Highlights
-
•
Neutrophils play important roles throughout the metastatic process.
-
•
Tumour progression and invasion are influenced by infiltrating neutrophils.
-
•
Signalling from neutrophils can enhance survival of circulating tumour cells.
-
•
Neutrophils play a key role in establishing the metastatic niche.
Abbreviations: APC, antigen presenting cell; CCL, C-C motif chemokine ligand; CCR, C-C motif chemokine receptor; CXCL, C-X-C motif chemokine ligand; CXCR, C-X-C motif chemokine receptor; ECM, extracellular matrix; EMT, epithelial to mesenchymal transition; FACS, fluorescence-activated cell sorting; FGF, fibroblast growth factor; G-CSF, granulocyte-colony stimulating factor; GM-CSF, granulocyte-macrophage colony stimulating factor; gMDSCs, granulocytic myeloid-derived suppressor cells; HOCL, hypochlorous acid; ICAM, intercellular adhesion molecule; IL, interleukin; IFN, interferon; iNOS, Inducible nitric oxide synthase; MAPK, mitogen-activated protein kinase; MET, mesenchymal-to-epithelial transition; MMP, matrix metalloproteinase; NET, neutrophil extracellular trap; NF-κB, nuclear factor kappa B subunit 1; NK, Natural Killer; NLR, neutrophil to lymphocyte ratio; PD1, programmed cell death protein 1; PI3K, phosphoinositide 3-kinase; ROS, reactive oxygen species; SDF1, stromal cell-derived factor 1; STAT3, signal transducer and activator of transcription 3; TGFβ, transforming growth factor beta; TAM, tumour-associated macrophages; TAN, tumour-associated neutrophils; TIMP-1, tissue inhibitor of metalloprotease 1; TLR, toll-like receptor; TNF, tumour necrosis factor; VEGF, vascular endothelial growth factor
Keywords: Epithelial cancer, Metastasis, Neutrophils, Myeloid cells, Tumour microenvironment
Abstract
The metastasis cascade is complex and comprises several stages including local invasion into surrounding tissue, intravasation and survival of tumour cells in the circulation, and extravasation and colonisation of a distant site. It is increasingly clear that these processes are driven not only by signals within the tumour cells, but are also profoundly influenced by stromal cells and signals in the tumour microenvironment. Amongst the many cell types within the tumour microenvironment, immune cells such as lymphocytes, macrophages and neutrophils play a prominent role in tumour development and progression. Neutrophils, however, have only recently emerged as important players, particularly in metastasis. Here we review the current evidence suggesting a multi-faceted role for neutrophils in the metastatic cascade.
1. Introduction
Metastasis, the systemic spread and establishment of foci of cancer cells within distant organ systems, is the cause of death in the majority of cancer patients, with survival rates alarmingly low for those diagnosed with metastatic disease (Steeg, 2016). In order to establish distant metastases, cancer cells must successfully navigate a sequence of challenges, which together form the metastatic cascade. These include: primary tumour progression with local tissue invasion; basement membrane and endothelial transmigration (vascular invasion); intravascular dissemination; vascular arrest and extravasation leading to the establishment of micrometastases; and final outgrowth to form macrometastases (Nguyen et al., 2009). At each step of this complex process, cancer cells must also remain capable of evading immune surveillance, recognition, and killing.
The complexity of the metastatic cascade described above is easily matched by the complexity and diversity of the cellular components that are recruited and collectively form the tumour microenvironment. These recruited cells and the factors they produce are not only central to the continued growth and viability of the primary tumour, but are also increasingly recognised as vital components that facilitate the metastatic spread of neoplastic cells (Joyce and Pollard, 2009). Amongst the many constituent lineages of stromal cells within the tumour microenvironment, innate and adaptive leukocytes such as macrophages, neutrophils, and lymphocytes play a prominent role in tumour development, growth, metastasis, and ongoing immune evasion (Joyce and Pollard, 2009; Nguyen et al., 2009).
Of these leukocyte subsets, much attention has been given to tumour associated macrophages (TAMs) and the role that they play supporting tumour progression (Noy and Pollard, 2014). Increasingly however another leukocyte population, the neutrophil, has been receiving attention due to the prominent role it plays, particularly in metastasis. Indeed we and others have highlighted the significant and central role that neutrophils play in the establishment of distant metastases in a number of animal models of cancer (Coffelt et al., 2015; Park et al., 2016; Pekarek et al., 1995; Shojaei et al., 2007; Spicer et al., 2012; Steele et al., 2016; Wu et al., 2015; Yamamoto et al., 2017). Interestingly, this important role highlighted in in vivo models, is mirrored in the clinical setting with ever more studies, in a multitude of human cancers, linking peripheral neutrophil counts and/or neutrophil to lymphocyte ratio (NLR) with poor prognosis and by extension metastasis (Aliustaoglu et al., 2010; Jung et al., 2011; Roxburgh et al., 2010; Tomita et al., 2011).
Neutrophils classically defined by their nuclear morphology and tinctorial affinity are the predominant leukocyte in the peripheral blood of humans (Coffelt et al., 2016). They play a central role in host defence against infection due to their ability to perform phagocytosis, produce cytokines and reactive oxygen species, which can promote inflammation, and, through degranulation, to release the contents of their granules into the inflammatory exudate. Importantly however, the classic view of the neutrophil, as a short-lived, innate “first-responder” is rapidly changing and the true complexity of their function is increasingly but incrementally coming to light.
Granulopoiesis occurs within the bone marrow in man, though during foetal development and in certain pathological processes it may also occur in sites outside the bone marrow. When this is the case, it most commonly occurs within the spleen and liver, and is referred to as extramedullary haematopoiesis. In rodents, particularly mice, extramedullary haematopoiesis is also commonly observed as a normal component of the splenic red pulp. Numerous factors are known to play a role in driving and modulating neutrophil production, however, the key factor central to this process is granulocyte-colony stimulating factor (G-CSF) (Lieschke et al., 1994). Once granulopoiesis is complete, mature neutrophils are released from the bone marrow as terminally differentiated effector cells. The bone marrow however retains a marginal pool of mature, terminally differentiated neutrophils, ready for release in cases of increased demand due to inflammatory stimuli. In cases of severe inflammation, demand outstrips supply and this pool becomes depleted. In such instances immature neutrophils, with characteristic band/horseshoe or ring shaped nuclei, will begin to be released from the bone marrow niche, a so called “left-shift”. Though this inflammatory “left-shift” is commonly seen in severe bacterial infections, it is also seen frequently in cases of cancer (Sagiv et al., 2015). Unsurprisingly therefore, in cancer, it has been shown that numerous factors involved in stimulating granulopoiesis, neutrophil release, and chemotaxis are produced directly by neoplastic cells, or indirectly through their induced production in other stromal cells. These factors include G-CSF, GM-CSF, CXCL1, CXCL2, CXCL5, CXCL8 and CCL3 (Dumitru et al., 2013; Mishalian et al., 2017; Sagiv et al., 2015).
As with macrophages, tumour associated neutrophils (TANs) have been shown to be capable of polarisation into either an anti-tumourigenic “N1” phenotype or, in response to TGFβ, a pro-tumorigenic “N2” phenotype (Fridlender et al., 2009; Shaul and Fridlender, 2017). This perhaps simplified classification is based on the context-dependent activation status of these neutrophils as evidenced by the expression of various surface markers, cytokines, and their immunosuppressive activity. “N1” neutrophils exhibit increased cytotoxicity and reduced immunosuppressive ability through the production of TNFα, Fas, ICAM-1, and ROS and through decreased arginase expression. “N2” neutrophils in contrast express high levels of arginase, MMP-9, VEGF, and numerous chemokines (e.g. CXCL4, CCL2 and CCL5) (Fridlender et al., 2009). Frustratingly, “N1” and “N2” neutrophils are both characterised, in mice, by the cell surface expression of CD11b and Ly6G. Indeed the expression of these markers is also shared by another population of myeloid cells, granulocytic myeloid-derived suppressor cells (gMDSCs), which are defined by their immunosuppressive activity (Coffelt et al., 2016; Fridlender et al., 2012). With the recognition of the complexity of their role, it has become apparent that the simplicity by which neutrophils had been previously defined has been eroded. Indeed, instead of the simple innate “foot soldier”, a heterogeneous population of cells with significant functional plasticity has been uncovered. In fact, due to the often shared cell morphology and the overlap of expression of these cell surface markers between different functional groups, no easily defined lines exist which clearly distinguish between neutrophils, TANs (“N1” and “N2”), and gMDSCs (Coffelt et al., 2016; Fridlender et al., 2012). Instead, these cell populations almost certainly represent granulocytic myeloid cells presenting along a continuous range of differentiation, maturity, and activation status. In this review we will therefore highlight the roles that this spectrum of granulocytic cells play in tumour progression and metastasis.
2. Neutrophils and the metastatic cascade
2.1. Early metastatic cascade – tumour cell invasion
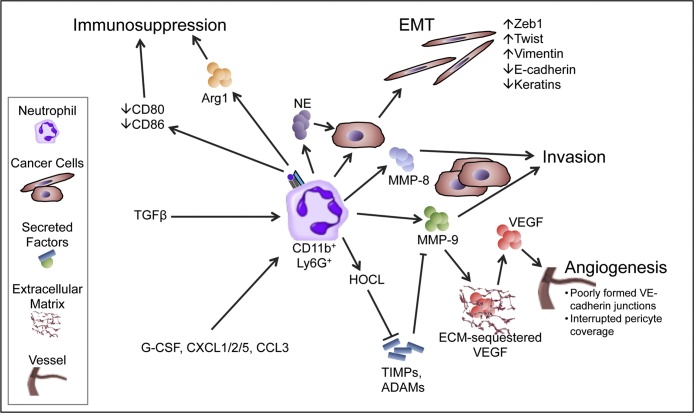
Although, as highlighted in other recent reviews, the roles that neutrophils play in supporting metastasis are likely weighted towards the earlier stages of the metastatic cascade, a growing body of literature increasingly points towards neutrophils playing important roles in all stages of metastasis (Coffelt et al., 2016, Coffelt et al., 2015; Cools-Lartigue et al., 2013; Spicer et al., 2012). Neutrophils perform numerous roles within the primary tumour that promote progression and metastasis (Fig. 1). One significant role, likely vital within the primary tumour and beyond, is the promotion of the invasive behaviour of tumour cells, a behaviour which is fundamental to any tumour attempting to progress to malignancy. Neutrophils can influence this behaviour due to their ability to produce and release a wide variety of proteins, in particular serine proteases, into the extracellular environment. These proteases are well established in their ability to degrade a variety of structural components of the extracellular matrix (ECM). Indeed, neutrophils have been suggested through the production of MMP-8 and MMP-9, Cathepsin G, and proteinase-3, to promote tumour cell invasion and migration (Benson et al., 2012; Dumitru et al., 2013). MMP-8, also known as neutrophil collagenase, degrades type I, II, and III collagen. As fibrillar collagens these collagen subtypes are central constituents of the ECM and represent a significant barrier to tumour invasion. In contrast, MMP-9 degrades a number of ECM proteins, but most importantly type IV collagen, which is the main constituent of the basement membrane through which tumour cells must pass in order to progress from carcinoma-in-situ to outright carcinoma. In a murine model of pancreatic ductal adenocarcinoma, neutrophils were found to be the major cell group producing MMP-9 and strikingly these neutrophils were predominantly present at the invasive fronts of metastatic tumours (Benson et al., 2012). Interestingly in infectious inflammatory conditions neutrophil entry into tissues relies on the production of MMP-9 by neutrophils which is induced in response to Cxcl2 signalling resulting from crosstalk between tissue resident and helper macrophages (Schiwon et al., 2014).
Fig. 1.
The role of neutrophils in the early metastatic cascade – primary tumour progression and invasion.
NE, neutrophil elastase; Arg1, arginase 1; TGFβ, Transforming growth factor beta; EMT, epithelial-mesenchymal transition; Zeb1, Zinc finger E-box-binding homeobox 1; Twist, Twist-related protein 1; MMP, matrix metalloproteinase; ECM, extracellular matrix; VEGF, Vascular endothelial growth factor; HOCL, Hypochlorous acid; TIMP, tissue inhibitor of metalloproteinases; ADAMs, a disintegrin and metalloproteinase; G-CSF, Granulocyte-colony stimulating factor; CXCL, Chemokine (C-X-C motif) ligand; CCL, Chemokine (C-C motif) ligand; VE-cadherin, vascular endothelial cadherin.
Although, as highlighted above, these serine proteases are classically produced by neutrophils, it has also been noted that neutrophils are capable of inducing the expression of a number of these proteins in tumour cells themselves. Indeed, in a murine orthotopic model of breast cancer, it has been demonstrated that the interaction between neutrophils and tumour cells results in production of large quantities of MMP-12 and MMP-13 (Yu et al., 2016). In this setting the influx of neutrophils is in response to CCR2 and CXCR2 ligands produced by mesenchymal stromal cells in response to TNFα produced by the tumour cells. Interestingly the functions of these MMPs mirror those of MMP-8 and MMP-9 mentioned previously. MMP-12 degrades elastin another component of the ECM and also a vital structural component of vascular walls; whilst MMP-13, mirroring the function of MMP8, is able to degrade type I, II, and III collagen.
Though neutrophils are capable of producing and inducing the production of numerous serine proteases, an additional layer of complexity is added by the fact that many, though not all, of these proteases are produced and secreted in an inactive form and must be activated in order to fulfil their potential. This again is a function which the neutrophil is capable of performing through the production of hypochlorous acid (HOCL) by neutrophil-derived myeloperoxidase (Larco et al., 2004). HOCL has been shown to activate MMP proenzymes (including MMP-2, MMP-7, MMP-8, and MMP-9) either directly via oxidation or indirectly through conformational change allowing activation by other neutrophil-derived proteases, such as neutrophil elastase (Larco et al., 2004). HOCL is also able to inactivate tissue inhibitor of metalloprotease 1 (TIMP-1) and thus maintain the activity of MMPs and distintegrin-metalloproteinases in the ECM (Larco et al., 2004).
Serine proteases, particularly MMP-9, also play a vital role in triggering the “angiogenic switch” during tumour progression, as observed in a murine model of pancreatic endocrine carcinogenesis (Nozawa et al., 2006). The mechanism by which MMP-9 contributes to the turning on of this “angiogenic switch” is likely through the indirect release of ECM-bound factors such as vascular endothelial growth factor (VEGF) (Nozawa et al., 2006). In addition to servicing the metabolic demands of the tumour, this induced angiogenesis likely has wider ranging effects. One such effect is the increase in size and permissive nature of the vascular window allowing tumour cell intravasation and therefore dissemination. Indeed neutrophils have been shown, through the delivery of MMP-9 into the tumour microenvironment, to support the formation of pro-metastatic vasculature in transplant models (Deryugina et al., 2014). Additionally neutrophil-derived MMP-9 can increase neoplastic cell intravasation secondary to the induction of angiogenesis (Bekes et al., 2011).
In addition to the production of factors that modulate the ECM, neutrophils can also promote epithelial to mesenchymal transition (EMT) in tumours via a number of mechanisms. Hu et al. showed that neutrophil-derived TGFβ is in part able to cause EMT in pulmonary adenocarcinoma cells through the downregulation of E-cadherin and upregulation of vimentin expression, and is associated with the enhanced migration of tumour cells in vitro (Hu et al., 2015). Neutrophil elastase is also capable of contributing to the process of neutrophil-induced EMT. Indeed, co-culture of pancreatic cancer cells results in tumour cell upregulation of TWIST and downregulation of keratins, two markers of EMT. This was shown to be dependent on neutrophil elastase, which the authors suggest likely resulted in EMT due to the induced loss of cell–cell contact. In the same study it was shown that the level of tumour neutrophil infiltration strongly correlated with nuclear β-catenin and ZEB-1 expression in cases of human pancreatic ductal adenocarcinoma supporting the notion of neutrophil-induced EMT in clinical samples (Grosse-Steffen et al., 2012). In a separate study, neutrophil infiltration at the invasive front, an area commonly associated with EMT, was shown to be a significant independent predictor of lymph node metastasis in patients with colorectal cancer (Akishima-Fukasawa et al., 2011).
Interestingly, limited evidence also points towards neutrophil-produced growth factors forming gradients resulting in the concentration-dependent migration of tumour cells particularly at the invasive front (Wislez et al., 2003). Finally in a zebrafish xenograft model, which allows the unparalleled ability to image tumour cells navigating the entirety of the metastatic cascade, He et al. revealed that neutrophils through normal migratory processes were unwittingly leaving tracks within the collagen matrix which allowed tumour cells to easily invade and form micrometastases although it remains to be confirmed whether this mechanism plays a role in clinical metastasis (He et al., 2012).
Neutrophils have also been shown, in a contact-dependent manner, to increase hepatocellular carcinoma cancer cell migration. In this context IL-17 production within the tumour resulted in the chemo-attraction of neutrophils, and contact-dependent signalling between hyaluronan on tumour cells and TLR4 on neutrophils. This activated PI3K/Akt signalling in neutrophils resulting in increased longevity and a contact-dependent increase in cancer cell motility (Wu et al., 2011). The increase in longevity in this system was at least in part due to upregulation of the anti-apoptotic protein Mcl-1 and downregulation of the pro-apoptotic protein Bax. Thus, the manner by which neutrophils may influence invasion may either be through secreted factors or through direct cell-to-cell contact with tumour cells (Wu et al., 2011).
2.2. Intermediate metastatic cascade – intracellular survival of tumour cells
Once within the vasculature, tumour cells have entered a completely new and hostile environment. Indeed the level of hostility that this environment poses was beautifully demonstrated in a study by Fidler, which showed that less than one in every hundred metastatic tumour cells survives to successfully form a metastasis (Fidler, 1970). The reason for this attrition is that tumour cells must cope with numerous previously unencountered factors such as; increased shear forces, the absence of cell–cell and cell-ECM interactions, and the presence of circulating leukocytes (particularly Natural Killer (NK) cells). As mentioned previously, given that neutrophils are the predominant circulating leukocyte in man, it is perhaps unsurprising that studies are beginning to show that they may contribute to tumour cell survival within the circulation and therefore successful completion of the metastatic cascade (Fig. 2).
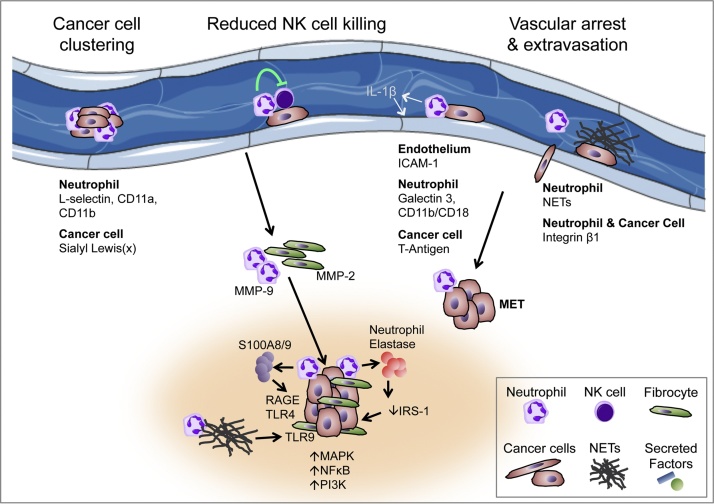
Fig. 2.
The role of neutrophils in the late stages of the metastatic cascade – intravascular dissemination, extravasation, and metastasis growth.
NETs, neutrophil extracellular traps; MMP, matrix metalloproteinase; ICAM-1, intercellular adhesion molecule 1; RAGE, receptor for advanced glycation endproducts; TLR, toll-like receptor; IRS-1, insulin receptor substrate 1; MET, mesenchymal-epithelial transition; IL-1β, interleukin 1 beta; MAPK, mitogen-activated protein kinase signalling; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; PI3K, Phosphatidylinositol-4,5-bisphosphate 3-kinase signalling.
Loss of cell–cell and cell-ECM contact, as alluded to above, is a significant challenge for malignant cells entering the circulation. There are a number of ways in which metastatic cells might overcome this obstacle, one of which is by avoiding it altogether through cell clustering and the formation of neoplastic cell aggregates. Indeed cell clustering has been shown to promote tumour cell survival and metastasis (Choi et al., 2015; Fabisiewicz and Grzybowska, 2017). Though unproven in vivo it is tempting to speculate that this may be a process enhanced by intravascular neutrophils given that neutrophils have been shown to promote breast cancer cell clustering in vitro, in a Cathepsin G-dependent manner (Morimoto-Kamata et al., 2012), and aggregation of colorectal carcinoma cells via binding of sialyl Lewis (x) on tumour cells to L-selectin, CD11a, and CD11b on neutrophils (Jadhav et al., 2001).
Tumour cells within the circulation are also exquisitely exposed to recognition and destruction by both the innate and adaptive immune system. Depending on their activation status it is known that neutrophils may function in an immune suppressive manner (Fridlender et al., 2009). Therefore given their presence within the peripheral circulation it is unsurprising that they have been shown to play a role in protecting tumour cells from leukocyte-mediated recognition and killing. One population of leukocytes present within the circulation are NK cells, which represent a population of innate cytotoxic lymphocytes that play a vital role in the immune surveillance and destruction of cancer cells both in the periphery and within the circulation (Morvan and Lanier, 2015). Spiegel and colleagues have elegantly shown that neutrophils mobilised by tumours impair NK cell function, thus increasing intraluminal tumour cell survival (Spiegel et al., 2016). In this study they showed that subcutaneous growth of a highly metastatic mammary cancer cell line (4T1) increased the incidence of metastasis of a rarely metastatic cell line (D2A1) growing subcutaneously in the contralateral flank. The authors were also able to increase the metastatic capability of D2A1 cells by inducing increased expression of G-CSF in the tumour cells. This process of metastasis however relied on the presence of neutrophils, as when neutrophils were depleted by 1A8 antibody treatment, metastasis was significantly decreased. Interestingly, when injected intravenously in high numbers, D2A1 cells were capable of lung colonisation, however, this process was again aided by either administration of G-CSF or 4T1-induced neutrophilia. Given the intravenous delivery of cells in this system these findings pointed towards neutrophils playing a pro-survival role post-intravasation. Indeed, Spiegel and colleagues were able to show that neutrophils increase short-term retention of circulating tumour cells within the pulmonary vasculature due to decreased clearance following injection. Through NK cell depletion they were able to show that NK cell killing was the process central to the clearance of circulating tumour cells and that this was successfully inhibited by G-CSF-induced neutrophils. This reduction in NK cell-mediated metastatic cell killing was not due to decreased numbers of circulating NK cells but due to a decrease in their ability to respond to signalling through their cell surface receptors and become functionally activated (Spiegel et al., 2016). Other studies support these findings, indeed, Zhang et al. have shown through co-culture and in vivo experiments that TANs are able to prevent the upregulation of membranous CD69, a marker of leukocyte activation (Zhang et al., 2015). Thus, there is growing evidence that neutrophils can influence the metastatic cascade in part by promoting intravascular survival through the induced suppression of leukocyte activation.
2.3. Intermediate metastatic cascade – extravasation
Having survived the hostile intravascular environment, metastatic tumour cells now face the same challenge faced by leukocytes when trying to marginate and transmigrate from the vessel. This is a significant challenge as cells within the vasculature attempting to adhere to the endothelium experience significant shear stresses that must be overcome if the cells are to complete the process of transmigration and successfully exit the vessel. Neutrophils again have been shown to play a significant role in these processes both directly and indirectly (Fig. 2). A number of studies utilising in vivo metastasis models and intravital microscopy have provided direct evidence of neutrophil co-localisation with tumour cells (McDonald et al., 2009; Spicer et al., 2012). In this context they showed that neutrophils were necessary for the formation of metastases through the facilitation of Lewis lung carcinoma cell adhesion to liver sinusoidal endothelium, which was dependent on neutrophil CD11b/Mac-1 and selectin expression (McDonald et al., 2009; Spicer et al., 2012). Numerous others studies have also demonstrated the role that neutrophils play in aiding cancer cell adhesion to the endothelium in metastatic sites. In these studies similar selectins were also involved, as were CD18 and Galectin 3 on neutrophils, ICAM-1 expression on endothelium, and T-Antigen expression on neoplastic cells (Dong et al., 2005; Huh et al., 2010; Liang and Dong, 2008; Reticker-Flynn and Bhatia, 2015). Disrupting these interactions not only decreased tumour cell adherence to the vascular endothelium but also reduced neutrophil-mediated extravasation of the tumour cells, confirming the importance that these interactions play in leading to establishment of metastases (Huh et al., 2010). It remains to be determined whether the role of the neutrophil is to locally activate the endothelium, or whether the neutrophil must directly interact simultaneously with the metastatic tumour cell and endothelium to form a complex capable of triggering arrest and adhesion to the endothelium, and subsequent transmigration.
In addition to the surface expression of selectins and integrins that aid in the endothelial arrest and adhesion of cancer cells, neutrophils have also been shown to cause vascular arrest via the production of neutrophil extracellular traps (NETs) (Cools-Lartigue et al., 2013; Najmeh et al., 2017; Tohme et al., 2016). NETs are extracellular web-like structures composed predominantly of neutrophil-derived DNA that complexes with a wide variety of neutrophil-derived proteins including a number of the serine proteases discussed previously (Park et al., 2016). NETs are produced in response to a variety of inflammatory cues and it has also been shown that NET production is increased within tumours due to hypoxia (Cools-Lartigue et al., 2013; Erpenbeck and Schön, 2016; Tohme et al., 2016). Using intravital imaging, Park et al. confirmed the presence of NET-like structures surrounding 4T1 breast cancer cells within the vasculature of the lung, and importantly also demonstrated the presence of NETs in clinical samples of breast cancer (Park et al., 2016). The importance of the role that NETs play in metastasis has also been demonstrated by a number of studies in which prevention of NET formation or promoting NET degradation via DNase I was able to prevent metastasis in a variety of in vivo tumour models (Cools-Lartigue et al., 2013; Najmeh et al., 2017; Park et al., 2016; Tohme et al., 2016). Interestingly, given the importance of cell adhesion molecules in vascular arrest, Najmeh et al. also showed that β1-integrin on both NETs and cancer cells is vital for the adhesion of cancer cells to NETs in vivo (Najmeh et al., 2017). Additional in vitro experiments tantalisingly point to cancer cell interactions with NETs resulting in increased cancer cell invasion and migration which may further aid cancer cell extravasation once vascular arrest has been achieved (Cools-Lartigue et al., 2013; Park et al., 2016; Tohme et al., 2016). Intriguingly given that NETs are primarily composed of DNA it has also been shown that NETs are capable of activating toll-like receptor 9 (TLR9) on colorectal cancer cells and in so doing promote cellular growth, proliferation, migration, and invasion via activation of MAPK signalling (Tohme et al., 2016).
In addition to the increased invasive and migratory behaviours induced by tumour cell interactions with NETs, neutrophils have also been shown to facilitate cancer cell extravasation via a number of other mechanisms. Indeed, Spiegel et al. showed that neutrophils aid tumour cell extravasation via endothelial activation elicited by neutrophil-derived IL-1β, as well as in a manner dependent on neutrophil-derived MMP-8 and MMP-9 (Spiegel et al., 2016). MMP-9 may play a central role in the mechanisms by which neutrophils aid tumour cell extravasation as other studies have similarly found that loss of MMP-9 significantly impairs metastasis (Yan et al., 2010). For example, Yan et al., demonstrated that loss of MMP-9 results in vascular normalisation in the pre-metastatic lungs of mammary tumour-bearing mice, with increased coverage of vessels by alpha smooth muscle actin-positive cells and formation of VE-cadherin junctions. These findings suggest that in the metastatic setting, neutrophil-derived MMP-9 is functioning to promote the formation of abnormal vasculature with decreased pericyte and smooth muscle coverage and disrupted inter-endothelial junctions (Yan et al., 2010).
2.4. Late metastatic cascade – metastatic outgrowth
Upon successful extravasation metastatic cells again find themselves in a novel environment and must rely on a wide variety of mechanisms to stimulate their growth and survival. In this setting it is increasingly established that metastatic cells land within a pre-established and favourable pre-metastatic niche (Peinado et al., 2017). Neutrophils play a central role in this niche and their presence before the arrival of tumour cells within metastatic sites has been repeatedly demonstrated and occurs in response to a variety of primary tumour-derived factors (Fig. 2). Cxcl1 produced by tumour-associated macrophages in response to tumour-derived VEGF-A has been shown to result in the Cxcr2-dependent accumulation of neutrophils within the pre-metastatic niche in a colorectal cancer model (Wang et al., 2017). We, and others, have also shown that Cxcr2-dependent neutrophil accumulation in the pre-metastatic liver is required for pancreatic cancer metastasis (Steele et al., 2016). Additionally, TIMP-1 has also been shown to contribute to the formation of the pre-metastatic niche in the liver via induction of hepatic SDF-1 resulting in Cxcr4-dependent chemotaxis of neutrophils into the hepatic parenchyma (Seubert et al., 2015). Exosomal RNAs from lung or melanoma tumour cells can also result in the chemo-attraction of neutrophils into the pre-metastatic niche via the TLR-3 signalling-promoted secretion of chemokines such as Cxcl1, Cxcl2, Cxcl5, and Cxcl12 (Liu et al., 2016).
Within the pre-metastatic niche neutrophils may perform a variety of pro-metastatic functions. For example, neutrophils have been shown to promote metastatic growth by inducing mesenchymal-to-epithelial transition (MET) and stimulating mammary cancer cell proliferation (Ouzounova et al., 2017). This finding is of note when we consider that neutrophils may also promote EMT in the context of the primary tumour invasive front. Neutrophil elastase has also been shown, through degradation of insulin receptor substrate-1, to enhance PI3K signalling, again promoting neoplastic cell proliferation (Houghton et al., 2010). Additionally Queen et al. as well as demonstrating a role for neutrophil-derived oncostatin M in inducing breast cancer cell detachment and invasion also highlighted its ability to trigger tumour cell proliferation via activation of STAT3 (Queen et al., 2005). Neutrophil-derived S100A8 and S100A9 have also been shown to promote tumour cell survival and proliferation by activating MAPK signalling in metastatic cells via RAGE, TLR-4, and carboxylated glycans which can also activate the NF-κB pathway (Acharyya et al., 2012; Ichikawa et al., 2011; Yu et al., 2016).
In addition to providing tumour trophic factors, neutrophils are also vital in supporting the formation of the pro-survival metastatic niche by attracting other cells vital for the successful support of the metastatic tumour cells. For example, Hirai et al. utilising murine metastasis models of colon cancer showed that Ccl9 secreted by cancer cells resulted in the early recruitment of Ccr1 expressing neutrophils into the liver to surround metastatic foci (Hirai et al., 2014). In turn they showed that this early recruitment of neutrophils was central to the mechanisms driving the later accumulation of “monocyte/fibrocytes”. These “fibrocytes” are so called because they express both monocytic and mesenchymal markers including CD45+, and CD11b+ and vimentin, and type I collagen respectively. In this study the two myeloid populations, neutrophils and monocytic “fibrocytes”, were shown to be vital for the support of establishment and growth of liver metastases. This requirement for neutrophils was due to their ability to attract the monocytic “fibrocytes” and produce MMP-9, and for “fibrocytes” due to their ability to produce of MMP-2 (Hirai et al., 2014).
Neutrophils have also been shown to be vital in driving metastatic progression via inducing angiogenesis. If we consider the essential role angiogenesis plays in primary tumours attempting to grow beyond 1–2 mm3 it is perhaps unsurprising that growing metastases will come under the same pressures and requirement for vascular supply (De Palma et al., 2017). Numerous studies have highlighted the role that neutrophils play in driving angiogenesis in the primary tumour (Bekes et al., 2011; Deryugina et al., 2014; Jablonska et al., 2010; Li et al., 2016; Shojaei et al., 2007). Whilst it is highly likely that similar mechanisms are at play in the metastatic niche, many of these mechanisms are yet to be confirmed. Some studies, however, are beginning to confirm a vital role for neutrophils in driving angiogenesis in the context of metastasis (Gordon-Weeks et al., 2017; Lim et al., 2015). In murine models of colorectal and pancreatic cancer metastasis, Gordon-Weeks and colleagues have shown that neutrophil depletion significantly reduces microvessel density and vascular branching in metastases and consequently inhibits their growth. FACS-sorted metastasis-associated neutrophils expressed significantly higher levels of proangiogenic fibroblast growth factor 2 (FGF2) but not VEGF, while inhibition of FGF2 signalling resulted in a decrease in microvessel density and vascular normalisation mirroring the exact effect that of neutrophil depletion in the same models (Gordon-Weeks et al., 2017). Work from the same group has also previously shown that CD11b+ myeloid cells are able to support angiogenesis in metastases by down-regulating antiangiogenic protein angiopoietin-like 7 (ANGPTL7) in colon and lung cancer cells. Indeed depletion of myeloid cells or overexpression of ANGPTL7 in these models was associated with reduced hepatic metastasis and angiogenesis (Lim et al., 2015). Together, the data suggest that tumour-mobilised neutrophils can induce a variety of pro-metastatic effects in distant sites.
3. Immunosuppression in the metastatic niche
As we have established, neutrophils are increasingly being proven to play significant roles in supporting the many stages of the metastatic cascade. In addition to this they are also well known for their ability to contribute to tumour survival through a multitude of immunosuppressive functions. Increasingly, these immunosuppressive mechanisms are being shown to be vital in the outgrowth of metastatic foci specifically. In our work we have shown that neutrophil depletion in models of metastatic pancreatic ductal adenocarcinoma results in increased T cell infiltration into both primary tumours and metastases as well as an increased response to PD-1 inhibition (Steele et al., 2016). Coffelt et al. elegantly showed in metastatic breast cancer models that neutrophils are able to suppress cytotoxic CD8+ T cell function in the early metastatic niche. In their models, IL-1β produced by macrophages elicits IL-17 expression by gamma delta T cells, which in turn results in a G-CSF-dependent increase in the number of cKIT+ neutrophils. These neutrophils are vital to the successful establishment of metastasis due to the production of iNOS and the suppression of the activation of CD8+ T cells to CD62L−CD44+IFNgamma+ T cells (Coffelt et al., 2015). Indeed neutrophils have been shown in melanoma patients to produce IL-10 in response to the systemic acute phase protein serum amyloid A 1. Produced systemically and signalling through the G-protein coupled receptor FPR2, SAA-1 results in neutrophils with an immunosuppressive phenotype capable of suppressing T cell proliferation via this IL-10 signalling. In these same patients it was also noted that the frequency of these neutrophils in the peripheral blood correlated with disease stage (De Santo et al., 2011). More recently, Wang and colleagues showed that neutrophils isolated from pre-metastatic livers were able to profoundly inhibit the proliferation and functional production of IFNγ by cytotoxic CD8+ T cells in models of colorectal cancer metastasis (Wang et al., 2017). Reduction of neutrophil infiltration into metastases via deletion of TNF receptor-2 has also been shown to correlate with reduced CD4+FoxP3+ regulatory T cells within the metastatic niche (Ham et al., 2015).
Interestingly, although all of the studies mentioned above show that neutrophils are capable of altering T cell dynamics within the primary tumour and metastatic context, none definitively confirm the location in which they are fulfilling this role. Given the central role of secondary lymphoid organs and tertiary lymphoid structures in activating and educating both B and T lymphocytes it is quite possible that neutrophils may be enacting their immunosuppressive roles in these locations. Again small numbers of studies are beginning to perhaps suggest that this may well be the case. One such study showed that neutrophils within liver metastases are able to inhibit the expression of CD80 on hepatic B cells by inhibiting STAT3 activation via a contact-dependent mechanism (Thorn et al., 2014). Given that B cells have the capacity to perform as antigen presenting cells (APCs) it is noteworthy that their expression of the vital co-stimulatory molecule CD80 is being prevented in this context. In so doing, neutrophils are likely compromising the ability of B cells as APCs within secondary lymphoid organs to stimulate adaptive immune responses. Indeed in this study B cells with down-regulated CD80 expression were unable to induce CD4+ T cell proliferation in vitro. Interestingly, neutrophils themselves are now believed to be able to function as APCs, in addition to their ability to influence the function of other APC populations (Singhal et al., 2016). Ban et al. have shown that Ccl5 signalling in murine models of breast cancer results in the generation of Ly6G+/MHCII+ cells with low levels of CD86. CD86, similarly to CD80, is a co-stimulatory molecule expressed by APCs vital for the activation of adaptive immune responses. Therefore, its absence in this context also correlates well with the finding of reduced tumour-infiltrating CD8+ T cells and increased regulatory T cells within lymph nodes in these models. Importantly, inhibition of Ccl5 signalling resulted in the increased expression of CD86 by these neutrophils functioning as APCs and was associated with the influx of CD8+ T cells into tumours and a reduction in the number of regulatory T cells within draining lymph nodes (Ban et al., 2017). Intriguingly inhibition of CCL5 signalling has been previously predicted to prevent metastasis (Capece et al., 2012).
In addition to altering T cell dynamics, neutrophils have been shown to modulate the innate immune response in the premetastatic niche through the inhibition of NK cell cytotoxic responses (Sceneay et al., 2012; Spiegel et al., 2016). As highlighted previously, Spiegel and colleagues demonstrated a vital role for neutrophils in preventing early NK cell killing of metastatic cells within the pulmonary vasculature, while an earlier, study also demonstrated that neutrophils within the premetastatic niche have the capacity to suppress NK cell cytotoxicity (Sceneay et al., 2012). Thus, targeting the immuno-suppressive signals that emanate from pro-tumourigenic neutrophils could provide opportunities for targeted therapy or immunotherapy that may have efficacy in both the primary tumour and distant sites.
4. Conclusions
Neutrophils are increasingly emerging as major cellular mediators that play significant roles at all stages of tumorigenesis and all steps of the metastatic cascade. These roles, as highlighted in this review, are mainly pro-tumoural, however, this is not exclusively the case. Indeed, whilst the majority of the literature points to a pro-tumoural role for neutrophils, it has also been shown in a small number of studies that neutrophils may promote tumour cell killing and prevent metastatic establishment (Granot et al., 2011). As alluded to in the introduction of this paper the variable role that neutrophils play is unsurprising given the ability of granulocytic myeloid cells to express such a wide and diverse spectrum of phenotypes. This spectrum of phenotypes and their context dependency increasingly necessitates the need to more completely understand the network of complementary and antagonistic factors controlling neutrophil development, polarisation, and activation both during granulopoiesis and within the peripheral circulation and tissues. Given the diverse range of effects neutrophils have been found to have it is unlikely that the simple depletion of neutrophils will have significant therapeutic benefit. Finally, as eloquently voiced by a number of recent reviews, the requirement for literature to more consistently, accurately, and precisely define granulocytic myeloid cell populations of interest beyond CD11b+Gr1+ will be central to unravelling the importance of neutrophils in cancer and metastasis (Coffelt et al., 2016; Mishalian et al., 2017). Indeed CD11b+, Ly6G+, cellular density, and nuclear morphology should/could perhaps be considered a minimum panel.
Author declaration
All authors have seen and approved the final version of the manuscript.
Conflicts of interest
The authors declare that they have no competing interests.
Funding
This work was supported by Cancer Research UK. JL is funded by an MRC Clinical Research Training Fellowship.
Glossary
- Metastatic niche
The (pre)-metastatic niche refers to the site in a distant organ that can be colonised by tumour cells. the microenvironment of the niche provides a supportive environment for the tumour cells to survive and grow in what would normally be hostile alien conditions.
- Invasion
The active infiltration by cancer cells into neighbouring healthy tissue.
- Angiogenic Switch
The stage at which a pre-vascular mass of tumour cells becomes vascularised through neo-angiogenesis (the growth of new blood vessels), enabling supply of oxygen and essential nutrients and the continued growth of the tumour.
- Intravasation
The process by which cells enter the bloodstream.
- Extravasation
The process by which cells exit the bloodstream.
- Immunotherapy
Therapy designed to harness the host immune system to fight cancer, either by blocking immune checkpoints, or by engineering host t cells to specifically recognize tumour cells.
Contributor Information
Jennifer P. Morton, Email: j.morton@beatson.gla.ac.uk.
Owen J. Sansom, Email: o.sansom@beatson.gla.ac.uk.
References
- Acharyya S., Oskarsson T., Vanharanta S., Malladi S., Kim J., Morris P.G., Manova-Todorova K., Leversha M., Hogg N., Seshan V.E., Norton L., Brogi E., Massagué J. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165–178. doi: 10.1016/j.cell.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akishima-Fukasawa Y., Ishikawa Y., Akasaka Y., Uzuki M., Inomata N., Yokoo T., Ishii R., Shimokawa R., Mukai K., Kiguchi H., Suzuki K., Fujiwara M., Ogata K., Niino H., Sugiura H., Ichinose A., Kuroda Y., Kuroda D., Ishii T. Histopathological predictors of regional lymph node metastasis at the invasive front in early colorectal cancer. Histopathology. 2011;59:470–481. doi: 10.1111/j.1365-2559.2011.03964.x. [DOI] [PubMed] [Google Scholar]
- Aliustaoglu M., Bilici A., Seker M., Dane F. The association of pre-treatment peripheral blood markers with survival in patients with pancreatic cancer. Hepatogastroenterology. 2010;57(99–100):640–645. [PubMed] [Google Scholar]
- Ban Y., Mai J., Li X., Mitchell-Flack M., Zhang T., Zhang L., Chouchane L., Ferrari M., Shen H., Ma X. Targeting autocrine CCL5–CCR5 axis reprograms immunosuppressive myeloid cells and reinvigorates antitumor immunity. Cancer Res. 2017;77:2857–2868. doi: 10.1158/0008-5472.CAN-16-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekes E.M., Schweighofer B., Kupriyanova T.A., Zajac E., Ardi V.C., Quigley J.P., Deryugina E.I., Bekes E.M., Schweighofer B., Kupriyanova T.A., Zajac E., Ardi V.C., Quigley J.P., Deryugina E.I. Tumor-recruited neutrophils and neutrophil TIMP-Free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am. J. Pathol. 2011;179(3):1455–1470. doi: 10.1016/j.ajpath.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson D.D., Meng X., Fullerton D.A., Moore E.E., Lee J.H., Ao L., Silliman C.C., Barnett C.C. Activation state of stromal inflammatory cells in murine metastatic pancreatic adenocarcinoma. Am. J. Physiol. – Regul. Integr. Comp. Physiol. 2012;302 doi: 10.1152/ajpregu.00320.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capece D., Verzella D., Fischietti M., Zazzeroni F., Alesse E. Targeting costimulatory molecules to improve antitumor immunity. J. Biomed. Biotechnol. 2012;2012:926321. doi: 10.1155/2012/926321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.W., Kim J.K., Yang Y.J., Kim P., Yoon K.H., Yun S.H. Urokinase exerts antimetastatic effects by dissociating clusters of circulating tumor cells. Cancer Res. 2015;75:4474–4482. doi: 10.1158/0008-5472.CAN-15-0684. [DOI] [PubMed] [Google Scholar]
- Coffelt S.B., Kersten K., Doornebal C.W., Weiden J., Vrijland K., Hau C.-S., Verstegen N.J.M., Ciampricotti M., Hawinkels L.J.A.C., Jonkers J., de Visser K.E. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–348. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffelt S.B., Wellenstein M.D., de Visser K.E. Neutrophils in cancer: neutral no more. Nat. Rev. Cancer. 2016;16:431–446. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- Cools-Lartigue J., Spicer J., McDonald B., Gowing S., Chow S., Giannias B., Bourdeau F., Kubes P., Ferri L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J. Clin. Invest. 2013;123:3446–3458. doi: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma M., Biziato D., Petrova T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer. 2017;17(8):457–474. doi: 10.1038/nrc.2017.51. [DOI] [PubMed] [Google Scholar]
- De Santo C., Arscott R., Booth S., Karydis I., Jones M., Asher R., Salio M., Middleton M., Cerundolo V., Europe PMC Funders Group Invariant NKT cells modulate the suppressive activity of Serum Amyloid A-differentiated IL-10-secreting neutrophils. Nat. Immunol. 2011;11:1039–1046. doi: 10.1038/ni.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deryugina E.I., Zajac E., Juncker-Jensen A., Kupriyanova T.A., Welter L., Quigley J.P. Tissue-Infiltrating neutrophils constitute the major In vivo source of angiogenesis-inducing MMP-9 in the tumor microenvironment. Neoplasia. 2014;16:771–788. doi: 10.1016/j.neo.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C., Slattery M.J., Liang S., Peng H.-H. Melanoma cell extravasation under flow conditions is modulated by leukocytes and endogenously produced interleukin 8. Mol. Cell. Biomech. 2005;2:145–159. [PMC free article] [PubMed] [Google Scholar]
- Dumitru C.A., Lang S., Brandau S. Modulation of neutrophil granulocytes in the tumor microenvironment: mechanisms and consequences for tumor progression. Semin. Cancer Biol. 2013;23:141–148. doi: 10.1016/j.semcancer.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Erpenbeck L., Schön M.P. Neutrophil extracellular traps: protagonists of cancer progression? Oncogene. 2016;36:2483–2490. doi: 10.1038/onc.2016.406. [DOI] [PubMed] [Google Scholar]
- Fabisiewicz A., Grzybowska E. CTC clusters in cancer progression and metastasis. Med. Oncol. 2017 doi: 10.1007/s12032-016-0875-0. [DOI] [PubMed] [Google Scholar]
- Fidler I.J. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2’-deoxyuridine. J. Natl. Cancer Inst. 1970;45:773–782. [PubMed] [Google Scholar]
- Fridlender Z.G., Sun J., Kim S., Kapoor V., Cheng G. Polarization of tumor-associated neutrophil phenotype by TGF-β:“N1” versus N2 TAN. Cancer Cell. 2009;16(3):183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlender Z.G., Sun J., Mishalian I., Singhal S., Cheng G., Kapoor V., Horng W., Fridlender G., Bayuh R., Worthen S.G., Albelda S.M. Transcriptomic analysis comparing tumor-associated neutrophils with granulocytic myeloid-derived suppressor cells and normal neutrophils. PLoS One. 2012;7(2):e31524. doi: 10.1371/journal.pone.0031524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Weeks A.N., Lim S.Y., Yuzhalin A.E., Jones K., Markelc B., Buzelli J.N., Fokas E., Cao Y., Smart S., Muschel R. Neutrophils promote hepatic metastasis growth through fibroblast growth factor (FGF)2-dependent angiogenesis. Hepatology. 2017;65(6):1920–1935. doi: 10.1002/hep.29088. [DOI] [PubMed] [Google Scholar]
- Granot Z., Henke E., Comen E.A., King T.A., Norton L., Benezra R. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell. 2011;20:300–314. doi: 10.1016/j.ccr.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse-Steffen T., Giese T., Giese N., Longerich T., Schirmacher P., Hänsch M.G., Gaida M.M. Epithelial-to-mesenchymal transition in pancreatic ductal adenocarcinoma and pancreatic tumor cell lines: the role of neutrophils and neutrophil-derived elastase. Clin. Dev. Immunol. 2012:720768. doi: 10.1155/2012/720768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham B., Wang N., D’Costa Z., Fernandez M., Bourdeau F., Auguste P., Illemann M., Eefsen R., Høyer-Hansen G., Vainer B., Evrard M., Gao Z.-H., Brodt P. TNF receptor-2 facilitates an immunosuppressive microenvironment in the liver to promote the colonization and growth of hepatic metastases. Cancer Res. 2015;75:5235–5247. doi: 10.1158/0008-5472.CAN-14-3173. [DOI] [PubMed] [Google Scholar]
- He S., Lamers G.E.M., Beenakker J.M., Cui C., Ghotra V.P.S., Danen E.H.J., Meijer A.H., Spaink H.P., Snaar-Jagalska E.B. Neutrophil-mediated experimental metastasis is enhanced by VEGFR inhibition in a zebrafish xenograft model. J. Pathol. 2012;227:431–445. doi: 10.1002/path.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H., Fujishita T., Kurimoto K., Miyachi H., Kitano S., Inamoto S., Itatani Y., Saitou M., Maekawa T., Taketo M.M. CCR1-mediated accumulation of myeloid cells in the liver microenvironment promoting mouse colon cancer metastasis. Clin. Exp. Metastasis. 2014;31:977–989. doi: 10.1007/s10585-014-9684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton A.M., Rzymkiewicz D.M., Ji H., Gregory A.D., Egea E.E., Metz H.E., Stolz D.B., Land S.R., Marconcini L.A., Kliment C.R., Jenkins K.M., Beaulieu K.A., Mouded M., Frank S.J., Wong K.K., Shapiro S.D. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat. Med. 2010;16:219–223. doi: 10.1038/nm.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P., Shen M., Zhang P., Zheng C., Pang Z., Zhu L., Du J. Intratumoral neutrophil granulocytes contribute to epithelial-mesenchymal transition in lung adenocarcinoma cells. Tumor Biol. 2015:7789–7796. doi: 10.1007/s13277-015-3484-1. [DOI] [PubMed] [Google Scholar]
- Huh S., Liang S., Sharma A., Dong C., Robertson G.P. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res. 2010;70:6071–6082. doi: 10.1158/0008-5472.CAN-09-4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa M., Williams R., Wang L., Vogl T., Srikrishna G. S100A8/A9 activate key genes and pathways in colon tumor progression. Mol. Cancer Res. 2011;9:133–148. doi: 10.1158/1541-7786.MCR-10-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonska J., Leschner S., Westphal K., Lienenklaus S., Weiss S. Neutrophils responsive to endogenous IFN-β regulate tumor angiogenesis and growth in a mouse tumor model. J. Clin. Invest. 2010;120:1151–1164. doi: 10.1172/JCI37223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav S., Bochner B.S., Konstantopoulos K. Hydrodynamic shear regulates the kinetics and receptor specificity of polymorphonuclear leukocyte-colon carcinoma cell adhesive interactions. J. Immunol. 2001;167:5986–5993. doi: 10.4049/jimmunol.167.10.5986. [DOI] [PubMed] [Google Scholar]
- Joyce J.A., Pollard J.W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M.R., Park Y.K., Jeong O., Seon J.W. Elevated preoperative neutrophil to lymphocyte ratio predicts poor survival following resection in late stage gastric cancer. J. Surg. Oncol. 2011;104(5):504–510. doi: 10.1002/jso.21986. [DOI] [PubMed] [Google Scholar]
- Larco J.E., Wuertz B.R.K., Furcht L.T. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin. Cancer Res. 2004:4895–4900. doi: 10.1158/1078-0432.CCR-03-0760. [DOI] [PubMed] [Google Scholar]
- Li T.-J., Jiang Y.-M., Hu Y.-F., Huang L., Yu J., Zhao L.-Y., Deng H.-J., Mou T.-Y., Liu H., Yang Y., Zhang Q., Li G.-X. Interleukin-17–producing neutrophils link inflammatory stimuli to disease progression by promoting angiogenesis in gastric cancer. Clin. Cancer Res. 2016;23:1575–1585. doi: 10.1158/1078-0432.CCR-16-0617. [DOI] [PubMed] [Google Scholar]
- Liang S., Dong C. Integrin VLA-4 enhances sialyl-Lewisx/a-negative melanoma adhesion to and extravasation through the endothelium under low flow conditions. AJP Cell Physiol. 2008;295:C701–C707. doi: 10.1152/ajpcell.00245.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieschke G.J., Grail D., Hodgson G., Metcalf D., Stanley E. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84(6):1737–1746. [PubMed] [Google Scholar]
- Lim S., Gordon-Weeks A., Allen D., Kersemans V., Beech J., Smart S., Muschel R.J. Cd11b+ myeloid cells support hepatic metastasis through down-regulation of angiopoietin-like 7 in cancer cells. Hepatology. 2015;62:521–533. doi: 10.1002/hep.27838. [DOI] [PubMed] [Google Scholar]
- Liu Y., Gu Y., Han Y., Zhang Q., Jiang Z., Zhang X., Huang B., Xu X., Zheng J., Cao X. Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. Cancer Cell. 2016;30:243–256. doi: 10.1016/j.ccell.2016.06.021. [DOI] [PubMed] [Google Scholar]
- McDonald B., Spicer J., Giannais B., Fallavollita L., Brodt P., Ferri L.E. Systemic inflammation increases cancer cell adhesion to hepatic sinusoids by neutrophil mediated mechanisms. Int. J. Cancer. 2009;125:1298–1305. doi: 10.1002/ijc.24409. [DOI] [PubMed] [Google Scholar]
- Mishalian I., Granot Z., Fridlender Z.G. The diversity of circulating neutrophils in cancer. Immunobiology. 2017;222:82–88. doi: 10.1016/j.imbio.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Morimoto-Kamata R., Mizoguchi S., Ichisugi T., Yui S. Cathepsin G induces cell aggregation of human breast cancer MCF-7 cells via a 2-step mechanism: catalytic site-independent binding to the cell surface and enzymatic activity-dependent induction of the cell aggregation. Mediators Inflamm. 2012;2012:456462. doi: 10.1155/2012/456462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morvan M.G., Lanier L.L. NK cells and cancer: you can teach innate cells new tricks. Nat. Rev. Cancer. 2015;16:7–19. doi: 10.1038/nrc.2015.5. [DOI] [PubMed] [Google Scholar]
- Najmeh S., Cools-Lartigue J., Rayes R.F., Gowing S., Vourtzoumis P., Bourdeau F., Giannias B., Berube J., Rousseau S., Ferri L.E., Spicer J.D. Neutrophil extracellular traps sequester circulating tumor cells via β1-integrin mediated interactions. Int. J. Cancer. 2017;140:2321–2330. doi: 10.1002/ijc.30635. [DOI] [PubMed] [Google Scholar]
- Nguyen D.X., Bos P.D., Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat. Rev. Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- Noy R., Pollard J.W. Review tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa H., Chiu C., Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc. Natl. Acad. Sci. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouzounova M., Lee E., Piranlioglu R., Andaloussi A., Kolhe R., Demirci M.F., Marasco D., Asm I., Chadli A., Hassan K.A., Thangaraju M., Zhou G., Arbab A.S., Cowell J.K., Korkaya H. Monocytic and granulocytic myeloid derived suppressor cells differentially regulate spatiotemporal tumour plasticity during metastatic cascade. Nat. Commun. 2017;8:14979. doi: 10.1038/ncomms14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Wysocki R.W., Amoozgar Z., Maiorino L., Fein M.R., Jorns J., Schott A.F., Kinugasa-Katayama Y., Lee Y., Won N.H., Nakasone E.S., Hearn S.A., Küttner V., Qiu J., Almeida A.S., Perurena N., Kessenbrock K., Goldberg M.S., Egeblad M. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci. Transl. Med. 2016;8:361ra138. doi: 10.1126/scitranslmed.aag1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H., Zhang H., Matei I.R., Costa-Silva B., Hoshino A., Rodrigues G., Psaila B., Kaplan R.N., Bromberg J.F., Kang Y., Bissell M.J., Cox T.R., Giaccia A.J., Erler J.T., Hiratsuka S., Ghajar C.M., Lyden D. Pre-metastatic niches: organ-specific homes for metastases. Nat. Rev. Cancer. 2017;17:302–317. doi: 10.1038/nrc.2017.6. [DOI] [PubMed] [Google Scholar]
- Pekarek L.A., Starr B.A., Toledano A.Y. Inhibition of tumor growth by elimination of granulocytes. J. Exp. Med. 1995;181(1):430–435. doi: 10.1084/jem.181.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen M.M., Ryan R.E., Holzer R.G., Keller-Peck C.R., Jorcyk C.L. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. 2005;65:8896–8904. doi: 10.1158/0008-5472.CAN-05-1734. [DOI] [PubMed] [Google Scholar]
- Reticker-Flynn N.E., Bhatia S.N. Aberrant glycosylation promotes lung cancer metastasis through adhesion to galectins in the metastatic niche. Cancer Discov. 2015;5:168–181. doi: 10.1158/2159-8290.CD-13-0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roxburgh C.S.D., Wallace A.M., Guthrie G.K. Comparison of the prognostic value of tumour‐and patient‐related factors in patients undergoing potentially curative surgery for colon cancer. Colorectal Dis. 12(10), 2010, 987-994 doi: 10.1111/j.1463-1318.2009.01961.x. [DOI] [PubMed] [Google Scholar]
- Sagiv J.Y., Michaeli J., Assi S., Mishalian I., Kisos H., Levy L., Damti P., Lumbroso D., Polyansky L., Sionov R.V., Ariel A., Hovav A.-H.H., Henke E., Fridlender Z.G., Granot Z. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015;10:562–573. doi: 10.1016/j.celrep.2014.12.039. [DOI] [PubMed] [Google Scholar]
- Sceneay J., Chow M.T., Chen A., Halse H.M., Wong C., Andrews D.M., Sloan E.K., Parker B.S., Bowtell D.D., Smyth M.J., Möller A. Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res. 2012;72:3906–3911. doi: 10.1158/0008-5472.CAN-11-3873. [DOI] [PubMed] [Google Scholar]
- Schiwon M., Weisheit C., Franken L., Gutweiler S., Dixit A., Meyer-Schwesinger C., Pohl J.M., Maurice N.J., Thiebes S., Lorenz K., Quast T., Fuhrmann M., Baumgarten G., Lohse M.J., Opdenakker G., Bernhagen J., Bucala R., Panzer U., Kolanus W., Gröne H.J., Garbi N., Kastenmüller W., Knolle P.A., Kurts C., Engel D.R. Crosstalk between sentinel and helper macrophages permits neutrophil migration into infected uroepithelium. Cell. 2014;156:456–468. doi: 10.1016/j.cell.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert B., Grünwald B., Kobuch J., Cui H., Schelter F., Schaten S., Siveke J.T., Lim N.H., Nagase H., Simonavicius N., Heikenwalder M., Reinheckel T., Sleeman J.P., Janssen K., Knolle P.A., Krüger A. Tissue inhibitor of metalloproteinases (TIMP)-1 creates a premetastatic niche in the liver through SDF-1/CXCR4-dependent neutrophil recruitment in mice. Hepatology. 2015;61:238–248. doi: 10.1002/hep.27378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul M.E., Fridlender Z.G. Neutrophils as active regulators of the immune system in the tumor microenvironment. J. Leukoc. Biol. 2017;102(2):343–349. doi: 10.1189/jlb.5MR1216-508R. [DOI] [PubMed] [Google Scholar]
- Shojaei F., Wu X., Zhong C., Yu L., Liang X.-H., Yao J., Blanchard D., Bais C., Peale F.V., van Bruggen N., Ho C., Ross J., Tan M., Carano R.A.D., Meng Y.G., Ferrara N. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450:825–831. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
- Singhal S., Bhojnagarwala P.S., O’Brien S., Moon E.K., Garfall A.L., Rao A.S., Quatromoni J.G., Stephen T., Litzky L., Deshpande C., Feldman M.D., Hancock W.W., Conejo-Garcia J.R., Albelda S.M., Eruslanov E.B. Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell. 2016;30:120–135. doi: 10.1016/j.ccell.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer J.D., McDonald B., Cools-Lartigue J.J., Chow S.C., Giannias B., Kubes P., Ferri L.E. Neutrophils promote liver metastasis via mac-1–mediated interactions with circulating tumor cells. Cancer Res. 2012;72:3919–3927. doi: 10.1158/0008-5472.CAN-11-2393. [DOI] [PubMed] [Google Scholar]
- Spiegel A., Brooks M.W., Houshyar S., Reinhardt F., Ardolino M., Fessler E., Chen M.B., Krall J.A., DeCock J., Zervantonakis I.K., Iannello A., Iwamoto Y., Cortez-Retamozo V., Kamm R.D., Pittet M.J., Raulet D.H., Weinberg R.A. Neutrophils suppress intraluminal NK cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov. 2016;6:630–649. doi: 10.1158/2159-8290.CD-15-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg P.S. Targeting metastasis. Nat. Rev. Cancer. 2016;16:201–218. doi: 10.1038/nrc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele C.W., Karim S.A., Leach J., Bailey P., Upstill-Goddard R., Rishi L., Foth M., Bryson S., McDaid K., Wilson Z., Eberlein C., Candido J.B., Clarke M., Nixon C., Connelly J., Jamieson N., Carter R.C., Balkwill F., Chang D.K., Evans T.R., Strathdee D., Biankin A.V., Nibbs R., Barry S.T., Sansom O.J., Morton J.P. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell. 2016;29:832–845. doi: 10.1016/j.ccell.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn M., Point G.R., Burga R.A., Nguyen C.T., Espat J.N., Katz S.C. Liver metastases induce reversible hepatic B cell dysfunction mediated by Gr-1 + CD11b+ myeloid cells. J. Leukoc. Biol. 2014:96. doi: 10.1189/jlb.3A0114-012RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohme S., Yazdani H.O., Al-Khafaji A.B., Chidi A.P., Loughran P., Mowen K., Wang Y., Simmons R.L., Huang H., Tsung A. Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res. 2016;76:1367–1380. doi: 10.1158/0008-5472.CAN-15-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita M., Shimizu T., Ayabe T., Yonei A. Preoperative neutrophil to lymphocyte ratio as a prognostic predictor after curative resection for non-small cell lung cancer. Anticancer Res. 2011;31(9):2995–2998. [PubMed] [Google Scholar]
- Wang D., Sun H., Wei J., Cen B., DuBois R.N. CXCL1 is critical for pre-metastatic niche formation and metastasis in colorectal cancer. Cancer Res. 2017;77(13):3655–3665. doi: 10.1158/0008-5472.CAN-16-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wislez M., Rabbe N., Marchal J., Milleron B., Crestani B., Mayaud C., Antoine M., Soler P., Cadranel J. Hepatocyte growth factor production by neutrophils infiltrating bronchioloalveolar subtype pulmonary adenocarcinoma: role in tumor progression and death. Cancer Res. 2003:1405–1412. [PubMed] [Google Scholar]
- Wu Y., Zhao Q., Peng C., Sun L., Li X., Kuang D. Neutrophils promote motility of cancer cells via a hyaluronan-mediated TLR4/PI3 K activation loop. J. Pathol. 2011;225:438–447. doi: 10.1002/path.2947. [DOI] [PubMed] [Google Scholar]
- Wu C.F., Andzinski L., Kasnitz N., Kröger A., Klawonn F., Lienenklaus S., Weiss S., Jablonska J. The lack of type i interferon induces neutrophil-mediated pre-metastatic niche formation in the mouse lung. Int. J. Cancer. 2015;137:837–847. doi: 10.1002/ijc.29444. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Kawada K., Itatani Y., Inamoto S., Okamura R., Iwamoto M., Miyamoto E., Chen-Yoshikawa T.F., Hirai H., Hasegawa S., Date H., Taketo M.M., Sakai Y. Loss of SMAD4 promotes lung metastasis of colorectal cancer by accumulation of CCR1+ tumor-associated neutrophils through CCL15-CCR1 axis. Clin. Cancer Res. 2017;23:833–844. doi: 10.1158/1078-0432.CCR-16-0520. [DOI] [PubMed] [Google Scholar]
- Yan H.H., Pickup M., Pang Y., Gorska A.E., Li Z., Chytil A., Geng Y., Gray J.W., Moses H.L., Yang L. Gr-1 + CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 2010;70:6139–6149. doi: 10.1158/0008-5472.CAN-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P.F., Huang Y., Han Y.Y., Lin L.Y., Sun W.H., Rabson A.B., Wang Y., Shi Y.F. TNFα-activated mesenchymal stromal cells promote breast cancer metastasis by recruiting CXCR2+ neutrophils. Oncogene. 2016;36:482–490. doi: 10.1038/onc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Qiao X., Shi H., Han X., Liu W., Tian X., Zeng X. Circulating tumor-associated neutrophils (cTAN) contribute to circulating tumor cell survival by suppressing peripheral leukocyte activation. Tumor Biol. 2015;37:5397–5404. doi: 10.1007/s13277-015-4349-3. [DOI] [PubMed] [Google Scholar]