Abstract
Disruptions in growth hormone/insulin-like growth factor-1 (GH/IGF-1) signaling have been linked to improved longevity in mice and humans. Nevertheless, while IGF-1 levels are associated with increased cancer risk, they have been paradoxically implicated with protection from other age-related conditions, particularly in the brain, suggesting that strategies aimed at selectively increasing central IGF-1 action may have favorable effects on aging. To test this hypothesis, we generated inducible, brain-specific (TRE-IGF-1 × Camk2a-tTA) IGF-1 (bIGF-1) overexpression mice and studied effects on healthspan. Doxycycline was removed from the diet at 12 weeks old to permit post-development brain IGF-1 overexpression, and animals were monitored up to 24 months. Brain IGF-1 levels were increased approximately twofold in bIGF-1 mice, along with greater brain weights, volume, and myelin density (P < 0.05). Age-related changes in rotarod performance, exercise capacity, depressive-like behavior, and hippocampal gliosis were all attenuated specifically in bIGF-1 male mice (P < 0.05). However, chronic brain IGF-1 failed to prevent declines in cognitive function or neurovascular coupling. Therefore, we performed a short-term intranasal (IN) treatment of either IGF-1 or saline in 24-month-old male C57BL/6 mice and found that IN IGF-1 treatment tended to reduce depressive (P = 0.09) and anxiety-like behavior (P = 0.08) and improve motor coordination (P = 0.07) and unlike transgenic mice improved motor learning (P < 0.05) and visuospatial and working memory (P < 0.05). These data highlight important sex differences in how brain IGF-1 action impacts healthspan and suggest that translational approaches that target IGF-1 centrally can restore cognitive function, a possibility that should be explored as a strategy to combat age-related cognitive decline.
Electronic supplementary material
The online version of this article (10.1007/s11357-019-00065-3) contains supplementary material, which is available to authorized users.
Keywords: IGF-1, Aging, Brain, Intransasal, Healthspan, Cognitive function, Cognitive and sensorimotor decline
Introduction
The growth hormone/insulin-like growth factor-1 (GH/IGF-1) signaling pathway has been strongly implicated in the aging process across diverse species (Bartke et al. 2003; Barzilai et al. 2012). Indeed, disruption of daf2 in Caenorhabditiselegans (Kenyon et al. 1993) or Chico in Drosophila results in extended longevity (Clancy et al. 2001), while lifespan is improved in insulin-like growth factor-1 receptor (IGF-1R) haploinsufficient mice or animals with isolated circulating IGF-1 deficiency but only in females (Ashpole et al. 2017; Bokov et al. 2011; Holzenberger et al. 2003; Xu et al. 2014). In humans, dampened IGF-1 signaling, resulting from a nonsynonymous mutation in the IGF1R gene, was observed to be enriched in humans with exceptional longevity (Suh et al. 2008), while lower IGF-1 levels predict increased survival specifically in female nonagenarians (Milman et al. 2014). Likewise, low circulating IGF-1 levels are associated with lower risk of malignancy, including breast, colorectal, and prostate cancers (Milman et al. 2016). Given the evidence linking reduced IGF-1 signaling to delayed aging, strategies designed to disrupt this pathway, such as IGF-1R monoclonal antibodies (Mao et al. 2018), might prove beneficial to promote healthy aging in humans. However, epidemiologic studies suggest IGF-1 may be paradoxically protective against cardiovascular disease, type 2 diabetes, and frailty, implying a far more complex interaction between this axis and age-related diseases in humans that has been inferred from aging studies in model organisms (Milman et al. 2016).
The beneficial effects of IGF-1 have also been extended to the central nervous system (CNS) where IGF-1 plays a critical role in CNS development, a time in which local production of IGF-1 is markedly increased (Zhang et al. 2007). In contrast, the age-related decline in circulating IGF-1 levels with age has been associated with cognitive deficits (Doi et al. 2015) and an increased risk for poor performance in humans with neurodegenerative diseases (Vidal et al. 2016) and stroke (Saber et al. 2017). Whether the neuroprotective effects of IGF-1 implicate mostly systemic or locally produced IGF-1 in the brain is unclear. Raising circulating IGF-1 levels increases their concentration in the cerebrospinal fluid (CSF), whereas IGF-1 concentrations in blood and CSF are concomitantly reduced with aging (Muller et al. 2012). Interestingly, long-lived Ames Dwarf mice, which are deficient in circulating GH and IGF-1, demonstrate normal cognitive function, which is better maintained with age than controls. However, studies examining local IGF-1 levels in the brain of these mice have produced conflicting results, with an early report suggesting upregulation of IGF-1 levels (Sun et al. 2005), while a subsequent study reported lower IGF-1 levels in the cortex and hippocampus of these mice compared to wild-type controls (Puig et al. 2016).
The mechanism(s) mediating uptake of circulating IGF-1 into the CNS have been purported to occur by at least a few distinct processes. Transport of IGF-1 through the choroid plexus has been demonstrated to occur by transcytosis involving multicargo protein transporter low-density lipoprotein receptor-related protein 2 (LRP2) and its cognate receptor, while uptake across the parenchyma involving LRP1 and IGF-1R has also been described (Fernandez and Torres-Aleman 2012). Interestingly, an increase in neurovascular activity, such as during exercise, has been shown to promote enhanced uptake of IGF-1 into the brain (Nishijima et al. 2010). Moreover, aged mice were shown to have an enhanced capacity for IGF-1 uptake from the circulation to the CSF (Muller et al. 2012). However, peripheral administration of IGF-1 is not a viable approach to raise CNS levels due to numerous potential side effects.
Preclinical studies acutely targeting IGF-1 to the CNS, thereby circumventing the periphery, have demonstrated a protective effect against cognitive and neurosensory deficits, depressive-like behavior, and neurodegeneration (Cai et al. 2011; Carro et al. 2005). Moreover, studies in aged rodents have observed that short-term central administration of IGF-1 via intracerebroventricular (ICV) infusion or viral approaches confers protection against toxic insults and restores neuronal and cognitive function in aged animals (Lichtenwalner et al. 2001; Pardo et al. 2016, 2018). In addition, we have found that acute administration of IGF-1 centrally can restore whole-body glucose homeostasis in old insulin-resistant animals (Huffman et al. 2016). Thus, given the apparent benefits of IGF-1 in the brain, we hypothesized that strategies aimed at increasing its actions in the CNS, rather than the periphery, may be a suitable approach to promote healthy aging. Here, we have utilized a combination of transgenic mouse models designed to drive IGF-1 overexpression with spatial and temporal control in the brain, as well as intranasal (IN) administration of IGF-1 to old mice, to determine the effects of central IGF-1 action on aspects of healthspan.
Methods
Animals
Brain-specific, IGF-1 overexpressing mice with temporal and spatial control were generated by crossing animals expressing human IGF-1 under the control of the TRE promoter (TRE-IGF-1) (Ye et al. 2004) (a kind gift of Dr. Ping Ye) with Camk2a-tTA animals (Jackson stock no. 007004) to create a doxycycline-off (DOX-off) system. Male TRE-IGF-1–Camk2a-tTA (bIGF-1) mice on C57BL/6J background were crossed with Balb/cByJ females (Jackson stock no. 001026) to generate bIGF-1 mice on a CB6F1 background. Female bIGF-1 mice on a CB6F1 background were then crossed with C3D2F1/J males (Jackson stock no. 100004) to generate study mice on a genetically heterogeneous background akin to UM-HET3 mice. For studies performed at the University of Oklahoma, bIGF-1 mice were backcrossed to C57BL/6J for eight generations at Einstein and males were exported at 12–16 weeks, where they were aged for planned experiments. Breeders and study mice were provided a DOX-supplemented diet (625 mg/kg) from Purina (Rodent Diet 2018) to silence the IGF-1 transgene during development. Mice were group housed four to five per cage, subjected to a 14:10 light/dark photoperiod at 22 °C, and provided ad libitum access to food and water. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the Albert Einstein College of Medicine and the IACUC of OUHSC.
Experimental design
At 12 weeks of age, male and female bIGF-1 (TRE-hIGF-1–Camk2a-tTA) and Control (TRE-hIGF-1) mice on a heterogeneous background (n = 24 Control Females, n = 25 bIGF-1 Females, n = 25 Control Males, n = 27 bIGF-1 Males) were switched from a DOX-containing diet to a matched regular chow in order to permit human IGF-1 transgene expression (Fig. 1a). At 6–7 months of age, a subset of mice (n = 10 Control Females, n = 14 bIGF-1 Females, n = 13 Control Males, n = 13 bIGF-1 Males) was sacrificed for collection of plasma and tissue. The remainder of the animals (n = 12 Control Females, n = 10 bIGF-1 Females, n = 13 Control Males, n = 10 bIGF-1 Males) were then monitored for up to 24 months of age for measures of healthspan, as described below.
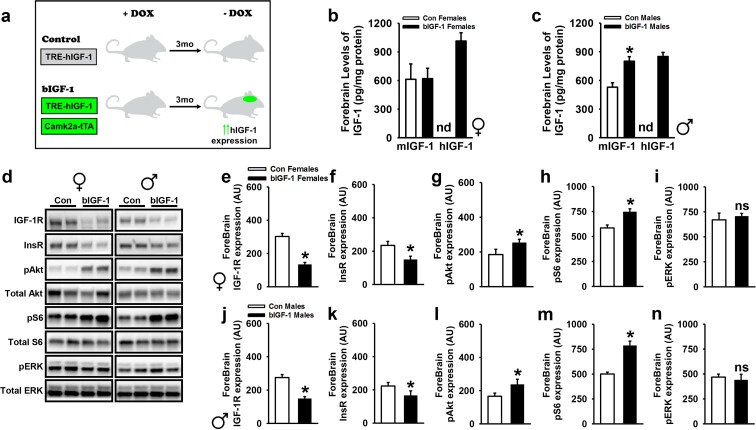
Fig. 1.
Characterization of brain IGF-1 expression and signaling in transgenic mice. a Experimental mouse model schematic indicates DOX-off system, thereby allowing transgenic expression upon DOX removal, after development. b, c IGF-1 protein levels were measured in forebrain tissues of females and males (n = 5 Control females, n = 7 bIGF-1 females, n = 7 Control males, n = 5 bIGF-1 males). d–n bIGF-1 mice showed a decrease in IGF-1R and InsR expression, while they showed an increase in p-Akt and p-S6 expression, and no significant change in p-ERK was seen (n = 9 Control females, n = 13 bIGF-1 females, n = 13 Control males, n = 11 bIGF-1 males). Logarithmically transformed data from male and female mice was analyzed using a one-way ANOVA. nd = not detectable. Bars represent mean ± SE. Asterisk: significantly different from controls, P ≤ 0.05
Body weight, body composition, and glucose metabolism
Body weight was monitored every 3 months and body composition (fat and lean mass) was measured by EchoMRI (qMR, Echo Medical Systems) in 6-month intervals for HET3 mice. At 6−7 months of age, glucose tolerance tests (GTTs) were performed in animals (n = 12 Control Females, n = 11 bIGF-1 Females, n = 11 Control Males, n = 15 bIGF-1 Males) following a 4–6-h fast (2 mg/kg dose IP) and insulin tolerance tests (ITTs) were performed in random-fed animals (n = 12 Control Females, n = 11 bIGF-1 Females, n = 12 Control Males, n = 12 bIGF-1 Males) early in the morning (1 U/kg dose IP), as described (Mao et al. 2018). A second round of GTTs and ITTs was subsequently performed in 20-month-old animals (n = 12 Control Females, n = 12 bIGF-1 Females, n = 14 Control Males, n = 11 bIGF-1 Males).
Indirect calorimetry
Whole-body energy metabolism was measured at 6–7 months of age in HET3 mice (n = 8 Control Females, n = 8 bIGF-1 Females, n = 8 Control Males, n = 7 bIGF-1 Males) by indirect calorimetry using an Oxymax System as described (Mao et al. 2018). Readouts included energy expenditure, substrate utilization (respiratory exchange ratio, RER), feeding behavior, and locomotor activity. Mice were initially allowed to acclimate in the metabolic cages for 48-72 h prior to measurements. Data was then collected over a 24-h period followed by a fast/re-feed challenge to evaluate metabolic flexibility.
Cognitive and behavioral function
At 15–16 months of age, a battery of cognitive and behavioral assessments were performed in both bIGF-1 and control HET3 animals (n = 5 Control Females, n = 7 bIGF-1 Females, n = 7 Control Males, n = 7 bIGF-1 Males) in the Einstein Cognitive/Behavioral Core. The Open Field (OF) was used to assess locomotor activity, anxiety, and exploration. Animals were placed in an opaque plastic arena and allowed to explore for 9 min, during which time the track length was recorded.
Visuospatial memory was assessed by the Object Recognition (OR) and Object Placement (OP) test. To this end, animals were placed in an opaque plastic arena and allowed to explore two identical objects. After a short or long retention interval, animals were returned to the arena in which one of the objects had been replaced with a new object (OR) or one of the objects had been moved (OP) and were allowed to explore for 4 min. Retention intervals were 3 or 24 h for OR and 45 min or 24 h for OP. Spatial working memory was assessed by spontaneous alternation during the Y-maze test. In brief, animals were placed in one of the arms and their spontaneous entries into different arms were counted for 5 min. The percent alternation was calculated as: [number of alternations / (total number of entries − 2)] × 100. In addition, the Zero Maze or the Elevated Plus Maze (EPM) was used to assess anxiety-like behavior. In brief, animals were placed either in the center of an elevated four-arm maze or in a closed arm of the round maze, which included two enclosed arms and two open arms, and time of exploration in either arm was recorded. To assess learning capacity, we used the published protocols of Tucsek et al. (2014). For this test, mice were placed individually at the end of an open arm with their back to the central platform. The time for mice to cross a line halfway along one of the closed arms was measured (transfer latency) on day 1 and day 2. Learning was defined as reduced transfer latency on day 2 compared to day 1. The Porsolt forced swim test was used to assess depressive-like behavior by placing animals in a tank half-filled with water (25 °C) for 10 min and time of immobility was recorded. Spatial memory was tested using the radial arms water maze as described (Ungvari et al. 2017). Motor coordination and motor learning were assessed by using an accelerated rotarod protocol as described (Tarantini et al. 2018), and performance was measured on four consecutive days.
Measurement of neurovascular coupling responses
For neurovascular coupling measurements, 20-month-old male Control and bIGF-1 mice (n = 4 Young Controls, n = 9 Old Controls, n = 6 Old bIGF-1) on a C57BL/6J background were anesthetized with isoflurane (4% induction and 1% maintenance), endotracheally intubated and ventilated, and were equipped with a closed cranial window. Neurovascular coupling responses were assessed by measuring changes in cerebral blood flow above the left barrel cortex using laser speckle contrast imaging in response to contralateral whisker stimulation according to a previously published protocol (Tarantini et al. 2017a, 2018). Changes in cerebral blood flow were averaged and expressed as percent (%) increase from the baseline value.
Neuromuscular function
Gross motor coordination was assessed by the balance beam test in young (6 months of age, n = 13 Control Females, n = 11 Control Males), middle-age (15 months of age, n = 5 Control Females, n = 7 bIGF-1 Females, n = 7 Control Males, n = 7 bIGF-1 Males), and old (24 months of age, n = 12 Control Females, n = 11 bIGF-1 Females, n = 13 Control Males, n = 13 bIGF-1 Males) HET3 animals (Mao et al. 2018). In brief, animals were first familiarized with the testing setup by walking twice across a 4-ft. plank. Animals were then challenged to transverse a 48-in.-long round beam of decreasing difficulty (0.5 in., difficult; 0.75 in., medium; 1 in., easy), with light and food cues as motivation to cross, and the number of slips was counted while crossing the beam. Grip strength was also assessed as a proxy of physical function. Animals were allowed to clasp a suspended wire or an inverted grid and the time to release (best of three trials) was recorded. Fine motor coordination and sensorimotor function were assessed by the tape removal test in 15-month-old mice (n = 5 Control Females, n = 7 bIGF-1 Females, n = 7 Control Males, n = 7 bIGF-1 Males). In brief, adhesive tape was placed on the front left paw and both the latency to contact and remove the tape were recorded. Endurance was determined by a single test on a treadmill (Exer 3/6, Columbus Instruments) on young (6 months of age, n = 13 Control Females, n = 11 Control Males) and old mice (24 months of age, n = 12 Control Females, n = 11 bIGF-1 Females, n = 13 Control Males, n = 13 bIGF-1 Males). Mice were first familiarized to the treadmill for three nonconsecutive days for 5 min at walking speed (8 m/min). Animals were then challenged with a graduated fatigue test, beginning at a 4% incline and 8 m/min for 3 min. The protocol used increased speed to 10 m/min at 3 min, 12 m/min at 4 min, and 15 m/min at 5 min which was maintained up to 30 min and 16 m/min at 30 min, 17 m/min at 40 min, 18 m/min at 50 min, and 19 m/min at 60 min (all animals voluntarily fatigued prior to 60 min).
Mitochondrial isolation and activities
Mitochondrial electron transport chain activities in skeletal muscle were performed by the UAB Bioanalytical Redox Biology Core (BARB). In brief, 50 mg of frozen gastrocnemius muscle (n = 8 Young Control Females, n = 9 Old Control Females, n = 8 Old bIGF-1 Females, n = 8 Young Control Males, n = 8 Old Control Males, n = 8 Old bIGF-1 Males) was pulverized, and mitochondria-enriched fractions isolated following a modification of Rasmussen et al. (1997). Pulverized samples were put into a 20:1 (volume/weight) solution of ice-cold Chappell-Perry (C/P) isolation buffer [100 mM KCl, 50 mM Tris-HCl, 1 mM Na-ATP, 5 mM MgSO4, 0.1 mM EGTA, 0.2% BSA, pH 7.4] + protease inhibitor cocktail (PIC, Roche, mini-complete). Samples were maintained at 0–1 °C while homogenized at 990 rpm using a customized Wheaton mortar and pestle. Homogenate was centrifuged at 600×g (10 min, 4 °C). The supernatant was then transferred to a separate ice-cold tube to be further centrifuged at 10,000×g (10 min, 4 °C). The resulting mitochondrial-enriched pellet was resuspended with 50 μL CP + PIC, and protein content was analyzed by Lowry. Complex I activity was immediately measured on a DU800 spectrophotometer using 2,6-dichloroindophenol (DCIP) as the terminal electron acceptor at 600 nm with the oxidation of NADH-reducing artificial substrate Coenzyme Q10 that then reduces DCIP as described (Krzywanski et al. 2016). Complex II activity was analyzed as the reduction of dichloroindophenol at 600 nm with succinate as the substrate (Fetterman et al. 2013). Complex III activity was measured in mitochondria by monitoring the conversion of cytochrome c in its oxidized form to its reduced form, as a linear increase in absorbance at 550 nm, with reduced ubiquinone as the substrate (Fetterman et al. 2013). Complex IV activity was measured by the oxidation of cytochrome c at 550 nm (Hinkle et al. 1967). Data are represented as the pseudo-first-order rate constant (k) divided by protein concentration. ATP synthase activity was determined by the continuous spectrophotometric monitoring of the oxidation of NADH, as described (Boveris et al. 1972). Citrate synthase activity was used as a surrogate measure of mitochondrial content (Hoppeler 1986) and measured using the coupled reaction with oxaloacetate, acetyl-CoA, and 5,5-dithiobis-(2,4-nitrobenzoic acid) (Raha et al. 2000).
Magnetic resonance imaging of brain
All MRI and 1H MRS data were acquired in a 9.4-T Varian Direct Drive system (Agilent Technologies, Inc., Santa Clara, CA) (Cui et al. 2017). A 14-mm-diameter receive-only surface RF coil (Doty Scientific Inc., Columbia, SC) along with a 7-cm ID 1H transmit and receive body coil (M2M Imaging Co., Cleveland, OH) was used. HET3 mice (n = 6 Young Control Females, n = 5 Old Control Females, n = 7 Old bIGF-1 Females, n = 6 Young Control Males, n = 7 Old Control Males, n = 5 Old bIGF-1 Males) were anesthetized with ~ 1.25% isoflurane mixed with room air. Respiration was monitored with a pressure pad (SA Instruments; Stony Brook, NY). Surface body temperature was maintained at 34–35 °C using warm air with feedback from a thermocouple placed beneath the body surface (SA Instruments; Stony Brook, NY). T2-weighted fast spin echo images were acquired for volume estimation: matrix 256 × 256, field of view (FOV) 25 mm2 over 30 slices, 0.5 mm thick, and no gap. Diffusion tensor imaging (DTI; 30 orthogonal directions (Jones et al. 1999)) was accomplished using b values of 939 s/mm2, a 128 × 128 matrix and FOV = 25 mm2, and 12 slices (0.6 mm thickness, 0.06 mm gap). Images of fractional anisotropy (FA) and mean diffusivity (MD) were calculated using the FMRIB FSL diffusion toolbox (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). DTI data were registered to anatomical data, which in turn were registered to a template (Paxinos-Franklin T2 atlas (Bai et al. 2012)) using FSL routines and scripts written in MATLAB.
1H MRS and brain metabolite quantification
1H MRS (Cui et al. 2017) was acquired via a LASER sequence (Garwood and DelaBarre 2001) with a 1.1 × 2.7 × 1.6-mm3 voxel positioned in the hippocampus. 1H MRS acquisition parameters were as follows: time of repetition (TR) = 3.5 s, time of echo (TE) = 36 ms, transients (NT) = 512, complex points = 1024, and spectral width (SW) = 4006 Hz. Water signal was efficiently suppressed with the WET sequence (Ogg et al. 1994). Shimming routinely resulted in unsuppressed voxel water signal line widths of 15–18 Hz, which was used as an internal reference for neurochemical concentration calculations from the same voxel. Metabolites were quantified using LCModel (Provencher 2001) to avoid subjective input, phasing or referencing, with a basis set kindly generated by Dr. S. Provencher using data acquired on our system. The coefficient of variation of metabolite concentrations reported in this study was similar to previously reported results at 9.4 T (Provencher 2001).
Hormone and cytokine assays
Endogenous IGF-1 concentrations in plasma were measured using a commercially available immunoassay (Quantikine ELISA, R&D Systems). To determine potential leak of transgenic IGF-1 in brain to the periphery, human IGF-1 levels in mouse plasma were performed using a specific “in-house” ELISA by the USC Aging Biomarker Core. In addition, mouse and human IGF-1 levels in forebrain were determined in 100 μL total protein extracts using specific, custom, ELISA-based assays by the USC Aging Biomarker Core. Briefly, 50 μL extraction buffer was used on 100 μL samples and incubated at room temperature for 30 min. Samples were then incubated for 30 min at room temperature in a neutralization buffer, centrifuged, and the supernatant containing the total protein extract was used for the subsequent IGF-1 ELISA. Finally, a Bio-Plex MAGPIX Multiplex Reader (Bio-Rad) was used to measure basal insulin levels and six inflammatory cytokines in plasma, including mouse IFN-γ, IL-10, IL-17A, IL-1β, IL-6, and TNF-α.
Intranasal administration of IGF-1
Awake 24-month-old C57BL/6 male mice (NIA) were habituated to the intranasal grip procedure over a 2-week period following a defined protocol (Hanson et al. 2013). Mice then received either recombinant human IGF-1 (25 μL at 2 μg/μL; 50 μg per dose) or an equal volume of saline (Control) every 48 h for at least 4 weeks, similar as described (Lopes et al. 2014; Vig et al. 2006). Specifically, mice were administered a total of 25 μL each session, giving 5 μL per nostril and alternating between nostrils, with 2 min pause in between each drop delivery. After 4 weeks of treatment, mice in the IN administration cohort were tested at 25–26 months of age (n = 14 IN Saline, n = 15 IN IGF-1) via a battery of physical and cognitive assessments while continuing to receive the assigned IN treatment. At the end of the study, animals were either sacrificed for blood and tissue collection or transcardially perfused with saline followed by ice-cold 4% paraformaldehyde for brain fixation and subsequent immunostaining.
Protein isolation and Western blotting
Western blotting was performed similarly as described (Huffman et al. 2016; Mao et al. 2018). In brief, tissues were homogenized in RIPA buffer and extracted protein concentration was determined using the BCA protein assay (Sigma, St. Louis, MO) (Huffman et al. 2008, 2016; Mao et al. 2018). For electrophoresis, 20 μg of total protein was separated on Criterion TGX Stain-Free gels (4–20%, Bio-Rad) at 120 V constant for 90 min (Huffman et al. 2008, 2016; Mao et al. 2018). Stain-free gels were then imaged prior to transfer on a Bio-Rad Chemidoc MP Imaging System (Bio-Rad, Hercules, CA) to confirm equal protein loading. Gels were then wet transferred onto PVDF membranes at 100 V constant for 1 h, and equal transfer was confirmed by Ponceau S stain as described (Huffman et al. 2016; Mao et al. 2018). Membranes were then blocked in 5% milk in TBST for 1 h at room temperature and then incubated overnight at 4 °C with primary antibodies from Cell Signaling against p-AktThr308 (1:1000; no. 13038), total Akt (1:1000; no. 4691), p-p44/42MAPKThr202/Tyr204 (1:1000; no. 9101), total p44/42 MAPK (1:1000; no. 4695), p-S6 (1:1000; no. 5364), total S6 (1:1000; no. 2217), total IGF-1R (1:1000; no. 9750), InsRβ (1:1000; no. 3025), total GluA1 (1:1000; no. 13185), and total GluA2 (1:1000; no. 5306). For OXPHOS content, Total OXPHOS Rodent WB Antibody Cocktail was used (Abcam, no. 110413). Following a 1-h incubation with the appropriate secondary antibody, Clarity Western ECL Substrate (Bio-Rad, Hercules, CA) was applied to the membrane and bands were visualized using a Bio-Rad Chemidoc MP bioimager to first pixel saturation. Densitometry was then performed using Image Lab software (Bio-Rad, Hercules, CA).
RNA isolation and expression
Total RNA from frozen tissues was isolated using the TRIzol® Reagent per the manufacturer’s instructions (Life Technologies). First-strand complementary DNA (cDNA) was synthesized with random primers using Bio-Rad iScript cDNA Synthesis Kit. All qPCR reactions were carried out using Bio-Rad SsoAdvanced SYBR Green mix on a Bio-Rad CFX384 qRT-PCR Machine, and all data were normalized to cyclophilin A (PPIA) (Supp. Table 1) (Thal et al. 2008).
Autophagy analysis
Freshly collected hippocampus and cortex tissues from mice treated with either IN saline or IGF-1 for 4 weeks (n = 6 IN IGF-1-treated, n = 5 IN saline-treated) were minced and incubated in DMEM with or without 20 mM NH4Cl and 100 μM Leupeptin for 4 h at 37 °C. Protein was isolated and subjected to Western blotting for p62 (Enzo, Cat. no. BML-PW9860-0100) and LC3 (Cell Signaling, no. 2775) in order to measure macroautophagy flux, as previously described (Walters et al. 2018).
BrdU labeling
For 5-bromo-2′-deoxyuridine (BrdU) labeling, mice that were assigned to the IN protocol received BrdU intraperitoneal injections (100 mg/kg/day) for three consecutive days prior to sacrifice. These mice (n = 7 IN IGF-1-treated, n = 7 saline-treated) were then transcardially perfused with 0.9% saline followed by ice-cold 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS, pH 7.4). Brains were removed, post-fixed overnight at 4 °C, and then infiltrated with 30% sucrose in 0.1 M PBS at 4 °C. Serial coronal sections of 30 μm thickness were cut on a cryostat (Leica CM1950; Leica, Wetzlar, Germany) and collected in PBS with 1% sodium azide until processing.
Immunohistochemistry
For immunostaining, free-floating sections were incubated in 0.3% Triton X-100 in 0.1 M PBS with 10% normal goat serum and 0.1% bovine serum albumin for 90 min at room temperature and then in rabbit polyclonal anti-GFAP (Abcam, no. 7260), rabbit polyclonal anti-NeuN (Abcam, no. 128886), or rabbit monoclonal MBP (Cell Signaling, no. 78896) at 4 °C overnight. DAB sections were then incubated in biotinylated goat anti-rabbit IgG (BA-1000; 1:300; Vector, Burlingame, CA) for 2 h, rinsed, and incubated in avidin-biotin complex (Vectastain Elite, Vector, Burlingame, CA) for 90 min. For BrdU staining, mouse monoclonal BrdU (Cell Signaling, no. 5292) was used, and M.O.M. immunodetection kit protocol was followed per manufacturer’s instructions (Vector Laboratories, Inc., Cat. no. BMK-2202). Rabbit polyclonal anti-NeuN (Abcam, no. 128886) was simultaneously incubated with BrdU at 4 °C overnight. Subsequently, sections were washed and then incubated in goat anti-mouse Alexa 488 (Thermo Fisher, Cat. no. A-11001) and goat anti-rabbit Alexa 568 (Thermo Fisher, no. A-11036) for 2 h, rinsed, and mounted with a mounting medium with DAPI (Vectashield, Vector Laboratories, Inc., Cat. no. H-1200). Imaging was conducted using the 3DHistec Panoramic 250 Flash II Slide Scanner (Tabrizian et al. 2017) for DAB images and immunofluorescent images were obtained using a Leica SP5 Confocal Microscope with a ×40 magnification. ImageJ software was used to count astrocyte numbers and BrdU-positive cells, and Case Viewer/Pannoramic Viewer (3dHistech, Ltd) was used to measure neuron and myelin density.
Statistics
Data were analyzed using SPSS (SPSS Inc., Chicago, IL) either by one-way ANOVA or two-way ANOVA (group × sex), while longitudinal measures were assessed by repeated measures ANOVA. Tukey post hoc adjustments were performed when appropriate. Data not normally distributed were log transformed to ensure normality of distribution. If normality was not achieved by log transformation, data were analyzed as non-parametric using Kruskal-Wallis and Mann-Whitney tests. All values reported here are means ± standard error (SE). P ≤ 0.05 was considered to be statistically significant.
Results
Characterization of transgene expression in bIGF-1 mice
To confirm spatial and temporal control of the human IGF-1 (hIGF-1) transgene in brain-specific IGF-1 transgenic mice (bIGF-1) (Fig. 1a), we measured expression in different regions of the brain in 12–14-week-old Control (TRE-IGF-1) and bIGF-1 mice (TRE-IGF-1–Camk2a-tTA), either on DOX diet or 2 weeks after DOX diet removal (Supp. Fig. 1a). Transgene expression was similarly low in Control and bIGF-1 mice on DOX diet but increased ~ 6000-fold in the forebrain, 4000-fold in cortex, and ~ 100–200-fold in cerebellum and brainstem of bIGF-1 animals upon DOX removal, confirming temporal control of the transgene was restricted to the appropriate brain region (Supp. Fig. 1a).
We next confirmed protein levels of endogenous (mIGF-1) and transgenic IGF-1 (hIGF-1) in forebrain tissue from male and female mice after 3-month DOX diet removal (6 months of age). In females, Control and bIGF-1 mice had comparable levels of endogenous IGF-1 (~ 600 pg/mg protein), while hIGF-1 levels were increased approximately by 1.5-fold over endogenous levels in bIGF-1 female mice (Fig. 1b). Interestingly, endogenous levels increased ~ 50% in bIGF-1 male mice, with comparable hIGF-1 levels (~ 850 pg/mg protein), resulting in a cumulative threefold increase in brain IGF-1 levels over male Controls (Fig. 1c). Furthermore, increased IGF-1 signaling in forebrain was evident in both sexes, as demonstrated by a reduction in total IGF-1R and total insulin receptor (InsR) protein expression, along with an increase in p-Akt and pS6, without effects on p-Erk (Fig. 1d–n). IGF-1 signaling in hypothalamus showed a similar protein expression pattern, with the exception of InsR, which did not significantly change between groups in either sex (Supp. Fig. 1b–l).
Effect of brain IGF-1 overexpression on energy balance with aging
Body weight and composition were monitored for up to 24 months of age in female and male bIGF-1 and Control HET3 animals. Female bIGF-1 tended to be heavier than Controls (Fig. 2a), which was attributed to an increase in adiposity (Fig. 2b, P < 0.05) rather than lean mass (Fig. 2c). On the other hand, body weight and composition were similar throughout the lifespan in male Control and bIGF-1 animals (Fig. 2d–f). Energy metabolism was assessed in both Control and bIGF-1 HET3 mice at 6–7 months of age. In females, central IGF-1 had no effect on energy balance, activity, or substrate utilization, compared to Controls (Supp. Fig. 2a–e). Likewise, bIGF-1 males had similar 24 h energy expenditure and intake as Controls, but feeding behavior, activity, as well as respiratory exchange ratio (RER) were increased during the light phase of their photoperiod (Supp. Fig. 2f–j).
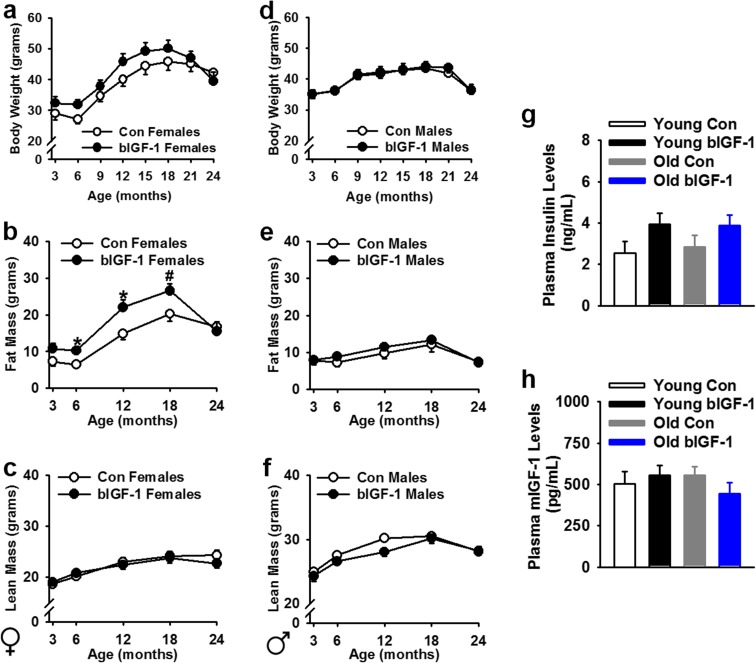
Fig. 2.
Effects of central IGF-1 overexpression on body weight, composition, and glucose homeostasis with age. a–c Female bIGF-1 mice had a numerical increase in body weight that could be attributed to an increase in fat mass, rather than lean mass (n = 12 Control females, n = 10 bIGF-1 females). d–f Body weight and composition were not changed with central IGF-1 overexpression in males (n = 13 Control males, n = 10 bIGF-1 males). g, h Insulin and IGF-1 levels were measured in plasma in male and female mice, but no significant differences were observed by age, sex, or overexpression [Young Control (n = 19 total; n = 9 females, n = 10 males), Young bIGF-1 (n = 22 total; n = 12 females, n = 10 males), Old Con (n = 19 total; n = 9 females, n = 10 males), Old bIGF-1 (n = 18 total; n = 9 females, n = 9 males)]. ns = not significant. Bars represent mean ± SE. Asterisk: significantly different from controls, P ≤ 0.05. #P = 0.074
Glucose metabolism and endocrine effects with chronic bIGF-1 overexpression
Since acute central IGF-1 has previously been shown to modulate glucose metabolism (Huffman et al. 2016), insulin and IGF-1 plasma levels, as well as glucose and insulin tolerance tests, were measured in young and old mice. Chronic brain overexpression of IGF-1 did not significantly change circulating levels of endogenous IGF-1 or insulin levels with age in either group (Fig. 2g, h). Additionally, hIGF-1 was undetectable in plasma of transgenic animals, suggesting no “leak” to the periphery (data not shown). Moreover, liver mIGF-1 gene expression was unchanged with age or bIGF-1 expression (Supp. Fig. 3a), while cortical expression of mIGF-1 was unchanged with age but slightly reduced in old bIGF-1 mice (Supp. Fig. 3a; P < 0.05). Furthermore, IGF-1R gene expression in cortex tended to go down with age (P = 0.075) and was significantly reduced in old bIGF-1 mice, as compared to young Controls (Supp. Fig. 3b; P < 0.001). Similarly, relative expression of InsR in cortex decreased with age (Supp. Fig. 3b; P < 0.05) and further decreased in old bIGF-1 mice (Supp. Fig. 3b; P < 0.05).
Glucose tolerance was slightly impaired in young bIGF-1 females (Supp. Fig. 3c, d; P < 0.05), but this difference disappeared in aged females (Supp. Fig. 3e, f). In males, no significant differences were observed for glucose or insulin tolerance in young or old animals (Supp. Fig. 3g–j). Further, both Old Control and bIGF-1 females demonstrated an age-related increase in plasma levels of IL-1β, IL-6, IFN-γ, and TNF-α, while bIGF-1 males showed increased plasma levels of IL-17A and IFN-γ levels as compared to age-matched controls (Table 1).
Table 1.
Effect of age and brain IGF-1 overexpression on plasma cytokine levels
| Cytokine (ng/mL) | Female | Male | ||||
|---|---|---|---|---|---|---|
| Young Con (n = 12) | Old Con (n = 10) | Old bIGF1 (n = 11) | Young Con (n = 13) | Old Con (n = 12) | Old bIGF1 (n = 10) | |
| IL-1β | 0.85 ± 0.10a | 1.87 ± 0.25b | 1.46 ± 0.10b | 18.10 ± 2.07 | 18.67 ± 2.78 | 16.32 ± 2.85 |
| IL-6 | 2.06 ± 0.20a | 4.98 ± 0.90b | 3.80 ± 0.46b | 26.72 ± 2.93 | 18.41 ± 4.35 | 20.92 ± 6.08 |
| IL-10 | 1.50 ± 0.26 | 1.38 ± 0.17 | 2.01 ± 0.34 | 9.04 ± 1.12 | 16.54 ± 3.33 | 14.17 ± 4.70 |
| IL-17A | 5.64 ± 0.59a | 7.34 ± 1.12ab | 9.48 ± 1.05b | 31.32 ± 6.16a | 31.53 ± 4.67a | 71.22 ± 17.95b |
| IFN-γ | 0.72 ± 0.07a | 1.47 ± 0.19b | 1.38 ± 0.12b | 10.50 ± 0.99ab | 8.96 ± 0.54a | 12.76 ± 1.53b |
| TNF-α | 0.59 ± 0.06a | 1.33 ± 0.19b | 1.19 ± 0.11b | 11.32 ± 1.05 | 10.68 ± 0.98 | 12.99 ± 2.34 |
Different superscript letters denote a significant difference between groups, P ≤ 0.05
Effects of IGF-1 on the brain phenotype are region- and sex-dependent
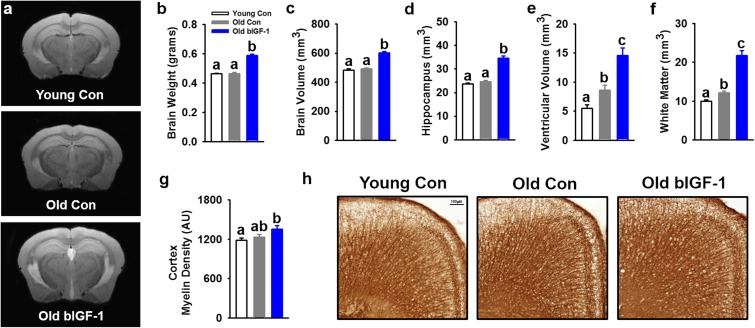
We next performed MRI scans to determine the effects of aging and IGF-1 on brain anatomical structure and related characteristics. Ventricular and white matter volumes were significantly increased with age, while bIGF-1 mice had a further increase in brain, hippocampal, ventricular, and white matter volume, as compared to Young and Old Controls (Fig. 3a–f; P < 0.05). Additionally, IGF-1 increased myelin density in Old bIGF-1 cortex, as compared to Young Controls (Fig. 3g, h), but it was not significant in hippocampus (Supp. Fig. 4a, b). Additionally, NeuN staining in the hippocampus and cortex to assess neuronal density revealed no significant changes with age or bIGF-1 overexpression (Supp. Fig. 4c–f).
Fig. 3.
Phenotyping of young, old, and old bIGF-1 mouse brains by MRI and immunostaining. a–f Representative MRI brain images from young controls, old controls, and old bIGF-1 HET3 mice and corresponding results for weight and volume. Old control mice showed an increase in ventricular and white matter volume compared to young controls, while bIGF-1 mice showed an increase in brain weight and volume, hippocampal volume, ventricular volume, and white matter volume compared to other groups. Both males and females were assessed but are presented together due to similar patterns in outcomes [Young Control (n = 11 total; n = 6 females, n = 5 males), Old Control (n = 12 total; n = 5 females, n = 7 males), Old bIGF-1 (n = 12 total; n = 7 females, n = 5 males)]. g, h Results and representative images from myelin immunostaining in cortex showed no difference with age but an increase in myelin density was observed in old bIGF-1 mice compared to young controls [Young Control (n = 16 total; n = 8 females, n = 8 males), Old Control (n = 17 total; n = 9 females, n = 8 males), Old bIGF-1 (n = 13 total; n = 6 females, n = 7 males)]. Bars represent mean ± SE. Letters indicate a significant difference between groups, P ≤ 0.05
DTI and 1H-MRS analysis were also performed to determine microstructure and metabolite changes in the brain. In females, Old Control and bIGF-1 mice had increased FA in white matter, cortex, hippocampus, and whole brain, when compared to Young Controls (Table 2). However, FA was significantly increased only in white matter and whole brain of Old bIGF-1 males (Table 2). MD did not show any significant differences with age or overexpression in female mice (Table 2). However, MD increased with age in hippocampus and whole brain of male mice, showing a further increase in white matter, cortex, hippocampus, and whole brain of old bIGF-1 male mice, when compared to controls (Table 2). In females, IGF-1 increased axial diffusivity (AD) only in hippocampus, while in males, IGF-1 significantly increased AD in white matter, hippocampus, and whole brain, compared to Old Controls (Table 2). Old Control and bIGF-1 females had a decrease in radial diffusivity (RD) in white matter, as compared to young Controls (Table 2). Meanwhile, brain IGF-1 increased RD in male mice, specifically in hippocampus and whole brain measures (Table 2).
Table 2.
DTI changes with age and IGF-1 overexpression in white matter, cortex, hippocampus, and whole brain
| Fractional anisotropy (FA) | ||||||
| Females | Males | |||||
| Young Con (n = 6) | Old Con (n = 5) | Old bIGF-1 (n = 7) | Young Con (n = 6) | Old Con (n = 7) | Old bIGF-1 (n = 5) | |
| White matter | 0.362 ± 8.0E-03a | 0.410 ± 5.0E-03b | 0.433 ± 1.1E-02b | 0.360 ± 5.0E-03a | 0.390 ± 8.0E-03a | 0.439 ± 1.8E-02b |
| Cortex | 0.188 ± 2.0E-03a | 0.202 ± 5.0E-03b | 0.204 ± 4.0E-03b | 0.193 ± 2.0E-03 | 0.197 ± 4.0E-03 | 0.211 ± 1.3E-02 |
| Hippocampus | 0.237 ± 5.0E-03a | 0.245 ± 1.0E-02ab | 0.264 ± 5.0E-03b | 0.237 ± 5.0E-03 | 0.235 ± 7.0E-03 | 0.256 ± 1.1E-02 |
| Whole brain | 0.237 ± 1.0E-03a | 0.252 ± 6.0E-03b | 0.264 ± 3.0E-03b | 0.242 ± 2.0E-03a | 0.248 ± 4.0E-03ab | 0.268 ± 1.0E-02b |
| Mean diffusivity (MD) (×10−4) | ||||||
| Females | Males | |||||
| Young Con | Old Con | Old bIGF-1 | Young Con | Old Con | Old bIGF-1 | |
| White matter | 5.71 ± 0.04 | 5.47 ± 0.07 | 5.54 ± 0.14 | 5.65 ± 0.09a | 5.70 ± 0.03a | 6.05 ± 0.02b |
| Cortex | 5.81 ± 0.03 | 5.66 ± 0.05 | 5.69 ± 0.12 | 5.74 ± 0.12a | 5.98 ± 0.05ab | 6.10 ± 0.08b |
| Hippocampus | 6.14 ± 0.06 | 6.25 ± 0.10 | 6.50 ± 0.21 | 5.98 ± 0.09a | 6.36 ± 0.05b | 6.93 ± 0.19c |
| Whole brain | 5.97 ± 0.04 | 5.89 ± 0.05 | 5.97 ± 0.12 | 5.86 ± 0.10a | 6.09 ± 0.02b | 6.34 ± 0.05c |
| Axial diffusivity (AD) (×10−4) | ||||||
| Females | Males | |||||
| Young Con | Old Con | Old bIGF-1 | Young Con | Old Con | Old bIGF-1 | |
| White matter | 7.98 ± 0.09 | 7.98 ± 0.10 | 8.30 ± 0.26 | 7.87 ± 0.13a | 8.16 ± 0.06a | 9.10 ± 0.14b |
| Cortex | 6.93 ± 0.04 | 6.83 ± 0.07 | 6.89 ± 0.15 | 6.85 ± 0.14a | 7.17 ± 0.04b | 7.42 ± 0.05b |
| Hippocampus | 7.69 ± 0.07a | 7.89 ± 0.18ab | 8.30 ± 0.24b | 7.49 ± 0.15a | 7.95 ± 0.07b | 8.82 ± 0.22c |
| Whole brain | 7.49 ± 0.05 | 7.47 ± 0.07 | 7.67 ± 0.17 | 7.38 ± 0.13a | 7.70 ± 0.02b | 8.16 ± 0.05c |
| Radial diffusivity (RD) (×10−4) | ||||||
| Females | Males | |||||
| Young Con | Old Con | Old bIGF-1 | Young Con | Old Con | Old bIGF-1 | |
| White matter | 4.57 ± 0.04a | 4.21 ± 0.05b | 4.16 ± 0.10b | 4.54 ± 0.09 | 4.47 ± 0.04 | 4.52 ± 0.08 |
| Cortex | 5.26 ± 0.03 | 5.08 ± 0.05 | 5.09 ± 0.11 | 5.18 ± 0.11 | 5.39 ± 0.05 | 5.45 ± 0.11 |
| Hippocampus | 5.36 ± 0.07 | 5.44 ± 0.07 | 5.60 ± 0.19 | 5.22 ± 0.07a | 5.56 ± 0.06b | 5.99 ± 0.19c |
| Whole brain | 5.21 ± 0.04 | 5.09 ± 0.05 | 5.12 ± 0.10 | 5.10 ± 0.08a | 5.29 ± 0.03b | 5.43 ± 0.07b |
Different superscript letters denote a significant difference between groups, P ≤ 0.05
The 1H-MRS analysis found no significant differences in a substantial number of metabolites in hippocampus with IGF-1, sex, or age, including alanine, total creatine (tCr), GABA, myo-inositol, or lactate (Table 3). However, glutamine and glutathione showed an age-related decrease in males (Table 3), while females showed a decrease in glutamate concentrations with age and IGF-1 (Table 3). Interestingly, total hippocampal choline (including glycerophosphocholine and phosphocholine) levels were significantly increased with age, an effect which was completely attenuated in Old bIGF-1 females (P < 0.05), while levels tended to be maintained in Old bIGF-1 males (Table 3; P = 0.08).
Table 3.
MRS analysis of metabolites in the hippocampus of young controls, old controls, and old bIGF-1 mice
| Metabolite | Females | Males | ||||
|---|---|---|---|---|---|---|
| Young Con (n = 6) | Old Con (n = 5) | Old bIGF-1 (n = 7) | Young Con (n = 6) | Old Con (n = 7) | Old bIGF-1 (n = 5) | |
| Alanine | 1.2 ± 0.1 | 1.2 ± 0.3 | 1.1 ± 0.2 | 1.0 ± 0.1 | 1.2 ± 0.2 | 0.9 ± 0.4 |
| Total creatine | 13.0 ± 0.3 | 13.4 ± 0.3 | 13.0 ± 0.3 | 12.9 ± 0.2 | 13.6 ± 0.4 | 13.9 ± 0.4 |
| GABA | 1.9 ± 0.1 | 1.9 ± 0.1 | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.3 ± 0.1 | 1.5 ± 0.2 |
| Glutamine | 2.4 ± 0.1 | 2.8 ± 0.4 | 2.2 ± 0.2 | 2.6 ± 0.3a | 1.5 ± 0.3b | 1.5 ± 0.2b |
| Glutamate | 10.0 ± 0.2a | 9.2 ± 0.2b | 8.0 ± 0.2c | 9.5 ± 0.3ab | 10.1 ± 0.4a | 8.8 ± 0.3b |
| GPC + Pcholine | 1.8 ± 0.0a | 2.0 ± 0.1b | 1.7 ± 0.1a | 1.7 ± 0.11a | 2.1 ± 0.1b | 1.9 ± 0.1ab |
| Myo-inositol | 5.6 ± 0.3 | 5.7 ± 0.3 | 6.2 ± 0.3 | 5.0 ± 0.1 | 5.3 ± 0.1 | 4.8 ± 0.4 |
| Lactate | 1.8 ± 0.2 | 1.2 ± 0.2 | 2.6 ± 0.6 | 1.6 ± 0.2 | 1.7 ± 0.4 | 1.7 ± 0.2 |
| NAA | 8.8 ± 0.2a | 8.7 ± 0.4a | 7.5 ± 0.3b | 8.6 ± 0.3 | 8.7 ± 0.3 | 8.8 ± 0.4 |
| NAAG | 0.7 ± 0.2 | 0.8 ± 0.2 | 0.6 ± 0.2 | 0.4 ± 0.2a | 0.9 ± 0.2b | 0.9 ± 0.1b |
| Taurine | 12.4 ± 0.3 | 11.8 ± 0.5 | 11.2 ± 0.4 | 13.8 ± 0.3a | 13.3 ± 0.5ab | 12.6 ± 0.2b |
| GSH | 2.0 ± 0.1 | 2.1 ± 0.2 | 1.7 ± 0.2 | 2.2 ± 0.1a | 1.6 ± 0.1b | 1.6 ± 0.1b |
Different superscript letters denote a significant difference between groups, P ≤ 0.05
Behavioral and cognitive changes with age and bIGF-1 overexpression
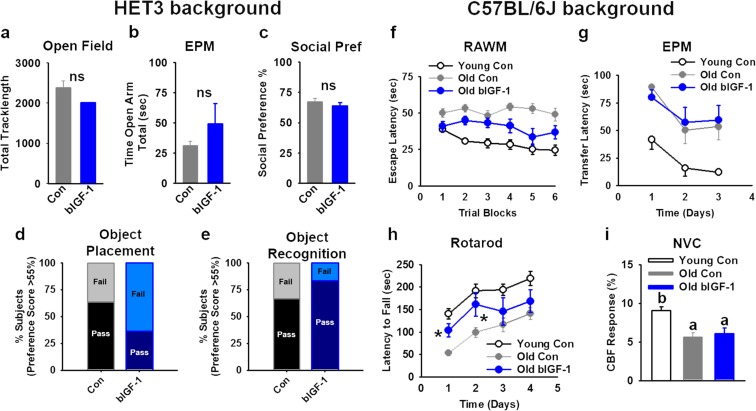
To determine if brain IGF-1 overexpression could modulate age-related changes in cognitive and behavioral characteristics, we performed a battery of assays to evaluate learning, memory, and anxiety. At 15–16 months of age, Control and bIGF-1 HET3 animals did not show differences in activity during OF, anxiety during EPM, social preference, or cognitive function, as assessed by OP and OR (Fig. 4a–e). Moreover, a separate cohort of 20-month-old bIGF-1 male mice on a C57BL/6J background also failed to demonstrate significant improvements in learning performance, as assessed either by the RAWM task or the EPM learning task (Fig. 4f, g). Motor skill learning was next assessed using a modified rotarod test. During a 4-day-long experimental period, Young Controls consistently had a higher latency to fall during a rotarod challenge as compared to Old Controls (Fig. 4h). Old bIGF-1 mice performed better on rotarod than their age-matched controls at days 1 and 2 (P < 0.05), but this difference was no longer apparent by days 3 and 4 (Fig. 4h). Age-related impairment of neurovascular coupling responses has been causally linked to impaired cognitive function by previous studies (Tarantini et al. 2015, 2018; Toth et al. 2017). Furthermore, circulating IGF-1 deficiency elicits significant impairment of neurovascular coupling responses in mice (Toth et al. 2015), which associates with cognitive decline. In line with outcomes from functional assays, Control aged mice demonstrated clear deficits in neuronal activity-induced cerebrovascular blood flow responses with age, extending previous findings (Tarantini et al. 2018; Toth et al. 2014). However, consistent with lack of IGF-1 effect on learning and memory, this reduction was not mitigated in Old bIGF-1 mice (Fig. 4i).
Fig. 4.
Effect of sustained IGF-1 overexpression in brain on cognitive function with age. a–e In HET3 background mice, no significant differences were found in activity during the Open Field (OF), anxiety during the Elevated Plus Maze (EPM) test, social preference, or learning and memory during Object Placement (OP) or Object Recognition (OR) tests at middle age. Both males and females were assessed but are presented together due to similar patterns in outcomes [Controls (n = 12 total; n = 5 females, n = 7 males), bIGF-1 (n = 14 total; n = 7 females, n = 7 males)]. f In older males on C57BL/6J background, there was an age-related decline in learning performance during the Radial Arm Water Maze (RAWM) task that was not improved in Old bIGF-1 male mice (n = 9 Young Control, n = 8 Old Control, n = 10 Old bIGF-1). g Using the EPM-based learning protocol, age-related cognitive impairment was also evident. There was no difference between the performance of Old Control and Old bIGF-1 male mice on this task (n = 10 Young Control, n = 8 Old Control, n = 8 Old bIGF-1). h Motor learning was assessed using the rotarod. Although there was a significant difference on day 1 and day 2 between Old bIGF-1 and Old Controls, this was not sustained over consecutive trials, suggesting no significant effects of bIGF-1 on motor learning (n = 10 Young Control, n = 8 Old Control, n = 10 Old bIGF-1). i Neurovascular coupling responses in the somatosensory whisker barrel cortex evoked by contralateral whisker stimulation and measured by laser speckle contrast imaging showed a significant decline with age, which remained unchanged in Old bIGF-1 male mice, compared to age-matched controls (n = 4 Young Control, n = 9 Old Control, n = 6 Old bIGF-1). Bars represent mean ± SE. Different letters denote a significant difference between groups. Asterisk: significantly different from age-matched controls, P ≤ 0.05
We next determined the effects of bIGF-1 overexpression on mood, by testing for behavioral despair using the Porsolt forced swim test. In 15–16-month-old bIGF-1 males, we detected a significant decrease in immobility, as compared to age-matched controls (Fig. 5a; P < 0.05), suggesting less depressive-like behavior in these mice, but this effect was not observed in females (Fig. 5b). Since hippocampal astrogliosis increases with age, GFAP was used as a marker for astrocyte cell number. Immunostaining in hippocampus detected a significant increase in GFAP for both Old males and females (P < 0.05), suggesting an increase in gliosis. However, hippocampal GFAP levels in Old bIGF-1 males were comparable to levels observed in Young Controls (Fig. 5c, d), while levels were similarly increased with age in Old Control and Old bIGF-1 females (Fig. 5e, f). However, these effects were not observed in cortex (Supp. Fig. 5a, b).
Fig. 5.
Mood changes with chronic IGF-1 overexpression. a, b Male bIGF-1 HET3 mice had significantly reduced percent immobility during the Porsolt forced swim test (n = 7 Control males, n = 7 bIGF-1 males), but no differences were observed in females (n = 5 Control females, n = 7 bIGF-1 females). c, d Representative images from GFAP immunostaining in male hippocampus and results show an increase in GFAP positive cells with age in the hippocampus of male mice, but chronic IGF-1 overexpression maintained more youthful levels of these cells (n = 8 Young Control males, n = 8 Old Control males, n = 7 Old bIGF-1 males). e, f Representative images from GFAP immunostaining in female hippocampus and results show an increase in GFAP positive cells in the hippocampus of female mice with age and were similarly increased in Old bIGF-1 mice (n = 8 Young Control females, n = 9 Old Control females, n = 6 Old bIGF-1 females). Bars represent mean ± SE. Different letters denote a significant difference between groups. Asterisk: significantly different from controls, P ≤ 0.05
Inflammatory cytokine expression in these regions also demonstrated sexually dimorphic patterns. While female mice showed an increase in hippocampal IL-6 expression with age and bIGF-1 (Supp. Fig. 5c), males had an increase in hippocampal IL-6 gene expression with age that tended to be decreased in bIGF-1 mice (Supp. Fig. 5d, P = 0.08). However, in cortex, both males and females showed an age-related IL-6 increase in expression that was not attenuated by IGF-1 (Supp. Fig. 5c, d), while IL-23 significantly increased with age in female hippocampus, but was abrogated by IGF-1 overexpression (Supp. Fig. 5c). Furthermore, Nrf2 target genes were also evaluated in hippocampus and cortex. Notably, in females, Txn1 was increased in both brain regions with age, which was not influenced by IGF-1, while Txn1 was markedly reduced in Old bIGF-1 male hippocampus but not cortex. Meanwhile, little to no effect was observed for Gclm, NQ01, GPX2, or HMOX1 (Supp. Fig. 5e, f).
Preservation of neuromuscular function by central IGF-1 with age is male-specific
Given that neuromuscular function and stamina are known to decrease with age, we evaluated exercise capacity by challenging mice to run on a motorized treadmill until voluntary fatigue. Both male and female Controls showed a marked decline in endurance capacity with age (P < 0.05). However, this age-related decline in exercise tolerance was completely attenuated in Old bIGF-1 male mice to levels comparable to Young Controls (Fig. 6a). In addition, grip strength was evaluated by wire hang and inverted grid, which revealed an age-related reduction in strength (Fig. 6b; P < 0.05). However, this age-related decline in strength in males tended to be attenuated by brain IGF-1, when compared to age-matched Controls (Fig. 6b; P = 0.09), and Old bIGF-1 males did not differ significantly from Young Controls. However, no such effects were observed in Old bIGF-1 female mice (Fig. 6c, d). However, there were no statistical differences in neither fine motor coordination, evaluated by the tape removal test, nor gross motor coordination, as determined by slips on a balance beam, between Controls and bIGF-1 mice, evaluated at 15–16 and at 24 months old (Supp. Fig. 6a–f).
Fig. 6.
Neuromuscular changes with age and chronic brain IGF-1 overexpression. a, b Chronic overexpression of brain IGF-1 profoundly attenuated the age-related decline in exercise performance in male HET3 mice (n = 11 Young Control males, n = 13 Old Control males, n = 13 Old bIGF-1 males) and tended to improve grip strength (P = 0.09, n = 11 Young Control males, n = 12 Old Control males, n = 13 Old bIGF-1 males). c, d In contrast, there was an age-related decline but no such benefit was noted for endurance or strength in Old bIGF-1 females (n = 12 Young Control females, n = 11 Old Control females, n = 11 Old bIGF-1 females). e–g Complex I activity (n = 8 Young Control males, n = 8 Old Control males, n = 8 Old bIGF-1 males) and VDAC protein expression in muscle of male mice showed no difference between groups (n = 7 Young Control males, n = 8 Old Control males, n = 8 Old bIGF-1 males), while citrate synthase levels were increased with age and restored to young control levels in old bIGF-1 mice. h–j In female muscle, citrate synthase showed no significant differences between groups, while Complex I activity was significantly decreased with age (n = 8 Young Control females, n = 9 Old Control females, n = 8 Old bIGF-1 females) and VDAC protein expression was significantly increased in bIGF-1 females, compared to age-matched controls (n = 8 Young Control females, n = 9 Old Control females, n = 6 Old bIGF-1 females). Bars represent mean ± SE. ns = not significant. Different letters denote a significant difference between groups, P ≤ 0.05
A hallmark of exercise training is an increase in mitochondrial function, including increased Complex I and citrate synthase activity in skeletal muscle. Thus, we evaluated several related markers of mitochondrial capacity in gastrocnemius muscle. In spite of their improved exercise tolerance and strength, bIGF-1 male mice did not demonstrate evidence of increased mitochondrial content or activities, as Complex I activity was not different from controls (Fig. 6e). Citrate synthase activity was increased in Old Controls (Fig. 6f; P < 0.05) but not in Old bIGF-1 males, while VDAC levels were unchanged (Fig. 6g). Meanwhile, the age-related decline in Complex I activity was partially attenuated in Old bIGF-1 females (Fig. 6h; P = 0.07), as well as the age-related reduction in Complex IV content and activity (Supp. Fig. 7e, l; P < 0.05) and VDAC content (Fig. 6j; P < 0.05), while no particular improvement was seen in other markers (Fig. 6i and Supp. Fig. 7).
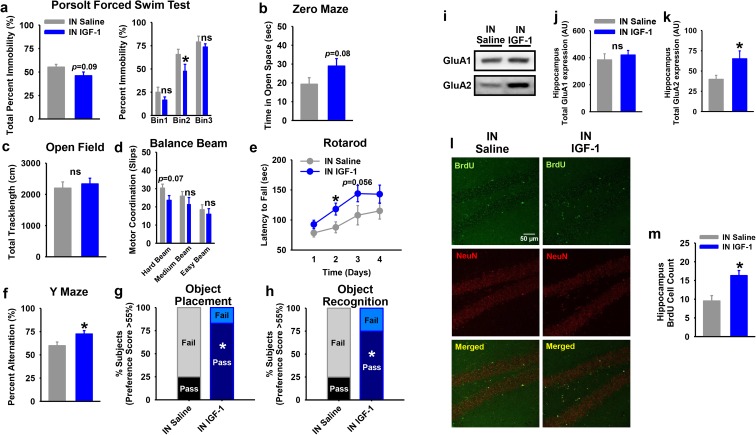
Intranasal IGF-1 restores cognitive and behavioral deficits in old male mice
Given that previous short-term administration studies to the CNS have reported beneficial effects of IGF-1 on cognition, we reasoned that the lack of effect in our transgenic model on learning and memory might have been due to chronic, oversaturated exposure to IGF-1 in the brain. Thus, to determine if a short-term pulsatile-like treatment regimen in aged animals could restore aspects of cognitive function and other healthspan parameters, we treated 24-month-old male mice with IN saline or IGF-1 every other day for at least 4 weeks and assessed several aspects of cognition, behavior, and physical function. Interestingly, IN IGF-1-treated mice showed a tendency to reduce percent immobility (P = 0.09), which was significant in the second bin of the Porsolt forced swim test (Fig. 7a; P < 0.05). Likewise, there was a tendency in IGF-1-treated mice to spend a higher amount of time in the open space of the zero maze (Fig. 7b; P = 0.08), suggesting an improvement in anxiety-like behavior, compared to the saline-treated group.
Fig. 7.
Intranasal IGF-1 benefited cognitive and behavioral aspects in old male mice. a Beginning at 24 months of age, C57BL/6 male mice administered IGF-1 intranasally tended to have a less depressive-like behavior, assessed by the Porsolt forced swim test, compared to saline-treated mice (IN Saline). There were no statistical differences between intranasal IGF-1-treated or saline-treated groups during the first 3 min or the last 3 min of the test. However, IN IGF-1-treated mice showed a significant decrease in immobility during the Porsolt forced swim test from 4 to 7 min, when compared to controls (n = 14 IN Saline, n = 15 IN IGF-1). b Mice treated intranasally with IGF-1 also showed a trend for less anxiety-like behavior, assessed by the zero maze (n = 14 IN Saline, n = 15 IN IGF-1). c IN IGF-1-treated male mice did not demonstrate significant differences in total locomotor activity in the Open Field. d, e IN IGF-1-treated mice showed a tendency for improved motor coordination on the hard beam and had better motor learning on days 2 and 3 of the rotarod protocol (n = 8 IN Saline, n = 9 IN IGF-1), as compared to saline-treated mice. f–h IN IGF-1-treated mice showed improved working memory during the Y-maze test (n = 14 IN Saline, n = 15 IN IGF-1) and improved visuospatial memory during OR and OP (n = 9 IN Saline, n = 9 IN IGF-1). i–k Hippocampus of IGF-1-treated mice showed no differences in total GluA1 but had a significant increase in total GluA2 expression, as compared to saline-treated (n = 7 IN Saline, n = 8 IN IGF-1). l, m IN IGF-1 treatment increased the number of total BrdU cells that colocalized with neurons within the dentate gyrus of the hippocampus, as compared to controls (n = 7 IN Saline, n = 7 IN IGF-1). ns = not significant. Bars represent mean ± SE. Asterisk: significantly different from Controls, P ≤ 0.05
No differences in locomotor activity were recorded during the Open Field total track length (Fig. 7c), and grip strength, as assessed by the wire or inverted grid test, or endurance on a treadmill were not impacted by IN IGF-1 (Supp. Fig. 8a, b). However, IGF-1-treated mice showed a tendency toward improved motor coordination during the hard balance beam (Fig. 7d; P = 0.07). Likewise, reminiscent of better initial rotarod performance in aged C57BL/6J mice, old C57BL/6 male mice treated with IN IGF-1 showed a numerical increase in latency to fall on day 1 of rotarod testing (Fig. 7e; P = 0.17), compared to saline controls. Moreover, when assessing motor learning on the rotarod over subsequent days, IN IGF-1-treated mice showed a significantly increased latency to fall on the accelerating rotarod on day 2 (Fig. 7e; P < 0.05), which was borderline significant on day 3 (P = 0.056), but this difference dissipated by day 4.
We next assessed visuospatial and working memory in mice. Short-term exposure to IN IGF-1 increased the spontaneous alternation during the Y-maze test, compared to saline, suggesting improved spatial working memory (Fig. 7f; P < 0.05). Similarly, IN IGF-1-treated mice showed a significant increase in preference scores of new objects (OR) or new placement of the objects (OP) with a 24-h retention time, compared to IN saline animals, further supporting improvements in memory with this short-term intervention (Fig. 7g, h; P < 0.05).
Furthermore, we investigated the effects of short-term exposure to IN IGF-1 in synaptic plasticity and neurogenesis within the hippocampus. Animals that were administered IN IGF-1 showed no difference in total GluA1 protein expression but had a significant increase in total GluA2 expression, as compared to saline-treated animals (Fig. 7i–k; P < 0.05). Also, short-term IN IGF-1 increased the total count of BrdU-labeled cells (colocalized with neurons) in the dentate gyrus of the hippocampus of old mice, as compared to controls (Fig. 7l, m; P < 0.05).
We further interrogated possible effects of pulsatile IN IGF-1 exposure on intracellular signaling pathways in hippocampus. Animals routinely received IN treatment late in their light cycle, and tissues were harvested from mice the next morning, approximately 15 h later. In contrast to the strong effects observed with chronic IGF-1 overexpression on downstream signaling in brain tissues, no significant differences were observed in IGF-1R, InsR, or downstream mediators with short-term IN IGF-1 administration (Supp. Fig. 8c–k). Finally, it has been shown that increased IGF-1 signaling via stimulation of PI3K-Akt signaling can impair autophagy (Bitto et al. 2010; Moll et al. 2016). Since autophagy serves as a mechanism to maintain healthy neurons and other CNS cell populations (Yang et al. 2014), we investigated macroautophagy flux in hippocampus and cortex of these mice. However, we observed no significant impact of IN IGF-1 on autophagic flux, as demonstrated by similar net flux of p62 and LC3 II in examined tissues between groups (Supp. Fig. 8l–p), suggesting that IN IGF-1 does not impair autophagy in hippocampus or cortex of aged mice.
Discussion
IGF-1 is critical for CNS development and maintenance, but the decline in systemic and presumably central IGF-1 levels with age has been proposed as a possible risk factor for cognitive decline and neurodegenerative diseases (Sonntag et al. 2013). At the tissue level, several studies have demonstrated that IGF-1 replacement in the CNS of aged animals by targeted delivery methods can rescue from features of cognitive impairment and related deficits (Markowska et al. 1998) and improve whole-body insulin action (Huffman et al. 2016). Further, at the cellular level, IGF-1 has been shown to be critical for maintaining homeostasis of neurons, endothelial cells, astrocytes, and glia. Therefore, given the reported salutary effects of IGF-1 in the brain, we reasoned that chronically elevating levels specifically in the CNS throughout adult life would be an effective strategy to mitigate age-related decrements in brain and potentially systemic aging. In spite of clear effects on neuroanatomical size, structure, and signaling, chronic IGF-1 failed to prevent declines in learning and memory, social preference, or glucose homeostasis in aged animals. However, short-term administration of IGF-1 to the CNS of aged mice via the IN route led to beneficial effects in learning and memory, suggesting that optimal dose and frequency of IGF-1 exposure may be imperative to achieve restorative effects.
Although sustained overexpression of central IGF-1 was ineffective at preserving several cognitive domains with aging, male bIGF-1 mice had reduced depressive-like behavior and preservation of motor performance, which was not observed in female mice. Remarkably, exercise capacity in old bIGF-1 male mice was comparable to young animals, but they did not show significant differences in exploration or voluntary locomotor activity compared to controls, suggesting that the decrease in immobility time during the Porsolt forced swim test was not a function of increased activity per se in these mice. Previous rodent studies have consistently noted a beneficial effect of IGF-1 on mood, although those reports were limited to younger animals and did not address the possible role of sex differences in this effect (Duman et al. 2009; Hoshaw et al. 2005; Malberg et al. 2007; Schilling et al. 2011; Sievers et al. 2014). Moreover, selective reductions in circulating and hippocampal IGF-1 have been shown to worsen depressive-like symptoms, while the present study shows that this age-related phenotype can be improved by raising central IGF-1 levels, suggesting the importance of local IGF-1 bioavailability on this phenotype. Furthermore, exercise has been shown to increase brain input of IGF-1, and a previous study has implicated IGF-1, along with estradiol, in the sexually dimorphic regulation of mood by exercise (Munive et al. 2016). In addition, hippocampal astrogliosis, which increases with age (Lindsey et al. 1979), was mitigated in male but not female bIGF-1 mice, which is consistent with a prior report in IGF-1 treated mice challenged with lipopolysaccharide (Park et al. 2011). However, long-term overexposure of IGF-1 centrally did not show a significant reduction in the expression of pro-inflammatory cytokines, unlike the previously mentioned study (Park et al. 2011).
In humans, data examining the possible relationship of IGF-1 with anxiety and depression have been less clear (Arwert et al. 2005). Initial reports suggested that low circulating IGF-1 levels were associated with increased depression, while others have reported that individuals with depression demonstrated higher IGF-1 levels, which were lowered by antidepressant medication (Bot et al. 2016). Interestingly, patients treated with antidepressants have not only reduced IGF-1 levels in circulation but also increased IGF-1 levels in the cerebral spinal fluid (Schilling et al. 2011). Although levels of IGF-1 in CSF were not measured in this study, our results support the notion that increased IGF-1 levels in the CNS are positively involved in reducing depressive-like behavior, at least in males. On the other hand, a population-based study that looked at both sexes has suggested that low IGF-1 levels in women and high IGF-1 levels in men were predictors of depression disorder development (Sievers et al. 2014). Moreover, a cross-sectional study showed a positive association between increased IGFBP-3 and well-being, while IGF-1 positively associated with depression in women, with no significant effects in males (Emeny et al. 2014). However, Chigogora et al. noted the existence of a U-shaped curve in the relationship of IGF-1 and depression in both men and women, with the lowest risk noted in those with moderate IGF-1 levels (Chigogora et al. 2016). Nevertheless, as discussed in detail elsewhere (Gubbi et al. 2018), there are several limitations in attempting to causally link circulating IGF-1 levels with features of cognitive function, and these barriers likely have contributed to the contradictory outcomes related to understanding IGF-1 and brain health in humans.
Age-related cognitive impairment, which is a growing concern among the older population, is associated with changes in IGF-1 across the life course in humans as well as in animal models (Frater et al. 2017). However, central administration of IGF-1 to old male rats was shown to restore spatial and working memory (Markowska et al. 1998) and neurogenesis (Lichtenwalner et al. 2001), while intracerebroventricular IGF-1 gene therapy was able to restore spatial memory in old female rats (Pardo et al. 2016). On the other hand, when systemic levels of IGF-1 are decreased, such as with liver-specific IGF-1-deficient mice, animals show an impairment in hippocampal-dependent cognitive function (Trejo et al. 2007) and functional hyperemia (Toth et al. 2015). While IGF-1 interventions on cognitive function in non-pathological aging have generally proven favorable, the role of IGF-1 signaling in neuropathologies, such as Alzheimer’s disease (AD), is less clear. In fact, several studies have now shown that reduced IGF-1 signaling slows progression of AD pathology and symptoms in mouse models. Indeed, heterozygous deletion of the IGF-1R or IRS-2 protected from AD-like symptoms, neuroinflammation, neuronal loss, and delayed proteotoxicity in APP and APP/PS1 models (Cohen et al. 2009; Freude et al. 2009; Gontier et al. 2015). In contrast, others have speculated that reductions in IGF-1 (and insulin) CSF concentrations with age could be causally linked to mild cognitive impairment (MCI) and AD risk, which is supported by studies showing beneficial effects in response to acute central replacement of these ligands in aged animals and has spurred clinical investigations with intranasal insulin for MCI and AD (Craft et al. 2017). However, similar to data on mood and depression in humans, the relationship of IGF-1 with human cognition is unclear. For instance, low IGF-1 levels have been associated with an increase in MCI in some studies (Doi et al. 2015) and with better cognitive function in others (Perice et al. 2016). Moreover, high IGF-1 levels have been associated with either better (Al-Delaimy et al. 2009) or worse cognitive function (Tumati et al. 2016) in older males, while intervention studies are also equivocal.
Remarkably, while studies on short-term IGF-1 interventions targeting the CNS in rodents have proven consistently favorable for learning and memory outcomes, our results detected no such benefit with long-term overexposure to IGF-1 in either sex or in two different strains. Nevertheless, consistent with previous studies, our short-term IN intervention restored aspects of cognition in old male mice. Moreover, these beneficial effects occurred without adversely affecting macroautophagy in either hippocampus or cortex of old male mice. Furthermore, GluA2-one subunit of amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), which are crucial for synaptic plasticity, was increased in aged IN IGF-1 treated-mice, in concordance with previous studies that have shown in young wild-type mice that high gene expression levels of GluA2 were associated with better long-term memory (Cantanelli et al. 2014). In contrast, reduced levels of GluA2 in the dentate gyrus of the hippocampus have been associated with memory loss in aged monkeys (Hara et al. 2012). Likewise, short-term IN IGF-1 showed beneficial effects on neurogenesis in old mice, similar to previous studies in which IGF-1 was introduced by different means in aged rodents (Lichtenwalner et al. 2001; Perez-Martin et al. 2010; Tang et al. 2017). Furthermore, based upon the ~ 30% increase in brain weight and volume in mice that were chronically overexposed to IGF-1 centrally, along with the marked increase in forebrain Akt and S6 activation, it seems plausible that the inability of IGF-1 to preserve cognitive function in these mice may have simply resulted from long-term overstimulation of this pathway. As the insulin/IGF-1 signaling pathway consists of distinct nodes and branches (Taniguchi et al. 2006), such overstimulation could have desensitized aspects of this signaling pathway among the key cell populations in the CNS thereby explaining the apparent discrepant effects between this model and more short-term strategies, such as our IN approach. Indeed, studies have shown that IN insulin treatment improved memory recall and plasticity and alleviated anxiety-like behavior in diverse young and old rodent models (Maimaiti et al. 2016; Mao et al. 2016), and early human trials exploring the potential of IN insulin to treat cognitive decline have demonstrated benefits in individuals with MCI or early-stage AD (Claxton et al. 2015).
Age-related declines in peripheral IGF-1, as well as IGF-1 deficiency, have been linked with human frailty, including gait abnormalities, decreases in walking endurance, and muscle strength (Tarantini et al. 2017b). Correspondingly, in the present study, physical performance as assessed by exercise capacity on a treadmill decreased with age in both males and females. However, our results show that old male mice with chronic brain IGF-1 overexpression had strikingly preserved exercise tolerance to levels observed in young controls and tended to have better forelimb grip strength than age-matched counterparts, while old bIGF-1 females showed no such effect. Moreover, bIGF-1 males on the C57BL/6 background had better initial performance on rotarod but consistent with other cognitive outcomes failed to demonstrate improved motor learning of this task following consecutive trials, a phenotype that was improved by IN IGF-1 treatment.
The mechanism(s) underlying the apparent benefit to physical performance in male bIGF-1 mice is not clear. It has been previously demonstrated that IGF-1 targeted to skeletal muscle or spinal cord motor neurons preserves neuromuscular junctions, muscle mass, and excitation-contraction coupling (Payne et al. 2007). However, human IGF-1 was undetectable in circulation of bIGF-1 mice, suggesting that expression was confined to the CNS and did not leak to the periphery. Furthermore, while increased citrate synthase and Complex I activity in skeletal muscle is a hallmark of endurance exercise training, these measures did not seem to provide any clues as to the enhanced exercise capacity in bIGF-1 male mice. Furthermore, as physiologic determinants of exercise capacity, including oxygen consumption (VO2 max) and cardiac output (Betik and Hepple 2008) were not directly measured, we cannot rule out other contributors to this effect, including enhanced motivation of aged male bIGF-1 to perform these tasks. In contrast, a prior study observed deleterious effects of sustained IGF-1 overexpression (IGF-2/1 Tg mice) in the CNS on muscle strength (Moreno et al. 2006). This report noted a reduction in circulating IGF-1 levels in transgenic mice, presumably by increased feedback inhibition on GH secretion by IGF-1 overexpression, while no such effect was observed in this study. However, other studies using an astrocyte-specific IGF-1 overexpression mouse model (Madathil et al. 2013) or ICV infusions of IGF-1 (Carlson and Saatman 2018) in the context of traumatic brain injury showed benefits in motor function.
In summary, while the effects of central IGF-1 on brain phenotypes were largely indistinguishable between males and females, we show that sustained, chronic overexpression of IGF-1 in the CNS preserves motor function and reduces depressive-like behavior preferentially in males, while only fat mass was specifically altered in female bIGF-1 mice, thereby further highlighting the importance of sex differences in this axis. Moreover, we also show that unlike chronic, sustained overexpression of IGF-1, a short-term IN IGF-1 intervention in old male mice had beneficial effects on motor learning and mood as well as working and visuospatial memory. Future efforts should determine if targeting the CNS with IGF-1 using clinically relevant strategies, such as IN delivery, might prove efficacious as a therapeutic approach in pathological contexts, such as MCI or AD, particularly given the potential for IGF-1 to activate both IGF-1R and InsR/IGF-1R hybrids, which are both abundant in brain. A better understanding of these and other facets involving IGF-1 signaling in the CNS could ultimately enable development of appropriate therapeutic strategies to target normal and its pathologic changes in the aging brain.
Electronic supplementary material
(PDF 2885 kb)
Acknowledgements
This work was supported by R00 AG037574, the American Federation for Aging Research (AFAR), and Einstein Startup Funds to D.M.H. This work was also supported by the Einstein Nathan Shock Center (P30 AG038072) and the Einstein-Sinai Diabetes Research Center (P30 DK020541). We would also like to acknowledge support from the NCI supported Einstein Cancer Center (P30 CA013330). This work was also supported by R01 AG055395 and R01 NS100782 to Z.U. and R01 AG 038747 and R01 NS 056218 to W.E.S. Mitochondrial assays were performed with assistance from the UAB Diabetes Research Center BARB Core (P30 DK079626). Einstein Analytical Imaging Core were supported by NIH SIG awards (no. 1S10OD019961-01; 1S10OD023591-01). We would also like to acknowledge Vera DesMarais and Hillary Guzik for expert advice/assistance in microscopy and software analysis. Finally, we would like to thank Drs. Pinchas Cohen and Junxiang Wan in the USC Aging Biomarker Core for technical advice and assistance with IGF-1 assays.
Author contribution
GEFQ, MHC, CAB, ZU, MG, WES, and DMH designed the experiments. GEFQ, KM, ZH, AN, MHC, KP, DRM, ST, TK, and AY performed the experiments. GEFQ, ZH, MHC, CAB, KP, DRM, ZU, and DMH analyzed and interpreted the data. GEFQ, ZU, and DMH wrote the manuscript and SG, WES, ST, TK, AY, and MHC revised the paper.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Al-Delaimy WK, von Muhlen D, Barrett-Connor E. Insulinlike growth factor-1, insulinlike growth factor binding protein-1, and cognitive function in older men and women. J Am Geriatr Soc. 2009;57:1441–1446. doi: 10.1111/j.1532-5415.2009.02343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arwert LI, Deijen JB, Drent ML. The relation between insulin-like growth factor I levels and cognition in healthy elderly: a meta-analysis. Growth Hormon IGF Res. 2005;15:416–422. doi: 10.1016/j.ghir.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Ashpole NM, Logan S, Yabluchanskiy A, Mitschelen MC, Yan H, Farley JA, Hodges EL, Ungvari Z, Csiszar A, Chen S, Georgescu C, Hubbard GB, Ikeno Y, Sonntag WE. IGF-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. Geroscience. 2017;39:129–145. doi: 10.1007/s11357-017-9971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J, Trinh TL, Chuang KH, Qiu A. Atlas-based automatic mouse brain image segmentation revisited: model complexity vs. image registration. Magn Reson Imaging. 2012;30:789–798. doi: 10.1016/j.mri.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Bartke A, Chandrashekar V, Dominici F, Turyn D, Kinney B, Steger R, Kopchick JJ. Insulin-like growth factor 1 (IGF-1) and aging: controversies and new insights. Biogerontology. 2003;4:1–8. doi: 10.1023/A:1022448532248. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61:1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betik AC, Hepple RT. Determinants of VO2 max decline with aging: an integrated perspective. Appl Physiol Nutr Metab. 2008;33:130–140. doi: 10.1139/H07-174. [DOI] [PubMed] [Google Scholar]
- Bitto A, Lerner C, Torres C, Roell M, Malaguti M, Perez V, Lorenzini A, Hrelia S, Ikeno Y, Matzko ME, McCarter R, Sell C. Long-term IGF-I exposure decreases autophagy and cell viability. PLoS One. 2010;5:e12592. doi: 10.1371/journal.pone.0012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokov AF, Garg N, Ikeno Y, Thakur S, Musi N, DeFronzo RA, Zhang N, Erickson RC, Gelfond J, Hubbard GB, Adamo ML, Richardson A. Does reduced IGF-1R signaling in Igf1r+/− mice alter aging? PLoS One. 2011;6:e26891. doi: 10.1371/journal.pone.0026891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bot M, Milaneschi Y, Penninx BW, Drent ML. Plasma insulin-like growth factor I levels are higher in depressive and anxiety disorders, but lower in antidepressant medication users. Psychoneuroendocrinology. 2016;68:148–155. doi: 10.1016/j.psyneuen.2016.02.028. [DOI] [PubMed] [Google Scholar]
- Boveris A, Oshino N, Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972;128:617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Fan LW, Lin S, Pang Y, Rhodes PG. Intranasal administration of insulin-like growth factor-1 protects against lipopolysaccharide-induced injury in the developing rat brain. Neuroscience. 2011;194:195–207. doi: 10.1016/j.neuroscience.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantanelli P, Sperduti S, Ciavardelli D, Stuppia L, Gatta V, Sensi SL. Age-dependent modifications of AMPA receptor subunit expression levels and related cognitive effects in 3xTg-AD mice. Front Aging Neurosci. 2014;6:200. doi: 10.3389/fnagi.2014.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SW, Saatman KE. Central infusion of insulin-like growth factor-1 increases hippocampal neurogenesis and improves neurobehavioral function after traumatic brain injury. J Neurotrauma. 2018;35:1467–1480. doi: 10.1089/neu.2017.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro E, Spuch C, Trejo JL, Antequera D, Torres-Aleman I. Choroid plexus megalin is involved in neuroprotection by serum insulin-like growth factor I. J Neurosci. 2005;25:10884–10893. doi: 10.1523/JNEUROSCI.2909-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chigogora S, Zaninotto P, Kivimaki M, Steptoe A, Batty GD. Insulin-like growth factor 1 and risk of depression in older people: the English Longitudinal Study of Ageing. Transl Psychiatry. 2016;6:e898. doi: 10.1038/tp.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- Claxton A, Baker LD, Hanson A, Trittschuh EH, Cholerton B, Morgan A, Callaghan M, Arbuckle M, Behl C, Craft S. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer's disease dementia. J Alzheimers Dis. 2015;44:897–906. doi: 10.3233/JAD-141791. [DOI] [PubMed] [Google Scholar]
- Cohen E, Paulsson JF, Blinder P, Burstyn-Cohen T, du D, Estepa G, Adame A, Pham HM, Holzenberger M, Kelly JW, Masliah E, Dillin A. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cell. 2009;139:1157–1169. doi: 10.1016/j.cell.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S, Claxton A, Baker LD, Hanson AJ, Cholerton B, Trittschuh EH, Dahl D, Caulder E, Neth B, Montine TJ, Jung Y, Maldjian J, Whitlow C, Friedman S. Effects of regular and long-acting insulin on cognition and Alzheimer's disease biomarkers: a pilot clinical trial. J Alzheimers Dis. 2017;57:1325–1334. doi: 10.3233/JAD-161256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui MH, Suzuka SM, Branch NA, Ambadipudi K, Thangaswamy S, Acharya SA, Billett HH, Branch CA (2017) Brain neurochemical and hemodynamic findings in the NY1DD mouse model of mild sickle cell disease. NMR Biomed 30. 10.1002/nbm.3692 [DOI] [PubMed]
- Doi T, Shimada H, Makizako H, Tsutsumimoto K, Hotta R, Nakakubo S, Suzuki T. Association of insulin-like growth factor-1 with mild cognitive impairment and slow gait speed. Neurobiol Aging. 2015;36:942–947. doi: 10.1016/j.neurobiolaging.2014.10.035. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Terwilliger R, Russell DS, Newton SS, Duman RS. Peripheral insulin-like growth factor-I produces antidepressant-like behavior and contributes to the effect of exercise. Behav Brain Res. 2009;198:366–371. doi: 10.1016/j.bbr.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeny RT, Bidlingmaier M, Lacruz ME, Linkohr B, Peters A, Reincke M, Ladwig KH. Mind over hormones: sex differences in associations of well-being with IGF-I, IGFBP-3 and physical activity in the KORA-Age study. Exp Gerontol. 2014;59:58–64. doi: 10.1016/j.exger.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Fernandez AM, Torres-Aleman I. The many faces of insulin-like peptide signalling in the brain. Nat Rev Neurosci. 2012;13:225–239. doi: 10.1038/nrn3209. [DOI] [PubMed] [Google Scholar]
- Fetterman JL, Zelickson BR, Johnson LW, Moellering DR, Westbrook DG, Pompilius M, Sammy MJ, Johnson M, Dunham-Snary KJ, Cao X, Bradley WE, Zhang J, Wei CC, Chacko B, Schurr TG, Kesterson RA, Dell’italia LJ, Darley-Usmar VM, Welch DR, Ballinger SW. Mitochondrial genetic background modulates bioenergetics and susceptibility to acute cardiac volume overload. Biochem J. 2013;455:157–167. doi: 10.1042/BJ20130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frater J, Lie D, Bartlett P, McGrath JJ. Insulin-like growth factor 1 (IGF-1) as a marker of cognitive decline in normal ageing: a review. Ageing Res Rev. 2017;42:14–27. doi: 10.1016/j.arr.2017.12.002. [DOI] [PubMed] [Google Scholar]
- Freude S, Hettich MM, Schumann C, Stöhr O, Koch L, Köhler C, Udelhoven M, Leeser U, Müller M, Kubota N, Kadowaki T, Krone W, Schröder H, Brüning JC, Schubert M. Neuronal IGF-1 resistance reduces Abeta accumulation and protects against premature death in a model of Alzheimer's disease. FASEB J. 2009;23:3315–3324. doi: 10.1096/fj.09-132043. [DOI] [PubMed] [Google Scholar]
- Garwood M, DelaBarre L. The return of the frequency sweep: designing adiabatic pulses for contemporary NMR. J Magn Reson. 2001;153:155–177. doi: 10.1006/jmre.2001.2340. [DOI] [PubMed] [Google Scholar]
- Gontier G, George C, Chaker Z, Holzenberger M, Aid S. Blocking IGF signaling in adult neurons alleviates Alzheimer's disease pathology through amyloid-beta clearance. J Neurosci Off J Soc Neurosci. 2015;35:11500–11513. doi: 10.1523/JNEUROSCI.0343-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbi S, Quipildor GF, Barzilai N, Huffman DM, Milman S. 40 years of IGF1: IGF1: the Jekyll and Hyde of the aging brain. J Mol Endocrinol. 2018;61:T171–T185. doi: 10.1530/JME-18-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson LR, Fine JM, Svitak AL, Faltesek KA (2013) Intranasal administration of CNS therapeutics to awake mice. J Vis Exp. 10.3791/4440 [DOI] [PMC free article] [PubMed]
- Hara Y, Punsoni M, Yuk F, Park CS, Janssen WG, Rapp PR, Morrison JH. Synaptic distributions of GluA2 and PKMzeta in the monkey dentate gyrus and their relationships with aging and memory. J Neurosci. 2012;32:7336–7344. doi: 10.1523/JNEUROSCI.0605-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle PC, Butow RA, Racker E, Chance B. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XV. Reverse electron transfer in the flavin-cytochrome beta region of the respiratory chain of beef heart submitochondrial particles. J Biol Chem. 1967;242:5169–5173. [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Géloën A, Even PC, Cervera P, le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Hoppeler H. Exercise-induced ultrastructural changes in skeletal muscle. Int J Sports Med. 1986;7:187–204. doi: 10.1055/s-2008-1025758. [DOI] [PubMed] [Google Scholar]
- Hoshaw BA, Malberg JE, Lucki I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Res. 2005;1037:204–208. doi: 10.1016/j.brainres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Huffman DM, Moellering DR, Grizzle WE, Stockard CR, Johnson MS, Nagy TR. Effect of exercise and calorie restriction on biomarkers of aging in mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1618–R1627. doi: 10.1152/ajpregu.00890.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman DM, Farias Quipildor G, Mao K, Zhang X, Wan J, Apontes P, Cohen P, Barzilai N. Central insulin-like growth factor-1 (IGF-1) restores whole-body insulin action in a model of age-related insulin resistance and IGF-1 decline. Aging Cell. 2016;15:181–186. doi: 10.1111/acel.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Lythgoe D, Horsfield MA, Simmons A, Williams SC, Markus HS. Characterization of white matter damage in ischemic leukoaraiosis with diffusion tensor MRI. Stroke. 1999;30:393–397. doi: 10.1161/01.STR.30.2.393. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Krzywanski DM, Moellering DR, Westbrook DG, Dunham-Snary KJ, Brown J, Bray AW, Feeley KP, Sammy MJ, Smith MR, Schurr TG, Vita JA, Ambalavanan N, Calhoun D, Dell’Italia L, Ballinger SW. Endothelial cell bioenergetics and mitochondrial DNA damage differ in humans having African or west Eurasian maternal ancestry. Circ Cardiovasc Genet. 2016;9:26–36. doi: 10.1161/CIRCGENETICS.115.001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603–613. doi: 10.1016/S0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- Lindsey JD, Landfield PW, Lynch G. Early onset and topographical distribution of hypertrophied astrocytes in hippocampus of aging rats: a quantitative study. J Gerontol. 1979;34:661–671. doi: 10.1093/geronj/34.5.661. [DOI] [PubMed] [Google Scholar]
- Lopes C, Ribeiro M, Duarte AI, Humbert S, Saudou F, Pereira de Almeida L, Hayden M, Rego AC. IGF-1 intranasal administration rescues Huntington's disease phenotypes in YAC128 mice. Mol Neurobiol. 2014;49:1126–1142. doi: 10.1007/s12035-013-8585-5. [DOI] [PubMed] [Google Scholar]
- Madathil SK, Carlson SW, Brelsfoard JM, Ye P, D'Ercole AJ, Saatman KE. Astrocyte-specific overexpression of insulin-like growth factor-1 protects hippocampal neurons and reduces behavioral deficits following traumatic brain injury in mice. PLoS One. 2013;8:e67204. doi: 10.1371/journal.pone.0067204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimaiti S, Anderson KL, DeMoll C, Brewer LD, Rauh BA, Gant JC, Blalock EM, Porter NM, Thibault O. Intranasal insulin improves age-related cognitive deficits and reverses electrophysiological correlates of brain aging. J Gerontol A Biol Sci Med Sci. 2016;71:30–39. doi: 10.1093/gerona/glu314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Platt B, Rizzo SJ, Ring RH, Lucki I, Schechter LE, Rosenzweig-Lipson S. Increasing the levels of insulin-like growth factor-I by an IGF binding protein inhibitor produces anxiolytic and antidepressant-like effects. Neuropsychopharmacology. 2007;32:2360–2368. doi: 10.1038/sj.npp.1301358. [DOI] [PubMed] [Google Scholar]
- Mao YF, Guo Z, Zheng T, Jiang Y, Yan Y, Yin X, Chen Y, Zhang B. Intranasal insulin alleviates cognitive deficits and amyloid pathology in young adult APPswe/PS1dE9 mice. Aging Cell. 2016;15:893–902. doi: 10.1111/acel.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao K, Quipildor GF, Tabrizian T, Novaj A, Guan F, Walters RO, Delahaye F, Hubbard GB, Ikeno Y, Ejima K, Li P, Allison DB, Salimi-Moosavi H, Beltran PJ, Cohen P, Barzilai N, Huffman DM. Late-life targeting of the IGF-1 receptor improves healthspan and lifespan in female mice. Nat Commun. 2018;9:2394. doi: 10.1038/s41467-018-04805-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL, Mooney M, Sonntag WE. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience. 1998;87:559–569. doi: 10.1016/S0306-4522(98)00143-2. [DOI] [PubMed] [Google Scholar]
- Milman S, Atzmon G, Huffman DM, Wan J, Crandall JP, Cohen P, Barzilai N. Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity. Aging Cell. 2014;13:769–771. doi: 10.1111/acel.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milman S, Huffman DM, Barzilai N. The somatotropic axis in human aging: framework for the current state of knowledge and future research. Cell Metab. 2016;23:980–989. doi: 10.1016/j.cmet.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll L, Ben-Gedalya T, Reuveni H, Cohen E. The inhibition of IGF-1 signaling promotes proteostasis by enhancing protein aggregation and deposition. FASEB J. 2016;30:1656–1669. doi: 10.1096/fj.15-281675. [DOI] [PubMed] [Google Scholar]
- Moreno RJ, Messi ML, Zheng Z, Wang ZM, Ye P, D'Ercole JA, Delbono O. Role of sustained overexpression of central nervous system IGF-I in the age-dependent decline of mouse excitation-contraction coupling. J Membr Biol. 2006;212:147–161. doi: 10.1007/s00232-006-0044-z. [DOI] [PubMed] [Google Scholar]
- Muller AP, Fernandez AM, Haas C, Zimmer E, Portela LV, Torres-Aleman I. Reduced brain insulin-like growth factor I function during aging. Mol Cell Neurosci. 2012;49:9–12. doi: 10.1016/j.mcn.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Munive V, Santi A, Torres-Aleman I. A concerted action of estradiol and insulin like growth factor I underlies sex differences in mood regulation by exercise. Sci Rep. 2016;6:25969. doi: 10.1038/srep25969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima T, Piriz J, Duflot S, Fernandez AM, Gaitan G, Gomez-Pinedo U, Verdugo JMG, Leroy F, Soya H, Nuñez A, Torres-Aleman I. Neuronal activity drives localized blood-brain-barrier transport of serum insulin-like growth factor-I into the CNS. Neuron. 2010;67:834–846. doi: 10.1016/j.neuron.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Ogg RJ, Kingsley PB, Taylor JS. WET, a T1- and B1-insensitive water-suppression method for in vivo localized 1H NMR spectroscopy. J Magn Reson B. 1994;104:1–10. doi: 10.1006/jmrb.1994.1048. [DOI] [PubMed] [Google Scholar]
- Pardo J, Uriarte M, Console GM, Reggiani PC, Outeiro TF, Morel GR, Goya RG. Insulin-like growth factor-I gene therapy increases hippocampal neurogenesis, astrocyte branching and improves spatial memory in female aging rats. Eur J Neurosci. 2016;44:2120–2128. doi: 10.1111/ejn.13278. [DOI] [PubMed] [Google Scholar]
- Pardo J, Abba MC, Lacunza E, Ogundele OM, Paiva I, Morel GR, Outeiro TF, Goya RG. IGF-I gene therapy in aging rats modulates hippocampal genes relevant to memory function. J Gerontol A Biol Sci Med Sci. 2018;73:459–467. doi: 10.1093/gerona/glx125. [DOI] [PubMed] [Google Scholar]
- Park SE, Dantzer R, Kelley KW, McCusker RH. Central administration of insulin-like growth factor-I decreases depressive-like behavior and brain cytokine expression in mice. J Neuroinflammation. 2011;8:12. doi: 10.1186/1742-2094-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne AM, Messi ML, Zheng Z, Delbono O. Motor neuron targeting of IGF-1 attenuates age-related external Ca2+-dependent skeletal muscle contraction in senescent mice. Exp Gerontol. 2007;42:309–319. doi: 10.1016/j.exger.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Martin M, Cifuentes M, Grondona JM, Lopez-Avalos MD, Gomez-Pinedo U, Garcia-Verdugo JM, Fernandez-Llebrez P. IGF-I stimulates neurogenesis in the hypothalamus of adult rats. Eur J Neurosci. 2010;31:1533–1548. doi: 10.1111/j.1460-9568.2010.07220.x. [DOI] [PubMed] [Google Scholar]
- Perice L, Barzilai N, Verghese J, Weiss EF, Holtzer R, Cohen P, Milman S. Lower circulating insulin-like growth factor-I is associated with better cognition in females with exceptional longevity without compromise to muscle mass and function. Aging (Albany NY) 2016;8:2414–2424. doi: 10.18632/aging.101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14:260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- Puig KL, Kulas JA, Franklin W, Rakoczy SG, Taglialatela G, Brown-Borg HM, Combs CK. The Ames dwarf mutation attenuates Alzheimer's disease phenotype of APP/PS1 mice. Neurobiol Aging. 2016;40:22–40. doi: 10.1016/j.neurobiolaging.2015.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raha S, McEachern GE, Myint AT, Robinson BH. Superoxides from mitochondrial complex III: the role of manganese superoxide dismutase. Free Radic Biol Med. 2000;29:170–180. doi: 10.1016/S0891-5849(00)00338-5. [DOI] [PubMed] [Google Scholar]
- Rasmussen HN, Andersen AJ, Rasmussen UF. Optimization of preparation of mitochondria from 25-100 mg skeletal muscle. Anal Biochem. 1997;252:153–159. doi: 10.1006/abio.1997.2304. [DOI] [PubMed] [Google Scholar]
- Saber H, Himali JJ, Beiser AS, Shoamanesh A, Pikula A, Roubenoff R, Romero JR, Kase CS, Vasan RS, Seshadri S. Serum insulin-like growth factor 1 and the risk of ischemic stroke: the Framingham study. Stroke. 2017;48:1760–1765. doi: 10.1161/STROKEAHA.116.016563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling C, Blum WF, Heuser I, Paslakis G, Wudy SA, Deuschle M. Treatment with antidepressants increases insulin-like growth factor-I in cerebrospinal fluid. J Clin Psychopharmacol. 2011;31:390–392. doi: 10.1097/JCP.0b013e3182189d86. [DOI] [PubMed] [Google Scholar]
- Sievers C, Auer MK, Klotsche J, Athanasoulia AP, Schneider HJ, Nauck M, Völzke H, John U, Schulz A, Freyberger HJ, Friedrich N, Biffar R, Stalla GK, Wallaschofski H, Grabe HJ. IGF-I levels and depressive disorders: results from the Study of Health in Pomerania (SHIP) Eur Neuropsychopharmacol. 2014;24:890–896. doi: 10.1016/j.euroneuro.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Deak F, Ashpole N, Toth P, Csiszar A, Freeman W, Ungvari Z. Insulin-like growth factor-1 in CNS and cerebrovascular aging. Front Aging Neurosci. 2013;5:27. doi: 10.3389/fnagi.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci U S A. 2008;105:3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LY, Al-Regaiey K, Masternak MM, Wang J, Bartke A. Local expression of GH and IGF-1 in the hippocampus of GH-deficient long-lived mice. Neurobiol Aging. 2005;26:929–937. doi: 10.1016/j.neurobiolaging.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Tabrizian T, Wang D, Guan F, Hu Z, Beck AP, Delahaye F, Huffman DM. Apc inactivation, but not obesity, synergizes with Pten deficiency to drive intestinal stem cell-derived tumorigenesis. Endocr Relat Cancer. 2017;24:253–265. doi: 10.1530/ERC-16-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JJ, Podratz JL, Lange M, Scrable HJ, Jang MH, Windebank AJ. Mechano growth factor, a splice variant of IGF-1, promotes neurogenesis in the aging mouse brain. Mol Brain. 2017;10:23. doi: 10.1186/s13041-017-0304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- Tarantini S, Hertelendy P, Tucsek Z, Valcarcel-Ares MN, Smith N, Menyhart A, Farkas E, Hodges EL, Towner R, Deak F, Sonntag WE, Csiszar A, Ungvari Z, Toth P. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J Cereb Blood Flow Metab. 2015;35:1871–1881. doi: 10.1038/jcbfm.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Fulop GA, Kiss T, Farkas E, Zölei-Szénási D, Galvan V, Toth P, Csiszar A, Ungvari Z, Yabluchanskiy A. Demonstration of impaired neurovascular coupling responses in TG2576 mouse model of Alzheimer's disease using functional laser speckle contrast imaging. Geroscience. 2017;39:465–473. doi: 10.1007/s11357-017-9980-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Springo Z, Fulop GA, Ashpole N, Gautam T, Giles CB, Wren JD, Sonntag WE, Csiszar A, Ungvari Z. Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell. 2017;16:469–479. doi: 10.1111/acel.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Fulop GA, Hertelendy P, Gautam T, Farkas E, Perz A, Rabinovitch PS, Sonntag WE, Csiszar A, Ungvari Z. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell. 2018;17:e12731. doi: 10.1111/acel.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal SC, Wyschkon S, Pieter D, Engelhard K, Werner C. Selection of endogenous control genes for normalization of gene expression analysis after experimental brain trauma in mice. J Neurotrauma. 2008;25:785–794. doi: 10.1089/neu.2007.0497. [DOI] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and down-regulation of NADPH oxidase. Am J Physiol Heart Circ Physiol. 2014;306:H299–H308. doi: 10.1152/ajpheart.00744.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell. 2015;14:1034–1044. doi: 10.1111/acel.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol. 2017;312:H1–H20. doi: 10.1152/ajpheart.00581.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Piriz J, Llorens-Martin MV, Fernandez AM, Bolós M, LeRoith D, Nuñez A, Torres-Aleman I. Central actions of liver-derived insulin-like growth factor I underlying its pro-cognitive effects. Mol Psychiatry. 2007;12:1118–1128. doi: 10.1038/sj.mp.4002076. [DOI] [PubMed] [Google Scholar]
- Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z, Csiszar A. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci. 2014;69:1339–1352. doi: 10.1093/gerona/glu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumati S, Burger H, Martens S, van der Schouw YT, Aleman A. Association between cognition and serum insulin-like growth factor-1 in middle-aged & older men: an 8 year follow-up study. PLoS One. 2016;11:e0154450. doi: 10.1371/journal.pone.0154450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Hertelendy P, Valcarcel-Ares MN, Fülöp GA, Logan S, Kiss T, Farkas E, Csiszar A, Yabluchanskiy A. Cerebromicrovascular dysfunction predicts cognitive decline and gait abnormalities in a mouse model of whole brain irradiation-induced accelerated brain senescence. GeroScience. 2017;39:33–42. doi: 10.1007/s11357-017-9964-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal JS, Hanon O, Funalot B, Brunel N, Viollet C, Rigaud AS, Seux ML, le-Bouc Y, Epelbaum J, Duron E. Low serum insulin-like growth factor-I predicts cognitive decline in Alzheimer's disease. J Alzheimers Dis. 2016;52:641–649. doi: 10.3233/JAD-151162. [DOI] [PubMed] [Google Scholar]
- Vig PJ, Subramony SH, D'Souza DR, Wei J, Lopez ME. Intranasal administration of IGF-I improves behavior and Purkinje cell pathology in SCA1 mice. Brain Res Bull. 2006;69:573–579. doi: 10.1016/j.brainresbull.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Walters RO, Arias E, Diaz A, Burgos ES, Guan F, Tiano S, Mao K, Green CL, Qiu Y, Shah H, Wang D, Hudgins AD, Tabrizian T, Tosti V, Shechter D, Fontana L, Kurland IJ, Barzilai N, Cuervo AM, Promislow DEL, Huffman DM. Sarcosine is uniquely modulated by aging and dietary restriction in rodents and humans. Cell Rep. 2018;25:663–676 e666. doi: 10.1016/j.celrep.2018.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Gontier G, Chaker Z, Lacube P, Dupont J, Holzenberger M. Longevity effect of IGF-1R(+/−) mutation depends on genetic background-specific receptor activation. Aging Cell. 2014;13:19–28. doi: 10.1111/acel.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Chu X, Yin M, Liu X, Yuan H, Niu Y, Fu L. mTOR and autophagy in normal brain aging and caloric restriction ameliorating age-related cognition deficits. Behav Brain Res. 2014;264:82–90. doi: 10.1016/j.bbr.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Ye P, Popken GJ, Kemper A, McCarthy K, Popko B, D'Ercole AJ. Astrocyte-specific overexpression of insulin-like growth factor-I promotes brain overgrowth and glial fibrillary acidic protein expression. J Neurosci Res. 2004;78:472–484. doi: 10.1002/jnr.20288. [DOI] [PubMed] [Google Scholar]
- Zhang J, Moats-Staats BM, Ye P, D'Ercole AJ. Expression of insulin-like growth factor system genes during the early postnatal neurogenesis in the mouse hippocampus. J Neurosci Res. 2007;85:1618–1627. doi: 10.1002/jnr.21289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 2885 kb)