Abstract
Objective
To investigate the development of a minimal traditional Chinese medicine (TCM) formula using selected TCM ingredients and evaluating their biological activity with bone-specific in vitro tests. Finally, determining if the minimal formula can maintain bone mineral density (BMD) in a low bone mass (LBM)/osteoporosis (OP) model system.
Methods and results
Sixteen different TCM plant extracts were tested for estrogenic, osteogenic and osteoclastic activities. Despite robust activation of the full-length estrogen receptors α and β by Psoralea corylifolia and Epimedium brevicornu, these extracts do not activate the isolated estrogen ligand binding domains (LBD) of either ERα or ERβ; estrogen (17-β estradiol) fully activates the LBD of ERα and ERβ. E. brevicornu and Drynaria fortunei extracts activated cyclic AMP response elements (CRE) individually and when combined these ingredients stimulated the production of osteoblastic markers Runx2 and Bmp4 in MC3T3-E1 cells. E. brevicornu, Salvia miltiorrhiza, and Astragalus onobrychis extracts inhibited the Il-1β mediated activation of NF-κβ and an E. brevicornu/D. fortunei combination inhibited the development of osteoclasts from precursor cells. Further, a minimal formula containing the E. brevicornu/D. fortunei combination with or without a third ingredient (S. miltiorrhiza, Angelica sinensis, or Lycium barbarum) maintained bone mineral density (BMD) similar to an estradiol-treated control group in the ovariectomized rat; a model LBM/OP system.
Conclusion
A minimal formula consisting of TCM plant extracts that activate CRE and inhibit of NF-κβ activation, but do not behave like estrogen, maintain BMD in a LBM/OP model system.
Keywords: Epimedium brevicornu, Drynaria fortunei, Osteoporosis, Estrogenic, Anti-inflammatory
Abbreviations: BMD, bone mineral density; Bmp4, bone morphogenic protein 4; BSA, bovine serum albumin; cAMP, cyclic adenosine monophosphate; CRE, cyclic adenosine monophosphate response element; CREB, cyclic adenosine monophosphate response element binding protein; DEXA, dual-energy X-ray absorptiometry; DMSO, Dimethyl sulfoxide; E2, estradiol; ER, estrogen receptor; ERE, estrogen response element; FBS, fetal bovine serum; Fsk, forskolin; Hprt, hypoxanthine-guanine phosphoribosyl-transferase; IL-1, interleukin 1; LBD, ligand binding domain; LBM, low bone mass; MAPK, mitogen activated protein kinase; M-CSF, macrophage colony-stimulating factor; NF-κβ, nuclear factor kappa beta; OP, osteoporosis; PTH, parathyroid hormone; PTHrp, PTH related peptide; qPCR, quantitative polymerase chain reaction; RANKL, receptor activator of nuclear factor kappa beta ligand; RLU, relative luminescence unit; ROI, region of interest; Runx2, runt-related transcription factor 2; SFM, serum free media; TCM, traditional Chinese medicine; TNFα, tumor necrosis factor alpha; TRAP, tartrate-resistant acid phosphatase; UAS, upstream activating sequence
Graphical abstract
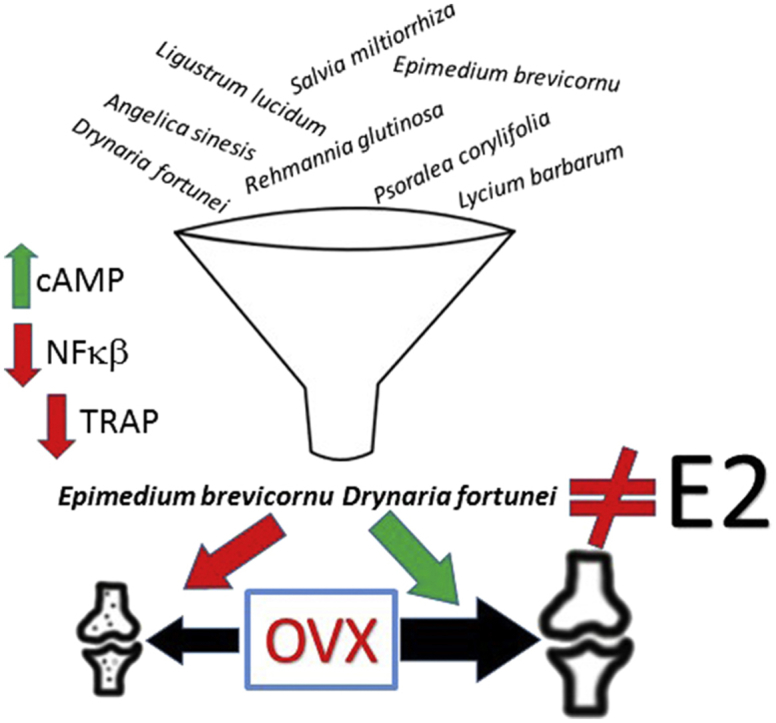
1. Introduction
Low bone mass (LBM) is a progressive, systemic skeletal disorder that may lead to the development of osteoporosis (OP), a disease characterized by osteopenia and degeneration of the bone's microstructure.1 In China alone, there are over 213 million people over the age of 50 affected by LBM; over 100 million of these are men. The development of OP and the concomitant fragility fractures are a worldwide health problem, affecting approximately 200 million people, a serious threat to the health and morbidity of middle-aged and elderly people.2 Postmenopausal women, lacking estrogen, are an especially susceptible population where it has been reported that one in three women over 50 has OP world-wide.3, 4
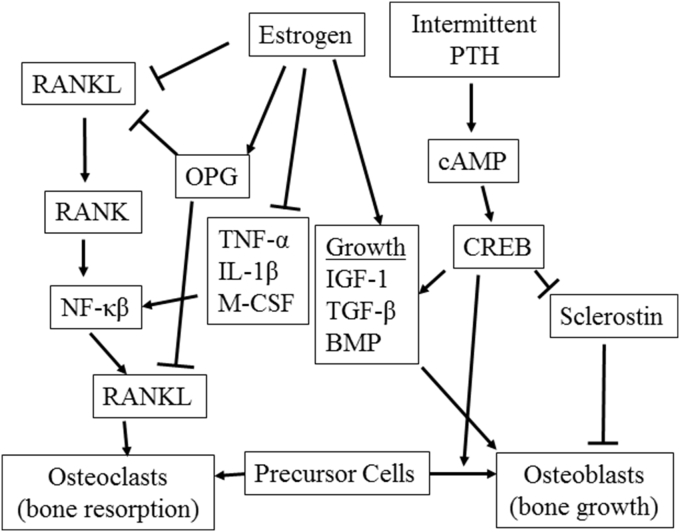
Clues to understanding the underlying causes of LBM may be found in the development of chronic inflammation and its associated decrease in bone density. Estrogen loss, as seen in postmenopausal women, has been described as an inflammatory event, which shifts the balance from bone mass maintenance to bone mass resorption (For review see Weitzmann and Pacifici5). Estrogen plays a central role in preventing inflammation thus inhibiting osteoclast development, bone reabsorbing cells. Key osteoclastic factors including tumor necrosis factor alpha (TNFα), interleukin-6, macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor kappa beta ligand (RANKL) are inhibited by estrogen.6, 7, 8 Estrogen binds to its receptor and inhibits a key inflammatory regulator, nuclear factor kappa beta (NF-κβ). Therefore, a significant means of preventing OP and, probably LBM, is the management of inflammation (Fig. 1).
Fig. 1.
Bone mineral density is affected by estrogen, parathyroid hormone and inflammatory pathways. Abbreviations are as follows: bone morphogenic protein (BMP); cyclic adenosine monophosphate (cAMP); cAMP response element-binding protein (CREB); insulin-like growth factor-1 (IGF-1); interleukin-1β (IL-1β); macrophage colony-stimulating factor (M-CSF); nuclear factor kappa-beta (NF-κβ); osteoprotegerin (OPG); parathyroid hormone (PTH); receptor activator of nuclear factor kappa-B (ligand; RANK(L)); tumor growth factor 1β (TGF-1β); tumor necrosis factor-α (TNF-α).
For building bone, the only United States Food and Drug Administration-approved method is the intermittent use of parathyroid hormone (PTH).9 While this effect of PTH has been known since 1929,10 the cellular targets and mechanisms of PTH on building bone remain controversial and high doses of PTH promote osteosarcomas.11 In a calcium regulated manner, PTH is secreted by the parathyroid gland and it maintains serum calcium homeostasis, renal phosphate resorption, and Vitamin D 1α hydroxylation.12 It activates the PTH/PTH-related receptor, a G-protein coupled receptor that upregulates cAMP and subsequent downstream pathways including the activation of cyclic AMP response element binding protein (CREB) which activates genetic targets through the cyclic AMP response element (CRE). Bone building targets affected by CREB have been identified13 as well as targets downstream of PTH/PTHrp receptor that affect bone mobilization factors such as RANKL and Sclerostin14, 15, 16, 17 (Fig. 1).
Plants, including those used in traditional Chinese medicines (TCM), are rich in anti-LBM/OP elements, and more researchers are exploring alternative therapies using these plant extracts.18 In classical Chinese documents, there are descriptions of medical conditions that are similar to LBM along with their treatments, so some traditional Chinese medicines may have the potential to maintain bone health.19
TCM formulas are generally prepared using more than 10 different ingredients in one preparation. There is little information on the development of a simple formulation using two or three TCM ingredients on bone mineral density (BMD) improvement and their biological activities on bone specific targets. Medicines containing fewer extracts would be more suitable for maintaining integrity and quality of the individual components and would also help in the standardization of TCM.
In this study, we selected 16 plant extracts used in TCM to improve bone health. These plants were evaluated for bone building potential by examining their ability to activate CRE, as well as their ability to block osteoclast development, and inflammation through NF-κβ inhibition. Additionally, we evaluated their effects on estrogen receptor (ER) activation wishing to avoid those plants act like estrogen and bind to and directly activate the isolated ligand binding domain (LBD) of ERα or ERβ. Further combinations of selected plants were tested in targeted bone assays and finally used in the ovariectomized rat model to evaluate their activity on BMD maintenance.
2. Materials and methods
2.1. Animals
Six-month-old female Sprague-Dawley rats weighing 250 ± 20 g were purchased from Shanghai Super-B&K Laboratory Animal Corp. Ltd. (License number: SYXK (Shanghai, CN) 2009-0069). Animals used in these experiments were treated according to the guidelines for the ethical treatment of experimental animals issued by the Chinese Ministry of Science and Technology in 2006.
2.2. Plant extracts
Plant extracts were prepared from feedstocks provided by Amway (China) R&D Center Co. LTD. Each feedstock was weighed and extracted with a 70% ethanol solution volume 10 times the feedstock mass overnight (∼18 h) at 22 °C with stirring. The extract was filtered using a GF/A Filter Paper and the filtrate was concentrated using a rotary evaporator to ∼1/50 the original volume at 55 °C, then dried to completion under N2 and vacuum desiccation. Final extract weight is determined for calculation of feedstock to extract ratio (Table 1).
Table 1.
Plant extracts used in this study.
| Abbra | Common name | Genus | Species | Plant part | Feedstock/Extractb |
|---|---|---|---|---|---|
| An | Angelica | Angelica | sinensis | Root | 20∖1 |
| As | Asiatic Cornelian Cherry | Cornus | mas | Fruit | 10∖1 |
| D | Danshen | Salvia | miltiorrhiza | Root | 7∖1 |
| Dr | Drynaria | Drynaria | fortunei | Root | 10∖1 |
| DS | Dodder Seed | Cuscuta | australis | Seed | 10∖1 |
| E | Epimedium | Epimedium | brevicornu | Leaf | 40∖1 |
| Eu | Eucommia | Eucommia | ulmoides | Leaf | 10∖1 |
| GP | Glossy Privet | Ligustrum | lucidum | Fruit | 10∖1 |
| IB | Indian Bread | Wolfiporia | extensa | Sclerotia | 40∖1 |
| M | Milkvetch | Astragalus | onobrychis | Root | 35∖1 |
| MC | Medicinal Cyathula | Cyathula | officinalis | Root | 20∖1 |
| P | Psoralea | Psoralea | corylifolia | Fruit | 10∖1 |
| R | Rehmannia | Rehmannia | glutinosa | Root | 12∖1 |
| TA | Two-toothed Achyranthes | Achyranthes | bidentata | Root | 10∖1 |
| WB | Wolfberry bark | Lycium | barbarum | Root | 15∖1 |
| Y | Common Yan Rhizome | Dioscorea | opposita | Root | 5∖1 |
Abbr, Abbreviations.
Feedstock Dry Mass (g)/Extract (g) Ratio.
2.3. Reagents, chemicals, and standards
Forskolin (Fsk), estradiol (E2; 17β-estradiol), and Acid Phosphatase (TRAP) kit were obtained from Sigma Corporation (St. Louis, MO). The NF-κβ antagonist (Bay 11-7085) was obtained from Tocris Biologicals (Minneapolis, MN). Recombinant human Interleukin 1β (IL-1β) and GW2580 were purchased from Calbiochem (Billerica, MA). RANKL and Human CSF-1 were purchased from R & D Systems (Minneapolis, MN). For the animal study, gentamycin was purchased from Southwest Pharmaceutical Co. LTD and E2 was from Bayer Pharmaceutical Co. Ltd. (Guangzhou, CN).
Vectors pGL4.29[luc2P/CRE/Hygro], pGL4.26 [luc2/minP/Hygro], pGL4.17 [luc2/Neo], pGL4.35 [luc2P/9XGAL4UAS/Hygro], pBIND-ERα-LBD (residues 299-595), and Fugene 6 and Fugene HD were purchased from Promega Inc. (Madison, WI). Vector pNF-κβ-Luc was from Clontech Laboratories (Mountain View, CA). Vectors pCMV6-XL4 ER β (Cat# SC119216), and pCMV6-NEO were purchased from Origene Technologies (Rockville, MD). Vector pCR BluntII TOPO ER α (Cat# MHS6278-211691051) was from Dharmacon (Lafayette, CO). Restriction enzymes were from New England Biolabs (Ipswich, MA) and D-Luciferin from Biotium (Hayward, CA).
Dulbecco's minimum essential media (DMEM), Ham's F-12 Kaighn's modification (F12K) media, Alpha MEM (αMEM; without ascorbic acid), Eagle's MEM (EMEM), OptiPRO SFM and l-glutamine Dulbecco's phosphate buffered saline (DPBS), fetal bovine Serum (FBS), penicillin/streptomycin, amphotericin B and WST-1 (cell proliferation reagent) were purchased from Fisher Scientific (Pittsburgh, PA). Hygromycin was from EMD Millipore (Temecula, CA). Bovine serum albumin essentially fatty acid free (BSA), G-418, and β-mercaptoethanol were from Sigma Corporation (St. Louis, MO).
QiaShredder, RNeasy Plus Mini kit, and quantitative polymerase chain reaction (PCR) primer sets for mouse Runt-related transcription factor 2 (Mm Runx2_1), bone morphogenic protein 4 (Mm Bmp4_1) and hypoxanthine-guanine phosphoribosyl-transferase (Mm Hprt1_1) were purchased from Qiagen (Toronto, ON). First strand cDNA synthesis kit, iScript, and realtime PCR kit, SsoFast™ EvaGreen®, were from Bio-Rad Laboratories (Hercules, CA).
2.4. Plasmid constructs
2.4.1. Estrogen response element construct
To evaluate full-length ER activation, an estrogen response element driven luciferase reporter construct was cloned. The estrogen response element (ERE) 5′-GTCAGGTCACAGTGACCTGAT-3’20 was repeated 4 times and cloned into pGL4.26 [luc2/minP/Hygro] (Promega Inc.) without spacers to create ERE luc2 vector.
2.4.2. Full-length estrogen receptor α construct
Full-length ERα was subcloned from pCR BluntII-TOPO into pCMV6-NEO using the EcoRI site and oriented using HindIII.
2.4.3. NF-κβ activation reporter construct
The NF-κβ response reporter construct was created using the NheI/HindIII fragment containing the kappa enhancer element and the pTal minimum promoter from pNF-κβ-Luc (Clontech) and ligated into pGL4.17 (Promega) to create NF-κβ Luc2 vector.
2.4.4. Estrogen receptor β constructs
To study the activation of ERβ LBD in a luciferase reporter assay, Gal4-ERβ-LBD vector was created in a modified pFN26A (BIND) vector (Promega Inc) as described previously.21 Briefly, the barnase gene was excised and a short cloning region (5′-cgcagagctcaaaagcg-3′) was inserted at the PvuI/EcoRI site to create pBIND3. The ERβ-LBD (residues 227-530) was PCR amplified from full-length human ERβ (SC119216, OriGene Technologies (Rockville, MD)) using forward and reverse primers 5′-CGATCGTTCGCCTTGTGCGGAGACAGAGAAGTGCC-3′ and 5′-TCTAGATTACTGAGACTGTGGGTTCTGGGAGCCCTC-3′, incorporating PvuI and XbaI sites at the 5′ and 3′ ends, respectively. The ERβ-LBD fragment was then ligated into the pBIND3 PvuI/XbaI site forming a Gal4- ERβ -LBD fusion protein.
2.5. Cell culture and stable cell lines
Chinese hamster ovary (CHO-K1), MC3T3-E1 subclone 14, HEK293T/17, SK-N-SH, and A549 were purchased from ATCC (Manasas, VA) and grown in in DMEM or αMEM or DMEM or EMEM or F12K media, respectively. All growth media was supplemented with 10% FBS, penicillin/streptomycin (100 U/100 μg/mL), and amphotericin B (250 μg/mL). Osteoclast precursor cells were purchased from Lonza (Basel, CH) and grown in DMEM with 10% FBS. All cells were grown in a 37○C, 5% CO2 containing incubator.
2.6. Response element reporter assays
Stable cell lines for the CRE and NF-κβ reporter assays were created by transfecting HEK293T/17 and A549 cells with vectors pGL4.29 CRE and pGL4.17 NF-κβ Luc2, respectively. Hygromycin (1200 μg/mL) and neomycin (400 μg/mL) supplemented DMEM and F12K media, respectively, were used to select monoclonal cell lines which were tested for assay responsiveness and selected for further development. The resulting stable cell lines were maintained in their respective growth media supplemented with 600 μg/mL hygromycin or 200 μg/mL neomycin.
The CRE activation assay was described previously.22 Briefly, ∼15,000 CRE reporter transfectant cells were plated per well in white walled 96 well poly-d-lysine coated plates (Corning™ Biocoat™). After 24 h, the media was changed to DMEM media lacking FBS but containing 0.5% BSA and incubated for another 24 h. Test ingredients were diluted in DMEM containing 0.5% BSA and added to the cells then incubated 6 h. Luciferase was quantified by activity assay described below.
To test for the inhibition of NF-κβ activation, ∼15,000 NF-κβ Luc2 reporter transfectant cells were plated per well in white walled 96 well plates in F12K growth media. After 24 h, the media was changed from F12K growth media to OptiPRO SFM supplemented with 4 mM l-glutamine, 0.5% BSA and incubated for another 24 h. Test ingredients were diluted in OptiPRO SFM/l-glutamine containing 0.5% BSA and added to the cells. After 20 min, NF-κβ activating compound, IL-1β, was added to each well at a concentration of 100 pg/mL and the treatments incubated for another 6 h. Luciferase was quantified by activity assay described below.
2.7. Ligand binding domain reporter assays
Cell lines containing two different vectors were needed to evaluate activation of the individual LBD of ERα and/or ERβ. These stable cell lines were generated by first transfecting CHO-K1 cells with pGL4.35 [luc2P/9XGAL4 UAS/Hygro] as described previously.21, 23 The ERα and ERβ LBD containing cell lines were then created by transfecting the stable pGL4.35 9XUAS Luc2P CHO-K1 cell line with the pBIND Gal4-ERα-LBD or pBIND3 Gal4-ERβ-LBD and selecting pBIND Gal4-ER-LBD containing cells with 600 μg/mL of G418. The resulting dual transfectants stable cells lines were maintained with growth media supplemented with 150 μg/mL hygromycin and 300 μg/mL G418.
2.8. Full length estrogen receptor activation reporter assay
To test activation of full-length ER, SK-N-SH cells, lacking ERα but having ERβ24 were transfected with empty construct or a full-length ERα or ERβ, and a luciferase reporter construct, ERE luc2. Briefly, ∼12000 SK-N-SH cells were plated per well of a white-walled 96 well plate in DMEM containing 10% FBS. After 24 h, 200 ng and 30 ng of ERE luc2 and pCMV6-NEO ERα or pCMV6-XL4 ERβ, respectively were transfected using 0.7 ul FugeneHD (Promega) per well. After 24 h, media were changed to DMEM/F12K, phenol red free, containing 0.5% BSA and incubated for another 24 h. Test samples were applied to the cells and incubated for another 24 h. Luciferase production was quantified using an activity assay described below.
2.9. Luciferase reporter assay
Luciferase was quantified using an activity assay from Biotium Inc. Briefly, at time of analysis, cells in white-walled 96-well plates were rinsed once using 50 μL of DPBS, then lysed with 20 μL of 1X passive lysis buffer. Lysis proceeded for ∼20 min at room temperature. Reconstituted, room temperature D-luciferin was added (100 μL per well) and light emission was immediately read using an M-5 spectrophotometer (Molecular Devices, Inc., Sunnyvale, CA).
2.10. Quantitative polymerase chain reaction
MC3T3-E1 cells were seeded at 1 × 106 cells/well in 6 well culture plates in αMEM containing 10% FBS, phenol-red and grown for 24 h, then the cells were treated with Fsk and the plant extracts. The media and treatments were changed daily. On day 7, cells were rinsed with 1X DPBS and total RNA was purified using the RNeasy mini plus kit per manufacturer's instructions. Total RNA was quantified by A260/A280, diluted to 1 μg per reaction, and reverse transcribed using the iScript cDNA synthesis kit.
Synthesized cDNA was diluted 20 fold with water prior to quantification reactions. Real time PCR reactions were completed using SsoFast™ EvaGreen® qPCR mix on a CFX96 Real Time Thermocycler (Bio-Rad). The reaction conditions were as follows: 95○C for 30 s; 40 cycles of 58○C for 5 s; and 95○C for 5 s. Fluorescent detection was completed following each cycle. Cycle times (Ct) of Runx2 and Bmp4 were normalized to housekeeping gene Hprt1 prior to comparisons with control samples.25
2.11. Tartrate-resistant acid phosphatase assay
Osteoclast precursor cells were plated in DMEM with 10% FBS containing 33 ng/mL CSF-1, 66 ng/mL RANKL and plant extract. Cells were grown in a 37○C, 5% CO2 incubator for 7–14 days until osteoclast formation occurred. Osteoclast differentiation was then detected by a dye prepared from the Acid Phosphatase kit. Briefly, dye solution was prepared by adding 0.2 mL Fast Garnet GBC to 0.2 mL sodium nitrite and allowing solution to stand 2 min, then 0.2 mL Naphthol AS-BI phosphate solution, 0.8 mL acetate solution, and 0.4 mL tartrate solution was added. The dye was warmed to 37○C, then added to assay plate and developed for 3–8 h in a 37○C, 5% CO2 incubator. Absorbance was measured at 550 nm.
2.12. In vivo bone mineral density
Female rats were ovariectomized to establish the LBM/OP model as described previously.26 After surgery the animals were administered 20000 U of gentamycin daily for 3 days and allowed to recover for another two days. After recovery period, the animals were randomly divided into 7 treatment groups (balanced by weight) and treated. The treatments for ovariectomized animals were as follows: untreated (Ovx); E2; Epimedium:Drynaria (E:Dr); Epimedium:Drynaria:Danshen (E:Dr:D); Epimedium:Drynaria:Wolfberry Bark (E:Dr:WB) and Epimedium:Drynaria:Angelica (E:Dr:An). A sham group that had intact ovaries, but underwent the operation and recovery period were used as the control group (Sham).
The number of animals that finished the trial in each group was as follows: Sham = 6; Ovx = 9; E2 = 10; E:Dr = 8; E:Dr:D = 9; E:Dr:WB = 9; and E:Dr:An = 6.
Experimental treatments were administered via oral gavage beginning the sixth day after ovariectomy. Each animal received 3 mL of treatment once a day for 12 consecutive weeks. Distilled water of the same volume was administered to the Sham group. The estrogen group received E2 (0.105 mg kg−1) daily. The total daily dose used for the plant extract test groups was 164 mg kg−1: 100 mg kg−1 of E, 32 mg kg−1 of Dr, and 32 mg kg−1 of either D, WB or An.
At the end of the trial, femur bones were excised and average bone mineral density (BMD) was determined using dual-energy X-ray absorptiometry (DEXA; Lunar Prodigy Advance; GE Healthcare, small animal scanning software). The region of interest (ROI) included the whole femur, femur head, and femur neck. The measurements were repeated three times within each ROI and the results of the whole femur, femur head, and femur neck were averaged and expressed as the BMD mean value.
2.13. Statistical analysis
Quantitative data are presented as mean ± standard deviation (SD). Comparisons between groups were made using one-way ANOVA with Dunnett's multiple comparisons test using GraphPad Prism version 6.01 (La Jolla, CA) and treatment means were compared with the untreated (Unt) group.
For the in vivo bone density study, the BMD mean values were compiled within each treatment group and analyzed by the nonparametric Kruskal-Wallis H test using IBM SPSS Statistics for Windows, Version 20.0 (Armonk, NY).27 A paired post-hoc comparison was done using a Dunn-Bonferroni test between the individual treatment groups and the ovariectomized group.28 An α level of 0.05 was used and considered statistically significant. Graph-Pad Prism version 6.01 software was used to generate graphs (La Jolla, CA).
3. Results
3.1. Estrogenic activity determination
Plant extracts evaluated in this study have been described as having estrogenic activity and yet the exact mechanism of this activity is not well understood.29, 30, 31, 32, 33 For this reason, we tested their activity on isolated LBD of ERα and ERβ as well as on full length receptors of ERα and ERβ. The results from using the isolated estrogen binding domains and the full length receptor helps to clarify how the extract might be activating the estrogen receptor and its downstream signaling pathways.
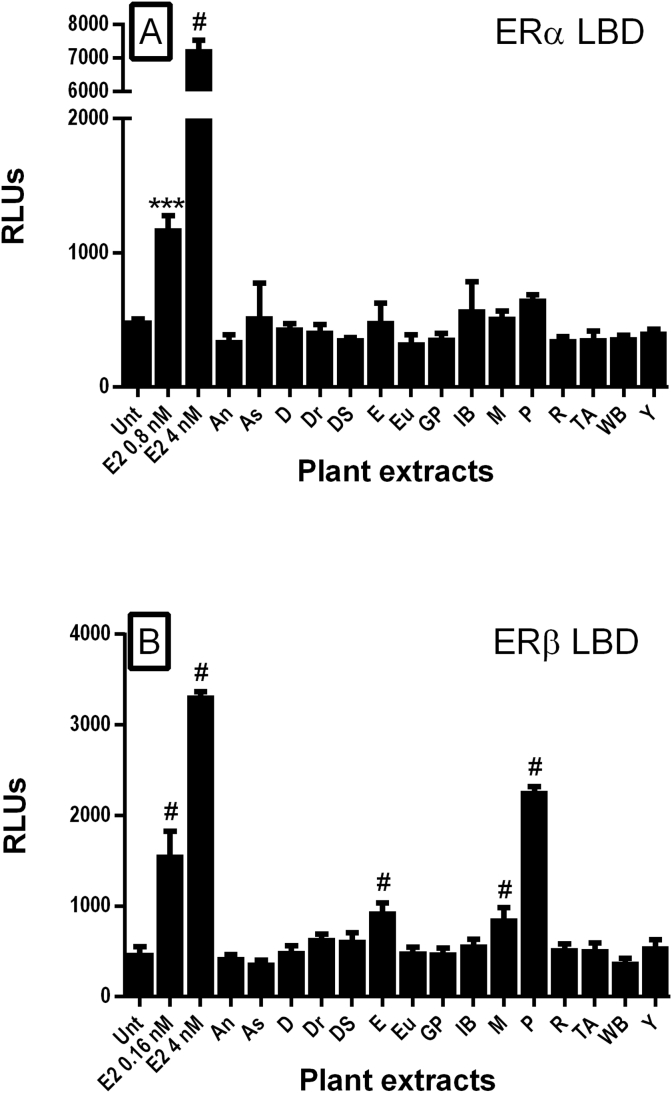
None of the extracts in this study activated the isolated ERα LBD (Fig. 2A). In this assay format, 0.8 nM of estradiol activated the LBD more than 2 fold and a dose of 4 nM activated the LBD more than 14 fold, so the assay was sensitive to extracts containing a minimum of 218 pg/mL of E2 activity. The extracts were tested at 40 μg/mL, which is a concentration difference (40 μg/ml to 218 pg/mL) of 180,000-fold.
Fig. 2.
Effect of selected TCM plant extracts on activation of estrogen α (A) or β (B) ligand binding domain assays. Plant extracts were resuspended in DMSO and added at a final concentration of 40 μg/mL to a Gal4-ERα- or ERβ-ligand binding domain (LBD) chimeric luciferase reporter assay. Estradiol (E2) was used as a positive control at 0.8, 1.6 or 4 nM. Cells were treated for 18 h. Extract abbreviations were as follows: As, Asiatic Cornelian Cherry; An, Angelica; Y, Common Yan Rhizome; D, Danshen; Dr, Drynaria; DS, Dodder Seed; E, Epimedium; Eu, Eucommia; GP, Glossy Privet; IB, Indian Bread; Mv, Milkvetch; P, Psoralea: R, Rehmannia; TA, Two-toothed Achyranthes; and WB, Wolfberry root bark. Data are represented as mean ± SD of relative luminescence units (RLUs (n = 3); ***p < 0.001, #p < 0.0001 compared to untreated (Unt) controls).
Psoralea, epimedium, and milkvetch extracts activated ERβ LBD (Fig. 2B). Psoralea extract (40 μg/mL) robustly activated the ERβ by 4 fold above control values. This response was approximately equal to an estradiol concentration of 1 nM (272 ng/mL); a 150,000-fold concentration difference (40 μg/mL versus 272 ng/mL). Epimedium and milkvetch extracts (40 μg/mL) activated the ERβ LBD to a lesser extent, approximately 2 fold. The response was similar to an estradiol dose of less than 0.16 nM (43 pg/mL), a 900,000-fold concentration difference (40 μg/mL versus 43 pg/mL). None of the plant extracts had activity in the control assay (UAS luciferase construct only, lacking an ER LBD; data not shown).
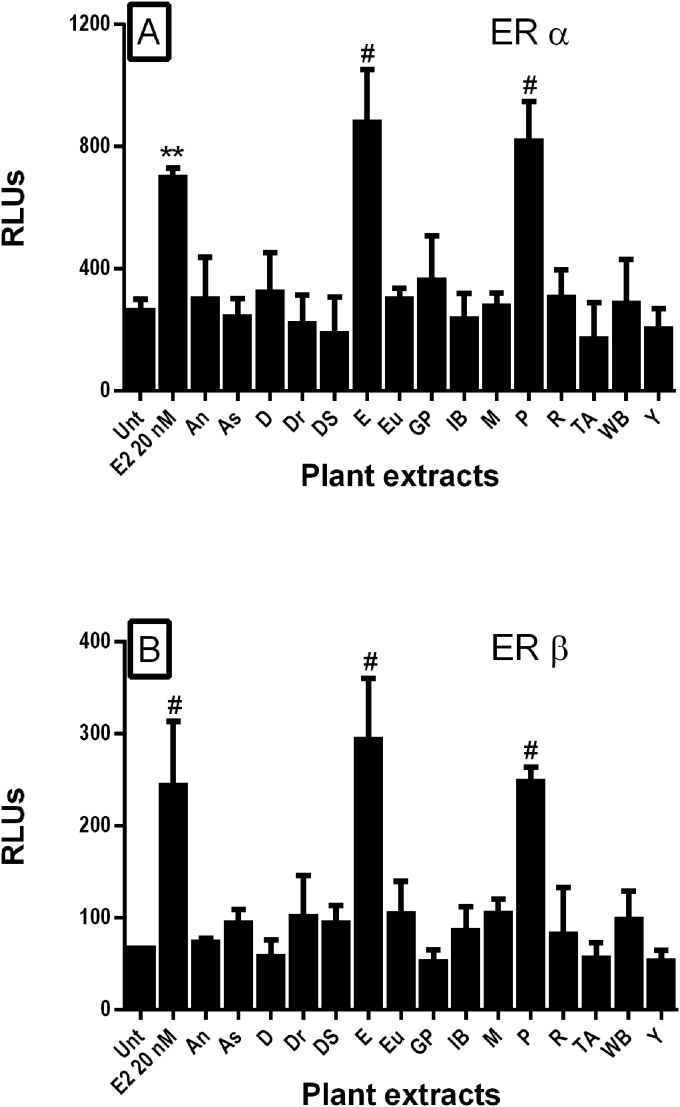
Psoralea and epimedium extracts robustly activated the full-length ERα and ERβ (Fig. 3). The other extracts did not demonstrate appreciable activity. Their apparent estrogenic activity on ERβ was approximately equal to an estradiol concentration of 20 nM (5.44 μg/mL). The estrogenic activity on full length ERβ was 20-fold higher for psoralea and 125-fold for epimedium, when compared to the estrogenic activity on the isolated ERβ LBD. These extracts have enhanced estrogenic activity on the full length ERα and ERβ versus the isolated LBDs. Psoralea or epimedium extracts did not activate the receptor, non-specifically; they had no activity in the control assay (SK-N-SH cells transfected with the ERE Luc2 construct alone without a transfected ER; data not shown).
Fig. 3.
Effect of selected TCM plant extracts on the activation of full-length estrogen receptor α (A) or β (B) as measured by an estrogen response element reporter assay. Plant extracts were resuspended in DMSO and added at a final concentration of 40 μg/mL to SK-N-SH cells transfected with full-length estrogen receptor α or β and a luciferase reporter construct under the control of 4X estrogen response elements. Estradiol (E2) was used as a positive control at 20 nM. Cells were treated for 18 h. Extract abbreviations were as follows: An, Angelica; As, Asiatic Cornelian Cherry; D, Danshen; Dr, Drynaria; DS, Dodder Seed; E, Epimedium; Eu, Eucommia; GP, Glossy Privet; IB, Indian Bread; M, Milkvetch; P, Psoralea: R, Rehmannia; TA, Two-toothed Achyranthes; WB, Wolfberry root bark; and Y, Common Yan Rhizome . Data are represented as mean ± SD of relative luminescence units (RLUs (n = 3); ***p < 0.001, #p < 0.0001 compared to untreated (Unt) controls).
3.2. Cyclic AMP activity
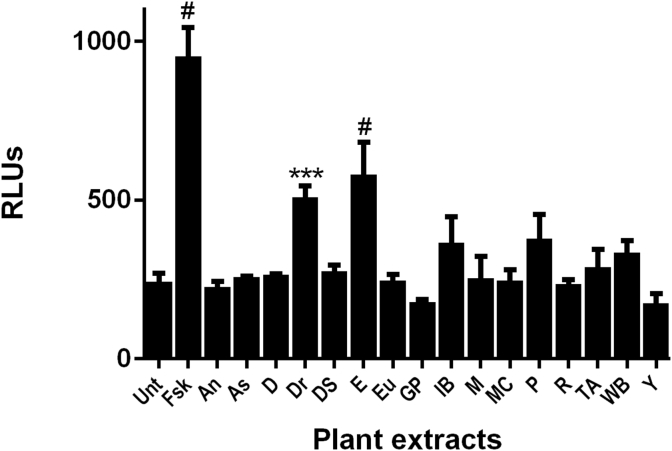
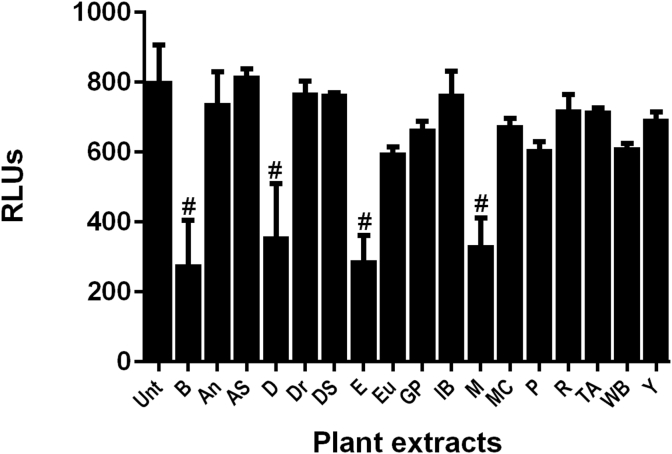
Evaluation of cyclic AMP upregulation and subsequent CREB activation was completed using a CRE luciferase reporter assay. Fsk, a known activator of adenylate cyclase was the positive control and 100 nM activated this assay approximately 4 fold. Drynaria and epimedium extracts (40 μg/mL) activated the CRE reporter assay approximately 2 fold above controls (Fig. 4). Indian bread and psoralea extracts were less active at the same concentration. .
Fig. 4.
Effect of selected TCM plant extracts on the activation of cyclic AMP response element binding protein (CRE) reporter assay. Plant extracts were resuspended in DMSO and added at a final concentration of 40 μg/mL to CHO cells stably transfected with a luciferase reporter construct under the control of 4X CRE response elements. Forskolin (Fsk) was used as a positive control at 100 nM. Cells were treated for 6 h. Extract abbreviations were as follows: An, Angelica; As, Asiatic Cornelian Cherry; D, Danshen; Dr, Drynaria; DS, Dodder Seed; E, Epimedium; Eu, Eucommia; GP, Glossy Privet; IB, Indian Bread; M, Milkvetch; MC, Medicinal Cyathula; P, Psoralea: R, Rehmannia; TA, Two-toothed Achyranthes; WB, Wolfberry root bark; and Y, Common Yan Rhizome. Data are represented as mean ± SD of relative luminescence units (RLUs (n = 3); ***p < 0.001, #p < 0.0001 compared to untreated (Unt) controls.
3.3. Anti-inflammatory activity determination
Pretreatment of A549 cells containing the NF-κβ activation reporter construct with either danshen, epimedium, or milkvetch extracts (40 μg/mL) inhibited IL-1β (100 pg/mL) activation of NF-κβ (Fig. 5). The other extracts did not demonstrate appreciable anti-inflammatory activity. The anti-inflammatory activity did not correlate to CRE activation. Drynaria did not inhibit NF-κβ activation by IL-1β despite activating CRE. Bay 11-7082 (12.5 μM) was used as a control inhibitor of IL-1β activated NF-κβ.34
Fig. 5.
Effect of selected TCM plant extracts on the inhibition of NF-κβ activation by IL-1β. Plant extracts were resuspended in DMSO and added at a final concentration of 40 μg/mL to A549 cells stably transfected with an NF-κβ responsive luciferase reporter construct. Cells were stimulated with IL-1β (100 pg/mL). Control treatments were IL-1β only and Bay11 (B; 12.5 μM). Cells were treated for 6 h. Extract abbreviations were as follows: An, Angelica; As, Asiatic Cornelian Cherry; D, Danshen; Dr, Drynaria; DS, Dodder Seed; E, Epimedium, Eu, Eucommia; GP, Glossy Privet; IB, Indian Bread; M, Milkvetch; MC, Medicinal Cyathula; P, Psoralea: R, Rehmannia; TA, Two-toothed Achyranthes; WB, Wolfberry root bark; and Y, Common Yan Rhizome. Data are represented as mean ± SD of relative luminescence units (RLUs (n = 3); #p < 0.0001 compared to untreated (Unt) controls).
3.4. Activation of bone specific genes Runx2 and Bmp-4
For subsequent evaluations, epimedium and drynaria were selected as companion ingredients and tested in additional bone related assays. Epimedium was selected as the primary (or monarch) ingredient for four reasons: 1) it activates the full-length ERα and ERβ (Fig. 3); but 2) it does not behave like estrogen because it does not activate either ERα LBD or ERβ LBD (Fig. 2); 3) it is a strong inhibitor of NF-κβ activation by IL-1β (Fig. 5); and 4) it activates CRE a possible bone building pathway. Drynaria was selected as a companion ingredient to epimedium because of its capability to activate CRE, a known downstream target of PTH, a bone building treatment.
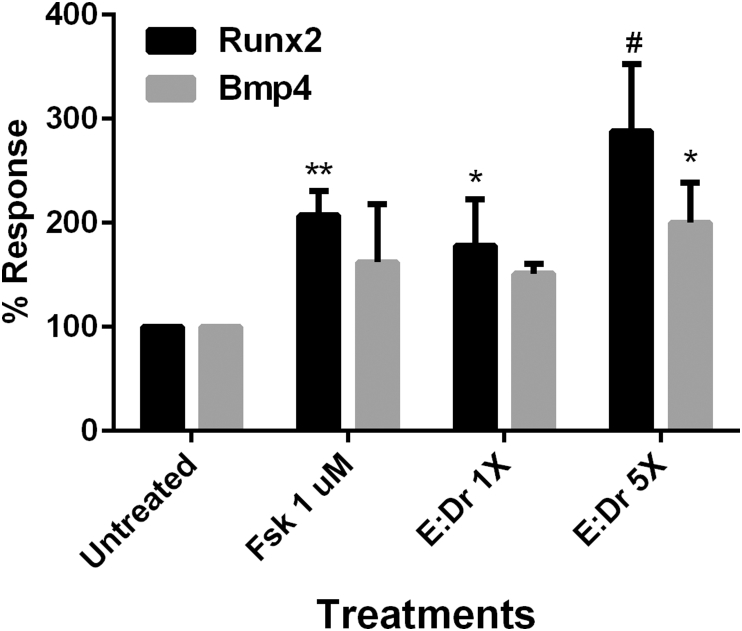
The epimedium and drynaria (E:Dr; 1:1) combination was tested in a bone-precursor, CRE-sensitive cell line, MC3T3-E1.35 Bone morphogenic signals in MC3T3-E1 cells,36 Runx2 (Runt-Related Transcription Factor 2) and Bmp4 (bone morphogenic peptide 4) were measured following treatment with different doses of a 1:1 combination of epimedium and drynaria. Forskolin, a known CRE activator, was used as a positive control. Treatment of MC3T3-E1 cells with Fsk (1 μM) for 7 days increased Runx2 transcription 207 % and Bmp4 162 %. Doses of 5 and 25 μg/mL of the epimedium/drynaria combination increased bone specific gene activation of Runx2, 178% and 288%, respectively and Bmp4, 151% and 200 %, respectively in a dose dependent manner (Fig. 6).
Fig. 6.
Effect of Forskolin, and epimedium/drynaria plant extract combinations on selected gene expression in MC3T3-E1 cells. Forskolin (Fsk, 1 μM) was used as a positive control. MC3T3-E1 cells were treated with 5:5 μg/mL epimedium/drynaria combination (E:Dr 1X) and 25:25 μg/mL (E:Dr 5X) for 7 days and mRNA expression levels of Runx2 and Bmp4 were measured as determined by qPCR and normalized to housekeeping gene Hprt1. The thermo-cycling conditions were as follows: 95○C for 30 s; 40 cycles of 58○C for 5 s; and 95○C for 5 s. Each column represents the mean response ± SD of 4 separate individual experiments (n = 4; compared to untreated (Unt) control group; *p < 0.05, **p < 0.01, and #p < 0.0001).
3.5. Inhibition of osteoclast development
The E:Dr combination was further evaluated in an osteoclast development model while testing the addition of a third ingredient. Danshen was selected as a possible third ingredient because of its robust anti-inflammatory activity (Fig. 5). Angelica and Wolfberry Bark were also selected as possible third ingredients to be tested because of their modest anti-inflammatory activity and their lack of activity on isolated ER LBDs. Psorelea and milkvetch were avoided as the third ingredients because of their minimal activity on isolated ERβ LBD (Fig. 2).
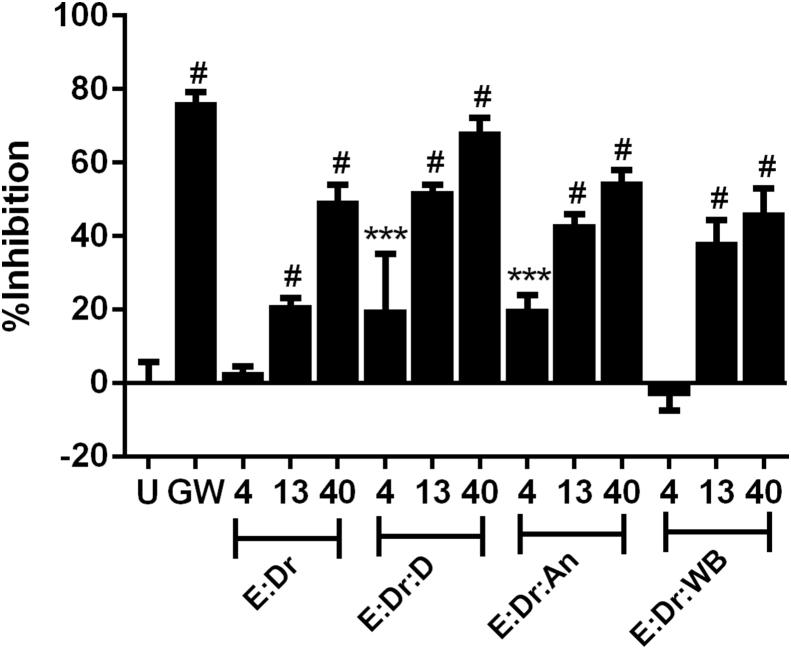
All treatments inhibited the differentiation of pre-osteoclast to osteoclasts containing the E:Dr combo alone or in combination with a third ingredient in a dose dependent manner as measured by the development of TRAP concentration, an indicator of osteoclast development (Fig. 7). At 13 and 40 μg/ml the group containing D as the third ingredient appeared to outperform the groups containing An or WB as a third ingredient which also had less anti-inflammatory activity than D. Further evaluation of these ingredient combinations were completed in the ovariectomized rat model to determine if inhibition of TRAP activity was a good indicator of in vivo activity.
Fig. 7.
Effect of epimedium/drynaria combinations with and without a third plant extract on the development of osteoclasts as determined by alkaline phosphatase levels. Osteoclast precursor cells were incubated with 4, 13 or 40 μg/mL of various combinations of epimedium/drynaria (E:Dr, 1:1) and a third plant extract (1:1:1), danshen (D), angelica (An) or wolfberry root bark (WB) in the presence of osteoclast activators RANKL (66 ng/mL) and CSF-1 (33 ng/mL) for 7–14 d. Percent inhibition is determined by the inhibition of alkaline phosphatase levels relative to the alkaline phosphatase activity in cells treated with RANKL and CSF-1 alone (untreated, U). Positive control inhibitor, GW2580 (GW), was used at 1 μM. Each column represents the mean response ± SD, (n = 4; compared to untreated (U) control group; ***p < 0.001, and #p < 0.0001).
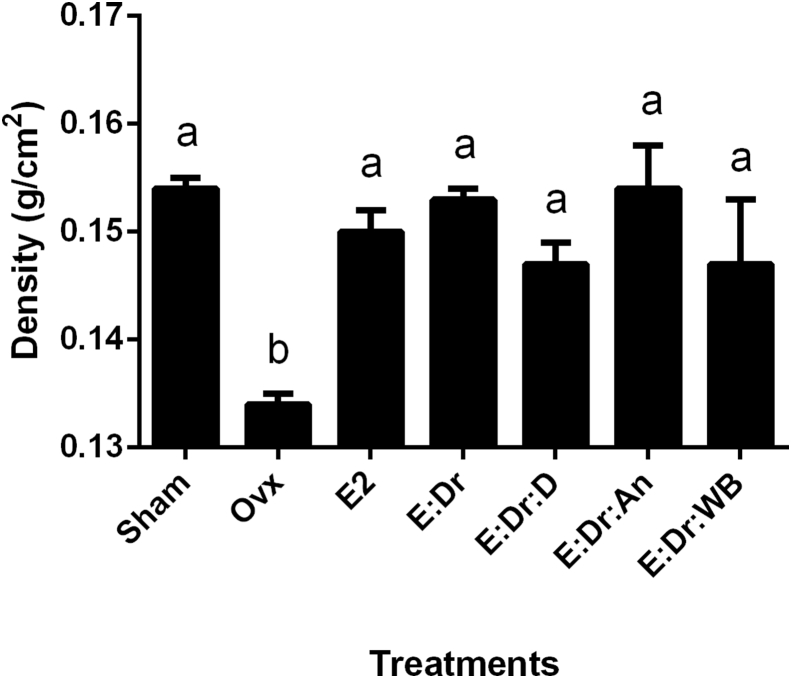
3.6. Maintenance of bone mineral density
Ovariectomized and sham operated animals were treated daily for 12 weeks with either water, E2 or the various combinations of plant extracts. The femurs of the animals were scanned using DEXA as described above. The animals treated with the E:Dr combination with or without either of the additional plant extracts D, An, or WB, maintained bone mass as measured by DEXA. Their BMD results were similar to the animals that had intact ovaries (Sham) or were treated with oral E2 (105 μg/kg; Fig. 8).
Fig. 8.
Bone Mineral Density (BMD) after 12 weeks of treatment. BMD values detected with dual-energy X-ray, after 12 weeks (g/cm2; n = 6–10). Treatment groups include the sham operative group (Sham) and the ovariectomized animal groups control (Ovx) or treated daily with estradiol (E2; 0.105 mg kg−1) or a base combination of epimedium (E; 100 mg kg−1) and drynaria (Dr; 32 mg kg−1) with a third ingredient of 32 mg kg−1 of either danshen (D), angelica (An), or wolfberry (WB) extract. The values are expressed as the mean ± SD (n = 6–10 animals), A Kruskal-Wallis H test for nonparametric data table was compiled. Bars labeled with different letters indicate that they are statistically different from each other. The treatment groups depicted with the letter “a” are statistically different from the Ovx group, depicted with the letter “b”, using Dunn-Bonferroni for pair-wise comparisons and an α level of 0.05 for mean comparisons.
4. Discussion
In TCM, LBM/OP is considered the result of a kidney deficiency (Kidney-Yang) accompanied by a weakened spleen-qi. Kidney governs the growth of bones and the cultivation of bone marrow. The fullness of the kidney essence gives rise to the proper development of bone marrow and strong bones. A kidney deficiency causes a depletion of bone marrow and under-nourishment of sinews and bones. The spleen is responsible for the digestive function to get the nutritive essence, like blood, to support bone strength.37 Thus, much of the traditional herbal method to support LBM is to properly nourish and invigorate the kidney and spleen.
Epimedium has been used to support kidney deficiency and subsequently to promote bone health in China for hundreds of years. In fact, a number of current TCM prescriptions use epimedium as the main ingredient or monarch such as the Xianling Gubao capsule, Er-Xian Decoction and Xian-Zhen Decoction. Other ingredients such as psoralea and drynaria rhizome serve as ministers or assistants to the monarch ingredient and are also included in these formulations.38
In this study, we present the development of a minimal TCM formula primarily containing two ingredients, epimedium and drynaria. Epimedium was chosen as the main or monarch ingredient partially because of its TCM warming properties but also because of its in vitro biological activities. Drynaria was chosen as a minister ingredient to the monarch to further strengthen the kidney and to support CRE activation and possible osteogenic activities. The other ingredients such as danshen were considered as assistants for invigorating the spleen and replenishment of qi as well as their anti-inflammatory activity. The selection of these ingredients were initially based on their use in TCM for LBM/OP, but their activity in a series of biological tests that evaluated estrogenic, osteogenic, and anti-osteoclastic activity were another reason for their selection for testing in the in vivo ovariectomized rat model of LBM/OP. Estrogenic activity has been a part of bone maintaining strategies for many years and this activity has been described for many traditional herbal ingredients.39 We examined whether the extracts in this study acted like estrogen by binding to the LBD of ERα or ERβ. We wanted to avoid those extracts that behaved like estrogen to avoid complication that may be associated with estrogen replacement therapy.40
Researchers using tissue culture31, 32, 41 and animal models33, 42 have previously suggested epimedium to be estrogenic, but they did not examine the mode of estrogen receptor activation. Our work shows that epimedium robustly activates full-length ERα and ERβ; however, it only minimally activates the estrogen binding domain of ERβ (∼2-fold) and does not activate the estrogen binding domain of ERα. From these data, we suggest that epimedium, like psoralea, does not work through the estrogen binding site, like estrogen. It may be binding to another portion of ERα and/or ERβ or it may be regulating cellular pathways that lead to ERα and ERβ activation, but epimedium is not behaving like estrogen. We believe this observation is important and suggest that epimedium activates ERα and ERβ differently than estrogen and as such may not have the same biological affects or concerns that estrogen has in other biological systems.40
Epimedium may be indirectly activating ERα or ERβ by affecting other cellular pathways that lead to ERα or ERβ activation; non-estrogenic activation of ERα has been described previously.43 Our study and others have demonstrated that epimedium activates CRE44 and inhibits NF-κβ activation.45, 46, 47, 48 However, drynaria another CRE activator and danshen and milkvetch, inhibitors of NF-κβ activation, do not activate full length ERα or ERβ. Thus, if epimedium is indirectly activating ERα or ERβ, it appears to be doing so in a manner other than the modulation of CRE or NF-κβ.
Osteogenic activity was evaluated measuring osteogenic genes, Runx2 and BMP-4 in the MC3T3-E1 model system. Epimedium and drynaria activated these targets despite the presence of estrogenic materials, phenol-red and FBS.49 We suggest that epimedium and drynaria may be upregulating these genes through their activation of CRE. Fsk, a adenylate cyclase activator and epimedium have previously been shown to upregulate CRE-related responses.44 Drynaria has not been shown previously to activate CRE.
Osteoclast development was inhibited by the epimedium and drynaria combination in a dose-dependent manner and the addition of a third ingredient, either danshen, angelica or wolfberry augmented the inhibition at selected doses. Previously, epimedium alone has been shown to inhibit NF-κβ activation as well as to stimulate p38 MAPK to inhibit NF-κβ during osteoclast differentiation.45, 46, 47, 48 The NF-κβ inhibition by epimedium does not appear to be the result of either CRE activation and/or ER activation50, 51, 52 because other extracts that activate these pathways (drynaria and psoralea, respectively) do not inhibit NF-κβ activation. Osteoclast development inhibition was observed in the presence of estrogenic compounds, phenol-red and FBS49; therefore, ER-related signaling pathways may not be needed in epimedium's effect on osteoclastogenesis.
The addition of a third ingredient, danshen, angelica or wolfberry to the base formula of epimedium and drynaria, had little if any additional effect on BMD in the ovarectomized rat model. All of the treatments containing the monarch, epimedium and its minister, drynaria maintained BMD when compared to the sham operated animals and the estrogen treated ovarectomized animals.
More research will be needed to understand the pathway(s) responsible for the exact mechanisms of osteogenic and anti-osteoclastic activity of epimedium and drynaria. More importantly, are there other mechanistic pathways that are important for the development and maintenance of strong bones? Additionally, can other TCM ingredients be used to identify other important mechanisms to maintain bone mass? Here we describe some biological mechanisms that may have an impact on maintaining BMD in the ovariectomized rat model of LBM/OP and we describe the development of a formula using these mechanisms to prevent the loss of BMD in this system.
Financial support
Research was wholly funded by the Nutrilite Health Institute, No. 6 Lane 720, Cailun Road Zhangjiang, High-Tech Park, Shanghai, 201203, China.
Author disclosure statement
Authors, Q.D., M.H., K.M.G., H.C., J.D.S., F.T., M.G., M.L., and J.F.R. are employees of Nutrilite Health Institute who funded this research.
Acknowledgements
We thank Dr. Rodney A. Velliquette for his critical review of this work and Dr. Mark L. Proefke for his support.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Nelson H.D., Helfand M., Woolf S.H., Allan J.D. Screening for postmenopausal osteoporosis: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137(6):529–541. doi: 10.7326/0003-4819-137-6-200209170-00015. [DOI] [PubMed] [Google Scholar]
- 2.Riancho J.A., Hernandez J.L. Pharmacogenomics of osteoporosis: a pathway approach. Pharmacogenomics. 2012;13(7):815–829. doi: 10.2217/pgs.12.50. [DOI] [PubMed] [Google Scholar]
- 3.Kanis J.A., McCloskey E.V., Johansson H., Oden A., Melton L.J., 3rd, Khaltaev N. A reference standard for the description of osteoporosis. Bone. 2008;42(3):467–475. doi: 10.1016/j.bone.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Jang S.N., Choi Y.H., Choi M.G. [Prevalence and associated factors of osteoporosis among postmenopausal women in Chuncheon: Hallym Aging Study (HAS)] J Prev Med public health = Yebang Uihakhoe chi. 2006;39(5):389–396. [PubMed] [Google Scholar]
- 5.Weitzmann M.N., Pacifici R. Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest. 2006;116(5):1186–1194. doi: 10.1172/JCI28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stein B., Yang M.X. Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta. Mol Cell Biol. 1995;15(9):4971–4979. doi: 10.1128/mcb.15.9.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srivastava S., Weitzmann M.N., Cenci S., Ross F.P., Adler S., Pacifici R. Estrogen decreases TNF gene expression by blocking JNK activity and the resulting production of c-Jun and JunD. J Clin Invest. 1999;104(4):503–513. doi: 10.1172/JCI7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srivastava S., Weitzmann M.N., Kimble R.B. Estrogen blocks M-CSF gene expression and osteoclast formation by regulating phosphorylation of Egr-1 and its interaction with Sp-1. J Clin Invest. 1998;102(10):1850–1859. doi: 10.1172/JCI4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finkelstein J.S., Klibanski A., Schaefer E.H., Hornstein M.D., Schiff I., Neer R.M. Parathyroid hormone for the prevention of bone loss induced by estrogen deficiency. N. Engl J Med. 1994;331(24):1618–1623. doi: 10.1056/NEJM199412153312404. [DOI] [PubMed] [Google Scholar]
- 10.Bauer W., Aub J.C., Albright F. Studies of calcium and phosphorus metabolism : V. A study of the bone Trabeculae as a readily available reserve supply of calcium. J Exp Med. 1929;49(1):145–162. doi: 10.1084/jem.49.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khosla S., Westendorf J.J., Oursler M.J. Building bone to reverse osteoporosis and repair fractures. J Clin Invest. 2008;118(2):421. doi: 10.1172/JCI33612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juppner H., Abou-Samra A.B., Freeman M. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science. 1991;254(5034):1024–1026. doi: 10.1126/science.1658941. [DOI] [PubMed] [Google Scholar]
- 13.Kim J.M., Choi J.S., Kim Y.H. An activator of the cAMP/PKA/CREB pathway promotes osteogenesis from human mesenchymal stem cells. J Cell physiology. 2013;228(3):617–626. doi: 10.1002/jcp.24171. [DOI] [PubMed] [Google Scholar]
- 14.Powell W.F., Jr., Barry K.J., Tulum I. Targeted ablation of the PTH/PTHrP receptor in osteocytes impairs bone structure and homeostatic calcemic responses. J Endocrinol. 2011;209(1):21–32. doi: 10.1530/JOE-10-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu Q., Jilka R.L., Manolagas S.C., O'Brien C.A. Parathyroid hormone stimulates receptor activator of NFkappa B ligand and inhibits osteoprotegerin expression via protein kinase A activation of cAMP-response element-binding protein. J Biol Chem. 2002;277(50):48868–48875. doi: 10.1074/jbc.M208494200. [DOI] [PubMed] [Google Scholar]
- 16.Huang J.C., Sakata T., Pfleger L.L. PTH differentially regulates expression of RANKL and OPG. J bone mineral Res official J Am Soc Bone Mineral Res. 2004;19(2):235–244. doi: 10.1359/JBMR.0301226. [DOI] [PubMed] [Google Scholar]
- 17.Saini V., Marengi D.A., Barry K.J. Parathyroid hormone (PTH)/PTH-related peptide type 1 receptor (PPR) signaling in osteocytes regulates anabolic and catabolic skeletal responses to PTH. J Biol Chem. 2013;288(28):20122–20134. doi: 10.1074/jbc.M112.441360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossouw J.E., Anderson G.L., Prentice R.L. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. Jama. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z-q, Li J-l, Sun Y-l. Chinese herbal medicine for osteoporosis: a systematic review of randomized controlled trails. Evidence-Based Complementary Altern Med. 2013:27–48. doi: 10.1155/2013/356260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klinge C.M. Estrogen receptor interaction with estrogen response elements. Nucleic acids Res. 2001;29(14):2905–2919. doi: 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rebhun J.F., Roloff S.J., Velliquette R.A., Missler S.R. Identification of evodiamine as the bioactive compound in evodia (Evodia rutaecarpa Benth.) fruit extract that activates human peroxisome proliferator-activated receptor gamma (PPARgamma) Fitoterapia. 2015;101:57–63. doi: 10.1016/j.fitote.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Rajgopal A., Rebhun J.F., Burns C.R., Scholten J.D., Balles J.A., Fast D.J. Immunomodulatory effects of Lippia sidoides extract: induction of IL-10 through cAMP and p38 MAPK-dependent mechanisms. J Med food. 2015;18(3):370–377. doi: 10.1089/jmf.2014.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rebhun J.F., Glynn K.M., Missler S.R. Identification of glabridin as a bioactive compound in licorice (Glycyrrhiza glabra L.) extract that activates human peroxisome proliferator-activated receptor gamma (PPARgamma) Fitoterapia. 2015;106:55–61. doi: 10.1016/j.fitote.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Ba F., Pang P.K., Davidge S.T., Benishin C.G. The neuroprotective effects of estrogen in SK-N-SH neuroblastoma cell cultures. Neurochem Int. 2004;44(6):401–411. doi: 10.1016/j.neuint.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Rajgopal A., Missler S.R., Scholten J.D. Magnolia officinalis (Hou Po) bark extract stimulates the Nrf2-pathway in hepatocytes and protects against oxidative stress. J Ethnopharmacol. 2016;193:657–662. doi: 10.1016/j.jep.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Kalu D.N. Evaluation of the pathogenesis of skeletal changes in ovariectomized rats. Endocrinology. 1984;115(2):507–512. doi: 10.1210/endo-115-2-507. [DOI] [PubMed] [Google Scholar]
- 27.SPSS Statistics for Windows, Version 20.0 [computer program]. Version 20.0. Armonk, NY2011.
- 28.Dunn O.J. Multiple comparisons using rank sums. Technometrics. 1964;6(3):241–252. [Google Scholar]
- 29.Xin D., Wang H., Yang J. Phytoestrogens from Psoralea corylifolia reveal estrogen receptor-subtype selectivity. Phytomedicine. 2010;17(2):126–131. doi: 10.1016/j.phymed.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Chopra B., Dhingra A.K., Dhar K.L. Psoralea corylifolia L. (Buguchi)—Folklore to modern evidence: review. Fitoterapia. 2013;90:44–56. doi: 10.1016/j.fitote.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 31.Songlin P., Ge Z., Yixin H. Epimedium-derived flavonoids promote osteoblastogenesis and suppress adipogenesis in bone marrow stromal cells while exerting an anabolic effect on osteoporotic bone. Bone. 2009;45(3):534–544. doi: 10.1016/j.bone.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 32.Mok S.K., Chen W.F., Lai W.P. Icariin protects against bone loss induced by oestrogen deficiency and activates oestrogen receptor-dependent osteoblastic functions in UMR 106 cells. Br J Pharmacol. 2010;159(4):939–949. doi: 10.1111/j.1476-5381.2009.00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang G., Qin L., Shi Y. Epimedium-derived phytoestrogen flavonoids exert beneficial effect on preventing bone loss in late postmenopausal women: a 24-month randomized, double-blind and placebo-controlled trial. J bone mineral Res official J Am Soc Bone Mineral Res. 2007;22(7):1072–1079. doi: 10.1359/jbmr.070405. [DOI] [PubMed] [Google Scholar]
- 34.Strickson S., Campbell D.G., Emmerich C.H. The anti-inflammatory drug BAY 11-7082 suppresses the MyD88-dependent signalling network by targeting the ubiquitin system. Biochem J. 2013;451(3):427–437. doi: 10.1042/BJ20121651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franceschi R.T., Xiao G., Jiang D., Gopalakrishnan R., Yang S., Reith E. Multiple signaling pathways converge on the Cbfa1/Runx2 transcription factor to regulate osteoblast differentiation. Connect tissue Res. 2003;44(1):109–116. [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao J., Ohba S., Shinkai M., Chung U-i, Nagamune T. Icariin induces osteogenic differentiation in vitro in a BMP-and Runx2-dependent manner. Biochem biophysical Res Commun. 2008;369(2):444–448. doi: 10.1016/j.bbrc.2008.02.054. [DOI] [PubMed] [Google Scholar]
- 37.Xu H., Lawson D., Kras A., Ryan D. The use of preventive strategies for bone loss. Am J Chin Med. 2005;33(02):299–306. doi: 10.1142/S0192415X05002916. [DOI] [PubMed] [Google Scholar]
- 38.Zhai Y.K., Guo X., Pan Y.L. A systematic review of the efficacy and pharmacological profile of Herba Epimedii in osteoporosis therapy. Die Pharm. 2013;68(9):713–722. [PubMed] [Google Scholar]
- 39.Glazier M.G., Bowman M.A. A review of the evidence for the use of phytoestrogens as a replacement for traditional estrogen replacement therapy. Archives Intern Med. 2001;161(9):1161–1172. doi: 10.1001/archinte.161.9.1161. [DOI] [PubMed] [Google Scholar]
- 40.Investigators WGftWsHI Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. Jama. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 41.Hsieh T.P., Sheu S.Y., Sun J.S., Chen M.H., Liu M.H. Icariin isolated from Epimedium pubescens regulates osteoblasts anabolism through BMP-2, SMAD4, and Cbfa1 expression. Phytomedicine. 2010;17(6):414–423. doi: 10.1016/j.phymed.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Zhang G., Qin L., Hung W. Flavonoids derived from herbal Epimedium Brevicornum Maxim prevent OVX-induced osteoporosis in rats independent of its enhancement in intestinal calcium absorption. Bone. 2006;38(6):818–825. doi: 10.1016/j.bone.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 43.Bunone G., Briand P.A., Miksicek R.J., Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J. 1996;15(9):2174–2183. [PMC free article] [PubMed] [Google Scholar]
- 44.Dell'Agli M., Galli G.V., Dal Cero E. Potent inhibition of human phosphodiesterase-5 by icariin derivatives. J Nat Prod. 2008;71(9):1513–1517. doi: 10.1021/np800049y. [DOI] [PubMed] [Google Scholar]
- 45.Hsieh T.-P., Sheu S.-Y., Sun J.-S., Chen M.-H. Icariin inhibits osteoclast differentiation and bone resorption by suppression of MAPKs/NF-κB regulated HIF-1α and PGE 2 synthesis. Phytomedicine. 2011;18(2):176–185. doi: 10.1016/j.phymed.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Choi H.J., Park Y.R., Nepal M. Inhibition of osteoclastogenic differentiation by Ikarisoside A in RAW 264.7 cells via JNK and NF-κB signaling pathways. Eur J Pharmacol. 2010;636(1):28–35. doi: 10.1016/j.ejphar.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 47.Zeng K.-W., Fu H., Liu G.-X., Wang X.-M. Icariin attenuates lipopolysaccharide-induced microglial activation and resultant death of neurons by inhibiting TAK1/IKK/NF-κB and JNK/p38 MAPK pathways. Int Immunopharmacol. 2010;10(6):668–678. doi: 10.1016/j.intimp.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 48.Jin Q., Lee C., Lee J.W. 2-Phenoxychromones and prenylflavonoids from Epimedium koreanum and their inhibitory effects on LPS-induced nitric oxide and interleukin-1β production. J Nat Prod. 2014;77(7):1724–1728. doi: 10.1021/np400831p. [DOI] [PubMed] [Google Scholar]
- 49.Berthois Y., Katzenellenbogen J.A., Katzenellenbogen B.S. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci. 1986;83(8):2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kameda T., Mano H., Yuasa T. Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J Exp Med. 1997;186(4):489–495. doi: 10.1084/jem.186.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakamura T., Imai Y., Matsumoto T. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. 2007;130(5):811–823. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 52.Ghisletti S., Meda C., Maggi A., Vegeto E. 17β-estradiol inhibits inflammatory gene expression by controlling NF-κB intracellular localization. Mol Cell Biol. 2005;25(8):2957–2968. doi: 10.1128/MCB.25.8.2957-2968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]