Abstract
Objective:
Anthracyclines are widely used to treat solid and hematologic malignancies, but are known to cause cardiotoxicity. As more childhood cancer survivors reach adulthood due to improvements in oncologic treatments, they become susceptible to late and progressive anthracycline-induced cardiotoxicity. Nonetheless, diagnostic criteria for early detection of cardiac dysfunction are not well defined in children, adolescent, and young adult group (CAYA, ages 1 to 40 years). We present a natural history of the changes in myocardial deformation in CAYA patients after anthracycline therapy.
Methods:
We performed a literature review search between 2001 and 2016 using Pubmed with the following search terms: strain (or deformation), torsion (or twist), children (or adolescent or young adult), cardiotoxicity (or dysfunction), and anthracyclines (or doxorubicin). A total of 23 articles were reviewed. Fourteen articles were incorporated in the meta-analysis.
Results:
Strain abnormalities are observed at both short-term and long-term follow-up. Global longitudinal strain (GLS) abnormalities are common during or early after chemotherapy, whereas changes in global circumferential strain (GCS) are more significant and consistent on long-term follow-up. Although global radial strain and torsional parameters are also often abnormal late after chemotherapy, there are few studies evaluating these parameters.
Conclusion:
There are significant abnormalities in GLS and GCS following anthracycline therapy acutely and late after treatment. The prognostic value of these strain abnormalities warrants further investigation.
Keywords: Cardiac toxicity, myocardial strain, transthoracic echocardiography
1. INTRODUCTION
Anthracyclines have been used since the 1950’s to treat many solid and hematologic malignancies, and are known to cause cardiotoxicity [1–3] in a dose-dependent relationship [4]. The risk of cardiovascular disease-related morbidity and mortality is 8 times higher in anthracycline-treated cancer survivors than in the general population, and persists up to 45 years after treatment [5]. As increasing numbers of childhood cancer survivors reach adulthood due to improved cancer treatments, more of these survivors will be affected by the long-term cardiac consequences of anthracycline therapy. As of 2008, there were 619,000 cancer survivors under the age of 40 in the United States, a number which is likely to increase with improvements in diagnosis and treatment protocols [6,7]. Despite the burden of cardiovascular morbidity and mortality in childhood cancer survivors, especially in the children, adolescent, and young adult group (CAYA – 1 to 40 years of age), sensitive diagnostic tools for evaluating subclinical dysfunction are not well defined.
Classification of cardiotoxicity can be based on chronology: acute (during treatment), early chronic (<1 year after treatment), and late chronic (>1 year after treatment). Subclinical myocardial dysfunction can develop during or after anthracycline treatment and is estimated to occur in 20 to 75% of survivors [8]. Acutely, anthracyclines cause transient electrophysiological changes and mild changes in myocardial contractility, which may be reversible after treatment [9,10]. Early- and late-onset cardiotoxicity are defined by heart failure, pericardial effusions, or dilated cardiomyopathy [9]. Children tend to present with asymptomatic restrictive and dilated cardiomyopathy [10].
The current paradigm for detection of chemotherapy-related cardiotoxicity is symptomatology of congestive heart failure or >10% decline in echo-derived left ventricular ejection fraction (LVEF) [11]. This practice has significant limitations because subclinical myocardial damage often occurs in the presence of a stable LVEF. The deterioration in LVEF is frequently only seen late when irreversible damage has already occurred [12]. Myocardial strain (or deformation) imaging has been proposed as a more sensitive surrogate for assessing myocardial function of cancer survivors [2].
Because large-scale studies to assess the natural history of anthracycline-related cardiotoxicity in the CAYA group are lacking, we aim to systematically summarize the effect of anthracyclines on myocardial deformation in CAYA with cancer or survivors of childhood cancers classified by timing of echocardiographic evaluation.
2. METHODS
2.1. Data sources and searches
A literature search was performed based on recommendations from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for systematic reviews [13]. Various combinations of the following terms were searched using PubMed: strain (or deformation), torsion (or twist), children (or adolescent or young adult), cardiotoxicity (or dysfunction), and anthracyclines (or doxorubicin). The time frame was limited to 2001–2016. The last date searched was April 25, 2016. For one article, unpublished data was obtained directly from the author [14]. Data was estimated from figures if numerical values were not provided.
2.2. Data selection, abstraction, synthesis, and analysis
The studies were limited to those focusing on the effects of anthracyclines on myocardial strain in subjects between 1 and 40 years of age, both during and after treatment. Two authors reviewed the titles and abstracts for appropriateness. The articles include observational, cross-sectional, and case-control studies. Studies of anthracycline exposure in CAYA were divided into three groups based on the duration of anthracycline exposure: during or less than one year after treatment (acute and early-chronic), 1–10 years after treatment (intermediate-late chronic), and ≥10 years after treatment (late-chronic). Because studies of anthracycline toxicity in the CAYA group described the risk of cardiotoxicity as a function of cumulative doxorubicin dosages, the total cumulative anthracycline dose was derived by taking the sum of the calculated doxorubicin-isotoxic dose equivalents [15]: [doxorubicin × 1] + [daunorubicin × 0.833] + [epirubicin × 0.67] + [idarubicin × 5] + [mitoxantrone × 4]. Cutoffs for anthracycline cardiotoxicity are as follows: doxorubicin >500 mg/m2, liposomal doxorubicin >900 mg/m2, epirubicin >720 mg/m2, mitoxantrone >120 mg/m2, and idarubicin >90 mg/m2. The meta-analysis portion was used to increase population size for two measures of myocardial deformation, GLS and GCS, which have been shown to be feasible and reproducible markers of myocardial injury [16] in adult patients. A meta-analysis was performed to evaluate the consistency of the change in GLS and GCS after chemotherapy across the available studies in the literature. Studies included in the meta-analysis were weighted based on the inverse of the reported standard error (and therefore indirectly to the sample size). Studies with smaller standard error and larger sample size were given more weight in calculating the pooled effect size. The heterogeneity among studies was determined using Cochran’s Q [17], which is based on Chi-square test with significance defined as p<0.10 [18]. Heterogeneity was also quantified using I2 [19]. Low, moderate, and high degree of inconsistency corresponds to I2 values of 25%, 50%, and 75%, respectively. A random-effects model was chosen to assess for standard mean difference (SMD) of global longitudinal strain (GLS) in two groups (pre- versus post-treatment and post-treatment versus normal controls) and global circumferential strain (GCS) in one group (post-treatment vs normal controls).
2.3. Myocardial deformation imaging parameters
Strain, defined as the percentage of change in myocardial wall length, was measured by using tissue Doppler imaging (TDI), speckle tracking echocardiography (STE) in both 2D and 3D, or velocity vector imaging (VVI) [16,20,21]. Longitudinal (LS) and circumferential (CS) strain describe active strain or shortening of the fibers while radial strain (RS) measures passive strain or thickening of the myocardium [16]. Global strain represents the average strain of the entire myocardium for each respective direction [16], whereas segmental strain refers to shortening or lengthening of a specific portion of the myocardium based on the 16- or 17-segment model [22]. Strain rate (Sr) is the rate of change in strain (reported as strain per second). Rotational mechanics represent myocardial rotation around the axis of the LV at the base and apex [16]. Twist is the absolute apex-to-base difference in rotation reported in degrees while torsion is the base-to-apex difference of the rotation angle divided by the axis of the LV and is reported in degrees per centimeter. Rotation and twisting velocity, reported in degrees per second, are calculated by dividing by the time-in-systole [16,23,24].
3. RESULTS
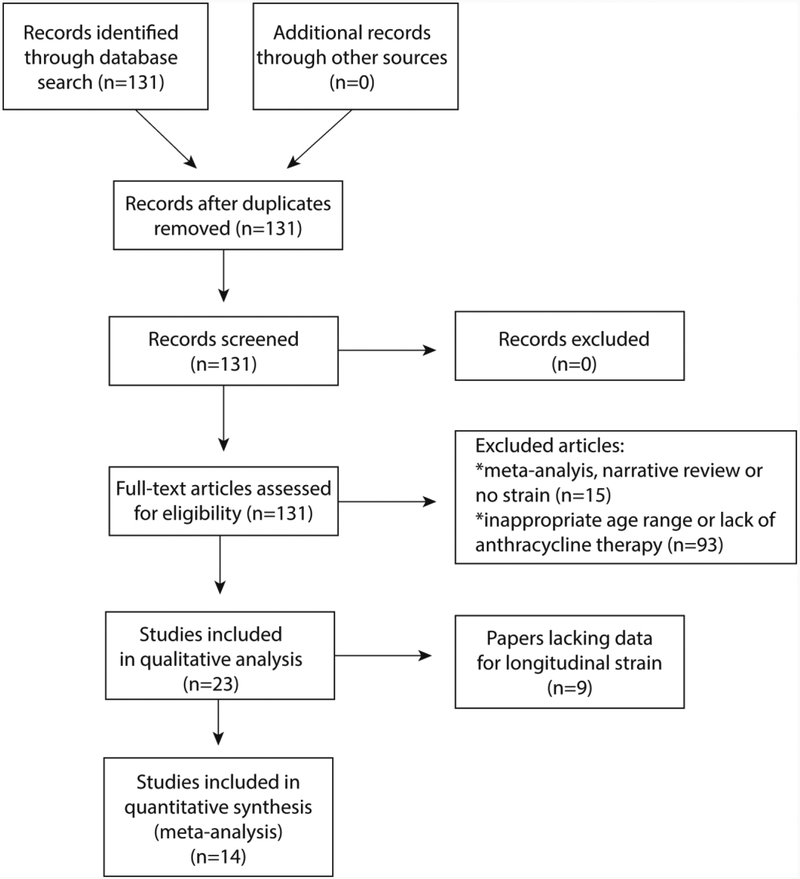
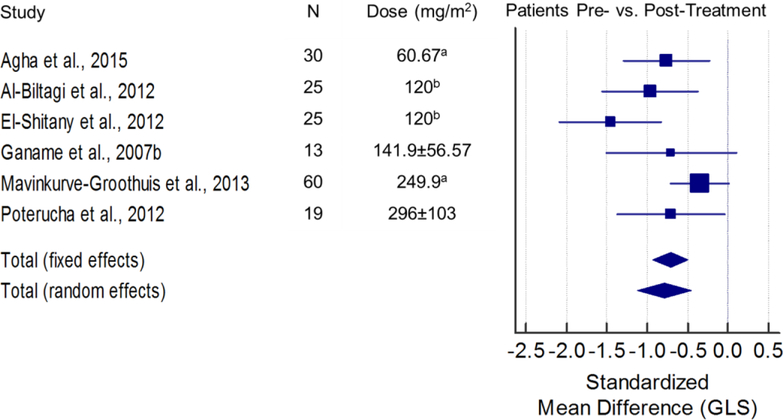
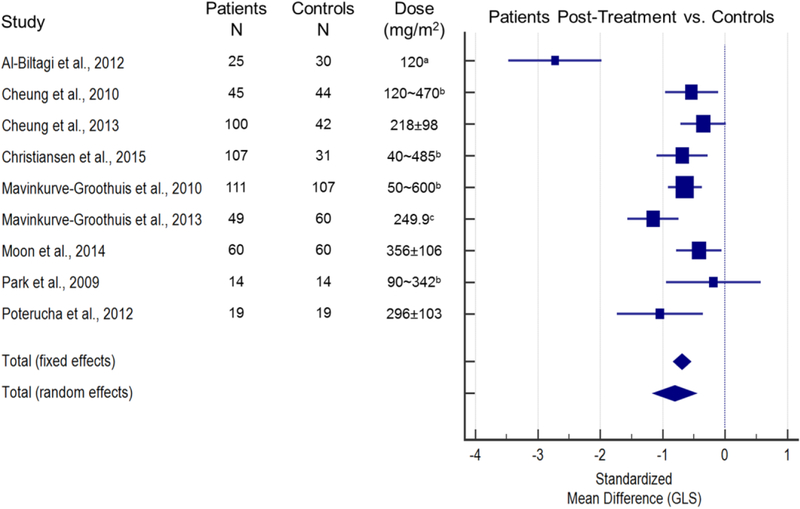
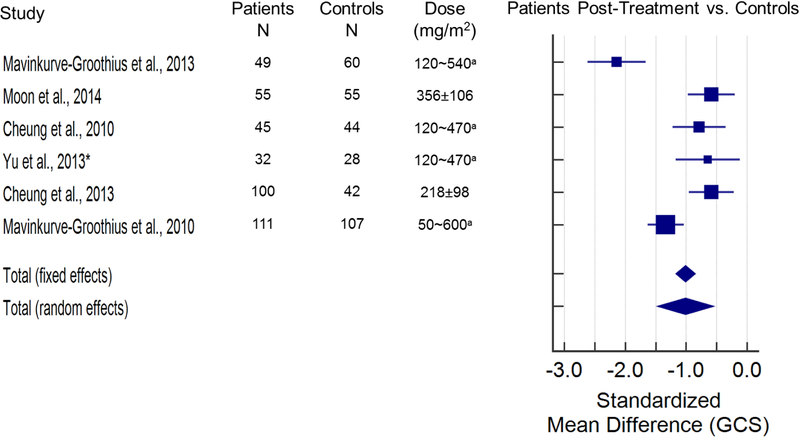
The search returned 131 articles, 23 of which were included in this review (Figure 1). Fourteen papers provided quantitative strain data and were used for the meta-analysis (Figures 2 and 3). Figure 2 illustrates the standardized mean difference (SMD) and 95% confidence intervals (CI) of GLS between patients pre- and post-anthracycline therapy. The pooled data in Figure 2 suggest that GLS is a suitable biomarker and can detect a change in myocardial function across a wide spectrum of anthracycline dosages with good agreement among the included studies. Figure 3 and figure 4 illustrate the SMD and 95% CI of GLS and GCS respectively in patients post-anthracycline therapy versus control subjects. Compared to the data in Figure 2, pooled data in Figure 3 and 4 show less agreement because of variations in the average anthracycline doses, time-to-evaluation, and technique for strain quantification.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flow Diagram [53].
Figure 2. Standardized mean difference (SMD) and 95% confidence intervals of global longitudinal strain (GLS) between patients at baseline (prior to anthracyclines) and patients within one year of anthracycline treatment.
The size of the square marker is proportional to the weight assigned to each study in the pooled estimate (diamond) using a random effects model. The weighing is related to the inverse of the standard error (and therefore indirectly to the sample size) reported in the studies. Studies with smaller standard error and larger sample size are given more weight in calculating the pooled effect size. SMD Total (fixed effects) = −0.714; SMD Total (random effects) = −0.788 (both p<0.001). Level II evidence. There was no statistically significant difference among the findings of the included 6 articles [27–31,33] (X2 (5)=10.32, p=0.067), and the inconsistency among included articles was quantified as I2=51.56% [95% CI=0–80.7]. The reported decreases in GLS after treatment based on the 6 included papers [27–31,33] are moderately heterogeneous. Doses are reported as mean ± SD unless noted otherwise. aaverage; bmedian.
Figure 3. Standardized mean difference (SMD) and 95% confidence intervals of global longitudinal strain (GLS) between normal controls and patients treated with anthracyclines.
The size of the square marker is proportional to the weight assigned to the study in the pooled estimate (diamond) using a random effects model. The weighing is related with the inverse of the standard error (and therefore indirectly to the sample size) reported in the studies. Studies with smaller standard error and larger sample size are given more weight in calculating the pooled effect size. The results indicate that GLS is lower in anthracycline treated patients as compared to a normal, age-matched population (SMD Total (fixed effects) = −0.695; SMD Total (random effects) = −0.810 (both p<0.001); Level II evidence). There was a significant difference among the findings of the included 9 articles [20,21,24,29,31,33,44,46,49] (X2 (8)=44.06, p<0.001), and the inconsistency among included articles was quantified as I2=81.84% [95% CI=66.7–90.1]. Doses are reported as mean ± SD unless noted otherwise. amedian; brange; caverage.
Figure 4. Standardized mean difference (SMD) and 95% confidence intervals of global circumference strain (GCS) between patients and controls following anthracycline-treatment.
The size of the square marker is proportional to the weight assigned to the study in the pooled estimate (diamond) using a random effects model. The weighing is related with the inverse of the standard error (and therefore indirectly to the sample size) reported in the studies. Studies with smaller standard error and larger sample size are given more weight in calculating the pooled effect size. The results indicate that GCS is lower in anthracycline treated patients as compared to a normal, age-matched population (SMD Total (fixed effects) = −1.013; SMD Total (random effects) = −1.010 (both p<0.001); Level II evidence). There was a significant difference among the findings of the included 6 articles [20,24,31,41,44,46] (X2 (5)=40.01, p<0.001), and the inconsistency among included articles was quantified as I2=87.50% [95% CI=75.2–93.7]. Doses are reported as mean ± SD unless noted otherwise. arange. *mid-papillary level GCS was used for analysis from the study by Yu et al[41].
3.1. Reference values for myocardial deformation in CAYA
Differences in strain between groups are described as absolute changes in the strain magnitude with the convention that LS and CS are negative and RS is positive. The following values were used as reference ranges for strain [25] (mean [95% CIs]): GLS (−20.2 [−20.8 to −19.6]), global CS (GCS) (−22.3 [−24.6 to −19.9]), global RS (GRS) (45.2 [38.8 to 51.7]). Levy et al [25] further separated the strain values by age (0–1, 2–9, 10–13, and 14–21 years-of-age), and age-specific values were used as reference when appropriate. For young adults older than 21 years of age, adult strain reference values were applied [26] (mean [95% CIs]): GLS (−19.7 [−20.4 to −18.9]), GCS (−23.3 [−24.6 to −22.1]), and GRS (46.3 [43.6 to 51.0]).
3.2. Acute and early (<1 year post-treatment) evaluation of strain
Seven studies [27–33] investigated strain during or less than 1 year after anthracycline exposure (Table 1). All studies assessed strain at baseline and over the course of chemotherapy [27–31,33], except Pignatelli et al [32] who measured myocardial strain only post-treatment. Several studies (3 of 7 studies) compared post-treatment patients to healthy controls [31,33] or reference values in healthy children [32]. Ganame et al [27] used TDI while 2D-STE was used in the other studies. The average anthracycline dose in the seven studies was below conventional and established thresholds for high-risk of cardiotoxicity (normalized to doxorubicin, <500 mg/m2). One study evaluated the relationship between change in strain with respect to patient age [32].
TABLE 1.
Summary of studies evaluating patients during and <1 year following anthracycline therapy.
| Study | Patient Age (years) | Timing of Measurements (years) | Anthracycline Dose (mg/m2) | Cancer Type | Software | Pre-chemotherapyc | Post-chemotherapyc | Controlsc |
|---|---|---|---|---|---|---|---|---|
| Agha et al [28] | 9.24 ± 4.14 | Baseline and 1 week after last dose | 60.67 ± 9.8 | AML, ALL, HL, nHL | EchoPAC, GE | GLS: −21.58 ± 2.54 | GLS: −19.18 ± 3.59 | - |
| Al-Biltagi et al [29] | 9.2 ± 2.9 | Baseline and within 1 week after last dose | 120 (median) | ALL | EchoPAC, GE | GLS: −18.65 ± 4.52 | GLS: −15.10 ± 2.45 | GLS: −21.5 ± 2.2 |
| El-Shitany et al [30] | 9.5 ± 2.6 | Baseline and within 1 week after last dose | 120 | ALL | EchoPAC, GE | GLS: −18.65 ± 2.9 | GLS: −15.1 ± 1.769 | - |
| Ganame et al [27] | 10.7 ± 3.8 | Baseline and within 2 hours of the first 3 doses | 141.9 ± 56.57 | LY, ALL, OS, ES, AML | Proprietary software | GLS: −27 ± 5 | GLS: −23 ± 6 | - |
| GRS: 74 ± 14 | GRS: 45 ± 11 | |||||||
| Mavinkurve-Groothuis et al [31] | 6 [2.2 – 15.4] | Baseline, after induction, and 1-year follow (>2 weeks after last dose) | 120 mg/m2 after induction; at 1-year follow-up; standard risk = 120, medium risk = 300, high risk = 540 | ALL | EchoPAC 6.1, GE | GLS: −18.2 ± 3.1 | GLS: −16.7 ± 5.2 | GLS: −20.9 ± 1.3a |
| GCS: −19.4 ± 4.3 | GCS: −16.9 ± 3.1 | GCS: −22.5 ± 2.1 | ||||||
| GRS: 66.8 ± 12 | GRS: 55.2 ± 16 | GRS: 54.3 ± 6a | ||||||
| Poterucha et al [33] | 15.3 ± 3 | 0, 4, and 8 months of treatment | 296 ± 103 | OS, ES, HL, BCL, RMS, MPNST, ALL | EchoPAC, GE | GLS: −19.9 ± 2.1 | GLS: −18.1 ± 2.8 | GLS: −20.5 ± 1.5 |
| Pignatelli et al [32]b | 9.8 ± 5.8 | 1-year follow up (>3 weeks since last dose) | 213.33 ± 124.4 | ALL, AML, NB, OS, ES | EchoPAC, GE | - | 1– 4 years: | 1– 4 years: |
| GLS: −19.31 ± 5.48 | GLS: −20.7 ± 1.30a | |||||||
| GCS: −15.88 ± 3.86 | GCS: erroneous value | |||||||
| 5 to 9 years: | 5 to 9 years: | |||||||
| GLS: −18.33 ± 4.02 | GLS: −21 ± 1.30a | |||||||
| GCS: −15.74 ± 2.08 | GCS: −20.90 ± 2.00 | |||||||
| 10 to 14 years: | 10 to 14 years: | |||||||
| GLS: −19.43 ± 1.4 | GLS: −21.8 ± 1.30 | |||||||
| GCS: −15.81 ± 1.77 | GCS: −21.5 ± 1.70 | |||||||
| 15–19 years: | 15–19 years: | |||||||
| GLS: −18.79 ± 1.4 | GLS: −22.5 ± 1.30 | |||||||
| GCS: −12.16 ± 2.09 | GCS: −21.90 ± 2.10 |
ALL=acute lymphoblastic leukemia; AML=acute myeloid leukemia; BCL=B-cell lymphoma; ES=Ewing sarcoma; HL=Hodgkin lymphoma; LY=lymphoma; MPNST=malignant peripheral nerve sheath tumor; NB=neuroblastoma;.nHL=non-Hodgkin lymphoma; OS=osteosarcoma; RMS=rhabdomyosarcoma;
no significant difference between control group and patients.
normal reference values were used as controls.
units, strain (%).
3.2.1. Longitudinal Strain.
The decrease in GLS during or immediately following treatment compared to baseline values ranged from 8.2% to 19% [27,28,30,31,33]. Post-treatment GLS was 6.7% to 20% lower compared to control values [31–33]. All post-treatment GLS values fall outside the reference range [25] except for the 1–4 year-old and 10–14 year-old groups in Pignatelli et al’s paper [32]. The majority of segmental LS values were significantly decreased as well. The left-ventricular (LV) basal LS decreased by 14% [27] to 16% [33], mid LS decreased by 11% [27] to 12% [33], and apical LS decreased by 11% [28]. Three papers [27,28,31] showed significant changes in GLS rate (GLSr) from 12% [28] to 18% [27], though most post-treatment values remained within reference range [34]. Two studies [29,30] showed that severity of LS abnormalities after chemotherapy varies based on the echocardiographic view; the most significant reduction was seen in the apical long axis view (40% decrease) with more modest changes in the apical 4 chamber and apical 2 chamber views (8.7%, and 13% decrease, respectively).
3.2.2. Circumferential Strain.
All post-treatment values of GCS [31,32] were outside the reference range [25], indicating that GCS in cancer survivors can deteriorate as early as 1 year after treatment. Mavinkurve-Groothuis et al [31] reported a 13% decrease in GCS and a 12% decrease in GCS rate (GCSr) from baseline to post-treatment in patients. Pignatelli et al [32] compared GCS values 1-year post-treatment to normal reference values [35] according to age (1–4, 5–9, 10–14, and 15–19 years of age). The reduction in GCS was greater with older age.
3.2.3. Radial Strain.
Ganame et al [27] and Mavinkurve-Groothius et al [31] compared baseline and post-treatment GRS, and GRS rate (GRSr) in patients; both studies showed a significant reduction in GRS (39% and 17% respectively), and GRSr (19% and 12% respectively) after anthracycline treatment. However, the post-treatment GRS was not significantly different when compared to healthy controls in one of the studies [31]. When compared to reference values [25], the patients in Ganame et al’s [27] study had markedly elevated baseline GRS (74 ± 14%) which decreased after each of the 3 doses of anthracycline administration (56 ± 11% to 52 ± 12% to 45 ± 11%).
3.3. Evaluation of strain 1 to 10 years post-treatment
Ten papers [14,20,21,24,36–41], nine of which were cross-sectional studies [14,21,24,36–41], assessed changes in strain parameters 1 to 10 years after anthracycline treatment in patients aged 6.9 to 24 years (Table 2). All studies compared patients treated with anthracyclines to healthy controls except Ryerson et al [14]. Patients were treated with average or median doses of anthracyclines (normalized to doxorubicin) ranging from 220 to 401.1 mg/m2. Strain was assessed in images captured by TDI [37,39], 2D-STE [14,24,36,38,41], 3D-STE [40] and VVI [20,21].
TABLE 2.
Summary of studies evaluating patients at 1 to 10 years following anthracycline therapy.
| Study | Patient Age (yrs) | Timing (yrs) | Anthracycline Dose (mg/m2) | Cancer Type | Software | Pre-chemotherapye | Post-chemotherapye | Controlse |
|---|---|---|---|---|---|---|---|---|
| Park et al [21] | 9.8 [6.1 – 17.5] | - | 90 – 342 | ALL, AML, NB, nHL, HL | Syngo US Workplace 3.0, Acuson | - | GLS: −22.89 ± 2.47 | GLS: −23.55 ± 4.19a |
| Moon et al [20] | 10.5 ± 4.7 | 3.9 ± 4.0 | 356 ± 106 | AML, BL, ES, OS | Syngo Velocity Vector Imaging, Siemens | GLS: −19.26 ± 3.78 | GLS: −18.27 ± 3.35 | GLS: −19.74 ± 3.63 |
| GCS: −26.84 ± 5.61 | GCS: −24.21 ± 3.74 | GCS: −26.48 ±4.0 | ||||||
| Ryerson et al [14]b | Low risk: 16 ± 2.89, Moderate risk: 14.5 ± 3.2, High risk: 16.2 ± 3.64 | Low risk: 8.0 ± 2.73, Moderate risk: 9.6 ± 3.43, High risk: 8.3 ± 3.03 | Low risk: 119.6 ± 43.29, Moderate risk: 171 ± 40.01, High risk: 344 ± 62.27 | LE, LY, SA, WT, NB, Other | EchoPAC, GE | - | Low risk: | Low risk: |
| GLS: −19.2 | GLS: −14.26 | |||||||
| Moderate risk: | Moderate risk: | |||||||
| GLS: −17.4 | GLS: −14.26 | |||||||
| High risk: | High risk: | |||||||
| GLS: −15.4 | GLS: −14.26 | |||||||
| Cheung et al [36] | 15.6 ± 5.5 | 7.0 [3.1 – 24.3] | 240 [120 – 470] | ALL | EchoPAC, GE | - | Twist: 8.0 ± 4.1 | Twist: 11.8 ± 4.5 |
| Twist velocity: 68.1 ± 20.3 | Twist velocity: 91.0 ± 22.3 | |||||||
| Untwisting velocity: −90.1 ± 34.3 | Untwisting velocity: −109.6 ± 33.4 | |||||||
| Cheung et al [24] | 15.3 ± 5.8 | 6.3 [2.7 – 19.8] | 240 [120 – 470] | ALL | EchoPAC, GE | - | GLS: −17.6 ± 3.0 | GLS: −19.0 ± 2.2 |
| GCS: −14.5 ± 2.9 | GCS: −17.4 ± 4.3 | |||||||
| GRS: 40.1 ± 15.6 | GRS: 50.0 ± 16.4 | |||||||
| Ganame et al [37] | 12.7 [4 – 28] | 5.2 [2.0 – 15.2] | 240 [90 – 300] | ALL, AML, LY, solid tumors | Proprietary software | - | GLS: shown graphically | GLS: shown graphically |
| Toro-Salazar et al [38] | 22 [12 – 42] | 9.6 [2.5 – 26.9] | 328 [200 – 600] | AML, OS, HL, ES | - | - | GLS: −18.5 ± 2.4 | GLS: data not provided |
| GCS: shown graphically | GCS: shown graphically | |||||||
| Yagci-Kupeli et al [39] | 14 | 5.8 ± 3.6 | 350 – 480 | nHL, HL, HB, WT, LS, NB, OS, NEDT, NC | EchoPAC, GE | - | no numerical data | no numerical data |
| Yu et al [40]c | 18.6 ± 5.1 | 7.2 [2.4 – 16.4] | 229 [40 – 644] | ALL, AML, OS, BL, HL, nHL, SS, NB, HB | Advanced Cardiology Package, Toshiba | - | Twist: 6.6 ± 2.5 | Twist: 9.9 ± 3.2 |
| Torsion: 1.3 ± 0.5 | Torsion: 1.9 ± 0.7 | |||||||
| Yu et al [41]d | 19.3 ± 5.4 | 6.9 [2.2 – 14.4] | 220 [120 – 470] | ALL, AML, OS, HL, nHL | Advanced Cardiology Package, Toshiba | - | Basal level: | Basal level: |
| GCS: 12.6 ± 5.0 | GCS: 15.6 ± 3.2 | |||||||
| Mid-papillary level: | Mid-papillary level: | |||||||
| GCS: 13.7 ± 1.9 | GCS: 15.2 ± 2.7 | |||||||
| GRS (inner): 33.8 ± 4.2 | GRS (inner): 39.7 ± 10.8 | |||||||
| GRS (outer): 23.0 ± 4.0 | GRS (outer): 29.1 ± 6.3 | |||||||
| Apical level: | Apical level: | |||||||
| GCS: 13.9 ± 2.9 | GCS: 15.9 ± 3.1 | |||||||
| GRS (inner): 25.7 ± 4.5 | GRS (inner): 29.1 ± 7.7 |
ALL=acute lymphoblastic leukemia; AML=acute myeloid leukemia; BL=Burkitt lymphoma; ES=Ewing sarcoma; HB=hepatoblastoma; HL=Hodgkin lymphoma; LE=leukemia; LS=liver sarcoma; LY=lymphoma; NB=neuroblastoma; NC=nasopharyngeal carcinoma; NEDT=neuroectodermal tumor; nHL=non-Hodgkin lymphoma; OS=osteosarcoma; RMS=rhabdomyosarcoma; SA=sarcoma; SS=synovial sarcoma WT=Wilms tumor.
no significant difference between control group and patients.
comparison group consists of anthracycline naïve cancer survivors.
3D measurements.
absolute strain values provided.
units, strain measurements (%), twist (°), torsion (°/cm), velocity (°/s).
3.3.1. Longitudinal Strain.
Eight articles [14,20,21,24,37–39,41] assessed GLS following anthracycline administration and showed inconsistent findings. Four of the 8 studies [20,24,37,39] found that anthracycline administration was associated with a significant decrease in GLS. Moon et al [20] and Cheung et al [24] reported similar findings where the GLS was on average 7.4% lower in the patient group compared to controls. Yagci-Kupeli et al [39] did not provide numerical data. Ganame et al [37] graphically demonstrated that, while there is a similar pattern of regional variation in the strain values from base to apex in the septum and lateral LV wall, the absolute strain values are approximately 25% lower in patients compared to the control group. In contrast to the studies mentioned above, Park et al [21] showed that GLS was not significantly different in patients compared to controls; however, patients did have significantly lower LS, diastolic Sr, and systolic Sr in the septum when compared to the lateral LV wall. Toro-Salazar et al [38] showed slightly increased GLS in patients versus controls; however, this trend was reversed with MRI-derived strain values. In contrast, Ryerson et al [14] showed a nonsignificant improvement in GLS in patients who received low-, moderate-, or high-dose anthracyclines compared to controls (35% increase, 22% increase, and 8.0% increase respectively), but GLS measurements were performed only in the apical 4-chamber view. The study by Ryerson et al [14] was unique because the control group consisted of 21 anthracycline-naïve cancer survivors, 15 of whom were overweight or obese which may account for the baseline lower strain values in the control group [42,43]. Lastly, Yu et al [41] found similar strain values between patients and controls, however no numerical values were provided.
Most of the studies also evaluated Sr and similarly found variable results [14,20,24,37,39]. Ganame et al [37] and Yagci-Kupeli et al [39] found that GLSr was significantly lower in patients compared to controls (data shown graphically in Ganame et al’s article, and not provided by Yagci-Kupeli et al). In contrast, Cheung et al [24] and Ryerson et al [14] showed that changes in GLSr were similar between patients and controls. Moon et al [20] found diastolic LSr, but not systolic LSr, to be significantly lower in patients (12% lower than controls).
3.3.2. Circumferential Strain.
Of the four studies which examined CS, all showed consistently abnormal values in patients 1 to 10 years post chemotherapy [20,24,38,41]. Yu et al [41] examined transmural strain at the basal, papillary muscle, and apical levels. Patients displayed significantly lower transmural CS gradients at all three levels compared to controls (19%, 9.9%, and 13% lower at the basal, papillary muscle, and apical levels respectively). Interestingly, the difference in CS between groups was only observed in the endocardial portion, but not in the epicardial portion. This finding was attributed to worsened subendocardial function with preserved subepicardial function. Cheung et al [24] showed that patients had reduced segmental CS in the anteroseptal, inferoseptal, inferior, and anterior segments as well as 17% reduction in GCS. Similar reductions in the anteroseptal and inferior segments, as well as GCS were reported by Toro-Salazar et al [38]; however, no numerical values were provided. In regards to Sr, Cheung et al [24] found that patients’ GCSr was significantly lower than controls’ by 15%. Moon et al [20] similarly reported that, compared to controls, GCS was reduced in patients by 8.6% while CSr was decreased by 8.8% for systolic CSr and 14% for diastolic CSr. The severity of these abnormalities correlated with increasing anthracycline doses.
3.3.4. Radial Strain.
Radial strain was evaluated in three studies [24,37,41]. In Yu et al’s [41] article, RS of the inner segment at the apex, and inner and outer segments of the papillary muscle level were significantly decreased in patients relative to control by 12%, 15%, 21% in the apical inner layer, mid-papillary inner level, mid-papillary outer level, respectively. There was no significant difference in the basal segments or the transmural radial strain in patients compared to controls. In contrast, Cheung et al [24] showed a decrease in GRS of 20% to just above reference values in patients compared to controls. The radial strain difference was present in all segments of myocardium. Statistically significant differences between patients and controls in both peak radial systolic Sr and strain in the inferolateral wall were also reported (no numerical values provided) [37].
3.3.5. Torsion and Twist.
Three papers examined twist, torsion (twist/LV length), and twisting/untwisting rate [36,40,41]. Yu et al [40] examined twist and torsion using 3D-STE, and found that both were reduced significantly compared to the healthy cohort (33%, and 32% decrease, respectively). Cheung et al [36] examined peak apical and basal rotation, twisting, and untwisting rates, and LV torsion, systolic twisting velocity, and diastolic untwisting velocity. Peak apical rotation, and untwisting rate were significantly reduced (24%, and 26% reduction respectively), while basal parameters showed no significant change between patients and controls. All three LV parameters in patients were significantly reduced as compared to controls (peak torsion: 32% reduction, peak systolic twisting velocity: 25% reduction, peak diastolic untwisting velocity: 18% reduction). Yu et al [41] used 2D-STE to examine transmural rotation, twisting, and untwisting velocity at the base and apex. At the base, both the subendocardial and subepicardial rotation, twisting velocity, and untwisting velocity were significantly reduced in patients; hence, there was no significant difference in the transmural gradient between patients and controls. However, at the apex, only the subendocardial layer showed significant changes in rotation, twisting velocity, and untwisting velocity, which led to a significantly reduced transmural rotation gradient when compared to controls (41% reduction).
3.4. Evaluation of strain >10 years post-treatment
Six articles examined strain measurements greater than 10 years after treatment [44–49] (Table 3). Time of follow-up ranged from 13.2 to 23.4 years on average. Most studies compared strain measurements between patients treated with anthracyclines to normal controls [44–46]. One study [49] compared patients to anthracycline naïve cancer survivors. Yu et al [48] and Armstrong et al [47] divided patients based on whether they received treatment with anthracyclines only or anthracyclines and mediastinal radiotherapy (MSRT).
TABLE 3.
Summary of studies evaluating patients at >10 years following anthracycline therapy.
| Study | Patient Age (years) | Timing of Measurements (years) | Anthracycline Dose (mg/m2) | Cancer Type | Software | Post-chemotherapyc | Controlsc |
|---|---|---|---|---|---|---|---|
| Cheung et al44 | 24.1 ± 4.2 | 15.3 ± 5.8 | 218 ± 98 | ALL, AML | EchoPAC, GE | GLS: −16.0 ± 3.1 | GLS: −17.1 ± 3.2 |
| GCS: −14.3 ± 3.5 | GCS: −16.6 ± 4.7 | ||||||
| GRS: 32.9 ± 10.9 | GRS: 42.3 ± 12.5 | ||||||
| Dietz et al45 | 27 [18 – 50] | 17 [5 – 30] | 440 [300 – 645] | OS, RMS, ES, SS, LY, WT | QLAB quantification, Phillips |
Radial displacement:
5.61 ± 1.16 |
Radial displacement:
6.73 ± 1.52 |
| Mavinkurve-Groothuis et al46 | 20 [5.6 – 37.4] | 13.2 [5 – 29.2] | 180 [50 – 600] | ALL, AML, EP, ES, HB, HL, NC, NB, nHL, OS, RMS, WT | EchoPAC, GE | GLS: −19.8 ± 2.6 | GLS: −21.2 ± 1.6 |
| GCS: −15.9 ± 6.7 | GCS: −22.6 ± 2.1 | ||||||
| GRS: 49 ± 12 | GRS: 57 ± 5 | ||||||
| Yu et al48, b | 31 [18 – 62] | 15 [2 – 39] | 300 [27 – 660] | SA, HL, ALL, AML, nHL | EchoPAC 12.0, GE | Anthracycline alone: | - |
| GLS: 19 [17 – 20] | |||||||
| GCS: 17.6 [16 – 19.7] | |||||||
| GRS: 42.0 [31.9 – 51.4] | |||||||
| Anthracycline and radiation: | |||||||
| GLS: 18 [16 – 19.5] | |||||||
| GCS: 16.1 [14.5 – 19.7] | |||||||
| GRS: 42.1 [26.5 – 55.2] | |||||||
| Armstrong et al47 | 31 [18 – 65] | 22.6 [10.4 – 48.3] | Median not given [0 – 600] | LE, ALL, AML, LY, nHL, HL, CNS tumor, Bone tumor, ES, OS, Soft tissue SA, RMS, Nonrhabdo SA, GC, Melanoma, NB, RB, WT, Carcinoma, Other | EchoPAC, GE | Anthracycline alone: | - |
| GLS: −19.3 ± 2.6 | |||||||
| GCS: −20.2 ± 5.0 | |||||||
| Anthracyclines and radiation: | |||||||
| GLS: −18.5 ± 2.8 | |||||||
| GCS: −20.1 ± 5.0 | |||||||
| Christiansen et al49, a | 28.6 [18.6 – 46.5] | 23.4 [7.4 – 40] | Low dose: 120 [40 – 120], Mod-high dose: 240 [173 – 485] |
ALL | EchoPAC 7, GE | Low dose: | Low dose: |
| GLS: −18.1 ± 2.3 | GLS: −19.7 ± 2.4 | ||||||
| Moderate dose: | Moderate dose: | ||||||
| GLS: −18.3 ±1.9 | GLS: −19.7 ± 2.4 |
ALL=acute lymphoblastic leukemia; AML=acute myeloid leukemia; EP=ependymoma; ES=Ewing sarcoma; GC=germ cell tumor; HB=hepatoblastoma; HL=Hodgkin lymphoma; LE=leukemia; LY=lymphoma; NB=neuroblastoma; NC=nasopharyngeal carcinoma; nHL=non-Hodgkin lymphoma; OS=osteosarcoma; RB=retinoblastoma; RMS=rhabdomyosarcoma; SA=sarcoma; SS=synovial sarcoma; WT=Wilms tumor.
comparison group consists of anthracycline naïve cancer survivors.
absolute strain values provided.
units, strain measurements (%), radial displacement (mm).
3.4.1. Longitudinal Strain.
Cheung et al [44], Mavinkurve-Groothuis et al [46], and Christiansen et al [49] all showed that GLS was significantly reduced in patients versus controls (between 6.4% to ~7.6% decrease). Changes in strain rate were variable: Cheung et al’s [44] paper showed no significant change in systolic or diastolic Sr while Mavinkurve-Groothuis et al’s [46] showed a significant 13% decrease in GLSr in patients. When comparing patients treated with anthracyclines-only to patients treated with anthracyclines and MSRT, Yu et al [48] showed that patients with dual-therapy had significantly lower GLS compared to those treated with mono-therapy; however GLSr showed no change between the two groups. Armstrong et al [47] presented that GLS was abnormal in 27% of anthracycline-only treated patients while LVEF was abnormal in only 4.3%. Abnormal GLS was associated with any dose of MSRT and anthracycline dose >300 mg/m2.
3.4.2. Circumferential Strain.
Abnormal GCS was common in patients treated with anthracycline therapy alone (23%) [47]. Cheung et al [44] and Mavinkurve-Groothuis et al [46] both showed significant reductions of GCS in patients compared to controls (14%, and 30% decrease respectively). Both Cheung’s and Mavinkurve-Groothius’ papers reported significant decreases in GCSr (11%, and 19% respectively). As with GLS, the mean value of GCS and GCSr in patients Cheung et al’s [44] article was lower than in Mavinkurve-Groothuis et al’s [46] article. Yu et al [48] showed that the GCS is abnormal in all patients treated with anthracyclines (average GCS 17.3 [15.2 – 19.7]); however unlike the change in GLS, there was no significant difference in the GCS with respect to radiation treatment.
3.4.3. Radial Strain.
Cheung et al [44] and Mavinkurve-Groothuis et al [46] both showed significant reductions in GRS (22%, and 14% respectively), and GRSr (11%, and 49% respectively) as compared to controls. However, despite the striking change, the GRS values in Mavinkurve-Groothuis’s [46] article remained in the normal range likely illustrating lack of standardization in measurements among software vendors. Dietz et al [45] substituted radial displacement for radial strain due to variability in strain measurements ultimately finding a significant reduction (17%) between patients and controls. Yu et al [48] found minimal, nonsignificant changes in GRS when comparing anthracycline-treated patients with and without MSRT, however the majority of patients in both groups had GRS values below the reference range [26].
4. CONCLUSION
Despite a high degree of heterogeneity among studies using GLS and GCS to compare patients with normal controls, myocardial strain by echocardiography appears to be useful for intra-individual evaluation subclinical myocardial injury in childhood cancer patients treated with anthracycline therapy. Based on a review of the current body of literature, we found that during and immediately (<1 year) after treatment, the GLS, GCS, and strain rate all show significant changes. Radial strain is decreased compared to baseline; however, these changes are not necessarily different from controls or below the normal range. In the 1–10 years post-treatment, circumferential strain and strain rate are the most consistently abnormal measurements, followed by radial strain measurements. Longitudinal strain measurements appear to be less reliable in this group, with some papers even showing increased absolute strain in patients compared to controls. Patients >10 years post-treatment continue to display significant reductions of circumferential strain that are greater than longitudinal strain. Radial strain shows similar reductions in long-term follow-up.
Previous studies addressing anthracycline therapy and strain have focused on the effects of anthracyclines in all age groups [50], which includes many breast cancer survivors who are treated with other cardiotoxic medications (such as trastuzumab) and often receive mediastinal radiotherapy. In Thavendiranathan’s review [50] for example, GLS was determined to be the most consistently affected measure during chemotherapy. In this article, where we focus on childhood cancer survivors only, we showed that GLS is most consistent for acute monitoring, but becomes less consistent >1 year after therapy. Our meta-analysis showed only moderate heterogeneity among studies when GLS is assessed in the same patient pre- and post-therapy and within 1 year of treatment.
Based of the available published data, it remains unclear which strain measurement is optimal for late follow-up in CAYA cancer survivors. As mentioned above, GCS was abnormal more often than GLS in patients who were >1 year post treatment, and hence may be an important measurement for long-term follow up in childhood cancer survivors. This is different from the adult literature where GLS appears to be a more consistent marker across many pathologies including restrictive cardiomyopathy, coronary artery disease, and some valvular disease [16,51]. We evaluated the consistency of both GLS and GCS abnormalities across studies in patients post-therapy compared to controls. While there was a difference in the SMD when comparing GLS and GCS values in patients following treatment and a control cohort, there was high heterogeneity among studies. The heterogeneity was likely due to different strain tracking methods and software algorithms, as well as variable time-to-evaluation. Lack of standardization in the optimal views used for measuring strain may also explain some of the observed variability in strain measurements between studies. For example, strain data derived from two apical long-axis views that are foreshortened may not be as accurate as those derived from three apical long-axis views (2-chamber, 3-chamber, 4-chamber). In summary, while the findings from our meta-analysis are promising, they also suggest that additional studies with larger sample sizes and standardized image acquisition will be helpful for demonstrating value when relating myocardial strain changes to clinical outcomes.
There were limitations encountered in the included studies. Although strain showed good inter- and intra-observer reliability, certain measurements did show increased variability and may partly be due to inconsistent techniques, vendor-specific strain algorithms, or strain derivation (TDI vs speckle-tracking). The American Society of Echocardiography, the European Association of Cardiovascular Imaging, and industry partners have established a task force to identify sources of variability in strain measurements in order to improve standardization [52]. The sample size of the papers was modest and in some cases images were not analyzable due to poor image quality. Some papers did not include data or listed data as figures only. Ganame et al [27,37] and Yagci-Kupeli et al [39] papers used TDI, which led to generally increased strain values in comparison to other articles.
Strain is more sensitive to myocardial changes than traditional echocardiographic measures (LVEF, LV fractional shortening) in both early [27–31] and late-term [44–47,49] follow-up. Although clinical guidelines recommend obtaining strain measurements in patients who are undergoing treatment with anthracyclines in order to identify early myocardial dysfunction, it is unknown to what extent clinical labs are equipped with technical training and stringent image acquisition protocols to ensure accurate and reproducible strain assessment. Further, clinically significant myocardial strain thresholds are needed for CAYA survivors of childhood cancer, and how these thresholds relate to future development of cardiomyopathy require additional investigation. Understanding the natural history is imperative for testing of preventive strategies and treatments.
Acknowledgements
The authors thank Dr. A. Blythe Ryerson for providing additional strain data needed for the review and analysis (Ryerson AB, Border WL, Wasilewski-Masker K, Goodman M, Meacham L, Austin H, Mertens AC. Assessing anthracycline‐treated childhood cancer survivors with advanced stress echocardiography. Pediatric Blood & Cancer 62.3: 502–508 (2015).
This study was supported by pilot funding from the UCLA Jonsson Comprehensive Cancer Center Foundation and the infrastructure afforded by UCLA Clinical and Translational Institute (CTSI Grant #ULTR001881).
Footnotes
Conflict of Interest Statement. The authors report no relevant financial conflict of interest.
REFERENCES
- 1.Lipshultz SE, Cochran TR, Franco VI MT. Treatment-related cardiotoxicity in survivors of childhood cancer. Nat Rev Clin Oncol. 2013;10:697–710. [DOI] [PubMed] [Google Scholar]
- 2.Bijnens B, Cikes M, Butakoff C, Sitges M CF. Myocardial motion and deformation: What does it tell us and how does it relate to function? Fetal Diagn Ther. 2012;32:5–16. [DOI] [PubMed] [Google Scholar]
- 3.Volkova M RR 3rd. Anthracycline cardiotoxicity: prevalence, pathogenesis and treatment. Curr Cardiol Rev. 2011;7:214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Marco A, Cassinelli G AF. The discovery of daunorubicin. Cancer Treat Rep. 1981;65:3–8. [PubMed] [Google Scholar]
- 5.Reulen RC, Winter DL, Frobisher C, Lancashire ER, Stiller CA, Jenney ME, Skinner R, Stevens MC HMBCCSSSG. Long-term cause-specific mortality among survivors of childhood cancer. JAMA. 2010;304:172–9. [DOI] [PubMed] [Google Scholar]
- 6.American Cancer Society. Cancer treatment and survivorship facts and figures. [Internet] 2016. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-treatment-and-survivorship-facts-and-figures/cancer-treatment-and-survivorship-facts-and-figures-2016-2017.pdf. Accessed October 3, 2017.
- 7.Parry C, Kent EE, Mariotto AB, Alfano CM RJ. Cancer survivors: a booming population. Cancer Epidemiol Biomarkers Prev. 2011;20:1996–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeung ST, Yoong C, Spink J, Galbraith A SP. Functional myocardial impairment in children treated with anthracyclines for cancer. Lancet. 1991;337:816–8. [DOI] [PubMed] [Google Scholar]
- 9.Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT, Criscitiello C, Goldhirsch A, Cipolla C RFEGWG. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2012;23:vii155–166. [DOI] [PubMed] [Google Scholar]
- 10.Adams MJ LS. Pathophysiology of anthracycline- and radiation-associated cardiomyopathies: implications for screening and prevention. Pediatr. Blood Cancer. 2005;44:600–6. [DOI] [PubMed] [Google Scholar]
- 11.Institute NC. Cancer therapy evaluation program, common terminology criteria for adverse events version 4.0 (ctcae v4.0). 2010. [Google Scholar]
- 12.Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, Rubino M, Veglia F, Fiorentini C CC. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55:213–20. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P SLP-PG. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryerson AB, Border WL, Wasilewski-Masker K, Goodman M, Meacham L, Austin H, et al. Assessing anthracycline-treated childhood cancer survivors with advanced stress echocardiography. Pediatr. Blood Cancer. United States; 2015;62:502–8. [DOI] [PubMed] [Google Scholar]
- 15.Children’s oncology group. Long-term follow-up guidelines for survivors of childhood, adolescent and young adult cancers. Version 4.0. [Internet] 2013. Available from: http://www.survivorshipguidelines.org. Accessed December 3, 2016.
- 16.Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, Galderisi M, Marwick T, Nagueh SF, Sengupta PP, Sicari R, Smiseth OA, Smulevitz B, Takeuchi M, Thomas JD, Vannan M, Voigt JU ZJ. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr. 2011;24:277–313. [DOI] [PubMed] [Google Scholar]
- 17.Cochran W The Combination of Estimates from Different Experiments. Biometrics. 1954;10:101–29. [Google Scholar]
- 18.Dickersin K BJ. Meta-analysis: state-of-the-science. Epidemiol Rev. 1992;154–76. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ AD. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moon TJ, Miyamoto SD, Younoszai AK, Landeck BF. Left ventricular strain and strain rates are decreased in children with normal fractional shortening after exposure to anthracycline chemotherapy. Cardiol. Young 2014;24:854–65. [DOI] [PubMed] [Google Scholar]
- 21.Park JH, Kim YH, Hyun MC, Kim HS. Cardiac functional evaluation using vector velocity imaging after chemotherapy including anthracyclines in children with cancer. Korean Circ. J. Korea (South); 2009;39:352–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, Nesser HJ, Khandheria B, Narula J SP. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23:351–69. [DOI] [PubMed] [Google Scholar]
- 23.Mele D, Rizzo P, Pollina AV, Fiorencis A FR. Cancer therapy-induced cardiotoxicity: role of ultrasound deformation imaging as an aid to early diagnosis. Ultrasound Med Biol. 2015;41:627–43. [DOI] [PubMed] [Google Scholar]
- 24.Cheung Y, Hong W, Chan GCF, Wong SJ, Ha S. Left ventricular myocardial deformation and mechanical dyssynchrony in children with normal ventricular shortening fraction after anthracycline therapy. Heart. 2010;96:1137–41. [DOI] [PubMed] [Google Scholar]
- 25.Levy PT, Machefsky A, Sanchez AA, Patel MD, Rogal S, Fowler S, Yaeger L, Hardi A, Holland MR, Hamvas A SG. Reference Ranges of Left Ventricular Strain Measures by Two-Dimensional Speckle-Tracking Echocardiography in Children: A Systematic Review and Meta-Analysis. J Am Soc Echocardiogr. 2016;29:209–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yingchoncharoen T, Agarwal S, Popović ZB MT. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr. 2013;26:185–91. [DOI] [PubMed] [Google Scholar]
- 27.Ganame J, Claus P, Eyskens B, Uyttebroeck A, Renard M, D’hooge J, et al. Acute Cardiac Functional and Morphological Changes After Anthracycline Infusions in Children. Am. J. Cardiol 2007;99:974–7. [DOI] [PubMed] [Google Scholar]
- 28.Agha H, Shalaby L, Attia W, Abdelmohsen G, Aziz OA, Rahman MYA. Early Ventricular Dysfunction After Anthracycline Chemotherapy in Children. Pediatr. Cardiol. United States; 2016;37:537–44. [DOI] [PubMed] [Google Scholar]
- 29.Al-Biltagi M, Abd Rab Elrasoul Tolba O, El-Shanshory MR, Abd El-Aziz El-Shitany N, El-Sayed El-Hawary E. Strain Echocardiography in Early Detection of Doxorubicin-Induced Left Ventricular Dysfunction in Children with Acute Lymphoblastic Leukemia. ISRN Pediatr. 2012;2012:870549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Shitany NA, Tolba OA, El-Shanshory MR, El-Hawary EE. Protective effect of carvedilol on adriamycin-induced left ventricular dysfunction in children with acute lymphoblastic leukemia. J. Card. Fail. Elsevier Inc; 2012;18:607–13. [DOI] [PubMed] [Google Scholar]
- 31.Mavinkurve-Groothuis AMC, Marcus KA, Pourier M, Loonen J, Feuth T, Hoogerbrugge PM, et al. Myocardial 2D strain echocardiography and cardiac biomarkers in children during and shortly after anthracycline therapy for acute lymphoblastic leukaemia (ALL): A prospective study. Eur. Hear. J. Cardiovasc. Imaging 2013;14:562–9. [DOI] [PubMed] [Google Scholar]
- 32.Pignatelli RH, Ghazi P, Reddy SCB, Thompson P, Cui Q, Castro J, et al. Abnormal Myocardial Strain Indices in Children Receiving Anthracycline Chemotherapy. Pediatr. Cardiol. 2015;36:1610–6. [DOI] [PubMed] [Google Scholar]
- 33.Poterucha JT, Kutty S, Lindquist RK, Li L EB. Changes in left ventricular longitudinal strain with anthracycline chemotherapy in adolescents precede subsequent decreased left ventricular ejection fraction. J Am Soc Echocardiogr. 2012;25:733–40. [DOI] [PubMed] [Google Scholar]
- 34.Jashari H, Rydberg A, Ibrahimi P, Bajraktari G, Kryeziu L, Jashari F HM. Normal ranges of left ventricular strain in children: a meta-analysis. Cardiovasc. Ultrasound 2015;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marcus KA, Mavinkurve-Groothuis AM, Barends M, van Dijk A, Feuth T, de Korte C KL. Reference values for myocardial two-dimensional strain echocardiography in a healthy pediatric and young adult cohort. J Am Soc Echocardiogr. 2011;24:625–36. [DOI] [PubMed] [Google Scholar]
- 36.Cheung YF, Li SN, Chan GCF, Wong SJ, Ha SY. Left ventricular twisting and untwisting motion in childhood cancer survivors. Echocardiography. 2011;28:738–45. [DOI] [PubMed] [Google Scholar]
- 37.Ganame J, Claus P, Uyttebroeck A, Renard M, D’hooge J, Bijnens B, et al. Myocardial Dysfunction Late After Low-Dose Anthracycline Treatment in Asymptomatic Pediatric Patients. J. Am. Soc. Echocardiogr 2007;20:1351–8. [DOI] [PubMed] [Google Scholar]
- 38.Toro-Salazar OH, Gillan E, O’Loughlin MT, Burke GS, Ferranti J, Stainsby J, et al. Occult cardiotoxicity in childhood cancer survivors exposed to anthracycline therapy. Circ. Cardiovasc. Imaging 2013;6:873–80. [DOI] [PubMed] [Google Scholar]
- 39.Yaǧci-Küpeli B, Varan A, Yorgun H, Kaya B, Büyükpamukçu M. Tissue Doppler and myocardial deformation imaging to detect myocardial dysfunction in pediatric cancer patients treated with high doses of anthracyclines. Asia. Pac. J. Clin. Oncol 2012;8:368–74. [DOI] [PubMed] [Google Scholar]
- 40.Yu HK, Yu W, Cheuk DKL, Wong SJ, Chan GCF, Cheung YF. New three-dimensional speckle-tracking echocardiography identifies global impairment of left ventricular mechanics with a high sensitivity in childhood cancer survivors. J. Am. Soc. Echocardiogr. Elsevier Inc; 2013;26:846–52. [DOI] [PubMed] [Google Scholar]
- 41.Yu W, Li S, Chan GCF, Ha S, Wong SJ, Cheung Y. Transmural strain and rotation gradient in survivors of childhood cancers. Eur. Hear. J. Cardiovasc. Imaging. England; 2013;14:175–82. [DOI] [PubMed] [Google Scholar]
- 42.Barbosa MM, Beleigoli AM, de Fatima Diniz M, Freire CV, Ribeiro AL NM. Strain imaging in morbid obesity: insights into subclinical ventricular dysfunction. Clin Cardiol. 2011;34:288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kibar AE, Pac FA, Ece İ, Oflaz MB, Ballı Ş, Bas VN AZ. Effect of obesity on left ventricular longitudinal myocardial strain by speckle tracking echocardiography in children and adolescents. Balk. Med J 2015;32:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheung Y fai, Yu W, Cheuk DK leung, Cheng FW tsoi, Yang JY kwan, Yau JP wa, et al. Plasma High Sensitivity Troponin T Levels in Adult Survivors of Childhood Leukaemias: Determinants and Associations with Cardiac Function. PLoS One. 2013;8:e77063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dietz AC, Sivanandam S, Konety S, Kaufman CL, Gage RM, Kelly AS, Neglia JP M DA. Evaluation of traditional and novel measures of cardiac function to detect anthracycline-induced cardiotoxicity in survivors of childhood cancer. J Cancer Surviv. 2014;8:183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mavinkurve-Groothuis AMC, Groot-Loonen J, Marcus KA, Bellersen L, Feuth T, Bökkerink JPM, et al. Myocardial strain and strain rate in monitoring subclinical heart failure in asymptomatic long-term survivors of childhood cancer. Ultrasound Med Biol. 2010;36:1783–91. [DOI] [PubMed] [Google Scholar]
- 47.Armstrong GT, Joshi VM, Ness KK, Marwick TH, Zhang N, Srivastava D, Griffin BP, Grimm RA, Thomas J, Phelan D, Collier P, Krull KR, Mulrooney DA, Green DM, Hudson MM, Robison LL PJ. Comprehensive Echocardiographic Detection of Treatment-Related Cardiac Dysfunction in Adult Survivors of Childhood Cancer: Results From the St. Jude Lifetime Cohort Study. J Am Coll Cardiol. 2015;65:2511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu AF, Raikhelkar J, Zabor EC, Tonorezos ES, Moskowitz CS, Adsuar R, Mara E, Huie K, Oeffinger KC, Steingart RM LJ. Two-Dimensional Speckle Tracking Echocardiography Detects Subclinical Left Ventricular Systolic Dysfunction among Adult Survivors of Childhood, Adolescent, and Young Adult Cancer. Biomed Res Int. 2016;2016:9363951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christiansen JR, Kanellopoulos A, Lund MB, Massey R, Dalen H, Kiserud CE, et al. Impaired exercise capacity and left ventricular function in long-term adult survivors of childhood acute lymphoblastic leukemia. Pediatr. Blood Cancer. United States; 2015;62:1437–43. [DOI] [PubMed] [Google Scholar]
- 50.Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick THJ. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63:2751–68. [DOI] [PubMed] [Google Scholar]
- 51.Smedsrud MK 1, e E, Gjesdal O, Svennevig JL, Andersen K, Ihlen HET. Detection of left ventricular dysfunction by global longitudinal systolic strain in patients with chronic aortic regurgitation. J Am Soc Echocardiogr. 2011;24:1253–9. [DOI] [PubMed] [Google Scholar]
- 52.Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, Pedri S, Ito Y, Abe Y, Metz S, Song JH, Hamilton J, Sengupta PP, Kolias TJ, d’Hooge J, Aurigemma GP, Thomas JD BL. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Hear. J Cardiovasc Imaging. 2015;16:1–11. [DOI] [PubMed] [Google Scholar]
- 53.Moher D, Liberati A, Tetzlaff JADPG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6. [DOI] [PMC free article] [PubMed] [Google Scholar]