Abstract
We hypothesized that balancing the content of exogenous amino acids, especially lysine, to reduce protein content in swine diets could reduce nitrogen (N) pollution associated with animal husbandry. Two experiments (45 d each experiment) were performed on weaned piglets (Duroc × Landrace × Yorkshire, 28 d of age) to test this and to determine the optimal lysine to crude protein (Lys:CP) ratio in diet. In Exp. 1, 12 piglets (6 replicates [n = 6]) were fed diets containing different levels of CP (17% and 20%) but the same level of Lys. Increased CP content resulted in significant increases (P < 0.05) of average daily gain (ADG), average daily feed intake (ADFI), and body weight (BW), but did not affect the feed to gain ratio. In Exp. 2, 24 piglets (8 replicates [n = 8]) were fed 1 of 3 diets as follows: 1) 20% CP with a regular Lys:CP ratio (6.23%, control); 2) 17% CP with a reduced Lys:CP ratio (6.14%, LL); or 3) 17% CP with a standard Lys:CP ratio (7.32%, SL). The ADG, final BW, serum concentrations of growth hormone and insulin-like growth factor-1, villus height in the jejunum, and villus height to crypt depth ratio were the lowest in piglets fed LL diet, whereas blood urea N concentration was the lowest and the value of lipase activity was the highest in the piglets fed SL diet. The SL diet did not affect growth performance, intestinal morphology, or serum hormone concentrations, indicating that reduced dietary N with a high Lys:CP ratio can efficiently reduce dietary N excretion without negatively affecting weaned piglets.
Keywords: Protein, Lysine, Feeding, Digestion, Nutrition, Genetic mapping
1. Introduction
The shortage of protein resources has become a major global concern, with one billion people are suffering from starvation, undernutrition, and malnutrition (Schade and Pimentel, 2010). Feed represents 70% of the total cost of production in the swine industry, of which 35% is attributed to the cost of protein ingredients. The progress and expansion of the industrial production of amino acids (AA) contribute to yearly decreases in the costs of exogenous AA. This makes it possible to reduce dietary protein content by balancing exogenous AA in swine diets. In addition, pollution from livestock and poultry restricts the development of animal husbandry, as nitrogen (N) emission in feces is approximately 25% of total N emissions, but that in urine is 75% (Bathurst, 1952). It has been shown that a 1% protein decrease in diets may lead to an 8% decrease in N emissions (Kerr and Easter, 1995, Heo et al., 2008, Świech et al., 2010). However, decreasing the protein content in diets may also restrict pig growth and health (Rist et al., 2013). A reduction in dietary protein levels may result in changes in the intestinal structure and function (Wang et al., 2008). The underlying molecular and cellular mechanisms regarding such changes are, however, largely unknown. Investigations into optimal diet formulations, therefore, not only aim to improve protein utilization efficiency, but also to reduce N emissions to the environment, as well as ensure sufficient growth and health of animal.
The theory of optimal AA supplementation for pigs was proposed in the 1970s (Fuller and Boyne, 1971, Fuller, 1977, Fuller and Crofts, 1977). Nowadays, researchers use synthetic AA to balance the limiting AA in swine diets and improve N utilization. The present study aimed to investigate the impact of reducing dietary protein in weaned piglets, using corn–soybean meals balanced with exogenous lysine (Lys), methionine (Met), threonine (Thr), and tryptophan (Trp). Specifically, this study evaluated the effects of reduced dietary protein on weaned piglets, and aimed to determine the optimal lysine to crude protein (Lys:CP) ratio for weaned piglets based on growth performance, gastrointestinal hormone, jejunal morphology analysis, and mucosal digestive enzymes activities.
2. Material and methods
The experiments were performed following the guidelines for animal welfare. The protocol was reviewed and approved by the Institute of Subtropical Agriculture (ISA), the Chinese Academy of Sciences (CSC, 2015050).
2.1. Animals and experimental design
Duroc × Landrace × Yorkshire piglets (weaned at 28 d of age) were used in this study. After an adaptation period of 7 d, piglets were fed experimental feeds for 45 d. The initial body weight (BW) was determined (9.51 ± 0.62 kg, 12 piglets for Exp. 1; 9.51 ± 0.13 kg, 24 piglets for Exp. 2). All diets were corn-soybean meal-based supplemented with the limiting Lys, Met, Thr, and Trp to meet the nutrient requirements for piglets with BW of 11 to 30 kg (NRC, 2012). The ingredients of each diet are shown in Table 1 (Exp. 1) and Table 2 (Exp. 2). The nutritional composition of the diets is shown in Table 3.
Table 1.
Ingredients and nutritional composition of the diets fed to piglets (10 to 30 kg BW) in Exp. 1 (as-fed basis, %).
| Item | Diets1 |
|
|---|---|---|
| CP17 | CP20 | |
| Ingredients | ||
| Corn | 66.50 | 63.70 |
| Soybean | 18.80 | 19.80 |
| Whey powder | 4.30 | 4.30 |
| Fishmeal | 4.00 | 9.00 |
| Soybean oil | 2.60 | 0.80 |
| Lysine | 0.62 | 0.38 |
| Hydroxy methionine | 0.19 | 0.10 |
| L-threonine | 0.21 | 0.09 |
| L-tryptophan | 0.04 | 0.01 |
| Monocalcium phosphate | 0.74 | 0.00 |
| Limestone | 0.70 | 0.52 |
| Sodium chloride | 0.30 | 0.30 |
| Premix vitamins2 | 1.00 | 1.00 |
| Total | 100.00 | 100.00 |
| Nutrients and energy3 | ||
| DE, MJ/kg | 14.60 | 14.62 |
| Crude fat | 4.31 | 4.33 |
| Total calcium | 0.59 | 0.58 |
| Total phosphorus | 0.49 | 0.50 |
| CP | 17.09 | 20.05 |
| Lysine | 1.23 | 1.23 |
| Methionine + Cystine | 0.67 | 0.68 |
| Threonine | 0.74 | 0.73 |
| Tryptophan | 0.20 | 0.20 |
| Lys:CP ratio | 7.20 | 6.13 |
DE = digestible energy; CP = crude protein.
CP20 is the diet containing 20% CP; CP17 is the diet containing 17% CP.
Premix vitamins supplied per kilogram of diet: 5 mg Cu, 80 mg Fe, 3 mg Mn, 85 mg Zn, 0.1 mg I, 0.3 mg Se, 50 mg nicotinic acid, 5 mg pantothenic acid, 2 mg folic acid, 0.2 mg biotin; 10,800 IU vitamin A, 4,000 IU vitamin D3, 40 IU vitamin E, 4 mg vitamin K3, 6 mg vitamin B1, 12 mg vitamin B2, and 0.05 mg vitamin B12.
Values are calculated.
Table 2.
Ingredients of diets used in Exp. 2 (as-fed basis, %).
| Ingredients | Diets1 |
||
|---|---|---|---|
| Control | LL | SL | |
| Puffed soybean (43% CP) | 7.00 | 7.00 | 7.00 |
| Corn | 12.30 | 15.50 | 14.00 |
| Fish meal (64% CP) | 4.00 | 3.00 | 3.00 |
| Puffed corn | 22.00 | 22.00 | 22.00 |
| Maize (7.8% CP) | 42.50 | 41.72 | 43.00 |
| Soybean oil | 1.50 | 1.00 | 0.63 |
| Monocalcium phosphate | 0.95 | 0.97 | 0.95 |
| Limestone | 0.40 | 0.50 | 0.50 |
| Sodium chloride | 0.40 | 0.40 | 0.40 |
| Whey Powder | 3.00 | 3.00 | 3.00 |
| Glucose | 1.00 | 1.00 | 1.00 |
| Sucrose | 3.00 | 2.00 | 2.00 |
| Citric acid | 0.80 | 0.80 | 0.80 |
| Zinc oxide | 0.30 | 0.30 | 0.30 |
| Choline chloride | 0.10 | 0.10 | 0.10 |
| Lysine hydrochloride | 0.34 | 0.34 | 0.63 |
| Hydroxy methionine | 0.12 | 0.08 | 0.23 |
| L-threonine | 0.09 | 0.08 | 0.22 |
| L-tryptophan | 0.01 | 0.02 | 0.05 |
| Premix vitamins 2 | 0.04 | 0.04 | 0.04 |
| Organic mineral essence 2 | 0.15 | 0.15 | 0.15 |
| Total | 100.00 | 100.00 | 100.00 |
CP = crude protein.
Control, a normal protein containing 20% CP; LL, a low ratio of Lys to CP diet containing 17% CP; SL, a standard ratio of Lys to CP diet containing 17% CP.
Premix vitamins supplied per kilogram of diet: 5 mg Cu, 80 mg Fe, 3 mg Mn, 85 mg Zn, 0.1 mg I, 0.3 mg Se, 50 mg nicotinic acid, 5 mg pantothenic acid, 2 mg folic acid, 0.2 mg biotin; 10,800 IU vitamin A, 4,000 IU vitamin D3, 40 IU vitamin E, 4 mg vitamin K3, 6 mg vitamin B1, 12 mg vitamin B2, and 0.05 mg vitamin B12.
Table 3.
Nutritional composition of piglet diets used in Exp. 2 (%).
| Item | Diets1 |
NRC2012 | ||
|---|---|---|---|---|
| Control | LL | SL | ||
| CP | 19.90 | 16.94 | 16.93 | 19.50 |
| DE | 3,488 | 3,434 | 3,424 | 3,400 to 3,450 |
| ME | 3,338 | 3,303 | 3,294 | 3,280 to 3,400 |
| NE | 2,501 | 2,508 | 2,507 | 2,412 |
| Ca | 0.55 | 0.54 | 0.53 | 0.68 to 0.70 |
| TP | 0.59 | 0.60 | 0.60 | 0.55 to 0.60 |
| Lysine | 1.35 | 1.14 | 1.33 | 1.40 |
| Methionine + Cysteine | 0.78 | 0.66 | 0.8 | 0.79 |
| Tyrosine | 0.84 | 0.72 | 0.83 | 0.87 |
| Tryptophan | 0.22 | 0.19 | 0.21 | 0.23 |
| SID M + C | 0.68 | 0.57 | 0.69 | 0.68 |
| SID Threonine | 0.73 | 0.62 | 0.74 | 0.73 |
| SID Tryptophan | 0.21 | 0.18 | 0.20 | 0.20 |
| SID Lysine | 1.24 | 1.04 | 1.24 | 1.23 |
| Lysine:CP | 6.23 | 6.14 | 7.23 | 6.15 |
DE = digestible energy; ME = metabolizable energy; NE = net energy; SID = standard ileal digestible; TP = total phosphorus; M + C = methionine and cysteamine; CP = crude protein.
Control, a normal protein containing 20% CP; LL, a low ratio of Lys to CP diet containing 17% CP; SL, a standard ratio of Lys to CP diet containing 17% CP.
The piglets in each replicate were housed in a nursery facility (1.98 m × 1.98 m) with plastic and slatted flooring, and given free access to water and diets during the 45 d of the experiments. Body weight and feed intake were recorded weekly. Average daily gain (ADG), average daily feed intake (ADFI), and feed to gain ratio (F:G) were calculated.
2.1.1. Experiment 1
Twelve piglets were randomly assigned to 1 of 2 treatments (n = 6 per treatment): regular diet containing 20% CP (control diet, CP20), or a test diet containing 27% CP (CP17). The control diet was formulated as recommended by the National Research Council (NRC, 2012).
Sample collection. At the end of the trial period, the piglets were sacrificed and sampled. Approximately 20 cm of intestinal segments were aseptically isolated from the middle sections of the jejunum. The isolated intestinal segments were used for mucosal sampling (Wu et al., 2012). Immediately after collection, the jejunum mucosal samples were frozen in liquid N and stored at −80 °C until further analysis, including RNA microarray and gene expression analysis.
Gene microarray analysis. Total RNA was isolated from the jejunum samples using TRIzol reagent (Invitrogen, Carlsbad, CA), according to the manufacturer's instructions. The quality and quantity of RNA were assessed by OD260/OD280. Five micrograms of total RNA was converted to double-stranded complementary DNA (cDNA) using a reverse transcription (RT) kit (QIAGEN, Shanghai, China) with an oligo (dT) primer containing a T7 RNA polymerase promoter. Biotin-labeled complementary RNA (cRNA) was synthesized from purified double-stranded cDNA using a bio-array high-yield RNA transcript labeling kit (QIAGEN). Approximately 20 mg cRNA was fragmented to 50 to 300 bases and hybridized to Porcine Oligo Microarray chips (Agilent, Santa Clara, CA). A total of 6 chips were used here: 3 replicates for piglets receiving diets containing 20% and 17% CP (mRNA was pooled for the 3 piglets in each replicate). The hybridized arrays were washed, stained, and scanned using Agilent Porcine Oligo Microarrays (4 × 44 K) containing more than 40,000 probes. Microarray data were collected and analyzed using Agilent G2567AA Feature Extraction software, following Agilent's direct labeling protocol. The quantile method was used to normalize the probe intensities across the whole set of arrays. Three criteria were used to determine significant differential expression of intestinal genes between piglets from the CP20 and CP17 groups: 1) significance: P-value < 0.05 as determined by t-test; 2) reliability: a spot quality flag P (“P” is a quality flag assigned by the software package); and 3) relevance: a minimal fold change between the means of the 2 groups > 2.0.
2.1.2. Experiment 2
Twenty-four piglets were randomly assigned to three treatments (n = 8 per treatment): normal (20%) CP diet with a regular Lys:CP ratio (6.23%, control treatment), low CP (17%) diet with a reduced Lys:CP ratio (6.14%, LL treatment), or low CP (17%) diet with a standard Lys:CP ratio (7.32%, SL treatment). The control diet was formulated based on the ratio of Lys to CP recommended by the National Research Council (NRC, 2012), i.e., 6.15 g standard ileal digestible (SID) Lys per 100 g CP. The LL diet was formulated by decreasing the SID Lys composition of the control diet by 1.04%, and the SL diet was formulated by increasing the Lys:CP ratio of the control diet by 1.18 times.
Sample collection. Fecal material was collected over the last 3 d of the experiment using the total fecal collection method and stored at −20 °C (Van Keulen and Young, 1977). At the end of the trial period, the piglets were sacrificed and sampled. Before slaughter, blood samples (approximately 5 mL from each piglet) were obtained by anterior vena cava puncture. Serum was then collected and stored at −20 °C for subsequent analysis.
Approximately 20 cm of intestinal segments were aseptically isolated from the middle sections of the jejunum. Isolated intestinal segments were then flushed with phosphate-buffered saline (PBS). A sample (approximately 2 cm in length) per segment was fixed using a 10% formaldehyde-phosphate buffer, and kept at 4 °C for intestinal morphology analysis. The remaining section was used for mucosal sampling (Wu et al., 2012). Immediately after collection, the jejunum mucosal samples were frozen in liquid N and stored at −80 °C until further analysis of digestive enzymes activity (trypsin, chymotrypsin, lipase, and amylase).
2.2. Blood biochemistry
Serum concentrations of albumin (ALB), total protein (TP), triglyceride (TG), glucose (GLU) and urea N (BUN) were quantified using a CX4 automatic biochemical analyzer (Beckman Coulter, Inc.) and commercial kits (Leadman Biochemistry Technology Company, Beijing, China), according to the manufacturers’ instructions.
Blood hormone concentrations. Serum samples from all the piglets in each group were collected and analyzed to determine concentrations of growth hormone (GH), insulin (INS), insulin-like growth factor (IGF-1), glucagon, cholecystokinin (CCK), leptin and ghrelin using a hormone ELISA kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Jejunum morphology analysis. The fixed jejunum samples were embedded using a paraffin wax. Jejunum segments (5 μm in thickness) were stained with hematoxylin and eosin (H&E). Villus height (VH) and crypt depth (CD) of each jejunum segment were measured at 40 × magnification using an image processing and analysis system (Version 1, Leica Imaging Systems Ltd., Cambridge, UK). At least 10 well-oriented intact villi and their associated crypts were examined per piglet. The mean VH and CD of each section were calculated and the VH:CD ratio was determined for each piglet (Tan et al., 2009).
2.3. Activities of mucosal digestive enzymes in small intestine
Trypsinogen and chymotrypsinogen in mucous of jejunum were activated as previously described (Hedemann et al., 2006). Nα-benzoyl-DL-arginine-4-nitroanilide hydrochloride and N-succinyl-Ala-Ala-Pro-Phep-nitroanilide were the substrates used to determine trypsin and chymotrypsin, respectively. One unit of trypsin or chymotrypsin activity was defined as the hydrolysis of 1 μmol of substrate per minute. Amylase activity was determined using cornstarch as the substrate, and one activity unit was defined as the amount of amylase that caused formation of reducing power equivalent to 1 mg of glucose in 1 h at room temperature (Somogyi, 1960). Lipase activity was assayed using olive oil as the substrate by the method previously described by Tietz and Fiereck (1966). One unit of lipase activity was defined as the volume (in milliliters) of 0.05 mol/L NaOH required to neutralize the fatty acid liberated during 2 h of incubation at 37 °C. A hormone ELISA kit (R&D Systems) was used to measure dipeptidyl peptidase 4 (DPP4). Hormone-sensitive lipase (HSL) activity was determined using [14C] cholesteryl oleate (PerkinElmer) as a substrate, according to the procedures previously described by Fredrikson et al. (1980).
2.4. Statistical analysis
Results were statistically analyzed by one-way ANOVA using SPSS version 19.0. Duncan's multiple range test was used to compare differences among the groups. The differences were declared significant at P < 0.05 and a trend at 0.05 < P < 0.1 in all analyses.
3. Results
3.1. Experiment 1
3.1.1. Growth performance
None of the piglets experienced diarrhea throughout the experimental period. The ADG (P = 0.003) and final BW (P = 0.039) increased with increasing dietary CP levels, while ADFI (P = 0.089) showed an increasing trend. However, only the ADG and final BW differed significantly (P < 0.05) between piglets (11 to 30 kg BW) in the CP17 and CP20 treatments (Table 4). Dietary CP did not affect F:G ratio and final BW.
Table 4.
Effects of crude protein (CP) proportion in diets on growth performance in weaned piglets.
| Item | Diets1 |
SEM | P-value | |
|---|---|---|---|---|
| CP17 | CP20 | |||
| Initial BW, kg | 9.56 | 9.48 | 0.142 | 0.972 |
| ADG, g | 446.6 | 505.4 | 18.5 | 0.003 |
| ADFI, g | 766.8 | 837.6 | 27.6 | 0.089 |
| F:G ratio | 1.71 | 1.65 | 0.0290 | 0.793 |
| Final BW, kg | 29.64 | 32.23 | 0.799 | 0.039 |
BW = body weight; ADG = average daily gain; ADFI = average daily feed intake; F:G ratio = feed to gain ratio.
CP20 is the diet containing 20% CP; CP17 is the diet containing 17% CP. Number of observation is 6.
3.1.2. Microarray analysis of jejunum
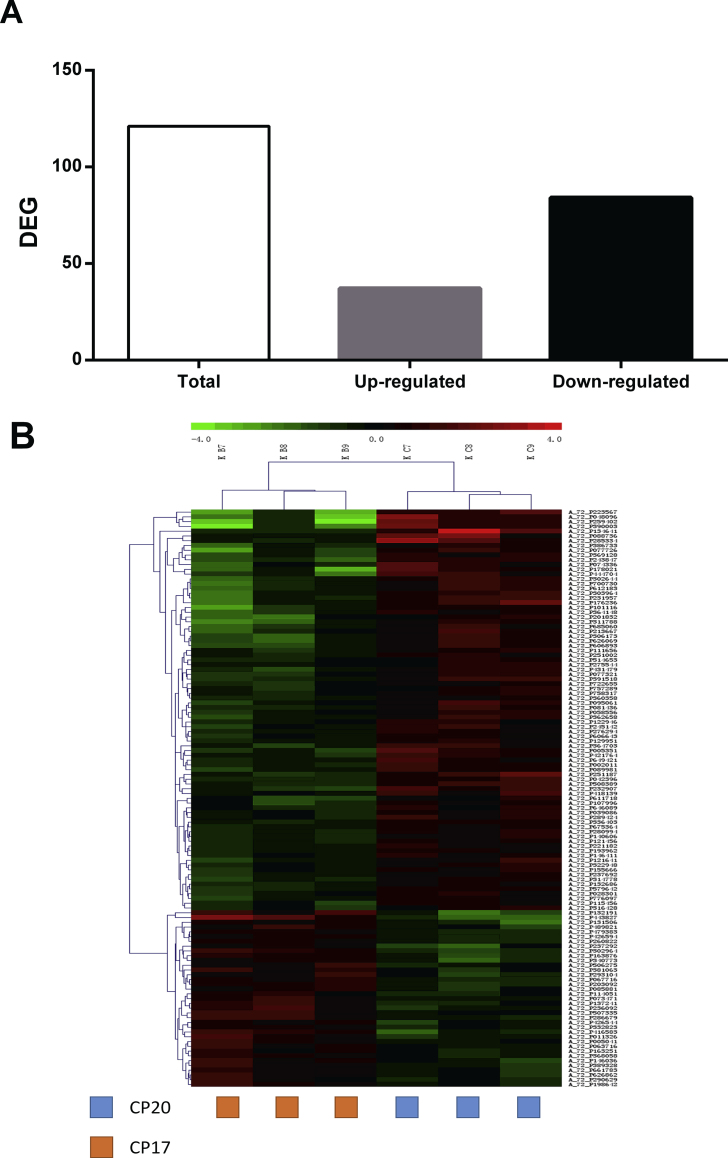
To better understand the mechanism of the effect of dietary protein content on intestinal development of the piglets, the jejunum mucosal samples of Exp.1 were examined by microarrays and SAS analysis with thresholds for low probability values (FDR) set at P < 0.05 and log-fold change > 1. Principal component analysis revealed differences among piglets fed diets with different levels of protein. The expression of 52 gene sets in piglets fed the CP17 diet differed significantly from that in piglets fed the CP20 diet (Fig. 1A, P < 0.05, fold change >2 or <0.5); 12 gene sets were up-regulated, and 40 gene sets were down-regulated (Table 5). The heatmap plot (Fig. 1B) shows that most of these genes were down-regulated in piglets fed the 17% CP diet compared with those fed the control diet (20% CP).
Fig. 1.
Visualization of differentially expressed genes (DEG). (A) total number of DEG, including those up- and down-regulated in the piglets in the CP17 compared to the CP20 group. (B) Heatmap of normalized expression level of the 900 DEG for each sample, represented as z-scores. CP20 = pigs fed the regular diet containing 20% crude protein; CP17 = pigs fed a diet containing 17% crude protein. Dendrograms represent relationships between samples (columns) and genes (rows) based on complete linkage clustering.
Table 5.
Microarray analysis of jejunum in piglets fed diets with different proportions of crude protein.
| Item | Regulation | Gene symbol | Genbank accession | Gene name | P-value1 |
|---|---|---|---|---|---|
| 1 | up | HTR1D | NM_214158 | 5-hydroxytryptamine (serotonin) receptor 1D, G protein-coupled | 0.0015 |
| 2 | up | BMP3 | NM_001206388 | bone morphogenetic protein 3 | 0.0423 |
| 3 | up | HMX3 | H6 family homeobox 3 | 0.0454 | |
| 4 | up | IFIT3 | NM_001204395 | interferon-induced protein with tetratricopeptide repeats 3 | 0.0475 |
| 5 | up | MMP11 | XM_001929445 | matrix metallopeptidase 11 (stromelysin 3) | 0.0485 |
| 6 | up | SLA-6 | NM_001113704 | MHC class I antigen 6 | 0.0281 |
| 7 | up | OBFC1 | NM_001243685 | oligonucleotide/oligosaccharide -binding fold containing 1 | 0.0425 |
| 8 | up | PGLYRP4 | NM_001244284 | peptidoglycan recognition protein 4 | 0.0392 |
| 9 | up | LOC100154160 | AK235490 | phosphoserine aminotransferase | 0.0437 |
| 10 | up | LOC100154160 | AK235490 | phosphoserine aminotransferase | 0.0499 |
| 11 | up | RUNX1T1 | AK396951 | runt-related transcription factor 1; translocated to, 1 (cyclin D-related) | 0.0230 |
| 12 | up | SEMA6D | XM_003121524 | sema domain, transmembrane domain (TM), and cytoplasmic domain, (semaphorin) 6D | 0.0498 |
| 13 | down | ANG1 | NM_001044573 | angiogenin | 0.0243 |
| 14 | down | ANG1 | NM_001044573 | angiogenin | 0.0329 |
| 15 | down | ANG1 | NM_001044573 | angiogenin | 0.0288 |
| 16 | down | ATP4B | NM_001001258 | ATPase, H+/K+ exchanging, beta polypeptide | 0.0347 |
| 17 | down | ATP4B | NM_001001258 | ATPase, H+/K+ exchanging, beta polypeptide | 0.0214 |
| 18 | down | BPI | NM_001159307 | bactericidal/permeability -increasing protein | 0.0386 |
| 19 | down | BPI | NM_001159307 | bactericidal/permeability -increasing protein | 0.0451 |
| 20 | down | BST2 | NM_001161755 | bone marrow stromal cell antigen 2 | 0.0136 |
| 21 | down | CCL2 | NM_214214 | chemokine (C-C motif) ligand 2 | 0.0030 |
| 22 | down | CCL28 | NM_001024695 | chemokine (C-C motif) ligand 28 | 0.0248 |
| 23 | down | CXCL2 | NM_001001861 | chemokine (C-X-C motif) ligand 2 | 0.0258 |
| 24 | down | CSF2 | NM_214118 | colony stimulating factor 2 (granulocyte-macrophage) | 0.0300 |
| 25 | down | LOC100037951 | NM_001097448 | complement component C9 | 0.0103 |
| 26 | down | CRISP3 | AK399726 | cysteine-rich secretory protein 3 | 0.0304 |
| 27 | down | CYTB | AK399730 | cytochrome b | 0.0336 |
| 28 | down | DUOX2 | NM_213999 | dual oxidase 2 | 0.0344 |
| 29 | down | DUOX2 | NM_213999 | dual oxidase 2 | 0.0093 |
| 30 | down | DUOXA2 | NM_001243550 | dual oxidase maturation factor 2 | 0.0334 |
| 31 | down | EGFL8 | NM_001123111 | EGF-like-domain, multiple 8 | 0.0488 |
| 32 | down | E3 | NM_001244471 | Epididymal secretory protein E3 | 0.0262 |
| 33 | down | FCN2 | NM_213868 | ficolin (collagen/fibrinogen domain containing lectin) 2 (hucolin) | 0.0402 |
| 34 | down | FXYD3 | NM_214208 | FXYD domain containing ion transport regulator 3 | 0.00515 |
| 35 | down | GIF | AK400404 | gastric intrinsic factor (vitamin B synthesis) | 0.0366 |
| 36 | down | GLUL | NM_213909 | glutamate-ammonia ligase | 0.0182 |
| 37 | down | GLUL | AK230941 | glutamate-ammonia ligase | 0.0360 |
| 38 | down | LOC100156419 | AK232803 | homer 2 | 0.0184 |
| 39 | down | HSD11B1 | NM_214248 | hydroxysteroid (11-beta) dehydrogenase 1 | 0.0330 |
| 40 | down | LTF | NM_214362 | lactotransferrin | 0.0433 |
| 41 | down | ND3 | AK391734 | NADH dehydrogenase subunit 3 | 0.0500 |
| 42 | down | ND4 | AK389834 | NADH dehydrogenase subunit 4 | 0.0124 |
| 43 | down | KCNA1 | EF424223 | potassium voltage-gated channel, shaker-related subfamily, member 1 (episodic ataxia with myokymia) | 0.0018 |
| 44 | down | REG3G | NM_001144847 | regenerating islet-derived 3 gamma | 0.0175 |
| 45 | down | REG4 | NM_001190251 | regenerating islet-derived family, member 4 | 0.0049 |
| 46 | down | RNASE1 | NM_001167655 | ribonuclease, RNase A family, 1 (pancreatic) | 0.0187 |
| 47 | down | SDS | AK233471 | serine dehydratase | 0.0207 |
| 48 | down | TCN1 | X52566 | transcobalamin I (vitamin B12 binding protein, R binder family) | 0.0199 |
| 49 | down | LOC100621891 | X52566 | transcobalamin-1-like | 0.0167 |
| 50 | down | LOC100621891 | AK391024 | transcobalamin-1-like | 0.0164 |
| 51 | down | LOC100516673 | XR_130576 | transmembrane protease serine 6-like | 0.0490 |
| 52 | down | TNNC2 | NM_001001862 | troponin C type 2 (fast) | 0.0488 |
Parametric P-value of the univariate test.
3.1.3. Functional annotation
Among the genes that were reported to be differentially expressed between diets with high or low CP content. Gene ontology (GO) terms are widely used for global interpretation of the functions of genes revealed by differential microarray analysis. According to GO, genes that were differentially expressed between piglets fed CP17 and CP20 diets participate in several biological processes that can be grouped in 5 main biological functions: respiratory chain, oxidoreductase activity, immune response, electron transport chain, and sugar binding (Table 6).
Table 6.
Relevant gene ontology identified from the annotation analysis.1.
| Category | P-value | Molecules |
|---|---|---|
| Respiratory chain | 0.009 | ND4, ND3, CYTB |
| Oxidoreductase activity | 0.009 | ND4, ND3, DUOX2 |
| Immune response | 0.017 | CSF2, CCL2, FCN2, CCL28, SLA-6 |
| Electron transport chain | 0.025 | ND4, ND3, CYTB |
| Sugar binding | 0.050 | REG4, FCN2, REG3G |
ND = NADH dehydrogenase; CYTB = cytochrome b; DUOX2 = dual oxidase 2; CSF2 = colony-stimulating factor 2; CCL2 = chemokines chemokine ligand 2; CCL28 = chemokines chemokine ligand 28; FCN2 = ficolin 2; SLA-6 = swine leukocyte antigen 6; REG4 = regenerating gene family 4; REG3G = regenerating islet-derived gene γ.
The biological interpretation of expressional data was performed using Genespring (12.5, Agilent). The genes included in the analysis were shown by microarray.
3.2. Experiment 2
3.2.1. Growth performance
As shown in Table 7, diets with different Lys:CP ratios changed final BW (P = 0.015), ADG (P = 0.20), and F:G ratios (P = 0.002) in pigs. Piglets fed the LL diet had lower final BW (P = 0.036) and ADG (P = 0.011), and higher F:G ratios (P = 0.010) than piglets fed the control and SL diets. The final BW, ADG, ADFI, and F:G ratios of piglets fed the SL diet were not significantly different from those of piglets fed the control diet.
Table 7.
Effects of diets on the growth performance of pigs.
| Item | Diets1 |
SEM | P-value | ||
|---|---|---|---|---|---|
| Control | LL | SL | |||
| Initial BW, kg | 9.60 | 9.42 | 9.53 | 0.64 | 0.893 |
| Final BW, kg | 31.83a | 29.42b | 32.02a | 1.86 | 0.015 |
| ADG, g | 635.25a | 571.45b | 642.38a | 51.56 | 0.020 |
| ADFI, g | 1,069.5 | 1,086.6 | 1,055.0 | 78.3 | 0.802 |
| F:G ratio | 1.69b | 1.90a | 1.64b | 0.12 | 0.002 |
SEM = standard error of the mean.
a, b Different letters within a row indicate significant differences at P < 0.05.
Control, a normal protein containing 20% CP; LL, a low ratio of Lys to CP diet containing 17% CP; SL, a standard ratio of Lys to CP diet containing 17% CP; number of observation is 8.
3.2.2. Jejunum morphology analysis
The LL diet decreased jejunum VH and VH:CD ratio in relation to the control diet (P < 0.05), whereas the SL diet had no significant effect on these indices (Table 8).
Table 8.
Effects of diets on jejunum morphology.
| Item | Diets1 |
SEM | P-value | ||
|---|---|---|---|---|---|
| Control | LL | SL | |||
| Villus height (VH), μm | 813.6a | 703.4b | 793.1ab | 33.8 | 0.050 |
| Crypt depth (CD), μm | 291.3 | 281.3 | 287.1 | 2.9 | 0.831 |
| VH:CD ratio | 2.78a | 2.51b | 2.77a | 0.09 | 0.043 |
SEM = standard error of the mean.
a, b Different letters within a row indicate significant differences at P < 0.05.
Control, a normal protein containing 20% CP; LL, a low ratio of Lys to CP diet containing 17% CP; SL, a standard ratio of Lys to CP diet containing 17% CP; number of observation is 8.
3.2.3. Blood biochemistry
Piglets fed the SL diet had significantly lower concentrations of BUN and GLU compared to the pigs fed control diet (P < 0.05; Table 9). There were no differences in concentrations of ALB, TG, and TP among piglets in the 3 treatment groups.
Table 9.
Effects of diets on parameters of blood serum.
| Item | Diets1 |
SEM | P-value | ||
|---|---|---|---|---|---|
| Control | LL | SL | |||
| ALB, g/L | 34.57 | 34.17 | 37.16 | 0.937 | 0.384 |
| BUN, mmol/L | 3.27a | 3.43a | 2.19b | 0.389 | <0.001 |
| GLU, mmol/L | 8.03a | 7.40ab | 7.14b | 0.264 | 0.096 |
| TG, mmol/L | 0.550 | 0.572 | 0.488 | 0.025 | 0.628 |
| TP, g/L | 48.86 | 49.25 | 44.74 | 1.443 | 0.209 |
SEM = standard error of the mean; ALB = albumin; BUN = blood urea nitrogen; GLU = glucose; TG = triglyceride; TP = Total phosphorus.
a, b Different letters within a row indicate significant differences at P < 0.05.
Control, a normal protein containing 20% CP; LL, a low ratio of Lys to CP diet containing 17% CP; SL, a standard ratio of Lys to CP diet containing 17% CP; number of observation is 8.
3.2.4. Blood hormone concentrations
Serum GH and IGF-1 concentrations were affected in the same trend by the diets, i.e., SL concentrations > control concentrations > LL concentrations (Table 10). However, the trend in GH affected by diets was significant (P = 0.018), and that shown in IGF-1 was not significant (P = 0.071). No significant effects were observed for insulin among treatment groups.
Table 10.
Effects of diets on concentrations of serum hormone.
| Item | Diets1 |
SEM | P-value | ||
|---|---|---|---|---|---|
| Control | LL | SL | |||
| GH, ng/mL | 1.25ab | 1.05a | 1.41b | 0.104 | 0.018 |
| Insulin, mIU/L | 7.42 | 7.46 | 7.59 | 0.051 | 0.943 |
| IGF-1, U/mL | 8.22ab | 7.37a | 8.66b | 0.379 | 0.071 |
| Glucagon, pg/mL | 132.3 | 138.3 | 136.3 | 3.521 | 0.972 |
| CCK, ng/L | 144.7a | 101.4b | 136.2ab | 23.60 | 0.029 |
| Leptin, ng/mL | 13.25a | 9.04b | 13.19a | 2.031 | 0.034 |
| Ghrelin, pg/mL | 237.7 | 263.3 | 257.6 | 17.81 | 0.079 |
SEM = standard error of the mean; GH = growth hormone; IGF-1 = insulin-like growth factors-1; CCK = cholecystokinin.
a, b Different letters within a row indicate significant differences at P < 0.05.
Control, a normal protein containing 20% CP; LL, a low ratio of Lys to CP diet containing 17% CP; SL, a standard ratio of Lys to CP diet containing 17% CP; number of observation is 8.
3.2.5. Activities of small intestinal mucosal digestive enzymes
Piglets fed LL and SL diets had significantly lower trypsin and chymotrypsin activities (Table 11, P < 0.05). Lipase activity tended to be affected by the treatments, although this effect was not significant (P = 0.0599). There were no differences in the activities of amylase, DPP4, and HSL in the jejunum mucosa of weaned piglets fed the 3 different diets.
Table 11.
Effects of diets on digestive enzyme activities in the jejunum mucosa (U/mg protein).
| Item | Diets 1 |
SEM | P-value | ||
|---|---|---|---|---|---|
| Control | LL | SL | |||
| Trypsin | 139.8a | 104.5b | 103.5b | 11.949 | 0.0077 |
| Chymotrypsin | 0.753a | 0.495ab | 0.337b | 0.121 | 0.0025 |
| Lipase | 94.886 | 89.77 | 108.414 | 5.562 | 0.0599 |
| Amylase | 0.894 | 0.581 | 0.706 | 0.091 | 0.5563 |
| DPP4 | 1.561 | 1.507 | 1.55 | 0.031 | 0.808 |
| HSL | 0.415 | 0.558 | 0.507 | 0.028 | 0.288 |
SEM = standard error of the mean; DPP4 = dipeptidyl peptidase 4; HSL = hormone-sensitive lipase.
a, b Different letters within a row indicate significant differences at P < 0.05.
Control, a normal protein containing 20% CP; LL, a low ratio of Lys to CP diet containing 17% CP; SL, a standard ratio of Lys to CP diet containing 17% CP; number of observation is 8.
4. Discussion
Reducing dietary protein is an effective way to decrease N emissions in the swine industry. However, a reduction in dietary protein level results in a concomitant decrease in the intake of AA and N (Portejoie et al., 2003). A previous investigation in our laboratory showed that a reduction of dietary CP by 6% limited growth performance (unpublished data). Moreover, a low-protein diet supplemented with deficient AA reduced the excretion of N into the environment without affecting weight gain in pigs with the BW of 60 to 90 kg (unpublished data). Weaned piglets are sensitive to AA diet contents and requiring more CP intake. Thus, based on the net energy system of weaned piglets, the diets used in the present study were formulated using fishmeal, soybean meal, and corn to adjust protein and energy levels and eliminate differences in dietary energy and flavor.
Strategies to alleviate the detrimental effects of reduced dietary protein on intestinal integrity are of great significance for the health of pigs. In this study, only 52 genes observed in the jejunum showed differences between groups supplemented with 20% or 17% CP. Expression of 40 genes was reduced in the small intestine of piglets in response to reduced dietary protein. These genes encode proteins that play crucial roles in regulating: 1) the respiratory chain (NADH dehydrogenase [ND] 4, ND3, cytochrome b [CYTB]); 2) oxidoreductase activity (ND4, ND3, dual oxidase [DUOX] 2); 3) immune response (colony-stimulating factor 2 [CSF2], colony-stimulating factor [CCL] 2, ficolin 2 [FCN2], CCL28, swine leukocyte antigen [SLA-6]); 4) electron transport chain (ND4, ND3, CYTB); and 5) sugar binding (regenerating gene family 4 [REG4], FCN2, regenerating islet-derived gene γ [REG3G]).
The Agricultural Research Council (ARC, 1981) concluded that pigs fed diets including 7 g Lys per 100 g CP achieved the best growth performance. According to Wang and Fuller (1989), the optimal Lys:CP ratio was 6.5, which was lower than that recommended by ARC. However, the ratio of other AA to CP recommended by Wang and Fuller (1989) was higher than that recommended by the ARC. Henry et al. (1992) indicated that the ideal Lys:CP ratio should be approximately 6.5% to 6.8%. To reduce N emissions in the swine industry, dietary AA, especially Lys, Met, Thr, and Trp, have to be efficiently balanced in diets. However, it was previously unclear what ratios of limited AA are required to optimize CP proportions in the diet of weaned piglets. Piglets fed the SL diet (i.e. the standard Lys to CP ratio) achieved the best growth performance, and had the highest weight gain and lowest F:G ratio, whereas the diet with the reduced Lys:CP ratio (the LL diet), resulted in the highest ADFI. This may be due to an insufficient content of essential AA in this diet, resulting in a higher food intake to meet nutritional demand. The imbalanced AA in the diet with the reduced Lys:CP ratio also led to an increase in the F:G ratio, and to a reduction in the final BW. A low protein diet with a balanced AA profile, i.e., the SL diet, therefore, does not affect the growth performance of piglets (Easter and Baker, 1980). Moreover, the optimal Lys:CP ratio of this diet provided better results than those reported from other diets in the literature, and provided better results than those of the diets based on ARC recommendations. The differences in the findings may be due to differences in pig breeds, the quality of raw materials, and/or husbandry management.
The jejunum is the main site for the digestion and absorption of dietary protein, and long-term changes in dietary protein affect intestinal morphology. Villus height and CD are indices used to evaluate the function of the intestine and its ability to maintain optimum nutrition absorption and digestive health (Manzanilla et al., 2006). The shorter the villus, the smaller its surface area, and therefore VH determines the absorption efficiency of the intestine. Changes in VH may thus lead to a high ADFI and slow growth rate in piglets fed a low Lys:CP ratio diet. The VH to CD ratio is a criterion used to estimate nutrient digestion and the absorption capacity of the small intestine (Montagne et al., 2003).
In general, reducing the CP content in diets decreases intestinal VH in piglets (Pluske et al., 1996, Guay et al., 2006) due to AA deficiency. In the current study, a 3% reduction in dietary CP using a standard Lys:CP ratio (the SL diet) did not change VH and CD in piglets, and did not affect intestinal morphology, as long as dietary Lys was sufficient. Ren et al. (2014) found that a protein-restricted diet (17.32% CP) did not affect VH because sufficient essential AA were provided in the diets fed to weaning piglets. Further studies are still required to determine the relationship between dietary essential AA and the development of intestinal villi.
Urea N is the final product of protein metabolism in animals. A reduced supply of dietary CP balanced by AA can significantly decrease the concentration of urea N and increase the AA utilization rate during protein synthesis in the body (Heo et al., 2008; Lopez et al., 1994, Shriver, 2003). Thus, urea N was used to estimate the balance of dietary AA (Kerr and Easter, 1995). In the present study, a decreased BUN concentration in piglets fed the SL feed indicated that the Lys:CP ratio (7.23%) used in this diet was optimal for protein synthesis in weaned piglets, and the lower N to urea conversion indicated that the protein utilization improved. On the other hand, the LL diet did not supply a sufficient amount of dietary Lys, leading to an imbalanced AA intake, which resulted in increasing BUN concentrations in the serum. These results corroborate the importance of dietary AA balance in the improvement of protein utilization and N emission.
Concentrations of insulin and IGF-1 were not significantly affected by LL or SL diets, indicating that the effects of dietary Lys:CP ratio on the secretion of these serum hormones were very small, but the inhibitory effects of dietary Lys:CP ratio on the secretion of digestive enzymes were not mediated by hormones. Lipase and DPP4 activities were positively correlated with insulin concentration. Increasing blood glucose stimulates the expression of lipase upstream of the stimulatory factor in the liver, and hepatic lipase transcription (Botma et al., 2001), and GH could increase lipase activity in vitro (Oscarsson et al., 1999). Although the HSL enzyme catalyzes intracellular triacylglycerol to release non-esterified fatty acids (Kratky, 2014, Morak et al., 2012), in the present study, changes in Lys:CP ratio were found to affect GH secretion without affecting insulin concentration and lipase activity. Thus, appropriate reductions in diet protein and Lys supply are likely to have no effect on lipid digestion ability.
Similarly, amylase activity was not significantly different between the treatment groups. The pancreas is able to adapt secretion of digestive enzymes to changes in dietary nutrients, especially in starch. Piglets fed diets containing 31% starch was found to show lower amylase activity (approximately 26.9%) than piglets fed diets containing 51% starch (Mourot et al., 1995). In addition, amylase activity in pigs fed a high-starch diet (80.8%) was 2.3 times as high as that in pigs fed a low-starch diet (21.8%) (Corring and Chayvialle, 1987). Pancreatic amylase activity was found to be positively related to blood insulin concentration (Ahrén et al., 1990). In our experiment, neither the amylase activity in jejunum mucosa nor serum insulin concentration were affected, indicating a consistent relationship between enzyme activity and insulin secretion.
Most protein is hydrolyzed to peptides and free AA by trypsin and chymotrypsin in the intestine. The secretion of these enzymes is influenced by the intake of protein and AA composition (Debray et al., 2003). A previous study showed that the activity of intestinal protease in piglets fed a 18% CP did not significantly differ from that of piglets fed a 20% CP diet (Hou et al., 1999). In the present study, both trypsin and chymotrypsin activities were reduced by low CP diets, but the activities of these proteases did not differ between piglets fed LL and SL diets. This indicates that the Lys:CP ratio has little impact on the secretion of trypsin and chymotrypsin, and that the secretion of both proteases depends on the protein content of the diet, not on the ratio of Lys to CP. Therefore, dietary protein level was positively correlated with the activities of these proteases.
5. Conclusions
Overall, the present study indicates that low CP diets with a reduced Lys:CP ratio promoted feed intake in weaned piglets, but decreased the N utilization and feed conversion. On the other hand, a reduced CP diet with a standard Lys:CP ratio improved gastrointestinal development. The results of the present study corroborate that a 17% CP diet supplemented with the standard Lys:CP ratio (7.23%) can reduce dietary N excretion without negative effects in weaned piglets, and could, therefore, be used to reduce N pollution associated with pig husbandry. The conversion efficiency of essential AA in a low CP diet could be studied further based on the results of the experiments performed here.
Conflict of interest
The authors declare that they have no conflict of interests.
Acknowledgements
This study was jointly supported by the China Basic Research Program (#2013CB127301); National Natural Science Foundation of China (31472106, 31501964), Natural Science Foundation of Hunan Province of China (2018JJ3579), Ministry of Science and Technology of the People's Republic of China (2013BAD21B04), Key Programs of frontier scientific research of the Chinese Academy of Sciences (QYZDY-SSW- SMC008), Youth Innovation Team Project of ISA, CAS (2017QNCXTD_TBE), China Agriculture Research System (CARS-35) and the Public Service Technology Center, Institute of Subtropical Agriculture, Chinese Academy of Sciences.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Jiming Yao, Email: wangdayaojiming@163.com.
Xingguo Huang, Email: huangxi8379@aliyun.com.
Tiejun Li, Email: tjli@isa.ac.cn.
References
- Ahrén B., Pierzynowski S.G., Weström B., Karlsson B. Pancreastatin inhibits insulin secretion and exocrine pancreatic secretion in the pig. Diabetes Res. 1990;14:93. [PubMed] [Google Scholar]
- ARC . In: The nutrient requirements of pigs. Agric C., editor. Bureaux; Slough, U.K: 1981. [Google Scholar]
- Bathurst N. The amino-acids of sheep and cow urine. J Agric Sci. 1952;42:476–478. [Google Scholar]
- Botma G.J., Verhoeven A.J., Jansen H. Hepatic lipase promoter activity is reduced by the C-480T and G-216A substitutions present in the common LIPC gene variant, and is increased by Upstream Stimulatory Factor. Atherosclerosis. 2001;154:625–632. doi: 10.1016/s0021-9150(00)00478-0. [DOI] [PubMed] [Google Scholar]
- Corring T., Chayvialle J.A. Diet composition and the plasma levels of some peptides regulating pancreatic secretion in the pig. Reprod Nutr Dev. 1987;27:967–977. doi: 10.1051/rnd:19870801. [DOI] [PubMed] [Google Scholar]
- Debray L., Le H.L.I., Gidenne T., Fortun-Lamothe L. Digestive tract development in rabbit according to the dietary energetic source: correlation between whole tract digestion, pancreatic and intestinal enzymatic activities. Comp Biochem Physiol Part A Mol Integr Physiol. 2003;135:443. doi: 10.1016/s1095-6433(03)00112-0. [DOI] [PubMed] [Google Scholar]
- Easter R.A., Baker D.H. Lysine and protein levels in corn-soybean meal diets for growing-finishing swine. J Anim Sci. 1980;50:467–471. doi: 10.2527/jas1980.503467x. [DOI] [PubMed] [Google Scholar]
- Fredrikson G., Strålfors P., Nilsson N., Belfrage P. Hormone-sensitive lipase from adipose tissue of rat. Methods Enzymol. 1980;71:636–646. doi: 10.1016/0076-6879(81)71076-0. [DOI] [PubMed] [Google Scholar]
- Fuller M.F. The protein-sparing effect of carbohydrate: 1. Nitrogen retention of growing pigs in relation to diet. Br J Nutr. 1977;38:479–488. doi: 10.1079/bjn19770113. [DOI] [PubMed] [Google Scholar]
- Fuller M.F., Boyne A.W. The effects of environmental temperature on the growth and metabolism of pis given different amountsof food. 1. Nitrogen metabolism, growth and body composition. Br J Nutr. 1971;28:373–384. doi: 10.1079/bjn19710087. [DOI] [PubMed] [Google Scholar]
- Fuller M.F., Crofts R.M. The protein. Br J Nutr. 1977;38 doi: 10.1079/bjn19770113. [DOI] [PubMed] [Google Scholar]
- Guay F., Donovan S.M., Trottier N.L. Biochemical and morphological developments are partially impaired in intestinal mucosa from growing pigs fed reduced-protein diets supplemented with crystalline amino acids. J Anim Sci. 2006;84:1749–1760. doi: 10.2527/jas.2005-558. [DOI] [PubMed] [Google Scholar]
- Hedemann M.S., Jensen B.B., Poulsen H.D. Influence of dietary zinc and copper on digestive enzyme activity and intestinal morphology in weaned pigs. J Anim Sci. 2006;84:3310–3320. doi: 10.2527/jas.2005-701. [DOI] [PubMed] [Google Scholar]
- Henry Y., Colleaux Y., Seve B. Effects of dietary level of lysine and of level and source of protein on feed intake, growth performance, and plasma amino acid pattern in the finishing pig. J Anim Sci. 1992;70:188–195. doi: 10.2527/1992.701188x. [DOI] [PubMed] [Google Scholar]
- Heo J.-M., Kim J.-C., Hansen C.F., Mullan B.P., Hampson D.J., Pluske J.R. Effects of feeding low protein diets to piglets on plasma urea nitrogen, faecal ammonia nitrogen, the incidence of diarrhoea and performance after weaning. Arch Anim Nutr. 2008;62:343. doi: 10.1080/17450390802327811. [DOI] [PubMed] [Google Scholar]
- Hou Y., Yu M., Zhou Y., Ji C., Liang D. A study of the optimum dietary level of protein and lysine for early-weaned piglets. Chinese J Anim Nutr. 1999;11:38–44. [Google Scholar]
- Kerr B.J., Easter R.A. Effect of feeding reduced protein, amino acid-supplemented diets on nitrogen and energy balance in grower pigs. J Anim Sci. 1995;73:3000. doi: 10.2527/1995.73103000x. [DOI] [PubMed] [Google Scholar]
- Kratky D. Pleiotropic regulation of mitochondrial function by adipose triglyceride lipase-mediated lipolysis. Biochimie. 2014;96:106. doi: 10.1016/j.biochi.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J., Goodband R., Allee G., Jesse G., Nelssen J., Tokach M. The effects of diets formulated on an ideal protein basis on growth performance, carcass characteristics, and thermal balance of finishing gilts housed in a hot, diurnal environment. J Anim Sci. 1994;72:367–379. doi: 10.2527/1994.722367x. [DOI] [PubMed] [Google Scholar]
- Manzanilla E., Nofrarias M., Anguita M., Castillo M., Perez J., Martin-Orue S. Effects of butyrate, avilamycin, and a plant extract combination on the intestinal equilibrium of early-weaned pigs. J Anim Sci. 2006;84:2743–2751. doi: 10.2527/jas.2005-509. [DOI] [PubMed] [Google Scholar]
- Montagne L., Pluske J.R., Hampson D.J. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals [Review] Anim Feed Sci Technol. 2003;108:95–117. [Google Scholar]
- Morak M., Schmidinger H., Riesenhuber G., Rechberger G.N., Kollroser M., Haemmerle G. Adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL) deficiencies affect expression of lipolytic activities in mouse adipose tissues. Mol Cell Proteomics. 2012;11:1777–1789. doi: 10.1074/mcp.M111.015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourot J., Camara M., Février C. Effects of dietary fats of vegetable and animal origin on lipid synthesis in pigs. C R Acad Sci Ser III Sci Vie. 1995;318:965. [PubMed] [Google Scholar]
- NRC . 12th ed. Natl. Acad. Press; Washington, DC: 2012. Nutrient requirements of swine. [Google Scholar]
- Oscarsson J., Ottosson M., Edén S. Effects of growth hormone on lipoprotein lipase and hepatic lipase. J Endocrinol Invest. 1999;22:2–9. [PubMed] [Google Scholar]
- Pluske J.R., Thompson MJAtwood C.S., Bird P.H., Williams I.H., Hartmann P.E. Maintenance of villus height and crypt depth, and enhancement of disaccharide digestion and monosaccharide absorption, in piglets fed on cows whole milk after weaning. Br J Nutr. 1996;76:409–422. doi: 10.1079/bjn19960046. [DOI] [PubMed] [Google Scholar]
- Portejoie S., Martinez J., Guiziou F., Coste C.M. Effect of covering pig slurry stores on the ammonia emission processes. Bioresour Technol. 2003;87:199–207. doi: 10.1016/s0960-8524(02)00260-2. [DOI] [PubMed] [Google Scholar]
- Ren M., Liu C., Zeng X., Yue L., Mao X., Qiao S. Amino acids modulates the intestinal proteome associated with immune and stress response in weaning pig. Mol Biol Rep. 2014;41:3611–3620. doi: 10.1007/s11033-014-3225-3. [DOI] [PubMed] [Google Scholar]
- Rist V., Weiss E., Eklund M., Mosenthin R. Impact of dietary protein on microbiota composition and activity in the gastrointestinal tract of piglets in relation to gut health: a review. Animal. 2013;7:1067–1078. doi: 10.1017/S1751731113000062. [DOI] [PubMed] [Google Scholar]
- Schade C., Pimentel D. Population crash: prospects for famine in the twenty-first century. Environ Dev Sustain. 2010;12:245–262. [Google Scholar]
- Shriver J.A. Effects of adding fiber sources to low crude protein, amino acid supplemented diets on nitrogen excretion, performance, and carcass traits of finishing pigs. J Anim Sci. 2003;81:492–502. doi: 10.2527/2003.812492x. [DOI] [PubMed] [Google Scholar]
- Somogyi M. Modifications of two methods for the assay of amylase. Clin Chem. 1960;6:23–35. [PubMed] [Google Scholar]
- Świech E., Buraczewska L., Tuśnio A., Taciak M. The effects of supplementing a low-protein threonine-deficient diet with different sources of non-essential amino acids on nitrogen retention and gut structure in young pigs. Arch Anim Nutr. 2010;64:22. doi: 10.1080/17450390903217523. [DOI] [PubMed] [Google Scholar]
- Tan B., Yin Y., Liu Z., Li X., Xu H., Kong X., Huang R., Tang W., Shinzato I., Smith S.B. Dietary L-arginine supplementation increases muscle gain and reduces body fat mass in growing-finishing pigs. Amino acids. 2009;37:169–175. doi: 10.1007/s00726-008-0148-0. [DOI] [PubMed] [Google Scholar]
- Tietz N.W., Fiereck E.A. A specific method for serum lipase∗ determination. Clin Chim Acta. 1966;13:352–358. doi: 10.1016/0009-8981(66)90215-4. [DOI] [PubMed] [Google Scholar]
- Van Keulen J., Young B. Evaluation of acid-insoluble ash as a natural marker in ruminant digestibility studies 1, 2. J Anim Sci. 1977;44:282–287. [Google Scholar]
- Wang J., Chen L., Li P., Li X., Zhou H., Wang F., Li D., Yin Y., Wu G. Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. J Nutr. 2008;138:1025–1032. doi: 10.1093/jn/138.6.1025. [DOI] [PubMed] [Google Scholar]
- Wang T.C., Fuller M.F. The optimum dietary amino acid pattern for growing pigs. 1. Experiments by amino acid deletion. Br J Nutr. 1989;62:77–89. doi: 10.1079/bjn19890009. [DOI] [PubMed] [Google Scholar]
- Wu X., Zhang Y., Liu Z., Li T., Yin Y. Effects of oral supplementation with glutamate or combination of glutamate and N-carbamylglutamate on intestinal mucosa morphology and epithelium cell proliferation in weanling piglets. J Anim Sci. 2012;90:337–339. doi: 10.2527/jas.53752. [DOI] [PubMed] [Google Scholar]