Abstract
Background
Research shows that stroke patients and their families are dissatisfied with the information provided and have a poor understanding of stroke and associated issues.
Objectives
To assess the effectiveness of information provision strategies in improving the outcome for stroke patients or their identified caregivers, or both.
Search methods
For this update we searched the Cochrane Stroke Group Trials Register (June 2012), the Cochrane Central Register of Controlled trials (CENTRAL), the Cochrane Database of Systematic Reviews (CDSR), the Database of Abstracts of Reviews of Effects (DARE), the NHS Economic Evaluation Database (EED), and the Health Technology Assessment (HTA) Database (The Cochrane Library June, 2012), MEDLINE (1966 to June 2012), EMBASE (1980 to June 2012), CINAHL (1982 to June 2012) and PsycINFO (1974 to June 2012). We also searched ongoing trials registers, scanned bibliographies of relevant articles and books and contacted researchers.
Selection criteria
Randomised trials involving patients or carers of patients with a clinical diagnosis of stroke or transient ischaemic attack (TIA) where an information intervention was compared with standard care, or where information and another therapy were compared with the other therapy alone.
Data collection and analysis
Two review authors independently assessed trial eligibility and methodological quality and extracted data. Primary outcomes were knowledge about stroke and stroke services, and impact on mood.
Main results
We have added four new trials to this update. This review now includes 21 trials involving 2289 patient and 1290 carer participants. Nine trials evaluated a passive and 12 trials an active information intervention. Meta‐analyses showed a significant effect in favour of the intervention on patient knowledge (standardised mean difference (SMD) 0.29, 95% confidence interval (CI) 0.12 to 0.46, P < 0.001), carer knowledge (SMD 0.74, 95% CI 0.06 to 1.43, P = 0.03), one aspect of patient satisfaction (odds ratio (OR) 2.07, 95% CI 1.33 to 3.23, P = 0.001), and patient depression scores (mean difference (MD) ‐0.52, 95% CI ‐0.93 to ‐0.10, P = 0.01). There was no significant effect (P > 0.05) on number of cases of anxiety or depression in patients, carer mood or satisfaction, or death. Qualitative analyses found no strong evidence of an effect on other outcomes. Post‐hoc subgroup analyses showed that active information had a significantly greater effect than passive information on patient mood but not on other outcomes.
Authors' conclusions
There is evidence that information improves patient and carer knowledge of stroke, aspects of patient satisfaction, and reduces patient depression scores. However, the reduction in depression scores was small and may not be clinically significant. Although the best way to provide information is still unclear there is some evidence that strategies that actively involve patients and carers and include planned follow‐up for clarification and reinforcement have a greater effect on patient mood.
Plain language summary
Information provision for stroke patients and their caregivers
Studies have shown that stroke survivors and their carers often report they have not been given enough information about stroke and feel unprepared for life after discharge from hospital. However, the best way to provide information after stroke is unclear. The authors of this review looked at the evidence for the effectiveness of providing information to patients, or carers of patients, who have had a stroke or transient ischaemic attack (TIA), sometimes called a mini‐stroke. They examined randomised trials (studies) in which one group of stroke patients or carers who were given the intervention being tested (such as a course of lectures) was compared with a group of stroke patients or carers who received standard care. Twenty‐one studies, involving 2289 patients and 1290 carers, are now included in this updated review. Overall, the studies showed that providing information to patients and carers improved their knowledge of stroke and increased patient satisfaction with some, but not all, of the information they received about stroke. There was also an effect on reducing patient depression, although the reduction was small and may not be enough to seem meaningful to patients. When information was provided in a way that more actively involved patients and carers, for example by offering repeated opportunities to ask questions, it had more effect on patient mood than information which was given on one occasion only. There is not much evidence that providing information had effects on other aspects of patient or carer stroke recovery such as independence or social activities.
Background
Every year approximately 110,000 people in England have a stroke (National Audit Office 2005) and at any one time over 300,000 people are living with moderate to severe disability as a result of a stroke (Adamson 2004). The provision of appropriate, accurate, timely information and advice about stroke has been recommended as a key component of service provision (Canadian Stroke Network 2006; RCP 2008; National Stroke Foundation Australia 2010). Information, combined with the right support, is the key to better care, better outcomes and reduced costs. Patients should have information and data on all aspects of health care, to enable them to share in decisions about their care and access appropriate services (Department of Health 2010). The information needs of people who have had a stroke and their carers are diverse and change over time. Information should be tailored to an individual's requirements and provided in a variety of formats (Department of Health 2007; Eames 2011), taking into account their stroke‐specific impairment and personal situation (RCP 2008).
There is a wide range of nationally and locally produced leaflets, booklets, videos and audio tapes available for patients and carers. However, despite this emphasis on giving information, research suggests that patients' understanding of stroke, its consequences and the support available, remains poor. A recent systematic review identified multiple and diverse unmet educational needs by stroke patients and their caregivers (Hafsteinsdottir 2011). In a survey of community dwelling adults who suffered a stroke at least one and up to five years previously, over half reported wanting more information about their stroke (McKevitt 2011).
In a UK study, carers of stroke patients reported that whilst leaflets were available, they were not always appropriate to the situation (Mackenzie 2007). A survey by primary care trusts in England, of the information provided to patients after stroke, reported that the majority provided good information. However, only 40% contained information relevant to local services. Furthermore, the size, content and organisation of the information varied extensively (Care Quality Commission 2011). Inadequate provision and receipt of appropriate information has important consequences for compliance with secondary prevention and the longer‐term psycho‐social outcome for patients and carers (O'Mahoney 1997). Enhanced knowledge of stroke care by carers may improve the quality of discharge home from hospital for stroke patients (Evans 1991). Despite the perceived and expressed need for information, successful strategies have not as yet been identified. In order to fully explore available evidence we have undertaken a systematic review of information provision for patients and their carers after stroke.
Description of the condition
A stroke is defined by the World Health Organization as: "Rapidly developing clinical signs of focal (or global) disturbance of cerebral function, lasting more than 24 hours or leading to death, with no apparent cause other than vascular origin" (Aho 1980). A transient ischaemic attack (TIA) is a brief reversible episode of focal, non‐convulsive ischaemic dysfunction of the brain with a duration of less than 24 hours (Adams 1998). Stroke can lead to death or physical and cognitive impairment (McKevitt 2011; Mukherjee 2011) and can have long lasting psychological and social implications (Knapp 2000).
Description of the intervention
The intervention is the provision of information for stroke survivors or their informal caregivers, or both, following a stroke or TIA. The intervention may be provided in a variety of formats such as leaflets, workbooks, or verbal communication including lectures or teaching sessions. Whilst the content of the intervention may vary, it is likely to contain at least one of the following components: information about the causes and nature of stroke; management and recovery from the effects of stroke; prevention or reducing the risk of future strokes; information on resources or services. Whilst the provision of information should be incorporated as standard practice following stroke, evidence suggests it is lacking or inconsistent (Mackenzie 2007; Care Quality Commission 2011).
How the intervention might work
If stroke survivors and carers are to be active in their decision making and management of the long‐term effects of stroke, appropriate information delivered in a timely and effective format is necessary. Information is considered necessary to recognise and act upon symptoms, manage disease exacerbation and to access effective treatments and medicines and produce better outcomes (Department of Health 2001; Department of Health 2010). Furthermore, inadequate provision of information has implications for compliance with secondary prevention and psycho‐social outcomes for stroke patients and carers (O'Mahoney 1997). Evidence from non‐stroke populations suggests providing written information improves adherence to hospital after‐care regimens (Gibbs 1989; Firth 1991) and may assist with self‐care (Coulter 1998), which may indirectly produce beneficial outcomes.
Why it is important to do this review
It has been proposed that information, combined with the right support, is the key to better care, better outcomes and reduced costs (Department of Health 2010). The information derived from this review has the potential to lead to the development of more effective information provision strategies and highlight which outcomes might be affected by such interventions.
Objectives
The objective of this review was to examine the effectiveness of information strategies provided with the intention of improving the outcome for stroke patients or their identified caregivers or both.
Methods
Criteria for considering studies for this review
Types of studies
We included unconfounded randomised trials where an information intervention was compared with standard care or where information and another therapy was compared with the other therapy alone.
Types of participants
Patients with a clinical diagnosis of stroke or TIA and their identified caregivers or both.
Types of interventions
Information provided with the intention of improving the outcome of patients or their caregivers or both. We excluded trials in which information‐giving was only one component of a more complex rehabilitation intervention, for example family support worker trials (Forster 1996; Dennis 1997; Mant 2000; Lincoln 2003; Ellis 2005), which are the subject of a separate Cochrane review (Ellis 2010).
Types of outcome measures
We considered that information provision would impact most directly on knowledge and patients' or carers' mood state (anxiety and depression) or both. Therefore, we used the following primary and secondary outcome measures to assess the effectiveness of information provision.
Primary outcomes
Patient or carer knowledge about stroke and stroke services or both.
Patient or carer impact on mood (e.g. Hospital Anxiety and Depression Scale).
Secondary outcomes
Activities of daily living (e.g. Barthel Index).
Participation (e.g. London Handicap Scale).
Social activities (e.g. Frenchay Activities Index).
Perceived health status (e.g. Short‐form 36, Nottingham Health Profile).
Quality of life (e.g. Dartmouth Coop Chart).
Satisfaction with information.
Hospital admissions, service contacts or health professional contacts.
Compliance with treatment/rehabilitation (e.g. Miller's Health Behaviour Scale).
Death or institutionalisation or both.
Resource outcomes
Cost to health and social services.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We searched for trials in all languages and arranged translation of papers published in languages other than English.
Electronic searches
For this update we searched the Cochrane Stroke Group Trials Register (last searched in June 2012). In addition, we searched the following electronic bibliographic databases and trials registers:
Cochrane Central Register of Controlled trials (CENTRAL) (The Cochrane Library 2012, Issue 4) (Appendix 1);
Cochrane Database of Systematic Reviews (CDSR) (The Cochrane Library 2012, Issue 4) (Appendix 1);
Database of Abstracts of Reviews of Effects (DARE) (The Cochrane Library 2012, Issue 4) (Appendix 1);
NHS Economic Evaluation Database (EED) (The Cochrane Library 2012, Issue 4) (Appendix 1);
Health Technology Assessment (HTA) Database (The Cochrane Library 2012, Issue 4) (Appendix 1);
MEDLINE (1966 to June 2012) (Appendix 2);
EMBASE (1980 to June 2012) (Appendix 3);
CINAHL (1982 to June 2012) (Appendix 4);
PsycINFO (1974 to June 2012) (Appendix 5);
Current Controlled Trials (http://www.controlled‐trials.com/) (June 2012);
National Rehabilitation Information Center (www.naric.com) (June 2012);
RePORT Expenditures and Results (RePORTER) query tool (http://projectreporter.nih.gov/reporter.cfm) (December 2012);
Internet Stroke Center stroke trials registry (www.strokecenter.org) (June 2012).
Searching other resources
In an effort to identify further published, unpublished and ongoing trials, we searched bibliographies of relevant articles and books and contacted authors of relevant research and previous articles on information provision.
For the previous version of the review we searched:
Science Citation Index and Social Science Citation Index (1981 to March 2007);
ASSIA (Applied Social Science Index and Abstracts) (1987 to March 2007);
Index to UK theses (1970 to March 2007);
Dissertation Abstracts (1961 to March 2007);
National Research Register (www.nrr.nhs.uk) (to September 2007);
Journal of Advanced Nursing (1996 to March 2007).
Data collection and analysis
Selection of studies
Two review authors independently assessed the titles and abstracts of records from the electronic searches and excluded obviously irrelevant studies. We obtained the full text of the remaining studies and at least two review authors assessed these against the review inclusion criteria to determine which trials would be eligible for inclusion. The review authors resolved disagreements by discussion with other members of the review team.
Data extraction and management
At least two review authors scrutinised all the eligible trials to grade methodological quality, patient selection, the intervention, outcome measures used, and length of follow‐up. We allocated studies to one of two categories ‐ passive information or active information ‐ according to the nature of the intervention. An intervention was classified as passive if the information was provided on a single occasion and there was no subsequent systematic follow‐up or reinforcement procedure. An intervention was classified as active if, following the provision of the information, there was a purposeful attempt to allow the participant to assimilate the information and a subsequent agreed plan for clarification and consolidation or reinforcement. We made this classification because it would inform future research and be helpful for service planners in terms of committing resources. Two review authors extracted data independently using piloted data extraction forms, and measured agreement. They resolved disagreement through group consensus. Where necessary, we contacted study authors for additional information and data.
Assessment of risk of bias in included studies
We assessed the methodological quality of selected studies using the tool for assessing risk of bias as described in section 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We scored each of the following domains as 'high risk of bias', 'low risk of bias', or 'unclear risk of bias' and reported them in the 'Risk of bias' tables.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other possible bias.
Measures of treatment effect
We compared studies based on end‐of‐study results. We used the mean difference (MD) or standardised mean difference (SMD) for continuous outcomes. We treated ordinal data as continuous data and combined them using the MD. We combined dichotomous data using the Peto odds ratio (OR).
Dealing with missing data
If data were missing, we performed an available case analysis. The proportion of participants in each study arm who did not provide data is shown in the 'Data and analyses' section.
Assessment of heterogeneity
We tested for the presence of heterogeneity between the trials using the I2 statistic. We used a fixed‐effect model if we detected no substantial heterogeneity (I2 < 50%). Where there was substantial heterogeneity (I2 ≥ 50%) we used a random‐effects model.
Assessment of reporting biases
We were able to reduce reporting bias by undertaking comprehensive searches of multiple databases and trials registers, and contacting authors. There were no restrictions based on language and translations were undertaken if required. It was not possible to detect reporting bias by the method of assessment of funnel plots as there were insufficient studies included in the meta‐analyses.
Data synthesis
We compared studies based on the end‐of‐study results. Meta‐analyses have been undertaken for the domains of knowledge, emotional outcome, death and for selected satisfaction questions. For the domain of knowledge, we combined data using the SMD as all the trials had used different knowledge questionnaires. We combined dichotomous data (domains of mood, satisfaction, death) using the Peto OR. For the domain of mood, we dichotomised the Hospital Anxiety and Depression Scale scores, the Geriatric Depression Scale scores and the General Health Questionnaire scores using the recommended cut‐off points (Zigmond 1983; Sheikh 1986; Goldberg 1988). We treated ordinal data (domain of patient mood) as continuous data and combined them using the MD. For a number of studies both ordinal and dichotomised patient Hospital Anxiety and Depression Scale data were available. If this was the case, we extracted and analysed both forms of data. For other outcomes, we present a narrative summary stratified by subgroup and a summary of the data is provided in the Data and analyses section.
Subgroup analysis and investigation of heterogeneity
We undertook post‐hoc subgroup analyses for the type of intervention (passive and active). We used the method described by Deeks et al (Deeks 2001) to compare the magnitude of treatment effect of the two subgroups.
Results
Description of studies
Results of the search
For this update we reviewed 28,110 titles; 134 papers were reviewed of which duplicate papers were identified for 14 studies, four new studies are included in the review (Johnston 2007; Chiu 2008; O'Connell 2009; Chinchai 2010). Of the 134 papers reviewed for this update, five were commentaries, reviews or meta‐analyses, seven trials included non‐stroke participants and six studies did not investigate the effectiveness of information provision after stroke; 43 studies have been added to the excluded studies section (details reported in Characteristics of excluded studies); 14 studies are currently pending assessment and 10 studies are currently ongoing.
Included studies
The current analysis includes 21 completed trials with 2289 patient and 1290 carer participants (Lomer 1987; Evans 1988; Pain 1990; Downes 1993; Banet 1997; Mant 1998; Rodgers 1999; Frank 2000; Johnson 2000; Kalra 2004; Smith 2004; Ellis 2005; Larson 2005; Draper 2007; Hoffmann 2007; Johnston 2007; Lowe 2007; Maasland 2007; Chiu 2008; O'Connell 2009; Chinchai 2010).
Setting
Three of the included trials were conducted in the USA (Evans 1988; Banet 1997; Johnson 2000), 11 in the UK (Lomer 1987; Pain 1990; Downes 1993; Mant 1998; Rodgers 1999; Frank 2000; Kalra 2004; Smith 2004; Ellis 2005; Johnston 2007; Lowe 2007), three in Australia (Draper 2007; Hoffmann 2007; O'Connell 2009), one in Sweden (Larson 2005), one in the Netherlands (Maasland 2007), one in Taiwan (Chiu 2008) and one in Thailand (Chinchai 2010).
Participants
In 19 trials the majority of patients were at least 60 years old (Evans 1988; Pain 1990; Downes 1993; Mant 1998; Rodgers 1999; Frank 2000; Johnson 2000; Kalra 2004; Smith 2004; Ellis 2005; Larson 2005; Draper 2007; Hoffmann 2007; Johnston 2007; Lowe 2007; Maasland 2007; Chiu 2008; O'Connell 2009; Chinchai 2010). Two trials did not report age (Lomer 1987; Banet 1997). Six trials reported carer age (Evans 1988; Downes 1993; Rodgers 1999; Smith 2004; Larson 2005; Draper 2007). Carers were younger than the patients, notably so in Evans 1988 where the mean age of the carers was under 50 years old. This study was carried out at a Veterans Administration Medical Centre and was also exceptional in that 94% of the stroke patients were male. In Larson 2005 the majority of spouses were female. Ten trials were concerned with the patient only (Pain 1990; Banet 1997; Frank 2000; Johnson 2000; Ellis 2005; Hoffmann 2007; Lowe 2007; Maasland 2007; Chiu 2008; O'Connell 2009) and in four trials the intervention involved the carer or spouse only (Evans 1988; Kalra 2004; Larson 2005; Draper 2007). In the remaining trials the focus of the intervention was the patient and carer (Lomer 1987; Downes 1993; Mant 1998; Rodgers 1999; Smith 2004; Johnston 2007; Chinchai 2010).
Interventions
Two of the included trials evaluated two interventions (Evans 1988; Downes 1993): one evaluated education and counselling (Evans 1988) and the other evaluated information provision plus counselling (Downes 1993). Only the data from the information/education group and the control groups have been analysed in this review.
Category
In nine studies we categorised the intervention as passive and in a further 12 studies we categorised the intervention as active. We considered that one of the 17 studies (Lowe 2007) exhibited features of both categories. We therefore sought further information from the lead author. Following discussion we agreed that it should be categorised as passive because information was provided on one occasion only with no subsequent opportunity for clarification and consolidation or reinforcement.
Content and administration
Nine trials evaluated a passive intervention (Lomer 1987; Pain 1990; Downes 1993; Banet 1997; Mant 1998; Hoffmann 2007; Lowe 2007; Maasland 2007; O'Connell 2009). In three trials (Lomer 1987; Downes 1993; Mant 1998) this comprised written generic information about stroke in the form of booklets and leaflets. In five studies (Pain 1990; Banet 1997; Hoffmann 2007; Lowe 2007; Maasland 2007) the information was tailored to be of relevance to the individual. In Pain 1990 and Hoffmann 2007 participants were provided with individualised booklets. In Banet 1997 the intervention group were given a copy of their medical history, clinical résumés, test results and leaflets. In Maasland 2007 information was delivered via an individualised multimedia computer programme. In Lowe 2007 the intervention comprised personalised information presented by a research registrar who explained its contents and addressed any additional concerns. In O'Connell 2009, the intervention group were given a patient‐held record that included telephone numbers, generic stroke information and fact sheets relevant to the patient's specific stroke problems.
Twelve trials evaluated an active intervention (Evans 1988; Rodgers 1999; Frank 2000; Johnson 2000; Kalra 2004; Smith 2004; Ellis 2005; Larson 2005; Draper 2007; Johnston 2007; Chiu 2008; Chinchai 2010). In four trials (Evans 1988; Rodgers 1999; Johnson 2000; Larson 2005) the intervention consisted of a programme of lectures providing information about stroke and services available and an opportunity to ask questions. In addition to this, the four‐week course evaluated by Johnson 2000 emphasised the importance of self‐esteem and coping strategies. Participants in the trial by Larson 2005 were also able to contact the stroke specialist nurse between sessions for extra information and support. Five studies (Frank 2000; Kalra 2004; Smith 2004; Ellis 2005; Draper 2007) evaluated a multi‐component intervention. Carers in Kalra 2004 received instruction on a range of topics plus hands‐on training. In Frank 2000 the intervention consisted of a recovery plan, an interactive workbook and a weekly phone call from the researcher. In Draper 2007 the programme for carers of aphasic patients included communication strategies, relaxation and stress management. Patients and carers in Smith 2004 were provided with an information manual supported by fortnightly pre‐arranged review meetings with their multidisciplinary team. In Ellis 2005, the intervention group patients received a monthly review by a stroke nurse specialist, specially selected relevant written information, and personalised records detailing their individual risk factors and recommended risk factor targets. In Johnston 2007, participants received a workbook which provided information about stroke, task material such as goal setting and an audio relaxation tape. In Chiu 2008, the intervention consisted of information delivered by a pharmacist over a course of six sessions. In Chinchai 2010, the intervention consisted of lectures delivered to carers with weekly follow‐up reinforcement at home by health service volunteers. Further details of the interventions are provided in the Characteristics of included studies table.
Timing
The intervention was implemented prior to discharge from hospital in nine trials (Lomer 1987; Evans 1988; Banet 1997; Rodgers 1999; Kalra 2004; Smith 2004; Hoffmann 2007; Lowe 2007; O'Connell 2009); within three weeks of discharge (Johnston 2007) and one month after stroke, or at discharge, which ever was sooner in one trial (Mant 1998). In the remaining trials the intervention was implemented at varying times post‐discharge: soon after discharge (Pain 1990; Downes 1993); within three months of stroke (Ellis 2005; Maasland 2007 ); within 12 months of stroke (Draper 2007); after 12 months since stroke (Chiu 2008); six months to three years after stroke (Johnson 2000); within 18 months of stroke (Chinchai 2010); within two years of stroke (Frank 2000), and a mean of 76 days after stroke onset (Larson 2005).
Outcomes measured
The studies measured a range of outcomes. Details of these are provided in the 'Characteristics of included studies' table.
Assessment of knowledge
Ten trials evaluated patient or carer knowledge or both. All used different questionnaires, the majority of which had been specifically developed for the study. The questionnaire used by Evans 1988 had been validated (Stroke Care Information Test, range 0 to 36) (Evans 1985). The 26‐item knowledge of stroke scale used by Rodgers 1999 and the 17‐item knowledge of stroke and services questionnaire used by Smith 2004 were based on instruments used in other studies (Wellwood 1994; Drummond 1996; Mant 1998), and the content of the specific educational programme under evaluation. In the study by Hoffmann 2007, the 25‐item knowledge of stroke questionnaire developed for the study was based partly on a previously validated measure (Sullivan 2004). The content validity and test‐retest reliability of this instrument were assessed prior to the commencement of the study. The questionnaire used by Lowe 2007 was developed from professionals' ideas of what patients should be aware of concerning secondary prevention of stroke and was piloted with 58 stroke patients. In Maasland 2007 the questionnaire was developed and validated in 42 partners of patients with TIA. None of the questionnaires in the remaining three studies (Lomer 1987; Pain 1990; Mant 1998) had been validated.
Excluded studies
We excluded 51 studies because the information/education was part of a multiple component, complex rehabilitation intervention (Linn 1979; Christie 1984; Printz‐Feddersen 1990; Friedland 1992; Forster 1996; Dennis 1997; Goldberg 1997; Hochstenbach 1999; McKinney 1999; Napolitan 1999; Chang 2000; Rimmer 2000; Andersen 2002; Grant 2002; Nour 2002; Clark 2003; Hartke 2003; Leathley 2003; Boter 2004; Glass 2004; Harari 2004; Burton 2005; Tilling 2005; Claiborne 2006; Grasel 2006; Harwood 2006; Nir 2006; Boysen 2007; Desrosiers 2007; Ertel 2007; Habibzadeh 2007; Kendall 2007; Pierce 2007; Bakas 2008; Redfern 2008; Shyu 2008; Allen 2009; Battersby 2009; Chaiyawat 2009; Sahebalzamani 2009; Winkens 2009; Gillham 2010; Harrington 2010; Mackay‐Lyons 2010; Bacchini 2011; Chang 2011; Cheng 2011; Chumbler 2011; Clarke 2011; Holzemer 2011; Nguyen 2011), nine studies did not include a random allocation procedure (Evans 1984; Folden 1993; Morrison 1998; Ayana 2000; van den Heuvel 2000; Sit 2007; Huijbregts 2009; Oupra 2010; Brier 2011), we excluded three trials because information provision was not the evaluated intervention (Towle 1989; Mant 2000; Lincoln 2003), three trials included participants with conditions other than stroke and the data for stroke were not available separately (Sanguinetti 1987; Dongbo 2003; Brotons 2007), three trials included motivational interviewing (Green 2006; Adie 2010; Byers 2010) and four lacked a suitable control (Lorenc 1992; Skidmore 2008; Jones 2009; Neubert 2011).
Ongoing studies
Ten studies of potential relevance to this review are ongoing (Damush 2006; Boden‐Albala 2007; Shaughnessy 2007; Young 2007; Rochette 2008; Dromerick 2008; Graven 2008; Hackett 2008; Hoffmann 2009; O'Carroll 2010).
Studies awaiting assessment
There are 14 trials currently awaiting assessment (Bonita 1995; Jian 1998; Heier 2002; Andrea 2003; Choi 2006; Tuncay 2006; Ostwald 2007; Eames 2008; Piano 2010; Bodin 2011; Cameron 2011; Kim 2011; Sun 2011; Aben 2012). We are awaiting further information from authors.
Risk of bias in included studies
Method of analyses
Twelve studies reported that an intention‐to‐treat analysis had been conducted (Pain 1990; Banet 1997; Mant 1998; Rodgers 1999; Johnson 2000; Kalra 2004; Smith 2004; Ellis 2005; Hoffmann 2007; Johnston 2007; Lowe 2007; Maasland 2007).
Allocation
Allocation was concealed in nine trials (Mant 1998; Rodgers 1999; Kalra 2004; Smith 2004; Ellis 2005; Hoffmann 2007; Lowe 2007; Maasland 2007; O'Connell 2009). Larson 2005 reported that the sequence could not be predicted but the method of allocation concealment was not reported. Allocation by random number sequence was reported in one study (Downes 1993). However, the method was not described. Johnson 2000 used a matched pair design with one member of each pair randomly assigned (unconcealed randomisation) to either the treatment or control group. The method of random sequence generation was unclear or not reported in 11 trials (Lomer 1987; Pain 1990; Banet 1997; Frank 2000; Smith 2004; Larson 2005; Draper 2007; Johnston 2007; Lowe 2007; Chiu 2008; Chinchai 2010). One study reported the use of minimisation (Evans 1988). Ellis 2005 reported the use of a computer‐generated random sequence procedure. However, they reported that three patients were entered twice in to the treatment group in error. Further details of allocation concealment and methods of randomisation are provided in the 'Risk of bias' tables.
Blinding
Blinding of both participants and personnel was not a feature in any of the trials or blinding was unclear. Blinding of outcome assessors was reported in 14 trials (Evans 1988; Pain 1990; Downes 1993; Mant 1998; Rodgers 1999; Kalra 2004; Smith 2004; Ellis 2005; Hoffmann 2007; Johnston 2007; Lowe 2007; Maasland 2007; O'Connell 2009; Chinchai 2010), not described in five (Lomer 1987; Banet 1997; Larson 2005; Draper 2007; Chiu 2008) and not undertaken in two (Frank 2000; Johnson 2000). Further details of blinding are provided in the 'Risk of bias' tables.
Incomplete outcome data
The studies ranged in sample size from 36 (Pain 1990) to 300 (Kalra 2004). Losses to follow‐up ranged from zero (Lomer 1987; Johnson 2000; Chinchai 2010) to more than 20% (Downes 1993; Mant 1998; Rodgers 1999; Smith 2004; O'Connell 2009). A sample size calculation was reported for nine trials (Downes 1993; Rodgers 1999; Kalra 2004; Smith 2004; Ellis 2005; Draper 2007; Hoffmann 2007; Maasland 2007; O'Connell 2009). Of these, Downes 1993 reported final follow‐up results for 62 couples rather than the estimated 165, and Draper 2007 recruited only 39 of the 60 caregivers required. Rodgers 1999 recruited the required number of patients and carers in each group but a larger than anticipated number of patients were unable to complete the main outcome measure (Short Form‐36) leading to a short fall in the number of patients required to meet the original power calculation (73%, 117 patients of 160). There was also a short fall in the number of carers at final follow‐up (106 of 216). In the O'Connell 2009 trial, a combination of recruitment and retention problems and non‐use of the intervention resulted in the trial being terminated prior to completion. Sample size was small in a number of trials, particularly: Pain 1990 (N = 36); Banet 1997 (N = 52); Frank 2000 (N = 41); Johnson 2000 (N = 41); and Draper 2007 (N = 39). Further details of attrition bias are provided in the 'Risk of bias' tables.
Selective reporting
Study protocols were not obtained for any of the studies. As a result, it is unclear if selective reporting contributed to bias in the majority of studies. Further details of selective reporting bias are provided in the 'Risk of bias' tables.
Other potential sources of bias
In the trial undertaken by Rodgers 1999, only 51 patients (42%) of those randomised attended three or more out of the six outpatient sessions provided. In Draper 2007, collection of baseline data occurred after randomisation (although participants were still blinded at that point). In O'Connell 2009, the trial was terminated early as it was reported that numerous participants could not remember receiving the information (a sample size of 240 was the initial target, however, the trial was stopped when 66 participants were recruited).
Effects of interventions
Results are reported separately for patients and carers. Resource outcomes are also presented separately. Meta‐analyses have been undertaken for the domains of knowledge, emotional outcome, death, and for selected satisfaction questions. For other outcomes we have presented a narrative summary stratified by subgroup, and a summary of the data are provided in the 'Data and analyses' section.
Patient outcomes
Knowledge
Seven trials (Lomer 1987; Mant 1998; Rodgers 1999; Smith 2004; Hoffmann 2007; Lowe 2007; Maasland 2007) evaluated the effect of a passive or active intervention on knowledge. All had used different questionnaires.
Patient knowledge
Data were available for 536 of 770 participants from six trials (Mant 1998; Rodgers 1999; Smith 2004; Hoffmann 2007; Lowe 2007; Maasland 2007). There was a statistically significant effect on patient knowledge in favour of the intervention (SMD 0.29, 95% CI 0.12 to 0.46, P < 0.001) (Analysis 1.1).
1.1. Analysis.
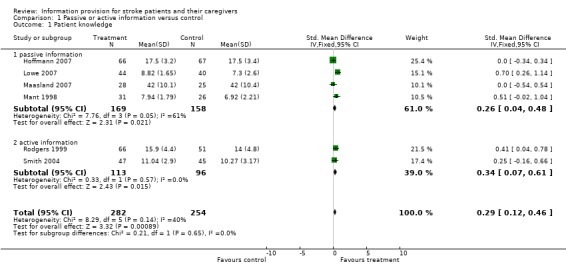
Comparison 1 Passive or active information versus control, Outcome 1 Patient knowledge.
Suitable data were not available for one trial (Lomer 1987). Patients followed up at one week knew significantly more about the aetiology of stroke and the treatment they were receiving than the controls (P < 0.05) but not about the specific prognosis or help and benefits available. This was a small trial, methods of randomisation and outcome assessment are unclear, and comparability of treatment groups is not reported. The results may therefore be subject to bias.
Subgroup analysis
Data were available from four passive information and two active information trials. There was no significant difference in the magnitude of effect between passive and active information (passive: SMD 0.26, 95% CI 0.04 to 0.48, active: SMD 0. 34, 95% CI 0.07 to 0.61, test for subgroup differences P = 0.65) (Analysis 1.1).
Emotional outcomes
We performed meta‐analyses for the outcomes of patient anxiety and patient depression using both dichotomous and continuous data. For each outcome we report the results of both meta‐analyses. A narrative summary is presented for other outcomes.
Anxiety
The majority of trials that evaluated patient anxiety used the anxiety subscale of the Hospital Anxiety and Depression Scale. We converted scale data to dichotomised data using an anxiety subscale cut‐off score of 10/11(Zigmond 1983). Johnston 2007 reported data from the anxiety sub‐scale of the Hospital Anxiety and Depression Scale at baseline only and a total anxiety and depression score post‐intervention.
Patient emotional outcome: anxiety (dichotomised data)
Dichotomous data were available for 681 of 975 participants from six trials (Downes 1993; Mant 1998; Rodgers 1999; Kalra 2004; Smith 2004; Hoffmann 2007). The pooled result for all trials showed no significant difference in the number of cases of anxiety between the intervention and control groups (OR 0.89, 95% CI 0.57 to 1.38, P = 0.60) (Analysis 1.2).
1.2. Analysis.

Comparison 1 Passive or active information versus control, Outcome 2 Patient emotional outcome: anxiety (dichotomised data).
Patient emotional outcome: anxiety (continuous data)
Continuous data were available for 720 of 1016 participants from seven trials (Downes 1993; Mant 1998; Rodgers 1999; Frank 2000; Kalra 2004; Smith 2004; Hoffmann 2007). The pooled result for all trials showed no significant difference in anxiety scores between the intervention and the control groups (MD ‐0.34, 95% CI ‐1.17 to 0.50, P = 0.43) (Analysis 1.3). Johnston 2007 was not included in the meta‐analysis as we were unable to obtain suitable data. However, they reported no significant difference between intervention and control at baseline (P > 0.05) and no significant effects post‐intervention (data and P value not reported).
1.3. Analysis.
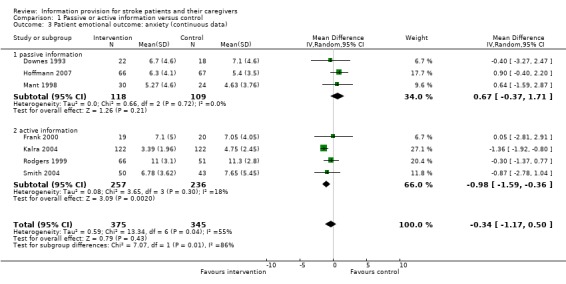
Comparison 1 Passive or active information versus control, Outcome 3 Patient emotional outcome: anxiety (continuous data).
Subgroup analysis
As there was no significant overall effect on anxiety, the following subgroup analyses may be unreliable.
Dichotomous data were available from three passive and three active information trials. The effect on anxiety was not significant in either subgroup (P > 0.05). However, there was a significant difference between active information and passive information on the number of cases of patient anxiety (passive: OR 1.64, 95% CI 0.80 to 3.37; active: OR 0.61 95% CI 0.35 to 1.07, test for subgroup differences P = 0.03). There was a trend towards an increase in anxiety from the passive information and a decrease from the active information.
Continuous data were available from three passive and four active information trials. The effect on anxiety was significant for the active information subgroup (P = 0.002) and not for the passive information subgroup (P = 0.21) There was a significant difference between active information and passive information on patient anxiety scores (passive: MD 0.67, 95% CI ‐0.37 to 1.71; active: MD ‐0.98 95% CI ‐1.59 to ‐0.36, test for subgroup differences P = 0.008). There was a trend towards an increase in anxiety from the passive information.
Depression
Twelve trials evaluated the effect of passive or active information on patient depression. Depression was measured using the depression subscale of the Hospital Anxiety and Depression Scale in eight trials (Downes 1993; Mant 1998; Rodgers 1999; Frank 2000; Kalra 2004; Smith 2004; Hoffmann 2007); Johnston 2007 reported the depression sub‐scale of the Hospital Anxiety and Depression Scale at baseline and a total score post intervention. Other measures included the Geriatric Depression Scale (short form) (Sheikh 1986) by (Ellis 2005); the Beck Depression Inventory (Gallagher 1982) by Johnson 2000, the Yale single question (Mahoney 1994) by Lowe 2007 and the emotions subscale of the Stroke Impact Scale (Duncan 1999) by O'Connell 2009. Scale data were converted to dichotomous data using the recommended cut‐off scores for each outcome measure (hospital anxiety and depression scale depression sub‐scale cut‐off score of 10/11 and a Geriatric Depression Scale score > 10).
Patient emotional outcome: depression (dichotomised data)
Dichotomous data were available for 956 of 1280 participants from eight trials (Downes 1993; Mant 1998; Rodgers 1999; Kalra 2004; Smith 2004; Ellis 2005; Hoffmann 2007; Lowe 2007). The pooled result for all trials showed no significant difference in the number of cases of depression between the intervention and control groups (OR 0.90, 95% CI 0.61 to 1.32, P = 0.59) (Analysis 1.4).
1.4. Analysis.

Comparison 1 Passive or active information versus control, Outcome 4 Patient emotional outcome: depression (dichotomised data).
Patient emotional outcome: depression (continuous data)
Continuous data (Hospital Anxiety and Depression Scale) were available for 720 of 1016 participants from seven trials (Downes 1993; Mant 1998; Rodgers 1999; Frank 2000; Kalra 2004; Smith 2004; Hoffmann 2007). There was a significant effect on depression scores in favour of the intervention (MD ‐0.52, 95% CI ‐0.93 to ‐0.10, P = 0.01) (Analysis 1.5).
1.5. Analysis.
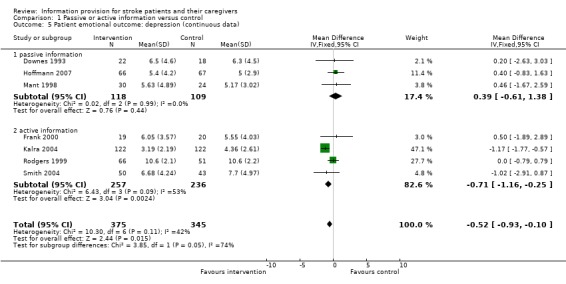
Comparison 1 Passive or active information versus control, Outcome 5 Patient emotional outcome: depression (continuous data).
Two trials were not included in the meta‐analysis as we were unable to obtain suitable data (Johnson 2000; Johnston 2007). Johnson 2000 reported no significant difference between the two groups at baseline but when all patients were reassessed one week after completion of the intervention phase (a four‐week education course), there was a significant difference in the mean depression scores measured by the Beck Depression Inventory (possible score range 0 to 63) (baseline: treatment group 12.52, control 12.94, F = 1.36, P < 0.53; follow‐up: treatment group 8.5, control 12.61, F = 2.79, P < 0.04). Johnston 2007 reported no significant difference between the intervention and the control at baseline (P > 0.05) and no significant effect post intervention (data and P value not reported).
Subgroup analyses
The following subgroup analyses for depression (dichotomous data) may be unreliable as there was no overall net effect.
Dichotomous data were available from four passive and four active information trials. The effect on depression was not significant in either subgroup (P > 0.05). However, there was a significant difference between active information and passive information on the number of cases of patient depression (passive: OR 1.57, 95% CI 0.85 to 2.93; active: OR 0.63, 95% CI 0.38 to 1.03, test for subgroup differences P = 0.02), with a trend in favour of active information.
Continuous data were available from three passive information and four active information trials. The effect on depression was significant in the active information subgroup (P = 0.002) and not in the passive information subgroup (P = 0.44). There was a significant difference between active information and passive information on patient depression scores (passive: MD 0.39, 95% CI ‐0.61 to 1.38; active: MD ‐0.71, 95% CI ‐1.16 to ‐ 0.25, test for subgroup differences P = 0.05).
Other emotional outcomes
An active information study (Johnson 2000) evaluated hope and hopelessness (Herth Hope Scale, score range 0 to 90) (Farran 1995) and coping (Ways of Coping‐Cardiovascular Accident Scale, score range 0 to 93, specifically developed for the study). There were no differences between the two groups at baseline for either outcome. At one week after completion of the intervention phase (a four‐week education course), there was a significant difference between the two groups in the mean hope scale scores (baseline: treatment group 68.89, control 69.2, P < 0.42; follow‐up: treatment group 73.68, control 66.33, P < 0.001). There was no significant difference in the coping scores.
A further active information study (Johnston 2007) evaluated perceived control over recovery utilising the Recovery Locus of Control Scale (Partridge 1989). Scores from nine items on a five‐point scale (strongly agree to strongly disagree) are combined such that higher scores indicate greater belief in personal control. A confidence in recovery scale was also administered (Lewin 1992). This scale measured patients' confidence in recovery from 0 (not at all confident) to 10 (totally confident). There was no significant difference (P > 0.05) between the intervention and the control for perceived control over recovery. There was a significant group by time interaction effect for patients' confidence in recovery, F(1, 197) = 10.67, P = 0.001. Confidence in recovery declined over time for control group patients but remained relatively stable for patients in the intervention group.
Activities of daily living
Passive information studies
There is no evidence of an effect of passive information on activities of daily living. There were no significant differences between the intervention and control groups in any of the four trials that evaluated this outcome (Pain 1990; Banet 1997; Mant 1998; O'Connell 2009).
Active information studies
There is no evidence of an effect of active information on activities of daily living. There were no significant differences between the intervention and control groups in any of the four trials that evaluated this outcome (Kalra 2004; Smith 2004; Draper 2007; Johnston 2007).
Participation
Passive information studies
There is no evidence of an effect of passive information on participation. There were no significant differences between the intervention and control groups in any of the three trials that evaluated this outcome (Mant 1998; Lowe 2007; Maasland 2007).
Active information studies
There is no evidence of an effect of active information on participation. There were no significant differences between the intervention and control groups in any of the trials that evaluated this outcome (Rodgers 1999; Kalra 2004; Smith 2004; Draper 2007).
Social activities
Passive information studies
There is no evidence of an effect of passive information on social activities. The one trial that evaluated this outcome (Pain 1990) reported no significant difference in social activities between the intervention and control groups as measured by the Frenchay activities index (Holbrook 1983).
Active information studies
There is no evidence of an effect of active information on social activities. The two trials that evaluated this outcome (Rodgers 1999; Smith 2004) reported no significant differences in social activities between the intervention and control groups as measured by the Nottingham extended activities of daily living (Nouri 1987) or the Frenchay activities index (Holbrook 1983) respectively.
Perceived health status and quality of life
Passive information studies
There is no evidence of an effect of passive information on patient health status or quality of life (Dartmouth COOP Charts) (Rowan 1994) in the two trials that measured this outcome (Mant 1998; Hoffmann 2007).
Active information studies
Four trials (Rodgers 1999; Frank 2000; Kalra 2004; Ellis 2005) evaluated this outcome. Kalra 2004 reported significantly improved quality in life as measured by the EuroQol visual analogue scale (EuroQol Group 1990) at both three and 12 months in patients whose caregivers had received training (intervention) compared with those who had received conventional care (control) (median score (range) at three months: intervention 60 (42 to 70), control 50 (40 to 90), P = 0.019: median score (range) at 12 months: intervention 65 (55 to 80), control 60 (41 to 80), P = 0.009). Three trials (Rodgers 1999; Frank 2000; Ellis 2005) found no significant difference between the intervention and control groups as measured by the MOS 36‐item short‐form health survey (SF36) (Ware 1992), the Functional Limitations Profile (Patrick 1989) or the EuroQol (EuroQol Group 1990) respectively. Chinchai 2010 investigated quality of life with the WHO Quality of Life Measure (WHOQOL‐BRIEF THAI) (Sakthong 2007). There were significant within group differences (P < 0.05) in the intervention group for the physical, psychological and environmental categories. No significant within‐group differences (P > 0.05) resulted in the control group. Between‐group differences were reported pre‐intervention only (P > 0.05).
Satisfaction with care and information received
Eight trials (Mant 1998; Rodgers 1999; Smith 2004; Ellis 2005; Hoffmann 2007; Johnston 2007; Lowe 2007; O'Connell 2009) evaluated patient satisfaction. Of these, four trials (Mant 1998; Rodgers 1999; Smith 2004; Ellis 2005) measured patient satisfaction using the Pound scale (Pound 1994) or a modified version of that scale. Additionally, the bespoke questionnaire used in the trial by Lowe 2007 included some common items. Meta‐analysis was performed for two questions that were considered to be most relevant to the review: (1) satisfaction with information about the causes and nature of stroke; and (2) satisfaction with information about allowances and services. Three trials did not contribute data to the meta‐analysis (Hoffmann 2007; Johnston 2007; O'Connell 2009). Hoffmann 2007 used a bespoke questionnaire, Johnston 2007 assessed satisfaction with treatment and advice using a 0 to 10 scale applied in a previous study (Morrison 2000). O'Connell 2009 evaluated whether participants in the intervention group recalled receiving and reading the information and taking action as a result of the information.
Patient satisfaction with information about causes and nature of the stroke
Data were available for 541 of 772 participants from five trials (Mant 1998; Rodgers 1999; Smith 2004; Ellis 2005; Lowe 2007). There was a significant difference in favour of the intervention in satisfaction with information about the causes and nature of stroke (OR 2.07, 95% CI 1.33 to 3.23, P = 0.001) (Analysis 1.9).
1.9. Analysis.
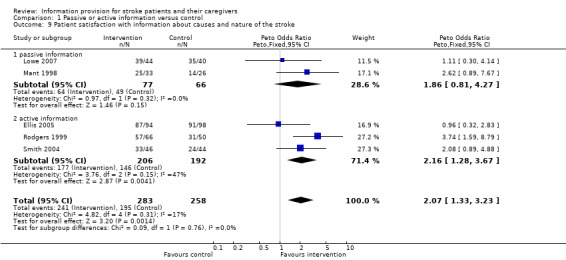
Comparison 1 Passive or active information versus control, Outcome 9 Patient satisfaction with information about causes and nature of the stroke.
Patient satisfaction with information about allowances and services
Data were available for 452 of 672 participants from four trials (Mant 1998; Rodgers 1999; Smith 2004; Ellis 2005). There was no significant difference in satisfaction with information about allowances and services (OR 1.18, 95% CI 0.76 to 1.83, P = 0.46) (Analysis 1.10).
1.10. Analysis.
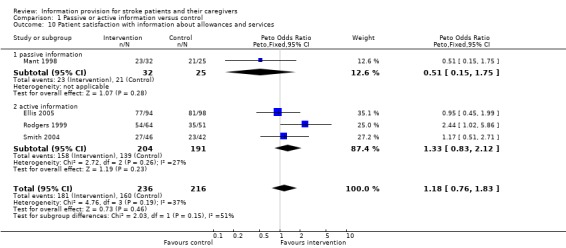
Comparison 1 Passive or active information versus control, Outcome 10 Patient satisfaction with information about allowances and services.
Subgroup analyses
Satisfaction with information about the causes and nature of the stroke
Data were available for two passive information and three active information trials. There was no significant difference in the magnitude of effect of passive compared to active information (passive: OR 1.86, 95% CI 0.81 to 4.27; active: OR 2.16, 95% CI 1.28 to 3.67, test for subgroup differences P > 0.2).
Satisfaction with information about allowances and services
There were insufficient data to perform a subgroup analysis.
Service use
Passive information studies
There is no evidence of an effect of passive information on service use in the one study (Mant 1998) that evaluated this outcome.
Active information studies
There is no evidence of an effect of active information on service use. The four trials that measured this outcome (Evans 1988; Rodgers 1999; Kalra 2004; Smith 2004) reported that there was no significant difference in service use between the intervention and control groups.
Modification of health‐related behaviours or risk reduction
Passive information studies
There is no evidence of an effect of passive information on the modification of health behaviours or risk reduction. Two trials (Banet 1997; Lowe 2007) evaluated this outcome. One trial (Banet 1997) reported no statistically significant difference in scores for diet or medication between the group who received their medical records and the group that received information leaflets only, although actual results were not reported. In the other study (Lowe 2007) there were no statistically significant differences in blood pressure between the intervention group and control groups. In Maasland 2007, those who regularly used tobacco or alcohol reduced these behaviours more in the intervention group, but differences were not significant. There was a decrease in systolic and diastolic blood pressure in the intervention and control group but no significant difference between the groups. Patients in neither group reduced their weight. Serum cholesterol dropped significantly in both the intervention and the control group with no differences between the groups.
Active information studies
Three trials evaluated this outcome (Rodgers 1999; Ellis 2005; Chiu 2008). There is limited evidence of an effect of active information on the modifications of health behaviours or risk reduction from one study. In Chiu 2008, there was a statistically significant difference (P < 0.001) between the intervention and the control group for satisfactory management of blood pressure. However, there was insufficient information reported to determine the effectiveness of the blinding of patients, personnel or outcomes assessment and if allocation concealment was undertaken. There was no significant difference for the management of glucose or lipids. Two trials found no significant difference between the intervention and the control group. In Ellis 2005, they reported that their initial (planned) analysis appeared to demonstrate a statistically significant reduction in systolic blood pressure in the intervention group compared with the control group (P value not reported). However, when the analysis was repeated with adjustment for baseline blood pressure the difference was not significant (P = 0.126). There were no statistically significant changes in other major modifiable risk factors: systolic and diastolic blood pressure; reported smoking rate; cholesterol; random blood glucose; or HbA1c. In the other trial (Rodgers 1999) there was no significant difference in the numbers of patients who stopped smoking after the stroke (intervention 9/25, control 3/17, P = 0.44).
Death
Mortality data were available for 1553 participants from nine trials (Evans 1988; Mant 1998; Rodgers 1999; Kalra 2004; Smith 2004; Ellis 2005; Hoffmann 2007; Lowe 2007; Johnston 2007). There was no significant difference in mortality between the intervention and control groups (OR 0.86 95% CI 0.59 to1.25, P = 0.43) (Analysis 1.13).
1.13. Analysis.
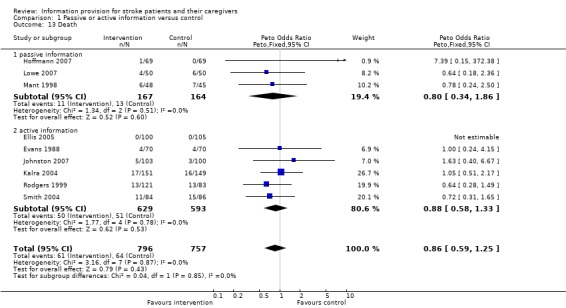
Comparison 1 Passive or active information versus control, Outcome 13 Death.
Subgroup analysis
Data were available from three passive information and six active information trials. There was no significant difference in the magnitude of effect of passive information compared with active information (passive: OR 0.80, 95% CI 0.34 to 1.86; active: OR 0.88, 95% CI 0.58 to1.33, test for subgroup differences P > 0.9).
Carer outcomes
Knowledge
Six trials (Lomer 1987; Evans 1988; Pain 1990; Mant 1998; Rodgers 1999; Smith 2004) evaluated the effect of a passive information or active information intervention on carer knowledge.
Carer knowledge
Data were available for 336 of 469 participants from four trials (Evans 1988; Mant 1998; Rodgers 1999; Smith 2004). There was a significant difference in carer knowledge between the intervention and control groups in favour of the intervention (SMD 0.74, 95% CI 0.06 to 1.43, P = 0.03) (Analysis 1.14).
1.14. Analysis.
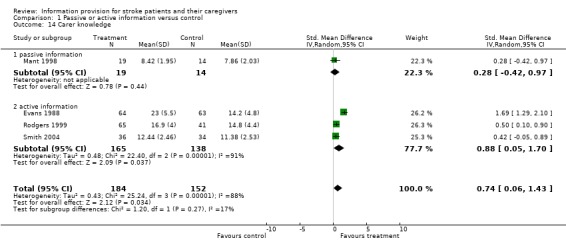
Comparison 1 Passive or active information versus control, Outcome 14 Carer knowledge.
Two small trials did not contribute data to the meta‐analysis (Lomer 1987; Pain 1990); Lomer 1987 found no significant difference in carer knowledge of stroke and no difference in the level of knowledge about the specific prognosis or help and benefits available. Pain 1990 reported that individualised information enhanced the carer's knowledge of how therapists had instructed the patient, although statistical significance was not reached.
Subgroup analysis
There were insufficient data to perform a sub‐group analysis.
Emotional outcomes
We conducted a meta‐analysis for the outcome of carer stress. As a variety of outcome measures were used to measure stress we only used dichotomous data in the analysis. A narrative summary is provided for the carer emotional outcomes of psychological distress, depression and burden.
Psychological distress
Psychological distress in caregivers was measured by Downes 1993, Kalra 2004 and Johnston 2007 using the Hospital Anxiety and Depression Scale (Zigmond 1983). Rodgers 1999 and Smith 2004 used the General Health Questionnaire‐30 or the General Health Questionnaire‐28 (Goldberg 1979). we converted scale data to dichotomous data using the recommended cut‐off scores of 10/11 for the Hospital Anxiety and Depression Scale and 4/5 for the General Health Questionnaire (Goldberg 1979; Zigmond 1983).
Suitable data were not available for Draper 2007 or Johnston 2007. Draper 2007 reported no significant difference in carer stress scores at final follow‐up (three months). Johnston 2007 reported baseline stress data for carers utilising the anxiety sub‐scale of the Hospital Anxiety and Depression Scale. Mean (SD): Intervention 7.64 (4.89), control 7.08 (4.01) and no significant effect of group by time interaction on total Hospital Anxiety and Depression Scale score.
Carer emotional outcome: Psycholgical distress
Dichotomous data were available for 498 of 643 participants from four trials (Downes 1993; Rodgers 1999; Kalra 2004; Smith 2004). There was no significant difference in carer stress between the intervention and the control group (OR 1.13, 95% CI 0.65 to 1.97, P = 0.65) (Analysis 1.16).
1.16. Analysis.
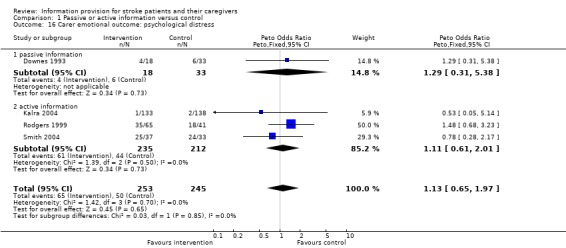
Comparison 1 Passive or active information versus control, Outcome 16 Carer emotional outcome: psychological distress.
Subgroup analysis
There were insufficient data to perform a subgroup analysis.
Depression
Passive information studies
In the Downes 1993 trial, there was no significant difference in depression as measured by the Hospital Anxiety and Depression Scale between carers in the intervention group and the control group (mean depression score at six months (SD): intervention 5.8 (5.2), control 5.1 (3.2). Johnston 2007 reported baseline data for depression of carers, measured by the depression subscale of the Hospital Anxiety and Depression Scale. Mean (SD): intervention 5.7 (4.3), control 4.8 (3.9) and reported no significant effect of group by time interaction on total Hospital Anxiety and Depression Scale score.
Active information studies
One trial (Kalra 2004) evaluated this outcome. Carers in the intervention group were significantly less depressed as measured by the Hospital Anxiety and Depression Scale than carers in the control group (median depression score at one year (IQR): intervention 2 (1 to 3), control 3 (2 to 5); P < 0.0001).
Burden
Passive information studies
In the one trial that evaluated this outcome (Mant 1998) there was no evidence of an effect of passive information on carer burden.
Active information studies
Two trials evaluated this outcome. In the study by Kalra 2004 caregiver burden was significantly reduced in carers in the intervention group compared with the control group at both three months and one year (median score at 12 months (IQR): intervention 32 (27 to 41), control 41 (36 to 50); P = 0.0001). In Draper 2007 there were no significant differences in pre to post‐treatment scores for either the intervention or wait control group.
Social Activities
Passive information studies
No trials evaluated this outcome.
Active information studies
There was no significant difference in carer social activities in the two trials (Kalra 2004; Draper 2007) that evaluated this outcome.
Perceived health status and quality of life
Passive information studies
From the one study (Mant 1998) that evaluated this outcome there is no evidence of an effect of passive information on carer perceived health and quality of life.
Active information studies
Three trials measured this outcome (Rodgers 1999; Kalra 2004; Larson 2005). The largest of these (Kalra 2004) reported that carers in the intervention group had a higher quality of life as measured by the EuroQol visual analogue scale (EuroQol Group 1990) than controls at both three months and one year (median score (IQR) at one year: intervention 80 (70 to 90), control 70 (60 to 80); P < 0.0001). In Rodgers 1999 there were no significant differences between carers in the intervention and the control groups on any of the domains of the SF36 except social functioning. This was significantly higher for carers in the control group (intervention group: mean 66.7 ± 29.8 SD; control group; mean 78.1 ± 27.4 SD; difference between means: 95% CI 11.3; 0.09 to 22.7; P = 0.04). This may be a chance finding due to multiple testing and the authors suggest that it should be interpreted with caution. In the trial by Larson 2005, there were no statistically significant differences between the groups over time.
Satisfaction
Five trials (Pain 1990; Mant 1998; Rodgers 1999; Kalra 2004; Smith 2004) evaluated carer satisfaction. Of these, three trials (Mant 1998; Rodgers 1999; Smith 2004) measured carer satisfaction using the Pound scale (Pound 1993) or a modified version of this scale. Meta‐analyses were performed for two questions considered to be of most relevance to the review: (1) satisfaction with information about recovery and rehabilitation; and (2) satisfaction with information about allowances and services. The remaining studies (Pain 1990; Kalra 2004) both evaluated aspects of carer satisfaction using a bespoke questionnaire and are not included in the meta‐analyses.
Carer satisfaction with information about recovery and rehabilitation
Data were available for 165 of 273 participants from two trials (Rodgers 1999; Smith 2004). There was no significant difference in satisfaction with information about recovery and rehabilitation (OR 1.78, 95% CI 0.88 to 3.60, P = 0.11) (Analysis 1.19).
1.19. Analysis.

Comparison 1 Passive or active information versus control, Outcome 19 Carer satisfaction with information about recovery and rehabilitation.
Carer satisfaction with information about allowances and services
Data were available for 214 of 322 participants from three trials (Mant 1998; Rodgers 1999; Smith 2004) for one question only: there was no significant difference in satisfaction between the groups (OR 1.30, 95% CI 0.71 to 2.37, P = 0.39) (Analysis 1.20).
1.20. Analysis.
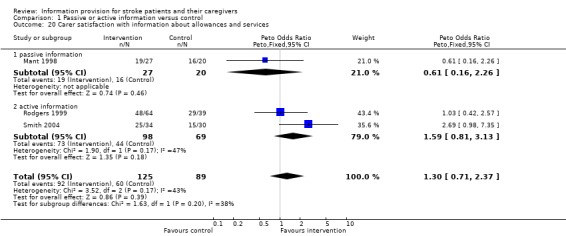
Comparison 1 Passive or active information versus control, Outcome 20 Carer satisfaction with information about allowances and services.
Subgroup analysis
There were insufficient data to perform a subgroup analysis.
Resource outcomes
Cost to health and social services
Passive information studies
No trials evaluated this outcome.
Active information studies
Only one study (Kalra 2004) evaluated resource use. Total health and social care costs over one year for patients whose carers received training (intervention) were significantly lower (MD ‐£4043 ($7249; EUR 6072), 95% CI to ‐£1595 to £6544). The cost differences were largely due to differences in length of hospital stay.
Discussion
This review has explored the effectiveness of information provision for stroke patients and their carers as a process of care aimed at improving stroke recovery. In order to summarise effectively the available evidence on the core concept of information provision, we categorised the studies according to the nature of the intervention using two categories: passive and active. Our intention was to differentiate between interventions where participation was largely passive with no subsequent systematic follow‐up or reinforcement procedure, and those in which there was active participation with a subsequent agreed plan for clarification and reinforcement. This classification was developed, agreed, and adopted prior to results synthesis.
We performed meta‐analyses for the outcomes of knowledge, mood and death, and for selected satisfaction questions. We carried out a qualitative analysis for all other outcomes. We performed meta‐analyses for the outcomes of patient anxiety and depression using both the reported mean and standard deviation of the Hospital Anxiety and Depression Scale scores and dichotomised data. An advantage of using dichotomised data is that it may provide more clinically meaningful results as it relates to 'cases' of depression and anxiety. However, it has been argued that collapsing ordinal stroke trial data in this way can result in a loss of discrimination between groups such that significant treatment effects are missed (OAST 2007).
It is worthy of comment that we undertook extensive searches for the update of this review, we reviewed over 20,000 titles, yet only four new studies are included. This reflects a lack of precision of the search strategies but may also be a reflection on research progress. We excluded 73 studies, many of which evaluated a complex intervention of which information provision and education are components. Information provision is acknowledged as a key component of stroke service delivery, provision of leaflets is not effective and it would seem that new multi‐faceted ways of addressing the information needs of patients and their carers are being developed and evaluated.
Summary of main results
We have identified a total of 21 trials involving 2289 patients and 1290 carer participants. We found some statistically significant but clinically small benefits supporting the general concept that information provision after stroke might improve outcomes. There was evidence of benefit in relation to improved patient and carer knowledge, some aspects of patient‐reported satisfaction and for depression scores in patients. Additionally, we found some evidence that interventions using active information provision may be more effective than passive information for the clinically important outcomes of patient depression and anxiety symptoms. However, as we saw no effect with the dichotomous endpoints of anxiety or depression, effects may be small. We found no evidence that information interventions are associated with improvements in activity limitation, participation or changes in service use.
Overall completeness and applicability of evidence
All included studies were relevant to the review question. There was extensive variation in the content and delivery format of the interventions. This appears to reflect the diversity of interventions provided within clinical practice. Whilst there were sufficient data to address the primary outcomes and the majority of secondary outcomes for this review, there were limited studies to address social activities in carers or resource outcomes. Current practice on information provision after stroke varies nationally and internationally. Our review identified studies from seven countries, thus drawing conclusions on overall applicability of findings internationally is limited.
Quality of the evidence
There was considerable variation in the interventions evaluated and the 21 included trials were of variable quality. Clearly concealed randomisation was achieved in only 10 trials (Mant 1998; Rodgers 1999; Kalra 2004; Smith 2004; Ellis 2005; Larson 2005; Hoffmann 2007; Lowe 2007; Maasland 2007; O'Connell 2009). The rate of attrition was over 20% in five trials (Downes 1993; Mant 1998; Rodgers 1999; Smith 2004; O'Connell 2009). In several trials the sample size was small: less than 75 participants in eight trials (Pain 1990; Downes 1993; Banet 1997; Frank 2000; Johnson 2000; Draper 2007; Maasland 2007; Chinchai 2010).
Our evaluation of the effect on passive or active information provision on the outcome of stroke knowledge was limited by a lack of a consistently‐used measure. Knowledge of stroke was assessed in nine of the 21 studies reviewed (Lomer 1987; Evans 1988; Pain 1990; Mant 1998; Rodgers 1999; Smith 2004; Hoffmann 2007; Lowe 2007; Maasland 2007) but as each study had used a different questionnaire, combining the results in a meta‐analysis was problematic. Our initial intention was to perform a meta‐analysis using dichotomised data (knowledge improved or not improved). However, this was not feasible as in some trials knowledge was measured on one occasion only. We therefore combined the data using the SMD wherein the MDs in outcome between the groups being studied are standardised to account for differences in scoring methods. A disadvantage with this method is that interpretation of the clinical relevance of the treatment effect is difficult as estimated effect sizes serve only as a qualitative measure of the strength of evidence against the null hypothesis (de Beurs 1999). The results should therefore be treated with some caution. In addition, for the majority of the bespoke questionnaires used to measure knowledge there was limited information available about the reliability of the questions they contained.
Potential biases in the review process
Our search strategy was comprehensive and as we were able to identify a number of unpublished studies, publication bias is unlikely. Study selection, data collection and analysis were undertaken by two people, with a third person or consensus meeting used to resolve differences. As a result we are confident of limited bias in the review process for this review.
Agreements and disagreements with other studies or reviews
The positive effects of information on knowledge and depression demonstrated in this review are supported by the findings of reviews of patient education interventions in other conditions. In a meta‐analysis, Brown reported that diabetes education had a moderate to large effect on improving patient knowledge (Brown 1990). A systematic review of education for adults with rheumatoid arthritis showed a small effect on depression (Riemsma 2003). In accord with our review, an overview of systematic reviews of educational interventions for healthcare professionals reported that passive approaches were generally ineffective and unlikely to result in changes in professionals' behaviour, whereas educational approaches involving active learning were more likely to be effective (Grimshaw 2001). A systematic review of education programmes for patients with diabetic kidney disease found education programmes have beneficial effects on improving patients' knowledge of diabetes and some self‐management behavioural changes (Li 2011). A meta‐analysis of patient teaching strategies showed that the greatest effect size was associated with reinforcement, independent study, and the use of multiple strategies (Theis 1995).
Future direction
This review has demonstrated some positive effects of information provision on patient and carer knowledge, aspects of satisfaction and depression. However, the effects, although statistically significant, were clinically small and more effective information provision strategies after stroke need to be developed. The results of the review suggest that a strategy based on an active, rather than passive intervention approach should be adopted. This is perhaps unsurprising as stroke is a complex condition with wide‐ranging effects and probably requires a more profound approach to promote recovery than can be achieved by the provision of passive information alone. The specific components of the active information provision (i.e. involving recipients, planned follow‐up or reinforcement), which resulted in modest beneficial effects on some outcomes, requires further investigation. Future work should focus on the further development of a generalisable active information intervention that could be robustly evaluated in a large multicentre study.
Authors' conclusions
Implications for practice.
There is evidence to support the routine provision of information to stroke patients and their families. Providing information has been shown to improve knowledge of stroke, increase some aspects of patient satisfaction, and reduce patient depression scores. However, the reduction in depression score (as measured by the Hospital Anxiety and Depression Scale) was small and may not be clinically significant. There is currently no evidence that providing information is effective in improving other patient and carer outcomes. Although the best way to provide information is still not clear, the results of the review suggest that strategies that actively involve patients and carers and include planned follow‐up for clarification and reinforcement should be used in routine practice.
Implications for research.
Future work should focus on the further development of a generalisable intervention which could be robustly evaluated in a large multicentre study. The evaluation of interventions is currently limited by the lack of a widely recognised measure of stroke knowledge. Attention should be given to given to the design, development and evaluation of a stroke knowledge questionnaire. Consideration should be given to the most appropriate outcome domains for this type of intervention.
What's new
| Date | Event | Description |
|---|---|---|
| 18 July 2012 | New citation required but conclusions have not changed | The results and the conclusions for this update are the same as for the previous version of the review. |
| 30 December 2011 | New search has been performed | This review update has added four new trials (Johnston 2007; Chiu 2008; O'Connell 2009; Chinchai 2010).The review now includes 21 trials involving 2289 patient and 1290 carer participants. Additional data have been added for analysis for the patient outcome for death. |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 3, 2001
| Date | Event | Description |
|---|---|---|
| 12 May 2008 | Amended | Converted to new review format. Additional text added to the 'Acknowledgements' section and a new 'External sources of support' included. |
Acknowledgements
Northern and Yorkshire NHS Executive Research and Development Directorate funded the original review. This publication presents independent research commissioned by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research funding stream (Grant Reference Number RP‐PG‐0606‐1128 ). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. We would like to thank the authors who provided additional information and data: H Rodgers, H Pain, R Evans, J Mant, L Lorenc, J Johnson, V Morrison, L Kalra, R Hartke, IW Miller, S Jian, D Lowe, T Hoffmann, L Kalra, G Ellis, Dongbo Fu, E Maasland, B Draper, B O'Connell and M Johnston. We also wish to thank Deirdre Andre (University of Leeds) who undertook the search strategies and Tom Crocker and Seline Ozer for assisting with reviewing the papers. We are also grateful for the ongoing support of Brenda Thomas and Hazel Fraser from the Cochrane Stroke Group.
Appendices
Appendix 1. Cochrane search strategy
#1MeSH descriptor Health Education, this term only #2MeSH descriptor Health Promotion, this term only #3MeSH descriptor Patient Education explode all trees #4MeSH descriptor Health Knowledge, Attitudes, Practice, this term only #5MeSH descriptor Telephone, this term only #6MeSH descriptor Pamphlets, this term only #7MeSH descriptor Books, this term only #8MeSH descriptor Manuals, this term only #9MeSH descriptor Audiovisual Aids, this term only #10MeSH descriptor Counseling, this term only #11MeSH descriptor Tape Recording, this term only #12MeSH descriptor Video Recording explode all trees #13MeSH descriptor Patient Participation explode all trees #14(patient *particip*):ti,ab,kw or (patient* complian*):ti,ab,kw #15MeSH descriptor Patient Compliance explode all trees #16MeSH descriptor Patient Satisfaction explode all trees#17(patient* satis*):ti,ab,kw #18(doctor* patient* communic*):ti,ab,kw or (patient* doctor* communic*):ti,ab,kw or (nurse* patient* communic*):ti,ab,kw or (patient* nurse* communic*):ti,ab,kw #19(professional* patient* communic*):ti,ab,kw or (patient* professional* communic*):ti,ab,kw #20(consumer* health inform*):ti,ab,kw #21(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20) #22(patient* ):ti,ab,kw or (inpatient*):ti,ab,kw or (care*):ti,ab,kw or (caregiver *):ti,ab,kw or (family):ti,ab,kw NEAR/5 (education):ti,ab,kw or (information):ti,ab,kw or (support):ti,ab,kw or (knowledge):ti,ab,kw or (counsel*):ti,ab,kw #23(patient* ):ti,ab,kw or (inpatient*):ti,ab,kw or (care*):ti,ab,kw or (caregiver *):ti,ab,kw or (family):ti,ab,kw NEAR/5 (book*):ti,ab,kw or (leaflet*):ti,ab,kw or (pack*):ti,ab,kw or (video*):ti,ab,kw or (tape*):ti,ab,kw or (manual*):ti,ab,kw or (advice*):ti,ab,kw #24(education):ti,ab,kw or (information):ti,ab,kw or (teach*):ti,ab,kw NEAR/5 (book*):ti,ab,kw or (leaflet*):ti,ab,kw or (pack*):ti,ab,kw or (video*):ti,ab,kw or (tape*):ti,ab,kw or (manual*):ti,ab,kw or (advice*):ti,ab,kw #25(education):ti,ab,kw or (information):ti,ab,kw or (teach*):ti,ab,kw NEAR/5 (program* ):ti,ab,kw or (intervention*):ti,ab,kw or (material*):ti,ab,kw or (resource*):ti,ab,kw #26(#21 OR #22 OR #23 OR #24 OR #25) #27MeSH descriptor Cerebrovascular Disorders explode all trees #28(stroke*):ti,ab,kw or (cerebrovascular*):ti,ab,kw or (transient ischemic attack*):ti,ab,kw or (transient ischaemic attack*):ti,ab,kw #29MeSH descriptor Hemiplegia, this term only #30(asphasi*):ti,ab,kw or (dysphasi*):ti,ab,kw or (hemianopi*):ti,ab,kw or (hemiplegi*):ti,ab,kw or (hemipar*):ti,ab,kw #31(#27 OR #28 OR #29 OR #30) #32(#26 AND #31) #33(#32), from 2011 to 2012 #34(#32), from 2011 to 2012 #35(#32), from 2011 to 2012 #36(#32), from 2011 to 2012 #37(#32), from 2011 to 2012
Appendix 2. MEDLINE search strategy
The following search strategy was used for MEDLINE and was modified to suit other databases ( / denotes MeSH headings, .tw denotes words in the title and abstract, $ is the truncation symbol and adj5 denotes word combinations within 5 words) 1. Health education / 2. Health promotion/ 3. Patient education/ 4. Knowledge attitudes, practice/ 5. Telephone/ 6. Pamphlets/ 7. Books/ 8. Manuals/ 9. Audiovisual aids/ 10. Counseling/ 11. Tape recording/ 12. Exp Video recording/ 13. Exp Patient participation/ 14. Patient$ particip$.tw. 15. Exp Patient compliance/ 16. Patient$ complian$.tw. 17. Exp Patient satisfaction/ 18. Patient$ satis$.tw. 19. (Doctor$ patient$ communic$ or Patient$ doctor$ communic$).tw. 20. (Nurse$ patient$ communic$ or Patient$ nurse$ communic$).tw. 21. (Professional$ patient$ communic$ or Patient$ professional$ communic$).tw. 22. Consumer$ health inform$.tw. 23. Or/1‐22 24. (Patient$ or inpatient$ or care$ or care?giver$ or family).tw. 25. (Education or information or support or knowledge or counsel$).tw. 26. 24 adj5 25 27. (Book$ or leaflet$ or pack$ or video$ or tape$ or telephone or manual$ or advice).tw. 28. 24 adj5 27 29. (Education$ or information$ or teach$).tw. 30. 29 adj5 27 31. (Program$ or intervention$ or material$ or resource$).tw. 32. 29 adj5 31 33. 23 or 26 or 28 or 30 or 32 34. Exp Cerebrovascular disorders/ 35. Stroke$.tw. 36. Cerebrovascular$.tw. 37. Transient Isch?emic attack$.tw 38. Hemiplegia/ 39. (Aphasi$ or dysphasi$ or hemianopi$).tw. 40. (Hemiplegi$ or hemipar$).tw. 41. Or/34‐40 42. 33 and 41
Appendix 3. EMBASE search strategy
cerebrovascular disease/
basal ganglion hemorrhage/
cerebral artery disease/
cerebrovascular accident/
stroke/
stroke patient/ or stroke unit/
vertebrobasilar insufficiency/
exp carotid artery disease/
exp brain hemangioma/
exp brain hematoma/
exp brain hemorrhage/
brain infarction/ or brain infarction size/ or brain stem infarction/ or cerebellum infarction/
exp Brain Ischemia/
exp Cerebrovascular Malformation/
exp intracranial aneurysm/
exp occlusive cerebrovascular disease/
brain injury/
brain stem injury/ or artery dissection/
cerebellum injury/
exp carotid artery/
exp carotid artery surgery/
carotid endarterectomy/
*heart atrium septum defect/ or heart foramen ovale/
*heart atrium fibrillation/
paradoxical embolism/
exp aphasia/ or hemiplegia/ or hemiparesis/ or paresis/ or spastic paresis/ or pseudobulbar palsy/ or hemianopia/ or homonymous hemianopia/ or dysphagia/ or dysarthria/ or dysphasia/ or spasticity/ or apraxia/ or dyspraxia/ or hemiballism/
(stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or isch?emi$ attack$ or tia$1 or neurologic$ deficit$ or SAH or AVM).tw.
((brain$ or cerebr$ or cerebell$ or cortical or vertebrobasilar or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or MCA or anterior circulation or posterior circulation or basal ganglia) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypox$ or vasospasm or obstruction or vasculopathy)).tw.
((lacunar or cortical) adj5 infarct$).tw.
((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or subarachnoid or putaminal or putamen or posterior fossa) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
((brain or cerebral or intracranial or communicating or giant or basilar or vertebral artery or berry or saccular or ruptured) adj5 aneurysm$).tw.
(vertebral artery dissection or cerebral art$ disease$).tw.
((brain or intracranial or basal ganglia or lenticulostriate) adj5 (vascular adj5 (disease$ or disorder or accident or injur$ or trauma$ or insult or event))).tw.
((isch?emic or apoplectic) adj5 (event or events or insult or attack$)).tw.
((cerebral vein or cerebral venous or sinus or sagittal) adj5 thrombo$).tw.
(CVDST or CVT).tw.
((intracranial or cerebral art$ or basilar art$ or vertebral art$ or vertebrobasilar or vertebral basilar) adj5 (stenosis or isch?emia or insufficiency or arteriosclero$ or atherosclero$ or occlus$)).tw.
((venous or arteriovenous or brain vasc$) adj5 malformation$).tw.
((brain or cerebral) adj5 (angioma$ or hemangioma$ or haemangioma$)).tw.
carotid$.hw.
(patent foramen ovale or PFO).tw.
((atrial or atrium or auricular) adj fibrillation).tw.
asymptomatic cervical bruit.tw.
(aphasi$ or apraxi$ or dysphasi$ or dysphagi$ or deglutition disorder$ or swallow$ disorder$ or dysarthri$ or hemipleg$ or hemipar$ or paresis or paretic or hemianop$ or hemineglect or spasticity or anomi$ or dysnomi$ or acquired brain injur$ or hemiball$).tw.
((unilateral or visual or hemispatial or attentional or spatial) adj5 neglect).tw.
or/1‐45
health education/
health promotion/
patient education/
exp patient attitude/
patient counseling/
patient guidance/
patient information/
exp information/
patient participation/
patient$ particip$.tw.
patient compliance/
patient complian$.tw.
patient satisfaction/
patient$ satis$.tw.
(doctor$ patient$ communic$ or patient$ doctor$ communic$).tw.
(nurse$ patient$ communic$ or patient$ nurse$ communic$).tw.
(professional$ patient$ communic$ or patient$ professional$ communic$).tw.
exp consumer health information/
consumer$ health inform$.tw.
attitude/
telephone/
book/
exp audiovisual equipment/
education program/
teaching/
education/
comprehension/
self help/
((written or printed or oral) adj1 information).tw.
(education$ adj1 method$).tw.
((patient$ or inpatient$ or carer$ or care?giver$ or care?provider or family) adj5 (education$ or information or support or knowledge or (understand$ or comprehen$ or counsel$ or self?help))).tw.
((patient$ or inpatient$ or carer$ or care?giver$ or care?provider$ or family) adj5 (book$ or leaflet$ or pack$ or video$ or work?book$ or tape$ or telephone or phone or manual$ or advice)).tw.
((education$ or information or teach$ or self?help) adj5 (book$ or leaflet$ or pack$ or video$ or work?book$ or tape$ or telephone or phone or manual$ or advice)).tw.
((education$ or information or teach$ or self?help) adj5 (program$ or intervention$ or material$ or resource$ or meeting$ or session$ or strateg$ or work?shop$ or visit$)).tw.
or/47‐80
randomized controlled trial/
randomization/
controlled study/
control group/
clinical trial/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ or controlled clinical trial/
crossover procedure/
double blind procedure/
single blind procedure/ or triple blind procedure/
latin square design/
parallel design/
placebo/
multicenter study/
experimental design/ or experimental study/ or quasi experimental study/
experimental therapy/
drug comparison/ or drug dose comparison/
drug screening/
evaluation/ or "evaluation and follow up"/ or evaluation research/ or clinical evaluation/
methodology/
"types of study"/
research subject/
comparative study/
"systematic review"/
meta analysis/
random$.tw.
(controlled adj5 (trial$ or stud$)).tw.
(clinical$ adj5 trial$).tw.
((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
(surgical adj5 (group$ or subject$ or patient$)).tw.
(quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
((multicenter or multicentre or therapeutic) adj5 (trial$ or stud$)).tw.
((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
(coin adj5 (flip or flipped or toss$)).tw.
latin square.tw.
versus.tw.
(cross‐over or cross over or crossover).tw.
placebo$.tw.
sham.tw.
(assign$ or alternate or allocat$ or counterbalance$ or multiple baseline).tw.
controls.tw.
(treatment$ adj6 order).tw.
(meta‐analy$ or metaanaly$ or meta analy$ or systematic review or systematic overview).tw.
or/82‐123
human/
nonhuman/
125 and 126
126 not 127
124 not 128
46 and 129
130 and 81
limit 131 to yr="2008 ‐ 2010"
limit 132 to english language
Appendix 4. CINAHL search strategy
S154 S63 and S128 and S153 S153 S129 or S130 or S131 or S132 or S133 or S134 or S135 or S136 or S137 or S138 or S139 or S140 or S141 or S142 or S143 or S144 or S145 or S146 or S147 or S148 or S149 or S150 or S151 or S152 S152 TX "meta*analys*" or TX "meta analys*" or TX "systematic review*" S151 TX counterbalance* or TX ("multiple baseline*") or TX("ABAB design*") S150 TX( clin* N10 trial*) or TX (intervention* N10 trial*) or TX (compar* N10 trial*) or TX (experiment* N10 trial*) or TX (preventive N10 trial*) or TX (therapeutic N10 trial*) S149 TX (crossover or "cross over" or placebo* or control* or factorial or sham*) S148 TX (trebl* N25 blind*) or TX (trebl* N25 mask*) S147 TX (tripl* N25 blind*) or TX(tripl* N25 mask*) S146 TX (doubl* N25 blind*) or TX (doubl* N25 mask*) S145 TX (singl* N25 blind*) or TX (singl* N25 mask*) S144 TX random* S143 PT ("clinical trial") or PT ("systematic review") S142 MH community trials or MH experimental studies or MH one‐shot case study or MH pretest‐posttest design or MH solomon four‐group design or MH static group comparison or MH study design S141 MH clinical nursing research or MH clinical research S140 MH meta analysis S139 MH placebos S138 MH nonrandomized trials S137 MH quasi‐experimental studies S136 MH factorial design S135 MH control group S134 MH "control (research)" S133 MH comparative studies S132 MH clinical trials+ S131 MH crossover design S130 MH random sample+ S129 MH random assignment S128 S64 or S65 or S66 or S67 or S68 or S71 or S74 or S75 or S78 or S79 or S81 or S82 or S85 or S87 or S88 or S94 or S98 or S99 or S100 or S102 or S103 or S104 or S105 or S106 or S107 or S108 or S109 or S110 or S111 or S112 or S113 or S114 or S115 or S116 or S117 or S118 or S119 or S120 or S123 or S124 or S125 or S126 or S127 S127 TX (occupational and therapy) or TX(activity and therapy) S126 MH cognition disorders+ S125 MH "activity therapy (iowa nic)" S124 MH home occupational therapy or MH occupational therapy S123 S121 and S122 S122 TX (disorder* or therap* or impair* or rehabilitation) S121 TX (speech or cognit* or language) S120 MH articulation disorders or MH fluency disorders or MH speech disorders S119 MH "impaired verbal communication (nanda)" S118 MH "rehabilitation, speech and language+" S117 MH language disorders S116 TX (swallow* and impair*) or TX (swallow* and disorder*) or TX (swallow* and problem*) or TX (swallow* and difficult*) S115 TX (dysarthri* or dysphag* or aprax* or dysprax*) S114 MH apraxia or MH neurologic manifestations S113 MH "swallowing therapy (iowa nic)" S112 (MH "Swallowing Impairment (Saba CCC)") S111 MH "impaired swallowing (nanda)" S110 MH deglutition disorders S109 MH communicative disorders S108 MH dysarthria S107 TX ("unilateral neglect" or "neglect syndrome*" or "visual neglect" or hemianop*) S106 TX (aphasi* or dysphasi* or hemipleg* or hemipar*) S105 MH "unilateral neglect (nanda)" S104 MH hemiplegia S103 MH aphasia+ S102 S93 and S101 S101 (MH "Embolism and Thrombosis (Non‐Cinahl)+") S100 TX atrial fibrillation S99 MH atrial fibrillation S98 S72 and S97 S97 S95 and S96 S96 TX (fistula* or malformation* or aneurysm*) S95 TX(arteriovenous or venous) S94 (S89 and S93) S93 (S90 or S91 or S92) S92 MH cerebral arteries S91 MH cerebral veins S90 MH brain+ S89 MH arteriovenous malformations+ S88 TX carotid* S87 (S83 and S86) S86 MH arterial occlusive diseases+ S85 S83 and S84 S84 MH endarterectomy S83 MH carotid arteries S82 (MH "Endarterectomy, Carotid") S81 (MH "Carotid Arteries/SU") S80 TX tia S79 TX ("trans* ischaemic attack*" or "trans* ischemic attack*") S78 S76 and S77 S77 TX (intracranial or venous N5 sinus or sagittal N5 venous or sagittal N5 vein or cranial N5 sinus) S76 TX thrombo* S75 TX "sinus thrombosis" S74 S72 and S73 S73 TX (haemorrhage or hemorrhage or haematoma or hematoma or bleed* or aneurysm) S72 TX (cerebral or intracerebral or intracranial or parenchymal or brain* or intraventricular or periventricular or cerebellar or infratentorial or supratentorial or subarachnoid) S71 S69 and S70 S70 TX (infarct* or ischaemi* or ischemi* or thrombo* or emboli* or vasospasm* or apople*) S69 TX (cerebral or cerebellar or brain* or vertebrobasilar) S68 TX "cerebral vascular*" S67 TX cerebrovasc* S66 TX cva* S65 TX stroke* S64 MH cerebrovascular disorders or MH carotid artery diseases+ or MH cerebral aneurysm or MH "cerebral embolism and thrombosis" or MH cerebral ischemia+ or MH cerebral vasospasm or MH intracranial hemorrhage+ or MH vertebral artery dissections or MH stroke patients or MH stroke units S63 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 or S48 or S49 or S50 or S51 or S52 or S53 or S54 or S55 or S56 or S57 or S58 or S59 or S60 or S61 or S62 S62 TX (education* N5 resource* or information* N5 resource*) S61 TX (education* N5 material* or information* N5 material*) S60 TX (education* N5 intervention* or information* N5 intervention*) S59 TX (education* N5 program* or information* N5 program*) S58 TX (education* N5 advice* or information* N5 advice* or material* N5 advice* or resource* N5 advice*) S57 TX (education* N5 manual* or information* N5 manual* or material* N5 manual* or resource* N5 manual*) S56 TX (education* N5 telephone* or information* N5 telephone* or material* N5 telephone* or resource* N5 telephone*) S55 TX (education* N5 phone* or information* N5 phone* or material* N5 phone* or resource* N5 phone*) S54 TX (education* N5 tape* or information* N5 tape* or material* N5 tape* or resource* N5 tape*) S53 TX (education* N5 video* or information* N5 video* or material* N5 video* or resource* N5 video*) S52 TX (education* N5 pack* or information* N5 pack* or material* N5 pack* or resource* N5 pack*) S51 TX (education* N5 leaflet* or information* N5 leaflet* or material* N5 leaflet* or resource* N5 leaflet*) S50 TX (education* N5 book* or information* N5 book* or material* N5 book* or resource* N5 book*) S49 TX (patient* N5 advice* or inpatient* N5 advice* or care* N5 advice* or caregiver* N5 advice* or "care giver*" N5 advice* or family N5 advice*) S48 TX (patient* N5 manual* or inpatient* N5 manual* or care* N5 manual* or caregiver* N5 manual* or "care giver*" N5 manual* or family N5 manual*) S47 TX (patient* N5 telephone* or inpatient* N5 telephone* or care* N5 telephone* or caregiver* N5 telephone* or care giver* N5 telephone* or family N5 telephone* ) S46 TX (patient* N5 phone* or inpatient* N5 phone* or care* N5 phone* or caregiver* N5 phone* or "care giver*" N5 phone* or family N5 phone*) S45 TX (patient* N5 video* or inpatient* N5 video* or care* N5 video* or caregiver* N5 video* or "care giver*" N5 video* or family N5 video*) S44 TX (patient* N5 video* or inpatient* N5 video* or care* N5 video* or caregiver* N5 video* or "care giver*" N5 video* or family N5 video*) S43 TX (patient* N5 pack* or inpatient* N5 pack* or care* N5 pack* or caregiver* N5 pack* or "care giver*" N5 pack* or family N5 pack*) S42 TX (patient* N5 leaflet* or inpatient* N5 leaflet* or care* N5 leaflet* or caregiver* N5 leaflet* or "care giver*" N5 leaflet* or family N5 leaflet*) S41 TX (patient* N5 book* or inpatient* N5 book* or care* N5 book* or caregiver* N5 book* or "care giver*" N5 book* or family N5 book*) S40 TX (Family N5 education or family N5 information or family N5 support or family N5 Knowledge or family N5 counsel*) S39 TX ("care giver" N5 education or "care giver" N5 information or "care giver" N5 support or "care giver" N5 Knowledge or "care giver" N5 counsel* ) S38 TX (caregiver N5 education or caregiver N5 information or caregiver N5 support or caregiver N5 Knowledge or caregiver N5 counsel* ) S37 TX (care* N5 education or care* N5 information or care* N5 support or care* N5 Knowledge or care* N5 counsel*) S36 TX (inpatient* N5 education or inpatient* N5 information or inpatient* N5 support or inpatient* N5 Knowledge or inpatient* N5 counsel* ) S35 TX (patient* N5 education or patient* N5 information or patient* N5 support or patient* N5 Knowledge or patient* N5 counsel*) S34 TX ("consumer* health inform*") S33 MH consumer health information S32 MH health information S31 TX (professional* patient* communic*) or TX (patient* nurse* communic*) S30 TX ("nurse* patient* communic*") or TX ("patient* nurse* communic*") S29 TX ("doctor* patient* communic*") or TX ("patient* doctor* communic*") S28 TX ("patient* satis*") S27 MH patient satisfaction S26 TX ("patient complian*") S25 MH patient compliance+ S24 TX (patient* N1 particip*) S23 TX (patient* N1 consumer*) S22 MH consumer participation S21 MH videorecording S20 MH audiorecording S19 (MH "Teaching, Guidance, and Counseling (Omaha)") S18 (MH "Counseling Service (Saba CCC)") S17 MH counseling "(Iowa Nic)" S16 MH counseling S15 MH optical disks+ S14 MH multimedia S13 MH teaching materials S12 MH audiovisuals S11 MH books S10 MH pamphlets S9 MH telephone information services S8 MH telephone consultation "(IOWA NIC)" S7 MH telephone S6 MH information resources S5 MH attitude to health S4 MH health knowledge S3 MH patient education S2 MH health promotion S1 MH health education
Appendix 5. PsycINFO search strategy
Health Education/
health promotion/
client education/
health knowledge/
instructional media/ or educational audiovisual aids/ or reading materials/
printed communications media/
books/
counseling/ or rehabilitation counseling/
internet/
television/
teaching machines/ or computer assisted instruction/ or programmed instruction/
videotapes/
computer mediated communication/
client attitudes/
group counseling/
written communication/ or verbal communication/
treatment compliance/
client participation/
client satisfaction/
((patient$ or client$) adj particip$).tw.
((patient$ or client$) adj complian$).tw.
((patient$ or client$) adj satisfact$).tw.
patient$ doctor$ communicat$.tw.
doctor$ patient$ communicat$.tw.
patient$ nurse$ communicat$.tw.
nurse$ patient$ communicat$.tw.
patient$ professional$ communicat$.tw.
(professional$ adj (client$ or patient$) adj communicat$).tw.
"provider to client communication".id.
"therapeutic communication".id.
educational programs/
teaching/
education/
exp verbal comprehension/
comprehension/
consumer$ health information.tw.
or/1‐36
((patient$ or client$ or inpatient$ or carer$ or care?giver$ or care?provider$ or family) adj3 (education or information or support or teach$ or training or knowledge or counsel$)).tw.
((patient$ or client$ or inpatient$ or carer$ or care?giver$ or care?provider$ or family) adj5 (book$ or leaflet$ or sheet$ or pack$ or video$ or tape$ or telephone or phone or internet or www or manual$ or advice)).tw.
((education or information or support or teach$ or training or knowledge or counsel$) adj5 (book$ or leaflet$ or sheet$ or pack$ or video$ or tape$ or telephone or phone or internet or www or manual$ or advice)).tw.
((program$ or intervention or material$1 or resource$1) adj5 (education or information or support or teach$ or training or knowledge or counsel$)).tw.
37 or 38 or 39 or 40 or 41
exp cerebrovascular disorders/
exp traumatic brain injury/
exp carotid arteries/
*"fibrillation (heart)"/
(stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or isch?emi$ attack$ or tia$1 or neurologic$ deficit$ or SAH or AVM).tw.
((brain$ or cerebr$ or cerebell$ or cortical or vertebrobasilar or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or MCA or anterior circulation or posterior circulation or basal ganglia) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypox$ or vasospasm or obstruction or vasculopathy)).tw.
((lacunar or cortical) adj5 infarct$).tw.
((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or subarachnoid or putaminal or putamen or posterior fossa) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
((brain or cerebral or intracranial or communicating or giant or basilar or vertebral artery or berry or saccular or ruptured) adj5 aneurysm$).tw.
(vertebral artery dissection or cerebral art$ disease$).tw.
((brain or intracranial or basal ganglia or lenticulostriate) adj5 (vascular adj5 (disease$ or disorder or accident or injur$ or trauma$ or insult or event))).tw.
((isch?emic or apoplectic) adj5 (event or events or insult or attack$)).tw.
((cerebral vein or cerebral venous or sinus or sagittal) adj5 thrombo$).tw.
(CVDST or CVT).tw.
((intracranial or cerebral art$ or basilar art$ or vertebral art$ or vertebrobasilar or vertebral basilar) adj5 (stenosis or isch?emia or insufficiency or arteriosclero$ or atherosclero$ or occlus$)).tw.
((venous or arteriovenous or brain vasc$) adj5 malformation$).tw.
((brain or cerebral) adj5 (angioma$ or hemangioma$ or haemangioma$)).tw.
carotid$.tw.
(patent foramen ovale or PFO).tw.
((atrial or atrium or auricular) adj fibrillation).tw.
asymptomatic cervical bruit.tw.
exp aphasia/ or hemiplegia/ or hemianopia/ or dysphagia/ or dysarthria/
(aphasi$ or apraxi$ or dysphasi$ or dysphagi$ or deglutition disorder$ or swallow$ disorder$ or dysarthri$ or hemipleg$ or hemipar$ or paresis or paretic or hemianop$ or hemineglect or spasticity or anomi$ or dysnomi$ or acquired brain injur$ or hemiball$ or pseudobulbar palsy or musc$ spas$).tw.
((unilateral or visual or hemispatial or attentional or spatial) adj5 neglect).tw.
or/43‐66
42 and 67
limit 68 to yr="2008 ‐ 2010"
limit 69 to english language
Data and analyses
Comparison 1. Passive or active information versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient knowledge | 6 | 536 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.29 [0.12, 0.46] |
| 1.1 passive information | 4 | 327 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.26 [0.04, 0.48] |
| 1.2 active information | 2 | 209 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.34 [0.07, 0.61] |
| 2 Patient emotional outcome: anxiety (dichotomised data) | 6 | 681 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.89 [0.57, 1.38] |
| 2.1 passive information | 3 | 227 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.64 [0.80, 3.37] |
| 2.2 active information | 3 | 454 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.61 [0.35, 1.07] |
| 3 Patient emotional outcome: anxiety (continuous data) | 7 | 720 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐1.17, 0.50] |
| 3.1 passive information | 3 | 227 | Mean Difference (IV, Random, 95% CI) | 0.67 [‐0.37, 1.71] |
| 3.2 active information | 4 | 493 | Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.59, ‐0.36] |
| 4 Patient emotional outcome: depression (dichotomised data) | 8 | 956 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.61, 1.32] |
| 4.1 passive information | 4 | 311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.57 [0.85, 2.93] |
| 4.2 active information | 4 | 645 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.63 [0.38, 1.03] |
| 5 Patient emotional outcome: depression (continuous data) | 7 | 720 | Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐0.93, ‐0.10] |
| 5.1 passive information | 3 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.39 [‐0.61, 1.38] |
| 5.2 active information | 4 | 493 | Mean Difference (IV, Fixed, 95% CI) | ‐0.71 [‐1.16, ‐0.25] |
| 6 Patient activities of daily living and participation: summary of results | Other data | No numeric data | ||
| 7 Patient social activities: summary of results | Other data | No numeric data | ||
| 8 Patient perceived health status and quality of life: summary of results | Other data | No numeric data | ||
| 9 Patient satisfaction with information about causes and nature of the stroke | 5 | 541 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.07 [1.33, 3.23] |
| 9.1 passive information | 2 | 143 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.86 [0.81, 4.27] |
| 9.2 active information | 3 | 398 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.16 [1.28, 3.67] |
| 10 Patient satisfaction with information about allowances and services | 4 | 452 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.18 [0.76, 1.83] |
| 10.1 passive information | 1 | 57 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.15, 1.75] |
| 10.2 active information | 3 | 395 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.33 [0.83, 2.12] |
| 11 Service use: summary of results | Other data | No numeric data | ||
| 12 Modification of health related behaviours: summary of results | Other data | No numeric data | ||
| 13 Death | 9 | 1553 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.86 [0.59, 1.25] |
| 13.1 passive information | 3 | 331 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.34, 1.86] |
| 13.2 active information | 6 | 1222 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.58, 1.33] |
| 14 Carer knowledge | 4 | 336 | Std. Mean Difference (IV, Random, 95% CI) | 0.74 [0.06, 1.43] |
| 14.1 passive information | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.42, 0.97] |
| 14.2 active information | 3 | 303 | Std. Mean Difference (IV, Random, 95% CI) | 0.88 [0.05, 1.70] |
| 15 Carer emotional outcome: summary of results | Other data | No numeric data | ||
| 16 Carer emotional outcome: psychological distress | 4 | 498 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.13 [0.65, 1.97] |
| 16.1 passive information | 1 | 51 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.29 [0.31, 5.38] |
| 16.2 active information | 3 | 447 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.11 [0.61, 2.01] |
| 17 Carer social activities: summary of results | Other data | No numeric data | ||
| 18 Carer perceived health status and quality of life: summary of results | Other data | No numeric data | ||
| 19 Carer satisfaction with information about recovery and rehabilitation | 2 | 165 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.78 [0.88, 3.60] |
| 19.1 active information | 2 | 165 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.78 [0.88, 3.60] |
| 20 Carer satisfaction with information about allowances and services | 3 | 214 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.30 [0.71, 2.37] |
| 20.1 passive information | 1 | 47 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.61 [0.16, 2.26] |
| 20.2 active information | 2 | 167 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.59 [0.81, 3.13] |
| 21 Cost to health and social services: summary of results | Other data | No numeric data | ||
| 22 Self management: summary of results | Other data | No numeric data | ||
| 23 Family functioning and patient adjustment: summary of results | Other data | No numeric data |
1.6. Analysis.
Comparison 1 Passive or active information versus control, Outcome 6 Patient activities of daily living and participation: summary of results.
| Patient activities of daily living and participation: summary of results | |||||
|---|---|---|---|---|---|
| Study | Follow‐up | Comparison | Outcome measure | Results | Notes |
| Banet 1997 | 6 months | Patient education packet and shared medical record versus patient education packet | Glasgow Outcome Scale Global Outcome Scale (not validated) | Number of participants with outcome data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: 24/number unclear, control 28/number unclear Comparison between shared record and control groups Glasgow Outcome Scale F < 0.01, P = 0.74 Global Outcome Scale F = 1.7, P = 0.20 | |
| Frank 2000 | 1 month | Workbook + home visits and telephone contact versus wait control | Functional Limitations Profile | Number of participants with outcome data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 19/20, control 20/21 Mean score before intervention: intervention 69.62 (SD 17.77), control 71.73 (SD 25.41) 1 month: intervention 64.03 (SD 20.96), control 66.89 (SD 22.87) | |
| Johnston 2007 | 8 weeks (post intervention) and 6 months from baseline | Post discharge workbook intervention including information and audio relaxation tape versus usual care | Barthel Index (transformed scores) | Number of participants with outcome data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 74/103, control 84/100
Mean score before intervention: intervention 1.57 (SD 0.73), control 1.50 (SD 0.63)
8 weeks: intervention 1.44 (SD 0.65), control 1.43 (SD 0.59) 6 months: intervention 1.43 (SD 0.68), control 1.39 (SD 0.61) |
Transformed scores: higher score = higher disability |
| Kalra 2004 | 3 and 12 months | Education sessions plus hands on training versus conventional care | Barthel Index Modified Rankin Scale | Number of participants with Barthel Index data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 134/151, control 134/149 Barthel Index score > 18 3 months: intervention 77/141, control 52/140 12 months: intervention 93/134, control 75/134 Number of participants Modified Rankin Score data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 133/151, control 134/149 Modified Rankin Scale score 0 to 2 3 months: intervention 80/141, control 63/140 12 months: intervention 100/134, control 87/134 | |
| Lowe 2007 | 3 and 6 months | CareFile (29‐page personalised information booklet) + discussion with research registrar versus usual information and follow‐up | Rankin Scale | Number of participants with outcome data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 44/50, control 40/50 Median (range) Before intervention: intervention 3 (2 to 4), control 2 (1 to 3) 3 months: intervention 3 (2 to 3), control 3 (2 to 3) 6 months: intervention 2 (1 to 3), control 2 (2 to 3) | |
| Maasland 2007 | 12 weeks | Health education plus computer programme versus health education alone | Rankin Scale | Number of participants with outcome data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 30/33, control 27/32 No significant differences between the groups | |
| Mant 1998 | 6 months | Information pack versus no intervention | London Handicap Scale Barthel Index | Number of participants with London Handicap Scale data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 34/48, control 34/45 Mean score London Handicap Scale: intervention 0.6 (SD 0.22), control 0.5 (SD 0.18) Number of participants with Barthel Index data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 37/48, control 34/45 Barthel Index: intervention 15.1 (SD 6.42), control 15.1 (SD 4.82) | |
| O'Connell 2009 | 4 weeks and 4 months | Patient Held Record (PHR) incorporating fact sheets with patient specific stroke information versus usual discharge information | ADL sub‐scale of the Stroke Impact Scale (SIS) | Number of participants with outcome data available at the end of 4 month follow‐up/number of participants in group at outset of the trial: intervention 28/46, control 38/47 Mean score SIS ADL sub‐scale: at 4 weeks: intervention 71.96 (SD 16.49), control 81.64 (SD 19.03) 4 months: intervention 74.91(SD 18.25), control 82.43 (SD 17.71) | |
| Pain 1990 | 3 months | Individualised information booklet + advice and information versus advice and information | Barthel Index | Number of participants with outcome data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 16/21, control 13/15 Mean score Barthel Index: at discharge: intervention 77.6 (SD 24.5), control 83.5 (SD 18.5) 3 months: intervention 75.1 (SD 26.0), control 85.0 (SD 17.6) | |
| Rodgers 1999 | 6 months | Education programme versus usual care | Oxford Handicap Scale | Number of participants with outcome data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 89/121, control 64/83 Mean score Oxford Handicap Scale: intervention 3.1 (SD 1.4), control 3.2 (SD 1.2) | |
| Smith 2004 | 3 and 6 months | Stroke Recovery Programme (information manual) + fortnightly meetings with multidisciplinary team versus usual care | Barthel Index London Handicap Scale | Number of participants with Barthel Index data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 69/84, control 64/86 Median change from baseline (range) Barthel Index: 3 months: intervention 6 (‐5 to 17), control 5 (‐5 to 20) 6 months: intervention 7 (‐13 to 17), control 5.5 (‐9 to 19) Median score (range) Number of participants with London Handicap data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 69/84, control 64/86 London Handicap Scale 3 months: intervention 57 (9 to 90), control 54 (25 to 95) 6 months: intervention 59 (20 to 94), control 57 (12 to 84) | |
1.7. Analysis.
Comparison 1 Passive or active information versus control, Outcome 7 Patient social activities: summary of results.
| Patient social activities: summary of results | |||||
|---|---|---|---|---|---|
| Study | Follow‐up | Comparison | Outcome measure | Results | Notes |
| Kalra 2004 | 12 months | Education sessions + hands on training versus conventional care | Frenchay Activities Index | Number of participants with outcome data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 134/151, control 133/149 Median Score at one year: intervention 15 (IQR 9 to 23), control 16 (IQR 8 to 22) | |
| Pain 1990 | 3 months | Individualised information booklet + advice and information versus advice and information | Frenchay Activities Index | Number of participants with outcome data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 16/21, control 13/15 Mean Score Discharge (baseline): intervention 21.4 (SD 10.6), control 23.8 (SD 7.5) 3 months: intervention 12.5 (SD 8.8), control 13.9 (SD 10.4) | |
| Rodgers 1999 | 6 months | Education programme versus usual care | Nottingham Extended ADL | Number of participants with outcome data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 84/121, control 61/83 Mean score: intervention 7.6 (SD 6.5), control 8.0 (SD 6.2) | |
| Smith 2004 | 3 and 6 months | Stroke Recovery Programme (information manual) + fortnightly meetings with multidisciplinary team versus usual care | Frenchay Activities Index | Number of participants with outcome data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 68/84, control 64/86 Median change from baseline (range) 3 months: intervention 1 (0 to 30), control 0 (0 to 23) 6 months: intervention 5 (0 to 32), control 3 (0 to 33) | |
1.8. Analysis.
Comparison 1 Passive or active information versus control, Outcome 8 Patient perceived health status and quality of life: summary of results.
| Patient perceived health status and quality of life: summary of results | |||||
|---|---|---|---|---|---|
| Study | Follow‐up | Comparison | Outcome measure | Results | Notes |
| Chinchai 2010 | 2 months | An education programme with follow up reinforcement versus usual care information | WHO Qualiy of Life Measure (Thai version) | Number of participants with outcome data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 30/30, control 30/30 Mean (SD) scores Physical: intervention 23.7 (2.2), control 20.5 (1.9) Psychological: intervention 20.9 (1.9), control 18.1 (2.4) Social relationship: intervention 8.6 (0.9), control 7.9 (1.4) Environmental: intervention 25.9 (2.2), control 23.7 (2.8) |
Between group differences reported pre‐intervention only (P = 0.23). Within group differences were statistically significant for the intervention group for physical, psychological and environmental categories (P<0.05). There were no statistically significant within group differences for the control group (P > 0.05). |
| Ellis 2005 | 5 months | Generic risk factor advice + stroke nurse specialist review and written advice versus generic risk factor advice | EuroQol Perceived Health Status | Number of participants with outcome data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 94/100, control 98/105 Number (%) with decrease in quality of life (score increase of 1 or more): Mobility: intervention 11 (12%), control 17(17%) Self‐care: intervention 8 (9%), control 16 (16%) Usual activities: intervention 14 (15%), control 22 (22%) Pain: intervention 18 (19%), control 25 (26%) Anxiety and depression: intervention 17 (18%), control 25 (26%) Percentage change (visual analogue scale): intervention 3.5 (‐0.9 to 7.9), control 1 (‐3.3 to 5.3) | |
| Hoffmann 2007 | 3 months | Computer‐generated tailored written information versus stroke fact sheets | Coop Charts (patient) | Number of participants with outcome data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 66/69, control 67/69. Change scores 0 to 3 months (95% confidence intervals) Physical fitness: intervention 0.4, control 0.4 (‐0.4 to 0.4) Feelings: intervention ‐0.2, control ‐0.3 (‐0.3 to 0.6) Daily activities: intervention ‐0.1, control ‐0.1 (‐0.5 to 0.6) Social activities: intervention 0.1, control ‐0.1 (‐0.4 to 0.8) Pain: intervention 0.1, control 0.2 (‐0.6 to 0.6) Change in health: intervention ‐1.4, control ‐1.1 (‐0.8 to 0.3) Overall health: intervention ‐0.4, control ‐0.1 ( ‐0.6 to 0.2) Social support: intervention ‐0.4, control ‐0.2 (‐0.6 to 0.2) Quality of life: intervention ‐0.2, control ‐0.5 ( ‐0.1 to 0.7) | |
| Kalra 2004 | 3 months and 1 year | Education sessions + hands on training versus conventional care | EuroQuol Visual Analogue Scale | Number of participants with outcome data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 112/151, control 112/149 Median (IQR) before intervention: intervention 85 (75 to 90), control 85 (75 to 95) 3 months: intervention 60 (42 to 70), control 50 (40 to 90)* 1 year: intervention 65 (55 to 80)†, control 60 (41 to 80)† | Statistically significant difference * P = 0.019 † P = 0.009 |
| Mant 1998 | 6 months | Information pack versus no intervention | Dartmouth Coop Chart (patient) | Number of participants with outcome data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 33/48, control 32/45 Mean score Physical fitness: intervention 4.2 (SD 0.89), control 4.4 (SD 0.76) Feelings: intervention 2.4 (SD 1.41), control 2.2 (SD 1.17) Daily activities: intervention 2.7 (SD 1.63), control 3.4 (SD 1.45) Social activities: intervention 2.2 (SD 1.49), control 2.7 (SD 1.45) Pain: intervention 2.7 (SD 1.66), control 2.9 (SD 1.54) Change in health: intervention 2.8 (SD 0.71), control 2.7 (SD 0.73) Overall health: intervention 3.2 (SD 1.11), control 3.2 (SD 1.07) Social support: intervention 1.3 (SD 0.65), control 1.3 (SD 0.81) Quality of life: intervention 2.2 (SD 0.68), control 2.4 (SD 0.79) | |
| Rodgers 1999 | 6 months | Education programme versus usual care | Short‐Form 36 (patient) | Number of participants with outcome data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 66/121, control 51/83 Mean score Physical functioning: intervention 33.3 (SD 33.3), control 27.8 (SD 26.0) Role physical: intervention 20.8 (SD 37.3), control 14.7 (SD 32.1) Bodily pain: intervention 61.0 (SD 30 .55), control 57.5 (SD 29.2) General health: intervention 47. 0 (SD 19.8), control 48.8 (SD 22.3) Vitality: intervention 35.4 (SD 24.7), control 43.7 (SD 24.0) Social functioning: intervention 47.2 (SD 33.2), control 44.4 (SD 30.9) Role emotional: intervention 34.4 (SD 43.2), control 36.6 (SD 45.8) Mental health: intervention 61.7 (SD 24.0), control 61.7 (SD 23.6) | |
1.11. Analysis.
Comparison 1 Passive or active information versus control, Outcome 11 Service use: summary of results.
| Service use: summary of results | |||||
|---|---|---|---|---|---|
| Study | Follow‐up | Comparison | Outcome measure | Results | Notes |
| Evans 1988 | 6 months | Education classes versus routine care | ESCROW (social resources use) | Number of participants with outcome data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 64/70, control 63/70 Mean scores before intervention: education 10.5 (SD 4.1), control 10.5 (SD 4.6) 6 months post stroke: education 9.2 (SD 2.3), control 9.6 (SD 3.3) 1 year post stroke: education 9.9 (SD 3.3), control 9.8 (SD 2.9) | |
| Kalra 2004 | 12 months | Education sessions + hands on training versus conventional care | Use of services/resources | Number of participants with outcome data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: numbers varied according to service evaluated: intervention 134 to 151/151, control 125 to 149/149 Resource use: there was a trend towards lesser use of personal and domestic care services in the intervention group but only significant for the use of day care mean difference ‐2.8, 95% CI ‐5.1 to ‐0.5 | |
| Mant 1998 | 6 months | Information pack versus no intervention | Number/type of healthcare professional contacts | Number of participants with outcome data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: data obtained for 67 families but no information available on group allocation Number of different types of health professionals seen*: intervention mean 4 median 3, control mean 3 median 2 Types of contact with support groups and healthcare facilities: no difference Contact with any particular type of healthcare professional or type of support group/healthcare facility: no significant differences | *P = 0.08 (Mann‐Whitney U) |
| Rodgers 1999 | 6 months | Education programme versus usual care | Resource utilisation | Number of participants with outcome data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 90/121, control 64/83 Residential care: intervention 9 (10%), control 4 (6%) Nursing home: intervention 9 (10%), control 5 (8%) Home care ‐ domestic: intervention 17 (24%), control 19 (35%) Home care ‐ personal: intervention 11 (15%), control 11 (20%) Home help ‐ private: intervention 3 (3%), control 3 (5%) Meals on Wheels: intervention 3 (3%), control 6 (11%) Nursing auxilliary for bath: intervention 3 (3%), control 2 (4%) Laundry services: intervention 3 (3%), control 0 (0%) Day centre/Luncheon club: intervention 3 (3%), control 7 (11%) Day Hospital: intervention 20 (22%), control 13 (20%) District Nurse: intervention 28 (31%), control 18 (28%) Health Visitor: intervention 0 (0%), control 0 (0%) Social Worker: intervention 15 (17%), control 7 (11%) Occupational therapist: intervention 9 (10%), control 7 (11%) Physiotherapist: intervention 32 (36%), control 18 (28%) Chiropodist: intervention 39 (43%), control 29 (45%) Continence Advisor: intervention 2 (2%), control 2 (3%) CPN: intervention 3 (3%), control 1 (2%) | Residential care: P = 0.38 Nursing home: P = 0.61 Home care ‐ domestic: P = 0.26 Home care ‐ personal: P = 0.61 Home help ‐ private: P = 0.79 Meals on Wheels: P = 0.17 Nursing auxiliary for bath: P = 0.83 Laundry services: P = 0.12 Day centre/Luncheon club: P = 0.10 Day Hospital: P = 0.66 District Nurse: P = 0.55 Social Worker: P = 0.26 Occupational Therapist: P = 0.93 Physiotherapist: P = 0.24 Chiropodist: P = 0.93 Continence advisor: P = 0.77 CPN: P = 0.47 |
| Smith 2004 | 6 months | Stroke Recovery Programme (information manual) + fortnightly meetings with multidisciplinary team versus usual care | Service use and receipt of benefits | Number of participants with outcome data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 64/84, control 61/86 6 months Number (%) of patients having contact: Social worker: intervention 20 (31%), control 19 (31%) Community nurse: intervention 36 (56%), control 32 (52%) General Practitioner: intervention 60 (94%), control 50 (82%) Therapist: intervention 47 (73%), control:34 (56%) Chiropodist: intervention 29 (45%), 23 (38%) Receipt of: Home support: intervention 18 (28%), control 20 (33%) Day centre attendance: intervention 8 (13%), control 3 (5%) Rehabilitation centre attendance: intervention 2 (3%), control 1 (2%) Receipt of financial and mobility benefits: Disability/mobility allowance: intervention 10 (16%), control 15 (25%) Attendance/carers allowance: intervention 21 (33%), control 15 (25%) Invalidity benefit: intervention 2 (3%), control 2 (3%) Blue badge scheme: intervention 27 (42%), control 20 (33%) | |
1.12. Analysis.
Comparison 1 Passive or active information versus control, Outcome 12 Modification of health related behaviours: summary of results.
| Modification of health related behaviours: summary of results | |||||
|---|---|---|---|---|---|
| Study | Follow‐up | Comparison | Outcome measure | Results | Notes |
| Banet 1997 | 6 months | Patient education packet and shared medical record versus patient education packet | Miller's Health Intention Scale Miller's Health Behaviour Scale 3 areas of compliance examined: smoking, diet and medication | Number of participants with outcome data available at the end of scheduled follow up/number of participants in group at outset of the trial: intervention 24/number unclear, control 28/number unclear Smoking: no analysis done Follow‐up variable comparisons between shared record and control groups: diet: F= 0.03, P = 0.85 medication: F=0.02, P = 0.87 | |
| Chiu 2008 | Pharmacist lead intervention providing information on drug effects, lifestyle modification, benefits of therapies, importance of compliance, drug interactions and adverse events versus control group (no information reported) | Proportion of subjects with satisfactory management of modifiable risk factors: Blood pressure (defined as <140/90mmHg) Lipids (defined as low‐density lipoprotein (LDL) cholesterol <100 mg dL or, if LDL was not available, total cholesterol (TC) <160 mg /dL) Glucose (defined as defined as glycosylated haemoglobin A1c (HbA1c) <7% or, if HbA1c not available, FBG <126 mg dL. When HbA1c or FBG were not available, random post‐prandial blood glucose less than 200 mg/dL was used to define adequate control |
Number of participants with outcome data available at the end of scheduled follow up/number of participants in group at outset of the trial: intervention 78/80, control 76/80 There was a statistically significant difference (P<0.001) between the groups for management of blood pressure. After the intervention, 65/78 (83.3%) of the intervention group and 33/76 (43.4%) of the control group had satisfactory management of blood pressure No statistically significant difference between the intervention and control groups for management of lipids or glucose. After the intervention, 21/53 (39.6%) of the intervention group and 13/49 (26.5%) of the control group had satisfactory management of lipids. After the intervention, 12/34 (53.5%) of the intervention group and 15/33 (45.5%) of the control group had satisfactory management of glucose |
||
| Ellis 2005 | 5 months | Generic risk factor advice + stroke nurse specialist review and written advice versus generic risk factor advice | Modifiable risk factors within the recommended treatment range according to the contemporary national and local treatment guidelines: blood pressure (< 140/85 mmHg), cigarette consumption (complete cessation), Random blood glucose (< 80 mmol/l), HbA1c (< 7.5%), Total cholesterol (< 5.0 mmol/l) | Number of participants with outcome data available at the end of scheduled follow up/number of participants in group at outset of the trial: intervention 94/100, control 98/105 Mean (95% confidence intervals) or number (%) 5 months: intervention n = 94, control n = 98 All relevant risk factors controlled: intervention 45 (46.4%), control 41 (41.7%) Individual risk factors: hypertension, change in systolic BP (mmHg): intervention ‐9.3 (‐15.0 to ‐3.5), control ‐1.0 (‐6.3 to 4.3) hypertension, change in diastolic BP(mmHg): intervention ‐2.1 (‐5.7 to 1.5), control ‐1.2 (‐4.5 to 4.5) smoking, change in number of cigarettes per day: intervention ‐1.6 (‐5.1 to 1.8), control ‐0.4 (‐3.7 to 2.8) diabetes, change in random blood glucose (mmol/l): intervention 0.92 (‐1.39 to 3.23), control 0.89 (‐2.09 to 3.87) diabetes, change in HbA1C (%): intervention ‐0.25 (‐0.57 to 0.08), control ‐0.78 (‐1.50 to 0.05) hypercholesterolaemia, total cholesterol (mmol/l): intervention ‐0.96 (‐1.20 to 0.71), control ‐0.87 (‐1.14 to 0.61) | |
| Lowe 2007 | 3 and 6 months | CareFile (29 page personalised information booklet) + discussion with research registrar versus usual information and follow up | Risk factor modification: blood pressure | Number of participants with outcome data available at the end of scheduled follow up/number of participants in group at outset of the trial: intervention 44/50, control 40/50 Median (mmHg) (IQR) Before intervention, systolic: intervention 137 (124 to 150), control 130 (116 to 149) Before intervention, diastolic: intervention 77 (70 to 83), control 71(65 to 80) 3 months, systolic: intervention 140 (130 to 160), control 140 (124 to 150) 3 months, diastolic: intervention 80 (70 to 85), control 76 (70 to 82) 6 months, systolic: intervention 149 (130 to 159), control 138 (130 to 150) 6 months, diastolic: intervention 80 (70 to 84), control 70 (70 to 80) | |
| Maasland 2007 | 12 weeks | Health education + computer programme versus health education alone | Risk factor modification: Blood pressure, Serum cholesterol, Serum Triglyceride, Serum LDL, Body mass Index, Number of cigarettes/smoker, Number of alcoholic drinks/drinker | Number of participants with outcome data available at the end of scheduled follow up/number of participants in group at outset of the trial: intervention 30/33, control 27/32 Change from baseline to 12 weeks (intervention effect and 95% CI) systolic blood pressure: intervention ‐8.4, control ‐6.9 (1.5 95% CI ‐7.7 to 10.8) diastolic blood pressure: intervention ‐5.4, control ‐6.2 (‐0.8 (95% CI ‐6.1 to 4.5) serum cholesterol: intervention ‐1.1, control ‐1.6 (‐0.5 95% CI ‐1.2 to 0.2) serum triglyceride: intervention ‐0.6, control ‐0.6 (00 95% CI‐0.7 to 0.7) serum LDL: intervention ‐1.2, control ‐1.4 (‐0.2 95% CI ‐1.0 to 0.5) body mass index: intervention 0.0, control 0.3 (0.3 95% CI ‐0.3 to 0.8) number of cigarettes/smoker: intervention ‐20.1, control ‐13.2 (6.9 95% CI ‐16.2 to 30.1) number of alcoholic drinks/drinker: intervention ‐0.8, control ‐0.6 (0.2 95% CI ‐0.6 to 1.0) | |
| Rodgers 1999 | 6 months | Education programme versus usual care | Lifestyle and risk factor modifications: smoking, blood pressure, and medication | Number of participants with outcome data available at the end of scheduled follow up/number of participants in group at outset of the trial: intervention 66/121, control 51/83 Smoking: present smoker: intervention 14 (21%), control 14 (28%) smoked 6 months ago: intervention 25 (38%), control 17 (33%) blood pressure, checked since leaving hospital: intervention 61 (92%), control 48 (94%) Medication aspirin: intervention 36 (62%), control 31 (72%) dipyridamole: intervention 2 (3%), control 2 (5%) warfarin: intervention 10 (17%), control 6 (14%) | Smoking: present smoking P = 0.44 Smoked six months ago: P = 0.61 Blood pressure checked since leaving hospital: P = 0.74 Medication: aspirin: P = 0.29, dipyridamole: P = 0.66, warfarin: P = 0.76 |
1.15. Analysis.
Comparison 1 Passive or active information versus control, Outcome 15 Carer emotional outcome: summary of results.
| Carer emotional outcome: summary of results | |||||
|---|---|---|---|---|---|
| Study | Follow‐up | Comparison | Outcome measure | Results | Notes |
| Downes 1993 | 6 months | Information pack versus no intervention | Hospital Anxiety and Depression Scale; Depression sub‐scale | Number of participants with outcome data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 22/?35, control 18/?35 Mean score Baseline: depression: intervention 6.3 (SD 4.0), control 5.4 (SD 3.7) 6 months: depression: intervention 5.8 (SD 5.2), control 5.1 (SD 3.2) | |
| Draper 2007 | 4 weeks and 3 months | Education programme versus usual care (wait control) | 28‐item General Health Questionnaire | Number of participants with outcome data at final follow‐up: intervention 17/19, wait control 18/20 Mean (SD) Intervention: baseline: 6.26 (5.67) week 4: 3.21 (4.20)* week 16: 4.26 (5.27) Control: baseline: 5.17 (4.11) week 4: not measured week 16: 6.28 (7.01) | * statistically significant result P = 0 .006 |
| Johnston 2007 | 8 weeks (post intervention) and 6 months from baseline | Post discharge workbook intervention including information and audio relaxation tape versus usual care | Hospital Anxiety and Depression scale (anxiety and depression sub‐scales reported separately) | Number of participants with outcome data at the outset of the trial: intervention 82, control 90 Pre‐ intervention Anxiety Mean (SD) Intervention 7.64 (4.89) Control 7.08 (4.01) Pre‐intervention depression Mean (SD) Intervention 5.65 (4.27) Control 4.77 (3.9) Post‐intervention data not reported |
Reported no significant group by time interaction (P > 0.05) on total HADS or on anxiety or depression sub‐scales |
| Kalra 2004 | 12 months | Education sessions + hands on training versus conventional care | Caregiver Burden Scale | Number of participants with outcome data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 123/151, control 128/149 Median scores at 12 months: intervention 32 (IQR 27 to 41), control 41 (IQR 36 to 50)* | * statistically significant result P = 0 .0001 |
| Mant 1998 | 6 months | Information pack versus no intervention | Caregiver Strain Index | Number of participants with outcome data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 27/32, control 19/24 Mean score: intervention 3.9 (SD 3.7), control 4.1 (SD 2.74) | |
1.17. Analysis.
Comparison 1 Passive or active information versus control, Outcome 17 Carer social activities: summary of results.
| Carer social activities: summary of results | |||||
|---|---|---|---|---|---|
| Study | Follow‐up | Comparison | Outcome measure | Results | Notes |
| Kalra 2004 | 12 months | Education sessions + hands on training versus conventional care | Frenchay Activities Index | Number of participants with outcome data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 133/151, control 133/149 Median score at 1 year: intervention 27 (23 to 30), control 26 (24 to 30) | |
1.18. Analysis.
Comparison 1 Passive or active information versus control, Outcome 18 Carer perceived health status and quality of life: summary of results.
| Carer perceived health status and quality of life: summary of results | |||||
|---|---|---|---|---|---|
| Study | Follow ‐up | Comparison | Outcome measure | Results | Notes |
| Kalra 2004 | 3 months and 1 year | Education sessions + hands on training versus conventional care | EuroQuol visual analogue scale Quality adjusted life years | Number of participants with outcome data available at the end of scheduled follow up/number of participants in group at outset of the trial: intervention 112/151, control 120/149 EuroQuol visual analogue scale Median (IQR) Before intervention: intervention 90 (80 to 95), control 85 (80 to 90) 3 months: intervention 80 (71 to 90), control 70 (60 to 80)* 1 year: intervention 80 (70 to 90), control 70 (60 to 80)† Quality adjusted life years mean (SD) Pre‐intervention: intervention 0.94 (SD 0.10), control 0.94 (SD 0.14) 1 year: intervention 0.91 (SD 0.11), control 0.90 (SD 0.14) | statistically significant difference *P = 0.0001 †P = 0.0001 |
| Larson 2005 | 6 months and 1 year | Education programme + support versus regular information and possibility of attending 1 open session | General quality of life visual analogue scale Bradley's well‐being questionnaire LISS questionnaire (life situation among spouses after stroke event) EuroQuol visual analogue scale | Number of participants with outcome data available at the end of scheduled follow up/number of participants in group at outset of the trial: intervention 46/50, control 45/50 Mean (SD) General quality of life visual analogue scale: Before intervention: intervention 60.08 (22.79), control 60.22 (22.57) 6 months: intervention 63.04 (22.35), control 63.87 (20.45) 1 year: intervention 68.00 (22.89), control 66.78 (20.22) Bradley's well‐being questionnaire General well being: Before intervention: intervention 25.28 (5.03), control 25.28 (5.33) 6 months: intervention 25.87 (6.57), control 24.14 (6.57) 12 months: intervention 24.57 (5.87), control 25.91 (6.20) LISS‐questionnaire: Life situation total score : Before intervention: intervention 46.47 (10.29), control 47.54 (9.42) 6 months: intervention 48.43 (9.82), control 47.48 (9.26) 12 months: intervention 48.59 (10.91), control 49.11 (9.66) EuroQol visual analogue scale: Before intervention: intervention 72.35 (18.54), control 77.98 (20.18) 6 months: intervention 76.91 (16.39), control 76.85 (18.02) 12 months: intervention 73.63 (17.98), control 77.27 (16.77) | |
1.21. Analysis.
Comparison 1 Passive or active information versus control, Outcome 21 Cost to health and social services: summary of results.
| Cost to health and social services: summary of results | |||||
|---|---|---|---|---|---|
| Study | Follow‐up | Comparison | Outcome measure | Results | Notes |
| Kalra 2004 | 12 months | Education sessions + hands on training versus conventional care | Costs in first year after onset of stroke | Costs: total health and social care costs over one year significantly lower for intervention group, mean difference £4043 (EUR 6072, $7249) 95% CI ‐£6544 to ‐ £1595 | |
1.22. Analysis.
Comparison 1 Passive or active information versus control, Outcome 22 Self management: summary of results.
| Self management: summary of results | |||||
|---|---|---|---|---|---|
| Study | Follow‐up | Comparison | Outcome measure | Results | Notes |
| Frank 2000 | 1 month | Workbook + home visits and telephone contact versus wait control | Recovery Locus of Control Scale (RLOC) Perceived Health Competencies Scale (PHCS) | Number of participants with outcome data available at the end of scheduled follow up/number of participants in group at outset of the trial: intervention 19/20, control 20/21 Mean (SD) RLOC Before intervention: intervention 36.10 (4.93), control 35.50 (5.23) 1 month: intervention 36.42 (5.56), control 37.55 (4.08) PHCS: Before intervention: intervention 28.05 (5.91), control 26.80 (5.23) 1 month: intervention 29.21 (5.97), control 26.95 (5.49) | |
| Hoffmann 2007 | 3 months | Computer‐ generated tailored written information versus stroke fact sheets | Self efficacy to perform self‐management behaviours scale | Number of participants with outcome data available at the end of scheduled follow‐up/number of participants in group at outset of the trial: intervention 66/69, control 67/69 Mean (SD) Before intervention: Section 1 (to get information about disease): intervention 8.1(2.3), control 7.9 (2.5) Section 2 (to obtain help from family, community and friends): intervention 7.9 (1.8), control 8.1 (1.5) Section 3 (to communicate with the doctor): intervention 8.6 (1.8), control 9.1 (1.7) Section 4 (to control/manage depression): intervention 7.7 (2.0), control 7.8 (1.8) Section 5 (to manage the disease in general): intervention 7.8 (1.8), control 8.0 (1.9) Section 6 (to manage symptoms): intervention 7.3 (2.0), control 7.7 (1.8) Mean change scores 0 to 3 months (95% confidence intervals) 3 months: Section 1 (to get information about the disease): intervention 0.2, control 1.6 (‐1.5 to 1.1) Section 2 (to obtain help form family , community, and friends): intervention 0.0, control 0.2 (‐0.8 to 0.3) Section 3 (to communicate with the doctor): intervention 0.3, control ‐0.1 (‐0.2 to 1.1) Section 4 (to control/manage depression): intervention 0.0, control 0.3 (‐0.8 to 0.2) Section 5 (to manage the disease in general): intervention 0.4, control 0.3 (‐0.3 to 0.7) Section 6 (to manage symptoms): intervention 0.0, control ‐0.2 ( ‐0.5 to 0.9) | |
1.23. Analysis.
Comparison 1 Passive or active information versus control, Outcome 23 Family functioning and patient adjustment: summary of results.
| Family functioning and patient adjustment: summary of results | |||||
|---|---|---|---|---|---|
| Study | Follow‐up | Comparison | Outcome measure | Results | Notes |
| Evans 1988 | 6 months and 1 year | Education versus routine care | Personal Adjustment and Role Skills Scale (PARS) (patient) Family Assessment Device (FAD) (carer) | Number of participants with outcome data available at the end of scheduled follow up/number of participants in group at outset of the trial: intervention 64/70, control 63/70 Mean scores PARS Before intervention: intervention 50.6 (SD 5.1), control 51.4 (SD 5.60) 6 months: intervention 40.1 (SD 5.8), control 37.6 (SD 5.1) 1 year: 40.5 (SD 6.1), control 39.1 (SD 5.7) FAD problem solving Before intervention: intervention 2.0 (SD 0.40), control 2.1 (SD 0.37) 6 months: intervention 2.2 (SD 0.33)*, control 2.3 (SD 0.35) 1 year: intervention 2.2 (SD 0.37)*, control 2.4 (SD 0.35) Role assignments Before intervention: intervention 2.0 (SD 0.33), control 2.0 (SD 0.35) 6 months: intervention 2.2 (SD 0.35), control 2.2 (SD 0.42) 1 year: intervention 2.3 (SD 0.39), control 2.3 (SD 0.39) Communication Before intervention: intervention 2.0 (SD 0.39), control 2.0 (SD 0.37) 6 months: intervention 2.2 (SD 0.33)*, control 2.3 (SD 0.35) 1 year: intervention 2.1 (SD 0.44)*, control 2.3 (SD 0.40) Behaviour control Before intervention: 2.00 (SD 0.36), control 2.0 (SD 0.36) 6 months: intervention 2.2 (SD 0.35), control 2.2 (SD 0.36) 1 year: intervention 2.2 (SD 0.39), control 2.2 (SD 0.44) Affective involvement Before intervention: intervention 2.0 (SD 0.36), control 2.0 (SD 0.35) 6 months: intervention 2.2 (SD 0.38), control 2.2 (SD 0.36) 1 year: intervention 2.1 (0.44)*, control 2.3 (SD 0.41) Affective responsiveness Before intervention: intervention 2.0 (SD 0.36), control 2.0 (SD 0.35) 6 months: intervention 2.2 (SD 0.41), control 2.2 (SD 0.37) 1 year: intervention 2.2 (SD 0.39), control 2.3 (0.53) Global family function Before intervention: 1.9 (SD 0.47), control 2.0 (SD 0.35) 6 months: 2.0 (SD 0.41)*, control 2.2 (SD 0.40) 1 year 2.1 (SD 0.42)*, control 2.3 (SD 0.37) | *P < 0.01 different from control |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Banet 1997.
| Methods | Patients who met all criteria and volunteered to participate were randomly assigned to treatment group; no further details given No stated blind outcome assessment 6 patients lost to follow‐up; no report of differential losses between groups 6‐month follow‐up |
|
| Participants | St Louis, Mo, USA 58 first‐ time stroke patients: number allocated to intervention or control not given No details of age Sex: women N = 28 Inclusion criteria: aged 18 years or older, first‐time stroke, medically stable, competent to give informed consent, ready for hospital discharge Exclusion criteria: aphasia or motor impairments that hindered ability to complete forms unless neurologist believed it did not interfere with giving consent and had a caregiver who could help complete forms, or could dictate answer to investigator N = 52 for final follow‐up |
|
| Interventions | Treatment: copy of medical history, clinical resumes, notes on outpatient visits, x‐ray, scan reports and pertinent laboratory results. Also received patient education packet containing leaflets on stroke care, stroke team, tests and procedures, community resources, defining terms, facts about stroke, how stroke affects behaviour and recovering from stroke Focus: patient Setting: hospital Administration: unclear who gave record Encouraged to maintain records by incorporating updated information by taking them to all appointments with physicians and all trips during the study 1 contact, length unknown Patients ready for discharge Control: given patient education packet containing leaflets on stroke care, stroke team, tests and procedures, community resources, defining terms, facts about stroke, how stroke affects behaviour and recovering from stroke |
|
| Outcomes | (1) Disability and handicap (baseline and 6 months) (2) Intention to modify health‐related behaviours and compliance (baseline and 6 months) Not included in the review: Global Outcome (baseline and 6 months) |
|
| Notes | Validity assessment: use of inappropriate statistical tests | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The procedure for generating a random sequence was not reported |
| Allocation concealment (selection bias) | Unclear risk | Reported that patients were randomly assigned but method not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No report of blinding of participants or personnel but as no intervention provided for the control group, group assignment would have been apparent |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not reported if outcome assessments were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "One volunteer died, and five provided incomplete data. Thus data from 52 subjects was available for analysis." Although losses were relatively small, it was not reported which groups they were from |
| Selective reporting (reporting bias) | Unclear risk | Quote: "Subjects reported their intentions to modify health‐related behaviours by completing the diet, smoking, and medication sub‐scales of Miller’s Health Intention Scale." Quote: "Because so few subjects smoked, this was not included as a variable in the analysis." Comment: note that this question was typically answered (i.e. data was not generally missing), but only 7 smoked at the time of their stroke |
| Other bias | Low risk | No other obvious sources of bias |
Chinchai 2010.
| Methods | Cluster RCT No participants lost to follow‐up |
|
| Participants | Chiang Mai, Thailand 60 stroke patients and their primary caregivers (N = 60) Patients: intervention N = 30; control N = 30 Caregivers: intervention N = 30; control N = 30 Age range of patients intervention (years): < 40 N = 9; 40 to 59 N = 8; 60 to 69 N = 9; 70 to 79 N = 7 Age range of patients control (years): < 40 N = 4; 40 to 59 N = 8; 60 to 69 N = 5; 70 to 79 N = 13 Sex of patients male: intervention 60%; control 53% Age range of carers intervention (years): < 40 N = 2; 40 to 59 N = 8; 60 to 69 N = 11; 70 to 79 N = 9 Age range of carers control (years): < 40 N = 5; 40 to 59 N = 12; 60 to 69 N = 6; 70 to 79 N = 7 Sex of carers male: intervention 47%; control 53% Inclusion criteria patient: discharged from hospital < 18 months, physical function recovery level 2 to 4 classified by Brunnstorm; communication (verbal, non‐verbal), no complications (e.g. bedsores, pain, fever during data collection), willingness to participate in the study Inclusion criteria carer: primary caregiver (family member or relative), not previously attended the home health care and stroke rehabilitation programme, minimum 8 hours a day caring, willingness to participate in the study Exclusion criteria: not reported |
|
| Interventions | Intervention: an education programme for caregivers with follow‐up reinforcement. Included lectures and active practice of activities of daily living and written information in guidebooks. Intervention started within 18 months of patient stroke. Carers attended a 1‐day, 7‐hour education session on 3 consecutive weeks and received weekly visits for reinforcement by health service volunteers Focus: patient and caregiver Setting: primary healthcare unit Administration: occupational therapists with a minimum of 2 years experience Control: usual care information from health stations located in the community |
|
| Outcomes | Patient outcomes: Quality of Life (7 days pre‐intervention and 2 months post intervention) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The procedure for generating a random sequence was not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although not specifically reported, control participants received usual care, therefore the (lack of) intervention could have been obvious |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research assistants blind to group assignment performed assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop outs or exclusions |
| Selective reporting (reporting bias) | High risk | When describing the WHOQOL‐BREF the authors report the individual items for overall health and overall QOL, as well as a total score (the summation of all items). However these were not presented in the results |
| Other bias | Low risk | No other obvious sources of bias |
Chiu 2008.
| Methods | RCT by simple random sampling. Patients were stratified by age (over 65 or not) and sex 4 patients from the control group and 2 patients from the intervention group lost to follow‐up |
|
| Participants | Kaohsiung, Taiwan 160 stroke patients (intervention N = 80, control N = 80) Mean age of patients: intervention 66 years; control 65 years Sex of patient male: 50% Inclusion criteria: stroke out‐patients who had visited clinics regularly after stroke (> 12 months) Exclusion criteria: enrolled in other studies, terminal illness, no consent |
|
| Interventions | Intervention: consultation (drug effects, lifestyle modification, benefits of therapies, importance of compliance, verification of drug interaction and reminder of adverse events). Focus: patient Setting: unclear Administration: intervention delivered by pharmacist over 6 x 1‐hour sessions over a 6‐month period Control: no information reported |
|
| Outcomes | (1) Management of hypertension (2) Management of lipids (3) Management of glucose |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as simple random sampling but method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | No information reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Small number lost to follow‐up (2 from intervention and 4 from the control) |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available so cannot assess reporting bias |
| Other bias | Low risk | No other obvious signs of bias |
Downes 1993.
| Methods | Allocation by random number sequence; no other details given Blinded outcome assessment Number lost to follow‐up unclear; no report of differential losses between groups 6‐month follow‐up |
|
| Participants | Birmingham, UK Stroke patients and carers (couples): number initially recruited to control and information groups unknown (105 couples recruited to the 3‐group trial) Information provided for N = 18 control group, N = 22 information group who completed 6‐month assessment Age of patient > 60 years: treatment 91%; control 89% Sex of patient male: treatment 55%: control 44% Inclusion criteria: stroke survivors living at home with their informal carers, recent stroke (not necessarily first) causing increase on modified Rankin Disability Scale and post‐stroke Rankin score of 2 to 5 Exclusion criteria: none stated |
|
| Interventions | Treatment: information pack designed for study containing information about physical, cognitive and emotional effects of stroke, carer well being and local services Focus: patient and carer Setting: home Administration: single visit by nurse counsellor who demonstrated how to access relevant information and answered questions. 1 x 1‐hour visit at least 2 weeks after discharge but exact time unknown Control: usual care, no intervention |
|
| Outcomes | (1) Emotional outcome (baseline and 6 months) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "they were allocated by a random number sequence generation." However, method not described. |
| Allocation concealment (selection bias) | Unclear risk | No information reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No evidence of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assesment was carried out by a research assistant who was blind to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 150 couples originally recruited in to the study but only 62 completed and were in the final analysis. Unclear how many from each group |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available so cannot assess reporting bias |
| Other bias | Low risk | Appears free from other sources of bias |
Draper 2007.
| Methods | Allocation by random selection of names by blinded investigator Postal outcome assessment 8 carers lost to follow‐up |
|
| Participants | Sydney, Australia 39 carers of aphasic stroke patients recruited from rehabilitation services of 3 public hospitals: treatment N = 19; control N = 20 Completed final follow‐up: N = 31 Mean age carer: treatment 64 years; control 60 years Mean age of patient: treatment 69 years; control 68 years Sex of carer: not reported Sex of patient: not reported |
|
| Interventions | Treatment: education programme covering the impact of stroke, managing the resulting life changes, communication strategies, relaxation and stress management, managing emotions, accessing community services and relapse prevention strategies. At the end of course the caregivers were encouraged to remain in contact as a self‐help group Focus: carer Setting: held in outpatient area of hospital rehabilitation department Administration: 4 x 1‐weekly group session, each session 2 hours, numbers in each group varied from 6 to 11, sessions run by a speech pathologist and social worker, clinical psychologist included for 1 session Control: usual care, wait‐list control commenced the treatment after a delay of 3 months |
|
| Outcomes | Carer Primary outcomes (1) Psychological distress (4 weeks and 3 months) (2) Caregiver burden (4 weeks and 3 months) (3) Communication (4 weeks and 3 months) Secondary outcomes (1) Attitudes towards care‐giving (4 weeks and 3 months) (2) Self ‐rated health (4 weeks and 3 months) (3) Social/recreational activities (baseline, 4 weeks and 3 months) (4) Social support (baseline, 4 weeks and 3 months) (5) Behaviour and mood disturbance Patient (1) Level of dependency in personal care (baseline, 4 weeks and 3 months) |
|
| Notes | Shortfall in recruitment: recruited 39/60 required | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as random but specific method not reported |
| Allocation concealment (selection bias) | Unclear risk | Reported as concealed but specific method for concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: ''Caregivers did not know which group they were in when the baseline measures were completed, however this blinding could not be subsequently maintained.'' |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was no external outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Control group lost 40% of participants |
| Selective reporting (reporting bias) | High risk | Intimate Bonds Measure not reported in results |
| Other bias | Unclear risk | Baseline data collected after randomisation |
Ellis 2005.
| Methods | Random allocation using a computer‐generated random sequence concealed in sequentially numbered opaque sealed envelopes Blinded outcome assessment Stated intention‐to‐treat analysis 13 patients (6 treatment, 7 control) lost to follow‐up 5‐month follow‐up |
|
| Participants | Glasgow, UK 205 patients at stroke clinic or geriatric day hospital: treatment N = 100; control N = 105; completed final follow‐up N = 192 Mean age of patient: treatment 64 years; control 66 years Sex of patient: male: treatment 54%, control 50% Inclusion criteria: clinical diagnosis of stroke, TIA or amaurosis fugax commencing in the previous 3 months; 1 or more risk factors from raised BP, history of concurrent smoking, high cholesterol, diabetes (regardless of their risk factor control) Exclusion criteria: patients with cognitive impairment (defined as AMT < 5 on screening) |
|
| Interventions | Treatment: monthly review with Stroke Nurse Specialist for 3 months at which individual given advice on lifestyle changes, the importance of medication compliance and relevance to secondary prevention Focus: patient Setting: outpatient consultation Administration: reviewed by Stroke Nurse Specialist in consultation lasting approximately 30 minutes. Lifestyle issues including diet, exercise or increased activity and medical services discussed in depth and tailored to the patient's circumstances and functional abilities. Verbal information backed up by written information selected by Stroke Nurse Specialist as relevant to the individual patient. Personalised patient‐held records, detailing their risk factors and the recommended risk factor targets given to the patient and updated at each visit (considered a key part of intervention). Patients given opportunity to bring up participants as appropriate. If risk factor (e.g. BP) deemed to be at unacceptable level, patients encouraged to consult their General Practitioner with that information Control: usual care including generic risk factor advice from medical staff as well as the Stroke Nurse Specialist |
|
| Outcomes | Primary (1) Proportion of patients whose risk factors were 'on target ' Secondary (1) Survival (2) Perceived health status (3) Mood (4) Satisfaction with stroke services |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "eligible patients were randomly allocated to treatment or control groups using a computer‐generated random sequence concealed in sequentially numbered opaque sealed envelopes." Quote: "Three patients were entered twice in error, each time to the treatment group. These subjects were analysed on their initial data only and subsequent data were excluded from the analysis." Comment: errors in sequence generation could have subverted randomisation |
| Allocation concealment (selection bias) | Low risk | Randomly allocated to treatment or control groups using a computer‐generated random sequence concealed in sequentially numbered opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single blinded trial with blinded assessment so presume unblinded participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Outcomes were recorded at 5 months by an independent blinded assessor." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low numbers lost to follow‐up and similar across groups |
| Selective reporting (reporting bias) | Unclear risk | All outcomes specified in the methods were reported in the results. However, study protocol not available so cannot assess reporting bias |
| Other bias | Low risk | No other obvious sources of bias |
Evans 1988.
| Methods | Allocation by method of Taves (minimisation) Blinded outcome assessment 13 patients and carers (6 treatment, 7 control) lost to follow‐up 6‐month and 1‐year follow‐up |
|
| Participants | Seattle, WA, USA 140 stroke patients and carers (majority couples) recruited: treatment N = 70; control N = 70; completed final follow‐up: N = 127 Mean age of patient: treatment 63 years; control 62 years Sex of patient male: treatment 95%; control 94% Inclusion criteria: all stroke patients on inpatient wards from any referring service, hospitalised primarily for stroke, living with primary caregiver Exclusion criteria: none stated |
|
| Interventions | Treatment: 2 classes: (1) lecture and video 'Living with stroke', followed specific outline of information developed by psychiatrists, included basic information about the consequences of stroke; (2) explanation of treatment unique to the family's situation and questions Focus: carer Setting: hospital Administration: occupational therapist (class 1), social worker (class 2). 2 x 1‐hour classes during third week of stroke; second class within 3 working days of the first Control: routine care |
|
| Outcomes | (1) Knowledge of stroke (6 months and 1 year) (2) Family function (6 months and 1 year) (3) Patient adjustment (6 months and 1 year) (4) Use of social resources (6 months and 1 year) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to conditions after minimizing the differences for variates known to predict stroke recovery: mood, self‐care ability (Barthel Index), mental status, age, and location of the lesion. The method of Taves[14] was used." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information reported. However, as no alternative intervention for control groups, blinding of participants not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No report of blinded assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Small numbers lost to follow‐up with similar reasons reported |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available so cannot assess reporting bias |
| Other bias | Unclear risk | Imbalance in reported baseline conditions (marital status and number in household) may mean choice of minimisation factors was incomplete |
Frank 2000.
| Methods | Randomisation using independently prepared envelopes Outcome assessment not blinded 2 patients (1 treatment, 1 control) lost to follow‐up 1 month follow‐up |
|
| Participants | Fife, UK 41 stroke patients: treatment N = 20; control N = 21; completed final follow‐up: N = 39 Mean age of patient: treatment 64 years; control 64 years Sex of patient: male: treatment 53%; control 50% Inclusion criteria: stroke within 24 months of recruitment, fluent in English, not aphasic, not cognitively impaired Exclusion criteria: none stated |
|
| Interventions | Treatment: workbook designed to increase perceptions of control by giving information, enhancing coping resources and rehearsing planning and problem‐solving skills. Recovery plan developed with researcher. Weekly phone call (over 3‐week period). First part of workbook dealt largely with information about stroke, causes, management, and recovery. Additional sections of relevance to the individual available (e.g. on diet, smoking). Second part introduced methods of coping and relaxation tape and instructions for use Focus: patient Setting: patients' home Administration: workbook introduced in 2 parts: part 1 introduced following baseline assessment; patients asked to work through the sections, answering quizzes and deciding which additional sections were relevant to them; part 2 introduced 1 week later along with relaxation tape and instructions for use. Requests for additional parts of the workbook met. A recovery plan consisting of a daily task with records made as joint exercise between researcher patient, and carer. Over next 3 weeks patient and carer worked independently on workbook and received weekly telephone call from researcher to enquire about progress and give opportunity to ask questions Control: wait control group received the workbook once the study was complete |
|
| Outcomes | (1) Functional limitations (1 month) (2) Mood (1 month) (3) Perceived control (1 month) |
|
| Notes | Validity assessment: No stated intention‐to‐treat analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Low risk | Used an enveloped prepared independently of the interviewer |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding; treatment group received a workbook and control group received nothing until end of trial – would have been obvious which group they were in |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The intervention and assessment were undertaken by the same individual |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 of 41 lost to follow‐up, 1 from each group, both unavailable |
| Selective reporting (reporting bias) | Unclear risk | All outcome measures specified in methods were reported. However, study protocol not available so cannot assess reporting bias |
| Other bias | Low risk | No other obvious sources of bias |
Hoffmann 2007.
| Methods | Randomisation using predetermined computer‐generated randomisation sequence Balanced block design where randomisation occurred in blocks of 4 Blinded outcome assessment Stated intention‐to‐treat analysis 5 patients (3 treatment, 2 control) lost to follow‐up 3‐month follow‐up |
|
| Participants | Brisbane, Australia 138 stroke patients: treatment N = 69; control N = 69. Completed final follow‐up N = 133 Mean age of patients: treatment 67 years; control 69 years Sex of patients male: treatment 64%; control 46% Inclusion criteria: diagnosed stroke or TIA, medically stable, reported English‐proficiency level, corrected hearing and vision and communication status adequate to participate in an interview and complete assessment tasks, no reported or observable dementia, living within 50 km of the hospital Exclusion criteria: none stated |
|
| Interventions | Treatment: computer‐generated tailored written information, customised according to patients' informational needs. 34 topics available covering such issues as: how stroke occurs, risk factors, understanding and managing the effects of stroke, reducing stroke risk, treatment and rehabilitation and managing after discharge Focus: stroke patients Setting: stroke unit Administration: within 1 day of baseline interview the research nurse completed the 'what you need to know about stroke' checklist with the patient. Further information given as needed about the scope and content in each of the available topics. Once the checklist completed, the research nurse entered topic selections, desired version of each topic (detailed, shortened) and desired font size into the database. Then generated and printed an individualised booklet and placed into a ring‐binder folder. Patient name written on booklet and given to the patient Control: Within 1 day of the baseline interview, provided by research nurse with a copy of the Stroke Association of Queensland fact sheet |
|
| Outcomes | (1) Knowledge of stroke (3 months) (2) Mood (3) Self efficacy (3 months) (4) Perceived health status (3 months) (5) Use of and satisfaction with information |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "..database randomly assigned the patient to either the intervention or control group." |
| Allocation concealment (selection bias) | Low risk | Quote: "One of the database tables contained a predetermined computer generated randomisation sequence, thus ensuring concealed allocation." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No report of blinding of participants and may not have been obvious to participants which group they were in as both received written information. However, remains unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An outcome assessor who was blind to patients’ group allocation, conducted baseline interviews while the patient was in hospital, and follow‐up interviews 3 months after discharge." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low number of losses to follow‐up and numbers balanced across groups, with similar reasons |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available so cannot assess reporting bias |
| Other bias | Low risk | No other obvious sources of bias |
Johnson 2000.
| Methods | Matched pairs design based on baseline scores on outcome measures, age, sex and side of stroke. Random assignment within each pair by tossing a coin Not blind outcome assessment All participants reassessed 1 week after intervention group completed a 4‐week course No losses to follow‐up |
|
| Participants | Minneapolis, USA *41 stroke patients identified from hospital‐based register of stroke survivors Treatment N = 21; control N = 20. Completed final follow‐up N = 41 Mean age: treatment 64.2 years; control 63.9 years Sex of patient male: * treatment 38%; control 50% Inclusion criteria: > 18 years of age, English speaking, community dwelling, stroke 6 months to 3 years earlier, gave informed consent |
|
| Interventions | Treatment: 8 x 2‐hour structured educational classes over a 4‐week period. Content included facts on stroke, living with disability, exploring spiritual wellness Control group offered the intervention after the end of the evaluation |
|
| Outcomes | (1) Self‐reported state of depression (2) Self‐reported sense of hope (3) Self‐reported ways of coping |
|
| Notes | Match pairs design then randomisation by toss of a coin, not concealed Unpaired participant assigned to the treatment group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported that one member of each pair was randomly assigned to either the treatment or control group but method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No report of blinding of participants and personnel. Control group received usual care (compared with structured education course) so may have been obvious they were not receiving an intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No report of blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if outcomes were reported for all participants |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available so cannot assess reporting bias |
| Other bias | Low risk | No other obvious sources of bias |
Johnston 2007.
| Methods | RCT. A statistician prepared 2 separate randomisations for patients with carers who also agreed to participate (carer‐patient subgroup) and for carers partnered with a patient who could not participate because of cognitive and communication impairments (carer‐only subgroup) Blinded outcome assessment Reported intention‐to‐treat analysis 45 patients (29 intervention, 16 control) and 42 carers (total across intervention and control groups) lost to follow‐up 6‐month follow‐up |
|
| Participants | Dundee, Scotland 203 acute stroke patients and 172 carers Patients: intervention N = 103; control N = 100 Carers: intervention N = 82; control N = 90 Mean age of patients: intervention 69 years; control 69 years Sex of patient male: intervention 61%; control 61% Mean age of carers: intervention 63 years; control 61 years Carer sex male: 35% (across intervention and control groups) Inclusion criteria patient: fluent in English; discharged from hospital following stroke. Carers identified by the patient as the person most involved in their care Exclusion criteria: not reported |
|
| Interventions | Intervention: post‐discharge workbook intervention delivered by a workbook implementer over a 5‐week period. The workbook provided information about stroke and recovery; guidance on coping skills; and self‐management instruction. Task materials (e.g. goal setting), diary sheets and an audio relaxation cassette tape that described simple body relaxation and breathing exercises. Intervention included 2 home visits and 2 telephone contacts. Intervention started within 3 weeks (approximately) of hospital discharge Focus: patient and carer Setting: home Administration: work book implementer Control: usual care |
|
| Outcomes | Patient outcomes (1) Disability (baseline, 8 weeks post‐intervention and 6 months after baseline) (2) Anxiety and depression (baseline, 8 weeks post‐intervention and 6 months after baseline) (3) Perceived control (completed by the patient and by the carer on behalf of the patient at baseline and 8 weeks) (4) Satisfaction with treatment and advice (8 weeks post‐intervention and 6 months after baseline) (5) Confidence in recovery (baseline) Carer outcomes (1) Anxiety and depression (baseline, 8 weeks post‐intervention and 6 months after baseline). (2) Health related quality of life (baseline) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients asked not to disclose group allocation though potentially broken. Participants would have been aware they were receiving the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research assistants blind to the process |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | More losses to follow‐up in the intervention group (29 out of 103) compared with the control group (16 out of 100). 42 carers lost (only reported across groups) |
| Selective reporting (reporting bias) | High risk | Observer Assessed Disability (OAD) not reported at baseline. Hospital and Anxiety Depression Scale (HADS) sub‐scales reported for anxiety and depression at baseline but combined post intervention |
| Other bias | Unclear risk | No other obvious signs of bias |
Kalra 2004.
| Methods | Block randomisation procedures, each block included 10 participants. Allocation schedule prepared in advance using computer‐generated random numbers. Allocation codes held in central office remote from study environment Blinded outcome assessment Stated intention‐to‐treat analysis 32 patients and caregivers lost to follow‐up: treatment N = 17; control N = 15 12‐month follow‐up |
|
| Participants | London, UK 300 patients and caregivers: treatment N = 151; control N = 149. Completed final follow‐up: N = 268 Median age of patient: treatment N = 76 years ; control N = 76 years Sex of patient male: treatment 57%; control 50% Inclusion criteria: patient ‐ independent in daily living activities before the stroke, medically and neurologically stable at time of baseline assessments, expected to return home with residual disability; carer ‐ no notable disability (Rankin score 0 to 2), willing and able to provide support after discharge Exclusion criteria: none stated |
|
| Interventions | Treatment: conventional care plus 3 to 5 sessions of 30 to 45 minutes comprising instruction by appropriate professional on common stroke‐related problems and their prevention, management of pressure areas and prevention of bed sores, continence, nutrition, positioning, gait facilitation, advice on benefits and services. Hands‐on training in lifting and handling techniques, facilitation of mobility and transfers, continence, assistance with personal activities of daily living and communication, tailored to the needs of the individual patients Focus: caregivers Setting: stroke rehabilitation unit Administration: training started when patients' rehabilitation needs stabilised and discharge contemplated. Caregivers competencies assessed at the end of training. Follow‐through session conducted by hospital team at home to adapt skills learnt to home environment Control: conventional care consisting of information on stroke and its consequences, prevention and management options; involvement in goal setting for rehabilitation and discharge planning; encouragement to attend nursing and therapy activities to learn about patient's abilities and informal instruction on facilitating mobility and activities of daily living tasks; advice on community services, benefits, and allowances including contact information for voluntary support services for caregivers |
|
| Outcomes | Patients (1) Death or institutionalisation (3 and 12 months) (2) Function (3 and 12 months) (3) Mood (3 and 12 months) (4) Quality of life (3 and 12 months) Caregiver (1) Function and social activities (3 and 12 months) (2) Emotional health (3 and 12 months) (3) Quality of life (3 and 12 months) Economic (1) Health and social care costs (12 months) (2) Informal care costs (12 months) (3) Quality adjusted life years in caregivers (over 1 year) |
|
| Notes | Validity assessment: Possibility of limited generalisability (setting was largely middle‐class suburban area) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation codes were held in a central office remote from the study environment" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were aware of training received |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reported that an observer who did not participate in allocation or management of patients assessed outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Some missing data. However, numbers and reasons for missing data relatively balanced across groups |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available so cannot assess reporting bias |
| Other bias | Low risk | No other obvious sources of bias |
Larson 2005.
| Methods | Randomisation performed by authors using blocks of 20 participants, where 10 would be allocated to each arm of the trial and the sequence could not be predicted Outcome assessment by self‐rated questionnaires 9 carers(4 treatment, 5 control) lost to follow‐up 6‐month and 1‐year follow‐up |
|
| Participants | Stockholm, Sweden 100 spouses of stroke patients: treatment N = 50; control N = 50. Completed final follow‐up: N = 91 Mean age of spouse: treatment 68 years; control 67 years Sex of spouse female: treatment 76%; control 84% Inclusion criteria: spouse of stroke patient (defined as person living in the same household as the stroke patient) Exclusion criteria: not possible to obtain information from the spouse and/or if patient not able to return home after hospitalisation |
|
| Interventions | Treatment: support and education programme led by stroke specialist nurses and group discussion with issues raised by participants. Topics included: the nature of stroke, treatment and recovery, psychological and social effects, how to prevent recurrence. Participants able to call the stroke specialist nurse between sessions to get extra information or support Focus: spouse of stroke patient Setting: hospital Administration: groups of 10, attended 6 times in 6 months. Session commenced with lecture on 1 of the topics for 20 to 30 minutes, followed by group discussion Control: regular information during hospitalisation and also at discharge. Possibility of attending 1 open session of 1.5 hours by a stroke physician on the ward (only 3 control participants chose this option) |
|
| Outcomes | (1) General Quality of Life (6 and 12 months) (2) Life situation (6 and 12 months) (3) General well‐being (6 and 12 months) (4) Perceived health state (6 and 12 months) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned to intervention or control group but method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Reported that sequence could not be predicted but method of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo intervention for control group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether an interviewer was used or whether questionnaires were self‐completed. No report of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up low (10% control and ˜2% treatment) but reasons not provided |
| Selective reporting (reporting bias) | Unclear risk | All outcomes described in the methods reported in the results. However, study protocol not available so cannot assess reporting bias |
| Other bias | Low risk | No other obvious sources of bias |
Lomer 1987.
| Methods | Patients randomly selected to receive leaflets, no further details given No stated blind outcome assessment No reported losses to follow‐up 1‐week follow‐up |
|
| Participants | Southampton, UK Numbers unclear; report states that 73 stroke incidents were assessed No participant characteristics reported Inclusion criteria: admission to medical or geriatric wards of the 2 major teaching hospitals in Southampton, clinical diagnosis of stroke Exclusion criteria: discharge within 7 days of admission, severe illness, aphasia or dysphasia that prevents response to interview, lack of awareness that have had a stroke |
|
| Interventions | Treatment: 12‐page leaflet prepared for study personalised with name, sections on basic pathologies of stroke, predisposing factors, treatment, recovery, facilities available in the community, and financial benefits available Focus: patient and relative Setting: hospital Administration: presented to patient by a medical student with no explanation other than the leaflet may be interesting for them and their relatives to read. 1 contact, length of time unknown, between 1 and 2 weeks after admission Control: usual care, no leaflet |
|
| Outcomes | (1) Knowledge of stroke (1 week) | |
| Notes | Validity assessment: comparability of treatment and control groups unknown as no reporting of participant characteristics | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method for allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No placebo intervention for control group. However, participants did not appear to be informed of the study when they were provided with the leaflet and staff were not informed who received the leaflet. However, blinding may have been broken |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if blinded outcome assessment was undertaken |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A number of exclusions and unclear at which time point in the study |
| Selective reporting (reporting bias) | Unclear risk | All outcomes described. However, study protocol not available so cannot assess reporting bias |
| Other bias | Low risk | No other obvious sources of bias |
Lowe 2007.
| Methods | Randomised using sealed opaque envelopes in blocks of 10 and 1 to 1 ratio. Envelopes prepared by independent researcher Blinded outcome assessment Stated intention‐to‐treat analysis 16 patients (6 treatment, 10 control) lost to follow‐up 3 and 6‐month follow‐up |
|
| Participants | Liverpool, UK 100 stroke patients: treatment N = 50; control N = 50. Completed final follow‐up: N = 84 Median age of patient: treatment 68 years; control 73 years Sex of patient female: treatment 42%; control 38% Inclusion criteria: Confirmed stroke, all ages, either sex, patients who are discharged home and who can complete a questionnaire, or who have a named carer who can do so Exclusion criteria: pre‐existing cognitive impairment, discharge to institutionalised care, discharge home but unable to self‐complete questionnaire and no named carer |
|
| Interventions | Treatment: CareFile (A5 size laminated 29 page booklet). Includes general information about stroke as well as information personal to the patient, secondary prevention measures, and personal goals aimed at reducing risk of further stroke. Also contains useful telephone numbers for all stroke‐related services and local support agencies. Design allows for removal of pages not relevant to the individual. Sections included for members of the multi‐disciplinary team to complete summaries of patient's achievements and future rehabilitation goals. Also provided with advice from therapists and offered leaflets from Chest, Heart and Stroke Association Focus: patient Setting: hospital ward Administration: interview arranged between researcher and patient when patient discharge date in place. Carer also invited to attend. The CareFile and its contents explained by the research registrar and any additional concerns or issues addressed in discussion lasting approximately 15 to 20 minutes. Patients advised to take the CareFile with them to all General Practitioner and clinic appointments Control: received the usual stroke information leaflets provided by the stroke unit and follow‐up in stroke review clinic |
|
| Outcomes | Primary (1) Knowledge of stroke (3 and 6 months) Secondary (1) Utilisation of CareFile (3 and 6 months) (2) Satisfaction with information given (3 and 6 months) (3) Blood pressure (3 and 6 months) (4) Participation (3 and 6 months) (5) Screening question for depression (3 and 6 months) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported that eligible patients were randomised but method not reported |
| Allocation concealment (selection bias) | Low risk | Reported to have used sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No report of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors do not appear to have been blinded – "those in the intervention group were asked if they had brought the CareFile to the Review Clinic and if they found it useful" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Almost twice as many lost to follow‐up in the control group (10/50) compared with the intervention group (6/50) |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available so cannot assess reporting bias |
| Other bias | Low risk | No other obvious sources of bias |
Maasland 2007.
| Methods | Random allocation in blocks of 10 using computer‐generated random numbers. Size of blocks unknown to investigators at time of trial Blinded outcome assessment Intention‐to‐treat analysis 7 patients lost to follow‐up plus 1 withdrawn (results not reported) as breached inclusion criteria. Differential losses between the groups unclear follow‐up at 1 and 12 weeks |
|
| Participants | Rotterdam, Netherlands 65 patients at TIA/minor stroke clinic: treatment N = 33; control N = 32. Completed final follow‐up: N = 58 (results reported for 57) Mean age of patient: treatment 65 years; control 63 years Sex of patient male; treatment 57%; control 63% Inclusion criteria: 18 years or older, TIA or minor Ischaemic stroke within last 3 months, speak/write Dutch fluently, modified Rankin score < 4 Exclusion criteria: professionally engaged in cardio‐vascular health education, aphasia, dementia, visual impairment to a degree that would interfere with health education delivery |
|
| Interventions | Treatment: discussion of test results and standard education by physician plus IMCP comprising of modules containing lay information for each of 8 modifiable risks. All modules highly structured and contained combinations of slides shows, background voice and personal address Focus: patient Setting: outpatient clinic Administration: after consultation with physician shown IMCP. Given brief introduction. 1 of 2 versions used according to age and educational level: general introduction of their personal diagnosis, explanation of the used or prescribed medications, then each patient shown 4 risk factor modules, or if has less than 4 risk factors general information about frequent vascular risk factor, printed summary of the information Control: discussion of test results and standard health education |
|
| Outcomes | (1) Knowledge at 1 (primary) and 12 weeks (secondary) post intervention (2) Function (12 weeks) (3) Changes in cholesterol level, weight, cigarette, and alcohol consumption and physical activity (12 weeks) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Treatment allocation was random, and based on computer‐generated random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No report of blinding of participants or personnel and no placebo intervention provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No report of blinded assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Eight of the 65 participants were lost in total and unclear which groups they were lost from |
| Selective reporting (reporting bias) | High risk | Blood pressure was not a pre‐specified outcome but has been reported |
| Other bias | Low risk | No other obvious sources of bias |
Mant 1998.
| Methods | Randomisation performed by telephone in computer‐generated blocks of 10 using sequentially‐numbered opaque envelopes Blinded outcome assessment Stated intention‐to‐treat analysis 22 patients (11 treatment, 11 control) and 7 carers (4 treatment, 3 control) lost to follow‐up 6‐month follow‐up |
|
| Participants | Oxford, UK 93 stroke patients: treatment N = 48; control N = 45. Completed final follow‐up: N = 71 56 carers of these patients: treatment N = 32; control N = 24. Completed final follow‐up: N = 49 Mean age of patient: treatment 70 years; control 76 years Sex of patient male: treatment 65%; control 65% Inclusion criteria: Oxfordshire resident, admission to any Oxford hospital, stroke within past month (could be recurrent) Exclusion criteria: identified over 1 month after stroke, death within 1 month of admission or considered likely to occur prior to follow‐up, taking part in another trial involving follow‐up interview, dysphasic with no close informal carer, stroke not the major medical problem, admitted from a nursing home, subdural, subarachnoid haemorrhage when no accompanying intracerebral haemorrhage, TIA |
|
| Interventions | Treatment: a collection of 8 leaflets published by the Stroke Association assembled in an A5 folder covering what a stroke is, effects, cause, problems that might be experienced and how they might be dealt with. An introductory leaflet was specially prepared plus leaflets giving local and national contact names and addresses of support groups and services Focus: patient and closest informal carer if available Setting: home Administration: pack addressed to both patient and carer (where applicable). No contact at delivery. Sent to home address 1 week after randomisation (4 to 5 weeks after stroke). Pack left with patient and carer for 6 months Control: received nothing |
|
| Outcomes | (1) Knowledge of stroke (6 months) (2) Emotional outcome (6 months) (3) Perceived health status and quality of life (6 months) (4) Satisfaction with information and care received (6 months) (5) Disability and Handicap (6 months) (6) Service use (6 months) |
|
| Notes | Validity assessment: treatment and control groups not balanced in respect of age | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence generation |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No intervention provided for the control group. As a result a high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "While in theory the interviewer was blinded to the treatment allocation, in practice she guessed the correct status of the patients more often than might be expected by chance." Correct guessing may indicate blinding was unsuccessful or that the outcome assessor was noticing real differences between participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up balanced in numbers and reasons across groups |
| Selective reporting (reporting bias) | Unclear risk | All measures described in the methods were reported in the results. However, study protocol not available so cannot assess reporting bias |
| Other bias | Low risk | No other obvious sources of bias |
O'Connell 2009.
| Methods | RCT utilising computer‐generated randomised sequence Blinded outcome assessment 27 patients (18 intervention, 9 control) lost to follow‐up |
|
| Participants | Melbourne, Australia 93 stroke patients (intervention N = 46; control N = 47) Age mean (range): 73 years (32.1 to 91.3) 33 males and 33 females completed second post intervention follow‐up (sex details not reported at baseline) Inclusion criteria: > 18 years, able to be discharged home, English proficiency, adequate communication for interview, corrected vision and hearing, no evidence of severe cognitive impairment Exclusion criteria: none reported |
|
| Interventions | Intervention: patient held‐record (PHR) which included contact details, questions for health professionals, notes on care, useful phone numbers, brochures from the national stroke foundation and fact sheets relating to specific problems associated with their stroke, level of disability and symptoms (movement and balance, swallowing difficulties, continence, driving and vision, mood changes, pain, sexuality, speech and communication). In addition, usual discharge information (health summary sheet listing medication) Focus: patient Setting: hospital prior to discharge Administration: trained health care researcher Control: usual discharge information (health summary sheet listing medication) |
|
| Outcomes | (1) Stroke Impact Scale (4 weeks and 4 months post intervention) (2) PHR evaluation questionnaire (unclear when administered) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation procedure |
| Allocation concealment (selection bias) | Low risk | Computer‐generated randomisation procedure. External researcher held randomisation codes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Whilst it was reported that the outcome assessor was blinded, one of the measures appeared to be given to the intervention group only which would have compromised assessor blinding (not explained how this was overcome). As a result unclear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | One‐third lost to follow‐up. More lost in the intervention group and reasons for losses not reported |
| Selective reporting (reporting bias) | High risk | Protocol unavailable so cannot determine if all outcomes have been reported. No pre‐intervention data reported |
| Other bias | High risk | This trial was terminated early as a number of the intervention participants were unable to recall receiving the information |
Pain 1990.
| Methods | Computer‐generated randomised allocation. Stratified for side of cerebrovascular accident Blinded outcome assessment 6 patients (2 treatment, 4 control) lost to follow‐up 3‐month follow‐up |
|
| Participants | Southampton, UK 36 stroke patients and carers (couples): treatment N = 21; control N = 15. Completed final follow‐up: N = 30 Age of patient: number < 65 years: treatment N = 8; control N = 4; number > 65 years: treatment N = 13, control N = 11 Sex of patient male: treatment N = 16; control N = 9 Inclusion criteria: admission to hospital with a CVA as defined by WHO, discharge home after a minimum period of treatment of 10 days to live with a relative or carer, agreement to participate in the study Exclusion criteria: none stated |
|
| Interventions | Treatment: individualised booklet containing information on persisting symptoms, current aims of rehabilitation, instructions concerning ADLs, description of exercises provided, pertinent photos, useful local and national addresses and contacts. Also provided with advice from therapists and offered leaflets from Chest, Heart and Stroke Association Focus: patient and carer Setting: home Administration: no contact at delivery. Sent within 7 days of discharge (> 17 days post‐stroke) by research therapist to home address Control: provided with advice from therapists and offered leaflets from Chest, Heart and Stroke Association |
|
| Outcomes | (1) Knowledge of stroke (3 months) (2) Satisfaction with information received (3 months) (3) Disability and Handicap (3 months) |
|
| Notes | Validity assessment: unequal numbers in treatment and control, participants in the treatment group had higher levels of impairment and co‐morbidity | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Therapists were not informed which patients were to receive the booklets. No report of blinding of participants, both groups were provided with advice and offered leaflets in hospital, only treatment group received booklets after discharge – may not have been obvious which group they were in |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Social services occupational therapists who were blind to the trial and control groupings undertook the interviews |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Slightly more losses to follow‐up in intervention group (4/21) than control group (2/15); reasons only given for group as a whole so cannot determine if reasons were similar between groups |
| Selective reporting (reporting bias) | Unclear risk | All outcomes described in the methods reported in the results. However, study protocol not available so cannot assess reporting bias |
| Other bias | Low risk | No other obvious sources of bias |
Rodgers 1999.
| Methods | Randomisation by a centralised telephone service. Randomised by computer initially in blocks of 8, stratified by presence of informal carer and incontinence of urine at 24 hours post stroke Blinded outcome assessment Stated intention‐to‐treat analysis 50 patients (31 treatment, 19 control) and 70 carers (42 treatment, 28 control) lost to follow‐up 6‐month follow‐up |
|
| Participants | North Tyneside, UK 204 stroke patients: treatment N = 121; control N = 83. Completed final follow‐up: N = 154 176 informal carers of these patients: treatment N = 107; control N = 69. Completed final follow‐up: N = 106 Median age of patients: treatment 74 years, control 76 years Sex of patient male: treatment 49%; control 46% Inclusion criteria: confirmed diagnosis of stroke, medically stable, normally resident in North Tyneside, not in residential home prior to admission, still in hospital within 48 hours of admission Exclusion criteria: none stated |
|
| Interventions | Treatment: 7 group sessions (1 during inpatient stay and 6 outpatient) covering the experience and nature of stroke, the role of physiotherapy and occupational therapy, psychological effects, caring, communication and swallowing problems, reducing risk. Leaflet with telephone number of stroke help line, Stroke Association, day hospital and stroke units Focus: patients and informal carer Setting: stroke unit and day hospital Administration: a rolling programme held 7 times during course of study. Presentation by speaker at each session followed by questions and discussion. Opportunity to ask questions at beginning or end of session. Inpatient session 1 x 1 hour, 6 x 1 hour outpatient sessions over 6‐week period Control: usual care. All given a basic 2‐sided leaflet about North Tyneside stroke service plus staff prompted to provide information about stroke on day of admission and at regular intervals throughout stay. Record of communication and Stroke Association literature available. Given details of telephone hotline run by the stroke service prior to discharge |
|
| Outcomes | Primary (1) Perceived health status (6 months) Secondary (1) Knowledge of stroke (6 months) (2) Emotional outcome (6 months) (3) Stress of care‐giving (6 months) (4) Satisfaction with hospital services and discharge (6 months) (5) Disability and handicap (6 months) (6) Service use (6 months) |
|
| Notes | Validity assessment: large losses to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence |
| Allocation concealment (selection bias) | Low risk | Central telephone service used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo intervention for the control group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "interviewed in their own homes at 6 months after stroke by a researcher who was blinded to the randomisation group." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Non‐attenders and attenders included in analysis as is appropriate. Approximately 25% lost to follow‐up with relatively similar numbers and reasons across groups. However, due to dysphasia or cognitive problems the primary outcome (SF‐36) could not be completed by another 37 patients (24%) meaning almost half of these outcomes were missing. Also, approximately 40% of carers were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | All outcomes described in the methods reported. However, study protocol not available so cannot assess reporting bias |
| Other bias | Unclear risk | Only 51 patients (42%) of those randomised attended 3 or more of the 6 outpatient sessions |
Smith 2004.
| Methods | Patients randomly allocated using random length restricted permuted blocks (block lengths of 2, 4, and 6). Randomisation carried out by independent research assistant by using sealed, numbered , opaque envelopes kept in a locked separate location. Stratified by Barthel Index scores of 0 to 4, 5 to 9, 10 to 14, 15 to 19, presence of aphasia, and presence of a carer Blinded outcome assessment Stated intention‐to‐treat analysis 37 patients (15 treatment, 22 control) and 21 carers (9 treatment, 12 control) lost to follow‐up 3 and 6‐month follow‐up |
|
| Participants | Bradford, UK 170 stroke patients: treatment N = 84; control N = 86. Completed final follow‐up: N = 133 97 carers of these patients: treatment N = 49; control N = 48. Completed final follow‐up: N = 76 Median age of patients: treatment 75 years; control 74 years Sex of patients female: treatment N = 46%; control N = 52% Inclusion criteria: all patients admitted to the stroke rehabilitation unit with a confirmed diagnosis of stroke Exclusion criteria: patients with receptive aphasia, cognitive impairment or who did not understand English and did not have a carer |
|
| Interventions | Treatment: provided with the Stroke Recovery Programme, a specifically devised manual containing information about causation and consequences of stroke, recovery, financial benefits, relevant services, and a specific section for carers. Also invited to attend specifically convened meetings with members of their multidisciplinary team (doctor, nurse, physiotherapist, occupational therapist). The intention of the meeting was to provide background information about stroke, discuss patient's progress, answer specific questions, and develop shared rehabilitation goals. Focus: patient but when the patient had receptive aphasia, cognitive impairment or did not understand English the carer was the main focus Setting: stroke unit Administration: Stroke Recovery Programme given by stroke unit staff following randomisation. Meetings scheduled to last approximately 20 minutes held in the ward dayroom fortnightly for duration of stroke unit stay. Guidelines developed for use by rehabilitation teams to ensure coverage of the of the key topics included in the Stroke Recovery Programme and record of matters discussed completed following each meeting. Agreed goals recorded in the manual and retained by the patient Control: Received usual practice. A folder of information about stroke causation, consequences and recovery previously devised by ward staff and stroke association leaflets were available |
|
| Outcomes | Primary (1) Knowledge of stroke (3 and 6 months) Secondary (1) Physical function (3 and 6 months) (2) Social function (3 and 6 months) (3) Handicap (3 and 6 months) (4) Patient mood (3 and 6 months) (5) Carer mood (3 and 6 months) (6) Patient and carer satisfaction (3 and 6 months) (7) Use of services and receipt of benefits (6 months) |
|
| Notes | Validity assessment: losses to follow‐up 22% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "Concealed randomisation was achieved using sealed, numbered, opaque envelopes kept in a locked separate location by an independent research assistant who carried out the randomisation and conveyed patient allocation information to the stroke unit co‐ordinator." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No report of blinding of participants and groupings probably obvious‐treatment group attended meetings, control group received usual care |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Patients and carers were followed up by a research nurse who was blind to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ˜22% lost to follow‐up with similar reasons and proportions across groups. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes described in the methods reported in the results. However, study protocol not available so cannot assess reporting bias |
| Other bias | High risk | Quote: "unavoidable contact and associated intervention contamination between the two groups of patients and relatives during the inpatient period" |
ADL: activities of daily living AMT: Abbreviated Mental Test BP: blood pressure CVA: cerebrovascular accident IMCP: individualised multimedia computer programme N: sample size QOL: quality of life RCT: randomised controlled trial TIA: transient ischaemic attack WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adie 2010 | The intervention included motivational interviewing |
| Allen 2009 | The information/education provision was part of a more complex rehabilitation intervention |
| Andersen 2002 | The information/education provision was part of a more complex rehabilitation intervention |
| Ayana 2000 | Non‐random |
| Bacchini 2011 | The information/education provision was part of a more complex rehabilitation intervention |
| Bakas 2008 | The information/education provision was part of a more complex rehabilitation intervention |
| Battersby 2009 | The information/education provision was part of a more complex rehabilitation intervention |
| Boter 2004 | The information/education provision was part of a more complex rehabilitation intervention |
| Boysen 2007 | The information/education provision was part of a more complex rehabilitation intervention |
| Brier 2011 | Non‐random |
| Brotons 2007 | The trial included participants with conditions other than stroke and the data were not available separately |
| Burton 2005 | The information/education provision was part of a more complex rehabilitation intervention |
| Byers 2010 | The intervention included motivational interviewing |
| Chaiyawat 2009 | The information/education provision was part of a more complex rehabilitation intervention |
| Chang 2000 | The information/education provision was part of a more complex rehabilitation intervention |
| Chang 2011 | The information/education provision was part of a more complex rehabilitation intervention |
| Cheng 2011 | The information/education provision was part of a more complex rehabilitation intervention |
| Christie 1984 | The information/education provision was part of a more complex rehabilitation intervention |
| Chumbler 2011 | The information/education provision was part of a more complex rehabilitation intervention |
| Claiborne 2006 | The information/education provision was part of a more complex rehabilitation intervention |
| Clark 2003 | The information/education provision was part of a more complex rehabilitation intervention |
| Clarke 2011 | The information/education provision was part of a more complex rehabilitation intervention |
| Dennis 1997 | The information/education provision was part of a more complex rehabilitation intervention |
| Desrosiers 2007 | The information/education provision was part of a more complex rehabilitation intervention |
| Dongbo 2003 | Study included both stroke and non‐stroke patients and data not available separately |
| Ertel 2007 | The information/education provision was part of a more complex rehabilitation intervention |
| Evans 1984 | Non‐random |
| Folden 1993 | (1) Not information/education; this was a study of goal setting (2) Non‐random |
| Forster 1996 | The information/education provision was part of a more complex rehabilitation intervention |
| Friedland 1992 | The information/education provision was part of a more complex rehabilitation intervention |
| Gillham 2010 | The information/education provision was part of a more complex rehabilitation intervention |
| Glass 2004 | The information/education provision was part of a more complex rehabilitation intervention |
| Goldberg 1997 | The information/education provision was part of a more complex rehabilitation intervention |
| Grant 2002 | The information/education provision was part of a more complex rehabilitation intervention |
| Grasel 2006 | The information/education provision was part of a more complex rehabilitation intervention |
| Green 2006 | The intervention included motivational interviewing |
| Habibzadeh 2007 | (1) The information/education was part of a more complex rehabilitation intervention (2) Non‐random |
| Harari 2004 | The information/education provision was part of a more complex rehabilitation intervention specifically targeted at improving bowel function |
| Harrington 2010 | The information/education provision was part of a more complex rehabilitation intervention |
| Hartke 2003 | The information/education provision was part of a more complex rehabilitation intervention |
| Harwood 2006 | The information/education provision was part of a more complex rehabilitation intervention |
| Hochstenbach 1999 | The information/education was part of a more complex rehabilitation intervention |
| Holzemer 2011 | The information/education provision was part of a more complex rehabilitation intervention |
| Huijbregts 2009 | (1) The information/education provision was part of a more complex rehabilitation intervention (2) Non‐random |
| Jones 2009 | (1) The information/education provision was part of a more complex rehabilitation intervention (2) No control group |
| Kendall 2007 | The information/education provision was part of a more complex rehabilitation intervention |
| Leathley 2003 | The information/education provision was part of a more complex rehabilitation intervention |
| Lincoln 2003 | Information provision was not the intervention evaluated The experimental condition was a support organiser |
| Linn 1979 | The information/education provision was part of a more complex intervention specifically targeted at management of medication |
| Lorenc 1992 | The study lacked a suitable control |
| Mackay‐Lyons 2010 | The information/education provision was part of a more complex rehabilitation intervention |
| Mant 2000 | Information provision was not the intervention evaluated. The experimental condition was a support worker |
| McKinney 1999 | The information/education provision was part of a more complex intervention focused on providing feedback of cognitive assessment to patients' carers and members of the multidisciplinary team |
| Morrison 1998 | Non‐random |
| Napolitan 1999 | The information/education provision was part of a more complex rehabilitation intervention |
| Neubert 2011 | No usual care group |
| Nguyen 2011 | The information/education provision was part of a more complex rehabilitation intervention |
| Nir 2006 | The information/education provision was part of a more complex rehabilitation intervention |
| Nour 2002 | The information/education provision was part of a more complex rehabilitation intervention |
| Oupra 2010 | Non‐random |
| Pierce 2007 | The information/education provision was part of a more complex rehabilitation intervention |
| Printz‐Feddersen 1990 | (1) The information/education was part of a more complex rehabilitation intervention (2) Non‐random |
| Redfern 2008 | The information/education provision was part of a more complex rehabilitation intervention |
| Rimmer 2000 | The information/education provision was part of a more complex rehabilitation intervention, which included classes in fitness and nutrition |
| Sahebalzamani 2009 | The information/education provision was part of a more complex rehabilitation intervention |
| Sanguinetti 1987 | The focus of the paper is head injury The data for stroke patients are not reported separately |
| Shyu 2008 | The information/education provision was part of a more complex rehabilitation intervention |
| Sit 2007 | Unacceptable randomisation procedure |
| Skidmore 2008 | No control group |
| Tilling 2005 | The information/education provision was part of a more complex rehabilitation intervention |
| Towle 1989 | Information provision was not the intervention evaluated The experimental condition was a support worker |
| van den Heuvel 2000 | (1) Non‐random (2) The information/education provision was part of a more complex rehabilitation intervention |
| Winkens 2009 | The information/education provision was part of a more complex intervention (psycho‐education) |
Characteristics of studies awaiting assessment [ordered by study ID]
Aben 2012.
| Methods | A randomised controlled trial |
| Participants | Stroke patients |
| Interventions | Education about memory after stroke, compensation strategies and psycho‐education. |
| Outcomes | Memory Self‐efficacy (MSE) and psychological quality of life, measured with the Metamemory In Adulthood questionnaire and the psychological domain of the WhoQol‐bref questionnaire |
| Notes |
Andrea 2003.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Bodin 2011.
| Methods | Controlled trial |
| Participants | Stroke patients |
| Interventions | The InfoCom booklet contains general information about aphasia, verbal and non‐verbal communications skills |
| Outcomes | Assessment of language deficiency (Montreal‐Toulouse‐1986) and communication skills (Test Lillois de Communication‐TLC and Protocole Toulousain d'Evaluation de la Communication au sein du Couple Aphasique‐ PTECCA). |
| Notes |
Bonita 1995.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Cameron 2011.
| Methods | Multi‐site mixed methodology pilot randomised controlled trial |
| Participants | Caregivers |
| Interventions | Stroke family support program |
| Outcomes | Outcome measures not reported |
| Notes |
Choi 2006.
| Methods | |
| Participants | Primary caregivers of stroke patients |
| Interventions | Education classes delivered by a researcher |
| Outcomes | Knowledge |
| Notes |
Eames 2008.
| Methods | Single blind randomised controlled trial |
| Participants | Clients or carers of clients with a current admission for stroke |
| Interventions | The education and support package consists of a written education booklet that provides tailored information, supplemented by verbal reinforcement and repetition of the information. Verbal reinforcement will occur face‐to‐face (prior to hospital discharge) and over the telephone (after hospital discharge) for up to 3 months post‐discharge. The written education booklet contains topics including the definition, causes, warning signs, risk factors, effects, diagnosis and treatment of stroke, as well as rehabilitation, recovery, returning to activities, going home, practical management strategies and services and support available after stroke |
| Outcomes | Primary outcome measures: stroke‐related knowledge as determined by a stroke knowledge questionnaire Secondary outcome measures: stroke‐risk factor awareness (as assessed by an opened ended question and a checklist of stroke‐related risk factors requiring a yes/no/unsure response), self‐efficacy (using measures designed for this study), stroke risk‐related behaviour change, Anxiety and depression (Hospital Anxiety and Depression Scale), client quality of life (using the Stroke and Aphasia Quality of life Scale‐39) and carer burden (Caregiver Strain Index), satisfaction (questions regarding satisfaction and usefulness of information received) |
| Notes |
Heier 2002.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Jian 1998.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Kim 2011.
| Methods | Controlled trial |
| Participants | Patients and family |
| Interventions | A web‐based secondary stroke prevention education program |
| Outcomes | Knowledge and health behaviour compliance |
| Notes |
Ostwald 2007.
| Methods | |
| Participants | Patients who have had a stroke within the last year, 50 years or older with a spouse or partner |
| Interventions | An interdisciplinary rehabilitation team will provide education, support, skill training, counselling, and social and community linkages to stroke survivors and their spouses for 6 months post‐hospital discharge |
| Outcomes | (1) function, quality of life and perceived health and depression in the stroke survivor; (2) unplanned clinic and emergency room visits, re‐hospitalisations and admissions to nursing homes; (3) depression, burden, stress and health of spousal caregivers and (4) cytokine imbalances related to the chronic stress of care‐giving among spouses |
| Notes |
Piano 2010.
| Methods | A prospective, randomised, open‐label controlled clinical trial |
| Participants | Stroke patients |
| Interventions | Video based stroke education programme |
| Outcomes | Stroke knowledge |
| Notes |
Sun 2011.
| Methods | Randomised controlled trial |
| Participants | Seventy patients with stroke |
| Interventions | Personalized health education |
| Outcomes | Hamilton anxiety scale (HAMA) to assess anxiety status, Barthel Index and Life Satisfaction Index to assess the life satisfaction of patients |
| Notes |
Tuncay 2006.
| Methods | |
| Participants | Patients with a new diagnosis of cerebrovascular disease |
| Interventions | A self‐care educational brochure |
| Outcomes | Barthel ADL |
| Notes |
ADL: activities of daily living
Characteristics of ongoing studies [ordered by study ID]
Boden‐Albala 2007.
| Trial name or title | Stroke Warning Information and Faster Treatment Study (SWIFT) |
| Methods | |
| Participants | Patients diagnosed with cerebral infarction or TIA |
| Interventions | Usual medical care (standard educational information on stroke, warning signs and risk factors) plus a 3‐session interactive stroke educational programme |
| Outcomes | Stroke knowledge and behaviour |
| Starting date | 2005 |
| Contact information | Dr Thania Perez, Columbia Presbyterian Hospital, Neurological Institute, 710 W 168th Street, 6th Floor, Room 640, New York, NY 10032, USA tperez@neuro.columbia.ed |
| Notes | This study is ongoing, but not recruiting participants (ClinicalTrials.gov, accessed December 2011) |
Damush 2006.
| Trial name or title | Adapting tools to implement stroke risk management to veterans (TOOLS) |
| Methods | Comparison of 2 regionally matched facilities on rates of secondary stroke prevention guideline care during the course of the study at the intervention sites Allocation: randomised Intervention model: single group assignment Masking: open label Primary purpose: health services research |
| Participants | Veterans 18 years or older hospitalised with stroke or TIA at Indianapolis VAMC and Houston VAMC |
| Interventions | Behavioural: physician stroke guideline adherence Behavioural: evaluation of stroke self management |
| Outcomes | Primary outcome measures: provider based outcomes: guideline adherent treatment, medication management at stroke discharge, 3 and 6 months. Risk factor screening, examination of CPRS records during hospitalisation or following 6 months. Lifestyle counselling, examination of CPRS records Secondary outcome measures: patient demographics at baseline, depression symptoms at baseline, 3 and 6 months; other co‐morbidities at 6 months |
| Starting date | July 2006 |
| Contact information | Teresa M Damush, Roudebush VA Medical Center Indianapolis, USA |
| Notes | This study is ongoing, but not recruiting participants (ClinicalTrials.gov, accessed December 2011) |
Dromerick 2008.
| Trial name or title | Preventing Recurrence Of Thromboembolic Events through Coordinated Treatment in the District of Columbia (PROTECT DC) |
| Methods | Randomised controlled trial |
| Participants | Hospitalised due to ischaemic stroke or intercurrent ischaemic stroke event within the past 30 days or TIA confirmed by stroke neurologist |
| Interventions | The program trains a lay person (stroke navigator) to provide participants with education on secondary prevention behaviour and to navigate the health and human service system, which will assist participants in obtaining the necessary services and programs to engage in secondary prevention behaviours |
| Outcomes | Primary outcome measures: low density lipoprotein value, systolic blood pressure value, haemoglobin A1C value, pill count of antiplatelet therapy medications Secondary outcome measures: smoking cessation status, AHA diet status, exercise status, stroke knowledge level |
| Starting date | June 2008 |
| Contact information | Alexander Dromerick, MD, National Rehabilitation Hospital, Georgetown University, USA |
| Notes |
Graven 2008.
| Trial name or title | From rehabilitation to recovery: a model to optimise consumer and carer involvement in the first year post stroke |
| Methods | Simple randomisation using a randomisation table created by a computer software (i.e., computerised sequence generation) Blinded (masking used) The people receiving the treatment/s The people assessing the outcomes The people analysing the results/data |
| Participants | Patient admitted for rehabilitation with a primary diagnosis of acute stroke Carers |
| Interventions | Collaborative goal setting with the patient and carer prior to discharge from rehabilitation Monitoring of goal achievement and barriers to goal achievement Collaborative problem solving to overcome barriers Facilitated referral to health and community agencies, tailored to needs Promotion of healthy and active lifestyles Promotion of self efficacy and self reliance Providing targeted carer support through information provision, emotional support and practical support tailored to needs over a 12‐month period Minimum of four interventions, maximum 12 |
| Outcomes | Primary outcome measures: Mean assessment of Quality of Life score for carers Mean Geriatric Depression Scale score for stroke survivors Secondary outcome measures: Zarit Caregiver Burden Scale ‐ carers Functional Independence Measure (motor subset) Minimental State Examination ‐ stroke survivors London Handicap Scale ‐ stroke survivors Activity Card Sort ‐ stroke survivors Strategies Used by People to Promote Health Scale ‐ stroke survivors |
| Starting date | January 2008 |
| Contact information | Christine Graven, Physiotherapy Department, St.Vincent's Health Melbourne, PO Box 2900, Fitzroy 3065, Victoria, Australia (03) 3288 3827 Christine.Graven@svhm.org.au |
| Notes |
Hackett 2008.
| Trial name or title | imProving Outcome after STroke (POST) |
| Methods | RCT |
| Participants | Recent (within 8 weeks) stroke Non‐depressed (< 8 on Hospital Anxiety and Depression Scale depression subscale at baseline) |
| Interventions | Participants will consent to a baseline screening assessment and interview, and contact from research staff for a period of up to 6 months following hospital discharge to determine their outcome and factors that might improve recovery. Specific information is not available for the general public to maintain blinding to treatment allocation and primary hypothesis |
| Outcomes | Psychosocial outcomes assessed using standard validated questionnaires |
| Starting date | 16 June 2008 |
| Contact information | Dr Maree Hackett, PO Box M201, Missenden Road, NSW 2050, Australia +61 2 9993 4593 mhackett@george.org.au |
| Notes |
Hoffmann 2009.
| Trial name or title | Evaluation of brief interventions for enhancing early emotional adjustment following stroke: a pilot randomised controlled trial |
| Methods | RCT |
| Participants | Particpants diagnosed with stoke, medically stable, adequate English and expressive and receptive communication skills and adequate cognitive capacity to provide informed consent |
| Interventions | The 8‐session self‐management intervention will be conducted by an occupational therapist and will include the provision and reinforcement of individualised written information, and activities that are aimed at assisting individuals to learn problem‐solving skills, perform functional tasks, and adjust to life post‐stroke |
| Outcomes | Primary outcome measure: Presence or absence, and severity of anxiety and depressive symptoms as determined by structured interview based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, and the Hospital Anxiety and Depression Scale Secondary outcome measures: Functional Performance as measured by the Modified Barthel Index and the Nottingham Extended Activities of Daily Living Scale Cognitive appraisal ability as measured by the Stress Appraisal Coping Measure (SAM) Self‐efficacy measured by a Self‐Efficacy Questionnaire Stroke knowledge measured by Knowledge of Stroke Questionnaire Quality of Life measured by the Stroke and Aphasia Quality of Life Scale (SAQOL) Treatment expectations measured by the Treatment Expectations Scale Self‐awareness of deficits as measured by the Self‐Perceptions in Rehabilitation Questionnaire (SPIRQ) |
| Starting date | 26/08/2009 |
| Contact information | Dr Tammy Hoffmann, Division of Occupational Therapy, School of Health and Rehabilitation Sciences, Services Road, University of Queensland, St Lucia, QLD 4072, Australia +61 7 3365 2306 t.hoffmann@uq.edu.au |
| Notes |
O'Carroll 2010.
| Trial name or title | Improving Adherence to Medication in Stroke Survivors (IAMSS) |
| Methods | 30 patients will be allocated to a brief intervention (2 sessions) and 30 to treatment as usual |
| Participants | First‐time stroke (ischaemic and haemorrhagic) or TIA patients (within 3 months discharged from a ward or clinic on any secondary preventative medication and living at home) Screening of 400 with an expected 75% response rate (300) |
| Interventions | 2 brief sessions (30 to 45 minutes) 2 weeks apart with a trained research fellow. Participants will be given the choice of having home visits or coming into a local hospital‐based Clinical Research Facility. Session 1 will focus on helping each patient draw up a specific plan, so as to establish a better medication‐taking. The effectiveness of the implementation intentions plan and any barriers/difficulties in following the plan will be reviewed in session 2, with individually tailored coping strategies/plans. Focus on eliciting and, if appropriate, challenging patients’ beliefs regarding their medication, e.g. beliefs regarding toxicity, dependence, fears regarding medications interacting harmfully |
| Outcomes | Primary outcome measures: Medication adherence recorded using Medication Event Monitoring System (MEMS) to report percentage of doses taken, percentage of days on which the correct number of doses was taken and percentage of doses taken on schedule Secondary outcome measures will include MARS self‐reported adherence of all secondary preventative medication and systolic and diastolic blood pressure |
| Starting date | Specific starting date not reported in the study protocol |
| Contact information | Ronan O’Carroll, Department of Psychology, Stirling University, Stirling, UK Correspondence: reo1@stir.ac.uk |
| Notes |
Rochette 2008.
| Trial name or title | You call‐we call trial |
| Methods | Baseline measures will be taken within the first month after stroke onset. Participants will be stratified according to comorbidity level and randomised to 1 of 2 groups: YOU CALL or WE CALL. Both interventions will be offered over a 6‐month period |
| Participants | 384 adults who meet inclusion criteria for a first mild stroke across 6 Canadian sites |
| Interventions | WE CALL is a multimodal (telephone, Internet and paper) support intervention provided to participants randomly allocated to the "WE CALL" group. The telephone component of the intervention is based on the Family Intervention Telephone Tracking model (FITT) YOU CALL group participants are provided with the name and phone number of a trained health care professional who is not involved in providing the "we call" intervention, whom they are free to contact should they feel the need |
| Outcomes | Primary outcome measures: unplanned use of health services for negative events and quality of life Secondary outcome measures: participation level, depressive symptoms and planned‐use of health services for health promotion and secondary prevention |
| Starting date | Decemeber 2008 |
| Contact information | annie.rochette@umontreal.ca |
| Notes |
Shaughnessy 2007.
| Trial name or title | Reshaping Exercise Habits And Beliefs (REHAB) |
| Methods | Allocation: randomised Endpoint classification: efficacy study Intervention model: parallel assignment Masking: open label |
| Participants | 40 to 85 years old ischaemic stroke patients |
| Interventions | Exercise: home‐based exercise prescriptions with weekly motivational telephone calls Stroke education program with matched attention phone calls |
| Outcomes | Ambulatory Activity Profile |
| Starting date | October 2006 |
| Contact information | Marianne Shaughnessy, RN PhD, VA Maryland Health Care System, Baltimore, USA |
| Notes |
Young 2007.
| Trial name or title | Education to increase self efficacy for inpatients having rehabilitation after monophasic neurological disability |
| Methods | |
| Participants | Adult patients with a monophasic disabling neurological condition admitted to a neurological rehabilitation unit |
| Interventions | Group education session and video |
| Outcomes | Self‐efficacy Mood Confidence and recovery Goals achieved variance Participation in therapy Practice with nursing staff |
| Starting date | 01 February 2006 |
| Contact information | Dr Carolyn Young, Walton centre for Neurology and Neurosurgery, Lower Lane, Fazkerley, Liverpool, L9 7LJ,UK |
| Notes |
AHA: American Heart Association CPRS: Computerised Patient Record System MARS: Medication Adherence Report Scale RCT: randomised controlled trial TIA: transient ischaemic attack
Contributions of authors
Anne Forster and John Young conceived and designed the review and wrote the research proposal to obtain funding for the original review and this update. John Wright assisted in designing the review and writing the research proposal. He also provided methodological expertise.
Peter Knapp, Anne Forster, John Wright, John Young, Allan House and Jane Smith all contributed to refining the original protocol. Jane Smith and Anne Forster co‐ordinated the first update of the review. Anne Forster and Lesley Brown co‐ordinated the review of papers, data extraction, obtaining additional information and writing of this second update.
Sources of support
Internal sources
R&D Levy Funding, UK.
Bradford Teaching Hospitals NHS Foundation Trust, UK.
External sources
NHS Executive Northern and Yorkshire R&D, UK.
Department of Health's NIHR Programme Grants for Applied Research, UK.
Declarations of interest
John Young, Jane Smith and Anne Forster have conducted a randomised trial evaluating an education programme for patients and carers after stroke. The trial is included in this review (Smith 2004).
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Banet 1997 {published data only}
- Banet GA, Felchlia MA. The potential utility of a shared medical record in a "first‐time" stroke population. Journal of Vascular Nursing 1997;15(1):29‐33. [DOI] [PubMed] [Google Scholar]
Chinchai 2010 {published data only}
- Chinchai P, Bunyamark T, Sirisatayawong P. Effects of caregiver education in stroke rehabilitation on the quality of life of stroke survivors. World Federation of Occupational Therapists Bulletin 2010;61:56‐63. [Google Scholar]
Chiu 2008 {published data only}
- Chiu C‐C, Wu S‐S, Lee P‐Y, Huang Y‐C, Tan T‐Y, Chang K‐C. Control of modifiable risk factors in ischemic stroke outpatients by pharmacist intervention: an equal allocation stratified randomized study. Journal of Clinical Pharmacy and Therapeutics 2008;33(5):529‐35. [DOI] [PubMed] [Google Scholar]
Downes 1993 {unpublished data only}
- Downes B, Rooney V, Roper‐Hall A, Oyebode J, Main A, Mayer P. The effectiveness of counselling stroke survivors and their carers in the community. Age and Ageing 1993;22 Suppl 3:28. [Google Scholar]
- Downes B, Rooney V, Roper‐Hall A, Oyebode J, Mayer P, Main A. The effect of giving information and counselling on depression and anxiety in stroke survivors and carers (The Birmingham Stroke Counselling Project). Unpublished.
Draper 2007 {published data only}
- Draper B, Bowring G, Thompson C, Heyst J, Conroy P, Thompson J. Stress in caregivers of aphasic stroke patients: a randomized controlled trial. Clinical Rehabilitation 2007;21:122‐30. [DOI] [PubMed] [Google Scholar]
Ellis 2005 {published data only}
- Ellis G, Rodger J, McAlpine C, Langhorne P. The impact of stroke nurse specialist input on risk factor modification: a randomised controlled trial. Age and Ageing 2005;34(4):389‐92. [DOI] [PubMed] [Google Scholar]
Evans 1988 {published data only}
- Evans RL, Matlock AL, Bishop DS, Stranahan S, Pederson C. Family intervention after stroke: does counselling or education help?. Stroke 1988;19:1243‐9. [DOI] [PubMed] [Google Scholar]
Frank 2000 {published data only}
- Frank G. Evaluation of a workbook‐based intervention for stroke patients. Diplomarbeit, Psychologisches Institut der Universitaet Tuebingen 1997.
- Frank G, Johnston M, Morrison V, Pollard B, MacWalter R. Perceived control and recovery from functional limitations: preliminary evaluation of a workbook‐based intervention for discharged stroke patients. British Journal of Health Psychology 2000;5:413‐20. [Google Scholar]
Hoffmann 2007 {published and unpublished data}
- Hoffmann T, McKenna K, Worrall L, Read S. Randomised trial of a computer‐generated tailored written education package for patients following stroke. Age and Ageing 2007;36:280‐6. [DOI] [PubMed] [Google Scholar]
- Hoffmann T, Russell T, McKenna K. Producing computer‐generated tailored written information for stroke patients and their carers: system development and preliminary evaluation. International Journal of Medical Informatics 2004;73:751‐8. [DOI] [PubMed] [Google Scholar]
Johnson 2000 {published and unpublished data}
- Johnson J, Pearson V. The effects of a structured education course on stroke survivors living in the community. Rehabilitation Nursing 2000;25(2):59‐65. [Google Scholar]
- Johnson J, Pearson V, McDivitt L. Stroke rehabilitation: assessing stroke survivors' long‐term learning needs. Rehabilitation Nursing 1997;22(5):243‐8. [DOI] [PubMed] [Google Scholar]
- Pearson V, Johnson J. Educational intervention reduces occurrence of depression in community‐dwelling stroke survivors. Stroke 2005;36(2):423. [Google Scholar]
Johnston 2007 {published and unpublished data}
- Johnston M, Bonetti D, Joice S, Pollard B, Morrison V, Francis J, et al. Recovery from disability after stroke as a target for a behavioural intervention: results of a randomized controlled trial. Disability and Rehabilitation 2007;29(14):1117‐27. [DOI] [PubMed] [Google Scholar]
Kalra 2004 {published and unpublished data}
- Kalra L, Evans A, Perez I, Melbourn A, Patel A, Knapp M, et al. Training carers of stroke patients: randomised controlled trial. BMJ 2004;328:1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Knapp M, Evans A, Perez I, Kalra L. Training care givers of stroke patients: economic evaluation. BMJ 2004;328:1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Larson 2005 {published data only}
- Larson J, Franzen‐Dahlin A, Billing E, Arbin M, Murray V, Wredling R. The impact of a nurse‐led support and education programme for spouses of stroke patients: a randomised controlled trial. Journal of Clinical Nursing 2005;14:995‐1003. [DOI] [PubMed] [Google Scholar]
Lomer 1987 {published data only}
- Lomer M, McLellan DL. Informing hospital patients and their relatives about stroke. International Journal of Rehabilitation Research 1986;9(1):103‐4. [Google Scholar]
- Lomer M, McLellen DL. Informing hospital patients and their relatives about stroke. Clinical Rehabilitation 1987;1:33‐7. [Google Scholar]
Lowe 2007 {published and unpublished data}
- Lowe D, Leathley M, Sharma AK. An assessment of the utility of an individualised information booklet in patients after stroke. Age and Ageing 2005;34 Suppl 1:i38. [DOI] [PubMed] [Google Scholar]
- Lowe D, Leathley M, Sharma AK. Assessment of stroke knowledge. Age and Ageing 2002;31 Suppl 1:42. [Google Scholar]
- Lowe D, Leathley M, Sharma AK. Patient education following stroke: the CareFile Project. Cerebrovascular Diseases 2002;13 Suppl 3:81. [Google Scholar]
- Lowe D, Leathley M, Sharma AK. The effects of an individualised information booklet in post‐stroke patients: the CareFile Project. Cerebrovascular Diseases 2003;14 Suppl 4:95. [Google Scholar]
- Lowe D, Sharma A, Leathley M. The CareFile project: a feasibility study to examine the effects of an individualised information booklet on patients after stroke. Age and Ageing 2007; Vol. 36:83‐9. [DOI] [PubMed]
Maasland 2007 {published data only}
- Maasland E, Koudstaal PJ, Habbema JDF, Dippel DWJ. Effects of an individualised multimedia computer programme for health education in patients with a recent minor stroke or transient ischemic attack ‐ a randomized controlled trial. Acta Neurologica Scandinavica 2007;115:41‐8. [DOI] [PubMed] [Google Scholar]
Mant 1998 {published and unpublished data}
- Mant J, Carter J, Wade DT, Winner S. The impact of an information pack on patients with stroke and their carers: a randomized controlled trial. Clinical Rehabilitation 1998;12:465‐76. [DOI] [PubMed] [Google Scholar]
O'Connell 2009 {published and unpublished data}
- O'Connell B, Hawkins M, Botti M, Buchbinder R, Baker L. Providing information to stroke survivors: lessons from a failed randomised controlled trial. Journal of the Australasian Rehabilitation Nurses' Association 2009;12:4‐6. [Google Scholar]
Pain 1990 {published data only}
- Pain HSB, McLellan DL. The use of individualised booklets after a stroke. Clinical Rehabilitation 1990;4:265‐72. [Google Scholar]
Rodgers 1999 {published and unpublished data}
- Atkinson C, Curless RH, Bond S, Suddes M, Dobson R, Rodgers H. Randomised controlled trial of a comprehensive stroke education programme for patients and carers. Proceedings of The Challenge of Stroke. The Lancet Conference. Montreal, Canada. October 1998.
- Rodgers H, Atkinson C, Bond S, Suddes M, Dobson R, Curless R. Randomised controlled trial of a comprehensive stroke education program for patients and caregivers. Stroke 1999;30:2585‐91. [DOI] [PubMed] [Google Scholar]
- Rodgers H, Curless R, Bond S, Suddes M. A randomised trial of a comprehensive stroke information service for patients and carers. Report to Northern and Yorkshire NHS Executive Unpublished. [Project Number: HSR/067]
Smith 2004 {published and unpublished data}
- Smith J, Forster A, Young J. A randomized trial to evaluate an education programme for patients and carers after stroke. Clinical Rehabilitation 2004;18(7):726‐36. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adie 2010 {published data only}
- Adie K, James M. Does telephone follow‐up improve blood pressure after minor stroke or TIA?. Age Ageing 2010;39(5):598‐603. [DOI] [PubMed] [Google Scholar]
Allen 2009 {published data only}
- Allen K, Hazelett S, Jarjoura D, Hua K, Wright K, Weinhardt J, et al. A randomized trial testing the superiority of a postdischarge care management model for stroke survivors. Journal of Stroke and Cerebrovascular Diseases 2009;18:443‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andersen 2002 {published data only}
- Andersen HE, Eriksen K, Brown A, Schultz‐Larsen K, Forchhammer BE. Follow‐up services for stroke survivors after hospital discharge ‐ a randomized control study. Clinical Rehabilitation 2002;16(6):593‐603. [DOI] [PubMed] [Google Scholar]
Ayana 2000 {published data only}
- Ayana M, Pound P, Ebrahim S. Development and use of a patient held record to improve the coordination of services between the hospital and the community after stroke. NHS Executive, London Regional Office, Commissioned R & D programme, 2000. [National Research Register N0256033066]
- Ayana M, Pound P, Lampe F, Ebrahim S. Improving stroke patients' care: a patient held record is not enough. BMC Health Services Research 2001; Vol. 1, issue 1. [DOI] [PMC free article] [PubMed]
Bacchini 2011 {published data only}
- Bacchini M, Rovacchi C, Chiampo F, Rossi, M. Gait analysis outcome following re‐education in patients poststroke. Gait and Posture 2011;33:S53‐S54. [Google Scholar]
Bakas 2008 {published data only}
- Bakas T, Farran C, Austin J, Given B, Johnson E, Williams L. Stroke caregiver outcomes from the Telephone Assessment and Skill‐Building Kit (TASK). Topics in Stroke Rehabilitation 2009;16(2):105‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakas T, Farran C, Austin J, Given B, Perkins S, Johnson E, et al. Stroke caregiver outcomes from the Telephone Assessment and Skill‐building Kit (TASK). Stroke 2008;39(2):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Battersby 2009 {published data only}
- Battersby M, Hoffmann S, Cadilhac D, Osborne R, Lalor E, Lindley R. Getting your life back on track after stroke: a phase II multi‐centered, single‐blind, randomized, controlled trial of the Stroke Self‐Management Program vs. the Stanford Chronic Condition Self‐Management Program or standard care in stroke survivors. International Journal of Stroke 2009;4(2):137‐44. [DOI] [PubMed] [Google Scholar]
- Cadilhac DA, Hoffmann S, Kilkenny M, Lindley R, Lalor E, Osborne RH, et al. A phase II multicentered, single‐blind, randomized, controlled trial of the stroke self‐management program. Stroke 2011;42(6):1673‐9. [DOI] [PubMed] [Google Scholar]
Boter 2004 {published data only}
- Boter H. Multicenter randomized controlled trial of an outreach nursing support program for recently discharge stroke patients. Stroke 2004;35(12):2867‐72. [DOI] [PubMed] [Google Scholar]
Boysen 2007 {published data only}
- Boysen G, Krarup L, Zeng X, Zeng X, Oskedra A, Korv J, et al. ExStroke pilot trial of the effect of repeated instructions to improve physical activity after ischaemic stroke: a multinational randomised controlled clinical trial. BMJ 2009;339:b2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brier 2011 {published data only}
- Brier J, Carpentier M, Moreau J. Helping patients learn new skills: does involving CVA patients and their families in the design of a patient education program improve outcomes?. Clinical Nurse Specialist 2011;25(2):85‐6. [Google Scholar]
Brotons 2007 {published data only}
- Brotons C, Soriano N, Moral I, Rodrigo MP, Kloppe P, Rodriguez AI, et al. Randomized clinical trial to assess the efficacy of a comprehensive programme of secondary prevention of cardiovascular disease in general practice: the PREseAP study. Revista Espanola de Cardiologia 2011;64(1):13‐20. [DOI] [PubMed] [Google Scholar]
Burton 2005 {published data only}
- Burton C, Gibbon B. Expanding the role of the stroke nurse: a pragmatic clinical trial. Journal of Advanced Nursing 2005;52:640‐50. [DOI] [PubMed] [Google Scholar]
Byers 2010 {published data only}
- Byers A, Lamanna L, Rosenberg A. Enhanced stroke education using motivational interviewing increases patient knowledge and satisfaction after discharge from an acute care setting. Canadian Journal of Neuroscience Nursing 2010;32(1):22. [Google Scholar]
Chaiyawat 2009 {published data only}
- Chaiyawat P, Kulkantrakorn K, Sritipsukho P. Effectiveness of home rehabilitation for ischemic stroke. Neurology International 2009;1(1):e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2000 {published data only}
- ChangT, Li I. The effect of social support intervention on home‐bound stroke patients' physical and mental health in the Ilan area. Journal of Nursing Research (China) 2000;8(4):423‐34. [Google Scholar]
Chang 2011 {published data only}
- Chang K, Zhang H, Xia Y, Chen C. Testing the effectiveness of knowledge and behavior therapy in patients of hemiplegic stroke. Topics in Stroke Rehabilitation 2011;18(5):525‐35. [DOI] [PubMed] [Google Scholar]
Cheng 2011 {published data only}
- Cheng EM, Cunningham WE, Towfighi A, Sanossian N, Bryg RJ, Anderson TL. Randomized, controlled trial of an intervention to enable stroke survivors throughout the Los Angeles County safety net to "stay with the guidelines". Circulation: Cardiovascular Quality and Outcomes 2011;4:229‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Christie 1984 {published data only}
- Christie D, Weigall D. Social work effectiveness in two‐year stroke survivors: a randomised controlled trial. Community Health Studies 1984;8:26‐32. [DOI] [PubMed] [Google Scholar]
Chumbler 2011 {published data only}
- Chumbler NR, Roudebush RL, Morey MC, Griffiths P, Quigley P, Rose DK, et al. The effects of a stroke telerehabilitation in‐home intervention on function and disability: preliminary results of a randomized clinical trial. Stroke 2011;42(3):e76‐7. [Google Scholar]
Claiborne 2006 {published data only}
- Claiborne N. Effectiveness of a care coordination model for stroke survivors: a randomized study. Health and Social Work 2006;31(2):87‐96. [DOI] [PubMed] [Google Scholar]
Clark 2003 {published data only}
- Clark MS, Rubenach S, Winsor A. A randomized controlled trial of an education and counselling intervention for families after stroke. Clinical Rehabilitation 2003;17(7):703‐12. [DOI] [PubMed] [Google Scholar]
Clarke 2011 {published data only}
- Clarke A, Barker‐Collo SL, Feigin VL. Educational group treatment for post‐stroke fatigue: a randomised pilot trial. Brain Impairment 2011;12:63. [Google Scholar]
- Clarke A, Barker‐Collo SL, Feigin VL. Poststroke fatigue: does group education make a difference? A randomized pilot trial. Topics in Stroke Rehabilitation 2012;19(1):32‐9. [DOI] [PubMed] [Google Scholar]
Dennis 1997 {published data only}
- Dennis M, O'Rourke S, Slattery J, Staniforth T, Warlow C. Evaluation of a stroke family care worker: results of a randomised controlled trial. BMJ 1997;314:1071‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, O'Rourke S, Staniforth T, Warlow C. Evaluation of a stroke family care worker: a randomised controlled trial. Cerebrovascular Diseases 1996;6 Suppl 2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke SJ, Dennis MS, Slattery J, Warlow CP. Preliminary results from a randomised trial of a stroke family care worker; patients outcome six months post stroke. Age and Ageing 1996;25 Suppl 1:32. [Google Scholar]
Desrosiers 2007 {published data only}
- Desrosiers J, Noreau L, Rochette A, Carbonneau H, Fontaine L, Viscogliosi C, et al. A home leisure education program may reduce depression after a stroke. Stroke 2007;38(2):473‐4. [DOI] [PubMed] [Google Scholar]
- Desrosiers J, Noreau L, Rochette A, Viscogliosi C, Bravo G. Effect of a home leisure education program after stroke: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2007;88(9):1095‐100. [DOI] [PubMed] [Google Scholar]
Dongbo 2003 {published data only}
- Dongbo F, Hua F, McGowan P, Yie S, Lizhen Z, Huiqin Y, et al. Implementation and quantitative evaluation of chronic disease self‐management programme in Shanghai, China: randomized controlled trial. Bulletin of the World Health Organization 2003;81:3. [PMC free article] [PubMed] [Google Scholar]
Ertel 2007 {published data only}
- Ertel K, Glymour M, Glass T, Berkman L. Frailty modifies effectiveness of psychosocial intervention in recovery from stroke. Clinical Rehabilitation 2007;21(6):511‐2. [DOI] [PubMed] [Google Scholar]
Evans 1984 {published data only}
- Evans RL, Held S. Evaluation of family stroke education. International Journal of Rehabilitation Research 1984;7(1):47‐51. [DOI] [PubMed] [Google Scholar]
Folden 1993 {published data only}
- Folden SL. The effect of supportive‐educative nursing interventions in poststroke older adults' self‐care perceptions. Miami, Florida: University of Miami, 1990. [Google Scholar]
- Folden SL. Effect of a supportive‐educative nursing intervention on older adults' perceptions of self‐care after a stroke. Rehabilitation Nursing 1993;18:162‐7. [DOI] [PubMed] [Google Scholar]
Forster 1996 {published and unpublished data}
- Dowswell G, Lawler J, Young J, Forster A, Hearn J. A qualitative study of specialist nurse support for stroke patients and care‐givers at home. Clinical Rehabilitation 1997;11:283‐301. [DOI] [PubMed] [Google Scholar]
- Forster A, Young J. Specialist nurse support of patients with stroke in the community: a randomised controlled trial. BMJ 1996;312:1642‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Friedland 1992 {published data only}
- Friedland J, McColl M. Social support for stroke survivors: development and evaluation of an intervention programme. Physical and Occupational Therapy in Geriatrics 1989;7(3):55‐69. [Google Scholar]
- Friedland JF, McColl M. Social support intervention after stroke: results of a randomised trial. Archives of Physical Medicine and Rehabilitation 1992;73:573‐81. [PubMed] [Google Scholar]
Gillham 2010 {published data only}
- Gillham S, Endacott R. Impact of enhanced secondary prevention on health behaviour in patients following minor stroke and transient ischaemic attack: a randomized controlled trial. Clinical Rehabilitation 2010;24(9):822‐30. [DOI] [PubMed] [Google Scholar]
Glass 2004 {published data only}
- Glass T, Berkman L, Hiltunen E, Furie K, Glymour M, Fay M, et al. The Families In Recovery from Stroke Trial (FIRST): primary study results. Psychosomatic Medicine 2004;66(6):889‐97. [DOI] [PubMed] [Google Scholar]
Goldberg 1997 {published data only}
- Goldberg G, Segal ME, Berk SN, Schall RR, Gershkoff AM. Stroke transition after inpatient rehabilitation. Topics in Stroke Rehabilitation 1997;4:64‐79. [DOI] [PubMed] [Google Scholar]
Grant 2002 {published data only}
- Grant JS, Elliott TR, Weaver M, Bartolucci AA, Giger JN. Telephone intervention with family caregivers of stroke survivors after rehabilitation. Stroke 2002;38(8):2060‐5. [DOI] [PubMed] [Google Scholar]
Grasel 2006 {published data only}
- Grasel E, Schmidt R, Biehler J, Schupp W. Long‐term effects of the intensification of the transition between inpatient neurological rehabilitation and home care of stroke patients. Clinical Rehabilitation 2006;20(7):577‐83. [DOI] [PubMed] [Google Scholar]
Green 2006 {published data only}
- Green T, Haley E, Eliasziw M, Hoyte K. Education in stroke prevention: efficacy of an educational counselling intervention to increase knowledge in stroke survivors. Canadian Journal of Neuroscience Nursing 2007;29(2):13‐20. [PubMed] [Google Scholar]
Habibzadeh 2007 {published data only}
- Habibzadeh H, Gofranipoor F, Ahmadi F. A study on the effect of self care plan on activity daily living status in patient with cerebrovascular accident. Journal of Medical Sciences 2007;7(1):126‐30. [Google Scholar]
Harari 2004 {published data only}
- Harari D, Norton C, Lockwood L, Swift C. Treatment of constipation and fecal incontinence in stroke patients: randomised controlled trial. Stroke 2004;35:2549‐55. [DOI] [PubMed] [Google Scholar]
Harrington 2010 {published data only}
- Harrington R, Taylor G, Hollinghurst S, Reed M, Kay H, Wood, V. A community‐based exercise and education scheme for stroke survivors: a randomized controlled trial and economic evaluation. Clinical Rehabilitation 2010;24(1):3‐15. [DOI] [PubMed] [Google Scholar]
Hartke 2003 {published and unpublished data}
- Hartke R, King R. Head trauma and stroke. The effectiveness of a telephone support group for stroke caregivers. Rehabilitation R&D Progress Reports May 1997:124‐5.
- Hartke RJ, King RB. Telephone group intervention for older stroke caregivers. Topics in Stroke Rehabilitation 2003;9(4):65‐81. [DOI] [PubMed] [Google Scholar]
Harwood 2006 {published data only}
- Harwood M. A randomised controlled study to measure the effectiveness of an educational video compared to a goal setting exercise and to a control (written pamphlet) in the treatment of stroke to improve stroke recovery for Maori and Pacific people and their families. http://www.anzctr.org.au/trial (accessed August 2011).
- Harwood M, Weatherall M, Talemaitoga A, Barber PA, Gommans J, Taylor W, et al. Taking charge after stroke: promoting self‐directed rehabilitation to improve quality of life – a randomized controlled trial. Clinical Rehabilitation 2012;26(6):493‐501. [DOI] [PubMed] [Google Scholar]
Hochstenbach 1999 {published data only}
- Hochstenbach J, Mulder T. The development and evaluation of a treatment program directed at the improvement of psychosocial behaviour following stroke. Veterans Administration Database of Research in Progress, 1999.
Holzemer 2011 {published data only}
- Holzemer E, Thanavaro J, Malmstrom T, Cruz‐Flores S. Modifying risk factors after TIA and stroke: the impact of intensive education. Journal for Nurse Practitioners 2011;7(5):372‐7. [Google Scholar]
Huijbregts 2009 {published data only}
- Huijbregts M, McEwen S, Taylor D. Exploring the feasibility and efficacy of a telehealth stroke self‐management programme: a pilot study. Physiotherapy Canada 2009;61(4):210‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2009 {published data only}
- Jones F, Mandy A, Partridge C. Changing self‐efficacy in individuals following a first time stroke: preliminary study of a novel self‐management intervention. Clinical Rehabilitation 2009;23(6):522‐33. [DOI] [PubMed] [Google Scholar]
Kendall 2007 {published data only}
- Kendall E, Catalano T, Kuipers P, Posner N, Buys N, Charker J. Recovery following stroke: the role of self‐management education. Social Science and Medicine 2007;64(3):735‐46. [DOI] [PubMed] [Google Scholar]
Leathley 2003 {published data only}
- Leathley M, Fall S, Sharma A, Watkins C, Barer D. Life after stroke: multicentre trial of psychosocial interventions after stroke. Cerebrovascular Diseases 2003;16 Suppl 4:70. [Google Scholar]
Lincoln 2003 {published data only}
- Lincoln NB, Francis VM, Lilley SA, Sharma JC, Summerfield M. Evaluation of a stroke family support organiser: a randomized controlled trial. Stroke 2003;34(1):116‐21. [DOI] [PubMed] [Google Scholar]
Linn 1979 {published data only}
- Linn JT. Medications for the elderly. Medical Journal of Australia 1979;1:315‐6. [DOI] [PubMed] [Google Scholar]
Lorenc 1992 {published and unpublished data}
- Lorenc L, Sturmey P, Brittain H. Evaluation of a meta‐cognitive strategy to improve the information gained from a stroke information pack. Stress Medicine 1992;8(2):111‐2. [Google Scholar]
Mackay‐Lyons 2010 {published data only}
- MacKay‐Lyons M, Gubitz G, Giacomantonio N, Wightman H, Marsters D, Thompson K, et al. Program of rehabilitative exercise and education to avert vascular events after non‐disabling stroke or transient ischemic attack (PREVENT Trial): a multi‐centred, randomised controlled trial. BMC Neurology 2010;10:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mant 2000 {published data only}
- Mant J, Carter J, Wade DT, Winner S. Family support for stroke: a randomised controlled trial. Lancet 2000;356:808‐13. [DOI] [PubMed] [Google Scholar]
McKinney 1999 {published and unpublished data}
- McKinney M, Blake H, Treece KA, Lincoln N, Playford D, Gladman JRF. Evaluation of a cognitive assessment in stroke rehabilitation. Clinical Rehabilitation 2002;16:129‐36. [DOI] [PubMed] [Google Scholar]
- McKinney M, Playford D, Lincoln N, Blake H, Treece K. Evaluation of cognitive assessment after stroke. Stroke Association Conference Proceedings. 2000.
Morrison 1998 {published and unpublished data}
- Morrison VL, Johnston M, MacWalter RS, Pollard BS. Improving emotional outcomes following acute stroke: a preliminary evaluation of a work‐book based intervention. Scottish Medical Journal 1998;43(2):52‐3. [DOI] [PubMed] [Google Scholar]
Napolitan 1999 {published data only}
- Napolitan SM. An evaluation of the effectiveness of early psychoeducational orientation and home visit intervention for first‐time caregivers of stroke patients. Dissertation Abstracts. Dissertation Abstracts, 1999. [ISBN 0‐599‐32429‐8]
Neubert 2011 {published data only}
- Neubert S, Sabariego C, Stier‐Jarmer M, Cieza A. Development of an ICF‐based patient education program. Patient Education & Counseling 2011;84(2):e13‐7. [DOI] [PubMed] [Google Scholar]
Nguyen 2011 {published data only}
- Nguyen V‐HV, Poon J, Tokuda L, Sayers J, Wallis R‐A, Dergalust S. Pharmacist telephone interventions improve adherence to stroke preventive medications and reduce stroke risk factors: a randomized controlled trial. Stroke 2011;42(3):e244. [Google Scholar]
Nir 2006 {published data only}
- Nir Z, Weisel‐Eichler A. Improving knowledge and skills for use of medication by patients after stroke: evaluation of a nursing intervention. American Journal of Physical Medicine and Rehabilitation 2006;85(7):582‐92. [DOI] [PubMed] [Google Scholar]
Nour 2002 {published data only}
- Nour K, Desrosiers J, Gauthier P, Carbonneau H. Impact of a home leisure educational program for older adults who have had a stroke. Therapeutic Recreation Journal 2002;36(1):48‐64. [Google Scholar]
Oupra 2010 {published data only}
- Oupra R, Griffiths R, Pryor J, Mott S. Effectiveness of Supportive Educative Learning programme on the level of strain experienced by caregivers of stroke patients in Thailand. Health and Social Care in the Community 2010;18(1):10‐20. [DOI] [PubMed] [Google Scholar]
Pierce 2007 {published data only}
- Pierce L, Steiner V, Khuder S, Govoni A, Horn L. The effect of a Web‐based stroke intervention on carers' well‐being and survivors' use of healthcare services. Disability and Rehabilitation 2009;31(20):1676‐84. [DOI] [PubMed] [Google Scholar]
- Steiner V, Pierce L, Drahuschak S, Nofziger E, Buchman D, Szirony T. Emotional support, physical help, and health of caregivers of stroke survivors. Journal of Neuroscience Nursing 2008;40(1):48‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner V, Pierce L, Windnagel F, Martincin K, Pawlak R, Salvador D. The impact of a web‐based caregiver intervention on the reasons stroke survivors use health care services during the first year post treatment. Topics in Stroke Rehabilitation 2009;16(2):122‐32. [DOI] [PubMed] [Google Scholar]
Printz‐Feddersen 1990 {published data only}
- Printz‐Feddersen V. Group process on caregiver burden. Journal of Neuroscience Nursing 1990;22(3):164‐8. [DOI] [PubMed] [Google Scholar]
Redfern 2008 {published data only}
- Redfern J, McKevitt C, Rudd A, Heuschmann P, Grieve A, Wolfe C. Stop stroke: cluster randomised controlled trial of a patient/carer and general practitioner intervention to improve risk factor management after stroke. Cerebrovascular Diseases 2009;27:66. [Google Scholar]
- Redfern J, Rudd A, Wolfe C, McKevitt C. Stop stroke: development of an innovative intervention to improve risk factor management after stroke. Patient Education and Counseling 2008;72(2):201‐9. [DOI] [PubMed] [Google Scholar]
- Wolfe C, Redfern J, Rudd A, Grieve A, Heuschmann P, McKevitt C. Cluster randomized controlled trial of a patient and general practitioner intervention to improve the management of multiple risk factors after stroke: stop stroke. Stroke 2010;41(11):2470‐6. [DOI] [PubMed] [Google Scholar]
Rimmer 2000 {published data only}
- Rimmer JH, Braunschweig C, Silverman K, Riley B, Creviston T, Nicola T. Effects of a short‐term health promotion intervention for a predominantly African‐American group of stroke survivors. American Journal of Preventive Medicine 2000;18(4):332‐8. [DOI] [PubMed] [Google Scholar]
Sahebalzamani 2009 {published data only}
- Sahebalzamani M, Aliloo L, Shakibi A. The efficacy of self‐care education on rehabilitation of stroke patients. Saudi Medical Journal 2009;30(4):550‐4. [PubMed] [Google Scholar]
Sanguinetti 1987 {published data only}
- Sanguentetti M, Cantanzaro M. A comparison of discharge teaching on the consequences of brain injury. Journal of Neuroscience Nursing 1987;19:271‐5. [DOI] [PubMed] [Google Scholar]
Shyu 2008 {published data only}
- Shyu Y, Chen M, Chen S, Wang H, Shao J. A family caregiver‐oriented discharge planning program for older stroke patients and their family caregivers. Journal of Clinical Nursing 2008;17(18):2497‐508. [DOI] [PubMed] [Google Scholar]
- Shyu Y, Kuo L, Chen M, Chen S. A clinical trial of an individualised intervention programme for family caregivers of older stroke victims in Taiwan. Journal of Clinical Nursing 2010;19(11‐12):1675‐85. [DOI] [PubMed] [Google Scholar]
Sit 2007 {published data only}
- Sit J, Yip V, Stanley K, Gun A, Lee J. A quasi‐experimental study on a community‐based stroke prevention programme for clients with minor stroke. Journal of Clinical Nursing 2007;16(2):272‐81. [DOI] [PubMed] [Google Scholar]
Skidmore 2008 {published data only}
- Skidmore ER, Koenig KL, Munin MC, Whyte E M, O'Donnell L, Penrod L, et al. Do clinical rehabilitation education programs really improve stroke‐related knowledge?. American Journal of Physical Medicine & Rehabilitation 87;8:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tilling 2005 {published data only}
- Tilling K, Coshall C, McKevitt C, Daneski K, Wolfe C. A family support organiser for stroke patients and their carers: a randomised controlled trial. Cerebrovascular Diseases 2004;20(2):85‐91. [DOI] [PubMed] [Google Scholar]
Towle 1989 {published data only}
- Towle D, Lincoln NB, Matfield LM. Service provision and functional independence in depressed stroke patients and the effects of social work intervention on these. Journal of Neurology Neurosurgery and Psychiatry 1989;52:519‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towle D, Lincoln NB, Mayfield LM. Evaluation of social work on depression after stroke. Clinical Rehabilitation 1989;3:89‐96. [Google Scholar]
van den Heuvel 2000 {published data only}
- Schure L, Heuvel E, Stewart R, Sanderman R, Witte L, Meyboom‐de Jong B. Beyond stroke: description and evaluation of an effective intervention to support family caregivers of stroke patients. Patient Education and Counseling 2006;62(1):46‐55. [DOI] [PubMed] [Google Scholar]
- Heuvel ETP, Witte LP, Nooyen‐Haazen I, Sanderman R, Meyboom‐de Jong B. Short‐term effects of a group support program and an individual support program for caregivers of stroke patients. Patient Education and Counseling 2000;40:109‐20. [DOI] [PubMed] [Google Scholar]
- Heuvel ETP, Witte LP, Stewart RE, Schure LM, Sanderman R, Meyboom‐de Jong B. Long‐term effects of a group support program and an individual support program for informal caregivers of stroke patients: which caregivers benefit the most?. Patient Education and Counselling 2002;47:291‐9. [DOI] [PubMed] [Google Scholar]
Winkens 2009 {published data only}
- Winkens I, Heugten C, Wade D, Habets E, Fasotti L. Efficacy of time pressure management in stroke patients with slowed information processing: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2009;90(10):1672‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Aben 2012 {published data only}
- Aben L, Heijenbrok‐Kal M, Ponds R, Busschbach J, Ribbers G. Long term effects of a memory self‐efficacy training program for stroke patients in the chronic stage: a randomized controlled trial. Brain Injury 2012;26(4‐5):414‐5. [Google Scholar]
Andrea 2003 {published data only}
- Andrea S, Kotzabassaki S, Bellou M, Vardaki Z. Evaluation of the effectiveness of a self‐care educational program on activities of daily living, performed by hemiplegic patients. ICUs and Nursing Web Journal 2003; Vol. Oct‐Dec, issue 16.
Bodin 2011 {published data only}
- Bodin S, Labrunee‐Prod'homme K, Delort‐Albrespit I, Castel‐Lacanal E, Labrunee M, Marque P, et al. Impact of early information of close relative about communication with an aphasic patient: InfoCom study. Annals of Physical and Rehabilitation Medicine 2011;54:e215. [Google Scholar]
Bonita 1995 {unpublished data only}
- Bonita R. Provision of an information leaflet. Unpublished.
Cameron 2011 {published data only}
- Cameron JI, Naglie G, Green T, Gignac MAM, Bayley M, Huijbregts M, et al. The Timing It Right Stroke Family Support Program: phase 2 pilot randomized controlled trial. Cerebrovascular Diseases 2011;31 Suppl 2:42. [Google Scholar]
Choi 2006 {published data only}
- Choi JS, Seo YM, Kwon IS. Effects of education on knowledge and practice of caregivers of the stroke patient. Taehan Kanho Hakhoe Chi 2006;36(7):1175‐82. [DOI] [PubMed] [Google Scholar]
Eames 2008 {published data only}
- Eames S. Evaluation of a post‐discharge education and support package for stroke clients and their carers: randomised control trial (Does providing information after stroke help?). http://www.anzctr.org.au/trial (accessed November 2011).
- Eames S, Hoffmann T, Worrall L, Wong A, Read S. Evaluation of an innovative post‐discharge education and support package for patients with stroke and their carers. International Journal of Stroke 2010;5 Suppl 2:190. [Google Scholar]
- Emmes S, Hoffman T, Worrall L, Read S, Wong A. Randomised controlled trial of a postdischarge education and support package for clients with stroke and their carers. Australian Occupational Therapy Journal 2011;58:51. [Google Scholar]
Heier 2002 {published data only}
- Heier H, Laemmler G, Steinhagen‐Thiessen E. Evaluation of a psychoeducative intervention program for caregivers of stroke patients. Zeitschrift Fuer Neuropsychiogie 2002;13(3):201‐9. [Google Scholar]
Jian 1998 {published data only}
- Jian S, Chi LI. A study of patient education for stroke survivors in Wuhan, China. Annals of Oncology 1998;9(53):119. [Google Scholar]
Kim 2011 {published data only}
- Kim CG, Park HA. Development and evaluation of a web‐based education program to prevent secondary stroke. Journal of Korean Academy of Nursing 2011;41(1):47‐60. [DOI] [PubMed] [Google Scholar]
Ostwald 2007 {published data only}
- Ostwald S. Intervention for stroke survivors and their spousal caregivers. http://clinicaltrials.gov/ (accessed 1 November 2011).
Piano 2010 {published data only}
- Piano AN, Roxas AA, Aganon F, Ticod MLJC, Sibulo JV, Cajucom T, et al. Stroke awareness among stroke victors: a randomized open label clinical trial comparing videobased stroke education program vs. nurse education. International Journal of Stroke 2010;5 Suppl 2:281. [Google Scholar]
Sun 2011 {published data only}
- Sun M, Ling XM, Yu DF. Effects of personalized health education on quality of life in patients with stroke. Journal of Shanghai Jiaotong University (Medical Science) 2011;31(7):996‐9. [Google Scholar]
Tuncay 2006 {published data only}
- Tuncay FO, Mollaoglu M. The effect of a self‐care education program on cerebrovascular disease patients' activities of daily living. Neurology Psychiatry and Brain Research 2006;13(2):83‐8. [Google Scholar]
References to ongoing studies
Boden‐Albala 2007 {published data only}
- Boden‐Albala B, Sacco R, Stillman JI, Wright CB. The Stroke Warning Information and Faster Treatment Study (SWIFT). http://clinicaltrials.gov/ct2/show/NCT00415389?term=SWIFT&rank=7(accessed December 2011).
- Boden‐Albala B, Stillman J, Chong J, Perez T, Rivolta J, Evenson L, et al. Transient cerebral ischemia is associated with earlier ED arrival times than ischemic stroke: the Stroke Warning Information and Faster Treatment (SWIFT) study. Stroke 2007;38(2):502. [Google Scholar]
- Stillman J, Chong J, Perez T, Cheung P, Paik MC, Sacco RL, et al. Are stroke patients knowledgeable and prepared for a recurrent event? Findings from the SWIFT study. Stroke 2007;38(2):527. [Google Scholar]
Damush 2006 {published data only}
- Damush TM. Adapting tools to implement stroke risk management to veterans (TOOLS). http://clinicaltrials.gov/ct2/show/NCT00355147?term=TOOLS&rank=1 (accessed November 2011).
Dromerick 2008 {published data only}
- Dromerick AW. Preventing Recurrence Of Thromboembolic Events through Coordinated Treatment in the District of Columbia (PROTECT DC). http://clinicaltrials.gov/ct2/show/NCT00703274?term=PROTECT+DC&rank=1 (accessed November 2011). [DOI] [PMC free article] [PubMed]
Graven 2008 {published data only}
- Graven C, Brock K, Hill K, Ames D, Cotton S, Joubert L. From rehabilitation to recovery: protocol for a randomised controlled trial evaluating a goal‐based intervention to reduce depression and facilitate participation post‐stroke. BMC Neurolgy 2011;11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hackett 2008 {published data only}
- Hackett ML, Carter G, Crimmins D, Clarke T, Maddock K, Sturm JW. imProving Outcomes after STroke clinical pilot trial protocol (POST). International Journal of Stroke 2010;5(10):52‐6. [DOI] [PubMed] [Google Scholar]
Hoffmann 2009 {published data only}
- Hoffmann T. Do stroke patients receiving either a self‐management or cognitive‐behavioural intervention demonstrate enhanced early emotional adjustment as compared with those receiving usual care?. http://www.anzctr.org.au/trial_view.aspx?ID=308355 (accessed Novemeber 2011).
O'Carroll 2010 {published data only}
- O'Carroll R, Dennis M, Johnston M, Sudlow C. Improving adherence to medication in stroke survivors (IAMSS): A randomised controlled trial: study protocol. BMC Neurology 2010;10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rochette 2008 {published data only}
- Rochette A, Komer‐Bitensky N, Bishop D, Teasell R, White C, Bravo G, et al. Study protocol of the YOU CALL ‐ WE CALL TRIAL: impact of a multimodal support intervention after a "mild" stroke. BMC Neurology 2010;10(3):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaughnessy 2007 {published data only}
- Shaughnessy M. Reshaping Exercise Habits and Beliefs (REHAB). http://www.clinicaltrial.gov/ (accessed November 2011).
Young 2007 {published data only}
- Young C. Education to increase self efficacy for patients having rehabilitation after monophasic neurological disability. www.nrr.nhs.uk/ (accessed March 2007).
Additional references
Adams 1998
- Adams RD, Victor M, Ropper, AH. Principles of Neurology: Companion Handbook. 6th Edition. McGraw‐Hill, 1998. [Google Scholar]
Adamson 2004
- Adamson J, Beswick A, Ibrahim S. Is stroke the most common cause of disability?. Journal of Stroke and Cerebrovascular Diseases 2004;13(4):171‐7. [DOI] [PubMed] [Google Scholar]
Aho 1980
- Aho K, Harmsen P, Hatano S, Marquardsen J, Smirnov V. Cerebrovascular disease in the community: results of a WHO Collaborative Study. Bulletin of the World Health Organization 1980;58:113‐30. [PMC free article] [PubMed] [Google Scholar]
Brown 1990
- Brown SA. Studies of educational interventions and outcomes in diabetic adults: a meta‐analysis revisited. Patient Education and Counseling 1990;16:189‐215. [DOI] [PubMed] [Google Scholar]
Canadian Stroke Network 2006
- Canadian Best Practice Recommendations for Stroke Care, Ottawa 2006. Canadian Stroke Network and the Heart and Stroke Foundation of Canada: Canadian Stroke Strategy.
Care Quality Commission 2011
- Care Quality Commission. Supporting life after stroke. Care Quality Commission, London 2011.
Coulter 1998
- Coulter A, Entwhistle V, Gilbert D. Informing patients: an assessment of the quality of patient information materials. Kings Fund, London 1998.
de Beurs 1999
- Beurs E, Bouter LM. Is the standardised mean difference a suitable measure of treatment effect?. Proceedings of the 7th Cochrane Colloquium, Universita S. Tommaso D'Aquino, Rome. 1999.
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐Analysis in Context. 2nd Edition. London: BMJ Publication Group, 2001. [Google Scholar]
Department of Health 2001
- The expert patient: a new approach to chronic disease management for the 21st century. Department of Health, London 2001.
Department of Health 2007
- Department of Health. National STROKE Strategy. Department of Health, London 2007.
Department of Health 2010
- Equity and Excellence: Liberating the NHS. Department of Health, London 2010.
Drummond 1996
- Drummond A, Lincoln N, Juby L. Effects of a stroke unit on knowledge of stroke and experiences in hospital. Health Trends 1996;28:26‐30. [Google Scholar]
Duncan 1999
- Duncan P, Wallace D, Lai S, Johnson D, Embretson S, Laster L. The stroke impact scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke 1999;30(10):2131‐40. [DOI] [PubMed] [Google Scholar]
Eames 2011
- Eames S, Hoffmann T, Worrall L, Read S. Delivery styles and formats for different stroke information topics: patient and carer preferences. Patient Education and Counseling 2011;84:e18‐e23. [DOI] [PubMed] [Google Scholar]
Ellis 2010
- Ellis G, Mant J, Langhorne P, Dennis M, Winner S. Stroke liaison workers for stroke patients and carers: an individual patient data meta‐analysis. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD005066.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
EuroQol Group 1990
- The Euroqol Group. EuroQol: a new facility for the measurement of health related quality of life. Health Policy 1990;16:199‐208. [DOI] [PubMed] [Google Scholar]
Evans 1985
- Evans RL, Pomeroy S, Weele T, Hammond MC. Reliability of a stroke care information test for family caretakers. International Journal of Rehabilitation Research 1985;8:199‐201. [Google Scholar]
Evans 1991
- Evans RL, Bishop DS, Haselkorn JK. Factors predicting satisfactory home care after stroke. Archives of Physical Medicine and Rehabilitation 1991;72:144‐7. [PubMed] [Google Scholar]
Farran 1995
- Farran CJ, Herth KA, Popovich JM. Hope and hopelessness: critical clinical constructs. Sage Publications, 1995. [Google Scholar]
Firth 1991
- Frith B. Giving information to radiotherapy patients. Nursing Standard 1991;5(34):33‐5. [DOI] [PubMed] [Google Scholar]
Gallagher 1982
- Gallagher D, Nies G, Thompson LW. Reliability of the Beck Depression Inventory with older adults. Journal of Consulting and Clinical Psychology 2000;50:152‐3. [DOI] [PubMed] [Google Scholar]
Gibbs 1989
- Gibbs S, Waters WE, George CF. The benefits of prescription information leaflets. British Journal of Clinical Pharmacology 1989;27(6):723‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldberg 1979
- Goldberg DP, Hillier VF. A scaled version of the General Health Questionnaire. Psychological Medicine 1979;9:139‐45. [DOI] [PubMed] [Google Scholar]
Goldberg 1988
- Goldberg D, Williams P. A User's Guide to the General Health Questionnaire. Windsor, UK: NFER‐Nelson Publishing Company Limited, 1988. [Google Scholar]
Grimshaw 2001
- Grimshaw J, Shirran L, Thomas R, Mowatt G, Fraser C, Bero L, et al. Changing provider behaviour. An overview of systematic reviews of interventions. Medical Care 2001;39(8 Suppl 2):II‐2‐II‐45. [PubMed] [Google Scholar]
Hafsteinsdottir 2011
- Hafsteinsdottir TB, Vergunst M, Lindeman E, Schuurmans M. Educational needs of patients with a stroke and their caregivers: a systematic review of the literature. Patient Education and Counseling 2011;85(1):14‐25. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holbrook 1983
- Holbrook M, Skilbeck CE. An activities index for use with stroke patients. Age and Ageing 1983;12:166‐70. [DOI] [PubMed] [Google Scholar]
Knapp 2000
- Knapp P, Young J, House A, Forster A. Non‐drug strategies to resolve psycho‐social difficulties after stroke. Age and Ageing 2000;29(1):23‐30. [DOI] [PubMed] [Google Scholar]
Lewin 1992
- Lewin B. A self‐help post‐MI rehabilitation package ‐The Heart Manual: effects on psychological adjustment, hospitalisation and GP consultation. Lancet 1992;339:1036‐40. [DOI] [PubMed] [Google Scholar]
Li 2011
- Li T, Wu HM, Wang F, Huang CQ, Yang M, Dong BR, et al. Education programmes for people with diabetic kidney disease. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD007374.pub2] [DOI] [PubMed] [Google Scholar]
Mackenzie 2007
- Mackenzie A, Perry L, Lockhart E, Cottee M, Cloud G, Mann H. Family carers of stroke survivors: needs, knowledge, satisfaction and competence in caring. Disability and Rehabilitation 2007;29(2):111‐21. [DOI] [PubMed] [Google Scholar]
Mahoney 1994
- Mahoney J, Drinka TJK, Abler R. Screening for depression: single question versus GDS. Journal of American Geriatric Society 1994;9:1006‐8. [DOI] [PubMed] [Google Scholar]
McKevitt 2011
- McKevitt C, Fudge N, Redfern J, Sheldenkar A, Crichton S, Rudd AR, et al. Self‐reported long‐term needs after stroke. Stroke 2011;42(5):1398‐403. [DOI] [PubMed] [Google Scholar]
Morrison 2000
- Morrison V, Johnston M, Walter RM. Predictors of distress following an acute stroke: disability, control cognitions, and satisfaction with care. Psychology and Health 2000;15(3):395‐408. [Google Scholar]
Mukherjee 2011
- Mukherjee D, Patil CG. Epidemiology and the global burden of stroke. World Neurosurgery 2011;76(6 Suppl):S85‐90. [DOI] [PubMed] [Google Scholar]
National Audit Office 2005
- National Audit Office. Reducing brain damage: faster access to better stroke care. London, NAO 2005.
National Stroke Foundation Australia 2010
- Clinical Guidelines for Stroke Management, Melbourne 2010. National Stroke Foundation Australia.
Nouri 1987
- Nouri FM, Lincoln NB. An extended activities of daily living for stroke patients. Clinical Rehabilitation 1987;1:301‐5. [Google Scholar]
O'Mahoney 1997
- O'Mahoney PG, Rodgers H, Thomson RG, Dobson R, James OFW. Satisfaction with information and advice received by stroke patients. Clinical Rehabilitation 1997;11:68‐72. [DOI] [PubMed] [Google Scholar]
OAST 2007
- The Optimising Analysis of Stroke Trials (OAST) Collaboration. Can we improve the statistical analysis of stroke trials? Statistical re‐analysis of functional outcomes in stroke trials. Stroke 2007;38:1911‐5. [DOI] [PubMed] [Google Scholar]
Partridge 1989
- Partridge C, Johnston M. Perceived control of recovery from physical disability: measurement and prediction. British Journal of Clinical Psychology 1989;28(Pt 1):53‐9. [DOI] [PubMed] [Google Scholar]
Patrick 1989
- Patrick DL, Peach H (Eds). Disablement in the community. Oxford: Oxford University Press, 1989. [Google Scholar]
Pound 1993
- Pound P, Gompertz P, Ebrahim S. Development and results of a questionnaire to measure carer satisfaction after stroke. Journal of Epidemiology and Community Health 1993;47:500‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pound 1994
- Pound P, Gompertz P, Ebrahim S. Patients' satisfaction with stroke services. Clinical Rehabilitation 1994;8:7‐17. [Google Scholar]
RCP 2008
- Royal College of Physicians. National clinical guideline for stroke, 3rd edition. Royal College of Physicians, London 2008.
Riemsma 2003
- Riemsma RP, Kirwan JR, Taal E, Rasker JJ. Patient education for adults with rheumatoid arthritis. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD003688] [DOI] [PubMed] [Google Scholar]
Rowan 1994
- Rowan K. Global questions and scores. In: Jenkinson C editor(s). Measuring Health and Medical Outcomes. London: UCL Press, 1994. [Google Scholar]
Sakthong 2007
- Sakthong P, Schommer J, Gross C, Sakulbumrungsil R, Prasithsirikul W. Psychometric properties of WHOQOL‐BREF‐Thai in patients with HIV/AIDS. Journal of Medical Association of Thailand 2007;90:2449‐57. [PubMed] [Google Scholar]
Sheikh 1986
- Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clinical Gerontology: A Guide to Assessment and Intervention. New York: The Haworth Press, 1986:165‐73. [Google Scholar]
Sullivan 2004
- Sullivan K, Dunton N. Development and validation of the Stroke Knowledge Test. Topics in Stroke Rehabilitation 2004;11:19‐28. [DOI] [PubMed] [Google Scholar]
Theis 1995
- Theis AL, Johnson JH. Strategies for teaching patients: a meta‐analysis. Clinical Nurse Specialist 1995;9:100‐5. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). Medical Care 1992;30:473‐83. [PubMed] [Google Scholar]
Wellwood 1994
- Wellwood I, Dennis MS, Warlow CP. Perceptions and knowledge of stroke amongst surviving patients with stroke and their carers. Age and Ageing 1994;23:293‐8. [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 1983;67:361‐70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Forster 2001
- Forster A, Smith J, Young J, Knapp P, House A, Wright J. Information provision for stroke patients and their caregivers. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD001919] [DOI] [PubMed] [Google Scholar]
Smith 2008
- Smith J, Forster A, House A, Knapp P, Wright J, Young J. Information provision for stroke patients and their caregivers. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD001919.pub2] [DOI] [PubMed] [Google Scholar]


