Abstract
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder caused by the interplay of multiple genetic and non-genetic factors. Hypertension is one of the AD risk factors that has been linked to underlying pathological changes like senile plaques and neurofibrillary tangles formation as well as hippocampal atrophy. In this study, we investigated the differences in the genetic architecture of AD between hypertensive and non-hypertensive subjects in four independent cohorts. Our genome-wide association analyses revealed significant associations of 15 novel potentially AD-associated polymorphisms (P < 5E−06) that were located outside the chromosome 19q13 region and were significant either in hypertensive or non-hypertensive groups. The closest genes to 14 polymorphisms were not associated with AD at P < 5E−06 in previous genome-wide association studies (GWAS). Also, four of them were located within two chromosomal regions (i.e., 3q13.11 and 17q21.2) that were not associated with AD at P < 5E−06 before. In addition, 30 genes demonstrated evidence of group-specific associations with AD at the false discovery rates (FDR) < 0.05 in our gene-based and transcriptome-wide association analyses. The chromosomal regions corresponding to four genes (i.e., 2p13.1, 9p13.3, 17q12, and 18q21.1) were not associated with AD at P < 5E−06 in previous GWAS. These genes may serve as a list of prioritized candidates for future functional studies. Our pathway-enrichment analyses revealed the associations of 11 non-group-specific and four group-specific pathways with AD at FDR < 0.05. These findings provided novel insights into the potential genetic heterogeneity of AD among subjects with and without hypertension.
Electronic supplementary material
The online version of this article (10.1007/s11357-019-00071-5) contains supplementary material, which is available to authorized users.
Keywords: Neurodegenerative disorders, Dementia, Aging, Cerebral microvasculature, Genomics, Transcriptomics, Pathway-enrichment analysis
Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disorder and the most common cause of dementia in elderly. In most cases, the exact biological processes resulting in AD are not clear and both genetic and non-genetic factors have been implicated in its pathogenesis (Alzheimer’s Association 2016; Raghavan and Tosto 2017). So far, many genetic variants have been associated with AD, of which those mapped to several genes such as the APOE cluster genes, CLU, CR1, GAB2, MS4A6A, PCDH11X, PICALM, and PPP1R37 demonstrated strong signals that were replicated in independent studies (Leslie et al. 2014; MacArthur et al. 2017; Raghavan and Tosto 2017).
Among non-genetic risk factors, age has the strongest association with AD as it is almost three times more prevalent among people over 85 years old compared with those in their early sixties (Alzheimer’s Association 2016). There are also reports on the potential links between AD and some vascular and life-style risk factors (e.g., cholesterol level, hypertension (HTN), and diabetes, inactivity, smoking, alcohol consumption, dietary habits, and obesity), although no causal relationships have been established yet (Daviglus et al. 2010; Alzheimer’s Association 2016). While some studies demonstrated that the modifiable risk factors may directly confer an aggregate AD risk of around 30–50% (Barnes and Yaffe 2011; Norton et al. 2014), the others suggested that the effects of such factors may be mediated by their contribution to cerebrovascular pathology, and subsequent hypoperfusion and metabolic stress of brain (Stampfer 2006; Power et al. 2011; Thorin 2015).
Among AD vascular risk factors, HTN, with a prevalence rate of 65% among those older than 60 years (Nwankwo et al. 2013), is of great importance and has been implicated in both initiation and progression of AD (Power et al. 2011; Csiszar et al. 2017). HTN may cause widespread damages to the cerebral microvasculature (e.g., blood-brain barrier (BBB) disruption, capillary loss, neurovascular uncoupling, microhemorrhages, and microinfarcts) leading to chronic brain hypoperfusion and hypoxic injuries, increased neuroinflammatory responses, decreased cerebral amyloid-beta (Aβ) clearance, and neuronal loss and dysfunction (Faraco and Iadecola 2013; Thorin 2015; Ungvari et al. 2017; Csiszar et al. 2017; Tarantini et al. 2017a; Tucsek et al. 2017). Therefore, the HTN-induced structural and functional damages to the cerebrovascular integrity, which can be superimposed on the microvascular damages caused by aging and AD amyloid pathologies, may initiate or exacerbate cognitive impairment and AD (Csiszar et al. 2017). They may also play a role in development of the classical pathologic findings in the brain of AD patients such as amyloid plaques and neurofibrillary tangles (Power et al. 2011; Faraco and Iadecola 2013; Csiszar et al. 2017). It was suggested that for a given level of such AD-induced pathologic changes in brain, dementia is more likely to appear in patients with more severe cerebrovascular diseases (Power et al. 2011). Several studies have reported that HTN may trigger or accelerate the Aβ deposition in the neurovascular unit, and may promote tau protein phosphorylation (Petrovitch et al. 2000; Díaz-Ruiz et al. 2009; Carnevale et al. 2012; Sagare et al. 2013; Schreiber et al. 2014; Faraco et al. 2016). These in turn can aggravate the cerebral microvascular injuries (Csiszar et al. 2017). HTN was also reported to induce synaptic loss and synaptic plasticity impairment in hippocampus neurons that can affect memory establishment and contribute to the AD pathogenesis (Tucsek et al. 2017).
Despite strong empirical evidence indicating associations between HTN and AD, it is not clear if the genetic mechanisms underlying AD might be, to some extent, different in patients who have ever suffered from HTN from those without any history of high blood pressure. In this study, we investigated if potentially different genetic makeup of hypertensive and non-hypertensive subjects may lead to some differences in the genetic bases of AD between them. The genetic analyses were performed in four independent cohorts using genetic information on ~ 2 million genotyped and imputed single-nucleotide polymorphism (SNPs). Each dataset was divided into two groups of subjects with and without HTN, and the genetic predisposition to AD was then investigated through genome-wide association (GWA), transcriptome-wide association (TWA), gene-based, and pathway-enrichment analyses in each group.
Methods
Study participants
The following four independent cohorts were used to investigate the genetic basis of AD in the context of HTN status of subjects: (1) the SNP Typing for Association with Multiple Phenotypes from Existing Epidemiologic Data (STAMPEED) from Cardiovascular Health Study (CHS) (Fried et al. 1991), (2) the SNP Health Association Resource (SHARe) from Framingham Heart Study (FHS) (Dawber et al. 1951; Feinleib et al. 1975), (3) the University of Michigan Health and Retirement Study (HRS) (Sonnega et al. 2014), and (4) the Late Onset Alzheimer’s Disease Family Study from National Institute on Aging (NIA-LOADFS) (Lee et al. 2008). Detailed information about the design, data gathering, and objectives of these studies can be found in the cited original literature and in the Supporting Acknowledgment section provided in the Additional File 1. All four studies were reviewed and approved by institutional review boards (IRBs). The CHS and HRS were population-based studies, and the LOADFS and FHS cohorts had family-based designs. In addition, all four predominantly sampled individuals of Caucasian ancestry. Therefore, only Caucasian subsets of these cohorts were included in our study in order to increase the power of analyses. Also, only original and offspring generations from the FHS were included in our study to make the age of participants comparable across all cohorts. Each dataset was divided into the hypertension-positive (HTN-P) and hypertension-negative (HTN-N) groups. Subjects with and without HTN were directly determined by the LOADFS. For the other three datasets, those with three or more readings of blood pressure ≥ 140/90, with history of taking anti-hypertensive therapy (FHS, CHS, and HRS), or with hypertension diagnosis in Medicare claims according to the International Classification of Disease codes, Ninth revision (ICD-9) (CHS and HRS), were considered as the HTN-P group. With respect to AD status, cases and healthy controls were directly identified by the LOADFS and FHS studies, and were determined by using proper ICD-9 codes in the CHS and HRS datasets. Table S1 contains demographic information about these four cohorts.
Genotype data and quality control
Since different genotyping platforms were used by four investigated cohorts, their genotype data were made comparable by imputing non-genotyped loci in each dataset to generate a common set of ~ 2 million SNPs. Details of the imputation procedure were explained elsewhere (Nazarian et al. 2019). Imputed SNPs with low imputation quality, measured as the squared correlation (r2) between the imputed and expected true genotypes < 0.7, were first excluded. The remaining imputed SNPs along with directly genotyped ones were subject to quality control (QC) procedure to further remove low-quality SNPs and subjects/families according to the following criteria: SNPs with minor allele frequencies < 0.01, SNPs and subject with missing values > 5%, SNPs with Hardy-Weinberg disequilibrium P value < 1E−06, and SNPs, subjects, and families with Mendel error rates > 2% (LOADFS and FHS cohorts). QC was performed using PLINK (Purcell et al. 2007). Table S2 summarizes information about the SNPs that passed QC measures in the four investigated datasets.
Genetic analysis
-
i.
GWA analysis
Discovery and replication steps: each of the four cohorts of interest was used as a discovery set to detect the significantly AD-associated SNPs at the genome-wide significance level (i.e., P < 5E−08) or suggestive levels of associations (i.e., 5E−08 < P ≤ 5E−06). These thresholds were selected to control for type I error rates arising from multiple testing issues. Significant association signals detected from each dataset in discovery step were then subject to a replication study in the other three datasets at significance level of 0.05.
Population structure: in each cohort, the confounding effects of potential population stratification were investigated by principal component analysis (PCA) of genotype data using a subset of unrelated individuals with identity-by-descent (IBD) values < 0.0884 and a subset of SNPs not in linkage disequilibrium (LD) (i.e., r2 < 0.2 within any windows of size 100 SNPs across autosomal chromosomes) (Verma et al. 2014). The KING (Manichaikul et al. 2010) and PLINK (Purcell et al. 2007) packages were used to obtain the subsets of unrelated subjects and low-LD SNPs, respectively, and the GENESIS R package (Conomos et al. 2015) was used to perform the PCA. The genomic inflation factors (λ values) based on the results from logistic regression models (see below) are shown in Table S3. The λ values were all smaller than 1.1 suggesting that population substructure had trivial confounding impact on our analyses (Wellcome Trust Case Control Consortium 2007).
Statistical models: logistic regression models were fitted using PLINK (Purcell et al. 2007) to explore the associations between SNPs and AD in each of the HTN-P and HTN-N groups. The top 5 PCs, sex, and birth cohort (i.e., birth year as a proxy to adjust for age and secular trends in AD prevalence) of subjects and the additive genetic effects of SNPs were considered as fixed-effects covariates. Since ignoring family relationships in family-based cohorts may lead to inflation of type I error rates (McArdle et al. 2007), the association signals of SNPs with P < 0.05 in the logistic regression analyses of the LOADFS and FHS were further validated by fitting generalized logistic mixed models (GLMMs) in which family IDs were considered as a random-effects covariate in addition to the aforementioned fixed-effects covariates. The GLMMs were fitted by the lme4 R package (Bates et al. 2015).
Meta-analysis: in each of the HTN-P and HTN-N groups, the GWA results from the four investigated cohorts were combined by a conventional inverse variance meta-analysis to obtain meta-statistics of interest using GWAMA package (Mägi and Morris 2010). The same significance thresholds used at the discovery phase were considered for interpreting the meta-analysis results.
Group-specific effects: for any SNPs that were specifically associated with AD in only one of the HTN-P and HTN-N groups, we further investigated if their odds ratios were significantly different between the two groups using a Wald’s chi-square test with 1 degrees of freedom (Allison 1999):
where bP and bN are the natural logarithm of odds ratios in the HTN-P and HTN-N groups, respectively, and seP and seN are their standard errors.
Analysis of the pooled samples of HTN-P and HTN-N groups: for SNPs that were significantly associated with AD in only one of the HTN-P and HTN-N groups and, in addition, had demonstrated significant group-specific effects in Wald’s chi-square tests of ORs, we investigated if their signals remain significant in pooled samples of the HTN-P and HTN-N groups. This was performed through a moderation analysis by fitting models that contained all aforementioned fixed- and random-effects covariates along with HTN status and SNP-by-HTN interaction term as fixed-effects covariates. A significant P value from the interaction term indicated that the association of a given SNP with AD status was dependent on (i.e., moderated by) the HTN status in the pooled samples. The results from analyses in the four cohorts of interest were again combined by a conventional meta-analysis.
Between-cohorts genetic differences: as the four cohorts under consideration contributed different proportions of cases and controls to the conducted meta-analyses (e.g., the LOADFS contained most AD cases), it may raise the question if the heterogeneous contributions might potentially have impacted the meta-analyses results due to a selection bias. To investigate this issue, we calculated the fixation index (FST) as a measure of between-cohorts genetic differences (Weir and Cockerham 1984; Nei 1987). The FST value may range between 0 and 1, with values greater than 0.05 implying that there is a moderate to substantial between-groups genetic diversity (i.e., population stratification) that can explain a sizable portion of the genetic variance. The FST values close to 0 indicate very small between-groups genetic differences and negligible population structure (Hartl and Clark 1997). In each of the HTN-P and HTN-N groups, the FST values were obtained using SNPs with Pmeta-analysis < 0.05. The two SNP sets were LD pruned (as explained above in the “Population structure” subsection) to obtain sets of SNPs that were in small LD. The FST values were estimated by the hierfstat R package (Goudet 2005).
Comparing significant findings with previous GWAS results: SNPs significantly associated with AD were mapped to genes and chromosomal regions (i.e., cytogenetic bands) using the genes and regions coordinates provided by PLINK (Purcell et al. 2007) and UCSC Genome Browser (Casper et al. 2018). The GRASP (Leslie et al. 2014) and NHGRI-EBI GWAS (MacArthur et al. 2017) catalogs were searched to determine which of the AD-associated SNPs in our study were newly detected (i.e., they were searched to find any evidence of AD-associated SNPs with P < 5E−06 in the genes and chromosomal regions corresponding to the SNPs detected in our study). The newly detected SNPs were considered informative AD markers if they were not in LD (i.e., had r2 < 0.4 and non-significant χ2 test of LD) with previously detected AD-associated SNPs that had P < 5E−06 and were located in their 1 Mb up-/downstream regions. The LDlink web-tool (Machiela and Chanock 2015) was used to obtain LD measures for the SNPs of interest in the CEU population (i.e., Utah Residents with Northern and Western European Ancestry).
-
ii.
Gene-based analysis
The results of conventional meta-analysis in each of the two studied groups were used to perform a gene-based analysis that combines the test statistics of SNPs within 50 kb of any gene into a single measure of association for that gene. Here, the HRS dataset was considered as reference for calculating LD (i.e., r2 metric) among SNPs corresponding to each gene, and subsequently pruning SNPs using a LD threshold of r2 > 0.9. The gene-based analysis was performed using GCTA package (v1.26.0) (Yang et al. 2011). The significant gene-AD associations were ranked and selected at false discovery rate (FDR) of 0.05 (Benjamini and Hochberg 1995).
-
iii.
TWA analysis
In each of the HTN-P and HTN-N groups, a TWA analysis was performed by integrating our meta-analysis results with the summary results from previous expression quantitative trait loci (eQTLs) studies on blood cells (Lloyd-Jones et al. 2017) and brain tissue (Qi et al. 2018). The TWA analyses were performed using SMR package (v0.68) (Zhu et al. 2016). Any probes with at least one significant eQTL (i.e., PeQTL < 5E−08) were included in our TWA analysis if the reported eQTLs were present in our study as well. This resulted in sets of ~ 8250 and ~ 7070 probes with cis-eQTLs when blood and brain-specific data were analyzed, respectively. In each group, the FDR threshold for SMR test was the significance level at which the number of possible false-positives was less than 1. Probes with significant PSMR (i.e., significant PSMR at FDR of 0.01 (HTN-N group) or 0.05 (HTN-P group)) were considered AD-associated if they had non-significant P values in the heterogeneity test (PHEIDI ≥ 0.05). A non-significant heterogeneity test indicates possible pleiotropic effects of a single locus that may confer AD risk and, at the same time, change the probe expression level (Zhu et al. 2016). Finally, if there was no AD-associated SNPs with P < 5E−08 within 1 Mb of genes with significant probes, they were considered potentially novel AD genes.
-
iv.
Pathway-enrichment analysis
Pathway-enrichment analysis was performed through the same method explained for the gene-based analysis. SNPs within 50 kb of any genes contributing to a given pathway were considered as a SNP set for that pathway. Pathways defined by REACTOME pathway knowledgebase (Fabregat et al. 2018) and the Pathway Interaction Database (PID) (Schaefer et al. 2009) were included in our analyses (674 and 196 pathways, respectively). Pathways definitions were obtained from the Broad Institute gene set enrichment analysis (GSEA) website (Subramanian et al. 2005). A FDR level of 0.05 was considered as the significance threshold.
Results
-
i.
GWA analysis
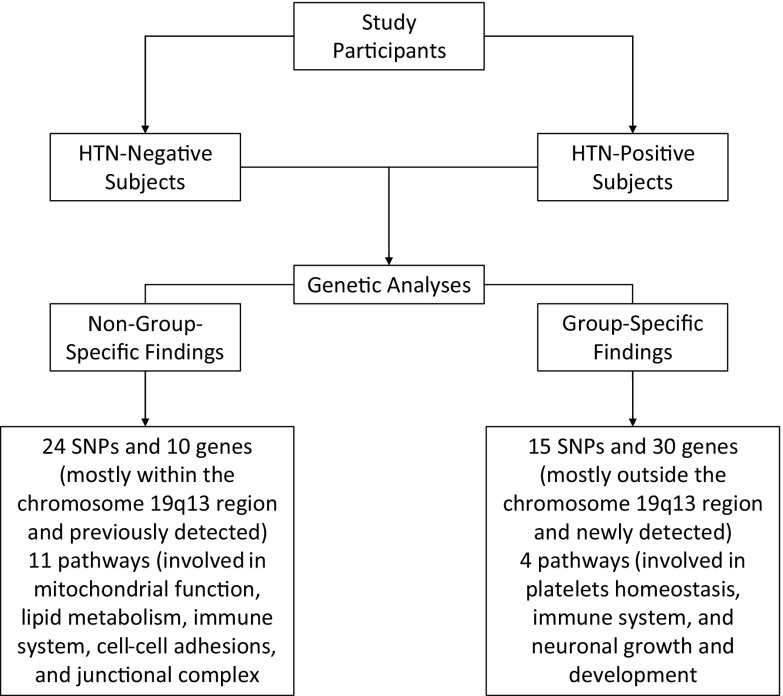
In each of the HTN-P and HTN-N groups, the genetic basis of AD was investigated by GWA analyses of the LOADFS, FHS, CHS, and HRS cohorts which were then combined through an inverse variance meta-analysis. These resulted in the replicated and meta-analysis sets of AD-associated SNPs that are listed in Tables S4 and S5. Also, an overview of our significant findings is presented in Fig. 1. The replicated SNPs were significantly associated with AD in a discovery dataset and their association signals were replicated in another cohort. The meta-analysis sets contained SNPs that were not among replicated sets, were genotyped in two or more cohorts, and had significant P values in conducted meta-analyses. Also, the Manhattan and QQ plots corresponding to these analyses are shown in Figures S1-S4. The Manhattan plots showed that in both studied groups and across all cohorts, the SNPs that were significantly associated with AD at the genome-wide significance level (i.e., P < 5E−08) were mostly located on the chromosome 19. In addition, as seen in Tables S4 and S5, the significant SNPs that were located within the chromosome 19q13 region (i.e., APOE cluster genes region) were mostly non-group-specific whereas those located outside this chromosomal region were group-specific.
Fig. 1.
An overview of the significant findings
(a) SNPs outside the chromosome 19q13 region: the summary information about the replicated and meta-analysis sets of AD-associated SNPs that were significant in either HTN-P or HTN-N groups and were located outside the chromosome 19q13 region is provided in Table 1. Detailed information about these SNPs, such as their allele frequencies, odds ratios (ORs), and P values in the four studied cohorts can be found in Table S6.
Table 1.
AD-associated SNPs outside the chromosome 19q13 region
| Chromosome | Closest gene | SNP | Position | A1 | Sig? | P min | Effects | A1.Freq | OR (se) | P meta | P q | i 2 | P abs | Proxy? | Gene? | Region? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HTN-P group | ||||||||||||||||
| 3q13.11 | MIR548AB | rs74528420 | 104209719 | T | YYNN | 4.63E−04 | −−−− | 0.867 | 0.716 (0.048) | 4.42E−06 | 6.68E−01 | 0 | 4.42E−06 | N | N | N |
| 3q13.11 | MIR548AB | rs28868104 | 104241993 | C | YYYY | 2.89E−03 | −−−− | 0.869 | 0.688 (0.047) | 3.51E−07 | 9.88E−01 | 0 | 3.50E−07 | N | N | N |
| 3q13.11 | MIR548AB | rs4501143 | 104256947 | T | YYYY | 1.59E−03 | ++++ | 0.126 | 1.437 (0.100) | 1.23E−06 | 8.86E−01 | 0 | 1.23E−06 | N | N | N |
| 6p22.3 | NRSN1 | rs1925458 | 23378723 | G | Y?YY | 4.27E−04 | −?−− | 0.883 | 0.644 (0.054) | 1.26E−06 | 9.36E−01 | 0 | 1.26E−06 | N | N | Y |
| 12p13.1 | GRIN2B | rs78210707 | 13763366 | G | N?YY | 2.02E−03 | −?−− | 0.989 | 0.375 (0.065) | 4.28E−06 | 9.73E−01 | 0 | 4.29E−06 | N | Y | Y |
| 12q12 | SLC2A13 | rs75036080 | 39882501 | G | YNYN | 2.13E−04 | −−−− | 0.98 | 0.486 (0.065) | 3.07E−06 | 3.22E−01 | 0.14 | 3.09E−06 | N | N | Y |
| HTN-N group | ||||||||||||||||
| 17q21.2 | SMARCE1 | rs11658260* | 40597298 | A | NYYN | 1.79E−06 | −−+− | 0.501 | 0.967 (0.070) | 6.65E−01 | 1.50E−06 | 0.899 | 2.75E−03 | N | N | N |
| 18q21.33 | RNF152 | rs72934313* | 61948797 | C | YYN? | 4.77E−06 | +−+? | 0.924 | 1.074 (0.147) | 6.55E−01 | 6.64E−07 | 0.93 | 6.05E−06 | N | N | Y |
| 2p16.1 | MIR4432 | rs243034 | 60375757 | C | YYYN | 2.54E−03 | ++++ | 0.394 | 1.521 (0.121) | 1.31E−06 | 1.58E−01 | 0.422 | 1.31E−06 | N | N | Y |
| 4q35.2 | LINC01060 | rs1353581 | 189068835 | G | YYYN | 3.00E−05 | −−−+ | 0.766 | 0.650 (0.056) | 4.80E−06 | 3.76E−03 | 0.777 | 4.54E−07 | N | N | Y |
| 9p21.1 | LINGO2 | rs10968750 | 28753984 | T | Y?NN | 1.90E−06 | +?+− | 0.941 | 2.633 (0.442) | 1.97E−06 | 1.97E−01 | 0.385 | 6.45E−07 | N | N | Y |
| 11p11.2 | MIR7154,CHST1 | rs1447575 | 45688426 | T | YNYN | 4.67E−05 | −−−− | 0.909 | 0.507 (0.061) | 6.42E−07 | 5.16E−01 | 0 | 6.42E−07 | N | N | Y |
| 11p11.2 | MIR7154,CHST1 | rs1447576 | 45689102 | G | YNYN | 4.67E−05 | −−−− | 0.909 | 0.505 (0.060) | 5.46E−07 | 5.54E−01 | 0 | 5.46E−07 | N | N | Y |
| 11p11.2 | MIR7154,CHST1 | rs2666895 | 45689480 | G | YNYN | 3.67E−05 | −−−− | 0.908 | 0.502 (0.060) | 4.48E−07 | 5.56E−01 | 0 | 4.48E−07 | N | N | Y |
| 14q32.12 | CATSPERB | rs3814837 | 91725006 | C | YNYN | 3.88E−05 | −−−+ | 0.753 | 0.643 (0.056) | 3.26E−06 | 3.68E−01 | 0.051 | 3.03E−06 | N | N | Y |
| 21q21.3 | ATP5J | rs11909995 | 25725147 | G | YYYN | 1.62E−03 | −−−− | 0.824 | 0.617 (0.059) | 4.41E−06 | 3.23E−01 | 0.14 | 4.41E−06 | Y | N | Y |
Abbreviations: AD, Alzheimer’s disease; SNP, single-nucleotide polymorphism; HTN-P, hypertension-positive; HTN-N, hypertension-negative
Chromosome: chromosomal region based on the cytogenetic bands; Position: position of SNP based on Human Genome version 38 (hg38); A1: effect allele; Sig?: if the SNP had a P value less than 0.05 in LOADFS, FHS, CHS, and HRS datasets, respectively (Yes, NO, Missing); Pmin: minimum P value detected for the SNP in the aforementioned datasets; Effects: direction of SNP’s effects in the aforementioned datasets (Positive, Negative, Missing); A1.Freq: frequency of effect allele based on meta-analysis; OR (se): odds ratio and its standard error based on meta-analysis; Pmeta: P value of the SNP in meta-analysis; Pq: P value of Q-statistics (Cochran’s heterogeneity test); i2: i-squared inconsistency metric; Pabs: P value of the SNP in meta-analysis on absolute values of effects (i.e., beta coefficients); Proxy?: if the SNP is in LD with any previously detected AD-associated loci whose P value is less than the one detected in this study (Yes, NO); Gene?: if previous studies have detected AD-associated SNPs with P < 5E−06 inside closest gene to the detected SNP (Yes, NO); Region?: if previous studies have detected AD-associated SNPs with P < 5E−06 inside chromosomal region in which the detected SNP is located (Yes, NO); *: Replicated SNPs
HTN-P group: there were no replicated SNPs with significant association signals in this group. However, we found that 6 SNPs had P < 5E−06 in meta-analysis of the four cohorts in the HTN-P group (Tables 1 and S6). These SNPs were not associated with AD in the HTN-N group. In addition, a Wald’s chi-square test revealed that their odds ratios were significantly different between the two groups with P < 0.05 (Table S7) indicating that they had group-specific effects.
The association signals of none of these SNPs were reported in previous GWAS. In addition, all of them were considered as informative potential AD markers because they were not in LD with any AD-associated SNPs within their 1 Mb flanking regions that had P < 5E−06 (Machiela and Chanock 2015). Among the closest genes to these SNPs, GRIN2B corresponding to rs78210707 was previously associated with AD at P < 5E−06. Also, the chromosomal regions corresponding to rs1925458 and rs75036080 (i.e., 6p22.3 and 12q12, respectively) contained previously reported AD-associated SNPs at P < 5E−06 (Leslie et al. 2014; MacArthur et al. 2017). There were no AD-associated SNPs within the 3q13.11 region (corresponding to rs74528420, rs28868104, and rs4501143) in previous GWAS. Detailed information about the genes/chromosomal regions with previously reported AD-associated SNPs can be found in Table S8.
HTN-N group: there were 2 SNPs (i.e., rs11658260 and rs72934313) that had significant P values at the suggestive level of associations at the discovery stage, and their associations were replicated in another dataset at P < 0.05 (Tables 1 and S6). None of them had significant meta-analysis P values which may be attributed to their heterogeneous effects across different cohorts reflected by their high i2 inconsistency metric (~ 0.9) and highly significant P values from Cochran’s heterogeneity tests (Pq < 1.5E−06). The impact of the heterogeneity of their effects was corroborated by the meta-analysis on the absolute values of the coefficients which resulted in considerably smaller P values than those from the conventional meta-analysis. There were 8 additional SNPs that were associated with AD at the suggestive level of significance in conducted meta-analysis (Tables 1 and S6). None of these 10 SNPs were associated with AD in the HTN-P group. Also, all of them had group-specific effects (i.e., their ORs were significantly different between the HTN-P and HTN-N groups with P < 0.05 in Wald’s chi-square tests for comparing ORs (Table S7)) .
None of these SNPs were associated with AD in previous GWAS, and rs11909995 was the only SNP that was in LD with several previously detected AD-associated SNPs (i.e., rs12386284, rs1783012, rs1783013, rs926963, rs1893650, rs2226326, rs2829803, rs2298369, rs2829823, rs2829832 (Nazarian et al. 2019)) within its 1Mb flanking regions (Machiela and Chanock 2015). The other 9 SNPs were not in LD with previously discovered AD-associated SNPs within their 1 Mb up-/downstream regions that had P < 5E−06, indicating that they were informative potential AD markers.
There was no previous evidence of associations of the closest genes to these SNPs with AD at P < 5E−06. However, AD-associated SNPs at the genome-wide significance level were previously reported within 2p16.1 and 14q32.12 regions corresponding to rs243034 and rs3814837, respectively. Also, AD-associated SNPs at P < 5E−06 were previously discovered inside the chromosomal regions corresponding to rs72934313 (18q21.33), rs1353581 (4q35.2), rs10968750 (9p21.1), rs1447575, rs1447576, rs2666895 (11p11.2), and rs11909995 (21q21.3). There were no AD-associated SNPs within the 17q21.2 region corresponding to rs11658260 in previous GWAS (Leslie et al. 2014; MacArthur et al. 2017). Further information about these genes/chromosomal regions can be found in Table S8.
Analysis of the pooled samples of HTN-P and HTN-N groups: the associations of 16 group-specific SNPs, listed in Tables 1 and S6, with AD were further investigated in the pooled samples of these groups by including the interaction term between HTN status and these SNPs in the models. The results from these moderation analyses are summarized in Table S7. We found that all these SNPs except rs1925458 (that was among the significant SNPs in the HTN-P group) were significantly associated with AD (P < 0.05) in interaction with HTN status. In other words, their associations were different across different levels of the HTN status indicating that their associations with AD were dependent on and moderated by the HTN status of subjects. These findings substantiate the group-specific significant associations of these 15 SNPs with AD that were discovered in the stratified analyses of the HTN-P and HTN-N groups. However, as expected, the resulting P values (between 2.43E−04 and 4.64E−02) were larger than those detected in the stratified analyses because they measured the significance of the differences of SNP’s effects between the two groups instead of measuring SNP’s effects in a particular group. In addition, it is worthy of mention that detecting significant interactions, in general, requires larger sample sizes (> 4 folds) compared with detecting the main effects of the same magnitudes (Leon and Heo 2009). The non-significant P value of the interaction between HTN status and rs1925458 (P = 1.19E−01) might indicate that the group-specific effect detected for this SNP in the stratified analysis was a false-positive finding arising from the insufficient sample sizes and power differences between the HTN-P or HTN-N groups.
(b) SNPs within the chromosome 19q13 region: the results regarding SNPs within the APOE cluster genes region that were mostly associated with AD in both HTN-P and HTN-N groups are discussed in the Additional File 1 and are summarized in Tables S9-S11.
(c) Between-cohorts genetic differences: the overall Nei’s (Nei 1987) and Weir-Cockerham’s (Weir and Cockerham 1984) FST values were 4.00E−04 and 5.64E−04, respectively, in the HTN-P group, and 3.00E−04 and 4.59E−04, respectively, in the HTN-N group. The estimated pairwise FST values for the four cohorts of interest (Table S12) were between 2.35E−04 and 7.00E−04 in the HTN-P group, and between 2.60E−04 and 6.00E−04 in the HTN-N group. In all cases, the FST values were very small indicating negligible between-cohorts genetic differences (i.e., only a tiny portion of the genetic variance could be attributed to the potential between-cohorts genetic differences). Therefore, despite the heterogeneous contributions of the four investigated cohorts to our meta-analyses with respect to their case-to-control ratios, it is unlikely that the population structure arising from such trivial genetic differences may have had a significant impact on the modeled genetic variance compromising our meta-analyses results due to a selection bias.
-
ii.
Gene-based analysis
Most of the genes significantly associated with AD at FDR level of 0.05 were located inside the chromosome 19q13 region (Table 2) which harbors many AD-associated SNPs with P < 5E−08 in current and previous studies (Leslie et al. 2014; MacArthur et al. 2017). The only significant gene outside this region was JAM2 gene (inside the chromosome 21q21 region) that was detected in the HTN-N group. No AD-associated SNPs with P < 5E−08 were found within 1 Mb of this gene in our study or previous reports, although rs11909995 which is ~ 7 kb apart from JAM2 gene was associated with AD at the suggestive level of associations (P = 4.41E−06) in our meta-analysis in the HTN-N group. Also, AD-associated SNPs with significant P values at the suggestive level of associations were previously reported in this chromosomal region (Leslie et al. 2014; MacArthur et al. 2017; Nazarian et al. 2019) (Table S13).
-
iii.
TWA analysis
Table 2.
AD-associated genes detected by the gene-based analyses
| Gene | Chromosome | Start | End | No. SNPs | HTN-P group | HTN-N group | ||
|---|---|---|---|---|---|---|---|---|
| Chi-square | P value | Chi-square | P value | |||||
| PVRL2 | 19 | 44846135 | 44889228 | 90 | 1654.52 | 1.14E−30 | 1461.30 | 1.83E−27 |
| TOMM40 | 19 | 44891219 | 44903689 | 73 | 1549.23 | 6.10E−36 | 1372.37 | 2.66E−32 |
| APOE | 19 | 44905781 | 44909393 | 70 | 1514.03 | 1.07E−35 | 1339.20 | 4.37E−32 |
| APOC1 | 19 | 44914663 | 44919349 | 67 | 1470.39 | 1.52E−36 | 1274.89 | 2.77E−32 |
| APOC1P1 | 19 | 44926802 | 44931386 | 57 | 1457.47 | 1.85E−39 | 1247.24 | 1.34E−34 |
| APOC4 | 19 | 44942237 | 44945496 | 46 | 1347.30 | 3.20E−39 | 1089.16 | 2.33E−32 |
| APOC4-APOC2 | 19 | 44942237 | 44949565 | 46 | 1347.30 | 3.20E−39 | 1089.16 | 2.33E−32 |
| APOC2 | 19 | 44945981 | 44949566 | 42 | 999.48 | 1.92E−32 | 785.17 | 2.77E−26 |
| CLPTM1 | 19 | 44954584 | 44993346 | 47 | 902.97 | 9.50E−27 | 716.92 | 9.92E−22 |
| CBLC | 19 | 44777868 | 44800646 | 52 | NS | NS | 210.55 | 2.01E−05 |
| BCAM | 19 | 44809058 | 44821421 | 44 | NS | NS | 221.74 | 1.63E−05 |
| JAM2 | 21 | 25639281 | 25717562 | 51 | NS | NS | 303.90 | 8.93E−06 |
Abbreviations: AD, Alzheimer’s disease; SNP, single-nucleotide polymorphism; HTN-P, hypertension-positive; HTN-N, hypertension-negative; NS, non-significant
Genomic coordinates are based on Human Genome version 38 (hg38)
There were 3 and 21 probes that passed both SMR and HEIDI tests in the HTN-P and HTN-N groups, respectively, when eQTLs data from blood cells was analyzed (Table 3). TPM2 and GAMT genes had more than one significant probe. Significantly detected genes were group-specific except for PADI4 gene that was significant in both HTN-P and HTN-N groups. Also, NDRG3 (significant in the HTN-P subjects) and AAR2 (significant in the HTN-N groups) were located ~ 0.4 Mb apart within the chromosome 20q11 region. When brain-specific eQTLs data was analyzed, 10 probes/genes passed both SMR and HEIDI tests in the HTN-N group (Table 4). The top eQTLs corresponding to these probes/genes were all nominally significant in our meta-analyses (1.55E−04 ≤ PGWAS ≤ 3.70E−02). Probes corresponding to ACAA1, SAAL1, and ST14 genes were significant in the analyses of both blood cells and brain-specific eQTLs data.
Table 3.
AD-associated probes/genes detected by the transcriptome-wide analyses of blood cells data
| Probe ID | Gene | Top eQTL | Chr | Pos | A1 | A1.Freq | P GWAS | P eQTL | b SMR | SESMR | P SMR | P HEIDI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HTN-P group | ||||||||||||
| ILMN_1807529 | PADI4 | rs16824888 | 1 | 17283250 | A | 0.05 | 3.98E−03 | 6.28E−87 | − 0.258 | 0.06 | 1.54E−05 | 2.74E−01 |
| ILMN_1664750 | TMBIM4 | rs35758766 | 12 | 66067239 | A | 0.063 | 3.72E−03 | 2.16E−61 | 0.334 | 0.082 | 4.36E−05 | 9.67E−02 |
| ILMN_1738229 | NDRG3 | rs118164920 | 20 | 36483848 | G | 0.044 | 5.88E−03 | 3.15E−51 | − 0.338 | 0.077 | 1.05E−05 | 6.80E−01 |
| HTN-N group | ||||||||||||
| ILMN_1807529 | PADI4 | rs16824888 | 1 | 17283250 | A | 0.05 | 1.12E−02 | 6.28E−87 | − 0.383 | 0.08 | 1.98E−06 | 3.76E−01 |
| ILMN_1681301 | AIM2 | rs76576833 | 1 | 159093183 | T | 0.047 | 7.48E−03 | 1.82E−17 | − 0.864 | 0.202 | 1.82E−05 | 5.97E−01 |
| ILMN_2242403 | DGUOK | rs7557705 | 2 | 73928514 | T | 0.049 | 4.94E−03 | 3.29E−11 | 1.276 | 0.301 | 2.18E−05 | 9.04E−02 |
| ILMN_1740216 | ERCC3 | rs4150436 | 2 | 127284258 | C | 0.025 | 4.03E−03 | 1.72E−21 | 0.82 | 0.151 | 5.85E−08 | 1.70E−01 |
| ILMN_1738921 | ACAA1 | rs61095322 | 3 | 38126634 | C | 0.045 | 7.36E−03 | 1.27E−23 | − 0.842 | 0.183 | 4.00E−06 | 4.52E−01 |
| ILMN_1843932 | SAP30L | rs6884266 | 5 | 154460912 | C | 0.119 | 9.86E−03 | 1.72E−71 | 0.393 | 0.1 | 7.95E−05 | 2.94E−01 |
| ILMN_1737847 | RMND5B | rs3812071 | 5 | 178148265 | C | 0.027 | 2.23E−02 | 8.86E−14 | − 0.858 | 0.215 | 6.47E−05 | 2.10E−01 |
| ILMN_1789196 | TPM2 | rs2295797 | 9 | 35722285 | C | 0.301 | 1.24E−03 | 6.45E−181 | − 0.303 | 0.066 | 4.39E−06 | 5.72E−02 |
| ILMN_1757604 | TPM2 | rs2295797 | 9 | 35722285 | C | 0.301 | 1.24E−03 | 3.22E−144 | − 0.339 | 0.074 | 4.72E−06 | 1.05E−01 |
| ILMN_1784661 | TMEM2 | rs7858861 | 9 | 71684384 | G | 0.038 | 1.41E−02 | 1.78E−15 | 0.918 | 0.205 | 7.56E−06 | 1.51E−01 |
| ILMN_2239754 | IFIT3 | rs41284944 | 10 | 89402425 | T | 0.026 | 1.37E−02 | 4.19E−39 | − 0.461 | 0.102 | 6.12E−06 | 3.16E−01 |
| ILMN_1739428 | IFIT2 | rs41284944 | 10 | 89402425 | T | 0.026 | 1.37E−02 | 1.65E−13 | 0.821 | 0.204 | 5.48E−05 | 6.93E−02 |
| ILMN_1658678 | SAAL1 | rs11024482 | 11 | 18099100 | C | 0.253 | 2.10E−04 | 2.25E−89 | − 0.52 | 0.096 | 5.85E−08 | 5.64E−01 |
| ILMN_1699887 | ST14 | rs12269733 | 11 | 130118110 | G | 0.11 | 5.37E−04 | 1.27E−17 | − 1.203 | 0.243 | 7.78E−07 | 4.26E−01 |
| ILMN_1761450 | DHRS4L2 | rs10483284 | 14 | 23932540 | T | 0.037 | 7.01E−03 | 5.36E−38 | − 0.497 | 0.103 | 1.31E−06 | 7.99E−02 |
| ILMN_1797046 | MTHFSD | rs8047645 | 16 | 86547585 | A | 0.048 | 2.38E−02 | 7.35E−45 | − 0.471 | 0.114 | 3.83E−05 | 3.79E−01 |
| ILMN_1720233 | CWC25 | rs12952045 | 17 | 38784819 | T | 0.013 | 3.70E−02 | 9.63E−44 | 0.493 | 0.099 | 6.07E−07 | 6.71E−02 |
| ILMN_1655422 | RPL17 | rs17656050 | 18 | 49495404 | G | 0.04 | 9.38E−03 | 3.87E−113 | 0.272 | 0.055 | 9.24E−07 | 8.25E−02 |
| ILMN_1756469 | GAMT | rs3786974 | 19 | 1400867 | T | 0.03 | 2.64E−02 | 1.35E−18 | 1.182 | 0.256 | 3.83E−06 | 3.72E−01 |
| ILMN_1794595 | GAMT | rs3786974 | 19 | 1400867 | T | 0.03 | 2.64E−02 | 5.93E−09 | 1.815 | 0.457 | 7.21E−05 | 5.20E−02 |
| ILMN_1721225 | AAR2 | rs41293100 | 20 | 36232134 | A | 0.034 | 3.09E−03 | 1.31E−35 | − 0.512 | 0.091 | 2.19E−08 | 1.29E−01 |
Abbreviations: AD, Alzheimer’s disease; eQTL, expression quantitative trait loci; HTN-P, hypertension-positive; HTN-N, hypertension-negative
Chr: top eQTL chromosome; Pos: position of top eQTL based on Human Genome version 38 (hg38); A1/A1.freq: effect allele and its frequency
The false discovery rates (FDR) thresholds for SMR test were 0.01 and 0.05 in the HTN-N and HTN-P groups, respectively
Table 4.
AD-associated probe/genes detected by the transcriptome-wide analyses of brain tissue data in the HTN-N group
| Probe ID | Gene | Top eQTL | Chr | Pos | A1 | Freq | P GWAS | P eQTL | b SMR | SESMR | P SMR | P HEIDI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ENSG00000060971 | ACAA1 | rs61095322 | 3 | 38126634 | A | 0.955 | 7.36E−03 | 2.12E−10 | − 0.639 | 0.159 | 5.79E−05 | 3.42E−01 |
| ENSG00000163945 | UVSSA | rs111388896 | 4 | 1166078 | C | 0.957 | 5.15E−03 | 2.27E−24 | 0.399 | 0.079 | 3.95E−07 | 9.30E−01 |
| ENSG00000087008 | ACOX3 | rs62286001 | 4 | 8377136 | C | 0.943 | 5.49E−03 | 7.84E−14 | 0.524 | 0.127 | 3.82E−05 | 1.00E−01 |
| ENSG00000223969 | CDK14 | rs17867001 | 7 | 90631335 | G | 0.947 | 1.01E−02 | 4.63E−17 | 0.239 | 0.061 | 7.72E−05 | 9.46E−01 |
| ENSG00000166788 | SAAL1 | rs7106970 | 11 | 18074035 | C | 0.407 | 5.88E−04 | 1.15E−43 | − 0.536 | 0.115 | 3.18E−06 | 8.85E−01 |
| ENSG00000086205 | FOLH1 | rs11602981 | 11 | 49980436 | C | 0.076 | 1.34E−03 | 0 | 0.002 | 0.0001 | 3.23E−146 | 4.92E−01 |
| ENSG00000149418 | ST14 | rs34008994 | 11 | 130165703 | C | 0.904 | 1.55E−04 | 2.79E−08 | − 1.072 | 0.246 | 1.30E−05 | 3.34E−01 |
| ENSG00000259065 | LOC100506476 | rs17182607 | 14 | 73809286 | A | 0.846 | 4.78E−03 | 0 | 0.178 | 0.043 | 3.20E−05 | 4.21E−01 |
| ENSG00000070761 | C16orf80 | rs74019790 | 16 | 58107923 | T | 0.931 | 5.00E−04 | 6.30E−09 | − 0.927 | 0.211 | 1.07E−05 | 1.57E−01 |
| ENSG00000105298 | CACTIN | rs12984273 | 19 | 3647903 | T | 0.571 | 2.71E−03 | 1.30E−21 | − 0.592 | 0.151 | 8.61E−05 | 5.74E−01 |
Please see the description provided below Table 3
The false discovery rates (FDR) threshold for SMR test was 0.025
No AD-associated SNPs with P < 5E−08 were found within 1 Mb up-/downstream regions of these genes in our GWA analyses. However, evidence of strong associations with AD at the genome-wide significance level was reported for SNPs within 1 Mb of CACTIN, ERCC3, GAMT, and SAP30L genes by previous GWAS. Also, AD-associated SNPs at P < 5E−06 were previously reported in the 1 Mb flanking regions of AAR2, ACAA1, AIM2, C16orf80, DHRS4L2, FOLH1, IFIT2, IFIT3, LOC100506476, MTHFSD, NDRG3, RMND5B, TMBIM4, and TMEM2 genes. In addition, PADI4, UVSSA, ACOX3, CDK14, SAAL1, and ST14 were located in chromosomal regions (i.e., 1p36.13, 4p16.3, 4p16.1, 7q21.13, 11p15.1, and 11q24.3, respectively) containing AD-associated SNPs at P < 5E−06 (Leslie et al. 2014; MacArthur et al. 2017). No association signals at P < 5E−06 were discovered within 2p13.1, 9p13.3, 17q12, and 18q21.1 regions corresponding to DGUOK, TPM2, CWC25, and RPL17, respectively, in previous GWAS. Detailed information about previously detected AD-associated SNPs in these genes and chromosomal regions can be found in Table S13.
-
iv.
Pathway-enrichment analysis
Our pathway-based analyses (Table 5) demonstrated that 15 pathways were significantly associated with AD at the FDR level of 0.05 that ensured the number of possible false-positives was < 1 among the significant findings. Of these, 11 pathways were significant in both HTN-P and HTN-N groups. The other 4 pathways were group-specific, as 1 of them was significant in the HTN-P group, and the other 3 were significant only in the HTN-N group.
Table 5.
AD-associated pathways detected by the pathway-enrichment analyses
| Pathway | Pathway GSEA ID | No. genes | HTN-P group | HTN-N group | ||
|---|---|---|---|---|---|---|
| Chi-square | P value | Chi-square | P value | |||
| Chylomicron-mediated lipid transport | M14162 | 16 | 2162.29 | 2.66E−16 | 1948.48 | 3.96E−14 |
| Mitochondrial protein import | M590 | 58 | 2980.09 | 1.99E−15 | 2825.24 | 7.28E−15 |
| HDL-mediated lipid transport | M5056 | 15 | 2259.68 | 3.26E−13 | 2146.58 | 1.79E−12 |
| Nectin adhesion pathway* | M72 | 30 | 3652.62 | 1.05E−11 | 3676.89 | 4.75E−13 |
| Lipoprotein metabolism | M3462 | 28 | 3096.39 | 2.26E−11 | 2910.74 | 3.07E−10 |
| Lipid digestion, mobilization, and transport | M1023 | 46 | 3727.71 | 5.37E−10 | 3590.66 | 7.71E−10 |
| E-cadherin stabilization pathway* | M232 | 42 | 4072.55 | 6.04E−08 | 3918.9 | 1.04E−07 |
| Immunoregulatory interactions between a lymphoid and a non-lymphoid cell | M8240 | 70 | 4149.51 | 8.13E−08 | 3865.09 | 7.93E−07 |
| Cell-cell junction organization | M820 | 56 | 7000.69 | 6.72E−07 | 6400.05 | 6.62E−05 |
| Adherens junctions interactions | M11980 | 27 | 5445.75 | 8.80E−07 | 5075.21 | 1.51E−05 |
| Cell junction organization | M19248 | 78 | 8439.37 | 4.00E−06 | 7908.32 | 7.06E−05 |
| Platelet homeostasis | M916 | 78 | 7404.97 | 1.77E−04 | NS | NS |
| Signal transduction by L1 | M878 | 34 | NS | NS | 2813.01 | 1.63E−04 |
| TAK1 activating NFKB by phosphorylation and activation of IKK complex | M852 | 23 | NS | NS | 1545.78 | 1.64E−04 |
| Nucleotide-binding domain leucine-rich repeat containing receptor NLR signaling pathways | M1074 | 46 | NS | NS | 2478.88 | 2.13E−04 |
Abbreviations: AD, Alzheimer’s disease; GSEA, gene set enrichment analysis platform; HTN-P, hypertension-positive; HTN-N, hypertension-negative; NS, non-significant
*The definition of pathway is based on the Pathway Interaction Database (PID). The other pathways are from the REACTOME pathway knowledgebase
Discussion
HTN is an important risk factor for AD which doubles the risk of disease incidence in elderly and contribute to its progression (Csiszar et al. 2017). An increased number of studies have examined the potential links between HTN and AD underlying mechanisms. It has been suggested that, in addition to the hallmark pathologic changes such as amyloid plaques and neurofibrillary tangles, cerebral microvascular pathologies (e.g., arising from Aβ deposition) play an important role in cognitive impairment and AD pathogenesis. The dysfunction or degeneration of cerebral microvasculature may lead to the cerebral hypoperfusion, hypoxic stress, impaired clearance and increased deposition of Aβ, increased neuroinflammatory responses, and neuronal dysfunction or loss (Zlokovic 2011; Csiszar et al. 2017). There is strong empirical evidence indicating that aging process and HTN per se can also cause structural and functional damages to the cerebral microvasculature that may lead to the cognitive impairment and/or AD onset, or may superimpose on AD-related cerebrovascular pathologies exacerbating AD progression (Iadecola et al. 2009; Csiszar et al. 2017). For instance, a marked capillary rarefaction and subsequent decline in cerebral perfusion has been reported in old ages and in the brain regions affected by amyloid and tau pathologies in early stages of AD (Buée et al. 1994; Johnson et al. 2005). HTN particularly in elderly may also cause cerebral capillary loss that can contribute to the AD pathogenesis (Suzuki et al. 2003; Tarantini et al. 2016). Decreased cerebral blood flow can reduce Aβ clearance and increase its deposition in cerebral vessels, further damaging the cerebral microvasculature (Csiszar et al. 2017). Also, HTN may damage the BBB structure and function, particularly in old ages (Zhang et al. 2010; Toth et al. 2013). BBB disintegration has been implicated in the onset and progression of AD (Zlokovic 2011; Sagare et al. 2013; van de Haar et al. 2016; Montagne et al. 2017). The BBB breakdown results in plasma proteins entering into brain parenchyma, and the subsequent activation of microglia and neuroinflammation. It also promotes amyloid angiopathy, tau pathology, and neuronal loss (Sagare et al. 2013; Csiszar et al. 2017). AD, aging, and HTN may also synergistically increase the risk of cerebral microhemorrhages (i.e., parenchymal hemorrhages with maximum diameters of less than 5–10 mm) which in turn can strongly exacerbate the cognitive impairment and AD progression (Jeerakathil et al. 2004; Yates et al. 2014; Ungvari et al. 2017). In addition, it has been reported that AD patients suffer from the impairment of autoregulation of cerebral perfusion in response to the metabolic demands of functioning cerebral tissue (i.e., neurovascular uncoupling). Aging and HTN may also act in concert with AD-induced pathologies to further impair neurovascular coupling mechanisms, compromise cerebral blood flow, and consequently worsen cognitive dysfunction (Tarantini et al. 2017b, a; Wiesmann et al. 2017; Csiszar et al. 2017).
In this work, we studied the potential heterogeneity of the genetic architecture of AD that may exist given the HTN status of subjects. Since the hypertension-positive subjects (HTN-P) are likely to have different genetic backgrounds from hypertension-negative individuals (HTN-N), it is also likely that the genetic mechanisms of AD represent some differences in these two groups. We investigated such potential differences through GWA, TWA, gene-based, and pathway-enrichment analyses which revealed both group-specific and non-group-specific associations of several SNPs, genes, and pathways with AD.
In addition to replicating the associations of a number of previously reported AD-associated SNPs, mostly within the chromosome 19q13 region, our GWA analyses identified 15 new associations at the suggestive level of significance (Tables 1 and S6). These newly detected AD-associated SNPs were all located outside the chromosome 19q13 region and were group-specific as they were significantly associated with AD in either HTN-P or HTN-N groups and, in addition, their ORs were significantly different between the two groups. Of these, 14 SNPs were considered informative and novel potential AD markers as there were no proxy SNPs within their 1 Mb up-/downstream regions that were in LD with them and were previously associated with AD at P < 5E−06. The overlapping or closest genes to some of these SNPs were previously implicated in AD by GWAS or candidate gene studies. For instance, the genetic variations in GRIN2B gene (corresponding to rs78210707 that was significant in the HTN-P group) were suggested to play a role in amyloid-β (Aβ)-mediated synaptic damage, neuronal loss, and impairment of memory and learning ability (Kessels et al. 2013; Andreoli et al. 2014). Also, SNPs mapped to this gene were associated with AD at the suggestive level of associations in previous GWAS (Leslie et al. 2014; MacArthur et al. 2017). In addition, SLC2A13 gene (corresponding to rs75036080 that was significant in the HTN-P group) may be involved in the Aβ production process because its silencing/overexpression resulted in the decrease/increase of Aβ production (Teranishi et al. 2015). Also, CHST1 gene (corresponding to rs1447575, rs1447576, and rs2666895 that were significant in the HTN-N group) that encodes a phase II drug metabolism enzyme was implicated in the variability of pharmacogenomics responses of patients to the medications for AD (Cacabelos 2016).
In gene-based analyses, besides several genes inside the APOE cluster genes region that were mostly significant in both investigated groups, JAM2 gene located inside the chromosome 21q21 region was associated with AD in the HTN-N group. Several SNPs in this region (e.g., rs1910621, rs2828893, rs2828897, rs12482241, rs2830165, rs2040280, rs13050454, rs219710, and rs7276641) were previously associated with diastolic blood pressure, plasma renin activity (Hiura et al. 2010), and coronary disease (Wen and Yeh 2014), with their minor allele having protective effects on these traits. This might indicate possible pleiotropic effects of the genetic variants in this region on both AD and HTN/cardiovascular diseases.
Our TWA analyses revealed evidence of associations of several probes corresponding to 28 genes with AD. Most of these associations were specifically observed only in one of the HTN-P and HTN-N groups, with PADI4 being the only significant gene in both groups. Also, ACAA1, SAAL1, and ST14 genes had significant probes in the analyses of eQTLs data from both blood cells and brain tissue. The corresponding top eQTLs were all nominally significant in our meta-analyses and, in addition, there were no AD-associated SNPs with P < 5E−08 within 1 Mb of these genes in our GWA analyses which might be due to the insufficient sample sizes in our study or the heterogeneity of SNP’s effects across the four investigated cohorts which may both compromise the power of conducted meta-analyses. It is also worthy of mention that the detected associations from TWA analyses did not imply on causal relationships between the significant genes and AD. Instead, they provided a list of prioritized targets for further candidate gene studies (Zhu et al. 2016).
These findings also provided additional support for GWAS and candidate gene studies that previously suggested some of these genes may be involved in AD pathology. For instance, previous GWAS have reported strong association signals (P < 5E−08) inside SAP30L and within 1 Mb of ERCC3, CACTIN, and GAMT genes (Leslie et al. 2014; MacArthur et al. 2017). ERCC3 and GAMT are located within the chromosome 2q14.3 and 19p13.3 regions in close proximity of two previously known AD genes, i.e., BIN1 and ABCA7, respectively. Also, CACTIN is located within the chromosome 19p13.3 region. Hermon et al. (1998) reported that the protein encoded by ERCC3 was increased in different regions of cortex and cerebellum of AD patients. Since ERCC3 is involved in DNA repair pathway, its upregulation might be indicative of a potential underlying oxidative stress (Hermon et al. 1998). Also, the enzyme encoded by GAMT is involved in biosynthesis of creatine, a critical energy provider for brain neurons. The impairment of creatine energy system has been previously implicated in AD pathogenesis (Bürklen et al. 2006).
Previous GWAS have also revealed suggestive association signals (P < 5E−06) within 1 Mb of AAR2, ACAA1, AIM2, C16orf80, DHRS4L2, FOLH1, IFIT2, IFIT3, LOC100506476, MTHFSD, NDRG3, RMND5B, TMBIM4, and TMEM2 genes (Leslie et al. 2014; MacArthur et al. 2017). In addition, DGUOK, IFIT2, IFIT3, and RPL17 were empirically implicated in AD by studies on human subjects. For example, the mitochondrial protein encoded by DGUOK was reported to decrease in the entorhinal and precuneus cortex of AD patients (Ansoleaga et al. 2015). IFIT2 and IFIT3 genes that encode two proteins involved in cytokine responses and innate immunity are located inside the chromosome 10q23 region that was linked to AD by linkage analyses of several independent studies (Grupe et al. 2006). In addition, the interaction between IFIT3 protein and three other (i.e., APP, CDC37, and ST13) proteins was implicated in AD susceptibility (Soler-López et al. 2011). The ribosomal protein encoded by RPL17 was found to be downexpressed in peripheral blood samples from patients with vascular dementia and AD (by ~ 4.8- and ~ 5.8-fold, respectively), compared with matched controls in a previous study (Luo et al. 2016).
The animal models and cell line studies have also revealed possible links between the cognitive impairment and/or AD pathology, and several other genes including AIM2 (Wu et al. 2016), CWC25 (Butzlaff et al. 2015), FOLH1 (Kim et al. 2010), ST14 (Wirz et al. 2013), and TMP2 (Hsu et al. 2017). There was also some indirect evidence linking ACAA1 gene to AD as the peroxisomal enzyme encoded by ACAA1 is involved in the biosynthesis of plasmalogens that were implicated in the metabolism and function of high-density lipoprotein cholesterol (HDL-C), a protective factor against AD (Maeba and Nakahara 2017). Plasmalogens deficiency was previously reported in diseases caused by oxidative stress and aging such as AD (Goodenowe et al. 2007; Maeba and Nakahara 2017).
Finally, our pathway-enrichment analyses revealed the associations of 15 pathways with AD at FDR level of 0.05. Eleven pathways were significantly associated with AD in both HTN-P and HTN-N groups. Four others were group-specific. These pathways were involved in processes such as mitochondrial function, lipid metabolism, immune system responses, cell-cell adhesion and junctional complex, and platelets homeostasis that have been linked to AD pathogenesis by previous studies (Querfurth and LaFerla 2010; Catricala et al. 2012; Liu and Zhang 2014; Leshchyns’ka and Sytnyk 2016; Stamatovic et al. 2016). For instance, mitochondrial dysfunction has been reported as an important pathologic finding in AD patients which may both result from and promote Aβ deposition (Swerdlow 2017). Dysfunctional mitochondria have also been implicated in augmenting oxidative stress (Querfurth and LaFerla 2010). Since the oxidative stress plays an important role in initiating and aggravating the cerebral microvascular pathologies observed in AD, aging, and HTN (Csiszar et al. 2017), the dysregulation of pathways that cause mitochondrial dysfunction may contribute to the molecular mechanisms underlying such cerebral microvasculature damages. As another example, the junctional complexes (e.g., tight junctions and adherens junctions) were identified as essential components of BBB (Stamatovic et al. 2016). Therefore, the dysregulation of proteins and pathways involved in cell adhesion and junctional complex may contribute to the functional and structural damages to the BBB integrity induced by AD, aging, and HTN (Zlokovic 2011; Montagne et al. 2015, 2017; Csiszar et al. 2017).
In conclusion, our GWA, TWA, gene-based, and pathway-based analyses revealed several group-specific and non-group-specific AD-associated SNPs, genes, and pathways. These findings provided novel insights into the possible heterogeneity of the genetic architecture of AD attributed to HTN. Further independent studies with larger sample sizes are necessary to replicate these findings and to obtain a more comprehensive view of such genetic heterogeneities, which in turn may have tremendous impact on the translation to AD treatment. Albeit beyond the scopes and design of our study, an essential issue to be addressed by future studies is to investigate the extent to which different genetic effects in the HTN-P and HTN-N groups might be mediated by their interactions with vascular risk factors (i.e., gene-by-environment interactions) or by the cerebral microvascular endophenotypes (e.g., capillary rarefaction, BBB disruption, neurovascular uncoupling, and microhemorrhages). As mentioned above, these cerebromicrovascular alterations result from complex synergistic interactions among aging process, AD (e.g., through Aβ-mediated damages), and HTN. Consequently, it is likely that the molecular mechanisms underlying their genetic bases would not be completely the same in the HTN-P and HTN-N subjects; instead, genetic heterogeneity might exist at the level of microvascular endophenotypes as well. Protecting cerebral microvascular system has been suggested as a potential strategy for improvement of cognition, and AD prevention and treatment (Sagare et al. 2013; Richardson et al. 2015; Lin et al. 2017; Csiszar et al. 2017). Therefore, exploring such potential genetic heterogeneities can be of great importance from a personalized medicine perspective as they may provide novel molecular targets for improving the cerebral microvasculature health and slowing down the AD progression.
Electronic supplementary material
(DOCX 165 kb)
Acknowledgments
This manuscript was prepared using limited access datasets obtained though dbGaP (accession numbers: phs000168.v2.p2 (LOADFS), phs000007.v28.p10 (FHS), phs000287.v5.p1 (CHS), and phs000428.v2.p2 (HRS)) and the University of Michigan. Phenotypic HRS data are available publicly and through restricted access from http://hrsonline.isr.umich.edu/index.php?p=data. See also Supporting Acknowledgment section in Additional File 1.
Funding
This research was supported by Grants from the National Institute on Aging (P01AG043352 and R01AG047310). The funders had no role in study design, data collection and analysis, decision to publish, or manuscript preparation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alireza Nazarian, Email: alireza.nazarian@duke.edu.
Alexander M. Kulminski, Email: kulminsk@duke.edu
References
- Allison PD. Comparing logit and probit coefficients across groups. Sociol Methods Res. 1999;28:186–208. doi: 10.1177/0049124199028002003. [DOI] [Google Scholar]
- Alzheimer’s Association 2016 Alzheimer’s disease facts and figures. Alzheimers Dement J Alzheimers Assoc. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Andreoli V, De Marco EV, Trecroci F, et al. Potential involvement of GRIN2B encoding the NMDA receptor subunit NR2B in the spectrum of Alzheimer’s disease. J Neural Transm Vienna Austria 1996. 2014;121:533–542. doi: 10.1007/s00702-013-1125-7. [DOI] [PubMed] [Google Scholar]
- Ansoleaga B, Jové M, Schlüter A, Garcia-Esparcia P, Moreno J, Pujol A, Pamplona R, Portero-Otín M, Ferrer I. Deregulation of purine metabolism in Alzheimer’s disease. Neurobiol Aging. 2015;36:68–80. doi: 10.1016/j.neurobiolaging.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57:289–300. [Google Scholar]
- Buée L, Hof PR, Bouras C, et al. Pathological alterations of the cerebral microvasculature in Alzheimer’s disease and related dementing disorders. Acta Neuropathol (Berl) 1994;87:469–480. doi: 10.1007/BF00294173. [DOI] [PubMed] [Google Scholar]
- Bürklen TS, Schlattner U, Homayouni R, Gough K, Rak M, Szeghalmi A, Wallimann T. The creatine kinase/creatine connection to Alzheimer’s disease: CK inactivation, APP-CK complexes, and focal creatine deposits. J Biomed Biotechnol. 2006;2006:1–11. doi: 10.1155/JBB/2006/35936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butzlaff M, Hannan SB, Karsten P, Lenz S, Ng J, Voßfeldt H, Prüßing K, Pflanz R, Schulz JB, Rasse T, Voigt A. Impaired retrograde transport by the dynein/dynactin complex contributes to tau-induced toxicity. Hum Mol Genet. 2015;24:3623–3637. doi: 10.1093/hmg/ddv107. [DOI] [PubMed] [Google Scholar]
- Cacabelos R. The complexity of Alzheimer’s disease pharmacogenomics and metabolomics in drug development. MetabolomicsOpen Access. 2016;6:e145. [Google Scholar]
- Carnevale D, Mascio G, D’Andrea I, et al. Hypertension induces brain β-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertens Dallas Tex 1979. 2012;60:188–197. doi: 10.1161/HYPERTENSIONAHA.112.195511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper J, Zweig AS, Villarreal C, et al. The UCSC Genome Browser database: 2018 update. Nucleic Acids Res. 2018;46:D762–D769. doi: 10.1093/nar/gkx1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catricala S, Torti M, Ricevuti G. Alzheimer disease and platelets: how’s that relevant. Immun Ageing A. 2012;9:20. doi: 10.1186/1742-4933-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conomos MP, Miller MB, Thornton TA. Robust inference of population structure for ancestry prediction and correction of stratification in the presence of relatedness. Genet Epidemiol. 2015;39:276–293. doi: 10.1002/gepi.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Tarantini S, Fülöp GA, Kiss T, Valcarcel-Ares MN, Galvan V, Ungvari Z, Yabluchanskiy A. Hypertension impairs neurovascular coupling and promotes microvascular injury: role in exacerbation of Alzheimer’s disease. GeroScience. 2017;39:359–372. doi: 10.1007/s11357-017-9991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviglus ML, Bell CC, Berrettini W, Bowen PE, Connolly ES Jr, Cox NJ, Dunbar-Jacob JM, Granieri EC, Hunt G, McGarry K, Patel D, Potosky AL, Sanders-Bush E, Silberberg D, Trevisan M. NIH state-of-the-science conference statement: preventing Alzheimer’s disease and cognitive decline. NIH Consens State Sci Statements. 2010;27:1–30. [PubMed] [Google Scholar]
- Dawber TR, Meadors GF, Moore FE. Epidemiological approaches to heart disease: the Framingham study. Am J Public Health Nations Health. 1951;41:279–286. doi: 10.2105/AJPH.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Ruiz C, Wang J, Ksiezak-Reding H, Ho L, Qian X, Humala N, Thomas S, Martínez-Martín P, Pasinetti GM. Role of hypertension in aggravating Abeta neuropathology of AD type and tau-mediated motor impairment. Cardiovasc Psychiatry Neurol. 2009;2009:107286. doi: 10.1155/2009/107286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabregat A, Jupe S, Matthews L, Sidiropoulos K, Gillespie M, Garapati P, Haw R, Jassal B, Korninger F, May B, Milacic M, Roca CD, Rothfels K, Sevilla C, Shamovsky V, Shorser S, Varusai T, Viteri G, Weiser J, Wu G, Stein L, Hermjakob H, D’Eustachio P. The reactome pathway knowledgebase. Nucleic Acids Res. 2018;46:D649–D655. doi: 10.1093/nar/gkx1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraco G, Iadecola C. Hypertension: a harbinger of stroke and dementia. Hypertension. 2013;62:810–817. doi: 10.1161/HYPERTENSIONAHA.113.01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraco G, Park L, Zhou P, Luo W, Paul SM, Anrather J, Iadecola C. Hypertension enhances Aβ-induced neurovascular dysfunction, promotes β-secretase activity, and leads to amyloidogenic processing of APP. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2016;36:241–252. doi: 10.1038/jcbfm.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham offspring study: design and preliminary data. Prev Med. 1975;4:518–525. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O’Leary DH, Psaty B, Rautaharju P, Tracy RP, Weiler PG. The cardiovascular health study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-W. [DOI] [PubMed] [Google Scholar]
- Goodenowe DB, Cook LL, Liu J, Lu Y, Jayasinghe DA, Ahiahonu PWK, Heath D, Yamazaki Y, Flax J, Krenitsky KF, Sparks DL, Lerner A, Friedland RP, Kudo T, Kamino K, Morihara T, Takeda M, Wood PL. Peripheral ethanolamine plasmalogen deficiency: a logical causative factor in Alzheimer’s disease and dementia. J Lipid Res. 2007;48:2485–2498. doi: 10.1194/jlr.P700023-JLR200. [DOI] [PubMed] [Google Scholar]
- Goudet J. hierfstat: a package for r to compute and test hierarchical F-statistics. Mol Ecol Notes. 2005;5:184–186. doi: 10.1111/j.1471-8286.2004.00828.x. [DOI] [Google Scholar]
- Grupe A, Li Y, Rowland C, Nowotny P, Hinrichs AL, Smemo S, Kauwe JSK, Maxwell TJ, Cherny S, Doil L, Tacey K, van Luchene R, Myers A, Wavrant-de Vrièze F, Kaleem M, Hollingworth P, Jehu L, Foy C, Archer N, Hamilton G, Holmans P, Morris CM, Catanese J, Sninsky J, White TJ, Powell J, Hardy J, O’Donovan M, Lovestone S, Jones L, Morris JC, Thal L, Owen M, Williams J, Goate A. A scan of chromosome 10 identifies a novel locus showing strong association with late-onset Alzheimer disease. Am J Hum Genet. 2006;78:78–88. doi: 10.1086/498851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl DL, Clark AG (1997) Principles of population genetics. Sinauer Associates
- Hermon M, Cairns N, Egly JM, Fery A, Olga Labudova, Lubec G. Expression of DNA excision-repair-cross-complementing proteins p80 and p89 in brain of patients with Down syndrome and Alzheimer’s disease. Neurosci Lett. 1998;251:45–48. doi: 10.1016/S0304-3940(98)00488-1. [DOI] [PubMed] [Google Scholar]
- Hiura Y, Tabara Y, Kokubo Y, et al. A genome-wide association study of hypertension-related phenotypes in a Japanese population. Circ J Off J Jpn Circ Soc. 2010;74:2353–2359. doi: 10.1253/circj.cj-10-0353. [DOI] [PubMed] [Google Scholar]
- Hsu WL, Ma YL, Liu YC, Lee EHY. Smad4 SUMOylation is essential for memory formation through upregulation of the skeletal myopathy gene TPM2. BMC Biol. 2017;15:112. doi: 10.1186/s12915-017-0452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Park L, Capone C. Threats to the mind: aging, amyloid, and hypertension. Stroke. 2009;40:S40–S44. doi: 10.1161/STROKEAHA.108.533638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeerakathil T, Wolf PA, Beiser A, Hald JK, Au R, Kase CS, Massaro JM, DeCarli C. Cerebral microbleeds: prevalence and associations with cardiovascular risk factors in the Framingham study. Stroke. 2004;35:1831–1835. doi: 10.1161/01.STR.0000131809.35202.1b. [DOI] [PubMed] [Google Scholar]
- Johnson NA, Jahng G-H, Weiner MW, Miller BL, Chui HC, Jagust WJ, Gorno-Tempini ML, Schuff N. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology. 2005;234:851–859. doi: 10.1148/radiol.2343040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels HW, Nabavi S, Malinow R. Metabotropic NMDA receptor function is required for β-amyloid-induced synaptic depression. Proc Natl Acad Sci U S A. 2013;110:4033–4038. doi: 10.1073/pnas.1219605110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M-J, Chae SS, Koh YH, Lee SK, Jo SA. Glutamate carboxypeptidase II: an amyloid peptide-degrading enzyme with physiological function in the brain. FASEB J. 2010;24:4491–4502. doi: 10.1096/fj.09-148825. [DOI] [PubMed] [Google Scholar]
- Lee JH, Cheng R, Graff-Radford N, Foroud T, Mayeux R, National Institute on Aging Late-Onset Alzheimer’s Disease Family Study Group Analyses of the national institute on aging late-onset Alzheimer’s disease family study: implication of additional loci. Arch Neurol. 2008;65:1518–1526. doi: 10.1001/archneur.65.11.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon AC, Heo M. Sample sizes required to detect interactions between two binary fixed-effects in a mixed-effects linear regression model. Comput Stat Data Anal. 2009;53:603–608. doi: 10.1016/j.csda.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshchyns’ka I, Sytnyk V. Synaptic cell adhesion molecules in Alzheimer’s disease. Neural Plast. 2016;2016:1–9. doi: 10.1155/2016/6427537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie R, O’Donnell CJ, Johnson AD. GRASP: analysis of genotype-phenotype results from 1390 genome-wide association studies and corresponding open access database. Bioinforma Oxf Engl. 2014;30:i185–i194. doi: 10.1093/bioinformatics/btu273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A-L, Jahrling JB, Zhang W, DeRosa N, Bakshi V, Romero P, Galvan V, Richardson A. Rapamycin rescues vascular, metabolic and learning deficits in apolipoprotein E4 transgenic mice with pre-symptomatic Alzheimer’s disease. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2017;37:217–226. doi: 10.1177/0271678X15621575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Zhang J. Lipid metabolism in Alzheimer’s disease. Neurosci Bull. 2014;30:331–345. doi: 10.1007/s12264-013-1410-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones LR, Holloway A, McRae A, Yang J, Small K, Zhao J, Zeng B, Bakshi A, Metspalu A, Dermitzakis M, Gibson G, Spector T, Montgomery G, Esko T, Visscher PM, Powell JE. The genetic architecture of gene expression in peripheral blood. Am J Hum Genet. 2017;100:228–237. doi: 10.1016/j.ajhg.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Han G, Wang J, Zeng F, Li Y, Shao S, Song F, Bai Z, Peng X, Wang YJ, Shi X, Lei H. Common aging signature in the peripheral blood of vascular dementia and Alzheimer’s disease. Mol Neurobiol. 2016;53:3596–3605. doi: 10.1007/s12035-015-9288-x. [DOI] [PubMed] [Google Scholar]
- MacArthur J, Bowler E, Cerezo M, Gil L, Hall P, Hastings E, Junkins H, McMahon A, Milano A, Morales J, Pendlington ZM, Welter D, Burdett T, Hindorff L, Flicek P, Cunningham F, Parkinson H. The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog) Nucleic Acids Res. 2017;45:D896–D901. doi: 10.1093/nar/gkw1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machiela MJ, Chanock SJ. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinforma Oxf Engl. 2015;31:3555–3557. doi: 10.1093/bioinformatics/btv402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeba R, Nakahara S (2017) Extrinsic effectors regulating genes for plasmalogen biosynthetic enzymes in HepG2 cells. Biomed Res Clin Pract 2. doi: 10.15761/BRCP.1000128
- Mägi R, Morris AP. GWAMA: software for genome-wide association meta-analysis. BMC Bioinformatics. 2010;11:288. doi: 10.1186/1471-2105-11-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manichaikul A, Mychaleckyj JC, Rich SS, Daly K, Sale M, Chen WM. Robust relationship inference in genome-wide association studies. Bioinforma Oxf Engl. 2010;26:2867–2873. doi: 10.1093/bioinformatics/btq559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle PF, O’Connell JR, Pollin TI, et al. Accounting for relatedness in family based genetic association studies. Hum Hered. 2007;64:234–242. doi: 10.1159/000103861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A, Zhao Z, Zlokovic BV. Alzheimer’s disease: a matter of blood-brain barrier dysfunction? J Exp Med. 2017;214:3151–3169. doi: 10.1084/jem.20171406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarian A, Yashin AI, Kulminski AM. Genome-wide analysis of genetic predisposition to Alzheimer’s disease and related sex disparities. Alzheimers Res Ther. 2019;11:5. doi: 10.1186/s13195-018-0458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M (1987) Molecular evolutionary genetics, Reprint edn. Columbia University Press, New York
- Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13:788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- Nwankwo T, Yoon SS, Burt V, Gu Q (2013) Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011–2012. Centers for Disease Control and Prevention
- Petrovitch H, White LR, Izmirilian G, Ross GW, Havlik RJ, Markesbery W, Nelson J, Davis DG, Hardman J, Foley DJ, Launer LJ. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Neurobiol Aging. 2000;21:57–62. doi: 10.1016/s0197-4580(00)00106-8. [DOI] [PubMed] [Google Scholar]
- Power MC, Weuve J, Gagne JJ, McQueen MB, Viswanathan A, Blacker D. The association between blood pressure and incident Alzheimer disease: a systematic review and meta-analysis. Epidemiol Camb Mass. 2011;22:646–659. doi: 10.1097/EDE.0b013e31822708b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, Sklar P, de Bakker PIW, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi T, Wu Y, Zeng J, et al. Identifying gene targets for brain-related traits using transcriptomic and methylomic data from blood. Nat Commun. 2018;9:2282. doi: 10.1038/s41467-018-04558-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Raghavan N, Tosto G. Genetics of Alzheimer’s disease: the importance of polygenic and epistatic components. Curr Neurol Neurosci Rep. 2017;17:78. doi: 10.1007/s11910-017-0787-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A, Galvan V, Lin A-L, Oddo S. How longevity research can lead to therapies for Alzheimer’s disease: the rapamycin story. Exp Gerontol. 2015;68:51–58. doi: 10.1016/j.exger.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagare AP, Bell RD, Zhao Z, Ma Q, Winkler EA, Ramanathan A, Zlokovic BV. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun. 2013;4:2932. doi: 10.1038/ncomms3932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Schaefer CF, Anthony K, Krupa S, Buchoff J, Day M, Hannay T, Buetow KH. PID: the pathway interaction database. Nucleic Acids Res. 2009;37:D674–D679. doi: 10.1093/nar/gkn653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S, Drukarch B, Garz C, Niklass S, Stanaszek L, Kropf S, Bueche C, Held F, Vielhaber S, Attems J, Reymann KG, Heinze HJ, Carare RO, Wilhelmus MMM. Interplay between age, cerebral small vessel disease, parenchymal amyloid-β, and tau pathology: longitudinal studies in hypertensive stroke-prone rats. J Alzheimers Dis JAD. 2014;42(Suppl 3):S205–S215. doi: 10.3233/JAD-132618. [DOI] [PubMed] [Google Scholar]
- Soler-López M, Zanzoni A, Lluís R, et al. Interactome mapping suggests new mechanistic details underlying Alzheimer’s disease. Genome Res. 2011;21:364–376. doi: 10.1101/gr.114280.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR. Cohort profile: the health and retirement study (HRS) Int J Epidemiol. 2014;43:576–585. doi: 10.1093/ije/dyu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatovic SM, Johnson AM, Keep RF, Andjelkovic AV. Junctional proteins of the blood-brain barrier: new insights into function and dysfunction. Tissue Barriers. 2016;4:e1154641. doi: 10.1080/21688370.2016.1154641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer MJ. Cardiovascular disease and Alzheimer’s disease: common links. J Intern Med. 2006;260:211–223. doi: 10.1111/j.1365-2796.2006.01687.x. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Masawa N, Sakata N, Takatama M. Pathologic evidence of microvascular rarefaction in the brain of renal hypertensive rats. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc. 2003;12:8–16. doi: 10.1053/jscd.2003.1. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH. Mitochondria and mitochondrial cascades in Alzheimer’s disease. J Alzheimers Dis. 2017;62:1403–1416. doi: 10.3233/JAD-170585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Fulop GA, Kiss T, Farkas E, Zölei-Szénási D, Galvan V, Toth P, Csiszar A, Ungvari Z, Yabluchanskiy A. Demonstration of impaired neurovascular coupling responses in TG2576 mouse model of Alzheimer’s disease using functional laser speckle contrast imaging. GeroScience. 2017;39:465–473. doi: 10.1007/s11357-017-9980-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Tran CHT, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer’s disease: contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol. 2017;94:52–58. doi: 10.1016/j.exger.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Tucsek Z, Valcarcel-Ares MN, Toth P, Gautam T, Giles CB, Ballabh P, Wei JY, Wren JD, Ashpole NM, Sonntag WE, Ungvari Z, Csiszar A. Circulating IGF-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the mouse hippocampus and retrosplenial cortex: implications for cerebromicrovascular and brain aging. Age (Dordr) 2016;38:273–289. doi: 10.1007/s11357-016-9931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teranishi Y, Inoue M, Yamamoto NG, Kihara T, Wiehager B, Ishikawa T, Winblad B, Schedin-Weiss S, Frykman S, Tjernberg LO. Proton myo-inositol cotransporter is a novel γ-secretase associated protein that regulates Aβ production without affecting Notch cleavage. FEBS J. 2015;282:3438–3451. doi: 10.1111/febs.13353. [DOI] [PubMed] [Google Scholar]
- Thorin E. Hypertension and Alzheimer disease: another brick in the wall of awareness. Hypertension. 2015;65:36–38. doi: 10.1161/HYPERTENSIONAHA.114.04257. [DOI] [PubMed] [Google Scholar]
- Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2013;33:1732–1742. doi: 10.1038/jcbfm.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z, Noa Valcarcel-Ares M, Tarantini S, Yabluchanskiy A, Fülöp G, Gautam T, Orock A, Csiszar A, Deak F, Ungvari Z. Hypertension-induced synapse loss and impairment in synaptic plasticity in the mouse hippocampus mimics the aging phenotype: implications for the pathogenesis of vascular cognitive impairment. GeroScience. 2017;39:385–406. doi: 10.1007/s11357-017-9981-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Kirkpatrick AC, Csiszar A, Prodan CI. Cerebral microhemorrhages: mechanisms, consequences, and prevention. Am J Physiol Heart Circ Physiol. 2017;312:H1128–H1143. doi: 10.1152/ajpheart.00780.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Haar HJ, Jansen JFA, van Osch MJP, van Buchem MA, Muller M, Wong SM, Hofman PAM, Burgmans S, Verhey FRJ, Backes WH. Neurovascular unit impairment in early Alzheimer’s disease measured with magnetic resonance imaging. Neurobiol Aging. 2016;45:190–196. doi: 10.1016/j.neurobiolaging.2016.06.006. [DOI] [PubMed] [Google Scholar]
- Verma SS, de Andrade M, Tromp G, Kuivaniemi H, Pugh E, Namjou-Khales B, Mukherjee S, Jarvik GP, Kottyan LC, Burt A, Bradford Y, Armstrong GD, Derr K, Crawford DC, Haines JL, Li R, Crosslin D, Ritchie MD. Imputation and quality control steps for combining multiple genome-wide datasets. Front Genet. 2014;5:370. doi: 10.3389/fgene.2014.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir BS, Cockerham CC. Estimating F-statistics for the analysis of population structure. Evolution. 1984;38:1358–1370. doi: 10.1111/j.1558-5646.1984.tb05657.x. [DOI] [PubMed] [Google Scholar]
- Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen S-H, Yeh J-I. Cohen’s h for detection of disease association with rare genetic variants. BMC Genomics. 2014;15:875. doi: 10.1186/1471-2164-15-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesmann M, Roelofs M, van der Lugt R, Heerschap A, Kiliaan AJ, Claassen JAHR. Angiotensin II, hypertension and angiotensin II receptor antagonism: roles in the behavioural and brain pathology of a mouse model of Alzheimer’s disease. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2017;37:2396–2413. doi: 10.1177/0271678X16667364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirz KTS, Bossers K, Stargardt A, Kamphuis W, Swaab DF, Hol EM, Verhaagen J. Cortical beta amyloid protein triggers an immune response, but no synaptic changes in the APPswe/PS1dE9 Alzheimer’s disease mouse model. Neurobiol Aging. 2013;34:1328–1342. doi: 10.1016/j.neurobiolaging.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Wu P-J, Liu H-Y, Huang T-N, Hsueh Y-P. AIM2 inflammasomes regulate neuronal morphology and influence anxiety and memory in mice. Sci Rep. 2016;6:32405. doi: 10.1038/srep32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88:76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates PA, Desmond PM, Phal PM, Steward C, Szoeke C, Salvado O, Ellis KA, Martins RN, Masters CL, Ames D, Villemagne VL, Rowe CC, For the AIBL Research Group Incidence of cerebral microbleeds in preclinical Alzheimer disease. Neurology. 2014;82:1266–1273. doi: 10.1212/WNL.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Mao Y, Ramirez SH, Tuma RF, Chabrashvili T. Angiotensin II induced cerebral microvascular inflammation and increased blood-brain barrier permeability via oxidative stress. Neuroscience. 2010;171:852–858. doi: 10.1016/j.neuroscience.2010.09.029. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Zhang F, Hu H, Bakshi A, Robinson MR, Powell JE, Montgomery GW, Goddard ME, Wray NR, Visscher PM, Yang J. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat Genet. 2016;48:481–487. doi: 10.1038/ng.3538. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 165 kb)