Abstract
The growing interest in the alterations of tumor cell metabolism and their possible therapeutic exploitation also spurred new complementary and integrative approaches such as treating patients with a ketogenic diet (KD). KDs aim at inhibiting glycolytic tumor metabolism and growth, and have therefore been proposed as adjuncts not only to standard-of-care, but also to other therapies targeting tumor metabolism. Here I describe the life and forgotten work of one of the earliest researchers who realized the importance of altered tumor cell metabolism and its possible exploitation through metabolic modifications: Wilhelm Brünings. Brünings was a German natural scientist and physician famous for his innovative contributions to the fields of physiology and otorhinolaryngology. Based on the findings of Otto Warburg and his physiological reasoning he started to experiment with insulin administration and KDs in his patients with head and neck cancers, aiming to maximally lower blood glucose concentrations. He obtained encouraging short-term results, although most tumors became refractory to treatment after several weeks. His pioneering work is worth revisiting, especially for an international readership that may be unaware of his efforts, as hypoglycemic treatments, including the use of insulin injections and KDs, are currently being re-investigated as complementary and integrative cancer treatments.
Keywords: Cancer, Hypoglycemia, Insulin, Ketogenic diet, Low carbohydrate diet
Graphical abstract
1. Introduction
There is an increasing interest in the connection between metabolism and cancer and its potential for therapeutic interventions.1, 2, 3, 4 Among these are several approaches targeting the glucose metabolism of tumors such as the application of ketogenic diets (KDs) to cancer patients. KDs are very low carbohydrate, high fat diets that mimic certain characteristics of fasting, in particular through reduction of plasma insulin with an accompanying elevation of the ketone bodies acetoacetate and β-hydroxybutyrate. The state of nutritional ketosis in adults is typically characterized by concentrations of β-hydroxybutyrate in the range 0.5–3 mmol/l5 and glucose in the range 85–95 mg/dl.6 However, with concurrent calorie restriction much lower blood glucose concentrations (55–80 mg/dl7) can be achieved, resulting in a higher ketone-to-glucose ratio. This may be desirable for an optimal chance of disease management,1 although in some animal models unrestricted KDs led to tumor growth retardation without lowering blood glucose levels.8,9
First clinical pilot studies on the KD and cancer have been conducted,7,10, 11, 12, 13, 14 and several others are planned or currently running.15, 16, 17 These studies are based on mainly three rationales.18 First, KDs have been shown to be capable of slowing down tumor growth in many, although not all, animal models.18,19 Second, the replacement of carbohydrates with fat that is characteristic for a KD is supposed to account for the increased fat oxidation rates in cancer patients that are due to tumor-induced insulin resistance, in this way possibly protecting against skeletal muscle loss.20, 21, 22 Third, KDs are expected to sensitize tumor cells to radio- and chemotherapy, principally opening up a very broad range of applications as complementary cancer treatments. This at least partly relates to their ability to impair tumor cell glycolysis, reducing ATP levels and the ability to utilize substrates of glycolytic metabolism for protection against reactive oxygen species.23, 24, 25
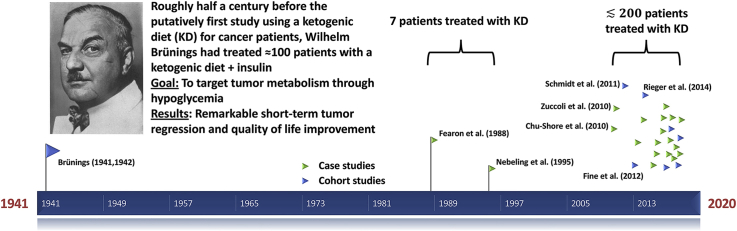
From a historical point of view it is interesting that the major discoveries underlying the above rationales for KDs in cancer treatment were made approximately 100 years ago. Otto Warburg and coworkers performed systematic investigations of the high rates of fermentation of glucose to lactate in tumor versus normal tissues in the 1920s.26, 27, 28 This is nowadays referred to as the Warburg effect and routinely employed via FDG-PET (2-deoxy-2-[18F]fluoro-d-glucose positron emission tomography) imaging for clinical tumor assessment. While first preclinical studies into the effect of different forms of diet or macronutrient manipulation on cancer growth have roughly paralleled the early studies on cancer and metabolism,29, 30, 31 the first clinical application of a KD to cancer patients is usually assumed to be much more recent, and usually attributed to Kenneth Fearon and co-workers who described the administration of a medium chain triglyceride-rich KD to five cachectic cancer patients in 1988.32 Others mention the case study of two pediatric brain tumor patients by Nebeling et al.33,34 from 1995 as the first clinical KD study (for example Hyde et al.,35 Poff et al.36 or we ourselves in our 2011 review22). Nevertheless, as I describe in the remainder of this paper, both assumptions are not correct: The first clinical study which included the description of a KD to treat cancer patients was published in 1941 by Wilhelm Brünings, a scientist and physician whose obituary has its 80th anniversary this year. His forgotten experiments are worth re-discovery and review for the international cancer and metabolism research community.
2. A brief review on the life and achievements of Wilhelm Brünings
Wilhelm Brünings was born on January 31st 1876 in Kustedt near Stade, Germany. He studied natural sciences, philosophy and medicine in Tubingen and later in Erlangen where he received his PhD in physics, chemistry and zoology in 1899. Afterwards, he turned to clinical research in Berlin and Tubingen, where he received his medical doctorate in 1901. After a round-the-world trip as a ship's doctor, Brunings joined the department of physiology at the university of Zurich, Switzerland, where he published several papers on electrophysiology37, 38, 39, 40 and instrumentation41 and habilitated in 1904. His shift towards otorhinolaryngology was made when external reasons led him to Freiburg where he became the assistant of the famous laryngologist Gustav Kilian and earned the venia legendi for this field in 1908.42 After World War I in which he served as a military doctor he became full professor and head of the university department for otorhinolaryngology first in Greifswald (1917), then Jena (1926) and finally Munich (1930).
Contemporaries described Brünings as an excellent lecturer, teacher, scientist, physician and mechanic.42,43 His deep knowledge of the natural sciences combined with great technical and manual skills would have allowed him an efficient and unusually multidimensional way to approach and solve scientific problems. Indeed, his research spanned a diverse range of topics including cell electricity, X-ray diagnostics, radium treatment for cancer and, most importantly, instrumentation. Brünings' endoscopic instruments became world-famous and developed widespread utilization.44 Among all these diverse activities, his passion was active patient care. When the Prussian Ministry of Culture offered Brünings his own Kaiser-Wilhelm research institute in Berlin after he had given a lecture on electroacoustic methods for treating amblyacousia, he declined because he preferred to continue treatment of patients.42
Until his death on October 3rd 1958 Brünings remained in Munich where he was full professor of otorhinolaryngology until his retirement in 1950 (Fig. 1).
Fig. 1.
Wilhelm Brünings as shown in a laudatory article by A. Greifenstein on the occasion of his 60th birthday celebration which was published in the journal Archiv für Ohren-, Nasen-und Kehlkopfheilkunde (now the European Archives of Oto-Rhino-Laryngology).42
It is against this background that we are going to examine Brünings' Beiträge zum Krebsproblem (“contributions to the cancer problem”, Fig. 2), which include what is likely the first detailed description of a very low carb (ketogenic) diet to treat cancer patients – almost half of a century prior to the clinical study of Fearon et al.32 that is usually cited as the first KD study on cancer patients.
Fig. 2.
An excerpt from Brünings’ first report.45
3. Brünings' ketogenic diet studies
In the years 1941 and 1942, Brünings published two reports and a separate conclusion paper in the journal Münchener Medizinische Wochenschrift describing his experimental treatment of head and neck cancer patients in his Munich clinic by using insulin together with a KD in order to maximally lower blood glucose levels.45, 46, 47 Having spent years studying parallels in the age-dependency, incidence rates and heritability among diabetes, hypertension and cancer, he developed a working hypothesis that there would likely also be a commonality concerning the etiology of these diseases. Combined with Warburg's findings this commonality would strongly implicate a disturbance of carbohydrate metabolism as a general factor necessary for cancer development. In the first report, titled “Ueber eine diätetisch-hormonale Beeinflussung des Krebses” (“On a dietetic-hormonal manipulation of cancer”), Brünings first described the case of a diabetic patient with a maxillary tumor who was referred to dietetic and insulin therapy for several weeks prior to planned surgery. During surgery, Brünings was completely surprised to find that large parts of the tumor had regressed and the remainder transformed into a viscous-elastic tissue.45 This observation prompted him to quickly initiate some experiments that he had apparently planned for a long time, yet was reluctant to start due to the circumstances of the war:
Die Versuche begannen zunächst mit Normalkost bei vorsichtig steigenden Insulindosen. In einer zweiten Reihe wurde lediglich Kh.-freie Ernährung ohne Insulin versucht. Es folgte eine Kh.-arme Diät (ca. 50 g oder 4 WBE Kohlehydrat) mit Steigerung des Insulins bis zur Toleranzgrenze und schließlich, als schärfste Form der Zuckerentziehung, eine praktisch kohlehydratfreie Ernährung mit gleichzeitig maximalen Insulingaben. Eine Beschreibung der ersten drei Behandlungsformen, ihrer Wirkungen und Ergebnisse ist unnötig, da ich mich schon nach kurzer Zeit auf die letzte, schärfste Form beschränkt habe, die sich als die wirksamste erwies.45, p. 119
(The experiments began with a normal diet and carefully increasing doses of insulin. In a second trial only a carbohydrate-free diet without insulin was tried. Subsequently, a low carbohydrate diet with increasing insulin doses until the tolerance limit and finally, as the strictest form of glucose deprivation, a practically carbohydrate-free diet with maximally tolerable doses of insulin was applied. A description of the results of the first three trials is unnecessary since after a short time of experimentation, I decided to restrict the trials to the strictest form which turned out to be the most effective)
Depot insulin was administered three times daily (at 7 a.m., 2 and 9 p.m.) by a nurse, starting with 0.3 cm3 per injection and increasing by 0.1 cm3 daily until signs of hypoglycemia appeared at which point the dose would be maintained. This usually occurred up to the fourth day.
Concerning the diet and its feasibility, Brünings wrote
Auch hat meine erfindungsreiche Küchenleiterin es verstanden, den Kranken aus Fleisch, Wurst, Fisch, Eiern, Fetten, Käse, Quark, Kh.-armen Koch-und Rohgemüsen, Sauerfrüchten, Nüssen, Sionon usw. eine abwechslungsreiche Kost herzustellen, gegen deren monatelange Verabreichung sich keiner von ihnen gesträubt hat.45, p.119
(My inventive kitchen leader figured out how to create a varied diet out of meat, sausages, fish, eggs, fat, cheese, curd, low-carbohydrate cooked, raw and fermented vegetables, nuts, Sionona etc. that none of the patients was reluctant to eat for several months.)b
Brünings pointed out the importance of consuming large amounts of vegetables as a source of fiber and protection against acidosis. The diet was supplemented with twice-weekly vitamin C injections and two drops of vitamin D oil daily (with the argument that patients would have to stay in their rooms during the low-light winter). He also pointed out that he and his staff had never seen a subjective or objective deterioration of any patient caused by the combined insulin and “carbohydrate-free” diet treatment that he referred to as Entzuckerungsmethode (“de-glycation method”).
His first report presents 14 cases with no further curative treatment options and tumors that could easily be measured by eye. Of these, 6 (43%) achieved a moderate remission, 7 (50%) an extensive remission and 1 (7%) a macroscopic complete remission.45 In addition, there was a general improvement in the physical and psychological conditions of the patients as early as 8–10 days after therapy initiation. However, while the rate of remission appeared to peak at about 2–3 weeks, there also was a rebound effect after 2–3 months of which Brünings was apparently very disappointed. He was neither able to explain the fast regression nor the late-onset regrowth, but his observations allowed him to rule out several theories. In particular, he mentioned that:
Die naheliegende, primitive Vorstellung, daß der Krebs zu seinem Wachstum „Zucker braucht“ und durch dessen Entziehung „ausgehungert werden kann“, erwies sich von vornherein als unzureichend.45, p. 122
(The obvious, primitive belief that cancer “needs glucose” for its growth and can be “starved” through its deprivation turned out as insufficient right from the beginning.)
He was also able to conclude that neither insulin nor the KD alone could be responsible for the observed effects – only their combination was sufficient for efficacy.
His second report “Klinische Anwendungen der diätetisch-hormonalen Krebsbeeinflussung (‘Entzuckerungsmethode’)”46 contained more practical details on the insulin dosing and diet.c Brünings was eager to emphasize that even though the circumstances of the war made the provision of adequate foods difficult, one should not make compromises in the diet concerning its carbohydrate limitation and caloric adequacy – it would be better to limit the number of applications of the method. The second report contained another 30 cases of patients with various tumors that Brünings sampled from a larger collective of roughly 100 cases he had treated. The increased sample size had also increased the variance in the observed effects, with a larger percentage of tumors being refractory to the treatment. This, together with the rebound effect in those tumors that had responded, had apparently shifted Brünings' focus from a potentially curative intent to the general management of clinical symptoms that – contrary to the response of the tumor – had shown improvements in every single patient within a few days. Therefore, Brünings concluded his reports with the following suggestions for using his de-glycation method47:
-
1.
Prior to surgery in order to improve post-surgery prognosis.
-
2.
As a means to diagnose occult cancer based on the positive response of a patients' general condition to the insulin and KD treatment – known as diagnosis ex juvantibus.d
-
3.
As palliative treatment of desperate cancer cases.
-
4.
As a supportive treatment during radiotherapy or to make a patient eligible for radiotherapy in the first place.e
The suggested treatment duration was 5–7 days for the applications 1 and 2 and up to 14 days for the applications 3 and 4. Finally, given the simplicity of the approach, Brünings closed with the proposition to adopt his method for general clinical usage and further research.47
4. Discussion
The above summary shows that the idea to alter cancer patients' metabolism through a very low carbohydrate (ketogenic) diet is much older than usually acknowledged. The aim of this review was to reveal, 80 years after his death, Brünings' forgotten ingenious and original experiments to an international audience, especially given the recent renewed interest in metabolic therapies against cancer.1,3,4
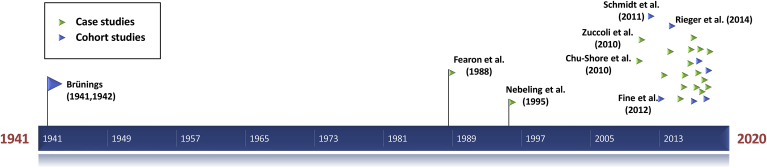
A list of relevant KD studies published in peer-reviewed journals is given in Table 1, and Fig. 3 shows their chronological appearance relating to Brünings’ publications.7,10, 11, 12, 13, 14,32,34,48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65 It took almost half a century after Brünings’ first publication until what is generally considered the first clinical study treating cancer patients with a KD was published (Fearon et al. 198832), and 70 years until the publication of a study with a comparable number of cancer patients (Schmidt et al. 201150). While Brünings’ diet was based on whole foods with an emphasis on vegetables and animal products, the studies that followed were apparently influenced by the application of KDs against epileptic seizures where such diets typically consisted “predominantly of heavy cream and butter fat” so that the “[u]se of vitamin/mineral supplements is essential to maintain nutrient adequacy in the ketogenic diet.”33 From the beginning, however, it was the goal to make the KDs for cancer patients more flexible by using ketogenic medium-chain triglycerides, and later allowing for a much larger percentage of whole foods and vegetables in so-called modified KDs. Together with other recent approaches such as the Paleolithic KD58,62,65 at least part of the research community therefore seems to appraise KDs not as per se unhealthy, but as an holistic and from an evolutionary perspective natural approach to dieting, much in line with Brünings' experiences.f
Table 1.
Human studies on the ketogenic diet and cancer published since Brünings' initial experiments. The list should contain all articles listed in PubMed upon a search using the keyword “ketogenic”. Only studies published as peer-reviewed journal articles have been listed, while those mentioned solely in abstracts, comments or posters have been left out. In case of more than two study participants, their ages are given either as median (range) or mean ± standard deviation. CT: Chemotherapy; MCT: Medium chain triglycerides; N: Number of subjects on a KD; RCT: Radio-chemotherapy; RT: Radiotherapy.
| Study author(s) | Year | Study type | N | Age [years] | Tumor | Concurrent treatment | KD/study duration | Concurrent calorie restriction prescribed | Supplements/artificial foods prescribed |
|---|---|---|---|---|---|---|---|---|---|
| Brünings45 | 1941 | Intra-cohort study | 14 | 56 (42–68) | Head and neck cancer | Insulin | ≈12 weeks | No | No |
| Brünings46 | 1942 | Intra-cohort study | 30 | 55 (38–70) | Various extra-cranial tumors | Insulin | 1.6 (0.3–3.0) weeks | No | No |
| Fearon et al.32 | 1988 | Case study | 5 | 59 (52–73) | Stage IV extra-cranial tumors | None | 6 days control diet, then 7 days KD | No | Yes (MCT, arginine D-3-hydroxybutyrate, whey protein) |
| Nebeling et al.34 | 1995 | Case study | 2 | 3 and 8.5 | Grade III anaplastic/cerebellar astrozytoma | None/CT | 8 weeks | No | Yes (MCT, artificial flavorings, protein powder, multivitamins, vitamin D, multiminerals) |
| Chu-Shore et al.48 | 2010 | Case study | 5 | 8 (2–47) | Renal angiomyolipomas/subependymal giant cell tumors | None | 50 (3–66) months | No | Probably yes (hospital-prescribed KD with specified ratios) |
| Zuccoli et al.49 | 2010 | Case study | 1 | 65 | Glioblastoma | RCT | 2 months | Yes | Yes (4:1 KetoCal®, MCT, multivitamins, multiminerals) |
| Schmidt et al.50 | 2011 | Intra-cohort study | 16 | 50 (30–65) | Stage IV extra-cranial tumors | None | 12 weeks | No | Yes (yoghurt drinks including MCT, vegetable oils and milk protein) |
| Fine et al.10 | 2012 | Intra-cohort study (registered) | 10 | 62 (52–73) | Stage IV extra-cranial tumors | None | 4 weeks | No | No |
| Schroeder et al.51 | 2013 | Intra-cohort study | 11 | 67 | Stage II-IV head and neck cancer | None | 4 days | No | No |
| Champ et al.52 | 2014 | Case study | 6 | 59 (34–62) | Glioblastoma | RCT | 32 (13–52) weeks | No (5), yes (1) | No |
| Rieger et al.11 | 2014 | Intra-cohort study (registered) | 20 | 57 (30–72) | Glioblastoma | None | 5 weeks | No | Yes (commercial yoghurt drinks and plant oils) |
| Branca et al.53 | 2015 | Case study | 1 | 66 | Grade 3 breast cancer | Vitamin D3 (10000 IU every other day) | 3 weeks | No | Yes (commercial preparation of oleic acid associated with glycosylated vitamin D-binding protein, branched chain amino acids) |
| Schwartz et al.7 | 2015 | Case study (registered) | 2 | 55 and 52 | Glioblastoma | None | 4 and 12 weeks | Yes | Yes (KetoCal®) |
| Strowd et al.54 | 2015 | Case study | 8 | 41 ± 10 | Low and high grade glioma | None/CT | 2-24 (mean 13.2) months | No | Yes (multivitamins, vitamin D, calcium) |
| Jansen & Walach55 | 2016 | Case study | 13 | 68 | Various curative (6)/palliative (6)/end stage (1) tumors | Mixed | ≈1 year | No | Yes (commercial artificial foods) |
| Klement & Sweeney56 | 2016 | Case study | 6 | 54 (40–74) | Stage I-IV extra-cranial | RCT (5), CT (1) | 6.6 (4.6–10.4) weeks | No | Yes (commercial crystalline amino acid formula) |
| Schwalb et al.57 | 2016 | Case study | 6 | 64 (55–73) | Stage IV extra-cranial | Complementary immunotherapeutic treatment | 4.5 (1–34) weeks | No | Yes (commercial crystalline amino acid formula, fermented milk and colostrum product, emulsion with chondroitin sulfate, vitamin D3 and oleic acid, vitamin D3, curcumin, omega-3 fatty acids, ubiquinol, arginine, multivitamins) |
| Tan-Shalaby et al.12 | 2016 | Intra-cohort study (registered) | 17 | 65 (42–87) | Stage IV various tumors | None | 16 weeks (with extension up to 131 weeks in 3 patients) | No | No |
| Tóth & Clemens58 | 2016 | Case study | 1 | 60 | Grade 2 myoepithelial soft palate tumor | None | 20 months | No | No |
| Artzi et al.59 | 2017 | Controlled case study (registered) | 5 | 42 (37–69) | Low and high grade glioma | None (1), bevacizumab (4) | 8 (2–31) months | No | Yes (4:1 KetoCal®) |
| İyikesici et al.60 | 2017 | Case study | 1 | 29 | Stage IV triple-negative breast cancer | Metabolically (insulin-) supported CT, hyperbaric oxygen, hyperthermia | 1 year | No | No |
| Santos et al.61 | 2017 | Case study | 1 | 54 | Recurrent glioblastoma | Intranasal perillyl alcohol |
3 months | No | No |
| Tóth & Clemens62 | 2017 | Case study | 1 | 62 | Rectal cancer | RT (6 weeks), then none | 24 months | No | No |
| Zahra et al.13 | 2017 | Intra-cohort study | 9 | 67 (51–83) | Stage III/IV NSCLC (7) and pancreatic cancer (2) | RCT | 1.1 (0–6) weeks | No | Yes (4:1 KetoCal®, artificial flavorings) |
| Elsakka et al.63 | 2018 | Case study | 1 | 38 | Glioblastoma | RCT, hyperbaric oxygen, metformin | 24 months | Yes (9 months), then no | Yes (multivitamins, vitamin D, multiminerals, phytochemicals, MCT) |
| Martin-McGill et al.64 | 2018 | Intra-cohort study (registered) | 6 | 46 (34–66) | High grade glioma | Lomustine (1), RCT (3), CT (2) | 3 months | No | No |
| Santos et al.14 | 2018 | Controlled cohort study (registered) | 9 | 53 (31–61) | Recurrent glioblastoma | Intranasal perillyl alcohol |
3 months | No | No |
| Tóth et al.65 | 2018 | Case study | 1 | 45 | High-grade cervical intraepithelial neoplasia |
None | 26 months | No | No |
Fig. 3.
Timeline of studies using ketogenic diets to treat cancer patients. The early studies are labeled with author names and the year of publication.
While Brünings' experiments probably constitute the first clinical cohort study involving a detailed description of and focus on a KD, there exists one earlier report from 1927 by Fritz Silberstein and co-workers66 involving 21 non-operable cancer patients who were also treated with insulin injections (partly into the tumor) and a concurrent high caloric, very low carbohydrate diet.g Again, beneficial effects of the insulin on the general condition of the patients were noted, and some cases responded with a local regression of their tumors. Silberstein et al. also compared blood glucose curves between the cancer patients and normal subjects and found not only elevated fasting glucose concentrations in the former, but also an abnormal postprandial elevation after a very low carbohydrate meal,h even after prior insulin administration, similar to what would be expected in diabetics. Indeed, later studies have justified the belief that insulin resistance is a general hallmark of advanced cancer patients.67, 68, 69, 70, 71
After Brünings had published his second report, there was a huge media coverage which motivated others to try reproducing his experiments. Among them were Schulte and Schütz in 1942, who were able to reproduce the short-term beneficial effects of insulin on the general condition of their patients, but not to induce macroscopic tumor regression or longer-term improvements, so that they discouraged clinicians from using the method.72 However, in their paper no details of the “carbohydrate-free” diet are given, and it appears possible that – given the difficulties of obtaining the appropriate foods during the war as mentioned by Brünings – their diet was not as strict as demanded by Brünings. In 1957 Joseph Weiss published his results of treating 90 incurable cancer patients with depot insulin and a calorie restricted low carbohydrate diet containing 124 g carbohydrates, 66 g fat and 56 g protein per day.73 He again reported pleiotropic effects such as pain reduction, euphoria and weight gain; 20 patients achieved stable disease and 9 patients tumor regression which lasted several months, in one case up to 4 years. This work was not directly comparable to Brünings', as the carbohydrate content was considerably higher and would certainly not have induced ketosis.
It is not straightforward to interpret Brünings' results and those of the replication studies in hindsight. The insulin administrations appear to have been responsible for short-term improvements in the general condition of the patients such as improved appetite, euphoria and weight gain. However, concerning pain reduction, at least in mice insulin does not appear to have analgesic effects.74 What further complicates the interpretation is that insulin in former times used to be contaminated with other peptides including glucagon to varying degrees.i For example, Bishop and Marks explicitly mention the use of glucagon-free insulin in a 1959 study that also dealt with the dysregulated glucose metabolism and insulin resistance in cancer patients.67 Still in 1985, substantial amounts of glucagon were found in insulin from three commercial manufactures.75
There is also the possibility that the diet itself mediated the pain reduction described by Brünings. Indeed, reduction in glycolytic metabolism and KDs are known to have anti-inflammatory and analgesic properties.76 Brünings’ observations concerning the superiority of a very strict carbohydrate restricted diet over milder versions provide some evidence that the diet has indeed played an important role. Concerning Brünings' descriptions of the influence on the tumor, two possible interpretations arise:
First, KDs by themselves have been shown to impair glycolytic tumor metabolism in humans, as demonstrated specifically in head and neck cancer patients in which tumor lactate levels dropped significantly after only a few days on the diet.51 As discussed elsewhere this could be indicative of impaired growth and increased susceptibility to cytotoxic treatments.24,25,77 One case study with 20 months follow-up even reports the halted progression of a soft palate tumor through initiation of an animal fat and meat-based Paleolithic KD with no further therapy.58 An indication of the mechanisms at work can be derived from in vitro experiments showing that both ketone bodies78,79 and free fatty acids80 are able to impair tumor cell glycolysis. This is problematic for these cells because they frequently lack metabolic flexibility. On the one hand, many studies have found that tumor cells display decreased expression of ketolytic enzymes,81, 82, 83, 84, 85 although more recently a few counterexamples were discovered.6,7 On the other hand, a large part of a solid tumor would consist of hypoxic areas in which oxygen concentrations are too low to efficiently metabolize ketone bodies and fatty acids, even if the necessary enzymes would be expressed.86 Furthermore, a hallmark of tumor cells appears to be dysfunctional mitochondria which generate large amounts of reactive oxygen and nitrogen species without effectively generating ATP.87, 88, 89, 90, 91 A ketone- and fatty acid-mediated inhibition of glucose-6-phosphate in these cells induces a reduction of NADPH (required for regeneration of reduced glutathione) and lactate (a free radical scavenger) and therefore an increase in oxidative stress (reviewed in23,24). Accordingly, mouse studies have shown that the combination of KDs with pro-oxidative therapies such as chemotherapy,92 ionizing radiation93,94 or hyperbaric oxygen95 works better than either of these treatments on its own, which emphasizes their role as complementary cancer treatments. Lastly, Martuscello et al. showed that KDs downregulated the mTOR (mammalian target of rapamycin) pathway in xenografted tumors derived from glioblastoma cells.96 Collectively these examples show that KDs can exert pleiotropic effects on tumor cell metabolism and signaling pathways, providing some justification for their supportive application.
A second interpretation that a very strict low carbohydrate diet was found necessary by Brünings is that a KD would minimally compromise, and even support, hypoglycemic treatments. The switch to ketone body and fatty acid metabolism (known as keto-adaption) is able to protect against signs of hypoglycemia. This was impressively demonstrated when Drenick et al. infused insulin into keto-adapted obese subjects, resulting in serum glucose levels as low as 9 mg/dl without symptoms.97 It is therefore possible that Brünings' diet contributed to improved tolerance of hypoglycemia, and that hypoglycemia in turn was the principle cause of the regression of tumors observed by Brünings and later by Joseph Weiss.
Proof-of-principle that hypoglycemia itself can induce tumor regression was provided in 1962 by Koroljow98 who reported the achievement of a one-year complete remission in two metastasized cancer patients who were put into an insulin coma (lowest blood glucose reading 22 mg/dl).j A multitude of recent in vitro experiments have shown that in contrast to normal cells, many tumor cells are very vulnerable to glucose withdrawal by mechanisms involving both energy stress99,100 and oxidative stress.101, 102, 103, 104, 105 Again, this confirms the metabolic inflexibility of tumor cells arising from their dysfunctional mitochondria as described above.
Today, insulin administration is still in use in some centers with the aim of inducing hypoglycemia prior to administration of chemotherapy in what is referred to as “insulin potentiation therapy” or “metabolically supported chemotherapy”. In a recently published combination of this concept with a KD, hyperthermia and hyperbaric oxygen, a metastasized breast cancer patient achieved a complete radiological and pathological response.60 Insulin administration in these applications is usually limited to pulsed doses since it is now established that insulin is also a growth factor for many tumor cells.106,107 Another drawback is that insulin also inhibits ketogenesis, reducing the possibility of utilizing ketone bodies as an alternative energy source for the brain, and exploiting their putative anti-tumor effects. Alternative proposals for hypoglycemic treatment of cancer patients therefore avoid using insulin; they include methods such as removal of glucose (and glutamine) through dialysis108 or application of gluconeogenesis inhibitors in patients who have been keto-adapted.109,110
The examples above highlight the renewed interest in metabolic therapies of cancer, including KD interventions. My hope is that the discussion of Brünings' scientific reasoning and experimental data will further strengthen this interest. The significance of altered tumor and patient metabolism for the treatment of cancer as well as the supportive role of very low carbohydrate diets were already recognized and realized many decades ago by a remarkable investigator ahead of his time.
Compliance with ethical standards
The author declares that he has no potential conflicts of interest. The reader should be aware that many of the historical articles discussed in this review were performed prior to the Declaration of Helsinki and therefore that the ethical standards of today do not apply to these experiments.
Data statement
All historical papers mentioned in this article are available from the author upon request.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. I am grateful to Dr. Michael K. Fink who revealed to me his collected copies of Brünings’ work. I also thank Dr. Eugene J. Fine for thoroughly reading and spell-checking this work. Finally, I would like to thank Dr. Ciro Isidoro and three anonymous reviewers for constructive comments that helped to improve an earlier version of this paper.
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Sionon was the trade name for D-sorbitol.111
This diet was estimated to contain <50 g carbohydrates and about 100 g fat and 200 g protein per day, although it seems that Brünings counted meat, sausages and eggs simply as “protein”.
Concerning beverages, it is interesting that Brünings wrote that no coffee or tea (which would be allowed) were available in Bavaria, but beer was. He cautioned against allowing beer which despite its “war dilution” with water still had too many carbohydrates.
An anonymous reviewer mentioned the potential role of bioactive peptides as anti-cancer agents.112 As these are derived from whole animal and plant foods, their concentration would also be much higher in diets containing large amounts of vegetables and animal foods of marine and terrestrial origin.
Brünings mentioned in an appendix that he became aware of Silberstein's work only after his experiments had been conducted.47
This meal consisted of 500 ml whipped cream and 2 eggs. A typical breakfast described by Silberstein et al.66 would consist of 250 ml whipped cream and one egg.
I thank Dr. Michael Fink for first pointing this out to me.
Koroljow's rationale was based on the theory that hypoglycemia reduces the oxidation of glucose, so that the unused oxygen raises the oxygen concentration which should retard tumor growth. Interestingly, Poff and colleagues have shown that only raising oxygen concentrations in tumor-bearing mice through hyperbaric oxygen breathing had no effect on tumor growth, but it augmented the anti-tumor effects of a KD.95
The general effects of the method appeared to be cancer-specific, since Brünings had seen no objective or subjective improvements in wellbeing, appetite or body weight in 20 healthy subjects and 6 non-cancerous patients.
Brünings had observed mixed results in the response of tumors to radiotherapy when patients had been pre-treated with his method, with some tumors apparently becoming more radioresistant.
References
- 1.Seyfried T.N., Flores R.E., Poff A.M., D'Agostino D.P. Cancer as a metabolic disease: implications for novel therapeutics. Carcinogenesis. 2014;35(3):515–527. doi: 10.1093/carcin/bgt480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez-Outschoorn U.E., Peiris-Pagés M., Pestell R.G., Sotgia F., Lisanti M.P. Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol. 2017;14(2) doi: 10.1038/nrclinonc.2017.1. 113–113. [DOI] [PubMed] [Google Scholar]
- 3.Winter S.F., Loebel F., Dietrich J. Role of ketogenic metabolic therapy in malignant glioma: a systematic review. Crit Rev Oncol Hematol. 2017;112:41–58. doi: 10.1016/j.critrevonc.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Hamaguchi R., Wada H. Paradigm shift in cancer treatment: cancer treatment as a metabolic disease − fusion of Eastern and Western medicine. J Tradit Chinese Med Sci. 2018 [Google Scholar]
- 5.Miller V.J., Villamena F.A., Volek J.S. Nutritional ketosis and mitohormesis: potential implications for mitochondrial function and human health. J Nutr Metab. 2018;2018:5157645. doi: 10.1155/2018/5157645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartmann C., Raman S.R.J., Flöter J. Beta-hydroxybutyrate (3-OHB) can influence the energetic phenotype of breast cancer cells, but does not impact their proliferation and the response to chemotherapy or radiation. Canc Metabol. 2018;6:8. doi: 10.1186/s40170-018-0180-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz K., Chang H.T., Nikolai M. Treatment of glioma patients with ketogenic diets: report of two cases treated with an IRB-approved energy-restricted ketogenic diet protocol and review of the literature. Canc Metabol. 2015;3:3. doi: 10.1186/s40170-015-0129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otto C., Kaemmerer U., Illert B. Growth of human gastric cancer cells in nude mice is delayed by a ketogenic diet supplemented with omega-3 fatty acids and medium-chain triglycerides. BMC Canc. 2008;8(1):122. doi: 10.1186/1471-2407-8-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hao G.-W., Chen Y.-S., He D.-M., Wang H.-Y., Wu G.-H., Zhang B. Growth of human colon cancer cells in nude mice is delayed by ketogenic diet with or without omega-3 fatty acids and medium-chain triglycerides. Asian Pac J Cancer Prev. 2015;16(5):2061–2068. doi: 10.7314/apjcp.2015.16.5.2061. [DOI] [PubMed] [Google Scholar]
- 10.Fine E.J., Segal-isaacson C.J., Feinman R.D. Targeting insulin inhibition as a metabolic therapy in advanced cancer: a pilot safety and feasibility dietary trial in 10 patients. Nutrition. 2012;28(10):1028–1035. doi: 10.1016/j.nut.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Rieger J., Bähr O., Maurer G.D. ERGO: a pilot study of ketogenic diet in recurrent glioblastoma. Int J Oncol. 2014;44(6):1843–1852. doi: 10.3892/ijo.2014.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan-Shalaby J.L., Carrick J., Edinger K. Modified Atkins diet in advanced malignancies - final results of a safety and feasibility trial within the Veterans Affairs Pittsburgh Healthcare System. Nutr Metab. 2016;13:52. doi: 10.1186/s12986-016-0113-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zahra A., Fath M.A., Opat E. Consuming a ketogenic diet while receiving radiation and chemotherapy for locally advanced lung cancer and pancreatic cancer: the University of Iowa experience of two phase 1 clinical trials. Radiat Res. 2017;187(6):743–754. doi: 10.1667/RR14668.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santos J.G., Da Cruz W.M.S., Schönthal A.H. Efficacy of a ketogenic diet with concomitant intranasal perillyl alcohol as a novel strategy for the therapy of recurrent glioblastoma. Oncol Lett. 2018;15(1):1263–1270. doi: 10.3892/ol.2017.7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin-McGill K.J., Marson A.G., Tudur Smith C., Jenkinson M.D. Ketogenic diets as an adjuvant therapy in glioblastoma (the KEATING trial): study protocol for a randomised pilot study. Pilot Feasibility Stud. 2017;3:67. doi: 10.1186/s40814-017-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freedland S.J., Allen J., Armstrong A.J. Interim analysis of a prospective randomized trial of dietary carbohydrate restriction for men with a rising PSA after failed primary treatment: Carbohydrate and Prostate Study 2 (CAPS2) J Clin Oncol. 2018;36(Suppl. 6S) https://meetinglibrary.asco.org/record/157555/abstract abstr 382. [Google Scholar]
- 17.Schwartz K.A., Noel M., Nikolai M., Chang H.T. Investigating the ketogenic diet as treatment for primary aggressive brain cancer: challenges and lessons learned. Front Nutr. 2018;5:11. doi: 10.3389/fnut.2018.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klement R.J. Beneficial effects of ketogenic diets for cancer patients: a realist review with focus on evidence and confirmation. Med Oncol. 2017;34:132. doi: 10.1007/s12032-017-0991-5. [DOI] [PubMed] [Google Scholar]
- 19.Weber D.D., Aminazdeh-Gohari S., Kofler B. Ketogenic diet in cancer therapy. Aging. 2018;10(2):164–165. doi: 10.18632/aging.101382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breitkreutz R., Tesdal K., Jentschura D., Haas O., Leweling H., Holm E. Effects of a high-fat diet on body composition in cancer patients receiving chemotherapy: a randomized controlled study. Wien Klin Wochenschr. 2005;117(19–20):685–692. doi: 10.1007/s00508-005-0455-3. [DOI] [PubMed] [Google Scholar]
- 21.Holm E., Kämmerer U. Lipids and carbohydrates in nutritional concepts for tumor patients. Aktuel Ernährungsmed. 2011;36:286–298. [Google Scholar]
- 22.Klement R.J., Kämmerer U. Is there a role for carbohydrate restriction in the treatment and prevention of cancer? Nutr Metab. 2011;8:75. doi: 10.1186/1743-7075-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen B.G., Bhatia S.K., Anderson C.M. Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox Biol. 2014;2:963–970. doi: 10.1016/j.redox.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klement R.J. The influence of ketogenic therapy on the 5 R's of radiobiology. Int J Radiat Biol. 2017 doi: 10.1080/09553002.2017.1380330. [DOI] [PubMed] [Google Scholar]
- 25.Klement R.J. Fasting, fats, and physics: combining ketogenic and radiation therapy against cancer. Complement Med Res. 2017 doi: 10.1159/000484045. [DOI] [PubMed] [Google Scholar]
- 26.Warburg O., Posener K., Negelein E. Über den Stoffwechsel der Carcinomzelle. Biochem Z. 1924;152:309–343. [Google Scholar]
- 27.Warburg O., Wind F., Negelein E. Über den Stoffwechsel der Tumoren im Körper. Klin Wochenschr. 1926;5:829–838. [Google Scholar]
- 28.Warburg O., Wind F., Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8(6):519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreschi C. Beziehungen zwischen Ernahrung und Tumorwachstum. Z Immunitätsforsch. 1909;2(6):651–675. [Google Scholar]
- 30.Van Alstyne E.V.N., Beebe S.P. Diet studies in transplantable tumors - I. The effect of non-carbohydrate DIet upon the growth of transplantable sarcoma in rats. J Media Res. 1913;29(2):217–232. [PMC free article] [PubMed] [Google Scholar]
- 31.Tannenbaum A. The dependence of tumor formation on the composition of the calorie-restricted diet as well as on the degree of restriction. Canc Res. 1945;5(11):616–625. [PubMed] [Google Scholar]
- 32.Fearon K.C., Borland W., Preston T., Tisdale M.J., Shenkin A., Calman K.C. Cancer cachexia: influence of systemic ketosis on substrate levels and nitrogen metabolism. Am J Clin Nutr. 1988;47(1):42–48. doi: 10.1093/ajcn/47.1.42. http://www.ncbi.nlm.nih.gov/pubmed/3122552 [DOI] [PubMed] [Google Scholar]
- 33.Nebeling L.C., Lerner E. Implementing a ketogenic diet based on medium-chain triglyceride oil in pediatric patients with cancer. J Am Diet Assoc. 1995;95(6):693–697. doi: 10.1016/S0002-8223(95)00189-1. http://www.ncbi.nlm.nih.gov/pubmed/7759747 [DOI] [PubMed] [Google Scholar]
- 34.Nebeling L., Miraldi F., Shurin S., Lerner E. Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: two case reports. J Am Coll Nutr. 1995;14(2):202–208. doi: 10.1080/07315724.1995.10718495. [DOI] [PubMed] [Google Scholar]
- 35.Hyde P.N., Lustberg M.B., Miller V.J., LaFountain R.A., Volek J.S. Pleiotropic effects of nutritional ketosis: conceptual framework for keto-adaptation as a breast cancer therapy. Cancer Treat Res Commun. 2017;12:32–39. [Google Scholar]
- 36.Poff A., Kourtnik A., Egan K.M., Sahebjum S., D'Agostino D., Kumar N.B. Targeting the Warburg effect for cancer treatment: ketogenic diets for management of glioma. Semin Canc Biol. 2018 doi: 10.1016/j.semcancer.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brünings W. Beiträge zur Physiologie des Tetanus. Erste Mitteilung. Ueber die Muskeltöne bei elektrischer Tetanisierung des ausgeschnittenen Froschgastrocnemius. Pflügers Arch - Eur J Physiol. 1903;93(7–8):302–326. https://link.springer.com/article/10.1007/BF01663253 [Google Scholar]
- 38.Brünings W. Beiträge zur Elektrophysiologie. I. Mitteilung. Vorbemerkungen - Ueber den Ruhestrom des Froschmuskels I. Pflügers Arch - Eur J Physiol. 1903;98(5–6):241–283. https://link.springer.com/article/10.1007/BF01663447 [Google Scholar]
- 39.Brünings W. Beiträge zur Elektrophysiologie. II. Mitteilung. Ueber Ruhestrom und Reizung. Pflügers Arch - Eur J Physiol. 1903;100(7–8):367–427. https://link.springer.com/article/10.1007/BF01663094 [Google Scholar]
- 40.Brünings W. Beiträge zur Elektrophysiologie. III. Mitteilung. Zur osmotischen Theorie der Zellelektrizität. Pflügers Arch - Eur J Physiol. 1907;117(7–9):409–460. [Google Scholar]
- 41.Brünings W. Ein neuer Apparat für Blutkörperchenzählung. Pflügers Arch - Eur J Physiol. 1903;93(9–10):377–411. [Google Scholar]
- 42.Greifenstein A. Wilhelm Brünings zum 60. Geburtstage. Arch f Ohren - Nasen- u Kehlkopfheilkd. 1936;141:1–4. doi: 10.1007/BF01583586. [DOI] [Google Scholar]
- 43.Kressner A. Professor Dr. Brünings − 80 Jahre alt. Bayer Ärzteblatt. 1956;11(3):57–58. [Google Scholar]
- 44.Condon H.A. Brünings' bronchoscopic mask. Anaesthesia. 1987;42:996–997. doi: 10.1111/j.1365-2044.1987.tb05375.x. [DOI] [PubMed] [Google Scholar]
- 45.Brünings W. Beiträge zum Krebsproblem. 1. Mitteilung: Ueber eine diätetisch-hormonale Beeinflussung des Krebses. Münchener Med Wochenschr. 1941;88(5):117–123. [Google Scholar]
- 46.Brünings W. Beiträge zum Krebsproblem. 2. Mitteilung: Klinische Anwendungen der diätetisch-hormonalen Krebsbeeinflussung (“Entzuckerungsmethode”) Münchener Med Wochenschr. 1942;89(4):71–76. [Google Scholar]
- 47.Brünings W. Beträge zum Krebsproblem - Schluß. Münchener Med Wochenschr. 1942;89(4):102–107. [Google Scholar]
- 48.Chu-Shore C.J., Thiele E.A. Tumor growth in patients with tuberous sclerosis complex on the ketogenic diet. Brain Dev. 2010;32(4):318–322. doi: 10.1016/j.braindev.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 49.Zuccoli G., Marcello N., Pisanello A. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: case report. Nutr Metab. 2010;7:33. doi: 10.1186/1743-7075-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmidt M., Pfetzer N., Schwab M., Strauss I., Kämmerer U. Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: a pilot trial. Nutr Metab. 2011;8(1):54. doi: 10.1186/1743-7075-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schroeder U., Himpe B., Pries R., Vonthein R., Nitsch S., Wollenberg B. Decline of lactate in tumor tissue after ketogenic diet: in vivo microdialysis study in patients with head and neck cancer. Nutr Canc. 2013;65(6):843–849. doi: 10.1080/01635581.2013.804579. [DOI] [PubMed] [Google Scholar]
- 52.Champ C.E., Palmer J.D., Volek J.S. Targeting metabolism with a ketogenic diet during the treatment of glioblastoma multiforme. J Neurooncol. 2014;117(1):125–131. doi: 10.1007/s11060-014-1362-0. [DOI] [PubMed] [Google Scholar]
- 53.Branca J.J.V., Pacini S., Ruggiero M. Effects of pre-surgical vitamin D supplementation and ketogenic diet in a patient with recurrent breast cancer. Anticancer Res. 2015;35(10):5525–5532. [PubMed] [Google Scholar]
- 54.Strowd R.E., Cervenka M.C., Henry B.J., Kossoff E.H., Hartman A.L., Blakeley J.O. Glycemic modulation in neuro-oncology: experience and future directions using a modified Atkins diet for high-grade brain tumors. Neuro-Oncology Pract. 2015;2(3):127–136. doi: 10.1093/nop/npv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jansen N., Walach H. The development of tumours under a ketogenic diet in association with the novel tumour marker TKTL1: a case series in general practice. Oncol Lett. 2016;11(11):584–592. doi: 10.3892/ol.2015.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klement R.J., Sweeney R.A. Impact of a ketogenic diet intervention during radiotherapy on body composition: I. Initial clinical experience with six prospectively studied patients. BMC Res Notes. 2016;9:143. doi: 10.1186/s13104-016-1959-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwalb M., Taubmann M., Hines S., Reinwald H., Ruggiero M. Clinical observation of a novel, complementary, immunotherapeutic approach based on ketogenic diet, chondroitin sulfate, vitamin D 3, oleic acid and a fermented milk and colostrum product. Am J Immunol. 2016;12(4):91–98. [Google Scholar]
- 58.Tóth C., Clemens Z. Halted progression of soft palate cancer in a patient treated with the paleolithic ketogenic diet alone. Am J Med Case Reports. 2016;4(8):288–292. [Google Scholar]
- 59.Artzi M., Liberman G., Vaisman N. Changes in cerebral metabolism during ketogenic diet in patients with primary brain tumors: 1H-MRS study. J Neurooncology. 2017;132(2):267–275. doi: 10.1007/s11060-016-2364-x. [DOI] [PubMed] [Google Scholar]
- 60.İyikesici M.S., Slocum A.K., Slocum A., Berkarda F.B., Kalamian M., Seyfried T.N. Efficacy of metabolically supported chemotherapy combined with ketogenic diet, hyperthermia, and hyperbaric oxygen therapy for stage IV triple-negative breast cancer. Cureus. 2017;9(7) doi: 10.7759/cureus.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Santos J.G., Da Cruz WMaS., Schönthal A.H. Patient with recurrent glioblastoma responding favorably to ketogenic diet combined with intranasal delivery of perillyl alcohol: a case report and literature review. Arq Bras Neurocir Braz Neurosurg. 2017;36(3):194–199. [Google Scholar]
- 62.Tóth C., Clemens Z. Treatment of rectal cancer with the paleolithic ketogenic diet: a 24-months follow-up. Am J Med Case Reports. 2017;5(8):205–216. [Google Scholar]
- 63.Elsakka A.M.A., Bary M.A., Abdelzaher E. Management of glioblastoma multiforme in a patient treated with ketogenic metabolic therapy and modified standard of care: a 24-month follow-up. Front Nutr. 2018;5:20. doi: 10.3389/fnut.2018.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martin-McGill K.J., Marson A.G., Smith C.T., Jenkinson M.D. The modified ketogenic diet in adults with glioblastoma: an evaluation of feasibility and deliverability within the National Health Service the modi fi ed ketogenic diet in adults with glioblastoma: an evaluation. Nutr Canc. 2018 doi: 10.1080/01635581.2018.1460677. [DOI] [PubMed] [Google Scholar]
- 65.Tóth C., Schimmer Zsófia Clemens M. Complete cessation of recurrent cervical intraepithelial neoplasia (CIN) by the paleolithic ketogenic diet: a case report. J Cancer Res Treat. 2018;6(1):1–5. [Google Scholar]
- 66.Silberstein F., Freud J., Révész T. Versuehe, inoperable Carcinome mit Insulin zu behandeln. Z Gesamte Exp Med. 1927;55(1):78–102. [Google Scholar]
- 67.Bishop J.S., Marks P.A. Studies on carbohydrate metabolism in patients with neoplastic disease. II. Response to insulin administration. J Clin Invest. 1959;38(4) doi: 10.1172/JCI103845. 668–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lundholm K., Hoim G., Scherstén T. Insulin resistance in patients with cancer. Canc Res. 1978;38(12):4665–4670. [PubMed] [Google Scholar]
- 69.Permert J., Adrian T.E., Jacobsson P., Jorfelt L., Fruin A.B., Larsson J. Is profound peripheral insulin resistance in patients with pancreatic cancer caused by a tumor-associated factor? Am J Surg. 1993;165(1):61–66. doi: 10.1016/s0002-9610(05)80405-2. 67. [DOI] [PubMed] [Google Scholar]
- 70.Yoshikawa T., Noguchi Y., Matsumoto A. Effects of tumor removal and body weight loss on insulin resistance in patients with cancer. Surgery. 1994;116(1):62–66. http://www.ncbi.nlm.nih.gov/pubmed/8023270 [PubMed] [Google Scholar]
- 71.Yoshikawa T., Noguchi Y., Doi C., Makino T., Okamoto T., Matsumoto A. Insulin resistance was connected with the alterations of substrate utilization in patients with cancer. Canc Lett. 1999;141(1–2):93–98. doi: 10.1016/s0304-3835(99)00086-5. http://www.ncbi.nlm.nih.gov/pubmed/10454248 [DOI] [PubMed] [Google Scholar]
- 72.Schulte G., Schütz H. Insulin in der Krebsbehandlung. Münchener Med Wochenschr. 1942;89(29):648–650. [Google Scholar]
- 73.Weiss J. Über Erfahrungen mit Insulin und kohlenhydratreduzierender Diät bei inkurablen Krebskranken. Med Klin. 1957;52(27):1190–1191. [PubMed] [Google Scholar]
- 74.Akanmu M.A., Nwabudike N.L., Ilesanmi O.R. Analgesic, learning and memory and anxiolytic effects of insulin in mice. Behav Brain Res. 2009;196(2):237–241. doi: 10.1016/j.bbr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 75.Adrian T.E., Bloom S.R. The continuing hormonal contamination of insulin. Pract Diabetes Int. 1985;2(6):34–36. [Google Scholar]
- 76.Masino S.A., Ruskin D.N. Ketogenic diets and pain. J Child Neurol. 2013;28(8):993–1001. doi: 10.1177/0883073813487595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seyfried T.N., Yu G., Maroon J.C., D'Agostino D.P. Press-pulse: a novel therapeutic strategy for the metabolic management of cancer. Nutr Metab. 2017;14:19. doi: 10.1186/s12986-017-0178-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fine E.J., Miller A., Quadros E.V., Sequeira J.M., Feinman R.D. Acetoacetate reduces growth and ATP concentration in cancer cell lines which over-express uncoupling protein 2. Canc Cell Int. 2009;9:14. doi: 10.1186/1475-2867-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shukla S.K., Gebregiworgis T., Purohit V. Metabolic reprogramming induced by ketone bodies diminishes pancreatic cancer cachexia. Canc Metabol. 2014;2:18. doi: 10.1186/2049-3002-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marchut E., Gumińska M., Kedryna T. The inhibitory effect of various fatty acids on aerobic glycolysis in Ehrlich ascites tumour cells. Acta Biochim Pol. 1985;33(1):7–16. http://europepmc.org/abstract/MED/2940781 [PubMed] [Google Scholar]
- 81.Tisdale M.J., Brennan R.A. Loss of acetoacetate coenzyme A transferase activity in tumours of peripheral tissues. Br J Canc. 1983;47(2):293–297. doi: 10.1038/bjc.1983.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Skinner R., Trujillo A., Ma X., Beierle E.A. Ketone bodies inhibit the viability of human neuroblastoma cells. J Pediatr Surg. 2009;44(1):212–216. doi: 10.1016/j.jpedsurg.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 83.Maurer G.D., Brucker D.P., Bähr O. Differential utilization of ketone bodies by neurons and glioma cell lines: a rationale for ketogenic diet as experimental glioma therapy. BMC Canc. 2011;11:315. doi: 10.1186/1471-2407-11-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chang H.T., Olson L.K., Schwartz K.A. Ketolytic and glycolytic enzymatic expression profiles in malignant gliomas: implication for ketogenic diet therapy. Nutr Metab. 2013;10:47. doi: 10.1186/1743-7075-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morscher R.J., Aminzadeh-Gohari S., Feichtinger R.G. Inhibition of neuroblastoma tumor growth by ketogenic diet and/or calorie restriction in a CD1-nu mouse model. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0129802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Otto C., Klingelhöffer C., Biggermann L. Analysis of the metabolism of ketone bodies and lactate by gastrointestinal tumor cells in vitro. Aktuelle Ernährungsmed. 2014;39:51–59. [Google Scholar]
- 87.Seyfried T.N., Shelton L.M. Cancer as a metabolic disease. Nutr Metab. 2010;7:7. doi: 10.1186/1743-7075-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Verschoor M.L., Ungard R., Harbottle A., Jakupciak J.P., Parr R.L., Singh G. Mitochondria and cancer: past, present, and future. BioMed Res Int. 2013;2013:612369. doi: 10.1155/2013/612369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sullivan L.B., Chandel N.S. Mitochondrial reactive oxygen species and cancer. Canc Metabol. 2014;2:17. doi: 10.1186/2049-3002-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gaude E., Frezza C. Defects in mitochondrial metabolism and cancer. Canc Metabol. 2014;2:10. doi: 10.1186/2049-3002-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Seyfried T.N. Cancer as a mitochondrial metabolic disease. Front Cell Dev Biol. 2015;3:43. doi: 10.3389/fcell.2015.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morscher R.J., Aminzadeh-Gohari S., Hauser-Kronberger C., Feichtinger R.G., Sperl W., Kofler B. Combination of metronomic cyclophosphamide and dietary intervention inhibits neuroblastoma growth in a CD1-nu mouse model. Oncotarget. 2016;7(13):17060–17073. doi: 10.18632/oncotarget.7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abdelwahab M.G., Fenton K.E., Preul M.C. The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0036197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Allen B.G., Bhatia S.K., Buatti J.M. Ketogenic diets enhance oxidative stress and radio-chemo-therapy responses in lung cancer xenografts. Clin Canc Res. 2013;19(14):3905–3913. doi: 10.1158/1078-0432.CCR-12-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Poff A.M., Ari C., Seyfried T.N., D'Agostino D.P. The ketogenic diet and hyperbaric oxygen therapy prolong survival in mice with systemic metastatic cancer. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0065522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martuscello R.T., Vedam-Mai V., McCarthy D.J. A supplemented high-fat low-carbohydrate diet for the treatment of glioblastoma. Clin Canc Res. 2016;22(10):2482–2495. doi: 10.1158/1078-0432.CCR-15-0916. [DOI] [PubMed] [Google Scholar]
- 97.Drenick E.J., Alvarez L.C., Tamasi G.C., Brickman A.S. Resistance to symptomatic insulin reactions after fasting. J Clin Invest. 1972;51(10):2757–2762. doi: 10.1172/JCI107095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koroljow S. Two cases of malignant tumors with metastases apparently treated successfully with hypoglycemic coma. Psychiatr Q. 1962;36(1–4):261–270. doi: 10.1007/BF01586115. [DOI] [PubMed] [Google Scholar]
- 99.Demetrakopoulos G.E., Linn B., Amos H. Rapid loss of ATP by tumor cells deprived of glucose: contrast to normal cells. Biochem Biophys Res Commun. 1978;82(3):787–794. doi: 10.1016/0006-291x(78)90851-3. [DOI] [PubMed] [Google Scholar]
- 100.Priebe A., Tan L., Wahl H. Glucose deprivation activates AMPK and induces cell death through modulation of Akt in ovarian cancer cells. Gynecol Oncol. 2011;122(2):389–395. doi: 10.1016/j.ygyno.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 101.Spitz D.R., Sim J.E., Ridnour L.A., Galoforo S.S., Lee Y.J. Glucose deprivation-induced oxidative stress in human tumor cells. A fundamental defect in metabolism? Ann N Y Acad Sci. 2000;899:349–362. doi: 10.1111/j.1749-6632.2000.tb06199.x. [DOI] [PubMed] [Google Scholar]
- 102.Ahmad I.M., Aykin-Burns N., Sim J.E. Mitochondrial O2- and H2O2 mediate glucose deprivation-induced stress in human cancer cells. J Biol Chem. 2005;280(6):4254–4263. doi: 10.1074/jbc.M411662200. [DOI] [PubMed] [Google Scholar]
- 103.Jelluma N., Yang X., Stokoe D., Evan G.I., Dansen T.B., Haas-Kogan D.A. Glucose withdrawal induces oxidative stress followed by apoptosis in glioblastoma cells but not in normal human astrocytes. Mol Canc Res. 2006;4(5):319–330. doi: 10.1158/1541-7786.MCR-05-0061. [DOI] [PubMed] [Google Scholar]
- 104.Aykin-Burns N., Ahmad I.M., Zhu Y., Oberley L.W., Spitz D.R. Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem J. 2009;418:29–37. doi: 10.1042/BJ20081258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Graham N.A., Tahmasian M., Kohli B., Komisopoulou E., Zhu M., Vivanco I. Glucose deprivation activates a metabolic and signaling amplification loop leading to cell death. Mol Syst Biol. 2012;8:589. doi: 10.1038/msb.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Canc. 2012;12(3):159–169. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 107.Klement R.J., Fink M.K. Dietary and pharmacological modification of the insulin/IGF-1 system: exploiting the full repertoire against cancer. Oncogenesis. 2016;5 doi: 10.1038/oncsis.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mathews E.H., Liebenberg L. Cancer control via glucose and glutamine deprivation. J Intern Med. 2013;274(5):492. doi: 10.1111/joim.12068. [DOI] [PubMed] [Google Scholar]
- 109.Oleksyszyn J. The complete control of glucose level utilizing the composition of ketogenic diet with the gluconeogenesis inhibitor, the anti-diabetic drug metformin, as a potential anti-cancer therapy. Med Hypotheses. 2011;77(2):171–173. doi: 10.1016/j.mehy.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 110.Kapelner A., Vorsanger M. Starvation of cancer via induced ketogenesis and severe hypoglycemia. Med Hypotheses. 2015;84(3):162–168. doi: 10.1016/j.mehy.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 111.Payne W.W., Lawrence R.D., Mccance R.A. Sorbitol (SIONON) for diabetics. Lancet. 1933;222(5753):1257–1258. [Google Scholar]
- 112.Cicero A.F.G., Fogacci F., Colletti A. Potential role of bioactive peptides in prevention and treatment of chronic diseases: a narrative review. Br J Pharmacol. 2017;174(11):1378–1394. doi: 10.1111/bph.13608. [DOI] [PMC free article] [PubMed] [Google Scholar]