Abstract
Objective
To assess the effect of statins compared with placebo on the risk of developing hypertransaminasemia.
Patients and Methods
We performed a systematic review of electronic databases and included articles published between January 1, 1965, and April 10, 2017. Randomized clinical trials (RCTs) comparing statins vs placebo were included. Odds ratios (ORs) were pooled in random-effect meta-analyses according to established methods recommended by the Cochrane Collaboration.
Results
Seventy-three eligible RCTs, comprising 123,051 patients, were identified. Statins associated with a significantly risk of hypertransaminasemia (OR 1.45; 95% confidence interval [CI], 1.24-1.69; P<.001). Atorvastatin showed the highest odds (OR 2.66; 95% CI, 1.74-4.06; P<.001) followed by rosuvastatin (OR 1.35; 95% CI, 1.06-1.70; P=.01) and lovastatin (OR 1.53; 95% CI, 1.03-2.28; P=.04). Pravastatin, fluvastatin, and simvastatin yielded no statistically different odds compared with placebo.
Conclusions
A dose-dependent risk of developing hypertransaminasemia occurs in patients taking atorvastatin, rosuvastatin, and lovastatin.
Abbreviations and Acronyms: ORs, odds ratios; RCTs, randomized controlled trials
Statins are the most common therapy used in the treatment of hypercholesterolemia and among the most used drugs in clinical practice. They have been shown to reduce cardiovascular events significantly compared with placebo.1, 2 Statins can lead to meaningless increased concentrations of liver-associated enzymes3 but a very low incidence of serious liver injury.4 However, these reports have generated controversy as to whether or not to recommend the monitoring of liver enzymes under statin treatment, as reflected by the contrasting indications promulgated by such international tasks forces or agencies as the Food and Drug Administration (FDA).5
It has been estimated that, in the United States, 1% to 10% of those taking statins (ie, 300,000 to 3,000,000) have been denied the benefit of statins as a result of unwarranted concern,6 and the annual cost of semiannual liver-test monitoring is estimated to be $3 billion a year.6, 7
Current statins package inserts prescribe liver-function tests before (all statins), at 12 weeks after initiation of therapy (rosuvastatin and fluvastatin), and when otherwise clinically indicated (all statins).8, 9 Given the established cardiovascular benefits of statins, and the likely increasing use of intensive statin regimens even in patients suffering with chronic liver diseases, it is pivotal to estimate the associated liver risks precisely, ultimately enabling physicians and patients to make informed choices.
To date, appropriately powered comparisons among statins with regard to the risk of developing hypertransaminasemia are lacking. The only comparative analysis was not designed to analyze the risk of elevation of transaminase during statin treatment and had significant limitations due to high heterogeneity that mitigated the clinical applicability of the result.10
One of the most relevant limits in the analysis of trials has been the different definition of liver toxicity, the different dose used, and the low frequency of events that makes any attempt of network analysis inconsistent. Accordingly, only a comprehensive meta-analysis of all randomized controlled trials (RCTs) may provide reliable conclusions in this debated scenario. Accordingly, we performed an updated meta-analysis of randomized and placebo-controlled clinical trials to investigate the potential risk of hypertransaminasemia after administration of statins.
Methods
We compared the risk of developing hypertransaminasemia in patients assuming statins vs placebo treatment and enrolled in RCTs. The following statins were included: atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, and simvastatin. We conducted the meta-analysis according to established methods recommended by the Cochrane Collaboration and reported our findings according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement.11, 12
Data Sources and Searches
We conducted systematic search in Pubmed Central, Scopus, the Cochrane Central Register of Controlled Trials, ClinicalTrials.gov, and major congress proceedings until April 10, 2017. The following key words were used: statins, liver, atorvastatin, rosuvastatin, simvastatin, pravastatin, fluvastatin, lovastatin, placebo, hepatotoxicity, transaminases, AST, ALT, aspartate aminotransferase, alanine aminotransferase, safety, and randomized controlled trial. For each RCT, the most updated or most inclusive data were used. Titles and abstracts were screened, and full-text articles were assessed if they were considered to be relevant.
Study Selection
The main inclusion criteria were (1) RCTs conducted in humans, (2) RCTs conducted in adults, (3) studies reporting data of hepatic safety, (4) duration of statin treatment of at least 4 weeks, (5) English language. Exclusion criteria were (1) non-RCTs, (2) RCTs conducted in patients with liver diseases, (3) concurrent administration of potentially hepatotoxic drugs, (4) crossover RCTs, (5) duration of statin treatment of less than 4 weeks, (6) RCTs not reporting safety data, (7) RCTs reporting hepatic adverse events but not criteria for severity. Internal validity was appraised according to the proper allocation sequence/concealment, patient blinding, investigator blinding, and complete outcome/full reporting. Primary clinical end point was significant serum liver enzyme alterations during statin treatment. Supplemental Table 1 summarizes the included RCTs and the criteria for the definition of significant liver injury.
Data Extraction and Quality Assessment
Two investigators not involved in any of the selected studies independently abstracted data by using prespecified forms. Two investigators then independently appraised the accuracy of the abstractions and resolved any discrepancies by consensus after discussion with a third investigator. High-dose statin therapy was defined as daily use of atorvastatin, 20 mg or more; rosuvastatin, 20 mg or more; simvastatin, 40 mg or more; fluvastatin 80 mg13; lovastatin and pravastatin 80 mg. Other treatment regimens were defined as low-dose or standard-dose statin treatment. Two unblinded investigators independently appraised the potential risk of bias of the RCTs by using methods described in the Cochrane Collaboration.11
The Grades of Recommendation, Assessment, Development and Evaluation (GRADE) system for rating the quality of evidence was also used to evaluate the strength of evidence.14 Accordingly, the absolute effect was also calculated for each statin.
Data Synthesis and Statistical Analysis
Data were analyzed according to the intention-to-treat principle. Odds ratios (ORs) with 95% confidence intervals (CIs) were used as summary statistics. Heterogeneity was assessed by the Cochran Q test and summarized by I2 statistic, which quantifies the percentage of variation in study results due to heterogeneity rather than chance.15, 16 Pooled ORs were calculated by using the more conservative random effect model with the Mantel-Haenszel method. The fixed effect model was applied when no or low-to-moderate heterogeneity (<50%) or not significant inconsistency (P>.05) was found.11, 16 The test for subgroup differences was performed to show interaction among subgroups and investigate potential different degrees of risk in different statin subgroups.
Results were considered statistically significant at 2-sided P≤.05. Analyses have been conducted using Review Manager 5.3 (Cochrane Collaboration, Copenhagen, Denmark).
Results
Study Selection and Patient Population
Figure 1 shows the PRISMA flow diagram of study selection. Seventy-three trials comprising a total of 123,051 patients were included in our final analysis. Study characteristics are shown in Supplemental Table 2. The longest follow-up was up to 5.3 years. Fifty-three trials were funded by industry. Most trials were performed after the year 2000.
Figure 1.
Flow diagram of the review process according to the Preferred Reporting Items for Systematic reviews and meta-analysis (PRISMA) statement.
Risk of Bias
The risks of bias of the included studies are shown in Supplemental Table 3. Funnel plots or Egger regression test did not reveal publication bias (Supplemental Figures 1 and 2). The RCTs were similar in that their risk of bias was low for most of the items in the majority of the included studies. Sixty-one RCTs were multicenter trials, and all studies included reported data according to the intention-to-treat principle, except for one. The certainty of evidence was also calculated according to grade profile and was reported for all statins in Supplemental Table 4.
Primary Clinical End Point: Overall Risk of Hypertransaminasemia
Seventy-three trials contributed to the final analysis. Two RCTs 17, 18 included data on more than one statin and were considered more than once during statistical analysis vs placebo.
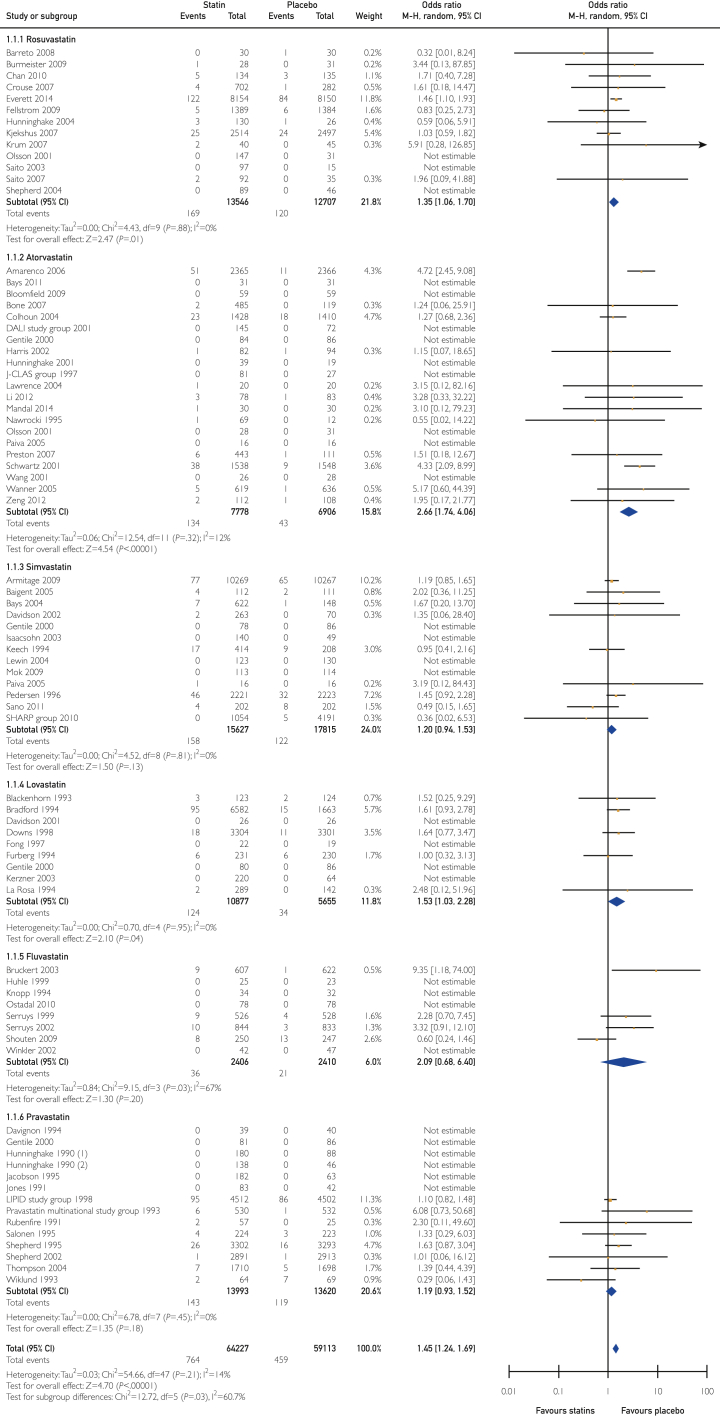
Overall, there was a statistically significant increase in hypertransaminasemia with statin treatment compared with placebo (OR 1.45; 95% CI, 1.24-1.69; P<.001; heterogeneity P=.21; I2=14%) (Figure 2).
Figure 2.
Analysis of odds ratios and 95% confidence intervals for overall risk of hypertransaminasemia.
Pravastatin, simvastatin, and fluvastatin did not increase the odds of developing high transaminase levels compared with placebo. Rosuvastatin and lovastatin treatment was significantly associated with a 35% and 53% odds increase of developing hypertransaminasemia, respectively.
Among all statins, atorvastatin yielded the highest odds (OR 2.66; 95% CI, 1.74-4.06; P<.001; absolute effect of 10 more per 1000 patients), followed by rosuvastatin (OR=1.35; 95% CI, 1.06-1.70; P<.01; 3 more per 1000 patients), and lovastatin (OR=1.53; 95% CI, 1.03-2.28; P<.04; absolute effect of 3 more per 1000 patients). However, the subgroup analysis showed heterogeneity (χ2 = 12.72, P=.03; I2 = 60.7%) that was fully resolved (χ2 = 2.48, P=.78, I2 = 0%) by excluding 3 studies of 73 that included patients with complicated conditions including stroke and acute coronary syndrome (ACS).
It is interesting that high doses of atorvastatin had an absolute effect of 48 more events per 1000 patients. Fluvastatin increased the absolute effect of developing hypertransaminases; however, the OR was not statistically significant, and the certainty of evidence was low (Supplemental Table 5).
Sensitivity Analysis of High Doses vs Standard Doses of Statins and Risk of Hypertransaminasemia
The overall class analysis was stratified by comparing high-dose statins and standard doses of statins vs placebo, according to the definition of a board of experts from the European Atherosclerosis Society.13
Forty-four RCTs, comprising 71,060 patients, were included, and treatment with high-dose statins resulted in a statistically significant increase in development of hypertransaminasemia compared with placebo (OR 1.64; 95% CI, 1.30-2.06; P<.001) (Figure 3). The odds did not differ significantly from placebo for fluvastatin, pravastatin, or simvastatin. The odds of developing hypertransaminasemia increased by 78%, 47%, and 53% during treatment with high doses of atorvastatin, rosuvastatin, and lovastatin, respectively.
Figure 3.
Analysis of odds ratios and 95% confidence intervals for hypertransaminasemia during high-dose statin therapy.
The same analysis was performed with standard doses of statins by comparing 34 RCTs including 52,265 patients, and results showed no difference compared with placebo (OR 1.19; 95% CI, 0.98-1.46; P=.08; heterogeneity P=.85; I2=0%) (Figure 4). Subgroup analysis of single statins confirmed these data.
Figure 4.
Analysis of odds ratios and 95% confidence intervals for hypertransaminasemia during low-dose statin therapy.
In trials exploring standard doses of lovastatin, pravastatin, and fluvastatin, hypertransaminasemia was not observed. Sensitivity analysis of atorvastatin showed that all subgroup analyses had increased risk to develop liver toxicity; the subgroups that developed the highest rates had ACS and acute cerebrovascular events (Supplemental Table 5).
A sensitivity analysis for atorvastatin was performed and showed that diabetes, hypercholesterolemia, or renal failure have no effects on the comparison (Supplemental Information Table 5). On the other hand, including only studies with ACS or cerebrovascular events significantly influences the overall risk (OR 4.49; 95% CI, 2.79-7.22; P<.001; I2=0%).
Discussion
The current study is the largest analysis of hypertransaminasemia developing in patients treated with statins. Data on whether—and to what extent—treatment with statins is associated with an increased risk of liver abnormalities remains an issue of debate.
Our systematic review and results of meta-analysis investigating the relationship between use of statins and occurrence of hypertransaminasemia is the most comprehensive overview on the liver safety of all commercialized statins from 1990. The main findings of our meta-analysis are as follows: (1) Irrespective of clinical significance, there is an increase in serum transaminases with statin treatment compared with placebo; (2) high-doses of atorvastatin, rosuvastatin, and lovastatin are associated with significant increase of odds of developing hypertransaminasemia compared with placebo; and (3) all analyzed low-dose statins showed a similar and nonsignificant risk compared with placebo.
The data from this large-scale analysis provide, for the first time, robust evidence of a gradient across statins in the induction of hypertransaminasemia with atorvastatin, rosuvastatin, and lovastatin—particularly at high doses—associated with the highest odds of liver-function test (LFT) abnormalities. Of note, a gradient across statins was found for all patients treated with statins. Patients affected by ACS or stroke greatly show the highest risk, but populations are heterogeneous. Probably, in such patients, the higher risk of developing hypertransaminasemia depends on assumption of atorvastatin 80 mg together with critical clinical conditions.
Our results support previous observations that pravastatin and simvastatin—both at standard and at high doses—do not induce hypertransaminasemia, and, accordingly, routine monitoring should be avoided for these statins. A previous meta-analysis on pravastatin, pooling 5 RCTs,19, 20, 21, 22 has shown no difference in the risk of developing hypertransaminasemia compared with placebo.23 Our analysis, which included 14 trials, confirmed such reports.
Simvastatin has, so far, not been analyzed in a meta-analysis study. We included 13 trials with 15,627 patients, and no difference was observed in terms of hypertransaminasemia when compared with placebo at any doses.
De Denus et al. showed that fluvastatin yielded an increase in the odds of hypertransaminasemia compared with placebo; however, the authors explained the observed increased risk as a consequence of the extremely low rate in the overall placebo group.23 In the current analysis, we considered 8 trials and 4816 patients (2406 treated with the statin), and the final results show no increased risk of hypertransaminasemia at any doses compared with placebo.
With high-dose atorvastatin (including 17 studies and 11,560 patients) the odds of hypertransaminasemia rises almost 3-fold compared with placebo, with an absolute effect of 48 more per 1000 patients, compared with placebo. In the analysis of rosuvastatin, 12 trials were included for a total of 26,253 patients. The overall risk of developing hypertransaminasemia was 1.35, which reaches 1.47 in the intensive regimen. There are several underlying mechanisms that might explain the highest degree of hypertransaminasemia found with certain types of statins vs others.
Atorvastatin is a synthetic hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase inhibitor, approved for use in the United States in 1996, and become one of the most commonly prescribed drugs in the United States, with more than 50 million prescriptions yearly.24 Atorvastatin is largely metabolized in the liver via CYP 3A4, excreted in bile, and usually induces a transient elevation of serum transaminase levels.25 The pathogenesis of atorvastatin-associated liver dysfunction is unclear. Some authors suggest that the induction of the CYP 450 system may be involved in the liver injury, and, indeed, genetic polymorphisms in CYP 3A4 may reflect differences in drug reactions.26 Idiosyncratic and clinically apparent liver injury has many features of autoimmunity and may be immune mediated.
In comparison with other statins, atorvastatin is significantly longer acting. It has been proposed that the longer exposure with atorvastatin could explain an increased risk of liver injury.27
Dujovne hypothesized that, as atorvastatin has more pronounced activity in lowering serum low-density lipoprotein, this, in turn, could influence the structure of cellular membranes, leading to greater leakage of cellular enzymes and increased incidence of LFT abnormalities without direct hepatotoxicity.28
Rosuvastatin was approved for use in the United States in 2003 and, at present, is one of the more potent available statins. The cause of hepatic injury from rosuvastatin is unknown because it is minimally (∼10%) metabolized in the liver (via CYP2C9), but, as with atorvastatin, rosuvastatin has been linked to hepatitis with autoimmune features29 including elevated immunoglobulin levels, ANA positivity, and a clinical response to corticosteroids.24
The study by Koh et al.30 showed that rosuvastatin is more potent and less hydrophilic than pravastatin and is associated with significant adverse metabolic effects such as insulin resistance.
Finally, lovastatin was approved for use in the United States in 1987, the first of this class of drugs to be commercially available. It is largely metabolized in the liver (via CYP 3A4), and metabolites are excreted in bile.
The pattern of injury is typically cholestatic, as described by several case reports,31, 32, 33 but can be also hepatocellular. Eosinophilia and autoimmune features are uncommon, and the idiosyncratic and clinically apparent liver injury associated with lovastatin may be due to failure of adaptation.24
Remarkably, our results indicate that the elevation of liver enzymes associated with administration of statins is not a homogeneous class-effect phenomenon dependent on the statin type and dose. The observations of the current report are pivotal in demonstrating that high-dose atorvastatin and rosuvastatin increase, to a greater extent, levels of LFTs when compared with low-dose statins or high doses of less potent statins (simvastatin, fluvastatin, lovastatin, or pravastatin). Therefore, hypertransaminasemia appears to occur mostly with the 2 most potent regimens now available for serum lipid lowering. Based on this article, it might be conceivable that the more drastic low-density lipoprotein-reduction levels—and then the effects of serum lipid lowering on the structure of cellular membranes—may be involved in the potential risk of the highest doses.
Sensitivity analysis of atorvastatin showed that all subgroup analyses had increased odds to develop hypertransaminasemia; however, the subgroups that developed the highest and most statistically significant rates of hypertransaminasemia included patients with ACS, acute cerebrovascular events, or persistent hypertransaminasemia (Supplemental Table 5).
Limitations
Our study has several limitations to be acknowledged. As with all meta-analyses of aggregated data, the availability of individual patient data would have further improved our findings. The results of this article, however, are robust and corroborated in several sensitivity analyses.
Another limitation is the use of different criteria for the definition of hypertransaminasemia used for study selection. However, sensitivity analyses conducted by removing one study at time did not reveal any differences with the overall findings, suggesting that the effect is stable and justified.
Conclusion
Different types and doses of statins display different potential to increase the incidence of hypertransaminasemia. High-dose atorvastatin, rosuvastatin, and lovastatin yielded higher risks of LFT abnormalities. These findings can have an impact on public health, particularly on management with statins of the population at risk, such as patients with ACS, acute cerebrovascular events, and persistent liver abnormalities.
Acknowledgments
Dr Serviddio was responsible for the conception and design of the work; Drs Villani, Serviddio, Cavallone, Bellanti, and Facciorusso for the acquisition, analysis, and interpretation of data.
Drs Villani Serviddio, Navarese, and Kubica did the statistical analysis. Drs Villani, Serviddio, Navarese, and Vendemiale drafted the article. All authors contributed to the critical revision of the manuscript for important intellectual content. Drs Serviddio and Vendemiale approved the version to be published.
Footnotes
Grant support: No funding has been received to conduct this meta-analysis.
Potential Competing Interests: Eliano Pio Navarese reports research funding from Amgen. The other authors report no competing interests.
Supplemental Online Material
Supplemental material can be found online at http://mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
References
- 1.Griffin S.J., Borch-Johnsen K., Davies M.J., et al. Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): a cluster-randomised trial. Lancet. 2011;378(9786):156–167. doi: 10.1016/S0140-6736(11)60698-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seshasai S.R., Kaptoge S., Thompson A., et al. Emerging Risk Factors Collaboration Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen D.E., Anania F.A., Chalasani N. An assessment of statin safety by hepatologists. Am J Cardiol. 2006;97(8):S77–S81. doi: 10.1016/j.amjcard.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Bjornsson E., Jacobsen E.I., Kalaitzakis E. Hepatotoxicity associated with statins: reports of idiosyncratic liver injury post-marketing. J Hepatol. 2012;56(2):374–380. doi: 10.1016/j.jhep.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 5.Bays H., Cohen D.E., Chalasani N., Harrison S.A., The National Lipid Association's Statin Safety Task Force An assessment by the Statin Liver Safety Task Force: 2014 update. J Clin Lipidol. 2014;8(3 suppl):S47–S57. doi: 10.1016/j.jacl.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Stagnitti M. Agency for Healthcare Research and Quality Statistical Brief; Rockville, MD: 2008. Trends in Utilization and Expenditures for the US Civilian Nonistitutionalized Population, 2000-2005. [PubMed] [Google Scholar]
- 7.Fallon Community Health Plan . 2009. Making More Cost-Effective Health Care Choices Starts Here. Worcester, MA. [Google Scholar]
- 8.U.S. Food & Drug Administration. Drugs@FDA: FDA Approved Drug Products. U.S. Food & drug Administration website http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm Accessed April 26, 2019.
- 9.Bader T. The myth of statin-induced hepatotoxicity. Am J Gastroenterol. 2010;105(5):978–980. doi: 10.1038/ajg.2010.102. [DOI] [PubMed] [Google Scholar]
- 10.Naci H., Brugts J., Ades T. Comparative tolerability and harms of individual statins: a study-level network meta-analysis of 246 955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcomes. 2013;6(4):390–399. doi: 10.1161/CIRCOUTCOMES.111.000071. [DOI] [PubMed] [Google Scholar]
- 11.Higgins J.P.T., Green S., editors. Cochrane Handbook for Systematic Review of Interventions. The Cochrane Collaboration; 2011. http://handbook.cochrane.org Version 5.1.0. [updated March 2011]. Available from. [Google Scholar]
- 12.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Laforest L., Moulin P., Souchet T., et al. Correlates of LDL-cholesterol goal attainment in patients under lipid lowering therapy. Atherosclerosis. 2008;199(2):368–377. doi: 10.1016/j.atherosclerosis.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 14.Murad M.H. Clinical practice guidelines: a primer on development and dissemination. Mayo Clin Proc. 2017;92(3):423–433. doi: 10.1016/j.mayocp.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleiss J.L. Analysis of data from multiclinic trials. Control Clin Trials. 1986;7(4):267–275. doi: 10.1016/0197-2456(86)90034-6. [DOI] [PubMed] [Google Scholar]
- 17.Gentile S., Turco S., Guarino G., et al. Comparative efficacy study of atorvastatin vs simvastatin, pravastatin, lovastatin and placebo in type 2 diabetic patients with hypercholesterolaemia. Diabetes Obes Metab. 2000;2(6):355–362. doi: 10.1046/j.1463-1326.2000.00106.x. [DOI] [PubMed] [Google Scholar]
- 18.Olsson A.G., Pears J., McKellar J., Mizan J., Raza A. Effect of rosuvastatin on low-density lipoprotein cholesterol in patients with hypercholesterolemia. Am J Cardiol. 2001;88(5):504–508. doi: 10.1016/s0002-9149(01)01727-1. [DOI] [PubMed] [Google Scholar]
- 19.Shepherd J., Cobbe S.M., Ford I., et al. West of Scotland Coronary Prevention Study Group Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333(20):1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 20.The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339(19):1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 21.Sacks F.M., Pfeffer M.A., Moye L.A., et al. Cholesterol and Recurrent Events Levels Trial investigators The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol. N Engl J Med. 1996;335(14):1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 22.Shepherd J., Blauw G.J., Murphy M.B., et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 23.de Denus S., Spinler S.A., Miller K., Peterson A.M. Statins and liver toxicity: a meta-analysis. Pharmacotherapy. 2004;24(5):584–591. doi: 10.1592/phco.24.6.584.34738. [DOI] [PubMed] [Google Scholar]
- 24.National Institutes of Health. Search the LiverTox Database. LIVERTOX website https://livertox.nih.gov/ Accessed April 26, 2019.
- 25.Perger L., Kohler M., Fattinger K., Flury R., Meier P.J., Pauli-Magnus C. Fatal liver failure with atorvastatin. J Hepatol. 2003;39(6):1095–1097. doi: 10.1016/s0168-8278(03)00464-1. [DOI] [PubMed] [Google Scholar]
- 26.Bernini F., Poli A., Paoletti R. Safety of HMG-CoA reductase inhibitors: focus on atorvastatin. Cardiovasc Drugs Ther. 2001;15(3):211–218. doi: 10.1023/a:1011908004965. [DOI] [PubMed] [Google Scholar]
- 27.Malinowski J.M. Atorvastatin: a hydroxymethylglutaryl-coenzyme A reductase inhibitor. Am J Health Syst Pharm. 1998;55(21):2253–2267. doi: 10.1093/ajhp/55.21.2253. quiz 2302-2253. [DOI] [PubMed] [Google Scholar]
- 28.Dujovne C.A. Side effects of statins: hepatitis versus “transaminitis”: myositis versus “CPKitis”. Am J Cardiol. 2002;89(12):1411–1413. doi: 10.1016/s0002-9149(02)02356-1. [DOI] [PubMed] [Google Scholar]
- 29.Wolters L.M., Van Buuren H.R. Rosuvastatin-associated hepatitis with autoimmune features. Eur J Gastroenterol Hepatol. 2005;17(5):589–590. doi: 10.1097/00042737-200505000-00019. [DOI] [PubMed] [Google Scholar]
- 30.Koh K.K., Quon M.J., Sakuma I., et al. Differential metabolic effects of rosuvastatin and pravastatin in hypercholesterolemic patients. Int J Cardiol. 2013;166(2):509–515. doi: 10.1016/j.ijcard.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 31.Grimbert S., Pessayre D., Degott C., Benhamou J.P. Acute hepatitis induced by HMG-CoA reductase inhibitor, lovastatin. Dig Dis Sci. 1994;39(9):2032–2033. doi: 10.1007/BF02088142. [DOI] [PubMed] [Google Scholar]
- 32.McQueen M.J. Cholestatic jaundice associated with lovastatin (Mevacor) therapy. CMAJ. 1990;142(8):841–842. [PMC free article] [PubMed] [Google Scholar]
- 33.Geddes J.A. Cholestatic jaundice associated with lovastatin (mevacor) therapy. CMAJ. 1990;143(1):13–14. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.