Abstract
Background
Components of substance use disorder (SUD) treatment have been shown to reduce inpatient and emergency department (ED) utilization. However, integrated treatment using pharmacotherapy and recovery coaches in primary care has not been studied.
Objective
To determine whether integrated addiction treatment in primary care reduces inpatient and ED utilization and improves outpatient engagement.
Design
A retrospective cohort study comparing patients in practices with and without integrated addiction treatment including pharmacotherapy and recovery coaching during a staggered roll-out period.
Participants
A propensity score matched sample of 2706 adult primary care patients (1353 matched pairs from intervention and control practices) with a SUD diagnosis code, excluding cannabis or tobacco only, matched on baseline utilization.
Intervention
A multi-modal strategy that included forming interdisciplinary teams of local champions, access to addiction pharmacotherapy, counseling, and recovery coaching. Control practices could refer patients to an addiction treatment clinic offering pharmacotherapy and behavioral interventions.
Main Measures
The number of inpatient admissions, hospital bed days, ED visits, and primary care visits.
Key Results
During the follow-up period, there were fewer inpatient days among the intervention group (997 vs. 1096 days with a mean difference of 7.3 days per 100 patients, p = 0.03). The mean number of ED visits was lower for the intervention group (36.2 visits vs. 42.9 per 100 patients, p = 0.005). There was no difference in the mean number of hospitalizations. The mean number of primary care visits was higher for the intervention group (317 visits vs. 270 visits per 100 patients, p < 0.001). Intervention practices had a greater increase in buprenorphine and naltrexone prescribing.
Conclusions
In a non-randomized retrospective cohort study, integrated addiction pharmacotherapy and recovery coaching in primary care resulted in fewer hospital days and ED visits for patients with SUD compared to similarly matched patients receiving care in practices without these services.
KEY WORDS: addiction, substance use disorder, recovery coach, primary care, buprenorphine, integrated addiction treatment, utilization
BACKGROUND
Untreated substance use disorder (SUD) results in substantial healthcare costs.1–3 Integrating addiction treatment into medical settings has been shown to be feasible and clinically effective. Previous research has demonstrated improved clinical outcomes with the integration of addiction pharmacotherapy or behavioral interventions into primary care, hospital settings, the emergency department (ED), and HIV treatment, among others.4–7
While numerous studies have looked at the impact of integrated addiction treatment in primary care on clinical outcomes, less is known about the impact on healthcare utilization. Individual components of SUD treatment including pharmacotherapy for alcohol and opioid use disorder that were offered within primary care have been shown to reduce inpatient and emergency department utilization.8–11 A 2001 study evaluated the opposite model of integrating primary care into addiction treatment and found that individuals randomly assigned to receive integrated primary care versus no primary care in an outpatient addiction treatment program had improvements in substance-related medical conditions.12 We are not aware of any studies that have examined the impact of integrated addiction treatment which includes pharmacotherapy and recovery coaches in primary care on acute care utilization. Recovery coaches, who are peers with a history of SUD, are increasingly being utilized to offer outreach, navigation, and support for patients with SUD. While the literature on the use of recovery coaches is limited, a 2016 systematic review suggested a positive impact. The systematic review, which included nine studies, examined the effectiveness of peer-delivered recovery support services and found that most studies showed significant improvements in abstinence and other recovery outcomes.13 While the range of peer-delivered services included recovery coaching, most looked at different types of peer support. The one study which looked specifically at the impact of recovery coaches in community-based recovery centers found that patients connected to a recovery coach had more primary care visits; fewer hospital, ED, and inpatient detoxification admissions; and significant improvements in recovery capital.13 The impact of recovery coaches based in primary care settings is unknown.
The goal of this study was to evaluate the impact of a new clinical initiative to integrate addiction treatment into primary care on acute care utilization for primary care patients with a SUD. Beginning in October 2014, our hospital launched a system-wide SUD initiative with a goal of increasing access to treatment across care settings. Our previous work demonstrated improvements in abstinence and reductions in addiction severity and self-reported acute care utilization among individuals receiving hospital-based addiction treatment through this new initiative.14 The goal of this study was to evaluate the impact of the outpatient components of this initiative which included integrated addiction pharmacotherapy and recovery coaches within select primary care practices. Our primary outcome was acute care utilization, measured by the number of inpatient admissions, hospital bed days, and ED visits. Our secondary outcome was the number of primary care visits. We chose to focus on acute care utilization given the growing recognition that ED visits and rehospitalizations are potentially preventable and always costly events and medical patients with SUD have higher rates of both types of acute care utilization.2 Our hypothesis was that patients with SUD receiving care in practices with this new model would have reduced inpatient and ED utilization and improved engagement in outpatient care.
METHODS
Study Design
A retrospective cohort study comparing patients in primary care practices with and without integrated addiction treatment and recovery coaching during a staggered roll-out period, with each site launching the intervention on different start dates.
Study Site
Massachusetts General Hospital (MGH) primary care practices. MGH provides primary care services for adults in 18 locations throughout the greater Boston area seeing over 160,000 patients and conducting roughly 500,000 visits per year. Between October 2014 and December 2015, four MGH primary care practices implemented a new integrated SUD care model. Patients from practices not receiving the intervention programs at the same time period served as the control group. The four intervention practices included three community health centers and one large hospital-based practice. These practices voluntarily agreed to participate. The control practices included hospital-based practices, community health centers, and satellite primary practices. Practices in both intervention and control groups varied in access to onsite behavioral health resources, but all could refer patients to a hospital-based specialty addiction clinic.
Participants
Adult patients with a SUD diagnosis code, excluding cannabis or tobacco only, receiving primary care at any MGH practice in a 9-month period prior to the site-specific launch of the intervention.
Intervention
The practice-level intervention was a multi-modal strategy that included the formation of interdisciplinary teams of local champions, access to pharmacotherapy with buprenorphine and extended-release naltrexone, counseling, and recovery coaching. The champion teams consisted of at least one representative from the primary care physicians, nursing staff, and administration at each practice. Teams met bi-monthly with an addiction specialist for case conferences. These conferences offered team members an opportunity to present complex SUD cases, get input from a specialist, and discuss care plans with the group. This provided support for providers as well as education and training. One recovery coach was included in each intervention practice to assist patients by addressing barriers to treatment and providing motivational support. Recovery coaches were required to have 2 years of sobriety, took a 5-day course in recovery coaching, worked full-time within the primary care site, were available during business hours, and provided in-person, telephonic, and text messaging support to patients. Recovery coaches also provided informal education to providers and joined all case conferences and champions’ meetings. Other staff in the practice were invited to the bi-monthly meetings and joined intermittently, often to present a challenging case. All four intervention sites were required to offer office-based addiction treatment with buprenorphine and extended-release naltrexone. Access to buprenorphine initiation was reviewed monthly at champions’ meetings with the expectation that sites would be able to offer immediate initiation for appropriate patients. While the exact number of buprenorphine-waivered providers was not pre-specified, all sites had at least three prescribers. Buprenorphine waiver trainings were held regularly and offered to all providers. Technical assistance was provided by the addiction specialists to get extended-release naltrexone injection protocols implemented. Recovery coaches helped facilitate referrals to more intensive treatment settings, such as opioid treatment programs, residential treatment, or intensive outpatient programs as clinically indicated. All sites had access to behavioral health services either on-site or in adjacent practices. There were no additional behavioral health clinicians added as a part of the intervention; however, existing behavioral health staff joined the champion teams in three of the four practices.
Control practices did not have recovery coaches or integrated addiction treatment within the practice. Control practices could refer patients to a separate, stand-alone, specialty, consultative addiction treatment clinic which offered pharmacotherapy and behavioral interventions.
This study was approved by the Partners Human Research Committee.
Statistical Analysis
The pre-specified outcomes were healthcare utilization measures, including the number of inpatient admissions, hospital bed days, the number of ED visits, and the number of primary care provider visits. Baseline was defined as a 9-month period prior to the site-specific launch of the intervention and the follow-up period was defined as the same nine calendar months 1 year after baseline to allow for a 3-month transition period. For each patient at an intervention practice, we selected a patient from a control practice corresponding to the same pre- and post-intervention time periods. To control for baseline differences between patients from intervention and control practices, we used a combination of a propensity score matching based on the probability of being at an intervention site and an exact coarsened matching based on baseline utilization rates to match patients from the intervention practices to patients from the control practices. We included age, sex, race, education level, Charlson score, type of substance use disorder, and timing of diagnosis in the propensity score model predicting the probability of being at an intervention practice. We first matched patients from intervention practices (cases) to patients from control practices (controls) on the logit scale of the propensity score and used calipers of width equal to 0.2 of the standard deviation. Among potential matches, we further matched cases to controls in a 1:1 ratio on all categories of baseline utilization measures using coarsened exact matching. With all factors being closely matched, we compared the differences in utilization during the follow-up period from the matched samples using Poisson models without further adjustment.
Analyses were done using SAS version 9.4 (The SAS Institute, Cary, NC). A two-sided p value of < .05 was considered statistically significant.
RESULTS
We identified 1868 patients from the four intervention practices and 5135 from the 14 control practices. Those in the intervention practices were younger, more likely to be racial minority, had lower education level, and more likely to have a SUD diagnosis of drug use disorder. These imbalances were significantly improved in the propensity score matched sample of 1353 pairs. The matched sample had an overall mean age of 49, 61% male, and predominantly non-Hispanic white (83%). Drug use disorder NOS (coded for 69% of patients) was the most common diagnosis. Among those with a specified type of drug use disorder, opioid use disorder was seen most frequently (45%), followed by cocaine (5.8%) and sedative/hypnotic (2.8%). Alcohol use disorder was diagnosed in 38% of the sample (Table 1).
Table 1.
Baseline Characteristics of Matched Intervention and Control Patients
| Matched sample* | |||
|---|---|---|---|
| Intervention | Control | p value | |
| N = 1353 | N = 1353 | ||
| Age, mean (SD) | 49.0 (14.5) | 49.2 (15.4) | 0.76 |
| Male, N (%) | 820 (60.6) | 834 (61.6) | 0.58 |
| Race White, N (%) | 924 (14.5) | (15.4) | 0.76 |
| SUD, N (%) | |||
| Alcohol | 505 (37.3) | 516 (38.1) | 0.66 |
| Drug NOS | 935 (69.1) | 931 (68.8) | 0.87 |
| Opioid | 613 (45.3) | 593 (43.8) | 0.44 |
| Sedative/hypnotic | 35 (2.6) | 40 (3.0) | 0.56 |
| Cocaine | 78 (5.8) | 78 (5.8) | 1.00 |
| Stimulant | 13 (1.0) | 18 (1.3) | 0.37 |
| LSD | 2 (0.1) | 2 (0.1) | 1.00 |
| Other/poly | 513 (37.9) | 504 (37.3) | 0.72 |
| Charlson comorbidity score, mean (SD) | 2.6 (2.5) | 2.5 (2.5) | 0.43 |
*The propensity score model included age, sex, race, education level, Charlson score, type of substance use disorder, and timing of diagnosis. Patients were matched on the logit scale of the propensity score using calipers of width equal to 0.2 of the standard deviation. Among potential matches, baseline utilization measures were further matched using coarsened exact matching
The two groups were matched on baseline utilization for all four utilization measures using coarsened exact match with a mean number of 7.0 inpatient admissions per 100 patients during the 9-month baseline period in each group. During the 9-month follow-up period, the mean number of inpatient admissions was 13.5 admissions per 100 patients for the intervention and 13.3 admissions per 100 patients for the control group (p = 0.92). The total number of inpatient days in the 9-month follow-up period was 997 days for intervention and 1096 days for control patients with a mean difference of 7.3 days per 100 patients (p = 0.03) (Fig. 1). During the 9-month baseline period, the mean number of emergency department visits was 34.9 visits per 100 patients for both groups. The mean number of ED visits during the 9-month follow-up was 36.2 visits per 100 patients for intervention and 42.9 visits per 100 patients for control patients (p = 0.005) (Fig. 2). The mean number of primary care visits during the 9-month baseline period was 370 visits per 100 patients for intervention and 361 visits per 100 patients for control patients. The mean number of primary care visits during the nine-month follow-up remained higher for the intervention group (317 visits per 100 patients vs. 270 visits per 100 patients, p < 0.001). (Table 2).
Fig. 1.
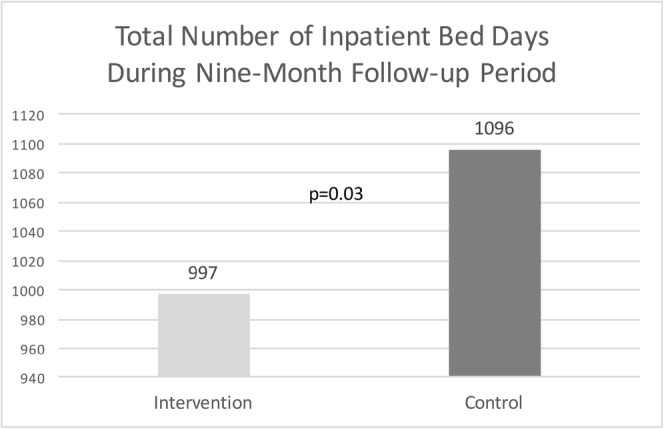
Total number of inpatient bed days during the 9-month follow-up period. Figures 1 and 2 contain text below the minimum required font size of 6pts inside the artwork, and there is no sufficient space available for the text to be enlarged. Please provide replacement figure files.I will provide replacement files.
Fig. 2.
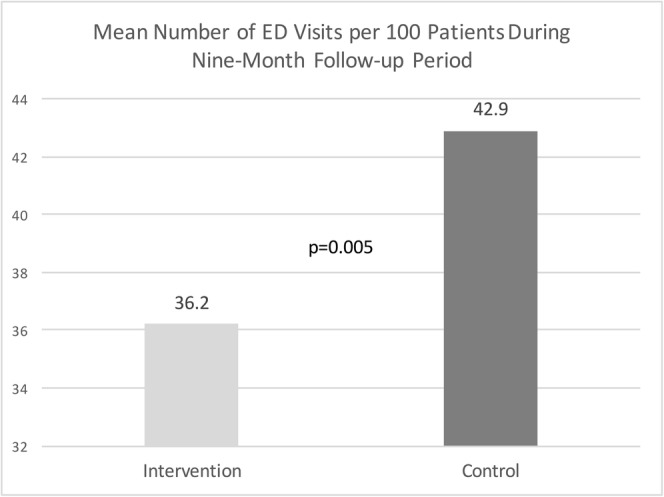
Mean number of ED visits per 100 patients during the 9-month follow-up period.
Table 2.
Utilization During the 9-Month Baseline and Follow-up Periods per 100 Patients
| Matched sample | |||
|---|---|---|---|
| Intervention | Control | P value* | |
| Inpatient admission, mean (SD) | |||
| Baseline | 7.0 (29.8) | 7.0 (29.8) | |
| Follow-up | 13.5 (54.6) | 13.3 (53.3) | 0.92 |
| Inpatient LOS, mean (SD) | |||
| Baseline | 33.2 (170.5) | 33.0 (169.0) | |
| Follow-up | 73.7 (377.5) | 81.0 (463.5) | 0.03 |
| ED visits, mean (SD) | |||
| Baseline | 34.9 (73.8) | 34.9 (73.8) | |
| Follow-up | 36.2 (95.8) | 42.9 (138.9) | 0.005 |
| Primary care visits, mean (SD) | |||
| Baseline | 370 (337) | 361 (324) | |
| Follow-up | 317 (347) | 270 (306) | < 0.001 |
*Chi-square p values from Poisson models comparing the differences in utilization during the follow-up period
The groups differed in the addiction services they received at follow-up (Table 3). At baseline, the mean number of recovery coach contacts was 0.023 for the intervention group and 0.016 for the control group. At follow-up, the mean number of recovery coach contacts increased more for the intervention group to 0.346 compared to 0.061 for the control group (p < 0.001). There was a greater increase in addiction pharmacotherapy among the intervention groups. At baseline, the mean number of prescriptions for buprenorphine was similar between groups at 0.013 in the intervention and 0.014 in the control group. At follow-up, the mean number of prescriptions for buprenorphine increased to 0.554 in the intervention and to 0.194 in the control group (p < 0.001). Similarly, for naltrexone (intramuscular or oral), the baseline mean number of prescriptions was 0.039 in the intervention and 0.055 in the control group and increased to 0.131 in the intervention and to 0.061 in the control group (p < 0.001). There was no difference between groups in the prescribing of acamprosate. Methadone was excluded since it cannot be prescribed in office-based settings.
Table 3.
Mean Number of Recovery Coach Contacts and the Mean Number of Prescriptions for Addiction Pharmacotherapy During the 9-Month Baseline and Follow-up Periods
| Type of addiction service | Time period | Intervention | Control | p value |
|---|---|---|---|---|
| Mean number of recovery coach contacts | Baseline | 0.023 | 0.016 | |
| Follow-up | 0.346 | 0.061 | < 0.001 | |
| Mean number of buprenorphine prescriptions | Baseline | 0.013 | 0.014 | |
| Follow-up | 0.554 | 0.194 | < 0.001 | |
| Mean number of naltrexone prescriptions | Baseline | 0.039 | 0.055 | |
| Follow-up | 0.131 | 0.061 | < 0.001 | |
| Mean number of acamprosate prescriptions | Baseline | 0.003 | 0.005 | |
| Follow-up | 0.01 | 0.01 | 0.99 |
DISCUSSION
In this retrospective cohort study, patients with SUD who received primary care in a practice with integrated addiction treatment and recovery coaching had fewer emergency department visits and hospital days than patients who received primary care in other practices. There was no difference between groups in the total number of hospitalizations. For every 1000 patients with SUD receiving primary care at an intervention practice compared to a control site, the expected benefits over 1 year would be 98 fewer hospital days and 90 fewer ED visits offset by an additional 627 primary care visits. In addition to receiving recovery coaching, patients in practices with integrated care received more treatment with buprenorphine and naltrexone compared to control practices.
Our findings add to the existing body of evidence showing the clinical effectiveness of addiction treatment integration in primary care. Although it is not possible in this study to determine the relative impact of the different components of the SUD initiative, access to pharmacotherapy in addition to recovery coaching likely played a role. In a recent study of people who use drugs, receiving medication for opioid use disorder with methadone and having a primary care physician were each associated with a roughly 50% lowered risk of having two or more ED visits in a year.15 Buprenorphine adherence has been associated with decreased healthcare costs, largely driven by reductions in hospitalizations and ED visits despite overall increases in pharmacy and outpatient costs.10 Pharmacotherapy in medical settings also offers an opportunity to reduce costs by improving access; a recent study modeled the societal cost savings associated with rapid initiation of medication for opioid use disorder and demonstrated nearly $40,000 in health resource cost savings for treated individuals.16 Our previous work found that internists are more likely to report offering medications for addiction treatment after receiving support from our SUD initiative.17
Increasing access to medication for addiction treatment has become a major focus amidst the ongoing overdose crisis. Studies examining buprenorphine prescribing among primary care physicians have identified several barriers. A survey found that physicians who do not prescribe buprenorphine were more likely to report a lack of institutional support and a lack of confidence as barriers to providing treatment.18 Another study found that non-waivered physicians were more likely to report a lack of belief in the efficacy of buprenorphine, concerns about being overwhelmed by requests for treatment, and a lack of education.19 This intervention, which provided practices with additional support from the institution in the form of recovery coaches, education, and input from an addiction specialist at bi-monthly conferences, was effective at increasing the frequency of prescribing for both buprenorphine and naltrexone.
The reduction in the length of hospital stay but not in the number of hospitalizations was interesting and unexpected. The reduction in the length of stay could be explained by greater access to primary care-based addiction resources facilitating a more rapid discharge. For example, when pharmacotherapy is initiated for opioid use disorder (OUD) during a hospital stay, the inpatient team must ensure a patient has a prescriber to continue therapy and a lack of access to community-based care is a common barrier which could delay discharge.20 In addition, recovery coaches are expected to provide continuity visits to hospitalized patients which may have helped coordinate care and facilitate timely discharge. The reason for a lack of impact on the number of hospital admissions is unclear from this study.
The increase in primary care visits among intervention patients is expected and has been previously described in analyses of the cost impact of office-based buprenorphine treatment.21 A study of Vermont’s approach to increase access to pharmacotherapy for OUD found that primary care visits increased significantly among the treated group but overall healthcare costs were decreased due to less acute care utilization.21 While this study was not designed to look at cost, previous research has demonstrated that reducing acute care utilization reduces costs even when outpatient utilization increases. A pilot intervention focused on care coordination and case management for high-risk Medicaid members, 95% of whom had active substance use, demonstrated reductions in ED visits and hospitalizations accompanied by a far greater increase in outpatient visits.22 Despite the significant increase in outpatient utilization, overall Medicaid costs decreased in the follow-up period. In addition to the potential cost savings from preventing ED visits and days of hospitalization, the increase in primary care visits may be a marker for greater linkage to important preventive health services. A prior study of patients with OUD in federally qualified health centers who were initiating buprenorphine treatment found that receiving buprenorphine from a primary care physician rather than a psychiatrist and being retained in buprenorphine treatment were both associated with higher rates of nationally recommended preventive health care screening.23 These findings emphasize the important role that primary care can play in improving treatment for patients with OUD. While the idea of increased visit frequency may be daunting, team-based care offers an effective and efficient model. In Massachusetts, increased access to office-based addiction treatment has been achieved through the use of nurse care managers who see patients more frequently, thus addressing the barrier posed by physicians’ competing activities.24
The impact of recovery coaching on healthcare utilization has not been previously studied. To our knowledge, our study is the first to evaluate the inclusion of recovery coaches as a component of integrated addiction treatment in primary care. Our findings suggest that recovery coaches may be one effective component of an integrated primary care model for patients with SUD, although this study was not designed to isolate this effect. The mechanism of recovery coach effect could be through greater engagement in primary care or a direct effect of reducing substance use severity or substance-related medical conditions. A qualitative study looking at the integration of recovery coaches into primary care identified the core activities of recovery coaching to be system navigation, supporting behavior change, harm reduction, and relationship building. Further, it found that patients perceived this to be a valuable role.25 More research is needed to isolate the effect of recovery coaches on SUD and other health outcomes.
A notable finding in our study was the increase in acute care utilization with both ED visits and hospitalizations in both groups over time, although the magnitude of increase was lower in the intervention group. This finding has been seen in other care management programs for high-risk patients and may be due to the medical and psychosocial complexity of patients with SUD identified in the healthcare setting.26 Patients who have unidentified SUD may be disengaged from care, and the months following the recognition of this diagnosis may be a period during which patients are experiencing greater medical consequences of SUD. Another explanation could be that because we limited our analysis to patients within our primary care practices, we identified patients who were newly engaging in our system and thus the increase in utilization could reflect a shift in care to MGH. Further study is needed to evaluate this increase in acute care utilization following a diagnosis of SUD.
There are several limitations to this study. This was a single institution, retrospective analysis. Practice assignment of a recovery coach was not random, so although we attempted to match patients on key variables, it is possible patients or practices had unmeasured differences which influenced these outcomes. In addition, it is impossible to isolate the impact of each component of the intervention. The majority of patients in this study had a drug use disorder and we did not separately evaluate the impact of the intervention on patients by type of substance use disorder. Treatment for different types of drug use disorders and for alcohol use disorder differs and it is possible that outcomes may have varied by type of substance. We were only able to look at data from within our health system; it is possible that patients in either group received addiction services elsewhere or had healthcare utilization outside of our system. We were unable to evaluate costs even for patients for whom we had access to claims data because of the federal privacy law 42 CFR part 2, which limits information sharing related to substance use disorder care.27 Future research is needed to prospectively evaluate the impact and the cost-effectiveness of integrated recovery coaching and pharmacotherapy in primary care.
CONCLUSION
Integrated addiction pharmacotherapy and recovery coaching in primary care resulted in fewer hospital days and fewer ED visits for patients with SUD compared to similarly matched patients receiving care in practices without these services.
Acknowledgements
All authors contributed to this work. This work was not externally funded. We received internal funding from the Massachusetts General Hospital Substance Use Disorder Initiative. Preliminary findings from this study were presented at the April 2018 American Society of Addiction Medicine Conference.
Compliance with Ethical Standards
This study was approved by the Partners Human Research Committee.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cherpitel CJ, Ye Y. Drug use and problem drinking associated with primary care and emergency room utilization in the US general population: data from the 2005 national alcohol survey. Drug Alcohol Depend. 2008;97(3):226–30. doi: 10.1016/j.drugalcdep.2008.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walley AY, Paasche-Orlow M, Lee EC, Forsythe S, Chetty VK, Mitchell S, Jack BW. Acute care hospital utilization among medical inpatients discharged with a substance use disorder diagnosis. J Addict Med. 2012;6(1):50–6. doi: 10.1097/ADM.0b013e318231de51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NIDA. Trends & statistics. 2017. Retrieved from https://www.drugabuse.gov/related-topics/trends-statistics, November 2, 2018.
- 4.Fiellin DA, Barry DT, Sullivan LE, Cutter CJ, Moore BA, O'Connor PG, Schottenfeld RS. A randomized trial of cognitive behavioral therapy in primary care-based buprenorphine. Am J Med. 2013;126(1):74.e11–7. doi: 10.1016/j.amjmed.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369–76. doi: 10.1001/jamainternmed.2014.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Onofrio G, O'Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015;313(16):1636–44. doi: 10.1001/jama.2015.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walley AY, Palmisano J, Sorensen-Alawad A, Chaisson C, Raj A, Samet JH, Drainoni ML. Engagement and substance dependence in a primary care-based addiction treatment program for people infected with HIV and people at high-risk for HIV infection. J Subst Abus Treat. 2015;59:59–66. doi: 10.1016/j.jsat.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Baser O, Chalk M, Fiellin DA, Gastfriend DR. Cost and utilization outcomes of opioid-dependence treatments. Am J Manag Care. 2011;17(Suppl 8):S235–48. [PubMed] [Google Scholar]
- 9.Bryson WC, McConnell J, Korthuis PT, McCarty D. Extended-release naltrexone for alcohol dependence: persistence and healthcare costs and utilization. Am J Manag Care. 2011;17(Suppl 8):S222–34. [PMC free article] [PubMed] [Google Scholar]
- 10.Tkacz J, Volpicelli J, Un H, Ruetsch C. Relationship between buprenorphine adherence and health service utilization and costs among opioid dependent patients. J Subst Abus Treat. 2014;46(4):456–62. doi: 10.1016/j.jsat.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Wei J, Defries T, Lozada M, Young N, Huen W, Tulsky J. An inpatient treatment and discharge planning protocol for alcohol dependence: efficacy in reducing 30-day readmissions and emergency department visits. J Gen Intern Med. 2015;30(3):365–70. doi: 10.1007/s11606-014-2968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weisner C, Mertens J, Parthasarathy S, Moore C, Lu Y. Integrating primary medical care with addiction treatment: a randomized controlled trial. JAMA. 2001;286(14):1715–23. doi: 10.1001/jama.286.14.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bassuk EL, Hanson J, Greene RN, Richard M, Laudet A. Peer-delivered recovery support services for addictions in the United States: a systematic review. J Subst Abus Treat. 2016;63:1–9. doi: 10.1016/j.jsat.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Wakeman SE, Metlay JP, Chang Y, Herman GE, Rigotti NA. Inpatient addiction consultation for hospitalized patients increases Post-discharge abstinence and reduces addiction severity. J Gen Intern Med. 2017;32(8):909–916. doi: 10.1007/s11606-017-4077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kendall CE, Boucher LM, Mark AE, Martin A, et al. A cohort study examining emergency department visits and hospital admissions among people who use drugs in Ottawa, Canada. Harm Reduct J. 2017;14:16. doi: 10.1186/s12954-017-0143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krebs E, Enns B, Evans E, et al. Cost-effectiveness of publicly funded treatment of opioid use disorder in California. Ann Intern Med 2017. 10.7326/M17-0611. [DOI] [PubMed]
- 17.Wakeman SE, Kanter GP, Donelan K. Institutional substance use disorder intervention improves general internist preparedness, attitudes, and clinical practice. J Addict Med. 2017;11(4):308–314. doi: 10.1097/ADM.0000000000000314. [DOI] [PubMed] [Google Scholar]
- 18.Hutchinson E, Catlin M, Andrilla CH, Baldwin LM, Rosenblatt RA. Barriers to primary care physicians prescribing buprenorphine. Ann Fam Med. 2014;12(2):128–33. doi: 10.1370/afm.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huhn AS, Dunn KE. Why aren’t physicians prescribing more buprenorphine? J Subst Abus Treat. 2017;78:1–7. doi: 10.1016/j.jsat.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassamal S, Goldenberg M, Ishak W, Haglund M, Miotto K, Danovitch I. Overcoming barriers to initiating medication-assisted treatment for heroin use disorder in a general medical hospital: a case report and narrative literature review. J Psychiatr Pract. 2017;23(3):221–229. doi: 10.1097/PRA.0000000000000231. [DOI] [PubMed] [Google Scholar]
- 21.Mohlman MK, Tanzman B, Finison K, Pinette M, Jones C. Impact of medication assisted treatment for opioid addiction on Medicaid expenditures and health services utilization rates in Vermont. J Subst Abus Treat. 2016;67:9–14. doi: 10.1016/j.jsat.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Raven MC, Doran KM, Kostrowski S, Gillespie CC, Elbel BD. An intervention to improve care and reduce costs for high-risk patients with frequent hospital admissions: a pilot study. BMC Health Serv Res. 2011;11:270. doi: 10.1186/1472-6963-11-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haddad MS, Zelenev A, Altice FL. Buprenorphine maintenance treatment retention improves nationally recommended preventive primary care screenings when integrated into urban federally qualified health centers. J Urban Health. 2015;92(1):193–213. doi: 10.1007/s11524-014-9924-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaBelle CT, Han SC, Bergeron A, Samet JH. Office-based opioid treatment with buprenorphine (OBOT-B): statewide implementation of the Massachusetts Collaborative Care Model in community health centers. J Subst Abus Treat. 2016;60:6–13. doi: 10.1016/j.jsat.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jack HE, Oller D, Kelly J, Magidson JF, Wakeman SE. Addressing substance use disorder in primary care: the role, integration, and impact of recovery coaches. Subst Abus. 2017;9:1–8. doi: 10.1080/08897077.2017.1389802. [DOI] [PubMed] [Google Scholar]
- 26.Sadowski LS, Kee RA, VanderWeele TJ, Buchanan D. Effect of a housing and case management program on emergency department visits and hospitalizations among chronically ill homeless adults: a randomized trial. JAMA. 2009;301(17):1771–1778. doi: 10.1001/jama.2009.561. [DOI] [PubMed] [Google Scholar]
- 27.Frakt AB, Bagley Nphysicians prescribing more buprenorphine Protection or harm? Suppressing substance-use data. N Engl J Med 2015;372(20):1879–81. [DOI] [PubMed]


