Abstract
Background
The EC/IC Bypass Study Group found no benefit of extracranial to intracranial (EC/IC) bypass surgery over medical therapy in patients with symptomatic carotid artery occlusion (sCAO). However, the study was criticised for many reasons and the real effect of this treatment is still not known conclusively.
Objectives
To determine whether bypass surgery plus medical care is superior to medical care alone in patients with sCAO.
Search methods
We searched the Cochrane Stroke Group Trials Register (last searched June 2009). In addition, we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 2, 2006), MEDLINE (1966 to June 2009) and EMBASE (1980 to June 2009). We also searched ongoing trials and research registers, checked reference lists of relevant articles, and contacted colleagues, trial authors and researchers.
Selection criteria
Randomised controlled trials (RCT) and non‐random studies of EC/IC bypass surgery plus best medical treatment compared with best medical treatment alone to prevent subsequent stroke, improve cerebral haemodynamics and reduce dependency after stroke.
Data collection and analysis
Two review authors independently selected studies for inclusion, and extracted data items on the number of outcome events onto a data extraction form. We only analysed secondary outcomes if the study provided information on at least one primary outcome. We also used intention‐to‐treat analysis where possible.
Main results
We included 21 trials, including two RCTs, involving 2591 patients. For all endpoints, no benefit of EC/IC bypass surgery was shown either in the RCTs (any death: odds ratio (OR) 0.81, 95% confidence interval (CI) 0.62 to 1.05, P = 0.11; stroke: OR 0.99, 95% CI 0.79 to 1.23, P = 0.91; death and dependency: OR 0.94, 95% CI 0.74 to 1.21, P = 0.64), or in the non‐RCTs (any death: OR 1.00, 95% CI 0.62 to 1.62, P = 0.99; stroke: OR 0.80, 95% CI 0.54 to 1.18, P = 0.25; death and dependency: OR 0.80, 95% CI 0.50 to 1.29, P = 0.37).
Authors' conclusions
EC/IC bypass surgery in patients with sCAO disease was neither superior nor inferior to medical care alone. However, most studies included patients irrespective of their cerebral haemodynamics. Participation in an ongoing RCT, which is restricted to patients with impaired haemodynamics, is recommended as these patients might benefit from bypass surgery.
Plain language summary
Extracranial‐intracranial arterial bypass surgery for occlusive carotid artery disease
Patients with symptomatic occlusion (obstruction) of the carotid artery have a high risk of subsequent stroke. Anticoagulant treatment and antiplatelet agents are not very effective in these patients and a surgical procedure known as extracranial‐intracranial (EC/IC) arterial bypass surgery has been a treatment option. In this review, we included 21 trials (two randomised controlled trials and 19 non‐random studies, with a total of 2591 patients). We found that EC/IC bypass surgery in patients with symptomatic carotid artery occlusive disease was no better or worse than medical care alone. A multi‐centre trial comparing EC/IC bypass surgery with best medical treatment in patients with both a high risk of stroke and haemodynamic compromise (impaired blood flow) is underway, and aims to discover whether EC/IC bypass surgery is beneficial in this specific group of patients.
Background
Up to 15% of patients presenting with anterior circulation ischaemia have complete occlusion of the ipsilateral carotid artery (Bozzao 1989; Pessin 1977; Thiele 1980). Their annual risk for subsequent stroke is 5% to 7% (Grubb 1986; Klijn 1997), and the risk of stroke ipsilateral to the occluded carotid artery is 2% to 6% per year (Hankey 1991; Klijn 1997). The efficacy of anticoagulant treatment or antiplatelet agents in patients with symptomatic occlusion of the carotid artery is small (Klijn 1997). Cerebral revascularisation of the anterior or posterior circulation by extracranial to intracranial (EC/IC) anastomosis is thought to be a therapeutic option for preventing subsequent transient ischaemic attack or stroke in patients with occlusive carotid disease.
EC/IC bypass surgery is an operative procedure which most commonly involves the anastomosis of the superficial temporal artery to the middle cerebral artery. Results of the first EC/IC bypass were published in 1967 (Donaghy 1967; Yasargil 1969) and it became a widespread method in the next decade. In 1985, the EC/IC Bypass Study Group showed no benefit of EC/IC bypass surgery over medical therapy in patients with symptomatic carotid occlusion (EC/IC Bypass Study 1985). However, the study was criticised for including all patients with occlusion of the carotid artery irrespective of their cerebral haemodynamics. No stratification was done to separate patients with embolic stroke but sufficient intracranial haemodynamics from those with ongoing haemodynamic compromise. Thus, the negative result of the EC/IC bypass study does not necessarily mean that EC/IC bypass is not beneficial for some patients with substantial haemodynamic compromise due to occlusion of the carotid artery (Ausman 1986; Day 1986; Sundt 1987; Vorstrup 1992).
Haemodynamic compromise of ipsilateral artery occlusion is divided into three stages (Derdeyn 2002; Powers 1987; Powers 1991):
Stage 0: normal cerebral haemodynamics;
Stage 1: autoregulatory vasodilation;
Stage 2: autoregulatory failure (increased oxygen extraction fraction (OEF)), also termed 'misery perfusion' (Baron 1981).
Several studies have been carried out to evaluate whether the subgroup of patients with haemodynamic compromise due to occlusion of the carotid artery might benefit from bypass surgery. In addition, more recently there have been two other randomised controlled trials (RCTs) of EC/IC bypass (COSS; JET 2006). With these considerations in mind, we tried to evaluate whether EC/IC bypass surgery plus best medical treatment compared with best medical treatment alone prevents subsequent stroke, improves cerebral haemodynamics and reduces dependency after stroke in all eligible patients for EC/IC bypass or only in patients with impaired haemodynamics.
Objectives
The objective was to determine whether bypass surgery and medical care in patients with a symptomatic carotid artery occlusion was superior to medical care alone, both in all patients and in the subgroup of patients with haemodynamic compromise.
A further purpose of this review was to determine to what degree the intervention resulted in the correction of haemodynamic compromise in the affected hemisphere.
Finally we aimed to assess safety: death (all causes, vascular, non‐vascular) and stroke (all); intracranial haemorrhage; major extracranial haemorrhage; myocardial infarction; non‐vascular complication of surgery; and infection.
Methods
Criteria for considering studies for this review
Types of studies
The review has two parts, Part 1 and Part 2, each with two divisions, labelled (a) and (b). Division (a) included all patients undergoing EC/IC bypass surgery without measurement of cerebral haemodynamics, while division (b) assessed only those patients with impaired haemodynamics measured by positron emission tomography (PET), single photon emission computed tomography (SPECT), perfusion magnetic resonance (PMR) imaging, and ultrasound.
Part 1
Only randomised controlled trials (RCTs) of EC/IC bypass surgery plus best medical treatment compared with best medical treatment alone to prevent subsequent stroke, improve cerebral haemodynamics and reduce dependency after stroke, including:
(a) all patients without measurement of haemodynamic status before the intervention;
(b) only patients with impaired haemodynamics before the intervention measured using a method mentioned above.
Part 2
All studies (except RCTs) of EC/IC bypass surgery plus best medical treatment compared with best medical treatment alone to prevent subsequent stroke, improve cerebral haemodynamics and reduce dependency after stroke, including:
(a) all patients without measurement of haemodynamic status before the intervention;
(b) only patients with impaired haemodynamics before the intervention measured using a method mentioned above.
Types of participants
Patients with symptomatic (transient ischaemic attack or stroke) occlusion of internal carotid arteries demonstrated by angiography and less than 50% stenosis of the contralateral internal carotid artery and, where available, measured haemodynamic compromise identified by PET, SPECT, PMR imaging and ultrasound. Patients with transient ischaemic monocular blindness were not eligible unless hemispheric symptoms also were present. Patients with asymptomatic occlusion of the internal carotid artery were not eligible because of the low risk of subsequent ischaemic stroke (Powers 2000).
Patients who had undergone thrombendarterectomy of the contralateral internal carotid artery prior to EC/IC bypass were eligible. We excluded all studies including patients with non‐atherosclerotic conditions causing or likely to cause cerebral ischaemia (including carotid dissection, fibromuscular dysplasia, Moyamoya disease, arteritis and other vasculopathy likely to cause cerebral events) from the analysis. If this information was not available, we only used the published data.
Types of interventions
We included any surgical bypass procedure for the treatment of patients with radiologically demonstrated unilateral occluded internal carotid artery, irrespective of the approach and type of graft employed. The bypass patients as well as the control patients should have received best medical treatment for preventing stroke.
Types of outcome measures
Primary outcomes
Death from all causes.
Any stroke during the follow‐up period. This combined outcome included ischaemic strokes, intracranial haemorrhage, or stroke of unknown aetiology.
Death or dependency at the end of follow up. This composite outcome included all patients who qualified either for death or dependency (handicap) which was defined according to the modified Rankin Scale (mRS): independency (mRS 0 to 2) was distinguished from dependency (3 to 5). If the Glasgow Outcome Scale (GOS) was used, we considered good recovery and moderate disability as surrogates for independence, and considered severe disability and persistent vegetative state as dependence. When mRS values were not available, we defined independence as recovery (that is, patients are able to look after their own affairs without assistance) or return to work. In cases of 'deterioration', 'worsened', 'fair' or 'poor' outcome, we assumed dependency as 'partial improvement', 'no improvement', 'no recovery', 'inability to walk without assistance', or 'requiring some help for bodily activities of daily living'. This algorithm was also used for the Cochrane Review of carotid artery dissection (Lyrer 2003).
Secondary outcomes
-
Vascular death, which we defined as death caused by:
stroke or a complication of stroke (e.g. brain herniation, status epilepticus);
coronary artery disease or a complication of it (e.g. myocardial infarction, congestive heart failure, arrhythmia);
sudden death;
pulmonary embolism;
peripheral vascular disease;
haemorrhage (intracranial or extracranial); or
other vascular causes (for example, rupture of aneurysm, dissection) including 'vascular death' mentioned without specification in the publications.
Serious vascular events (during follow‐up period) or vascular death. This composite outcome included all patients who qualified for outcome events 1, 3, 4 or 5.
Myocardial infarction. All patients with fatal or non‐fatal myocardial infarction. Patients who died of occlusive coronary artery disease as reported by autopsy, are classified as 'fatal myocardial infarction'.
Ischaemic stroke (during follow‐up period). We defined ischaemic stroke as any neurological deficit due to cerebral ischaemia lasting longer than 24 hours, and showing no evidence of any other underlying pathology (e.g. haemorrhage, tumour).
Intracranial haemorrhage. Intracranial haemorrhage included any subarachnoid haemorrhage, subdural haemorrhage, epidural haematoma, or parenchymatous intracerebral haemorrhage, as confirmed by neuroradiological investigations or by autopsy. We did not consider haemorrhagic transformation of an ischaemic infarction as intracranial haemorrhage.
Major extracranial haemorrhage. We took the definition of a major extracranial haemorrhage from the original publication. If no definition was given, we defined major as a fatal bleeding, or one requiring surgery, transfusion or (prolonged) hospitalisation. If a haemorrhage was declared 'serious' or 'severe' in the publication, we considered it a major haemorrhage for the purpose of this review.
Local haemorrhage requiring surgery. All patients with local haematoma requiring surgical exploration, haematoma evacuation, or the application of a suture, a patch, or a bypass met the criteria for this outcome event.
Transient ischaemic attack or amaurosis fugax.
Normalisation of cerebral haemodynamics, using PET, computerised tomography (CT), SPECT or ultrasound criteria, which we defined as a normalisation of hemispheric oxygen extraction fraction (OEF) ratios or decreased OEF ratio after EC/IC‐bypass surgery.
Non‐vascular complications of surgery (for example, wound infection, limited function of the EC/IC bypass).
For all outcome events, we only included patients once. If one patient experienced more than one non‐fatal event, we only recorded the first one.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module.
We searched the Cochrane Stroke Group Trials Register, which was last searched by the Managing Editor in June 2009. In addition, we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 2, 2006), MEDLINE (1966 to June 2009) (Appendix 1) and EMBASE (1980 to June 2009) (Appendix 2). In an effort to identify further published, unpublished and ongoing studies we searched reference lists of relevant papers, contacted colleagues, trial authors and researchers, and searched the following clinical trials and research registers:
Current Controlled Trials (http://www.controlled‐trials.com/);
National Institutes of Health ClinicalTrials.gov (http://clinicaltrials.gov/);
Stroke Trials Registry (http://www.strokecenter.org/trials/).
Data collection and analysis
Two review authors (FF, SE) independently selected studies for inclusion. The same two review authors independently extracted data items on the number of outcome events onto a data extraction form. In cases of disagreement, the review authors reached consensus by discussion.
The data that we analysed are listed in the 'Types of outcome measures' section. We only analysed secondary outcomes if the study provided information on at least one primary outcome. We used intention‐to‐treat analysis. For the EC/IC bypass study, we also included the 118 patients who did not meet the inclusion criteria and, thus, were excluded from the analysis published in 1985 (EC/IC Bypass Study 1985).
Statistical methods
We calculated a weighted estimate of the odds for each outcome event across studies using the Peto odds ratio (OR) method.
Results
Description of studies
See the 'Characteristics of included studies' and the' Characteristics of excluded studies' sections.
We have identified a total of two randomised controlled trials (RCTs) comparing EC/IC bypass and best medical treatment with medical care alone and fulfilling the inclusion criteria (EC/IC Bypass Study 1985; JET 2006). One RCT is still in progress (COSS). For Part 1a, we found a total of 1691 patients, whereas for Part 1b we only included data for 195 patients from the JET study (JET 2006). Length of follow up in the EC/IC bypass study was 55.8 months, and in the JET study was 25 months. We identified 118 drop‐outs in the EC/IC bypass study, whereas in the JET study 10 patients dropped out. Furthermore, detailed patient data were only given as percentages in the EC/IC bypass study, thus we had to translate these percentages into patient numbers, a procedure which is only approximate (our efforts to get absolute numbers for all necessary outcome variables by contacting the investigators were unsuccessful). In the EC/IC bypass study, only 78% of all randomised patients had a brain CT. In Part 1b, haemodynamic compromise was determined by measuring quantitatively cerebral blood flow using PET, SPECT and the Xenon inhalation method in the JET study.
For Part 2a, we identified 19 non‐randomised trials (900 patients), which reported at least one primary outcome variable. Part 2b included three studies, involving 65 patients. The methods of measuring haemodynamic compromise as a selection criteria for EC/IC bypass varied widely: PET study (Ishikawa 1992), acetazolamide test (Karnik 1992) and Xenon/CT cerebral blood flow measurements (Yonas 1996).
Risk of bias in included studies
See 'Characteristics of included studies' section.
Quality assessment of trials
We obtained details regarding blinding, randomisation (generation and concealment of randomisation sequence) and number of randomised patients, as well as the number of drop‐outs, withdrawals, cross‐over treatments and those lost to follow up. If information was missing, we tried to contact the corresponding authors. All three review authors independently performed quality assessment and, if there was disagreement, reached consensus by discussion.
Effects of interventions
Part 1a
Primary outcomes
Death from all causes
We obtained data from two included trials for 1691 participants. The EC/IC bypass study had a trend towards fewer fatal outcome events in the group who had EC/IC bypass surgery than in the control group (EC/IC Bypass Study 1985). In the JET study, death from all causes as an outcome event was uncommon and similar in the EC/IC bypass group (two events) to the group with best medical treatment only (one event) (JET 2006). Across both studies we observed no significant difference between treatment and control groups regarding death from all causes (OR 0.81, 95% CI 0.62 to 1.05, P = 0.11) (Analysis 1.1).
1.1. Analysis.
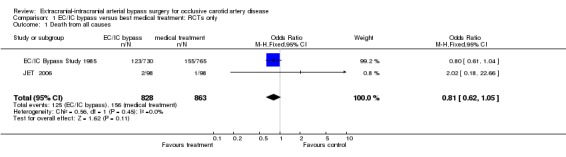
Comparison 1 EC/IC bypass versus best medical treatment: RCTs only, Outcome 1 Death from all causes.
Any stroke during follow up
Analysis of 'any stroke during follow up' was based on two trials of 1691 participants. The OR of 0.99 (95% CI 0.79 to 1.23, P = 0.91) indicated neither harm nor benefit of EC/IC bypass for any stroke during follow up (Analysis 1.2).
1.2. Analysis.
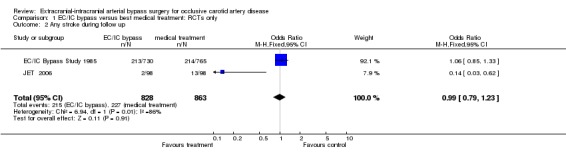
Comparison 1 EC/IC bypass versus best medical treatment: RCTs only, Outcome 2 Any stroke during follow up.
Death or dependency
Only the EC/IC bypass study reported on death or dependency (1377 participants). We observed no significant difference between treatment and control groups for 'death or dependency' (OR 0.94, 95% CI 0.74 to 1.21) (Analysis 1.3).
1.3. Analysis.
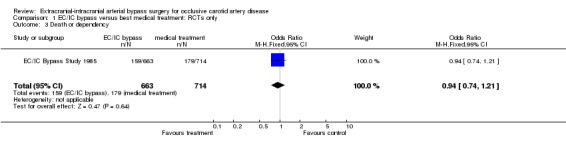
Comparison 1 EC/IC bypass versus best medical treatment: RCTs only, Outcome 3 Death or dependency.
Secondary outcomes
Vascular death
One trial (EC/IC Bypass Study 1985) reported data enabling us to analyse vascular death at the end of the follow‐up period (1377 participants). Data analysis revealed no significant difference between patients who underwent EC/IC bypass surgery and the control group regarding vascular death (OR 0.96, 95% CI 0.71 to 1.29, P = 0.77) (Analysis 1.4).
1.4. Analysis.
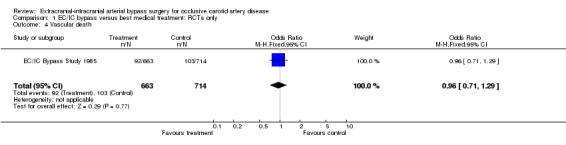
Comparison 1 EC/IC bypass versus best medical treatment: RCTs only, Outcome 4 Vascular death.
Stroke, serious vascular events or vascular death
Both RCTs provided data enabling the analysis of this composite endpoint at the end of the follow‐up period (1573 participants). Across both studies, the OR of 0.68 (95% CI 0.51 to 0.91) indicated a statistically significant beneficial effect in favour of surgery in reducing events at the end of the follow‐up period (P = 0.009) (Analysis 1.5).
1.5. Analysis.
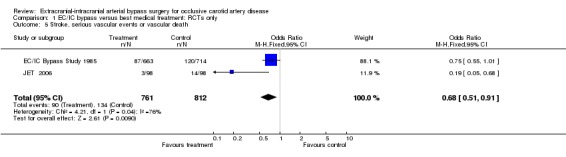
Comparison 1 EC/IC bypass versus best medical treatment: RCTs only, Outcome 5 Stroke, serious vascular events or vascular death.
Myocardial infarction
Both RCTs reported myocardial infarction (1522 patients). No significant difference between the treatment groups was shown (OR 0.78, 95% CI 0.46 to 1.32, P = 0.35) (Analysis 1.6).
1.6. Analysis.
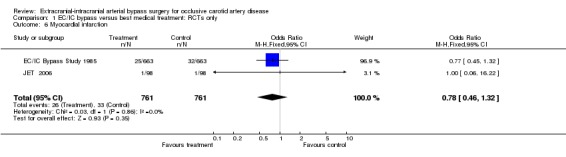
Comparison 1 EC/IC bypass versus best medical treatment: RCTs only, Outcome 6 Myocardial infarction.
Ischaemic stroke
Both RCTs reported ischaemic stroke at the end of the follow‐up period (1573 participants). No statistically significant difference between the surgical group and the group with best medical treatment was shown (OR 0.69, 95% CI 0.44 to 1.08) (Analysis 1.7).
1.7. Analysis.
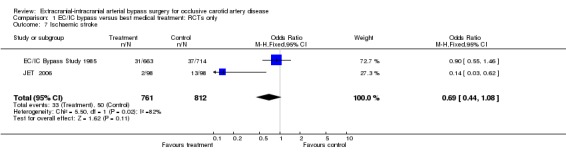
Comparison 1 EC/IC bypass versus best medical treatment: RCTs only, Outcome 7 Ischaemic stroke.
Intracranial haemorrhage
Neither RCT reported on the occurrence of intracranial haemorrhage after EC/IC bypass surgery.
Major extracranial haemorrhage
Neither RCT collected data about major extracranial haemorrhage.
Local haemorrhage requiring surgery
Neither trial reported any data for this outcome event.
Transient ischaemic attack or amaurosis fugax
Neither trial reported on the occurrence of transient ischaemic attack or amaurosis fugax after EC/IC bypass surgery or best medical treatment alone.
Normalisation of cerebral haemodynamics
Normalisation of cerebral haemodynamics was not measured in either trial.
Non‐stroke complication of surgery
No data about non‐stroke complication of surgery are available.
Part 1b
Only the JET study randomised patients exclusively with haemodynamic compromise for EC/IC bypass surgery (JET 2006), thus it was not possible for us to conduct a meaningful meta‐analysis.
Part 2a
Primary outcomes
Death from all causes
We obtained data from 19 trials (900 participants). Only 11 studies reported on such events. Data analysis indicated neither benefit nor harm of EC/IC bypass surgery (OR 1.00, 95% CI 0.62 to 1.62) (Analysis 2.1).
2.1. Analysis.
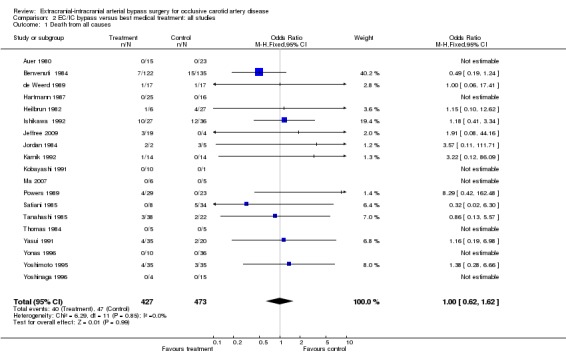
Comparison 2 EC/IC bypass versus best medical treatment: all studies, Outcome 1 Death from all causes.
Any stroke during follow up
Analysis of 'any stroke during follow up' was based on 18 trials (881 participants). We did not observe any significant difference between the treatment and control groups regarding stroke (OR 0.80, 95% CI 0.54 to 1.18) (Analysis 2.2).
2.2. Analysis.
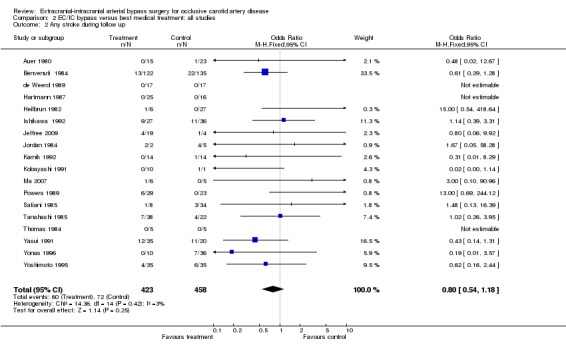
Comparison 2 EC/IC bypass versus best medical treatment: all studies, Outcome 2 Any stroke during follow up.
Death or dependency
Eight trials reported on death or dependency (346 participants). We did not observe any significant difference between the treatment and control groups regarding death or dependency (OR 0.80, 95% CI 0.50 to 1.29) (Analysis 2.3).
2.3. Analysis.
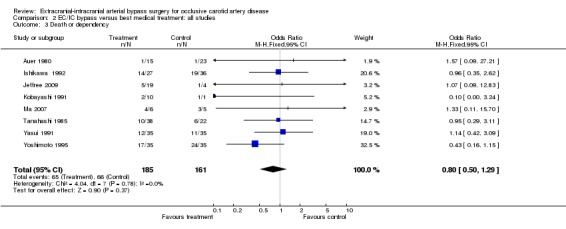
Comparison 2 EC/IC bypass versus best medical treatment: all studies, Outcome 3 Death or dependency.
Secondary outcomes
Vascular death
Analysis of 'vascular death' was based on 19 trials (900 participants) and indicated no significant benefit of EC/IC bypass surgery compared with the group undergoing best medical treatment only (OR 0.95, 95% CI 0.56 to 1.63) (Analysis 2.4).
2.4. Analysis.
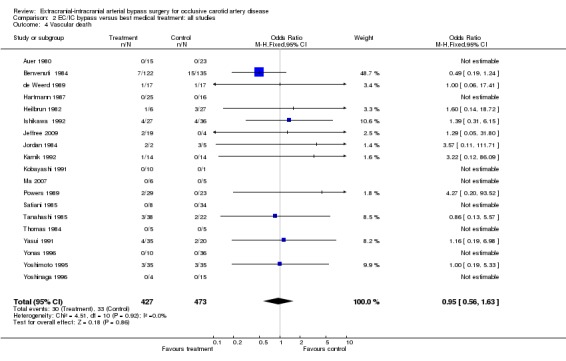
Comparison 2 EC/IC bypass versus best medical treatment: all studies, Outcome 4 Vascular death.
Stroke, serious vascular events or vascular death
Thirteen trials provided data enabling the analysis of this composite endpoint at the end of the follow‐up period (673 participants). Across all studies a trend was indicated in favour of EC/IC bypass surgery (OR 0.69, 95% CI 0.45 to 1.04) (Analysis 2.5).
2.5. Analysis.
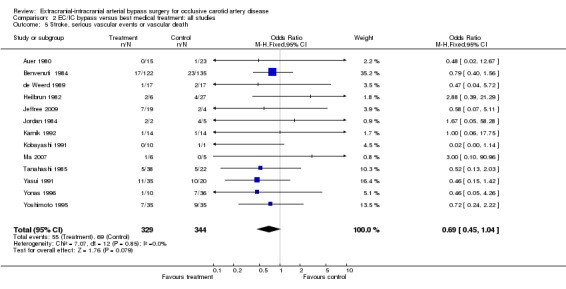
Comparison 2 EC/IC bypass versus best medical treatment: all studies, Outcome 5 Stroke, serious vascular events or vascular death.
Myocardial infarction
Two studies reported on myocardial infarction (79 participants). No statistically significant difference between both treatment groups was shown (OR 2.67, 95% CI 0.41 to 17.60). Furthermore, due to the small number of events (N = 3), meaningful interpretation was not possible (Analysis 2.6).
2.6. Analysis.
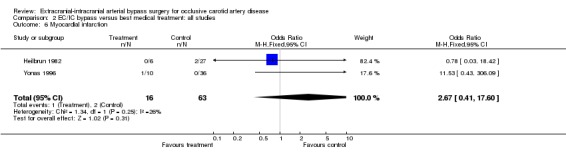
Comparison 2 EC/IC bypass versus best medical treatment: all studies, Outcome 6 Myocardial infarction.
Ischaemic stroke
Thirteen trials reported ischaemic stroke at the end of the follow‐up period (640 participants). The OR was 0.72 in favour of EC/IC bypass surgery, but the 95% CI of 0.44 to 1.18 indicated no statistical significance (Analysis 2.7).
2.7. Analysis.
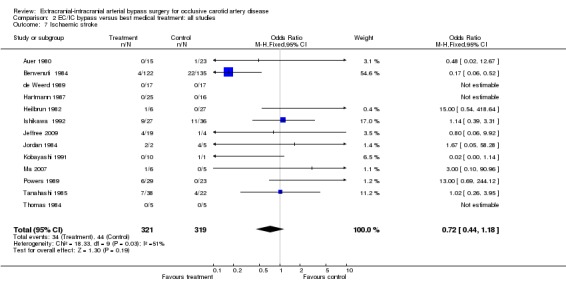
Comparison 2 EC/IC bypass versus best medical treatment: all studies, Outcome 7 Ischaemic stroke.
Intracranial haemorrhage
Data on this outcome event were sparse; only four trials provided data (361 participants). There was no statistically significant difference between the treated and the control group (OR 1.14, 95% CI 0.44 to 2.93) (Analysis 2.8).
2.8. Analysis.
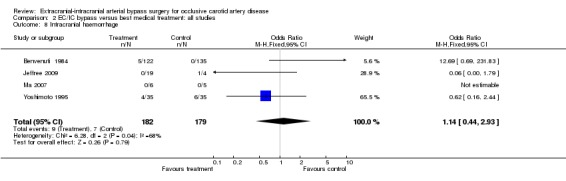
Comparison 2 EC/IC bypass versus best medical treatment: all studies, Outcome 8 Intracranial haemorrhage.
Major extracranial haemorrhage
No trials reported data for this outcome.
Local haemorrhage requiring surgery
No trials contained data for this outcome event.
Transient ischaemic attack or amaurosis fugax
For the outcome 'transient ischaemic attack or amaurosis fugax' at the end of the follow‐up period, we obtained data from 11 trials (524 participants). Across trials a statistically significant beneficial effect in favour of EC/IC bypass surgery in reducing transient ischaemic attack or amaurosis fugax was indicated (OR 0.34, 95% CI 0.16 to 0.69, P = 0.003) (Analysis 2.9).
2.9. Analysis.
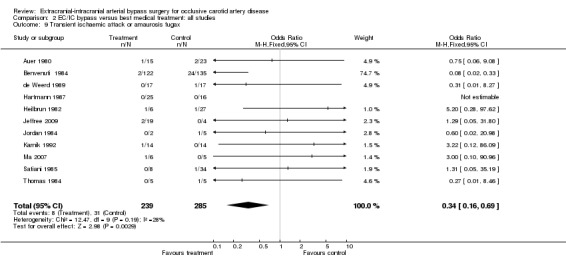
Comparison 2 EC/IC bypass versus best medical treatment: all studies, Outcome 9 Transient ischaemic attack or amaurosis fugax.
Normalisation of cerebral haemodynamics
Analysis was based on three trials (56 participants) showing a lack of normalisation of cerebral haemodynamics after EC/IC bypass surgery (OR 6.63, 95% CI 1.85 to 23.78) (Analysis 2.10).
2.10. Analysis.
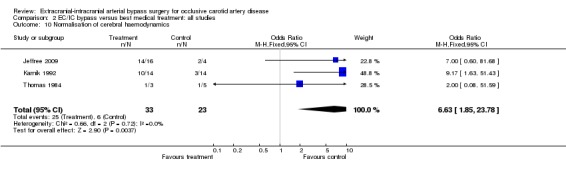
Comparison 2 EC/IC bypass versus best medical treatment: all studies, Outcome 10 Normalisation of cerebral haemodynamics.
Non‐stroke complication of surgery
No data were available for this outcome.
Part 2b
Three studies formed a subgroup of patients with haemodynamic compromise as a selection criterion for EC/IC bypass surgery. We only found data for the endpoints 'death from all causes' and 'any stroke during follow up' (Analysis 3.1; Analysis 3.2). No statistically significant difference between treatment groups was shown for these endpoints.
3.1. Analysis.
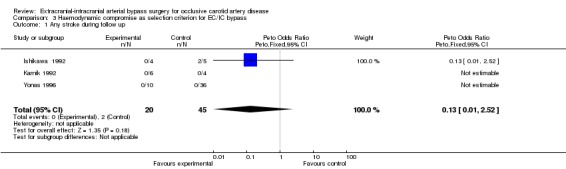
Comparison 3 Haemodynamic compromise as selection criterion for EC/IC bypass, Outcome 1 Any stroke during follow up.
3.2. Analysis.
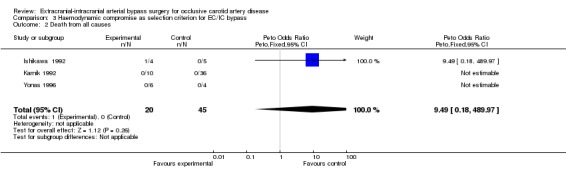
Comparison 3 Haemodynamic compromise as selection criterion for EC/IC bypass, Outcome 2 Death from all causes.
Discussion
We identified 21 trials (including two RCTs) involving 2591 patients. For all primary endpoints, neither benefit nor harm from EC/IC bypass surgery could be shown either in the RCTs ('any death': OR 0.81, 95% CI 0.62 to 1.05, P = 0.11; 'stroke': OR 0.99, 95% CI 0.79 to 1.23, P = 0.91; 'death and dependency': OR 0.94, 95% CI 0.74 to 1.21, P = 0.64), or in the non‐RCTs ('any death': OR 1.00, 95% CI 0.62 to 1.62, P = 0.99; 'stroke': OR 0.80, 95% CI 0.54 to 1.18, P = 0.25; 'death and dependency': OR 0.80, 95% CI 0.50 to 1.29, P = 0.37). One possible explanation for these neutral results was the inclusion in most studies of patients with occlusion of the carotid artery irrespective of their cerebral haemodynamics. No stratification was done to separate patients with embolic stroke but sufficient intracranial haemodynamics from those with ongoing haemodynamic compromise. After the data analysis of studies selecting patients for EC/IC bypass without measurement of haemodynamic compromise, it still remained unclear whether EC/IC bypass was beneficial or not for some patients with substantial haemodynamic compromise due to occlusion of the carotid artery (Ausman 1986; Day 1986; Sundt 1987; Vorstrup 1992). In order to address this question, we carried out a sub‐analysis of the randomised controlled trials (RCTs) (Part 1b) and of the non‐RCTs (Part 2b). For the RCTs, only the JET study had randomised patients with haemodynamic compromise for EC/IC bypass surgery (JET 2006). However, this study did not compare surgically treated patients showing haemodynamic compromise with those with normal or almost normal haemodynamics. Of the non‐RCTs, only three studies qualified for Part 2b. However, there were very few endpoints, preventing meaningful analysis. In summary, neither the RCTs nor the non‐RCTs showed a benefit of EC/IC bypass, probably because haemodynamic compromise was not taken into account in most cases.
The findings about normalisation of impaired haemodynamics across the non‐RCTs confirms that EC/IC bypass is effective in restoring normal haemodynamics (Yonas 1996). However, whether this surrogate marker translates into a clinical benefit is unproven. The fact that the JET trial (JET 2006) failed to show a clinical benefit for the primary outcomes ('death from all causes' or 'any stroke') prompts scepticism as to whether the criteria 'impaired haemodynamics' is clinically important enough to predict a potentially beneficial effect of EC/IC bypass surgery. In addition, the best method of determining the presence or absence of impaired haemodynamics remains to be established and the variability between methods used to determine haemodynamic compromise has yet to be defined. The ongoing COSS trial, which uses a more sophisticated measure of impaired haemodynamics, may give clearer answers (COSS).
As a limitation, only two RCTs (EC/IC Bypass Study 1985; JET 2006) have been identified for this meta‐analysis. Furthermore, the inclusion criteria of both RCTs differ, thus a meta‐analysis across both studies could produce misleading conclusions. Potential benefit or harm cannot be excluded definitively. For the non‐RCTs, the most important limitation is the non‐randomised treatment allocation, which involves a high risk of bias. In addition, the majority of studies available for this meta‐analysis were single group reports of small numbers of participants without sub‐stratification by haemodynamic compromise. In the studies reported, only 255 of 2591 patients (9.8%) had pre‐operative assessments of cerebral haemodynamics, while 2336 of 2591 patients (90.2%) did not. Even fewer data were available comparing pre and post‐operative measurements with clinical outcome parameters. Overall the quality of most studies was poor.
Authors' conclusions
Implications for practice.
EC/IC‐bypass surgery in patients with a symptomatic carotid artery occlusive disease was neither superior nor inferior to medical care alone. Thus, the role of EC/IC bypass surgery in symptomatic carotid disease remains undetermined. However, most studies included patients irrespective of their cerebral haemodynamics. Patients currently being submitted for EC/IC bypass procedures need protocol‐driven assessment delivered by multi‐disciplinary teams. Participation in an ongoing randomised controlled trial (RCT), which is restricted to patients with impaired haemodynamics, is recommended as these patients might benefit from bypass surgery.
Implications for research.
The optimum bypass has yet to be determined and whether a branch of the superficial temporal artery is sufficient in all cases to reverse the degree of measured haemodynamic compromise and provide a measurable clinical benefit is unknown. In addition, establishing a reliable, widely accessible and cost‐effective method of assessing impaired cerebral haemodynamics is of paramount importance.
The data suggest the necessity of a multicentre, prospective RCT comparing EC/IC bypass with the best medical treatment in a selected subgroup of patients at high risk of stroke and with identified haemodynamic compromise. One such study is still ongoing; it remains to be seen whether measuring haemodynamic compromise will help to determine whether patients with symptomatic carotid artery occlusive disease benefit from EC/IC bypass surgery. Establishing the safety, efficacy and benefit of surgical bypass can only be established by the future use of multicentre RCTs.
History
Protocol first published: Issue 2, 2006 Review first published: Issue 2, 2010
| Date | Event | Description |
|---|---|---|
| 22 September 2008 | Amended | Converted to new review format. |
Acknowledgements
We wish to thank Hazel Fraser and Brenda Thomas for searching and providing us with lists of the relevant trials from the Cochrane Stroke Group Trials Register. We thank Graeme Hankey, Kameshwar Prasad, Brenda Thomas and David Porter for their helpful comments on an earlier version of this manuscript.
Appendices
Appendix 1. MEDLINE search strategy
The following search strategy was used for MEDLINE (Ovid) and was modified for the other databases.
1. cerebrovascular disorders/ or basal ganglia cerebrovascular disease/ or exp brain ischemia/ or carotid artery diseases/ or carotid artery thrombosis/ or carotid stenosis/ or cerebrovascular accident/ or exp brain infarction/ or exp hypoxia‐ischemia, brain/ or exp intracranial arterial diseases/ or cerebral arterial diseases/ or exp "intracranial embolism and thrombosis"/ or vasospasm, intracranial/ 2. (stroke$ or cva).tw. 3. ((cerebr$ or brain$ or carotid or cerebellar or intracranial or vertebrobasilar or MCA) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or occlud$ or arteriosclero$ or stenosis or steno‐occlus$ or obstruct$)).tw. 4. transient isch$.tw. 5. 1 or 2 or 3 or 4 6. cerebral revascularization/ 7. exp cerebral arteries/su 8. arterial occlusive diseases/su 9. *vascular surgical procedures/ 10. *anastomosis, surgical/ 11. (extra?cranial adj5 intra?cranial).tw. 12. ((cerebral or brain or arterial or surgical or microsurgical) adj5 (anastomosis or revascular$ or bypass or graft)).tw. 13. (temporal artery adj5 middle cerebral artery).tw. 14. ((temporal or occipital) adj5 intracranial).tw. 15. (EC‐IC or ECIC or EC#IC or extra‐intracranial or STA‐MCA).tw. 16. or/6‐15 17. 5 and 16 18. limit 17 to human
Appendix 2. EMBASE search strategy
The following search strategy was used for EMBASE (Ovid).
1. cerebrovascular disease/ or cerebral artery disease/ or cerebrovascular accident/ or stroke/ or vertebrobasilar insufficiency/ or carotid artery disease/ or exp carotid artery obstruction/ or exp brain infarction/ or exp brain ischemia/ or exp occlusive cerebrovascular disease/ 2. (stroke$ or cva).tw. 3. ((cerebr$ or brain$ or carotid or cerebellar or intracranial or vertebrobasilar or MCA) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or occlud$ or arteriosclero$ or stenosis or steno‐occlus$ or obstruct$)).tw. 4. transient isch$.tw. 5. brain atherosclerosis/ 6. exp carotid artery/ or exp brain artery/ 7. artery occlusion/ or exp thromboembolism/ 8. 6 and 7 9. 1 or 2 or 3 or 4 or 5 or 8 10. cerebrovascular surgery/ or brain artery bypass/ or extraintracranial anastomosis/ 11. artery anastomosis/ or artery bypass/ 12. *bypass surgery/ or *artery graft/ or *revascularization/ 13. *superficial temporal artery/ 14. (extra?cranial adj5 intra?cranial).tw. 15. ((cerebral or brain or arterial or surgical or microsurgical) adj5 (anastomosis or revascular$ or bypass or graft)).tw. 16. (temporal artery adj5 middle cerebral artery).tw. 17. ((temporal or occipital) adj5 intracranial).tw. 18. (EC‐IC or ECIC or EC#IC or extra‐intracranial or STA‐MCA).tw. 19. or/10‐18 20. 9 and 19 21. limit 20 to human
Data and analyses
Comparison 1. EC/IC bypass versus best medical treatment: RCTs only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death from all causes | 2 | 1691 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.62, 1.05] |
| 2 Any stroke during follow up | 2 | 1691 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.79, 1.23] |
| 3 Death or dependency | 1 | 1377 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.74, 1.21] |
| 4 Vascular death | 1 | 1377 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.71, 1.29] |
| 5 Stroke, serious vascular events or vascular death | 2 | 1573 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.51, 0.91] |
| 6 Myocardial infarction | 2 | 1522 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.46, 1.32] |
| 7 Ischaemic stroke | 2 | 1573 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.44, 1.08] |
Comparison 2. EC/IC bypass versus best medical treatment: all studies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death from all causes | 19 | 900 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.62, 1.62] |
| 2 Any stroke during follow up | 18 | 881 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.54, 1.18] |
| 3 Death or dependency | 8 | 346 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.50, 1.29] |
| 4 Vascular death | 19 | 900 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.56, 1.63] |
| 5 Stroke, serious vascular events or vascular death | 13 | 673 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.45, 1.04] |
| 6 Myocardial infarction | 2 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.67 [0.41, 17.60] |
| 7 Ischaemic stroke | 13 | 640 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.44, 1.18] |
| 8 Intracranial haemorrhage | 4 | 361 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.44, 2.93] |
| 9 Transient ischaemic attack or amaurosis fugax | 11 | 524 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.16, 0.69] |
| 10 Normalisation of cerebral haemodynamics | 3 | 56 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.63 [1.85, 23.78] |
Comparison 3. Haemodynamic compromise as selection criterion for EC/IC bypass.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any stroke during follow up | 3 | 65 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 2.52] |
| 2 Death from all causes | 3 | 65 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 9.49 [0.18, 489.97] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Auer 1980.
| Methods | Non‐random | |
| Participants | 38 | |
| Interventions | EC/IC bypass | |
| Outcomes | Death from all causes, any stroke during follow up, death or dependency | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Benvenuti 1984.
| Methods | Non‐random, retrospective | |
| Participants | 257 | |
| Interventions | EC/IC bypass | |
| Outcomes | Death from all causes, any stroke during follow up | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
de Weerd 1989.
| Methods | Non‐random | |
| Participants | 34 | |
| Interventions | EC/IC bypass | |
| Outcomes | Death from all causes, any stroke during follow up | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
EC/IC Bypass Study 1985.
| Methods | RCT | |
| Participants | 1377 | |
| Interventions | EC/IC bypass | |
| Outcomes | Death from all causes, any stroke during follow up, death or dependency | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Hartmann 1987.
| Methods | Non‐random, retrospective | |
| Participants | 41 | |
| Interventions | EC/IC bypass | |
| Outcomes | Increased mean rCBF after EC/IC bypass | |
| Notes | Main focus: CBF measurement | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Heilbrun 1982.
| Methods | Non‐random | |
| Participants | 49 | |
| Interventions | EC/IC bypass | |
| Outcomes | Death from all causes, any stroke during follow up | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Ishikawa 1992.
| Methods | Non‐random | |
| Participants | 63 | |
| Interventions | EC/IC bypass | |
| Outcomes | Death from all causes, any stroke during follow up | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Jeffree 2009.
| Methods | Non‐random | |
| Participants | 23 | |
| Interventions | EC/IC bypass | |
| Outcomes | Death from all causes, any stroke during follow up, death or dependency | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
JET 2006.
| Methods | RCT | |
| Participants | 206, report on 196, 1 excluded due to protocol violation | |
| Interventions | EC/IC bypass | |
| Outcomes | Death from all causes, re‐stroke with disability | |
| Notes | Randomisation procedure not described in detail | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Jordan 1984.
| Methods | Non‐random | |
| Participants | 34 | |
| Interventions | EC/IC bypass | |
| Outcomes | Death from all causes, any stroke during follow up | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Karnik 1992.
| Methods | Non‐random | |
| Participants | 104: 14 underwent EC/IC bypass, 14 received best medical treatment Blood flow velocity measurement was assessed in 6 patients with EC/IC bypass surgery and in 4 controls | |
| Interventions | EC/IC bypass | |
| Outcomes | Death from all causes, any stroke during follow up, improvement of vasomotor reactivity in patients with EC/IC bypass after acetazolamide application | |
| Notes | Randomisation procedure not described in detail | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Kobayashi 1991.
| Methods | Non‐random | |
| Participants | 11 | |
| Interventions | EC/IC bypass | |
| Outcomes | Any stroke during follow up, death or dependency | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Ma 2007.
| Methods | Non‐random | |
| Participants | 11 | |
| Interventions | EC/IC bypass | |
| Outcomes | Death from all causes, any stroke during follow up, death or dependency | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Powers 1989.
| Methods | Non‐random | |
| Participants | 52 | |
| Interventions | EC/IC bypass | |
| Outcomes | Death from all causes, any stroke during follow up | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Satiani 1985.
| Methods | Non‐random | |
| Participants | 42 | |
| Interventions | EC/IC bypass | |
| Outcomes | Death from all causes, any stroke during follow up | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Tanahashi 1985.
| Methods | Non‐random | |
| Participants | 60 | |
| Interventions | EC/IC bypass | |
| Outcomes | Death from all causes, any stroke during follow up, death or dependency | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Thomas 1984.
| Methods | Non‐random | |
| Participants | 11 | |
| Interventions | EC/IC bypass | |
| Outcomes | Death from all causes, any stroke during follow up, improvement of CBF after EC/IC bypass | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Yasui 1991.
| Methods | Non‐random, retrospective | |
| Participants | 55 | |
| Interventions | EC/IC bypass | |
| Outcomes | Death from all causes, any stroke during follow up, death or dependency | |
| Notes | Only patients with viable brain tissue | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Yonas 1996.
| Methods | Non‐random, retrospective | |
| Participants | 46 | |
| Interventions | EC/IC bypass | |
| Outcomes | Death from all causes, any stroke during follow up | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Yoshimoto 1995.
| Methods | Non‐random, retrospective | |
| Participants | 70 | |
| Interventions | EC/IC bypass | |
| Outcomes | Death from all causes, any stroke during follow up, death or dependency | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Yoshinaga 1996.
| Methods | Non‐random | |
| Participants | 19 | |
| Interventions | EC/IC bypass | |
| Outcomes | Death from all causes, any stroke during follow up | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
CBF: cerebral blood flow EC/IC bypass: extracranial to intracranial bypass rCBF: regional cerebral blood flow RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Binder 1982 | The study compared neuropsychological function between patients with EC/IC bypass and patients with aspirin and dipyridamole treatment, while data about primary and secondary outcome events defined for this review were not given |
| Danaila 1984 | In this study, the authors present a mixture of Moyamoya disease, fibromuscular and atherosclerotic steno‐occlusive ICA and MCA disease |
| Fields 1976 | The surgical group had only thrombendarterectomy of occluded carotid artery rather than EC/IC bypass, and were compared with patients receiving only best medical treatment |
| Lenzi 1988 | Patients who underwent EC/IC bypass procedure were not compared with a control group receiving best medical treatment |
| McCormick 1991 | No comparison of surgically‐treated patients with a medically‐treated group |
| Meyer 1982 | Patients with Moyamoya disease are mixed with atherosclerotic ICA occlusion |
| Sunada 1989 | EC/IC bypass patients were compared with normal volunteers |
| Wu 1986 | Patients with Moyamoya disease are mixed with atherosclerotic ICA occlusion |
EC/IC bypass: extracranial to intracranial bypass ICA: internal carotid artery MCA: middle cerebral artery
Characteristics of ongoing studies [ordered by study ID]
COSS.
| Trial name or title | Carotid Occlusion Surgery Study |
| Methods | — |
| Participants | Patients with atherosclerotic occlusion of one or both carotid arteries, with hemispheric TIA or mild‐to‐moderate stroke (Barthel Index > 60) in the territory of an occluded carotid artery within 120 days and with increased OEF measured by PET ipsilateral to the symptomatic carotid artery occlusion |
| Interventions | EC/IC bypass |
| Outcomes | All strokes in patients with symptomatic carotid occlusion and high OEF |
| Starting date | February 2000 |
| Contact information | http://www.cosstrial.org/coss/contact.asp |
| Notes | — |
EC/IC bypass: extracranial to intracranial bypass OEF: oxygen extraction fraction PET: positron emission tomography TIA: transient ischaemic attack
Differences between protocol and review
None.
Contributions of authors
Felix Fluri: developing the protocol, writing the protocol, extracting data, drafting the review. Stefan Engelter: developing the protocol, extracting data, revising the review draft. Philippe Lyrer: developing the protocol, writing the protocol, fundraising, revising the review draft.
Sources of support
Internal sources
Scientific Fund of the Stroke Programme, Neurology Department, University Hospital, Basel, Switzerland.
External sources
No sources of support supplied
Declarations of interest
The authors received an in‐house grant from the Department of Neurology, University Hospital Basel, to perform the review.
New
References
References to studies included in this review
Auer 1980 {published data only}
- Auer L, Gallhofer B, Ladurner G, Ott E, Heppner F, Lechner H. Medical versus surgical treatment of patients with cerebrovascular insufficiency. European Neurology 1980;19:152‐62. [DOI] [PubMed] [Google Scholar]
Benvenuti 1984 {published data only}
- Benvenuti l, Gagliardi R, Giombini SM, Andreoli A, Limoni P, Piazza I, et al. Long‐term follow up in 257 ICA occlusion: comparison between EIAB‐treated and untreated patients. Neurological Research 1984;6:181‐3. [DOI] [PubMed] [Google Scholar]
de Weerd 1989 {published data only}
- Weerd AW, Veldhuizen RJ, Veering MM, Poortvliet DCJ, Jonkman EJ. Long‐term clinical and neurophysiological effects of reconstructive vascular surgery for cerebral ischemia. Acta Neurologica Scandinavica 1989;79:311‐5. [DOI] [PubMed] [Google Scholar]
EC/IC Bypass Study 1985 {published data only}
- EC/IC Bypass Study Group. Failure of extracranial‐intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. New England Journal of Medicine 1985;313:1191‐200. [DOI] [PubMed] [Google Scholar]
Hartmann 1987 {published data only}
- Hartmann A, Rommel T, Winter R, Tsuda Y, Menzel J. Measurements of regional cerebral blood flow in patients following superficial temporal artery‐middle cerebral artery anastomosis. Acta Neurochirurgica 1987;89:106‐11. [DOI] [PubMed] [Google Scholar]
Heilbrun 1982 {published data only}
- Heilbrun MP. Overall management of vascular lesions considered treatable with extracranial‐intracranial bypass: part 1. Neurosurgery 1982;11:239‐46. [DOI] [PubMed] [Google Scholar]
Ishikawa 1992 {published data only}
- Ishikawa T, Yasui N, Suzuki A, Hadeishi H, Shishido F, Uemura K. STA‐MCA bypass surgery for internal carotid artery occlusion ‐ comparative follow‐up study. Neurologia Medico Chirurfica (Tokyo) 1992;32:5‐9. [DOI] [PubMed] [Google Scholar]
Jeffree 2009 {published data only}
- Jeffree RL, Stoodley MA. STA–MCA bypass for symptomatic carotid occlusion and haemodynamic impairment. Journal of Clinical Neurosciences 2009;16:226‐35. [DOI] [PubMed] [Google Scholar]
JET 2006 {published data only}
- Ogasawara K, Okawa A. JET‐Study (Japanese EC‐IC Bypass Trial). Nippon Rinsho 2006;64 Suppl 7:524‐7. [PubMed] [Google Scholar]
Jordan 1984 {published data only}
- Jordan BP, Mayschak DT, Flye MW. Treatment of the totally occluded carotid artery. Archives of Surgery 1984;119:952‐5. [DOI] [PubMed] [Google Scholar]
Karnik 1992 {published data only}
- Karnik R, Valentin A, Ammerer HP, Donath P, Slany J. Evaluation of vasomotor reactivity by transcranial Doppler and acetazolamide test before and after extracranial‐intracranial bypass in patients with internal carotid artery occlusion. Stroke 1992;23:812‐7. [DOI] [PubMed] [Google Scholar]
Kobayashi 1991 {published data only}
- Kobayashi H, Hayashi M, Kawano H, Handa Y, Kabuto M, Maeda H, et al. Evaluation of extracranial‐to‐intracranial bypass surgery using iodine 123 iodoamphetamine single‐photon emission computed tomography. Surgical Neurology 1991;35:436‐40. [DOI] [PubMed] [Google Scholar]
Ma 2007 {published data only}
- Ma J, Mehrkens JH, Holtmannspoetter M, Linke R, Schmid‐Elsaesser R, Steiger HJ, et al. Perfusion MRI before and after acetazolamide administration for assessment of cerebrovascular reserve capacity in patients with symptomatic internal carotid artery (ICA) occlusion: comparison with 99mTc‐ECD SPECT. Neuroradiology 2007;49:317‐26. [DOI] [PubMed] [Google Scholar]
Powers 1989 {published data only}
- Powers WJ, Grubb RL Jr, Raichle ME. Clinical results of extracranial‐intracranial bypass surgery in patients with hemodynamic cerebrovascular disease. Journal of Neurosurgery 1989;70:61‐7. [DOI] [PubMed] [Google Scholar]
Satiani 1985 {published data only}
- Satiani B, Burns J, Vasko JS. Surgical and nonsurgical treatment of total carotid artery occlusion. American Journal of Surgery 1985;149:362‐7. [DOI] [PubMed] [Google Scholar]
Tanahashi 1985 {published data only}
- Tanahashi N, Meyer JS, Rogers RL, Kitagawa Y, Mortel KF, Kandula P, et al. Long‐term assessment of cerebral perfusion following STA‐MCA by‐pass in patients. Stroke 1985;16:85‐91. [DOI] [PubMed] [Google Scholar]
Thomas 1984 {published data only}
- Thomas M, Hennerici M, Marshall J. Cerebral blood flow after carotid occlusion and extracranial‐intracranial bypass. Journal of Neurology, Neurosurgery & Psychiatry 1984;47:148‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yasui 1991 {published data only}
- Yasui N, Suzuki A, Sayama I, Kawamura S, Shishido F, Uemura K. Comparison of the clinical results of STA‐MCA anastomosis and the medical treatment in the cerebral low perfusion patients with viable brain tissue. Neurology Research 1991;13:84‐8. [DOI] [PubMed] [Google Scholar]
Yonas 1996 {published data only}
- Yonas H, Przybylski GJ, Webster MW, Smith HA, Johnson DW. Diagnosis and treatment of high‐risk patients from symptomatic carotid occlusive disease with STA‐MCA bypass. Acta Neurologica Scandinavica Supplementum 1996;166:114. [Google Scholar]
Yoshimoto 1995 {published data only}
- Yoshimoto Y, Kwak S. Superficial temporal artery ‐ middle cerebral artery anastomosis for acute cerebral ischemia: the effect of small augmentation of blood flow. Acta Neurochirurgica 1995;137:128‐37. [DOI] [PubMed] [Google Scholar]
Yoshinaga 1996 {published data only}
- Yoshinaga S, Tanaka A, Kumate S, Nakayama Y, Tomonaga M. A comparison of hemodynamic effect on an STA/MCA bypass with that of anti‐platelet therapy. Acta Neurologica Scandinavica Supplementum 1996;166:113. [Google Scholar]
References to studies excluded from this review
Binder 1982 {published data only}
- Binder LM, Tanabe CT, Waller FT, Wooster NE. Behavioral effects of superficial temporal artery to middle cerebral artery bypass surgery: preliminary report. Neurology 1982;32:422‐4. [DOI] [PubMed] [Google Scholar]
Danaila 1984 {published data only}
- Danaila L, Olarescu A, Gheorghitescu L, Lungu A, Bratu S. Extra‐intracranial anastomosis between the superficial temporal artery and a cortical branch of the middle cerebral artery. Neurologie et Psychiatrie 1984;22:251‐61. [PubMed] [Google Scholar]
Fields 1976 {published data only}
- Fields WS, Lemak NA. Joint study of extracranial arterial occlusion. X. Internal carotid artery occlusion. JAMA 1976;235:2734‐8. [PubMed] [Google Scholar]
Lenzi 1988 {published data only}
- Lenzi B, Fiori L, Marconi F, Parenti G. Cerebral ischemia in young adults. Minerva Medica 1988;79:707‐10. [PubMed] [Google Scholar]
McCormick 1991 {published data only}
- McCormick PW, Tomecek FJ, McKinney J, Ausman JI. Disabling cerebral transient ischemic attacks. Journal of Neurosurgery 1991;75:891‐901. [DOI] [PubMed] [Google Scholar]
Meyer 1982 {published data only}
- Meyer JS, Nakajima S, Okabe T, Amano T, Centeno R, Len YY, et al. Redistribution of cerebral blood flow following STA‐MCA by‐pass in patients with hemispheric ischemia. Stroke 1982;13:774‐84. [DOI] [PubMed] [Google Scholar]
Sunada 1989 {published data only}
- Sunada I. Measurement of cerebral blood flow by single photon emission computed tomography in cases of internal carotid artery occlusion. Neurologia Medico Chirurfica (Tokyo) 1989;29:496‐502. [DOI] [PubMed] [Google Scholar]
Wu 1986 {published data only}
- Wu RQ, Zhu JK, Zhang C, Zhang YJ, Zhang SM, Zhao MX. Follow‐up study on 250 patients with extra‐intracranial arterial bypass operation for ischemic stroke. Chinese Medical Journal 1986;99:703‐7. [PubMed] [Google Scholar]
References to ongoing studies
COSS {published data only}
- Grubb RL Jr, Powers WJ, Derdeyn CP, Adams HP Jr, Clarke WR. The Carotid Occlusion Surgery Study. Neurosurgical Focus 2003;14(3):e9. [DOI] [PubMed] [Google Scholar]
Additional references
Ausman 1986
- Ausman JI, Diaz FG. Critique of the extracranial‐intracranial bypass study. Surgical Neurology 1986;26(3):218‐21. [DOI] [PubMed] [Google Scholar]
Baron 1981
- Baron JC, Bousser MG, Rey A, Guillard A, Comar D, Castaigne P. Reversal of focal "misery‐perfusion syndrome" by extra‐intracranial arterial bypass in hemodynamic cerebral ischemia. A case study with 15O positron emission tomography. Stroke 1981;12(4):454‐9. [DOI] [PubMed] [Google Scholar]
Bozzao 1989
- Bozzao L, Fantozzi LM, Bastianello S, Bozzao A, Fieschi C. Early collateral blood supply and late parenchymal brain damage in patients with middle cerebral artery occlusion. Stroke 1989;20(6):735‐40. [DOI] [PubMed] [Google Scholar]
Day 1986
- Day AL, Rhoton AL Jr, Little JR. The extracranial‐intracranial bypass study. Surgical Neurology 1986;26(3):222‐6. [DOI] [PubMed] [Google Scholar]
Derdeyn 2002
- Derdeyn CP, Videen TO, Yundt KD, Fritsch SM, Carpenter DA, Grubb RL, et al. Variability of cerebral blood volume and oxygen extraction: stages of cerebral haemodynamic impairment revisited. Brain 2002;125(3):595‐607. [DOI] [PubMed] [Google Scholar]
Donaghy 1967
- Donaghy RMP. Patch and bypass in microangional surgery. In: Donaghy RMP, Yasargil MG editor(s). Microvascular Surgery. St. Louis: CV Mosby, 1967:75‐86. [Google Scholar]
Grubb 1986
- Grubb RL Jr. Management of the patient with carotid occlusion and a single ischemic event. Clinical Neurosurgery 1986;33:251‐80. [PubMed] [Google Scholar]
Hankey 1991
- Hankey GJ, Warlow CP. Prognosis of symptomatic carotid occlusion: an overview. Cerebrovascular Disease 1991;1:245‐56. [Google Scholar]
Klijn 1997
- Klijn CJ, Kappelle LJ, Tulleken CA, Gijn J. Symptomatic carotid artery occlusion. A reappraisal of hemodynamic factors. Stroke 1997;28(10):2084‐93. [DOI] [PubMed] [Google Scholar]
Lyrer 2003
- Lyrer P, Engelter S. Antithrombotic drugs for carotid artery dissection. Cochrane Database of Systematic Reviews 2003, Issue 3. [Art. No.: CD000255. DOI: 10.1002/14651858.CD000255] [DOI] [PubMed] [Google Scholar]
Pessin 1977
- Pessin MS, Duncan GW, Mohr JP, Poskanzer DC. Clinical and angiographic features of carotid transient ischemic attacks. New England Journal of Medicine 1977;296(7):358‐62. [DOI] [PubMed] [Google Scholar]
Powers 1987
- Powers WJ, Press GA, Grubb RL Jr, Gado M, Raichle ME. The effect of hemodynamically significant carotid artery disease on the hemodynamic status of the cerebral circulation. Annals of Internal Medicine 1987;106(1):27‐34. [DOI] [PubMed] [Google Scholar]
Powers 1991
- Powers WJ. Cerebral hemodynamics in ischemic cerebrovascular disease. Annals of Neurology 1991;29(3):231‐40. [DOI] [PubMed] [Google Scholar]
Powers 2000
- Powers WJ, Derdeyn CP, Fritsch SM, Carpenter DA, Yundt KD, Videen TO, et al. Benign prognosis of never‐symptomatic carotid occlusion. Neurology 2000;54(4):878‐82. [DOI] [PubMed] [Google Scholar]
Sundt 1987
- Sundt TM Jr. Was the international randomized trial of extracranial‐intracranial arterial bypass representative of the population at risk?. New England Journal of Medicine 1987;316(13):814‐6. [DOI] [PubMed] [Google Scholar]
Thiele 1980
- Thiele BL, Young JV, Chikos PM, Hirsch JH, Strandness DE Jr. Correlation of arteriographic findings and symptoms in cerebrovascular disease. Neurology 1980;30(10):1041‐6. [DOI] [PubMed] [Google Scholar]
Vorstrup 1992
- Vorstrup S, Paulson OB. Extracranial‐intracranial bypass revisited. Cerebrovascular Diseases 1992;2:261‐2. [Google Scholar]
Yasargil 1969
- Yasargil MG. Anastomosis between the superficial temporal artery and a branch of the middle cerebral artery. Microsurgery Applied to Neurosurgery. Stuttgart: Georg Thieme, 1969:105‐15. [Google Scholar]


