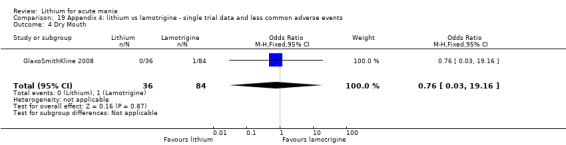
Comparison 19 Appendix 4: lithium vs lamotrigine ‐ single trial data and less common adverse events, Outcome 4 Dry Mouth.
19.5. Analysis.
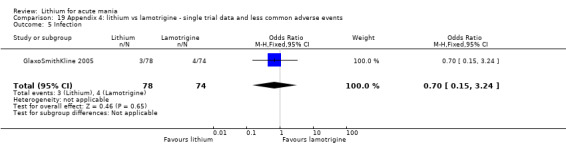
Comparison 19 Appendix 4: lithium vs lamotrigine ‐ single trial data and less common adverse events, Outcome 5 Infection.
19.6. Analysis.
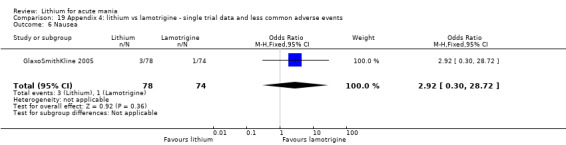
Comparison 19 Appendix 4: lithium vs lamotrigine ‐ single trial data and less common adverse events, Outcome 6 Nausea.
19.7. Analysis.
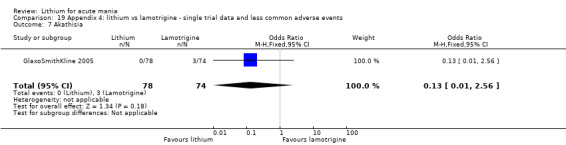
Comparison 19 Appendix 4: lithium vs lamotrigine ‐ single trial data and less common adverse events, Outcome 7 Akathisia.
19.8. Analysis.
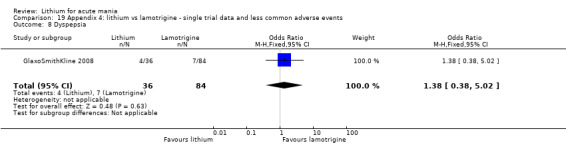
Comparison 19 Appendix 4: lithium vs lamotrigine ‐ single trial data and less common adverse events, Outcome 8 Dyspepsia.
19.9. Analysis.
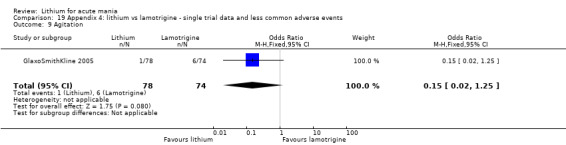
Comparison 19 Appendix 4: lithium vs lamotrigine ‐ single trial data and less common adverse events, Outcome 9 Agitation.
19.10. Analysis.
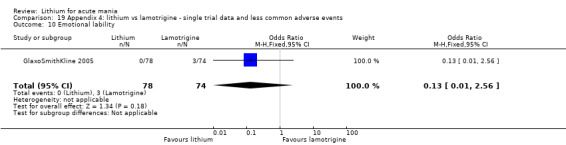
Comparison 19 Appendix 4: lithium vs lamotrigine ‐ single trial data and less common adverse events, Outcome 10 Emotional lability.
19.11. Analysis.
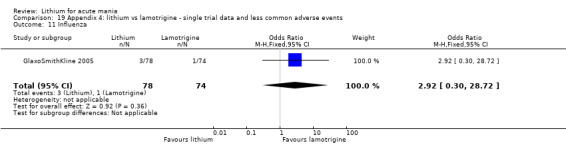
Comparison 19 Appendix 4: lithium vs lamotrigine ‐ single trial data and less common adverse events, Outcome 11 Influenza.
19.12. Analysis.
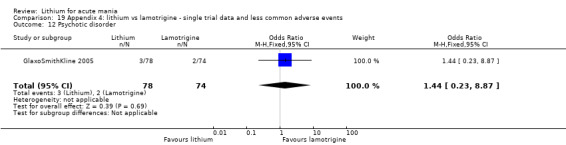
Comparison 19 Appendix 4: lithium vs lamotrigine ‐ single trial data and less common adverse events, Outcome 12 Psychotic disorder.
19.13. Analysis.
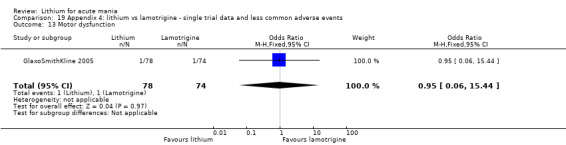
Comparison 19 Appendix 4: lithium vs lamotrigine ‐ single trial data and less common adverse events, Outcome 13 Motor dysfunction.
19.14. Analysis.
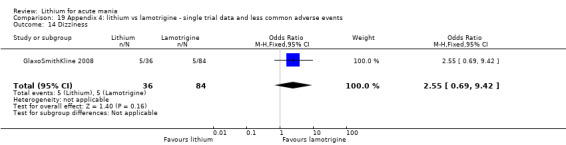
Comparison 19 Appendix 4: lithium vs lamotrigine ‐ single trial data and less common adverse events, Outcome 14 Dizziness.
19.15. Analysis.
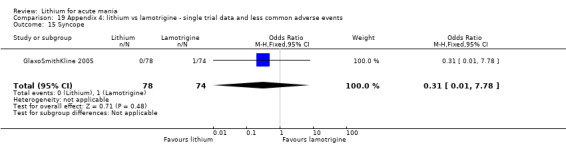
Comparison 19 Appendix 4: lithium vs lamotrigine ‐ single trial data and less common adverse events, Outcome 15 Syncope.
19.16. Analysis.
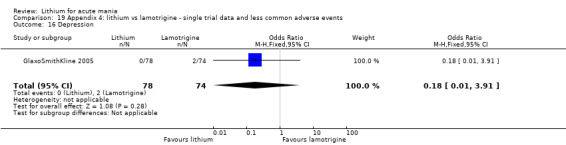
Comparison 19 Appendix 4: lithium vs lamotrigine ‐ single trial data and less common adverse events, Outcome 16 Depression.
19.17. Analysis.
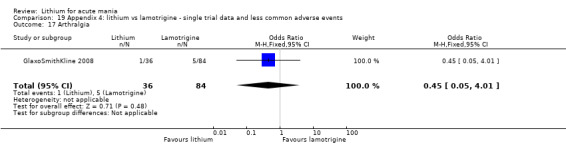
Comparison 19 Appendix 4: lithium vs lamotrigine ‐ single trial data and less common adverse events, Outcome 17 Arthralgia.
19.18. Analysis.
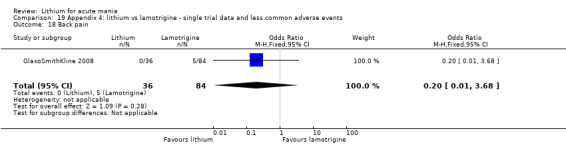
Comparison 19 Appendix 4: lithium vs lamotrigine ‐ single trial data and less common adverse events, Outcome 18 Back pain.
19.19. Analysis.
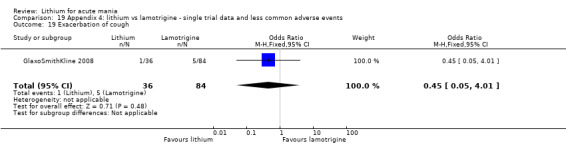
Comparison 19 Appendix 4: lithium vs lamotrigine ‐ single trial data and less common adverse events, Outcome 19 Exacerbation of cough.
19.20. Analysis.
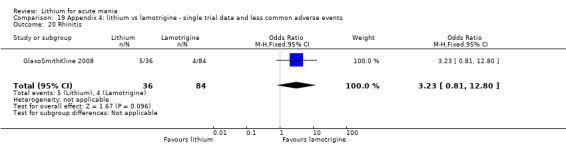
Comparison 19 Appendix 4: lithium vs lamotrigine ‐ single trial data and less common adverse events, Outcome 20 Rhinitis.
19.21. Analysis.
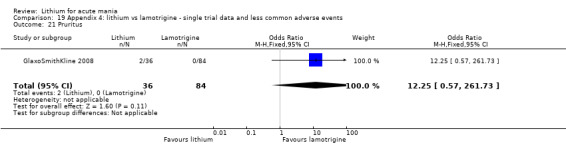
Comparison 19 Appendix 4: lithium vs lamotrigine ‐ single trial data and less common adverse events, Outcome 21 Pruritus.
19.22. Analysis.
Comparison 19 Appendix 4: lithium vs lamotrigine ‐ single trial data and less common adverse events, Outcome 22 Non fatal serious adverse events.
Comparison 20. Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tremor | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.40] |
| 2 Any adverse event | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.31, 1.16] |
| 3 Nausea | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.86 [0.59, 5.85] |
| 4 Constipation | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.08, 1.14] |
| 5 Nasopharyngitis | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.23, 4.04] |
| 6 Somnolence | 1 | 123 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.05, 1.46] |
| 7 Vomiting | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.15, 3.33] |
| 8 Diarrhoea | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.08, 2.66] |
| 9 Dizziness | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.08, 2.66] |
| 10 Cough | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.13, 7.09] |
| 11 Restlessness | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.93] |
| 12 Tachycardia | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.03, 3.10] |
| 13 Fatigue | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.62] |
| 14 Headache | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.40] |
| 15 Increased appetite | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.62] |
| 16 Tonsillitis | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.17, 22.25] |
| 17 URTI | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.17, 22.25] |
| 18 Dry Mouth | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.00] |
| 19 Gingivitis | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.24, 106.05] |
| 20 Haemorrhoids | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.00] |
| 21 Adverse effects possibly related to drug use | 1 | 123 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.12, 0.59] |
| 22 Metabolism and nutrition disorders | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.03, 2.13] |
| 23 Nervous system disorders | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.12, 1.36] |
| 24 Abnormal hepatic function | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.96 [0.12, 73.85] |
| 25 EPSE | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.17, 22.25] |
| 26 Significant high cholesterol | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.03, 3.10] |
| 27 Significant high glucose | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.97] |
| 28 Weight gain > 7% | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.14 [1.31, 28.83] |
20.2. Analysis.
Comparison 20 Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events, Outcome 2 Any adverse event.
20.3. Analysis.
Comparison 20 Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events, Outcome 3 Nausea.
20.4. Analysis.
Comparison 20 Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events, Outcome 4 Constipation.
20.5. Analysis.
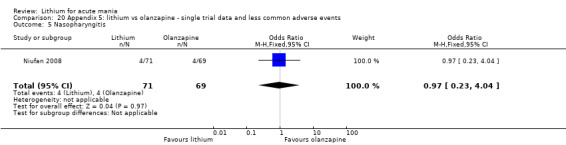
Comparison 20 Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events, Outcome 5 Nasopharyngitis.
20.6. Analysis.
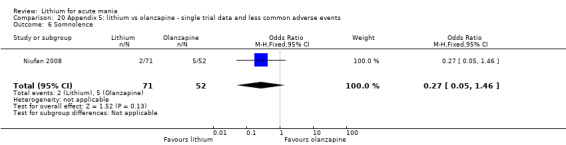
Comparison 20 Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events, Outcome 6 Somnolence.
20.7. Analysis.
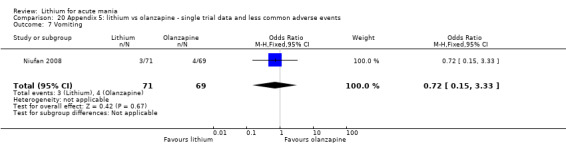
Comparison 20 Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events, Outcome 7 Vomiting.
20.8. Analysis.
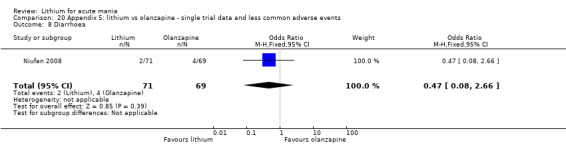
Comparison 20 Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events, Outcome 8 Diarrhoea.
20.9. Analysis.
Comparison 20 Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events, Outcome 9 Dizziness.
20.10. Analysis.
Comparison 20 Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events, Outcome 10 Cough.
20.11. Analysis.
Comparison 20 Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events, Outcome 11 Restlessness.
20.12. Analysis.
Comparison 20 Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events, Outcome 12 Tachycardia.
20.13. Analysis.
Comparison 20 Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events, Outcome 13 Fatigue.
20.14. Analysis.
Comparison 20 Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events, Outcome 14 Headache.
20.15. Analysis.
Comparison 20 Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events, Outcome 15 Increased appetite.
20.16. Analysis.
Comparison 20 Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events, Outcome 16 Tonsillitis.
20.17. Analysis.
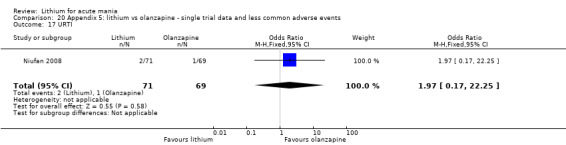
Comparison 20 Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events, Outcome 17 URTI.
20.18. Analysis.
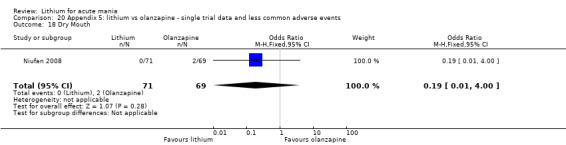
Comparison 20 Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events, Outcome 18 Dry Mouth.
20.19. Analysis.
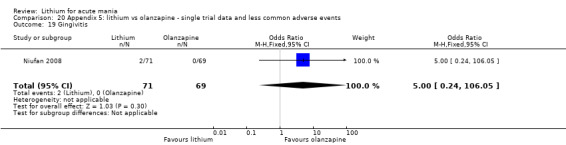
Comparison 20 Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events, Outcome 19 Gingivitis.
20.20. Analysis.
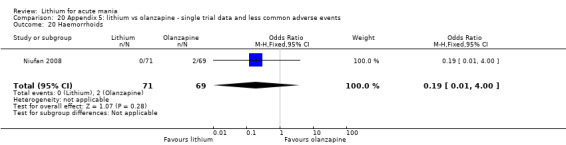
Comparison 20 Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events, Outcome 20 Haemorrhoids.
20.21. Analysis.
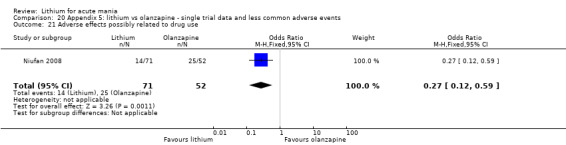
Comparison 20 Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events, Outcome 21 Adverse effects possibly related to drug use.
20.22. Analysis.
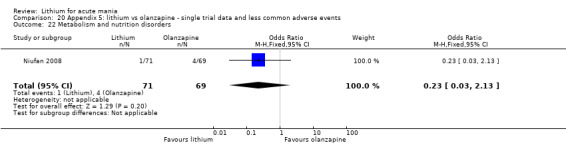
Comparison 20 Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events, Outcome 22 Metabolism and nutrition disorders.
20.23. Analysis.
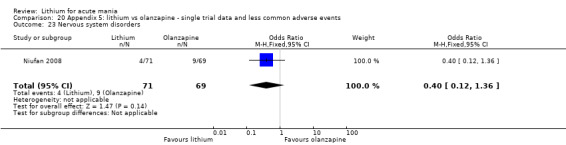
Comparison 20 Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events, Outcome 23 Nervous system disorders.
20.24. Analysis.
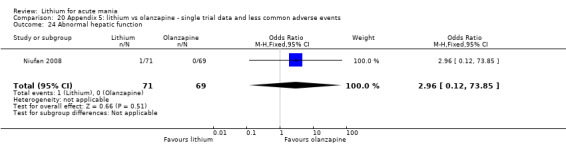
Comparison 20 Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events, Outcome 24 Abnormal hepatic function.
20.25. Analysis.
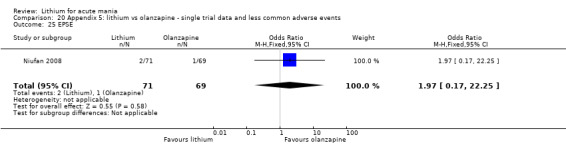
Comparison 20 Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events, Outcome 25 EPSE.
20.26. Analysis.
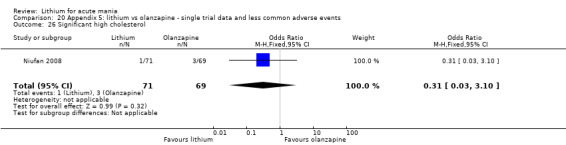
Comparison 20 Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events, Outcome 26 Significant high cholesterol.
20.27. Analysis.
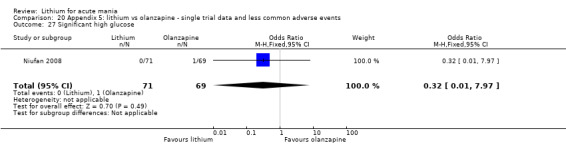
Comparison 20 Appendix 5: lithium vs olanzapine ‐ single trial data and less common adverse events, Outcome 27 Significant high glucose.
Comparison 21. Appendix 6: lithium vs risperidone ‐ single trial studies and less common adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ≥ 50% change in YMRS | 1 | 32 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.01, 0.49] |
| 2 YMRS ≤ 12 | 1 | 32 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.0 [1.10, 22.82] |
| 3 Tremor | 1 | 46 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.06, 1.99] |
| 4 Adverse events | 1 | 46 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.21, 2.28] |
| 5 Dizziness | 1 | 46 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.14, 3.61] |
| 6 Dyspepsia | 1 | 46 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.71 [0.66, 20.76] |
| 7 EPSE | 1 | 46 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.25] |
| 8 Proportion of patients requiring seclusion | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.41 [0.52, 11.10] |
| 9 AIMS score | 1 | 313 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.11, ‐0.07] |
| 10 YMRS change from baseline at week 3 | 1 | 309 | Mean Difference (IV, Random, 95% CI) | 0.60 [0.42, 0.78] |
21.1. Analysis.
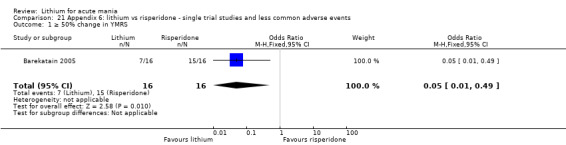
Comparison 21 Appendix 6: lithium vs risperidone ‐ single trial studies and less common adverse events, Outcome 1 ≥ 50% change in YMRS.
21.2. Analysis.
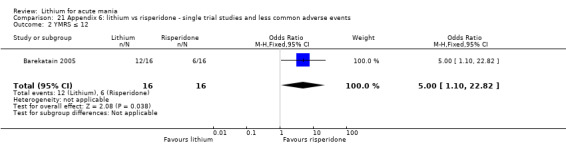
Comparison 21 Appendix 6: lithium vs risperidone ‐ single trial studies and less common adverse events, Outcome 2 YMRS ≤ 12.
21.3. Analysis.
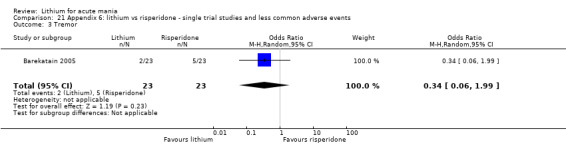
Comparison 21 Appendix 6: lithium vs risperidone ‐ single trial studies and less common adverse events, Outcome 3 Tremor.
21.4. Analysis.
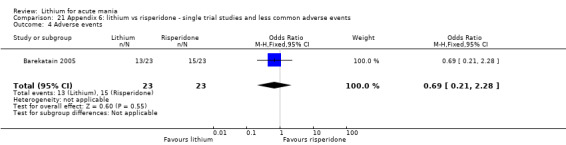
Comparison 21 Appendix 6: lithium vs risperidone ‐ single trial studies and less common adverse events, Outcome 4 Adverse events.
21.5. Analysis.
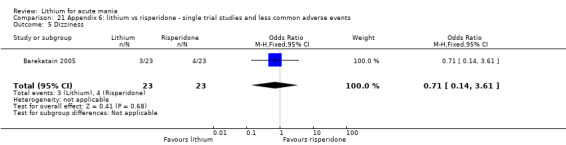
Comparison 21 Appendix 6: lithium vs risperidone ‐ single trial studies and less common adverse events, Outcome 5 Dizziness.
21.6. Analysis.
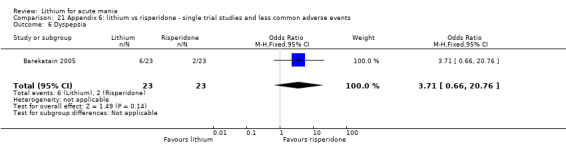
Comparison 21 Appendix 6: lithium vs risperidone ‐ single trial studies and less common adverse events, Outcome 6 Dyspepsia.
21.7. Analysis.
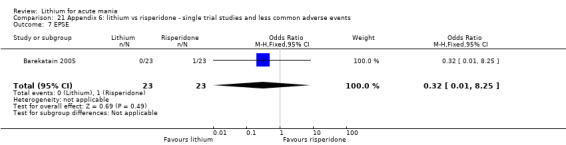
Comparison 21 Appendix 6: lithium vs risperidone ‐ single trial studies and less common adverse events, Outcome 7 EPSE.
21.8. Analysis.
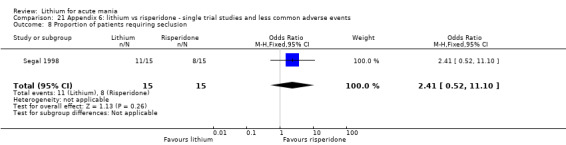
Comparison 21 Appendix 6: lithium vs risperidone ‐ single trial studies and less common adverse events, Outcome 8 Proportion of patients requiring seclusion.
21.9. Analysis.
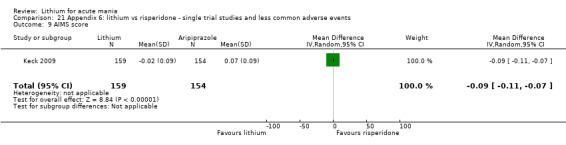
Comparison 21 Appendix 6: lithium vs risperidone ‐ single trial studies and less common adverse events, Outcome 9 AIMS score.
21.10. Analysis.
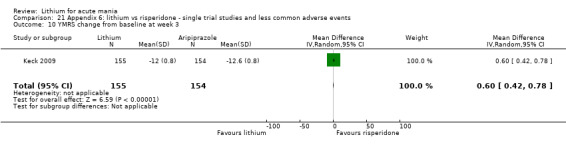
Comparison 21 Appendix 6: lithium vs risperidone ‐ single trial studies and less common adverse events, Outcome 10 YMRS change from baseline at week 3.
Comparison 22. Appendix 7: lithium vs chlorpromazine ‐ adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Toxicity | 1 | 118 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.36, 5.58] |
| 2 Intercurrent illness | 1 | 255 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.16, 3.37] |
| 3 Side effects total: mildly active group | 1 | 130 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.13, 0.92] |
| 4 Side effects total: highly active group | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.86, 4.56] |
| 5 Anorexia: mildly active group | 1 | 130 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.56 [0.21, 96.77] |
| 6 Anorexia: highly active group | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.24 [0.42, 162.91] |
| 7 Nausea: mildly active group | 1 | 130 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.56 [0.21, 96.77] |
| 8 Nausea: highly active group | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 10.78 [0.57, 204.68] |
| 9 Vomiting: mildly active group | 1 | 130 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.56 [0.21, 96.77] |
| 10 Vomiting: highly active group | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.78 [0.27, 122.94] |
| 11 Diarrhoea: highly active group | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.41 [0.14, 85.34] |
| 12 Dry mouth: mildly active group | 1 | 130 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.81] |
| 13 Dry mouth: highly active group | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.05, 6.25] |
| 14 Constipation: mildly active group | 1 | 130 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.01, 2.38] |
| 15 Constipation: highly active group | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.78 [0.27, 122.94] |
| 16 Abdominal pain: highly active group | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.07, 18.33] |
| 17 Muscle weakness: mildly active group | 1 | 130 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 7.26] |
| 18 Ataxia: highly active group | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.48 [0.35, 34.43] |
| 19 Tremor: mildly active group | 1 | 130 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.56 [0.21, 96.77] |
| 20 Tremor: highly active group | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.36 [0.86, 63.04] |
| 21 Facial twitching; highly active group | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.41 [0.14, 85.34] |
| 22 Parkinsonism: highly active group | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.41 [0.14, 85.34] |
| 23 Somnolence: mildly active group | 1 | 130 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.45] |
| 24 Somnolence; highly active group | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.13, 2.25] |
| 25 Confusion: mildly active group | 1 | 130 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.69 [0.40, 33.97] |
| 26 Confusion: highly active group | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.48 [0.35, 34.43] |
| 27 Slurred speech: mildly active group | 1 | 130 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.01, 2.38] |
| 28 Slurred speech: highly active group | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.07, 18.33] |
| 29 Blurred vision: mildly active group | 1 | 130 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 7.26] |
| 30 Dizziness: mildly active group | 1 | 130 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 3.64] |
| 31 Dizziness: highly active group | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.41 [0.14, 85.34] |
| 32 Seizures: highly active group | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.41 [0.14, 85.34] |
| 33 Hypotensive reaction: mildly active group | 1 | 130 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.01, 2.38] |
| 34 Hypotensive reaction: highly active group | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.01, 9.18] |
| 35 Pruritus: highly active group | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.41 [0.14, 85.34] |
| 36 Polyuria: mildly active group | 1 | 130 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.11, 67.35] |
| 37 Severe reaction: mildly active group | 1 | 130 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.13, 0.92] |
| 38 Severe reaction: highly active group | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.86, 4.56] |
22.1. Analysis.
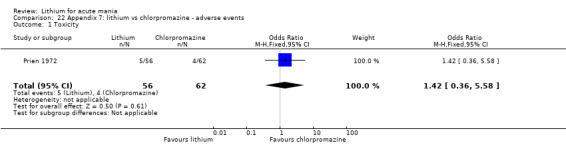
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 1 Toxicity.
22.2. Analysis.
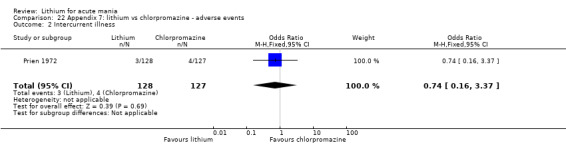
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 2 Intercurrent illness.
22.3. Analysis.
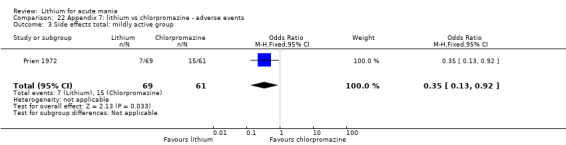
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 3 Side effects total: mildly active group.
22.4. Analysis.
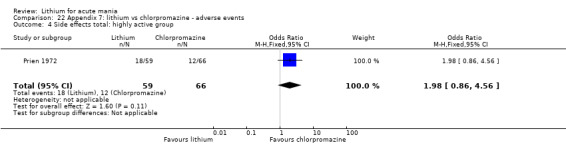
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 4 Side effects total: highly active group.
22.5. Analysis.
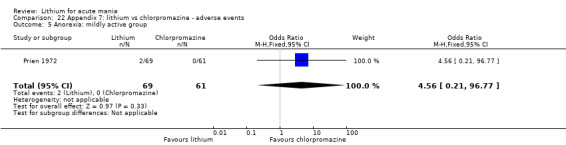
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 5 Anorexia: mildly active group.
22.6. Analysis.
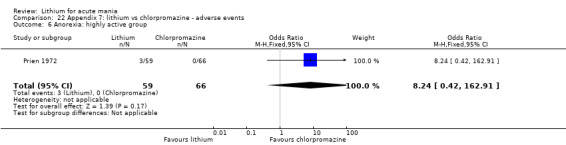
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 6 Anorexia: highly active group.
22.7. Analysis.
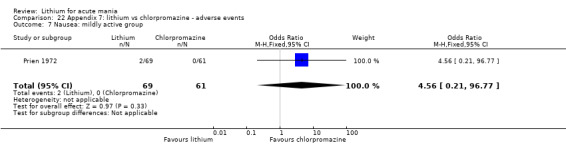
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 7 Nausea: mildly active group.
22.8. Analysis.
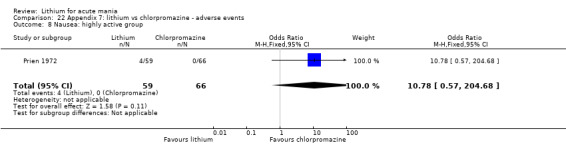
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 8 Nausea: highly active group.
22.9. Analysis.
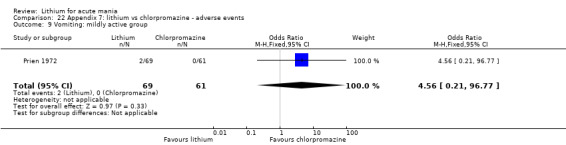
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 9 Vomiting: mildly active group.
22.10. Analysis.
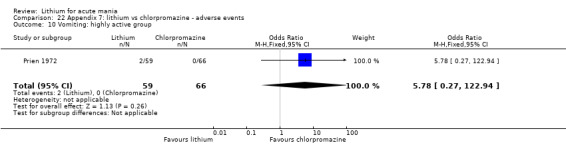
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 10 Vomiting: highly active group.
22.11. Analysis.
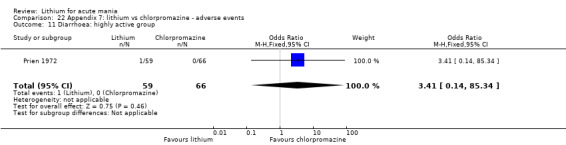
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 11 Diarrhoea: highly active group.
22.12. Analysis.
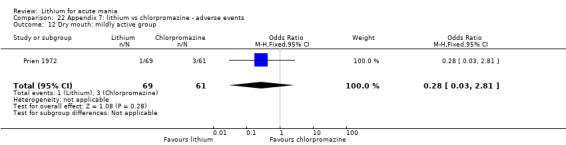
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 12 Dry mouth: mildly active group.
22.13. Analysis.
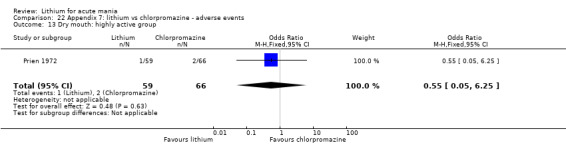
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 13 Dry mouth: highly active group.
22.14. Analysis.
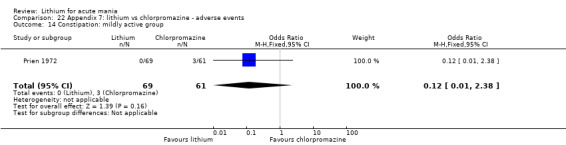
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 14 Constipation: mildly active group.
22.15. Analysis.
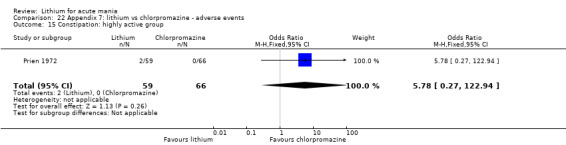
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 15 Constipation: highly active group.
22.16. Analysis.
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 16 Abdominal pain: highly active group.
22.17. Analysis.
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 17 Muscle weakness: mildly active group.
22.18. Analysis.
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 18 Ataxia: highly active group.
22.19. Analysis.
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 19 Tremor: mildly active group.
22.20. Analysis.
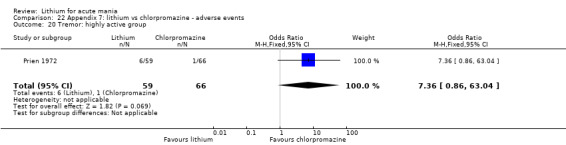
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 20 Tremor: highly active group.
22.21. Analysis.
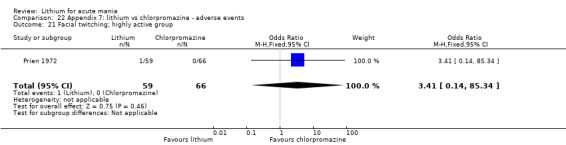
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 21 Facial twitching; highly active group.
22.22. Analysis.
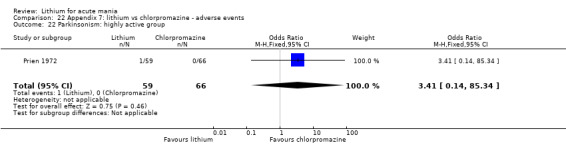
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 22 Parkinsonism: highly active group.
22.23. Analysis.
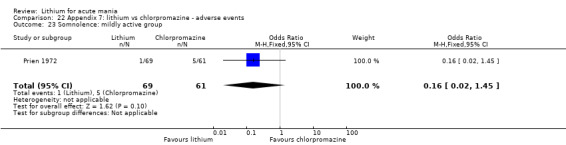
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 23 Somnolence: mildly active group.
22.24. Analysis.
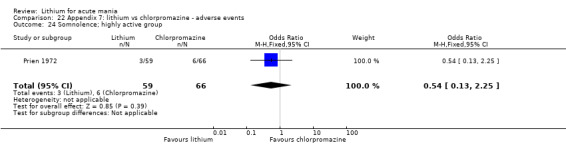
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 24 Somnolence; highly active group.
22.25. Analysis.
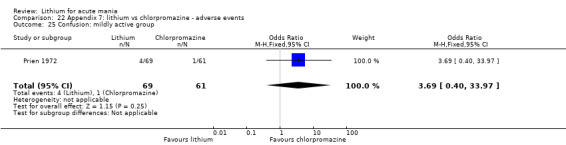
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 25 Confusion: mildly active group.
22.26. Analysis.
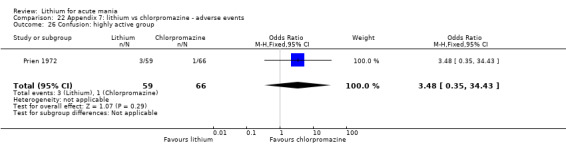
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 26 Confusion: highly active group.
22.27. Analysis.
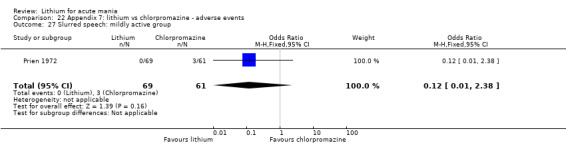
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 27 Slurred speech: mildly active group.
22.28. Analysis.
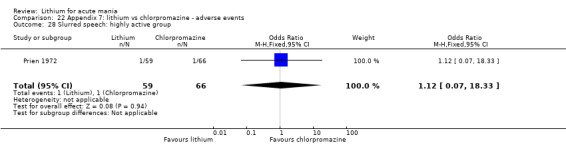
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 28 Slurred speech: highly active group.
22.29. Analysis.
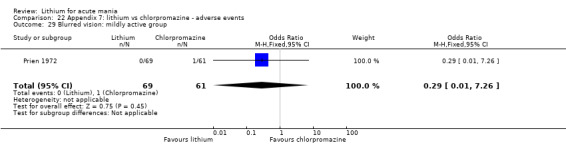
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 29 Blurred vision: mildly active group.
22.30. Analysis.
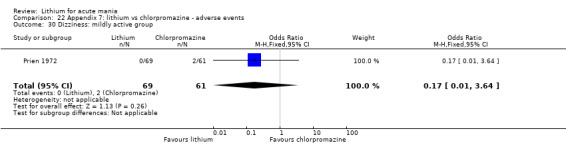
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 30 Dizziness: mildly active group.
22.31. Analysis.
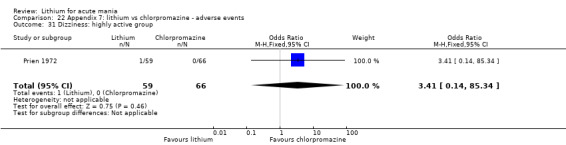
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 31 Dizziness: highly active group.
22.32. Analysis.
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 32 Seizures: highly active group.
22.33. Analysis.
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 33 Hypotensive reaction: mildly active group.
22.34. Analysis.
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 34 Hypotensive reaction: highly active group.
22.35. Analysis.
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 35 Pruritus: highly active group.
22.36. Analysis.
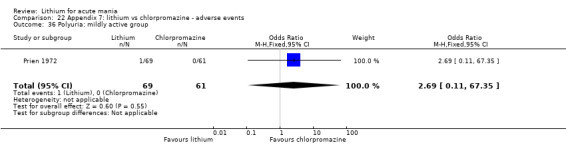
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 36 Polyuria: mildly active group.
22.37. Analysis.
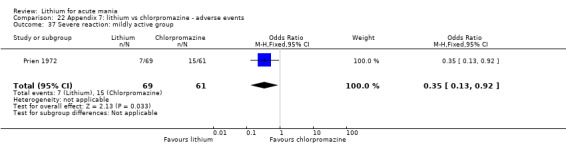
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 37 Severe reaction: mildly active group.
22.38. Analysis.
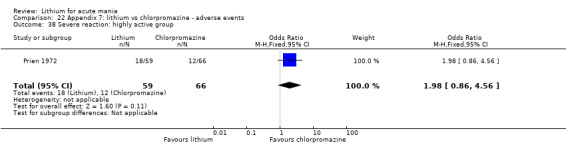
Comparison 22 Appendix 7: lithium vs chlorpromazine ‐ adverse events, Outcome 38 Severe reaction: highly active group.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Astra Zeneca 2009.
| Methods | Study design: 6‐week, multicentre, double‐blind, randomised, parallel‐group, placebo‐controlled study with a flexible dose design | |
| Participants |
Diagnosis: bipolar I disorder with current (or most recent) episode manic or mixed Method of diagnosis: amended version of the SCID Age: for lithium + quetiapine, median = 37.9 (SD = 12.71) years; for placebo + quetiapine, median = 38.8 (SD = 12.09) years Sex: for lithium + quetiapine, 72 women; 101 men; for placebo + quetiapine, 62 women; 121 men Location: 38 study centres in 8 countries (India 11, Russia 7, Bulgaria 7, Ukraine 6, Poland 3, Germany 1, South Africa 2, and Belgium 1) Co‐morbidities: not described Adjunctive therapy: not described Adjunctive medication: not described |
|
| Interventions | Participants were randomly assigned to either: Experimental arm n = 173 Duration: 43 days Treatment protocol: lithium as an add‐on to quetiapine Therapist/face‐to‐face contact: not described Comparator arm n = 183 Duration: 43 days Treatment protocol: placebo as add‐on to quetiapine Therapist/face‐to‐face contact: not described |
|
| Outcomes |
Primary outcome
Secondary outcome
|
|
| Notes |
Date of study: 2009‐2010 Funding source: Astra Zeneca, Quintiles Inc Declarations of interest among the primary researchers: unpublished study with no listed authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" was the only description given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masking: double‐blind (participant, caregiver, investigator, outcomes assessor) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lithium – 26 did not complete study Placebo – 39 did not complete study |
| Selective reporting (reporting bias) | Low risk | All 21 outcomes reported |
| Other bias | Unclear risk | None identified |
Banga 2003.
| Methods | Study design: prospective, randomised, clinical study | |
| Participants |
Diagnosis: acutely manic participants Method of diagnosis: not described Age: age 36.3 years +/‐ 10.8 years Sex: not described Location: Lady Hardinge Medical College, New Delhi Co‐morbidities: not described Adjunctive therapy: not described Adjunctive medication: not described |
|
| Interventions | Participants were randomly assigned to either: lithium or valproate Experimental arm n = 15 Duration: 21 days Treatment protocol: lithium was started in a dose of 300 mg twice daily, which was increased to 450 mg twice daily in the first week. Further change in doses were carried out at weekly intervals, guided by clinical improvement and treatment‐emergent adverse events . Therapist/face‐to‐face contact: not described Comparator arm n = 15 Duration: 21 days Treatment protocol: valproate was started in dose of 20 mg/kg/day. Further change in doses were carried out at weekly intervals, guided by clinical improvement and treatment‐emergent adverse events. Therapist/face‐to‐face contact: not described |
|
| Outcomes |
Primary outcome: > 50% fall in total YMRS from baseline to end of study was taken as a response Secondary outcomes: number and type of adverse events |
|
| Notes | Nil | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | High risk | Primary outcome data well reported. Adverse events outcomes poorly reported. |
| Other bias | Unclear risk | None identified |
Barekatain 2005.
| Methods | Study design: 2‐week, double‐blind, randomised, parallel‐group study | |
| Participants |
Diagnosis: BMD‐I most recent episode manic, hospitalised for treatment Method of diagnosis: expert psychiatrist diagnosed BMD‐I (manic episode) based on DSM‐IV and CIDI Age: for valproate + lithium, median = 29.8 (SD = 10.3) years; for valproate + risperidone, median = 31.4 (SD = 10.1) years; range = not described (18‐65 years allowed) Sex: females; males Location: Noor University hospital, Isfahan, Iran Co‐morbidities: substance abuse in (8 for lithium, 13 for risperidone) Adjunctive therapy: not described Adjunctive medication: in addition to the main drugs, only 1‐4 mg/day oral clonazepam (Sobhan Darou Iran) or lorazepam (Wyeth‐Ayerst Lab USA) were permitted to be administered during the study. For severe agitation, intramuscular lorazepam was allowed. |
|
| Interventions | Participants were randomly assigned to either: Experimental arm n = 23 Duration: 2 weeks Treatment protocol: 20 mg/kg per day sodium valproate three times daily at days 1‐14. Lithium capsules (300 mg) were administered three times daily at days 1‐5. If the participant's body weight was below 45 kg, lithium was used twice daily. After measuring serum lithium concentration at days 5 and 10, if lithium level was below 0.8 mEq/L, the dosage of lithium was adjusted by 300 mg increment in dosage at days 6 and 11 Therapist/face‐to‐face contact: not described Comparator arm n = 23 Duration: 2 weeks Treatment protocol: 20 mg/kg per day sodium valproate three times daily at the days 1‐14. Risperidone was administered once daily in 2 mg capsules (matching those capsules used for lithium) at days 1‐2, and twice daily at days 3‐14." Therapist/face‐to‐face contact: not described |
|
| Outcomes |
Timepoints for assessment: 4, 8, 14 days Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Date of study: 2003 Funding source: not described Declarations of interest among the primary researchers: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized" ‘46 cases were enrolled in two groups, equally |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind Total number of placebo capsules (matched for lithium and risperidone) were equally ad‐ ministered per day for each participant in both groups. Assessment by "by a trained blind psychiatry resident |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | During the study, a total number of 7 participants (30.4%) dropped out in each group: between 4th and 7th days, four participants (17.4%) discontinued the study in each group; |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | None identified |
Berk 1999.
| Methods | Study design: double‐blind, parallel, randomised controlled study | |
| Participants |
Diagnosis: bipolar disorder, manic phase Method of diagnosis: DSM‐IV criteria. Participants were interviewed using the structured clinical interview, MINI Age: for lithium, median = 31.9 years (SD =not provided); for olanzapine, median = 29.4 years (SD =not provided); range = 20‐59 years Sex: lithium 7 women; 8 men, olanzapine 6 women; 9 men, lamotrigine ‐ data not provided. Location: not described Co‐morbidities: not described Adjunctive therapy: none Adjunctive medication: lorazepam (4–12 mg daily) was available for the treatment of restlessness or disruptive behaviour. No other psychotropic medication was permitted during the course of the study. Anticholinergic medication (biperiden 2‐6 mg daily) was allowed for acute dystonia, and severe parkinsonian symptoms. |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium N = 15 Duration: 4 weeks Treatment protocol: lithium was maintained at a constant dose of 400 mg twice daily, resulting in a mean serum concentration of 0.743 mmol/L. Therapist/face‐to‐face contact: not described Comparator arm 1 ‐ lamotrigine N = 15 Duration: 4 weeks Treatment protocol: dosing schedule for lamotrigine was 25 mg daily during week 1, 50 mg daily during week 2 and 100 mg daily during weeks 3 and 4. This was a more rapid titration schedule than recommended to minimise the risk of skin rash, but was necessary due to the short treatment period. Therapist/face‐to‐face contact: not described Comparator arm 2 ‐ olanzapine N = 15 Duration: 4 weeks Treatment protocol: dose of olanzapine was 10 mg daily. Trial medication was administered as a twice daily dose, with a morning placebo in the olanzapine group Therapist/face‐to‐face contact: not described |
|
| Outcomes |
Timepoints for assessment: baseline and weekly Primary outcome:
Secondary outcome:
|
|
| Notes |
Date of study: unknown Funding source: Eli Lilly South Africa for the supply of sample olanzapine Declarations of interest among the primary researchers: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" ‐ only description |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | High risk | Poorly reported |
| Other bias | Unclear risk | None identified |
Bowden 1994.
| Methods | Study design: randomised, double‐blind, parallel‐group study | |
| Participants |
Diagnosis: manic disorder Method of diagnosis: met Research Diagnostic Criteria based on the structured interview and rating scale of SADS Age: for lithium, median = 39.1 (SD = 11.2) years; for divalproex, median = 40.4 (SD = 12.8) years; for placebo, median = 39.0 (SD = 10.0) years; range = not specified Sex: lithium 28% women; 72% men, divalproex 48% women; 52% men, placebo 43% women; 57% men. Location: Audie L. Murphy Memorial Veterans Hospital, San Antonio, Texas and San Antonio State Hospital (44 participants); Harris County Psychiatric Center, Houston, Texas (49 participants); Larue D. Carter Memorial Hospital, Indianapolis (19 participants); Emory University Hospital, Atlanta, Georgia (6 participants); University Hospitals of Cleveland (Ohio) (22 participants); Veterans Affairs Medical Center, Dallas, Texas (13 participants); Jackson Memorial Hospital, Miami, Florida (7 participants); and Illinois State Psychiatric Center, Chicago (16 participants). Co‐morbidities: not described Adjunctive therapy: none Adjunctive medication: the protocol allowed the use of adjunctive chloral hydrate or lorazepam as needed for control of agitation, irritability, restlessness, insomnia, and hostile behaviours. |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium N = 35 Duration: 3 weeks Treatment protocol: lithium carbonate was administered at an initial dose of 900 mg/d, dispensed in divided doses three times daily. On day 3, the total daily dosages of divalproex or lithium were increased to 1000 mg and 1200 mg respectively, and trough serum concentrations of both drugs were determined. On day 5, an unblended physician at each centre reviewed the serum concentration and adjusted the dosage of active medication. Thereafter, trough concentrations were measured on days 8, 10, 12, 15, and 18. Medication adjustments were made on days 7, 10, 12, 14, and 17. Drug dosage was raised on each adjustment day unless precluded by an adverse event or a serum concentration of valproate or lithium exceeding 1041 μ/L (150 μg/mL) or 1.5 mmol/L, respectively. Therapist/face‐to‐face contact: not described Comparator arm ‐ divalproex sodium N = 68 Duration: 3 weeks Treatment protocol: divalproex sodium was administered at an initial dose of 750 mg/day, dispensed in divided doses three times daily. On day 3, the total daily dosages of divalproex or lithium were increased to 1000 mg and 1200 mg respectively, and trough serum concentrations of both drugs were determined. On day 5, an unblended physician at each centre reviewed the serum concentration and adjusted the dosage of active medication. Thereafter, trough concentrations were measured on days 8, 10, 12, 15, and 18. Medication adjustments were made on days 7, 10, 12, 14, and 17. Drug dosage was raised on each adjustment day unless precluded by an adverse event or a serum concentration of valproate or lithium exceeding 1041 μ/L (150 μg/mL) or 1.5 mmol/L, respectively. Therapist/face‐to‐face contact: not described Comparator arm ‐ placebo N = 73 Duration: 3 weeks Treatment protocol: placebo, dispensed in divided doses three times daily. Comparable adjustments were made in the dosage of placebo according to blinded protocol‐specified dosing schedules. Therapist/face‐to‐face contact: not described |
|
| Outcomes |
Timepoints for assessment: 5, 10, 15, and 21 days. Primary outcome:
Secondary outcome:
|
|
| Notes |
Date of study: not clear Funding source: this study was funded in part by a grant from Abbott Laboratories, North Chicago, 111. Declarations of interest among the primary researchers: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A separate randomization schedule for each center was generated prior to the study start. Randomized in blocks of 5" |
| Allocation concealment (selection bias) | Low risk | "The post‐study treatment was individually determined for each patient without breaking the study blind". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "On day 5, an unblinded physician at each center reviewed the serum concentration and adjusted the dosage of active medication. Whenever possible, the same blinded investigator rated the patient throughout the study". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Sixty‐four percent of participants in the placebo group and 61% in the lithium‐treated group failed to complete all 21 days of treatment compared with only 48% in the divalproex‐treated group |
| Selective reporting (reporting bias) | Unclear risk | Incomplete data provided |
| Other bias | Unclear risk | None identified |
Bowden 2005.
| Methods | Study design: randomised, placebo‐controlled study | |
| Participants |
Diagnosis: bipolar I disorder, current episode manic Method of diagnosis: according to the DSM‐IV Age: for lithium, median = 38.0 years (SD = not given); for quetiapine, median = 41.3 years (SD = not given); for placebo, median = 38.8 years (SD = not given) range = 18 ‐ 73 years Sex: for lithium 40.8% women; 59.2% men, for quetiapine 43.9% women; 56.1% men, placebo 42.1% women; 57.9% men. Location: 24 centres in Europe and Asia Co‐morbidities: not described Adjunctive therapy: not described Adjunctive medication: "The following sedatives/hypnotics were permitted during the study for insomnia, providing the maximum doses were not exceeded and only 1 was used on any study day: zolpidem tartrate (maximum dose = 10 mg/day), chloral hydrate (maximum dose = 2 g/day from days 1 to 7 and 1 g/day from days 8 to 84), zopiclone (maximum dose = 7.5 mg/day), and zaleplon (maximum dose = 20 mg/day). Use of concomitant anti‐ cholinergic medications was not allowed after randomisation unless in relation to an adverse event of extrapyramidal symptoms (EPS). Lorazepam treatment for agitation (but not insomnia) was allowed as follows: up to 6 mg/day from screening to day 4, up to 4 mg/day from days 5 to 7, up to 2 mg/day from days 8 to 10, and up to 1 mg/day from days 11 to 14. Lorazepam was withheld for 6 hours before psychiatric assessments were conducted and was not permitted by the protocol after day 14. Within these guidelines, treatment was at the discretion of the physician, and if a participant experienced insomnia and agitation concurrently, lorazepam plus one of the permitted sedatives/hypnotics could be co‐administered". |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium N=67 Duration: 12 weeks Treatment protocol: all medication was administered twice daily in a double‐blind fashion. Double‐blinding was maintained throughout the study. Lithium dosing was initiated on day 1 at a dose of 900 mg/day. Dose adjustment between days 5 and 84 was at the discretion of the investigator in order to optimise efficacy and tolerability. The target trough serum lithium concentration was between 0.6 and 1.4 mEq/L and was monitored throughout the study by an investigator inde‐ pendent of the dosing investigator. Study blinding was maintained by collecting blood samples from all participants at least 12 hours after administration of the previous dose of study medication, and serum lithium concentrations were determined on days 4, 7, 14, 21, 28, 42, 56, 70, and 84 (or final visit). Additional tests were conducted as needed, at the discretion of the investigator, to assess lithium toxicity. The mean serum lithium concentrations in lithium‐treated participants were within the target range of 0.6 to 1.4 mEq/L at all assessments from day 4 onward. The median serum lithium concentration was 0.73 mEq/L at day 14, 0.80 mEq/L at day 21, and 0.80 mEq/L at day 84". Therapist/face‐to‐face contact: not described Comparator arm ‐ quetiapine N = 72 Duration: 12 weeks Treatment protocol: all medication was administered twice daily in a double‐blind fashion. Double‐blinding was maintained throughout the study. Quetiapine was flexibly dosed and initiated at target doses of 100 mg on day 1, 200 mg on day 2, 300 mg on day 3, and 400 mg on day 4. Quetiapine dose could be adjusted up to 600 mg/day on day 5 and up to 800 mg/day thereafter (days 6 to 84). At day 21, 90% of participants who responded to quetiapine were taking doses between 400 and 800 mg/day. At day 84, 91% of responders were taking doses in this range. The mean quetiapine dose for responders was 586 mg/day in the last week of treatment prior to day 21 and 618 mg/day prior to day 84 (mean doses calculated by averaging the median dose for responders in the last week of treatment). |
|
| Outcomes |
Timepoints for assessment: days 1 (baseline), 4, 7, 14, 21, 28, 42, 56, 70, and 84. Primary outcome:
Secondary outcome:
|
|
| Notes |
Date of study: April 2001‐May 2002 Funding source: this study was supported by AstraZeneca Pharmaceuticals, Wilmington, Del. Declarations of interest among the primary researchers: AstraZeneca Pharmaceuticals, Wilmington, Del. (Drs. Mullen and Brecher and Mr. Jones); and AstraZeneca Pharmaceuticals, Södertälje, Sweden (Dr. Paulsson, Mr. Vågerö, and Ms. Svensson). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "On day 1, participants were randomly assigned to treat‐ ment with quetiapine or lithium or their matching placebos". |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Study blinding was maintained by collecting blood samples from all participants. In addition, all investigators and individuals who administered psychiatric rating scales remained blinded to treatment for the duration of the study". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | More participants treated with quetiapine (67.3%) or lithium (68.4%) completed the study at day 84 compared with those treated with placebo (36.1%). |
| Selective reporting (reporting bias) | Low risk | Comprehensively reported |
| Other bias | Low risk | None identified |
Bowden 2010.
| Methods |
Study design: an international, multicentre, randomised, open‐label, parallel‐group, equivalence study |
|
| Participants |
Diagnosis: bipolar I participants experiencing a manic or a mixed episode Method of diagnosis: according to the DSM IV Age: for lithium, median = 38.2 (SD = 13.1) years; for valproate, median =38.8 (SD = 12.0) years; range = (18 ‐ 75 years accepted) Sex: lithium 85 women; 50 men, valproate 66 women; 56 men Location: the study was conducted in 21 centres in six countries (Bulgaria, Hong Kong, Malaysia, Russia, Taiwan, and Thailand) Co‐morbidities: only minor anxiety disorders, no axis 2 or substance misuse problems Adjunctive therapy: none Adjunctive medication: concomitant administration of lorazepam (up to 8 mg/day from day 0 to day 7 and up to 4 mg/day after day 7) or equivalent (diazepam or other benzodiazepine) was permitted to manage agitation, irritability, restlessness, insomnia, or hostility. In addition, nonbenzodiazepine hypnotics (zolpidem 10 mg or zopiclone 7.5 mg per night) or antidepressants other than fluoxetine could be given if needed for the management of insomnia or emergent depression. |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium N = 132 Duration: 12 weeks Treatment protocol: "After a screening visit at which inclusion criteria were assessed, a 3‐day wash‐out period was initiated for all psychotropic drugs, except benzodiazepines at doses of 8 mg/day lorazepam equivalents. This could be reduced to 1 day in case of worsening severity of mania. lithium was provided as scored tablets of 250, 300, 400, and 500 mg of lithium carbonate. A sustained release form was recommended when this was available. lithium was started at the nearest dose to 800 mg/day orally (600–900 mg/day depending on the available formulations in individual countries), divided into two daily doses, for the first 5 days, after which dose adjustment was permitted. Target serum concentrations were 0.8–1.2 mmol/L for lithium and 70–125 mg/mL for valproate. If major side effects occurred, dose reduction to 15 mg/kg/day for valproate and to achieve serum concentrations of 0.6–0.8 mmol/L for lithium was allowed". Therapist/face‐to‐face contact: not described Comparator arm ‐ valproate N = 122 Duration: 12 weeks Treatment protocol: "After a screening visit at which inclusion criteria were assessed, a 3‐day wash‐out period was initiated for all psychotropic drugs, except benzodiazepines at doses of 8 mg/day lorazepam equivalents. This could be reduced to 1 day in case of worsening severity of mania. valproate was provided as a sustained‐release formulation in 200, 250, 300, and 500 mg tablets depending on the country. valproate was started at the nearest dose to 20 mg/kg/day orally for the first 5 days, after which the dose was adjusted as a function of response and serum concentration of valproic acid at day 5 and at any subsequent study visit at the discretion of the investigator. If the total dose did not exceed 1000 mg/day, valproate was administered once a day, otherwise a twice daily regimen was implemented". |
|
| Outcomes |
Timepoints for assessment: baseline, 5 , 10, 21, 56, 84 days Primary outcome:
Secondary outcome:
|
|
| Notes |
Notes Date of study: January 2004‐February 2006 Funding source: "This study was supported, sponsored, and funded by sanofi‐aventis, manufacturer of sodium valproate. The study sponsor, sanofi‐aventis, initiated the study, chose the study investigators, provided study medication, coordinated the data analysis, and provided financial support for the conduct of the study and the preparation of a draft version of this manuscript by a medical communications agency (SARL FOXYMED, Fresnes, France). THERAMIS/MEDISCIS, a contract research organization, was responsible for data management, statis‐ tical analyses and production of the study report". Declarations of interest among the primary researchers: "The authors of this manuscript constituted the Steering Committee of the study. Sergey Mosolov, Luchezar Hranov, Eric Chen, Hussain Habil, Ronnachai Kongsakon, and Hsin‐Nan lithiumn also participated in the study as investigators. All academic authors have received honoraria from the sponsor for participation in the study as well as, in some cases, consultancy fees in the previous 3 years. Robert Manfredi is an employee of the study sponsor. The corresponding author, Charles L. Bowden, chairman of the Steering Committee, had full access to all data from the study, and was responsible for the decision to submit the finalized manuscript for publication". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization code was generated centrally. Investigators were provided with randomization numbers in sealed envelopes in blocks of four". |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blind. Wherever possible, raters were to be blinded to treatment. Although it was intended that patient assessment would be conducted by blinded raters, this was not always possible given the pragmatic, clinical practice base of the study, which could introduce a source of evaluation bias". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One hundred and eighty‐five participants completed the study as planned (69.0% of randomised participants). |
| Selective reporting (reporting bias) | Low risk | Good reporting |
| Other bias | Low risk | None identified |
Chouinard 1983.
| Methods | Study design: randomised, double‐blind cross‐over study | |
| Participants |
Diagnosis: mania Method of diagnosis: confirmed by the research psychiatrist on the basis of the Research Diagnostic Criteria Age: 25 – 63 years old, median 43 years Sex: 4 women; 9 men Location: Hopital Louis‐H Lafontaine Co‐morbidities: not described Adjunctive therapy: none Adjunctive medication: "For ethical reasons haloperidol was administered in cases of agitation that could not be controlled by the study medications. Thus, PRN medication presented an indirect measure of efficacy, and reflected the judgement of the nurses in direct contact with the participants. A standard procedure was used and nurses needed to obtain the approval of the investigating psychiatrist before giving the PRN medication. The need for haloperidol PRN was assessed on each occasion and it was never prescribed on a regular basis. Procyclidine HCL was administered with the haloperidol for control of parkinsonian symptoms." |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐lithium N = 6 Duration: 10 days + 10 days Treatment protocol: "Patients were treated with clonazepam or lithium carbonate in a double blind crossover design: six participants chosen randomly received 10 days of treatment with clonazepam followed immediately by 10 days of treatment with lithium, while the others received the same treatments in reverse order. Patients were started on the experimental treatment as soon as the evaluations and laboratory tests were completed (1 or 2 days after admission. Before the study started, participants were given haloperidol on a PRN basis whenever their behaviour was uncontrolled. After treatment with the research drug had begun, no other medications (including hypnotics) were administered, expect for haloperidol in cases of severe agitation. Clonazepam and lithium carbonate were administered under double‐blind conditions. While participants were treated with lithium, they received clonazepam placebos and vice versa. Both medications were given in an equally divided four time daily regimen. Clonazepam was administered in 2 mg tablets and lithium carbonate in 300 mg tablets. To ensure compliance the tablets were taken with water in the presence of a nurse the initial doses were chosen on the basis of an equivalency of 2mg of clonazepam for 300mg lithium carbonate. Dosages were subsequently adjusted according to therapeutic effect and side effects. lithium plasma levels were sent to an internist who could request substitution of lithium by placebo if abnormally high lithium concentrations occurred. However, this never happened. The daily dosages of clonazepam given on day 1 varied from x to 8mg (mean 4.2 mg) and on day 10 from 4 to 16 mg (mean 10.4 mg), the daily doses of lithium given on day 1 varied from 900 to 1500 mg (mean 1118 mg) and on day 10 from 900 to 2100 mg (mean 1691 mg)." Therapist/face‐to‐face contact: not described Comparator arm ‐ clonazepam N = 6 Duration: 10 days + 10 days Treatment protocol: "Patients were treated with clonazepam or lithium carbonate in a double blind crossover design: six participants chosen randomly received 10 days of treatment with clonazepam followed immediately by 10 days of treatment with lithium, while the others received the same treatments in reverse order. Patients were started on the experimental treatment as soon as the evaluations and laboratory tests were completed (1 or 2 days after admission. Before the study started, participants were given haloperidol on a PRN basis whenever their behaviour was uncontrolled. After treatment with the research drug had begun, no other medications (including hypnotics) were administered, expect for haloperidol in cases of severe agitation. Clonazepam and lithium carbonate were administered under double‐blind conditions. While participants were treated with lithium, they received clonazepam placebos and vice versa. Both medications were given in an equally divided four times daily regimen. Clonazepam was administered in 2 mg tablets and lithium carbonate in 300 mg tablets. To ensure compliance the tablets were taken with water in the presence of a nurse the initial doses were chosen on the basis of an equivalency of 2 mg of clonazepam for 300 mg lithium carbonate. Dosages were subsequently adjusted according to therapeutic effect and side effects. lithium plasma levels were sent to an internist who could request substitution of lithium by placebo if abnormally high lithium concentrations occurred. However, this never happened. The daily dosages of clonazepam given on day 1 varied from x to 8 mg (mean 4.2 mg) and on day 10 from 4 to 16 mg (mean 10.4 mg), the daily doses of lithium given on day 1 varied from 900 to 1500 mg (mean 1118 mg) and on day 10 from 900 to 2100 mg (mean 1691 mg)". Therapist/face‐to‐face contact: not described |
|
| Outcomes |
Timepoints for assessment: 0, 10, (+20) days Primary outcome:
Secondary outcome:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly" ‐ no further description |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Clonazepam and lithium carbonate were administered under double‐blind conditions, Assessment of symptoms was based on clinical interviews conducted by the psychiatrist. Performance = low Detection = unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Good completion rate (11/12) |
| Selective reporting (reporting bias) | Low risk | Comprehensive reporting |
| Other bias | Low risk | None identified |
Clark 1996.
| Methods | Study design: single‐blind, parallel, randomised controlled study | |
| Participants |
Diagnosis: bipolar affective disorder manic phase Method of diagnosis: DSM IV Age: "matched" Sex: "matched" Location: not given Co‐morbidities: not described but exclusion criteria included other axis 1&2 disorders Adjunctive therapy: none Adjunctive medication: in the event of agitation not being controlled on the study medication, clothiapine, a low potency neuroleptic, at a dose of 40 ± 120 mg was given on an as‐needed basis to a maximum of 240 mg daily. Controlled seclusion periods were also an option in this situation. Extrapyramidal symptoms were treated with orphenadrine 50 ± 150 mg daily on as‐needed basis. |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium N = 20 Duration: 4 weeks Treatment protocol: participants were started on lithium at a dose of 250 mg three times a day, which was adjusted on an individual basis to achieve a blood level of 0.6 ± 1.2 mmol/L to a maximum of 1800 mg per day. Once the regimen was commenced, no other routine psychotropic medications were administered. Therapist/face‐to‐face contact: not described Comparator arm ‐ clonazepam N = 20 Duration: 4 weeks Treatment protocol: participants were started on clonazepam at a dose of 2 mg four times daily increasing as needed to a maximum of 16 mg per day. Once the regimen was commenced, no other routine psychotropic medications were administered |
|
| Outcomes |
Timepoints for assessment: 3, 10, 21, 28 days Primary outcome:
Secondary outcome:
|
|
| Notes |
Date of study: not described Funding source: not described Declarations of interest among the primary researchers: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Authors admitted that it was possible blinding had been broken at times |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study sample consisted of 15 participants on lithium and 15 participants on clonazepam. One participant in the clonazepam group absconded from the hospital on day 10 but was included in the analysis on an intend‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | Well reported |
| Other bias | Low risk | None identified |
Findling 2015.
| Methods | Study design: multicentre, randomised, double‐blind, placebo‐controlled study | |
| Participants |
Diagnosis: bipolar affective disorder type 1, currently in manic or mixed episode Method of diagnosis: children aged 7 to 17 years meeting unmodified DSM IV, criteria for BP‐I currently in a manic or mixed episode, scoring 20 on the YMRS Age: for lithium, median 11.5 (SD = 2.9) years, for placebo, median 11.2 (SD = 3.0) years Sex: lithium: 31 female; 22 male, placebo: 13 female; 15 male Location: outpatient participants were enrolled at 1 of 10 academic medical centres in the United States that are experienced in paediatric psychiatric care Co‐morbidities: children were ineligible if they: were clinically stable on a medication regimen for BP‐I; diagnosed with schizophrenia or schizoaffective disorder, a pervasive developmental disorder, anorexia nervosa, bulimia nervosa, obsessive compulsive disorder, substance dependence, symptoms of mania that were attributable to a general medical condition or secondary to use of medications or general medical condition including neurologic disease, diabetes mellitus, thyroid dysfunction, or renal dysfunction that might be adversely affected by lithium; had clinically significant abnormal laboratory assessments that could influence the efficacy or safety of lithium or would complicate interpretation of study results; had evidence of serious homicidal/suicidal ideation or active hallucinations and delusions such that in the treating physician’s opinion it would not be appropriately safe for the participant to participate in this study; or had concomitant prescription of overthe‐counter medication or nutritional supplements that would interact with lithium or affect the participant’s physical or mental status. Adjunctive therapy: none Adjunctive medication: "participants with comorbid attention‐deficit/hyperactivity disorder were able to receive psychostimulants after 4 weeks of double‐blind therapy at the treating physician’s discretion. Melatonin (up to 3 mg) at bedtime was permitted to treat insomnia." |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium N = 53 Duration: 8 weeks Treatment protocol: "Starting dose of lithium (supplied as 300 mg, regular‐release capsules) was either 600 or 900 mg/d. Participants weighing, 30 kg started with 600 mg/day; all other participants began lithium therapy with 900 mg/day. Dose increases of 300 mg/day could occur at study visits and via telephone call during the middle of the first week of randomised treatment unless the participant had the following: had met dosing response criteria (defined as a CGI 25 score ≥2 and a 50% decrease in the YMRS score from baseline assessment); experienced1 adverse effect that significantly affected functioning that was at least of moderate severity; had a serum lithium level 1.4 mEq/L; or if the dose exceeded 40 mg/kg/day (with the exception of participants weighing, 23 kg, who could receive up to 900 mg/day)." Therapist/face‐to‐face contact: not described Comparator arm ‐ placebo N = 28 Duration: 8 weeks Treatment protocol: "Participants randomised to receive placebo were pre‐assigned to a maximum dose at randomisation to maintain the integrity of the blind. Adherence to study medication was monitored by using a dosing diary and pill counts." |
|
| Outcomes |
Timepoints for assessment: weekly Primary outcome:
Secondary outcome:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were enrolled into the study and were randomized to receive lithium or matching placebo in a 2 (lithium): 1 (placebo) allocation ratio. Stratification factors included study site, age at randomization (7–11 years and 12–17 years), and gender (male and female). The randomization list was created by an unblinded BPCA data coordinating centre (DCC) statistician. Unblinded site staff members were provided randomization assignments via an electronic data capture system." |
| Allocation concealment (selection bias) | Low risk | double‐blinded |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Participants randomized to receive placebo were preassigned to a maximum dose at randomization to maintain the integrity of the blind." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis lithium 16/53 did not complete 8 weeks ‐ all reasons given Placebo 7/28 did not complete 8 weeks ‐ all reasons given |
| Selective reporting (reporting bias) | Low risk | Comprehensively reported |
| Other bias | Low risk | None identified |
Freeman 1992.
| Methods | Study design: double‐blind, parallel, randomised controlled study | |
| Participants |
Diagnosis: DSM‐III‐R diagnosis of manic episode Method of diagnosis: "confirmed independently by two board‐certified psychiatrists using a semi‐structured interview and a DSM‐III‐R checklist." Age: not described Sex: women = 21; men = 6. Location: Clinical Research Unit of the Harris County Psychiatric Center Co‐morbidities: 8 participants assigned to valproate and seven participants assigned to lithium had histories of drug or alcohol abuse at some point during the course of their bipolar disorder Adjunctive therapy: none Adjunctive medication: "Rescue medication, including chloral hydrate and lorazepam, was allowed for extreme behavioural problems not responding to non‐pharmacological interventions." |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium N = 13 Duration: 3 weeks Treatment protocol: "The lithium group, consisting of 10 women and three men, received lithium citrate as the elixir, starting at 0.5 meq/kg per day. The dose of lithium was increased until either a maximum dose of 1800 mg/day, level of 1.5 mmol/plasma lithium, dose‐limiting side effects, or clinical improvement occurred. Lithium levels at the end of the study ranged from 0.8 to 1.4 mmol/plasma lithium. Plasma drug levels were monitored on days 5‐7 of each week, the results were sent to the research pharmacist, and the dose was adjusted by a non‐blinded, non‐treating physician." Therapist/face‐to‐face contact: not described Comparator arm ‐ valproate N = 14 Duration: 3 weeks Treatment protocol: "The valproate group, consisting of 11 women and three men, was started on a fixed‐dose regimen of valproate as the elixir. Valproate participants received 1500 mg/day during the first week, 2250 mg/day during the second week, and 3000 mg/day during the third week, unless their symptoms had remitted or they experienced dose‐limiting side effects. Plasma drug levels were monitored on days 5‐7 of each week, the results were sent to the research pharmacist, and the dose was adjusted by a non‐blinded, non‐treating physician." Therapist/face‐to‐face contact: not described |
|
| Outcomes |
Timepoints for assessment: weekly Primary outcome:
Secondary outcome:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Assigned randomly" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5/27 withdrew |
| Selective reporting (reporting bias) | High risk | No SADS total, no BPRS subscales, unclear which week reported + data from other weeks. Change from baseline data. Varied presentation of data from responders. |
| Other bias | Low risk | Non identified |
Garfinkel 1980.
| Methods | Study design: double‐blind, parallel, randomised controlled study | |
| Participants |
Diagnosis: bipolar disorder, manic episode Method of diagnosis: past history of bipolar illness and met criteria of Feighner for mania by unanimous agreement of the 3 investigating psychiatrists. Age: for lithium, median = 41.5 (SD = 5.8) years; for haloperidol, median = 37.0 (SD = 5.3) years; for lithium and haloperidol, median = 37.0 (SD = 6.1) years Sex: lithium 5 women; 2 men; haloperidol 3 women; 4 men; haloperidol and lithium 4 women; 3 men. Location: Clarke Institute of Psychiatry Co‐morbidities: not described Adjunctive therapy: none Adjunctive medication: chloral hydrate was the only bedtime hypnotic permitted. |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium N = 7 Duration: 3 weeks Treatment protocol: "Subjects (in each group on day 1) received 3 capsules of lithium carbonate (300 mg each) and 3 placebo capsules identical in size, shape and colour to the haloperidol capsules, After the first day the dosage of these medications was varied according to the response or the appearance of untoward effects (Table 2). lithium blood levels were obtained on all participants at weekly intervals." Therapist/face‐to‐face contact: N/A Comparator arm ‐ haloperidol N = 7 Duration: 3 weeks Treatment protocol: "3 capsules of haloperidol (10 mg each) and 3 placebo capsules identical to the lithium capsules, After the first day the dosage of these medications was varied according to the response or the appearance of untoward effects (Table 2). lithium blood levels were obtained on all participants at weekly intervals." Therapist/face‐to‐face contact: not described Comparator arm ‐ lithium and haloperidol N = 7 Duration: 3 weeks Treatment protocol: "3 capsules of haloperidol (l0 mg each) and 3 capsules of lithium carbonate (300 mg). After the first day the dosage of these medications was varied according to the response or the appearance of untoward effects (Table 2). lithium blood levels were obtained on all participants at weekly intervals." Therapist/face‐to‐face contact: not described |
|
| Outcomes |
Timepoints for assessment: day 1, twice weekly for 3 weeks Primary outcome:
Secondary outcome:
|
|
| Notes |
Date of study: Funding source: McNeil Laboratories kindly provided haloperidol, matching placebo and financial assistance for the investigation Declarations of interest among the primary researchers: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Twenty‐one subjects who met these criteria were randomly assigned to one of 3 treatment groups" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "lithium blood levels were obtained on all participants at weekly intervals. All personnel involved in patient ratings were unaware of the lithium blood levels, these being known only to the laboratory staff and the non‐blind psychiatrist who was allowed to recommend changes in lithium or placebo dose to the blind (treating) psychiatrist. Ratings of the patient’s clinical state were made by an off ward research nurse blind to medication groups" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Low risk | None identified |
Geller 2012.
| Methods |
Study design: parallel, randomised controlled study |
|
| Participants |
Diagnosis: DSM‐IV diagnosis of bipolar I disorder, manic or mixed episode, for at least 4 consecutive weeks Method of diagnosis: CGAS 15 score of 60 or less Age: for lithium, median = 9.7 (SD = 2.7) years; for risperidone, median = 11.0 (SD = 3.0) years; for divalproex sodium, median = 9.7 (SD = 2.4) years; range = 6.0 to 15.11 years Sex: lithium 37 girls; 53 boys, risperidone 47 girls; 42 boys, valproate 56 girls; 44 boys. Location: 5 sites participated: the Children’s National Medical Center in Washington, DC; the Johns Hopkins Medical Institutions in Baltimore, Maryland; the University of Pitts‐ burgh in Pennsylvania; the University of Texas Medical Branch in Galveston and the University of Texas Southwestern in Dallas; and Washington University in St Louis, Missouri. Co‐morbidities: disruptive disorders, anxiety disorders, sleep disorders and elimination disorders Adjunctive medication: ten doses of chlorpromazine at 25 mg each were allowed as rescue medications during weeks 1 to 4. |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium N = 93 Duration: 8 weeks Treatment protocol: titration schedules for twice‐a‐day dosing, which included lithium at 1.1‐1.3 mEq/L (to convert to millimoles/L, multiply by 1.0). Blood levels were obtained 10‐12 h after the dose and were titrated (Table 1) using weekly Clinical Global Impressions for Bipolar Illness Improvement–Mania (CGI‐BP‐IM) and adverse events scores. Therapist/face‐to‐face contact: not described Comparator arm ‐ risperidone N = 93 Duration: 8 weeks Treatment protocol: titration schedules for twice‐a‐day dosing, which included risperidone at 4 to 6 mg. Therapist/face‐to‐face contact: not described Comparator arm ‐ divalproex sodium N = 104 Duration: 8 weeks Treatment protocol: titration schedules for twice‐a‐day dosing, which included divalproex sodium at 111 to 125 μg/mL (to convert to micromoles/L, multiply by 6.934) blood levels were obtained 10 to 12 hours after the dose and were titrated (Table 1) using weekly Clinical Global Impressions for Bipolar Illness Improvement–Mania (CGI‐BP‐IM) and adverse events scores. Therapist/face‐to‐face contact: not described |
|
| Outcomes |
Timepoints for assessment: baseline and endpoint Primary outcome:
Secondary outcome:
|
|
| Notes |
Date of study: 2003‐2008 Funding source: This work was supported by NIMH grants U01 MH064846, U01 MH064850, U01 MH064851, U01 MH064868, U01 MH064869, U01 MH064887, U01 MH064911, and R01 MH051481. Role of Sponsor: The NIMH program staff participated in the conception and design of the study, in the analysis and interpretation of data, in the critical revision of the manuscript for important intellectual content, and in administrative, technical, and material support. During the first 2 years of study, Abbott supplied Depakote but had no other input and no knowledge of study data or conduct. There were 2 sites at Washington University in St Louis. One was the data coordinating, management, and statistical analysis site (principal investigator (PI): Dr Geller). The data coordinating site did not participate in data collection and, therefore, did not receive study medication from Abbott. The second site at Washington University in St Louis was a data collection site (PI: Dr Luby). Declarations of interest among the primary researchers: Financial Disclosure: Dr Geller reports the following for the work under consideration: a grant from NIMH; sup‐ port for travel to meetings from NIMH; payment for writ‐ ing or reviewing the manuscript from NIMH; and pro‐ vision of writing assistance, equipment, or administrative support from NIMH. Dr Geller also reports the follow‐ ing from outside the submitted work: consultancy for NIMH and the US Food and Drug Administration (FDA) Federal Advisory Committees; employment at Washing‐ ton University in St Louis, Missouri; grants from NIMH; payment for lectures from Vanderbilt University and the International Review of Bipolar Disorder; payment for manuscript preparation from NIMH; royalties from Guil‐ ford Press; travel, accommodations, and meeting expenses from NIMH and FDA for service on Federal Advisory Committees; payment from Massachusetts Medical Society for Journal Watch in Psychiatry Associate Editorship. Dr Luby reports the following for the work un‐ der consideration: grant from NIMH and provision of medicines from Abbott. Dr Luby also reports the follow‐ ing from outside the submitted work: employment at Washington University School of Medicine in St Louis, Missouri; grants/grants pending from NIMH, National Alliance for Research on Schizophrenia and Depression, and CHADS; and royalties from Guilford Press. Dr Joshi re‐ ports the following from the work under consideration: a grant from NIMH; support for travel to meetings from NIMH; provision of medicines from Abbott. Dr Joshi also... etc |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was stratified by age group (6‐12 vs 13‐15 years) and by the presence or absence of the following characteristics: mixed mania, psychosis, and daily rapid cycling. A separate random list of medication assignments was created for each site based on these stratifiers. |
| Allocation concealment (selection bias) | Unclear risk | Randomization was performed at the co‐ordinating site, and a form identifying the randomised medication was e‐mailed to the site’s non‐blinded staff members |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants, family members, and treating clinicians were aware of treatment assignment. Independent evaluators (IEs) who were blinded to medication status administered baseline and end‐ point assessments. Masking of the treatment assignment to the IEs was strictly enforced by using staff who were totally uninvolved with the participants’ treatment. Families were instructed not to reveal either the medication or adverse events to the blinded end‐point raters |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 80 withdrawals/290 randomised |
| Selective reporting (reporting bias) | Low risk | Data comprehensively reported |
| Other bias | Low risk | None identified |
GlaxoSmithKline 2005.
| Methods |
Study design: double‐blind, parallel, placebo‐controlled study |
|
| Participants |
Diagnosis: people who were bipolar I who were currently experiencing an acute manic or mixed episode according to DSM‐IV criteria Method of diagnosis: score on the MRS from SADS‐C of ≥ 18. The current manic or mixed episode was to have a duration of at least 1 week, but no greater than 3 months. Age: for lithium, median = 35.7 (SD = 13.8) years; for lamotrigine, median = 39.0 (SD = 13.1) years; for placebo, median = 38 (SD = 14.5) years; at least 18 years old Sex: lithium 39 women; 39 men, lamotrigine 37 women; 37 men, placebo 32 women; 45 men. Location: 38 centres in Australia (3), Austria (1), Bulgaria (4), Croatia (4), Czech Republic (4, France (3), Hungary (5), India (4), New Zealand (1), Poland (2), Russia (1), Singapore (1), South Africa (3) and UK (2) Co‐morbidities: not described Adjunctive therapy: none Adjunctive medication: not described |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium N = 78 Duration: 42 days Treatment protocol: lithium (dosed to therapeutic serum levels 0.7‐ 1.3 mEq/L) Therapist/face‐to‐face contact: not described Comparator arm ‐ lamotrigine N = 74 Duration: 42 days Treatment protocol: lamotrigine (weeks 1 and 2, 25 mg; weeks 3 and 4, 50 mg; week 5, 100 mg; week 6, 200 mg) Therapist/face‐to‐face contact: not described Comparator arm ‐ placebo N = 77 Duration: 42 days Treatment protocol: placebo as monotherapy Therapist/face‐to‐face contact: not described |
|
| Outcomes |
Timepoints for assessment: Primary outcome:
Secondary outcome:
|
|
| Notes |
Date of study: 27 January 1998‐8 March 1999 Funding source: GSK Declarations of interest among the primary researchers: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised …in a randomised manner using a balanced design (i.e. 1:1:1 ratio). |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 50% completion |
| Selective reporting (reporting bias) | Low risk | Only end of study data reported |
| Other bias | Low risk | None identified |
GlaxoSmithKline 2008.
| Methods |
Study design: double‐ blind, parallel, placebo‐controlled study |
|
| Participants |
Diagnosis: bipolar disorder and currently experiencing an acute manic or mixed episode Method of diagnosis: DSM‐IV, have a score on items 1‐11 of the MRS from SADS‐C of ≥ 18 at baseline Age: for lithium, median = 41.8 (SD = 13.1) years; for lamotrigine, median = 37.4 (SD = 10.0) years; for placebo, median = 36.9 (SD = 11.4) years Sex: lithium 17 women; 19 men, lamotrigine 39 women; 46 men, placebo 46 women; 49 men. Location: 47 centres in the US (37), India (4), Hungary (2), South Africa (2) and Canada (2) Co‐morbidities: not described Adjunctive therapy: none Adjunctive medication: not described |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium N = 36 Duration: 3 weeks Treatment protocol: lithium (serum therapeutic level 0.8 and 1.3 mEq/L) as monotherapy Therapist/face‐to‐face contact: not described Comparator arm ‐ lamotrigine N = 85 Duration: 3 weeks Treatment protocol: lamotrigine (Week 1, 25 mg; Week 2, 25 mg; Week 3, 50 mg) as monotherapy Therapist/face‐to‐face contact: not described Comparator arm ‐ placebo N = 95 Duration: 3 weeks Treatment protocol: placebo as monotherapy Therapist/face‐to‐face contact: not described |
|
| Outcomes |
Timepoints for assessment: Day 22 Primary outcome:
Secondary outcome:
|
|
| Notes |
Date of study: 8 January 1998‐23 July 1999 Funding source: GSK Declarations of interest among the primary researchers: none described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 130/216 completed |
| Selective reporting (reporting bias) | Low risk | All reported |
| Other bias | Low risk | None identified |
Gouliaev 1996.
| Methods |
Study design: single‐blind, parallel, randomised controlled study |
|
| Participants |
Diagnosis: DSM‐111‐R episode with or without psychotic symptoms in need of antimanic treatment were included. Method of diagnosis: SADS Age: for lithium + clonazepam, median = 41.6 (SD = 11.9); for zuclopenthixol + clonazepam, median = 36.9 (SD = 11.9); range = 21 ‐ 64 Sex: lithium + clonazepam 9 women; 6 men, zuclopenthixol + clonazepam 8 women; 5 men. Location: psychiatric Hospital in Aarhus, Denmark Co‐morbidities: not described Adjunctive therapy: none Adjunctive medication: clonazepam as needed |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium + clonazepam N = 14 Duration: 28 days Treatment protocol: "Patients received initially 12 mEq lithium citrate twice daily, and subsequent dosing adjustment was made to achieve serum lithium level of 0.9‐1 mEq/L. Clonazepam was given twice daily at a fixed dose of 1mg in the morning and 2 mg in the evening. Clonazepam as additional per need medication was allowed in the first week only with doses up to extra 2 mg daily. No other drugs were allowed." Therapist/face‐to‐face contact: not described Comparator arm ‐ zuclopenthixol + clonazepam N = 14 Duration: 28 days Treatment protocol: "Patients received oral zuclopenthixol at a fixed daily dose of 10 mg in the morning and 10 mg in the evening. Serum levels of zuclopenthixol were measured weekly. Clonazepam was administered the same way, as described for the other group. In case of extrapyramidal symptoms additional medication with orphenadrine was allowed. After 28 days of treatment participants were referred to a standard treatment regime." Therapist/face‐to‐face contact: not described |
|
| Outcomes |
Timepoints for assessment: 0, 3 , 6, 13, 20, 27 days Primary outcome:
Secondary outcome:
|
|
| Notes |
Date of study: 1990 ‐ 1992 Funding source: this study was supported by the Danish Trust for Psychiatric Research. Declarations of interest among the primary researchers: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly" allocated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | The study design was single‐blind, with the rater being blind to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/28 withdrew |
| Selective reporting (reporting bias) | High risk | Poor coverage of reporting |
| Other bias | Low risk | None described |
Hirschfeld 1999.
| Methods |
Study design: double‐blind, randomised, parallel‐group, study |
|
| Participants |
Diagnosis: a DSM‐IV diagnosis of bipolar disorder (manic or mixed) and hospitalized for treatment of an acute manic episode Method of diagnosis: participants were required to have manic symptoms of sufficient severity to have a total YMRS score ≥ 14, as assessed by SADS Age: for lithium, median = 36.4 (SD = 8.4) years; for divalproex loading, median = 36.0 (SD = 9.4) years; for divalproex non‐loading, median = 32.4 (SD = 9.1) years; range = 18 ‐ 60 years accepted Sex: lithium 8 women; 11 men, divalproex loading 9 women; 11 men, divalproex non‐loading 8 women; 12 men. Location: not described Co‐morbidities: not described Adjunctive therapy: none Adjunctive medication: lorazepam was allowed to manage agitation, insomnia, restlessness, irritability, and hostility (4 mg/day on days 1‐4 and 2 mg/day on days 5‐7) |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium N = 19 Duration: 10 days Treatment protocol: "After a drug washout period of no more that 72 hours and confirmation of the diagnosis of acute mania and of sub‐therapeutic serum concentration of valproate (< 20 ug/mL) and lithium (< 0.2 mEq/L, participants were randomly assigned to 1 of 3 groups. The lithium group (n = 19) received lithium carbonate at the usual initial dose of 300 mg three times daily on days 1 and 2 followed by gradual dose titration on days 3 through 10." Therapist/face‐to‐face contact: not described Comparator arm ‐ divalproex loading N = 20 Duration: 10 days Treatment protocol: "After a drug washout period of no more that 72 hours and confirmation of the diagnosis of acute mania and of sub‐therapeutic serum concentration of valproate (< 20 ug/mL) and lithium (< 0.2 mEq/L, participants were randomly assigned to 1 of 3 groups. The divalproex loading group (N = 20) received oral divalproex administered via a rapid stabilization schedule: 30 mg/kg/day on days 1 and 2 and 20 mg/kg/day on days 3 through 10." Therapist/face‐to‐face contact: not described Comparator arm ‐ divalproex non‐loading N = 20 Duration: 10 days Treatment protocol: "After a drug washout period of no more that 72 hours and confirmation of the diagnosis of acute mania and of sub‐therapeutic serum concentration of valproate (< 20 ug/mL) and lithium (< 0.2 mEq/L, participants were randomly assigned to 1 of 3 groups. The divalproex non‐loading group (n = 20) received oral divalproex at the usual dose of 150 mg three times daily on days 1 and 2 followed by gradual dose titration for the remaining 8 days." Therapist/face‐to‐face contact: not described |
|
| Outcomes |
Timepoints for assessment: on days 2 through 6 and on days 8 and 10 Primary outcome:
Secondary outcome:
|
|
| Notes |
Date of study: Funding source: sponsored by Abbott Laboratories Declarations of interest among the primary researchers: none given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned to 1 of 3 groups" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Blinded medication was provided. Blinded raters evaluated the participants." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Seven participants (35%) in each of the divalproex‐treated groups and 9 (47%) in the lithium standard‐titration group discontinued the study medication before the end of the study" |
| Selective reporting (reporting bias) | Unclear risk | Some missing data |
| Other bias | Low risk | None identified |
Ichim 2000.
| Methods | Study design: a double‐blind, parallel, randomised controlled trial | |
| Participants |
Diagnosis: an acute manic episode Method of diagnosis: DSM‐ IV criteria for the manic phase of bipolar disorder on a structured clinical interview Age: for lithium, median = 31.9 years (SD =not given); for lamotrigine, median = 33.6 years (SD =not given); range = between 20 ‐ 59 years. Sex: for lithium 7 women; 8 men, for lamotrigine 7 women, 8 men. Location: South Africa Co‐morbidities: no information provided Adjunctive therapy: none Adjunctive medication: lorazepam (4–12 mg daily) was given when necessary for the control of aggression. No other psychotropic medication was permitted during the course of the study. |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium N = 15 Duration: 28 days Treatment protocol: lithium was administered at a dose of 400 mg twice daily. Mean lithium level was 0.743 mmol/L. Therapist/face‐to‐face contact: not described Comparator arm ‐ lamotrigine N = 15 Duration: 28 days Treatment protocol: "Lamotrigine was given once daily at night, with a titration schedule consisting of a daily dose of 25 mg for 1 week, increasing to 50 mg in the second week and then to 100 mg in the third week. To maintain study blinding, the lamotrigine group also received a morning placebo" Therapist/face‐to‐face contact: not described |
|
| Outcomes |
Timepoints for assessment: week 0, 1, 2, 3, 4 Primary outcome:
Secondary outcome:
|
|
| Notes |
Date of study: not given Funding source: the authors wish to thank… GlaxoWellcome for the supply of lamotrigine samples. Declarations of interest among the primary researchers: none described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "To maintain study blinding, the lamotrigine group also received a morning placebo, and lithium monitoring was carried out by an independent clinician. No other description. Not described." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/30 discontinued |
| Selective reporting (reporting bias) | Low risk | Well described |
| Other bias | Low risk | None identified |
Keck 2009.
| Methods |
Study design: A randomised, double‐blind, placebo‐ and lithium‐controlled study with crossover of placebo participants to aripiprazole |
|
| Participants |
Diagnosis: acute mania in participants with bipolar I disorder Method of diagnosis: "met criteria for bipolar I disorder, type I, as defined by the DSM‐IV and were experiencing an acute manic or mixed episode that required hospitalisation. Diagnosis was confirmed by MINI Age: for lithium, median = 39.6 (SD = 10.5) years; for aripiprazole, median = 39.6 (SD = 10.6) years; for placebo, median = 39.8 (SD =11.3) years; range = 18‐69 years Sex: lithium 52% men, aripiprazole 51% men, placebo 5% men. Location: 46 study centres in the United States Co‐morbidities: not described Adjunctive therapy: none Adjunctive medication: "Concomitant benzodiazepines were allowed as needed to ameliorate anxiety, agitation or insomnia, although the dose was tapered as follows: ≤ 4 mg/day lorazepam (or equivalents) on days 1–4; ≤ 2 mg/day lorazepam on days 5–10; ≤ 1 mg/day lorazepam on days 11–14; no lorazepam (or equivalents) was permitted from day 15 onwards. Benztropine ≤ 4 mg/ day was permitted to treat EPS, but its use was not permitted as prophylactic medication. Propranolol was permitted at a maximum dose of 60 mg/day for the treatment of akathisia or tremor." |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium N = 160 Duration: 3 weeks Treatment protocol: 900‐1500 mg per day as three divided doses, mean serum lithium 0.76 mmol/L at end of week 3. Therapist/face‐to‐face contact: not described Comparator arm ‐ aripiprazole N = 155 Duration: 3 weeks Treatment protocol: 15 mg daily for 3 days, increased to 30 mg daily from day 4 Therapist/face‐to‐face contact: not described Comparator arm ‐ placebo N = 165 Duration: 3 weeks Treatment protocol: placebo matched in look to active drugs Therapist/face‐to‐face contact: not described |
|
| Outcomes |
Timepoints for assessment: the YMRS and MADRS scale scores were recorded at Day 2, Day 4, Weeks 1, 2, 3, 4, 5, 6, 8, 10, and 12; while the PANSS scores were only assessed at Weeks 3 and 12. Primary outcome:
Secondary outcome:
|
|
| Notes |
Date of study: 20 April 2004‐9 October 2006 Funding source: this study was supported by Bristol‐Myers Squibb (Princeton, NJ) and Otsuka Pharmaceutical Co., Ltd. (Tokyo, Japan). Bristol‐Myers Squibb had a role in the study design, analysis interpretation of the data, writing of report and decision to submit the paper for publication. Editorial support for the preparation of this manuscript was provided by Michelle O'Donovan, PhD, Ogilvy Healthworld Medical Education; funding was provided by Bristol‐Myers Squibb. Declarations of interest among the primary researchers : Paul E Keck is a principle or co‐investigator on research studies sponsored by Abbott Laboratories, American Diabetes Association, AstraZeneca, Bristol‐Myers Squibb, GlaxoSmithKline, Eli Lilly, Janssen Pharmaceutica, National Institute of Mental Health, National Institue of Drug Abuse, Pfizer, the Stanley Medical Research Institute and UCB Pharma. Paul J Orsulak is an employee of Medtox Laboratories, Inc. Andrew J Cutler has received grant/research support from, has been a speaker for, and/or has been a consultant for Abbott, Acadia, AstraZeneca, Bristol‐Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen, Jazz, Johnson and Johnson, McNeil, MediciNova, Merck, Novartis, Organon, Otsuka America, Pfizer, Sanofi, Sepracor, Shire, Solvay, Vanda, and Wyeth. Raymond Sanchez is a former employee of Bristol‐Myers Squibb and is now an employee of Otsuka Pharmaceutical Development & Commercialization, Inc. Anne Torbeyns and Ronald N Marcus are employees of Bristol‐Myers Squibb. Robert D McQuade and William H Carson are employees of Otsuka Pharmaceutical Development & Commercialization, Inc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised ‐ only description |
| Allocation concealment (selection bias) | Unclear risk | Poor description |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind Blind drug switching Sham lithium levels |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 50% discontinuation |
| Selective reporting (reporting bias) | Low risk | Well reported |
| Other bias | Low risk | None identified |
Kowatch 2000.
| Methods |
Study design: parallel, open‐label, randomised controlled study |
|
| Participants |
Diagnosis: bipolar I or II children and adolescents during a mixed or manic episode. Method of diagnosis: participants had to meet DSM‐IV inclusion criteria for bipolar I or II disorder during a mixed or manic episode and be 6‐18 years old. They had to score ≥ 14 on the YMRS Age: median = 11.4 (SD =3.0) years Sex: 26 boys, 16 girls Location: paediatric Psychiatry Center at Children's Medical Center of Dallas Co‐morbidities: there were high rates of other psychiatric disorders, particularly ADHD (71%), oppositional defiant disorder (38%), and anxiety disorders (17%). Adjunctive therapy: Adjunctive medication: "Chlorpromazine, 10 to 50 mg/day, was allowed as a "rescue medication" 2 to 3 times per week for sleep or agitation during the first 2 weeks of treatment." |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium N = 13 Duration: 6 weeks Treatment protocol: "After a 2‐week assessment period during which all medications were tapered off. lithium dosage was selected on the basis of the weight algorithms with a starting dose of approximately 30 mg/kg per day in 3 divided doses. Serum levels of the different mood stabilizers were measured after 1 week of treatment, and the dosages were then titrated until the following serum levels were reached: lithium, 0.8 to 1.2 mEq/L; carbamazepine, 7 to 10 µg/L; divalproex sodium, 85 to 110 µg/L." Therapist/face‐to‐face contact: not described Comparator arm ‐ carbamazepine N = 13 Duration: 6 weeks Treatment protocol: "After a 2‐week assessment period during which all medications were tapered off, The initial dose of carbamazepine was 15 mg/kg per day in 3 divided doses, Serum levels of the different mood stabilizers were measured after 1 week of treatment, and the dosages were then titrated until the following serum levels were reached: lithium, 0.8 to 1.2 mEq/L; carbamazepine, 7 to 10 µg/L; divalproex sodium, 85 to 110 µg/L." Therapist/face‐to‐face contact: not described Comparator arm ‐ divalproex N = 15 Duration: 6 weeks Treatment protocol: "After a 2‐week assessment period during which all medications were tapered off, the initial dose of divalproex sodium was 20 mg/kg per day, also in 3 divided doses. Serum levels of the different mood stabilizers were measured after 1 week of treatment, and the dosages were then titrated until the following serum levels were reached: lithium, 0.8 to 1.2 mEq/L; carbamazepine, 7 to 10 µg/L; divalproex sodium, 85 to 110 µg/L." Therapist/face‐to‐face contact: not described |
|
| Outcomes |
Timepoints for assessment: weekly Primary outcome:
Secondary outcome:
|
|
| Notes |
Date of study: not given Funding source: "The authors gratefully acknowledge support from the NAMI/Stanley Foundation Research Awards Program and NIMH grants K07‐MH01057 (Dr. Kowatch) and MH53799 (Dr. Rush). Dr. Weinberg was funded by The Caleb C. and Julia W. Dula Education and Charitable Foundations, Mr. and Mrs. Woody Hunt, Mr. and Mrs. Morton Myerson, and the Azoulay family. The authors acknowledge the administrative support of Kenneth Z. Altshuler, M.D., Stanton Sharp Distinguished Chair, Professor and Chairman, Department of Psychiatry." Declarations of interest among the primary researchers: |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "they were randomly assigned, using the minimization method, to treatment with either lithium, carbamazepine, or divalproex sodium. The minimization method is based on the idea that the next patient to enter the study is given whichever treatment would minimize the overall imbalance between groups at that stage of the study (Altman, 1991). Subjects were stratified on the basis of age (< 13 years and > 13 years), gender, and the presence or absence of ADHD. Dose and serum level ranges were monitored with levels after 1, 2, and 4 weeks of treatment. After randomization, subjects returned weekly for 6 consecutive weeks of open treatment". |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind‐ only description. Also, the child and adolescent psychiatrists completing the outcome ratings were not blind to the participant's medication status |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of 42 participants randomised, 6 completed less than 4 weeks of treatment, 10 completed 5 weeks, 13 completed 6 weeks, 10 completed 7 weeks, and 3 completed 8 weeks ‐ they are all accounted for |
| Selective reporting (reporting bias) | High risk | Not all outcomes reported |
| Other bias | Low risk | None identified |
Kushner 2006.
| Methods |
Study design: multicentre randomised, double‐ blind, placebo‐controlled, parallel‐group study |
|
| Participants |
Diagnosis: acute manic or mixed episodes of bipolar I disorder. Method of diagnosis: "a primary DSM‐IV diagnosis of bipolar I disorder and hospitalised with an acute manic or mixed episode, confirmed by a SCID Axis I Disorders" Age: for lithium, median = 42 (SD = 13) years; for topiramate 200, median = 42 (SD = 14) years; for topiramate 400, median = 43 (SD = 14) years; for placebo, median = 43 (SD = 14) years Sex: lithium 49% women; 51% men, topiramate 200 55% women; 45% men, topiramate 400 46% women; 54% men, placebo 59% women; 41% men. Location: USA and 19 other countries (Eastern and Western Europe, Argentina, Australia, India, Israel, Latin America, South Africa) Co‐morbidities: not described Adjunctive therapy: none Adjunctive medication: "during the first 14 days of double‐blind treatment, chloral hydrate, non‐benzodiazepine short‐acting sedative hypnotics, and short‐acting benzodiazepine anxiolytics could be used as rescue medication. Patients requiring antipsychotics or mood stabilizers were discontinued. Non‐pharmacologic interventions other than supportive or educational psychotherapy were prohibited." |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium Duration: 3 weeks (core study) Treatment protocol: "Each of the four studies included a screening phase during which previous psychotropic medications were discontinued, followed by randomisation to double‐blind treatment for 3 weeks (core study); in three studies, double‐blind treatment was continued for a total of 12 weeks (3‐week core study + 9‐ week double‐blind extension). Screening/washout. The screening‐phase duration varied according to the time needed for medication washout. A 48‐hour washout was allowed if YMRS score worsened by ‡25% compared with screening. Otherwise, the washout period was equivalent to five half‐lives of the medication in use or one treatment cycle for depot antipsychotic medications The washout period was extended for 1 week if YMRS score improved ≥ 25% from the screening score; if the patient’s YMRS score was ≥ 20 after the 2‐week washout, the patient could be randomised to double‐blind treatment. Randomization/titration. Patients eligible after washout were randomised to topiramate, placebo, or lithium. To maintain study blinding, investigators received instructions from the central laboratory to increase/decrease the mid‐day lithium dose to achieve target levels, with sham adjustments in the mid‐day (inactive) dose for participants in topiramate and placebo groups. In studies with 9‐week double‐blind extensions, placebo‐treated participants were crossed over to lithium (target: 1500 mg/day; PDMD‐004) or to topiramate (target: 150 mg/day; PDMD‐008). Dose titration and adjustments for lithium‐treated participants followed the same schedule as in the core double‐blind study. For participants converted to topiramate, the starting dose was 50 mg/day increased in 50 mg increments on days 2 and 3." Therapist/face‐to‐face contact: not described Comparator arm ‐ topiramate 200 Duration: 3 weeks (core study) Treatment protocol: "Each of the four studies included a screening phase during which previous psychotropic medications were discontinued, followed by randomization to double‐blind treatment for 3 weeks (core study); in three studies, double‐blind treatment was continued for a total of 12 weeks (3‐week core study + 9‐ week double‐blind extension). Screening/washout. The screening‐phase duration varied according to the time needed for medication washout. A 48‐h washout was allowed if YMRS score worsened by ≥ 25% compared with screening. Otherwise, the washout period was equivalent to five half‐lives of the medication in use or one treatment cycle for depot antipsychotic medications The washout period was extended for 1 week if YMRS score improved ≥ 25% from the screening score; if the patient’s YMRS score was ⪕ 20 after the 2‐week washout, the patient could be randomised to double‐blind treatment. Randomization/titration. Patients eligible after washout were randomised to topiramate, placebo, or lithium. The starting dose of 50 mg/day topiramate was increased to 100 mg/day at day 2 and in 100 mg increments each day for the next 1–5 days until the target dose (200, 400, or 600 mg/day) or the maximally tolerated dose was achieved. Investigators could slow titration by withholding doses or could reduce the dosage, with a maximum reduction of two tablets or capsules (100 mg/day topiramate) to improve tolerability To maintain study blinding, investigators received instructions from the central laboratory to increase/decrease the mid‐day lithium dose to achieve target levels, with sham adjustments in the mid‐day (inactive) dose for participants in topiramate and placebo groups. In studies with 9‐week double‐blind extensions, placebo‐treated participants were crossed over to lithium (target: 1500 mg/day; PDMD‐004) or to topiramate (target: 150 mg/day; PDMD‐008). Dose titration and adjustments for lithium‐treated participants followed the same schedule as in the core double‐blind study. For participants converted to topiramate, the starting dose was 50 mg/day increased in 50 mg increments on days 2 and 3." Therapist/face‐to‐face contact: not described Comparator arm ‐ topiramate 400 Duration: 3 weeks (core study) Treatment protocol: "Each of the four studies included a screening phase during which previous psychotropic medications were discontinued, followed by randomization to double‐blind treatment for 3 weeks (core study); in three studies, double‐blind treatment was continued for a total of 12 weeks (3‐week core study + 9‐ week double‐blind extension). Screening/washout. The screening‐phase duration varied according to the time needed for medication washout. A 48‐h washout was allowed if YMRS score worsened by ≥25% compared with screening. Otherwise, the washout period was equivalent to five half‐lives of the medication in use or one treatment cycle for depot antipsychotic medications The washout period was extended for 1 week if YMRS score improved ≥ 25% from the screening score; if the patient’s YMRS score was ≥ 20 after the 2‐week washout, the patient could be randomised to double‐blind treatment. Randomization/titration. Patients eligible after washout were randomised to topiramate, placebo, or lithium. The starting dose of 50 mg/day topiramate was increased to 100 mg/day at day 2 and in 100‐mg increments each day for the next 1–5 days until the target dose (200, 400, or 600 mg/day) or the maximally tolerated dose was achieved. Investigators could slow titration by withholding doses or could reduce the dosage, with a maximum reduction of two tablets or capsules (100 mg/day topiramate) to improve tolerability. To maintain study blinding, investigators received instructions from the central laboratory to increase/decrease the mid‐day lithium dose to achieve target levels, with sham adjustments in the mid‐day (inactive) dose for participants in topiramate and placebo groups. In studies with 9‐week double‐blind extensions, placebo‐treated participants were crossed over to lithium (target: 1500 mg/day; PDMD‐004) or to topiramate (target: 150 mg/day; PDMD‐008). Dose titration and adjustments for lithium‐treated participants followed the same schedule as in the core double‐blind study. For participants converted to topiramate, the starting dose was 50 mg/day increased in 50 mg increments on days 2 and 3." Therapist/face‐to‐face contact: not described Comparator arm ‐ placebo Duration: 3 weeks (core study) Treatment protocol: "Each of the four studies included a screening phase during which previous psychotropic medications were discontinued, followed by randomization to double‐blind treatment for 3 weeks (core study); in three studies, double‐blind treatment was continued for a total of 12 weeks (3‐week core study + 9‐ week double‐blind extension). Screening/washout. The screening‐phase duration varied according to the time needed for medication washout. A 48‐h washout was allowed if YMRS score worsened by ≥ 25% compared with screening. Otherwise, the washout period was equivalent to five half‐lives of the medication in use or one treatment cycle for depot antipsychotic medications The washout period was extended for 1 week if YMRS score improved ≥25% from the screening score; if the patient’s YMRS score was ≥ 20 after the 2‐week washout, the patient could be randomised to double‐blind treatment. Randomization/titration. Patients eligible after washout were randomised to topiramate, placebo, or lithium. In studies with lithium as an active comparator (PDMD‐004 and PDMD‐008), the starting dose of 300 mg/day lithium was increased daily in 300‐mg increments to 1200 mg/day at day 4 and 1500 mg/day at day 6; lithium dosage could be reduced a maximum of 600 mg/day. lithium dosage was individualized based on target serum levels (titration, 0.8–1.2 mEq/L; stabilization, 0.6– 1.2 mEq/L; maximum 1800 mg/day). To maintain study blinding, investigators received instructions from the central laboratory to increase/decrease the mid‐day lithium dose to achieve target levels, with sham adjustments in the mid‐day (inactive) dose for participants in topiramate and placebo groups. In studies with 9‐week double‐blind extensions, placebo‐treated participants were crossed over to lithium (target: 1500 mg/day; PDMD‐004) or to topiramate (target: 150 mg/day; PDMD‐008). Dose titration and adjustments for lithium‐treated participants followed the same schedule as in the core double‐blind study. For participants converted to topiramate, the starting dose was 50 mg/day increased in 50 mg increments on days 2 and 3." Therapist/face‐to‐face contact: not described Participants randomised in each part of the study: 004: placebo = 111, topiramate = 220, lithium = 113 008: placebo = 112, topiramate = 116, lithium = 114 |
|
| Outcomes |
Timepoints for assessment: 3 Weeks + 12 weeks Primary outcome:
Secondary outcome:
|
|
| Notes |
Date of study: not described Funding source: these studies were sponsored by Johnson & Johnson Pharmaceutical Research & Development, L.L.C. SFK, AK and RL are employees of Johnson & Johnson Pharmaceutical Research & Development. WHO is an employee of Ortho‐McNeil Janssen Scientific Affairs, L.L.C. Declarations of interest among the primary researchers: none given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Study treatment was blinded to participants, investigators and clinical staff, study monitors, data reviewers, and data entry personnel until the double‐blind phase was completed and the data‐ base was finalized. The central laboratory had access to the randomization code in order to identify blood samples for assaying lithium blood levels. To maintain study blinding, investigators received instructions from the central laboratory to increase/decrease the mid‐day lithium dose to achieve target levels, with sham adjustments in the mid‐day (inactive) dose for participants in topiramate and placebo groups. Topiramate and placebo were supplied as matching 50‐ or 100‐mg tablets (PDMD‐005 and PDMD‐006) or as identical‐appearing capsules (PDMD‐004 and PDMD‐008) that contained two 25‐mg topiramate tablets, placebo, or 300‐mg lithium capsules. Study treatment was blinded to participants, investigators and clinical staff, study monitors, data reviewers, and data entry personnel until the double‐blind phase was completed and the data‐ base was finalized." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clearly described. ‘Premature discontinuations from the core studies plus double‐ blind extensions were related to adverse events in 7% of placebo‐treated participants, 4% with placebo/ topiramate, 8–11% with topiramate, 13% with lithium, and 13% with placebo/lithium.’ |
| Selective reporting (reporting bias) | Unclear risk | Not clear if all data reported or not. |
| Other bias | Low risk | None identified |
Lerer 1987.
| Methods |
Study design: parallel, double‐blind, randomised controlled study |
|
| Participants |
Diagnosis: bipolar disorder, manic. Method of diagnosis: according to DSM‐III criteria Age: for lithium, median = 37 years (SD =not given); for carbamazepine, median = 44 years (SD = not given); range = 23 ‐ 65 years Sex: lithium 9 women; 5 men, carbamazepine 6 women; 8 men. Location: Lafayette Clinic, Detroit and Northville Regional Psychiatric Hospital, Northville, Michigan Co‐morbidities: not described Adjunctive therapy: none given Adjunctive medication: "psychotropic medications other than chloral hydrate or barbiturates for nocturnal or daytime sedation were not permitted throughout the drug‐free and study periods." |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium N = 14 Duration: 4 weeks Treatment protocol: "Lithium treatment commenced with 900 mg/day and was increased gradually to attain serum levels as close to 1.0 mEq/L as possible. In addition, participants treated with carbamazepine received lithium placebo tablets; participants treated with lithium received carbamazepine placebo tablets. This “double placebo” design resulted in participants being treated as if they were on both drugs, with “dummy” serum levels being supplied for the appropriate placebo. Thus, participants, ward staff, and the clinical raters were blind to the true nature of the treatment. A psychiatrist and a research nurse who were aware of the treatment allocation determined weekly serum levels. To maintain integrity of the double blind, potential toxic levels were reported as if for both drugs rather than for only the actual treatment." Therapist/face‐to‐face contact: not described Comparator arm ‐ carbamazepine N = 14 Duration: 4 weeks Treatment protocol: "Patients were randomly assigned to treatment with carbamazepine or lithium. Carbamazepine treatment commenced with 600 mg/day in divided doses and was increased gradually to a dosage yielding carbamazepine levels of 8 to 12 ug/mL. In addition, participants treated with carbamazepine received lithium placebo tablets; participants treated with lithium received carbamazepine placebo tablets. This “double placebo” design resulted in participants being treated as if they were on both drugs, with “dummy” serum levels being supplied for the appropriate placebo. Thus, participants, ward staff, and the clinical raters were blind to the true nature of the treatment. A psychiatrist and a research nurse who were aware of the treatment allocation determined weekly serum levels. To maintain integrity of the double blind, potential toxic levels were reported as if for both drugs rather than for only the actual treatment." |
|
| Outcomes |
Timepoints for assessment: weekly Primary outcome:
Secondary outcome:
|
|
| Notes |
Date of study: not given Funding source: carbamazepine and placebo were supplied by Ciba‐Gelgy, USA Declarations of interest among the primary researchers: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "random" ‐ only description |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "In addition, participants treated with carbamazepine received lithium placebo tablets; participants treated with lithium received carbamazepine placebo tablets. This “double placebo” design resulted in participants being treated as if they were on both drugs, with “dummy” serum levels being supplied for the appropriate placebo. Thus, participants, ward staff, and the clinical raters were blind to the true nature of the treatment. A psychiatrist and a research nurse who were aware of the treatment allocation determined weekly serum levels. To maintain integrity of the double blind, potential toxic levels were reported as if for both drugs rather than for only the actual treatment." Clinical raters were blind to the true nature of the treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 28/35 completed |
| Selective reporting (reporting bias) | Low risk | Majority at least reported in figures. |
| Other bias | Low risk | None identified |
Li 2008.
| Methods |
Study design: multicentre, randomised, double‐blind, lithium‐controlled, parallel‐group study |
|
| Participants |
Diagnosis: acute manic episode, bipolar disorder Method of diagnosis: CCMD‐3 criteria Age: for lithium, median = 33.6 (SD = 11.41) years; for quetiapine, median = 32.7 (SD = 11.51) years; range = 18 ‐ 63 years Sex: females, males Location: centres in China Co‐morbidities: "Participants were fit and healthy aside from bipolar disorder. Other exclusion criteria included pregnancy or lactation, childbearing potential without appropriate birth control measures, substance or alcohol dependence (except for nicotine dependence), as defined in CCMD‐3, within 1 month before randomization; thyroid‐stimulating hormone concentration more than 10% above the upper limit of the normal range, regardless of treatment for hypothyroidism or hyperthyroidism; renal, cardiovascular, hepatic, hematological, endocrine, or other disease or clinical finding that was unstable; medical conditions that would affect absorption, distribution, metabolism, or excretion of the study treatment; and participation." Adjunctive therapy: no Adjunctive medication: "Previously prescribed medications for stable medical, non‐psychiatric illnesses were permitted during the study. A routine dose of sleep medication (estazolam, zolpidem tartrate, midazolam maleate, triazolam, zopiclone, clonazepam, alprazolam, lorazepam) at night to help sleep could be accepted during the study. Anticholinergic medications should only be initiated in relation to an EPS adverse event. Lorazepam treatment for agitation was only allowed as follows: up to 6 mg/day from screening to day 4; up to 4 mg/day from day 5 to day 7; up to 2 mg/day from day 8 to day 10; and up to 1 mg/day from day 11 to day 14. Use of lorazepam was not allowed after day 14. Lorazepam was withheld for 6 h before psychiatric assessments were conducted and was not used to treat insomnia." |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium N = 63 Duration: 28 days Treatment protocol: "After giving informed consent and undergoing screening procedures, the participants were randomised into the quetiapine or lithium group on day 1. Patients were required to be hospitalised for the treatment and assessment defined in the protocol. Once a patient was assigned to randomised study treatment, dose titration with blinded quetiapine or lithium (or their matching placebo) began. lithium treatment began on day 1 with a dose of 250–500 mg/day, which was increased to 500–2000 mg/day on day 4. From days 5 to 28, the dose was adjusted at the discretion of the investigator (to a maximum dose of 2000 mg/day) to achieve symptom control, minimize side effects and achieve target trough serum lithium concentration of 0.6–1.2 mmol/L. Serum lithium concentration was measured on days 4, 7, 14, 21, and 28 (or final visit). Additional tests of lithium concentration were conducted as needed, at the discretion of the investigator, to assess whether the lithium concentration exceeded a safe range. If the patient’s serum lithium concentration exceeded 1.2 mmol/L, the investigator reduced the dosage to ensure the patient’s safety. Tests for lithium concentration were conducted by a local laboratory. Blood samples were withdrawn at least 12 h after administration of the previous dose of study medication." Therapist/face‐to‐face contact: not described Comparator arm ‐quetiapine N = 72 Duration: 28 days Treatment protocol: "After giving informed consent and undergoing screening procedures, the participants were randomised into the quetiapine or lithium group on day 1. Patients were required to be hospitalized for the treatment and assessment defined in the protocol. Once a patient was assigned to randomised study treatment, dose titration with blinded quetiapine or lithium (or their matching placebo) began. Quetiapine treatment began on day 1 with a dose of 100–200 mg/day and was increased to 200–600 mg/day on day 4. On days 5–28, the quetiapine dose was adjusted, at the investigator’s discretion, up to a maximum of 800 mg/day." Therapist/face‐to‐face contact: not described |
|
| Outcomes |
Timepoints for assessment: days 4, 7, 14, 21, and 28. Primary outcome:
Secondary outcome:
|
|
| Notes |
Date of study: September 2005‐August 2006 Funding source: this study was sponsored by AstraZeneca Pharmaceuticals. Huafang Li, Cui Ma, Gang Wang and Niufan Gu have all worked as consultants for AstraZeneca Pharmaceuticals. Xiaotong Zhu and Mengye Peng are full‐time employees of AstraZeneca Pharmaceuticals. Declarations of interest among the primary researchers: none given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" ‐ only description |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Once a patient was assigned to randomised study treatment, dose titration with blinded quetiapine or lithium (or their matching placebo) began. To maintain the study blind, all participants had blood samples collected for the determination of serum lithium concentrations. To maintain the study blind two investigators at each centre worked together. One investigator (the doser) was responsible for reviewing serum lithium concentrations, adjusting the dosage of study medication, dispensing study medication, and performing drug accountability procedures. The second investigator (the rater) administered the psychiatric and safety assessments and was blinded to serum lithium concentrations." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 155 participants recruited, 154 participants (77 quetiapine‐treated participants and 77 lithium‐treated participants) were analyzed for efficacy in an ITT analysis set. |
| Selective reporting (reporting bias) | Low risk | Well reported |
| Other bias | Low risk | None identified |
Lusznat 1988.
| Methods | Study design: parallel, double‐blind randomised controlled study | |
| Participants |
Diagnosis: presumptive diagnosis of mania or hypomania Method of diagnosis: assessed by a Research Registrar, scored 10 or more on the BRMS Age: aged 17‐65 years (no breakdown provided) Sex: women; men. Location: Department of Psychiatry, Southampton. Co‐morbidities: not described Adjunctive therapy: no Adjunctive medication: "In the setting of an ordinary acute admission unit it proved impossible to manage severely manic participants without additional medication, so nearly all of then (n = 52) received variable amounts of neuroleptics during the acute study. It had initially been hoped to give chlorpromazine as the only rescue medication of this type, but several participants had known chlorpromazine sensitivity or were known to respond better to other neuroleptics such as haloperidol, son on ethical grounds this intended restriction had to be dropped." |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium N = 27 Duration: 6 weeks Treatment protocol: "Patients were started on study medication as soon as their initial assessment, including DST, had been completed. They were randomly assigned to be given either tablets containing 200 mg of carbamazepine plus placebo lithium carbonate tablets, or tablets containing 400 mg lithium plus placebo carbamazepine tablets. This ‘double dummy" technique was used since it had proved impossible to obtain tablets containing lithium and tablets containing carbamazepine that looked alike. Medication was started in a dose of one active tablet plus one placebo tablet daily and was increased by one tablet of each type every second day until a serum carbamazepine level 0.6‐1.2 mg per 100ml was attained, or a serum lithium concentration of 0.6‐1.4 mmol/L. Serum drug levels were estimated at each follow‐up assessment in order to check on correctness of dosage and compliance. Medication and day‐to‐day aspects of the clinical care of each patient were managed by the Research registrar, who carefully concealed serum drug estimations from those making follow‐up ratings. Therapist/face‐to‐face contact: not described Comparator arm ‐ carbamazepine N = 27 Duration: 6 weeks Treatment protocol: Patients were started on study medication as soon as their initial assessment, including DST, had been completed. They were randomly assigned to be given either tablets containing 200 mg of carbamazepine plus placebo lithium carbonate tablets, or tablets containing 400 mg lithium plus placebo carbamazepine tablets. This ‘double dummy' technique was used since it had proved impossible to obtain tablets containing lithium and tablets containing carbamazepine that looked alike. Medication was started in a dose of one active tablet plus one placebo tablet daily and was increased by one tablet of each type every second day until a serum carbamazepine level 0.6‐1.2 mg per 100 mL was attained, or a serum lithium concentration of 0.6‐1.4 mmol/L. Serum drug levels were estimated at each follow‐up assessment in order to check on correctness of dosage and compliance. Medication and day‐to‐day aspects of the clinical care of each patient were managed by the Research registrar, who carefully concealed serum drug estimations from those making follow‐up ratings." Therapist/face‐to‐face contact: not described |
|
| Outcomes |
Timepoints for assessment: Primary outcome:
Secondary outcome:
|
|
| Notes |
Date of study: not given Funding source: RL and PM were supported by a grant from Ciba‐Geigy. Declarations of interest among the primary researchers: none given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | This ‘double dummy" technique Follow‐up assessments were made by a rater blind to the participant's study medication. Medication and day‐to‐day aspects of the clinical care of each participant were managed by the Research registrar, who carefully concealed serum drug estimations from those making follow‐up ratings. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10/54 withdrew |
| Selective reporting (reporting bias) | High risk | Poor reporting |
| Other bias | Low risk | None identified |
Niufan 2008.
| Methods | Study design: double‐blind, parallel, randomised, controlled study | |
| Participants |
Diagnosis: bipolar disorder, manic or mixed episodes. Method of diagnosis: DSM‐ IV criteria for an index manic or mixed episode of bipolar disorder (with or without psychotic features), based on a clinical assessment. Participants were required to have a YMRS total score ≥ 20 Age: for lithium, median =34.0 (SD = 13.77) years; for olanzapine, median = 31.2 (SD = 12.55) years Sex: lithium 49.3% women, olanzapine 56.5% women. Location: seven sites in China Co‐morbidities: not described Adjunctive therapy: none given Adjunctive medication: "Concomitant medications with primarily central nervous system activity were excluded; however, the use of lorazepam (or lorazepam equivalent) up to 2 mg/ day was permitted to alleviate manic agitation, but not within 8 h of a psychiatric evaluation. Benzhexol hydrochloride (or equivalent) was permitted up to 6 mg/ day to alleviate extrapyramidal symptoms." |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium N = 71 Duration: 4 weeks Treatment protocol: "Patients who met all enrolment criteria after a 2– 7 day screening period were randomised in a 1:1 ratio to double‐blind treatment with either oral olanzapine (5– 20 mg/day, starting dose 15 mg/day; n = 69) or lithium carbonate (600–1800 mg/day in a divided dose, starting dose 300–600 mg/day; n = 71) over a 4‐week period. After 1 day on therapy, the olanzapine starting dose could be adjusted within the allowed dose range of 5– 20 mg/day, based on response (i.e., adverse events and efficacy). Those participants who could not tolerate olanzapine 5 mg/day or lithium carbonate 600 mg/day after Day 3 of the double‐blind treatment phase were discontinued from the study. In order to maintain double‐blinding of non‐identical medications, participants also received placebo tablets similar in appearance to the treatment they had not been randomised to receive (double‐dummy treatment). To further maintain the double‐blind nature of the study, all participants had serum specimens collected to test or lithium blood levels throughout the study, regardless of which treatment they were randomised to. An independent laboratory performed lithium blood level monitoring only for those participants randomised to receive lithium, although all participants received a laboratory report. Lithium blood levels were reported as “well below target” (below 0.60 mEq/L), “below target” (0.61–0.80 mEq/L), “within target” (0.81– 1.20 mEq/L), “above target” (1.21–1.40 mEq/L), or “well above target” (1.41–2.0 mEq/L), and corresponding recommendations were made to increase, decrease, or leave unchanged the daily “lithium” dose (regardless of whether the patient was actually receiving active lithium treatment). Olanzapine participants were randomly assigned to the various report categories." Therapist/face‐to‐face contact: not described Comparator arm ‐ olanzapine N = 69 Duration: 4 weeks Treatment protocol: "Patients who met all enrolment criteria after a 2– 7 day screening period were randomised in a 1:1 ratio to double‐blind treatment with either oral olanzapine (5– 20 mg/day, starting dose 15 mg/day; n = 69) or lithium carbonate (600–1800 mg/day in a divided dose, starting dose 300–600 mg/day; n = 71) over a 4‐week period. After 1 day on therapy, the olanzapine starting dose could be adjusted within the allowed dose range of 5– 20 mg/day, based on response (i.e., adverse events and efficacy). Those participants who could not tolerate olanzapine 5 mg/day or lithium carbonate 600 mg/day after Day 3 of the double‐blind treatment phase were discontinued from the study. In order to maintain double‐blinding of non‐identical medications, participants also received placebo tablets similar in appearance to the treatment they had not been randomised to receive (double‐dummy treatment). To further maintain the double‐blind nature of the study, all participants had serum specimens collected to test or lithium blood levels throughout the study, regardless of which treatment they were randomised to. An independent laboratory performed lithium blood level monitoring only for those participants randomised to receive lithium, although all participants received a laboratory report. Lithium blood levels were reported as “well below target” (below 0.60 mEq/L), “below target” (0.61–0.80 mEq/L), “within target” (0.81– 1.20 mEq/L), “above target” (1.21–1.40 mEq/L), or “well above target” (1.41–2.0 mEq/L), and corresponding recommendations were made to increase, decrease, or leave unchanged the daily “lithium” dose (regardless of whether the patient was actually receiving active lithium treatment). Olanzapine participants were randomly assigned to the various report categories." |
|
| Outcomes |
Timepoints for assessment: at randomization, and at days 3, 7, 14, 21 and 28 Primary outcome:
Secondary outcome:
|
|
| Notes |
Date of study: December 2003‐June 2005 Funding source: "Conflict of interest. This study was sponsored by Eli Lilly and Company. M. Tohen, A. Qiuqing, H. McElroy, and E. Pope are employees of Eli Lilly and Company. G. Niufan, Y. Fude, L. Ming, W. Gaohua, Z. Xinbao, L. Huichun, and S. Liang are clinical investigators for the F1D‐GH‐LOBV study (upon which the manuscript is based), for which their institutions were funded by Eli Lilly and Company for the time spent and assessment cost. The authors have no other potential conflicts of interest to disclose. Contributors. M. Tohen and A. Qiuqing were involved in the conception and design of the study. H. McElroy undertook the statistical analyses. E. Pope conducted the literature searches, and drafted the manuscript. G. Niufan, Y. Fude, L. Ming, W. Gaohua, Z. Xinbao, L. Huichun, and S. Liang were involved in the acquisition of data. All authors contributed to the interpretation of the data, critical revision of the manuscript for important intellectual content, and have approved the final manuscript. Role of funding source. Eli Lilly and Company (Lilly) provided funding for this study and was involved in the study conception and design. Lilly had no role in the collection of data for this particular manuscript, beyond the provision of clinical report forms and undertaking clinical monitoring of study sites. Lilly was involved in the analysis and interpretation of data for this manuscript, and in the drafting of the manuscript. Outside of the Lilly employees listed as authors (M. Tohen, A. Qiuqing, H. McElroy, and E. Pope), Lilly had no role in the decision to submit the paper for publication." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind; "To further maintain the double‐blind nature of the study, all participants had serum specimens collected to test for lithium blood levels throughout the study, regardless of which treatment they were randomised to." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15% did not complete the study |
| Selective reporting (reporting bias) | Low risk | Well reported |
| Other bias | Low risk | None identified |
Platman 1970.
| Methods | Study design: parallel, randomised controlled study | |
| Participants |
Diagnosis: the manic phase of manic‐depressive disease Method of diagnosis: clinical Age: for lithium, median = 56.7 (SD = 12.5) years; for chlorpromazine, median = 44.9 (SD = 10.3) years Sex: lithium 9 women; 4 men, chlorpromazine 9 women; 1 men. Location: Downstate Medical Centre, Brooklyn, New York Co‐morbidities: not described Adjunctive therapy: not described Adjunctive medication: not described |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐lithium N = 13 Duration: two weeks on placebo treatment followed by three week drug treatment Treatment protocol: "On admission participants were placed on placebo. However, all 30 participants who entered the initial random selection could not complete the minimum placebo period and seven were dropped from the study, In fact, the initial plan was to maintain participants on placebo for three weeks, but this would have meant that only 14 participants would have entered the study over a three‐year period. All 23 subjects reported in this study received at least a two‐week placebo period. The initial dose of lithium carbonate was 1200 mg and this was titrated to a target plasma level of 0.8‐1.0mmol/l. Mean maximum dose reached was 1823mg/day with a mean plasma level of 0.8mmol/l. Therapist/face‐to‐face contact: as above Comparator arm ‐ chlorpromazine N = 10 Duration: two weeks on placebo treatment followed by three week drug treatment Treatment protocol: "On admission participants were placed on placebo. However, all 30 participants who entered the initial random selection could not complete the minimum placebo period and seven were dropped from the study, In fact, the initial plan was to maintain participants on placebo for three weeks, but this would have meant that only 14 participants would have entered the study over a three‐year period. All 23 subjects reported in this study received at least a two‐week placebo period. The initial dose of chlorpromazine was 400 mg and this was titrated according to clinical response. The mean maximum dose reached at the end of the study was 870mg/day. |
|
| Outcomes | Psychiatric evaluation scale PEF scale |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Anyone who has administered lithium carbonate and chlorpromazine is fully aware that the term “double‐blind” is nonsense in a study between these two drugs." [Authors' comment: This probably refers either to the practice of chlorpromazine tablets being orange in colour in the 1950s‐70s, or to the fact that chlorpromazine was fairly commonly given as suppositories during this period. Completed by ward staff supposedly blind to the medication." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 7 drop outs |
| Selective reporting (reporting bias) | Low risk | Well reported |
| Other bias | Unclear risk | Study published in 1970 so methodology is less well described than in modern studies |
Prien 1972.
| Methods | Study design: parallel, double‐blind, randomised controlled study | |
| Participants |
Diagnosis: diagnosis of manic‐depressive psychosis, manic type, or schizoaffective psychosis, manic type Method of diagnosis: "each patient was independently interviewed by two psychiatrists and classified as (1) manic if both psychiatrists diagnosed the patient as manic‐depressive, manic state, (2) schizo‐affective if both psychiatrists diagnosed the patient as schizo‐affective, manic state, or (3) mixed if one psychiatrist diagnosed the patient as manic and the other as schizo‐affective. Any other diagnosis excluded the patient from the study. The diagnoses of manic‐depressive psychosis, manic type, and schizo‐affective psychosis, manic type, were based on criteria presented by Mayer‐ Gross'" and the American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders.1" Age: for (lithium), M = unknown (SD = unknown ); for (chlorpromazine), M = unknown (SD = unknown); whole sample: range = 17 to 60, median age was 44 years Sex: 92 women; 163 men. Location: 12 Veterans Administration hospitals and six public or private hospitals |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium N = 125 Duration: 3 weeks Treatment protocol: "Patients were randomly assigned to lithium carbonate or chlorpromazine. Medication was dispensed in identical‐appearing capsules containing 250 mg of lithium carbonate or 200 mg of chlorpromazine. Medication was administered for three weeks. On the first day, dosage was fixed at three capsules. Thereafter, the physician adjusted dosage according to the patient's clinical condition and occurrence of side effects. Serum levels, obtained three times each week, were used as a guide in establishing lithium carbonate dosage. The level was not to exceed 2 mEq/L. A complete blood count and urinalysis were performed weekly during treatment. Serum determinations were performed on blood drawn by veni‐ puncture before the morning dose and about 8 to 12 hours after the last dose. All examinations were repeated at the end of the study. Patients were not given any psychotropic drug other than their coded capsules during the treatment period. If a patient required another, known, medication he was dropped from the study." Therapist/face‐to‐face contact: as above Comparator arm ‐ chlorpromazine N = 123 Duration: 3 weeks Treatment protocol: "Patients were randomly assigned to lithium carbonate or chlorpromazine (Thorazine). Medication was dispensed in identical‐appearing capsules containing 250 mg of lithium carbonate or 200 mg of chlorpromazine. Medication was administered for three weeks. On the first day, dosage was fixed at three capsules. Thereafter, the physician adjusted dosage according to the patient's clinical condition and occurrence of side effects. Serum levels, obtained three times each week, were used as a guide in establishing lithium carbonate dosage. The level was not to exceed 2 mEq/L. A complete blood count and urinalysis were performed weekly during treatment. Serum determinations were performed on blood drawn by venipuncture before the morning dose and about 8 to 12 hours after the last dose. All examinations were repeated at the end of the study. Patients were not given any psychotropic drug other than their coded capsules during the treatment period. If a patient required another, known, medication he was dropped from the study." |
|
| Outcomes |
Timepoints for assessment: Primary outcome:
Secondary outcome:
|
|
| Notes |
Funding source: "This study was supported in part by grants MH14437, MH10892, MH14613, MH14611, MH14699, MH10295, and MH16069 from the Public Health Service. Lithium carbonate (Eskalith) and chlorpromazine (Thorazine) were sup¬ plied by John Buckley of Smith Kline & French Laboratories, Philadelphia" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Only the treatment physician knew the identity of the patient's study medication. Other treatment personnel and the clinical raters operated under double‐blind conditions." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Twenty‐two percent of the participants receiving lithium carbonate and 14% of the participants receiving chlorpromazine were dropped from the study" |
| Selective reporting (reporting bias) | Unclear risk | Unclear when ratings were carried out. No overall score data. |
| Other bias | Unclear risk | Study published in 1972 so methodology is less well described than in modern studies ‐ no biases recognised though |
Segal 1998.
| Methods |
Study design: double‐blind, randomised, controlled‐design study, parallel |
|
| Participants |
Diagnosis: bipolar disorder, manic phase Method of diagnosis: DSM‐IV criteria for bipolar disorder, manic phase, on a structured clinical interview. Age: for lithium, median = 37.2 years (SD = not given); for haloperidol, median = 29.5 years (SD = not given); for risperidone, median = 34.3 years (SD = not given); range = not given Sex: lithium 12 women; 3 men, haloperidol 10 women; 5 men, risperidone 13 women; 2 men. Comorbidities: not described Adjunctive medication: "Lorazepam was given when necessary to control aggression. No other psychotropic medication was permitted during the course of the study. Anticholinergic medication (orphenadrine) was allowed for acute dystonia and severe parkinsonian symptoms. The use of these agents was a secondary outcome measure." Adjunctive therapy: not described |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium N = 15 Duration: 28 days Treatment protocol: "Existing psychotropic medication was discontinued before the first day of the study. All participants were treated with either risperidone, 6 mg daily, haloperidol 10 mg daily, or lithium 800 to 1200 mg daily, with the lithium blood levels between 0.6 and 1.2 mmol/L. The starting dose of lithium was 400 mg twice a day. To facilitate blinding, lithium monitoring was down on all participants by an independent, blinded clinician." Therapist/face‐to‐face contact: not described Comparator arm ‐ haloperidol N = 15 Duration: 28 days Treatment protocol: "Existing psychotropic medication was discontinued before the first day of the study. All participants were treated with either risperidone, 6 mg daily, haloperidol 10 mg daily, or lithium 800 to 1200 mg daily, with the lithium blood levels between 0.6 and 1.2 mmol/L. The starting dose of lithium was 400 mg twice a day. To facilitate blinding, lithium monitoring was down on all participants by an independent, blinded clinician." Therapist/face‐to‐face contact: not described Comparator arm ‐ risperidone Duration: 28 days Treatment protocol: "Existing psychotropic medication was discontinued before the first day of the study. All participants were treated with either risperidone, 6mg daily, haloperidol 10 mg daily, or lithium 800 to 1200 mg daily, with the lithium blood levels between 0.6 and 1.2 mmol/L. The starting dose of lithium was 400 mg twice a day. To facilitate blinding, lithium monitoring was down on all participants by an independent, blinded clinician." |
|
| Outcomes |
Primary outcome:
Secondary outcome:
|
|
| Notes | Funding source: supported in part by Janssen Pharmaceuticals, South Africa | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participants were randomly assigned consecutively to treatment with lithium haloperidol or risperidone in a double‐blind fashion. |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | lithium monitoring was done on all participants by a independent, blinded clinician. All medication was administered in two divided doses. Blinding of outcome assessment not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 participants did not complete |
| Selective reporting (reporting bias) | Low risk | Well reported |
| Other bias | Unclear risk | Blinding (performance bias and detection bias) |
Shafti 2008.
| Methods | Study design: a randomised, double‐blind, parallel‐group study, parallel | |
| Participants |
Diagnosis: bipolar I disorder, manic episode Method of diagnosis: according to the DSM‐IV Age: not described Sex: 30 women; 0 men Location: not described Co‐morbidities: not described Adjunctive therapy: not described Adjunctive medication: "Although using benzodiazepine (lorazepam) and typical antipsychotic (haloperidol) as adjunctive agents were permissible during study, neither combining anticonvulsant nor atypical antipsychotic was prescribed throughout the aforesaid assessment. In addition, no psychosocial intervention other than ordinary care was used through this phase." |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium N = 15 Duration: 3 weeks Treatment protocol: "The first group (n = 15) was designated to lithium carbonate (300 mg uncoated tablets) as the solitary antimanic agent, whereas the second one (n = 15) was chosen for prescription of valproate sodium (200 mg coated tablets) for the same aim. Both drugs were prescribed according to practice guidelines and standard‐titration protocols The tablets were prescribed while previously inserted into empty and similar capsules, which were prepared in this regard to make participants blind with respect to the procedure. The evaluators were also unaware concerning the aforesaid partition and the type of medications arranged for each group.Mean serum level of lithium was 0.87 mmol/L." Therapist/face‐to‐face contact: as above Comparator arm ‐ valproate N = 15 Duration: 3 weeks Treatment protocol: "The first group (n = 15) was designated to lithium carbonate (300 mg uncoated tablets) as the solitary antimanic agent, whereas the second one (n = 15) was chosen for prescription of valproate sodium (200 mg coated tablets) for the same aim. Both drugs were prescribed according to practice guidelines and standard‐titration protocols The tablets were prescribed while previously inserted into empty and similar capsules, which were prepared in this regard to make participants blind with respect to the procedure. The evaluators were also unaware concerning the aforesaid partition and the type of medications arranged for each group." |
|
| Outcomes |
Timepoints for assessment: baseline and weekly Primary outcome:
Secondary outcome:
|
|
| Notes |
Date of study: not given Funding source: the authors declare no funding or conflicts of interest. Declarations of interest among the primary researchers: the authors declare no funding or conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random, 1:1 ratio – only description |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The tablets were prescribed while previously inserted into empty and similar capsules, which were prepared in this regard to make participants blind with respect to the procedure. The evaluators were also un‐ aware concerning the aforesaid partition and the type of medications arranged for each group." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no premature discontinuation in neither of groups. |
| Selective reporting (reporting bias) | Low risk | Well reported |
| Other bias | Low risk | None identified |
Shafti 2010.
| Methods |
Study design: parallel group, double‐blind, randomised study |
|
| Participants |
Diagnosis: bipolar I disorder, manic episode Method of diagnosis: according to DSM‐IV‐TR diagnostic criteria Age: not described Sex: 40 women; 0 men Location: Tehran, Iraq Co‐morbidities: not described Adjunctive therapy: no Adjunctive medication: prescription of lorazepam, as a sedating agent, was allowed during assessment |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium N = 20 Duration: 3 weeks Treatment protocol: "after 3–5 days washout period Patients in the first group (n = 20) were given lithium carbonate (300 mg uncoated tablets).The cases in the second group (n = 20) were prescribed olanzapine (5 mg uncoated tablets). Both of theses drugs were prescribed according to practice guidelines and standard titration protocols. no supplementary anticonvulsant or additional antipsychotic was permissible through‐ out the appraisal. Also no psychosocial intervention, except for usual care, was acceptable during evaluation. Upon completion of all baseline assessments, the tablets were prescribed. Identical‐looking capsules were prepared with the separate medication(s) to ensure the subjects in each group and the evaluator(s) were unaware of any distinction in the visual appearance, in the types of medication given in this study.Mean serum level of lithium 0.78 mmol/L." Therapist/face‐to‐face contact: as above Comparator arm ‐olanzapine N = 20 Duration: 3 weeks Treatment protocol: "after 3–5 days washout period Patients in the first group (n = 20) were given lithium carbonate (300 mg uncoated tablets).The cases in the second group (n = 20) were prescribed olanzapine (5 mg uncoated tablets). Both of theses drugs were prescribed according to practice guidelines and standard titration protocols. no supplementary anticonvulsant or additional antipsychotic was permissible through‐ out the appraisal. Also no psychosocial intervention, except for usual care, was acceptable during evaluation. Upon completion of all baseline assessments, the tablets were prescribed. Identical‐looking capsules were prepared with the separate medication(s) to ensure the subjects in each group and the evaluator(s) were unaware of any distinction in the visual appearance, in the types of medication given in this study." |
|
| Outcomes |
Primary outcome:
Secondary outcome:
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment to olanzapine or lithium carbonate in a 1:1 ratio. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three participants (15%) in the olanzapine group and two participants (10%) in the lithium group left the appraisal in the second half of the study due to unwillingness or adverse events of the prescribed drugs. |
| Selective reporting (reporting bias) | Low risk | Well reported |
| Other bias | Low risk | None identified |
Shopsin 1975.
| Methods | Study design: parallel, randomised controlled study | |
| Participants |
Diagnosis: manic illness Method of diagnosis: not described Age: not described Sex: women; men. but no figures provided Location: Psychiatric Hospital of New York, Bellevue Medical Centre` Co‐morbidities: not described Adjunctive therapy: not described Adjunctive medication: not described |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium N = 10 Duration: 21 days Treatment protocol: not described Therapist/face‐to‐face contact: not described Comparator arm ‐ chlorpromazine N = 10 Duration: 21 days Treatment protocol: not described Therapist/face‐to‐face contact: not described Comparator arm ‐ haloperidol N = 10 Duration: 21 days Treatment protocol: not described |
|
| Outcomes |
Primary outcome:
Secondary outcome:
|
|
| Notes | Funding source: "This investigation was supported in part by Public Health Service… MH17436 and MH04669" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "random" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "All personnel involved in patient ratings were unaware of the lithium… levels, these being only known to the laboratory staff… psychiatrist assigning treatment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | Well reported |
| Other bias | Unclear risk | Poorly described methodology so cannot rule out other biases |
Small 1988.
| Methods | Study design: randomised controlled study | |
| Participants |
Diagnosis: of bipolar disorder presenting in manic or mixed phases Method of diagnosis: DMS‐III criteria for a manic episode Age: mean age of 37.4 years Sex: women; men. but no figures provided Location: USA Co‐morbidities: not described Adjunctive therapy: not described Adjunctive medication: not described |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium N = 17 Duration:8 weeks Treatment protocol: A 10‐14 day washout of prior psychotropic medications was undertaken. Lithium carbonate was titrated to achieve plasma levels between 0.6‐1.5mmol/l. Therapist/face‐to‐face contact: not described Comparator arm ‐ Electroconvulsive therapy N = 17 Duration: 8 weeks Treatment protocol: "After completion of the baseline evaluations, participants were randomly assigned to treatment with lithium carbonate or ECT. At first, unilateral non‐dominant ECT was the form of treatment prescribed with the option of the attending psychiatrist to switch to bilateral treatment if therapeutic response was judged inadequate or difficulties were encountered with seizure induction. However, the first six manic participants randomised to ECT demonstrated little or no therapeutic benefit, and some participants’ condition actually worsened with unilateral ECT. At that point, the design was changed so that bilateral ECT was administered form the onset of treatment. The participants who underwent ECT received a series mean of nine treatments over three to five weeks." Therapist/face‐to‐face contact: not described |
|
| Outcomes |
Timepoints for assessment: weeks 0‐8 Primary outcome:
Secondary outcome:
|
|
| Notes | Funding source: this study was supported in part by grant MH40930 from… Institute of Mental Health, Bethseda, Md (Dr J G Small) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "random" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | ECT – not possible. The raters tried to avoid learning which treatment the participants received, but this was not always possible. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Low risk | Well reported |
| Other bias | Low risk | None identified |
Small 1991.
| Methods |
Study design: double‐blind, randomised controlled study, parallel |
|
| Participants |
Diagnosis: bipolar disorder, manic or mixed phases Method of diagnosis: examination of the patient and independent interviews with relatives using SADS. Patients met DSM‐ III‐R" criteria for a manic episode with or without coexisting symptoms of depression. Age: for lithium, median = 42.6 years (SD = not described); for carbamazepine, median = 34.3 years (SD = not described ) range = 22‐73 years Sex: lithium 13 women; 11 men, carbamazepine 14 women; 10 men. Location: Indiana University School of Medicine, Indianapolis Comorbidities: not described Adjunctive medication: "As during the washout phase, the only adjuvant medications that were allowed were chloral hydrate and amobarbital with repeated efforts to withdraw them after the first 3 weeks with double‐blind medications." Adjunctive therapy: not described |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium N = 24 Duration: 8 weeks Treatment protocol: "2 week washout were randomly assigned to treatment with lithium carbonate (300 mg) plus carbamazepine placebo tablets Medications were prescribed in initial dosages of one or two capsules and tablets daily, with dosage increments every 3 to 4 days until trough plasma lithium ion levels (10 to 12 hours after the last dose) were between 0.6 and 1.5 mmol/L and plasma carbamazepine levels were between 25 and 50 μ/L. Dosages were increased to ceiling levels or dosage‐limiting side effects. The "blind" of the attending clinicians was preserved by providing dummy plasma levels of lithium or carbamazepine, as was done in the study by Lerer et al." Therapist/face‐to‐face contact: as above Comparator arm ‐ carbamazepine N = 24 Duration: 8 weeks Treatment protocol: 2 week washout "Patients were randomly assigned to carbamazepine (200 mg tablets) and lithium placebo capsules. Medications were prescribed in initial dosages of one or two capsules and tablets daily, with dosage increments every 3 to 4 days until trough plasma lithium ion levels (10 to 12 hours after the last dose) were between 0.6 and 1.5 mmol/L and plasma carbamazepine levels were between 25 and 50 μ/L. The latter was primarily a measure of the parent compound without separation of the epoxide metabolite. Dosages were increased to ceiling levels or dosage‐limiting side effects. The "blind" of the attending clinicians was preserved by providing dummy plasma levels of lithium or carbamazepine, as was done in the study by Lerer et al." |
|
| Outcomes |
Timepoints for assessment: weekly Primary outcome:
Secondary outcome:
|
|
| Notes | Funding source: this study was supported in part MH40930 from the National Institute of Mental Health, Bethseda, Md. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo tablets "The "blind" of the attending clinicians was preserved by providing dummy plasma levels of lithium or carbamazepine, as was done in the study by Lerer et al. The clinical ratings of psychopathology were done by blinded clinicians who were not informed of the randomised treatment that was assigned nor were these clinicians involved in patient management." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 48/52 completed |
| Selective reporting (reporting bias) | Low risk | Well reported |
| Other bias | Low risk | None identified |
Spring 1970.
| Methods |
Study design: double‐blind, quasi‐randomised, crossover study. |
|
| Participants |
Diagnosis: mania Method of diagnosis: a team of three psychiatrist then independently evaluated the participants according to our criteria of “pure” mania Age: not described Sex: women; men. No figures given. Location: not described Co‐morbidities: not described Adjunctive therapy: not described Adjunctive medication: "The participants received no other psychotropic drugs. The only sedative allowed was amobarbital for sleep or, on temporary basis, for severe agitation." |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium N = 7 Duration: 3 weeks until crossover Treatment protocol: "The participants were treated with the study drug according to accepted clinical standards for a three week period. Typically, in the group treated with lithium the course of the treatment consisted of 1800 mg a day for the first week; if there was no response, the dose was increased to a maximum of 3.00 g a day. If, after the initial three‐week period, the treater felt that a complete or near complete remission had not been achieved, the participants were crossed over to the other drug for an additional three weeks. After three weeks or, if a crossover took place, after six weeks, the code was broken and the participants were put on a lithium carbonate maintenance dose. Blood levels were then maintained in the 0.6 to 1.3 mEq/L range and the participants was flowed on an outpatient basis." Therapist/face‐to‐face contact: not described Comparator arm ‐ chlorpromazine N = 7 Duration: 3 weeks until crossover Treatment protocol: "The participants were treated with the study drug according to accepted clinical standards for a three week period. In the chlorpromazine treated group the drug was rapidly increased depending on the severity of the mania; 1600 mg was the maximum dose. If, after the initial three‐week period, the treater felt that a complete or near complete remission had not been achieved, the participants were crossed over to the other drug for an additional three weeks. After three weeks or, if a crossover took place, after six weeks, the code was broken and the participants were put on a lithium carbonate maintenance dose. Blood levels were then maintained in the 0.6 to 1.3 mEq/L range and the participants was flowed on an outpatient basis." Therapist/face‐to‐face contact: not described |
|
| Outcomes |
Timepoints for assessment: weeks 3 and 6 Primary outcome:
Secondary outcome:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Flip of coin" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The third psychiatrist regulated the drugs. Neither the participants, the evaluators, nor any other staff members except the treating psychiatrist knew which drug was being given. The third psychiatrist regulated the drugs. Neither the participants, the evaluators, nor any other staff members except the treating psychiatrist knew which drug was being given" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/9 completion |
| Selective reporting (reporting bias) | Low risk | Well reported |
| Other bias | Low risk | None identified |
Trivedi 1996.
| Methods | Study design: open‐label, randomised study | |
| Participants |
Diagnosis: diagnosed as bipolar disorder ‐ mania Method of diagnosis: according to DSM‐III‐R criteria Age: "adults" Sex: female 8; male 35 Location: Department of Psychiatry, K.G.'s Medical College, Lucknow Co‐morbidities: not described Adjunctive therapy: not described Adjunctive medications: not described |
|
| Interventions | Participants were randomly assigned to either: Experimental arm ‐ lithium N = 11 Duration: 28 days Treatment protocol: "On day 'O' the selected participants were started on lithium (900 mg/day). Oral diazepam was given on SOS basis. The participants continued on the same medication, subject to appearance of serious side‐effects when the dosage were reduced, till day 21. By this time, if a reduction of 50% of the initial BRMS score was not achieved, the dose was increased to 1200 mg/day Therapist/face‐to‐face contact: not described Comparator arm ‐ carbamazepine N = 14 Duration: 28 days Treatment protocol: "On day 'O' the selected participants were started on carbamazepine (200 mg/day) which was built up to 800 mg/day by the end of first week. Oral diazepam was given on SOS basis. The participants continued on the same medication, subject to appearance of serious side‐effects when the dosage were reduced, till day 21. By this time, if a reduction of 50% of the initial BRMS score was not achieved, the dose was increased to 1200 mg/day. Therapist/face‐to‐face contact: not described Comparator arm ‐ haloperidol Duration: 28 days Treatment protocol: "On day 'O' the selected participants were started on haloperidol (15 mg/day). Oral trihexyphenidyl (6 mg/day) was also given. Oral diazepam was given on SOS basis. The participants continued on the same medication, subject to appearance of serious side‐effects when the dosage were reduced, till day 21. By this time, if a reduction of 50% of the initial BRMS score was not achieved, the dose ws increased to 20 mg/day. |
|
| Outcomes |
Timepoints for assessment: days 7, 14, 21, and 28 Primary outcome:
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "random" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Out of 61 participants selected for the study only 43 (male = 35, female = 8) completed the study |
| Selective reporting (reporting bias) | Unclear risk | Well reported |
| Other bias | Low risk | None identified |
AIMS: Abnormal Involuntary Movement Scale; BMD‐I: bipolar mood disorder‐I; BMI: body mass index; BPRS: Brief Psychiatric Rating Scale; BRMS: Bech‐Rafaelsen Mania Scale; CGI‐C; Clinical Global Impression‐change; CGI‐S: Clinical Global Impression‐severity; CIDI: Composite International Diagnostic Interview; CDRS: Children's Depression Rating Scale; CGAS: Children's Global Assessment Scale; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, fourth edition; ECT: electroconvulsive therapy; EPS: extrapyramidal symptoms; g: gram; GAF: Global Assessment of Functioning; GAS: Global Assessment Scale; IMPS: Inpatient Multidimensional Psychiatric Scale; kg: kilogram; KMRS: School‐Age Children Mania Rating Scale; Li: lithium; MADRS: Montgomery‐Åsberg Depression Rating Scale; MAS: Modified Ashworth Scale; mEq/L: milliequivalents per litre; mg: milligram; mg/mL: milligram per millilitre; MINI: Mini International Neuropsychiatric Interview; mmol/L: millimoles per litre; MRS: Mania Rating Scale; MSRS: Manic‐State Rating Scale; n: number; NOSIE: Nurses' Observation Scale for Inpatient Evaluation; N/A: not applicable; PANSS: Positive and Negative Syndrome Scale; PEF: peak expiratory flow; PIP: Personal Independence Payment; SADS: Schedule for Affective Disorders and Schizophrenia; SAS: Simpson Angus Scale; SCID: Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, fourth edition; SD: standard deviation; μg/mL: microgram per millilitre; VPA: valproate; YMRS: Young Mania Rating Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Axelson 2011 | Unclear diagnostic criteria, unclear participant characteristics, unclear if open or blinded, unclear randomisation |
| Bowden 2008 | Open‐label study |
| Brockington 1978 | Nonstandard diagnostic process. Not clearly participants with bipolar disorder. Unclear if randomised or not |
| Calabrese 2002 | Mixture of depressed, euthymic and manic participants. No subgroup of manic participants presented |
| Calabrese 2005 | Complex study. Recruited participants who were either euthymic or manic. Those who were manic had an open‐label stabilisation prior to randomisation. Then were randomised for maintenance therapy. Not an acute mania study. |
| Chou 2009 | Lithium in both arms of the study |
| Christie 1989 | Open‐label study |
| El‐Mallakh 2012 | Maintenance study ‐ participants not acutely manic |
| Giannini 1984 | Not a RCT |
| Giannini 1986 | Clonidine is not one of the agreed comparators |
| Goodwin 1979 | Lithium vs 'neuroleptics'. No individual drug data |
| Johnson 1971 | No standard diagnostic criteria/method of diagnosis |
| Kwon 2001 | Mixture of depressed and manic participants |
| NCT00314184 | Maintenance study ‐ participants not acutely manic |
| NCT01166425 | Study protocol. Appears to have either not occurred or was replaced by the study which is Findling 2015 |
| Nieto 2014 | Naturalistic study, non‐randomised |
| Okuma 1990 | Unclear diagnostic criteria |
| Pavuluri 2004 | Open‐label study and group with Lithium only n = 4 (valproate vs placebo vs placebo + lithium) |
| Pokorny 1974 | Mixture of participants ‐ unipolar and bipolar disorders in all mood states. Unclear diagnostic criteria |
| Swann 2001 | Randomisation occurred after mood stabilisation ‐ so this was a maintenance study |
| Takahashi 1975 | Unclear diagnostic criteria. Unblinded. Non validated outcome measures |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Grunze 2006.
| Methods | This is a conference abstract for a study examining the effects of valproate and lithium in acute and continuation treatment of bipolar mania. The abstract doesn't include any results data, randomisation or blinding strategies and we were unable to get any further details of this study. |
| Participants | Adults with a diagnosis of bipolar disorder in a manic or mixed episode |
| Interventions | Lithium carbonate or sodium valproate |
| Outcomes | CHange in YMRS from baseline to end of study. Other outcomes not reported. |
| Notes | ‐ |
Itoh 1974.
| Methods | This appears to be a double‐blind comparison of lithium carbonate and chlorpromazine in mania but we were unable to retrieve the full‐text. It may be the same study as Takahashi (excluded) as this was the same research group. |
| Participants | Chinese adults with bipolar disorder in a manic episode |
| Interventions | Lithium vs. chlorpromazine |
| Outcomes | Unknown |
| Notes | ‐ |
Kumar 2009.
| Methods | Kumar 2009 is a conference abstract for a study comparing the efficacy and side effects of lamotrigine compared with lithium in acute mania. The methodology is unclear and there is no efficacy data reported, only side effects. We were unable to get further information. |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Maggs 1963.
| Methods | This is a comparative study of lithium carbonate in the treatment of manic illness but the full‐text report is ambiguous in methodology. This is likely to be related to the publication date of 1963. It seems to be a lithium/placebo cross‐over study but this is only vaguely described. It is unclear what form of randomisation (if done as now understood) occurred. |
| Participants | Male and female inpatients admitted to a single hospital ward. Exact number enrolled unclear. |
| Interventions | Lithium ‐ in a liquid form and a tablet form, vs. a 'placebo' of syrup of ginger |
| Outcomes | Clinical improvement (qualitative) and ratings on Wittenborn scale. |
| Notes | Would need further detailed methodology to clear up if fitted inclusion criteria. Doesn't use any of stated outcome measures. |
NCT00183443.
| Methods | A 12‐week, double‐blind, placebo‐controlled ambulatory study |
| Participants | n = 75 with bipolar I disorder with hypomanic, manic, or mixed episodes |
| Interventions | Open‐label divalproex plus adjunctive blinded lithium carbonate, quetiapine or placebo |
| Outcomes | Symptoms of mania, as measured by YMRS (time frame: week 12) |
| Notes |
NCT00183443 (study results available January 2019) |
NCT00893581.
| Methods | Randomised, double‐blind, controlled treatment study (6 weeks) |
| Participants | Youth with first‐episode mania (n = 110; ages 10‐17 years) Group‐matched healthy comparison youth (n = 62) were also recruited |
| Interventions | Lithium vs quetiapine |
| Outcomes | Neurofunctional effects: fMRI scans; event‐related, voxel‐wise group comparisons |
| Notes | NCT00183443 |
Penick 1971.
| Methods | Penick 1971 is another conference abstract for a study comparing lithium carbonate and chlorpromazine in the treatment of manic states, but we were unable to contact the authors or to get further details of this study. |
| Participants | Unknown |
| Interventions | Unknown |
| Outcomes | Unknown |
| Notes | Penick SB, Prien RF. Controlled evaluation of lithium carbonate and chlorpromazine in the treatment of manic states. V World Congress of Psychiatry, 1971 Nov 28‐Dec 4, Ciudad De Mexico. 1971:941. |
Young 2017.
| Methods | Double‐blind, RCT |
| Participants | n = 224 inpatients and outpatients aged ≥ 60 years with bipolar I disorder who presented with a manic, hypomanic, or mixed episode |
| Interventions | Lithium (target serum concentration, 0.80‐0.99 muq/L) or divalproex (target serum valproate concentration, 80‐99 mug/mL) for 9 weeks |
| Outcomes | YMRS scores |
| Notes |
NCT00254488/ GERI‐BD Trial (study results available Jan 2019) |
fMRI: functional magnetic resonance imaging
n: number
RCT: randomised controlled trial
YMRS: Young Mania Rating Scale
Characteristics of ongoing studies [ordered by study ID]
NCT01893229.
| Trial name or title | Comparative efficacy and acceptability of antimanic drugs in acute mania |
| Methods | RCT |
| Participants | "Participants with a DSM‐IV diagnosis of bipolar I disorder, manic or mixed episode were randomly assigned to a treatment of lithium, valproate, oxcarbazepine, quetiapine, olanzapine, or ziprasidone. In the following conditions, participants took another antimanic drug as a combination medication: 1) those who have a reduction in YMRS scores less than 25% after one week of treatment; 2) those who have a reduction in YMRS scores less than 50% after two weeks of treatment; or 3) those who have a increase in YMRS more than 30% at day 4. An antipsychotic (quetiapine, olanzapine or ziprasidone) were added on for those who use lithium, valproate or oxcarbazepine as a first attempted medication; while lithium, valproate, or oxcarbazepine was added on for those who use an antipsychotic as a first attempted medication. Those participants who were recognized as non‐response/partial response to two combined medications after 6 weeks of treatment switched to MECT." |
| Interventions | As above |
| Outcomes |
"Primary outcomes
Secondary outcomes
Response criteria: < 25% reduction in YMRS scores or ≥ 4 scores of CGI is defined as non‐response. 25%‐49% reduction in YMRS scores from baseline as well as ≤ 3 scores of CGI is recognised as partial response ≥ 50% reduction in YMRS as well as 1 (very much improved) or 2 scores (much improved) of CGI is recognised as response. Remission is defined as a YMRS score ≤ 12 and CGI score 1 or 2" |
| Starting date | 2015 |
| Contact information | Contact: Guiyun Xu, MD, 86(02081891425) ext 8111 |
| Notes | ‐ |
BPRS: Brief Psychiatric Rating Scale
CGI: Clinical Global Impressions
DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, fourth edition
GAS: Global Assessment Scale
MECT: modified electroconvulsive therapy
RCT: randomised controlled trial
TESS: Treatment Emergent Symptom Scale
YMRS: Young Mania Rating Scale
Differences between protocol and review
Not all studies reported outcomes that were within our predetermined list of primary and secondary outcomes: this was especially the case for studies published a long time ago. In that situation, we extracted important efficacy and adverse events data in the units in which the study authors had chosen to express them (e.g. different scales, length of weeks of treatment).
-
Our protocol included that we would complete sensitivity analyses in the areas listed ‐ we did not do this for the reasons given below.
Excluding studies that recruited participants with treatment‐resistant mania: we did not find any studies that gave us this information
Excluding studies with lithium as add‐on treatment: we did not find any studies that used lithium as an add‐on and also fitted all our inclusion criteria
Excluding studies with a dropout rate greater than 20%: as the number of studies we found in the majority of our outcomes was very small, excluding these from the sensitivity analysis would have meant not being able to meta‐analyse at all, but we have acknowledged this within the GRADE ratings
We also planned to do the following sensitivity analyses.
Lithium alone and studies using lithium with a mood stabiliser or antipsychotic: we did not include any studies using lithium plus a mood stabiliser or antipsychotic
If data were available, analysis by length of treatment to ascertain whether any treatment differences detected vary with time ‐ there was not adequate data to do this, except for lithium versus carbamazepine where we have included the data for mean length of treatment in weeks.
Our comparators included ECT. We had prespecified we would only include blinded studies but this was the only available evidence for lithium versus ECT and it was single‐blinded, so for completeness of the review we decided to include this but make it clear within the GRADE ratings.
We pre‐specified time points of 3, 6, 8 and 12 weeks for data analysis. As the data in many areas were extremely limited, we included data at the end point of the study, as long as this was at least seven days. The majority were 21 to 28 days.
A peer reviewer suggested including an analysis of lithium versus all comparators. This was included as comparison 15.
-
We intended to included four outcomes in the 'Summary of findings' tables. As the outcomes actually available differed from those outlined in the protocol, we included the most important for each comparison, trying to stick to the four categories below as far as possible:
response;
remission;
main adverse events;
total withdrawal from the study.
Many of the analyses had moderate to high heterogeneity; for these, we used a random‐effects model. For those with low heterogeneity we were able to use a fixed‐effect model.
Contributions of authors
RMK screened all abstracts and full texts, extracted data, interpreted results and drafted the manuscript. EC and BA screened abstracts/full texts until 2014 and extracted data relating to that period. SdLM screened abstracts/full texts pertaining to 2014 to 2018, extracted data relating to that period and helped with drafting of the manuscript. AC and JG initiated the review. AC also provided guidance and support with following methodology and drafting the manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
RM is an NIHR Academic Clinical Fellow. JRG is an NIHR Senior Investigator. AC is supported by the NIHR Oxford Cognitive Health Clinical Research Facility, by an NIHR Research Professorship (grant RP‐2017‐08‐ST2‐006) and by the NIHR Oxford Health Biomedical Research Centre (grant BRC‐1215‐20005).
Declarations of interest
Rebecca F McKnight: no conflicts of interest Saïk JGN de La Motte de Broöns de Vauvert: no conflicts of interest Edward Chesney: no conflicts of interest Ben H Amit: no conflicts of interest John Geddes: no conflicts of interest Andrea Cipriani: no conflicts of interest
New
References
References to studies included in this review
Astra Zeneca 2009 {published data only}
- Astra Zeneca. An international, multicenter, double‐blind, randomized, placebo‐controlled, phase IV study of the safety and efficacy of lithium versus placebo as an add on to SEROQUEL XR (quetiapine fumarate) in adult patients with acute mania. AstraZeneca Clinical Trials 2009.
Banga 2003 {published data only}
- Banga N, Goswami U, Kohli K. A comparison of efficacy and tolerability of lithium monotherapy and valoproate monotherapy in mania. Journal of the Association of Physicians of India: Scientific Abstracts of the 59th Annual conference of the association of physicians of India. 2003;51:1158 (Abstract no, 37, pg 10). [Google Scholar]
Barekatain 2005 {published data only}
- Barekatain M, Fatemi A, Bashardoost N, Darougheh A, Salehi M, Asadollahi GH. Valproate‐risperidone valproate‐lithium combination in acute mania. Journal of Research in Medical Sciences 2005;10(5):274‐80. [Google Scholar]
Berk 1999 {published data only}
- Berk M, Ichim L, Brook S. Olanzapine compared to lithium in mania: a double‐blind randomized controlled trial. International Clinical Psychopharmacology 1999;14:339‐43. [DOI] [PubMed] [Google Scholar]
Bowden 1994 {published data only}
- Bowden CL, Brugger AM, Swann AC, Calabrese JR, Janicak PG, Petty F, et al. Efficacy of divalproex vs lithium and placebo in the treatment of mania. The Depakote Mania Study Group. Journal of the American Medical Association 1994;271:918‐24. [PubMed] [Google Scholar]
- Bowden CL, Calabrese JR, Wallin BA, Swann AC, McElroy SL, Risch SC, et al. Illness characteristics of patients in clinical drug studies of mania. Psychopharmacology Bulletin 1995;31(1):103‐9. [PubMed] [Google Scholar]
Bowden 2005 {published data only}
- Bowden CL, Grunze H, Mullen J, Brecher M, Paulsson B, Jones M, et al. A randomized, double‐blind, placebo‐controlled efficacy and safety study of quetiapine or lithium as monotherapy for mania in bipolar disorder. Journal of Clinical Psychiatry 2005;66:111‐21. [DOI] [PubMed] [Google Scholar]
Bowden 2010 {published data only}
- Bowden CL, Mosolov S, Hranov L, Chen E, Habil H, Kongsakon R, et al. Efficacy of valproate versus lithium in mania or mixed mania: a randomized, open 12‐week trial. International Clinical Psychopharmacology 2010;25(2):60‐70. [DOI] [PubMed] [Google Scholar]
Chouinard 1983 {published data only}
- Chouinard G, Young SN, Annable L. Antimanic effect of clonazepam. Biological Psychiatry 1983;18:451‐66. [PubMed] [Google Scholar]
- Chouinard, D. Antimanic effects of clonazepam. Psychosomatics 1985;26(12):7‐12. [DOI] [PubMed] [Google Scholar]
Clark 1996 {published data only}
- Clark HM, Berk M, Brook S. A randomized controlled single blind study of the efficacy of clonazepam and lithium in the treatment of acute mania. Human Psychopharmacology 1997;12:325‐8. [Google Scholar]
Findling 2015 {published data only}
- Findling RA, Robb A, McNamara NK, Pavuluri MN, Kafantaris V, Scheffer R, et al. Lithium in the acute treatment of bipolar I disorder: a double‐blind, placebo‐controlled study. Pediatrics 2015;136:885‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freeman 1992 {published data only}
- Freeman TW, Clothier JL, Pazzaglia P, Lesem MD, Swann AC. A double‐blind comparison of valproate and lithium in the treatment of acute mania. American Journal of Psychiatry 1992;149:108‐11. [DOI] [PubMed] [Google Scholar]
Garfinkel 1980 {published data only}
- Garfinkel PE, Stancer HC, Persad E. A comparison of haloperidol, lithium carbonate and their combination in the treatment of mania. Journal of Affective Disorders 1980;2:279‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Geller 2012 {published data only}
- Geller B, Luby JL, Joshi P, Wagner KD, Emslie G, Walkup JT, et al. A randomized controlled trial of risperidone, lithium, or divalproex sodium for initial treatment of bipolar I disorder, manic or mixed phase, in children and adolescents. Archives of General Psychiatry 2012;69(5):515‐28. [10.1001/archgenpsychiatry.2011.1508] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00057681. Study of outcome and safety of lithium, divalproex and risperidone for mania in children and adolescents (TEAM). clinicaltrials.gov/ct2/show/NCT00057681 (first received 7 April 2003).
- Salpekar JA, Joshi PT, Axelson DA, Reinblatt SP, Yenokyan G, Sanyal A, et al. Depression and suicidality outcomes in the treatment of early age mania study. Journal of the American Academy of Child and Adolescent Psychiatry 2015;54(999–1007):e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello B, Riddle MA, Yenokyan G, Axelson DA, M, Wagner KD, Joshi P, et al. Treatment moderators and predictors of outcome in the Treatment of Early Age Mania (TEAM) study. Journal of the American Academy of Child and Adolescent Psychiatry 2012;51:867‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
GlaxoSmithKline 2005 {published data only}
- GlaxoSmithKline. A six‐week, multicenter, double‐blind, placebo‐controlled, fixed‐dose evaluation of the safety and efficacy of lamotrigine compared to placebo and lithium in the treatment of an acute manic episode in patients who have bipolar disorder [SCAB2009]. GSK ‐ clinical study register (www.gsk‐studyregister.com) 2005.
- Goldsmith DR, Wagstaff AJ, Ibbotson T, Perry CM. Lamotrigine: a review of its use in bipolar disorder. Drugs 2003;63(19):2029‐50. [DOI] [PubMed] [Google Scholar]
GlaxoSmithKline 2008 {published data only}
- GlaxoSmithKline. A 3 week multicenter, double‐blind, placebo‐controlled evaluation of the safety and efficacy of LAMICTAL (lamotrigine) compared to placebo in the treatment of an acute manic or mixed episode in patients who have bipolar disorder. GSK ‐ clinical study register (www.gsk‐clinicalstudyregister.com) 2008.
Gouliaev 1996 {published data only}
- Gouliaev G, Licht RW, Vestergaard P, Merinder L, Lund H, Bjerre L. Treatment of manic episodes: zuclopenthixol and clonazepam versus lithium and clonazepam. Acta Psychiatrica Scandinavica 1996;93(2):119‐24. [DOI] [PubMed] [Google Scholar]
Hirschfeld 1999 {published data only}
- Hirschfeld RM, Allen MH, McEvoy JP, Keck PE Jr, Russell JM. Safety and tolerability of oral loading divalproex sodium in acutely manic bipolar patients. Journal of Clinical Psychiatry 1999;60:815‐8. [DOI] [PubMed] [Google Scholar]
Ichim 2000 {published data only}
- Ichim L, Berk M, Brook S. Lamotrigine compared with lithium in mania: a double‐blind randomized controlled trial. Annals of Clinical Psychiatry 2000;12:5‐10. [DOI] [PubMed] [Google Scholar]
Keck 2009 {published data only}
- Keck PE, Orsulak PJ, Cutler AJ, Sanchez R, Torbeyns A, Marcus RN, et al. The CN138‐135 Study Group. Aripiprazole monotherapy in the treatment of acute bipolar I mania: a randomized, double‐blind, placebo‐ and lithium‐controlled study. Journal of Affective Disorders 2009;112:36‐49. [DOI: 10.1016/j.jad.2008.05.014] [DOI] [PubMed] [Google Scholar]
Kowatch 2000 {published data only}
- Kowatch RA, Suppes T, Carmody TJ, Bucci JP, Hume JH, Kromelis M, et al. Effect size of lithium, divalproex sodium, and carbamazepine in children and adolescents with bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2000;39:713‐20. [DOI] [PubMed] [Google Scholar]
Kushner 2006 {published data only}
- Kushner SF, Khan A, Lane R, Olson WH. Topiramate monotherapy in the management of acute mania: results of four double‐blind placebo‐controlled trials. Bipolar disorders 2008;8(1):15‐27. [DOI] [PubMed] [Google Scholar]
- NCT00035230 [Kushner 2006 PDMD‐008]. A study on the safety and efficacy of topiramate in the treatment of patients with bipolar I disorder [A randomized, double‐blind, multicenter, placebo‐controlled 12‐week study of the safety and efficacy of topiramate in patients with acute manic or mixed episodes of bipolar I disorder with an optional open‐label extension]. clinicaltrials.gov/ct2/show/NCT00035230 (first received 3 May 2002).
- NCT00037674 [Kushner 2006 PDMD‐004]. A study on the safety and efficacy of topiramate in the treatment of patients with Bipolar I Disorder [A randomized, double‐blind, multicenter, placebo‐controlled 12‐week study of the safety and efficacy of two doses of topiramate for the treatment of acute manic or mixed episodes in patients with bipolar i disorder with an optional open‐label extension]. clinicaltrials.gov/ct2/show/NCT00037674 (first received 21 May 2002).
Lerer 1987 {published data only}
- Lerer B, Moore N, Meyendorff E, Cho SR, Gershon S. Carbamazepine versus lithium in mania: a double‐blind study. Journal of Clinical Psychiatry 1987;48:89‐93. [MEDLINE: ] [PubMed] [Google Scholar]
Li 2008 {published data only}
- Li H, Ma C, Wang G, Zhu X, Peng M, Gu N. Response and remission rates in Chinese patients with bipolar mania treated for 4 weeks with either quetiapine or lithium: a randomized and double‐blind study. Current Medical Research and Opinion 2008;24(1):1‐10. [DOI] [PubMed] [Google Scholar]
- NCT00448578. Efficacy and safety of seroquel and lithium as monotherapy in acute mania treatment in bipolar disorder patients (STAR). clinicaltrials.gov/ct2/show/record/NCT00448578 (first received 19 March 2007).
Lusznat 1988 {published data only}
- Lusznat RM, Murphy DP, Nunn CMH. Carbamazepine vs lithium in the treatment and prophylaxis of mania. British Journal of Psychiatry 1988;153:198‐204. [DOI] [PubMed] [Google Scholar]
Niufan 2008 {published data only}
- NCT00485680. Olanzapine versus comparator in the treatment of bipolar disorder. clinicaltrials.gov/ct2/show/record/NCT00485680 (first received 13 June 2007).
- Niufan G, Tohen M, Qiuqing A, Fude Y, Pope E, McElroy H, et al. Olanzapine versus lithium in the acute treatment of bipolar mania: a double‐blind, randomized, controlled trial. Journal of Affective Disorders 2008;105(1‐3):101‐8. [DOI] [PubMed] [Google Scholar]
Platman 1970 {published data only}
- Platman SR. A comparison of lithium carbonate and chlorpromazine in mania. American Journal of Psychiatry 1970;127:351‐3. [DOI] [PubMed] [Google Scholar]
Prien 1972 {published data only}
- Prien RF, Caffey EM Jr, Klett CJ. Comparison of lithium carbonate and chlorpromazine in the treatment of mania. Report of the Veterans Administration and National Institute of Mental Health Collaborative Study Group. Archives of General Psychiatry 1972;26:146‐53. [DOI] [PubMed] [Google Scholar]
Segal 1998 {published data only}
- Segal J, Berk M, Brook S. Risperidone compared with both lithium and haloperidol in mania: a double‐blind randomized controlled trial. Clinical Neuropharmacology 1998;21:176‐80. [PubMed] [Google Scholar]
Shafti 2008 {published data only}
- Shafti SS, Shahveisi B. Comparison between lithium and valproate in the treatment of acute mania. Journal of Clinical Psychopharmacology 2008;28(6):718‐20. [DOI] [PubMed] [Google Scholar]
Shafti 2010 {published data only}
- Shafti SS. Olanzapine vs. lithium in management of acute mania. Journal of Affective Disorders 2010;122(3):273‐6. [DOI] [PubMed] [Google Scholar]
Shopsin 1975 {published data only}
- Shopsin B, Gershon S, Thompson H, Collins P. Psychoactive drugs in mania. A controlled comparison of lithium carbonate, chlorpromazine, and haloperidol. Archives of General Psychiatry 1975;32:34‐42. [DOI] [PubMed] [Google Scholar]
Small 1988 {published data only}
- Small JG, Klapper MH, Kellams JJ, Miller MJ, Milstein V, Sharpley PH, et al. Electroconvulsive treatment compared with lithium in the management of manic states. Archives of General Psychiatry 1988;45(8):727‐32. [DOI] [PubMed] [Google Scholar]
Small 1991 {published data only}
- Small JG, Klapper MH, Milstein V, Kellams JJ, Miller MJ, Marhenke JD, et al. Carbamazepine compared with lithium in the treatment of mania. Archives of General Psychiatry 1991;48:915‐21. [DOI] [PubMed] [Google Scholar]
Spring 1970 {published data only}
- Spring G, Schweid D, Gray C, Steinberg J, Horwitz M. A double‐blind comparison of lithium and chlorpromazine in the treatment of manic states. American Journal of Psychiatry 1970;126:1306‐10. [DOI] [PubMed] [Google Scholar]
Trivedi 1996 {published data only}
- Trivedi JK, Lata A, Dalal PK, Sinha PK, Srivastava SA. Comparative study of side‐effects of lithium, carbamazepine and haloperidol in acute mania. Indian Journal of Psychiatry 1996;38(4):248‐9. [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Axelson 2011 {published data only}
- Axelson D. The treatment of early‐age mania study (TEAM): primary outcomes conference abstract. Biological Psychiatry (abstracts from the 66th Annual Meeting of the Society of Biological Psychiatry San Francisco, CA United States) 2011;69(9):S1. [Google Scholar]
Bowden 2008 {published data only}
- Bowden C, Gogus A, Grunze H, Haggstrom L, Rybakowski J, Vieta E. A 12‐week, open, randomized trial comparing sodium valproate to lithium in patients with bipolar I disorder suffering from a manic episode. International Clinical Psychopharmacology 2008;23(5):252‐62. [DOI] [PubMed] [Google Scholar]
Brockington 1978 {published data only}
- Brockington IF, Kendell RE, Curry SH, Wainwright S. Trials of lithium, chlorpromazine and amitriptyline in schizoaffective patients. British Journal of Psychiatry 1978;133:162‐8. [DOI] [PubMed] [Google Scholar]
Calabrese 2002 {published data only}
- Calabrese JR. Lamotrigine maintenance treatment in bipolar disorder. XII World Congress of Psychiatry 2002.
Calabrese 2005 {published data only}
- Calabrese JR, Shelton MD, Rapport DJ, Youngstrom EA, Jackson K, Bilali S, et al. A 20‐month, double‐blind, maintenance trial of lithium versus divalproex in rapid‐cycling bipolar disorder. American Journal of Psychiatry 2005;162(11):2152‐61. [DOI] [PubMed] [Google Scholar]
Chou 2009 {published data only}
- Chou JC, Czobor P, Charles O, Tuma I, Winsberg B, Allen MH. Acute mania: haloperidol dose and augmentation with lithium or lorazepam. Journal of Clinical Psychopharmacology 1999;19(6):500‐5. [DOI] [PubMed] [Google Scholar]
Christie 1989 {published data only}
- Christie JE, Whalley LJ, Hunter R, Bennie J, Fink G. Sulpiride treatment of acute mania with a comparison of the effects on plasma hormone concentrations of lithium and sulpiride treatment. Journal of Affective Disorders 1989;16(2‐3):115‐20. [DOI] [PubMed] [Google Scholar]
El‐Mallakh 2012 {published data only}
- El‐Mallakh RS, Marcus R, Baudelet C, McQuade R, Carson WH, Owen R. A 40‐week double‐blind aripiprazole versus lithium follow‐up of a 12‐week acute phase study (total 52 weeks) in bipolar I disorder. Journal of Affective Disorders 2012;136(3):258‐66. [DOI] [PubMed] [Google Scholar]
Giannini 1984 {published data only}
- Giannini AJ, Houser WL Jr, Loiselle RH, Giannini MC, Price WA. Antimanic effects of verapamil. American Journal of Psychiatry 1984;141:1602‐3. [DOI] [PubMed] [Google Scholar]
Giannini 1986 {published data only}
- Giannini AJ, Pascarzi GA, Loiselle RH, Price WA, Giannini MC. Comparison of clonidine and lithium in the treatment of mania. American Journal of Psychiatry 1986;143:1608‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Goodwin 1979 {published data only}
- Goodwin FK, Zis AP. Lithium in the treatment of mania: comparisons with neuroleptics. Archives of General Psychiatry 1979;36:840‐4. [DOI] [PubMed] [Google Scholar]
Johnson 1971 {published data only}
- Johnson G, Gershon S, Burdock EI, Floyd A, Hekimian L. Comparative effects of lithium and chlorpromazine in the treatment of acute manic states. British Journal of Psychiatry 1971;119(550):267‐76. [DOI] [PubMed] [Google Scholar]
Kwon 2001 {published data only}
- Kwon YJ, Jeong HY, Park IJ. Lamotrigine and lithium in the treatment of acute bipolar disorder. Journal of the Korean Neuropsychiatric Association 2001;40(5):885‐92. [Google Scholar]
NCT00314184 {published and unpublished data}
- NCT00314184. Multicenter, randomized, parallel‐group, double‐blind, placebo‐controlled phase 3 study of the efficacy & safety of quetiapine fumarate & lithium as monotherapy in 28‐104 weeks maintenance treatment of Bipolar I. clinicaltrials.gov/ct2/show/NCT00314184 (first received 13 April 2006).
NCT01166425 {published data only}
- NCT01166425. A randomized, double‐blind, placebo controlled study of the efficacy of lithium for the treatment of pediatric mania followed by an open label long‐term safety period, double‐blind, placebo‐controlled discontinuation phase, and open label restabilization period. clinicaltrials.gov/show/NCT01166425 (first received 21 July 2010). [PubMed]
Nieto 2014 {published data only}
- Nieto E, Plans L, Carreras J, Gomez A. Naturalistic study of either lithium or valproate in combination treatment with antipsychotics for pure manic inpatients. European Neuropsychopharmacology 2009;24(S2):S427. [Google Scholar]
Okuma 1990 {published data only}
- Okuma T, Yamashita I, Takahashi R, Itoh H, Otsuki S, Watanabe S, et al. Comparison of the antimanic efficacy of carbamazepine and lithium carbonate by double‐blind controlled study. Pharmacopsychiatry 1990;23(3):143‐50. [DOI] [PubMed] [Google Scholar]
Pavuluri 2004 {published data only}
- Pavuluri MN, Henry DB, Carbray JA, Sampson G, Naylor MW, Janicak PG. Open‐label prospective trial of risperidone in combination with lithium or divalproex sodium in pediatric mania. Journal of Affective Disorders 2004;82 Suppl 1:S103‐11. [PUBMED: 15571784] [DOI] [PubMed] [Google Scholar]
Pokorny 1974 {published data only}
- Pokorny AD, Prien RF. Lithium in treatment and prevention of affective disorder: a VA NIMH collaborative study. Diseases of the Nervous System 1974;35(7):327‐33. [PubMed] [Google Scholar]
Swann 2001 {published data only}
- Swann AC. Prediction of treatment response in acute mania: controlled clinical trials with divalproex. Encephale 2001;27(3):277‐9. [PubMed] [Google Scholar]
Takahashi 1975 {published data only}
- Takahashi R, Sakuma A, Itoh K, Itoh H, Kurihara M. Comparison of efficacy of lithium carbonate and chlorpromazine in mania. Report of collaborative study group on treatment of mania in Japan. Archives of General Psychiatry 1975;32(10):1310‐8. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Grunze 2006 {published and unpublished data}
- Grunze H. Efficacy and safety of valproate and lithium in acute and continuation treatment of bipolar mania. Abstract from 2nd Biennial Conference of the Association of Bipolar Disorders. 2006.
Itoh 1974 {published data only}
- Itoh K. Comparison of efficacy of lithium carbonate and chlorpromazine in mania by double‐blind controlled study. Rinsho Hyoka 1974;2(1):23‐45. [Google Scholar]
Kumar 2009 {published data only}
- Kumar M. The efficacy and side effect profile of lamotrigine in acute mania: a double‐blind comparison with lithium conference abstract. Indian Journal of Psychiatry (abstracts from the 61st Annual National Conference of Indian Psychiatric Society, ANCIPS Agra India) 2009;Not stated:Not stated. [Google Scholar]
Maggs 1963 {published data only}
- Maggs R. Treatment of manic illness with lithium carbonate. British Journal of Psychiatry 1963;109:56‐65. [Google Scholar]
NCT00183443 {published data only}
- Fischer E, Cosgrove V, Suppes T, Lange NK, Gwizdowski I, Doan G, et al. Efficacy and tolerability of divalproex sodium plus adjunctive quetiapine, lithium, or placebo for hypomanic or manic episodes in outpatients with bipolar I disorder [abstract P‐140]. Bipolar Disorders (20th annual conference of the international society for bipolar disorders) 2018; Vol. 20:116.
- NCT00183443. Treatment of mania symptoms with drug therapy [Divalproex extended release and placebo, lithium, or quetiapine for mania]. clinicaltrials.gov/ct2/show/NCT00183443 (first received 16 September 2005).
NCT00893581 {published data only}
- DelBello M, Duran L, Strawn J, Klein C, Welge J, Blom T, et al. Neural markers of treatment effects and response in youth with first‐episode mania [64th Annual Meeting American Academy of Child and Adolescent Psychiatry, AACAP 2017]. Journal of the American Academy of Child and Adolescent Psychiatry 2017;56(10):S137. [Google Scholar]
- NCT00893581. Multimodal neuroimaging of treatment effects in adolescent mania. clinicaltrials.gov/ct2/show/NCT00893581 (first received 6 May 2009).
Penick 1971 {published data only}
- Penick SB, Prien RF. Controlled evaluation of lithium carbonate and chlorpromazine in the treatment of manic states. World Congress of Psychiatry 1971;Not stated:Not stated. [V World Congress of Psychiatry, 1971 Nov 28‐Dec 4, Ciudad De Mexico: 941] [Google Scholar]
Young 2017 {published data only}
- Gyulai L. GERI‐BD: first randomized, double blind controlled trial comparing lithium and valproate in late‐life mania [conference abstract]. Bipolar Disorders (10th international conference on bipolar disorder of the international‐society‐for‐bipolar‐disorders). 2013; Vol. 15:30.
- NCT00254488. Treatment of bipolar mania in older adults (GERI‐BD). clinicaltrials.gov/ct2/show/NCT00254488 (first received 16 November 2005).
- Young RC, Mulsant BH, Sajatovic M, Gildengers AG, Gyulai L, Al Jurdi RK, et al. GERI‐BD: A randomized double‐blind controlled trial of lithium and divalproex in the treatment of mania in older patients with bipolar disorder. American Journal of Psychiatry 2017;174(11):1086‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT01893229 {unpublished data only}
- NCT01893229. Comparative efficacy and acceptability of antimanic drugs in acute mania [Comparative efficacy and acceptability of lithium, valproate, oxcarbazepine, quetiapine, olanzapine, and ziprasidone in bipolar I disorder, manic or mixed phase]. clinicaltrials.gov/ct2/show/record/NCT01893229 (first received 8 July 2013).
Additional references
Ahluwalia 1982
- Ahluwalia P, Grewaal DS, Singhal RL. Brain gabaergic and dopaminergic systems following lithium treatment and withdrawal. Progress in Neuropsychopharmacology 1981;5(5‐6):527‐30. [DOI] [PubMed] [Google Scholar]
Alsonso 2011
- Alonso J, Petukhova M, Vilagut G, Chatterji S, Heeringa S, Üstün TB, et al. Days out of role due to common physical and mental conditions: results from the WHO World Mental Health surveys. Molecular Psychiatry 2011;16(12):1234‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV). 5th Edition. Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Balazs 2006
- Balazs J, Benazzi F, Rihmer Z, Rihmer A, Akiskal KK, Akiskal HS. The close link between suicide attempts and mixed (bipolar) depression: implications for suicide prevention. Journal of Affective Disorders 2006;91(2‐3):133‐8. [DOI] [PubMed] [Google Scholar]
Bauer 2006
- Bauer M, Grof P, Muller B. Lithium in neuropsychiatry: the comprehensive guide. Abingdon, UK: Informa Healthcare, 2006. [Google Scholar]
Bech 1979
- Bech P, Bolwig TG, Kramp P, Rafaelsen OJ. The Bech‐Rafaelsen Mania Scale and the Hamilton Depression Scale. Acta Psychiatrica Scandinavica 1979;59:420‐30. [DOI] [PubMed] [Google Scholar]
Berk 2007
- Berk M, Dodd S, Callaly P, Berk L, Fitzgerald P, Castella AR, et al. History of illness prior to a diagnosis of bipolar disorder or schizoaffective disorder. Journal of Affective Disorders 2007;103:181‐6. [DOI] [PubMed] [Google Scholar]
Berk 2009
- Berk M. Neuroprogression: pathways to progressive brain changes in bipolar disorder. International Journal of Neuropsychopharmacology 2009;12(4):441‐5. [DOI] [PubMed] [Google Scholar]
BNF 2016
- Joint Formulary Committee. British National Formulary. Vol. 73, London: BMJ Group and Pharmaceutical Press, 2017. [Google Scholar]
BNF 2017
- Joint Formulary Committee. British National Formulary. 70. BMJ Group and Pharmaceutical press, 2015. [Google Scholar]
Bocchetta 2016
- Bocchetta A. Reconsidering risk factors for renal dysfunction in lithium‐treated patients. Evidence‐Based Mental Health 2016;19(4):e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brookes 2004
- Brookes ST, Whitely E, Egger M, Smith GD, Mulheran PA, Peters TJ. Subgroup analyses in randomized trials: risks of subgroup‐specific analyses; power and sample size for the interaction test. Journal of Clinical Epidemiology 2004;57(3):229‐36. [DOI] [PubMed] [Google Scholar]
Burgess 2001
- Burgess SS, Geddes J, Hawton KK, Taylor MJ, Townsend E, Jamison K, et al. Lithium for maintenance treatment of mood disorders. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD003013] [DOI] [PubMed] [Google Scholar]
CANMAT 2018
- Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Bond DJ, Frey BN, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disorders 2018;20(2):97‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 1996
- Chen Y, Dilsaver S. Lifetime rates of suicide attempts among subjects with bipolar and unipolar disorders relative to subjects with other axis I disorders. Biological Psychiatry 1996;39(10):896‐9. [DOI] [PubMed] [Google Scholar]
Cipriani 2011
- Cipriani A, Barbui C, Salanti G, Rendell J, Brown R, Stockton S, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: multiple‐treatments meta‐analysis. Lancet 378;9799:1306‐15. [DOI] [PubMed] [Google Scholar]
Cipriani 2013
- Cipriani A, Hawton K, Stockton S, Geddes JR. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta‐analysis. BMJ 2013;346:f3646. [DOI] [PubMed] [Google Scholar]
De Fazio 2017
- De Fazio, P. Lithium in late‐life mania: a systematic review. Neuropsychiatric Disease and Treatment 2017;13:755‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Zelicourt 2003
- Zelicourt M, Dardennes R, Verdoux H. Frequency of hospitalisations and inpatient care costs of manic episodes. Pharmacoeconomics 2003;21(15):1081‐90. [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Endicott 1978
- Endicott V, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Archives of General Psychiatry 1978;35:837‐48. [DOI] [PubMed] [Google Scholar]
Furukawa 2002
- Furukawa TA, Guyatt GH, Griffith LE. An empirical study of summary effect measures in meta‐analyses. International Journal of Epidemiology 2002;31(1):72‐6. [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7‐10. [DOI] [PubMed] [Google Scholar]
Geddes 2010
- Geddes JR, Goodwin GM, Rendell J. Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): a randomised open‐label trial. Lancet 2010;375(9712):385‐95. [DOI] [PubMed] [Google Scholar]
Geddes 2015
- Geddes JR, Rendell J, Cipriani A, Saunders KE, Attenburrow MJ, Bilderbeck AC, et al. Rationale and design of OxLith: a randomised placebo controlled trial exploring the short‐term physical and psychological effects of lithium on mood instability. Bipolar Disorders 2015;17(S1):110. [Google Scholar]
Goldstein 2015
- Goldstein BI, Carnethon MR, Matthews KA, McIntyre RS, Raghuveer G, Stoney CM, et al. Major depressive disorder and bipolar disorder predispose youth to accelerated atherosclerosis and early cardiovascular disease a scientific statement from the American Heart Association. Circulation 2015;132(10):965‐86. [DOI] [PubMed] [Google Scholar]
Goodwin 1994
- Goodwin G. Recurrence of mania after lithium withdrawal. Implications for the use of lithium in the treatment of bipolar affective disorder. British Journal of Psychiatry 1994;164:149‐52. [DOI] [PubMed] [Google Scholar]
Goodwin 2016
- Goodwin GM, Haddad PM, Ferrier IN, Aronson JK, Barnes T, Cipriani A, et al. Evidence‐based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. Journal of Psychopharmacology 2016;30(6):495‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEproGDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEproGDT. Version accessed 14 April 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guelen 1992
- Guelen PJ, Janssen TJ, DeWitte TC. Bioavailability of lithium from lithium‐citrate syrup versus conventional lithium‐carbonate tablets. Biopharmaceutics & Drug Disposition 1992;13(7):503‐11. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Revised. Rockville, MD: National institute of Mental Health, 1976. [Google Scholar]
Hamilton 1960
- Hamilton M. A Rating Scale for Depression. Journal of Neurology, Neurosurgery and Psychiatry 1960;23:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hayes 2016
- Hayes JF, Marston L, Walters K, Geddes JR, King M, Osborn DP. Lithium vs. valproate vs. olanzapine vs. quetiapine as maintenance monotherapy for bipolar disorder: a population‐based UK cohort study using electronic health records. World Psychiatry 2016;15(1):53‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Kay 1987
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin 1987;13(2):261‐76. [DOI] [PubMed] [Google Scholar]
Laursen 2011
- Laursen TM. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophrenia Research 2011;131(1‐3):101‐4. [DOI] [PubMed] [Google Scholar]
Lorr 1966
- Loor M, Klett CJ. Inpatient Multidimensional Psychiatric Scale. Palo Alto, California: Consulting Psychiatrists' Press, 1966. [Google Scholar]
Malhi 2013
- Malhi GS, Tanious M, Das P, Coulston C, Berk M. Potential mechanisms of action of lithium in bipolar disorder. CNS Drugs 2013;27:135‐53. [DOI] [PubMed] [Google Scholar]
Malhi 2017
- Malhi G, Masson M, Bellivier F. The Science and Practice of Lithium Therapy. 1st Edition. Springer, 2017. [Google Scholar]
Manji 2000
- Manji HK, Lenox RH. Signaling: cellular insights into the pathophysiology of bipolar disorder. Biological Psychiatry 2000;48(6):518‐30. [DOI] [PubMed] [Google Scholar]
McKnight 2012
- McKnight RF, Adida M, Budge K, Stockton S, Goodwin GM, Geddes JR. Lithium toxicity profile: a systematic review and meta‐analysis. Lancet 2012;379(9817):721‐8. [DOI] [PubMed] [Google Scholar]
McKnight 2017
- McKnight RF, Bilderbeck AC, Miklowitz DJ, Hinds C, Goodwin GM, Geddes JR. Longitudinal mood monitoring in bipolar disorder: course of illness as revealed through a short messaging service. Journal of Affective Disorders 2017;223:139‐45. [DOI] [PubMed] [Google Scholar]
MHRA 2018
- The Medicines and Healthcare products Regulatory Agency (MHRA). Valproate use by women and girls. Drug Safety Update 2018;11(9):1. [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Annals of Internal Medicine 2009;151(4):264‐9. [DOI] [PubMed] [Google Scholar]
Montgomery 1979
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134(4):382. [DOI] [PubMed] [Google Scholar]
Montgomery 2000
- Montgomery SA, Keck PE. First international exchange on bipolar disorder. Journal of Affective Disorders 2000;59(Suppl 1):81‐88. [DOI] [PubMed] [Google Scholar]
Ng 2009
- Ng WX, Lau IY, Graham S, Sim K. Neurobiological evidence for thalamic, hippocampal and related glutamatergic abnormalities in bipolar disorder: a review and synthesis. Neuroscience and Biobehavioral Reviews 2009;33(3):336‐54. [DOI] [PubMed] [Google Scholar]
NICE 2014
- National Institute for Health and Care Excellence (NICE). Bipolar disorder: the assessment and management of bipolar disorder in adults, children and young people in primary and secondary care. Clinical Guideline 185. www.nice.org.uk/guidance/cg185 (accessed before 14 February 2019).
NIMH‐NIH 1985
- NIMH‐NIH Consensus Group. Mood disorders: pharmacologic prevention of recurrences. American Journal of Psychiatry 1985;142(4):469‐72. [DOI] [PubMed] [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports 1962;10:799. [Google Scholar]
Pallaskorpi 2017
- Pallaskorpi S, Suominen K, Ketokivi M, Valtonen H, Arvilommi P, Mantere O, et al. Incidence and predictors of suicide attempts in bipolar I and II disorders: a 5‐year follow‐up study. Bipolar Disorders 2017;19(1):13‐22. [DOI] [PubMed] [Google Scholar]
Philips 2013
- Phillips ML, Kupfer DJ. Bipolar disorder diagnosis: challenges and future directions. Lancet 2013;381(9878):1663‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Post 1980
- Post RM, Jimerson DC, Bunney WE. Dopamine and mania: behavioral and biochemical effects of the dopamine receptor blocker pimozide. Psychopharmacology 1980;67(3):297‐305. [DOI] [PubMed] [Google Scholar]
Quiroz 2010
- Quiroz JA, Machado‐Vieira R, Zarate JC. Novel insights into lithium’s mechanism of action: neurotrophic and neuroprotective effects. Neuropsychobiology 2010;62(1):50‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riblet 2017
- Riblet NB, Shiner B, Young‐Xu Y, Watts BV. Strategies to prevent death by suicide: meta‐analysis of randomised controlled trials. British Journal of Psychiatry 2017;210(6):396‐402. [DOI] [PubMed] [Google Scholar]
Samara 2017
- Samara MT, Goldberg Y, Levine SZ, Furukawa TA, Geddes JR, Cipriani A, et al. Initial symptom severity of bipolar I disorder and the efficacy of olanzapine: a meta‐analysis of individual participant data from five placebo‐controlled studies. Lancet Psychiatry 2017;4(11):859‐67. [DOI: 10.1016/S2215-0366(17)30331-0] [DOI] [PubMed] [Google Scholar]
Schou 1993
- Schou M. Is there a lithium withdrawal syndrome? An examination of the evidence. British Journal of Psychiatry 1993;163:514‐8. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials.. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Severus 2014
- Severus E, Taylor MJ, Sauer C, Pfennig A, Ritter P, Bauer M, et al. Lithium for prevention of mood episodes in bipolar disorders: systematic review and meta‐analysis. International Journal of Bipolar Disorders 2014;20(2):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shelley 1986
- Shelley RK, Silverstone T. Single dose pharmacokinetics of 5 formulations of lithium: a controlled comparison in healthy subjects. International Clinical Psychopharmacology 1986;1(4):324‐31. [DOI] [PubMed] [Google Scholar]
Shine 2015
- Shine B, McKnight R, Leaver L, Geddes J. Long‐term effects of lithium on renal, thyroid, and parathyroid function: a retrospective analysis of laboratory data. Lancet 2015;386:461‐8. [DOI] [PubMed] [Google Scholar]
Smith 2007
- Smith LA. Acute bipolar mania: a systematic review and meta‐analysis. Acta Psychiatrica Scandinavia 2007;115:12‐20. [DOI] [PubMed] [Google Scholar]
Smith 2017
- Smith KA, Cipriani A. Lithium and suicide in mood disorders: updated meta‐review of the scientific literature. Bipolar Disorder 2017;19(7):575‐86. [DOI: 10.1111/bdi.12543] [DOI] [PubMed] [Google Scholar]
Spitzer 1970
- Spitzer RL, Gibbon M, Endicott J. ECDEU Assessment Manual for Psychopharmacology. Washington DC: US Department of Health, 1976. [Google Scholar]
Sterne 2017
- Sterne JA, Egger M, Moher D, Boutron I (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Taylor 2014
- Taylor DM. Comparative efficacy and acceptability of drug treatments for bipolar depression: a multi‐treatments meta‐analysis. Acta Psychiatrica Scandinavia 2014;130:452‐69. [DOI] [PubMed] [Google Scholar]
Tsapakis 2002
- Tsapakis EM, Travis MJ. Glutamate and psychiatric disorders. Advances in Psychiatric Treatment 2002;8:189‐97. [Google Scholar]
WHO 1986
- World Health Organization (WHO). WHO Multicentric Collaborative Study. Geneva: World Health Organization, 1986. [Google Scholar]
WHO 1992
- World Health Organization (WHO). The Tenth Revision of the International Classification of Diseases and Related Jealth Problems. 10th Edition. Geneva: World Health Organization, 1992. [Google Scholar]
Yildiz 2011
- Yildiz A, Vieta E, Leucht S, Baldessarini AJ. Efficacy of antimanic treatments: meta‐analysis of randomized, controlled trials. Neuropsychopharmacology 2011;36(2):375‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yildiz 2015
- Yildiz A, Nikodem M, Vieta E, Correll CU, Baldessarini RJ. A network meta‐analysis on comparative efficacy and all‐cause discontinuation of antimanic treatments in acute bipolar mania. Psychological Medicine 2015;45(2):299‐317. [DOI] [PubMed] [Google Scholar]
Young 1978
- Young RC, Biggs JT, Zeigler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry 1978;133:429‐35. [DOI] [PubMed] [Google Scholar]


