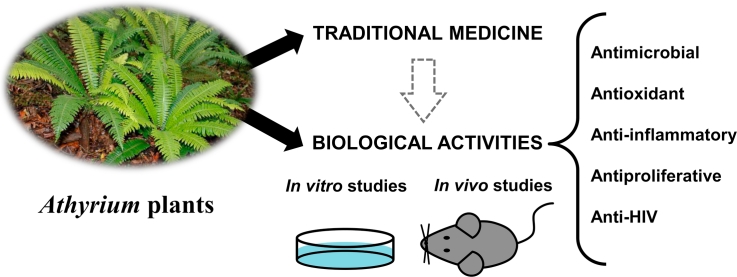
Abstract
Athyrium plants consist of more than 230 species that are largely distributed in the Sino-Himalayan region and the Western Pacific islands. Athyrium species are being used in traditional medicine worldwide to treat various ailments such as cough, rheumatic pain, scorpion stings, sores, burns and scalds, intestinal fever, pain, specifically breast pain during child birth, to increase milk flow, as an antiparasitic, anthelmintic, and carminative. A deep look in the literature has revealed that Athyrium species have been poorly investigated for their food preservative applications and in vivo and in vitro biological and phytochemical studies. However, some Athyrium species have demonstrated antimicrobial, anti-inflammatory, antioxidant, antiproliferative and anti-HIV potential. Athyrium multidentatum (Doll.) Ching is the most investigated species and the biological activities of their extracts, such as they antioxidant properties, seem to be related to the sulfate contents of their polysaccharides. This review provides an update on the ethnopharmacology, phytochemistry and biological properties of Athyrium plants that might be useful for further research. Of course, well-designed clinical trials will be required for some species to be used as therapy.
Keywords: Athyrium, Ethnobotany, Ethnopharmacology, Biological activities, Phytoconstituents
Graphical abstract
1. Introduction
Athyrium or ferns, in the family Athyriaceae Alston, is a genus composed of ca. 230–300 species of terrestrial or epilithic plants with mostly erect or occasionally creeping or ascending rhizomes. Athyrium are mainly distributed in the Sino-Himalayan region including Southwest China, Sichuan Basin, Tibet-Yunnan Plateau and Nepal and secondarily in the Western Pacific islands such as Japanese Archipelago, the Ryukyu Islands, Taiwan, and the Philippines.1, 2, 3 Athyrium species are being used in traditional medicine throughout their range to treat various ailments such as cough4 and sores,5,6 and as antiparasitic,7, 8, 9 anthelmintic6, 7, 8, 9, 10, 11, 12, 13, 14, 15 and diuretic agents.6,16, 17, 18
Their phytochemical and biological properties have been poorly explored and scientific knowledge of their chemical constituents is limited.18 The great medicinal potency of Athyrium plants has been associated with the presence of several classes of natural compounds such as flavonoids, phenols, alkaloids, steroids, triterpenes and polysaccharides.17,18 In addition, Athyrium plants have been shown to contain nutrients, such as protein, carbohydrates, fats, antioxidants and vitamins, which benefit health in many ways.17
Several extracts from ferns exhibited remarkable antioxidant capacity, comparable to vitamin C, making them good food preservatives. They have also exhibited a promising effect on the inhibition of cell proliferation and stimulated apoptosis in the human liver cancer cell line (HepG2).19 The strong superoxide radical-scavenging and reducing power of Athyrium genus have been attributed to its polysaccharides.19,20
A. multidentatum (Döll) Ching (AMC) is the most investigated species. It is a well-known nutritious potherb and traditional Chinese medicine native to northeast China, especially in the Changbai Mountain area. Moreover, Liu et al.,19 reported that polysaccharides extracted from AMC possess high antioxidant activity as mentioned above. Also, it possesses high antiproliferative activity.19,20 The present review provides an overview of the ethnopharmacy, phytochemistry and biological properties of Athirium plants.
2. Traditional medicine uses of Athyrium plants
Athyrium species have been used in traditional medicine systems in different parts of the world (Table 1). For instance, A. felix-femina (L.) Roth has been used along with honey to cure cough in Diano valley (Province of Salerno, Campania region, Italy),4 while a decoction of A. felix-femina has been used in decoction as an antiparasitic and anthelmintic agent.8,9 In Iran, the rhizome is used also used as an antiparasitic and anthelmintic.7 The Rajasthan Bheels (a tribe from India) used the rhizome of A. pectinatum (Wall. ex Mett.) T. Moore as a strong anthelmintic.13,15 The people of Mymensing district of Bangladesh used fresh leave juice of A. asperum (Blume) Milde in children as an anthelmintic and carminative.14 In Maharastra (India), fronds and rhizome of A. hohenackerianum T. Moore are used as decoction against rheumatic pain as well as an anthelmintic.10 In addition, the rhizome paste has been used against scorpion sting.10 A. falcatum Bedd. has been used by the people of Madhya Pradesh (India) as an anthelmintic.11 The people of Palani Hills (Western Ghats of South India) used its fronds and roots in their traditional medicine practices. For instance, the young fronds were consumed to treat internal ailments such as cancer and the roots consumed as an anthelmintic.6 A. lanceum T. Moore roots are used to overcome pain, specifically breast pain during child birth. It also increases milk flow and the dried powder when applied to sores is found to be useful.6 Moreover, it has been used as a remedy for burns and scalds, against intestinal fever,21 and anti-inflammatory herb for the treatment of ascariasis in Malaysia.12 In New Guinea, Athyrium is used to treat sores.5 AMC has also been used in traditional Chinese medicine for its tranquilizer, antihypertensive and diuretic properties.16, 17, 18
Table 1.
Traditional uses and Biological activities of Athyrium spp.
| Traditional use | Reference |
|---|---|
| Antihypertensive | 16, 17, 18 |
| Antiparasitic and anthelmintic | 6, 7, 8, 9, 10, 11,13, 14, 15 |
| Burns and scalds | 21 |
| Cancer | 6 |
| Carminative | 14 |
| Cough | 4 |
| Diuretic | 6,16, 17, 18 |
| Increases milk flow | 6 |
| Intestinal fever | 21 |
| Scorpion bite | 10 |
| Rheumatic pain | 10 |
| Sores | 5,6 |
| Tranquilizer | 16, 17, 18 |
| Biological activity | |
| Anti-HIV | 22 |
| Anti-inflammatory | 23 |
| Antioxidant | 1,17, 18, 19,24, 25, 26, 27 |
| Antiproliferative | 17,20,28 |
| Antimicrobial | 7,26,29, 30, 31, 32 |
3. Food preservative applications of Athyrium plants
Microbial food safety is a global problem with significant effect on human health.33, 34, 35 In fact, food-borne pathogens are important causes of illness and death.36,37 According to the World Health Organization, unsafe food results in illnesses of at least 2 billion people worldwide annually and can be deadly.33,38 In addition, oxidative deterioration of food may also cause lipid peroxidation, nutritional loss, off-flavor and color impairment. Both microbial and oxidative deterioration decrease functional and nutritional value of food and consumers’ acceptance.38
To ensure food safety and prevent spoilage of food, a number of physical and chemical preservation techniques have been developed. Thermal processing destroys vegetative microorganisms but this technique may lead to undesirable organoleptic and nutritional effects.33,39 Several synthetic food preservatives (benzoic acid, sorbic acid, propionic acid, salts, BHA, BHT etc.) are commonly used to reduce the microbial growth rate or viability and extend the shelf life. Because of increasing regulatory restrictions, consumer awareness and microbial resistance, alternative natural sources of new antimicrobial compounds including plants, must be investigated.33,38, 39, 40, 41, 42, 43
Since ancient time, plants and their derived essential oils and extracts have been used for many purposes, especially in medicine and food industry as flavoring agents and food preservatives.33,36,44, 45, 46 Plants contain a diversity of bioactive compounds47 including polyphenols, flavonoids, terpenoids, carotenoids, sterols, peptides and polysaccharides etc., showing many biological properties38,48, 49, 50, 51, 52, 53 such as antioxidant54 and antimicrobial activity.34,39,47
The use of Athyrium plants and their derivatives for food preservation and safety as alternative sources of natural antimicrobial and antioxidant agents have been poorly investigated. However, polysaccharides and their derivatives found in Athyrium plants have been explored for their multipharmacological activities and applications in food and pharmaceutical industries especially as antioxidants.54 Of note, the antioxidant activities of polysaccharides depend on type of sugar, glycosidic branching, flexibility and configuration of the chains.55 Liu et al.19 prepared the polysaccharide extract from AMC and found it to be a neutral heteropolysaccharide, mainly made of glucose, galactose, rhamnose, mannose, and arabinose. The polysaccharide extract showed strong reducing power and scavenging activities on hydroxyl and superoxide radicals, and chelating abilities and the authors suggested a significant correlation between antioxidant activities with molecular weight and sulfate content.19 Notably, high molecular weight polysaccharides are less active than low molecular weight ones due to their poor penetration capability on cell-membranes.56 In addition, another study demonstrated the strong antioxidant and reducing power of the acetylated, sulfated and phosphorylated derivatives of polysaccharides from the same plant.24 Low molecular weight polysaccharide derivatives from AMC rhizome also showed strong scavenging ability on 1,1-diphenyl-2-picrylhydrazyl (DPPH), superoxide and hydroxyl radicals.25 Superoxide anion and hydroxyl radicals are reactive oxygen species (ROS) that can cause oxidative damage to biomolecules including DNA, lipids and proteins, and induce severe tissue damage or cell death.57,58
Soare et al.26 reported a good antioxidant activity of the methanolic extract of A. filix-femina leaves that positively correlated with the total phenolic compounds. In another study, A. filix-femina exhibited strong 2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical-scavenging activity.1
The antimicrobial and antioxidant potencies of Athyrium suggest it could be used as alternative natural preservatives to ensure food safety and meet the consumer demands related to natural food additives.
4. Biological activities of Athyrium plants
Only a few Athyrium plants have been investigated for their biological activities, mostly focused on antibacterial and antioxidant properties (Table 1).
4.1. In vitro activity
4.1.1. Antimicrobial assays
A. filix-femina (L.) Roth had good antimicrobial activity in acetone extract for both Gram-negative and Gram-positive bacteria with zones of inhibition of 7–20 mm diameter against Escherichia coli and Bacillus megaterium.29 A. filix-femina extract showed low antimicrobial activity with inhibition zone of 7–8 mm extract against Staphyllococcus aureus and Enterococcus faecalis and a minimum inhibitory concentration (MIC) value of 600 μg/mL against Bacillus cereus and Saccharomyces cerevisiae.26 However, the methanol extracts obtained from rhizome and leaves of A. filix-femina were found to have strong antibacterial potential against E. coli, S. aureus, and B. megaterium with MIC value ranging from 8 to 32 μg/mL.7,30
Parihar et al.31,32 showed that the leaf extract of A. pectinatum inhibited the growth of Salmonella arizonae, but did not inhibit the growth of E. coli or Salmonella typhi. Aqueous and alcoholic extracts of A. pectinatum were found effective against E. coli, S. arizonae, S. typhi and S. aureus.31,32
4.1.2. Antioxidant activity
Liu et al.19 reported that the antioxidant activity of AMC is related to the amount of peptides, present in the form of polysaccharide–peptide complexes. This study suggested that the molecular weight and sulfate content of AMC polysaccharides played very important roles on antioxidant activity.19 Moreover, Yuan et al.59 found that the presence of sulfate, acetyl or phosphate could enhance the antioxidant activity of polysaccharide in vitro. Similarly, Behera et al.60 found that polysaccharides having small polysaccharide/peptide ratios showed higher scavenging activities. Chen et al.61 also suggested that the mechanism of antioxidant action of polysaccharides might be linked to the supply of hydrogen from the polysaccharide–peptide complexes, or the combination of the complexes with the radical ions, where the reaction was then terminated.
Ferrous ions are considered the most important pro-oxidants in food systems,62 so the chelating effect on ferrous ions is widely used to evaluate antioxidant activity. Liu et al.19 reported high chelating effect on ferrous ions of the AMC polysaccharides.
As Liu et al.19 noticed, the chelating power could be related to the sulfate contents of the AMC polysaccharides. Sulfated polysaccharides are often involved in many biological activities in animal cells, such as cell recognition, cell adhesion or regulation of receptor functions, which are of interest in medicine.63 Qi et al.64 also observed that the higher the sulfate content of a polysaccharide, the stronger the antioxidant activity and antihyperlipidemic activity. The hydroethanolic extract of the dried aerial parts of AMC, defatted with petroleum ether and purified using an AB-8 macroporous resin, showed significant DPPH, ABTS, •OH scavenging activity and ferric reducing power with EC50 of 15.0, 4.6, 48.0 and 13.2 μg/mL, respectively.17 The extract also protected protein oxidation in a dose-dependent manner at concentration of 5–100 μg/mL.17 Besides protein fragmentation, the oxidation of arginine, threonine, lysine and proline may also generate carbonyl derivatives considered to be a marker of ROS-mediated protein oxidation.65 Similarly, AMC purified extract in the range of 5–100 μg/mL efficiently inhibited 2,2′-azobis (2-amidinopropane) dihydrochloride (AAPH) -induced oxidation of albumin from bovine serum (BSA) carbonyl in a dose-dependent manner. In addition, AMC also inhibited lipid peroxidation in a dose-dependent manner and had the greatest cellular antioxidant activity, with cellular antioxidant activity values of 88.1 μM quercetin equivalents per g extract. AMC had powerful protective effects on biological macromolecules including proteins and lipids, indicating their potential use in the chemoprevention of diseases related to ROS.
Sheng et al.18 also reported that the n-butanol fraction of the methanol extract of AMC exhibited strong superoxide anion-scavenging capacity and strong reducing power. In another study, Sheng and Sun25 degraded AMC rhizomes by using hydrogen peroxide and ascorbic acid mixtures to obtain four low molecular polysaccharides derivatives. Their results showed that high molecular weight polysaccharides showed prominent reducing power and DPPH activity, whereas low molecular weight polysaccharides exhibited relatively stronger free-radical scavenging activity.25 Total sugar and sulfate content could affect the scavenging capability of the samples against superoxide radicals.25 Wang et al.66 found over-sulfated fucoidan, a sulfated polysaccharide found mainly in various species of brown algae and brown seaweed, had much greater antioxidant activity than fucoidan itself.
The oxidative stress theory of aging is based on the hypothesis that age-associated functional losses are due to the accumulation of ROS-induced damages. Anti-aging effects of AMC have been reported and linked to its polysaccharide content and could thus be used as a promising adjuvant agent for aging prevention.27
4.1.3. Antiproliferative activity
AMC significantly inhibited the tumor growth of hepatocellular carcinoma,20,28 suggesting that it could be a promising agent for treatment of cancer. The purified AMC extract showed antiproliferative potency on HepG2 cells with IC50 values of 220 μg/mL after 24 h and 114 μg/mL after 48 h.20 A non-significant toxic effect was observed on human liver cell line, HL-7702, with IC50 values of 332 μg/mL after 24 h and 304 μg/mL after 48 h. On the other hand, AMC extract showed strong inhibition of cell growth and induced apoptosis and cell cycle arrest in HepG2 cells by significantly upregulating the protein expressions of Fas and its ligand Fas-L, which initiated the extrinsic pathway of apoptosis. In addition, AMC extract also suppressed the expression level of anti-apoptotic protein (Bcl-2) and triggered the translocation of Bax from cytoplasm to mitochondria, and the Bcl-2/Mito-Bax ratio gradually decreased in a concentration-dependent manner. Moreover, the expression levels of cleaved caspase-3 and the cleavage of PARP were increased in a dose-dependent manner after treatment with AMC for 24 h. This means that AMC induces apoptosis in HepG2 cells through both intrinsic and extrinsic pathways. AMC evoked apoptosis via modulation of the phosphatidylinositol-3-kinase and protein kinase B (PI3K/Akt), mitogen-activated protein kinase (MAPK), nuclear factor kappa B (NFκB) and nuclear factor erythroid 2–related factor 2 (Nrf2) pathways and their downstream transcriptional cascades.20 AMC treatment also resulted in an increase in the percentage of cells in the G2/M phase in a concentration-dependent manner. Protein expressions of cyclin-dependent kinases (CDKs) CDK1, CDK2 and cyclin D1 were both markedly decreased on exposure of the cells to AMC, which suggested that AMC could induce cell cycle arrest in G2/M phase associated with decreases of CDK1, CDK2 and cyclin D1.17,20
Striatisporolide A (SA) has been isolated from the rhizomes of A. multidentatum. This is a simple butenolide derivative with a fragrant odor.67,68 It was reported to possess cytotoxic activity on the human lung carcinoma cell line (A549) with an IC50 value of 36.5 μM (7.75 μg/mL).28
SA at 100 μM increased cell viability rate in human umbilical vein endothelial cells (HUVECs) to 128%. Meanwhile, in H2O2-treated HUVECs, SA pre-incubated at 50 μM enhanced cell viability by 56.9%. In both cases, the cell apoptosis rates were reduced to 2.2% and 3.1%, respectively. SA prevented the overproduction of ROS in HUVECs induced by H2O2 and the fluorescent intensity was abated after pre-incubation with 100 μM SA.28
4.1.4. Anti-HIV activity
Mimhina et al.22 isolated three sulfolipids from the pteridophyte A. niponicum (Mett.) Hance that were evaluated on human immunodeficiency virus (HIV). The sulfolipids at 6 pg/mL were found to significantly inhibit the activities of both calf DNA polymerase α (pol. α) and rat DNA polymerase β (pol. β). Calf thymus terminal deoxynucleotidyl transferase was inhibited moderately by the sulfolipids. The sulfolipids appear to be selective inhibitors of the mammalian DNA polymerases in vitro. Sulfolipids also inhibited the mammalian pol. α, pol. β, and HIV-RT in a concentration-dependent manner; IC50 for inhibition of pol. α and pol. β was 1.5 and 3 μg/mL, respectively, and almost complete inhibition (more than 90–95%) was achieved at 6 and 8 μg/mL, respectively. The sulfolipids were slightly more effective on pol. α than pol. β.22
4.2. In vivo studies
The in vivo anti-inflammatory activity of the 95% ethanol extract of AMC dried aerial parts has been reported in a lipopolysaccharide (LPS)-induced inflammatory model in mice.23 AMC extract inhibited NO and prostaglandin E2 (PGE2) production by suppressing their inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX-2) gene expression in LPS-induced peritoneal macrophages. The secretion of interleukins (IL-6 and IL-1β), and tumor necrosis factor (TNF-α) were also decreased through the down-regulation of mRNA levels. The AMC anti-inflammatory effects seem to be mediated via suppression of the toll-like receptor 4 (TLR4) signaling. In in-vivo experiments, the cell count and cytokines (TNF-α, IL-1β and IL-6) levels in bronchoalveolar lavage fluid (BALF) decreased significantly in a dose-dependent manner in the AMC extract groups. In particular, the effect of 10 mg/kg AMC extract was similar to that of the positive control dexamethasone.23
Qi et al.17,20 also studied the in-vivo antitumor activity of AMC purified extract in a xenograft tumor model in which HepG2 cells were injected subcutaneously into nude mice. Mice were treated with AMC (25 or 100 mg/kg) daily for 31 days. It was noticed that AMC had a significant inhibitory effect on tumor size compared to the control group. Caspase-3 and Ki-67 staining confirmed an increase in apoptosis and a reduction in proliferative cells in AMC-treated tumor animals in comparison to the untreated control.17,20
5. Conclusions and future perspectives
Overall, this review summarized the ethnophamacological, pharmacological, and phytochemical aspect of Athyrium plants. Only three Athyrium species have been investigated for their phytochemical constitution and biological activities. The demonstrated antimicrobial, anti-inflammatory, antioxidant, and anti-HIV in vitro and in vivo potencies warrant further clinical investigations in order to be used as therapy. However, other species from the Athyrium genus require a thorough investigation to reveal their real clinical value.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Contributor Information
Mehdi Sharifi-Rad, Email: mehdi_sharifirad@yahoo.com.
Miquel Martorell, Email: martorellpons@gmail.com.
Javad Sharifi-Rad, Email: javad.sharifirad@gmail.com.
References
- 1.Valizadeh H., Sonboli A., Kordi F.M., Dehghan H., Bahadori M.G. Cytotoxicity, antioxidant activity and phenolic content of eight fern species from North of Iran. Pharmaceut Sci. 2015;21(1):18–24. [Google Scholar]
- 2.Wei R., Zhang X.C. Athyrium sessilipinnum: a new lady fern (Athyriaceae) from southern China. Brittonia. 2016;68(4):440–447. [Google Scholar]
- 3.Wood K.R., Wagner W.L. Athyrium haleakalae (Athyriaceae), a new rheophytic fern species from East Maui, Hawaiian Islands: with notes on its distribution, ecology, and conservation status. PhytoKeys. 2017;76:115–124. doi: 10.3897/phytokeys.76.11637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Novella R., Di Novella N., De Martino L., Mancini E., De Feo V. Traditional plant use in the National Park of Cilento and Vallo di Diano, Campania, Southern, Italy. J Ethnopharmacol. 2013;145(1):328–342. doi: 10.1016/j.jep.2012.10.065. [DOI] [PubMed] [Google Scholar]
- 5.Powell J.M. vol. 106. New Guinea Vegetation; New Guinea: 1976. (Ethnobotany). [Google Scholar]
- 6.Sathiyaraj G., Muthukumar T., Ravindran K.C. Ethnomedicinal importance of fern and fern allies traditionally used by tribal people of Palani Hills (Kodaikanal), Western Ghats, South India. J Med Herbs Ethnomed. 2015;1(1):4–9. [Google Scholar]
- 7.Bahadori M.B., Kordi F.M., Ahmadi A.A., Bahadori S., Valizadeh H. Antibacterial evaluation and preliminary phytochemical screening of selected ferns from Iran. Res J Pharmacogn. 2015;2(2):53–59. [Google Scholar]
- 8.Eskandari M., Riazi B., Shirzadian S., Mazooji A. Investigation of fern species in Guilan province. Rostaniha. 2012;13:1–9. [Google Scholar]
- 9.Mozaffarian V.A. first ed. Farhange Moaser Press; Tehran: 2012. Identification of Iranian Medicinal and Aromatic Plants. [Google Scholar]
- 10.Shaikh S.D., Masal V.P., Shaikh A.S. Ethnomedicinal uses of some pteridophytes from Konkan region of Maharashtra, India. Int J Pharmaceut Chem Sci. 2014;3(1):62–65. [Google Scholar]
- 11.Singh B.P., Upadhyay R. Medicinal pteridophytes of Madhya Pradesh. J Med Plants Stud. 2014;2(4):65–68. [Google Scholar]
- 12.Chai T.-T., Elamparuthi S., Yong A.-L., Quah Y., Ong H.-C., Wong F.-C. Antibacterial, anti-glucosidase, and antioxidant activities of selected highland ferns of Malaysia. Bot Stud. 2013;54(1):55. doi: 10.1186/1999-3110-54-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parihar P., Parihar L. Some pteridophytes of medical importance from Rajasthan. Nat Product Radiance. 2006;5(4):297–301. [Google Scholar]
- 14.Sarker S.K., Hossain A.B.M.E. Pteridophytes of greater Mymensingh district of Bangladesh used as vegetables and medicines. Bangladesh J Plant Taxon. Jun 2009;16(1):47–56. [Google Scholar]
- 15.Vyas M.S., Sharma B.D. Today and tomorrow's printers and publishers; New Delhi: 1988. Ethnobotanical Importance of the Ferns of Rajasthan. Indigenous Medicinal Plants Including Microbes and Fungi. [Google Scholar]
- 16.Liu Y., Wujisguleng W., Long C. Food uses of ferns in China: a review. Acta Soc Bot Pol. 2012;81(4):263–270. [Google Scholar]
- 17.Qi G., Yang L., Xiao C., Shi J., Mi Y., Liu X. Nutrient values and bioactivities of the extracts from three fern species in China: a comparative assessment. Food Funct. 2015;6(9):2918–2929. doi: 10.1039/c5fo00510h. [DOI] [PubMed] [Google Scholar]
- 18.Sheng J.W., Liu D.M., Li Z.J., Qi L., Zhang W.F. Bioactivity-guided fractionation for antioxidant property of Athyrium multidentatum (Doll) Ching. rhizome. J Med Plants Res. 2011;5(32):7000–7005. [Google Scholar]
- 19.Liu D., Sheng J., Li Z. Antioxidant activity of polysaccharide fractions extracted from Athyrium multidentatum (Doll.) Ching. Int J Biol Macromol. 2013;56:1–5. doi: 10.1016/j.ijbiomac.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 20.Qi G., Liu Z., Fan R. Athyrium multidentatum (Doll.) Ching extract induce apoptosis via mitochondrial dysfunction and oxidative stress in HepG2 cells. Sci Rep. 2017;7(1):2275. doi: 10.1038/s41598-017-02573-8. 2017/05/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corrigan D. Medicinal plants in folk Tradition−an ethnobotany of Britain and Ireland by David E. Allen and Gabrielle Hatfield (natural history museum, London, and the royal botanic gardens, Kew). Timber press, Portland, OR. J Nat Prod. 2004/12/01 2004;67(12):2154-2154.
- 22.Mimhina Y., Watanabe I., Ohru K. Studies on inhibitors of mammalian DNA polymerase α and β sulfolipids prom a pteridcphyte, Athymum niponeum. Biochem Pharmacol. 1998;55:537–541. doi: 10.1016/s0006-2952(97)00536-4. [DOI] [PubMed] [Google Scholar]
- 23.Han X.Z., Ma R., Chen Q. Anti-inflammatory action of Athyrium multidentatum extract suppresses the LPS-induced TLR4 signaling pathway. J Ethnopharmacol. 2018;217:220–227. doi: 10.1016/j.jep.2018.02.031. [DOI] [PubMed] [Google Scholar]
- 24.Sheng J.W. Preparation and antioxidant activity of Athyrium multidentatum (Doll.) Ching polysaccharide derivatives. J Chem Pharmaceut Res. 2014;6(6):1129–1135. [Google Scholar]
- 25.Sheng J., Sun Y. Antioxidant properties of different molecular weight polysaccharides from Athyrium multidentatum (Doll.) Ching. Carbohydr Polym. 2014;108:41–45. doi: 10.1016/j.carbpol.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Soare L.C., Ferdes M., Stefanov S. Antioxidant activity, polyphenols content and antimicrobial activity of several native pteridophytes of Romania. Not Bot Horti Agrobot. 2012;40(1):53–57. [Google Scholar]
- 27.Liu D.M., Sheng J.W., Mu J., Li W.Z., Li Z.J., Sun Y.L. Antioxidant activities of an active fraction from Athyrium multidentatum (Doll.) Ching. J Chem Pharmaceut Res. 2016;8(6):458–462. [Google Scholar]
- 28.Liu D.M., Sheng J.W., Wang S.H., Zhang W.F., Zhang W., Zhang D.J. Cytoprotective effects of striatisporolide a isolated from rhizomes of Athyrium multidentatum (Doell.) ching on human umbilical vein endothelial cells. Molecules (Basel, Switzerland) 2016;21(10) doi: 10.3390/molecules21101280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pal S.K. Study of solvent extracts of some selected ferns for antimicrobial activity. Indian J Biol Sci. 2012;18:33–37. [Google Scholar]
- 30.Kumarpal S. Study of activity of some medicinal ferns of darjeeling. Int J Sci Res Publ. 2013;3(8) [Google Scholar]
- 31.Parihar P., Parihar L., Bohra A. Antibacterial activity of Athyrium pectinatum (Wall.) Presl. Nat Prod Radance. 2006;5(4):262–265. [Google Scholar]
- 32.Parihar P., Parihar L., Bohra A. In vitro antibacterial activity of fronds (leaves) of some important pteridophytes. J Microbiol Antimicrob. 2010;2(2):19–22. [Google Scholar]
- 33.Hintz T., Matthews K.K., Di R. The use of plant antimicrobial compounds for food preservation. BioMed Res Int. 2015;2015:246264. doi: 10.1155/2015/246264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raeisi S., Ojagh S.M., Sharifi-Rad M., Sharifi-Rad J., Quek S.Y. Evaluation of Allium paradoxum (M.B.) G. Don. and Eryngium caucasicum trauve. Extracts on the shelf-life and quality of silver carp (Hypophthalmichthys molitrix) fillets during refrigerated storage. J Food Saf. 2017;37(3) [Google Scholar]
- 35.Raeisi S., Sharifi-Rad M., Quek S.Y., Shabanpour B., Sharifi-Rad J. Evaluation of antioxidant and antimicrobial effects of shallot (Allium ascalonicum L.) fruit and ajwain (Trachyspermum ammi (L.) Sprague) seed extracts in semi-fried coated rainbow trout (Oncorhynchus mykiss) fillets for shelf-life extension. Lwt-Food Sci Technol. 2016;65:112–121. [Google Scholar]
- 36.Mohanka R., Priyanka Plant extract as natural food preservative against spoilage fungi from processed food. Int J Curr Microbiol Appl Sci. 2014;3(8):91–98. [Google Scholar]
- 37.Sharifi-Rad M., Nazaruk J., Polito L. Matricaria genus as a source of antimicrobial agents: from farm to pharmacy and food applications. Microbiol Res. 2018:76–88. doi: 10.1016/j.micres.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 38.Prakash B., Kujur A., Singh P., Kumar A., Yadav A. Plants-derived bioactive compounds as functional food ingredients and food preservative. J Nutr Food Sci. 2017;1:1–7. [Google Scholar]
- 39.Tiwari B.K., Valdramidis V.P., O'Donnell C.P., Muthukumarappan K., Bourke P., Cullen P.J. Application of natural antimicrobials for food preservation. J Agric Food Chem. 2009;57(14):5987–6000. doi: 10.1021/jf900668n. [DOI] [PubMed] [Google Scholar]
- 40.Sharifi-Rad M., Roberts T.H., Matthews K.R. Ethnobotany of the genus Taraxacum—phytochemicals and antimicrobial activity. Phytother Res. 2018 doi: 10.1002/ptr.6157. [DOI] [PubMed] [Google Scholar]
- 41.Mishra A.P., Sharifi-Rad M., Shariati M.A. Bioactive compounds and health benefits of edible Rumex species-A review. Cell Mol Biol. 2018;64(8):27–34. [PubMed] [Google Scholar]
- 42.Mishra A.P., Saklani S., Salehi B. Satyrium nepalense, a high altitude medicinal orchid of Indian Himalayan region: chemical profile and biological activities of tuber extracts. Cell Mol Biol. 2018;64(8):35–43. [PubMed] [Google Scholar]
- 43.Mishra A.P., Saklani S., Sharifi-Rad M. Antibacterial potential of Saussurea obvallata petroleum ether extract: a spiritually revered medicinal plant. Cell Mol Biol. 2018;64(8):65–70. [PubMed] [Google Scholar]
- 44.Salehi B., Ayatollahi S.A., Segura-Carretero A. Bioactive chemical compounds in Eremurus persicus (Joub. & Spach) Boiss. essential oil and their health implications. Cell Mol Biol. 2017;63(9):1–7. doi: 10.14715/cmb/2017.63.9.1. [DOI] [PubMed] [Google Scholar]
- 45.Sharifi-Rad J., Ayatollahi S.A., Varoni E.M. Chemical composition and functional properties of essential oils from nepeta schiraziana boiss. Farmacia. Sep-Oct 2017;65(5):802–812. [Google Scholar]
- 46.Abdolshahi A., Naybandi-Atashi S., Heydari-Majd M. Antibacterial activity of some Lamiaceae species against Staphylococcus aureus in yoghurt-based drink (Doogh) Cell Mol Biol. 2018;64(8):71–77. [PubMed] [Google Scholar]
- 47.Sharifi-Rad M., Iriti M., Sharifi-Rad M., Gibbons S., Sharifi-Rad J. Anti-methicillin-resistant Staphylococcus aureus (MRSA) activity of Rubiaceae, Fabaceae and Poaceae plants: a search for new sources of useful alternative antibacterials against MRSA infections. Cell Mol Biol. 2016;62(9):39–45. [PubMed] [Google Scholar]
- 48.Sharifi-Rad J., Salehi B., Schnitzler P. Susceptibility of herpes simplex virus type 1 to monoterpenes thymol, carvacrol, p-cymene and essential oils of Sinapis arvensis L., Lallemantia royleana Benth. and Pulicaria vulgaris Gaertn. Cell Mol Biol. 2017;63(8):42–47. doi: 10.14715/cmb/2017.63.8.10. [DOI] [PubMed] [Google Scholar]
- 49.Sharifi-Rad J., Salehi B., Varoni E.M. Plants of the Melaleuca genus as antimicrobial agents: from farm to pharmacy. Phytother Res. 2017;31(10):1475–1494. doi: 10.1002/ptr.5880. [DOI] [PubMed] [Google Scholar]
- 50.Sharifi-Rad M., Varoni E.M., Salehi B. Plants of the genus Zingiber as a source of bioactive phytochemicals: from tradition to pharmacy. Molecules (Basel, Switzerland) 2017;22(12) doi: 10.3390/molecules22122145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salehi B., Mishra A.P., Shukla I. Thymol, thyme, and other plant sources: health and potential uses. Phytother Res. 2018;32(9):1688–1706. doi: 10.1002/ptr.6109. [DOI] [PubMed] [Google Scholar]
- 52.Sharifi-Rad M., Varoni E.M., Iriti M. Carvacrol and human health: a comprehensive review. Phytother Res. 2018;32(9):1675–1687. doi: 10.1002/ptr.6103. [DOI] [PubMed] [Google Scholar]
- 53.Salehi B., Zucca P., Sharifi-Rad M. Phytotherapeutics in cancer invasion and metastasis. Phytother Res. 2018;32(8):1425–1449. doi: 10.1002/ptr.6087. [DOI] [PubMed] [Google Scholar]
- 54.Wang J., Hu S., Nie S., Yu Q., Xie M. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxid Med Cell Longev. 2016;2016 doi: 10.1155/2016/5692852. 5692852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu Y, Wang D, Hu Y, Huang X, Wang J. Sulfated modification of epimedium polysaccharide and effects of the modifiers on cellular infectivity of IBDV. Carbohydr Polym. 2008/01/24/2008;71(2):180-186.
- 56.Wu Q., Zheng C., Ning Z.-X., Yang B. Modification of low molecular weight polysaccharides from tremella fuciformis and their antioxidant activity in vitro. Int J Mol Sci. 2007;8(7):670–679. [Google Scholar]
- 57.Spencer J.P., Jenner A., Aruoma O.I. Intense oxidative DNA damage promoted by L-dopa and its metabolites. Implications for neurodegenerative disease. FEBS Lett. 1994;353(3):246–250. doi: 10.1016/0014-5793(94)01056-0. [DOI] [PubMed] [Google Scholar]
- 58.Sharifi-Rad J., Sharifi-Rad M., Salehi B. In vitro and in vivo assessment of free radical scavenging and antioxidant activities of Veronica persica Poir. Cell Mol Biol. 2018;64(8):57–64. [PubMed] [Google Scholar]
- 59.Yuan H., Zhang W., Li X. Preparation and in vitro antioxidant activity of kappa-carrageenan oligosaccharides and their oversulfated, acetylated, and phosphorylated derivatives. Carbohydr Res. 2005;340(4):685–692. doi: 10.1016/j.carres.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 60.Behera B.C., Verma N., Sonone A., Makhija U. Evaluation of antioxidant potential of the cultured mycobiont of a lichen Usnea ghattensis. Phytother Res. 2005;19(1):58–64. doi: 10.1002/ptr.1607. [DOI] [PubMed] [Google Scholar]
- 61.Chen Y., Xie M.Y., Nie S.P., Li C., Wang Y.X. Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum. Food Chem. 2008;107:231–241. [Google Scholar]
- 62.Yamauchi R., Tatsumi Y., Asano M., Kato K., Ueno Y. Effect of metal salts and fructose on the autoxidation of methyl linoleate in emulsions. Agric Biol Chem. 1988;52(3):849–850. [Google Scholar]
- 63.Ellouali M., Boisson-Vidal C., Durand P., Jozefonvicz J. Antitumor activity of low molecular weight fucans extracted from brown seaweed Ascophyllum nodosum. Anticancer Res. 1993;13(6a):2011–2019. [PubMed] [Google Scholar]
- 64.Qi H., Huang L., Liu X., Liu D., Zhang Q., Liu S. Antihyperlipidemic activity of high sulfate content derivative of polysaccharide extracted from Ulva pertusa (Chlorophyta) Carbohydr Polym. 2012;87(2):1637–1640. [Google Scholar]
- 65.Levine R.L., Stadtman E.R. Oxidative modification of proteins during aging. Exp Gerontol. 2001;36(9):1495–1502. doi: 10.1016/s0531-5565(01)00135-8. [DOI] [PubMed] [Google Scholar]
- 66.Wang J., Zhang Q., Zhang Z., Zhang J., Li P. Synthesized phosphorylated and aminated derivatives of fucoidan and their potential antioxidant activity in vitro. Int J Biol Macromol. 2009;44(2):170–174. doi: 10.1016/j.ijbiomac.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 67.Liu D.S., Huang Y.L., Ma L.Y., Lu C.J., Liu W.Z. Chemical constituents and their cytotoxic activities of the secondary metabolites of Penicillium janthinellum. Chin Tradit Patent Med. 2016;38:830–834. [Google Scholar]
- 68.Stewart M., Capon R.J., Lacey E., Tennant S., Gill J.H. Calbistrin E and two other new metabolites from an Australian isolate of Penicillium striatisporum. J Nat Prod. 2005;68(4):581–584. doi: 10.1021/np049614y. [DOI] [PubMed] [Google Scholar]