Abstract
Background
Crohn's disease has a high morbidity and there is no known cure. Current treatments have multiple side effects and an effective treatment with minimal side effects is desired. Probiotics have been proposed as such a treatment but their efficacy is undetermined. There is some evidence that probiotics are effective in other conditions affecting the gastrointestinal tract and they are popular with patients. They are thought to work through competitive action with commensal and pathogenic flora, influencing the immune response.
Objectives
To determine if there is any evidence for the efficacy of probiotics for the induction of remission in Crohn's disease.
Search methods
The Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 1, 2007), MEDLINE (1966 to 2007), Excerpta Medica/EMBASE (1974 to 2007), CINAHL (1982‐2007) and the Cochrane Inflammatory Bowel Disease and Functional Bowel Disorders Group Specialised Trial Register were searched. Manufacturers of probiotics were also contacted to identify any unpublished trials. References of trials were also searched for any additional trials.
Selection criteria
Randomised controlled trials (RCTs) that compared probiotics against placebo or any other intervention for the induction of remission in Crohn's disease were eligible for inclusion.
Data collection and analysis
Data extraction and assessment of methodological quality of included studies were independently performed by two authors. The main outcome measure was the occurrence of clinical remission. Odds ratios and 95% confidence intervals were calculated for dichotomous outcomes.
Main results
One small study (n = 11) met the inclusion criteria and was included in the review. There were some methodological concerns with this study. Four of 5 patients in the probiotic group achieved remission compared to 5 of 6 in the placebo group (OR 0.80; 95% CI 0.04 to 17.20).
Authors' conclusions
There is insufficient evidence to make any conclusions about the efficacy of probiotics for induction of remission in Crohn's disease. There is a lack of well designed RCTs in this area and further research is needed.
Keywords: Humans, Crohn Disease, Crohn Disease/therapy, Probiotics, Probiotics/therapeutic use, Randomized Controlled Trials as Topic, Remission Induction
Probiotics for treatment of active Crohn's disease
Crohn's disease is a chronic inflammatory disease of the intestines which has periods of inactivity and periods when it flares up. Crohn's disease can affect any part of the digestive tract, from the mouth to the anus. The most common symptoms of Crohn's disease are abdominal pain, and diarrhoea. Probiotics are living microorganisms that are thought to benefit health by altering the growth of bacteria in the intestines thereby reducing inflammation. Only one study was identified and this did not show that probiotics had any effect in treating active Crohn's disease. However this study was only small (11 patients) and no definite conclusion can be made regarding the effectiveness of probiotics. Probiotics were generally well tolerated and no side effects were reported. There is insufficient evidence to make any conclusions about the effectiveness of probiotics for treatment of active Crohn's disease.
Background
Crohn's disease (CD) is a chronic relapsing condition with a high morbidity. There is no known cure for CD and treatment is aimed at inducing and maintaining remission, correcting malnutrition, addressing complications, and thereby improving the quality of life of patients. In children, a major additional goal is to facilitate normal growth and pubertal development which are frequently impeded.
Although effective, current treatments may have serious adverse events. New treatments with minimal adverse effects would be welcomed by both patients and physicians. Any new therapy, ideally, would have the same or better efficacy as current modalities but without causing the adverse events associated with steroids and immunosuppressive agents. Probiotics have been proposed as a possible treatment (Malin 1996), as they seem to have few adverse effects but their efficacy for induction of remission in Crohn's disease is yet to be ascertained.
Probiotics consist of a group of live microorganisms that are thought to be beneficial to health. Micro‐organisms that have been used as probiotics include Lactobacillus spp, Bifidobacterium spp, Streptococcus salivarius spp, VSL#3 (a combination of 8 probiotic bacterial species), yeasts (e.g. Saccharomyces boulardii) and E. Coli Nissle 1917 (Sartor 2005).
Probiotics are thought to work through competitive action with commensal and pathogenic flora and by influencing the immune response (Shanahan 2000). Efficacy has been examined in diseases related to gut barrier dysfunction and probiotics seem to have some effect in childhood diarrhoea (Hove 1999, Pedone 1999, Pochapin 2000), atopic eczema (Isolauri 2000, Majamaa 1997) and milk hypersensitivity (Pelto 1998). There is limited evidence of efficacy of probiotics in ulcerative colitis (Kruis 1997, Rembacken 1994), pouchitis (Gionchetti 2000) and Crohn's disease, (Malchow 1997, Guslandi 2000, Campieri 2000 ). If probiotics were shown to be an effective treatment for inflammatory bowel disease, it is feasible that complications from the use of immunosuppressives and steroids could be reduced.
The aim of this review is to summarise the available evidence for the use of probiotics for induction of remission in CD.
Objectives
1. To evaluate the efficacy of probiotic supplementation for the induction of remission in CD. 2. To evaluate adverse events associated with the use of probiotics.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials.
Types of participants
Patients of any age with Crohn's disease whose disease was active at the time of entry into the study, as defined by a recognised Crohn's disease activity index.
Types of interventions
Intervention Probiotics, either singly or in combination, administered orally in any form (e.g. a drink, a powder or a capsule).
Control No intervention, placebo or other interventions.
Types of outcome measures
The primary outcome measure was induction of clinical remission, as defined by the primary studies.
We planned to include the following secondary outcomes but due to lack of available data these outcomes were not assessed: 1. Endoscopic remission; 2. Clinical response rates as defined by the primary studies; 3. Clinical, histological or endoscopic improvement as defined by the primary studies; 4. Quality of life as measured by a validated quality of life tool; and 5. Adverse events (e.g. sepsis).
Search methods for identification of studies
See: Inflammatory Bowel Disease and Functional Bowel Disorders Group methods used in reviews.
A. Electronic search The following electronic databases were searched for relevant studies:
1. MEDLINE via PUBMED (1966‐2007); 2. EMBASE (1974‐2007); 3. Cochrane Central Register of Controlled Trials (Issue 1, 2007); 4. CINAHL (1982‐2007); and 5. Cochrane Inflammatory Bowel Disease and Functional Bowel Disorders Group Specialised Trial Register.
B. Reference searching The references of all identified studies were inspected for more trials.
C. Manufacturers of probiotic agents were contacted to identify other studies.
MEDLINE on PUBMED was searched using the following search strategy:
1. Crohn* disease 2. crohn disease (MeSH) 3. regional enteritis 4. ileitis 5. ileitis (MeSH) 6. inflammatory bowel disease 7. inflammatory bowel diseases (MeSH) 8. 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 9. probiotic* 10. probiotics (MeSH) 11. Commensal 12. Complementary medicine 13. Lactobacillus OR lactobacilli 14. Bifidobacteria OR bifidobacterium 15. E coli Nissle 16. Yeast OR Saccharomyces boulardii 17. Fungus 18. Streptococcus salivarius 19. VSL#3 OR VSL 3 20. 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19 21. 8 AND 20
The above search strategy was then adapted to search the other databases. The search strategy was not limited by language.
Data collection and analysis
Step 1: Using the above search strategy, papers that appeared to be potentially relevant were identified by two authors.
Step 2: The authors, after reading the full texts, independently assessed the eligibility of all trials identified using an ad hoc eligibility, based on the inclusion criteria above. Disagreement among authors was discussed and agreement was reached by consensus.
Step 3. The methodological quality of selected trials was assessed independently by two authors using the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Alderson 2004) and the Jadad scale (Jadad 1996). The former is based on the evidence of a strong relationship between allocation concealment and direction of effect. The categories are defined below:
A. adequate allocation concealment; B. allocation concealment unclear; and C. inadequate allocation concealment.
The Jadad scale is a validated five point scale which measures some important factors that impact on the quality of a trial. This is summarised below:
a. was the study described as randomised? b. was the method of randomisation well described and appropriate? c. was the study described as double blind? d. was the double blinding well described and appropriate? e. were withdrawals and dropouts described?
Each item was given a score of 1 if the answer was 'yes' and 0 if the answer was 'no'. One point was deducted if the described method of randomisation or blinding was inappropriate.
DATA COLLECTION A data extraction form was developed and used to extract information on relevant features and results of included studies. Two authors independently extracted and recorded the data on the predefined checklist. Extracted data included the following items:
a. characteristics of patients: age, sex, disease distribution, disease duration, disease activity index; b. total number of patients originally assigned to each intervention group; c. intervention: type and amount of probiotic d. control: no intervention, placebo or other interventions; e. concurrent medications; and f. outcomes: time of assessment, length of follow up, type of Crohn's disease activity index used, definitions of remission and relapse, relapse rates, time to relapse, quality of life assessment, adverse events.
STATISTICAL ANALYSIS The Cochrane Collaboration review manager (RevMan) software (version 4.2.9) was used for data analysis. Data were analysed according to the intention to treat principle. Patients with final missing outcomes were assumed to be treatment failures. Analyses were planned to be grouped by length of follow up but, as there were limited data, this was not possible.
Dichotomous variables Odds ratios (OR) and their 95% confidence interval (CI) were calculated based on the fixed effects model.
Heterogeneity Heterogeneity among trial results were planned to be assessed by inspection of graphical presentations and by calculating the chi square test of heterogeneity but this was not possible because of insufficient data.
Publication Bias Publication bias was to be investigated with funnel plots (trial effects versus trial size). This was not done due to a lack of studies.
Subgroup analysis A subgroup analysis was planned but was not done due to insufficient data.
Results
Description of studies
Twelve potentially relevant studies on the use of probiotics for inducing remission in Crohn's disease were identified. Eleven studies were excluded for various reasons: four studies related to maintenance of remission only (Barclay 1992, Bousvaros 2005, Marteau 2006, Prantera 2002); four were review articles (Chapman 2006, Ewaschuk 2006, Gionchetti 2001, Mach 2006); two were open label uncontrolled studies (Guandalini 2002, Gupta 2000); one was excluded because all patients were given probiotics during the induction phase (Plein 1993) and one was a study on collagenous colitis (Wildt 2006). Only one study was identified that satisfied the inclusion criteria (Schultz 2004).
Schultz 2004 In this study, 11 adults were recruited from one centre in Germany. Participants were patients with active Crohn's disease, (defined as a Crohn's disease activity index (CDAI) of 150 to 300)). The trial encompassed both induction and maintenance of remission with patients receiving one week of antibiotics (ciprofloxacin 500 mg twice daily and metronidazole 250 mg three times daily) and corticosteroids (60 mg initially and then tapering down). After one week, patients were randomised to receive either Lactobacillus GG (2 billion colony forming units per day) or a corn starch placebo. After one further week the antibiotics were stopped leaving the patients on corticosteroids plus the probiotic or placebo for the remainder of the trial. Remission was defined as a CDAI of less than 150 at twelve weeks and was the primary outcome for the induction stage. The maintenance stage of the trial continued for a total of six months and any relapses were recorded.
Risk of bias in included studies
Schultz 2004 was a very small involving 11 patients and no power calculation was reported. Allocation to the groups was random, blinding was double‐blind and the trial was placebo‐controlled. However, further details on methods were not provided. The authors were contacted for further information. Randomisation was performed using a computer generated randomisation table. Blinding was performed by a trial nurse who had no contact with the patients and the placebo consisted of corn starch placed in a capsule similar to that used for the probiotics. There were no patients lost to follow up. The Jadad score was four. Allocation concealment was unclear.
It was not clear in the paper when or how induction of remission was assessed. On contacting the authors, they stated that remission was assessed at 12 weeks. During the induction phase two patients in the treatment group and two in the control group were reported as relapsing. This appears to contradict the author's statement that remission was assessed at 12 weeks. Another concern with the trial design was the fact that corticosteroids were used concurrently in the two groups. Corticosteroids are potent inducers of remission and could have been a confounding factor in this trial.
Effects of interventions
Efficacy There was no statistically significant difference between probiotics and placebo for induction of remission in Crohn's disease. However, due to the small sample size, there was a high risk of a type 2 error. Meta analysis was not possible as only one study was included in the review. In the probiotics group 4 of 5 patients (80%) achieved remission compared to 5 of 6 (83%) in the placebo group (OR 0.80; 95% CI 0.04 to 17.20). The wide confidence interval reflected the small sample size.
Safety No adverse events were reported in the 11 patients who took part during the induction phase and also during the follow up period while the maintenance study was taking place.
Discussion
In some patients, Crohn's disease can be refractory to conventional therapy such as corticosteroids, and immune‐modulators (including azathioprine, 6‐mercaptopurine and methotrexate). Some patients may become dependent on corticosteroids thereby increasing the risk of developing steroid‐related adverse events. In these situations, it is important that other treatment options are considered. Probiotics have a favorable adverse effects profile and have been proposed as an alternative treatment option for patients with mild Crohn's disease (Malin 1996).
There is a paucity of RCTs assessing probiotics treatment for induction of remission in Crohn's disease. Only one small study (n = 11) was identified that met the inclusion criteria (Schultz 2004) and this study had methodological flaws. This study did not show any statistically significant benefit for probiotics. However, given the weaknesses of this study, it cannot be conclusively stated that probiotics are ineffective. Appropriately powered, well‐designed RCTs are needed to address this question.
Probiotics have also been investigated as maintenance therapy for CD. A recent Cochrane review concluded there is no evidence to suggest that probiotics are beneficial for the maintenance of remission in CD (Rolfe 2006). All of the studies included in this review enrolled small numbers of patients and may have lacked statistical power to show differences should they exist. Larger trials are required to determine if probiotics are of benefit for maintenance treatment in Crohn's disease.
In conclusion, there is insufficient evidence to make any conclusions regarding the efficacy of probiotics treatment for induction of remission in Crohn's disease.
Authors' conclusions
There is currently no evidence to support the use of probiotics for the induction of remission in Crohn's disease. The use of probiotics as induction therapy for Crohn's disease cannot be recommended at this time.
This review highlights the need for good quality, adequately powered RCT's to investigate the efficacy and safety of probiotics for the induction of remission in Crohn's disease.
Acknowledgements
Funding for the IBD/FBD Review Group (October 1, 2005 ‐ September 30, 2010) has been provided by the Canadian Institutes of Health Research (CIHR) Knowledge Translation Branch; the Canadian Agency for Drugs and Technologies in Health (CADTH); and the CIHR Institutes of Health Services and Policy Research; Musculoskeletal Health and Arthritis; Gender and Health; Human Development, Child and Youth Health; Nutrition, Metabolism and Diabetes; and Infection and Immunity.
Miss Ila Stewart has provided support for the IBD/FBD Review Group through the Olive Stewart Fund.
Data and analyses
Comparison 1.
Probiotics versus placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Induction of remission | 1 | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.04, 17.20] |
Analysis 1.1.
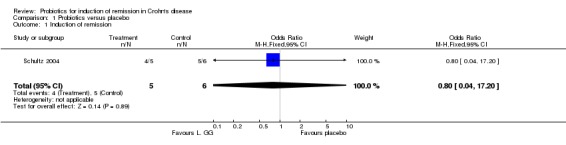
Comparison 1 Probiotics versus placebo, Outcome 1 Induction of remission.
What's new
| Date | Event | Description |
|---|---|---|
| 12 May 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 3, 2007 Review first published: Issue 3, 2008
| Date | Event | Description |
|---|---|---|
| 21 April 2008 | New citation required and conclusions have changed | Substantive amendment |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Single‐centre study. Described as randomised: Yes. Randomisation method described: Yes. Described as double blind: Yes. Blinding method described: Yes. Follow‐ups described: Yes. | |
| Participants | Inclusion criteria: Age >18 years. CDAI 150 ‐ 300. | |
| Interventions | 11 patients had one week of corticosteroids and antibiotics and then allocated to L.GG or placebo. Remission assessed at 12 weeks. | |
| Outcomes | Remission: defined as CDAI<150 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barclay 1992 | Maintenance study |
| Bousvaros 2005 | Maintenance study |
| Chapman 2006 | Review article |
| Ewaschuk 2006 | Review article |
| Gionchetti 2001 | Review article |
| Guandalini 2002 | Open label study |
| Gupta 2000 | Open label study |
| Mach 2006 | Review article |
| Marteau 2006 | Maintenance study |
| Plein 1993 | All patients received probiotics during the induction phase |
| Prantera 2002 | Maintenance study |
| Wildt 2006 | Study not on Crohn's disease |
Declarations of interest
None known.
New
References
References to studies included in this review
- Schultz M, Timmer A, Herfarth HH, Sartor RB, Vanderhoof JA, Rath HC. Lactobacillus GG in inducing and maintaining remission of Crohn's disease. BMC Gastroenterol 2004;4(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
- Barclay GR, McKenzie H, Pennington J, Parratt D, Pennington CR. The effect of dietary yeast on the activity of stable Crohn's disease. Scand J Gastroenterol 1992;27:196‐200. [DOI] [PubMed] [Google Scholar]
- Bousvaros A, Guandalini S, Baldassano RN, Botelho C, Evans J, Ferry GD, et al. A randomized, double‐blind trial of Lactobacillus GG versus placebo in addition to standard maintenance therapy for children with Crohn's disease. Inflamm Bowel Dis 2005;11:833‐8. [DOI] [PubMed] [Google Scholar]
- Chapman T, Plosker GL, Figgitt DP. VSL#3 Probiotic mixture. A review of its use in chronic inflammatory bowel diseases. Drugs 2006;66(10):1371‐87. [DOI] [PubMed] [Google Scholar]
- Ewaschuk JB, Dieleman LA. Probiotics and prebiotics in chronic inflammatory bowel diseases. World J Gastroenterol 2006;12(37):5941‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gionchetti P, Rizello F, Campieri M. Probiotics and antibiotics in inflammatory bowel disease. Curr Opin Gastroenterol 2001;17:331‐5. [DOI] [PubMed] [Google Scholar]
- Guandalini S. Use of Lactobaccilus‐GG in paediatric Crohn's disease. Digest Liver Dis 2002;34(Suppl.2):s63‐5. [DOI] [PubMed] [Google Scholar]
- Gupta P, Andrew A, Kirschner BS, Guandalini S. Is Lacotbaccillus GG helpful in children with Crohn's disease? Results of a preliminary, open‐label study. J Pediatr Gastroenterol Nutr 2000;31:453‐7. [DOI] [PubMed] [Google Scholar]
- Mach T. Clnical usefulness of probiotics in inflammatory bowel diseases. J Physiol Pharmacaol 2006;57(Suppl. 9):23‐33. [PubMed] [Google Scholar]
- Marteau P, Lemann M, Seksik P, Laharie D, Colombei JF, Bouhnik Y, et al. Ineffectiveness of Lactobacillus johnsonii LA1 for prophylaxis of postoperative recurrence in Crohn's disease: a randomised, double blind, placebo controlled GETAID trial. Gut 2006;55:842‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plein K, Hotz J. Therapeutic effects if Saccharomyces boulardii on mild residual symptoms in a stable phase of Crohn's disease with special respect to chronic diarrhoea ‐ a pilot study. Z Gastroenterol 1993;31:129‐34. [PubMed] [Google Scholar]
- Prantera C, Scribano ML, Falasco G, Andreoli A, Luza C. Ineffectiveness of probiotics in preventing recurrence after curative resection for Crohn's disease: a randomised controlled trial with Lactobacillus GG. Gut 2002;51:405‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildt S, Munck LK, Vinter‐Jensen L, Hansen BF, Nordgaard‐Lassen I, Christensen S, et al. Probiotic Treatment of Collagenous Colitis: A randomised, double‐blind, placebo controlled trial with Lactobaccillus acidophilus and Bifidobacterium animalis subsp. Lactis. Inflamm Bowel Dis 2006;12(5):395‐401. [DOI] [PubMed] [Google Scholar]
Additional references
- Alderson P, Green S, Higgins JPT. Assessment of study quality. Cochrane Reviewers' Handbook 4.2.2 [updated December 2003]; Section 6. In: The Cochrane Library, Issue 1. Chichester, UK: John Wiley & Sons, Ltd, 2004. [Google Scholar]
- Campieri M, Rizzello F, Venturi A, Poggioli G, Ugolini F, Helwig U, et al. Combination of antibiotic and probiotic treatment is efficacious in prophylaxis of post‐operative recurrence of Crohn's disease: a randomized controlled study vs mesalamine. Gastroenterology 2000;118:A4179. [Google Scholar]
- Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double‐blind, placebo‐controlled trial. Gastroenterology 2000;119:305‐9. [DOI] [PubMed] [Google Scholar]
- Guslandi M, Mezzi G, Sorghi M, Testoni PA. Saccharomyces boulardii in maintenance treatment of Crohn's disease. Dig Dis Sci 2000;45:1462‐4. [DOI] [PubMed] [Google Scholar]
- Hove H, Norgaard H, Mortensen PB. Lactic acid bacteria and the human gastrointestinal tract.. Eur J Clin Nutr 1999;53:339‐50. [DOI] [PubMed] [Google Scholar]
- Isolauri E, Arvola T, Sutas Y, Moilanen E, Salminen S. Probiotics in the management of atopic eczema. Clin Exp Allergy 2000;30:1605‐10. [DOI] [PubMed] [Google Scholar]
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Control Clin Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
- Kruis, W. Schutz, E. Fric, P. Fixa, B. Judmaier, G. Stolte, M. Double‐blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther 1997;11:853‐8. [DOI] [PubMed] [Google Scholar]
- Majamaa H, Isolauri E. Probiotics: a novel approach in the management of food allergy. J Allergy Clin Immunol 1997;99:179‐86. [DOI] [PubMed] [Google Scholar]
- Malchow HA. Crohn's disease and Escherichia coli. A new approach in therapy to maintain remission of colonic Crohn's disease?. J Clin Gastroenterol 1997;25:653‐8. [DOI] [PubMed] [Google Scholar]
- M Malin, H Suomalainen, M Saxelin, E Isolauri. Promotion of IgA immune response in patients with Crohn's disease by oral bacteriotherapy with Lactobacillus GG. Ann Nutr Metab 1996;40:137‐45. [DOI] [PubMed] [Google Scholar]
- Pedone CA, Bernabeu AO, Postaire ER, Bouley CF, Reinert P. The effect of supplementation with milk fermented by Lactobacillus casei (strain . DN‐114 001) on acute diarrhoea in children attending day care centres. Int J Clin Pract 1999;53:179‐84. [PubMed] [Google Scholar]
- Pelto L, Isolauri E, Lilius EM, Nuutila J, Salminen S. Probiotic bacteria downregulate the milk‐induced inflammatory response in milk‐hypersensitive subjects but have an immunostimulatory effect in healthy subjects. Clin Exp Allergy 1998;28:1474‐9. [DOI] [PubMed] [Google Scholar]
- Pochapin M. The effect of probiotics on Clostridium difficile diarrhea. Am Gastroenterol 2000;95(1 Suppl):S11‐13. [DOI] [PubMed] [Google Scholar]
- Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon AT. Non‐pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet 1994;344:1046‐9. [DOI] [PubMed] [Google Scholar]
- Rolfe VE, Fortun PJ, Hawkey CJ, Bath‐Hextall F. Probiotics for maintenance of remission in Crohn's disease. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI] [PubMed] [Google Scholar]
- Sartor RB. Probiotic therapy of intestinal inflammation and infections. Curr Opin Gastroenterol 2005;21:44‐50. [PubMed] [Google Scholar]
- Shanahan F. Probiotics and inflammatory bowel disease: is there a scientific rationale?. Inflamm Bowel Dis 2000;6:107‐15. [DOI] [PubMed] [Google Scholar]


