Abstract
Sarcopenia, the age-related loss of muscle mass and strength, contributes to frailty, functional decline, and reduced quality of life in older adults. Exercise is a recognized therapy for sarcopenia and muscle dysfunction, though not a cure. Muscle power declines at an increased rate compared to force, and force output declines earlier than mass. Thus, there is a need for research of exercise focusing on improving power output and functionality in older adults. Our primary purpose was proof-of-concept that a novel individualized power exercise modality would induce positive adaptations in adult mice, before the exercise program was applied to an aged cohort. We hypothesized that after following our protocol, both adult and older mice would show improved function, though there would be evidence of anabolic resistance in the older mice. Male C57BL/6 mice (12 months of age at study conclusion) were randomized into control (n = 9) and exercise (n = 6) groups. The trained group used progressive resistance (with a weighted harness) and intensity (~ 4–10 rpm) on a custom motorized running wheel. The mice trained similarly to a human workout regimen (4–5 sets/session, 3 sessions/week, for 12 weeks). We determined significant (p < 0.05) positive adaptations post-intervention, including: neuromuscular function (rotarod), strength/endurance (inverted cling grip test), training physiology (force/power output per session), muscle size (soleus mass), and power/velocity of contraction (in vitro physiology). Secondly, we trained a cohort of older male mice (28 months old at conclusion): control (n = 12) and exercised (n = 8). While the older exercised mice did preserve function and gain benefits, they also demonstrated evidence of anabolic resistance.
Electronic supplementary material
The online version of this article (10.1007/s11357-019-00069-z) contains supplementary material, which is available to authorized users.
Keywords: Sarcopenia, Mice, Exercise, Muscle, Power
Introduction
Exercise is a proven therapy used to maintain function in older adults. In order to evaluate the efficacy and safety of novel interventions and their synergistic effects with exercise training programs prior to clinical testing in humans, animal models of exercise training are used. There are several established rodent models of resistance training such as the squat rack mimic device (Tamaki et al. 1992; Drummond et al. 2010), a weighted backpack and standing model (Farrell et al. 1999; Wirth et al. 2003), and the tail weight ladder climb (Hornberger Jr and Farrar 2004). These models are able to follow the principles of progressive resistance training (e.g., increasing weights at specific time points). Endurance training is also established in rodent models by running on treadmills or swimming for long periods of time (Kregel et al. 2006; Kilikevicius et al. 2013). These training programs result in exercise training-specific skeletal muscle adaptations. Resistance training results primarily in muscle hypertrophy and an increase in strength; whereas, endurance training results primarily in improvements in aerobic capacities and cardiovascular function.
In contrast to resistance training and endurance training, power training (resistance training at relatively high concentric velocity) is used to improve functional tasks of daily living and explosive movements (e.g., such as opening a pickle jar or quickly accelerating walking speed to avoid an oncoming car) (Bean et al. 2009; Morgan et al. 2015; Sayers and Gibson 2014). The formula for power is strength multiplied by velocity. Thus, power training enhances functional ability, or movements, because it improves both the velocity and force-generating capacity of muscle contraction. Functional tasks and movements require endurance, too. In fact, improved physical movements or function resulting from an exercise modality encompassing strength, endurance, and power simultaneously would be optimal for an individual (e.g., older adult) seeking to maximize the cost/benefit of a training program. Typical training techniques that incorporate functional, power, resistance, and endurance aspects include the walking lunge (exaggerated steps under load), the farmers walk (walking while holding dumbbells), and running with a weighted pack.
Older adults may face a reduction in the ability to perform the basic activities of daily living (e.g., shopping, cooking, cleaning, grooming, getting out to go to the doctor) because of chronic illness burden, frailty, and sarcopenia. Power is a critical factor for movement-based activities, and power training has previously been shown to be efficacious in improving function in older adults (McKinnon et al. 2017; Reid and Fielding 2012; Bean et al. 2009). Thus, to maximize the functional benefit of a given exercise program, it should both improve power output (e.g., of carrying bags of groceries up a flight of stairs) and increase endurance at the same time (e.g., ascending the stairs without having to stop and rest!).
Adaptation of an exercise protocol to an individual’s needs and strengths/weaknesses is the hallmark of personal training (individualized training), i.e., one size fits all is not an optimal program. To our knowledge, there is a dearth of individualized power training programs available in rodent models. Moreover, the animal exercise programs described above are not fully voluntary as participation by the animal is induced via operant condition (punishment avoidance) or by external stressors. An electric shock to either the tail or the feet is used to motivate animals in the squat, backpack, and treadmill running models. A spray of cold water is used in the ladder climb.
Because of the multiple benefits associated with human power training programs, the main purpose of this study was to design an individualized power training protocol for mice using principles of progressive resistance/power exercise, with elements of endurance, that mimic the human functional exercises described above, hypothesizing that the training would improve physical function and muscle contractility. To test our hypothesis, we assessed a group of exercised and control adult mice with a carefully selected battery of functional, contractile, and morphological outcome measures. The training modality consisted of running on a powered wheel while wearing an increasingly heavy weighted harness. Because this mouse model has potential to be a resource for researchers to investigate mechanisms contributing to age-associated sarcopenia, and strategies to restore age-related loss of physical function, we also report herein on a second study where we evaluated instituting this power training program in a group of older mice.
Methodology
Animal model
In study 1, C57BL/6 male mice from the NIA Aging Colony (adult, 12 months old at endpoint) were randomly selected to control (n = 9) or exercise (n = 6) groups. In study 2, 24-month-old C57BL/6 male mice from the NIA Aging Colony (28 months old at endpoint) were randomly selected to control (n = 12) or exercise (n = 8) groups. In both studies, the mice were group housed with 12-h light cycle at 22 °C and fed ad libitum. All animals were treated humanely in accordance with an approved IACUC protocol. Mice were weighed before and after the training period.
Training equipment and protocol
Two pieces of equipment were custom-designed and fabricated (Fig. 1a, b: a Velcro weight harness and a powered running wheel). In week 2, the mice underwent baseline testing for functional performance, and then the mice underwent through two different training periods: (1) acclimation training (1 week) and (2) individualized power training (12 weeks) (Fig. 1c). This was followed by post-intervention functional performance testing. Specifically, acclimation training introduced both the weight harness and running wheel, and the mice were trained to use the equipment. During individualized power training, both the resistance (mass of weights added) and the intensity (velocity at which the mice ran) were carefully adjusted over the time of the training period for progressive intensity/resistance according to the ability of each mouse. Principles of exercise training were incorporated into the program: progressive resistance/intensity, training frequency, number of sets per day, warm-up sets, set duration, and rest between sets (for a more detailed description of the protocol, see the Online Resource Video 1, and further details in the Online Resource Methods Section, as well as Tables S1 and S2).
Fig. 1.
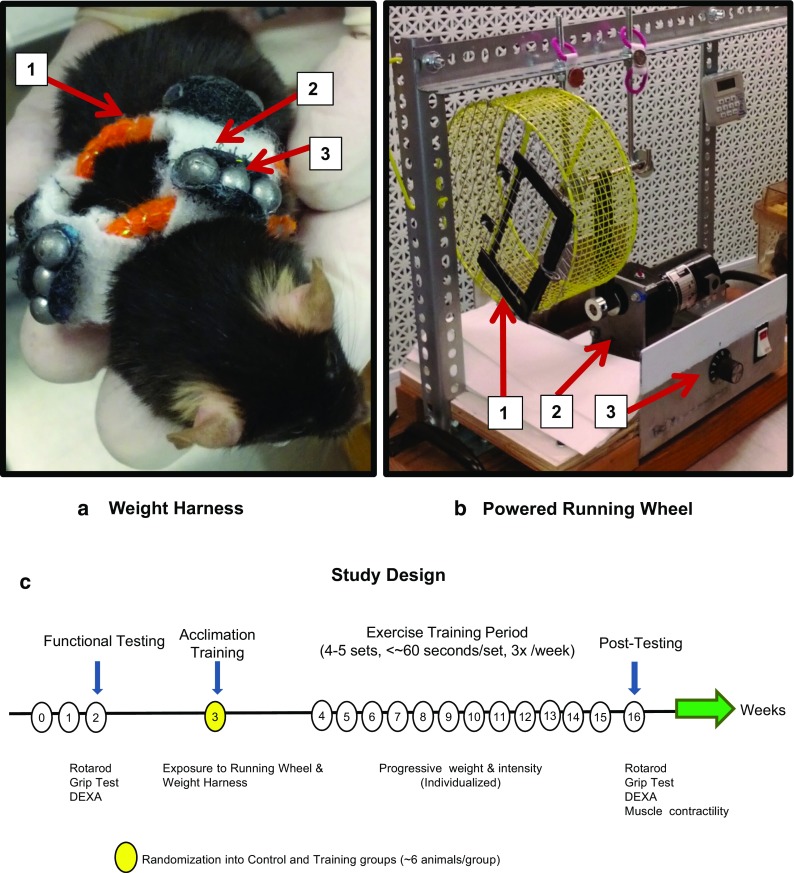
Training equipment and study design. a Weight harness. A Velcro weight harness to which small lead weights were applied with three main components: (1) padded elastic band, (2) Velcro strips connecting the bands, and (3) lead weights that can be added in various combinations to increase resistance. b Powered running wheel. A powered running wheel engineered by mounting a steel caged-in 27.5 cm diameter running wheel to a rotary electric motor with a speed controller that could rotate between 1 and 10 rpm. Three main components: (1) enclosed running wheel with door, (2) electric motor, and (3) speed controller (1–10 rpm). c Study design and training schedule. Functional testing consisted of rotarod and grip test. Acclimation training started in week 3 and consisted of exposure to the running wheel and weight harness. The training period begins in week 4 and was individualized to the performance of each mouse, using progressive weight and intensity. Post-testing followed the training period and consisted of post-intervention rotarod, grip test, and muscle contractile physiology
ESM 1.
(MP4 11,251 kb)
Outcome measurements
Animal functional performance and training effect
Animal performance measures were described in detail previously (Graber et al. 2013, 2018). The individual performance of each mouse was evaluated at each training session to determine adherence to exercise principles and to calculate increases in power output (intensity of effort = work/time) (Table S2).
Rota-Rod tested overall motor function (balance, coordination, stamina, power) using the acceleration mode, from 4 to 40 rpm over 5 min (Lsi Letica Rota-Rod R/S). The latency to fall from the device was recorded. The best of three trials was the outcome measure. Mice were trained on the device by doing random sessions 3×/day for 2 days prior to the testing day.
Grip Test is an indicator of muscle strength and stamina, determined by the inverted cling grip test (custom testing device). The animal was placed on a wire grid lid and the lid was then closed to invert the mouse. The latency to fall from the lid (to the padded floor of the device 20 cm below) was recorded as the outcome measure. The best of the two trials was the outcome.
Training effect—whole-body training physiology
The individual performance of the mice was monitored over the training period to determine both adherence to the principle of progressive resistance and to examine whether power output would increase as the load was increased. Training force, normalized per gram of body mass, and normalized training power were all tracked through the duration of the study. The expression of this data is described by using the common units of millinewtons for force and milliwatts for power (see Table S2 for definitions and calculation equations).
Tissue and cellular response to training
Muscle contractility and hypertrophy
We used in vitro contractile physiology to determine the contractile properties of isometric force (tetanic force), velocity, the force-velocity curve, maximal power output, and the force-power curve in the soleus (SOL) muscle; methods have been previously published (Graber et al. 2013, 2015a). The SOL, quadriceps (in adult mice only) and plantaris were blotted dry and massed to determine wet weight. We also normalized the SOL mass to mouse body weight in grams (normalized mass). Plantaris muscles were sectioned and the serial cross sections (n = 4 per group) were stained with hematoxylin and eosin and then assessed for single fiber cross-sectional area using previously published methodology (Graber et al. 2015b).
Muscle function—contractility
In brief: The SOL was isolated and perfused with 95% O2/5% CO2 in Krebs/Ringer buffer (at 25 °C). The muscle was tied to a force transducer (Aurora 300b) with 4-gauge silk suture line attached at either myotendinous junction and suspended between two platinum electrodes. Using an Aurora High Power Bi-Phase Current Stimulator and a Dual-System Signal Interface controlled by DMC (Dynamic Muscle Control, v.4.1.6) software, the muscles were stimulated with protocols to determine peak twitch force (Pt), optimal length (L0), pre-load tension, and then maximum isometric contractile force (P0) was determined using the force/frequency curve from 10 to 180 Hz stimulus. We also normalized P0 (P0/gbm, gbm = grams body mass) to the body mass.
To determine the velocity of contraction, the load clamp technique was used to generate a force-velocity curve. The force transducer was set at various percentages of P0 (10–90%); the SOL was set to L0 and then maximally stimulated at the frequency determined to produce P0. Upon producing enough force to overcome the set load (clamped at 10–90% P0), the muscle concentrically contracted against the load. Velocity of contraction was determined by finding the first derivative of the time-distance curve derived from the muscle contraction. Maximum unloaded velocity was calculated using a derivation of the Hill equation [(V + b)(P/P0 + a/P0) = b(1 + a/P0), a and b are constants, V = maximum velocity at fractional load]. The data was curve fit (only r2 > 0.98) using a customized MatLab program. After establishing the force-velocity curve, the force-power curve was derived by multiplying the maximum velocity at each %P0 by the force output at each %P0 and fitting the data to a 5th degree polynomial. Pmax was the highest number on the curve (first derivative = 0), and the %P0@Pmax was the value at the x-axis where Pmax was the y-axis value. We determined physiological cross-sectional area (PCSA) of the SOL with the average density of skeletal muscle by using the standard formula: PCSA (cm2) = Muscle mass (g) / [L0 (cm) × 1.06 (g/cm3)].
Fiber size—histochemistry of plantaris muscle
A subset (n = 4 trained and n = 4 control; of both adult and older mice) of plantaris muscles was flash-frozen in liquid nitrogen cooled isopentane. An average of n = 793, 440, 683, 592 cells (adult control, adult exercise, older control, and older exercise, respectively) was analyzed per muscle, and the fibers were pooled for analysis. Ten-micrometer sections were sliced as cross sections on a cryostat and subsequently stained with hematoxylin and eosin (H&E). The sections were imaged with a microscope at × 100 total magnification and then the perimeter of each was circled using ImageJ to determine cross-sectional area (CSA).
DEXA (dual x-ray absorptiometry)
In study 2, using a DEXA system (GE Lunar PixiMus I), we measured lean mass and fat mass percentages from the older control, older exercise, and a cohort of adult (12-month-old) control mice. The mice were anesthetized with a ketamine/xylazine (1:10 ratio) mixture (~ 100 mg/kg) and oriented on the machine, the x-rays were taken, and the mice were then allowed to recover from anesthesia fully in a recovery cage heated to ~ 37 °C with a heating pad (indirectly placed underneath).
Statistics
Data are presented as means ± standard error, as appropriate. To compare means between the mice, repeated measures general linear model, general linear model, t tests (independent and paired), and ANCOVAs used α < 0.05 as significant and 0.05 < α < 0.10 as a trend. ANCOVA was adjusted for body mass in grip test and rotarod. Linear regression determined relationships between variables. Two-sample Kolmogorov-Smirnov, Mann-Whitney U test, and the independent samples median test compared distributions, means, and medians. Symbols “*” denote p < 0.05, significant, and “#” denote 0.05 < p < 0.10, trend. IBM SPSS v20 and v24 were used for statistical analysis.
Results
Study 1—power training in adult mice
Overall the results demonstrate that the adult mice in the exercise group received tangible and significant functional, contractile, and morphological benefit from the power-training protocol when compared to the control sedentary group.
Evidence of whole-body adaptations to power training
Animal functional performance preserved with power training (Fig. 2)
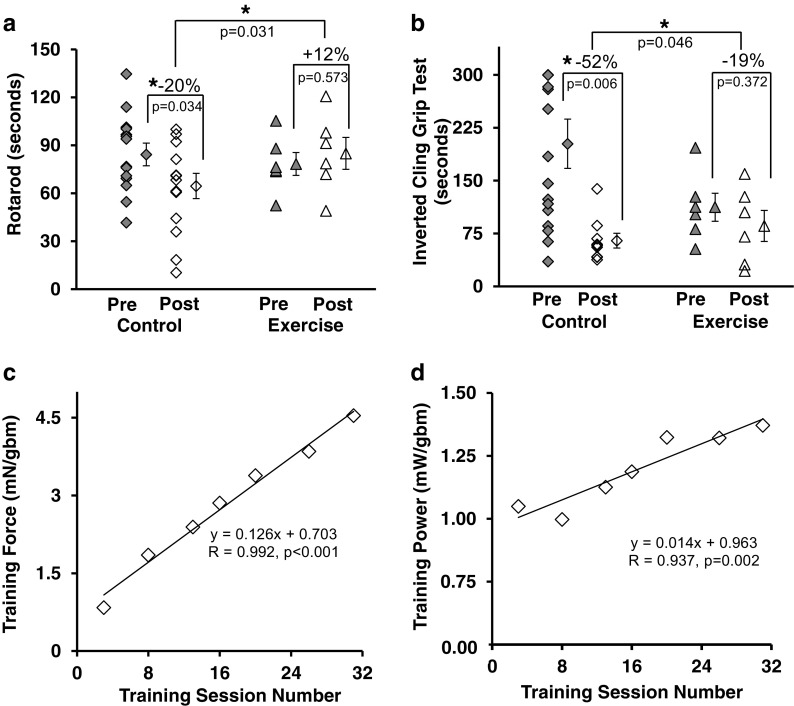
Fig. 2.
Exercise preserved functional performance. a Rotarod performance preserved with training. b Grip function preserved with exercise. c Training force, the amount of force produced by the mice/gram of body mass, increased over the course of the training. d Training power, the amount of power produced/gram of body mass increased over the course of the training. Symbols: *p < 0.05, each symbol in scatter plots is the result from an individual mouse (except for “means”). Filled symbols are before the training intervention period and open symbols are after the intervention period. Individual symbols to the right of the data spread represent the mean ± SE. Lines delineate significance. Diamonds = control mice; triangles = trained mice. Statistics: a, b 2 × 2 repeated measures ANCOVA, adjusted for body mass of mice. c, d Each symbol in the regression plots is the mean of all mice at the given session. mN = millinewtons, gbm = grams body mass, mW = milliwatts, equation is simple linear regression
In general, the functional performance of the adult mice in the control group declined over the 12-week training period, whereas the performance of the mice in the power-training group was maintained. Notably, overall rotarod ability was preserved with training, whereas control mice function was reduced by 20%. The difference between the two groups was significant (Fig. 2a). The exercise mice also retained grip performance, compared to a 52% loss of strength in the control group, once again with a significant difference between the two groups. (Fig. 2b).
Training effect—force and power production increased over the power training period (Fig. 2c, d):
There was a significant correlation between the extent of training adaptation (training force and power) and the duration of power training (Fig. 2c, d). Mean force and power generation were significantly increased post training. Mean normalized training force increased by 443% (Fig. 2c). Mean normalized training power increased 23.4% (Fig. 2d).
Evidence of positive tissue and cellular response to training
Power training induced muscle hypertrophy (Fig. 3)
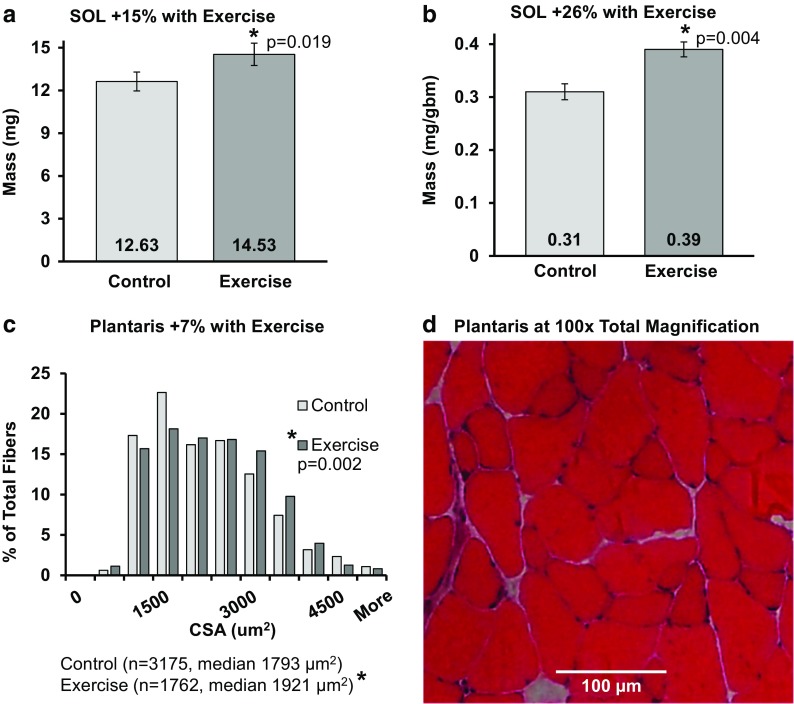
Fig. 3.
Training induced hypertrophy in adult mice. a Soleus (SOL) mass increased with exercise. b SOL mass normalized to body size increased with exercise. c Median cross-sectional area of pooled individual fibers from the plantaris muscle significantly increased (p<0.05) and there was a significant size distribution shift to the right in exercised mice (p=0.002). d Representative image of the plantaris muscle and cross-sectional area, hematoxylin and eosin. Frequency % = 100 × [(number of fibers in each bin) / (total number of fibers measured)]. Symbols: *p < 0.05, numbers at the base of the bar graphs are the mean, CSA = cross-sectional area in micrometers2, mg = milligrams, gbm = grams body mass. Statistics: a, b Student’s t tests; c two-sample Kolmogorov-Smirnov, independent samples median test
There was an increase in both the SOL absolute wet mass (15%) and normalized mass (26%) with power training (Fig. 3a, b). The wet mass of the quadriceps increased by 21.5% (from 148.14 mg to 179.90 mg, p = 0.009) and the normalized quadriceps mass increased by 26.9% (from 3.76 ± 0.21 mg/gbm to 4.77 ± 0.30 mg/gbm, p = 0.016) (data not shown). In contrast, the wet mass and the normalized mass of the plantaris were not significantly different between the two groups. The CSA distribution of the single fibers within the plantaris muscles shifted right with training (2-sample Kolmogorov-Smirnov, p = 0.001) indicating more large cells were present in the exercise group (Fig. 3c). The median CSA increased 7% with training (Fig. 3c). The plantaris mean CSA increased 4% from 1933.7 (control group) to 2006.4 μm2 (exercise group) (t test, p < 0.001, data not shown).
Power training improved contractile velocity and power (Fig. 4)
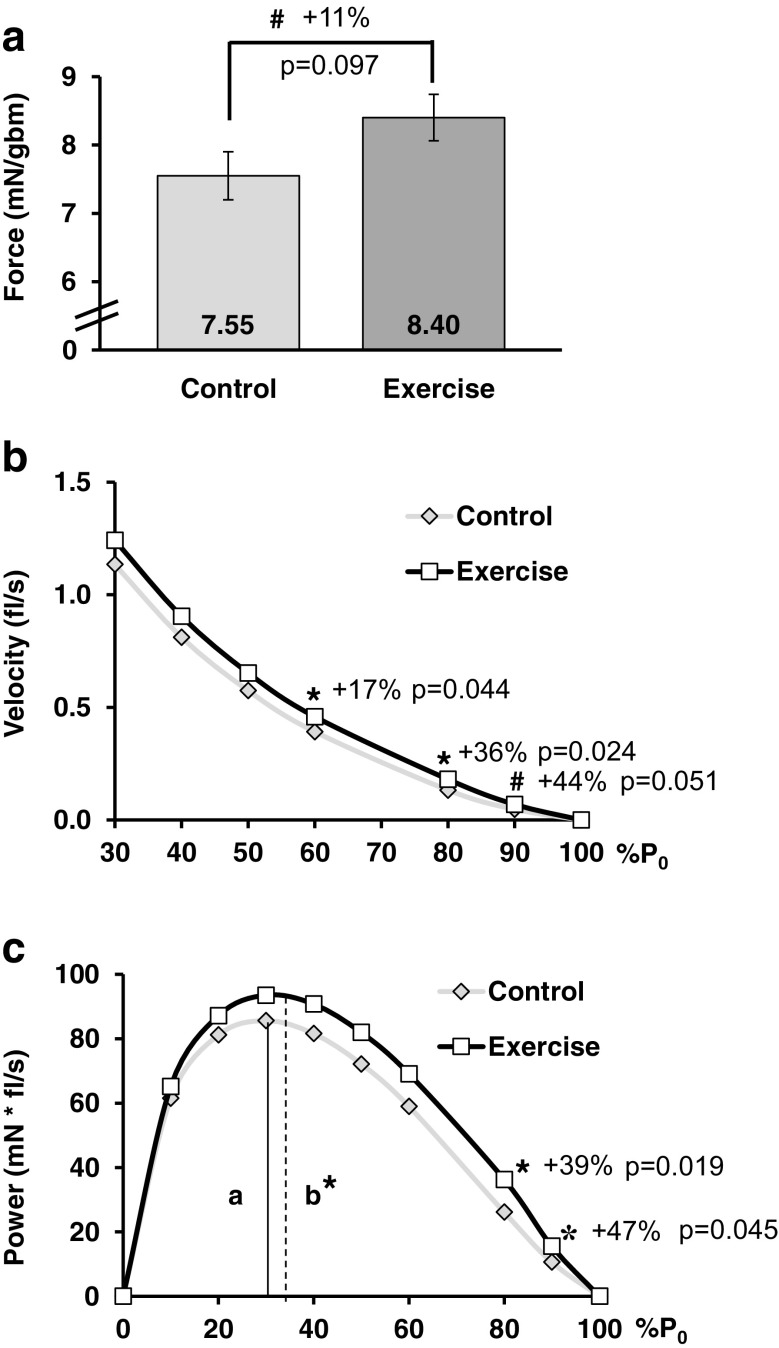
Fig. 4.
Training improved SOL contraction. a Soleus muscle normalized force (mN/gbm) tended to increase. b Contractile velocity increased under heavy load (curve displayed truncated at 30% P0 to highlight differences, no difference from 0 to 20%). c Power output improved under heavy load. a, b %P0 at Pmax for control and exercise, respectively. Symbols: *p < 0.05, #0.05 < p < 0.10, numbers within bar graphs are the mean, each symbol in the plots is the mean from each group at the given %P0, P0 = peak tetanic force, mN = millinewtons, gbm = grams body mass, fl/s = fiber lengths per second. Statistics: Student’s t test
The SOL P0/gbm tended to be 11% greater in the exercise group. The velocity of contraction in the exercise group was faster when contracting against the higher percentages (higher loads) of P0 (Fig. 4b). The force-velocity curve tended to shift up with exercise indicating increased contractile velocity over the curve (a/P0 was 21% lower in the exercise group, 0.018 ± 0.002 compared to the control group, 0.023 ± 0.002; p = 0.055). The shape of the force-power curve shifted up and to the right, indicating increased power production. With exercise, power production increased 39% at 80%P0 and 47% at 90%P0 (Fig. 4c), representing the upward shift. The %P0 where peak power occurred increased 7% with exercise (control 27.3 ± 0.6 % P0 and exercise 29.2 ± 0.5 % P0, p = 0.045), representing the rightward shift (Fig. 4c). Pmax was not statistically increased with exercise (+ 10% with exercise, 97.7 ± 10.5 mN * fl/s, and control, 89.1 ± 4.7 mN * fl/s, p = 0.47).
Study 2—power training in older mice
Overall, the results demonstrate that the older mice in the exercise group received tangible and significant functional and morphological benefit, with improved body composition, from the power-training protocol when compared to the control sedentary group, though contractile parameters were not significantly improved.
Evidence of whole-body adaptations to power training (Fig. 5)
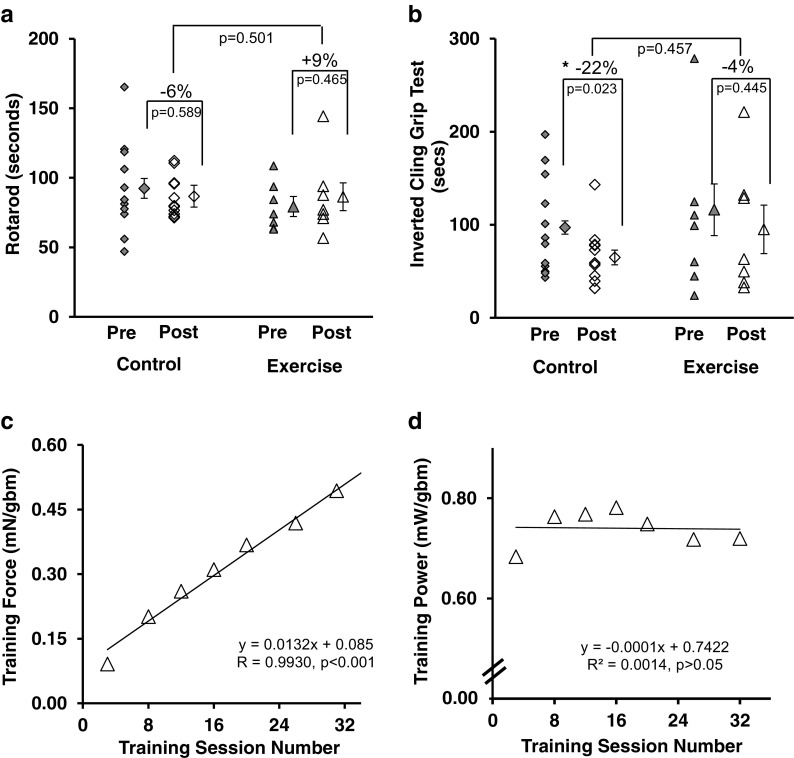
Fig. 5.
Exercise preserved functional performance. a While training did not improve rotarod performance in older mice, function was preserved. b Grip function preserved with exercise and grip function was lost in control mice. c Training force, the amount of force produced/gram of body mass, increased over the course of the training. d Training power, the amount of power produced/gram of body mass remained static over the course of the training. Symbols: *p < 0.05, each symbol in scatter plots is the result from an individual mouse (except for “means”). Filled symbols are before the training intervention period and open symbols are after the intervention period. Individual symbols to the right of the data spread represent the mean ± SE. Lines delineate significance. Diamonds = control mice; triangles = trained mice. Statistics: a, b 2 × 2 repeated measures ANCOVA, adjusted for body mass of mice. c, d Each symbol in the regression plots is the mean of all mice at the given session. mN = millinewtons, gbm = grams body mass, mW = milliwatts, equation is simple linear regression
Animal functional performance with power training
Overall, the functional performance of the older mice in the control group declined over the 12-week training period, whereas the performance of the mice in the power training group was maintained, or the loss of function was minimized. Within the exercise group, rotarod ability was numerically 9% larger following training (not significant) but the control mice were reduced 6% (not significant). There was no significant rotarod difference between control and exercise (Fig. 5a). Within the exercise group, the mice retained grip performance; however, there was a significant 22% loss of strength in the control group over the same time period (Fig. 5b).
Training effect: force and power production increased over the power training period
There was a significant correlation between the extent of training adaptation to produce force (training force) and the duration of training (Fig. 5c). Mean normalized training force increased by 442% over the training period (Fig. 5C). However, mean normalized training power did not change over the course of the study (Fig. 5d).
Body composition (Fig. 6)
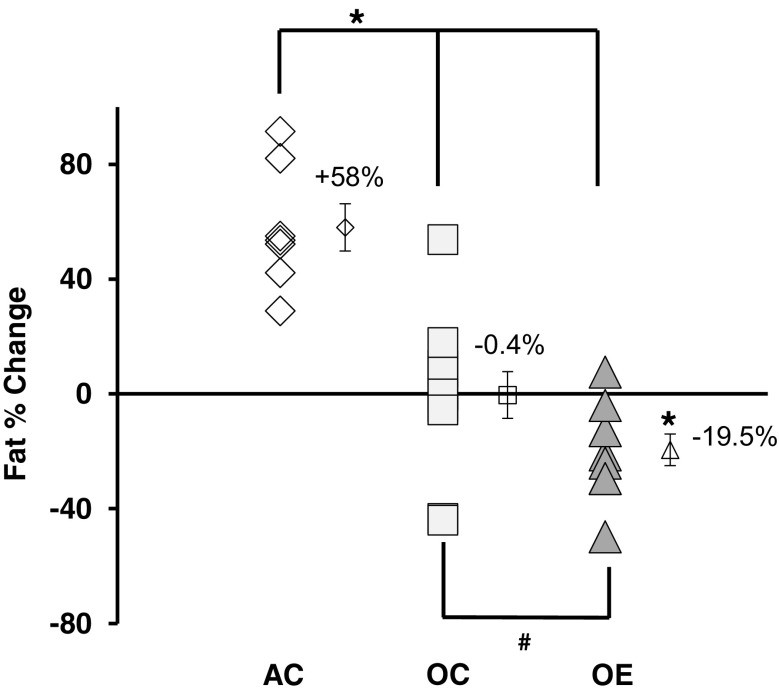
Fig. 6.
Older mice improve body composition with training. Fat percentage was reduced with training in old exercised animals by 19.5% (paired t test, before and after training, data not shown, p = 0.013, represented by *). Adult control mice gained fat while older control mice maintained fat level. No data available for adult exercise mice. Symbols: Symbol with error bars to the right of each group are the mean ± standard error. Diamonds are adult control (AC, n = 12), squares are old controls (OC, n = 7), and triangles are old exercise mice (OE, n = 11). * p < 0.05 and # 0.05 < p < 0.10. Lines indicate significance from one-way ANOVA comparing percent change: f = 24.403, p < 0.001. AC > OC, p < 0.001; AC > OE, p < 0.001; OC > OE, p = 0.072
Exercise training reduced body fat accumulation in older mice. Within the groups, a paired measures t test demonstrated that the older exercise mice had a significant 19.5% loss in body fat after training (p = 0.013), whereas the older control mice essentially maintained fat percentage (lost 0.4%; p > 0.10). However, in a one-way ANOVA of the percent change between the groups, while the older exercise group tended to lose more fat than the older control group, a group of adult control mice (age 12 months at final DEXA) significantly gained 58% more fat over the same period when compared to both older groups.
Evidence of positive tissue and cellular response to power training
Training induced muscle hypertrophy (Fig. 7)
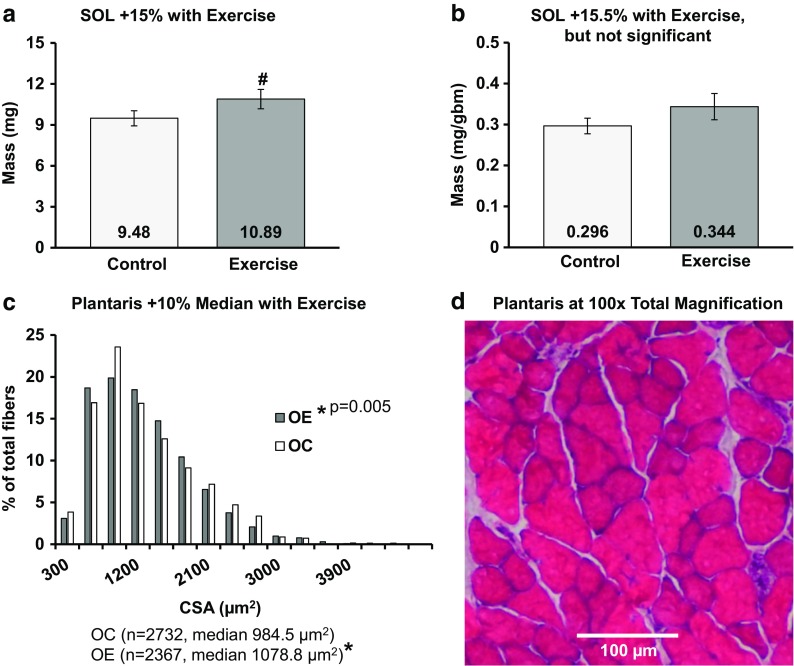
Fig. 7.
Training induced hypertrophy in old mice, a tendency. a Soleus mass tended to increase (p = 0.089). b Soleus mass normalized to body size increased numerically, but not significantly (p > 0.05). c Median cross-sectional area of individual fibers from the plantaris muscle significantly increased (p < 0.05) with a significant size distribution shift right in exercised mice (p<0.005). d Representative image of the plantaris muscle and cross-sectional area, hematoxylin and eosin. Frequency % = 100 × [(number of fibers in each bin) / (total number of fibers measured)]. Symbols: *p < 0.05, #0.05 < p < 0.10, numbers at base in bar graphs are the mean, CSA = cross-sectional area in micrometers2, mg = milligrams, gbm = grams body mass, OC = older control, OE = older exercise. Statistics: a, b Student’s t tests; c two-sample Kolmogorov-Smirnov, independent samples median test
The SOL absolute wet mass tended to increase with training by 15% (Fig. 7a), but the normalized mass did not significantly alter with training (Fig. 7b). In contrast, neither the wet mass nor the normalized mass of the plantaris were significantly different between the two older groups. We also examined the pooled plantaris fiber data of the two groups (histogram, Fig. 7c). The single fiber CSA of old exercise mice (1203.9 ± 17.9 μm2) was 4.8% larger than the old control (1148.0 ± 11.5 μm2) (Student’s t test p = 0.033). The distributions of the single fiber CSA were significantly different, with the control group skewed left. The median CSA significantly increased 9.6% with training.
Power training did not statistically improve contractile velocity and power in older mice (Fig. 8)
Fig. 8.
Training did not improve SOL contraction in older mice. a SOL (soleus) muscle normalized force (mN/gbm) did not alter significantly. b Contractile velocity did not alter significantly (curve truncated at 30% P0, no significant differences anywhere on curve). c Power output did not change significantly. a, b %P0 at Pmax for control and exercise, respectively. Symbols: numbers within bar graphs are the mean, each symbol in the plots is the mean from each group at the given %P0, P0 = peak tetanic force, mN = millinewtons, gbm = grams body mass, fl/s = fiber lengths per second. Statistics: Student’s t test
SOL P0/gbm was not greater in the exercise group (Fig. 8a). The velocity of contraction in the exercise group was not different from the controls (Fig. 8b). The shape of the force-power curve shifted up and to the right, indicating increased power production; however, the differences at the individual %P0 were not significant (Fig. 8c). Pmax was not statistically increased with exercise (+ 13% with exercise, 62.0 ± 6.3 mN fl/s, and control, 54.7 ± 5.3 mN fl/s, p = 0.408).
Discussion
The main goal of Study 1—power training in adult mice was to create a novel voluntary individualized power training protocol for mice using principles of progressive resistance/power exercise, with elements of endurance training, that mimic human functional exercise (refer to the Online Resource for more details). In order to determine whether the exercise-induced adaptations in the mice were equivalent to improvements observed in humans undertaking similar power training programs, we selected outcome measures validated to quantify gains in function, muscle contractility, and muscle hypertrophy. This study had two main findings: (1) The individualized power training protocol induced positive adaptations in the accepted outcome measurements of muscle hypertrophy, muscle force, velocity and power, neuromotor function (rotarod), and in the derived measurements of training physiology; (2) These results demonstrated the protocol is a mimic of human power training exercise, with both face and construct validity. In Study 2—power training in older mice, our goal was to evaluate the effect of the exercise protocol designed in study 1 in a cohort of older mice. This study had the following main findings: (1) The mice experienced evidence of positive outcomes from the exercise training. (2) The adaptations were not as extensive as those observed in the adult mice. (3) This exercise protocol has potential for use in mechanistic studies to tease out causes of age-related anabolic resistance.
Study 1—power training in adult mice
Adult mice post-training improvements mirror expected exercise-induced outcomes
The individualized power training resulted in muscle hypertrophy, increased contractile force, velocity and power, and improved physical function. These types of improvements have, of course, been reported in humans after resistance and power training programs (Romero-Arenas et al. 2018; Kraemer and Ratamess 2005; Mitchell et al. 2013; McKinnon et al. 2017; McCall et al. 1996; Tesch 1988; Burkholder et al. 1994; Morgan et al. 2015). Similar outcomes are observed in various animal models of exercise as well (Chen et al. 2016; Tamaki et al. 1992; Drummond et al. 2010; Krisan et al. 2004; Farrell et al. 1999; Fluckey et al. 1995; Deschenes et al. 2000; Wirth et al. 2003; Call et al. 2010; Alway et al. 2005). (For a more in-depth discussion of other rodent exercise protocols, how they compare to our new protocol, and training specificity principles see the Online Resource Discussion.)
Over the course of the 12-week training program, quantitatively, the mice adapted to moving heavier and heavier weights (produced more force) and they produced greater power output (velocity×force). These adaptations are consistent with the theory of training volume and exercise responsiveness (Schoenfeld 2017; American College of Sports Medicine position stand 2009). Anecdotally, mice improved functionally from being unbalanced and having poor gaits at slow speed with light weights in the beginning of the training program to eventually quickly running confidently, and fast, with weights greater than 50% of their body mass loaded on their vests (see Online Resource Video 1). These observations demonstrate, qualitatively, that the mice gained functional aptitude as a result of the training sessions. We also observed similar adaptations in the older mice in study 2. Because this power exercise protocol improved movement capability in both younger and older mice, the findings herein and future mechanistic studies using this protocol apply translationally to human exercise regimens designed to improve functional movement.
While positive functional/movement adaptations were documented in the adult mice, they also had numerous muscle contractility improvements and evidence of muscle hypertrophy following the training period. The hallmark of many power training programs is to facilitate performance/power production under increasingly heavy loads. Notably, with our individualized power training program, the increases in SOL velocity and power output occurred at the upper limits (above 60% P0) of the force-velocity and the force-power curves. These improvements in muscle function represent enhanced ability to move while exerting a high percentage of maximum force. The training program produces expected results similar to power training programs instituted in humans, such as Olympic weightlifting or strongman training. Collectively, based on the improvements spanning from how the mice moved to changes within the individual muscle fiber, we conclude that our validated individualized power training protocol for mice is a valuable resource for pre-clinical and basic science exercise adaptation research.
Study 2—power training in older mice
Older mice exhibited some post-training improvements
In study 2, we applied our individualized training protocol from study 1 to a cohort of older mice to determine the effect of training in aged animals. Our hypothesis was that the older mice would have multiple areas of improvement after training for 12 weeks, but that the effect would be modulated by the age of the mice. Based on published literature reporting age-dependent responses to exercise training, we expected evidence of resistance to anabolic stimuli manifested by a reduced or non-response in some of the outcome measures. One interesting observation was the wide spread of the data (variance) in the outcome measurements in both age-groups. This variance suggests same chronologically aged individuals may experience differential functional/biological aging; i.e., some mice declined in function more rapidly than others in a given age group. Although the two studies were done separately, it is clear that both ages of mice received some benefit from the exercise protocol. However, the overall effect was lessened or attenuated in the older cohort (see Table 1, and in the Online Resource: Tables S4 and S5; and Figs. S1–S2 where we do compare the results of the two studies), potentially as a result of anabolic resistance.
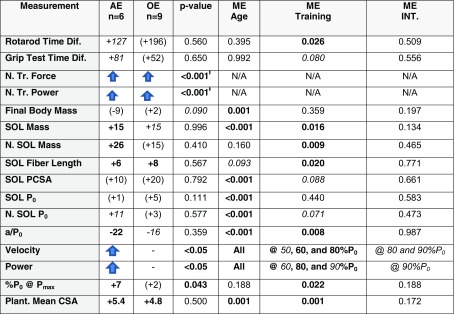
Table 1.
Summary of anabolic response findings. The adult mice demonstrated improvement in all categories, but the old had a more limited response. The percentage change for each exercise group in comparison to the age-matched controls is listed in the appropriate cell. The p value compares the fold change in each category, normalized by the mean of the age-matched control, in the adult exercise group to the change in the old exercise group. Main effects are from 2 × 2 ANOVA or ANCOVA (see Online Resource for details), with p values bolded if significant. Total significance adds the number of categories in which there was a significant change or trend (p < 0.10). Abbreviations: Diff difference in seconds; N. TR Power normalized training power; N. TR. Force normalized training force, p values from general linear model comparison of the regression lines; SOL soleus muscle; N normalized to grams of body mass; PCSA physiological cross-sectional area; P0 maximum isometric force; a/P0 measurement of shape of the force-velocity graph; Velocity contractile velocity improved at, at least, one percentage of P0; Power contractile power improved at, at least, one measured percentage of P0; %P0@Pmax percentage of P0 where maximum power is produced; Plant. plantaris; CSA cross-sectional area; ME Age main effect of age; ME Training main effect of training; ME Int. interaction term of age×training, from 2 × 2 ANOVA or ANCOVA (details of statistical methods and results found in Online Resource); N/A not applicable. Other symbols: parenthesis indicate a non-significant change; “Up Arrow” = significant improvement in at least one subcategory; “Dash” = no significant improvements; ƚ = from general linear model comparison of regression curves; items bolded if the difference in means was significant at p > 0.05, in italics if p < 0.10
Anabolic resistance
Anabolic resistance represents the blunted responses to stimuli (e.g., nutrient signaling and exercise) that normally induce anabolism. Anabolic resistance has been chronicled in numerous skeletal muscle studies demonstrating a general lack of responsiveness with age to nutrient sensing pathways and exercise (Burd et al. 2013; Koopman and van Loon 2009; Kumar et al. 2009; Degens and Alway 2000; Francaux et al. 2016; Drummond et al. 2008; Guillet et al. 2004; Fry and Rasmussen 2011; Cuthbertson et al. 2005). However, a few studies have indicated little difference between young and older individuals (Chevalier et al. 2011; Symons et al. 2011). Potential causes of an age-related blunted response to anabolic stimuli are numerous and include interactions between factors such as senescent cell secretory profiles (e.g., cytokines), satellite cell dysfunction, disrupted autophagy, overproduction of reactive oxygen species, mitochondrial abnormalities, and insulin resistance (Garcia-Prat et al. 2016; Gomes et al. 2017; Guillet et al. 2004; Morais et al. 2018). Two age-associated systemic dysfunctions, enhanced global inflammation and endocrine system dysregulation, likely also contribute to anabolic resistance. The inflammatory response post-exercise is an important signal for muscle to begin to initiate repair, yet, elevated age-related global inflammation may obscure this signal, thus contributing to a reduced exercise response (Merritt et al. 2013; Dalle et al. 2017). Older adults experience alterations in hormonal production, including a global reduction in free testosterone and somatotropin, each being important anabolic hormones, that when sufficiently produced allow for muscle growth (Sipilä et al. 2013; Gray et al. 1991; van den Beld et al. 2000). Thus, older adults having less free testosterone or growth hormone in circulation creates a less permissive environment for muscle hypertrophy. Collectively, these observations suggest the overall systemic physiological environment may be less conducive towards anabolism in the elderly.
In addition to and perhaps partially as a result of systemic changes in older adults, changes at the cellular and molecular level also occur that negatively affect anabolic responses. Impaired protein synthesis signaling with age has been implicated as a possible source of anabolic resistance (Fry and Rasmussen 2011). The cell signaling cascades associated with muscle hypertrophy (for example nutrient sensing, exercise, and growth factors) all feed through mammalian target of rapamycin complex 1 (mTORC1) (Glass 2010), and may be less responsive to a given stimuli in older versus younger subjects (Burd et al. 2013; Dickinson et al. 2013). For instance, there is evidence that activation of mTORC1, by resistance training, is compromised in aging (Fry et al. 2011). Another example of cellular/molecular factors of anabolic resistance would be that the ability of satellite cells to activate, proliferate, differentiate, and ultimately fuse with existing fibers to promote muscle repair is also compromised with age, which would reduce the ability of muscle to recover and regenerate after heavy exercise sessions (Conboy et al. 2005; Gopinath and Rando 2008). Throughout the literature, there is a theme that age results in a compromised ability to respond to anabolic stimuli with the same robustness experienced by younger adults, due to systemic factors and/or molecular/cellular changes.
In contrast, it has been suggested that the elderly possess potentially equivalent response mechanisms but may require a greater stimulus to achieve the same benefit as younger adults (Symons et al. 2011; Moore et al. 2015; Bickel et al. 2011; Shad et al. 2016). For example, Moore and colleagues demonstrated that to obtain a comparable level of mTORC1 signaling and protein synthesis observed in younger adults, older adults required a larger intake of protein.
There are many modifiable cofactors that might have played a role in the reduced response to training that we observed in our older mice, as just mentioned in our discussion of anabolic resistance. Potentially, an improved response could be achieved by further tailoring the exercise protocol to older mice. During study 2, we observed that the older mice sometimes were unable to increase resistance (weights) after three sessions of training, but instead, had to wait until the fourth session. Thus, they had lower volume of training overall compared to the adult mice, which might have resulted in reduced adaptation to the exercise. One important change to counteract this would be to extend the training period to 16 weeks rather than 12, as it may simply take longer for the older mice to adapt to the exercise. In addition, to overcome the lower response in older animals, synergistic treatments to exercise training might have an additive effect. An example would be nutrient support, such as increased dietary protein, which might help to improve muscle hypertrophy and strength gains in the older animals. In a second example, reduced levels of testosterone in the older mice may have reduced the rate at which muscle mass could be added. Thus, testosterone supplementation, in conjunction with the exercise, might also improve anabolic response. In essence, a multifactorial problem (e.g., anabolic resistance or sarcopenia) requires a multifactorial solution. Thus, we are proposing a paradigm shift towards personalized (individualized) precision exercise.
Precision exercise
A key concept for maximizing the benefit of any exercise is tailoring the exercise program to the individual. We consider exercise to be a form of regenerative medicine and thus believe the future of individualized exercise training is in the same arena as individualized medicine. Precision medicine is an approach to healthcare in which treatments are tailored to the individual needs and genetics of the patient. We suggest precision exercise is a needed advance in rehabilitation and general exercise physiology, because in any given population, there will be low responders, mid responders, and high responders to exercise prescriptions (Thalacker-Mercer et al. 2013). Thus, by individually tailoring exercise/rehabilitation prescriptions to the genetic, epigenetic, metabolic, hormonal, and other profiles of each patient, we may improve response to exercise and hasten acquisition of beneficial effect. To this end, both basic science and translational work are needed to determine biomarkers that predict exercise response and what modalities can be prescribed to improve response at the individual level. Future studies can examine these questions in more detail.
Functional preservation
In study 1, we demonstrated first that the individualized power training effectively stimulated positive adaptation in the adult mice, but we also demonstrated that the adaptations were less robust in the older mice in study 2. When tracking whole-body training physiology (force and power output) over each training session, we observed evidence of blunted exercise response: the older mice certainly did not progress as quickly or to the extent as observed in the adult mice. This is a typical result observed in human exercise trials when older adults (Moro et al. 2018) undergoing a similar exercise protocol are compared to younger adults (Reidy et al. 2016). We also observed limited evidence of tissue/cellular exercise adaptations in the older mice when compared to the adult mice (see Table 1). This is not surprising because human training literature has reported a reduced hypertrophic response to exercise in older subjects (Kumar et al. 2009; Welle et al. 1995).
However, functionally, both in the rotarod (overall neuromuscular motor function) and inverted cling grip test (strength/endurance), the old mice showed some evidence of improvement (main effect of training, when collapsed across age). Without exercise, older mice tended to decline (see the control groups in both current studies), but with exercise the decline in function has potential to be mitigated. In previous work (Graber et al. 2015b), we demonstrated that voluntary wheel running resulted in similar adaptations of increase rotarod ability in both adult and old mice, and decreased incidence of frailty in older mice. Therefore, we conclude that older animals can improve functionally given sufficient exercise stimulus. This has been reported in human studies of exercise and function as well (Marini et al. 2008; Fiatarone et al. 1990; Capodaglio et al. 2007). Thus, exercise in older individuals may not produce increases in positive adaptations to the same extent as it might in younger individuals, or may not produce improvements at all. Nonetheless, it is important to acknowledge and embrace that preservation of physical function or fitness is a goal when it comes to improving the lives of older adults.
While this was a study of the benefits of a relatively short-term acute bout of exercise intervention, we hypothesize that lifelong precision exercise training designed to serve the specific needs of a given individual would promote greater health benefits and positive adaptations. We know that both resistance and aerobic exercise can help ameliorate some of the molecular hallmarks of aging such as genomic instability, telomere attrition, age-associated epigenetic alterations, loss of proteostasis, and mitochondrial dysfunction (Rebelo-Marques et al. 2018). Our opinion is that one should start an exercise program, and maintain it, early in life to gain maximum benefit against age-related loss of functional capacity. However, it is important to note that any age and fitness level, even those who are already frail, can benefit greatly from initiating an exercise regimen (Fiatarone et al. 1990).
In humans, it has been shown in numerous studies of endurance-style training that sustained lifelong exercise can help to promote skeletal muscle and cardiovascular health and preserve physical function (Landi et al. 2018; Shibata et al. 2018; Campbell et al. 2019). Resistance training in both sustained long-term well-trained and lifelong weight lifters has been much less well studied. However, it could be expected that both sustained long-term and lifelong well-designed resistance training exercise programs would certainly preserve muscle mass, strength, function, and delay/retard the downward trajectory of sarcopenia (Aartolahti et al. 2019; Landi et al. 2018; Law et al. 2016). An individual practicing resistance exercise long-term over the lifespan would not suffer as much from disuse atrophy, might preserve the larger type 2 muscle motor units longer (use it or lose it principle), and would have increased lean muscle mass during the adult life such that there would be that much more muscle to lose during older adulthood before the amount lost would trigger maladaptive functional alterations (Lavin et al. 2019; Botero et al. 2013). Thus, a long-term sustained exercise program combining endurance, strength, and power adaptations, such as the protocol in the current study, would enable the maintenance of physical function in older adults better than a single acute training period or even sustained training comprising only a single element of exercise paradigm (i.e., aerobic or resistance training only).
Overall conclusion
The adult trained mice demonstrated evidence of improvement in all measured areas. However, when we applied the training protocol to the cohort of older mice, although we observed multiple areas of improvement with training, the effect was modulated by the age of the mice. In the older cohort of mice, there was evidence of resistance to anabolic stimuli manifested in a reduced or non-response in some of the outcome measures. Overall, the specific adaptations expected and observed from the exercise were similar to human studies (Miszko et al. 2003).
In our future direction, we will be determining what mechanisms contribute to the reduced level of exercise response in older mice. In addition, we will seek to determine what mechanisms cause the wide individual variability of response within age groups, i.e., why do some mice (or humans for that matter) respond at a high level to exercise while others have a lessened or null response? We conclude that our validated individualized power training protocol for mice is a valuable resource for pre-clinical and basic science translational/reverse translational exercise adaptation research in both adult and older mice.
Electronic supplementary material
(PDF 252 kb)
Author contributions
Conceptualization: TGG, LVT. Methodology: TGG. Validation: TGG. Formal analysis: TGG. Investigation: TGG, KRF. Resources: LVT. Writing (original draft): TGG, LVT. Writing (review and editing): TGG, LVT, KRF. Supervision: LVT, TGG. Project administration: TGG. Funding acquisition: LVT.
Funding
This study received support from the National Institute of Health: NCATS CTSA NRSA Fellowship TL1 TR001440 and NIA F31 AG044108 (TGG), R01 AG017768 (LVT); and the Travis M Roy Endowed Professorship (LVT).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
For Technical Assistance: Janice Shoeman, Rachel Borgstahl, Windy Torgerud, Alicia Behr, Nathan Proft, Steven Quiring, and Amanda Richabaught (for histology/CSA); and Haiming Liu (for assistance during tissue collection).
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ted G. Graber, Email: thgraber@utmb.edu
Katie R. Fandrey, Email: kfandrey03@gmail.com
LaDora V. Thompson, Email: lvthomp@bu.edu
References
- Aartolahti E, Lönnroos E, Hartikainen S, Häkkinen A (2019) Long-term strength and balance training in prevention of decline in muscle strength and mobility in older adults. Aging Clin Exp Res. 10.1007/s40520-019-01155-0 [DOI] [PMC free article] [PubMed]
- Alway SE, Siu PM, Murlasits Z, Butler DC (2005) Muscle hypertrophy models: applications for research on aging. Can J Appl Physiol 30(5):591–624 [DOI] [PubMed]
- American College of Sports Medicine position stand (2009) Progression models in resistance training for healthy adults. Med Sci Sports Exerc 41(3):687–708. 10.1249/MSS.0b013e3181915670 [DOI] [PubMed]
- Bean JF, Kiely DK, LaRose S, O'Neill E, Goldstein R, Frontera WR. Increased velocity exercise specific to task training versus the National Institute on Aging’s strength training program: changes in limb power and mobility. J Gerontol A Biol Sci Med Sci. 2009;64(9):983–991. doi: 10.1093/gerona/glp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel CS, Cross J, Bamman M. Exercise dosing to retain resistance training adaptations in young and older adults. Med Sci Sports Exerc. 2011;43(7):1177–1187. doi: 10.1249/MSS.0b013e318207c15d. [DOI] [PubMed] [Google Scholar]
- Botero JP, Shiguemoto GE, Prestes J, Marin CT, Do Prado WL, Pontes CS, Guerra RL, Ferreia FC, Baldissera V, Perez SE. Effects of long-term periodized resistance training on body composition, leptin, resistin and muscle strength in elderly post-menopausal women. J Sports Med Phys Fitness. 2013;53(3):289–294. [PubMed] [Google Scholar]
- Burd N, Gorissen S, van Loon Luc JC. (2013) anabolic resistance of muscle protein synthesis with aging. Exerc Sport Sci Rev. 2013;41(3):169–173. doi: 10.1097/JES.0b013e318292f3d5. [DOI] [PubMed] [Google Scholar]
- Burkholder TJ, Fingado B, Baron S, Leiber RL. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J Morphol. 1994;221(2):177–190. doi: 10.1002/jmor.1052210207. [DOI] [PubMed] [Google Scholar]
- Call JA, McKeenan JN, Novotny SA, Lowe DA. Progressive resistance voluntary wheel running in the mdx mouse. Muscle Nerve. 2010;42(6):871–880. doi: 10.1002/mus.21764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A, Grace F, Ritchie L, Beaumont A, Sculthorpe N. Long-term aerobic exercise improves vascular function into old age: a systematic review, meta-analysis and meta regression of observational and interventional studies. Front Physiol. 2019;10:31. doi: 10.3389/fphys.2019.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capodaglio P, Capodaglio Edda M, Facioli M, Saibene F. Long-term strength training for community-dwelling people over 75: impact on muscle function, functional ability and life style. Eur J Appl Physiol. 2007;100(5):535–542. doi: 10.1007/s00421-006-0195-8. [DOI] [PubMed] [Google Scholar]
- Chen Y-M, Lin C-L, Wei L, Hsu Y-J, Chen K-N, Huang CC, Kao C_H. Sake protein supplementation affects exercise performance and biochemical profiles in power-exercise-trained mice. Nutrients. 2016;8(2):106. doi: 10.3390/nu8020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier S, Goulet ED, Burgos SA, Wykes LJ, Morais JA. Protein anabolic responses to a fed steady state in healthy aging. J Gerontol A Biol Sci Med Sci. 2011;66(6):681–688. doi: 10.1093/gerona/glr036. [DOI] [PubMed] [Google Scholar]
- Conboy I, Conboy M, Wagers A, Girma E, Weissman I, Rando T. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19(3):422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- Dalle S, Rossmeislova L, Koppo K. The role of inflammation in age-related sarcopenia. Front Physiol. 2017;8:1045. doi: 10.3389/fphys.2017.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degens H, Alway SE. Skeletal muscle function and hypertrophy are diminished in old age. Muscle Nerve. 2000;27(3):339–347. doi: 10.1002/mus.10314. [DOI] [PubMed] [Google Scholar]
- Deschenes MR, Judelson DA, Kraemer WJ, Meskaitis VJ, Volek JS, Nindl BC, Harman FS, Deaver DR. Effects of resistance training on neuromuscular junction morphology. Muscle Nerve. 2000;23(10):1576–1581. doi: 10.1002/1097-4598(200010)23:10<1576::AID-MUS15>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Dickinson J, Drummond M, Coben J, Volpi E, Rasmussen B. Aging differentially affects human skeletal muscle amino acid transporter expression when essential amino acids are ingested after exercise. Clin Nutr. 2013;32(2):273–280. doi: 10.1016/j.clnu.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dill EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol. 2008;104(5):1452–1461. doi: 10.1152/japplphysiol.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Conlee RK, Mack GW, Sudweeks S, Schaalje GB, Parcell AC. Myogenic regulatory factor response to resistance exercise volume in skeletal muscle. Eur J Appl Physiol. 2010;108(4):771–778. doi: 10.1007/s00421-009-1279-z. [DOI] [PubMed] [Google Scholar]
- Farrell PA, Fedele MJ, Hernandez J, Fluckey JD, Miller JL, III, Lang CH, Vary TC, Kimball SR, Jefferson LS. Hypertrophy of skeletal muscle in diabetic rats in response to chronic resistance exercise. J Appl Physiol. 1999;87(3):1075–1082. doi: 10.1152/jappl.1999.87.3.1075. [DOI] [PubMed] [Google Scholar]
- Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lisitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA. 1990;263(22):3029–3034. doi: 10.1001/jama.1990.03440220053029. [DOI] [PubMed] [Google Scholar]
- Fluckey JD, Kraemer WJ, Farrell PA. Pancreatic islet insulin secretion is increased after resistance exercise in rats. J Appl Physiol. 1995;79(4):1100–1105. doi: 10.1152/jappl.1995.79.4.1100. [DOI] [PubMed] [Google Scholar]
- Francaux M, Demeulder B, Naslain D, Fortin R, Lutz O, Caty G, Deldicque L. Aging reduces the activation of the mTORC1 pathway after resistance exercise and protein intake in human skeletal muscle: potential role of REDD1 and impaired anabolic sensitivity. Nutrients. 2016;8(1):47. doi: 10.3390/nu8010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry C, Rasmussen B. Skeletal muscle protein balance and metabolism in the elderly. Curr Aging Sci. 2011;4(3):260–268. doi: 10.2174/1874609811104030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry C, Drummond M, Glynn E, et al. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle. 2011;1(1):11–11. doi: 10.1186/2044-5040-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Prat L, Martinez-Vicente M, Perdiguero E, et al. Autophagy maintains stemness by preventing senescence. Nature. 2016;529:37–42. doi: 10.1038/nature16187. [DOI] [PubMed] [Google Scholar]
- Glass DJ. PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. In: Rommel C, Vanhaesebroeck B, Vogt P, editors. Phosphoinositide 3-kinase in health and disease. Current topics in microbiology and immunology. Berlin, Heidelberg: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- Gomes MJ, Martinez PF, Pagan LU, Damatto RL, Cezar MDDM, Lima ARR, Okoshi K, Okoshi MP. Skeletal muscle aging: influence of oxidative stress and physical exercise. Oncotarget. 2017;8(12):20428–20440. doi: 10.18632/oncotarget.14670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath SD, Rando TA. Stem cell review series: aging of the skeletal muscle stem cell niche. Aging Cell. 2008;7(4):590–598. doi: 10.1111/j.1474-9726.2008.00399.x. [DOI] [PubMed] [Google Scholar]
- Graber TG, Ferguson-Stegall L, Kim J-H, Thompson LV. C57BL/6 neuromuscular healthspan scoring system. J Gerontol A Biol Sci Med Sci. 2013;68(11):1326–1336. doi: 10.1093/gerona/glt032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber TG, Kim J-H, McLoon LK, Grange RW, Thompson LV. C57BL/6 lifespan study: age-related declines in muscle power production and contractile velocity. Age. 2015;37:36. doi: 10.1007/s11357-015-9773-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber TG, Ferguson-Stegall L, Liu H, Thompson LV. Voluntary aerobic exercise reverses frailty in old mice. J Gerontol A Biol Sci Med Sci. 2015;70(9):1045–1058. doi: 10.1093/gerona/glu163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber TG, Rawls BL, Tian B, Durham WJ, Brightwell CR, Brasier AR, Rasmussen BB, Fry CS. Repetitive TLR-3-mediated lung damage induces skeletal muscle adaptations and cachexia. Exp Gerontol pii. 2018;S0531-5565(17):30667–30668. doi: 10.1016/j.exger.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray A, Feldman HA, McKinlay JB, Longcope C. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts male aging study. J Clin Endocrinol Metab. 1991;73:1016–1025. doi: 10.1210/jcem-73-5-1016. [DOI] [PubMed] [Google Scholar]
- Guillet C, Prod’homme M, Balage M, Gachon P, Giraudet C, Morin L, Grizard J, Boirie Y. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J. 2004;18(13):1586–1587. doi: 10.1096/fj.03-1341fje. [DOI] [PubMed] [Google Scholar]
- Hornberger TA, Jr, Farrar RP. Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Can J Appl Physiol. 2004;29(1):16–31. doi: 10.1139/h04-002. [DOI] [PubMed] [Google Scholar]
- Kilikevicius A, Venckunas T, Zelniene R, Carroll A, Lionikaite S, Ratkevicius A, et al. Divergent physiological characteristics and responses to endurance training among inbred mouse strains. Scand J Med Sci Sports. 2013;23(5):657–668. doi: 10.1111/j.1600-0838.2012.01451.x. [DOI] [PubMed] [Google Scholar]
- Koopman R, van Loon L. Aging, exercise, and muscle protein metabolism. J Appl Physiol. 2009;106(6):2040–2048. doi: 10.1152/japplphysiol.91551.2008. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, & Ratamess NA (2005) Progression and resistance training. President’s council on physical fitness and sports research digest. Ed. Young D, Pangrazi RP, Ainsworth, B. 6(3) Sept. 2005. Accessed via WWW on 10/29/18 https://www.presidentschallenge.org/informed/digest/docs/200509digest.pdf
- Kregel KC, Allen DL, Booth FW, Fleshner MR, Henriksen EJ, Musch T et al (2006) Resource book for the design of animal exercise protocols. Am Physiol Soc:152
- Krisan AD, Collins DE, Crain AM, Kwong CC, Mohenish KS, Bernard JR, Yaspelkis BB., III Resistance training enhances components of the insulin signaling cascade in normal and high-fat-fed rodent skeletal muscle. J Appl Physiol. 2004;96(5):1691–1700. doi: 10.1152/japplphysiol.01054.2003. [DOI] [PubMed] [Google Scholar]
- Kumar V, Selby A, Rankin D, Patel R, Atherton P, Williams J, Smith K, Seynnes O, Hicock N, Rennie MJ. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol Lond. 2009;587(1):211–217. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi F, Calvani R, Picca A, Tosato M, Martone AM, D’Angelo E, Serafini E, Bernabei R, Marzetti E. Impact of habitual physical activity and type of exercise on physical performance across ages in community-living people. PLoS One. 2018;13(1):e0191820. doi: 10.1371/journal.pone.0191820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin KM, Roberts BM, Fry CS, Moro T, Rasmussen BB, Bamman MM. The importance of resistance exercise training to combat neuromuscular aging. Physiology (Bethesda) 2019;34(2):112–122. doi: 10.1152/physiol.00044.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law TD, Clark LA, Clark BC. Resistance exercise to prevent and manage sarcopenia and dynapenia. Annu Rev Gerontol Geriatr. 2016;36(1):205–228. doi: 10.1891/0198-8794.36.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini M, Sarchielli E, Brogi L, Lazzeri R, Salerno R, Sgambati E, Monaci M. Role of adapted physical activity to prevent the adverse effects of the sarcopenia. A pilot study. Ital J Anat Embryol. 2008;113(4):217–225. [PubMed] [Google Scholar]
- McCall GE, Byrnes WC, Dickinson A, Pattany PM, Fleck SJ. Muscle fiber hypertrophy, hyperplasia, and capillary density in college men after resistance training. J Appl Physiol. 1996;81(5):2004–2012. doi: 10.1152/jappl.1996.81.5.2004. [DOI] [PubMed] [Google Scholar]
- McKinnon NB, Connelly DM, Rice CL, Hunter SW, Doherty TJ. Neuromuscular contributions to the age-related reduction in muscle power: mechanisms and potential role of high velocity power training. Ageing Res Rev. 2017;35:147–154. doi: 10.1016/j.arr.2016.09.003. [DOI] [PubMed] [Google Scholar]
- Merritt E, Stec M, Thalacker-Mercer A, Windham S, Cross J, Shelley D, Tuggle C, Kosek S, Kim J-S, Bamman MM. Heightened muscle inflammation susceptibility may impair regenerative capacity in aging humans. J Appl Physiol. 2013;115(6):937–948. doi: 10.1152/japplphysiol.00019.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miszko TA, Cress E, Slade J, Covey CJ, Agrawal SK, Doerr CE. Effect of strength and power training on physical function in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2003;58(2):171–175. doi: 10.1093/gerona/58.2.M171. [DOI] [PubMed] [Google Scholar]
- Mitchell CJ, Churchward-Venne TA, Bellamy L, Parise G, Baker SK, Phillips SM. Muscular and systemic correlates of resistance training-induced muscle hypertrophy. PLoS One. 2013;8(10):e78636. doi: 10.1371/journal.pone.0078636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D, Churchward Venne T, Witard O, Breen L, Burd NA, Tipton KD, Phillips SM. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci. 2015;70(1):57–62. doi: 10.1093/gerona/glu103. [DOI] [PubMed] [Google Scholar]
- Morais JA, Jacob KW, Chevalier S. Effects of aging and insulin resistant states on protein anabolic responses in older adults. Exp Gerontol. 2018;15(108):262–268. doi: 10.1016/j.exger.2018.04.025. [DOI] [PubMed] [Google Scholar]
- Morgan P, Embry A, Perry L, Holthaus K, Gregory CM (2015) Feasibility of lower-limb muscle power training to enhance locomotor function poststroke. J Rehabil Res Dev 52 (1):77–84 [DOI] [PubMed]
- Moro T, Brightwell CR, Deer RR, Graber TG, Galvan E, Fry CS, Volpi E, Rasmussen BB. Muscle protein anabolic resistance to essential amino acids does not occur in healthy older adults before or after resistance exercise training. J Nutr. 2018;148(6):900–909. doi: 10.1093/jn/nxy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebelo-Marques A, De Sousa Lages A, Andrade R, Ribeiro CF, Mota-Pinto A, Carrilho F, Espregueira-Mendes J (2018) Aging hallmarks: the benefits of physical exercise. Front Endocrinol 9 [DOI] [PMC free article] [PubMed]
- Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev. 2012;40(1):4–12. doi: 10.1097/JES.0b013e31823b5f13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy PT, Borack MS, Markofski MM, Dickinson JM, Deer RR, Husaini SH, Walker DK, Igbinigie S, Robertson SM, Cope MB, Mukherjea R, Hall-Porter JM, Jennings K, Volpi E, … Rasmussen BB (2016) Protein supplementation has minimal effects on muscle adaptations during resistance exercise training in young men: a double-blind randomized clinical trial. J Nutr 146(9):1660–1669. 10.3945/jn.116.231803 [DOI] [PMC free article] [PubMed]
- Romero-Arenas S, Ruiz R, Vera-Ibáñez A, Colomer-Poveda D, Guadalupe-Grau A, Márquez G. Neuromuscular and cardiovascular adaptations in response to high-intensity interval power training. J Strength Cond Res. 2018;32(1):130–138. doi: 10.1519/JSC.0000000000001778. [DOI] [PubMed] [Google Scholar]
- Sayers SP, Gibson K. High-speed power training in older adults: a shift of the external resistance at which peak power is produced. J Strength Cond Res. 2014;28(3):616–621. doi: 10.1519/JSC.0b013e3182a361b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld Dose-response relationship between weekly resistance training volume and increases in muscle mass: a systematic review and meta-analysis. J Sports Sci. 2017;35(11):1073–1082. doi: 10.1080/02640414.2016.1210197. [DOI] [PubMed] [Google Scholar]
- Shad BJ, Thompson JL, Breen L. Does the muscle protein synthetic response to exercise and amino acid-based nutrition diminish with advancing age? A systematic review. Am J Physiol Endocrinol Metab. 2016;311(5):E803–E817. doi: 10.1152/ajpendo.00213.2016. [DOI] [PubMed] [Google Scholar]
- Shibata S, Fujimoto N, Hastings JL, Carrick-Ranson G, Bhella PS, Hearon CM, Jr, Levine BD. The effect of lifelong exercise frequency on arterial stiffness. J Physiol. 2018;596(14):2783–2795. doi: 10.1113/JP275301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipilä S, Narici M, Kjaer M, Pöllänen E, Atkinson RA, Hansen M, Kovanen V. Sex hormones and skeletal muscle weakness. Biogerontology. 2013;14(3):231–245. doi: 10.1007/s10522-013-9425-8. [DOI] [PubMed] [Google Scholar]
- Symons TB, Sheffield-Moore M, Mamerow MM, Wolfe RR, Paddon-Jones D. The anabolic response to resistance exercise and a protein-rich meal is not diminished by age. J Nutr Health Aging. 2011;15(5):3763–3781. doi: 10.1007/s12603-010-0319-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki T, Uchiyama S, Nakano S. A weight-lifting exercise model for inducing hypertrophy in the hindlimb muscles of rats. Med Sci Sports Exerc. 1992;24(8):881–886. doi: 10.1249/00005768-199208000-00009. [DOI] [PubMed] [Google Scholar]
- Tesch PA. Skeletal muscle adaptations consequent to long-term heavy resistance exercise. Med Sci Sports Exerc. 1988;20:S132–S134. doi: 10.1249/00005768-198810001-00008. [DOI] [PubMed] [Google Scholar]
- Thalacker-Mercer A, Stec M, Cui X, Cross J, Windham S, Bamman M. Cluster analysis reveals differential transcript profiles associated with resistance training-induced human skeletal muscle hypertrophy. Physiol Genomics. 2013;45(12):499–507. doi: 10.1152/physiolgenomics.00167.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Beld AW, de Jong FH, Grobbee DE, Pols HA, Lamberts SW. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metabol. 2000;85(9):3276–3282. doi: 10.1210/jcem.85.9.6825. [DOI] [PubMed] [Google Scholar]
- Welle S, Thornton C, Statt M. Myofibrillar protein synthesis in young and old human subjects after three months of resistance training. Am J Phys. 1995;268(3):E422–E427. doi: 10.1152/ajpendo.1995.268.3.E422. [DOI] [PubMed] [Google Scholar]
- Wirth O, Gregory EW, Cutlip RG, Miller GR. Control and quantitation of voluntary weight-lifting performance of rats. J Appl Physiol. 2003;95(1):402–412. doi: 10.1152/japplphysiol.00919.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 252 kb)