Abstract
This study aims to investigate the effects of MaquiBright®, also known as BrightSight®, a standardized maqui berry extract, on improving eye dryness and fatigue in Japanese subjects (aged 30–60 years) experiencing eye dryness, eye fatigue, and ≥4 h of visual display terminal (VDT) work daily. Seventy-four participants were equally but randomly assigned to either a MaquiBright® (MB) or a placebo (P) group, wherein each participant consumed one capsule daily for 4 weeks of the appropriate treatment (MaquiBright® 60 or 0 mg). Eye dryness and fatigue were measured using the Schirmer's test, tear break-up time (BUT) test, pupillary response, and flicker test before intake and 4 weeks after intake. Furthermore, subjective symptoms were assessed using the Visual Analogue Scale (VAS) method and the Dry Eye–related Quality of Life Score (DEQS) questionnaire. The MB group demonstrated a significantly higher lacrimal fluid production in both eyes (increased 6.4 ± 8.1 mm, P = 0.005) in Schirmer's test compared to the P group before VDT load (playing a video game) at 4 weeks after intake. In the VAS method after VDT load, the reduction of subjective symptoms in eye fatigue (P = 0.047) and stiff shoulders (P = 0.035) were significantly higher in the MB group than in the P group as well as bothersome ocular symptoms (P = 0.037) by the DEQS. No adverse events were reported. Thus, the consumption of 60 mg of MaquiBright® per day for 4 weeks reduced eye dryness and seemed to alleviate eye fatigue.
Keywords: Maqui berry, Delphinidin, Schirmer's test, DEQS questionnaire, Lacrimal fluid production
Abbreviation: VDT, Visual Display Terminal; QOL, Quality of life; ROS, Reactive oxygen species; SD, Standard deviation; MB group, MaquiBright® group; P group, Placebo group; BUT, Break-up time; VAS, Visual Analogue Scale; DEQS, Dry Eye-related Quality of Life Score; BMI, Body mass index
Graphical abstract
1. Introduction
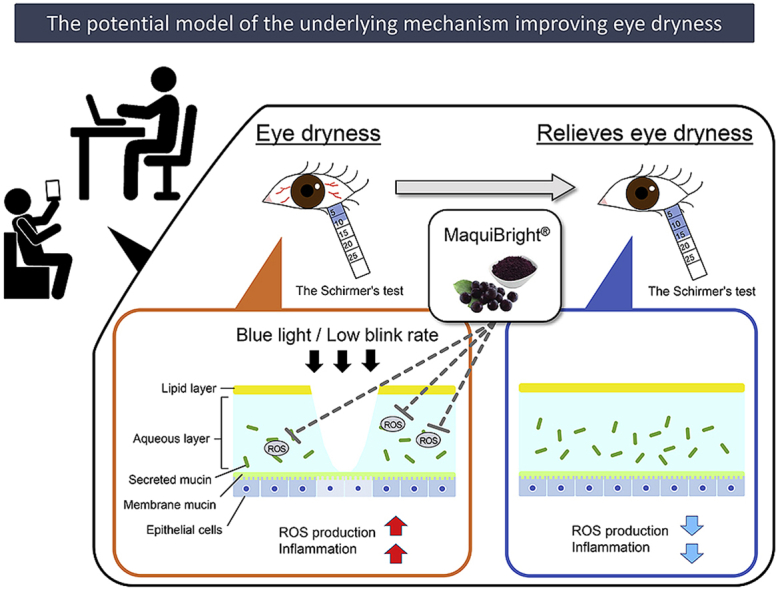
With changing environmental conditions, such as air conditioners, and dry air, dry eye syndrome has become a public health concern.1,2 Several epidemiological studies on Japanese employees3, 4, 5 have revealed that an upsurge in Visual Display Terminal (VDT) work by using a computer, cell phone, and other screen-based devices has increased the risk factor for dry eye syndrome. Reportedly, dry eye syndrome due to VDT work adversely affects eye functions causing eye dryness, eye fatigue, and blurry vision, affecting the quality of life (QOL).6, 7, 8, 9, 10, 11, 12 The onset of dry eye is attributed to an inflammation resulting from an increased amount of reactive oxygen species (ROS) in the corneal epithelial cells; the damage to corneal epithelial cells decreases the stability of the tear film layer.13,14 Thus, antioxidants have the potential to reduce dry eye symptoms.
Maqui berry (Aristotelia chilensis) growing in the Patagonia region of Chile is rich in anthocyanins, and has been used as a traditional medicine to treat inflammation since ancient times.15,16 Major anthocyanins contained in maqui berry are delphinidin derivatives with potent antioxidant activities. In particular, delphinidin-3,5-O-diglucoside is a specific compound in maqui berries.17 MaquiBright®, also known under the trade name BrightSight®, is a maqui berry extract standardized to bear not less than 35% of total anthocyanins and at least 25% of delphinidins. A pre-clinical study demonstrated that the consumption of MaquiBright® inhibited the decline in the lacrimal fluid production and prevented damages of corneal and lacrimal fluid tissues in dry-eyed rats.17 Additionally, the consumption of MaquiBright® has been demonstrated to increase the lacrimal fluid production, thereby improving QOL-related eye symptoms in a previous human pilot study.18 However, owing to the lack of a placebo group in the pilot trial, the evidence related to the beneficial effects of MaquiBright® on the lacrimal fluid production remains insufficient.
Hence, this study was conducted to investigate the effects of MaquiBright® capsules on eye dryness and fatigue in a randomized, double-blind, placebo-controlled trial.
2. Materials and methods
2.1. Study design
This randomized, double-blind, placebo-controlled trial was approved by the Takara Clinic (Medical Corporation Seishinkai, Tokyo, Japan) on November 28, 2016 (Approval ID: 1611-1610-OZ01-01-TC), and was conducted in compliance with the ethical principles of the Declaration of Helsinki modified in 2013 as well as the ethical guidelines for medical and health research involving human subjects completely adhering to medical ethics. All participants in this study were enrolled through the Go106 website (https://www.go106.jp/) operated by ORTHOMEDICO Inc. (Tokyo, Japan) and received a comprehensive explanation about the study before obtaining written informed consent from them. Each participant was examined at the Ario Nishiarai Eye Clinic (Tokyo, Japan). The temporal scope of the study comprised duration from November 30, 2016, to July 22, 2017. Furthermore, the protocol was registered at the University Hospital Medical Information Network Clinical Trials Registry (UMIN000025565).
2.2. Participants
The selection criteria of this study were as follows: (a) Japanese males and females aged 30–60 years; (b) presence of eye dryness symptoms; (c) complaint of eye fatigue; (d) playing video games, use of PCs, and/or a mobile phone, work at VDT for ≥4 h/day; and (e) vision correction of both eyes with ≥1.0 diopter without using contact lenses or able to switch to using eyeglasses during the test period. The exclusion criteria were as follows: (a) a medical history of a malignant tumor, heart failure, or myocardial infarction; (b) undergoing treatment for any of the following chronic diseases: arrhythmia, liver failure, kidney failure, cerebrovascular disease, rheumatism, diabetes mellitus, hyperlipidemia, hypertension, or any other chronic disease; (c) diagnosed with dry eye syndrome; (d) changing the frequency of using artificial lacrimal fluid, exceeding six times per day during the test period or using artificial lacrimal fluid seven times or more per day; (e) taking medication for dry eye syndrome (provided that a patient has not taken the medication for 2 weeks before the study and does not take the medication during the study period); (f) current eye disease (including conjunctivochalasis) or a history of eye disease; (g) currently using eye drops for the treatment of eye diseases; (h) the presence of Sjögren's syndrome; (i) pollen allergy or chronic asthma; (j) current pharmacotherapy or requiring pharmacotherapy; (k) meibomian gland dysfunction; (l) working in a late-night shift or having irregular lifestyle; (m) regular use of medications, including herbal medicines or/and supplements; (n) daily consumption of “Foods for Specified Health Uses” or “Foods with Functional Claims” regulated in Japan; (o) allergies to medications and/or products related to the study substance; (p) pregnancy, lactation, or expected pregnancy during the study period; (q) participation in another clinical study within 3 months of signing the informed consent form for this study; and (r) considered inappropriate for this study by the primary investigator.
All participants in this study were required to adhere to the following activities during the study period: (a) consume the prescribed amount of test food daily; (b) avoid consuming of “Foods for Specified Health Uses,” “Foods with Functional Claims,” or other functional food/beverages; (c) not to use contact lenses; (d) avoid consuming excess food and drink and maintain the usual lifestyle until study completion; (e) not to drink alcohol or exercise excessively the day before a study examination; (f) not to use artificial lacrimal fluid (eye drops) on the day of an examination; and (g) contact the study operating organization in case of an adverse event without delay for instructions.
Furthermore, participants were considered as dropouts for the following reasons: (a) voluntary withdrawal for personal reasons; (b) not adhering to the procedures required by the study investigators; (c) lifestyle deviation from that described in the test protocol; and (d) if the principal investigator decided that it is appropriate to remove the participant from the study. The safety evaluation data were individually evaluated. No participant belonged to any companies that were sponsors or funders of this study.
2.3. Determination of the sample size
Participants who initially scored 5–10 mm/5 min in Schirmer's test (normal: >10 mm) increased at the most 5 mm to be in the normal range. Thus, the 5 mm/5 min increase, at least, implied clinically significant differences in an intervention group compared to a placebo group. On the basis of the consumption of MaquiBright® for 8 weeks in a previous study,18 the standard deviation (SD) was set at ± 6.7 in this study. In addition, the study population calculation assumed a two-sided significance level of 5%, a detection level of 80%, and a dropout rate of 20%. On the basis of these factors, 37 participants were selected for each group.
2.4. Selection, randomization, and blinding
Of 196 participants who agreed to participate and provided informed consent, 122 participants were excluded; the remaining 74 participants with 5–10 mm/5 min in Schirmer's test before the VDT load (playing a video game) and ≤35 Hz in the flicker test were included. Then, participants were randomly assigned on the basis of Schirmer's test value in each eye and average of both eyes, the mean values in the flicker test, gender, and ages into two groups, a MaquiBright® group (MB group, n = 37) and a placebo group (P group, n = 37) in a 1:1 ratio. In this study, the allocation was performed using StatLight #11 Version 2.10 (Yukms Co., Ltd., Kanagawa, Japan) by an allocation controller who was blinded to the study. Likewise, neither participants, physicians, assessors of outcomes, nor others associated with the study were aware of the groups or were involved in their allocation. The number of participants was the maximum number of people by the trial budget, and those who violated the compliance were excluded from the analysis.
2.5. Preparation of the test capsules and intervention
The test supplements, indistinguishable brown capsules containing MaquiBright® or placebo, were provided by Oryza Oil & Fat Chemical Co., Ltd. (Aichi, Japan). Of note, the capsules were approved as identical to each other in color, odor, and flavor by the ethics committee, although active capsules contained 120 mg of dextrin and 60 mg of MaquiBright® (BrightSight®, maqui berry extract in powder containing 21 mg of total anthocyanins, 15 mg of total delphinidins, and 4 mg delphinidin-3,5-O-diglucoside), while placebo capsules contained 180 mg of dextrin. All participants were administrated one capsule per day of either MaquiBright® or placebo before breakfast for 4 weeks.
2.6. Examination items
In this study, all participants were examined before intake and 4 weeks after the first intake. At the clinic, participants were asked to play a video game for 45 min as the VDT load. The assessments associated with the eyes were the average in both eyes, dominant, non-dominant, right, and left eyes.
2.6.1. Primary outcomes
The amount of lacrimal fluid was measured using Schirmer's test strips (Katena Products Inc., New Jersey, U.S.A.). The 5 mm width long strip is inserted into the lower eyelid, and the length of wetting of the strips is measured after five minutes, providing mean values for left/right eyes.19 As the primary outcome, the values before and after the VDT load are considered to assess the amount of tear fluid as a parameter for eye dryness.
2.6.2. Secondary outcomes
The mean value in mm/5 min of lacrimal fluid was assessed using Schirmer's test in the dominant/non-dominant and left/right eyes as the secondary parameter.
The tear break-up time (BUT) examination, measured the tear film stability time used to assess eye dryness.20,21 After administering fluorescein into the subject's eyes, subjects are forbidden to blink. The time between the last blinking and the appearance of the first dry spot is observed and measured in seconds using a slit lamp. The BUT was administered before the VDT load.
The pupillary response was evaluated with TriIRIS C9000 (Hamamatsu Photonics K.K., Shizuoka, Japan) to assess the eye fatigue level, by calculating and assessing the pupillary near reflex.22 In addition, eye fatigue and the sensitivity of the ophthalmic nerve were measured with Handy Flicker HF-II (Neitz Instruments Co., Ltd., Tokyo, Japan) after the VDT load.23,24 While blinking at a visual target if a subject does not identify the flicker, the frequency of blinking is defined as the flicker value. Subjective symptoms related to eye conditions were measured with the Visual Analogue Scale (VAS) method25 and the dry eye-related quality of life score (DEQS) questionnaire.26 In the VAS method, a 100 mm long horizontal line was printed, whereas the left end (0 mm) was defined as “the worst condition,” and the right end (100 mm) as “the best condition.” Subjects expressed their feelings for the following symptom items: 1) eye dryness; 2) rough feeling of the eyes; 3) pain in the eyes; 4) blurred eyes; 5) eye fatigue; 6) stiff shoulders; 7) headache; 8) dimmed eyes; 9) clearness of vision; 10) inflamed eyes; and 11) physical fatigue. The VAS assessment was conducted before and after the VDT load.
The DEQS is a comprehensive QOL questionnaire showing impairment levels calculated from the response of six items of eye conditions and nine items of the effect on ordinary life. Subjects are required to answer to 15 questions, items 1–6 are related to Bothersome Ocular Symptoms, while items 7–15 are related to the Impact on Daily Life, by selecting a frequency grade from 0 to 4 (0 is never to 4 is always). The fifteen questions are the following: 1) grittiness (sensation of something in your eyes); 2) dry eyes; 3) sore eyes; 4) tired eyes; 5) heavy eyelids; 6) red eyes; 7) difficulty keeping eyes open (due to symptoms); 8) vision became blurry when engaged in activities that required sustained visual attention (e.g., computer work, reading, knitting, etc.); 9) light was too bright; 10) eye symptoms worsened when reading newspapers, magazines, or books; 11) eye symptoms worsened when watching TV or when using a computer/mobile phone; 12) eye symptoms reduced the ability to concentrate; 13) eye symptoms interfered with work, housework, or studying; 14) tended to avoid leaving the house because of eye symptoms; and 15) fell down due to eye symptoms. The DEQS was implemented before the VDT load.
2.6.3. Safety evaluation
Height, body weight, body mass index (BMI), and body fat percentage were measured in each participant. The height was measured using the manual stadiometer (TSUTSUMI, Tokyo, Japan) once before intake. The body weight and fat percentage were evaluated using TANITA digital health meter HD-654 (TANITA Co., Tokyo, Japan). The BMI was calculated from weight (kg) divided by the body height (m) squared. In addition, all measurements were implemented at ORTHOMEDICO Inc. (Tokyo, Japan), and the ophthalmological examination was conducted by the visual acuity test and tonometry.
2.7. Statistical analysis
All tests in this study were two sided, and the significance level was set at 5% with no adjustment for multiple comparisons. The data analysis was performed using the Windows SPSS Version 23.0 (IBM Japan, Ltd., Tokyo, Japan). The data were analyzed using ANCOVA in Schirmer's test at 4 weeks after ingestion and used Schirmer's test before ingestion as covariates. Furthermore, all data are expressed as mean and SD. Regarding secondary outcomes, the VAS and DEQS questionnaire were analyzed using the Mann–Whitney U test, and the data revealed the median and interquartile range.
3. Results
3.1. Participants
Supplementary Figure 1 shows the study flowchart and participant disposition. As all participants in this study had an ingestion rate >90%, the full analysis was set at 37 participants (9 male and 28 female; age: 45.2 ± 7.1 years) in the MB group and 37 participants (7 male and 30 female; age: 44.5 ± 7.8 years) in the P group. Supplementary Table 1 summarizes participants' demographic characteristics.
3.2. Schirmer's test
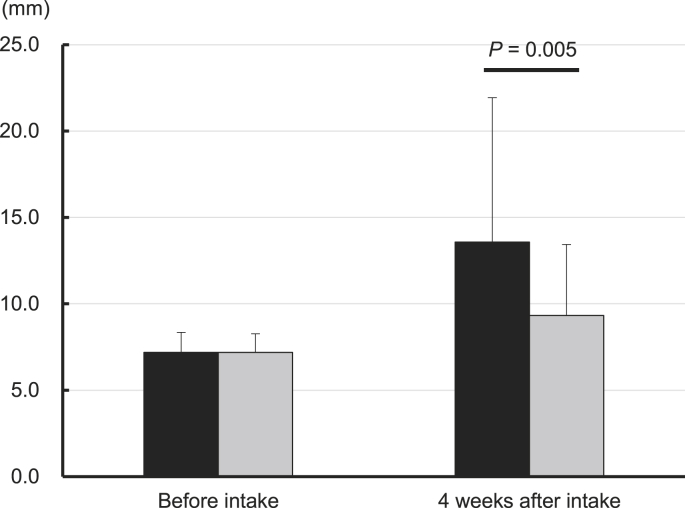
Supplementary Table 2 summarizes the results of Schirmer's test, and Fig. 1 displays the mean tear fluid average in both eyes in the MB and P groups before the VDT load. There were no significant differences between the MB and P group before intake, thus indicating intergroup homogeneity of the subjects at starting point. The MB group exhibited a significantly higher tear fluid generation compared to the P group before the VDT load at 4 weeks after intake in the left eye from 7.2 ± 1.8 mm to 14.3 ± 9.1 mm (P = 0.001), in the average of both eyes from 7.2 ± 1.2 mm to 13.6 ± 8.4 mm (P = 0.005), in the dominant eye from 7.2 ± 1.8 mm to 13.6 ± 8.9 mm (P = 0.022), and in the non-dominant eye from 7.2 ± 1.6 mm to 13.5 ± 8.5 mm (P = 0.003). Moreover, the MB group presented a significantly higher tear fluid production than the P group, before the VDT load on the change in the left eye [7.1 ± 8.8 mm (P = 0.001)], in the average of both eyes [6.4 ± 8.1 mm (P = 0.005)], in the dominant eye [6.4 ± 8.8 mm (P = 0.022)], and in the non-dominant eye [6.4 ± 8.2 mm (P = 0.003)]. After the VDT load, the MB group showed a significant increase of tear fluid in the left eye and non-dominant eye only, and 4 weeks after intake in the left eye from 9.9 ± 6.5 mm to 14.3 ± 8.5 mm (variation, 4.4 ± 8.5 mm; P = 0.032) and in the non-dominant eye from 9.4 ± 6.2 mm to 13.8 ± 7.9 mm (variation, 4.4 ± 7.9 mm; P = 0.035).
Fig. 1.
The average tear fluid variation of the MB and P groups measured in mm/5 min in both eyes with the Schirmer's test before the VDT load. The bar graph displays the mean value and SD, comparing the MB group (black bar) with the P group (gray bar) before intake and at 4 weeks after intake. Tear fluid of >15 mm/5 min is in the normal range and >10 mm/5 min is mild eye dryness. The data analyses were performed using ANCOVA, and change was assessed before intake.
3.3. BUT test, pupillary response, and flicker test
The BUT test, pupillary response, and Flicker test revealed no significant differences between the MB and P groups (Supplementary Tables 3–5, respectively).
3.4. Subjective symptoms
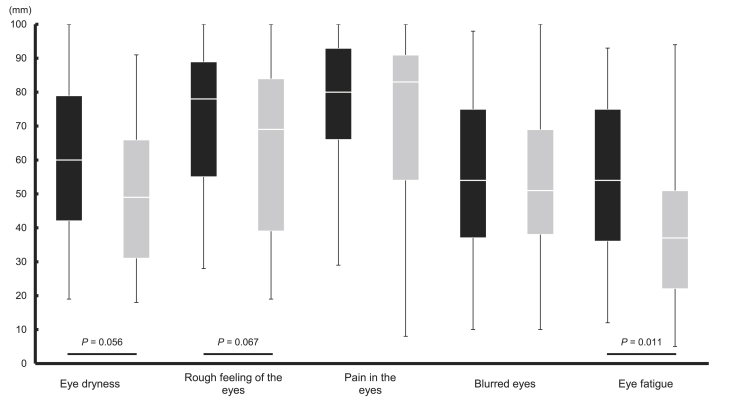
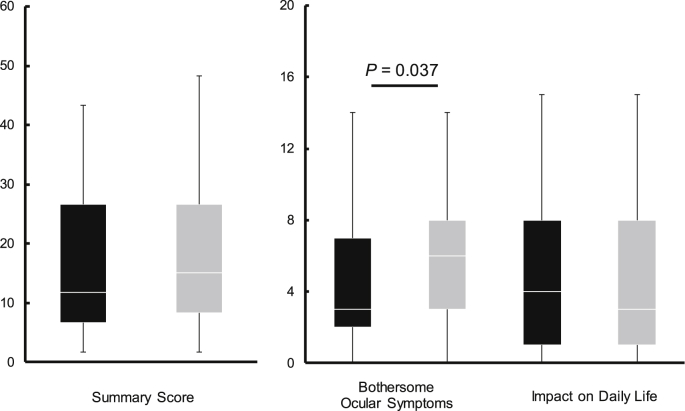
The VAS and DEQS questionnaire were used to assess subjective symptoms related to the eye (Supplementary Tables 6 and 7, respectively). Fig. 2, Fig. 3 show the VAS scores related to the eyes and the DEQS scores at 4 weeks after intake, respectively. No significant differences were observed between the MB and P groups before intake in the VAS and DEQS questionnaire revealing equivalent subjective symptoms among the subjects in both groups at the beginning of the trial.
Fig. 2.
The VAS scores of the MB and P groups related to subjective eye dryness before the VDT load at 4 weeks after intake. The box plot is shown in quartiles comparing the results of the MB group (black box) with the P group (gray box) a higher score indicates improved symptoms. The data analyses were performed using the Mann–Whitney U test.
Fig. 3.
The DEQS scores of the MB and P groups at 4 weeks after intake. The box plot is shown in quartiles comparing the MB group (black box) with the P group (gray box), a lower score indicates improved symptoms. The data analyses were performed using the Mann–Whitney U-test.
In the VAS method, the MB group exhibited a significant improvement before the VDT load at 4 weeks after intake for symptoms of eye fatigue increasing from 27.0 mm to 54.0 mm (P = 0.011) and of stiff shoulders from 33.0 mm to 75.0 mm (P < 0.001). After the VDT load, the MB group demonstrated a notable increase in VAS scores at 4 weeks after intake reducing symptoms of eye fatigue, from 23.0 mm to 41.0 mm (P = 0.047) and of stiff shoulders from 32.0 mm to 49.0 mm (P = 0.035). Additionally, another subjective symptom assessment, the results of the DEQS questionnaire, showed a significantly lower value for bothersome ocular symptoms in the MB group in comparison to the P group, decreasing scores from 7.0 to 3.0 (P = 0.037).
3.5. Safety evaluation
Table 1 shows the results of the physical and ophthalmology examinations of the subjects in this study. No significant intergroup differences between the MB and P groups were observed before intake. The MB group had significantly lower values in the intraocular pressure in the right eye than that in the P group (P = 0.035) at 4 weeks after intake.
Table 1.
Safety evaluations results of the MB and P groups before intake and four weeks after intake.
| Item | Unit | Group | Before intake | 4 weeks after intake |
|---|---|---|---|---|
| Weight | kg | MB group (n = 37) | 57.5 ± 9.2 | 57.3 ± 9.0 |
| P group (n = 37) | 56.4 ± 8.7 | 56.2 ± 8.8 | ||
| BMI | kg/m2 | MB group (n = 37) | 22.1 ± 2.6 | 22.0 ± 2.4 |
| P group (n = 37) | 21.7 ± 2.5 | 21.6 ± 2.6 | ||
| Body fat percentage | % | MB group (n = 37) | 26.5 ± 6.5 | 25.7 ± 6.4 |
| P group (n = 37) | 25.5 ± 7.2 | 25.1 ± 7.1 | ||
| Visual acuity (Right eye) | – | MB group (n = 37) | 0.470 ± 0.436 | 0.507 ± 0.485 |
| P group (n = 37) | 0.659 ± 0.483 | 0.741 ± 0.534 | ||
| Visual acuity (Left eye) | – | MB group (n = 37) | 0.468 ± 0.436 | 0.509 ± 0.538 |
| P group (n = 37) | 0.683 ± 0.529 | 0.727 ± 0.521 | ||
| Visual acuity (Average of both eyes) | – | MB group (n = 37) | 0.469 ± 0.429 | 0.508 ± 0.505 |
| P group (n = 37) | 0.671 ± 0.499 | 0.734 ± 0.518 | ||
| Intraocular pressure (Right eye) | mmHg | MB group (n = 37) | 14.9 ± 2.8 | 12.9 ± 2.8∗ |
| P group (n = 37) | 14.4 ± 3.4 | 13.7 ± 3.1 | ||
| Intraocular pressure (Left eye) | mmHg | MB group (n = 37) | 14.1 ± 2.9 | 13.2 ± 3.0 |
| P group (n = 37) | 14.5 ± 3.4 | 13.4 ± 3.0 | ||
| Intraocular pressure (Average of both eyes) | mmHg | MB group (n = 37) | 14.5 ± 2.6 | 13.1 ± 2.8 |
| P group (n = 37) | 14.4 ± 3.3 | 13.5 ± 2.9 |
Mean values and SD are presented. ∗P < 0.05 vs. P group.
4. Discussion
In this study, the consumption of a standardized maqui berry extract, MaquiBright®, also noted BrightSight®, resulted in an increase in the lacrimal fluid production. Several studies have demonstrated that the consumption of MaquiBright® improves the lacrimation capacity in murine models17, 27 and humans.18 Nakamura et al. reported that the oral administration of a maqui berry extract prevented a decline in the lacrimation secretion resulting from dry air and partial damage to the cornea and lacrimal gland tissue in vitro.17 Moreover, Hitoe et al. suggested that the intake of both 30 and 60 mg/day of MaquiBright® considerably enhanced the amount of lacrimal fluid after 4 and 8 weeks in humans.18 It can be concluded that an increase in lacrimal fluid might be stimulated by delphinidin-3,5-O-diglucoside in MaquiBright®,28 a compound known to inhibit the production of ROS in the lacrimal gland tissue and known to suppress corneal damage as well as lacrimal gland tissue dysfunction, thereby preventing eye dryness.17 The power analysis revealed that 5 mm/5 min in Schirmer's test was the condition of a significant difference in this study. Hence, the increase in average in both eyes before the VDT load in the Schirmer's test implies a clinically significant improvement in the lacrimal fluid production. Compared with the P group, however, the differences in the left and non-dominant eyes after the VDT load were <5 mm increase, which was not a clinically significant improvement. Although the consumption of MaquiBright® enhanced the lacrimal fluid production, its effect on lacrimal fluid was very small regarding the VDT load.
On the basis of the DEQS in this study, MaquiBright® intake alleviated subjective symptoms associated with eye dryness. A previous study has validated the reliability, validity, specificity, and reproducibility of the DEQS questionnaire for evaluating subjective symptoms.26 Hitoe et al. investigated the effects of MaquiBright® by assessing subjective eye dryness with the DEQS questionnaire, demonstrating that both 30 and 60 mg/day intake of MaquiBright® improved the QOL for all eye conditions.18 In this study, the DEQS results are consistent with those of the previous MaquiBright® pilot trial and demonstrated improvement in bothersome ocular symptoms. In addition, the VAS responses revealed a reduction in subjective symptoms associated with eye fatigue and stiff shoulders, demonstrating that MaquiBright® alleviated eye fatigue and its related symptoms caused by the VDT load. Furthermore, alleviations of eye fatigue and stiff shoulders were confirmed even after the VDT load. Assumedly, MaquiBright® might reduce accumulated fatigue from the VDT load. The rise in the lacrimation secretion observed by the consumption of MaquiBright® probably reduced general subjective symptoms associated with eye discomfort.
Previous studies investigating the onset mechanism of dry eyes have attributed the condition not only to lacrimal dysfunction but also to external factors such as a decrease in blinking, VDT use, and several other factors.29, 30, 31, 32 Apparently, most VDT light sources are light-emitting diodes that result in an exposure of excessive blue light on photoreceptor cells; blue light is known to induce a time-dependent production of ROS.33,34 Thus, an increase in ROS causes inflammation in corneal epithelial cells, which, in turn, decreases the stability of the tear film layer.35 Some animal studies have confirmed a reduction of blinking frequency in animals with dry eyes, further augmenting the ROS production13,27 and the presence of ROS in the tear film. Hence, the consumption of MaquiBright®, a botanical extract with antioxidant properties, causes (a) an increase in the amount of lacrimal fluid, (b) a reduction of ROS formation in the lacrimal gland tissue, and (c) an alleviation of subjective symptoms of eye discomfort and fatigue.
Although the consumption of MaquiBright® increased the amount of lacrimal fluid based on the results to the Schirmer's test, no significant changes were observed in the BUT, accommodative function, and flicker tests. This lack of effect might have resulted from different types of eye dryness. Dry eyes are typically divided into two types, aqueous deficient and evaporative,36 and both differ in the underlying mechanism of the onset. Although participants in this study might not have had evaporative dry eyes, their Schirmer's test results revealed their lacrimal fluid quantity between 5 and 10 mm/5 min, which is still lower than normal (moderate eye dryness). Thus, the effect of MaquiBright® intake on the BUT time was not statistically significant. In addition, the pupillary accommodation near responses was accompanied by convergence. To correct image blur, the lens thickens to adjust the focus, and the pupillary diameter decreases to alleviate spherical and chromatic aberration; this cascade is related to the central nervous system, and central fatigue is suspected when the near responses do not occur smoothly. Reportedly, the red color of the flicker test is an effective indicator of central fatigue,37 and the smaller flicker test value indicates higher fatigue.23,24 Indeed, participants who experienced a reduction in eye fatigue demonstrated a decrease in the flicker value and the percentage of pupil constriction.38,39 These studies used the Handy Flicker HF-II38,39 to assess eye fatigue. In the present study, the result values were between 29 and 50 Hz, which is in the reference range based on the instruction in the Appendix, indicating normality. The lack of a significant effect on the percentage change of the pupil diameter, which is linked with the central nervous system, could be illustrated by the characteristics of study participants. Although we enrolled participants who were exposed to VDT equipment for ≥4 h/day, those experiencing eye dryness were prioritized for enrollment in this study. In addition, participants with eye fatigue might have mild centralized fatigue, but the central nervous system typically controls the adjustment of focus. Thus, there was no fluctuation in the flicker value. Research has established the effects of antioxidants intake on tears in patients with dry eyes. Reportedly, the break-up of the tear film layer is delayed after antioxidants intake, which enhances stability.40 Accommodative function tests and assessments of subjective symptoms have demonstrated that anthocyanins, which are enriched and standardized in MaquiBright®, can reduce eye fatigue.38,39 Therefore, further studies on MaquiBright® are needed to investigate the BUT and to increase the percentage of pupil constriction in participants experiencing excessive evaporative dry eye type or central fatigue.
Regarding the safety evaluation, although a significant decrease was measured in the intraocular pressure in the right eye in the MB group compared with that of the P group, the intraocular pressure was within the reference range (the Japanese Ophthalmological Society reference value of the normal intraocular pressure is 19.9–20.0 mmHg).41 In this study, the examinations revealed no medically significant changes related to MaquiBright®.
Taken together, the ingestion of 60 mg of MaquiBright® per day for 4 weeks increased the lacrimal fluid generation, diminished subjective symptoms of eye fatigue, and alleviated eye dryness. Although MaquiBright® did not exert a significant effect on accommodative function or flicker test results, it demonstrated a positive and significant effect on eye fatigue and stiff shoulders subjective symptoms. MaquiBright® may reduce eye fatigue. Thus, future studies are planned to investigate the impact of MaquiBright® on eye fatigue, the tear film layer, and central fatigue. Furthermore, a crossover trial is endeavored to select appropriate participants and eliminate individual differences.
5. Conclusions
This study deduces that the consumption of MaquiBright® (BrightSight®, 60 mg/day) for 4 weeks alleviated eye dryness and most likely relieved eye fatigue in 30- to 60-year-old healthy participants. Overall, MaquiBright® was found to be safe for consumption under the study conditions.
Conflicts of interest
The sponsors of the study, ORYZA OIL & FAT CHEMICAL CO., LTD., ANKLAM EXTRAKT GMBH, and MAQUI NEW LIFE S.A. entrusted ORTHOMEDICO Inc., which conducted the study. S.Y., N.S., K.Y., and S.I. all belong to ORTHOMEDICO Inc. T. Y. (MD) is the principal investigator and received the medical facility fee to conduct the study.
Contributors
Concerning authors’ contributions, S.Y. designed the study and wrote the initial draft of the manuscript. N.S. contributed to the analysis and interpretation of data, and assisted in the preparation of the manuscript. All other authors have participated to data collection and interpretation, and critically reviewed the manuscript. All authors approved the final version of the manuscript, and agree to be accountable for all aspects of the work, thus in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgment
We would like to thank ORYZA OIL & FAT CHEMICAL CO., LTD., ANKLAM EXTRAKT GMBH, and MAQUI NEW LIFE S.A. for their support in this study. The authors would also like to thank all the participants and staffs who cooperated in this study.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2018.11.001.
Contributor Information
Shin-ichiro Yamashita, Email: shin@orthomedico.jp.
Naoko Suzuki, Email: nao@orthomedico.jp.
Kazuo Yamamoto, Email: kazu@orthomedico.jp.
Shin-ichiro Iio, Email: shinichiro@orthomedico.jp.
Takahiro Yamada, Email: dr_yamada@orthomedico.jp.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Uchino M., Schaumberg D.A. Dry eye disease: impact on quality of life and vision. Curr Ophthalmol Rep. 2013;1(2):51–57. doi: 10.1007/s40135-013-0009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paschides C.A., Stefaniotou M., Papageorgiou J., Skourtis P., Psilas K. Ocular surface and environmental changes. Acta Ophthalmol Scand. 1998;76(1):74–77. doi: 10.1034/j.1600-0420.1998.760113.x. [DOI] [PubMed] [Google Scholar]
- 3.Uchino M., Schaumberg D.A., Dogru M. Prevalence of dry eye disease among Japanese visual display terminal users. Ophthalmology. 2008;115(11):1982–1988. doi: 10.1016/j.ophtha.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 4.Uchino M., Nishiwaki Y., Michikawa T. Prevalence and risk factors of dry eye disease in Japan: koumi study. Ophthalmology. 2011;118(12):2361–2367. doi: 10.1016/j.ophtha.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 5.Uchino M., Yokoi N., Uchino Y. Prevalence of dry eye disease and its risk factors in visual display terminal users: the Osaka study. Am J Ophthalmol. 2013;156(4):759–766. doi: 10.1016/j.ajo.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 6.Pouyeh B., Viteri E., Feuer W. Impact of ocular surface symptoms on quality of life in a United States veterans affairs population. Am J Ophthalmol. 2012;153(6):1061–1066. doi: 10.1016/j.ajo.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 7.Mizuno Y., Yamada M., Miyake Y. Association between clinical diagnostic tests and health-related quality of life surveys in patients with dry eye syndrome. Jpn J Ophthalmol. 2010;54(4):259–265. doi: 10.1007/s10384-010-0812-2. [DOI] [PubMed] [Google Scholar]
- 8.Li M.-Y., Gong L. Progress of research on quality of life of dry eye patients. Chin J Ophthalmol. 2011;47(2):185–188. [PubMed] [Google Scholar]
- 9.García-Catalán M.R., Jerez-Olivera E., Benítez-Del-Castillo-Sánchez J.M. Dry eye and quality of life. Arch Soc Esp Oftalmol. 2009;84(9):451–458. doi: 10.4321/s0365-66912009000900004. [DOI] [PubMed] [Google Scholar]
- 10.Friedman N.J. Impact of dry eye disease and treatment on quality of life. Curr Opin Ophthalmol. 2010;21:310–316. doi: 10.1097/ICU.0b013e32833a8c15. [DOI] [PubMed] [Google Scholar]
- 11.Miljanović B., Dana R., Sullivan D.A., Schaumberg D.A. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143(3):409–415. doi: 10.1016/j.ajo.2006.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada M., Mizuno Y., Shigeyasu Impact of dry eye on work productivity. Clin Outcomes Res. 2012;4:307–312. doi: 10.2147/CEOR.S36352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura S., Shibuya M., Nakashima H., Imagawa T., Uehara M., Tsubota K. D-β-hydroxybutyrate protects against corneal epithelial disorders in a rat dry eye model with jogging board. Investig Opthalmology Vis Sci. 2005;46(7):2379–2387. doi: 10.1167/iovs.04-1344. [DOI] [PubMed] [Google Scholar]
- 14.Foulks G.N. The correlation between the tear film lipid layer and dry eye disease. Surv Ophthalmol. 2007;52(4):369–374. doi: 10.1016/j.survophthal.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Misle E., Garrido E., Contardo H., González W. Maqui [Aristotelia chilensis (Mol.) Stuntz]-the amazing chilean tree: a review. J Agric Sci Technol B1. 2011:473–482. [Google Scholar]
- 16.Muñoz O., Christen P., Cretton S. Chemical study and anti-inflammatory, analgesic and antioxidant activities of the leaves of Aristotelia chilensis (Mol.) Stuntz, Elaeocarpaceae. J Pharm Pharmacol. 2011;63(6):849–859. doi: 10.1111/j.2042-7158.2011.01280.x. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura S., Tanaka J., Imada T., Shimoda H., Tsubota K. Delphinidin 3,5-O-diglucoside, a constituent of the maqui berry (Aristotelia chilensis) anthocyanin, restores tear secretion in a rat dry eye model. J Funct Foods. 2014;10:346–354. [Google Scholar]
- 18.Hitoe S., Tanaka J., Shimoda H. MaquiBright® standardized maqui berry extract significantly increases tear fluid production and ameliorates dry eye-related symptoms in a clinical pilot trial. Panminerva Med. 2014;56(3 suppl 1):1–6. [PubMed] [Google Scholar]
- 19.Schirmer O. Studien zur physiologie und pathologie der tranenabsonderung und tranenabfuhr (in German) Arch Klin Exp Ophthalmol. 1903;56:197–291. [Google Scholar]
- 20.Lemp M.A. Breakup of the tear film. Int Ophthalmol Clin. 1973;13(1):97–102. doi: 10.1097/00004397-197301310-00008. [DOI] [PubMed] [Google Scholar]
- 21.Lemp M.A., Hamill J.R. Factors affecting tear film breakup in normal eyes. Arch Ophthalmol. 1973;89(2):103–105. doi: 10.1001/archopht.1973.01000040105007. [DOI] [PubMed] [Google Scholar]
- 22.Ito M., Nakamura T., Yoshida Y. Utility of TriIRIS C9000® in diagnosis and treatment for patients with asthenopia. JAPANESE Orthopt J. 2007;36:73–80. [Google Scholar]
- 23.National Institute of Vocational Rehabilitation . 1993. Research Reports and Material Series No.7: Basic Research on Aging and Fatigue of Handicapped. [Google Scholar]
- 24.Nishimura T., Morimoto K., Kishimoto T., Nii M. Correlation between subjective and objective rating of fatigue in VDT operations. J Inst Telev Eng Japan. 1986;40(12):1239–1244. [Google Scholar]
- 25.Hayes M., Paterson D. Experimental development of the graphic rating method. Psychol Bull. 1921;18:98–99. [Google Scholar]
- 26.Sakane Y., Yamaguchi M., Yokoi N. Development and validation of the dry eye–related quality-of-life score questionnaire. JAMA Ophthalmol. 2013;131(10):1331–1338. doi: 10.1001/jamaophthalmol.2013.4503. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura S., Shibuya M., Nakashima H. Involvement of oxidative stress on corneal epithelial alterations in a blink-suppressed dry eye. Investig Opthalmology Vis Sci. 2007;48(4):1552–1558. doi: 10.1167/iovs.06-1027. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka J., Kadekaru T., Ogawa K., Hitoe S., Shimoda H., Hara H. Maqui berry (Aristotelia chilensis) and the constituent delphinidin glycoside inhibit photoreceptor cell death induced by visible light. Food Chem. 2013;139(1-4):129–137. doi: 10.1016/j.foodchem.2013.01.036. [DOI] [PubMed] [Google Scholar]
- 29.Tsubota K., Nakamori K. Dry eyes and video display terminals. N Engl J Med. 1993;328(8):584. doi: 10.1056/NEJM199302253280817. [DOI] [PubMed] [Google Scholar]
- 30.Tsubota K., Toda I., Nakamori K. Poor illumination, VDTs, and desiccated eyes. Lancet. 1996;347:768–769. doi: 10.1016/s0140-6736(96)90122-1. [DOI] [PubMed] [Google Scholar]
- 31.Schlote T., Kadner G., Freudenthaler N. Marked reduction and distinct patterns of eye blinking in patients with moderately dry eyes during video display terminal use. Graefe’s Arch Clin Exp Ophthalmol. 2004;242(4):306–312. doi: 10.1007/s00417-003-0845-z. [DOI] [PubMed] [Google Scholar]
- 32.Wolkoff P., Nøjgaard J.K., Troiano P., Piccoli B. Eye complaints in the office environment: precorneal tear film integrity influenced by eye blinking efficiency. Occup Environ Med. 2005;62(1):4–12. doi: 10.1136/oem.2004.016030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tosini G., Ferguson I., Tsubota K. Effects of blue light on the circadian system and eye physiology. Mol Vis. 2016;22(August 2015):61–72. [PMC free article] [PubMed] [Google Scholar]
- 34.Kuse Y., Ogawa K., Tsuruma K., Shimazawa M., Hara H. Damage of photoreceptor-derived cells in culture induced by light emitting diode-derived blue light. Sci Rep. 2014;4:5223. doi: 10.1038/srep05223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Augustin A., Spitznas M., Kaviani N. Oxidative reactions in the tear fluid of patients suffering from dry eyes. Graefes Arch Clin Exp Ophthalmol. 1995;233(11):694–698. doi: 10.1007/BF00164671. [DOI] [PubMed] [Google Scholar]
- 36.Lemp M.A., Crews L.A., Bron A.J., Foulks G.N., Sullivan B.D. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472–478. doi: 10.1097/ICO.0b013e318225415a. [DOI] [PubMed] [Google Scholar]
- 37.Suzumura A. Eyestrain (in Japanese) Jpn J Ophthalmol. 1981;23(8):799–804. [Google Scholar]
- 38.Ozawa Y., Kawashima M., Inoue S. Bilberry extract supplementation for preventing eye fatigue in video display terminal workers. J Nutr Heal Aging. 2015;19(5):548–554. doi: 10.1007/s12603-014-0573-6. [DOI] [PubMed] [Google Scholar]
- 39.Nakata A., Yamashita S., Suzuki N., Liang T., Yang J., Yamada T. The improvement effect of bilberry extract (BILBERON®) –containing diet on eye fatigue and eye dryness -a randomized, double-blind, placebo-controlled parallel-group comparison study. Jpn Pharmacol Ther. 2016;44(12):1773–1783. [Google Scholar]
- 40.Drouault-Holowacz S., Bieuvelet S., Burckel A. Antioxidants intake and dry eye syndrome: a crossover, placebo-controlled, randomized trial. Eur J Ophthalmol. 2009;19(3):337–342. doi: 10.1177/112067210901900302. [DOI] [PubMed] [Google Scholar]
- 41.The Japan Glaucoma Society . third ed. 2011. Glaucome Clinical Practice Guideline. (in Japanese) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.