Abstract
Background
Pain is the most common symptom in the emergency setting; however, timely management of acute pain in children continues to be suboptimal. Intranasal drug delivery has emerged as an alternative method of achieving quicker drug delivery without adding to the distress of a child by inserting an intravenous cannula.
Objectives
We identified and evaluated all randomized controlled trials (RCTs) and quasi‐randomized trials to assess the effects of intranasal fentanyl (INF) versus alternative analgesic interventions in children with acute pain, with respect to reduction in pain score, occurrence of adverse events, patient tolerability, use of "rescue analgesia," patient/parental satisfaction and patient mortality.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 1); MEDLINE (Ovid SP, from 1995 to January 2014); EMBASE (Ovid SP, from 1995 to January 2014); the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO Host, from 1995 to January 2014); the Latin American and Caribbean Health Science Information Database (LILACS) (BIREME, from 1995 to January 2014); Commonwealth Agricultural Bureaux (CAB) Abstracts (from 1995 to January 2014); the Institute for Scientific Information (ISI) Web of Science (from 1995 to January 2014); BIOSIS Previews (from 1995 to January 2014); the China National Knowledge Infrastructure (CNKI) (from 1995 to January 2014); International Standard Randomized Controlled Trial Number (ISRCTN) (from 1995 to January 2014); ClinicalTrials.gov (from 1995 to January 2014); and the International Clinical Trials Registry Platform (ICTRP) (to January 2014).
Selection criteria
We included RCTs comparing INF versus any other pharmacological/non‐pharmacological intervention for the treatment of children in acute pain (aged < 18 years).
Data collection and analysis
Two independent review authors assessed each title and abstract for relevance. Full copies of all studies that met the inclusion criteria were retrieved for further assessment. Mean difference (MD), odds ratio (OR) and 95% confidence interval (CI) were used to measure effect sizes. Two review authors independently assessed and rated the methodological quality of each trial using the tool of The Cochrane Collaboration to assess risk of bias, as per Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions.
Main results
Three studies (313 participants) met the inclusion criteria. One study compared INF versus intramuscular morphine (IMM); another study compared INF versus intravenous morphine (IVM); and another study compared standard concentration INF (SINF) versus high concentration INF (HINF). All three studies reported a reduction in pain score following INF administration. INF produced a greater reduction in pain score at 10 minutes post administration when compared with IMM (INF group pain score: 1/5 vs IMM group pain score: 2/5; P value 0.014). No other statistically significant differences in pain scores were reported at any other time point. When INF was compared with IVM and HINF, no statistically significant differences in pain scores were noted between treatment arms, before analgesia or at 5, 10, 20 and 30 minutes post analgesia. Specifically, when INF was compared with IVM, both agents were seen to produce a statistically significant reduction in pain score up to 20 minutes post analgesia. No further reduction in pain score was noted after this time. When SINF was compared with HINF, a statistically and clinically significant reduction in pain scores over study time was observed (median decrease for both groups 40 mm, P value 0.000). No adverse events (e.g. opiate toxicity, death) were reported in any study following INF administration. One study described better patient tolerance to INF compared with IMM, which achieved statistical significance. The other studies described reports of a “bad taste” and vomiting with INF. Overall the risk of bias in all studies was considered low.
Authors' conclusions
INF may be an effective analgesic for the treatment of patients with acute moderate to severe pain, and its administration appears to cause minimal distress to children. However, this review of published studies does not allow any definitive conclusions regarding whether INF is superior, non‐inferior or equivalent to intramuscular or intravenous morphine. Limitations of this review include the following: few eligible studies for inclusion (three); no study examined the use of INF in children younger than three years of age; no study included children with pain from a "medical" cause (e.g. abdominal pain seen in appendicitis); and all eligible studies were conducted in Australia. Consequently, the findings may not be generalizable to other healthcare settings, to children younger than three years of age and to those with pain from a "medical" cause.
Keywords: Child; Humans; Acute Pain; Acute Pain/drug therapy; Administration, Intranasal; Analgesics, Opioid; Analgesics, Opioid/administration & dosage; Fentanyl; Fentanyl/administration & dosage; Morphine; Morphine/administration & dosage; Pain Measurement; Randomized Controlled Trials as Topic
Plain language summary
Intranasal fentanyl for the treatment of children in acute pain
Background
Pain is the most common reason why patients are seen in emergency departments (EDs). The challenging nature of treating children in acute severe pain is reflected in the medical literature by poor pain management in this population. We reviewed evidence on the effect of intranasal fentanyl (INF) (a strong pain relief drug, similar to morphine) compared with any other pain‐relieving technique for treatment of children in acute severe pain.
Study characteristics
We included studies with children (younger than 18 years of age) suffering from acute severe pain as a result of injury or medical illness. The target intervention was INF administered for pain relief compared with any other drug intervention for pain relief (e.g. intravenous morphine) or non‐drug intervention (e.g. limb splinting, wound dressing) provided in the emergency setting. The evidence is current to January 2014.
Key results
We identified three studies that included 313 children with acute severe pain resulting from broken bones of the upper and lower limbs. These trials compared INF versus morphine administered by a needle into a muscle (intramuscular morphine) or via a drip into a vein (intravenous morphine), as well as standard concentration INF versus high concentration INF. The collective study population in these trials consisted of children three to 15 years of age. Males accounted for approximately two‐thirds of the overall study population. The review concluded that INF may be an effective analgesic for the treatment of children in acute moderate to severe pain, and its administration appears to cause minimal distress to children; however, the evidence is insufficient to permit judgement of the effects of INF compared with intramuscular or intravenous morphine. No serious adverse events (e.g. opiate toxicity, death) were reported.
Limitations
Limitations of this review include the following: Few studies (three) were eligible for inclusion; no study examined the use of INF in children younger than three years of age; no study included children with pain resulting from a "medical" cause (e.g. abdominal pain seen in appendicitis); and all eligible studies were conducted in Australia. Consequently, the findings may not be generalizable to other healthcare settings, to children younger than three years of age and to those with pain from a "medical" cause.
Summary of findings
for the main comparison.
| Intranasal fentanyl compared with intravenous morphine for the management of acute moderate to severe pain in children | ||||||
|
Patient or population: children (aged < 18 years) with acute severe pain Settings: emergency department Intervention: intranasal fentanyl Comparison: intravenous morphine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous morphine | Intranasal fentanyl | |||||
|
Pain reduction (mean VAS) Pain assessed before analgesia (0 min) and at 5, 10, 20 and 30 min after analgesia |
0 min = 67 5 min = 42 10 min = 41 20 min = 35 30 min = 33 |
0 min = 68 5 min = 55 10 min = 46 20 min = 37 30 min = 37 |
67 participants (1 study) |
⊕⊕⊕⊕ high | Given no statistically significant difference between treatment arms, VAS scores were combined to form an overall VAS score for each time point. Combined VAS scores produced statistically significant reductions in pain at 5, 10 and 20 min after analgesia | |
| Respiratory depression | No cases were reported in this study | No cases were reported in this study | 67 participants (1 study) |
⊕⊕⊕ moderate | Dosage regimen for this study was calculated for 3 weight intervals. Inclusion of 21 children outside the weight intervals (1 less than 20 kg and 20 greater than 50 kg) may have resulted in most of these children receiving smaller per‐kilogram doses of IV morphine and INF, thereby reducing the potential occurrence of adverse events listed | |
| Hypotension | No cases were reported in this study | No cases were reported in this study | 67 participants (1 study) |
⊕⊕⊕ moderate | Dosage regimen for this study was calculated for 3 weight intervals. Inclusion of 21 children outside the weight intervals (1 less than 20 kg and 20 greater than 50 kg) may have resulted in most of these children receiving smaller per‐kilogram doses of IV morphine and INF, thereby reducing the potential occurrence of adverse events listed | |
| Decreased level of consciousness | No cases were reported in this study | No cases were reported in this study | 67 participants (1 study) |
⊕⊕⊕ moderate | Dosage regimen for this study was calculated for 3 weight intervals. Inclusion of 21 children outside the weight intervals (1 less than 20 kg and 20 greater than 50 kg) may have resulted in most of these children receiving smaller per‐kilogram doses of IV morphine and INF, thereby reducing the potential occurrence of adverse events listed | |
| Intolerance to analgesia | 1 participant complained of a momentary flush at the IV site following administration of morphine | 4 participants; 3 participants reported a "bad taste" following INF administration, 1 participant vomited 20 min following INF administration | 67 participants (1 study) |
⊕⊕⊕⊕ high | ||
| Use of ED "rescue" analgesia | 1 participant required 5 additional doses of IV morphine (protocol violation) | 1 participant required 6 additional doses of INF (protocol violation) | 67 participants (1 study) |
⊕⊕⊕ moderate | Protocol violation in control and intervention arms of this trial. As per protocol, participants should receive only 4 additional doses of either agent | |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
ED: Emergency department.
IV: Intravenous.
INF: Intranasal fentanyl.
VAS: Visual analogue scale.
Background
Description of the condition
Pain is the most common presenting symptom in the emergency setting and remains a challenging clinical problem for healthcare providers in both prehospital and emergency department (ED) environments (Alonso‐Serra 2003; Cordell 2002; Groenewald 2012; Paris 1988; Verghese 2010). Timely management of pain in children in the emergency care setting continues to be suboptimal (Rupp 2004; Wilsey 2004; Wilson 1989). Some studies have identified a significant disparity in the assessment and management of acute pain between adults and children, with adults twice as likely as children to receive appropriate analgesia for similar pain scores (Hennes 2005; Schechter 1989). Pain in the very young or in those with neurodevelopmental or cognitive delay has been associated with the worst pain management in this setting (Friedland 1994; Izsak 2008), and evidence shows that more than one‐third of children attending the ED via ambulance report acute pain as a chief complaint (Galinski 2011).
Description of the intervention
Management of acute pain in children in the emergency setting involves both pharmacological and non‐pharmacological interventions (Berde 2002; Kart 1997; Probst 2005; MacLean 2007). Examples of non‐pharmacological interventions to relieve pain in children include verbal reassurance, distraction techniques, wound dressings and splinting of fractures. Pharmacological agents may be administered by the oral, inhalational (e.g. nitrous oxide) or parenteral route. Commonly used oral analgesics include paracetamol (acetaminophen), non‐steroidal anti‐inflammatory drugs (NSAIDs) (e.g. ibuprofen) and opioids (e.g. codeine, morphine). Parenterally administered analgesia (e.g. morphine) is indicated for acute moderate to severe pain.
Although intravenous morphine traditionally has been considered the 'gold standard' analgesic for moderate to severe pain, the skills required to establish vascular access in children, in particular in the prehospital setting, are not universally available. Furthermore, insertion of an intravenous line invariably adds to the distress of most children. Intranasal fentanyl (INF) is increasingly employed as an acceptable alternative to intravenous morphine for the management of moderate or severe acute pain in children in prehospital, primary care and ED settings (Bendall 2011; Borland 2007; Borland 2008; Saunders 2010). The easily accessible rich vascular plexus of the nasal mucosa is an attractive route for drug delivery because it facilitates rapid drug absorption into the systemic circulation (by avoiding gastrointestinal degradation and hepatic first pass metabolism), resulting in an onset of action that compares favourably with intravenously administered analgesics. INF has a bioavailability of 89% with a short onset of action (˜7 minutes) (Panagiotou 2010). Duration of effect is directly related to INF dose, with pain scores returning to predose values at approximately 120 to 200 minutes after a single dose (Foster 2008). Pragmatically the intranasal route of administration is quicker than the intravenous route for all types of drug administration when the time required to insert an intravenous cannula is considered.
How the intervention might work
INF may offer emergency healthcare providers an acceptable alternative to intravenous opiates for achieving earlier effective pain relief for the child in pain, obviating the need for an intravenous cannula.
Why it is important to do this review
The intranasal route of analgesic administration offers several advantages over the intravenous route in the emergency care setting. These include reducing possible distress to the child, minimizing the risk of needle‐stick injuries, reducing staff training needed to undertake the procedure, providing a faster method of drug delivery and providing more rapid drug absorption than is achieved by the intravenous route.
Objectives
We identified and evaluated all randomized controlled trials (RCTs) and quasi‐randomized trials to assess the effects of INF versus alternative analgesic interventions in children with acute pain, with respect to reduction in pain score, occurrence of adverse events, patient tolerability, use of "rescue analgesia," patient/parental satisfaction and patient mortality.
Methods
Criteria for considering studies for this review
Types of studies
We included all RCTs and quasi‐randomized trials, with an RCT defined as a study in which participants were allocated to treatment groups on the basis of a random method (e.g. using random number tables, hospital number, date of birth).
Types of participants
We included children (< 18 years of age) with acute moderate to severe pain caused by injury (e.g. burns, wounds, suspected fractures) or medical illness.
We excluded from the review patients who received INF for the preemptive treatment of pain (i.e. patients who received INF as part of procedural sedation in the emergency setting). We also excluded children younger than three months of age because of opiate sensitivity.
Types of interventions
The target intervention was INF administered (via droplet, atomizer or spray) for pain relief in children with painful clinical conditions. INF concentration was noted. This treatment was compared with the following interventions.
Administration of other pharmacological interventions for pain relief (e.g. intravenous morphine) as an active control (including 'double‐dummy' study designs).
Non‐pharmacological intervention for pain relief (e.g. limb splinting).
Placebo administration.
Types of outcome measures
Primary outcomes
Reduction in pain score or intensity assessed by validated age‐appropriate pain scores (e.g. 'Faces, Legs, Activity, Cry, Consolability' (FLACC); Wong Baker; numerical rating scale (NRS); visual analogue scale (VAS)).
We sought outcome assessments at multiple time points post INF administration, if reported. We also sought to identify reductions in reported pain in terms of reductions across the spectrum of pain, that is, mild, moderate or severe pain, and no pain, such as reductions in pain from severe to moderate, or reductions in pain from pain to no pain (VAS < 2), as reported by study authors.
Secondary outcomes
Occurrence of all adverse events associated with INF (e.g. opiate toxicity).
Participant tolerance of INF including the incidence of nausea, vomiting or reported discomfort.
Use of 'rescue' analgesia before and after hospital arrival.
Participant satisfaction as defined by study authors.
Parental satisfaction as defined by study authors.
Cost as defined by study authors.
Mortality.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 1; see Appendix 1); MEDLINE (Ovid SP, from 1995 to January 2014; see Appendix 2); EMBASE (Ovid SP, from 1995 to January 2014; see Appendix 3); the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO Host, from 1995 to January 2014; see Appendix 4); Commonwealth Agricultural Bureaux (CAB) Abstracts (from 1995 to January 2014); the Institute for Scientific Information (ISI) Web of Science (from 1995 to January 2014; see Appendix 5); the Latin American and Caribbean Health Science Information Database (LILACS) (BIREME, from 1995 to January 2014; see Appendix 6); BIOSIS Previews (from 1995 to January 2014); the China National Knowledge Infrastructure (CNKI) (from 1995 to January 2014); International Standard Randomized Controlled Trial Number (ISRCTN) (from 1995 to January 2014); ClinicalTrials.gov (from 1995 to January 2014); and the International Clinical Trials Registry Platform (ICTRP) (to January 2014).
We identified published, unpublished and ongoing studies by searching these databases from 1995, as we understand no studies were conducted on the use of INF in children before this date. We modelled subject strategies for databases on the search strategy designed for MEDLINE (see Appendix 2). When appropriate, we combined subject strategies with adaptations of the highly sensitive search strategy designed by The Cochrane Collaboration for identifying randomized controlled trials and controlled clinical trials, as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2, Box 6.4.b (Higgins 2011).
We imposed no language restrictions.
Searching other resources
We searched the reference lists of review articles and relevant trials, textbooks and abstracts of scientific meetings to identify further RCTs. We reviewed the titles and abstracts to identify all potentially eligible RCTs. We obtained the full‐text versions of these articles. We made additional efforts to identify RCTs potentially relevant to the topic by using the following data sources.
Foreign language literature.
Grey literature (theses, internal reports, non–peer‐reviewed journals).
References (and references of references) cited in primary sources.
Other unpublished sources known to experts in the speciality (to be sought by personal communication).
Raw data from published trials (sought by personal communication).
Data collection and analysis
Selection of studies
Two independent review authors (JC and MB) assessed each title and abstract for relevance. Disagreements on eligibility were resolved by discussion or by referral to a third party (ROS). If no abstract was available, we obtained and assessed the full paper. We obtained the full copies of all studies that met the inclusion criteria for further assessment.
Data extraction and management
Two review authors (JH and SMC) independently extracted the data; two separate review authors (JC and NK) entered the data into Review Manager software (RevMan 5.2) for statistical analysis (see Appendix 7).
Assessment of risk of bias in included studies
Two review authors (JC and MB) independently undertook assessment of risk of bias of the included trials. They took the following into consideration, guided by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Random sequence generation.
Allocation concealment.
Blinding of personnel, participants and outcome assessment.
Incomplete outcome data.
Selective reporting.
Other bias.
We used the Cochrane risk of bias tool in RevMan 5.2, which involves describing each of the domains as reported in the trial and then assigning a judgement about the adequacy of each entry (low risk of bias, high risk of bias or unclear (or unknown) risk of bias).
We considered a trial as having low risk of bias when all domains were assessed as adequate. We considered a trial as having high risk of bias when one or more domains were assessed as inadequate or unclear.
We included the 'Risk of bias' table as part of the Characteristics of included studies section and present a 'Risk of bias summary' figure, which details all judgements made for all studies included in the review.
Measures of treatment effect
When the measure of the outcome was sufficiently consistent across trials, we used odds ratios (ORs) for dichotomous data and mean differences (MDs) for continuous data with corresponding 95% confidence intervals (CIs).
Unit of analysis issues
The unit of analysis is based on the individual participant (the unit to be randomly assigned for interventions to be compared). For included trials using a cross‐over design, we used only pre‐cross‐over data.
Dealing with missing data
When data seemed to be missing from a study, they were obtained, if possible, through correspondence with study authors. By using sensitivity analysis, we explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect. For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, that is, we attempted to include in the analyses all participants randomly assigned to each group and to analyse all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomly assigned minus the number of participants whose outcomes were known to be missing.
Assessment of heterogeneity
We evaluated clinical heterogeneity (differences between studies in key characteristics of participants, interventions or outcome measures) (Deeks 2001). In the absence of clinical heterogeneity, we used the I2 statistic to describe the percentage of total variation across studies that is due to heterogeneity rather than to chance. We considered an I2 value > 50% to represent significant statistical heterogeneity. We also used visual inspection of the graphical representation of study results with their 95% CIs to assess heterogeneity. We analysed results using both fixed‐effect and random‐effects model analysis because for each model, the result is counterintuitive in some situations. In the presence of significant statistical heterogeneity (I2 > 50%) and when differences in results were of practical importance, we gave greater emphasis to the random‐effects model, which takes into account between‐study variability as well as within‐study variability. We also used the fixed‐effect model to test the robustness of the analysis and to look for outliers.
Assessment of reporting biases
Detecting publication bias is difficult, and avoidance is a better strategy (Glasziou 2001). We avoided publication bias by comprehensively searching the literature and by using study registries (Glasziou 2001). Publication bias is associated with asymmetry (Light 1984). We looked for asymmetry and explored potential reasons other than publication bias, for example, high risk of bias of smaller studies, true heterogeneity, artefact or chance (Egger 1997).
Data synthesis
We performed meta‐analysis using RevMan software (RevMan 5.2). Our primary outcome measure was a reduction in pain score or intensity using a validated age‐appropriate pain score. For dichotomous (or binary) data, we described results both as a relative measure (risk ratio (RR)) and as an absolute measure (number needed to treat for an additional beneficial outcome (NNTB) and risk difference (RD)). Relative measures can be used to combine studies, but absolute measures can be more informative than relative measures because they reflect baseline risk as well as changes in risk associated with the intervention. For continuous data, we used the mean difference (MD) when outcomes were measured in a standard way across studies. This provided the advantage of summarizing results in natural units that are easily understood. When it was desirable to summarize results across studies with outcomes that are conceptually the same but are measured in different ways, we used standardized mean differences (SMDs).
Subgroup analysis and investigation of heterogeneity
We performed a subgroup analysis on the use of intranasal fentanyl in the prehospital environment.
Sensitivity analysis
We carried out a sensitivity analysis to test how sensitive study results were to reasonable changes in the assumptions made and in the protocol for combining data (Lau 1998). We performed sensitivity analysis for 'randomized versus quasi‐randomized' and 'low risk of bias' versus 'high risk of bias' studies.
Summary of findings
We used the principles of the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system (Guyatt 2008) to assess the quality of the body of evidence associated with specific outcomes. We included the following outcomes in our review and constructed a 'Summary of findings' (SoF) table using RevMan (Table 1).
Pain score reduction (using age‐appropriate validated pain scales) following administration of INF at multiple time points.
Occurrence of adverse events post INF administration.
Acceptability of INF administration among participants.
Use of 'rescue analgesia' post INF administration.
Participant and parental satisfaction as defined by study authors.
Cost as defined by study authors.
Mortality.
The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. Assessment of the quality of a body of evidence considers within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The search of electronic databases yielded a total of 4875 publications. After titles and abstracts of all studies had been reviewed, six full papers were retrieved for possible inclusion. After the full texts had been examined, three papers were excluded and three studies were included. The study selection process is summarized in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow diagram shown in Figure 1.
1.
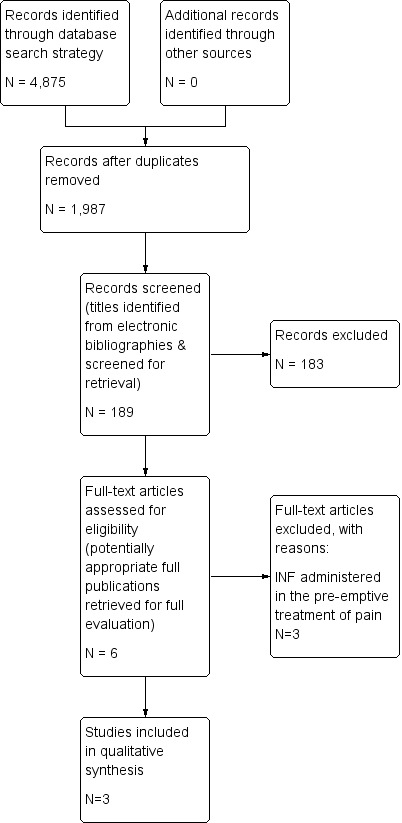
Study flow diagram.
Included studies
We included three trials (313 participants) comparing INF with alternative interventions for the treatment of children in acute pain (Borland 2007; Borland 2011; Younge 1999). All three studies included two comparison arms and were conducted at single sites in Australia. One study compared INF versus intramuscular morphine (IMM) (Younge 1999); another study compared INF versus intravenous morphine (IVM) (Borland 2007); and the final included study compared two different concentrations of INF for the treatment of children in acute pain (Borland 2011).
Each of the three selected studies included children who had experienced pain as a result of suspected limb fracture. One study recruited participants three to 10 years of age (Younge 1999), another study recruited participants three to 15 years of age (Borland 2011) and the final study recruited participants seven to 15 years of age (Borland 2007). Participant sex was not considered among the inclusion criteria. However, the intervention arm of one study consisted of 62.5% male participants (Younge 1999) and 62.9% male participants in another study (Borland 2011). Borland 2007 indicated that baseline characteristics were similar in control and intervention arms. Exclusion criteria were similar for all three studies (head injury impairing judgement, known allergy to opiates, blocked/traumatized nose, participants requiring immediate IV access, inability to perform pain scoring).
All three studies described reduction in pain intensity as the primary outcome measure. Pain scores were documented at five‐minute intervals for 30 minutes (Borland 2007; Younge 1999) and at 10‐minute intervals for 30 minutes (Borland 2011). Secondary outcome measures included participant tolerance to the medication administered (all studies); occurrence of opiate toxicity (respiratory depression, hypotension or decreased level of consciousness) was documented in two studies (Borland 2011; Younge 1999), and the use of "rescue analgesia" was identified in two studies (Borland 2007; Borland 2011).
Pooling of data was not possible, given that no studies employed the same comparator arms. Details of included studies are listed in Characteristics of included studies and Figure 1.
Excluded studies
Three trials were excluded because each involved preemptive treatment of children in pain in advance of lumbar puncture/bone marrow aspiration (Sandler 1992), children requiring a dressing change for burn injury (Borland 2005) and children undergoing catheterization for voiding cystourethrography (Chung 2010).
Risk of bias in included studies
Allocation
Borland 2011 and Younge 1999 appeared to provide sufficient detail in terms of random sequence generation and allocation of concealment. However, Borland 2007 was considered to have unclear risk of selection bias (Figure 2; Figure 3).
2.
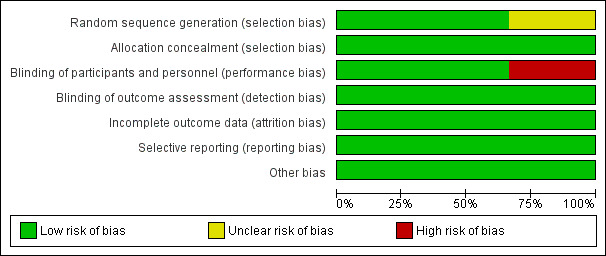
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Blinding
Blinding of participants and personnel was reported in two studies (Borland 2007; Borland 2011).
Incomplete outcome data
Two studies were limited by incomplete outcome data (Borland 2007; Borland 2011).
Selective reporting
Reporting bias was considered of low risk in all studies, given that each study's prespecified outcomes of interest in the review have been reported in the prespecified way.
Other potential sources of bias
No other potential sources of bias were identified in either study.
Effects of interventions
See: Table 1
Primary outcome
Reduction in pain score
All three studies reported a reduction in pain score/intensity—the primary outcome measure of this review.
Borland 2007 utilized a 100‐mm unmarked VAS for pain assessment. This study reported no statistically significant differences in visual analogue scale scores between treatment arms (INF or intravenous morphine) before analgesia or at 5, 10, 20 and 30 minutes post analgesia (P value 0.333). Statistically significant reductions in the combined VAS score were noted in both treatment arms at 5 minutes post analgesia of 20 mm (P value 0.000), at 10 minutes of 4 mm (P value 0.012) and at 20 minutes of 8 mm (P value 0.000). No further significant reductions in VAS score were reported beyond 20 minutes (P value 0.753) (Table 2). See also Table 1.
1. Intranasal fentanyl (INF) versus intravenous morphine (IVM).
| 0 min | 5 min | 10 min | 20 min | 30 min | |
| Intravenous morphine (mm) | 67 | 42 | 41 | 35 | 33 |
| Intranasal fentanyl (mm) | 68 | 55 | 46 | 37 | 37 |
| Difference (mm) (95% CI) | ‐1 (‐12 to 9) | ‐13 (‐23 to ‐3) | ‐5 (‐16 to 7) | ‐2 (‐13 to 10) | ‐4 (‐16 to 8) |
Borland 2007: Mean visual analogue score (mm) over time.
Borland 2011 reported no statistically significant differences in median pain scores between treatment arms (high concentration INF vs standard concentration INF) at any of the study time points. Each agent demonstrated a statistically and clinically significant reduction in pain scores over the duration of the study (median decrease for both groups 40 mm, P value 0.000) (Table 3).
2. High concentration intranasal fentanyl (HINF) versus standard concentration intranasal fentanyl (SINF).
| SINF | HINF | P value | |
| Before analgesia | 80.0 (60.0‐95.5) | 77.5 (60.0‐100) | 0.881 |
| 10 min | 49.5 (26.5‐68.5) | 43.0 (15.2‐66.0) | 0.176 |
| 20 min | 27.5 (18.5‐56.5) | 35.0 (9.0‐57.0) | 0.758 |
| 30 min | 20.0 (10.0‐46.0) | 21.5 (4.75‐51.0) | 0.662 |
Borland 2011: Median visual analogue pain score (mm) over time.
Younge 1999 assessed pain on a six‐point pain scale (0 = no pain, 5 = worst pain). No significant differences in presenting pain scores were noted between treatment arms (P value 0.46). This study reported a significant difference in pain scores at 10 minutes, with a lower pain score seen in the INF group (pain score of 1 vs pain score of 2 for INF vs intramuscular morphine, respectively; P value 0.014). The median pain score difference at 20 minutes did not reach statistical significance (P value 0.64), and no further difference in pain scores was noted at any other time (Table 4).
3. Intranasal fentanyl (INF) versus intramuscular morphine (IMM).
| 0 min | 5 min | 10 min | 20 min | 30 min | |
| Intranasal fentanyl | 4 | 3 | 1 | 1 | 1 |
| Intramuscular morphine | 4 | 3 | 2 | 2 | 1 |
Younge 1999: Median pain score (Wong Baker Faces, ordinal scoring 0‐5) over time.
Secondary outcomes
Occurrence of all adverse events associated with INF
No adverse events (e.g. opiate toxicity, death) were reported in any study following INF administration.
Participant tolerance of INF (including incidence of nausea, vomiting or reported discomfort)
Younge 1999 described better tolerance to INF (P value < 0.001), with one child crying during administration and a second child vomiting following administration.
Borland 2007 described three children who reported a bad taste in their mouth and one child who experienced an episode of vomiting 20 minutes post INF administration. One child complained of a momentary flush at the intravenous line site following administration of morphine.
Similarly, Borland 2011 identified one child who reported dislike of the taste of INF solution when swallowed.
Use of 'rescue' analgesia before and after hospital arrival
Younge 1999 identified one child and Borland 2007 identified one child who required rescue analgesia following INF administration.
Borland 2011 did not impose a restriction on participants receiving more than one additional analgesic agent (e.g. oral paracetamol, ibuprofen), and the decision to administer additional analgesia was made at the discretion of the treating nurse. Additional analgesia was offered as per standard fracture management in that ED, with a desire to have these agents active before the effects of fentanyl had worn off. More than one‐third (35.4%) of the overall study population (67/189) was administered additional analgesia. In all, 42 participants received standard concentration INF and 25 received high concentration INF. The standard concentration INF group was given significantly more additional analgesia compared with the high concentration INF group (P value 0.028). Given no demonstrable difference in pain scores between treatment arms, the clinical significance of this finding is difficult to determine.
Participant satisfaction as defined by study authors
No study addressed this outcome.
Parental satisfaction as defined by study authors
No study addressed this outcome.
Cost as defined by study authors
No study addressed this outcome.
Mortality
No study participant died as a result of INF administration.
Discussion
Summary of main results
This review assessed RCT evidence comparing outcomes of INF and alternative analgesic therapy in the treatment of children in acute pain. Our review found no evidence to support a difference in the primary outcome measure—relief of pain—between INF and either intravenous morphine or high concentration INF (Borland 2007; Borland 2011). One study, however, did show a statistically significant reduction in pain intensity at 10 minutes post drug administration when INF was compared with intramuscular morphine (Younge 1999). It was not possible to pool the results of these three trials because the comparator interventions were different, and timing of pain score assessment was inconsistent between two of the three studies. All three trials enrolled children with clinically deformed closed long bone fractures.
This review found no evidence of adverse events (e.g. opiate toxicity, anaphylaxis) associated with administration of INF in any of the included studies. One study described improved acceptability of INF administration compared with administration of intramuscular morphine (Younge 1999), in contrast to four participants in the other trials who reported a "bad taste" following administration of INF. "Rescue analgesia" was administered to three children in two studies (Borland 2007; Younge 1999), whereas the third study (Borland 2011) posed no limitation on participants receiving more than one additional analgesic agent. In this study, more than one‐third (35.4%) of study participants were administered additional analgesia. No study documented participant or parental satisfaction with INF. No participant died as a result of INF administration.
Overall completeness and applicability of evidence
We were unable to perform a valid sensitivity analysis as planned a priori because all RCTs included in this review were randomized (no quasi‐randomized trials were eligible for inclusion).
Quality of the evidence
Some limitations were noted in the design and implementation of all three studies. Borland 2007 used a convenience sample for enrolments that was dependent on identification of suitable participants at triage. No record was kept of potential participants who were not enrolled, so no conclusion can be drawn about potential selection bias. Enrolment in Borland 2011 was not compulsory but was actively encouraged by study investigators. The fact that not all potential participants were screened for inclusion in the study may influence external validity. However, patients meeting inclusion criteria through the "Drugs of Dependence" register in the study ED were recorded and reasons for failure to enrol in the study documented. Based on similarities between cohorts of included and non‐included patients, this potential source of selection bias was minimized. Younge 1999 was limited in design by the open nature of the trial and did not meet the criteria for a good quality study (one that includes all of the following domains: adequate allocation concealment, blinding of outcome assessment and analysis performed according to intention‐to‐treat principles), suggesting a likely potential source of bias.
We identified neither indirectness of evidence (indirect population, intervention, control, outcomes) nor unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses) in any included study. Similarly no evidence suggested imprecision of results (wide confidence intervals) in any included study. The overall risk of publication bias was thought to be low in all included studies.
Potential biases in the review process
We encountered no potential bias in the review process.
Agreements and disagreements with other studies or reviews
Our search yielded two other systematic reviews on the use of intranasal fentanyl in the context of acute pain management ( Hansen 2013, Mudd 2010). Hansen et al (2013) conducted a systematic review of INF trials (randomized and non‐randomized) completed in emergency department and prehospital settings and imposed no age restriction. These review authors concluded that only two of the 12 studies identified were adequately randomized and double‐blinded (Borland 2007; Borland 2011), emphasizing the fact that "further well‐performed double‐blinded randomized controlled trials are required to thoroughly validate the use of INF in this context." Nevertheless, the review authors acknowledged the fact that these studies demonstrated the non‐inferiority of INF compared with intravenous and intramuscular morphine.
Mudd (2010) conducted a systematic review of INF trials (randomized and non‐randomized) in children. Similarly, this review concluded that INF was "equivalent or superior to morphine that is administered orally, intravenously, and intramuscularly." In addition, this review illustrates that INF may be more favourable over intravenous or intramuscular morphine, given the painless nature of its administration.
Authors' conclusions
Implications for practice.
Our review found no clear evidence to support differences in short‐term pain relief or occurrence of adverse events when INF was compared with intravenous morphine or high concentration INF. Evidence was insufficient to permit definitive conclusions regarding whether INF is superior, non‐inferior or equivalent to intramuscular morphine. INF was generally well accepted by study participants. The risk of bias in one RCT was determined to be high because of the open‐label nature of the study. Existing RCT evidence was derived from trials conducted in Australia, which were mainly conducted in injured males older than three years of age, and so may not be generalizable to other healthcare settings in children with pain resulting from illness.
Implications for research.
Further adequately powered studies of high methodological quality are required to determine whether there is any difference in clinical outcomes between INF and all other forms of analgesic treatment of children in acute pain. Future research should focus on the treatment of children of all ages in acute severe pain (regardless of cause), with collaboration from emergency healthcare providers in multiple clinical settings.
Feedback
Risk of bias for allocation concealment and selective reporting as 'low risk' across all three studies included in the review, 8 May 2015
Summary
Murphy A et al. in their systematic review (Murphy 2014) reported the risk of bias for allocation concealment and selective reporting as 'low risk' across all three studies included in their review.
1.The studies, (Borland 2007; Borland 2011; Younge 1999) report using 'sealed envelopes in study packs' and 'concealed consecutive recruitment' as part of their allocation concealment methods. Borland 2007 used 'randomly allocated study packs supplied to the department in blocks of 10.'
Using the Cochrane risk of bias tool, we classified these studies as having an 'unclear risk of bias'. While the three studies (Borland 2007; Borland 2011; Younge 1999) used a sealed envelope/pack system, they were not transparent about whether or not envelopes were opaque or if packs were sequentially numbered. Overall, we feel that their methods of concealment are not described in sufficient detail to allow a definite judgment.
2. Furthermore, we assessed the risk of selective outcome reporting in these studies. While we were able to find a protocol for Borland 2011, and agreed that the risk of bias here was low, we were unable to find protocols for the other two studies (Borland 2007; Younge 1999 ). Therefore, we could not evaluate the risk of selective outcome reporting bias. As part of our feedback for the next update, we ask the authors of this review to provide clarity on how they assessed risk of selective outcome reporting bias for the other two studies as they were generally rated as a low risk of bias (i.e. were authors contacted for protocols?).
While we agree with the conclusions of this study, we feel that the risk of bias assessment be reconsidered for allocation concealment and transparency be provided for assessment of selective outcome reporting in the included studies.
We hope this provides some constructive feedback for your next review
Reply
Reply to point 1
We will contact Borland and Young and seek clarification on the above in advance of the next update for this review. If we can definitively describe a low risk of bias based on their response (based on the opacity of envelopes/numbering etc.), the risk of bias will remain unchanged. However, if that transpires not to be the case, we will amend to indicate an 'unclear risk of bias'.
Reply to point 2
We will contact Borland and Young and request protocols for all three studies ahead of the next update. We will incorporate justification for our risk of bias assessment (selective outcome reporting) having reviewed all protocols.
Contributors
Summary authors
Kieran Shah, BSc. (Pharm), Pharmacy Resident France Carriere, BSc. (Pharm), Pharmacy Resident
Angus Kinkade, BSc. (Pharm), ACPR, PharmD, MSc
We agree with the conflict of interest statement.
We certify that we have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
Adrian Murphy (contact person; Murphy 2014)
What's new
| Date | Event | Description |
|---|---|---|
| 13 August 2015 | Feedback has been incorporated | Feedback incorporated August 2015 (see Feedback) |
Notes
Feedback incorporated August 2015 (see Feedback)
Acknowledgements
We would like to thank Andy Smith (content editor), Cathal Walsh (statistical editor) and David Herd and Meredith Borland (peer reviewers) for help and editorial advice provided during preparation of the protocol for the systematic review and the full systematic review.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Fentanyl explode all trees #2 fentan?l*:ti,ab #3 (#1 OR #2) #4 MeSH descriptor Acute Pain explode all trees #5 MeSH descriptor Pain, this term only #6 MeSH descriptor Wounds and Injuries, this term only #7 MeSH descriptor Burns explode all trees #8 MeSH descriptor Pain Measurement, this term only #9 MeSH descriptor Emergencies explode all trees #10 pain*:ti or (pain near (acute or moderate or severe or relief or scor*)):ti,ab or (fracture* or burn* or injur* or emergency*):ti,ab #11 (#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10) #12 (#3 AND #11) #13 adult* #14 (child* or pediat*) #15 (#13 AND NOT ( #14 AND #13 )) #16 (#12 AND NOT #15)
Appendix 2. MEDLINE (Ovid SP) search strategy
1. exp Fentanyl/ or fentan?l*.af. 2. exp Acute Pain/ or exp Pain/co, pc or "Wounds and Injuries"/ or Burns/ or Pain Measurement/ or Emergencies/ or pain*.ti,ab. or (pain adj3 (acute or moderate or severe or relief or scor*)).af. or (fracture* or burn* or injur* or emergency*).ti,ab. 3. 1 and 2 4. adult*.af. 5. (child* or pediat*).af. 6. 4 not (4 and 5) 7. 3 not 6 8. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 9. 7 and 8
Appendix 3. EMBASE (Ovid SP) search strategy
1 exp fentanyl/ or fentan?l*.ti. 2 pain/co, pc or wound/ or injury/ or burn/ or pain assessment/ or emergency/ or pain*.ti. or (pain adj3 (acute or moderate or severe or relief or scor*)).ti,ab. or (fracture* or burn* or injur* or emergency*).ti. 3 1 and 2 4 adult*.af. 5 (child* or p?ediat*).af. 6 4 not (5 and 4) 7 3 not 6 8 (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab.) not (animals not (humans and animals)).sh. 9 7 and 8
Appendix 4. CINAHL (EBSCO Host) search strategy
S1 (MM "Fentanyl") OR "Fentanyl" S2 ( (MH "Acute Pain (Saba CCC)") OR (MH "Pain") OR (MH "Wounds and Injuries") OR (MH "Burns") OR (MH "Pain Measurement") OR (MH "Emergencies") ) OR TI pain OR AB ( pain and (acute or moderate or severe or relief or scor*) ) OR TI ( fracture* o.r burn* or injur* or emergency* ) S3 S1 and S2 S4 adult* S5 child* or p?ediat* S6 S4 not (S4 and S5) S7 S3 not S6 S8 ( (MM "Randomized Controlled Trials") OR (MH "Random Assignment") OR (MH "Clinical Trial Registry") OR (MH "Prospective Studies") OR (MH "Placebos") OR (MH "Double‐Blind Studies") OR (MH "Single‐Blind Studies") OR (MH "Triple‐Blind Studies") ) OR AB ( random* or ((controlled or clinical) and trial*) ) S9 S7 and S8
Appendix 5. ISI Web of Science and BIOSIS Citations Index SM search strategy
#1 TI=fentan?l* #2 TI=pain* or TS=(pain SAME (acute or moderate or severe or relief or scor*)) or TI=(fracture* or burn* or injur* or emergency*) #3 #1 AND #2 #4 TS=adult* #5 TS=(child* or p?ediat*) #6 #4 not (#4 and #5) #7 #3 not #6 #8 TS=(random* or ((controlled or clinical) SAME trial*) or prospective or multicenter) or TS=((mask* or blind*) SAME (single or double or triple or treble)) #9 #7 and #8
Appendix 6. LILACS search strategy
"fentanyl$" or "fentanil$" and ("pain" and ("acute" or "moderate" or "severe" or "relief" or "scor$")) or ("fracture$" or "burn$" or "injur$" or "emergency$")
Appendix 7. Data extraction form
DATA EXTRACTION FORM
| Paper Title/Reference | |||
| Outcome measure | Intranasal fentanyl (n = X) |
Alternative (n = X) |
|
| Pain reduction using age‐appropriate validated pain scale (e.g. FLACC (Faces, Legs, Activity, Cry, Consolability), Wong Baker, NRS (Numerical Rating Scale), VAS (Visual Analogue Scale)) | |||
| Occurrence of opiate toxicity I: Respiratory depression |
|||
| Occurrence of opiate toxicity II: Hypotension |
|||
| Occurrence of opiate toxicity III: Decreased level of consciousness |
|||
| Patient tolerance to analgesia (i.e. nausea or vomiting or reported patient discomfort) |
|||
| Use of “rescue analgesia” | Prehospital | ||
| Emergency department | |||
| Participant satisfaction | |||
| Parental satisfaction | |||
| Participant mortality | |||
Characteristics of studies
Characteristics of included studies [ordered by year of study]
Younge 1999.
| Methods | Prospective, randomized, open‐label, 2‐arm study at a single site (paediatric emergency department) in Australia | |
| Participants | 47 patients (3‐10 years of age) with clinically suspected fracture of the upper and/or lower limbs | |
| Interventions | Intervention 1: single dose of INF (50 mcg/mL) at a dose of 1 mcg/kg Intervention 2: single dose of IMM (10 mg/mL) at a dose of 0.2 mg/kg |
|
| Outcomes | Primary outcome measure: reduction in pain using Wong Baker Faces (ordinal scale 0‐5). Pain intensity was measured at 0, 5, 10, 20 and 30 minutes Secondary outcome measures: occurrence of opiate toxicity (sedation, respiratory or cardiovascular depression) and participant tolerance of analgesia (nausea/vomiting and reported discomfort) |
|
| Notes | Both interventions produced a reduction in pain score. "Rescue analgesia" was administered to 1 participant in the INF group and to no participants in the IMM group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Included in the study were children aged between 3 and 10 years who were otherwise healthy and who presented to the ED with clinical fracture of the upper or lower limbs" |
| Allocation concealment (selection bias) | Low risk | Quote: "Once parents had given informed consent, patients were randomized via a sealed envelope system" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment/Quote: Participants were randomly assigned "to receive a single dose of either 1.0 μg/kg INF or 0.2 mg/kg IMM" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Pain was assessed by the children at 0, 5, 10, 20 and 30 min after treatment administration using Wong Baker Faces (ordinal scoring 0–5) and also by their parents using a visual analogue score (continuous 0–10)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Forty‐seven children were recruited into the study, 24 into the INF arm and 23 into the IMM arm, with no parents refusing consent" Comment: No participant was withdrawn from this study following enrolment |
| Selective reporting (reporting bias) | Low risk | Comment: All of the study's prespecified outcomes of interest in the review have been reported in the prespecified way |
| Other bias | Low risk | Comment: The study appears to be free of other sources of bias |
Borland 2007.
| Methods | Prospective, double‐blind, randomized, placebo‐controlled clinical trial at a single site (tertiary paediatric emergency department) in Australia | |
| Participants | 67 patients (7‐15 years of age) with clinically deformed closed long bone fractures were enrolled | |
| Interventions | Intervention 1: active INF (150 mcg/mL) at a dose of 1.4 mcg/kg AND intravenous placebo Intervention 2: active IVM (10 mg/mL) at a dose of 0.1 mg/kg AND intranasal placebo |
|
| Outcomes | Primary outcome measure: reduction in pain using a 100‐mm unmarked visual analogue scale. Pain intensity was measured at 0, 5, 10, 20 and 30 minutes Secondary outcome measures: occurrence of opiate toxicity (sedation, respiratory or cardiovascular depression) and participant tolerance of analgesia (nausea/vomiting and reported discomfort) |
|
| Notes | Both interventions produced a reduction in pain score. "Rescue analgesia" was administered to no participants in the group who received active INF and to 2 participants in the group who received active IVM | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A convenience sample of children aged 7 to 15 years, presenting with clinically deformed closed long‐bone fractures, was identified at triage and invited to join the study" |
| Allocation concealment (selection bias) | Low risk | Quote: "The study packs were randomly allocated in the pharmacy and supplied to the department in blocks of 10, and the next available pack was taken on enrolment of the patient" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Study packs contained either the concentrated fentanyl solution or normal saline solution in identical containers plus a 1‐mL ampoule of morphine (10 mg/mL) or normal saline solution also in identical containers" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The patient provided a pain score with the visual analogue scale at 0, 5, 10, 20, and 30 minutes after the administration of analgesia. They also completed a second assessment to compare their current pain with the previous rating verbally as 'much better,' 'little better,' 'the same,' 'little worse,' or 'much worse.' The child was blinded to previous scores" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Of the 67 participants enrolled in the study, 2 were withdrawn (1 participant in each study arm) Quote: "1 child was withdrawn when IV access failed and intramuscular analgesia was administered; 1 child received 1 dose of intranasal fentanyl and withdrew at 5 minutes" |
| Selective reporting (reporting bias) | Low risk | Comment: All of the study's prespecified outcomes of interest in the review have been reported in the prespecified way |
| Other bias | Low risk | Comment: The study appears to be free of other sources of bias |
Borland 2011.
| Methods | Prospective, double‐blind, randomized, controlled trial at a single site (tertiary paediatric emergency department) in Australia | |
| Participants | 189 patients (3‐15 years of age) with clinically deformed closed long bone fractures were enrolled | |
| Interventions | Intervention 1: SINF (50 mcg/mL) at a dose of 1.5 mcg/kg Intervention 2: HINF (300 mcg/mL) at a dose of 1.5 mcg/kg |
|
| Outcomes | Primary outcome measure: reduction in pain using a 100‐mm VAS for participants >7 years or Faces Pain Scale for those participants incapable of using the VAS. Pain intensity was measured at 0, 10, 20 and 30 minutes Secondary outcome measures: occurrence of opiate toxicity (sedation, respiratory or cardiovascular depression) and participant tolerance of analgesia (nausea/vomiting and reported discomfort) |
|
| Notes | Both interventions produced a reduction in pain score. "Rescue analgesia" was administered to 42 participants in the SINF group and to 25 participants in the HINF group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Children aged 3–15 years inclusive presenting to the ED with clinically deformed closed long bone fractures were included. The patients were identified by the triage nurse as requiring urgent analgesia" |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomized using a computer‐generated programme in blocks of 10 stratified with age brackets of 3–5 years, 6–10 years and 11–15 years with allocation made by a sealed envelope in the study pack" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All patients received an initial standard dose of 1.5 mcg/kg (either SINF or HINF) administered with the MAD device (Wolfe Tory Medical, Salt Lake City, UT, USA) with volumes >0.2 mL alternated between nostrils. A nurse, blinded to the solution of fentanyl administered, undertook observations including pain scores at 0, 10, 20 and 30 min post initial INF dose" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Outcome measures included pain scores using either a 100 mm visual analogue pain scale (VAS) for patients >7 years of age if the patient was deemed capable by the observation nurse, or Faces Pain Scale–Revised (FPS‐R) for those patients incapable of using VAS" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 199 participants were enrolled in this study. 10 were withdrawn during the study (HINF, N = 6; SINF, N = 4) for the following reasons: no written consent, no pain score documented, prehospital administration of opiates, INF not required |
| Selective reporting (reporting bias) | Low risk | Comment: All of the study's prespecified outcomes of interest in the review have been reported in the prespecified way |
| Other bias | Low risk | Comment: The study appears to be free of other sources of bias |
ED: Emergency department.
HINF: High concentration intranasal fentanyl.
IMM: Intramuscular morphine.
INF: Intranasal fentanyl.
IVM: Intravenous morphine.
SINF: Standard concentration intranasal fentanyl.
VAS: Visual analogue scale.
Characteristics of excluded studies [ordered by year of study]
| Study | Reason for exclusion |
|---|---|
| Sandler 1992 | Preemptive treatment of children in pain in advance of lumbar puncture or bone marrow aspiration |
| Borland 2005 | Preemptive treatment of children in pain requiring a change in burn dressing |
| Chung 2010 | Preemptive treatment of children in pain during catheterization for voiding cystourethrography |
Characteristics of ongoing studies [ordered by study ID]
Barrett 2012.
| Trial name or title | Intranasal fentanyl versus intravenous morphine in the emergency department treatment of severe painful sickle cell crises in children: study protocol for a randomized controlled trial |
| Methods | Randomized, double‐blind, double‐dummy, active control trial |
| Participants | Children (weighing more than 10 kg) between 1 year and 21 years of age with severe painful sickle cell crisis |
| Interventions | Each participant will receive a single active agent and a single placebo via intravenous and intranasal routes |
| Outcomes | The primary endpoint is severity of pain scored at 10 minutes from administration of study medications. Secondary endpoints include pain severity measured at 0, 5, 15, 20, 30, 60 and 120 minutes after administration of analgesia, proportion of participants requiring rescue analgesia and incidence of adverse events |
| Starting date | March 2012 |
| Contact information | Michael Joseph Barrett: mjjbarrett@hotmail.com |
| Notes | Trial Registration: Current Controlled Trials ISRCTN67469672 and EudraCT no. 2011‐005161‐20 |
Differences between protocol and review
We made the following changes to the published protocol (Murphy 2012).
Byline: Nandini Kandamany joined the review team.
Searching other resources: We did not contact pharmaceutical companies.
-
In the published protocol for this review, we intended to document the following in the 'Summary of findings' (SoF) table.
Pain score reduction (using age‐appropriate validated pain scales) following administration of INF at multiple time points.
Occurrence of adverse events post INF administration.
Acceptability of INF administration among participants.
Use of 'rescue analgesia' post INF administration.
Participant and parental satisfaction as defined by study authors.
Cost as defined by study authors.
Mortality
However, the included studies did not document participant/parental satisfaction nor cost; therefore these were not included in the SOF table.
Contributions of authors
Conceiving of the review: Ronan O'Sullivan (ROS)
Co‐ordinating the review: Adrian Murphy (AM)
Undertaking manual searches: AM
Screening search results: AM
Organizing retrieval of papers: Siobhan McCoy (SMC)
Screening retrieved papers against inclusion criteria: John Cronin (JC) and Michael J Barrett (MB)
Appraising quality of papers: JC and MB
Abstracting data from papers: Jeffrey Hom (JH) and SMC
Writing to authors of papers for additional information: AM
Providing additional data about papers: AM
Obtaining and screening data on unpublished studies: AM
Managing data for the review: AM
Entering data into Review Manager (RevMan 5.2): JC and Nandini Kandamany (NK)
Analysing RevMan statistical data: Timothy Grant (TG)
Performing other statistical analysis not using RevMan: TG
Interpreting data: ROS and Abel Wakai (AW)
Making statistical inferences: TG
Writing the review: AM
Securing funding for the review: ROS
Performing previous work that served as the foundation of the present study: AM
Serving as guarantor for the review (one review author): ROS
Taking responsibility for reading and checking the review before submission: ROS and AM
Sources of support
Internal sources
National Children's Research Centre, Ireland.
External sources
No sources of support supplied
Declarations of interest
Adrian Murphy: none known.
Ronan O'Sullivan: none known.
Abel Wakai: none known.
Timothy Grant: I began working at ICON Clinical Research on December 1, 2012. None of my work at ICON involves related areas, and all work is performed independently of the work I had previously completed in preparation of this review. My current work involves primarily oncology and vaccines, and no activities focus on anaesthesia or pain management. Neither I, Tim Grant, nor my employer, ICON Clinical Research, is in a position to benefit financially from this review; I have no conflicts of interest. This work was performed independently of my employment, and this work is related in no way to my employer.
Michael J Barrett: none known.
John Cronin: none known.
Siobhan C McCoy: none known.
Jeffrey Hom: none known.
Nandini Kandamany: none known.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Borland 2007 {published data only}
- Borland M, Jacobs I, King B, O'Brien D. A randomized controlled trial comparing intranasal fentanyl to intravenous morphine for managing acute pain in children in the emergency department. Annals of Emergency Medicine 2007;49(3):335‐40. [DOI] [PubMed] [Google Scholar]
Borland 2011 {published data only}
- Borland M, Milsom S, Esson A. Equivalency of two concentrations of fentanyl administered by the intranasal route for acute analgesia in children in a paediatric emergency department: a randomized controlled trial. Emergency Medicine Australasia 2011;23(2):202‐8. [DOI: 10.1111/j.1742-6723.2011.01391.x] [DOI] [PubMed] [Google Scholar]
Younge 1999 {published data only}
- Young PA, Nicol MF, Kendall JM, Harrington AP. A prospective randomized pilot comparison of intranasal fentanyl and intramuscular morphine for analgesia in children presenting to the emergency department with clinical fractures. Emergency Medicine 1999;11(2):90‐4. [Google Scholar]
References to studies excluded from this review
Borland 2005 {published data only}
- Borland ML, Bergesio R, Pascoe EM, Turner S, Woodger S. Intranasal fentanyl is an equivalent analgesic to oral morphine in paediatric burns patients for dressing changes: a randomised double blind crossover study. Burns 2005;31(7):831‐7. [PUBMED: 16005154] [DOI] [PubMed] [Google Scholar]
Chung 2010 {published data only}
- Chung S, Lim R, Goldman RD. Intranasal fentanyl versus placebo for pain in children during catheterization for voiding cystourethrography. Pediatric Radiology 2010;40(7):1236‐40. [DOI: 10.1007/s00247-009-1521-1] [DOI] [PubMed] [Google Scholar]
Sandler 1992 {published data only}
- Sandler ES, Weyman C, Conner K, Reilly K, Dickson N, Luzins J, et al. Midazolam versus fentanyl as premedication for painful procedures in children with cancer. Pediatrics 1992;89(4):631‐4. [PUBMED: 1557241] [PubMed] [Google Scholar]
References to ongoing studies
Barrett 2012 {published data only}
- Intranasal fentanyl versus intravenous morphine in the emergency department treatment of severe painful sickle cell crises in children: study protocol for a randomized controlled trial. Ongoing study March 2012. [DOI] [PMC free article] [PubMed]
Additional references
Alonso‐Serra 2003
- Alonso‐Serra H, Wesley K. Position paper ‐ Prehospital pain management. Prehospital Emergency Care 2003;7:482‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bendall 2011
- Bendall JC, Simpson PM, Middleton PM. Effectiveness of prehospital morphine, fentanyl, and methoxyflurane in pediatric patients. Prehospital Emergency Care 2011;15(2):158‐65. [DOI] [PubMed] [Google Scholar]
Berde 2002
- Berde CB, Sethna NF. Analgesics for the treatment of pain in children. New England Journal of Medicine 2002;347:1094‐103. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Borland 2008
- Borland ML, Clark LJ, Esson A. Comparative review of the clinical use of intranasal fentanyl versus morphine in a paediatric emergency department. Emergency Medicine Australasia 2008;20(5):515‐20. [DOI] [PubMed] [Google Scholar]
Cordell 2002
- Cordell WH, Keene KK, Giles BK, Jones JB, Jones JH, Brizendine EJ. The high prevalence of pain in emergency care. American Journal of Emergency Medicine 2002;20:165‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Dave‐Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context. 2nd Edition. London: BMJ Publishing Group, 2001:285‐312. [Google Scholar]
Egger 1997
- Egger M, Smith GD, Philips AN. Meta‐analysis: principles and procedures. BMJ 1997;315(7121):1533‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Foster 2008
- Foster D, Upton R, Christrup L, Popper L. Pharmacokinetics and pharmacodynamics of intranasal versus intravenous fentanyl in patients with pain after oral surgery. Annals of Pharmacotherapy 2008;42(10):1380‐7. [DOI: 10.1345/aph.1L168] [DOI] [PubMed] [Google Scholar]
Friedland 1994
- Friedland LR, Kilick RM. Emergency department analgesia use in pediatric trauma victims with fractures. Annals of Emergency Medicine 1994;23(2):203‐7. [DOI] [PubMed] [Google Scholar]
Galinski 2011
- Galinski M, Picco N, Hennequin B, Raphael V, Ayachi A, Beruben A, et al. Out‐of‐hospital emergency medicine in pediatric patients: prevalence and management of pain. American Journal of Emergency Medicine 2011;29(9):1062‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Glasziou 2001
- Glasziou P, Irwing L, Bain C, Colitz G. Systematic Reviews in Healthcare: a Practical Guide. Systematic Reviews in Healthcare: A Practical Guide. 1st Edition. Cambridge: Cambridge University Press, 2001. [Google Scholar]
Groenewald 2012
- Groenewald C, Rabbitts J, Schroeder D, Harrison T. Prevalence of moderate–severe pain in hospitalized children. Pediatric Anesthesia 2012;27(7):661‐8. [DOI: 10.1111/j.1460-9592.2012.03807.x] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians. BMJ 2008;336:995‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hansen 2013
- Hansen MS, Dahl JB. Limited evidence for intranasal fentanyl in the emergency department and the prehospital setting ‐ a systematic review. Danish Medical Journal 2013;60(1):1‐6. [PUBMED: 23340187] [PubMed] [Google Scholar]
Hennes 2005
- Hennes H, Kim M, Pirrallo R. Prehospital pain management: a comparison of providers’ perceptions and practices. Prehospital Emergency Care 2005;9(1):32–9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Izsak 2008
- Izsak E, Moore JL, Stringfellow K, Oswanski MF, Lindstrom DA, Stombaugh HA. Prehospital pain assessment in pediatric trauma. Prehospital Emergency Care 2008;12(2):182‐6. [DOI] [PubMed] [Google Scholar]
Kart 1997
- Kart T, Christrup LL, Rasmussen M. Recommended use of morphine in neonates, infants, and children based on a literature review, part 2: clinical use. Paediatric Anaesthesia 1997;7:93‐101. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lau 1998
- Lau J, Loannidis JPA, Schmid CH. Quantitative synthesis in systematic reviews. In: Mulrow C, Cook D editor(s). Systematic Reviews: Synthesis of Best Evidence in Healthcare Decisions. 1st Edition. Vol. 1, Philadelphia: American College of Physicians, 1998:91‐110. [Google Scholar]
Light 1984
- Light RJ, Pillemer DB. Summing Up: The Science of Reviewing Research. 1st Edition. Vol. 1, Cambridge, Massachusetts: Harvard University Press, 1984. [Google Scholar]
MacLean 2007
- MacLean S, Obispo J, Young KD. The gap between pediatric emergency department procedural pain management treatments available and actual practice. Pediatric Emergency Care 2007;23(2):87‐93. [PUBMED: 17351407] [DOI] [PubMed] [Google Scholar]
Mudd 2010
- Mudd S. Intranasal fentanyl for pain management in children: a systematic review of the literature. Journal of Pediatric Health Care September‐October;25(5):316‐22. [DOI: 10.1016/j.pedhc.2010.04.011] [DOI] [PubMed] [Google Scholar]
Panagiotou 2010
- Panagiotou I, Mystakidou K. Intranasal fentanyl: from pharmacokinetics and bioavailability to current treatment applications. Expert Review of Anticancer Therapy 2010;10(7):1009‐21. [DOI: 10.1586/era.10.77] [DOI] [PubMed] [Google Scholar]
Paris 1988
- Paris PM, Stewart RD. Pain Management in Emergency Medicine. 1st Edition. Vol. 1, Norwalk, Connecticut: Appleton‐Lange, 1988:p xiii. [Google Scholar]
Probst 2005
- Probst BD, Lyons E, Leonard D, Esposito TJ. Factors affecting emergency department assessment and management of pain in children. Pediatric Emergency Care 2005;21(5):298‐305. [PUBMED: 15874811 ] [DOI] [PubMed] [Google Scholar]
RevMan 5.2 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rupp 2004
- Rupp T, Delaney KA. Inadequate analgesia in emergency medicine. Annals of Emergency Medicine 2004;43(4):494‐503. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saunders 2010
- Saunders M, Adelgais K, Nelson D. Use of intranasal fentanyl for the relief of pediatric orthopedic trauma pain. Academic Emergency Medicine 2010;17(11):1155‐61. [DOI] [PubMed] [Google Scholar]
Schechter 1989
- Schechter N. The under‐treatment of pain in children: an overview. Pediatric Clinics of North America 1989;36:781–94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Verghese 2010
- Verghese S, Hannallah R. Acute pain management in children. Journal of Pain Research 2010;3:105‐23. [PUBMED: 3004641] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilsey 2004
- Wilsey B, Fishman S, Rose JS, Papazian J. Pain management in the ED. American Journal of Emergency Medicine 2004;22(1):51‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wilson 1989
- Wilson JE, Pendleton JM. Oligoanalgesia in the emergency department. American Journal of Emergency Medicine 1989;7(6):620‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Murphy 2012
- Murphy A, O'Sullivan R, Wakai A, Grant T, Barrett MJ, Cronin J, et al. Intranasal fentanyl for the management of acute pain in children. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD009942] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2014
- Murphy A, O'Sullivan R, Wakai A, Grant TS, Barrett MJ, Cronin J, et al. Intranasal fentanyl for the management of acute pain in children. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD009942.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


