Abstract
Background
Tiotropium is a new anticholinergic therapy for chronic obstructive pulmonary disease (COPD) that differs from ipratropium by its functional relative selectivity for muscarinic receptor subtypes and which allows once‐per‐day dosing.
Objectives
To determine the efficacy of tiotropium on clinical endpoints such exacerbations and hospitalisations, symptom scales and pulmonary function compared to placebo and other bronchodilators used for stable COPD.
Search methods
Randomised controlled trials (RCTs) were identified from the Cochrane Airways Review Group Specialised Register, a compilation of systematic searches of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and CINAHL, and hand searching of 20 respiratory journals. Bibliographies from included studies and reviews were searched. The date of the last search was October 2004.
Selection criteria
Randomised clinical trials comparing tiotropium with placebo, ipratropium bromide, or long‐acting ß2‐agonists for greater than, or equal to, one month's duration.
Data collection and analysis
Two reviewers independently extracted data. Missing data were obtained from authors or the manufacturer of tiotropium. The data were analysed using the Cochrane Review Manager RevMan 4.2. Studies were pooled to yield weighted mean differences (WMD) or odds ratios (OR) and reported using 95% confidence intervals (CI).
Main results
From 69 identified references, nine RCTs (6,584 patients) met inclusion criteria. Tiotropium reduced the odds of a COPD exacerbation (OR 0.74; 95% CI 0.66 to 0.83) and related hospitalisations (OR 0.64; 95% CI 0.51 to 0.82) compared to placebo or ipratropium. When applied to an annual baseline risk of 45% for exacerbations and 10% for hospitalisation, the number of patients needed to treat with tiotropium for one year were 14 (95% CI 11 to 22) to prevent one exacerbation and 30 (95% CI 22 to 61) to prevent one hospitalisation compared to placebo and ipratropium. Reductions in these endpoints compared to long‐acting ß2‐agonists were not statistically significant. Similar patterns were evident for quality‐of‐life and symptom scales. Increases in FEV1 and FVC from baseline were significantly larger with tiotropium than with placebo, ipratropium and long‐acting ß2‐agonists over 6 to 12 months. The decline in trough FEV1 from steady state was 30 ml (95% CI 7 to 53 ml) less with tiotropium than with placebo or ipratropium over one year; no data on decline in FEV1 from steady state were available for long‐acting ß2‐agonists. Dry mouth was increased by tiotropium.
Authors' conclusions
Tiotropium reduced COPD exacerbations and related hospitalisations compared to placebo and ipratropium. It also improved health‐related quality‐of‐life and symptom scores among patients with moderate and severe disease, and may have slowed decline in FEV1. Additional long‐term studies are required to evaluate its effect on mortality and change in FEV1 to clarify its role in comparison to, or in combination with, long‐acting ß2‐agonists and to assess its effectiveness in mild and very severe COPD.
Keywords: Humans; Administration, Inhalation; Cholinergic Antagonists; Cholinergic Antagonists/administration & dosage; Ipratropium; Ipratropium/administration & dosage; Pulmonary Disease, Chronic Obstructive; Pulmonary Disease, Chronic Obstructive/drug therapy; Randomized Controlled Trials as Topic; Scopolamine Derivatives; Scopolamine Derivatives/administration & dosage; Spirometry; Tiotropium Bromide
Plain language summary
Tiotropium for stable chronic obstructive pulmonary disease
Tiotropium (Spiriva) is a bronchodilator drug that has been developed to open the airways in the lungs effectively with once daily dosing. The main aims of therapy in COPD are to reduce exacerbations and related hospitalisations, improve quality of life, and reduce the rate of decline in lung function. The evidence from the trials in the review indicates that, compared with a placebo and ipratropium, tiotropium does reduce exacerbations and related hospitalisations and improves quality of life and symptoms in people with moderately severe COPD, although the evidence with regards to decline in lung function is less clear. Tiotropium caused dry mouth. Compared with other commonly used drugs in COPD, such as long‐acting beta agonists (including salmeterol), there is not enough evidence for us to draw reliable conclusions. In order to better understand the effects of this drug we need long‐term studies (over several years), studies conducted in mild and severe COPD, and additional studies that measure outcomes in relation to other agents used in the treatment of this condition.
Background
Current recommendations for the therapy of patients with chronic obstructive pulmonary disease (COPD) suggest that anticholinergic drugs should play a prominent role (Ferguson 1993). The development of safe, effective, quaternary anticholinergic compounds that have functional relative selectivity for muscarinic receptor subtypes has generated renewed interest in anticholinergic bronchodilator therapy.
Tiotropium has a quaternary ammonium structure related to that of ipratropium bromide. Similar to ipratropium, tiotropium binds M1, M2 and M3 muscarinic receptors. Unlike ipratropium, tiotropium has kinetic selectivity for these receptors, dissociating slowly from M1 and M3 receptors but rapidly from M2 receptors (Haddad 1994). The slow dissociation of tiotropium from M1 and M3 receptors produces a bronchodilating effect which lasts over 24 hours, allowing once‐daily dosing (Disse 1999). The rapid dissociation from M2 receptors may also be advantageous since these are feedback inhibitory receptors. Blocking M2 receptors, therefore, paradoxically increases acetylcholine release whereas unblocking them decreases acetylcholine release (Barnes 2000). Tiotropium may, therefore, have clinical benefits over ipratropium, one of the current first‐line therapies for stable COPD.
Objectives
The aim of this review is to evaluate the efficacy of tiotropium on clinical endpoints, quality‐of‐life and symptom scales and pulmonary function from available randomised trial data comparing tiotropium to placebo, ipratropium bromide and long‐acting ß2‐agonists.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials comparing tiotropium with placebo, ipratropium bromide, or long‐acting ß2‐agonists. Since the primary purpose of the review was to evaluate long‐term clinical responses to tiotropium, we excluded studies that followed patients for less than one month after randomisation.
Types of participants
Adult patients aged greater than 35 years with known stable COPD fulfilling American Thoracic Society (ATS), European Respiratory Society (ERS), or GOLD diagnostic criteria were included. COPD patients with partial reversibility on pulmonary function testing were included, consistent with these criteria. Patients had clinically stable disease without evidence of an exacerbation for one month prior to study entry. Patients with significant diseases other than COPD, a diagnosis of asthma, cystic fibrosis, bronchiectasis, or other lung diseases were excluded.
Types of interventions
Comparisons were made between the administration of: 1) tiotropium versus placebo; 2) tiotropium versus ipratropium bromide; 3) tiotropium versus long‐acting ß2‐agonists (salmeterol or formoterol).
Types of outcome measures
Primary outcomes
1) clinical outcomes ‐ exacerbations, hospitalisations and mortality.
Secondary outcomes
1) health‐related quality‐of‐life scales; 2) self‐rated symptom score/symptoms of breathlessness; 3) change in forced expiratory volume in one second (FEV1) and change in forced ventilatory capacity (FVC): trough, peak and average; and other measures of pulmonary function, from baseline and from steady state (measured at 8 to 15 days after randomisation); 4) exercise performance ‐ six‐minute walk and other measures; 5) inhaled rescue medication used during the treatment period, and other concomitant medication usage including antibiotics and steroids; 6) adverse events ‐ palpitations, dry mouth, blurred vision, urinary obstruction, and constipation.
Search methods for identification of studies
Searches were current as of October 2004.
Electronic searches
The Cochrane Airways Review Group Specialised Register of COPD trials is a compilation of references to reports of controlled clinical trials assembled from systematic searches of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and CINAHL and supplemented by hand searching of leading respiratory journals and conference abstracts. It is not limited by language of publication. The Register was searched using the following terms:
tiotropium OR "Ba 679 BR" OR Spiriva OR oxitropium.
In addition, a search of LILACS and the Cochrane Central Register of Controlled Trials (CENTRAL) was performed.
Searching other resources
Reference lists of all primary studies and review articles were reviewed for additional references. Authors of identified randomised trials were asked about knowledge of other published and unpublished studies. The manufacturer of tiotropium (Boehringer Ingelheim) was contacted to clarify potential overlap between studies and to request results from unpublished studies and supplemental data from published studies.
Data collection and analysis
Selection of studies
Trials which appeared potentially relevant were identified independently by two reviewers. Using the full text of each study, two reviewers independently selected trials for inclusion in the review. Agreement was measured using simple agreement and kappa; third‐party adjudication was used to resolve differences.
Data extraction and management
Two reviewers independently extracted data from included trials according to intention‐to‐treat principles and entered results into the Cochrane Collaboration software program (RevMan). In some cases information regarding outcomes was estimated from graphs. This was performed independently by the two reviewers. Data extraction included the following items:
Population: age, gender, smoking status, study setting (country, practice setting), inclusion and exclusion criteria. Intervention: dose, duration. Control comparisons: placebo, ipratropium, long‐acting ß2‐agonists. Concurrent treatments: ipratropium, short and long‐acting ß2‐agonists, theophylline, inhaled and systemic corticosteroids. Outcomes: exacerbations (defined as a complex of respiratory symptoms (new onset or an increase in at least one of cough, sputum, dyspnoea, wheeze or chest discomfort) lasting at least three days and usually associated with a therapeutic intervention), related hospitalisations, self‐rated symptom score/symptoms, quality‐of‐life instruments, pulmonary function measures (change in FEV1 and FVC), timing of pulmonary function measures, 6‐minute walk, adverse events (palpitations, dry mouth, blurred vision, urinary obstruction and constipation), assessors, adjudicator of clinical endpoints Design: method of randomisation, presence and type of run‐in period, study design (parallel, cross‐over).
Assessment of risk of bias in included studies
After a preliminary review of all studies to confirm the basic requirements, two reviewers assessed the methodological quality of the included trials with particular emphasis on the concealment of allocation, which was ranked using Cochrane criteria (grade A: adequate concealment; grade B: uncertain; grade C: clearly inadequate concealment).
In addition, each study was assessed using the 0 to 5 scale described by Jadad, as summarised below. 1) Was the study randomised? (1=yes; 0=no) 2) Was the study double‐blind? (1=yes; 0=no) 3) Were withdrawals and dropouts described? (1=yes; 0=no) 4) Was the method of randomisation well described and appropriate? (1=yes; 0=no) 5) Was the double blinding well described and appropriate? (1=yes; 0=no) 6) Deduct one point if methods for randomisation or blinding were inappropriate Inter‐rater reliability was measured for both quality scales by using kappa (weighted or unweighted) statistics.
Assessment of reporting biases
Funnel plots were constructed to check for the presence of publication bias.
Data synthesis
Trials were combined using RevMan (Version 4.2) and analyses were intention‐to‐treat whenever possible. When follow‐up data was known to be missing (e.g., for change analyses), all available data were included and the number of persons included in the trial was considered to be the number of persons who completed the trial for that particular measure. For continuous variables, a fixed‐effect weighted mean difference (WMD) and 95% confidence interval (CI) was calculated for each study. Studies were pooled using fixed‐effect WMD and 95% CIs. For dichotomous variables, a fixed‐effect odds ratio (OR) with 95% confidence intervals (95% CI) was calculated for individual studies. Heterogeneity was tested using the Breslow‐Day test; a P value less than 0.1 was considered statistically significant. If heterogeneity was found, a random‐effects model was used. Numbers needed to treat (NNT) were calculated from the pooled OR and its 95% CI applied to the risk in the placebo group using an online calculator (Visual Rx at www.nntonline.net). This calculator converts the risk in the placebo group to the corresponding odds, applies the OR to estimate the odds in the tiotropium group, converts that odds to the corresponding risk and calculates the risk difference, the inverse of which is the NNT.
For each outcome, trials were pooled within categories of control group (placebo, ipratropium or long‐acting ß2‐agonists). Results of the one study that had two control groups (placebo and salmeterol) were entered into the two corresponding categories. Heterogeneity was tested both within and across categories of control group, so defined. Since an earlier large randomised clinical trial showed that ipratropium does not reduce health outcomes or slow decline in FEV1 relative to placebo (Anthonisen 1994; Anthonisen 2002), summary estimates were calculated comparing tiotropium with placebo or ipratropium for each these endpoints, as long as there was statistical homogeneity across categories of control group. This summary estimate was calculated by pooling trials across categories of placebo and ipratropium control groups; it was not specified in the original protocol.
Subgroup analysis and investigation of heterogeneity
Subgroup and sensitivity analyses: Heterogeneity was to be examined using the following subgroups, which were established a priori: 1) disease severity (related to baseline FEV1 and placebo‐group exacerbation rate); 2) prior ipratropium use (dichotomised as yes or no); 3) concurrent therapy with routine beta‐agonist use (short or long‐acting) and corticosteroid (systemic or inhaled) use (both dichotomised as yes or no); 4) reversibility of airflow obstruction with ß2‐agonist therapy (dichotomised as partial or none); 5) dose, duration and delivery method of therapy; 6) clinical description of COPD (dichotomised as emphysema or chronic bronchitis).
Sensitivity analysis
Quality weighting was also used to test the robustness of the results, and additional sensitivity analyses were performed comparing random‐effects and fixed‐effect models.
Results
Description of studies
Results of the search
A total of 69 articles were identified in the Cochrane Collaboration Airways COPD Registry and from other sources. The review of titles and abstracts yielded 20 articles that possibly fulfilled inclusion criteria. Among these, 12 met inclusion criteria and were included in the analysis. Some of the included articles overlapped, such that three of these articles reported the combined results of pairs of separate trials: Casaburi (Casaburi 2002) reported results for participants enrolled in his previously reported trial (Casaburi 2000) and a previously unpublished trial; Vincken (Vincken 2002) reported results for participants enrolled in the trial reported by van Noord (van Noord 2000) and a previously unpublished trial; and Brasasco (Brusasco 2003) reported results for participants enrolled in the trial reported by Donohue (Donohue 2002) and a previously unpublished trial. The net number of included trials was therefore nine, with total number of patients who were randomised of 6,584.
Included studies
Details of individual trials are given in Characteristics of included studies.
Seven of the studies included in the overview were published in the peer‐reviewed literature and two were published in abstract form. The characteristics of the studies are described in the Table of included studies. The protocols of all of these studies were extremely similar. All studies enrolled patients with COPD that met standardised criteria, that was moderately severe (GOLD Stage IIb, on average), and that was clinically stable. All studies enrolled patients regardless of response to bronchodilators but excluded patients with a prior history of asthma, atopy, allergic rhinitis and elevated eosinophil count.
Of the nine included studies, seven compared tiotropium to placebo, one compared tiotropium to ipratropium, and one compared tiotropium to placebo and to salmeterol. All studies prohibited the use of ipratropium and allowed the use of short‐acting ß2‐agonists and inhaled corticosteroids as co‐therapies in the intervention group. Eight of nine studies prohibited the use of long‐acting ß2‐agonists (the exception being Niewoehner 2005 where long‐acting ß2‐agonists were permitted). Thirty‐nine to 60% of randomised patients were taking ipratropium at enrolment. Of permitted co‐therapies, 66 to 99% of patients were taking ß2‐agonists at enrolment and 42 to 80% were taking inhaled corticosteroids. Use of long‐acting ß2‐agonists at enrolment was generally not reported separately from use of short‐acting ß2‐agonists.
Excluded studies
Eight studies were excluded from the review for the reasons listed in Characteristics of excluded studies.
Risk of bias in included studies
Overall, the methodological quality of the studies was good although not excellent (Table 1). Concealment of allocation was not described (Grade B) in nine and adequately described (Grade A) in three studies. Using the criteria of Jadad, two studies scored 5 out of a possible 5, seven studies scored 4 out of 5, and three studies scored 3 out of 5 for reporting of blinding, allocation and follow up.
Effects of interventions
Clinical outcomes
We extracted and analysed results for cumulative incidence of COPD exacerbations, related hospitalisations, and all‐cause mortality over the duration of the trials, which ranged from one to twelve months (weighted mean duration 6.3 months).
Exacerbations
The overall cumulative incidence of COPD exacerbations was 32% in the control groups (those not receiving tiotropium) over the duration of the trials. Tiotropium reduced the primary endpoint of COPD exacerbations compared to placebo (OR 0.75; 95% CI 0.66 to 0.85) and compared to ipratropium (OR 0.64; 95% CI 0.44 to 0.92). The cumulative incidence of exacerbations was lower in the tiotropium group than the salmeterol group but the difference was somewhat smaller and not statistically significant (OR 0.88; 95% CI 0.65 to 1.17). The treatment effect of tiotropium was statistically homogeneous across the control groups (P value 0.85) and the summary OR for tiotropium compared to placebo or ipratropium was 0.74 (95% CI 0.66 to 0.83), Figure 1.
1.
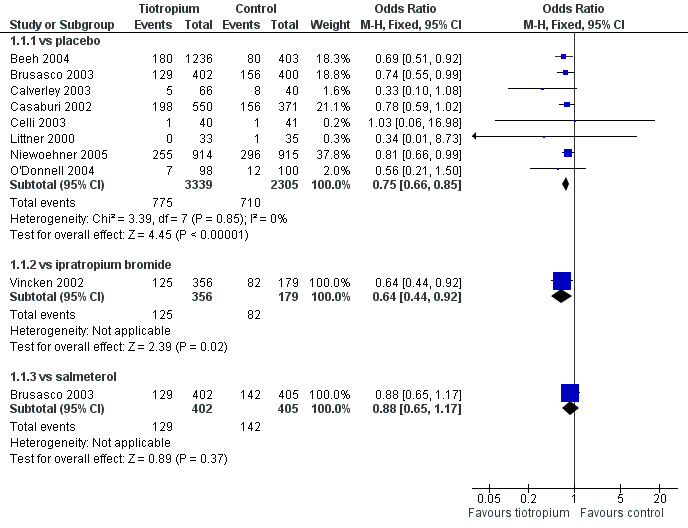
Forest plot of comparison: 1 Health outcomes, outcome: 1.1 Exacerbations.
This summary OR was applied to a risk of exacerbation of 45% over one year (the weighted average of the risk in the placebo and ipratropium arms of two trials of 12 months duration (Casaburi 2002; Vincken 2002) to yield a NNT for tiotropium over 12 months of 14 (95% CI: 11 to 22; Figure 2).
2.
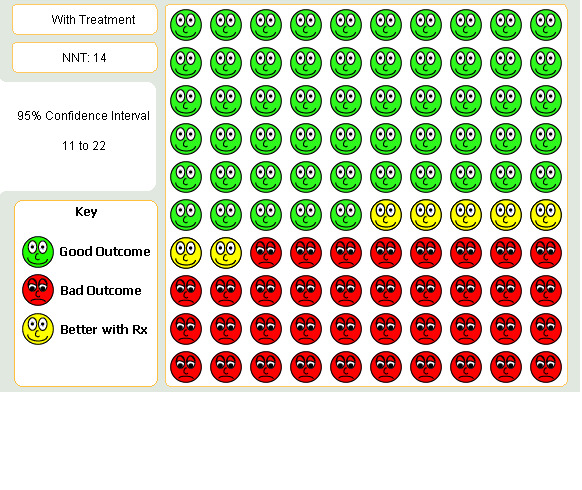
Patients suffering COPD exacerbation when given Tiotropium for one year in comparison to placebo or Ipratropium
Hospitalisations
The overall cumulative incidence of exacerbation‐related hospitalisations was 8% in the control groups over the duration of the trials. As shown in Detail 1:3, tiotropium reduced the proportion hospitalised for COPD exacerbations compared to placebo (OR 0.65; 95% CI 0.50 to 0.85). Similar reductions in hospitalisations were observed for tiotropium compared to ipratropium (OR 0.59; 95% CI 0.32 to 1.09) and compared to salmeterol (OR 0.59; 95% CI 0.29 to 1.23) but neither of these estimates were statistically significant, which may be due the smaller sample sizes. The treatment effect of tiotropium was statistically homogeneous across the control groups (P value 0.83) and the summary estimate for tiotropium compared to placebo or ipratropium was OR 0.64 (95% CI 0.51 to 0.82), Figure 3. When this OR was applied to a one year risk of hospitalisation of 10% in the two 12 month trials (Casaburi 2002; Vincken 2002), the NNT for tiotropium was 30 (95% CI 22 to 61; Figure 4).
3.
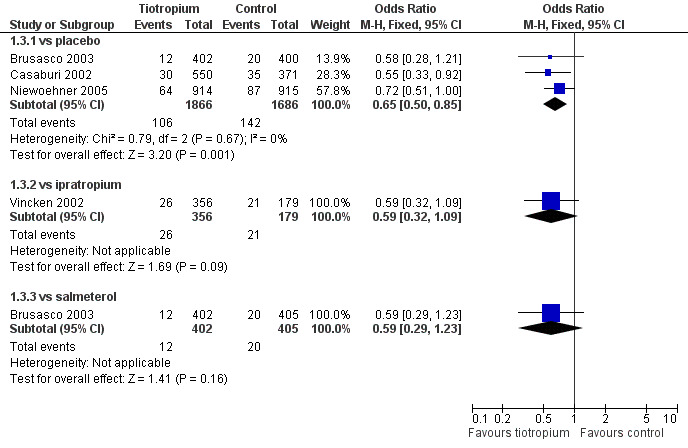
Forest plot of comparison: 1 Health outcomes, outcome: 1.3 Hospitalisations for COPD.
4.
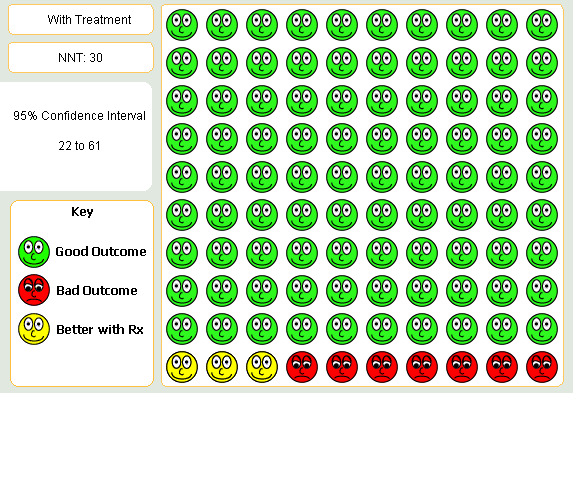
Patients with hospitalisation over one year on Tiotropium compared to placebo or Ipratropium
All‐cause mortality
Cumulative all‐cause mortality was 1.5% in the control groups over the duration of the trials. There were no statistically significant differences between tiotropium and placebo, ipratropium, or salmeterol (Detail 1:5). The trials were statistically homogeneous across the control groups (P value 0.22) and the summary estimate for tiotropium compared to other treatments was not significant; with a wide confidence interval due to small numbers (OR 0.73; 95% CI 0.35 to 1.49), Figure 5.
5.
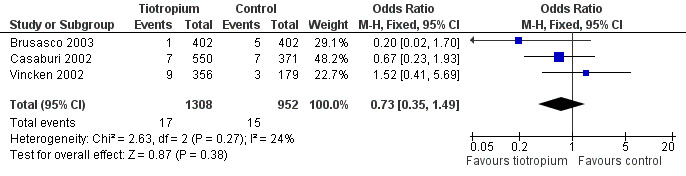
Forest plot of comparison: 1 Health outcomes, outcome: 1.6 All‐cause mortality ‐‐ summary estimate.
Health‐related quality of life and symptom scales
We extracted and analysed the mean change in health‐related quality of life, measured by the St George's Respiratory Questionnaire (SGRQ), and the proportion with a clinically significant improvement in the SGRQ and Transitional Dyspnea Index (TDI) over the duration of the trials. We were not able to obtain adequate reports of mean change in TDI to allow its combination.
St George's Respiratory Questionnaire
As shown in Detail 2:1, the mean change in SGRQ over the course of the trials was larger with tiotropium than with placebo (WMD ‐3.3; 95% CI ‐4.6 to ‐0.8) and ipratropium (WMD ‐3.3; 95% CI ‐5.6 to ‐1.0). A smaller and non‐significant difference was observed compared to salmeterol (WMD ‐1.4; 95% CI ‐3.2 to 0.4). The trials were statistically homogeneous across the control groups (P value 0.31) and the summary estimate for tiotropium compared to placebo or ipratropium was an improvement of WMD ‐3.3 (95% CI ‐4.7 to ‐2.2). The results for the proportion with a clinically significant change in SGRQ were similar to results for mean change in SGRQ. Statistically significant differences were observed for tiotropium compared to placebo and compared to ipratropium but not for tiotropium compared to salmeterol (Detail 2:3). There was evidence of statistical heterogeneity across the control groups (P value 0.03).
Transitional Dyspnea Index
As shown in Detail 2:4, the proportion with a clinically significant change in TDI over the course of the trials was greater with tiotropium than with placebo (OR 1.96; 95% CI 1.58 to 2.44) and ipratropium (OR 2.01; 95% CI 1.26 to 3.20) but was not significantly different from salmeterol (OR 1.08; 95% CI 0.80 to 1.46). There was evidence of statistical heterogeneity across the control groups (P value 0.07).
Spirometric indices
We extracted and analysed change in spirometric indices from baseline (prior to first drug dose) to the end of the trials, and change in spirometric indices from steady state (8 to 15 days after first drug dose) to the end of the trials for all available data.
Change in spirometric indices from baseline
As shown in Detail 3:1, the mean change in trough FEV1 from baseline was greater with tiotropium than with placebo (WMD 140 ml; 95% CI 118 to 162 ml) and ipratropium (WMD 150 ml; 95% CI 106 to 193 ml). A smaller but statistically significant difference was observed compared to salmeterol (WMD 40 ml; 95% CI 12 to 68 ml). There was evidence of statistical heterogeneity across the control groups (P value less than 0.0001), which arose from the smaller mean difference when tiotropium was compared to salmeterol. Similar results were observed for change in mean FEV1 and change in peak FEV1 (Details 3:2 and 3:3).
As shown in Detail 3:4, the mean change in trough FVC from baseline was greater with tiotropium than with placebo (WMD 278 ml; 95% CI 208 to 348) and ipratropium (WMD 210 ml; 95% CI 112 to 308 ml). A smaller but statistically significant difference was observed compared to salmeterol (WMD 90 ml; 95% CI 35 to 145 ml). There was evidence of statistical heterogeneity across the control groups (P value less than 0.0001). Similar results were observed for change in mean FEV1 and change in peak FVC except that the difference between tiotropium and ipratropium did not attain statistical significance (Details 3:5 and 3:6).
As shown in Detail 3:7, the mean change in morning peak flow from baseline was greater with tiotropium than with placebo (WMD 21 ml; 95% CI 15 to 28 ml) and ipratropium (WMD 16 ml; 95% CI 7 to 25 ml). No difference was observed compared to salmeterol (WMD 0 ml; 95% CI ‐8 to 9 ml). There was evidence of statistical heterogeneity across the control groups (P value 0.005). Similar results were observed for change in evening peak flow except that the difference between tiotropium and salmeterol was statistically significant (Detail 3:8).
Change in spirometric indices from steady state
Data on decline in FEV1 and FVC from steady state to the end of the trial were available for two 12‐month trials, one comparing tiotropium with placebo and the other comparing tiotropium with ipratropium. As shown in Detail 3:9, the mean decline in trough FEV1 from steady state was statistically significantly slower with tiotropium than placebo (WMD 30 ml; 95% CI 2 to 56 ml) and statistically insignificantly slower with tiotropium than ipratropium (WMD 30 ml; 95% CI ‐14 to 74 ml). The trials were statistically homogeneous across the control groups (Pvalue greater than 0.99) and the summary estimate showed a WMD of 30 ml (95% CI 7 to 53 ml) slower decline in FEV1 for tiotropium compared to placebo or ipratropium. Results for decline in mean FEV1 and peak FEV1 were variable and showed non‐significant differences for tiotropium compared to placebo or ipratropium (Details 3:10 and 3:11). No statistically significant differences were observed between tiotropium and either control group for decline in trough, mean and peak FVC (Details 3:12, 3:13 and 3:14).
Adverse events
We attempted to extract data on all available adverse events, including palpitations, dry mouth, blurred vision, urinary obstruction, constipation, and cardiovascular events. The only adverse event which was reported with sufficient consistency to allow combination was dry mouth. As shown in Detail 7:1, dry mouth was significantly increased with tiotropium compared to placebo (OR 5.4; 95% CI 3.3 to 8.8), ipratropium (OR 2.1; 95% CI, 1.05 to 4.2), and salmeterol (OR 5.1; 95% CI 2.2 to 12). The statistical homogeneity of the effect of tiotropium across the control groups was borderline (P value 0.11).
Sensitivity analyses
We attempted to perform sensitivity analyses as specified in the methods but found that the trials were very similar with respect to disease severity, prior ipratropium use, concurrent therapies, reversibility of airflow obstruction, dose and method of delivery, and clinical description of COPD. In an attempt to characterise the effect of tiotropium on exacerbations in milder COPD, we restricted the analysis to the three trials with the highest mean baseline FEV1 (Beeh 2004; O'Donnell 2004; Vincken 2002), which produced a similar summary estimate (OR 0.66; 95% CI 0.53 to 0.83) to the overall estimate.
The effect of tiotropium on exacerbations in the one trial that permitted concurrent use of long‐acting ß2‐agonists (Niewoehner 2005: OR 0.81; 95% CI 0.66 to 0.99) was statistically similar to the eight others that withheld long‐acting ß2‐agonists (OR 0.71; 95% CI 0.61 to 0.82). A similar pattern was evident for hospitalisations: the OR for hospitalisations was 0.72 (95% CI 0.51 to 1.00) with concurrent use of long‐acting ß2‐agonists permitted and 0.57 (95% CI 0.41 to 0.81) with concurrent use of long‐acting ß2‐agonists prohibited.
Analyses restricted to trials of long‐term duration (six months or more) yielded either identical or highly consistent results. The above‐reported results for hospitalisation, mortality, SGRQ, TDI and change in spirometric indices from steady state were all based on long‐term trials. The OR for exacerbations in the long‐term trials was 0.78 (95% CI 0.69 to 0.88) and results for change in spirometric indices from baseline were also similar.
Additional sensitivity analyses by random‐effects instead of fixed‐effect models generally produced identical results since in the setting of homogeneity the random‐effects model reduces to a fixed‐effect model.
Funnel plots were checked for primary endpoints. The funnel plot for exacerbations showed asymmetry and a relative absence of small studies with results that did not favour tiotropium, suggesting the possibility of some publication bias (Figure 6). Funnel plots for other outcomes showed no clear evidence of publication bias, although the ability to detect such a bias was limited by the small number of included studies.
6.
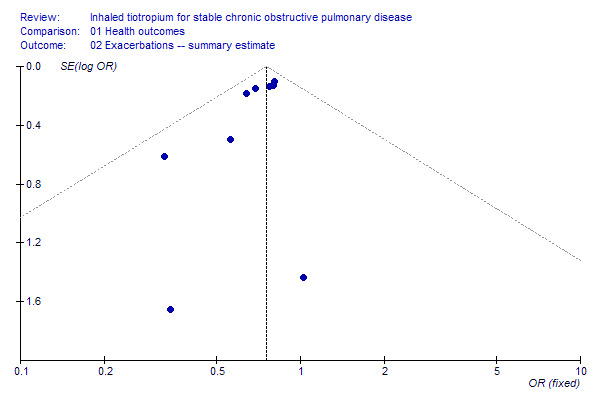
Funnel plot of included studies for COPD exacerbations
Discussion
This systematic review of the currently available randomised trials of tiotropium for stable COPD demonstrated that tiotropium reduced COPD exacerbations and related hospitalisations compared to placebo or ipratropium. Similar improvements were evident for quality‐of‐life and symptom scales. Increases in FEV1 and FVC from baseline were significantly larger with tiotropium than with placebo, ipratropium and long‐acting ß2‐agonists. Although the total number of patients contributing data at one‐year follow up in these trials was modest, there was a statistically significant difference in the decline in trough FEV1 from steady state with tiotropium compared to placebo or ipratropium.
The benefits observed with tiotropium for exacerbations and related hospitalisations were large and clinically important. COPD exacerbations were responsible for 8 million outpatient visits, 1.5 million emergency room visits, and 726,000 hospitalisations in the US in 2000 (Mannino 2002). A one‐third reduction in hospitalisations could potentially yield significant reductions in morbidity and cost. Consistent with this expectation, tiotropium has been shown to be cost‐effective, although not cost‐saving, compared to ipratropium, in Europe (Oostenbrink 2004). The magnitude of the reduction in exacerbation‐related hospitalisations with tiotropium was similar in comparison to placebo and ipratropium and was similar in the large placebo‐controlled trials that did, and did not, permit continuation of long‐acting ß2‐agonists during the trial.
Changes in quality of life, symptom scales and spirometric indices also appeared clinically significant. Compared to placebo and to ipratropium, the mean change in the SGRQ across all participants was close to the SGRQ's clinically significant change of 4 units, and substantially more participants on tiotropium achieved a clinically significant change in SQRQ and TDI compared to placebo and ipratropium. Improvements in spirometric indices from baseline were large and clinically significant compared to placebo and to ipratropium, and smaller but statistically significant compared to salmeterol.
The results of this meta‐analysis were consistent with a prior overview (Sin 2003) with respect to exacerbations and quality of life. They extend that review with more than twice as many patients and additional outcomes of hospitalisations, mortality, symptom scales, spirometric indices and adverse events. Most striking of these is the observation that the decline in trough FEV1 from steady state was slower with tiotropium than with placebo or ipratropium. This difference was large relative to the differences observed in a meta‐analysis of inhaled corticosteroids in COPD (Sutherland 2003). However, this observation should be interpreted with caution considering that it might also be due to: 1) incomplete attainment of steady state of tiotropium at 8 days; 2) chance, given that multiple spirometric indices were measured and that the duration of the relevant trials were only one year; and 3) bias, given that not all trial results for this index were available for inclusion in this summary estimate. Larger, longer‐term trials are necessary to assess the validity of this result, which would be of major clinical relevance if replicated.
The trials included in this review were of good to excellent quality and used almost identical designs with regard to inclusion and exclusion criteria. The clinical characteristics of the patients recruited into the trials was, therefore, fairly homogeneous, and disease severity as measured by baseline spirometry and event rates in the control groups was also similar between trials. The clinical homogeneity of the trials resulted in statistical homogeneity for many outcome measures across the trials. Meta‐analysis of these trials was, therefore, justified on clinical and statistical grounds.
We calculated summary estimates of the effects of tiotropium on clinical outcomes compared to placebo and ipratropium. Although on the face of it this approach may introduce some heterogeneity, we believe that it was justified on two grounds. First, heterogeneity would be introduced only if the control therapy (ipratropium) had an effect on the outcome. Ipratropium has been shown to not alter long‐term decline in FEV1 (Anthonisen 1994), hospitalisations or survival (Anthonisen 2002) compared to placebo; long‐acting ß2‐agonists, on the other hand, have been shown to reduce exacerbations and hospitalisations compared to placebo. Second, there was very little statistical evidence of heterogeneity across trials with varying control groups. The effect of tiotropium on hospitalisations, for example, was the same for comparison groups of placebo and ipratropium.
Potential limitations of meta‐analyses include double‐counting of patients from overlapping publications, publication bias, and selection bias. A recent overview of therapies for COPD (Sin 2003), which meta‐analysed SGRQ and COPD exacerbation results for tiotropium, erroneously included two pairs of trials as separate trials. The consequent double‐counting of patients yielded an analysis based on 3,574 patients instead of the 2,663 patients that were actually randomised in the trials cited in that review. We avoided such double‐counting by discussing trial overlap with the primary authors and with the manufacturer of tiotropium.
We evaluated for publication bias by checking funnel plots and found evidence of publication bias for exacerbations. Given the relatively small weight of the smaller studies for this outcome (less than 5%), it is unlikely that any small unpublished studies severely biased our results. Large unpublished studies would pose a bigger threat to the pooled results. In order to attempt to minimise such publication bias, we contacted the manufacturer of tiotropium to obtain a list of the published, unpublished, and ongoing trials of tiotropium. The manufacturer was very helpful in providing additional information on completed published trials but did not provide information on completed but unpublished trials. Publication bias, therefore, remains a potential concern. Two completed trials that compared tiotropium to salmeterol were published in a combined form (Brusasco 2003) that showed no difference between tiotropium and salmeterol. One of these trials showed that tiotropium was superior to salmeterol for exacerbations. It was published a year earlier (Donohue 2002) than the combined version; the other, which presumably showed that tiotropium was not superior to salmeterol for exacerbations is, as of this writing, yet to be published as a unique publication. We will continue to update this review in an attempt to minimise potential publication bias.
An additional possible concern with meta‐analyses is selection bias, which refers to bias from non‐differential selection of available trials for inclusion into the meta‐analysis. To avoid selection bias a systematic and comprehensive search was conducted and two reviewers independently evaluated trials for inclusion into the meta‐analysis using standardised pre‐specified inclusion and exclusion criteria.
Authors' conclusions
Implications for practice.
Tiotropium reduced COPD exacerbations and exacerbation‐related hospitalisations compared to placebo or ipratropium. It improved health‐related quality‐of‐life and symptom scores among patients with moderate and severe disease compared to placebo and ipratropium and can be recommended for the treatment of stable COPD. There was a suggestion from this review that tiotropium may slow decline in FEV1, although this finding requires confirmation.
Implications for research.
Additional long‐term studies are required to establish the effect of tiotropium on mortality and decline in FEV1, to evaluate its effectiveness in comparison to, and combination with, long‐acting ß2‐agonists, and to establish its role in mild and very severe COPD.
What's new
| Date | Event | Description |
|---|---|---|
| 21 November 2012 | Review declared as stable | This review is no longer being updated. It has been replaced by three new Cochrane reviews (tiotropium versus placebo (Karner 2012); tiotropium versus LABA (Chong 2012) and tiotropium verus ipratropium bromide (Cheyne 2011)). |
History
Protocol first published: Issue 1, 2001 Review first published: Issue 2, 2005
| Date | Event | Description |
|---|---|---|
| 3 July 2008 | Amended | Converted to new review format. |
| 31 January 2005 | New citation required and conclusions have changed | Substantive amendment |
Notes
This review is no longer being updated. It has been replaced by three new Cochrane reviews (tiotropium versus placebo (Karner 2012); tiotropium versus LABA (Chong 2012) and tiotropium verus ipratropium bromide (Cheyne 2011)).
Acknowledgements
We thank Brenda Taveras for assistance with manuscript preparation, various individuals at Boehringer‐Ingelheim who helped provide unpublished data to strengthen this review, and Phillippa Poole for editorial guidance.
Data and analyses
Comparison 1. Health outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exacerbations | 9 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 vs placebo | 8 | 5644 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.66, 0.85] |
| 1.2 vs ipratropium bromide | 1 | 535 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.44, 0.92] |
| 1.3 vs salmeterol | 1 | 807 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.65, 1.17] |
| 2 Exacerbations ‐‐ summary estimate | 9 | 6179 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.66, 0.83] |
| 3 Hospitalisations for COPD | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 vs placebo | 3 | 3552 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.50, 0.85] |
| 3.2 vs ipratropium | 1 | 535 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.32, 1.09] |
| 3.3 vs salmeterol | 1 | 807 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.29, 1.23] |
| 4 Hospitalisations for COPD ‐‐ summary estimate | 4 | 4087 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.51, 0.82] |
| 5 All‐cause mortality | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 vs placebo | 2 | 1723 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.20, 1.23] |
| 5.2 vs ipratropium bromide | 1 | 535 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.41, 5.69] |
| 5.3 vs salmeterol | 1 | 807 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.38] |
| 6 All‐cause mortality ‐‐ summary estimate | 3 | 2260 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.35, 1.49] |
1.1. Analysis.

Comparison 1 Health outcomes, Outcome 1 Exacerbations.
1.2. Analysis.

Comparison 1 Health outcomes, Outcome 2 Exacerbations ‐‐ summary estimate.
1.3. Analysis.

Comparison 1 Health outcomes, Outcome 3 Hospitalisations for COPD.
1.4. Analysis.

Comparison 1 Health outcomes, Outcome 4 Hospitalisations for COPD ‐‐ summary estimate.
1.5. Analysis.

Comparison 1 Health outcomes, Outcome 5 All‐cause mortality.
1.6. Analysis.

Comparison 1 Health outcomes, Outcome 6 All‐cause mortality ‐‐ summary estimate.
Comparison 2. Quality of life and symptom scales.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean change in SGRQ | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 vs placebo | 2 | 1522 | Mean Difference (IV, Random, 95% CI) | ‐3.27 [‐4.50, ‐2.04] |
| 1.2 vs ipratropium bromide | 1 | 486 | Mean Difference (IV, Random, 95% CI) | ‐3.3 [‐5.63, ‐0.97] |
| 1.3 vs salmeterol | 1 | 710 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐3.24, 0.44] |
| 2 Mean change in SGRQ ‐‐ summary estimate | 3 | 2008 | Mean Difference (IV, Fixed, 95% CI) | ‐3.28 [‐4.37, ‐2.19] |
| 3 Clinically significant change in SGRQ | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 vs placebo | 2 | 1522 | Odds Ratio (M‐H, Random, 95% CI) | 1.83 [1.21, 2.76] |
| 3.2 vs ipratropium | 1 | 486 | Odds Ratio (M‐H, Random, 95% CI) | 1.99 [1.35, 2.94] |
| 3.3 vs salmeterol | 1 | 710 | Odds Ratio (M‐H, Random, 95% CI) | 1.26 [0.93, 1.69] |
| 5 Clinically significant change in TDI focal score | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 vs placebo | 2 | 1489 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.96 [1.58, 2.44] |
| 5.2 vs ipratropium | 1 | 479 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.01 [1.26, 3.20] |
| 5.3 vs salmeterol | 1 | 688 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.80, 1.46] |
2.1. Analysis.

Comparison 2 Quality of life and symptom scales, Outcome 1 Mean change in SGRQ.
2.2. Analysis.

Comparison 2 Quality of life and symptom scales, Outcome 2 Mean change in SGRQ ‐‐ summary estimate.
2.3. Analysis.

Comparison 2 Quality of life and symptom scales, Outcome 3 Clinically significant change in SGRQ.
2.5. Analysis.

Comparison 2 Quality of life and symptom scales, Outcome 5 Clinically significant change in TDI focal score.
Comparison 3. Spirometry.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in trough FEV1 from baseline | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 vs placebo | 4 | 1735 | Mean Difference (IV, Random, 95% CI) | 139.96 [118.28, 161.64] |
| 1.2 vs ipratropium bromide | 1 | 490 | Mean Difference (IV, Random, 95% CI) | 150.0 [106.16, 193.84] |
| 1.3 vs salmeterol | 1 | 774 | Mean Difference (IV, Random, 95% CI) | 40.0 [12.31, 67.69] |
| 2 Change in mean FEV1 from baseline | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 vs placebo | 4 | 1735 | Mean Difference (IV, Random, 95% CI) | 203.94 [184.76, 223.11] |
| 2.2 vs ipratropium bromide | 1 | 490 | Mean Difference (IV, Random, 95% CI) | 100.0 [56.16, 143.84] |
| 2.3 vs salmeterol | 1 | 774 | Mean Difference (IV, Random, 95% CI) | 70.0 [42.31, 97.69] |
| 3 Change in peak FEV1 from baseline | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 vs placebo | 3 | 1669 | Mean Difference (IV, Fixed, 95% CI) | 220.55 [201.08, 240.02] |
| 3.2 vs ipratropium bromide | 1 | 490 | Mean Difference (IV, Fixed, 95% CI) | 100.0 [56.16, 143.84] |
| 3.3 vs salmeterol | 1 | 774 | Mean Difference (IV, Fixed, 95% CI) | 80.0 [52.31, 107.69] |
| 4 Change in trough FVC from baseline | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 vs placebo | 4 | 1735 | Mean Difference (IV, Random, 95% CI) | 278.10 [208.37, 347.84] |
| 4.2 vs ipratropium bromide | 1 | 490 | Mean Difference (IV, Random, 95% CI) | 210.0 [112.08, 307.92] |
| 4.3 vs salmeterol | 1 | 774 | Mean Difference (IV, Random, 95% CI) | 90.0 [34.56, 145.44] |
| 5 Change in mean FVC from baseline | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 vs placebo | 4 | 1735 | Mean Difference (IV, Random, 95% CI) | 387.20 [343.45, 430.94] |
| 5.2 vs ipratropium bromide | 1 | 490 | Mean Difference (IV, Random, 95% CI) | 80.0 [‐17.92, 177.92] |
| 5.3 vs salmeterol | 1 | 774 | Mean Difference (IV, Random, 95% CI) | 140.0 [84.56, 195.44] |
| 6 Change in peak FVC from baseline | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 vs placebo | 3 | 1669 | Mean Difference (IV, Random, 95% CI) | 405.08 [337.12, 473.03] |
| 6.2 vs ipratropium bromide | 1 | 490 | Mean Difference (IV, Random, 95% CI) | 90.0 [‐7.92, 187.92] |
| 6.3 vs salmeterol | 1 | 774 | Mean Difference (IV, Random, 95% CI) | 150.0 [94.56, 205.44] |
| 7 Change in morning PEFR from baseline | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 vs placebo | 4 | 1723 | Mean Difference (IV, Random, 95% CI) | 21.42 [14.83, 28.02] |
| 7.2 vs ipratropium bromide | 1 | 504 | Mean Difference (IV, Random, 95% CI) | 15.6 [6.53, 24.67] |
| 7.3 vs salmeterol | 1 | 772 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐8.31, 8.91] |
| 8 Change in evening PEFR from baseline | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 vs placebo | 3 | 1542 | Mean Difference (IV, Random, 95% CI) | 30.34 [23.19, 37.49] |
| 8.2 vs ipratropium bromide | 1 | 503 | Mean Difference (IV, Random, 95% CI) | 13.40 [3.95, 22.85] |
| 8.3 vs salmeterol | 1 | 765 | Mean Difference (IV, Random, 95% CI) | 11.0 [2.35, 19.65] |
| 9 Change in trough FEV1 from day 8, with summary estimate | 2 | 1336 | Mean Difference (IV, Random, 95% CI) | 30.00 [6.56, 53.44] |
| 9.1 vs placebo | 1 | 846 | Mean Difference (IV, Random, 95% CI) | 30.0 [2.27, 57.73] |
| 9.2 vs ipratropium bromide | 1 | 490 | Mean Difference (IV, Random, 95% CI) | 30.0 [‐13.84, 73.84] |
| 9.3 vs salmeterol | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Change in mean FEV1 from day 8, with summary estimate | 2 | 1336 | Mean Difference (IV, Random, 95% CI) | 15.72 [‐7.72, 39.15] |
| 10.1 vs placebo | 1 | 846 | Mean Difference (IV, Random, 95% CI) | 10.0 [‐17.73, 37.73] |
| 10.2 vs ipratropium bromide | 1 | 490 | Mean Difference (IV, Random, 95% CI) | 30.0 [‐13.84, 73.84] |
| 10.3 vs salmeterol | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Change in peak FEV1 from day 8, with summary estimate | 2 | 1336 | Mean Difference (IV, Random, 95% CI) | 10.00 [‐17.72, 37.72] |
| 11.1 vs placebo | 1 | 846 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐27.73, 27.73] |
| 11.2 vs ipratropium bromide | 1 | 490 | Mean Difference (IV, Random, 95% CI) | 30.0 [‐13.84, 73.84] |
| 11.3 vs salmeterol | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Change in trough FVC from day 8 | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 vs placebo | 1 | 846 | Mean Difference (IV, Random, 95% CI) | 40.0 [‐15.41, 95.41] |
| 12.2 vs ipratropium bromide | 1 | 490 | Mean Difference (IV, Random, 95% CI) | ‐40.0 [‐110.73, 30.73] |
| 12.3 vs salmeterol | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Change in mean FVC from day 8, with summary estimate | 2 | 1336 | Mean Difference (IV, Random, 95% CI) | ‐3.80 [‐47.42, 39.81] |
| 13.1 vs placebo | 1 | 846 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐55.41, 55.41] |
| 13.2 vs ipratropium bromide | 1 | 490 | Mean Difference (IV, Random, 95% CI) | ‐10.0 [‐80.73, 60.73] |
| 13.3 vs salmeterol | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Change in peak FVC from day 8, with summary estimate | 2 | 1336 | Mean Difference (IV, Random, 95% CI) | 24.99 [‐24.98, 74.97] |
| 14.1 vs placebo | 1 | 846 | Mean Difference (IV, Random, 95% CI) | 20.0 [‐50.63, 90.63] |
| 14.2 vs ipratropium bromide | 1 | 490 | Mean Difference (IV, Random, 95% CI) | 30.0 [‐40.73, 100.73] |
| 14.3 vs salmeterol | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.

Comparison 3 Spirometry, Outcome 1 Change in trough FEV1 from baseline.
3.2. Analysis.

Comparison 3 Spirometry, Outcome 2 Change in mean FEV1 from baseline.
3.3. Analysis.

Comparison 3 Spirometry, Outcome 3 Change in peak FEV1 from baseline.
3.4. Analysis.

Comparison 3 Spirometry, Outcome 4 Change in trough FVC from baseline.
3.5. Analysis.

Comparison 3 Spirometry, Outcome 5 Change in mean FVC from baseline.
3.6. Analysis.

Comparison 3 Spirometry, Outcome 6 Change in peak FVC from baseline.
3.7. Analysis.

Comparison 3 Spirometry, Outcome 7 Change in morning PEFR from baseline.
3.8. Analysis.

Comparison 3 Spirometry, Outcome 8 Change in evening PEFR from baseline.
3.9. Analysis.

Comparison 3 Spirometry, Outcome 9 Change in trough FEV1 from day 8, with summary estimate.
3.10. Analysis.

Comparison 3 Spirometry, Outcome 10 Change in mean FEV1 from day 8, with summary estimate.
3.11. Analysis.

Comparison 3 Spirometry, Outcome 11 Change in peak FEV1 from day 8, with summary estimate.
3.12. Analysis.

Comparison 3 Spirometry, Outcome 12 Change in trough FVC from day 8.
3.13. Analysis.

Comparison 3 Spirometry, Outcome 13 Change in mean FVC from day 8, with summary estimate.
3.14. Analysis.

Comparison 3 Spirometry, Outcome 14 Change in peak FVC from day 8, with summary estimate.
Comparison 7. Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dry mouth | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 vs placebo | 3 | 1791 | Odds Ratio (M‐H, Random, 95% CI) | 5.35 [3.27, 8.76] |
| 1.2 vs ipratropium bromide | 1 | 535 | Odds Ratio (M‐H, Random, 95% CI) | 2.10 [1.05, 4.18] |
| 1.3 vs salmeterol | 1 | 807 | Odds Ratio (M‐H, Random, 95% CI) | 5.08 [2.22, 11.64] |
7.1. Analysis.

Comparison 7 Adverse events, Outcome 1 Dry mouth.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Beeh 2004.
| Methods | Type: Parallel group. Duration: 3 months. Pre‐randomization run‐in period: 1 week wash‐out period. Randomisation method: Not stated. Blinding: Double‐blind. Co‐interventions allowed during trial: short‐acting B‐agonist, inhaled corticosteroid, prednisone <= 10 mg/day, theophylline. Co‐interventions NOT allowed during trial: ipratropium, long‐acting B‐agonist. Confounders: None noted. Assessment score: 4 | |
| Participants | Setting: Outpatients, referral source not stated. Inclusion criteria: Prior diagnosis of COPD, FEV1 <= 70% predicted, ratio <=70%, age >40 years, smoking history > 10 pack years. Exclusion criteria: asthma, allergic rhinitis, atopy, use of daytime oxygen, recent history of myocardial infarction, drug treatment for arrhythmia, or recent hospitalisation for congestive heart failure Number recruited: 1,639 Mean age: 62 years Gender: 75% male Baseline FEV1: 1.3 +/‐ 0.5 L; FVC 2.4 +/‐ 0.7 L; Ratio NA Baseline co‐interventions allowed during trial: short‐acting B‐agonist 76%, inhaled corticosteroid 57%, prednisone 16%, theophylline 52%. Baseline co‐interventions NOT allowed during trial: ipratropium 69%, long‐acting B‐agonist 50%. | |
| Interventions | Experimental: tiotropium 18ug qD by handihaler. Control: Placebo. | |
| Outcomes | Analysed: Cumulative incidence of exacerbations; Reported: Difference in FEV1 and FVC between intervention and control groups. | |
| Notes | Analysed: Cumulative incidence of exacerbations; Reported: Difference in FEV1 and FVC between intervention and control groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Brusasco 2003.
| Methods | Type: Parallel group. Duration: 6 months. Pre‐randomisation run‐in period: 2 week wash‐out period. Randomization method: Not stated. Blinding: Double‐blind. Co‐interventions allowed during trial: Not stated, but ?same as Donohue (2002): short‐acting B‐agonist, inhaled corticosteroid, prednisone <= 10 mg/day, theophylline. Co‐interventions NOT allowed during trial: Not stated, but ?same as Donohue (2002): ipratropium, long‐acting B‐agonist. Confounders: None noted. Assessment score: 4 | |
| Participants | Setting: Outpatients (18 countries), referral source not stated. Inclusion criteria: Prior diagnosis of COPD (ATS), FEV1 <= 65% predicted, ratio <=70%, age >40 years, smoking history > 10 pack years. Exclusion criteria: asthma, allergic rhinitis, atopy, total eosinophil count >= 600/mm³, use of supplemental oxygen or upper respiratory infection within 6 weeks, significant disease other than COPD. Number recruited: 1,207 Mean age: 64 years Gender: 76% male Baseline FEV1: 1.1 +/‐ 0.4 L; FVC 2.6 +/‐ 0.7 L; Ratio 43 +/‐ 10 % Baseline co‐interventions allowed during trial: Not reported. Baseline co‐interventions NOT allowed during trial: Not reported. | |
| Interventions | Experimental: tiotropium 18ug qD by handihaler. Control1: Salmeterol 50ug BID by metered‐dose inhaler. Control2: Placebo. | |
| Outcomes | Analysed: Cumulative incidence of exacerbations, hospitalisations, and all‐cause mortality; change in St. George's Respiratory Questionnaire and Transitional Dyspnea Index; change in trough FEV1 and FVC; adverse events Reported: rescue B‐agonist use. | |
| Notes | Reported combined results of Donohue (2002) and a similar unpublished trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Calverley 2003.
| Methods | Type: Parallel group. Duration: 6 weeks. Pre‐randomisation run‐in period: 1 week. Randomisation method: Not stated. Blinding: Double‐blind. Co‐interventions allowed: Inhaled corticosteroid, oral corticosteroid (doses fixed throughout study period), short‐acting B‐agonist PRN. Co‐interventions NOT allowed: ipratropium, long‐acting B‐agonist, oral B‐agonist, theophylline. Confounders: None noted. Assessment score: 3 | |
| Participants | Setting: Outpatients in UK+Netherlands, referral population. Inclusion criteria: Prior diagnosis of COPD (ATS), FEV1 25‐65% predicted, ratio <=70%, age >40 years, smoking history > 10 pack years. Exclusion criteria: asthma, allergic rhinitis, atopy, total eosinophil count >= 600/mm³, COPD exacerbation in prior 4 weeks, significant disease other than COPD. Number recruited: 121 Mean age: 66 years Gender: 75% male Baseline FEV1: 1.1 +/‐ 0.4 L; FVC 2.2 +/‐ 0.7 L; Ratio 41 +/‐ 12 %. Baseline co‐interventions allowed during trial: short‐acting B‐agonist 85%; inhaled corticosteroid 78%; oral corticosteroid 2%. Baseline co‐interventions NOT allowed during trial: ipratropium 39%; long‐acting B‐agonist ‐‐ not reported; oral B‐agonist 9%; theophylline 4%. | |
| Interventions | Experimental1: tiotropium 18 ug qAM by handihaler. Experimental2: tiotropium 18 ug qPM by handihaler. Control: Placebo. | |
| Outcomes | Analysed: Cumulative incidence of exacerbations, hospitalisations; change in FEV1 and FVC for groups. | |
| Notes | The original report showed no difference in clinical outcomes between intervention groups receiving morning and evening tiotropium; these groups were therefore combined for analyses of clinical outcomes. For analyses of spirometric indices, data were included for morning tiotropium and placebo groups only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Casaburi 2000.
| Methods | Type: Parallel group. Duration: 3 months. Pre‐randomisation run‐in period: 2 week wash‐out period. Randomisation method: By order of entry within each study centre. Blinding: Double‐blind. Co‐interventions allowed: Inhaled corticosteroid, prednisone <= 10 mg/day, theophylline (doses fixed throughout study period), short‐acting B‐agonist PRN. Co‐interventions NOT allowed: ipratropium, long‐acting B‐agonist. Confounders: None noted. Assessment score: 3 | |
| Participants | Setting: Outpatients in US, referral source not stated. Inclusion criteria: Prior diagnosis of COPD (ATS), FEV1 <= 65% predicted, ratio <=70%, age >=40 years old, smoking history > 10 pack years, 'clinically stable airway obstruction'. Exclusion criteria: asthma, allergic rhinitis, atopy, total eosinophil count >= 600/mm³, regular use of daytime supplemental oxygen, >=10 mg prednisone/day for COPD symptoms in prior month, MI within prior year, CHF within 3 years, use of anti‐arrhythmic drug. Number recruited: 470 Mean age: 65 years Gender: 65% male Baseline FEV1: 1.0 +/‐ 0.4 L; FVC not stated; Ratio 46 +/‐ 12 %. Baseline co‐interventions allowed during trial: Not reported. Baseline co‐interventions NOT allowed during trial: Not reported. | |
| Interventions | Experimental: tiotropium 18ug qD by handihaler. Control: Placebo. | |
| Outcomes | Analysed: Change in trough, peak and average FEV1 and FVC. Adverse Events: deaths: 1. | |
| Notes | Redundant with Casaburi (2002). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Casaburi 2002.
| Methods | Type: Parallel group. Duration: 12 months. Pre‐randomisation run‐in period: 2 week wash‐out period. Randomisation method: Not stated but ?same as Casaburi (2000): by order of entry within each study centre. Blinding: Double‐blind. Co‐interventions allowed: Inhaled corticosteroid, prednisone <= 10 mg/day, theophylline (doses fixed throughout study period), short‐acting B‐agonist PRN. Co‐interventions NOT allowed: ipratropium, long‐acting B‐agonist. Confounders: None noted. Assessment score: 3 | |
| Participants | Setting: Outpatients in US, referral source not stated. Inclusion criteria: Prior diagnosis of COPD (ATS), FEV1 <= 65% predicted, ratio <=70%, age >=40 years old, smoking history > 10 pack years, 'clinically stable airway obstruction'. Exclusion criteria: asthma, allergic rhinitis, atopy, total eosinophil count >= 600/mm³, regular use of daytime supplemental oxygen, >=10 mg prednisone/day for COPD symptoms in prior month, MI within prior year, CHF within 3 years, use of anti‐arrhythmic drug. Number recruited: 921 Mean age: 65 years Gender: 65% male Baseline FEV1: 1.0 +/‐ 0.4 L; FVC 2.3 +/‐ 0.8 L; Ratio 46 +/‐ 12 %. Co‐interventions at baseline allowed during trial: short‐acting B‐agonist 99%; inhaled corticosteroid 42%; oral corticosteroid 7%; theophylline 23%. Co‐interventions at baseline NOT allowed during trial: ipratropium 57%; long‐acting B‐agonist ‐‐ not reported. | |
| Interventions | Experimental: tiotropium 18ug qD by handihaler. Control: Placebo. | |
| Outcomes | Analysed: Change in FEV1, FVC and PEFR; cumulative incidence of COPD exacerbations, increase B‐ agonist use. Adverse Events: Deaths: 7. | |
| Notes | Reported combined results of Casaburi (2000) and a similar unpublished trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Celli 2003.
| Methods | Type: Parallel group. Duration: 1 month. Pre‐randomisation run‐in period: 2 week wash‐out period. Randomisation method: Not stated. Blinding: Double‐blind. Co‐interventions allowed: Not stated. Co‐interventions NOT allowed: ipratropium, long‐acting B‐agonist. Confounders: None noted. Assessment score: 4 | |
| Participants | Setting: Outpatients in US, referral source not stated. Inclusion criteria: Prior diagnosis of COPD (ATS), FEV1 30‐65% predicted, ratio <=70%, TGV >= 120%, age >40 years, smoking history > 10 pack years. Exclusion criteria: asthma, allergic rhinitis, atopy, total eosinophil count >= 600/mm³, recent upper respiratory infection. Number recruited: 81 Mean age: 64 years Gender: 61% male Baseline FEV1: 1.1 +/‐ 0.4 L; FVC 2.5 +/‐ 0.8 L; Ratio 47 +/‐ 10 %, TGV 5.5 +/‐ 1.4 L. Baseline co‐interventions allowed during trial: Not reported. Baseline co‐interventions NOT allowed during trial: Not reported. | |
| Interventions | Experimental: tiotropium 18ug qD by handihaler. Control: Placebo. | |
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Donohue 2002.
| Methods | Type: Parallel group. Duration: 6 months. Pre‐randomisation run‐in period: 2 week wash‐out period. Randomisation method: Not stated. Blinding: Double‐blind. Co‐interventions allowed: Inhaled corticosteroid, prednisone <= 10 mg/day, theophylline, short‐acting B‐agonist PRN Co‐interventions NOT allowed: ipratropium, long‐acting B‐agonist. Confounders: None noted. Assessment score: 4 | |
| Participants | Setting: Outpatients (12 countries), referral source not stated. Inclusion criteria: Prior diagnosis of COPD (ATS), FEV1 <= 60% predicted, ratio <=70%, age >40 years, smoking history > 10 pack years. Exclusion criteria: asthma, allergic rhinitis, atopy, total eosinophil count >= 600/mm³, use of daytime supplemental oxygen, recent upper respiratory infection, significant disease other than COPD. Number recruited: 623 Mean age: 65 years Gender: 75% male Baseline FEV1: 1.1 +/‐ 0.4 L; FVC 2.6 +/‐ 0.7 L; Ratio 42 +/‐ 9 %. Co‐interventions at baseline allowed during trial: Short‐acting B‐agonist 66%; inhaled corticosteroid 66%; oral corticosteroid 6%; theophylline 21%. Co‐interventions at baseline NOT allowed during trial: Ipratropium 53%; long‐acting B‐agonist ‐‐ not reported. | |
| Interventions | Experimental: tiotropium 18ug qD by handihaler. Control1: salmeterol 50ug BID by metered‐dose inhaler. Control2: Placebo. | |
| Outcomes | Analysed: Cumulative incidence of exacerbations, hospitalisations, and all‐cause mortality; change in St. George's Respiratory Questionnaire and Transitional Dyspnea Index; change in trough FEV1 and FVC; adverse events Others: None Mortality: None Morbidity: None. | |
| Notes | Redundant with Brasasco (2003). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Littner 2000.
| Methods | Type: Parallel group. Duration: 1 month. Pre‐randomisation run‐in period: 2 week wash‐out period. Randomisation method: Not stated. Blinding: Double‐blind. Co‐interventions allowed: Inhaled corticosteroid, theophylline (doses fixed throughout study period), short‐acting B‐agonist PRN. Co‐interventions NOT allowed: ipratropium, long‐acting B‐agonist, oral corticosteroid, cromolyn. Confounders: None noted. Assessment score: 4 | |
| Participants | Setting: Outpatients in US, referral source not stated. Inclusion criteria: Prior diagnosis of COPD (ATS), FEV1 30‐65% predicted, ratio <=70%, age >=40 years old, smoking history > 10 pack years. Exclusion criteria: asthma, allergic rhinitis, atopy, total eosinophil count >= 600/mm³, use of supplemental oxygen or upper respiratory infection within 6 weeks or during run‐in period, significant disease other than COPD. Number recruited: 169 Mean age: 66 years Gender: 57% male Baseline FEV1: 1.1 +/‐ 0.3 L; FVC not stated; Ratio not stated. Baseline co‐interventions allowed during trial: Not reported. Baseline co‐interventions NOT allowed during trial: Not reported. | |
| Interventions | Experimental: tiotropium 18ug qD by handihaler (n=33). Control: Placebo (n=35). | |
| Outcomes | Analysed: Change in trough FEV1 and FVC. Adverse Events: | |
| Notes | Data were extracted from this dose‐ranging study for patients randomised to the clinical dose of tiotropium (18ug) and placebo only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Niewoehner 2005.
| Methods | Type: Parallel group. Duration: 6 months. Pre‐randomisation run‐in period: No. Randomisation method: Block. Blinding: Double‐blind. Co‐interventions allowed during trial: Inhaled corticosteroid, theophylline (doses fixed throughout study period), long‐acting B‐agonist, short‐acting B‐agonist PRN. Co‐interventions NOT allowed during trial: ipratropium. Confounders: None noted. Assessment score: 4 | |
| Participants | Setting: Outpatients at 26 US Veterans' Administration medical centers. Inclusion criteria: Prior diagnosis of COPD, FEV1 <= 60% predicted, ratio <=70%, age >40 years, smoking history > 10 pack years. Exclusion criteria: Prior diagnosis of asthma, significant disease other than COPD Number recruited: 1,829 Mean age: 68 years Gender: 99% male Baseline FEV1: 1.0 L Baseline co‐interventions allowed during trial: Not reported. Baseline co‐interventions NOT allowed during trial: Not reported. | |
| Interventions | Experimental: tiotropium 18ug qD by handihaler. Control1: Placebo. | |
| Outcomes | Analysed: Cumulative incidence of exacerbations, hospitalisations. | |
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
O'Donnell 2004.
| Methods | Type: Parallel group. Duration: 6 weeks. Pre‐randomisation run‐in period: 2 week wash‐out period and to "familiarise [participants] with all testing procedures and to establish a standardised training history" Randomisation method: Not stated. Blinding: Double‐blind. Co‐interventions allowed: short‐acting B‐agonist (rescue), inhaled corticosteroid, prednisone, theophylline, mycolytic agents Co‐interventions NOT allowed: ipratropium, oral and long‐acting B‐agonists. Confounders: None noted. Assessment score: 4 | |
| Participants | Setting: Outpatients, referral source not stated. Inclusion criteria: Prior diagnosis of stable COPD, FEV1 <= 65% predicted, FRC >=120% predicted, age 40‐70 years, smoking history > 10 pack years. Exclusion criteria: asthma, allergic rhinitis, or atopy, significant disease other than COPD, important contraindications to exercise testing, exercise limitation not related to fatigue or exertional dyspnoea, participation in pulmonary rehabilitation program within 6 weeks Number recruited: 198 Mean age: 61 years Gender: 69% male Baseline FEV1: 1.3 +/‐ 0.5 L; FVC 2.8 +/‐ 0.8 L; Ratio 45 +/‐ 10 % Baseline co‐interventions allowed during trial: Not reported. Baseline co‐interventions NOT allowed during trial: Not reported. | |
| Interventions | Experimental: tiotropium 18ug qD by handihaler. Control: Placebo. | |
| Outcomes | Analysed: Cumulative incidence of exacerbations; change in St. George's Respiratory Questionnaire and Transitional Dyspnea Index; change in trough FEV1 and FVC; adverse events Reported: Difference between tiotropium and placebo in other pulmonary function (FEV1/FVC, SG, IC, RV, FRV, RV/TLC, FRC/TLC, TLC) and exercise capacity (Borg, IC, VT, fR, VE, IRV at rest, isotime and peak exercise). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
van Noord 2000.
| Methods | Type: Parallel group. Duration: 3 months. Pre‐randomisation run‐in period: 2 week wash‐out period. Randomisation method: Block (2:1) Blinding: Double‐blind. Co‐interventions allowed: Inhaled corticosteroid, prednisone <= 10 mg/day, theophylline (doses fixed throughout study period), short‐acting B‐agonist (PRN). Co‐interventions NOT allowed: ipratropium, long‐acting B‐agonists, oral B‐agonists, cromolyn. Confounders: None noted. Assessment score: 5 | |
| Participants | Setting: Outpatients at 14 centres in the Netherlands, referral source not stated. Inclusion criteria: Prior diagnosis of COPD (ATS), FEV1 <= 65% predicted, ratio <=70%, age >=40 years old, smoking history > 10 pack years, 'stable airway obstruction'. Exclusion criteria: asthma, allergic rhinitis, atopy, total eosinophil count >= 600/mm³, supplemental oxygen, upper respiratory tract infection in prior 6 weeks, MI in prior year, CHF within 3 years, use of anti‐arrhythmic drug, known hypersensitivity to anticholinergic drugs, known symptomatic prostatic hypertrophy, narrow angle glaucoma. Number recruited: 228 Mean age: 64 years Gender: 84% male Baseline FEV1: 1.2 +/‐ 0.4 L; FVC 2.8 +/‐ 0.8 L; Ratio 45 +/‐ 11 %. Baseline co‐interventions allowed during trial: short‐acting B‐agonist 69%; inhaled corticosteroid 76%; oral corticosteroid 9%; theophylline 13%. Baseline co‐interventions NOT allowed during trial: ipratropium 56%; long‐acting B‐agonist ‐‐ not reported. | |
| Interventions | Experimental: tiotropium 18ug qD by handihaler. Control: ipratropium 40 ug QID by MDI. | |
| Outcomes | Analysed: Change in trough FEV1 and FVC. Reported: Rescue B‐agonist use. Others: None Mortality: None Morbidity: None. | |
| Notes | Redundant with Vincken (2002). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Vincken 2002.
| Methods | Type: Parallel group. Duration: 12 months. Pre‐randomisation run‐in period: 2 week wash‐out period. Randomisation method: Block (2:1) Blinding: Double‐blind. Co‐interventions allowed: Inhaled corticosteroid, prednisone <= 10 mg/day, theophylline (doses fixed throughout study period), short‐acting B‐agonist (PRN). Co‐interventions NOT allowed: ipratropium, long‐acting B‐agonists, oral B‐agonists, cromolyn. Confounders: None noted. Assessment score: 5 | |
| Participants | Setting: Outpatients in Netherlands and Belgium, referral source not stated. Inclusion criteria: Prior diagnosis of COPD (ATS), FEV1 <= 65% predicted, ratio <=70%, age >=40 years old, smoking history > 10 pack years. Exclusion criteria: asthma, allergic rhinitis, atopy, total eosinophil count >= 600/mm³, regular use of supplemental oxygen, recent upper respiratory tract infection, 'significant disease other than COPD.' Number recruited: 535 Mean age: 64 years Gender: 85% male Baseline FEV1: 1.2 +/‐ 0.4 L; FVC 2.7 +/‐ 0.8; Ratio 46 +/‐ 10 %. Baseline co‐interventions allowed during trial: short‐acting B‐agonist 76%; inhaled corticosteroid 80%; oral corticosteroid 9%; theophylline 16%. Baseline co‐interventions NOT allowed during trial: ipratropium 60%; long‐acting B‐agonist ‐‐ not reported. | |
| Interventions | Experimental: tiotropium 18ug qD by handihaler. Control: ipratropium 40 ug QID by MDI. | |
| Outcomes | Analysed: Cumulative incidence of exacerbations, hospitalisations; change in Dyspnea Index; change in trough FEV1 and FVC; change in PEFR, QOL. Adverse Events: | |
| Notes | Reported combined results of van Noord (2000) and a similar unpublished trial. Some patients received 9 months of treatment instead of 12 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cazzola 2004 | Duration less than one month |
| Donohue 2003 | Same participants as Donohue 2002 |
| Maesen 1993 | Not randomised |
| Maesen 1995 | Duration less than one month |
| O'Connor 1996 | Asthma not COPD |
| Tashkin 2003 | Same participants as Casaburi 2002 |
| van Noord 2002 | Duration less than one month |
| Witek 2003 | Same participants as Brasasco 2003 |
Contributions of authors
RGB: trial identification and selection, quality scoring, data extraction, attainment of unpublished data, manuscript drafting and revision, statistical expertise JB: trial identification and selection, quality scoring, data extraction, attainment of unpublished data, manuscript revision CAC: quality scoring, data extraction, attainment of unpublished data, manuscript revision (Previous aurthor: Felix Ram: trial identification and selection, quality scoring, data extraction, manuscript revision, statistical expertise)
Sources of support
Internal sources
No sources of support supplied
External sources
Robert Wood Johnson Generalist Faculty Scholar Award (RG Barr), USA.
HL‐63841 NIH (CA Camargo Jr), USA.
Declarations of interest
Dr Bourbeau has received unrestricted educational grants for research from Boehringer‐Ingelheim and GlaxoSmithKline. Dr Camargo has received financial support from government agencies, private foundations, professional organizations, and manufacturers of pharmaceuticals and medical devices including Boehringer‐Ingelheim and GlaxoSmithKline.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Beeh 2004 {published data only}
- Beeh KM, Beier J, Stark‐Lorenzen P, Gerken F, Metzdorf N. Efficacy of tiotropium (Spiriva) in COPD of different severities (abstract) [Wirksamkeit von Tiotropium (Spiriva) bei verschiedenen Schweregraden der COPD]. Pneumologie 2004;58:S43. [DOI] [PubMed] [Google Scholar]
- Beeh KM, Beier J, Stark‐Lorenzen P, Gerken F, Metzdorf N. Efficacy of tiotropium in patients with mild‐to‐moderate COPD (abstract). Amercian Journal of Respiratory and Critical Care Medicine 2004;169:A519. [Google Scholar]
Brusasco 2003 {published and unpublished data}
- Brusasco V, Hodder R, Miravitlles M, Korducki L, Towse L, Kesten S. Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax 2003;58:399‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calverley 2003 {published and unpublished data}
- Calverley PMA, Lee A, Noord J, Witek TJ Jr, Kelsen S. Effect of tiotropium bromide on circadian variation in airflow limitation in chronic obstructive pulmonary disease. Thorax 2003;58:855‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Casaburi 2000 {published data only}
- Casaburi R, Briggs Jr DD, Donohue JF, Selby CW, Menjoge SS, Witek Jr TJ. The spirometric efficacy of once‐daily dosing with tiotropium in stable COPD: a 13‐week multicenter trial. The US Tiotropium Study Group. Chest 2000;118(5):1294‐302. [DOI] [PubMed] [Google Scholar]
Casaburi 2002 {published and unpublished data}
- Casaburi R, Malher DA, Jones PW, Wanner A, San PG, ZuWallack RL, Menjoge SS, Serby CW, Witek Jr T. A long‐term evaluation of once‐daily inhaled tiotropium in chronic obstructive pulmonary disease. European Respiratory Journal 2002;19:217‐24. [DOI] [PubMed] [Google Scholar]
Celli 2003 {published data only (unpublished sought but not used)}
- Celli B, Wallack RZ, Wang S, Kesten S. Improvement in resting inspiratory capacity and hyperinflation with tiotropium in COPD patients with increased static lung volumes. Chest 2003;124:1743‐48. [DOI] [PubMed] [Google Scholar]
Donohue 2002 {published data only}
- Donohue JF, Noord JA, Bateman ED, Lanley SJ, Lee A, Witek TJ Jr, Kesten S, Towse L. A 6‐month, placebo‐controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest 2002;122:47‐55. [DOI] [PubMed] [Google Scholar]
Littner 2000 {published data only}
- Littner MR, Ilowite JS, Tashkin DP, Friedman M, Serby CW, Menjoge SS, Witek Jr TJ. Long‐acting bronchodilation with once‐daily dosing of tiotropium (Spiriva) in stable chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2000;161:1136‐42. [DOI] [PubMed] [Google Scholar]
Niewoehner 2005 {published data only}
- Niewoehner D, Rice K, Cote C, Paulson D, Cooper JA, Korducki L, Cassino C, Kestern S. Reduced COPD exacerbations and associated health care utilization with once‐daily tiotropium in the VA Medical System [abstract]. American Journal of Respiratory Critical Care and Medicine 2004; Vol. 169:A207.
- Niewoehner DE, Rice K, Cote C, Paulson D, Cooper JA Jr, Korducki L, et al. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once‐daily inhaled anticholinergic bronchodilator: a randomized trial. Annals of internal medicine 2005;143(5):317‐26. [DOI] [PubMed] [Google Scholar]
O'Donnell 2004 {published data only (unpublished sought but not used)}
- O'Donnell DE, Fluge T, Gerken F, Hamilton A, Webb K, Aguilaniu B, Make B, Magnussen H. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. European Respiratory Journal 2004;23:832‐40. [DOI] [PubMed] [Google Scholar]
van Noord 2000 {published data only}
- Noord JA, Bantje TA, Eland ME, Korducki L, Cornelissen PJ. A randomised controlled comparison of tiotropium and ipratropium in the treatment of chronic obstructive pulmonary disease. The Dutch Tiotropium Study Group. Thorax 2000;55(4):289‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vincken 2002 {published and unpublished data}
- Vincken W, Noord JA, Greefhorst AP, Bante TA, Kesten S, Korducki L, et al. Dutch/Belgium Tiotropium Study Group. Improved health outcomes in patients with COPD during 1 yr's treatment with tiotropium. European Respiratory Journal 2002;19:209‐16. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Cazzola 2004 {published data only}
- Cazzola M, DiMarco F, Santus P, Boveri B, Verga M, Matera MG, Centanni S. The pharmacodynamic effects of single inhaled doses of formoterol, tiotropium and their combination in patients with COPD. Pulmonary Pharmacology and Therapeutics 2004;17(1):35‐39. [DOI] [PubMed] [Google Scholar]
Donohue 2003 {published data only}
- Donohue JF, Menjoge S, Kesten S. Tolerance to bronchodilating effects of salmeterol in COPD. Respiratory Medicine 2003;97:1014‐20. [DOI] [PubMed] [Google Scholar]
Maesen 1993 {published data only}
- Maesen FP, Smeets JJ, Costongs MAL, Wald FDM, Cornelissen PJG. Ba 679 Br, a new long‐acting antimuscarinic bronchodilator: a pilot dose‐escalation study in COPD. Eur Respir J 1993;6:1031‐36. [PubMed] [Google Scholar]
Maesen 1995 {published data only}
- Maesen FPV, Smeets JJ, Sledsens TJH, Wald FDM, Cornelissen PJG. Tiotropium bromide, a new long‐acting anti‐muscarinic bronchodilator: a pharmacodynamic study in patients with chronic obstructive pulmonary disease(COPD). Dutch Study Group. Eur Respir J 1995;8:1506‐13. [PubMed] [Google Scholar]
O'Connor 1996 {published data only}
- O'Connor BJ, Towse LJ, Barnes PJ. Prolonged effect of tiotropium bromide on methacholine‐induced bronchoconstriction in asthma. Am J Respir Crit Care Med 1996;154:876‐80. [DOI] [PubMed] [Google Scholar]
Tashkin 2003 {published data only}
- Tashkin D, Kesten S. Long‐term treatment benefits with tiotropium in COPD patients with and without short‐term bronchodilator responses. Chest 2003;123:1441‐49. [DOI] [PubMed] [Google Scholar]
van Noord 2002 {published data only}
- Noord JA, Smeets JJ, Custers FLJ, Korducki L, Cornelissen PJG. Pharmacodynamic steady state of tiotropium in patients with chronic obstructive pulmonary disease. Eur Respir J 2002;19:639‐44. [DOI] [PubMed] [Google Scholar]
Witek 2003 {published data only}
- Witek TJ Jr, Mahler DA. Minimal important difference of the transitional dyspnoea index in a multinational clinical trial. European Respiratory Journal 2003;21:267‐72. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
205.215 {unpublished data only}
- Boehringer Ingelheim. Effect of a 12‐week treatment with inhaled tiotropium (18 mcg once daily) on lung function and static lung volumes in stable, moderate to severe COPD patients. Correlation to dyspnoea scales. http://trials.boehringer‐ingelheim.com (accessed 31st January 2008). [Google Scholar]
205.234 {unpublished data only}
- Boehringer Ingelheim. A comparison of the effects of Tiotropium (18 mcg) Inhalation Capsule q.d. and Salmeterol (50 mcg) Inhalation Aerosol b.i.d. on arterial blood gases in a double‐blind, double‐dummy, 4‐week crossover study in patients with chronic obstructive pulmonary disease (COPD). http://trials.boehringer‐ingelheim.com (accessed 31st January 2008).
205.244 {unpublished data only}
- 205.244. A comparison of 18 mcg of Tiotropium inhalation capsules and atrovent metered dose inhaler (2 puffs of 20 mcg, 4 times daily) in a double‐blind, double‐dummy, efficacy and safety study in adults with chronic obstructive pulmonary disease (COPD). http://trials.boehringer‐ingelheim.com (accessed 31st January 2008). [Google Scholar]
205.247 {unpublished data only}
- 205.247. A randomized, double blind, placebo controlled trial to compare the effect of tiotropium inhalation capsules on exercise tolerance in patients with COPD participating in 8 weeks of pulmonary rehabilitation. http://trials.boehringer‐ingelheim.com (accessed 31st January 2008). [Google Scholar]
205.254 {unpublished data only}
- Boehringer Ingelheim. A randomised, double‐blind placebo controlled, parallel group efficacy and safety comparison of one‐year treatment of two doses (5 mcg [2 actuations of 2.5 mcg] and 10 mcg [2 actuations of 5 mcg]) of tiotropium inhalation solution delivered by the Respimat® inhaler in patients with chronic obstructive pulmonary disease (COPD). http://trials.boehringer‐ingelheim.com (accessed 31st January 2008).
205.255 {unpublished data only}
- Boehringer Ingelheim. A randomised, double‐blind, placebo‐controlled, parallel group efficacy and safety comparison of one‐year treatment of two doses (5 mcg [2 actuations of 2.5 mcg] and 10 mcg [2 actuations of 5 mcg]) of Tiotropium Inhalation Solution Delivered by the Respimat® Inhaler in Patients with Chronic Obstructive Pulmonary Disease (COPD). http://trials.boehringer‐ingelheim.com (accessed 31st January 2008).
205.257 {unpublished data only}
- Boehringer Ingelheim. Acute and long‐term effects of once daily oral inhalation of tiotropium 18 mcg dry powder inhalation capsules in a placebo controlled parallel group design study in patients with Chronic Obstructive Pulmonary Disease (COPD) of different severity. http://trials.boehringer‐ingelheim.com (accessed 31st January 2008). [Google Scholar]
205.270 {unpublished data only}
- Boehringer Ingelheim. A randomised, double‐blind, parallel‐group, placebo‐controlled study to evaluate the changes in inflammatory markers and in induced sputum following treatment with Tiotropium inhalation capsules 18 mcg once daily in patients with chronic obstructive pulmonary disease (COPD). http://trials.boehringer‐ingelheim.com/Trial_Results (accessed 31st January 2008).
Briggs 2005 {published data only}
- Briggs D, Covelli H, Lapidus R, Bhattacharya S, Kesten S, Cassino C. Improved daytime spirometric efficacy of tiotropium compared to salmeterol in COPD patients. American Thoracic Society 100th International Conference, May 21‐26. 2004:C22 Poster 511.
- Briggs Jr DD, Covelli H, Lapidus R, Bhattycharya S, Kesten S, Cassino C. Improved daytime spirometric efficacy of tiotropium compared with salmeterol in patients with COPD. Pulmonary Pharmacology & Therapeutics 2005;18(6):397‐404. [DOI] [PubMed] [Google Scholar]
Casaburi 2005 {published data only}
- Casaburi R, Kukafka D, Cooper CB, Kesten S. Improvement in exercise endurance with the combination of tiotropium and rehabilitative exercise training in COPD patients. American Thoracic Society 100th International Conference, May 21‐26 2004:D14. [Google Scholar]
- Casaburi R, Kukafka D, Cooper CB, Witek TJ, Kesten S. Improvement in exercise tolerance with the combination of Tiotropium and pulmonary rehabilitation in patients with COPD. Chest 2005;127(3):809‐17. [DOI] [PubMed] [Google Scholar]
- Cooper CB, Kukafka D, Casburi R, Kesten S. Tiotropium augments improvements in dyspnea and health‐related quality of life following pulmonary rehabilitation in COPD patients. American Thoracic Society 100th International Conference, May 21‐26, 2004, Orlando. 2004:C22 Poster 507.
Covelli 2005 {published data only}
- Covelli H, Bhattacharya S, Cassino C, Conoscenti C, Kesten S. Absence of electrocardiographic findings and improved function with once‐daily tiotropium in patients with chronic obstructive pulmonary disease. Pharmacotherapy 2005;25(12):1708‐18. [DOI] [PubMed] [Google Scholar]
- Kesten S, Cassino C, Conoscenti C. Absence of electrocardiographic findings with daily Tiotropium in patients with chronic obstructive pulmonary disease. Chest 2004;126(Suppl 4):837s. [DOI] [PubMed] [Google Scholar]
Criner 2006 {published data only}
- Criner GJ, Johnson P, Cassino C, Conoscenti C. Efficacy of tiotropium inhalation powder in COPD patients of African descent. Proceedings of the American Thoracic Society. 2006:A112; Poster J19.
EVEREST {published data only}
- Guia T, Punzal P, Canizares L, Sy‐Naval S, Salonga R, Tan D, et al. Evaluation of the response of Filipino COPD patients to Tiotropium (EVEREST STUDY). American Thoracic Society 100th International Conference, May 21‐26 2004:C47 Poster F78. [Google Scholar]
Hsu 2006 {published data only}
- Hsu JY, Perng RP, Lu JY, Wu CP, Huang MS, Luh KT, et al. Double‐blind randomized parallel group study comparing the efficacy and safety of tiotropium and ipratropium in the treatment of COPD patients in Taiwan. Journal of the Formosan Medical Association 2006;105(9):708‐14. [DOI] [PubMed] [Google Scholar]
Incorvaia 2007 {published data only}
- Incorvaia C, Galeazzo Riario‐Sforza G, Pravettoni C, Dugnani N, Paterniti F, Pessina L, et al. Effects of replacing oxitropium with tiotropium on pulmonary function in patients with COPD: A randomized study. Respiratory Medicine 2007;101(3):476‐80. [DOI] [PubMed] [Google Scholar]
Langley 2004 {published data only}
- Langley J, Briggs D, Bhattacharya S, Kesten S, Cassino C. Spirometric outcomes of COPD patients with tiotropium compared with salmeterol according to previous maintenance long acting beta‐agonist use. European Respiratory Journal 2004;24(Suppl 48):289s. [Google Scholar]
Lindsay 2005 {published data only}
- Lindsay M, Lee A, Chan K, Poon P, Han LK, Wong WC, et al. Does pulmonary rehabilitation give additional benefit over tiotropium therapy in primary care management of chronic obstructive pulmonary disease? Randomized controlled clinical trial in Hong Kong Chinese. Journal of Clinical Pharmacology and Therapeutics 2005;30(6):567‐73. [DOI] [PubMed] [Google Scholar]
Maltais 2005 {published data only}
- Maltais F, Hamilton A, Marciniuk D, Hernandez P, Sciurba FC, Richter K, et al. Improvements in symptom‐limited exercise performance over 8 h with once‐daily tiotropium in patients with COPD. Chest 2005;128(3):1168‐78. [DOI] [PubMed] [Google Scholar]
- O'Donnell D, Hamilton A, Kesten S. Influence of the exercise‐limiting symptom on the relationship between lung hyperinflation and endurance time in COPD patients treated with tiotropium. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:B23 Poster 502.
- O'Donnell D, He Z, Lam M, Webb K, Fluge T, Hamilton A. Reproducibility of measurements of inspiratory capacity dyspnea intensity and exercise endurance in multicentre trials in COPD. European Respiratory Journal 2004;24(Suppl 48):323s. [Google Scholar]
- O'Donnell DE, Johnson B, Richter K, Kesten S, Hamilton A. Inspiratory capacity (IC) and dyspnea during recovery from symptom‐limited exercise in COPD patients treated with tiotropium. European Respiratory Journal 2004;24(Suppl 48):214s. [Google Scholar]
MISTRAL {published data only}
- Dusser D, Bravo ML, Iacono P. The effect of tiotropium on exacerbations and airflow in patients with COPD. European Respiratory Journal 2006;27(3):547‐55. [DOI] [PubMed] [Google Scholar]
- Dusser D, Bravo ML, Iacono P. Tiotropium reduces health resource utilization associated with COPD exacerbations. European Respiratory Journal 2004;24(Suppl 48):513s. [Google Scholar]
- Dusser D, Bravo ML, Ianono P. Tiotropium COPD exacerbations the MISTRAL study. European Respiratory Journal 2004;24(Suppl 48):513s. [Google Scholar]
- Dusser D, Viel K, Bravo ML, Iacono P. Tiotropium reduces moderate‐to‐severe exacerbations in COPD patients irrespective of concomitant use of inhaled corticosteroids (ICS). Proceedings of the American Thoracic Society. 2006:A603.
- Dusser D, Viel K, Rouyrre N, Iancono P. Tiotropium reduces COPD exacerbations requiring treatment with systemic corticosteroids, antibiotics, or hospitalizations. Proceedings of the American Thoracic Society 2006:A603. [Google Scholar]
Moita 2008 {published data only}
- Moita J, Bárbara C, Cardoso J, Costa R, Sousa M, Ruiz J, et al. Tiotropium improves FEV(1) in patients with COPD irrespective of smoking status. Pulmonary Pharmacology & Therapeutics 2008;21(1):146‐51. [DOI] [PubMed] [Google Scholar]
- Moita J, Costa R, Barbara C, Cardosa J, Rulz J, Sousa M. The effect of tiotropium bromide in a stable cohort of COPD patients in Portugal. European Respiratory Journal 2005;26(Suppl 49):Abstract No: 1955. [Google Scholar]
Powrie 2006 {published data only}
- Powrie D, Wilkinson T, Donaldson G, Viel K, Jones P, Scrine K, et al. The effect of tiotropium on diary based exacerbation frequency in COPD. Proceedings of the American Thoracic Society. 2006:A604.
- Powrie DJ, Wilkinson TM, Donaldson GC, Jones P, Scrine K, Viel K, et al. Effect of tiotropium on sputum and serum inflammatory markers and exacerbations in COPD. European Respiratory Journal 2007;30(3):472‐8. [DOI] [PubMed] [Google Scholar]
SAFE {unpublished data only}
- 205.259. Spiriva® Assessment of FEV1 (SAFE). The effect of inhaled tiotropium bromide (18 mcg once daily) on the change in FEV1 during long‐term treatment in patients with COPD. A one‐year parallel group, double‐blind, randomised, placebo‐controlled study. http://trials.boehringer‐ingelheim.com (31st January 2008). [Google Scholar]
- Chan CK, Maltais F, Sigouin C, Haddon JM, Ford GT, on behalf of the SAFE Study Group. A randomized controlled trial to assess the efficacy of tiotropium in Canadian patients with chronic obstructive pulmonary disease. Canadian Respiratory Journal 2007;14(8):465‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
SPRUCE {published data only}
- Freeman D, Lee A, Price D. Efficacy and safety of tiotropium in COPD patients in primary care ‐ the SPiRiva Usual CarE (SPRUCE) study. Respiratory Research 2007;8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman D, Sarno M, White L, Lee A, Price D. Spruce: tiotropium in a UK primary care. Thorax 2004;59(Suppl ii):100. [Google Scholar]
- Price D, Sarno M, Lee A, Freeman D. SPiRiva usual carE‐ SPRUCE ‐ tiotropium in a UK primary care COPD population other regular inhaled treatment. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:A43 Poster: F26.
TIPHON {published data only}
- Tonnel AB, Bravo M‐L, Brun M. Clinically significant improvements of health status of COPD patients after 9 months treatment with tiotropium bromide the TIPHON stud. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:B93 Poster: 302.
- Tonnel AB, Perez T, Grosbois JM, Bravo ML, Brun M. Improvement in HRQoL of COPD patients after 9 months treatment with tiotropium bromide: use of a new scale for daily medical practice. European Respiratory Journal 2005;26(Suppl 49):Abstract No: 1934. [Google Scholar]
UPLIFT {published data only}
- Celli B, Decramer M, Tashkin D, Cassino C, Burkhart D, Kesten S. Frequency of acute flow and volume responses to bronchodilator administration in patients with COPD. European Respiratory Journal 2007;30(Suppl 51):525s. [Google Scholar]
- Celli B, Tashkin D, Decramer M, Burkhart D, Cassino C, Kesten S. Acute bronchodilator responsiveness in a global respiratory trial (UPLIFT) following maximal inhaled bronchodilators. European RespiratorY Journal 2005;26(Suppl 49):716s. [Google Scholar]
- Decramer M, Celli B, Tashkin DP, Burkhart D, Kesten S, Cassino C. Regional differences in the treatment of patients with moderate COPD enrolled in a global clinical trial. European Respiratory Journal 2006;28(Suppl 50):526s. [Google Scholar]
- Decramer M, Celli B, Tashkin DP, Pauwels RA, Burkhart D, Cassino C, et al. Clinical trial design considerations in assessing long‐term functional impacts of tiotropium in COPD: the UPLIFT trial. Journal of Chronic Obstructive Pulmonary Disease 2004;1(2):303‐12. [DOI] [PubMed] [Google Scholar]
- Decramer M, Pauwels R, Celli B, Tashkin D, Burkhart D, Cassino C, et al. Effectiveness of a spirometric quality assurance program in a large‐scale global COPD trial (UPLIFT). European Respiratory Journal 2004;24(Suppl 48):32s. [Google Scholar]
- Rutten‐van Molken M, Oostenbrink J, Monz B. EQ‐5D discriminates between COPD patients of different severity. European Respiratory Journal 2005;26(Suppl 49):470s. [Google Scholar]
- Rutten‐van Mölken MP, Oostenbrink JB, Tashkin DP, Burkhart D, Monz BU. Does quality of life of COPD patients as measured by the generic EuroQol five‐dimension questionnaire differentiate between COPD severity stages?. Chest 2006;130(4):1117‐28. [DOI] [PubMed] [Google Scholar]
- Tashkin D, Celli B, Decramer M, Burkhart D, Kesten S, Cassino C. Comorbid conditions reported in patients with COPD recruited into a global clinical trial (UPLIFT). European Respiratory Journal 2005;26(Suppl 49):Abstract No. 4203. [Google Scholar]
van Noord 2005 {published data only}
- Noord JA, Aumann J, Janssens E, Mueller A, Cornelissen PJG. A comparison of the 24 hour bronchodilator effect of tiotropium QD [T10] salmeterol BID [SALM] or their combination in COPD. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California 2005:B93 Poster: 311. [Google Scholar]
- Noord JA, Aumann J‐L, Janssens E, Smeets JJ, Verhaert J, Disse B, et al. Comparison of tiotropium once daily, formoterol twice daily and both combined once daily in patients with COPD. European Respiratory Journal 2005;26(2):214‐22. [DOI] [PubMed] [Google Scholar]
- Noord JA, Smeets JJ, Otte A, Bindels H, Mueller A, Cornellissen PJG. The effect of tiotropium, salmeterol and it's combination on dynamic hyperinflation in COPD. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:B93 Poster: 310.
Verkindre 2006 {published data only}
- Verkindre C, Bart F, Aguilaniu B, Fortin F, Guerin J‐C, Merre C, et al. The effect of tiotropium on hyperinflation and exercise capacity in chronic obstructive pulmonary disease. Respiration 2006;73(4):420‐7. [DOI] [PubMed] [Google Scholar]
Voshaar 2008 {unpublished data only}
- Boehringer Ingelheim. A randomised, double‐blind, double‐dummy, placebo‐ and active‐controlled, parallel group efficacy and safety comparison of 12‐week treatment of two doses [5 mg (2 actuations of 2.5 mg) and 10 mg (2 actuations of 5 mg)] of tiotropium inhalation solution delivered by the Respimat Inhaler, placebo and ipratropium bromide inhalation aerosol (MDI) in patients with Chronic Obstructive Pulmonary Disease (COPD). http://trials.boehringer‐ingelheim.com (accessed 31st January 2008). [Google Scholar]
- Boehringer Ingelheim. A randomised, double‐blind, double‐dummy, placebo‐ and active‐controlled, parallel group efficacy and safety comparison of 12‐week treatment of two doses [5 mg (2 actuations of 2.5 mg) and 10 mg (2 actuations of 5 mg)] of tiotropium inhalation solution delivered by the Respimat Inhaler, placebo and ipratropium bromide inhalation aerosol (MDI) in patients with chronic obstructive pulmonary disease (COPD). http://trials.boehringer‐ingelheim.com (accessed 31st January 2008).
- Voshaar T, Lapidus R, Maleki‐Yazdi R, Timmer W, Rubin E, Lowe L, et al. A randomized study of tiotropium Respimat® Soft MistTM Inhaler versus ipratropium pMDI in COPD. Respiratory Medicine 2008;102(1):32‐41. [DOI] [PubMed] [Google Scholar]
Additional references
Anthonisen 1994
- Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, Conway WA Jr, Enright PL, Kanner RE, O'Hara P. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA 1994;272:1497‐505. [PubMed] [Google Scholar]
Anthonisen 2002
- Anthonisen NR, Connett JE, Enright PL, Manfreda J for the Lung Health Study. Hospitalizations and Mortality in the Lung Health Study. Am J Respir Crit Care Med 2002;166:333‐339. [DOI] [PubMed] [Google Scholar]
Barnes 2000
- Barnes PJ. The pharmacological properties of tiotropium. Chest 2000;117(2 Suppl):63S‐66S. [DOI] [PubMed] [Google Scholar]
Cheyne 2011
- Cheyne L, Irvin‐Sellers MJ, White J. Tiotropium versus ipratropium bromide for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD009552] [DOI] [Google Scholar]
Chong 2012
- Chong J, Karner C, Poole P. Tiotropium versus long‐acting beta‐agonists for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD009157] [DOI] [PMC free article] [PubMed] [Google Scholar]
Disse 1999
- Disse B, Speck GA, Rominger KL, Witek TJ, Jr, Hammer R. Tiotropium (Spiriva): mechanistical considerations and clinical profile in obstructive lung disease. Life Sci 1999, (64):457‐64. [DOI] [PubMed] [Google Scholar]
Ferguson 1993
- Ferguson G, Cherniack R. Management of chronic obstructive pulmonary disease. N Engl J Med 1993, (328):1017‐22. [DOI] [PubMed] [Google Scholar]
Haddad 1994
- Haddad E, Mak J, Barnes P. Characterization of 3H Ba 679 BR, a slowly dissociation muscarinic antagonist, in human lung: radioligand binding and autoradiographic mapping. Mol Pharmacol 1994, (45):899‐907. [PubMed] [Google Scholar]
Karner 2012
- Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD009285.pub2] [DOI] [PubMed] [Google Scholar]
Mannino 2002
- Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance ‐‐ United States, 1971‐2000. MMWR 2002;51(SS‐6):1‐8. [PubMed] [Google Scholar]
Oostenbrink 2004
- Oostenbrink JB, Rutten‐van Molken MP, Al MJ, Noord JA, Vincken W. One‐year cost‐effectiveness of tiotropium versus ipratropium to treat chronic obstructive pulmonary disease. European Respiratory Journal 2004;23:241‐9. [DOI] [PubMed] [Google Scholar]
Sin 2003
- Sin DD, McAlister FA, Paul Man SF, Anthonisen NR. Contemporary management of chronic obstructive pulmonary disease: scientific review. JAMA 2003;290:2301‐12. [DOI] [PubMed] [Google Scholar]
Sutherland 2003
- Sutherland ER, Allmers H, Ayas NT, Venn AJ, Martin RJ. Inhaled corticosteroids reduce the progression of airflow limitation in chronic obstructive pulmonary disease: a meta‐analysis. Thorax 2003;58:937‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]


