Abstract
Background
Hyperthyroidism is a disease in which excessive amounts of thyroid hormones circulate in the blood. Patients, among other things suffer from tachycardia, warm moist skin and raised body temperature. The treatment of hyperthyroidism includes symptom relief and therapy with antithyroid medications, radioiodine and thyroidectomy. Medicinal herbs are used alone or in combination with antithyroid agents to treat hyperthyroidism in China and some other countries.
Objectives
To assess the effects of Chinese herbal medicines for treating hyperthyroidism.
Search methods
Studies were obtained from computerised searches of MEDLINE, EMBASE, The Cochrane Library, the Chinese Biomedical Database.
Selection criteria
Randomised controlled trials comparing the effects of Chinese herbal medicines alone with Chinese herbal medicines combined with antithyroid drugs, radioiodine or both.
Data collection and analysis
Three authors interviewed authors of all potentially relevant studies by telephone to verify randomisation procedures. One author entered data into a data extraction form and another author verified the results of this procedure.
Main results
Thirteen relevant trials with 1770 participants were included. All of them were of low quality. Fifty‐two studies still need to be assessed because the original authors could not be interviewed. None of these trials analysed mortality, health related quality of life, economic outcomes or compliance. Compared to antithyroid drugs alone the results showed that Chinese herbal medicines combined with antithyroid drugs may offer benefits in lowering relapse rates, reducing the incidence of adverse effects, relieving symptoms, improving thyroid antibody status and thyroid function. Two trials investigated Chinese herbal medicine versus radioiodine and reported improvements in anxiety, tachycardia and heat intolerance. However, thyroid function ‐ with the exception of restored thyroid stimulating hormone (TSH) ‐ was not significantly altered.
Authors' conclusions
The results suggest that traditional Chinese herbal medicines added to other routine treatment have a therapeutic potential for people with hyperthyroidism. However, due to methodological limitations, we could not identify a well‐designed trial to provide strong evidence for Chinese traditional herbal medicine in the treatment of hyperthyroidism. Thus, we currently cannot recommend any single preparation or formulation for clinical use.
Plain language summary
Chinese herbal medicines for hyperthyroidism
Hyperthyroidism is a common illness in which excessive amounts of thyroid hormones circulate in the blood. Affected people, among other things suffer from increased heart beats, warm moist skin and raised body temperature. A large number of Chinese herbal medicines are used to treat this condition in China. Thirteen relevant trials with 1770 participants were analysed. All of them were of low quality. None of these trials analysed mortality, health related quality of life, economic outcomes or compliance with treatments. Some of these herbs may show benefits in improving symptoms, thyroid function and adverse effects. Unfortunately, we were unable to find reliable evidence to recommend a specific herbal preparation from 103 investigated formulations.
Background
Description of the condition
Both hyperthyroidism and thyrotoxicosis describe a syndrome in which excessive amounts of thyroid hormones circulate in the blood because of an overactive thyroid gland or due to other causes leading to tachycardia, warm moist skin and raised body temperature.
The most frequent cause of hyperthyroidism is Graves's disease followed by non‐cancerous growths of the thyroid or pituitary gland, tumours of the testes or ovaries, inflammation of the thyroid due to viral infections or other causes, ingestion of excessive amounts of thyroid hormone and ingestion of excessive amounts of iodine. Graves's disease accounts for 85% of all cases of hyperthyroidism (Brown 2002).
Hyperthyroidism is a common disorder: Unsuspected and undiagnosed hyperthyroidism was found in roughly 0.5% of women in a large population‐based British survey, done in the 1970s (Cooper 2003). Approximately 2% of women and 0.2% of men in the general population are affected by hyperthyroidism (Farling 2000). Hyperthyroidism can occur at all ages, but is less common before the age of 15. At the other extreme, hyperthyroidism is one of the major causes of morbidity in the elderly. Graves's disease can occur at any age, whereas toxic multinodular goitre is more common in people older than 60 years (John 1997).
Clinical features of hyperthyroidism are all mainly caused by an excess of thyroid hormones. The most common manifestations include hyperkinesis, weight loss, sweating, palpitations and nervousness. Other manifestations include atrial fibrillation, cardiac failure, tremor and weakness with general fatigue etc. However, not all the patients show these symptoms or signs but present with varying disorders like menstrual irregularities, vomiting or diarrhoea, psychotic illness, proximal myopathy or pruritus. A proportion of patients have negligible or no symptoms with the diagnosis discovered only during screening (John 1997). Ophthalmic and cardiovascular complications are two clinically significant complications caused by hyperthyroidism. Thyroid‐associated eye disease may occur before, after or contemporary with hyperthyroidism in different patients. It may result in diplopia, increased intraocular pressure, soft‐tissue signs, marked congestive ophthalmopathy and even sight loss (John 1997). Clinically evident ophthalmopathy occurs in about 50% of patients, however, imaging studies like ultrasonography and computed tomography reveal evidence of ophthalmopathy, in the form of enlarged extraocular muscles, in most patients without clinical signs (Weetmen 2000). The cardiovascular complications of hyperthyroidism are common and sometimes life‐threatening. Most patients with hyperthyroidism complain of palpitations and breathlessness on exertion. A variety of atrial and ventricular tachycardias have been described in hyperthyroidism and the most common one is atrial fibrillation. Overt cardiac failure is rare in hyperthyroidism and usually occurs in the context of rapid atrial fibrillation in elderly patients with pre‐existing ischaemic or vulvar heart disease (Toft 2000).
The actual diagnosis of hyperthyroidism is easy to establish once its possibility is entertained. Accurate and widely available blood tests can confirm or rule out the diagnosis. In most patients, free tri‐iodothyronine (FT3) and thyroxine (FT4) concentrations in serum are raised and serum thyroid‐stimulating hormone (TSH) or thyrotropin might be undetectable. The 24‐hour radioactive iodine uptake is a measure of the iodine avidity of the thyroid gland. In most hyperthyroid diseases, including Graves's disease, toxic multinodular goitre and toxic adenoma, the results are at the higher end of normal or raised. But this test is generally not needed to arrive at the diagnosis. However, it can be very useful in distinguishing mild Graves's disease from silent or postpartum thyroiditis in which the 24‐hour uptake of radioactive iodine will be low (Cooper 2003).
Description of the intervention
Description of treatment options
The treatment of hyperthyroidism includes symptom relief and therapy with antithyroid medications, use of radioiodine (radioactive iodine 131 or I‐131) and thyroidectomy. The extrathyroidal manifestations are not improved by treatment directed at hyperthyroidism and specific treatment is needed (McKenna 2001).
Medications for symptom relief
Many of the neurological and cardiovascular symptoms such as tremor, palpitation and anxiety following thyrotoxicosis are relieved by beta‐blocker therapy because these medicines can block the peripheral effects of the excess amounts of thyroid hormones (Cheetham 1998). Those with a longer duration of action such as propranolol, metoprolol, atenolol and nadolol are usually preferred. Beta‐antagonists are generally well tolerated, but can cause depression, nausea, headache, and fatigue. Furthermore, these drugs should be used cautiously in patients with asthma, congestive heart failure, or Raynaud's phenomenon (Cooper 2003). Calcium channel blockers can be used for the same purposes when beta‐blockers are contraindicated or poorly tolerated.
Antithyroid medications
Antithyroid drugs (thioamides: carbimazole, methimazole or propylthiouracil) are used for almost all patients with thyrotoxicosis in European countries. Carbimazole 0.5 to 1.0 mg/kg/day or propylthiouracil 5 to 10 mg/kg/day in divided doses usually control hyperthyroidism in about 4 to 8 weeks. In practice, antithyroid drugs are usually given for 12 to 24 months when used alone. When the circulating thyroid hormone levels are restored to normal, treatment can be reduced to a single daily dose and titrated against regular thyroid function tests to maintain euthyroidism (McKenna 2001). As with any long term treatment, patients' compliance is not good because many patients and their families find it remarkably difficult to remember to take the drugs regularly. Some 2% to 5% of patients will develop minor side effects with an antithyroid drug, such as rash, nausea, headache, or arthralgia. Usually, such symptoms are transient but if they persist the patient may take another major agent, as cross sensitivity is unusual. Serious adverse effects are rare, but include neutropenia, agranulocytosis (which is nearly always reversible) and hepatotoxicity, all of which need to be dealt with immediately once the effect is discovered (Cheetham 1998).
Radioactive iodine
Radioactive iodine therapy is the most common treatment of hyperthyroidism in US adults. It is easy to administrate, lacks significant adverse effects and is relatively inexpensive. Those who relapse after a course of antithyroid drugs are usually prescribed radioactive iodine or undergo partial thyroidectomy. About 20% of patients will require a second dose of radioactive iodine. Around 50% become hypothyroidal within five years irrespective of the dose (McKenna 2001).
Thyroidectomy
Surgery for hyperthyroidism is used infrequently all over the world (Cooper 1998). Thyroidectomy is mainly used in specific situations including patient preference, poor response to antithyroid drugs (especially in pregnancy), presence of a very large goitre and the presence of a co‐existing potentially malignant thyroid nodule (Cooper 2003). The amount of thyroid tissue to be removed is not easy to decide on: A too conservative operation will result in a high rate of recurrent hyperthyroidism and an overly radical thyroidectomy in hypothyroidism. Either of these two problems could occur unpredictably many years later. Besides, a short hospital stay is needed and potential problems of the operation include the discomfort of the operation, the scar (which may form keloid), the risk of general anaesthesia and surgical complications like the risk of damage to the recurrent laryngeal nerves and transient or permanent hypoparathyroidism (Cheetham 1998).
All currently available therapies are effective, but all the three options have problems as well. Antithyroid drugs usually do not cure the patient, need to be used for long time and have rare but potentially life‐threatening adverse effects. Surgery is expensive, may cause permanent hypothyroidism and can be a source of significant morbidity from complications. Radioiodine necessitates radiation exposure, may almost inevitably cause hypothyroidism and may be associated with exacerbation of Graves ophthalmopathy in certain patient subsets (Cooper 1998).
Medicinal herbs
Medicinal herbs are widely used to treat many diseases including hyperthyroidism in China and many other countries. Chinese herbs including membranous milk vetch root, begonia, tangshen, common anemarrhena rhizome, dwarf lilyturf tuber, Chinese magnoliavine fruitfigwort, rehmannia dride rhizome, common selfheal fruit‐spike etc. have been used to treat hyperthyroidism. These herbs are able to decrease the raised affinity of alpha‐adrenergic receptors in hyperthyroidism, weaken the biological effects of thyroxine and inhibit the transformation of T4 to T3 (Yao 1998). Some are said to be able to modulate the function of sympathetic nerves or the immune system (Li 1998). The contents of traditional Chinese herbal preparations are variable depending on traditional Chinese medicine syndromes of patients. They are used to treat hyperthyroidism along or in combination with other anti‐hyperthyroidism drugs.
Adverse effects of the intervention
There is evidence that not all herbs are risk‐free. In particular, there are concerns about allergic reactions as well as nephropathy (Lampert 2002; Lord 2001; Nortier 2000).
Why it is important to do this review
Up to now, a lot of studies have been published about the effects of Chinese herbal medicines for hyperthyroidism. These studies showed marked benefits with treatment and indicated that various Chinese herbal medicine preparations are widely used clinically. However, the quality and the effects of these trials have not been systematically reviewed and assessed. Therefore, this review aims to summarise the existing evidence of the comparative effects and safety of Chinese herbal medicines in the treatment of hyperthyroidism.
Objectives
To assess the effects of Chinese herbal medicines for treating hyperthyroidism.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled clinical trials, irrespective of blinding, publication status or language.
Types of participants
Participants were males or females of any age or ethnic origin with hyperthyroidism. Participants were excluded if they had acute myocardial infarction, heart failure, hepatic or renal failure.
Diagnostic criteria:
clinical manifestations, such as weight loss, heat intolerance, irritability, anxiety, palpitations and tremor;
laboratory abnormalities, such as increase of free triiodothyronine (FT3), free thyroxine (FT4) and decrease of thyroid‐stimulating hormone (TSH) in the serum;
complications, such as ophthalmopathy and pretibial myxoedema.
Types of interventions
Therapy with Chinese medicinal herbs alone or Chinese medicinal herbs in combination with antithyroid drugs or radioactive iodine. The following comparisons were acceptable for evaluation:
antithyroid drugs without Chinese herbal medicines;
radioactive iodine without Chinese herbal medicines;
another Chinese herbal medicines preparation.
Types of outcome measures
Primary outcomes
mortality;
relapse rates (recurrence of hyperthyroidism) at least one year after completion of drug treatment;
incidence of hypothyroidism.
Secondary outcomes
course of ophthalmopathy (need for corticosteroids, radiotherapy, visual compromise);
adverse effects (for example agranulocytosis, drug rash, hepatitis, vasculitis);
symptoms of hyperthyroidism (for example anxiety, tachycardia, heat intolerance, diarrhoea, oligomenorrhoea);
thyroid antibody status;
weight change;
thyroid function tests and serum levels of thyroid hormones;
health‐related quality of life;
economic outcomes;
compliance rates (for example by calculations of pharmacy prescription, pill counts).
Search methods for identification of studies
Electronic searches
We used the following sources for the identification of trials:
The Cochrane Library (issue 2, 2006);
MEDLINE (until July 2006);
EMBASE (until July 2006);
Science Citation Index Expanded (until July 2006);
The Chinese Biomedical Database (until August 2006);
VIP Chinese Science and Technique Journals Database (until August 2006);
China National Infrastructure (CNKI) (until August 2006).
The described search strategy (see Appendix 1 for a detailed search strategy) was used for MEDLINE. For use with EMBASE and the other databases this strategy was slightly adapted. There were no language restrictions when searching for trials.
Data collection and analysis
Selection of studies
Zeng Xiaoxi (ZXX) scanned the results of the search strategy for potentially relevant studies and retrieved the full articles for all potentially relevant trials. We scrutinized each trial report for multiple publications from the same data set. ZXX, Yuan Yong (YY) and Liu Yan (LY) interviewed the original authors by telephone in order to find out whether participants were really randomised. ZXX and YY independently assessed each of these trials for inclusion in the review using an eligibility form based on the contents of the section 'Criteria for considering studies for this review'. We excluded studies that failed to meet the inclusion criteria and stated the reason in Characteristics of excluded studies. There was no disagreement for study selection.
Data extraction and management
ZXX entered data into a data extraction form and YY examined these. We extracted data on study characteristics including methods, participants, interventions and outcomes. We resolved any disagreements by referring back to the original trial report and through discussion. If data from the trial reports were insufficient or missing, we contacted the authors for additional information. Where possible, we extracted data to allow an intention‐to‐treat analysis. For binary outcomes, we recorded the number of participants experiencing the event in each group of the trial. For continuous outcomes, we extracted the arithmetic means and standard deviations for each group.
Assessment of risk of bias in included studies
According to empirical evidence (Jadad 1996; Juni 2001; Kjaergard 2001; Moher 1998; Schulz 1995), we assessed the methodological quality as described in Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2005):
generation of the allocation sequence: adequate (computer generated random numbers, table of random numbers or similar) or inadequate (other methods or not described);
allocation concealment: adequate (central independent unit, sealed envelopes or similar) or inadequate (not described or open table of random numbers or similar);
double blinding: adequate (identical placebo tablets or similar) or inadequate (not performed or tablets versus injections or similar);
follow up: adequate (number and reasons for dropouts and withdrawals described) or inadequate (number or reasons for dropouts and withdrawals not described).
Based on these criteria, we assigned studies to one of the following three categories: A ‐ all quality criteria met: low risk of bias; B ‐ one or more of the quality criteria only partly met: moderate risk of bias; C ‐ one or more criteria not met: high risk of bias.
This classification was used as the basis of a sensitivity analysis. Additionally, we assessed individual quality criteria.
Each trial was assessed independently by two authors (ZXX, LY). There was no disagreement.
Data synthesis
We did not perform meta‐analysis since the herbal medicines used in the evaluated studies were different to each other. We provide a qualitative description of studies relating to adverse effects.
Results
Description of studies
Results of the search
The electronic searches revealed 635 studies. After reading the titles and abstracts, 103 potential RCTs were retrieved for further assessment. We interviewed the original authors of these 103 studies and discovered that 13 trials turned out to be real RCTs. The authors of 52 studies could not be contacted, these are named under 'Studies awaiting assessment'. Thirty‐eight studies were excluded, the reasons for exclusion are listed under 'Characteristics of excluded studies'. In total, 13 trials were included in this review. For an overview (Figure 1), please see the adapted QUOROM (quality of reporting of meta‐analyses) flow‐chart of study selection (Moher 1999).
1.
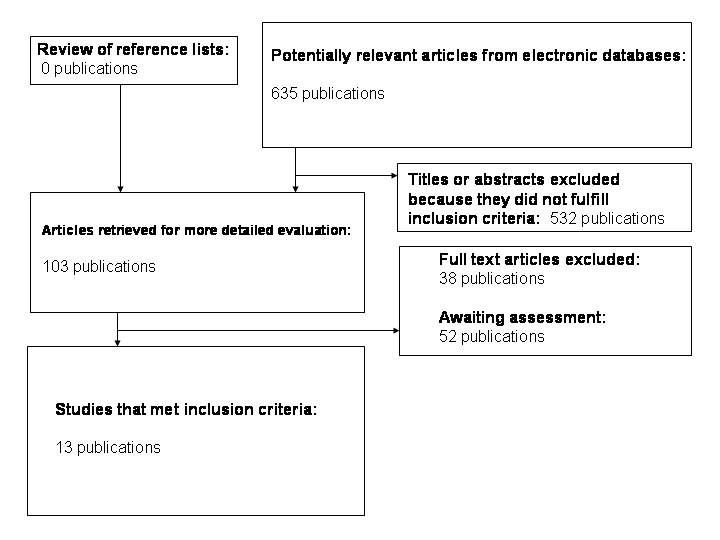
Adapted QUOROM (quality of reporting of meta‐analyses) flow‐chart of study selection
Included studies
Participants
Altogether 1770 patients with hyperthyroidism participated in the 13 trials. The proportion of males to females was 437 to 1333. All of them were Chinese with an average age of 32 years, ranging from 12 to 68 years. Seven studies (Chen 2004, Dai 2000, Ding 2001a, Ding 2005, Huang 2003, Zhang 2003a and Zhu 2005) described disease duration, which ranged from one month to longer than 10 years. The average number of participants was 136 individuals (ranging from 62 to 368 participants per trial). Eleven trials enrolled patients with hyperthyroidism alone. Among them, the participants were diagnosed with Graves's disease in two trials (Chen 2004, Fang 2003), diffuse goitre in one trial (Ding 2001b) and diffuse toxic goitre or subacute thyroiditis in one trial (Ding 2005). Two trials enrolled patients with hyperthyroidism accompanied by other co‐morbidities(Graves's disease with liver impairment (Yu 2002a) and Graves's disease with proteinuria (Yu 2002b)). The diagnostic criteria were based on clinical symptoms, physical signs and laboratory tests (mainly thyroid gland function tests). However, four studies (Huang 2003, Qiu 2003, Yan 1999 and Yu 2002b) did not describe exact diagnostic criteria.
Interventions
The 13 included RCTs used the following Chinese herbal preparations: Chen 2004: Consisted of a three‐arm comparison. The therapeutic effects of herbal oral liquid Jiakangxin Koufuye combined with radioiodine versus methimazole and radioiodine were tested . Jiakangxin koufuye was prepared by the trialists' hospital. Dai 2000: The therapeutic effects of self‐prepared herbal tea (Jia 1 formulation) combined with methimazole versus methimazole were compared. Ding 2001a: The therapeutic effects of self‐prepared herbal medicine (herbal tea in the beginning and later on Longdanxiegan Wan, Chenxiangshuqi Wan, Zhibaidihuang Wan) combined with radioiodine versus radioiodine were evaluated. Ding 2001b: The therapeutic effects of self‐prepared herbal tea (Jiakangping formulation) combined with methimazole versus methimazole were compared. Ding 2005: The therapeutic effects of the herbal capsule Jiakangmianyi Jiaonang combined with propylthiouracil versus another herbal pill Jiaokangning Pian combined with propylthiouracil were analysed. Jiakangmianyi Jiaonang was prepared by the trialists' hospital, while Jiakangning Pian was manufactured by a pharmaceutical company. Fang 2003: Consisted of a three‐arm comparison. The therapeutic effects of Huangqi injection combined with methimazole versus another herbal medicine Jiakangling Pian and methimazole were tested. Huang 2003: The therapeutic effects of the herbal medicine pill Yikang Wan which was manufactured by a pharmaceutical company combined with methimazole, propranolol and thyroxine were compared versus methimazole, propranolol and thyroxine. Qiu 2003: The therapeutic effects of self‐prepared herbal tea Erdongtang with Xiaoyingwan Jiawei (Yangyinqingre and huatansanjie formulation) combined with methimazole or propylthiouracil versus methimazole or propylthiouracil were evaluated. Yan 1999: The therapeutic effects of self‐prepared herbal tea combined with methimazole versus methimazole were compared. Yu 2002a: The therapeutic effects of self‐prepared herbal medicine (Qingganjieyu formulation) combined with methimazole or propylthiouracil versus methimazole or propylthiouracil were investigated. The intervention group used herbal tea in the beginning, later on herbal pills. Yu 2002b: The therapeutic effects of self‐prepared herbal pills (Qinglihuoxue formulation) combined with propylthiouracil versus propylthiouracil were investigated. Zhang 2003a: The therapeutic effects of Huangqi and Shengmai injection combined with methimazole versus methimazole were compared. Zhu 2005: The therapeutic effects of self‐prepared herbal tea (Jiakangxiao formulation) combined with methimazole and propranolol versus methimazole and propranolol were analysed.
The formulations, dosages, methods of administration and course of treatment varied in these trials. The raw Chinese herbal medicines used, formulations and methods of administration are listed in Appendix 2 and 'Chinese herbs terminology in three languages' in Appendix 3, as well as in the table Characteristics of included studies.
Outcomes
The outcome 'relapse rates' was reported in three trials (Ding 2001b, Qiu 2003 and Zhu 2005). The incidence of hypothyroidism was reported in one trial (Ding 2001a). Five trials reported adverse effects (Ding 2001b, Ding 2005, Huang 2003, Qiu 2003 and Zhang 2003a). Clinical symptoms, physical signs (ophthalmopathy and weight change) and thyroid function tests (including free serum thyroxine (FT3), free triiodothyronine (FT4) and thyroid‐stimulating hormone (TSH)) were reported in every trial. The outcomes were divided into recovery, marked improvement, improvement and inefficiency in three trials (Chen 2004, Ding 2005 and Zhu 2005), while division into recovery or control, improvement and inefficiency was observed in four trials (Dai 2000, Ding 2001a, Ding 2001b and Zhang 2003a). Five trials tested the thyroid antibody status (Ding 2005, Fang 2003, Qiu 2003, Yu 2002a and Yu 2002b). No trial reported on the outcomes mortality, health‐related quality of life, costs and compliance rates.
Risk of bias in included studies
None of the included trials was considered as a high quality study with regards to the components allocation concealment, double blinding and dropouts. As the publications did not provide enough information about design and methodology, we tried to acquire additional data by interviewing the authors, but some of them did not supply further relevant facts. Details of the individual studies are listed in Characteristics of included studies.
Due to the overall low methodological quality we did not perform sensitivity analyses on the basis of quality.
Allocation
Three of the 13 studies used computer software to generate randomisation (Ding 2005; Fang 2003; Qiu 2003), while ten studies used random number tables. None of the 13 trials used allocation concealment.
Blinding
None of the trials used double blinding. Only four studies used a single blind design (Ding 2005; Fang 2003; Yu 2002a; Yu 2002b). In these four trials, the patients were not aware of their allocated treatment.
Incomplete outcome data
Withdrawals and losses to follow‐up were described in one study (Fang 2003). None of the studies performed an intention‐to‐treat analysis.
Other potential sources of bias
Assessment of compliance
None of the studies mentioned any method of assessing compliance.
Similarity of comparison groups at baseline
Age and gender were considered important factors and appeared to be balanced across study groups.
Effects of interventions
Primary outcomes
Mortality
None of the studies analysed mortality.
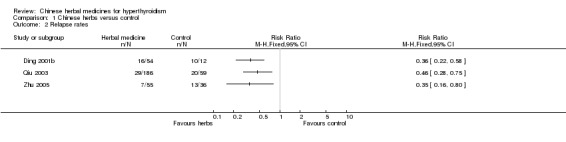
Relapse rates
Three studies showed significant differences in relapse rates which were lower after treatment with traditional Chinese herbal medicines in combination with antithyroid drugs than after antithyroid drugs alone:
self‐prepared herbal tea Jiakangping formulation combined with methimazole versus methimazole (Ding 2001b): RR 0.36, 95% confidence interval (CI) 0.22 to 0.58;
self‐prepared herbal tea Erdongtang with Xiaoyingwan Jiawei (Yangyinqingre and huatansanjie formulation) combined with methimazole or propylthiouracil versus methimazole or propylthiouracil (Qiu 2003): RR 0.46, 95% CI 0.28 to 0.75;
Jiakangxiao formulation combined with methimazole and propranolol versus methimazole and propranolol (Zhu 2005): RR 0.35, 95% CI 0.16 to 0.80.
Incidence of hypothyroidism
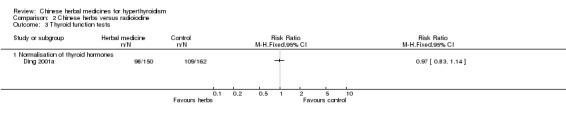
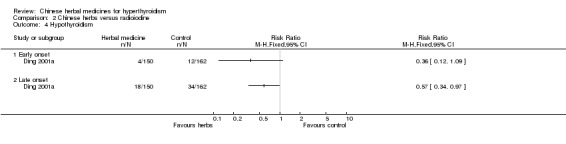
Weak evidence from one study (Ding 2001a) showed that self‐preparation of herbal tea (in the beginning, later on Longdanxiegan Wan, Chenxiangshuqi Wan, Zhibaidihuang Wan) combined with radioiodine was better at lowering the incidence of late‐onset hypothyroidism than radioiodine alone (RR 0.57, 95% CI 0.34 to 0.97). However, the same study showed no statistical significance at reducing the sub‐category of early‐onset hypothyroidism (RR 0.36, 95% CI 0.12 to 1.09).
Secondary outcomes
Course of ophthalmopathy
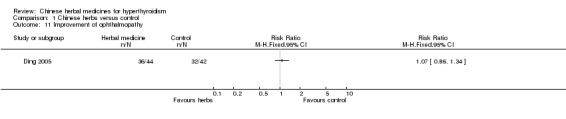
One study listed ophthalmopathy as an outcome and showed that Chinese herbal medicines combined with antithyroid drugs were not significantly better at modifying ophthalmopathy: Jia 1 formulation combined with methimazole versus methimazole alone (Dai 2000): RR 1.07, 95% CI 0.86 to 1.34.
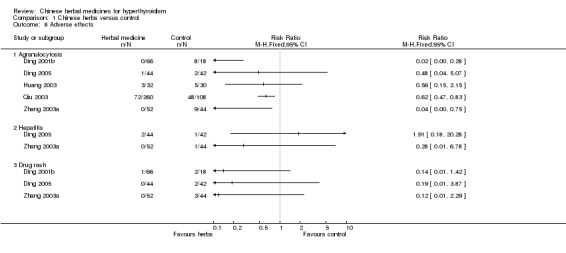
Adverse effects
Three studies showed borderline trends or statistical significant differences in reducing the incidence of agranulocytosis with Chinese herbal medicines combined with antithyroid drugs compared to antithyroid drugs alone:
self‐prepared herbal tea Jiakangping formulation combined with methimazole versus methimazole (Ding 2001b): RR 0.02, 95% CI 0.00 to 0.28;
self‐prepared herbal tea Erdongtang with Xiaoyingwan Jiawei (Yangyinqingre and huatansanjie formulation) combined with methimazole or propylthiouracil versus methimazole or propylthiouracil (Qiu 2003): RR 0.62, 95% CI 0.47 to 0.83;
Huangqi and Shengmai injection combined with methimazole versus methimazole (Zhang 2003a): RR 0.04, 95% CI 0.00 to 0.75.
However, two other studies did not demonstrate statistical significant differences in reducing the agranulocytosis rate:
herbal medicine pill Yikang Wan combined with methimazole, propranolol and thyroxine versus methimazole, propranolol and thyroxine (Huang 2003): RR 0.56, 95% CI 0.15 to 2.15;
Jiakangmianyi Jiaonang capsule combined with propylthiouracil versus Jiaokangning Pian pill combined with propylthiouracil (Ding 2005): RR 0.48, 95% CI 0.04 to 5.07.
Data from two studies showed that traditional Chinese herbal medicines combined with antithyroid drugs had no significantly better effect in reducing the incidence of hepatitis than antithyroid drugs:
Huangqi and Shengmai injection combined with methimazole versus methimazole (Zhang 2003a): RR 0.28, 95% CI 0.01 to 6.78;
Jiakangmianyi Jiaonang capsule combined with propylthiouracil versus Jiaokangning Pian pill combined with propylthiouracil (Ding 2005): RR 1.91, 95% CI 0.18 to 20.28.
Chinese herbal medicines combined with antithyroid drugs did not significantly reduce the incidence of drug rash compared to antithyroid drugs alone:
self‐prepared herbal tea Jiakangping formulation combined with methimazole versus methimazole (Ding 2001b): RR 0.14, 95% CI 0.01 to 1.42;
Huangqi and Shengmai injection combined with methimazole versus methimazole (Zhang 2003a): RR 0.12, 95% CI 0.01 to 2.29;
Jiakangmianyi Jiaonang capsule combined with propylthiouracil versus Jiaokangning Pian pill combined with propylthiouracil (Ding 2005): RR 1.91, 95% CI 0.01 to 3.87.
Symptoms of hyperthyroidism (anxiety, tachycardia, heat intolerance, diarrhoea)
Category 'symptoms subsided'
Four studies of traditional Chinese herbal medicines in combination with antithyroid drugs versus antithyroid drugs alone did not show significant differences:
Jia 1 formulation combined with methimazole versus methimazole alone (Dai 2000): RR 1.40, 95% CI 0.92 to 2.13;
self‐prepared herbal tea Jiakangping formulation combined with methimazole versus methimazole (Ding 2001b): RR 1.23, 95% CI 0.87 to 1.73;
Jiakangmianyi Jiaonang capsule combined with propylthiouracil versus Jiaokangning Pian pill combined with propylthiouracil (Ding 2005): RR 1.10, 95% CI 0.60 to 2.03;
Jiakangxiao formulation combined with methimazole and propranolol versus methimazole and propranolol (Zhu 2005): RR 1.71, 95% CI 0.61 to 4.81.
Two studies demonstrated significant effects in favour of the control intervention:
self‐prepared herbal tea Erdongtang with Xiaoyingwan Jiawei (Yangyinqingre and huatansanjie formulation) combined with methimazole or propylthiouracil versus methimazole or propylthiouracil (Qiu 2003): RR 1.31, 95% CI 1.08 to 1.58;
Huangqi and Shengmai injection combined with methimazole versus methimazole (Zhang 2003a): RR 2.47, 95% CI 1.52 to 4.02.
Category 'symptoms were markedly improved'
Traditional Chinese herbal medicines in combination with antithyroid drugs had no significant better effect than antithyroid drugs alone:
Jiakangmianyi Jiaonang capsule combined with propylthiouracil versus Jiaokangning Pian pill combined with propylthiouracil (Ding 2005): RR 1.06, 95% CI 0.67 to 1.66;
One study showed better control of symptoms after the control intervention: Jiakangxiao formulation combined with methimazole and propranolol versus methimazole and propranolol (Zhu 2005): RR 2.34, 95% CI 1.37 to 4.01.
Category 'symptoms were improved'
One study showed statistical significant differences in favour of herbal medicines: Huangqi and Shengmai injection combined with methimazole versus methimazole (Zhang 2003a): RR 0.47; 95% CI 0.25 to 0.86. However, the other four studies showed no statistical significant differences in improving symptoms:
Jia 1 formulation combined with methimazole versus methimazole alone (Dai 2000): RR 0.80, 95% CI 0.52 to 1.24;
self‐prepared herbal tea Jiakangping formulation combined with methimazole versus methimazole (Ding 2001b): RR 0.55, 95% CI 0.19 to 1.61;
Jiakangmianyi Jiaonang capsule combined with propylthiouracil versus Jiaokangning Pian pill combined with propylthiouracil (Ding 2005): RR 0.95, 95% CI 0.30 to 3.06;
Jiakangxiao formulation combined with methimazole and propranolol versus methimazole and propranolol (Zhu 2005): RR 0.74, 95% CI 0.041 to 1.35.
Category 'symptoms were improved'
Evidence from two studies showed statistical significant differences in the number of participants whose symptoms remained unimproved being smaller in the intervention than in the control groups:
Huangqi and Shengmai injection combined with methimazole versus methimazole (Zhang 2003a): RR 0.23, 95% CI 0.07 to 0.78;
Jiakangxiao formulation combined with methimazole and propranolol versus methimazole and propranolol (Zhu 2005): RR 0.29, 95% CI 0.13 to 0.62.
Three other studies revealed no statistical significant differences:
Jia 1 formulation combined with methimazole versus methimazole alone (Dai 2000): RR 0.40, 95% CI 0.08 to 1.97;
self‐prepared herbal tea Jiakangping formulation combined with methimazole versus methimazole (Ding 2001b): RR 0.55, 95% CI 0.11 to 2.74;
Jiakangmianyi Jiaonang capsule combined with propylthiouracil versus Jiaokangning Pian pill combined with propylthiouracil (Ding 2005): RR 0.57, 95% CI 0.15 to 2.25.
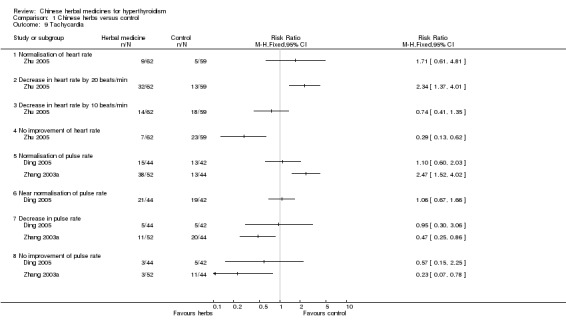
Heart rate
One study (Zhu 2005) investigated heart rates. Jiakangxiao formulation combined with methimazole and propranolol was significantly better than methimazole and propranolol in reducing heart rates by 20 beats/min (RR 2.34, 95% CI 1.37 to 4.01); however, there was no statistically significant difference in normalisation of heart rates (RR 1.71, 95% CI 0.61 to 4.81) and reduction of heart rates by 10 beats/min (RR 0.74, 95% CI 0.41 to 1.35).
Pulse rate
One study (Ding 2005) showed that Jiakangmianyi Jiaonang capsule combined with propylthiouracil versus Jiaokangning Pian pill combined with propylthiouracil had similar effects in restoring pulse rates to normal (RR 1.10, 95% CI 0.60 to 2.03), near normal (RR 1.06, 95% CI 0.67 to 1.66) and in reducing pulse rates (RR 0.95, 95% CI 0.30 to 3.06).
Data from another study (Zhang 2003a) showed statistically significant differences: Huangqi and Shengmai injection combined with methimazole had better effects in restoring pulse rates to normal levels (RR 2.47, 95% CI 1.52 to 4.02) and was less likely not to improve pulse rates (RR 0.23, 95% CI 0.07 to 0.78) than methimazole alone. However, there was no statistically significant difference in reducing pulse rates (RR 0.47, 95% CI 0.25 to 0.86).
Anxiety, tachycardia, heat intolerance, diarrhoea
One study (Ding 2001a) showed that self‐prepared herbal medicine (herbal tea in the beginning and later Longdanxiegan Wan, Chenxiangshuqi Wan, Zhibaidihuang Wan) combined with radioiodine was statistically significantly better in improving the symptoms of anxiety (RR 1.63, 95% CI 1.38 to 1.93), tachycardia (RR 1.21, 95% CI 1.06 to 1.38) and heat intolerance (RR 1.45, 95% CI 1.23 to 1.71) than radioiodine alone.
Another study (Chen 2004) showed no statistically significant differences in improving anxiety and tachycardia by treatment of Jiakangxin Koufuye combined with radioiodine versus methimazole and radioiodine or radioiodine alone(RR 1.05, 95% CI 0.94 to 1.16 and RR 1.04, 95% CI 0.94 to 1.16, respectively).
One study (Dai 2000) showed that the Jia 1 formulation combined with methimazole was better in improving the symptoms of tachycardia than methimazole. However, the same could not be demonstrated for weight change and diarrhoea.
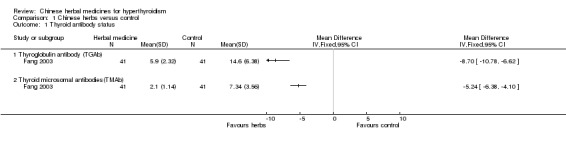
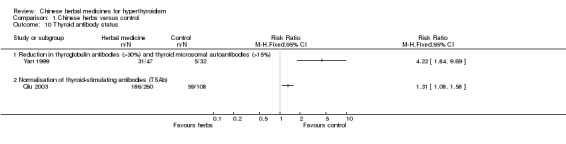
Thyroid antibody status
Several studies investigated the effects of herbal medicine on thyroid antibodies:
one study (Yan 1999) showed statistically significant restoration of thyroglobulin antibodies (TGAb) and thyroid microsomal autoantibodies (TMAb) after self‐prepared herbal‐tea combined with methimazole compared to methimazole alone (RR 4.22, 95% CI 1.84 to 9.69);
statistically significant improvements of TGAb after herbal medicine were demonstrated by two further studies: Self‐prepared herbal‐tea combined with methimazole versus methimazole (Yan 1999) and Huangqi injection combined with methimazole versus Jiakangling Pian and methimazole (Fang 2003);
two studies did not show significant improvements in TGAb after herbal medicine: Self‐prepared Qingganjieyu formulation combined with methimazole or propylthiouracil versus methimazole or propylthiouracil (Yu 2002a) and self‐prepared herbal pills Qinglihuoxue formulation combined with propylthiouracil versus propylthiouracil (Yu 2002b);
regarding restoration of TMAb, two studies revealed significant differences: Self‐prepared herbal‐tea combined with methimazole versus methimazole (Yan 1999) and Huangqi injection combined with methimazole versus Jiakangling Pian and methimazole (Fang 2003); self‐prepared Qingganjieyu formulation combined with methimazole or propylthiouracil versus methimazole or propylthiouracil (Yu 2002a) did not show significant differences;
one study (Fang 2003) showed that Huangqi injection combined with methimazole was significantly better at restoration of TGAb and TMAb than Jiakangling Pian and methimazole;
another study (Qiu 2003) showed that self‐prepared herbal tea erdongtang with Xiaoyingwan Jiawei (Yangyinqingre and huatansanjie formulation) combined with methimazole or propylthiouracil was followed by a statistically better restoration of thyroid stimulating antibodies (TSAb) than methimazole or propylthiouracil (RR 1.31, 95% CI 1.08 to 1.58).
Weight change
Traditional Chinese herbal medicines combined with antithyroid drugs were significantly better in reducing weight than antithyroid drugs in two studies:
Huangqi and Shengmai injection combined with methimazole versus methimazole (Zhang 2003a): RR 2.47, 95% CI 1.52 to 4.02;
Jiakangxiao formulation combined with methimazole and propranolol versus methimazole and propranolol (Zhu 2005): RR 2.17, 95% CI 1.42 to 3.31.
Two studies did not demonstrate significant differences:
Jiakangmianyi Jiaonang capsule combined with propylthiouracil versus Jiaokangning Pian pill combined with propylthiouracil (Ding 2005): RR 1.07, 95% CI 0.86 to 1.34;
Another study (Dai 2000) showed that Jia 1 formulation combined with methimazole was not statistically significantly better at improving a weight index than methimazole alone.
Thyroid function tests and thyroid hormone levels
Several studies investigated a variety of outcomes of thyroid function tests as well as thyroid hormone levels.
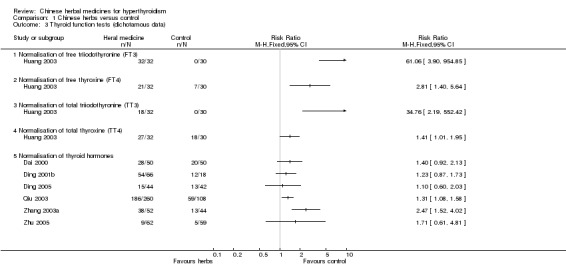
One study (Huang 2003) showed that herbal medicine pill Yikang Wan combined with methimazole, propanolol and thyroxine was better than methimazole, propanolol and thyroxine at restoration of free triiodothyronine(FT3) (RR 61.06, 95% CI 3.90 to 954.85), free thyroxine (FT4) (RR 2.81; 95% CI 1.40 to 5.64), total triiodothyronine(TT3) (RR 34.76, 95% CI 2.19 to 552.42) and total thyroxine (TT4) (RR 1.41, 95% CI 1.01 to 1.95).
Significant restoration of thyroid stimulating hormone (TSH) was shown in the following studies:
Huangqi injection combined with methimazole versus Jiakangling Pian and methimazole (Fang 2003);
Jiakangxin Koufuye combined with radioiodine versus methimazole and radioiodine(Chen 2004);
Jiakangmianyi Jiaonang capsule combined with propylthiouracil versus Jiaokangning Pian pill combined with propylthiouracil (Ding 2005).
Other studies did not demonstrate significant differences:
self‐prepared herbal‐tea combined with methimazole versus methimazole (Yan 1999);
self‐prepared Qingganjieyu formulation combined with methimazole or propylthiouracil versus methimazole or propylthiouracil (Yu 2002a).
Significant restoration of FT3 was shown in the following studies:
Jia 1 formulation combined with methimazole versus methimazole alone (Dai 2000);
Huangqi injection combined with methimazole versus Jiakangling Pian and methimazole (Fang 2003);
Huangqi and Shengmai injection combined with methimazole versus methimazole (Zhang 2003a);
Jiakangxin Koufuye combined with radioiodine versus methimazole and radioiodine (Chen 2004).
Other studies did not demonstrate significant differences:
Self‐prepared Qingganjieyu formulation combined with methimazole or propylthiouracil versus methimazole or propylthiouracil (Yu 2002a);
Jiakangmianyi Jiaonang capsule combined with propylthiouracil versus Jiaokangning Pian pill combined with propylthiouracil (Ding 2005).
Significant restoration of FT4 was shown in the following studies:
Jia 1 formulation combined with methimazole versus methimazole alone (Dai 2000);
Huangqi injection combined with methimazole versus Jiakangling Pian and methimazole (Fang 2003);
Huangqi and Shengmai injection combined with methimazole versus methimazole (Zhang 2003a);
Jiakangxin Koufuye combined with radioiodine versus methimazole and radioiodine (Chen 2004).
Other studies did not demonstrate significant differences:
Self‐prepared Qingganjieyu formulation combined with methimazole or propylthiouracil versus methimazole or propylthiouracil (Yu 2002a);
Jiakangmianyi Jiaonang capsule combined with propylthiouracil versus Jiaokangning Pian pill combined with propylthiouracil (Ding 2005).
Significant restoration of total triiodothyronine(TT3) and thyroxine (TT4) was shown in the following study:
Huangqi and Shengmai injection combined with methimazole versus methimazole (Zhang 2003a).
Another study did not demonstrate significant differences:
Self‐prepared herbal‐tea combined with methimazole versus methimazole (Yan 1999).
Significant normalisation of thyroid hormone levels was demonstrated in two studies:
Self‐prepared herbal tea Erdongtang with Xiaoyingwan Jiawei (Yangyinqingre and huatansanjie formulation) combined with methimazole or propylthiouracil versus methimazole or propylthiouracil (Qiu 2003): RR 1.31, 95% CI 1.08 to 1.58;
Huangqi and Shengmai injection combined with methimazole versus methimazole (Zhang 2003a): RR 2.47, 95% CI 1.52 to 4.02.
However, several studies showed no statistically significant differences:
Jia 1 formulation combined with methimazole versus methimazole alone (Dai 2000): RR 1.40, 95% CI 0.92 to 2.13;
self‐prepared herbal tea Jiakangping formulation combined with methimazole versus methimazole (Ding 2001b): RR 1.23, 95% CI 0.87 to 1.73;
Jiakangmianyi Jiaonang capsule combined with propylthiouracil versus Jiaokangning Pian pill combined with propylthiouracil (Ding 2005): RR 1.10, 95% CI 0.06 to 2.03;
Jiakangxiao formulation combined with methimazole and propranolol versus methimazole and propranolol (Zhu 2005): RR 1.71, 95% CI 0.61 to 4.81;
self‐prepared herbal medicine (herbal tea in the beginning and later Longdanxiegan Wan, Chenxiangshuqi Wan, Zhibaidihuang Wan) combined with radioiodine ((Ding 2001a) (RR 0.97, 95% CI 0.83 to 1.14).
In one study (Yu 2002b), FT3 and FT4 were measured twice, after 4 and 8 weeks respectively. No statistically significant differences could be demonstrated.
One study (Fang 2003) showed that Huangqi injection combined with methimazole was significantly better at restoration of TSH, FT3 and FT4 than Jiakangling Pian combined with methimazole.
One study (Chen 2004) showed that Jiakangxin Koufuye combined with radioiodine versus methimazole and radioiodine were significantly better at restoration of TSH and FT4 but not at restoration of FT3 compared to radioiodine alone.
Health‐related quality of life
None of the studies analysed health‐related quality of life.
Economic outcomes
None of the studies analysed economic outcomes.
Compliance rates
None of the studies analysed compliance rates.
Discussion
Studies of Chinese herbal medicine for hyperthyroidism lack sufficient power to provide reliable estimates of the effects of treatment with herbal medicines, due to poor study design and low methodological quality of publications.
Certain important clinical criteria were not clearly defined in our included studies. Except for three studies (Dai 2000, Ding 2005 and Zhu 2005), which provided diagnostic criteria, most included studies did not mention or failed to provide sufficient details on diagnosis. Only two studies (Ding 2005 and Fang 2003) supplied exclusion criteria.
Furthermore, the included studies assessed different outcome measures and some endpoints were only mentioned in one or two publications. Often, the descriptions of the effects were ambiguous and qualitative, which made comparisons difficult.
Formulations of herbal medicines were prepared by the trial authors themselves or associates in ten studies (Chen 2004; Dai 2000; Ding 2001a; Ding 2001b; Ding 2005; Qiu 2003; Yan 1999; Yu 2002a; Yu 2002b; Zhu 2005). The fact that in these ten studies, the trialists acted as the main players, including formulation designer, trial designer and performer, could have introduced bias
Although Chinese herbal medicine as a treatment for hyperthyroidism and its method of manufacturing are widely accepted in China, most of the constituents of the pharmacologically prepared drugs used in trials cannot be clearly specified. In contrast, in pharmacological agents like antithyroid drugs, the chemical constituents, their quantities, the percentage of any impurities or contaminants are precisely known; and the variation between different production batches is kept within specified limits. Variation between formulations and batches of herbal treatments are an inevitable consequence of Traditional Chinese Medicine (TCM), though the Chinese Government also specifies tolerable limits of variation. This variation is a factor that may contribute to heterogeneity between the studies. One formulation of Chinese herbal medicine was tested by one study only.
Furthermore, one must accept that the overall treatment concept of TCM is different to that used in a pharmacological approach by means of antithyroid drugs. When a study uses a self‐prepared herbal formulation, the quality of herbs and the methods of preparation should nevertheless be stated in detail, in order to achieve consistent effects.
A large number of the trials claimed to be RCTs, but when we phoned the trial authors and asked them about the method of randomisation they used, we found that in about 88% (93/106) of publications the authors misunderstood the concept of randomisation. In addition, some of the studies were conducted several years ago and the trial authors may have forgotten the details of the methodology they employed, again increasing the probability of bias.
Two studies (Ding 2001b; Qiu 2003) used unequal arms in their design. Of these studies, Ding 2001b applied a ratio of 3:1 and only 18 patients were included in the control group (66:18). In addition, calculation of sample sizes was not reported.
Hyperthyroidism is a pathological syndrome caused by different causes. Only four studies (Chen 2004, Ding 2005, Yu 2002a and Yu 2002b ) pointed out the exact causes. Therefore, it was difficult to associate the effects of herbal therapy with different causes of hyperthyroidism.
On the other hand, traditional Chinese herbal medicine should be used according to the "bian zheng lun zhi" rule. According to TCM theory, hyperthyroidism is recognised as nearly 40 types of "zheng". These different types of "zheng" need to be treated by different herbal medicine formulations. But no study reported in detail what type of "zheng" led to certain preparations making it difficult to judge whether the formulations used were appropriate or not.
Finally, half of studies (52/103) could not be evaluated because the original authors could not be interviewed with regards to the methodological issues. There is a potential risk of analysis bias regarding the results, although we think that only a few of these studies were real RCTs. We will try to contact the authors in the future and will update this review as soon as the interviews are completed.
Authors' conclusions
Implications for practice.
According to our findings, traditional Chinese herbal medicines in combination with other routine treatment may show some benefit for people with hyperthyroidism in improving their symptoms, signs, thyroid function and in avoiding or reducing some adverse effects, such as agranulocytosis, drug rash caused by antithyroid drugs and hypothyroidism caused by treatment with radioiodine and thyroidectomy.
However, due to methodological limitations, we could not find a well‐designed trial to provide strong evidence for Chinese traditional herbal medicine in the treatment of hyperthyroidism. Thus, we currently cannot recommend any single preparation or formulation for clinical use.
Implications for research.
High quality randomised controlled trials (RCT) are stilled needed for assessing the effects of Chinese herbal medicines combined with other routine treatment for hyperthyroidism. Especially, randomisation and allocation concealment should be done by independent individuals like a statistician. The authors should report the exact methods of randomisation and blinding (in particular who was blinded, for example outcomes assessors) as well as for allocation concealment to ensure quality and more rigorous reporting according to the CONSORT statement. The formulation for which "zheng" and the patients' "zheng" as well as details about the herbal medicines should be clearly specified and reported.
What's new
| Date | Event | Description |
|---|---|---|
| 7 July 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank all the original authors who agreed to be interviewed by telephone.
Appendices
Appendix 1. Search strategy
| Search terms |
| Unless otherwise stated, search terms are free text terms; MesH = Medical subject heading (Medline medical index term); exp = exploded MeSH; the dollar sign ($) stands for any character(s); the question mark (?) = to substitute for one or no characters; ti = title; ab = abstract; tw = text word; pt = publication type; sh = MeSH; adj = adjacent. #1 Complementary Therapies #2 alternative medicine$.ti,ab. #3 Plant Extracts #4 plant extract$.ti,ab. #5 botanical extract$.ti,ab. #6 Plants, Medicinal #7 medicinal plant$.ti,ab. #8 Medicine, Kampo/ or Phytotherapy/ or Drugs, Chinese Herbal #9 Medicine, Chinese Traditional/ or Medicine, Oriental Traditional #10 (Chinese adj3 medicin$).ti,ab. #11 phytodrug$.mp. #12 phytomedicine$.ti,ab. #13 phytopharmaceutical$.ti,ab. #14 herbal medicine$.ti,ab. #15 (complementary adj3 medicine$).ti,ab. #16 non‐prescription drug$.mp. or Drugs, Non‐Prescription #17 (Chinese adj3 herb$).mp. #18 herbal remed$.mp. #19 herbal extract$.mp. #20 herbal preparation$.mp. #21 botanical preparation$.mp. #22 (herb$ adj3 mixture$).mp. #23 exp medicine, traditional/ or exp medicine, african traditional/ or exp medicine, arabic/ or exp medicine, unani/ or exp medicine, ayurvedic/ or exp medicine, kampo/ or exp medicine, oriental traditional/ or exp medicine, chinese traditional/ or exp medicine, tibetan traditional/ or exp shamanism #24 #1‐ #23/or #25 exp hyperthyroidism #26 exp thyrotoxicosis #27 exp Graves' disease #28 #25 ‐ #27/or #29 #24 and #28 |
Appendix 2. Contents of the formulations used in the included studies
| Study ID | Contents | Treatment |
| Ding 2005 | Jiakangmianyi Jiaonang: Chuanshanlong, Huangqi, Huangjing, Xiakucao, Cebaiye | p.o.,t.i.d.,4 capsules each time,for 90 days |
| Zhu 2005 | Chaihu 10 g, Zhishi 10 g, Baishao 12 g, Shengmuli (fried) 20 g, Baitouweng 20 g, Zhebeimu 20 g, Zidanshen 20 g, Zhimu 15 g, Huangbai 15 g | 1 ampoule/d, decocted with water and getting 400mL liquid from each ampoule, orally drank 200ml in the morning and evening respectively. |
| Chen 2004 | Jiakangxin Koufuye: Taizishen 6 g, Shengshigao 10 g, Zhimu 6 g, Maidong 6 g, Shanyao 7 g, Huangqi 8 g, Wuweizi 5 g, Gegen 8 g, Cishi 7 g | P.o.,t.i.d.,20 ml each time,for 2 weeks |
| Zhang 2003a | The author didn't mention contents of Huangqi Injection and Shengmai Injection. | 1.Huangqi injection:20mL huangqi injection was diluted with 250mL 5% GNS and transfused intravenously once a day;2.Shengmai injection:30mL shengmai injection was diluted with 250 5% GNS and transfused intravenously once a day. |
| Fang 2003 | Jiakangning pian: Mohanlian, Danshen, Shaoyao, Longgu, Muli. The author didn't mention contents of shengmai injection. | 1.Jiakangling pian:p.o.,t.i.d.,2 tablets each time;2.Shengmai injetion:60mL shengmai injection was diluted with 500mL 5% GNS and transfused intravenously for 15 days. |
| Qiu 2003 | Erdong Tang with Xiaoluowan Jiawei: Tiandong 15 g, Maidong 15 g, Shashen 15 g, Tianhuafen 15 g, Zhimu 15 g, Shengdihuang 15 g, Xuanshen 15 g, Gancao 6 g, Longdancao 10 g, Xiakucao 10 g, Wuweizi 10 g, Xiyangshen 10 g, Zhebeimu 5 g | 1 ampoule/day was decocted with water and orally taken in the morning and evening.When the symptoms were modified,decrease the dosage to 1 ampoule/3 days and kept the minimum dosage of 1 ampoule/15 days for 1.5˜2 years. |
| Huang 2003 | Yikang wan: Lingyangjiao, Shengbaoshao, Shengdi, Xiangfu, Tiandong, Huangjing, Shijueming, Xuanshen, Chaihu, Nvzhenzi | The materials were made into pills.1 pill contained 9g raw material.p.o.,t.i.d.,1 pill for each time. |
| Dai2000 | Fabanxia 15 g, Fuling 15 g, Zhebei 12 g, Gualoupi 15 g, Hanliancao 15 g, Danshen 30 g, Tianqipian 5 g, Maozhuacao 15 g, Yujin 12 g | 1 ampoule/day,decocted with water. |
| Yan 1999 | Xuanshen 15 g, Huangqi 15 g, Xiakucao 15 g, Chuanshanjia 15 g, Taizishen 15 g, Sanleng 12 g, Eshu 12 g, Maidong 15 g, Huhuanglian 12 g, Wuweizi 10 g | 1 ampoule/day,decocted with water,one course of treatment was 28 days. |
| Yu 2002a | Zhizi 10 g, Pugongying 30 g, Banzhilian 30 g, Lianqiao 9 g, Yujin 10 g, Wuweizi 15 g, Chuanlianzi 10 g, Zhike 9 g, Juhua 10 g, Gouqizi 12 g, Baihuashehecao 30 g | 1 ampoule of raw material was needed for a day ,decocted with water,for 4 weeks.Then orally took pills made by the raw materials,1 amoule/day,for 4˜8 weeks. |
| Ding 2001a | chaihu 12 g, baishao 12 g, zhizi 10 g, shandougen 10 g, Shashen 15 g, Maidong 15 g, Gouqizi 15 g, Wuweizi 10 g, Baijili 10 g, Juemingzi 10 g, Chaozaoren 30 g, Gancao 6 g | 1 ampoule/day decocted with water,p.o.,b.i.d. |
| Yu 2002b | Liuyuexue 30 g, Fuling 10 g, Banzhilian 30 g, Shengdi 10 g, Yimucao 20 g, Baihuashehecao 30 g, Danshen 12 g, Danggui 15 g | Raw material was made into granules,1 ampoule/day,for 8 weeks. |
| Ding 2001b | Shenghuangqi 15˜30 g, Taizishen 10 g, Shengdi 10 g, Shengbaishao 10 g, Xiakucao 10 g, Maidong 10 g, Wuweizi 10 g, Chaozaoren 10 g, Jiugancao 10 g, Shenglonggu 25 g, Shengmuli 25 g, Caojueming 25 g | The authors didn't give details on the usage of the Chinese medicine used in the intervention group. |
Appendix 3. Chinese herbs terminology in three languages
| Pinyin name | Latin name | English name |
| Chaihu | Radix Bupleuri | Chinese Thorowax Root |
| Baishao | Radix Paeoniae Alba | White Paeony Root |
| Zhizi | Fructus gardeniae | Cape Jasmine Fruit |
| Shandougen | Radix Sophorae Tonkinensis | Vietnamese Sophora Root |
| Shashen | Radix Adenophorae | Ladybell Root |
| Maidong | Radix Ophiopogonis | Dwarf Lilyturf Tuber |
| Gouqizi | Fructus Lycil | Barbary Wolfberry Fruit |
| Wuweizi | Fructus Schisandrae | Chinese Magnoliavine Fruit |
| Baijili | Fructus Tribuli | Puncturevine Caltrop Fruit |
| Juemingzi | Semen Gassiae | Cassia Seed |
| Chaozaoren | Stir‐baked Semen Ziziphi Spinosae | |
| Gancao | Radix Glycyrrhizae | Liquoric Root |
| Taizishen | Radix Pseudostellariae | Heterophylly Falsestarwort Root |
| Zhimu | Rhizoma Anemarrhenae | Common Anemarrhena Rhizome |
| Shengshigao | Gypsum Fibrosum | Gypsum |
| Shanyao | Rhizoma Diosscoreae | Common Yam Rhizome |
| Huangqi | Radix Astragali | Membranous Milkvetch Root |
| Gegen | Radix Puerariae | Kudzuvine Root |
| Lingyangjiao | Cornu Saigae Tataricae | Antelope Horn |
| Cishi | Magnetitum | Magnetite |
| Shengbaishao | ||
| Shengdi | Rehmannia Dride Rhizome | |
| Xiangfu | Rhizoma Cyperi | Nutgrass Galingale Rhizoma |
| Tiandong | Radix Asparagi | Cochinchnese Asparagus Root |
| Huangjing | Rhizoma Polygonati | Manyflower Solomonseal Rhizome |
| Shijueming | Concha Haliotidis | Sea‐ear Shell |
| Xuanshen | Radix Scrophulariae | Figwort Root |
| Nvzhenzi | Fructus Liqustri Lucidi | Glossy Privet Fruit |
| Mohanlian | Herba Ecliptae | Yerbadetajo Herb |
| Danshen | Radix Salviae Miltiorrhizae | Danshen Root |
| Xiakucao | Spica Prunellae | Common Selfheal Fruit‐spike |
| Longgu | Os Draconis | Drgon's Bones |
| Muli | Concha Ostreae | Oyster Shell |
| Chuanshanjia | Squama Manitis | Pangolin Scales |
| Sanling | Rhizoma Sparganii | Common Burreed Rhizome |
| Huhuanglian | Rhizoma Picrorhizae | FigwortflowerPicrorhizaRhizome |
| Eshu | Rhizoma Curcumae | Zedoary |
| Pugongying | Herba Taraxaci | Mongolian Dandelion Herb |
| Banzhilian | Herba Scutellariae Barbatae | Barbed Skullcap Herb |
| Lianqiao | Fructus Forsythiae | Forsythia Suspensa |
| Yujin | Radix Curcumae | Turmeric Root‐tuber |
| Zhike | Fructus Aurantii | Bitter Orange |
| Chuanlianzi | Fructus Toosendan | Szechwan Chinaberry Fruit |
| Juhua | Flos Chrysanthemi | Chrysanthemum |
| Baihuasheshecao | Herba Hedyotidis Diffusae | Spreading Hedyotis Herb |
| Liuyuexue | Herba Serissae | Snow of June Herb |
| Fuling | Poria | Indian Buead |
| Yimucao | Herba Leonuri | Motherwort Herb |
| Danggui | Radix Angelicae Sinensis | Chinese Angelica |
| Tianhuafen | Radix Trichosanthis | Mongolian Snakegourd Root |
| Shengdihuang | Radix Rehmanniae | Rehmannia Root |
| Longdancao | Radix Gentianae | Chinese Gentian |
| Xiyangshen | Radix Panacis Quinquefolii | American ginseng |
| Zhebeimu | Bulbus Fritillariae Thunbergii | Thunberg Fritillary Bulb |
| Zhishi | Fructus Aurantii Immaturus | Immature Bitter Orange |
| Baitouweng | Radix Pulsatillae | Chinese Pulsatilla Root |
| Zidanshen | ||
| Huangbai | Cortex Phellodendri | Amur Corktree Bark |
| Chuanshanlong | Rhizoma Dioscoreae Nipponicae | Dioscorea Nipponica Dioscorea nipponica |
| Cebaiye | Cacumen Platycladi | Chinese Arborvitae Tops |
| Fabanxia | Rhizoma Pinelliae | Pinellia Tuber |
| Gualoupi | Pericarpium Trichosanthis | |
| Hanliancao | Herba Ecliptae | Yerbadetajo Herb |
| Maozhuacao | Radix Ranunculus Ternati | |
| Tianqi | Radix Notoginseng | Panax Pseudo‐ginseng |
Data and analyses
Comparison 1. Chinese herbs versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Thyroid antibody status | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Thyroglobulin antibody (TGAb) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Thyroid microsomal antibodies(TMAb) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Relapse rates | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Thyroid function tests (dichotamous data) | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Normalisation of free triiodothyronine (FT3) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Normalisation of free thyroxine (FT4) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Normalisation of total triiodothyronine (TT3) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Normalisation of total thyroxine (TT4) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Normalisation of thyroid hormones | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Thyroid function tests (continuous data) | 8 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Thyroid‐stimulating hormone (TSH) | 5 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Thyroid‐stimulating hormone (TSH)(Huangqi injection versus Jiahangling) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Free triiodothyronine (FT3) | 6 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Free triiodothyronine (FT3) (Huangqi injection versus Jiakangling) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Free triiodothyronine (FT3) at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Free triiodothyronine (FT3) at 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Free thyroxine (FT4) | 6 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.8 Free thyroxine (FT4) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.9 Free thyroxine (FT4) at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.10 Free thyroxine (FT4) at 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.11 Total triiodothyronine (TT3) | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.12 Total thyroxine (TT4) | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Thyroid antibody status | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Thyroglobulin antibodies (TGAb) | 4 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Thyroglobulin antibody (TGAb) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Thyroid microsomal autoantibodies (TMAb) | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Thyroid microsomal antibodies(TMAb) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Symptoms of hyperthyrodism | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Symptoms subsided | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Symptoms were markedly improved | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Symptoms were improved | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 No improvement of symptoms | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Weight change (improvement) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Adverse effects | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Agranulocytosis | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Hepatitis | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Drug rash | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Tachycardia | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 Normalisation of heart rate | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Decrease in heart rate by 20 beats/min | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Decrease in heart rate by 10 beats/min | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 No improvement of heart rate | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.5 Normalisation of pulse rate | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.6 Near normalisation of pulse rate | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.7 Decrease in pulse rate | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.8 No improvement of pulse rate | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Thyroid antibody status | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.1 Reduction in thyroglobulin antibodies (>30%) and thyroid microsomal autoantibodies (>15%) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Normalisation of thyroid‐stimulating antibodies (TSAb) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Improvement of ophthalmopathy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12 Weight change ( weight index ) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13 Symptoms of hyperthyroidism | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 Tachycardia (heart rate) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Diarrhoea ( frequency of stools) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
Comparison 1 Chinese herbs versus control, Outcome 1 Thyroid antibody status.
1.2. Analysis.
Comparison 1 Chinese herbs versus control, Outcome 2 Relapse rates.
1.3. Analysis.
Comparison 1 Chinese herbs versus control, Outcome 3 Thyroid function tests (dichotamous data).
1.4. Analysis.
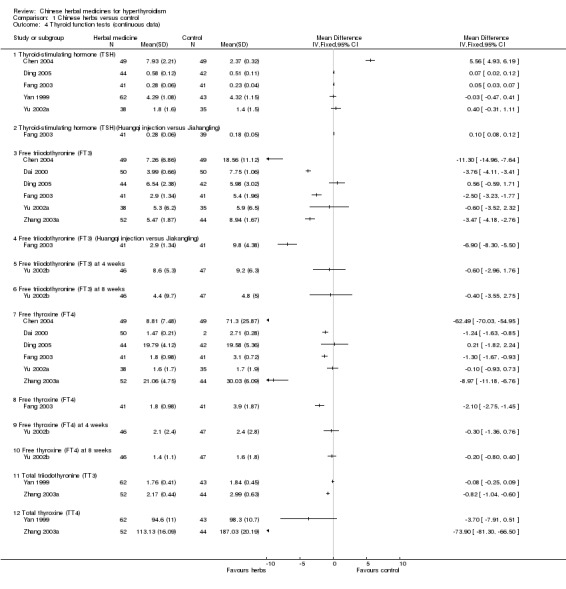
Comparison 1 Chinese herbs versus control, Outcome 4 Thyroid function tests (continuous data).
1.5. Analysis.
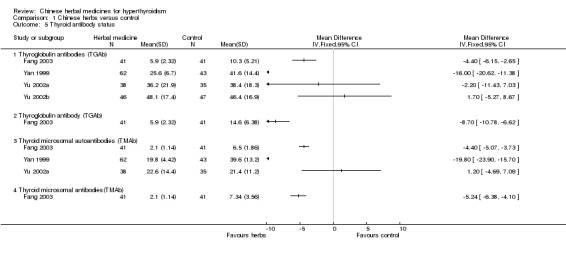
Comparison 1 Chinese herbs versus control, Outcome 5 Thyroid antibody status.
1.6. Analysis.
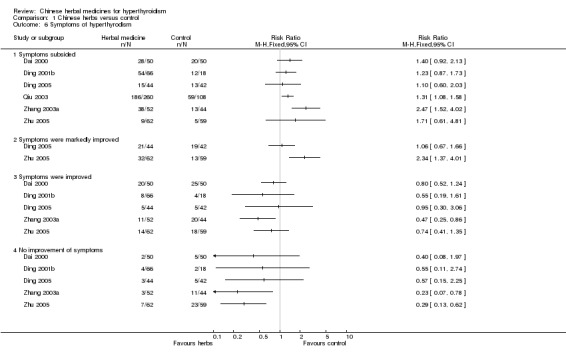
Comparison 1 Chinese herbs versus control, Outcome 6 Symptoms of hyperthyrodism.
1.7. Analysis.
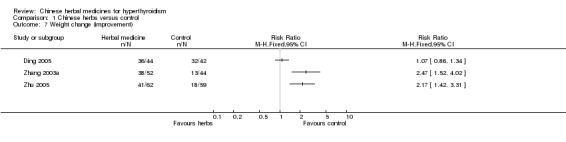
Comparison 1 Chinese herbs versus control, Outcome 7 Weight change (improvement).
1.8. Analysis.
Comparison 1 Chinese herbs versus control, Outcome 8 Adverse effects.
1.9. Analysis.
Comparison 1 Chinese herbs versus control, Outcome 9 Tachycardia.
1.10. Analysis.
Comparison 1 Chinese herbs versus control, Outcome 10 Thyroid antibody status.
1.11. Analysis.
Comparison 1 Chinese herbs versus control, Outcome 11 Improvement of ophthalmopathy.
1.12. Analysis.
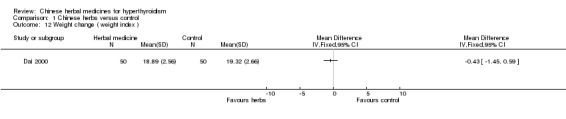
Comparison 1 Chinese herbs versus control, Outcome 12 Weight change ( weight index ).
1.13. Analysis.
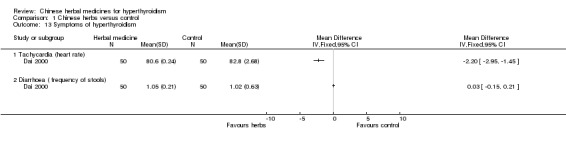
Comparison 1 Chinese herbs versus control, Outcome 13 Symptoms of hyperthyroidism.
Comparison 2. Chinese herbs versus radioiodine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptoms of hyperthyroidism | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Improvement of anxiety | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Improvement of tachycardia | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Improvement of heat intolerance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Thyroid function tests | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Free triiodothyronine (FT3) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Free thyroxine (FT4) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Thyroid‐stimulating thyroxine (TSH) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Thyroid function tests | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Normalisation of thyroid hormones | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Hypothyroidism | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Early onset | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Late onset | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
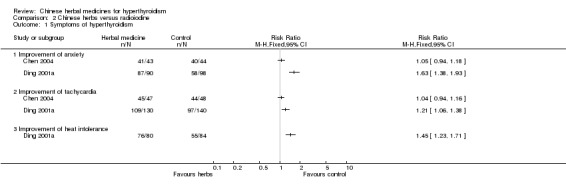
Comparison 2 Chinese herbs versus radioiodine, Outcome 1 Symptoms of hyperthyroidism.
2.2. Analysis.
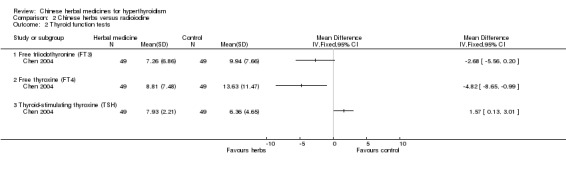
Comparison 2 Chinese herbs versus radioiodine, Outcome 2 Thyroid function tests.
2.3. Analysis.
Comparison 2 Chinese herbs versus radioiodine, Outcome 3 Thyroid function tests.
2.4. Analysis.
Comparison 2 Chinese herbs versus radioiodine, Outcome 4 Hypothyroidism.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chen 2004.
| Methods | Parallel design. Due to the randomisation method was not mentioned in original article, we telephone interviewed the trialist. The randomisation method was made clear that allocation sequence was generated by random number table. No blinding was performed. | |
| Participants | 147 cases of Grave's disease were included (M/F=38/109, aged between 21 and 68, 31.4+/‐4.8 years old on average, with the length of the disease between 2 months and 6 years, 17 months on average). 49 cases were in radioiodine group (I), 49 cases were in tapazole group (T) and 49 cases were in jiakangxin with radioiodine group (J). | |
| Interventions | Radioiodine was used in both I and J groups, p.o., 3.70˜5.55 MBq, only once. The second day after taking radioiodine, Jiakangxin Koufuye was additionally given to the latter group, p.o., t.i.d., 20 mL each time, for 2 weeks. Tapazole was used in T group, p.o., 15˜30 mg/d, decreased the dosage 2 months later according to the changes of thyroxin. | |
| Outcomes | The outcomes were evaluated by total effective rate, on the basis of improvement of principal symptoms, body weight, thyroid hormones after 6 months. 1. Recovery: T/I/J=10/23/24; 2. Improvement: T/I/J=7/18/19; 3. Moderate improvement: T/I/J=11/1/4; 4. No improvement: T/I/J=21/7/2; 5. Total effective rate: T/I/J=57.1%/85.7%/91.7%. I group and J group had better effect than T group (P<0.05 ); 6. Improvement of principal symptoms (including hidrosis, inertia, hyposomnia, dysphoria and limbs jitter): there was statistical significance between two groups, showing that 1˜3 months after the treatment, J group had better effects than I group. But there was no statistical significance between two groups 6 months after the treatment; 7. The level of FT3, FT4, TSH was better improved in I and J group than T group. There was statistical significance between two groups. | |
| Notes | 1. Jiakangxin Koufuye was prepared by the author's hospital; 2. There's potential conflict of interest in the study. | |
Dai 2000.
| Methods | Parallel design. Due to the randomisation method was not mentioned in original article, we telephone interviewed the original author. The randomisation method was made clear that allocation sequence was generated by random number table. No blindness. | |
| Participants | 100 patients of hyperthyroidism were included. 50 cases were in intervention group (M/F=9/41, aged between 16 and 65, 35 years old on average, with the length of disease between 1 month and 2 years, 1.2 years on average). 50 cases were in control group (M/F=11/39, aged between 15 and 66, 36 years old on average, with the length of disease between 1 month and 2 years, 1.1 years on average). Diagnosis criterion: symptoms: heat intolerances, polyphagia, emaciation, fatigue, sweating, palpitation, increased frequency of defecation, irregular menstruation; signs: tachycardia, enhanced heart sound, increased pulse pressure, enlarged thyroid gland, vascular murmur and tremor, hand and tongue tremor, damp skin and exophthalmos, obviousely increased TT3,TT4,FT3,FT4 concentration ,decreased TSH concentration, increased 99MTc intaking function, no tuberosity or tumor. Including criterion: according with diagnosis criterion, with no severe diseases of heat, brain and kidney, aged between 12 and 75. Excluding criterion: suffering from cerebrovascular accidents in half a year, with severe heart or kidney diseases. | |
| Interventions | Tapazole was used in both groups, p.o., 10 mg each time, t.i.d., for 2 months. Intervention group additionally used Chinese medicine, 1 ampoule/day, decocted with water, for 2 months. | |
| Outcomes | 1. Clinical recovery: the symptoms and signs subsided completely, concentration of FT3, FT4, TT3, TT4 came to the normal level, radioiodine intaking rate turned to be normal and/or T3 suppressive test was suppressed (I/C=28/20); 2. Improvement: the symptoms and signs were improved, not all the concentration of FT3, FT4, TT3, TT4 reached normal level (I/C=20/25); 3. No improvement: no change on the symptoms, sighs and the concentration of TT3, TT4, FT3, FT4 (I/C=2/5); 4. Total effective rate: I/C=96%/90%. There was statistical significance between two groups, showing that the effect of intervention group was better than control group; 5. The effect of intervention group on improving three symptoms, including tachycardia, body weight and frequency of defecation, was better than control group. There was statistical significance between two groups; 6. The effect of intervention group on decreasing concentration of FT3, FT4 was better than control group. | |
| Notes | 1. The formulation of Chinese medicine used in the intervention group was provided by the trialist; 2. There was potential conflict of interest in the study. | |
Ding 2001a.
| Methods | Parallel design. Due to the randomisation method was not mentioned in original article, we telephone interviewed the original author. The randomisation method was made clear that allocation sequence was generated by random number table. No blindness. | |
| Participants | 312 cases which were diagnosed as diffuse goitre by clinical symptoms and laboratory examination (including radioiodine, TT3, TT4, FT3, FT4, TSH, TGA, TMA, ect.) were included. Cases with obvious exophthalmos and hyperthyroidism complication were excluded. 150 cases were in the intervention group (M/F=48/114, patients' age: 20˜29/30˜39/40˜49/50˜59=23/45/63/19, first onset/recurrence after operation/recurrence after treatment with drugs=72/22/56). 162 cases were in the control group (M/F=38/112, patients' age: 20˜29/30˜39/40˜49/50˜59=24/45/66/27, first onset/recurrence after operation/recurrence after treatment with drugs=78/26/58). Baseline of two groups were similar. | |
| Interventions | Radioiodine and Chinese medicine was used in the intervention group. The radioiodine was given once, p.o., 74˜148 MBq. The 2nd week after taking radioiodine, Chinese medicine was used, p.o., for 3 months. Then Chinese formulated products Longdanxiegan Wan, Chenxiangshuqi Wan, Zhibaidihuang Wan was used. Control group used radioactive iodine. The whole course of treatment lasted 6 months˜1 year. The patients were followed up in 1˜ 4 years. | |
| Outcomes | 1. Recovery: symptoms, signs almost subsided or subsided completely, the results of laboratory examination turned normal (I/C=98/109); 2. Improvement: symptoms almost subsided, signs were markedly lightened, the results of laboratory examination were almost normal (I/C=40/38); 3. No improvement: symptoms was lightened, but signs and the results of laboratory examination remained the same (I/C=12/15); 4. Improvement of symptoms (including palpation, hyposomnia, polyphagia, restlessness, heat intolerance, sweating, ect.): there was statistical significance between two groups, showing that the effect of the intervention group was better than the control group; 5. The incidence of hypothyroidism: there was statistical significance between two groups, showing that the incidence of the intervention group was lower than that of the control group. | |
| Notes | 1. The formulation of Chinese medicine used in the intervention group was provided by the original authors themselves; 2. There was potential conflict of interest in the study. | |
Ding 2001b.
| Methods | Parallel design. Due to the randomisation method was not mentioned in original article, we telephone interviewed the original author. The randomisation method was made clear that allocation sequence was generated by random number table. No blindness. | |
| Participants | 84 patients of hyperthyroidism were included. The symptoms and laboratory examinations: polyphagia, irritability or depression, emaciation, fatigue, sweating, palpitation, dizziness, enlarged thyroid gland, thyroid murmur and vascular murmur, hand tremor, tachycardia (HR>90/min), exophthalmos, carmoisine tongue, increased T3,T4 and decreased TSH concentration. 36 cases were with liver function impairment, 24 were with decrease of WBC, 18 were with decrease of platelets. 66 cases were in the intervention group (M/F=12/54, 30.8 years old on average, 1.4 years on average of the length of disease). 18 were in the control group (M/F=3/15, 31.4 years old on average, 1.6 years on average of the length of disease). | |
| Interventions | Tapazole and Chinese medicine was used in the intervention group, 1 ampoule/day, decocted with water, b.i.d.; Tapazole was used in the control group, 5˜10 mg each time, t.i.d., for 2 months, examined FT3, FT4, TSH, liver function and blood routine test once a month. | |
| Outcomes | 1. Control: FT3, FT4 turned to normal level and symptoms of hyperthyroidism disappeared (I/C=54(81.8%)/12(22.2%)); 2. Improvement: FT3, FT4 decreased by more than 50%, symptoms improved obviousely (I/C=8(12.1%)/4(22.2%)); 3. No improvement: FT3, FT4 decreased by less than 50% or increased, symptoms didn't improve (I/C=4(6.1%)/2(11.1)); 4. Total effective rate: I/C=93.9%/88.9%. There was no statistical significance between two groups; 5. Adverse effects: in the control group, WBC decrease/platelets decrease/tetter and exophthalmos aggravation=8/3/2; in the intervention group, only 1 case appeared to have tetter and exophthalmos aggravation; 6. Relapse rate within 1 year: I/C=16(29.6%)/10(83.3%). There was statistical significance between two groups, showing that the recurrence rate of the intervention group was lower than the control group. | |
| Notes | 1. Chinese medicine used in the intervention group was prepared by the trialist; 2. There was potential conflict of interest in the study; 3. Two cases in control group were withdrawn from the study for aggravation of the disease. | |
Ding 2005.
| Methods | Parallel design. Due to the randomisation method did not be mentioned in original article, we telephone interviewed the original author. The randomisation method was made clear that allocation sequence was generated by randomisation computer software. Single blindness: the patients didn't know which group they were in. | |
| Participants | 86 diagnosed patients were included . Among them, 76 were suffered from diffuse toxic goitre and the rest were at the early stage of subacute thyroiditis. 44 cases were in the intervention group (M/F=18/26, aged between 18 and 65, 35.6+/‐12.0 years old on average, with the length of the disease between 1 month and 8 years, 4.5+/‐8.6 months on average). 42 cases were in the control group (M/F=15/27, aged between 15 and 67, 30.6+/‐14.1 years old on average, with the length of disease between 2 months and 5 years, 3.5+/‐6.8 months on average). Baseline of two groups were similar. | |
| Interventions | Propylthiouracil was used in both groups, t.i.d., 100 mg each time. Decreased the dosage to 50˜100 mg/d. Jiakangmianyi Jiaonang were used in the intervention group, p.o., t.i.d., 4 capsules each time, for 90 days. The control group used Jiakangning Pian, p.o., t.i.d., 6 tablets each time, for 90 days. | |
| Outcomes | 1. Clinical control: the symptoms subsided completely, body weight increased, sphygmus turned to be normal, tremor of the thyroid gland and vascular murmur disappeared, goitre and exophthalmos was lightened and the concentration of TSH, FT3, FT4 came to the normal level (I/C=15/13); 2. Improvement: Main symptoms subsided, body weight increased, sphygmus was almost normal, thyroid tremor and vascular murmur disappeared, goitre and exophthalmos was lightened and the concentration of TSH, FT3, FT4 became almost normal (I/C=21/19); 3. Moderate improvement: The symptoms were improved, sphygmus slowed down, the vascular murmur was lightened, goitre became smaller and the concentration of TSH, FT3, FT4 was almost normal (I/C=5/5); 4. No improvement: No change on the symptoms, signs and laboratory examination (I/C=5/5); 5. Total effective rate: I/C=93.2%/88.1% and there was no statistical significance between two groups; 6. Adverse effects: Decreasing of WBC and impairment of the liver function was observed in one and two cases in the intervention group respectively. In the control group, two patients got iatric tetters, two had WBC decreasing and one patient's liver function was impaired. | |
| Notes | 1. The drug was made and provided by the author's hospital; 2. It was a local government supported project. | |
Fang 2003.
| Methods | Parallel design. Due to the randomisation method wasn't mentioned in original article, we telephone interviewed the original author and made clear that the allocation sequence was generated by computer software. Single blindness: the patients didn't know which group they were in. | |
| Participants | 123 diagnosed GD patients (M/F=33/90, 32+/‐7 years old on average) were included. The diagnoses was based on typical hypermetabolic symptoms, enlargement of thyroid gland, exophthalmos and high concentration of T3,T4. All the patients had light symptoms and short length of disease. The enlargement of thyroid gland was within ¢ò degree and the increase of T3, T4 was less than 20%. Excluding criterion: hyperthyroidism crisis, recurrence cases after treatment of radioiodine or operation, pregnancy, cases accompanied by heart disease, ¢ó degree or more severe exophthalmos and cases with other chronic and endocrine diseases. The patients were divided into three groups. 41 cases for each group. Baseline of two groups were similar. | |
| Interventions | Tapazole and Huangqi injection was given in the intervention group. The usage of Tapazole was similar to control 2 group, 60 ml/day Huangqi injection diluted in 500 ml 5% glucose infusion, venous transfusing for 15 days. In diapause, Tapazole was used; Jiakangling was used in control 1 group, p.o., t.i.d., 2 tablets each time; Tapazole was used in control 2 group, p.o., t.i.d., 10 mg each time. | |
| Outcomes | The outcomes were evaluated by function of thyroid gland (FT3,FT4,TSH), TGA, TMA, T lymphocytes, blood routine test and liver function. 1. The changes of WBC amount, liver function and thyroid gland function: The liver function of the patients in control 1 group improved, but little improvement was seen on their WBC amount and thyroid gland function; in control 2 group, liver function and thyroid gland function of the patients improved; in the intervention group, liver function, thyroid gland function, WBC amount and concentration of TGA, TMA improved obviousely. And the result showed that the effects of intervention group were better than other groups; 2. The changes of T lymphocytes: In control 1 group, the T lymphocytes almost stayed the same; in control 2 group, CD4 decreased and CD8 increased a little so that CD4/CD8 decreased; in the intervention group, CD4 and CD8 improved so that CD4/CD8 decrease obviousely. The result showed that there was statistical significance between intervention group and other groups. | |
| Notes | 1. 'Positive drug' jiakangling was used to be the control but there was no any record about its effect; 2. Two cases in control 1 group quit the study for aggravation of the disease. | |
Huang 2003.
| Methods | Parallel design. Due to the randomisation method was not mentioned in original article, we telephone interviewed the original author. The randomisation method was made clear that allocation sequence was generated by random number table. No blindness. | |
| Participants | All patients included were inpatients or outpatients who were diagnosed as hyperthyroidism by clinical and laboratory examination. 32 cases were in the intervention group (M/F=8/24, aged between 15 and 61, 26+/‐9.8 years old on average, with the length of disease between 2 and 36 months, 14+/‐5.2 months on average). 30 cases were in the control group (M/F=7/23, aged between 19 and 57, 25+/‐9.2 years old on average, with the length of disease between 2 and 24 months, 12+/‐5.6 months on average). Baseline of two groups were similar. | |
| Interventions | Yikang Wan and Tapazole was used in the intervention group, Yikang Wan: p.o., 1 pill(9 g) each time, t.i.d., Tapazole: p.o., 5 mg each time, t.i.d. Tapazole was used in the control group, p.o., 5 mg each time, t.i.d. Propranolol (10 mg each time, t.i.d.) and Jiazhuangxian Pian (30 mg each time, q.d.) was used in both groups. | |
| Outcomes | The outcomes were evaluated by clinical symptoms, signs, blood routine test, FT3, FT4, TT4, TT3 concentration. They were examined 2, 3, 4 weeks after the treatment and before the treatment. 1. The hyperthyroidism diagnoses index of the intervention group was lower than the control group. There was statistical significance; 2. Two and three weeks after treatment, FT3 concentration of the intervention group turned to be normal, while FT3 concentration of the control group was still above the normal level; 3. FT4 concentration of 21 cases in the intervention group turned to be normal, while only 7 cases in the control group was normal; 4. TT3 concentration of 18 cases in the intervention group turned to be normal, while no one in the control group turned to be normal; 5. TT4 concentration of 27 cases in the intervention group turned to be normal, while 18 cases in the control group turned normal; 6. Adverse effect: temple WBC increasing was observed in 3 cases in the intervention group and 5 cases in the control group. Taking drugs that could increase WBC, the symptom subsided and the cases continued the study. | |
| Notes | 1. Yikang Wan was provided by the company that was the sponsor of the magazine in which the article was published; 2. There was potential conflict of interest in the study. | |
Qiu 2003.
| Methods | Parallel design. Due to the randomisation method wasn't mentioned in original article, we telephone interviewed the original author and made clear that the allocation sequence was generated by computer software. No blindness. | |
| Participants | 368 cases were included (M/F=82/286, aged between 18 and 62, 35.6 years old on average). The diagnoses criterion included FT3>10 pmol/L, FT4>30 pmol/L, or TT3>3.0 pmol/L, TT4>155 nmol/L and sTSH<0.5 mU/L. 260 were in the intervention group (34.8 years old on average). 108 were in control group (36.2 years old on average). Baseline of two groups were similar. | |
| Interventions | The intervention group was treated by Erdong Tang with Xiaoluowan Jiawei (ETXJ) on the basis of west medicine treatment. 1 ampoule/day, decocted with water, b.i.d. When the symptoms were lightened, decreased the dosage to 1 ampoule/3‐day and keep the minimum dosage of 1 ampoule/15‐day for 1.5˜2 years; Tapazole (p.o., 30˜40 mg/day) or Propylthiouracil (p.o., 300˜450 mg/day) was used in control group. The dosage was decreased when the symptoms were lightened. Then the patients were treated by the minimum dosage (tapazole: 5 mg/day, propylthiouracil: 50 mg/day) for 1.5˜2 years. | |
| Outcomes | 1. Recovery: The symptoms subsided completely, thyroid gland shank, negative TSAb and T3, T4, TSH sustained the normal level for 1 year after discontinuing drugs. (I/C=186(71.5%)/59(54.6%)) There was statistical significance between two groups, showing that the effect of intervention group was better than control group; 2. Relapse Rate: Hyperthyroidism was ameliorated but then relapsed within 1 year after discontinuing drugs, symptoms recurred and FT3, FT4 (or TT3,TT4) increased. (I/C=29(15.6%)/20(33.9%)) There was statistical difference between two groups that was the recurrence rate of intervention group was lower than control group; 3. Granulocytes decreasing: WBC<4*109/L and neutrophils were the mainly cells that decreased. (I/C=72(27.7%)/48(44.4%)) There was statistical difference between two groups, showing that the adverse effect of intervention group was less than control group. | |
| Notes | 1. Funded by patients themselves. 2. Both the Xiaying Wan and Erdongtang decoction were prepared by the original authors themselves; 3. There was potential conflict of interest in the study. | |
Yan 1999.
| Methods | Parallel design. Due to the randomisation method wasn't mentioned in original article, we telephone interviewed the original author. The randomisation method was made clear that allocation sequence was generated by random number table. No blindness. | |
| Participants | 105 in‐patients of hyperthyroidism were included. 62 cases were in intervention group (M/F=22/40, aged between 15 and 57, 29.1+/‐10.8 years old on average). 43 cases were in control group (M/F=14/29, aged between 14 and 56, 28.3+/‐11.2 years old on average). Baseline of two groups were similar. | |
| Interventions | Tapazole was used in both groups, 30 mg/day, for 28 days. Besides tapazole, Chinese medicine was additionally used in the intervention group, 1 ampoule/day, decocted with water. | |
| Outcomes | 1. Symptoms (including palpitation, fatigue, emaciation, heat intolerance) of both groups improved obviousely. There was statistical significance between two groups; 2. Concentration of T3, T4 decreased and concentration of TSH increased obviousely in both groups. There was no statistical significance between two groups; 3. TGAb (TGAb>30%) / TMAb (TMAb>15%) negative turning rate of intervention group was better than control group. There was statistical significance between two groups. (I/C=31 (65.9%) /5 (15.6%) ) | |
| Notes | 1. The formulation of Chinese medicine used in the intervention group was provided by the original authors; 2. There was potential conflict of interest in the study. | |
Yu 2002a.
| Methods | Parallel design. Due to the randomisation method wasn't mentioned in original article, we telephone interviewed the original author. The randomisation method was made clear that allocation sequence was generated by random number table. Single blindness: the patients didn't know which group they were in. | |
| Participants | 73 patients that suffered from Grave's disease with light to moderate liver impairment were included. The patients whose liver were impairment by virus hepatitis or the causes were excluded. 38 cases were in the intervention group (M/F=8/30, aged between 18 and 60, 31.2 years old on average). 35 cases were in the control group (M/F=6/29, aged between 19 and 63, 32.7 years old on average). Baseline of two groups were similar. | |
| Interventions | Chinese medicine and anti‐thyroid drugs (ATD, including Tapazole and Propylthiouracil) was used in the intervention group. Chinese medicine was decocted with water, 1 ampoule/d, for 4 weeks. Then take the pills made by Chinese herb, 1 ampoule/d, for 4˜8 weeks; Tapazole (5 mg/d, t.i.d.) or Propylthiouracil (50 mg/d, t.i.d.) was also used in the control group. Change the dosage from the 5th weeks after the function of thyroid gland turned to normal. The control group only used ATD. The method was similar to the intervention group. | |
| Outcomes | Thyroid gland function (including T3, T4, TSH, FT3, FT4), thyroid gland auto‐antibody (including TGA, TMA) and liver function (including ALT, AST, TBil) was taken into consideration. 1. The decrease of ALT and AST: The intervention group (ALT: from 67.2+/‐20.3 IU/L to 30.6+/‐4.5 IU/L, AST: from 54.6+/‐13.7 IU/L to 29.3+/‐5.1 IU/L, TBil: from 23.1+/‐7.3 IU/L to 12.3+/‐4.7 IU/L) had better effects than the control group after 12 weeks; 2. The time needed for liver function's improvement: The intervention group (it took 25.7+/‐9.2 days to be normal and 94% cases turned to be normal within 8 weeks.) was better than the control group (It took 36.8+/‐12.4 days to be normal and only 57.1% cases turned to be normal within 8 weeks); 3. The improvement of the thyroid gland and the changes of relative antibody: The improvement of FT3, FT4, TGA, TMA and TSH showed no statistical significance between two groups. | |
| Notes | 1. We telephone interviewed the pharmaceutical company and learned that the formulation of the Chinese medicine and the pills made by Chinese herb used in the intervention group was provided by the authors themselves, and the company provided processed raw material needed; 2. There was potential conflict of interest in the study. | |
Yu 2002b.
| Methods | Parallel design. Due to the randomisation method wasn't mentioned in original article, we telephone interviewed the original author. The randomisation method was made clear that allocation sequence was generated by random number table. Single blindness: the patients didn't know which group they were in. | |
| Participants | 93 patients who were diagnosed as Grave's disease and PCI>150 were included. The patients whose kidney were impaired other causes were excluded. 46 cases were in the intervention group (M/F=9/37, aged between 18 and 71, 38.1+/‐12.4 years old on average). 47 cases were in the control group (M/F=8/39, aged between 17 and 68, 36.4+/‐11.2 years old on average). Baseline of two groups were similar. | |
| Interventions | Propylthiouracil was used in both groups, p.o., 100 mg/day, b.i.d. Chinese medicine was additionally used in the intervention group. Chinese medicine was made into pills, 1 ampoule/d, for 4 weeks. | |
| Outcomes | The outcomes were evaluated by PCI=(uria protein/uria creatinine)*10, uria NAG/PNP method, blood/uria ¦Â2‐MG, thyroid gland function and relative antibody (FT3,FT4,TGA). 1. The changes of uria protein and NAG: After 4 and 8 weeks, uria protein and NAG of both groups decreased. There was statistical significance between two groups, showing that the decrease of intervention group was more obviousely than control group. And the decrease of PCI was in correlation of NAG; 2. The changes of blood/uria ¦Â2‐MG: ¦Â2‐MG of both groups decreased to normal level. After 4 weeks, both blood and uria ¦Â2‐MG of the intervention group decreased more obviousely than control group; 3. The changes of thyroid gland function: After 8 weeks, FT3, FT4 of both groups improved obviousely. | |
| Notes | 1. We telephone interviewed the pharmaceutical company and learned that the formulation of the Chinese medicine and the pills made by Chinese herb used in the intervention group was provided by the authors themselves, and the company provided processed raw material needed; 2. There was potential conflict of interest in the study. | |
Zhang 2003a.
| Methods | Parallel design. Due to the randomisation method wasn't mentioned in original article, we telephone interviewed the original author. The randomisation method was made clear that allocation sequence was generated by random number table. No blindness. | |
| Participants | 96 diagnosed patients were included. 52 cases were in the intervention group (M/F=11/41, aged between 18 and 66, 38.5+/‐3.6 years old on average; the length of disease within 0.5 year /within 10 years/longer than 10 years=38/11/3; first on set/recurrence after discontinuing drugs=42/10). 44 cases were in the control group (M/F=9/35, aged between 19 and 65, 40.5+/‐2.4 years old on average, with the length of disease between 2 months and 5 years, 3.5+/‐6.8 months on average; the length of disease within 0.5 year /within 10 years/longer than 10 years=32/10/2; first on set/recurrence after discontinuing drugs=36/8). Baseline of two groups were similar. | |
| Interventions | Tapazole was used in both groups, p.o., t.i.d., 10 mg each time, decreased the dosage to 5˜15 mg/day; Tapazole, Huangqi injection and Shengmai injection was given in the intervention group. The usage of Tapazole was similar to control 2 group. 20 ml/day Huangqi injection diluted in 250 ml 5% GNS infusion and 30 mL/day Shengmai injection diluted in 250 5% GNS was transfused through veins. | |
| Outcomes | 1. Clinical recovery: The symptoms subsided completely, body weight increased, sphygmus turned to be normal, thyroid gland function and the results of immunology test turned to be normal. (I/C=38 (73.1%) / 13 (29.5%) ) 2. Improvement: The symptoms were improved, sphygmus slowed down, thyroid gland function was almost normal. (I/C=11 (94.23%) / 20 (45.5%) ) 3. Inefficacy: The results didn't reach improvement criterion or discontinued the drugs because of adverse effects or accepted other treatment. (I/C=3 (5.7%) / 11 (25%) ) 4. Total effective rate: I/C=94.23%/75.00%. There was statistical significance between two groups, showing that the effect of intervention group was better than control group; 5. Comparison of changes of T3, T4, FT3, FT4 before and after the treatment: both groups decreased. There was statistical between two groups, showing that intervention group decreased more obviousely than control group; 6. Adverse effects: Decreasing of WBC, tetter and impairment of the liver function was observed in 9, 3 and 1 cases in the intervention group respectively. In the control group, no adverse effect was observed; 7. The dates for thyroid gland function turned to be normal: I/C=25.96+/‐3.84/30.77+/‐3.61. There was statistical significance between two groups, showing that the time needed of intervention group was shorter than control group. | |
| Notes | 1. The study was a local government support project. 2. Both Huangqi injection and Shengmai injection were made by pharmaceutic company. | |
Zhu 2005.
| Methods | Parallel design. Due to the randomisation method wasn't mentioned in original article, we telephone interviewed the original author. The randomisation method was made clear that allocation sequence was generated by random number table. No blindness. | |
| Participants | 121 cases that were diagnosed as hyperthyroidism were included. 62 cases were in the intervention group (M/F=15/47, aged between 12 and 53, 30 years old on average; with the length of disease between 3 months and 6 years, 1.2 years on average; ¢Ù all had the symptoms of sweating,dysphoria, palpitation, hard breath and emaciation; ¢Ú 41 cases had thyroid gland tremor; ¢Û The cases of¢ñ, ¢ò, ¢ó degree of thyroid gland enlargement=12/48/2; ¢Ü 53 cases were accompanied by sinus arrythmia, and ¢Ý 1 cases was atrial fibrillation; ¢Þ The concentration of TSH, T3, T4, FT3 and FT4 increased lightly in 11 cases, moderately in 31 cases and obviousely in 20 cases; ¢ß 20 cases' BMR>30%, 42 cases' BMR>60%.) 59 were in the control group (M/F=13/46, aged between 13 and 51, 29.5 years old on average; with lengths of disease between 4 months and 5 years, 1.3 years on average; ¢Ù all had the symptoms of sweating, dysphoria, palpitation, hard breath and emaciation; ¢Ú 40 cases had thyroid gland tremor; ¢Û The cases of¢ñ, ¢ò, ¢ó degree of thyroid gland enlargement=11/46/2; ¢Ü 48 cases were accompanied by sinus arrythmia, and ¢Ý 2 cases was atrial fibrillation; ¢Þ The concentration of TSH, T3, T4, FT3 and FT4 increased lightly in 10 cases, moderately in 30 cases and obviousely in 19 cases; ¢ß 21 cases' BMR>30%, 38 cases' BMR>60%. ) Baseline of two groups were similar. | |
| Interventions | Tapazole and Propranolol was used in the control group. Tapazole: 30 mg/d, t.i.d. The dosage was decreased to 5˜15 mg/d. For 1.5˜2 years. Propranolol:30 mg/d,t.i.d. and discontinued using it when HR<80/min; Besides the drugs listed above, Chinese medicine was used in the intervention group. 1 ampoule/d, decocting with water, and orally drank 200 ml in the morning and evening respectively. | |
| Outcomes | The outcomes were evaluated after 50 days. The patients were followed up for 1 year. 1. Clinical recovery: The symptoms subsided completely, body weight increased, the size of tyroid gland reduced by 30% or more, the concentration of thyroxin came to the normal level and gained the normal ECG and BMR. (I/C=9/5) 2. obviousely improvement: Symptoms were obviousely alleviated, body weight increased, sphygmus reduced by more than 20/min, the size of thyroid gland reduced by 20% or more, the concentration of thyroxin obviousely decreased and BMP<=30%. (I/C=32/13) 3. Improvement: The symptoms were improved, sphygmus slowed down by more than 10/min, the concentration of thyroxin decreased and BMR<=40%. (I/C=14/18) 4. Inefficacy: No change on the symptoms, the concentration of thyroxin and BMR stay the same. (7/23) 5. Total effective rate: I/C=88.6%/36.1%. There's statistical significance between two groups, showing that the effect of intervention group was better than the control group's. 6. Relapse Rate: After taking Chinese medicine and using tapazole alone, the symptoms recurred in once recovery and obviousely improving cases or the improving cases' weight gained and concentration of thyroxin increased. (I/C=7/13) There was statistical significance between two groups, showing that the recurrence of intervention group was lower than control group. | |
| Notes | 1. The formulation of Chinese medicine used in the intervention group was provided by the original authors; 2. There was potential conflict of interest in the study. | |
Abbreviations P.O.=per os (orally) B.i.d.=Eis in die (two times a day) T.i.d.=Ter in die (three time a day) Q.d.=Quaque die (one time a day) MBq = megabecquerel T3 = triiodothyronine T4 = thyroxine FT3 = free triiodothyronine FT4 = free thyroxine TT3 = total triiodothyronine TT4 = total thyroxine TSH = thyroid stimulating hormone TGA = anti‐thyroglobulin antibody TMA = anti‐thyroid‐microsome antibody ATD = anti‐thyroid drugs GNS = glucose and sodium chloride NAG = N‐acetyl‐¦Â‐D‐glucosaminidase BMR = basic metabolic rate WBC = white blood cell GD = grave's disease
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Du 2003 | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The patients were alternately allocated to the intervention and the control group by their visiting sequence. |
| Fei 2004 | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The allocation procedure was described as that the patients decided which treatment would be accepted by themselves. |
| Feng 1998 | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The patients were alternately allocated to the intervention and the control group by their visiting sequence. |
| Ge 2004 | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The trialist decided which remedy would be allocated to the patient in a non‐random way. |
| Hu 2005 | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. Chinese medicine was used by in‐patients and western medicine was used by out‐patients. |
| Huang 2002 | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The trialist decided which remedy would be allocated to the patient in a non‐random way. |
| Huang 2004 | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The patients were alternately allocated to the intervention and the control group by their visiting sequence. |
| Huang 2005 | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The patients were allocated to the intervention and control group by their registration sequence, depending on the even or odd number. |
| Li 2003a | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. Which remedy would be taken was decided by the patients. |
| Liang 2000 | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The patients were alternately allocated to the intervention and the control group by their visiting sequence. |
| Liao 1999 | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The trialist decided which remedy would be allocated to the patient in a non‐random way. |
| Liao 2000 | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The trialist decided which remedy would be allocated to the patient in a non‐random way. |
| Lv 1997 | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The patients were alternately allocated to the intervention and the control group by their visiting sequence. |
| Nie 1997 | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The patients were alternately allocated to the intervention and the control group by their visiting sequence. |
| Shi 2002 | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The patients were allocated to the intervention and control group by their registration number at libitum. |
| Sun 1999 | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The patients were alternately allocated to the intervention and the control group by their visiting sequence. |
| Tang 2003 | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The patients were alternately allocated to the intervention and the control group by their visiting sequence. |
| Wang 1999a | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The trialist decided which remedy would be allocated to the patient in a non‐random way. |
| Wang 2001a | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The patients were allocated to the intervention group and the control group by the method of self‐drawing lots. |
| Wang 2004a | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The patients were alternately allocated to the intervention and control group by their registration sequence. |
| Xiao 2004 | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. Which remedy would be taken was decided by the patients. |
| Xing 2003 | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The patients were alternately allocated to the intervention and the control group by their visiting sequence. |
| Xu 2003a | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The patients were alternately allocated to the intervention and the control group by their visiting sequence. |
| Yang 2004a | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The patients were allocated to the intervention and the control group by the date of visit. |
| Yao 1997 | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The patients were alternately allocated to the intervention and control group. |
| Yuan 2002 | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The trialist decided which remedy would be allocated to the patient in a non‐random way. |
| Zeng 1998 | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The patients were allocated to the intervention and the control group by the method of drawing lots. |
| Zhang 2000 | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The patients were alternately allocated to the intervention and the control group by their visiting sequence. |
| Zhang 2001 | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The patients were alternately allocated to the intervention and the control group by their visiting sequence. |
| Zhang 2002 | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The patients were alternately allocated to the intervention and the control group by their visiting sequence. |
| Zhang 2003b | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The trialist decided which remedy would be allocated to the patient in a non‐random way. |
| Zhang 2004a | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. Which remedy would be taken was decided by the patients. |
| Zhang 2005 | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The patients were allocated to the intervention and control group by their registration sequence depending on the even or odd number. |
| Zhao 2001 | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The patients were alternately allocated to the intervention and the control group by their visiting sequence. |
| Zhao 2003 | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The patients were alternately allocated to the intervention and the control group by the date on which they visited the doctor. |
| Zhao 2004a | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The patients were alternately allocated to the intervention and the control group by the date on which they visited the doctor. |
| Zhao 2004b | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The patients were alternately allocated to the intervention and the control group by the date on which they visited the doctor. |
| Zhao 2005 | Authors described their study as a randomised controlled trial. We interviewed the original author(s) by telephone to identify which method of randomisation was used. According to authors' description, we decided that this trial was of a non‐randomised nature. The patients were alternately allocated to the intervention and the control group by the date on which they visited the doctor. |
Contributions of authors
Han S and Wu TX contributed to develop the protocol Zen XX, Yuan Y and Liu Y interviewed the original authors of claimed randomised controlled trials and contributed to data extraction, quality assessment, data input and analysis Zen XX and Wu TX were responsible for developing the review
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Chen 2004 {published data only}
- Chen Y, Qiu L, Zhang CY, Zhang SS, Li WP. Combined treatment of Graves' disease with radioiodine and a Tratitional Chinese Medicine herb preparation Jiakangxin. Lin Chuang Hui Cui (Clinical Focus) 2004;19(18):1032,4. [Google Scholar]
Dai 2000 {published data only}
- Dai LY. Observation on Curative Effect of Treatment of TCM Combined with Western Medicien for Hyperthyroidism. Zhe Jiang Zhong Xi Yi Jie He Za Zhi (Zhejiang Journal of Integrated Traditional Chinese and Western Medicine) 2000;10(12):731. [PubMed] [Google Scholar]
Ding 2001a {published data only}
- Ding ML, Zhang RX. Observation on Curative Effect of Treatment of TCM plus Small Dosage of Radioactive Iodine 131 for Hyperthyroidism. Shi Yong Zhong Yi Za Zhi (Joural Of Practical Tranditional Chinese Medicine) 2001;17(6):27. [Google Scholar]
Ding 2001b {published data only}
- Ding YY, Zhang CX. Observation on Curative Effect of Treatment of TCM Combined with Western Medicine for Hyperthyroidism. Zhong Guo Zhong Yi Yao Xin Xi Za Zhi (Chinese Journal of Information on Traditional Chinese Medicine) 2001;8(4):66. [Google Scholar]
Ding 2005 {published data only}
- Ding P, Huang QY. Treatment on 44 Cases of Hyperthyroidism with Jiakang Mianyi Jiaonang. Zhong YI Yan Jiu (Research of Traditional Chineses Medicine) 2005;18(10):31. [Google Scholar]
Fang 2003 {published data only}
- Fang XM, Ye WC. Treatment on 41 Cases of Diffuse Toxic Goiter with Methimazole and Huangqi Injection Treatment on 41 Cases of Diffuse toxic goiter methimazole Diffuse toxic goiter. Herald of Medicine 2003;22(4):254. [Google Scholar]
Huang 2003 {published data only}
- Huang FQ. Clinical Observation on Treatment of Hyperthyroidism with TCM Combined with Western Medicine. Zhong Guo Di Fang Bing Fang Zhi Za Zhi (Chinese Journal of Control of Endemic Diseases) 2003;18(2):117. [Google Scholar]
Qiu 2003 {published data only}
- Qiu WY, Gu XW. An Analysis on Long‐Term Curative Effect of TCM Combined with Western Medicine for Hyperthyroidism. Xin Zhong Yi (New Chinese Medicine) 2003;35(5):43. [Google Scholar]
Yan 1999 {published data only}
- Yan H, Zhang SL, Jiang YS, Zhang DH. Treatment of Hyperthyroidism with Tapazole plus TCM. Qian Wei Yi Yao Za Zhi (Qianwei Journal OF Medicine & Pharmacy ) 1999;16(1):24. [Google Scholar]
Yu 2002a {published data only}
- Yu JY, Liu F. Clinical Research on Treatment of Grave's Disease Accompanied by Heptic Impairment with TCM Combined with Western Medicine. Jiang Su Zhong Yi Yao (Jiangsu Journal of Traditional Chinese Medicine) 2002;23(11):13. [Google Scholar]
Yu 2002b {published data only}
- Yu JY, Liu F. Clinical Research on Treatment of Grave's Disease Accompanied by Proteinuria with TCM Combined with Western Medicine. Zhong Guo Zhong Xi Yi Jie He Shen Bing Za Zhi (Chinese Journal of Integrated Traditional and Western Nephrology) 2003;4(7):416. [Google Scholar]
Zhang 2003a {published data only}
- Zhang HW. Treatment of 52 Cases of Hyperthyroidism with Traditional Chinese Medicine Combined with Western Medicine. Nanjing Zhong Yi Yao Da Xue Xue Bao(Joural of Nanjing TCM University) 2003;19(6):369. [Google Scholar]
Zhu 2005 {published data only}
- Zhu XN, Cao JH, Ou XM. Treatment on 62 Cases of Hyperthyroidism with Jiakangxiao. Shan XI Zhong Yi (Shanxi Journal of Traditional Chinese Medicine) 2005;26(9):926. [Google Scholar]
References to studies excluded from this review
Du 2003 {published data only}
- Du YM, Sui SL, Ren XY. Clinical Observation on 31 Cases of Adolesent Hyperthyrodism Treated with Combination of Chinese and Western Medicine. Zhong Guo Di Fang Bing Fang Zhi Za Zhi (Chinese Journal Control of Endemic Diseases) 2003;18(4):251. [Google Scholar]
Fei 2004 {published data only}
- Fei DD. Treatment on 35 Cases of Ophthalmopathy Caused by Hyperthyroidism with Traditional Chinese Medicine Combined with Western Medicine. Shan Dong Zhong Yi Za Zhi (Shandong Journal of Traditional Chinese Medicine) 2004;23(8):485. [Google Scholar]
Feng 1998 {published data only}
- Feng LH, Li WQ, Xiong YX, Jia JX, Li AL. Clinical Practical Research on Treatment of Hyperthyroidism with Jiakangshu plus Radioactive Iodine 131. Guang Xi Yi Xue (Guangxi Medical Journal) 1998;20(4):534. [Google Scholar]
Ge 2004 {published data only}
- Ge AH, Zhang QX. Observation on Curative Effect of Treatment for Hyperthyroidism with TCM Combined with Western Medicine. Si Chuan Zhong Yi (Journal of Sichuan of Traditional Chinese Medicine) 2004;22(3):51. [Google Scholar]
Hu 2005 {published data only}
- Hu XF. Treatment of Hyperthyroidism with Radioactive Iodine 131 Combined with TCM. Si Chan Zhong Yi (Journal of Sichuan Traditional Chinese Medicine) 2005;23(11):60. [Google Scholar]
Huang 2002 {published data only}
- Huang YM, Chen ZL. Effect of Jiazhongxiao on Iterleukin in Patients with Graves Disease. Zhong Guo Zhong Xi Yi Jie He Za Zhi (Chinese Journal of Integrated Traditional and Western Medicine) 2002;22(10):738. [Google Scholar]
Huang 2004 {published data only}
- Huang J. Chang XR, Wang C, Chen T, Zhao GW. Treatment on 36 Cases of Hyperthyroidism with Catgut Implantation at Acupoint plus Jiakangning Tang. Hu Nan Zhong Yi Za Zhi (Journal Of Hu Nan Traditional Chinese Medicine) 2004;20(2):28. [Google Scholar]
Huang 2005 {published data only}
- Huang JL. Clinical Observation on treatment of 30 cases of hyperthyroidism with Huangying Tang. Zhong Guo Zhong Yi Yao Ke Ji (Chinese Journal of Traditional Medical Science and Technology) 2005;12(4):242. [Google Scholar]
Li 2003a {published data only}
- Li FH. Observation of Curative Effect on 30 cases of Grave's Disease with TCM combined with Western Medicine. Xian Dai Zhong Xi Yi Jie He Za Zhi (Modern Journal of Integrated Traditional Chinese And West Medicine) 2003;12(1):31. [Google Scholar]
Liang 2000 {published data only}
- Liang JG, Jiang NY, Lu B, Lu XP, Liu S. Clinical Observation on Treatment of Jiakangshu Combined with Radioactive Iodine 131 for Hyperthyroidism. Zhong Guo Zhong Xi Yi Jie He Za Zhi (Chinese Journal of Integrated Traditional and Western Medicine) 2000;20(10):774. [Google Scholar]
Liao 1999 {published data only}
- Liao SH, Huang YM, Lin CS. Clinical Research on Treatment of Jiakangxiao for Hyperthyroidism. Liao Ning Zhong Yi Za Zhi (Liaoning Journal of Traditional Chinese Medicine) 1999;26(9):416. [Google Scholar]
Liao 2000 {published data only}
- Liao SH, Huang YM, Li LX, Du QH, Liu QP. Clinical Observation on treatment of Jiayanxiao Combined with Tapazole for Hyperthyroidism. Zhong Guo Zhong Xi Yi Jie He Za Zhi (Chinese Journal of Integrated Traditional and Western Medicine) 2000;20(6):433. [PubMed] [Google Scholar]
Lv 1997 {published data only}
- Lv WM. Clinical Observation of Agranulocytosis Caused by Thiourea Homologues Treated with TCM Combined with Western Medicine on 30 Cases of Hyperthyroidism. Gan Nan Yi Xue Yuan Xue Bao (Journal of Gannan Medical College) 1997;17(4):294. [Google Scholar]
Nie 1997 {published data only}
- Nei YZ, Zhao YM, Li ST. Observation of Curative Effect on Treatment of TCM combined with Western Medicine for Hyperthyroidism. Zhong Guo Yi Kan (Chinese Journal of Medicine) 1997;32(8):61. [Google Scholar]
Shi 2002 {published data only}
- Shi KW, Lin ZZ. Treatment of 40 Cases of Hyperthyroidism with Xuejie Combined with Tapazole. Fu Jian Yi Yao Za Zhi (Fujian Medical Journal) 2002;24(3):56. [Google Scholar]
Sun 1999 {published data only}
- Sun P. Treatment of 30 Cases of Grave's Disease with Agranulocytosis with Yiqishengbai Tang. Shan Xi Journal of Traditional Chinese Medicine 1999;15(4):28. [Google Scholar]
Tang 2003 {published data only}
- Tang L, Liao ZQ, Su K. Influence of Tripterygium Glycosides on Thyroid Gland Function and Autoantibody of Grave's Disease. Zhong Guo Zhong Xi Yi Jie He Za Zhi (Chinese Journal of Integrated Traditional and Western Medicine) 2003;23(4):294. [PubMed] [Google Scholar]
Wang 1999a {published data only}
- Wang J, Wang ZL, Du H, Zhao M, Feng GB, Xu RJ. Comparision on Curative Effect of Treatment for Severe Hyperthyroidism with Tripterygium Wilfordii and Glucocorticoid. Yi Xue Yan Jiu Sheng Bao (Journal Of Medical Postgraduate) 1999;12(4):218. [Google Scholar]
Wang 2001a {published data only}
- Wang S. Clinical Observation on Treatment of Hyperthyroidism Combined with Agranulocytosis with Shenqi Pian. He Bei Zhong Yi (Hebei J TCM) 2001;23(1):61. [Google Scholar]
Wang 2004a {published data only}
- Wang YG, Wang W, Zhao WJ. Randomised Double Blinded Paralled Observation with Placebo on the effect of Diyushengbai Pian to Elevating WBC Level in Antithyroid Therapy for Patients with Grave's Disease. Zhong Guo Zhong Yi Yao Xin Xi Za Zhi (Chinese Journal of Information on Traditional Chinese Medicine) 2004;11(2):158. [Google Scholar]
Xiao 2004 {published data only}
- Xiao L, Su B, He J, Zhou H. Treatment of 40 Cases of Refractory Hyperthyroidism with TCM Combined with Western Medicine. Ji Lin Zhong Yi Yao (Journal of Traditional Chinese Medicine and Chinese Materia Medica of Jilin) 2004;24(9):44. [Google Scholar]
Xing 2003 {published data only}
- Xing YX. Treatment of 41 Cases of Hyperthyroidism with TCM plus Tapazole. Shan Dong Yi Yao ( Shandong Medical Journal) 2003;43(4):72. [Google Scholar]
Xu 2003a {published data only}
- Xu ZB, Sun SF. Clinical Observation On Treatment Of Graves Disease With Yi Kang Wan plus Propylthiouracil. Zhong Guo Di Fang Bing Fang Zhi Za Zhi (Chinese Journal of Control of Endemic Disease) 2003;18(1):56. [Google Scholar]
Yang 2004a {published data only}
- Yang SJ, Fu WL. Clinical Research for self‐prepared Jiakangling Mixture in the treatment of 40 cases of Hyperthyroidism. Journal of Medicine & Pharmacy of Chinese Minorities 2004;S1:77‐8. [Google Scholar]
Yao 1997 {published data only}
- Yao DG, Zhu XH. Comparison of Curative Effect between Lithium Salt and TCM in Adjunctive Therapy for Hyperthyroidism. Zhe Jiang Zhong Xi Yi Jie He Za Zhi (Zhejiang Journal of Integrated Traditional Chinese and Western Medicine) 1997;7(2):79. [Google Scholar]
Yuan 2002 {published data only}
- Yuan Q. Clinical Observation on 53 Cases of Hyperthyroidism with TCM Combined with Western Medicine. Shi Zhen Guo YI Guo Yao (Lishizhen Medicine and Materia Medica Research) 2002;13(7):440. [Google Scholar]
Zeng 1998 {published data only}
- Zeng SF. Tripterygium Glycosides combined with Tapazole in the treatment of 89 cases of Grave's disease. Yi Xue Li Lun Yu Shi Jian (The Journal of Medical Therory and Practice ) 1998;11(11):502‐3. [Google Scholar]
Zhang 2000 {published data only}
- Zhang YX. Treatment on 34 Cases of Hyperthyroidism with Yiqiyangyin Huatanjiesan Tang. Zhong Guo Zhong Yi Yao Xin Xi Za Zhi (Chinese Journal of Information on Traditional Chinese Medicine) 2000;7(6):58. [Google Scholar]
Zhang 2001 {published data only}
- Zhang LP, Zhao P, Liao SH. Observation of Ultrasound on Treatment for Hyperthyroidism with Jiakangling Jiaonang. Guang Zhou Zhong Yi Yao Da Xue Xue Bao (Journal of Guang Zhou University of Traditional Chinese Medicine) 2001;18(3):211. [Google Scholar]
Zhang 2002 {published data only}
- Zhang MS. Clinical Observation on Treatment of 129 Cases of Grave's Disease with TCM Combined with Wetern Medicine. Shan Xi Zhong Yi (SHANXI J OF TCM) 2002;18(2):36. [Google Scholar]
Zhang 2003b {published data only}
- Zhang XJ. Observation on Curative Effect of Treatment for Adult Grave's Disease with TCM Combined with Western Medicine. Chong Qing Yi Xue (Chongqing Medical Journal ) 2003;32(1):98. [Google Scholar]
Zhang 2004a {published data only}
- Zhang LQ, Cao S, Chen LL, Liu XJ, Huang QL. Clinical Observation On hyerthyroidism Complex Treated With TCM and Western Medicine. Hei Long Jiang Yi Xue (HEI LONG JIANG MEDICAL JOURNAL) 2004;28(7):491. [Google Scholar]
Zhang 2005 {published data only}
- Zhang J, Lu B, Zhuang QZ. Treatment on 60 Cases of Hyperthyroidism with Shenqi Wuweizi Pan Combined with Tapazole. Shan Dong Zhong Yi Za Zhi (Shandong Journal of Traditional Chinese Medicine) 2005;24(3):161. [Google Scholar]
Zhao 2001 {published data only}
- Zhao P, Zhang LP, Liao SH. color Doppler Observation of Color Doppler on Treatment for Hyperthyroidism with Jiakangling Jiaonang. Xian Dai Zhong Xi Yi Jie He Za Zhi (Modern Journal of Integrated Traditional Chinese and Western Medicine) 2001;19(10):1815. [Google Scholar]
Zhao 2003 {published data only}
- Zhao LM. Observation on Curative Effect of Treatment with Acupuncture Combined with Medicine for Hyperthyroid Ophthalmopathy. Liao Ning Zhong Yi Za Zhi (Liaoning Journal of Traditional Chinese Medicine) 2003;30(10):848. [Google Scholar]
Zhao 2004a {published data only}
- Zhao LM. Treatment on 155 Cases of Hyperthyroid Cardiac Disease with Acupuncture Combined with Medicine. Zhen Jiu Lin Chuang Za Zhi (Journal of Clinical Acupuncture and Moxibustion) 2004;20(8):32. [Google Scholar]
Zhao 2004b {published data only}
- Zhao LM. Observation on Curative Effect of Treatment with Acupuncture Combined with Medicine for Hyperthyroidism. Liao Ning Zhong Yi Za Zhi (Liaoning Journal of Traditional Chinese Medicine) 2004;31(7):572. [Google Scholar]
Zhao 2005 {published data only}
- Zhao LM. Treatment for Chronic Hyperthyroidism Myopathy with Acupuncture Combined with Medicine. Zhen Jiu Lin Chuang Za Zhi (Journal of Clinical Acupuncture and Moxibustion) 2005;21(7):30. [Google Scholar]
References to studies awaiting assessment
Cai 2004 {published data only}
Cha 1997 {published data only}
Chang 2005 {published data only}
Chen 1998 {published data only}
Chen 1999 {published data only}
Cheng 2003 {published data only}
Diao 2002 {published data only}
Fang 2002 {published data only}
Fu 2001 {published data only}
Guo 2000 {published data only}
Guo 2002 {published data only}
Hua 2005 {published data only}
Li 2002 {published data only}
Li 2003b {published data only}
Li 2003c {published data only}
Lin 1999 {published data only}
Liu 1998 {published data only}
Liu 1999 {published data only}
Liu 2000 {published data only}
Liu 2003 {published data only}
Liu 2005 {published data only}
Long 2001 {published data only}
Men 1997 {published data only}
Niu 1999 {published data only}
Ouyang 2003 {published data only}
Qin 2000 {published data only}
Ren 2002 {published data only}
Sun 2004a {published data only}
Sun 2004b {published data only}
Tang 2005 {published data only}
Tao 2005 {published data only}
Tian 2002 {published data only}
Wang 1999 {published data only}
Wang 2000 {published data only}
Wang 2001b {published data only}
Wang 2003a {published data only}
Wang 2003b {published data only}
Wang 2004b {published data only}
Wang 2004c {published data only}
Wu 2000 {published data only}
Wu 2004 {published data only}
Xiong 2003 {published data only}
Xu 2003b {published data only}
Yang 1997 {published data only}
Yang 2004b {published data only}
Yang 2005 {published data only}
Yin 1999 {published data only}
Zhai 1999 {published data only}
Zhang 2004 {published data only}
Zheng 2003 {published data only}
Zheng 2005 {published data only}
Zhu 2001 {published data only}
Additional references
Brown 2002
- Todd T. Brown. Hyperthyroidism. http://medlineplus.gov/ 2002.
Cheetham 1998
- T D Cheetham. Treatment of hyperthyroidism in young people. Archives of Disease in Childhood 1998;78(3):207, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 1998
- David S Cooper. Editorial: Radioiodine for hyperthyroidism: Where do we stand after 50 years?. JAMA 1998;280(4):375,2. [DOI] [PubMed] [Google Scholar]
Cooper 2003
- David S Cooper. Hyperthyroidism. Lancet 2003;362:459‐68. [DOI] [PubMed] [Google Scholar]
Farling 2000
- P. A. Farling. Thyroid disease. British Journal of Anaesthesia 2000;85(1):14‐15. [DOI] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. In: The Cochrane Library, Issue 3, 2005. Chichester, UK: John Wiley & Sons, Ltd.
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
John 1997
- Lazarus JH. Hyperthyroidism. The Lancet 1997;349(9048):339‐44. [DOI] [PubMed] [Google Scholar]
Juni 2001
- Juni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. British Medical Journal 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Lampert 2002
- Lampert N, Xu Y. Chinese herbal nephropathy. Lancet 2002;359(9308):796‐7. [DOI] [PubMed] [Google Scholar]
Li 1998
- Li MD. Progress of reserach in Chinese medicine for hyperthyroidism. Journal of Chinese Medicine Information 1998;5(10):15‐16. [Google Scholar]
Lord 2001
- Lord GM, Cook T, Arlt VM, Schmeiser HH, Williams G, Pusey CD. Urothelial malignant disease and Chinese herbal nephropathy. Lancet 2001;358(9292):1515‐6. [DOI] [PubMed] [Google Scholar]
McKenna 2001
- T Joseph McKenna. Graves' disease. The Lancet 2001;357(9270):1793, 4. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad A, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analysis. The Lancet 1998;352:609‐13. [DOI] [PubMed] [Google Scholar]
Moher 1999
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta‐analyses. Lancet 1999;354(9193):1896‐900. [DOI] [PubMed] [Google Scholar]
Nortier 2000
- Nortier JL, Martinez MC, Schmeiser HH, Arlt VM, Bieler CA, Petein M, et al. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi) Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi). NEJM 2000;342(23):1686‐92. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Journal of the American Medical Association 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Toft 2000
- A D Toft. Thyroid disease and the heart. Heart 2000;84(4):455‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weetmen 2000
- Anthony P Weetmen. Graves' disease. The New England Journal of Medicine. 2000;343(17):1236‐13. [DOI] [PubMed] [Google Scholar]
Yao 1998
- Yao J. General Situation of Chinese Herbs for Hyperthyroidism. Journal of Chinese Medicine Information 1998;5(9):17‐19. [Google Scholar]


