Abstract
Saccharicterpenin is a new green additive agent that is derived from the extract of Theaceae plants and has the ability to improve immunity and meat quality, increase the digestive enzyme activity, and enhance the intestinal development and growth of animals. However, the antioxidant status and systematic changes in metabolic biochemistry associated with saccharicterpenin supplementation in animals are still unknown. This study examined the effects of saccharicterpenin on the antioxidant status and urinary metabolic profile of rats. Sixteen rats were randomly distributed to 2 groups. One group was treated with 400 mg/kg body weight of saccharicterpenin, and the other group was treated with equal amount of saline. Results revealed that saccharicterpenin significantly increased the capacities of anti-hydroxyl radical (13.18%) and anti-superoxide anion (14.36%), the total antioxidant capacity (48.27%), and the activities of total superoxide dismutase (3.68%), catalase (21.52%), glutathione peroxidase (5.83%) and glutathione S-transferase (29.59%) (P < 0.05). By contrast, the contents of malondialdehyde and glutathione were not significantly affected by saccharicterpenin (P > 0.05). Saccharicterpenin supplementation significantly increased the urinary levels of bile acids, ethanol, α-ketoglutarate, and α-hydroxybutyrate but decreased the level of N-acetylglutamate (P < 0.05). In summary, saccharicterpenin can enhance the antioxidant capacity and modulate the metabolism in rats.
Keywords: Saccharicterpenin, Antioxidant, Antioxidant status, Metabolic profile, Metabolism, Urine
1. Introduction
Saccharicterpenin is a new green additive derived from the extract of Theaceae plants as recognized by the Ministry of Agriculture of the People's Republic of China and is primarily composed of polysaccharides and triterpenoid saponins (Liu et al., 2016). Saccharicterpenin exerts many functions, such as improving immunity (Shen, 2010, Zhang, 2010) and meat quality (Huang, 2017), increasing digestive enzyme activity (Liu et al., 2016), and enhancing intestinal development (Huang et al., 2011) and growth (Tang et al., 2007, Fu et al., 2008, Huang, 2017) in animals. The growth and development of tissues in animals depend on the structure of the cells that are partly affected by the antioxidant status of the animal itself (Kisaoglu et al., 2013).
The antioxidant defense systems of animals consist of enzymatic and non-enzymatic antioxidants (Sies, 1997). Enzymatic antioxidants include total superoxide dismutase (T-SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione S-transferase (GST). Total superoxide dismutase and CAT are the 2 primary antioxidant enzymes that can eliminate superoxide anions and hydroxyl free radicals (Wu et al., 2014). Furthermore, GPx and GST are glutathione-dependent enzymes that can reduce hydroperoxides and lipid peroxides (Feng et al., 2014). The activity of T-SOD in fish serum was significantly improved by saccharicterpenin (Hao et al., 2014). However, whether or not saccharicterpenin can improve the performance of enzyme antioxidants in animals remains unclear. In addition to enzymatic antioxidants, non-enzymatic antioxidants are important in eliminating free radicals. Glutathione (GSH) is a major non-enzymatic antioxidant that can remove free radicals. The content of GSH was improved by triterpenoid saponins (a component of saccharicterpenin) in the liver of rats as reported by Gutierrez (2016), who suggested that non-enzymatic antioxidants were increased by triterpenoid saponins. However, the effect of saccharicterpenin on non-enzymatic components is unknown.
Metabolomics offers a novel strategy in understanding nutritional interventions inducing metabolomic changes in animal biofluids and tissues. However, no information is available about the response of animal or human biological systems to saccharicterpenin supplementation. In this study, we tested the hypothesis that saccharicterpenin supplementation may improve the antioxidant status and modulate the metabolic profiles of rats. The results of this study can offer a theoretical basis for the development of saccharicterpenin as a stress-resistant component in feeds and to explore the connection between saccharicterpenin supplementation and health or disease risk.
2. Materials and methods
2.1. Animal experiment and sample collection
The experimental protocol used in this study was approved by Sichuan Agricultural University Animal Care and Use Committee. Sixteen Sprague–Dawley (SD) rats weighing 214.5 ± 8.5 g were randomly distributed to 2 groups (n = 8 per group), namely, control and saccharicterpenin groups. The saccharicterpenin group received an intragastric supplementation of saccharicterpenin (400 mg/kg body weight) once a day at 09:00 for 28 d. Saccharicterpenin was purchased from Hangzhou Tangtian Technology Co., Ltd. (Hangzhou, China). The dosage of saccharicterpenin was selected according to the result of the preliminary experiment in our laboratory. The control group received normal saline (same amount of saccharicterpenin in the saccharicterpenin group). Ambient temperatures and relative humidity were controlled between 24 and 25 °C and 50% and 70%, respectively. Urine samples were collected in ice-cooled vessels and were added with 30 μL of sodium azide solution (1.0% wt/vol) from d 26 to 27 of the treatment period (24 h). The samples were collected between 08:30 and 12:00 to avoid potential metabolic variations due to the differences in diurnal rhythm. All urine samples were stored at −80 °C until subjected to nuclear magnetic resonance (NMR) spectroscopic test. The rats were sacrificed after 28 d, and blood samples were collected from the eyes of the rats administered with anesthesia with ether. The blood samples were placed in Eppendorf tubes, incubated for 30 min, and centrifuged at 4 °C with a speed of 3,500 × g for 10 min to obtain serum.
2.2. Antioxidant parameters in serum
To investigate the antioxidant status in rat serum, we detected the following parameters: the content of malondialdehyde (MDA); the capacities of anti-hydroxyl radicals (AHR) and anti-superoxide anions (ASA); the activities of several enzymatic antioxidants including catalase (CAT), total superoxide dismutase (T-SOD), glutathione peroxidase (GPx), and GST; the content of non-enzymatic antioxidants such as glutathione (GSH); and total antioxidant capacity (T-AOC). All antioxidative reagents and enzymes detection kits used in this experiment were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
2.3. Sample preparation and nuclear magnetic resonance spectroscopy
Urine samples were prepared by mixing 630 μL of urine and 70 μL of Anachro-certified 2,2-dimethyl-2-silapentane-5-sulfonate-d6 (DSS) standard solution containing 99.9% (vol/vol) D2O, 0.02% (wt/vol) NaN3, and 4.08 mmol/L DSS as a reference at 0 part per million. The samples with the standard solution were vortexed and centrifuged, and 550 μL of samples were transferred into NMR tubes for NMR analysis. The standard 1 D NMR spectrum nuclear overhauser effect spectroscopy (NOESY) of urine provides a general representation of the total biochemical composition of the samples and was recorded using the first increment of the NOESY pulse sequence to achieve water presaturation (recycle delay–90 °–t1–90 °–tm–90 °–acquire data). The recycle delay, t1, mixing time, and 90 ° pulse length were 2 s, 3 μs, 100 ms, and 13.70 μs, respectively. A total of 128 transients were acquired into 49,178 data points using a spectral width of 9,590 Hz and an acquisition time of 2.56 s. Metabolite assignments were achieved by considering chemical shifts, coupling constants, and relative intensities (Nicholson and Foxall, 1995, Fan, 1996, Wishart et al., 2013).
2.4. Nuclear magnetic resonance spectroscopic processes and analysis
The urine spectra were phased, and the baseline was corrected manually for the spectral analysis of the urine samples. The spectral region of urine at δ 0.5 to 9.5 was integrated into regions with an equal width of 0.01 part per million by using Mestrenova 8.1.2 software (Mestrelab Research S.L., Spain). The regions include δ 4.50 to 5.30 for H2O and δ 5.45 to 6.00 for urea were discarded in the urine spectra. Each integral bin was then normalized to the total sum of all integral regions for each spectrum prior to data analysis.
Multivariate data analysis was performed on normalized NMR datasets using the software package SIMCA-P+ (version 11.0, Umetrics, Sweden). Principal component analysis (PCA) was conducted on the dataset to provide an overview of the intrinsic similarities/dissimilarities within the dataset. Projection to latent structure-discriminant analysis (PLS-DA) and orthogonal projection to latent structure-discriminant analysis (OPLS-DA) were further performed with unit-variance scaling (UV) to identify the metabolites that significantly contributed to group differentiation. Projection to latent structure-discriminant analysis models were calculated with one predictive and orthogonal component using the NMR data as the X-matrix and the class information as the Y-matrix. The quality of the model was assessed by parameter R2X, which represents the total explained variations for the X matrix and Q2 reveals the predictability of the model. The models were evaluated using sevenfold cross validation and a permutation test. A model is significant when its Q2 value is significant (P < 0.05) as analyzed through permutation. Appropriate correlation coefficients, which were obtained by evaluating the significance of Pearson's product-moment correlation coefficient, were used as cut-off values.
2.5. Statistical analysis
Data on the antioxidant status and integral of altered metabolites were analyzed by T test using SPSS 22.0 software (SPSS Inc., Chicago, IL, USA). Results were considered statistically significant at P < 0.05. Experimental data were expressed as means ± standard error.
3. Results
3.1. Serum antioxidant status
Table 1 reveals the antioxidant indicators in serum. The capacities of AHR and ASA in the saccharicterpenin group were significantly increased by 13.18% and 14.36%, respectively, compared with those in the control group. By contrast, the T-SOD, CAT, GPx, and GST activities and T-AOC were increased by 3.68%, 21.52%, 5.83%, 29.59% and 48.27%, (P < 0.05), respectively. However, the contents of MDA and GSH in serum were not affected (P > 0.05) by saccharicterpenin.
Table 1.
Effect of saccharicterpenin on the antioxidant status of serum in rats.
| Parameters | Treatments |
|
|---|---|---|
| Control | Saccharicterpenin | |
| MDA, nmol/mg protein | 4.26 ± 0.13 | 4.12 ± 0.19 |
| ASA, U/g protein | 117.18 ± 4.44a | 134.01 ± 4.64b |
| AHR, U/mg protein | 188.56 ± 5.75a | 213.42 ± 3.90b |
| CAT, U/mg protein | 46.01 ± 0.62a | 55.91 ± 1.37b |
| T-SOD, U/mg protein | 69.81 ± 1.04a | 72.38 ± 0.49b |
| T-AOC, U/g protein | 3.46 ± 0.084a | 5.13 ± 0.12b |
| GSH, mg/g protein | 1.53 ± 0.09 | 1.85 ± 0.20 |
| GPx, U/mg protein | 284.43 ± 6.71a | 301.02 ± 3.05b |
| GST, U/mg protein | 109.61 ± 2.33a | 142.04 ± 5.64b |
MDA = malondialdehyde; ASA = anti-superoxide anion; AHR = anti-hydroxyl radical; CAT = catalase; T-SOD = total superoxide dismutase; T-AOC = total antioxidant capacity; GSH = glutathione content; GPx = glutathione peroxidase; GST = glutathione S-transferase.
a, b Values are shown as means ± SEM. Within a row, means with different superscript letters are significantly different (P < 0.05).
3.2. Multivariate data analysis of the nuclear magnetic resonance spectra
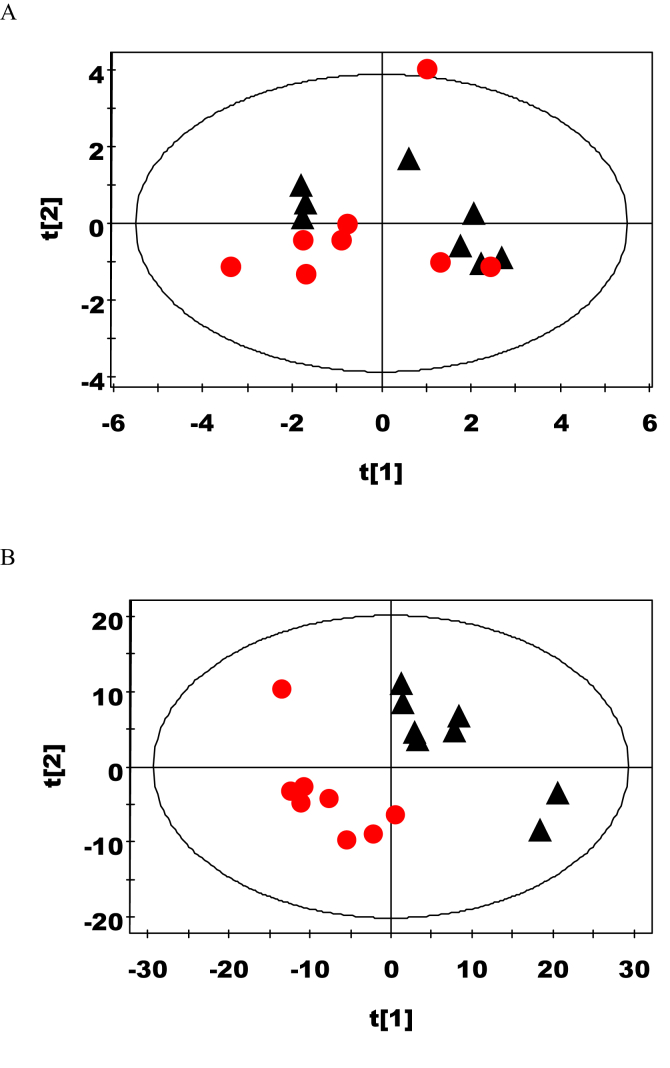
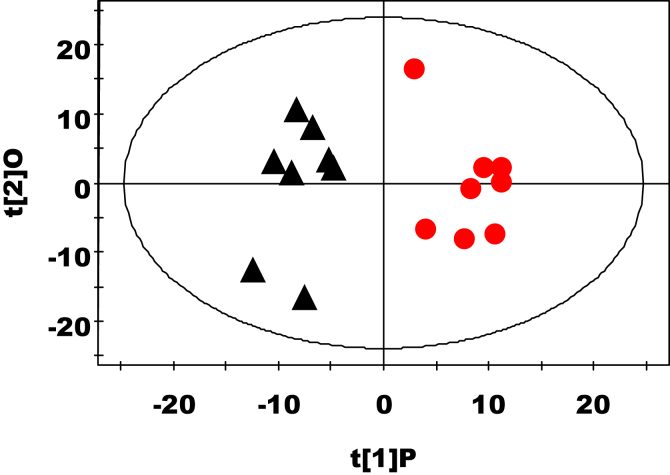
Principal component 1 (PC1) and principal component 2 (PC2) were used to explain 34.4% and 17.1% of the variables, respectively. Principal component analysis results (Fig. 1A) showed no difference in the metabolic urine profiles of rats from the saccharicterpenin and control groups. The score plots of PLS-DA obtained (Fig. 1B) highlighted 2 clusters corresponding to the 2 groups. OPLS-DA analysis identified important urinary metabolic changes induced by saccharicterpenin supplementation. The urinary levels of bile acids, ethanol, α-ketoglutarate, and α-hydroxybutyrate were increased due to the treatment of saccharicterpenin; however, the level of N-acetylglutamate was decreased (P < 0.05, Fig. 2 and Table 2).
Fig. 1.
Multivariate data analysis of the nuclear magnetic resonance spectra. (A) Principal component analysis (PCA) (R2X = 0. 643, Q2 = 0.139) and (B) projection to latent structure-discriminant analysis (PLS-DA) score plots (R2X = 0.251, R2Y = 0.897, Q2 = 0.138) based on the 1D nuclear magnetic resonance (NMR) spectra of the urine obtained from urinary metabolites from the control (black triangles) and saccharicterpenin (red circles) groups.
Fig. 2.
Orthogonal projection to latent structure-discriminant analysis (OPLS-DA) score plots of urinary metabolites derived from the control (black triangles), and saccharicterpenin (red circles) (R2X = 0.251, R2Y = 0.897, Q2 = 0.201) groups.
Table 2.
Orthogonal projection to latent structure-discriminant analysis (OPLS-DA) coefficients derived from the nuclear magnetic resonance (NMR) data of urine metabolites obtained from the control (A) and saccharicterpenin groups (B).
| Metabolite | OPLS-DA correlation coefficient (r)1 |
Relative integrals, %2 |
|
|---|---|---|---|
| B (vs. A) | B | A | |
| Bile acids | 0.695 | 0.033a | 0.025b |
| Ethanol | 0.666 | 0.135a | 0.111b |
| N-acetylglutamate | −0.693 | 0.351a | 0.485b |
| α-ketoglutarate | 0.770 | 0.324a | 0.217b |
| α-hydroxyburyrate | 0.779 | 0.157a | 0.132b |
| Unknown | −0.750 | 0.437a | 0.543b |
a, b Within a row, mean values with different superscript letters are significantly different (P < 0.05).
Correlation coefficients were obtained from the OPLS-DA results. The positive and negative signs suggest the positive and negative correlation, respectively. The correlation coefficient of |r| higher than 0.666 represents the cutoff value.
Normalized integral of metabolites in the spectrum (normalized to 100).
4. Discussion
Saccharicterpenin effectively improved the growth of animals (Tang et al., 2007, Fu et al., 2008). To further investigate the effect of saccharicterpenin on the antioxidant status of animals, we detected serum antioxidant indicators, such as T-AOC, the capacity of scavenging free radicals (ASA and AHR), and the activity of enzymatic (T-SOD, CAT, GPx, and GST) and non-enzymatic (GSH) antioxidants.
Reactive oxygen species (ROS) are natural results of aerobic respiration and cellular metabolism. The antioxidant status is mainly influenced by ROS, which can be categorized into O2− and OH− radicals (Lin and Yen, 1999). ASA and AHR are the 2 indicators that can reflect the total capacity of scavenging superoxide anion and hydroxyl free radicals (Cao et al., 2015). In this study, the capacities of ASA and AHR were significantly improved by saccharicterpenin. This result was similar to that of a previous study, wherein arginine significantly increased the capacities of ASA and AHR in the serum of rats (Xiao et al., 2016). These results suggest that saccharicterpenin can increase the capacity of scavenging free radicals, and this ability is related to the enzymatic antioxidant defense system in animals (Fang et al., 2002). Catalase and T-SOD are the 2 primary enzymes of the enzymatic components that exhibit catalytic activity with oxygen radicals. Catalase is an important endogenous antioxidant enzyme that can eliminate organic OH− and decompose H2O2 into O2 and H2O (Fang et al., 2017). Superoxide dismutase can collaboratively convert superoxide anions into H2O and O2 (Cao et al., 2015). In this study, the serum CAT activity was increased by saccharicterpenin. Similar results were obtained from the CAT activity of saponins in rats as reported by Liu et al. (2016). Triterpenoid saponins are the primary component of saccharicterpenin. Triterpenoid and soy saponins are both members of saponins. Thus, the improved activity of CAT by saccharicterpenin may be due to the role of triterpenoid saponins, which need further investigation. In addition, the activity of T-SOD was also improved, which was similar to the result of saccharicterpenin treatment in fish (Chen et al., 2014). Glutathione peroxidase and GST can reduce the production of free radicals. Glutathione peroxidase is involved in the detoxification in organic particles and can decrease the production of hydroperoxides and H2O2 (Jiang et al., 2009). Glutathione S-transferase plays an antioxidant role by conjugating the cleavage products of lipid peroxides to promote their separation from the oxidative metabolism in cells (Chen et al., 2009). In this study, the activities of GST and GPx were improved by saccharicterpenin. This enhanced activity of GPx was similar to the consequence of supplementing alfalfa saponin extract in piglets (Shi et al., 2014). Glutathione is the most important antioxidant agent of non-enzymatic antioxidant components (Wu et al., 2016). In this study, the content of GSH was not significantly affected by saccharicterpenin. This result was different from a previous study, which stated that triterpenoid saponins (a component of saccharicterpenin) can significantly increase the content of GSH in the pancreas of rats (Gutierrez, 2016). This conflict may be because of the difference in tissues. Total antioxidant capacity is an important comprehensive index used to reflect the total antioxidant status of the body (Ren et al., 2012). The serum T-AOC was improved by saccharicterpenin, which has a similar effect to Radix Trichosanthis saponins (Chen et al., 2014). This result is consistent with the previous attempts for antioxidant enzyme improvement. Collectively, these results suggest that saccharicterpenin can improve free radical scavenging and enhance enzymatic antioxidant activities.
Saccharicterpenin supplementation regulated the urinary metabolism of rats and changed the bile acid metabolism. The body needs cholesterol to produce bile acids, which are secreted via the bile into the intestines. Bile acids can help the formation of micelles that enhances the processing of dietary fat. In this study, the urinary bile acid levels were increased by saccharicterpenin supplementation, revealing that the total excretion of bile acids was promoted. N-acetylglutamate is required for the normal function of the urea cycle (Meijer and Verhoeven, 1984). When the α-ketoglutarate level was increased, the serum citrate levels were also increased by saccharicterpenin administration (data not shown). This finding implies that the tricarboxylic acid cycle was altered.
5. Conclusion
This study revealed that saccharicterpenin effectively promoted the antioxidant defense capacity in rat serum by enhancing the free radical-scavenging ability and enzymatic antioxidant activity. Moreover, saccharicterpenin supplementation modulated the urinary metabolism. Further studies should explore the molecular mechanism of saccharicterpenin with regard to the antioxidant status of serum in animals.
Acknowledgements
We thank our team members for their industrious assistance. We also thank the Specific Research Supporting Program for Discipline Construction in Sichuan Agricultural University (No: 03570126) for financial support.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Cao W., Liu G., Fang T., Wu X., Jia G., Zhao H. Effects of spermine on the morphology, digestive enzyme activities, and antioxidant status of jejunum in suckling rats. RSC Adv. 2015;5:76607–76614. [Google Scholar]
- Chen J., Zhou X., Feng L., Liu Y., Jiang J. Effects of glutamine on hydrogen peroxide-induced oxidative damage in intestinal epithelial cells of Jian carp (Cyprinus carpio var. Jian) Aquaculture. 2009;288:285–289. [Google Scholar]
- Chen Y., Miao Y., Huang L., Li J., Sun H., Zhao Y. Antioxidant activities of saponins extracted from Radix Trichosanthis: an in vivo and in vitro evaluation. BMC Complement Altern Med. 2014;14:86–93. doi: 10.1186/1472-6882-14-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan T.W.M. Metabolite profiling by one- and two-dimensional NMR analysis of complex mixtures. Prog Nucl Magn Reson Spectrosc. 1996;28:161–219. [Google Scholar]
- Fang T., Wu X., Cao W., Jia G., Zhao H., Chen X. Effects of dietary fiber on the antioxidant capacity, immune status, and antioxidant-relative signaling molecular gene expression in rat organs. RSC Adv. 2017;7:19611–19620. [Google Scholar]
- Fang Y., Yang S., Wu G. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18:872–879. doi: 10.1016/s0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- Feng L., Zhao S., Chen G., Jiang W., Liu Y., Jiang J. Antioxidant status of serum, muscle, intestine and hepatopancreas for fish fed graded levels of biotin. Fish Physiol Biochem. 2014;40:499–510. doi: 10.1007/s10695-013-9861-z. [DOI] [PubMed] [Google Scholar]
- Fu L., Zhang A., Jiang N., Song Y. Effect of Saccharicterpenin on growth performance, intestinal microflora and meat qualities for Royal Chicken in growing period. Acta Ecol Anim Domastici. 2008;29:48–52. [Google Scholar]
- Gutierrez R.M.P. Antidiabetic andantioxidant properties, and α-amylase and α-glucosidase inhibition effects of triterpene saponins from Piper auritum. Food Sci Biotechnol. 2016;25:229–239. doi: 10.1007/s10068-016-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao T., Wang J., Li B., Zhang D., Sun J., Sun Y. Effect of dietary supplementation of Saccharicterpenin on growth, immunity and heat shock protein 70 content of juvenile turbot (Scophthalmus maximus) J Zhejiang Univ. 2014;40:338–347. [Google Scholar]
- Huang F. Zhejiang University; 2017. Effect of saccharicterpenin on growth performance and meat quality in broilers. [Master degree thesis dissertation] [Google Scholar]
- Huang P., Zhao S., Zhang X., Zhang T., Xiao C. Effects of dietary Saccharicterpenin on mucosal structure of small intestine in Gushi chickens. Chin J Anim Nutr. 2011;23:2016–2023. [Google Scholar]
- Jiang W., Feng L., Liu Y., Jiang J., Zhou X. Myo-inositol prevents oxidative damage, inhibits oxygen radical generation and increases antioxidant enzyme activities of juvenile Jian carp (Cyprinus carpio var. Jian) Aquac Res. 2009;40:1770–1776. [Google Scholar]
- Kisaoglu A., Borekci B., Yapca O., Bilen H., Suleyman H. Tissue damage and oxidant/antioxidant balance. EAJM. 2013;45:47–49. doi: 10.5152/eajm.2013.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M., Yen C. Reactive oxygen species and lipid peroxidation product-scavenging ability of yogurt organisms. J Dairy Sci. 1999;82:1629–1634. doi: 10.3168/jds.S0022-0302(99)75391-9. [DOI] [PubMed] [Google Scholar]
- Liu J., Zeng T., Du X., Li G., Yu Y., Lu L. Effects of water supplemented with Saccharicterpenin and photosynthetic bacteria on egg production, egg quality, serum immunoglobulins, and digestive enzyme activities of ducks. Can J Anim Sci. 2016;96:397–403. [Google Scholar]
- Liu Z., Liu Y., Xiong Z., Feng Y., Tang W. Total soy saponins improve the antioxidant capacity of the myocardium and exercise ability in exhausted rats. J Sport Health Sci. 2016;5:424–429. doi: 10.1016/j.jshs.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer A.J., Verhoeven A.J. N-acetylglutamate and urea synthesis. Biochem J. 1984;223:559–560. doi: 10.1042/bj2230559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson J.K., Foxall P.J.D. 750 MHz 1H and 1H-13C NMR spectroscopy of human blood plasma. Anal Chem. 1995;67:793–811. doi: 10.1021/ac00101a004. [DOI] [PubMed] [Google Scholar]
- Ren W., Yin Y., Liu G., Yu X., Li Y., Yang G. Effect of dietary arginine supplementation on reproductive performance of mice with porcine circovirus type 2 infection. Amino Acids. 2012;42:2089–2094. doi: 10.1007/s00726-011-0942-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W. Zhejiang University; 2010. Effect of saccharicterpenin on growth performance and defensive system in broilers. [Master degree thesis dissertation] [Google Scholar]
- Shi Y., Wang J., Guo R., Wang C., Yan X., Xu B. Effects of alfalfa saponin extract on growth performance and some antioxidant indices of weaned piglets. Livest Sci. 2014;167:257–262. [Google Scholar]
- Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- Tang X., Liu X., Zhang S. Studies of Saccharicterpenin and Flavomycin on the production and intestinal bacilli of early weaned piglets. China Anim Husb Vet Med. 2007;34:15–18. [Google Scholar]
- Wishart D.S., Jewison T., Guo A.C., Wilson M., Knox C., Liu Y. HMDB 3.0-the human metabolome database in 2013. Nucleic Acids Res. 2013;41:D801–D807. doi: 10.1093/nar/gks1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P., Jiang W., Liu Y., Chen G., Jiang J., Li S. Effect of choline on antioxidant defenses and gene expressions of Nrf2 signaling molecule in the spleen and head kidney of juvenile Jian carp (Cyprinus carpio var. Jian) Fish Shellfish Immunol. 2014;38:374–382. doi: 10.1016/j.fsi.2014.03.032. [DOI] [PubMed] [Google Scholar]
- Wu X., Cao W., Jia G., Zhao H., Chen X., Wu C. New insights into the role of spermine in enhancing the antioxidant capacity of rat spleen and liver under oxidative stress. Anim Nutr. 2016;3:85–90. doi: 10.1016/j.aninu.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L., Cao W., Liu G., Fang T., Wu X., Jia G. Dietary arginine and N-carbamylglutamate supplementation enhance the antioxidant statuses of the liver and plasma against oxidative stress in rats. Food Funct. 2016;7:2303–2311. doi: 10.1039/c5fo01194a. [DOI] [PubMed] [Google Scholar]
- Zhang H. Henan Agricultural University; 2010. Effects of Saccharicterpenin on the T lymphocyte subpopulations, the development of intestine and intestinal mucosal immunity in Gushi chickens. [Master degree thesis dissertation] [Google Scholar]