Abstract
Background
Cough is the most common symptom presenting to primary healthcare services. Cough in children is associated with significant morbidity for both children and their parents. While inhaled corticosteroids (ICS) can potentially reduce cough associated with airway inflammation and airway hyper‐reactivity, use of ICS in children is not without potential adverse effects. Therefore, it would be beneficial to clinical practice to evaluate the evidence for the efficacy of ICS in reducing the severity of cough in children with subacute cough (defined as cough duration of two to four weeks) systematically.
Objectives
To evaluate the efficacy of ICS in reducing the severity of cough in children with subacute cough.
Search methods
The Cochrane Register of Controlled Trials (CENTRAL), the Cochrane Airways Group Specialised Register, MEDLINE, EMBASE, review articles and reference lists of relevant articles were searched. The latest searches were performed in November 2011.
Selection criteria
All randomised controlled trials (RCTs) comparing ICS with a control group in children with subacute cough were considered for inclusion.
Data collection and analysis
Search results were reviewed against pre‐determined criteria for inclusion. Two sets of review authors independently selected, extracted and assessed the data for inclusion. Study authors were contacted for further information where required. Data were analysed as 'intention to treat'.
Main results
The search identified 1178 potentially relevant titles; however, there were no published studies that were specifically designed to answer this question. Two studies met criteria for inclusion in the review and 98 children were included in the meta‐analysis. There was no significant difference between groups in the proportion of children 'not cured' at follow‐up (primary outcome measure), with a pooled odds ratio (OR) of 0.61 (95% confidence interval (CI) 0.24 to 1.55). However, the included studies were limited in their ability to answer the review question by the fact that all participants were infants, post acute bronchiolitis illness, and cough duration at the start of study medication was ill‐defined.
Authors' conclusions
There is currently no evidence to support the use of ICS for treatment of subacute cough in children. However, this systematic review is limited by the small number of studies available for analysis and the size, quality and design of these studies. Further well‐designed RCTs are required to support or refute the efficacy of treatment with ICS in children with subacute cough.
Keywords: Humans; Infant; Acute Disease; Administration, Inhalation; Adrenal Cortex Hormones; Adrenal Cortex Hormones/administration & dosage; Cough; Cough/drug therapy; Treatment Outcome
Plain language summary
Inhaled corticosteroids for subacute cough in children
Cough is the most common symptom presenting to doctors. Cough in children negatively impacts on both children and their families, therefore any improvement would be beneficial. Treatment with inhaled corticosteroids may reduce the severity of subacute cough (coughing for two to four weeks) in children by reducing airway inflammation. Data from two small studies were available for inclusion in this review; however, both studies were in infants following hospitalisation for an acute bronchiolitis illness (98 infants in total). There was no difference between groups in the proportion of children 'not cured' at follow‐up. There were no significant side effects in either of these studies. Without further available evidence, recommendations for the use of inhaled corticosteroids for the treatment of subacute cough in children cannot be made. Further well‐designed studies, including children over 12 months of age, are required to determine whether treatment with inhaled corticosteroids can safely and effectively reduce the severity of subacute cough in children.
Summary of findings
Summary of findings for the main comparison. Inhaled corticosteroids for subacute cough.
| Inhaled corticosteroids for subacute cough | ||||||
| Patient or population: children with subacute cough following acute bronchiolitis Settings: treatment given at discharge Intervention: inhaled corticosteroids compared to placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Inhaled corticosteroids | |||||
| Clinical failure (proportion of participants who were not cured or substantially improved (> 70% reduction in cough severity measure) at follow‐up). Follow‐up: 3 to 4 weeks | 41 per 100 | 30 per 100 (14 to 52) | OR 0.61 (0.24 to 1.55) | 98 (2 studies) | ⊕⊕⊝⊝ low1,2,3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Allocation concealment and blinding was unclear in both included studies. Randomisation sequence generation was not described. 2 In both included studies there was a limitation in the directness of the answers to the review question, due to the distinct patient population as defined by age (< 12 months) and illness (post acute bronchiolitis). 3 Both included studies had relatively few patients and few study events, resulting in wide CIs.
Background
Cough is the most common symptom presenting to primary healthcare services in Australia (Britt 2009) and worldwide (Cherry 2008; Irwin 2006a). The symptom of cough is also one of the most frequent reasons for referrals to paediatricians and respiratory physicians (Chang 2006a). In the US, the number of doctor visits per year for cough exceeds 27 million (Cherry 2008). Cough accounts for 6.8 of every 100 visits to general practitioners in Australia (Britt 2009). Cough in children is not only a common problem, but one that impacts at both an individual level with reduced quality of life (Marchant 2008), as well as at a population level due to the considerable expense of treatment (Irwin 2006a). Irrespective of the aetiology or cough duration, the symptom of cough in children is associated with significant morbidity to parents (Cornford 1993; Fuller 1998) and children as it disrupts usual daily activities including school and sleep (Faniran 1998). Cough was the most common reason for school absenteeism in a large community‐based study in the UK (Doull 1996). In the US, one in 10 children receives medication for their acute cough at any one time (Vernacchio 2008). This reflects the anxiety and distress to parents caused by the symptom of cough in their child (Cornford 1993). In addition, the use of unnecessary or inappropriate medications for cough is associated with adverse effects (Thomson 2002).
Description of the condition
Cough duration is variably defined. In the Australian and US, paediatric cough guidelines, subacute cough is defined as cough present for two to four weeks (Chang 2006a; Chang 2006b). Acute cough in children is defined as cough duration of less than two weeks, with chronic cough defined as cough duration of longer than four weeks (Chang 2006a). The paediatric definitions are different to the adult definitions (with chronic cough defined as cough lasting longer than eight weeks), due to the natural history of acute upper respiratory tract infections in children (Hay 2002) and the knowledge that cough in children differs from cough in adults (Chang 2006b; Chang 2005). Cough related to an acute upper respiratory tract infection resolves within one to three weeks in most pre‐school aged children presenting to primary care; however, this cough persists for up to three weeks following an acute upper respiratory tract infection in 10% of young children (Hay 2002).
Description of the intervention
Corticosteroids are a commonly used medication for eosinophilic dominated airway diseases such as asthma. For asthma, oral corticosteroids are used predominantly during periods of exacerbations, while inhaled corticosteroids (ICS) are used mainly for maintenance or preventive therapy (BTS SIGN 2012). In children, ICS can be delivered via a metered dose inhaler (MDI) with or without a spacer, dry powder inhalation (DPI) or through nebulisation.
How the intervention might work
Short‐term treatment with ICS reduces cough frequency in adults with post‐infectious cough (Gillissen 2007). Cough is the dominant symptom of airway inflammation, and airway hyper‐reactivity is also associated with cough (Nair 2010). ICS can ameliorate airway inflammation and airway hyper‐reactivity (at least in some people) (Rytila 2008), thus ICS treatment can potentially reduce the severity of cough in children with sub‐acute cough.
Why it is important to do this review
ICS is recommended as an empirical treatment in guidelines on adults with chronic cough (Irwin 2006b). Although ICS is not recommended in children with isolated chronic cough (i.e. cough without any other symptoms) (Chang 2006b), many doctors continue to use ICS in children with cough of various durations. Evidence examining the use of ICS for non‐specific chronic cough in children has been addressed in a Cochrane systematic review (Tomerak 2005) and an examination of the use of ICS for acute cough is embedded within another Cochrane review in preparation. Although physicians often think "it's only a cough", the symptom of cough is burdensome and substantially reduces the quality of life of parents (Marchant 2008). Due to the significant impact of cough in children, improvement from ICS treatment and other therapies would be beneficial. However, as with all interventions, adverse events also need to be considered. Given the knowledge that high‐dose ICS treatment is associated with significant adverse effects in children, this is a particularly important consideration in this review. A systematic review of the benefits (or otherwise) of ICS on subacute cough would therefore be useful to help guide clinical practice.
Objectives
To evaluate the efficacy of ICS in reducing the severity of cough in children with subacute cough (cough duration of two to four weeks).
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) comparing ICS with a control group in children with subacute cough.
Types of participants
Children (under 18 years of age) with subacute cough (cough duration of two to four weeks).
Exclusion criteria: participants with known chronic respiratory disease (such as cystic fibrosis, asthma, bronchiectasis, aspiration lung disease). Children with cough post acute respiratory infections such as croup were not excluded.
Types of interventions
All randomised controlled comparisons of any type of ICS (MDI, DPI or nebulised). Trials comparing two or more medications without a placebo comparison group were not included. Trials that included the use of other medications or interventions were only to be included if all participants had equal access to such medications or interventions. Treatment with ICS had to be inclusive of the subacute cough phase (two to four weeks) but could have commenced during the first two weeks of illness and continued beyond the four‐week mark.
Types of outcome measures
Reporting of one or more outcomes of interest was not an inclusion criterion.
Primary outcomes
Attempts were made to obtain data on at least one of the following outcome measures:
Primary outcome:
Proportion of participants who were not cured or not substantially improved (> 70% reduction in cough severity measure) at follow‐up (clinical failure).
The following hierarchy of assessment measures for cough severity was to be used (i.e. where two or more assessment measures are reported in the same study, the outcome measure that is listed first in the hierarchy was to be used):
objective measurements of cough indices (cough frequency, cough receptor sensitivity);
symptomatic (quality of life, Likert scale, visual analogue scale, level of interference of cough, cough diary) ‐ assessed by the patient (child);
symptomatic (quality of life, Likert scale, visual analogue scale, level of interference of cough, cough diary) ‐ assessed by the parents/carers;
symptomatic (Likert scale, visual analogue scale, level of interference of cough, cough diary) ‐ assessed by clinicians.
Secondary outcomes
Proportion of participants who were not cured at follow‐up.
Proportion of participants who were not substantially improved at follow‐up.
Mean difference in cough indices (cough diary, cough frequency, cough scores, quality of life).
Proportion of participants experiencing adverse effects of the intervention.
Proportion of participants experiencing complications (e.g. requirement for medication change).
The same hierarchy of assessment measures for cough severity was to be used for secondary outcomes one and two.
Search methods for identification of studies
Electronic searches
RCTs were identified using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts (please see the Airways Group Module on The Cochrane Library for further details). Additional searches of CENTRAL, MEDLINE and EMBASE were also conducted. The full search strategies are detailed in Appendix 1. Databases were searched from their inception up to November 2011, and there was no restriction on the language of publication.
Searching other resources
We handsearched references from identified papers and reviews for further references. We contacted authors to request their identification of any unpublished or missed trials.
Data collection and analysis
Selection of studies
Two sets of review authors (SA, AC for initial search; SA, JM for subsequent search) independently assessed for inclusion all the potentially relevant studies identified as a result of the search strategy. It was planned that any disagreement would be resolved through discussion or, if required, adjudication by a third review author.
Data extraction and management
Trials that satisfied the inclusion criteria were independently reviewed and the following information recorded: study setting, year of study, source of funding, participant recruitment details (including number of eligible people), inclusion and exclusion criteria, other symptoms, randomisation and allocation concealment method, number of participants randomised, blinding (masking) of participants, care providers and outcome assessors, duration of intervention, co‐interventions, number of participants not followed up, reasons for withdrawals from study protocol (clinical, side effects, refusal and other), details on side effects of therapy, and whether intention‐to‐treat analyses were possible. Data were extracted for the outcomes described above and any follow‐up data provided in the following four weeks post intervention were sought. Further information was requested from the study authors where required.
Assessment of risk of bias in included studies
Two sets of review authors (SA, AC for initial search; SA, JM for subsequent search) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). It was planned that any disagreement would be resolved by discussion or by involving a third review author. We assessed the risk of bias according to the following domains:
allocation sequence generation;
concealment of allocation;
blinding of participants and investigators;
incomplete outcome data;
selective outcome reporting.
We also noted other sources of bias. Each potential source of bias was graded as high risk, low risk or unclear risk, relating to whether the potential for bias was high, low or unknown, respectively.
Measures of treatment effect
An initial qualitative comparison of all the individually analysed studies examined whether pooling of results (meta‐analysis) was reasonable. This took into account differences in study populations, inclusion and exclusion criteria, interventions and outcome assessment. The results from studies that met the inclusion criteria and reported any of the outcomes of interest were included in the subsequent meta‐analyses.
For the dichotomous outcome variables of each individual study, we calculated the odds ratio (OR) and 95% confidence intervals (CIs) using a modified intention‐to‐treat analysis (modified if there were missing values due to drop‐outs). We used the Cochrane statistical package Review Manager 5 (RevMan 2011). Numbers needed to treat for an additional beneficial effect (NNTB) were to be calculated from the pooled OR and its 95% CI applied to a specified baseline risk (from the control group) using an online calculator (Cates 2003).
For continuous outcomes we planned to calculate the mean difference and 95% CIs using RevMan 2011. If studies reported outcomes using different measurement scales, the standardised mean difference was to be estimated.
Unit of analysis issues
Cross‐over trials are not appropriate for this intervention duration and thus were not included in any meta‐analysis but were to be described in the text.
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where possible.
Assessment of heterogeneity
We used the I2 statistic to measure heterogeneity among the trials in each analysis. If we had identified substantial heterogeneity, we planned to explore it by using pre‐specified subgroup analysis. We described any heterogeneity between the study results and tested this to see if it reached statistical significance using the Chi2 test. We considered heterogeneity to be significant when the P value was less than 0.10 (Higgins 2011). We categorised heterogeneity such that a value of under 25% was considered low, around 50% was considered moderate and over 75% was considered a high degree of heterogeneity.
Assessment of reporting biases
Where we suspected reporting bias (see 'Selective reporting bias' below), we attempted to contact study authors asking them to provide missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, the impact of including such studies in the overall assessment of results was to be explored by a sensitivity analysis.
If combination of data and meta‐analysis (with at least five studies) was possible, we planned to assess publication bias using a funnel plot. We planned to try and identify and report on any selective reporting in the included trials, ideally by comparing the trial protocol with the final published paper, but alternatively by comparing the 'Methods' and 'Results' sections of the published studies.
Data synthesis
We determined the summary OR and mean differences with their 95% CIs using a fixed‐effect model. We planned to use a random‐effects model whenever there were concerns about statistical heterogeneity.
Subgroup analysis and investigation of heterogeneity
type of control arm (placebo/no treatment);
children in different age groups (younger than six years, six to 14 years and 15 years and above) (as older children are more likely to have adult‐like cough responses);
doses of ICS (low to moderate defined as < 800 µg/day budesonide equivalent versus high defined as ≥ 800 µg/day budesonide equivalent).
Sensitivity analysis
Sensitivity analyses were also planned to assess the impact of the potentially important factors on the overall outcomes:
variation in the inclusion criteria;
risk of bias in the included studies (i.e. double versus single blinded or unblinded; allocation clearly concealed versus unclear or no concealment);
analysis using random‐effects model;
analysis by 'treatment received' or 'intention‐to‐treat'
nebulised ICS versus MDIs.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
The Airways Group register/search identified 1178 potentially relevant titles (see Appendix 1 for search strategy). After assessment of the abstracts, 14 papers were obtained for consideration for inclusion into the review. Two studies were included in the final review (see Figure 1). Both papers were published in English. There were no RCTs comparing ICS for subacute cough in children over 12 months of age.
1.
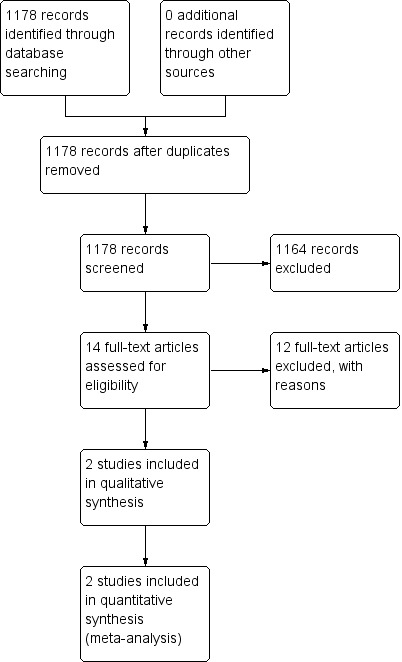
Study flow diagram.
Included studies
Of the two studies included, one was a single‐centre study (Wong 2000) and one was a dual‐centre study (Fox 1999). One study (Wong 2000) received support from a commercial interest (GlaxoWellcome). Both studies were conducted in hospitals within the UK and recruited infants admitted to paediatric wards with acute bronchiolitis, and the studies commenced when the infants were 'ready for discharge'. In both studies, cough duration at commencement of study medication was not specifically defined. The median age of participants in Fox 1999's study was 11 weeks. The mean ages in Wong 2000's study were 3.8 months (treatment group) and 3.9 months (placebo group). All participants in both studies were aged under 10.9 months, and the study populations appeared very similar.
Both studies were double‐blind, parallel group RCTs using twice daily ICS delivered via MDI with spacer and face mask compared to placebo. One study (Fox 1999) used budesonide 200 µg or one puff twice daily for eight weeks, and one study (Wong 2000) used fluticasone propionate 150 µg (3 puffs of a 50 µg inhaler) twice daily for three months, which are considered comparable ICS doses. As both these studies incorporate use of ICS beyond the acute two‐week period (i.e. within the subacute definition of cough), both fulfilled the eligibility criteria of this review.
Outcomes were available at three weeks (Wong 2000) and four weeks (Fox 1999). An objective outcome measurement of cough indices (overnight cough recording using a voice‐activated tape recorder) was used in one study (Wong 2000); however, as these were overnight cough recordings only, there were no objective day‐time cough symptom data available. In the same study (Wong 2000), additional symptomatic cough data (parent‐recorded diary card) was only available at three months, therefore this outcome could not be included in this review. No differentiated cough symptom data were available in one study (Fox 1999), which also used parent‐recorded respiratory symptom cards (combining cough and wheeze) as a subjective outcome measure. Only episodes of cough and wheeze that required treatment by a general practitioner (GP) or emergency department were included in the statistical analysis by Fox 1999.
Excluded studies
Twelve papers were excluded as they did not fulfil the criteria for the review. The main reasons studies were excluded were cough duration (chronic cough rather than subacute cough) and age of participants (adults rather than children), see Characteristics of excluded studies. Other reasons included physician‐diagnosed asthma, non‐randomised studies, cross‐over study design and non‐ICS treatment.
Risk of bias in included studies
This is summarised in the 'Risk of bias' summary (Figure 2).
2.
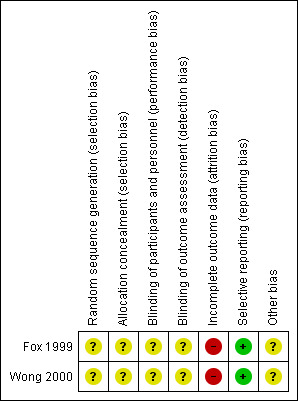
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Allocation was unclear in both included studies. Although both included studies stated that they were randomised, the methods of sequence generation and allocation concealment were not described in either paper.
Blinding
Blinding was unclear in both included studies. Although both included studies stated they were double blind, the methods used for blinding were not described in either paper. We could not determine who collected data in either study or how they were blinded.
Incomplete outcome data
The total number of participants withdrawn and lost to follow‐up from both included studies were described. The treatment allocation for each withdrawal was only reported for one study (Wong 2000). Participants with no overnight cough data following the baseline cough recordings were eliminated from the analysis (Wong 2000).
Selective reporting
Limitations of both studies were discussed by the authors. There was no suggestion that selective reporting had occurred.
Other potential sources of bias
The number of potentially eligible participants was not described in either study, resulting in an unclear assessment of recruitment selection bias.
Both studies included participants within a distinct patient population with a small age range. Participants were not specifically recruited for cough, cough duration at commencement of study medication was ill‐defined, and studies include limited objective cough measures.
Effects of interventions
See: Table 1
The two studies included 104 infants. Both studies used twice daily ICS treatment via MDI, spacer and face mask. Outcomes for the two studies were available at three weeks (Wong 2000) and four weeks (Fox 1999). Follow‐up data at these time points were available for 98 infants.
Primary outcome
Our primary outcome was the proportion of participants who were not cured or substantially improved (> 70% reduction in cough severity measure) at follow‐up (clinical failure).
Data from 98 infants in both studies were combined for this outcome measure. The number of children not cured at follow‐up was 36, using an 'intention‐to‐treat' analysis. The control event rate was 69.57% (Wong 2000) and 17.86% (Fox 1999) in the two studies. There was no significant difference between groups in the proportion of children 'not cured' at follow‐up, with a pooled OR of 0.61 (95% CI 0.24 to 1.55), see Figure 3 and Table 1.
3.
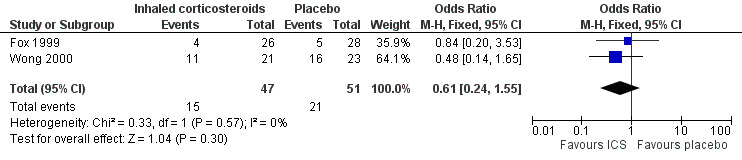
Forest plot of comparison: 1 Inhaled corticosteroids versus placebo, outcome: 1.1 Clinical failure.
Both included studies used a placebo control, thus subgroup analysis of type of control arm could not be performed. All participants were infants aged less than 12 months, therefore subgroup analysis of children in different age groups could not be performed. Both included studies used a low‐to‐moderate dose of ICS, hence subgroup analysis of doses of ICS could not be performed.
Secondary outcomes
Proportion of participants who were not cured at follow‐up was the same as the primary outcome measure as there were no data available for the proportion of participants who were not substantially improved at follow‐up in either study. There were no available data to suggest the severity of ongoing symptoms. Follow‐up data for both studies, at three weeks (Wong 2000) and four weeks (Fox 1999), were presented as 'cured' versus 'not cured'.
Mean difference in cough indices (cough diary, cough frequency, cough scores, quality of life): in the Wong 2000 study, cough recordings at the different time points were reported as group median values of weighted mean changes from baseline, hence data could not be entered into a forest plot. Authors of the study (Wong 2000) reported improvements in both groups but no significant difference between the ICS and placebo groups; at three weeks, the change in cough events per hour were ‐0.12 (95% CI ‐0.69 to 0) in the ICS group and ‐0.27 (95% CI ‐0.44 to ‐0.01) in the placebo group, and at six weeks, the respective values were ‐0.57 (95% CI ‐2.05 to ‐0.04) and ‐0.76 (95% CI ‐1.64 to ‐0.15). Symptomatic cough data (parent‐recorded diary card) in the study by Wong 2000 were only available at three months, therefore could not be included in this review. No differentiated cough symptom data were available in the study by Fox 1999, which also used parent‐recorded respiratory symptom cards (combining cough and wheeze) as a subjective outcome measure.
Proportion of participants experiencing adverse effects of the intervention: Wong 2000 reported that two infants in the fluticasone treatment group developed oral candidiasis during follow‐up; however, no fungal infections occurred during the treatment period. Fox 1999 reported two adverse events; however, these were unrelated to the study treatment (one infant in the placebo group was admitted to hospital with viral gastroenteritis and one infant in the budesonide group was re‐admitted with mild coughing and wheezing).
Proportion of participants experiencing complications (e.g. requirement for medication change): there were no reported complications in either study.
Sensitivity analyses
As there were only two studies included in this review, re‐analysis using a random‐effects model or by 'treatment received' or 'intention‐to‐treat' was not possible.
Inclusion criteria were similar in both studies, therefore re‐analysis by variation in the inclusion criteria could not be performed.
Risk of biases in the included studies (i.e. double versus single blinded or unblinded; allocation clearly concealed versus unclear or no concealment) was considered to be similar between the two available studies, as both studies were double blinded; however, allocation concealment was not described in either study.
Both studies used MDIs, therefore sensitivity analysis comparing nebulised ICS versus MDIs was unable to be performed.
Discussion
Summary of main results
A total of 98 infants were included in the meta‐analysis. There was no significant difference between groups in the proportion of children 'not cured' (primary outcome measure), see Table 1. There is currently no evidence to support the use of ICS in subacute cough in children. The evidence is limited by the small number of studies available for analysis as well as the quality and design of included studies.
Overall completeness and applicability of evidence
Evidence is limited by the small number of studies eligible for inclusion in this review. The two included studies both recruited a distinct patient population with a small age range, as all participants were less than 12 months of age. Further, while it is clear that treatment with ICS was administered in the non‐acute phase, it is unclear exactly when the intervention was commenced. It also remains unknown if ICS commenced early (i.e. during the acute phase) prevents ongoing symptoms, and differs from later use of ICS for treatment of cough.
All participants were infants recruited post hospitalisation for an acute bronchiolitis illness, so are a select subgroup of the general paediatric population. Studies have shown that infants with bronchiolitis have increased likelihood of ongoing recurrent episodes of cough and wheeze in the following 12 to 24 months, suggesting some ongoing airway pathology post this illness (Wennergren 2001). Studies on the use of ICS in subacute cough, limited to this distinct patient population, that is post acute bronchiolitis, are likely to be biased and not applicable to the paediatric population as a whole.
Both of these studies did not specifically recruit for the symptom of cough, did not have a clearly defined cough duration at the start of study treatment and included limited objective cough measures. In terms of cough indices, Fox 1999 did not differentiate cough symptom data and Wong 2000 measured overnight cough only. While outcomes for both studies were available in the short term, both studies were designed as longer‐term interventions with a longer follow‐up period, limiting completeness and applicability when assessing only for shorter‐term outcomes.
Further, clinicians should be cognisant that cough is a symptom and not a disease, and that use of ICS is not without potential adverse events. Both included studies were small and short term thus unlikely to define important yet uncommon adverse events associated with ICS such as growth failure and adrenal suppression (Patel 2001).
Quality of the evidence
Both included studies were double‐blind RCTs; however, neither the sequence generation or allocation concealment methods were adequately described in either study, therefore the quality of the included studies is unclear. Cough recordings reported by Wong 2000 were overnight recordings only, with no objective day‐time cough data available, and available data were only presented as 'cured' versus 'not‐cured', which are both potential weaknesses of this study. No differentiated cough symptom data were available in the study by Fox 1999, which was confirmed via written correspondence with the study investigator. In addition, the difference in control event rates between the two included studies (69.57% in Wong 2000 compared to 17.86% in Fox 1999) suggests that there may have been differences between the two included study populations, despite the apparent similarities.
Potential biases in the review process
There were no perceived biases in the review process.
Agreements and disagreements with other studies or reviews
There are no known other reviews or studies available for comparison.
Authors' conclusions
Implications for practice.
Due to the significant impact of cough in children, improvement from ICS treatment or other therapies would be beneficial. Without further available evidence, recommendations for the use of ICS for the treatment of subacute cough in children cannot be made. This review is limited by the small number of studies available for inclusion, the characteristics of the included studies, and the data available for analysis. The two studies included in this review describe a small number of participants from a distinct patient population within a small age range. The quality of the included studies was limited by the unclear risk of allocation concealment biases.
Implications for research.
Further well‐designed double‐blind parallel RCTs, specifically powered to answer this question, and using appropriate randomisation sequence generation and allocation concealment are required to support or refute the efficacy of treatment with ICS in children with subacute cough, and make valid conclusions in respect to the safety of this treatment. These studies should include children over 12 months, without acute bronchiolitis and with clearly defined cough duration at commencement of study treatment, to assess the role of ICS treatment in children with subacute cough. Future RCTs should be designed to include objective cough outcome measures such as cough frequency recordings, or validated symptomatic measures such as a cough score diary or visual analogue scale as assessed by the child, if age permits, and the parent/guardian. The study design should include a clear and appropriate definition of clinical improvement, utilising these objective measures.
Acknowledgements
We thank Dr Emma Welsh and Dr Chris Cates from the Cochrane Airways Group, and Dr Kerry‐Ann O'Grady from the Queensland Children's Medical Research Institute, for their advice, supportive role and comments to the protocol and review. We also thank Elizabeth Stovold for performing the searches. We are grateful to Dr Fox and Dr Wong for responding to our queries on their original studies needed to complete this review. Finally we thank the Australian Cochrane Airways Group and Scholarship for providing funding for SA to complete this review and present its findings at an international meeting.
Appendices
Appendix 1. Database search strategies
Airways Group Register
#45=COUGH and (((steroid* or corticosteroid* or glucocorticosteroid* or glucocorticoid* or corticoid*) AND (inhal*)) or (beclomethasone or budesonide or fluticasone or ciclesonide or mometasone or flunisolide or mometasone))
CENTRAL (on The Cochrane Library)
#1 MeSH descriptor Cough explode all trees
#2 cough*
#3 (#1 OR #2)
#4 MeSH descriptor Adrenal Cortex Hormones explode all trees
#5 (steroid* or corticosteroid* or glucocorticosteroid* or glucocorticoid* or corticoid*) AND (inhal*)
#6 beclomethasone or budesonide or fluticasone or ciclesonide or mometasone or flunisolide or mometasone
#7 (#4 OR #5 OR #6)
#8 (#3 AND #7)
#9 paediatric* or paediatric* or child* or adolescen* or infant* or young* or preschool* or pre‐school* or newborn* or new‐born* or neonat* or neo‐nat*
#10 MeSH descriptor Child explode all trees
#11 MeSH descriptor Pediatrics explode all trees
#12 MeSH descriptor Infant explode all trees
#13 MeSH descriptor Adolescent explode all trees
#14 (#9 OR #10 OR #11 OR #12 OR #13)
#15 (#8 AND #14)
MEDLINE (Ovid)
1. Cough/
2. cough$.mp. 3. 1 or 2
4. exp Adrenal Cortex Hormones/
5. ((steroid$ or corticosteroid$ or glucocorticosteroid$ or glucocorticoid$ or corticoid) adj5 inhal$).mp.
6. (beclomethasone or budesonide or fluticasone or ciclesonide or mometasone or flunisolide or mometasone).mp.
7. 4 or 5 or 6
8. 3 and 7
9. (clinical trial or controlled clinical trial or randomised controlled trial).pt.
10. (randomised or randomised).ab,ti.
11. placebo.ab,ti.
12. dt.fs.
13. randomly.ab,ti.
14. trial.ab,ti.
15. groups.ab,ti.
16. or/9‐15
17. Animals/
18. Humans/
19. 17 not (17 and 18)
20. 16 not 19
21. 8 and 20
22. exp Child/
23. exp Pediatrics/
24. exp infant/
25. exp adolescent/
26. (paediatric$ or paediatric$ or child$ or adolescen$ or infant$ or young$ or preschool$ or pre‐school$ or newborn$ or new‐born$ or neonat$ or neo‐nat$).mp.
27. 22 or 23 or 24 or 25 or 26
28. 21 and 27
EMBASE (Ovid)
1. exp COUGHING/
2. cough$.mp.
3. 1 or 2
4. exp corticosteroid/
5. ((steroid$ or corticosteroid$ or glucocorticosteroid$ or glucocorticoid$ or corticoid$) adj5 inhal$).mp.
6. (beclomethasone or budesonide or fluticasone or ciclesonide or mometasone or flunisolide or mometasone).mp.
7. 4 or 5 or 6
8. 3 and 7
9. Randomized Controlled Trial/
10. Controlled Study/
11. randomisation/
12. Double Blind Procedure/
13. Single Blind Procedure/
14. Clinical Trial/
15. Crossover Procedure/
16. follow up/
17. exp prospective study/
18. or/9‐17
19. (clinica$ adj3 trial$).mp.
20. ((singl$ or doubl$ or trebl$ or tripl$) adj5 (mask$ or blind$ or method$)).mp.
21. exp Placebo/
22. placebo$.mp.
23. random$.mp.
24. (latin adj3 square$).mp.
25. exp Comparative Study/
26. ((control$ or prospectiv$ or volunteer$) adj3 (trial$ or method$ or stud$)).mp.
27. (crossover$ or cross‐over$).mp.
28. or/19‐27
29. 18 or 28
30. exp ANIMAL/
31. Nonhuman/
32. Human/
33. 30 or 31
34. 33 not 32
35. 29 not 34
36. 8 and 35
37. child/
38. exp pediatrics/
39. infant/
40. adolescent/
41. (paediatric$ or paediatric$ or child$ or adolescen$ or infant$ or young$ or preschool$ or pre‐school$ or newborn$ or new‐born$ or neonat$ or neo‐nat$).mp.
42. 37 or 38 or 39 or 40 or 41
43. 36 and 42
Data and analyses
Comparison 1. Inhaled corticosteroids versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure | 2 | 98 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.24, 1.55] |
1.1. Analysis.
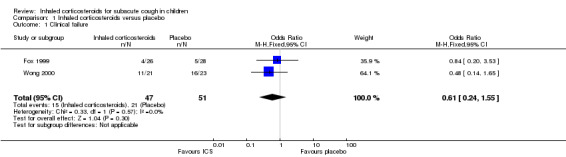
Comparison 1 Inhaled corticosteroids versus placebo, Outcome 1 Clinical failure.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Fox 1999.
| Methods | Dual‐centre double‐blind randomised controlled trial comparing budesonide 200 µg or 1 puff twice daily (via metered dose inhaler (MDI) with modified spacer and mask) versus placebo in infants admitted to hospital with acute bronchiolitis At baseline participants had a medical history, nasopharyngeal swab and clinical examination performed Randomisation occurred when infants were considered ready for discharge. Method of sequence generation and allocation concealment not described |
|
| Participants | 60 infants, aged median (range) 11 (1 to 42) weeks, with clinical diagnosis of acute viral bronchiolitis requiring hospital admission were included. There were no significant differences in any patient characteristics between the 2 groups Inclusion criteria: infants aged less than 12 completed months with a clinical diagnosis of acute viral bronchiolitis Exclusion criteria: children with underlying cardiopulmonary disease, including congenital heart disease, bronchopulmonary disease and cystic fibrosis, and those who experienced respiratory problems in the neonatal period, or required mechanical ventilation during current illness Follow‐up data at 1 month were available for 54 of the 60 infants initially randomised. Budesonide group N = 26 (20 males), placebo group N = 28 (14 males) 1 participant was excluded after randomisation but before receiving any study medication as required mechanical ventilation, 1 participant was excluded at the first follow‐up appointment due to poor compliance, and 4 additional participants failed to attend any follow‐up appointments 8 included infants had been born prematurely between 32 and 37 weeks' gestation, with 6 randomised to the placebo group; however, the difference between groups was non‐significant |
|
| Interventions | Treatment group received inhaled budesonide 200 µg or 1 puff twice daily (via MDI with spacer and mask) for 8 weeks, versus placebo control group Additional medications received by participants during the next 12‐month follow‐up period included cough suppressants, oral and inhaled bronchodilators, and inhaled and systemic corticosteroids. |
|
| Outcomes | Primary outcome: reduction in incidence of coughing and wheezing episodes requiring treatment by a general practitioner (GP) or emergency department during a 12‐month follow‐up period Parent‐completed diary card record of respiratory symptoms (episodes of coughing and wheezing), GP and hospital visits, and medication prescribed and used over a 12‐month follow‐up period Clinical examinations occurred at 1, 2, 6 and 12 months post discharge. 2 adverse events were recorded; however, these were unrelated to study medication. 1 infant was admitted to hospital with viral gastroenteritis and 1 infant was re‐admitted with mild coughing and wheezing |
|
| Notes | Only episodes of cough and wheeze that required treatment by a GP or emergency department were included in the statistical analysis Written communication with the author did not provide further differentiated cough symptom data Study funded by grants from the National Asthma Campaign and The St Thomas's Hospital Special Trustees |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised to receive either budesonide or placebo (30 to each group). Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Stated double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who was the assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reasons for withdrawals and missing data described; however, not included in final analysis |
| Selective reporting (reporting bias) | Low risk | Limitations of study discussed. Authors suggested possible Type 1 error due to more males in the treatment group |
| Other bias | Unclear risk | Possible selection bias in recruitment, as number of potentially eligible participants not described. Additional medications allowed during the follow‐up period included inhaled and systemic corticosteroids |
Wong 2000.
| Methods | Single‐centre, double‐blind randomised controlled trial comparing fluticasone propionate 150 µg (3 puffs of a 50 µg inhaler) twice daily (via metered dose inhaler (MDI) with low volume spacer and mask) versus placebo, in infants admitted to hospital for first documented episode of acute bronchiolitis Nasopharyngeal aspirates were sent for immunofluorescent study and viral culture. A detailed history was obtained and documented with examination findings and treatments Randomisation occurred when infants were considered ready for discharge. Method of sequence generation and allocation concealment not described Statistical analysis was performed on an intention‐to‐treat basis |
|
| Participants | 48 infants aged 2 to 52 weeks, with first documented episode of acute bronchiolitis requiring admission Inclusion criteria: infants admitted with first episode of lower respiratory tract infection, diagnosed by 1 investigator as having acute bronchiolitis using COURT criteria. Specific inclusion ages not stated Exclusion criteria: birth before 36 weeks' gestation; congenital heart disease or syndromic abnormalities; established systemic or chronic illnesses; treatment with corticosteroids or mechanical ventilation before entering the study. Those who were unable to master the required medication delivery technique after education were also excluded Follow‐up data at 3 weeks were available for 44 of the 48 infants initially randomised. Fluticasone propionate group N = 21, placebo group N = 23. The demographic data were similar in the 2 groups 2 participants in the treatment group were withdrawn due to distress resulting from the application of the face mask, with a third participant withdrawn due to social reasons. 1 participant in the placebo group was withdrawn for non‐compliance after the treatment period |
|
| Interventions | Treatment group received 150 µg fluticasone propionate via MDI with low‐volume spacer and mask for 3 months, versus placebo control group Prescription of additional medications by independent doctors included beta2‐agonists, corticosteroids and antibiotics |
|
| Outcomes | Primary outcome: reduction in overnight cough rate from pre‐treatment baseline levels Overnight cough recordings using a voice‐activated tape recorder, attempted at baseline and at each of the 6 follow‐up visits. During the treatment period, 87% of attempted cough recordings were technically successful. Not attempted on 10 occasions. Weighted mean change in cough rate was used to compare reductions in cough rate between treatment groups Percentages of infants cough free (based on overnight recording) at each home visit were reported Overnight oxygen saturation measurements were also conducted at time of cough recording Data on symptom frequency, use of rescue respiratory medications, hospital admissions was collected. Follow‐up clinical examinations occurred on 6 occasions over 12 months. Clinical decisions about need for additional treatments were made by family practitioners and hospital doctors. Family doctor and hospital records were examined at the end of the study for collaborative information Parents completed symptom diaries scoring cough, wheeze and general well‐being for both day and night; however, data were reported as percentage of days over 3 months for each symptom score Lung function was measured 6 months after hospital discharge (3 months after the treatment period), therefore could not be included in this review |
|
| Notes | Written communication with the study author did not provide further differentiated cough symptom data Project funded by GlaxoWellcome |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; however, method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Stated double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Primary outcome measure overnight cough rate (objective measure); however, unclear who was the assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop‐outs and withdrawals described, and included in final analysis up until withdrawal date. Participants with no overnight cough data following the baseline cough recordings were eliminated from the analysis |
| Selective reporting (reporting bias) | Low risk | Limitations of study discussed |
| Other bias | Unclear risk | Possible selection bias in recruitment, as number of potentially eligible participants not described. Prescription of additional medications by independent doctors included bronchodilators, corticosteroids and antibiotics. More infants in the placebo group received bronchodilators/corticosteroids, but not antibiotics |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Davies 1999 | Double‐blind randomised controlled trial of inhaled fluticasone propionate versus placebo. Cough duration was ≥ 6 weeks, although inclusion criteria was > 3 weeks. Excluded from review as chronic cough, not subacute cough |
| Evald 1989 | Randomised controlled trial of inhaled beclomethasone dipropionate versus placebo. Cross‐over occurred at 2 weeks. Excluded from review as chronic cough, not subacute cough, cross‐over study design, and participants were adults, not children |
| Jartti 2007 | Randomised controlled trial of oral prednisolone versus placebo. Excluded from review as oral prednisolone, not inhaled corticosteroids, and investigating effect on wheezing in virus‐positive children, not cough |
| Kooi 2008 | Double‐blind randomised controlled trial of inhaled fluticasone propionate, montelukast or placebo in children with asthma‐like symptoms. Study inclusion criteria does not comply as no separate cough symptom data, and daily symptoms were not required for inclusion. Run‐in period of 2 weeks implemented. Excluded from review as chronic cough, not subacute cough |
| Kwon 2006 | Not a randomised controlled trial (RCT). Study of adults with cough duration of 3 to 8 weeks who underwent bronchoprovocation and induced sputum tests to determine treatment with inhaled corticosteroids. Excluded from review as not an RCT, subacute and chronic cough, and adults, not children |
| Moskovljevic 2009 | Double‐blind randomised controlled trial of inhaled fluticasone propionate, montelukast or placebo in children aged 8 to 18. Excluded from review as study inclusion criteria do not comply, as participants had asthma‐like symptoms, and no short‐term data were available (collected at baseline and after 3 months) |
| Pelkonen 2009 | Randomised controlled trial of inhaled budesonide or placebo in children aged 3 to 26 months with abnormal lung function. Lower range of cough duration was 2 months, therefore excluded from review as chronic cough, not subacute cough, and abnormal lung function suggesting underlying condition |
| Ponsioen 2005 | Double‐blind randomised controlled trial of inhaled fluticasone propionate versus placebo in adults with cough duration ≥ 2 weeks. Excluded as adult participants, not children |
| Pornsuriyasak 2005 | Double‐blind randomised controlled trial of inhaled budesonide or placebo in participants with persistent post upper respiratory tract infection cough. All participants were aged ≥ 18 years, although inclusion criteria was > 15 years (as per written communication with author). Excluded from review as adult participants, not children |
| Profita 2010 | Double‐bind randomised controlled trialof inhaled beclomethasone dipropionate versus placebo in children with clinical diagnosis of intermittent asthma. Excluded from review as study inclusion criteria does not comply as participants had asthma |
| Puhakka 1998 | Double‐blind randomised controlled trial of intranasal fluticasone propionate versus placebo in adults for common cold. Excluded from review as adults, not children, and intranasal not inhaled corticosteroids, cough symptom data not differentiated |
| Ribeiro 2007 | Double‐blind randomised controlled trial of inhaled beclomethasone dipropionate versus placebo in adults with chronic cough (duration of ≥ 8 weeks). Excluded from review as chronic cough, not subacute cough and adults, not children |
| Rytila 2000 | Single‐blind randomised controlled trial of inhaled beclomethasone dipropionate versus placebo in adults with asthma. Excluded from review as adults, not children, and participants with asthma |
| Yuksel 1992 | Double‐blind randomised controlled trial of inhaled beclomethasone dipropionate versus placebo in premature infants. Cough duration was greater than 4 weeks. Excluded from review as chronic cough, not subacute |
Differences between protocol and review
Risk of bias categories were updated from 'yes', 'no' and 'unclear' to 'high risk', 'low risk' and 'unclear risk'.
Review Manager software version changed from 5.0 to 5.1.
Contributions of authors
SA and AC wrote the protocol, based on our previous protocols. Both selected articles from the search. SA and JM double entered data. SA, JM and AC drafted the manuscript. JA and CT reviewed the protocol and commented on the manuscript.
Sources of support
Internal sources
-
Royal Children's Hospital Foundation, Australia.
Program support for research team
-
Queensland Children's Medical Research Institute, Australia.
Program fund to research group
External sources
-
National Health and Medical Research Council, Australia.
Practitioner Fellowship for AC (grant number 545216)
-
Australian Cochrane Airways Group, Australia.
Support to SA
Declarations of interest
None of the authors has any conflict of interest.
New
References
References to studies included in this review
Fox 1999 {published data only (unpublished sought but not used)}
- Fox GF, Everard ML, Marsh MJ, Milner, AD. Randomised controlled trial of budesonide for the prevention of post‐bronchiolitis wheezing. Archives of Disease in Childhood 1999;80:343‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2000 {published data only (unpublished sought but not used)}
- Wong JYW, Moon S, Beardsmore C, O'Callaghan C, Simpson H. No objective benefit from steroids inhaled via a spacer in infants recovering from bronchiolitis. European Respiratory Journal 2000;15:388‐94. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Davies 1999 {published data only}
- Davies MJ, Fuller P, Picciotto A, McKenzie SA. Persistent nocturnal cough: randomised controlled trial of high dose inhaled corticosteroid. Archives of Disease in Childhood 1999;81:38‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Evald 1989 {published data only}
- Evald T, Munch EP, Kok‐Jensen A. Chronic non‐asthmatic cough is not affected by inhaled beclomethasone dipropionate. A controlled double blind clinical trial. Allergy 1989;44:510‐4. [DOI] [PubMed] [Google Scholar]
Jartti 2007 {published data only}
- Jartti T, Lehtinen P, Vanto T, Vuorinen T, Hartiala J, Hiekkanen H, et al. Efficacy of prednisolone in children hospitalised for recurrent wheezing. Pediatric Allergy & Immunology 2007;18:326‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kooi 2008 {published data only (unpublished sought but not used)}
- Kooi EM, Schokker S, Marike Boezen H, Vries TW, Vaessen‐Verberne AA, Molen T, et al. Fluticasone or montelukast for preschool children with asthma‐like symptoms: randomised controlled trial. Pulmonary Pharmacology and Therapeutics 2008;21:798‐804. [DOI] [PubMed] [Google Scholar]
Kwon 2006 {published data only}
- Kwon NH, Oh MJ, Min TH, Lee BJ, Choi DC. Causes and clinical features of subacute cough. Chest 2006;129:1142‐47. [DOI] [PubMed] [Google Scholar]
Moskovljevic 2009 {published data only}
- Moskovljevic J. Therapy effect of fluticasone and montelukast in school‐children with asthma. Allergy: European Journal of Allergy and Clinical Immunology 2009;64(Suppl 90):183‐4. [Google Scholar]
Pelkonen 2009 {published data only}
- Pelkonen AS, Malmstrom K, Malmberg LP, Sarna S, Turpeinen M, Kajosaari M, et al. Budesonide improves decreased airway conductance in infants with respiratory symptoms. Archives of Disease in Childhood 2009;94:536‐41. [DOI] [PubMed] [Google Scholar]
Ponsioen 2005 {published data only}
- Ponsioen BP, Hop WC, Vermue NA, Dekhuijzen PN, Bohnen AM. Efficacy of fluticasone on cough: a randomised controlled trial. European Respiratory Journal 2005;25:147‐52. [DOI] [PubMed] [Google Scholar]
Pornsuriyasak 2005 {published data only (unpublished sought but not used)}
- Pornsuriyasak P, Charoenpan P, Vongvivat K, Thakkinstian A. Inhaled corticosteroid for persistent cough following upper respiratory tract infection. Respirology 2005;10:520‐24. [DOI] [PubMed] [Google Scholar]
Profita 2010 {published data only}
- Profita M, Riccobono L, Liotta G, Bonanno A, Gjomarkaj M, Cibella F, et al. Nebulised beclomethasone in the treatment of childhood mild intermittent asthma with seasonal allergic rhinitis. A randomised, double‐blind, placebo controlled study. Allergy: European Journal of Allergy and Clinical Immunology 2010;64(Suppl 90):679. [Google Scholar]
Puhakka 1998 {published data only}
- Puhakka T, Makela MJ, Malmstrom K, Uhari M, Savolainen J, Terho EO, et al. The common cold: effects of intranasal fluticasone propionate treatment. The Journal of Allergy and Clinical Immunology 1998;101:726‐31. [DOI] [PubMed] [Google Scholar]
Ribeiro 2007 {published data only}
- Ribiero M, Pereira CADC, Nery LE, Beppu OS, Silva COS. High‐dose inhaled beclomethasone treatment in patients with chronic cough: a randomised placebo‐controlled study. Annals of Allergy, Asthma and Immunology 2007;99:61‐8. [DOI] [PubMed] [Google Scholar]
Rytila 2000 {published data only}
- Rytila P, Metso T, Heikkinen K, Saarelainen P, Helenius IJ, Haahtela T. Airway inflammation in patients with symptoms suggesting asthma but with normal lung function. European Respiratory Journal 2000;16:824‐30. [DOI] [PubMed] [Google Scholar]
Yuksel 1992 {published data only}
- Yuksel B, Greenough A. A randomised trial of inhaled steroids in preterm infants with respiratory symptoms at follow up. Thorax 1992;47:910‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Britt 2009
- Britt H, Miller GC, Charles J, Henderson J, Bayram C, Pan Y, et al. General Practice Activity in Australia 2008‐2009. Australian Institute of Health and Welfare 2009; Vol. AIHW Cat. No. GEP 25.
BTS SIGN 2012
- British Thoracic Society (BTS) and Scottish Intercollegiate Guidelines Network (SIGN). British Guideline on the Management of Asthma, revised 2012. www.sign.ac.uk/pdf/sign101.pdf. (accessed 10 December 2012).
Cates 2003 [Computer program]
- Cates C. Visual Rx v3. Online NNT Calculator [Computer program]. Cates C . London: http://www.nntonline.net/, 2003.
Chang 2005
- Chang AB. Cough: are children really different to adults?. Cough 2005;1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2006a
- Chang AB, Landau LI, Asperen PP, Glasgow NJ, Robertson CF, Marchant JM, et al. Thoracic Society of Australia and New Zealand. Position statement. Cough in children: definitions and clinical evaluation. Medical Journal of Australia 2006;184:398‐403. [DOI] [PubMed] [Google Scholar]
Chang 2006b
- Chang AB, Glomb WB. Guidelines for evaluating chronic cough in children: American College of Chest Physicians Guidelines for the management of cough. Chest 2006;129:S260‐83. [DOI] [PubMed] [Google Scholar]
Cherry 2008
- Cherry DK, Hing E, Woodwell DA, Rechtsteiner EA. National Ambulatory Medical Care Survey: 2006 Summary. National Health Statistics Reports No. 3 2008. [PubMed]
Cornford 1993
- Cornford CS, Morgan M, Ridsdale L. Why do mothers consult when their children cough?. Family Practice 1993;10:193‐96. [DOI] [PubMed] [Google Scholar]
Doull 1996
- Doull IJ, Williams AA, Freezer NJ, Holgate ST. Descriptive study of cough, wheeze and school absence in childhood. Thorax 1996;51:630‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Faniran 1998
- Faniran AO, Peat JK, Woolcock AJ. Persistent cough: is it asthma?. Archives of Disease in Childhood 1998;79:411‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fuller 1998
- Fuller P, Picciotto A, Davies M, McKenzie SA. Cough and sleep in inner‐city children. European Respiratory Journal 1998;12:426‐31. [DOI] [PubMed] [Google Scholar]
Gillissen 2007
- Gillissen A, Richter A, Oster H. Clinical efficacy of short‐term treatment with extra‐fine HFA beclomethasone dipropionate in patients with post‐infectious persistent cough. Journal of Physiology and Pharmacology 2007;58 (Suppl 5):223‐32. [PubMed] [Google Scholar]
Hay 2002
- Hay AD, Wilson AD. The natural history of acute cough in children aged 0 to 4 years in primary care: a systematic review. British Journal of General Practice 2002;52:401‐9. [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Irwin 2006a
- Irwin RS. Introduction to the diagnosis and management of cough: ACCP Evidence‐Based Clinical Practice Guidelines. Chest 2006;129:S25‐7. [DOI] [PubMed] [Google Scholar]
Irwin 2006b
- Irwin RS, Baumann MH, Bolser DC, Boulet LP, Braman SS, Brightling CE, et al. Diagnosis and Management of Cough: ACCP Evidence‐Based Clinical Practice Guidelines. Chest 2006;129:S1‐S23. [DOI] [PubMed] [Google Scholar]
Marchant 2008
- Marchant JM, Newcombe PA, Juniper EF, Sheffield JK, Stathis SL, Chang AB. What is the burden of chronic cough for families?. Chest 2008;134:303‐9. [DOI] [PubMed] [Google Scholar]
Nair 2010
- Nair P, Hargreave FE. Measuring bronchitis in airway diseases: clinical implementation and application: airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest 2010;138:38S‐43S. [DOI] [PubMed] [Google Scholar]
Patel 2001
- Patel L, Wales JK, Kibirige MS, Massarano AA, Couriel JM, Clayton PE. Symptomatic adrenal insufficiency during inhaled corticosteroid treatment. Archives of Disease in Childhood 2001;85:330‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre: The Cochrane Collaboration. Review Manager (RevMan) Version 5.1. Copenhagen: The Nordic Cochrane Centre: The Cochrane Collaboration, 2011.
Rytila 2008
- Rytila P, Ghaly L, Varghese S, Chung W, Selroos O, Haahtela T. Treatment with inhaled steroids in patients with symptoms suggestive of asthma but with normal lung function. European Respiratory Journal 2008;32:989‐96. [DOI] [PubMed] [Google Scholar]
Thomson 2002
- Thomson F, Masters IB, Chang AB. Persistent cough in children ‐ overuse of medications. Journal of Paediatrics and Child Health 2002;38:578‐81. [DOI] [PubMed] [Google Scholar]
Tomerak 2005
- Tomerak AAT, McGlashan J, Lakhanpaul M, Vyas HHV, McKean MC. Inhaled corticosteroids for non‐specific chronic cough in children. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD004231.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vernacchio 2008
- Vernacchio L, Kelly JP, Kaufman DW, Mitchell AA. Cough and cold medication use by US children, 1999‐2006: results from the Slone Survey. Pediatrics 2008;122:323‐9. [DOI] [PubMed] [Google Scholar]
Wennergren 2001
- Wennergren G, Kristjansson S. Relationship between respiratory syncytial virus bronchiolitis and future obstructive airway diseases. European Respiratory Journal 2001;18:1044‐58. [DOI] [PubMed] [Google Scholar]


