Abstract
Background
Endometrial polyps, which are benign growths of the endometrium, may be a factor in female subfertility. Possible mechanisms include physical interference with gamete transport, alteration of the endometrial milieu and unresponsiveness to the cyclical global endometrial changes. As such polyps remain mostly asymptomatic, their diagnosis is often incidental during routine investigations prior to embarking on assisted reproductive treatment. Transvaginal sonography, hysterosalpingography and saline infusion sonography are the diagnostic tools most commonly employed. However, hysteroscopy remains the gold standard for diagnosis, as well as for treatment. Due to the possible effect of endometrial polyps on fertility, their removal prior to any subfertility treatment is widely practiced.
Objectives
To determine the effectiveness and safety of removal of endometrial polyps in subfertile women.
Search methods
Electronic databases were searched, including the Cochrane Menstrual Disorders and Subfertility Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, PsycINFO, CINAHL and trial registers. The reference lists of identified articles were checked. The last search was performed on 30 July 2014.
Selection criteria
Only randomised controlled trials, reporting pregnancy or live birth rates and complication rates as primary or secondary outcomes, in which polyps were removed surgically prior to treatment of subfertility were eligible for inclusion. The diagnosis of endometrial polyps was required to be made by transvaginal ultrasound, hysterosalpingography, saline infusion, sono‐hysterography or hysteroscopy. Any surgical technique of polyp removal was acceptable, with no intervention in the control groups.
Data collection and analysis
Two review authors independently screened the titles, abstracts and full articles to assess their suitability for inclusion in this review. Quality assessment was attempted independently by two authors with discrepancies being settled by consensus or consultation with a third review author.
No data extraction was performed due to the absence of useable data in the one eligible study. If there had been data to include, two review authors would have independently extracted the data from the studies using a data extraction form designed and pilot tested by the authors. Any disagreements would have been resolved by discussion or by a third review author.
Main results
Only one randomised controlled trial of endometrial polypectomy was identified for inclusion. However, a single set of data could not be extracted from this study due to internal inconsistencies of the results reported. Attempts to contact the authors to resolve the issue were unsuccessful, by phone, post and e‐mail.
Authors' conclusions
Removal of endometrial polyps in subfertile women is commonly being performed in many countries with an aim to improve the reproductive outcome. We did not identify any analysable randomised trials which would allow us to reach any sound scientific conclusions on the efficacy of endometrial polypectomy in subfertile women. Well designed, methodologically sound, randomised controlled trials are urgently needed.
Plain language summary
Removal of endometrial polyps prior to infertility treatment
Review question
Cochrane authors investigated whether the removal of endometrial polyps in women presenting with subfertility was safe and whether it improved the chance of pregnancy.
Background
Endometrial polyps, which are benign and often asymptomatic growths of the lining of the womb, have the potential to interfere with female fertility. This can be due to alteration of the micro‐environment of the womb or due to physical interference with sperm transport impeding fertilization and subsequent implantation of the embryo. Diagnosis of these growths is mainly through using ultrasound during routine investigations prior to treatment for infertility. Removal of these polyps prior to embarking on any fertility treatment has been suggested as a way to improve the overall outcome of the treatment.
Study characteristics
The authors did not identify any analysable studies that were of sufficient quality to draw any conclusions. The searches are current to July 2014.
Key results and quality of evidence
Due to the lack of available randomised evidence, the authors of this review are unable to draw any conclusions on the routine removal of endometrial polyps prior to treatments for infertility. To answer this question, large and well designed studies are required.
Background
Description of the condition
Endometrial polyps are localized overgrowths of uterine mucosa that are of unknown aetiology. They may result from altered expression of the estrogen receptor in the endometrium, leading to excessive local endometrial growth in response to circulating estrogen. The polyps are commonly associated with irregular or abnormal ovulation (Lopez 2007; Mittal 1996) and are made up of irregular proliferative glands and stroma around a vascular pedicle originating from a spiral artery. Diagnosis is usually by transvaginal ultrasound, hysterosalpingography or saline infusion sonography, although the gold standard strategy is hysteroscopy (Taylor 2008). They are mostly asymptomatic but there is evidence in subfertile women that polyps may adversely affect fertility, although the mechanism is poorly understood (Taylor 2008). Proposed mechanisms include mechanical interference with sperm transport; anatomical interference with implantation (Spiewankiewicz 2003); increased production of inhibitory factors such as glycodelin, which can inhibit natural killer cell function (Richlin 2002); reduced secretion of implantation factors such as insulin‐like growth factor‐binding protein‐1 (IGFBP‐1), tumour necrosis factor (TNF‐alpha) and osteopontin (Ben‐Nagi 2009); and unresponsiveness to cyclical hormonal changes (Mittal 1996). It is plausible that removal of the polyps might improve fertility.
Subfertility is defined as failure to achieve a clinical pregnancy despite regular unprotected sexual intercourse for 12 months or more.
Description of the intervention
Polypectomy under general anaesthesia; or in an office setting performed without direct visualisation using a transcervical sharp curette; or hysteroscopy‐directed polypectomy using scissors, a loop electrode, electric probe or a morcellator (Taylor 2008).
How the intervention might work
Polypectomy reverses mechanical and anatomical distortions within the uterine cavity and this may potentially improve the chances of embryo implantation and a successful pregnancy outcome. A recent study has shown that the levels of endometrial implantation factors, such as mid‐secretory concentrations of IGFBP‐1, TNFa and osteopontin, are increased following the surgical removal of polyps (Ben‐Nagi 2009). The increase in these implantation factors enhances the implantation rates.
Why it is important to do this review
Embryo implantation is a critical step in achieving a successful pregnancy and involves a series of complex interactions between the developing blastocyst and the endometrium. A normal endometrium, physiologically and structurally, is essential and physiological and structural abnormalities may lead to adverse reproductive outcomes. While physiological abnormalities of the endometrium are mostly unresponsive to therapeutic manipulation, structural abnormalities such as uterine fibroids, endometrial polyps, intrauterine adhesions and Mullerian anomalies are potentially amenable to surgical treatment. Endometrial polyps are the most common structural abnormalities, with the prevalence ranging from 10% in asymptomatic women to 26% in women with unexplained subfertility (de Sa Rosa e de Silva 2005) and up to 47% in women with endometriosis‐associated subfertility (Kim 2003).
Subfertility has significant psychological and financial implications for a couple. As polyps are relatively frequent in subfertile women, most clinicians recommend removal prior to commencement of any fertility treatment. However, there is no robust evidence to support this and the procedure has risks of uterine perforation, bleeding, and infection together with associated anaesthetic risks. It is important to provide evidence‐based recommendations for the treatment of endometrial polyps in subfertile women.
Objectives
The aim of this review was to determine the effectiveness and safety of removal of endometrial polyps in subfertile women.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) in which polyps were removed surgically for the treatment of subfertility. Only trials that were either clearly randomised or claimed to be randomised and did not have evidence of inadequate sequence generation were eligible for inclusion.
Types of participants
Women with subfertility of more than 12 months' duration and who were diagnosed with one or more endometrial polyps detected by transvaginal ultrasound, hysterosalpingography, saline infusion sono‐hysterography or hysteroscopy.
Types of interventions
Surgical removal of endometrial polyps by any technique
Expectant management as the control
Types of outcome measures
Primary outcomes
1. Live birth rates
2. Reported surgical complications (e.g. infection; bleeding; injury to uterus, bowel, bladder, blood vessels)
Secondary outcomes
1. Clinical pregnancy rates (evidence of pregnancy by ultrasound visualization of a gestational sac)
2. Ongoing pregnancy rates
3. First trimester miscarriage
4. Second trimester miscarriage
5. Preterm delivery
Search methods for identification of studies
Electronic searches
The search strategy was designed in consultation with the Menstrual Disorders and Subfertility Group (MDSG) Trials Search Co‐ordinator. We searched the following electronic databases with no language restriction, from inception to the present with the Cochrane highly sensitive search strategy for identifying randomised trials that appears in the Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0, chapter 6, 6.4.11).
1. Cochrane MDSG Specialised Register (from inception to present).
2. Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, latest issue).
3. The English language electronic databases MEDLINE, EMBASE and PsycINFO.
4. The Cochrane Library (http://www.thecochranelibrary.com) for the Database of Abstracts of Reviews of Effects (DARE) (non‐Cochrane reviews on similar topics).
5. Current Controlled Trials (www.controlled-trials.com).
6. World Health Organization International Clinical Trials Registry Platform search portal (www.who.int/trialsearch/Default.aspx).
Searching other resources
We performed a search of the references lists of all included studies and relevant reviews to identify further relevant articles. We have contacted the authors and experts in the relevant field to aid in identification of potential studies. The data contained within this review are current to July 2014.
Data collection and analysis
We planned to perform the statistical analysis in accordance with the Cochrane Handbook for Systematic Reviews of Interventions and to use Review Manager 5.1 for input and analysis of data.
Selection of studies
The title, abstract and keywords of every record retrieved were scrutinized independently by two review authors to determine which studies required further assessment.
The full text was retrieved when the information given in the titles, abstracts and keywords suggested that the study was randomised and the intervention was a surgical polypectomy.
If there were any doubts regarding whether the study met the criteria for inclusion from scanning the titles and abstracts, the full article was retrieved for clarification. Disagreements were resolved by discussion with a third review author, when necessary. We attempted to contact the authors of potentially eligible trials in order to obtain missing data.
Data extraction and management
No data extraction was performed due to the absence of eligible studies.
If eligible studies are found when updating this review, two review authors will independently extract the data from these studies using a data extraction form designed and pilot tested by the authors. Any disagreements will be resolved by discussion or by a third review author. Where studies have multiple publications, the main trial report will be used as the reference and additional details supplemented from the secondary papers.
Assessment of risk of bias in included studies
We planned that risk of bias would be assessed using the Cochrane Collaboration tool for assessing risk of bias. We planned that two review authors would independently perform assessment of risk of bias in the included studies; disagreements would be noted and resolved by a third review author. We planned that the risk of bias table would be included in the table 'Characteristics of included studies’. The following risk of bias domains were to be assessed according to the quality criteria specified by the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0.
1. Sequence generation: low risk of bias, method clearly described (e.g. computer generated, random number tables, or drawing lots); unclear risk of bias, methods not fully described. 2. Allocation concealment: low risk of bias, method clearly described in detail (e.g. third party, sealed opaque consecutively numbered envelopes); high risk of bias (e.g. open list of allocation codes); unclear risk of bias (e.g. not stated). 3. Blinding of outcome assessors. 5. Completeness of outcome data. 6. Selective outcome reporting. 7. Any other sources of potential bias identified.
Measures of treatment effect
We planned that collected data would be dichotomous. The numbers of events in the control and intervention groups of each study would be used to calculate Peto odds ratios, and 95% confidence intervals will be presented for all outcomes.
Unit of analysis issues
We planned that the primary unit of analysis would be per woman randomised and not per cycle.
Dealing with missing data
We planned that the data would be analysed on an intention‐to‐treat basis (that is analysing all randomised participants in the original randomly assigned groups), as far as possible. We planned to contact the authors of the RCTs to source any missing data or to resolve any queries that might arise. If the participant numbers randomised and the numbers analysed were inconsistent we planned to use the data available, and the percentage loss to follow up would be calculated and reported in the 'Characteristics of included studies' table.
Assessment of heterogeneity
We planned to assess clinical heterogeneity and to carry out tests for statistical heterogeneity using the Chi2 test, with significance set at P < 0.1. We planned to use the I2 statistic to estimate the total variation across studies that was due to heterogeneity, with a value < 25% considered as low level, 25% to 50% as moderate level, and > 50% as high level heterogeneity. If high levels of heterogeneity (I2 > 50%) were seen for the primary outcomes, we planned to explore possible sources of heterogeneity using sensitivity analyses.
Assessment of reporting biases
We planned to assess potential publication bias using a funnel plot, or other corrective analytical methods, if there were sufficient included studies (10 or more).
Data synthesis
We planned that a meta‐analysis would be performed if the included studies were sufficiently similar. As all the planned outcomes were dichotomous variables, the results would be expressed as odds ratios (OR) with 95% confidence intervals (CI), calculated using Review Manager 5. As we anticipated heterogeneity amongst studies, we planned to use a the random‐effects model with inverse variance weighting. This method incorporates heterogeneity in the analysis of the overall efficacy of treatment by making adjustments to the study weights according to the extent of variation.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses.
1. Efficacy of surgical polypectomy in women treated with ovulation induction and timed intercourse.
2. Efficacy of surgical polypectomy in women treated with intrauterine insemination (IUI).
3. Efficacy of surgical polypectomy in women treated with in vitro fertilization treatment.
4. Efficacy of different surgical methods of polypectomy versus conservative management.
5. Efficacy of surgical polypectomy depending on polyp sizes (< 1 cm, 1 to 2 cm and > 2 cm).
6. Efficacy of surgical polypectomy in women undergoing differing assisted reproduction treatments.
We planned to perform subgroup analysis only if there were a substantial number of studies in each subgroup. Factors such as age, length of follow up and adjusted or unadjusted analysis would be considered in the interpretation of any heterogeneity.
Sensitivity analysis
We planned to perform sensitivity analyses by repeating the analysis in order to explore the influence of the following factors on effect size:
1. restriction of analysis to published studies; 2. restriction of analysis to high quality studies, with adequate reporting of allocation methods, blinding and numbers lost to follow up.
Overall quality of the body of evidence: summary of findings table
We planned to prepare a summary of findings table using Guideline Development Tool software. This table would evaluate the overall quality of the body of evidence for the main review outcomes (live birth, complications and clinical pregnancy) using the GRADE criteria (study limitations (that is risk of bias), consistency of effect, imprecision, indirectness and publication bias). Judgements about evidence quality (high, moderate or low) would be justified, documented and incorporated into reporting of results for each outcome.
Results
Description of studies
Results of the search
The electronic search was conducted on 30 July 2014 and a total of 306 citations were identified. From these, 10 studies were read in their entirety. The study selection flow diagram is shown in Figure 1.
1.
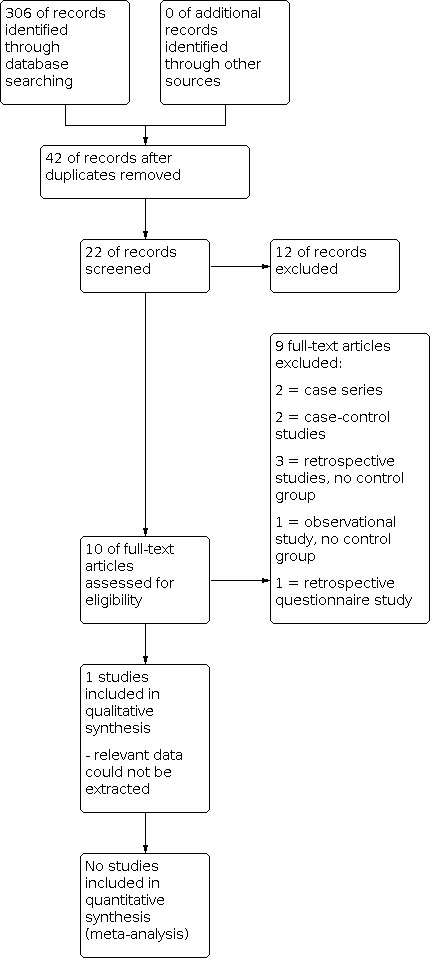
Study flow diagram.
Included studies
Only one relevant randomised trial was found (Perez‐Medina 2005). However, no results can be presented because we could not extract a single set of results. Specifically, we could not resolve the internal inconsistencies reported.
1. In the results section of the paper (paragraph 6, page 1634) a 51% pregnancy rate in the treatment (study) group and 25% in the control group after four cycles of IUI were reported. However, Kaplan‐Meier survival analysis curves (Figure 1 of the paper on the same page) show higher 'survival' in the treatment group than in the control group. The Y axis on the figure is not labelled, but if we assume the Y axis indicates ‘survival’ and ‘survival’ means participants remain non‐pregnant after each IUI cycle, then figure 1 is showing a lower pregnancy rate in the treatment group than in the control group.
2. In paragraph 3, page 1634, it was stated that 11 patients were excluded post‐randomisation. In one section of the same sentence it was implied they were all lost to follow up. In another it was stated that four (three treatment, one control) were lost to follow up, that three (one treatment, two control) were excluded because a polyp was not confirmed on histology, and four (two treatment, two control) because the pathology report showed a myoma. The reasons for excluding these latter four (or seven) are unclear.
Attempts by phone, e‐mail and post to contact the authors to resolve these queries were unsuccessful. Accordingly we concluded that we could not present any data.
Excluded studies
We excluded 9 controlled studies that we identified. Two of the potential articles were retrospective case control studies (Isikoglu 2006; Lass 1999), three were retrospective studies without a control population (Spiewankiewicz 2003; Stamatellos 2008; Yanaihara 2008), two were descriptions of case series (Batioglu 2005; Madani 2009), one was a retrospective questionnaire study (Varasteh 1999) and one was an observational study (Valle 1984).
Risk of bias in included studies
We present our assessments below.
Allocation
Random sequence generation
We assessed Perez‐Medina 2005 as at low risk of this bias, as a computerised random number table was used.
Allocation concealment
We assessed Perez‐Medina 2005 as at unclear risk of this bias; the authors state they used 'an opaque envelope technique' but do not mention numbering of the envelopes.
Blinding
We felt the trial was at unclear risk of these biases, owing to insufficient information apart from the statement that all hysteroscopies were performed by the same clinician.
Incomplete outcome data
We could not reconcile the attrition data reported by the trial authors so assessed this risk as unclear.
Selective reporting
At least one outcome of interest was reported incompletely so data could not be entered into a meta‐analysis. We assessed this risk as high.
Other potential sources of bias
Table I of the study shows some significant differences between the intervention and control groups at baseline, suggesting a potential source of bias related to the study design. In addition the trial authors have stated that the majority of pregnancies occurred before the IUI took place. We consider the trial at high risk of this bias.
Effects of interventions
We were unable to extract data for any of the outcomes reported in Perez‐Medina 2005, so there are no results to present in this review.
Discussion
Summary of main results
We found no eligible randomised controlled trials (RCTs) comparing hysteroscopic removal of endometrial polyps with expectant management in determining the effectiveness and safety of removal of endometrial polyps in subfertile women that had relevant data for inclusion. The one relevant RCT identified had internal inconsistencies in the results reported. In view of the lack of data, no sound scientific conclusions can be drawn on the efficacy and safety of endometrial polypectomy in subfertile women diagnosed to have endometrial polyps. Nevertheless, hysteroscopic removal of endometrial polyps in women with subfertility is commonly practiced considering that it is a minor in‐patient or out‐patient procedure. While its efficacy and safety have been favourably reported in many controlled studies, a sound evidence‐based conclusion could not be drawn due to the lack of data from any well conducted RCTs.
Potential biases in the review process
All steps of the review process were conducted in accordance with the Cochrane Handbook for Systematic Reviews of Interventions in order to minimize potential bias. Our searches were comprehensive.
Agreements and disagreements with other studies or reviews
A recent systematic review (Afifi 2010) included retrospective studies and, due to the heterogeneity of the data, the authors were unable to draw any conclusions regarding the effectiveness of polypectomy on the outcomes of artificial reproductive treatments (ART). However, the authors of the review suggested that women should be advised on hysteroscopy and contemporaneous polypectomy prior to embryo replacement if an endometrial polyp was identified.
Authors' conclusions
Implications for practice.
There is no evidence on the efficacy and safety of removal of endometrial polyps in subfertile women to support the routine practice of surgical intervention for endometrial polyps that are incidentally found while evaluating women for subfertility. On the other hand, the procedure is minimally invasive and hysteroscopic polypectomy provides an opportunity for a histological diagnosis. We have been unable to substantiate external evidence that endometrial injury during hysteroscopy prior to in vitro fertilization (IVF) treatment improves the chances of live births. Well designed, methodologically sound, randomised controlled trials are warranted to provide evidence‐based recommendations on managing endometrial polyps in subfertile women.
No good quality data exist supporting the routine treatment of endometrial polyps that are identified while women are undergoing artificial reproductive treatments (ARTs) such as IVF or intracytoplasmic sperm injection (ICSI).
When endometrial malignancy arising from the polyp is suspected, appropriate investigations and treatment should be carried out without undue delay and in accordance with local guidelines.
Implications for research.
Due to the paucity of available good quality data, many uncertainties and clinical queries exist. Besides the primary research question of whether endometrial polypectomy is effective and safe in subfertile women, there are various associated clinical dilemmas that need answers. What is the optimal timing of endometrial polypectomy? Is there a size effect and should every polyp be removed irrespective of size? If a polyp is identified during controlled ovarian stimulation, should it be removed and the embryo transfer deferred to another cycle? What is the effect of polypectomy on the treatment outcome of fresh IVF and ICSI cycles and frozen embryo replacement cycles? What is the effect of polypectomy on implantation rates, miscarriage rates, multiple pregnancy rates, and pregnancy complication rates? What is the complication rate of polypectomies in the subfertile population?
All these questions need to be answered by means of a well designed, large, randomised controlled trial. It is also worth investigating if just the physical injury to the endometrium performed while sampling the polyp to exclude dysplasia or malignancy, without complete polypectomy, can elicit a favourable or at least similar cycle outcome when compared to the intervention or placebo treatment. Data from studies where endometrial biopsy has been performed prior to ART in women with no endometrial pathology seem to support the last notion (Nastri 2012). However, the presence of the polyp might negate this benefit and the relationship should be further investigated.
What's new
| Date | Event | Description |
|---|---|---|
| 19 January 2021 | Review declared as stable | A scoping search in January 2018 did not identify any new trials. |
History
Protocol first published: Issue 1, 2012 Review first published: Issue 8, 2014
Acknowledgements
We thank Jane Clarke (former Managing Editor), Helen Nagels (Managing Editor), Marian Showell (Trials Search Co‐ordinator) and the editorial board of the Cochrane MDSG for their invaluable assistance in developing this review.
Appendices
Appendix 1. MDSG search strategy
Menstrual Disorders and Subfertility Group search strategy for BM623
Keywords CONTAINS "polyp removal" or "polypectomy" or "polyps"or "uterine polyps"or "endometrial polyps" or Title CONTAINS"polyp removal" or "polypectomy" or "polyps"or "uterine polyps"or "endometrial polyps"
Appendix 2. Appendix 1: EBM Reviews ‐ Cochrane Central Register of Controlled Trials search strategy
1 exp Polyps/ (457) 2 polyp$.tw. (2457) 3 or/1‐2 (2527) 4 exp Infertility/ (1455) 5 exp Infertility, Female/ (796) 6 infertil$.tw. (1730) 7 subfertil$.tw. (128) 8 vitro fertili?ation.tw. (1298) 9 (ivf or icsi).tw. (2131) 10 exp reproductive techniques, assisted/ or exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ or exp insemination, artificial/ or exp ovulation induction/ (2156) 11 assisted reproduct$.tw. (378) 12 (embryo transfer$ or ET).tw. (5047) 13 intracytoplasmic sperm injection$.tw. (398) 14 artificial insemination$.tw. (55) 15 ovulation induc$.tw. (429) 16 intra‐uterine insemination.tw. (26) 17 intrauterine insemination.tw. (376) 18 iui.tw. (278) 19 or/4‐18 (9082) 20 3 and 19 (35)
Appendix 3. Appendix 2: EMBASE search strategy
1 polyp/ or exp endometrium polyp/ (10005) 2 (endometri$ adj3 polyp$).tw. (1561) 3 (uter$ adj3 polyp$).tw. (343) 4 or/1‐3 (10664) 5 exp INFERTILITY/ or exp FEMALE INFERTILITY/ or exp INFERTILITY THERAPY/ (120768) 6 infertil$.tw. (43846) 7 subfertil$.tw. (3547) 8 vitro fertili?ation.tw. (17316) 9 (ivf or icsi).tw. (22018) 10 exp fertilization in vitro/ (33072) 11 exp embryo transfer/ (16728) 12 exp intracytoplasmic sperm injection/ (9560) 13 exp intrauterine insemination/ (2279) 14 exp ovulation induction/ (9658) 15 (embryo transfer$ or ET).tw. (299704) 16 intracytoplasmic sperm injection$.tw. (5083) 17 artificial insemination$.tw. (4252) 18 ovulation induc$.tw. (3777) 19 intra‐uterine insemination.tw. (230) 20 intrauterine insemination.tw. (1930) 21 iui.tw. (1440) 22 AIH.tw. (1796) 23 or/5‐22 (423403) 24 4 and 23 (572) 25 Clinical Trial/ (821915) 26 Randomized Controlled Trial/ (293827) 27 exp randomization/ (55111) 28 Single Blind Procedure/ (14524) 29 Double Blind Procedure/ (101992) 30 Crossover Procedure/ (31323) 31 Placebo/ (188741) 32 Randomi?ed controlled trial$.tw. (66936) 33 Rct.tw. (8137) 34 random allocation.tw. (1072) 35 randomly allocated.tw. (15908) 36 allocated randomly.tw. (1722) 37 (allocated adj2 random).tw. (690) 38 Single blind$.tw. (11292) 39 Double blind$.tw. (119567) 40 ((treble or triple) adj blind$).tw. (251) 41 placebo$.tw. (162223) 42 prospective study/ (177986) 43 or/25‐42 (1161007) 44 case study/ (14063) 45 case report.tw. (211040) 46 abstract report/ or letter/ (801560) 47 or/44‐46 (1022539) 48 43 not 47 (1127409) 49 24 and 48 (99) 50 limit 49 to yr="2010 ‐Current" (29)
Appendix 4. Appendix 3: MEDLINE search strategy
1 exp Polyps/ (23727) 2 polyp$.tw. (190927) 3 or/1‐2 (197943) 4 exp Infertility/ (49117) 5 exp Infertility, Female/ (21926) 6 infertil$.tw. (37462) 7 subfertil$.tw. (3029) 8 vitro fertili?ation.tw. (15103) 9 (ivf or icsi).tw. (16364) 10 exp reproductive techniques, assisted/ or exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ or exp insemination, artificial/ or exp ovulation induction/ (47341) 11 assisted reproduct$.tw. (7401) 12 (embryo transfer$ or ET).tw. (151367) 13 intracytoplasmic sperm injection$.tw. (4266) 14 artificial insemination$.tw. (4477) 15 ovulation induc$.tw. (3135) 16 intra‐uterine insemination.tw. (147) 17 intrauterine insemination.tw. (1537) 18 iui.tw. (1021) 19 or/4‐18 (248556) 20 randomized controlled trial.pt. (323396) 21 controlled clinical trial.pt. (84105) 22 randomized.ab. (239614) 23 placebo.tw. (139055) 24 clinical trials as topic.sh. (159707) 25 randomly.ab. (175370) 26 trial.ti. (102764) 27 (crossover or cross‐over or cross over).tw. (53018) 28 or/20‐27 (794163) 29 exp animals/ not humans.sh. (3724073) 30 28 not 29 (733436) 31 3 and 19 and 30 (58)
Appendix 5. Appendix 4: PsycINFO search strategy
1 polyp$.tw. (2641) 2 exp infertility/ (1485) 3 infertil$.tw. (2180) 4 subfertil$.tw. (51) 5 vitro fertili?ation.tw. (442) 6 (ivf or icsi).tw. (320) 7 exp reproductive technology/ (1119) 8 assisted reproduct$.tw. (394) 9 embryo transfer$.tw. (80) 10 intracytoplasmic sperm injection$.tw. (30) 11 artificial insemination$.tw. (211) 12 ovulation induc$.tw. (14) 13 intra‐uterine insemination.tw. (0) 14 intrauterine insemination.tw. (12) 15 iui.tw. (18) 16 or/2‐15 (3304) 17 1 and 16 (3)
Data and analyses
Comparison 1. Clinical pregnancy rates following hysteroscopic polypectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Pregnancy rates following hysteroscopic polypectomy | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
1.1. Analysis.

Comparison 1: Clinical pregnancy rates following hysteroscopic polypectomy, Outcome 1: Pregnancy rates following hysteroscopic polypectomy
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Perez‐Medina 2005.
| Study characteristics | ||
| Methods | Patients were randomized to one of the two groups with use of an opaque envelope technique, with assignment determined by a computerized random number table. | |
| Participants | 215 women with at least 24 months' history of infertility, with a sonographic evidence of an endometrial polyp undergoing IUI attending the infertility unit of our reference centre hospital during a 50‐month period (January 2000 to February 2004) agreed to participate and informed consent was obtained. Inclusion criteria were women with at least 24 months of sterility, with a sonographic diagnosis of EP and who were candidates for IUI. Exclusion criteria were patients.39 years of age, those with anovulation, azoospermia, uncorrected tubal disease or previous unsuccessful use of r‐FSH. |
|
| Interventions | The study group was composed of 107 women; the polypectomy was performed using rigid 5 Fr scissors and forceps during office hysteroscopy. When resection was not possible during the diagnostic hysteroscopy, the patient was scheduled for operative hysteroscopy under anaesthesia. The control group was composed of 108 women in whom only a biopsy of the polyp was performed during a diagnostic hysteroscopy. Women were scheduled to receive four cycles of IUI, and the first IUI was planned for three cycles after hysteroscopy in both groups. |
|
| Outcomes | Clinical pregnancy demonstrated on a TVUS 30 days after IUI was the main outcome measure analysed to determine the effectiveness of treatment. We studied the crude pregnancy rate in both groups. The secondary outcomes were to compare the time for success in each group and to determine whether the size of the EP influenced the pregnancy rate. | |
| Notes | Some pregnancies were the result of a spontaneous conception in the interim period between hysteroscopy and IUI. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized to one of the two groups with use of an opaque envelope technique, with assignment determined by a computerized random number table." "Subjects were randomized into one of two groups in a 1:1 ratio using a restricted randomization." |
| Allocation concealment (selection bias) | Unclear risk | "Patients were randomized to one of the two groups with use of an opaque envelope technique, with assignment determined by a computerized random number table." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The authors did not comment on blinding of the participants of the researchers, other than stating: "All the hysteroscopies were performed by T.P.‐M." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The authors did not comment on blinding of the outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Eleven patients were lost from the study, six in the study group [three lost to follow‐up, two pathologic reports of submucosal myoma and in one patient in whom the polyp was not confirmed (pathologic report of secretory endometrium)] and five in the control group (one lost to follow‐up, two patients in whom the polyp was not confirmed and two pathologic reports of myoma), and were excluded of the study, leaving 101 patients in the study group and 103 in the control group." Missing outcome data appear to be balanced in numbers across intervention groups, with similar reasons for missing data across groups but we could not confirm this. |
| Selective reporting (reporting bias) | High risk | One or more outcomes of interest (pregnancy rates in the control group in relation to polyp size) in the review are reported incompletely so that they cannot be entered in a meta‐analysis. |
| Other bias | High risk | Table I of the study shows some significant differences between the intervention and control groups at baseline. The majority of the pregnancies in the study population were as a result of a spontaneous conception and not IUI. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Batioglu 2005 | Case series of six patients |
| Isikoglu 2006 | Retrospective, case control study |
| Lass 1999 | Retrospective, case control study |
| Madani 2009 | Case series of nine patients |
| Spiewankiewicz 2003 | Retrospective study. No control group |
| Stamatellos 2008 | Retrospective study. No control group |
| Valle 1984 | Observational study without control groups |
| Varasteh 1999 | Retrospective, questionnaire study |
| Yanaihara 2008 | Retrospective study. No control group |
Differences between protocol and review
There were no differences between the protocol and the review.
Contributions of authors
Kannamannadiar Jayaprakasan (first author): all correspondence with drafting of the protocol, developing a search strategy, searching for trials, obtaining copies of trials, selecting which trials to include, extracting data from trials, entering data into RevMan, carrying out the analysis, interpreting the analysis, drafting the final review and updating the review.
Lukasz Polanski: extracting data from trials, entering data into RevMan, carrying out the analysis, interpreting the analysis, drafting the final review and updating the review.
Banchhita Sahu: drafting the protocol, searching for trials, obtaining copies of trials, selecting which trials to include, extracting data from trials, entering data into RevMan, carrying out the analysis, interpreting the analysis, drafting the final review and updating the review.
Nick Raine‐Fenning: drafting of the protocol, selecting which trials to include, interpreting the analysis, drafting the final review and updating the review.
Jim G Thornton: drafting of the protocol, selecting which trials to include, help in carrying out the analysis, interpreting the analysis, drafting the final review and updating the review.
Sources of support
Internal sources
-
Clinical Trials Unit, Nottingham University Hospitals NHS Trust, UK
Administrative support
External sources
-
Cochrane group (Menstrual Disorders and Subfertility), Other
Editorial support
Declarations of interest
The authors have no commercial interest to disclose.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Perez‐Medina 2005 {published data only}
- Pérez-Medina T, Bajo-Arenas J, Salazar F, Redondo T, Sanfrutos L, Alvarez P, Engels V. Endometrial polyps and their implication in the pregnancy rates of patients undergoing intrauterine insemination. Human Reproduction June 2005;20(6):1632-5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Batioglu 2005 {published data only}
- Batioglu S, Kaymak O. Does hysteroscopic polypectomy without cycle cancellation affect IVF? Reproductive Biomedicine Online Jun 2005;10(6):767-9. [DOI] [PubMed] [Google Scholar]
Isikoglu 2006 {published data only}
- Isikoglu M, Berkkanoglu M, Senturk Z, Coetzee K, Ozgur K. Endometrial polyps smaller than 1.5 cm do not affect ICSI outcome. Reproductive Biomedicine Online Feb 2006;12(2):199-204. [DOI] [PubMed] [Google Scholar]
Lass 1999 {published data only}
- Lass A, Williams G, Abusheikha N, Brinsden P. The effect of endometrial polyps on outcomes of in vitro fertilization (IVF) cycles. Journal of Assisted Reproduction and Genetics Sept 1999;16(8):410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Madani 2009 {published data only}
- Madani T, Ghaffari F, Kiani K, Hosseini F. Hysteroscopic polypectomy without cycle cancellation in IVF cycles. Reproductive Biomedicine Online Mar 2009;18(3):412-5. [DOI] [PubMed] [Google Scholar]
Spiewankiewicz 2003 {published data only}
- Spiewankiewicz B, Stelmachów J, Sawicki W, Cendrowski K, Wypych P, Swiderska K. The effectiveness of hysteroscopic polypectomy in cases of female infertility. Clinical and Experimental Obstetrics & Gynecology 2003;30(1):23-5. [PubMed] [Google Scholar]
Stamatellos 2008 {published data only}
- Stamatellos I, Apostolides A, Stamatopoulos P, Bontis J. Pregnancy rates after hysteroscopic polypectomy depending on the size or number of the polyps. Archives of Gynecology and Obstetrics May 2008;277(5):395-9. [DOI] [PubMed] [Google Scholar]
Valle 1984 {published data only}
- Valle RF. Therapeutic hysteroscopy in infertility. International Journal of Fertility 1984;29(3):143-8. [PubMed] [Google Scholar]
Varasteh 1999 {published data only}
- Varasteh NN, Neuwirth RS, Levin B, Keltz MD. Pregnancy rates after hysteroscopic polypectomy and myomectomy in infertile women. Obstetrics and Gynecology Aug 1999;94(2):168-71. [DOI] [PubMed] [Google Scholar]
Yanaihara 2008 {published data only}
- Yanaihara A, Yorimitsu T, Motoyama H, Iwasaki S, Kawamura T. Location of endometrial polyp and pregnancy rate in infertility patients. Fertility and Sterility Jul 2008;90(1):180-2. [DOI] [PubMed] [Google Scholar]
Additional references
Afifi 2010
- Afifi K, Anand S, Nallapeta S, Gelbaya TA. Management of endometrial polyps in subfertile women: a systematic review. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2010;151:117-21. [DOI] [PubMed] [Google Scholar]
Ben‐Nagi 2009
- Ben-Nagi J, Miell J, Yazbek J, Holland T, Jurkovic D. The effect of hysteroscopic polypectomy on the concentrations of endometrial implantation factors in uterine flushings. Reproductive BioMedicine Online 2009;19:737-44. [DOI] [PubMed] [Google Scholar]
de Sa Rosa e de Silva 2005
- Sa Rosa e de Silva AC, Rosa e Silva JC, Candido dos Reis FJ, Nogueira AA, Ferriani RA. Routine office hysteroscopy in the investigation of infertile couples before assisted reproduction. Journal of Reproductive Medicine 2005;50:501-6. [PubMed] [Google Scholar]
Kim 2003
- Kim MR, Kim YA, Jo MY, Hwang KJ, Ryu HS. High frequency of endometrial polyps in endometriosis. The Journal of the American Association of Gynecologic Laparoscopists 2003;10:46-8. [DOI] [PubMed] [Google Scholar]
Lopez 2007
- Lopes RG, Baracat EC, Albuquerque Neto LC, Ramos JF, Yatabe S, Depesr DB, Lippi UG. Analysis of estrogen- and progesterone-receptor expression in endometrial polyps. Journal of Minimally Invasive Gynecology 2007;14:300-3. [DOI] [PubMed] [Google Scholar]
Mittal 1996
- Mittal K, Schwartz L, Goswami S, Demopoulos R. Estrogen and progesterone receptor expression in endometrial polyps. International Journal of Gynecological Pathology 1996;15:345-8. [DOI] [PubMed] [Google Scholar]
Nastri 2012
- Nastri CO, Gibreel A, Raine-Fenning N, Maheshwari A, Ferriani RA, Bhattacharya S, Martins WP. Endometrial injury in women undergoing assisted reproductive techniques. Cochrane Database of Systematic Reviews 11.07.2012;7:CD009517. doi: 10.1002/14651858.CD009517.pub2.. [DOI] [PubMed] [Google Scholar]
Richlin 2002
- Richlin SS, Ramachandran S, Shanti A, Murphy AA, Parthasarathy S. Glycodelin levels in uterine flushings and in plasma of patients with leiomyomas and polyps: implications for implantation. Human Reproduction 2002;17:2742-7. [DOI] [PubMed] [Google Scholar]
Spiewankiewicz 2003
- Spiewankiewicz B, Stelmachow J, Sawicki W, Cendrowski K, Wypych P, Swiderska K. The effectiveness of hysteroscopic polypectomy in cases of female infertility. Clinical and Experimental Obstetrics & Gynecology 2003;30:23-5. [PubMed] [Google Scholar]
Taylor 2008
- Taylor E, Gomel V. The uterus and fertility. Fertility and Sterility 2008;89:1-16. [DOI] [PubMed] [Google Scholar]


