Abstract
Background
Lower limb peripheral arterial disease (PAD) is a common, important manifestation of systemic atherosclerosis. Stenoses or occlusions in the superficial femoral artery may result in intermittent claudication or even critical ischaemia, which may be treated by balloon angioplasty with or without stenting. This is the first update of a review published in 2009.
Objectives
The primary aim was to determine the effect of percutaneous transluminal angioplasty (PTA) compared with PTA with bare metal stenting for superficial femoral artery (SFA) stenoses on vessel patency in people with symptomatic (Rutherford categories1 to 6; Fontaine stages II to IV) lower limb peripheral vascular disease.
In addition, we assessed the efficacy of PTA and stenting in improving quality of life, ankle brachial index and treadmill walking distance.
Search methods
For this update the Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator searched the Specialised Register (last searched August 2013) and the Cochrane Central Register of Controlled Trials (CENTRAL) (2013, Issue 6).
Selection criteria
Randomised trials of angioplasty alone versus angioplasty with bare metal stenting for the treatment of superficial femoral artery stenoses.
Data collection and analysis
Two review authors (MC, CT) independently selected suitable trials, assessed trial quality and extracted data. Furthermore, these two review authors performed assessments of methodological quality and wrote the final manuscript. The third review author (ADM) cross‐checked all stages of the review process.
Main results
We include three new studies in this update, making a total of 11 included trials with 1387 participants. The average age was 69 years and all trials included men and women. Participants were followed for up to two years. There was an improvement in primary duplex patency at six and 12 months in participants treated with PTA plus stent over lesions treated with PTA alone (six months: odds ratio (OR) 2.90, 95% confidence interval (CI) 1.17 to 7.18, P = 0.02, six studies, 578 participants; 12 months: OR 1.78, 95% CI 1.02 to 3.10, P = 0.04, nine studies, 858 participants). This was lost by 24 months (P = 0.06). There was a significant angiographic patency benefit at six months (OR 2.49, 95% CI 1.49 to 4.17, P = 0.0005, four studies, 329 participants) which was lost by 12 months (OR 1.30, 95% CI 0.84 to 2.00, P = 0.24, five studies, 384 participants). Ankle brachial index (ABI) and treadmill walking distance showed no improvement at 12 months (P = 0.49 and P = 0.57 respectively) between participants treated with PTA alone or PTA with stent insertion. Three trials (660 participants) reported quality of life, which showed no significant difference between participants treated with PTA alone or PTA with stent insertion at any time interval. Antiplatelet therapy protocols and inclusion criteria regarding affected arteries between trials showed marked heterogeneity.
Authors' conclusions
Although there was a short‐term gain in primary patency there was no sustained benefit from primary stenting of lesions of the superficial femoral artery in addition to angioplasty. Future trials should focus on quality of life for claudication and limb salvage for critical ischaemia.
Plain language summary
Angioplasty versus angioplasty plus stenting for lesions of the superficial femoral artery
Intermittent claudication is pain in the leg that is brought on by walking and which is relieved by rest. The pain is a result of insufficient blood flow to the muscles of the leg due to narrowing of the arteries by atherosclerosis. People who have narrowing of the main artery in the thigh, the superficial femoral artery, and intermittent claudication which severely restricts their quality of life or causes dangerous tissue changes in the leg may undergo a procedure known as angioplasty to widen this narrowing. This procedure involves passing a balloon into the narrowed segment and inflating the balloon to push the artery open. In addition to this, a cylindrical piece of metal mesh called a stent may be inserted at the site where the artery has been pushed open with the aim of holding the narrowing open in the future. While stents work well in the arteries of the heart and in other arteries, it is not clear whether adding stents following angioplasty to narrowings of the superficial femoral artery gives any benefit to the patient.
We identified 11 randomised controlled trials with a total of 1387 participants. Their average age was 69 years and all trials included men and women. The participants were randomised to have either balloon angioplasty alone or balloon angioplasty with stent placement. At two years, blood flowing through the narrowing in the arteries was no greater in participants with a stent inserted than in those without. There was a small improvement in the distance that the participants with a stent could walk up to one year later. However, when asked about their quality of life there was no improvement, whether a stent was placed or not, up to one year later. There were differences in the included trials; in some trials people with narrowings in other leg arteries were included. There were also differences between trials in the blood thinning drugs given after stent placement, which may change results, as these agents are important in keeping stents working in other parts of the body. These factors led us to the conclusion that there is a small benefit to adding a stent when performing balloon angioplasty for people in whom balloon angioplasty fails. However, there is insufficient evidence to support this approach as routine practice for everyone and future trials should examine whether subgroups of patients may benefit from stenting.
Background
Description of the condition
Lower limb peripheral arterial disease (PAD) is a common, important manifestation of systemic atherosclerosis. It occurs in 3% to 10% of the population, increasing to 15% to 20% in people over 70 years of age (Selvin 2004). The most common site of PAD is the superficial femoral artery (SFA) (Fowkes 1991). While most people with PAD are asymptomatic, many have intermittent claudication, chronic critical limb ischaemia or acute critical limb ischaemia. As a result, PAD significantly impairs quality of life and is the most common cause of lower limb amputation in the western world. Successful treatment of PAD is therefore of the utmost importance.
Description of the intervention
Superficial femoral artery disease may be treated by a number of modalities depending on the length of lesion. Exercise therapy and best medical therapy are the best suggested methods of initial therapy for people with claudication (NICE 2012), while rest pain and tissue loss is treated more aggressively with early intervention. For lesions less than 10 cm long in symptomatic patients, the most common treatment is currently percutaneous transluminal angioplasty (PTA) with or without the use of stents. While PTA can result in initial technical success rates of more than 95%, late clinical failure remains an important concern.
Why it is important to do this review
The recognition that treatment for PAD varies by centre has led to the creation of continually updated international guidelines (NICE 2012; Norgren 2007). These guidelines currently recommend the use of PTA as a primary treatment in SFA lesions less than 10 cm long with stenting for acute primary failure. However, recent randomised control trials have results conflicting with these recommendations and, in some cases, with one another (Vienna Absolute Trial; Zdanowski 1999).
Objectives
The primary aim was to determine the effect of percutaneous transluminal angioplasty (PTA) compared with PTA with stenting for superficial femoral artery (SFA) stenoses on vessel patency in people with symptomatic (Rutherford categories 1 to 6; Fontaine stages II to IV) lower limb peripheral vascular disease.
In addition, we assessed the efficacy of PTA and stenting in improving quality of life as assessed by the trialists, ankle brachial index (ABI) and treadmill walking distance.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) that compared percutaneous angioplasty alone with percutaneous angioplasty plus stenting.
Types of participants
People with intermittent claudication or critical limb ischaemia (Rutherford categories 1 to 6; Fontaine stages II to IV) (Fontaine 1954; Rutherford 1997). We considered people with TASC (Norgren 2007) A and B superficial femoral artery (SFA) lesions. TASC A lesions are single stenoses ≤ 10 cm in length or single occlusions ≤ 5 cm in length, while TASC B lesions are multiple stenoses or occlusions each ≤ 5 cm, a single stenosis or occlusion ≤ 15 cm not involving the infrageniculate popliteal artery, and heavily calcified occlusions ≤ 5 cm in length. The definition of TASC B lesions also includes single or multiple lesions in the absence of continuous tibial vessels and single popliteal stenoses, which are not considered by interventions included in this review.
Types of interventions
The angioplasty or stent insertion had to have been performed percutaneously or though a limited groin incision. The treatment had to have been primary unless angioplasty failed and stenting was then performed as part of the primary procedure. We noted antiplatelet therapy preceding and following intervention. We did not consider drug‐eluting balloons, stents and stent grafts.
We did not consider uncommon percutaneous interventions such as atherectomy. Secondary and primary assisted patency rates were only considered as aims in some trials, and we therefore did not include them.
Types of outcome measures
Primary outcomes
Rates of restenosis (in trials with at least six months follow‐up and using techniques directly imaging the treatment site ‐ duplex ultrasound and angiography).
Secondary outcomes
Clinical outcomes; improvement in ankle brachial index (ABI) or treadmill walking distance, and quality of life scores as assessed by the trialists.
Search methods for identification of studies
Electronic searches
For this update the Cochrane Peripheral Vascular Diseases (PVD) Group Trials Search Co‐ordinator (TSC) searched the Specialised Register (last searched August 2013) and the Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 6, part of The Cochrane Library (www.thecochranelibrary.com). See Appendix 1 for details of the search strategy used to search CENTRAL. The Specialised Register is maintained by the TSC and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used are described in the Specialised Register section of the Cochrane Peripheral Vascular Diseases Group module in The Cochrane Library (www.thecochranelibrary.com).
Searching other resources
We examined bibliographies of papers found through the searches to identify further trials.
Data collection and analysis
Selection of studies
Two review authors (MC and CT) independently selected trials for inclusion in the review. These trials were sent to a third review author (ADM) who confirmed they were acceptable for inclusion. The section Criteria for considering studies for this review details the inclusion criteria used for the selection process.
Data extraction and management
Two review authors (MC and CT) collected data on each trial, including information on the participants (age and sex distribution, measures of severity of claudication such as walking distance, ABI), the interventions (angioplasty and stent type, control intervention, usual care in both groups) and the outcomes (as specified in Types of outcome measures). Data were independently extracted by MC and CT, and then cross‐checked by ADM.
Assessment of risk of bias in included studies
Two review authors (MC and CT) assessed the methodological quality of each trial independently according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), resolving discrepancies by discussion.
Two review authors (MC and CT) examined six key domains of the Cochrane 'Risk of bias' tool. We assessed and classified these domains as being at either a low risk of bias or a high risk of bias. Where insufficient detail was reported in a study to assess the risk, we reported this as 'unclear'. In addition to the six key domains, we reported any other form of bias noted in the study.
Measures of treatment effect
We synthesised data by comparing group results. We did not combine individual data from different trials. We used Mantel‐Haenszel odds ratios (ORs) and 95% confidence intervals (CIs) for dichotomous variables, and mean differences and 95% CIs for continuous variables.
Unit of analysis issues
For the primary outcome rates of restenosis the unit of analysis was the limb rather than the individual participant.
For the secondary outcomes ABI, walking distance and quality of life score the unit of analysis was the participant.
Dealing with missing data
All analyses were based on endpoint data from the individual clinical trials, which all quoted intention‐to‐treat results. We contacted individual trial authors for missing data where necessary.
Assessment of heterogeneity
We explored clinical heterogeneity in the studies using the previously identified characteristics of the studies and the quality of the included studies. We used the Chi² test to test for heterogeneity where data were pooled. A P value of < 0.10 was deemed to indicate heterogeneity.
Assessment of reporting biases
We tested for publication bias using the funnel plot for those meta‐analyses where sufficient studies were included.
Data synthesis
The data analysis comprised a comparison of group results where feasible. Statistical analyses followed the standard methods of the Cochrane PVD Group. All analyses were based on endpoint data from the individual clinical trials, which all quoted intention‐to‐treat results.
We used a fixed‐effect Mantel‐Haenszel meta‐analysis model, but a random‐effects model for analyses showing significant heterogeneity (P < 0.10).
Subgroup analysis and investigation of heterogeneity
We had planned to stratify the data for severity of disease (claudication and critical limb ischaemia) but due to lack of available data we were unable to perform this analysis.
Sensitivity analysis
We did not perform any sensitivity analyses.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
See Figure 1.
1.
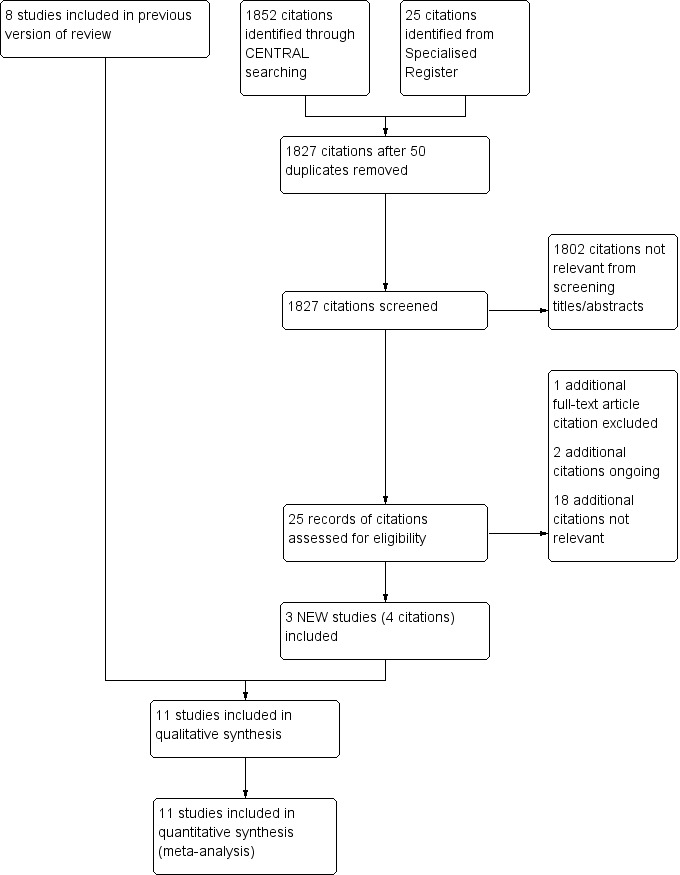
Study flow diagram.
Included studies
Summarised details of included studies can be found in the Characteristics of included studies table.
We included three new studies for this update (Dick 2009; RESILIENT; SUPER study), making a total of 11 randomised controlled trials (RCTs) which met the criteria for inclusion (Becquemin 2003; Cejna 2001; Dick 2009; Grenacher 2004; Grimm 2001; FAST Trial; RESILIENT; SUPER study; Vienna Absolute Trial; Vroegindeweij 1997; Zdanowski 1999). In all trials men and women with Fontaine stages II to IV were randomly allocated to percutaneous transluminal angioplasty (PTA) alone or to balloon angioplasty and stenting. Follow‐up was reported up to 12 months (Becquemin 2003; Cejna 2001; Dick 2009; FAST Trial (see Krankenberg 2007); RESILIENT; SUPER study; Zdanowski 1999) and up to 24 months (Grenacher 2004; Grimm 2001; Vienna Absolute Trial (24 month data reported as Schillinger 2007 and Sabeti 2007); Vroegindeweij 1997). However, RESILIENT only reported 'freedom from target lesions revascularisation' and 'clinical success' at 24 and 36 months, so no new data could be added to any analysis from the three‐year follow up data.
While PTA and stenting techniques were relatively consistent between trials, some trials used stainless steel stents (Becquemin 2003; Cejna 2001; Grenacher 2004; Grimm 2001; Vroegindeweij 1997) and some nitinol (Dick 2009; FAST Trial; RESILIENT; SUPER study; Vienna Absolute Trial; Zdanowski 1999).
Anticoagulation protocols varied extensively between trials, and in some cases even within trials (FAST Trial; Vienna Absolute Trial). No anticoagulant compliance checks were carried out.
There were no statistically significant differences in the major confounders (sex, age, smoking, dyslipidaemia (abnormal concentrations of lipids or lipoproteins in the blood), diabetes or hypertension) between the PTA and PTA with stent groups reported in any trial apart from Zdanowski 1999, where there was a slightly lower prevalence of men in the PTA‐alone group.
Excluded studies
For this update we excluded one additional study (Brancaccio 2012).
Detailed reasons for trials being excluded can be found in the Characteristics of excluded studies table. In summary, the five studies were excluded for using drug‐eluting stents (Duda 2006), covered stents (Saxon 2003),and for examining peripheral vascular disease that did not involve the superficial femoral artery (Ahn 1992). Brancaccio 2012 and the VascuCoil Trial were excluded because the outcomes reported were not relevant for this review.
Risk of bias in included studies
See Figure 2 and the risk of bias tables in Characteristics of included studies.
2.
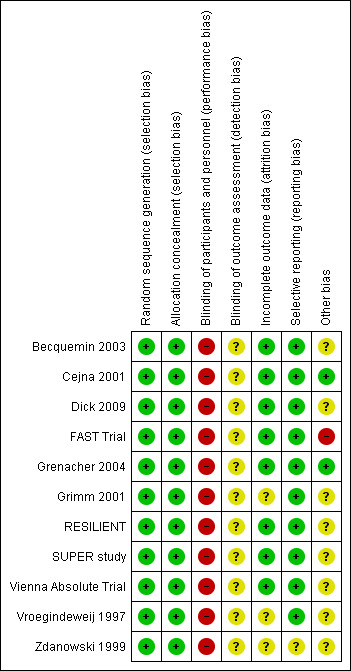
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
We deemed all the included studies to be at low risk of bias for random sequence generation and for allocation concealment.
Blinding
Blinding is not possible in this type of trial, so we deemed all the included studies to be at high risk of performance bias. We rated the studies at unclear risk of performance bias as radiological investigations for the primary outcome would show stent placement. According to the study publication, primary outcome assessment was blinded in Becquemin 2003, but it is unclear how this was achieved.
Incomplete outcome data
Attrition bias was low in all trials except for Grimm 2001, Vroegindeweij 1997 and Zdanowski 1999, where there was no statement on attrition or numbers.
Selective reporting
All but one (Zdanowski 1999) of the 11 included trials were deemed to be at low risk of reporting bias because all stated outcomes were accounted for. Zdanowski 1999 was deemed to be at unclear risk of bias because trial outcomes were not predefined.
Other potential sources of bias
Two trials were deemed to be at low risk of bias (Cejna 2001; Grenacher 2004).
Two trials (FAST Trial; Vienna Absolute Trial) quoted median values for treadmill distance and quality of life scores. These values were used as means for the purpose of analysis, with the standard deviation calculated from the interquartile range or confidence intervals assuming normal distribution of data. These data may have been skewed, and are therefore reported as medians.
Zdanowski 1999 did not specifically state inclusion and exclusion criteria for participants.
Three trials (FAST Trial; Grimm 2001; Vienna Absolute Trial) included participants with ipsilateral iliac artery stenoses, possibly biasing secondary outcomes such as quality of life data.
Becquemin 2003 reporting on drug prescription and salvage stenting in the PTA arm was unclear. Dick 2009 and FAST Trial did not report on adherence to medication protocol. Grimm 2001 did not assess medication, while RESILIENT did not have a medication compliance protocol. Vroegindeweij 1997 reported initial use of warfarin but medication protocol after that is unclear.
Effects of interventions
Primary outcomes
Rates of restenosis
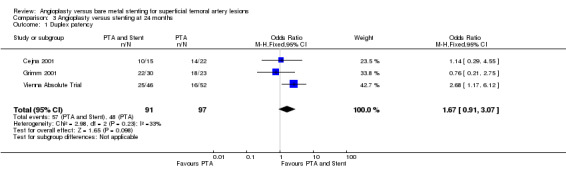
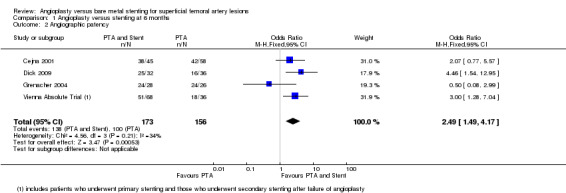
At six months follow‐up both primary duplex and angiographic patency were higher in the PTA plus stent group than the PTA alone group. The overall effect was significant (Figure 3; Mantel‐Haenszel odds ratio (OR) 2.90, 95% confidence interval (CI) 1.17 to 7.18, Z = 2.31, P < 0.02, six trials, 578 participants), and Figure 4; OR 2.49, 95% CI 1.49 to 4.17, Z = 3.47, P = 0.0005, four trials, 329 participants for duplex and angiography respectively). This significant finding was only sustained at 12 months of follow‐up (Figure 5) with regard to duplex patency with significant results with the inclusion of RESILIENT and the SUPER study (OR 1.78 95% CI 1.02 to 3.10, Z = 2.03, P < 0.04, nine trials, 858 participants). The significantly higher angiographic patency in the stent group at six months was lost by 12 months (five trials, 384 participants, Analysis 2.2; Figure 6). Twenty‐four months follow up also showed no significant difference in duplex or angiographic patency (Analysis 3.1; Analysis 3.2).
3.
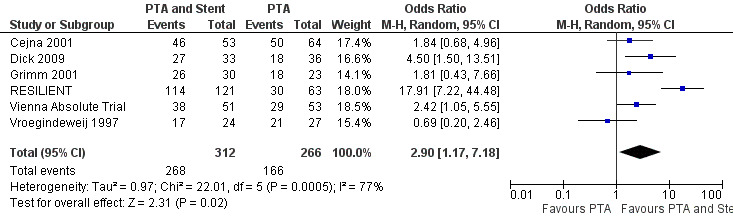
Forest plot of comparison: 1 Angioplasty versus stenting at 6 months, outcome: 1.1 Duplex patency.
4.
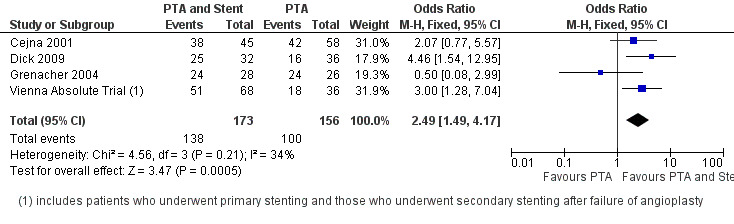
Forest plot of comparison: 1 Angioplasty versus stenting at 6 months, outcome: 1.2 Angiographic patency.
5.
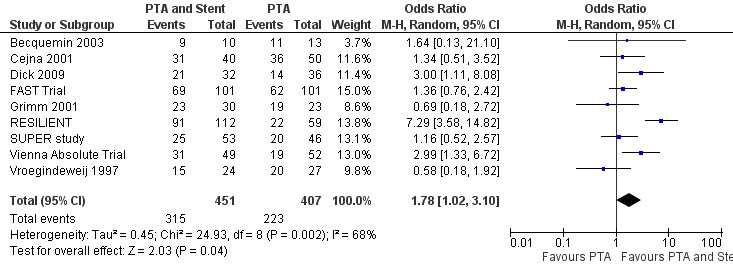
Forest plot of comparison: 2 Angioplasty versus stenting at 12 months, outcome: 2.1 Duplex patency.
2.2. Analysis.
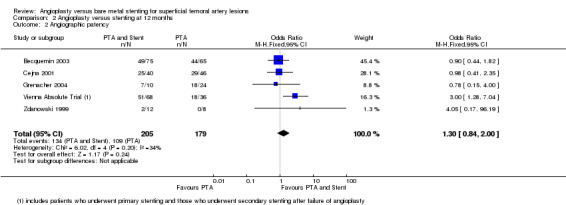
Comparison 2 Angioplasty versus stenting at 12 months, Outcome 2 Angiographic patency.
6.
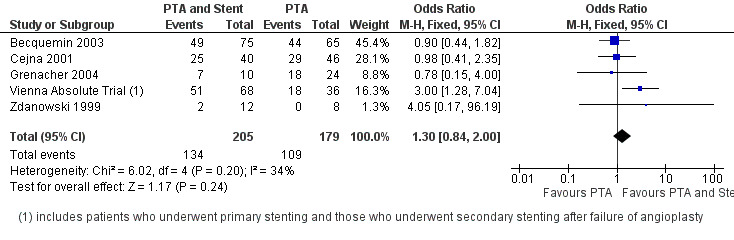
Forest plot of comparison: 2 Angioplasty versus stenting at 12 months, outcome: 2.2 Angiographic patency.
3.1. Analysis.
Comparison 3 Angioplasty versus stenting at 24 months, Outcome 1 Duplex patency.
3.2. Analysis.
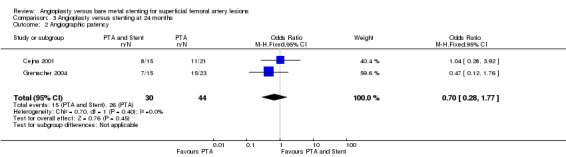
Comparison 3 Angioplasty versus stenting at 24 months, Outcome 2 Angiographic patency.
For restenosis rates at 12 months we created a funnel plot to check for publication bias (Figure 7). The plot showed asymmetry, consistent with the significant heterogeneity seen in the analysis (Chi² test for heterogeneity P = 0.002).
7.
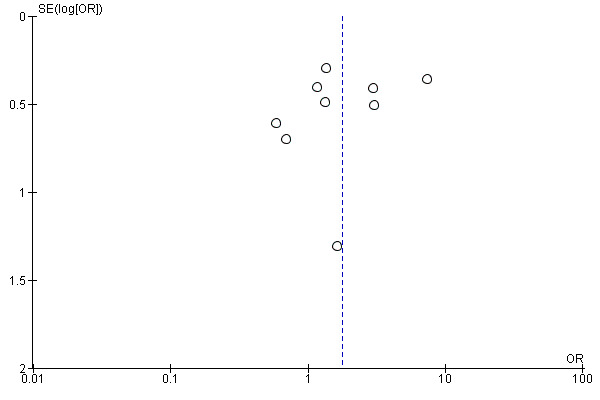
Funnel plot of comparison: 2 Angioplasty versus stenting at 12 months, outcome: 2.1 Duplex patency.
Secondary outcomes
Clinical outcomes
Ankle brachial index
Cejna 2001; Grimm 2001; Vroegindeweij 1997 reported significant improvement in ABI in both groups when compared to pre‐intervention ABI, with no difference between groups immediately post‐intervention. By 12 months there was no difference in ABI between the treatment groups (Analysis 2.3), even with the addition of the SUPER study in this updated review (OR 0.03, 95% CI ‐0.05 to 0.11, P = 0.49, four trials, 440 participants). There was significant heterogeneity between studies (Chi² test for heterogeneity P < 0.00001).
2.3. Analysis.
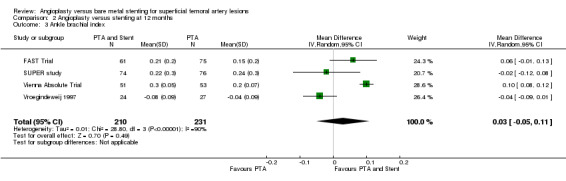
Comparison 2 Angioplasty versus stenting at 12 months, Outcome 3 Ankle brachial index.
One study (Vienna Absolute Trial; 98 participants) reported ABI at 24 months showing no difference between the PTA and PTA plus stenting groups (Analysis 3.3).
3.3. Analysis.
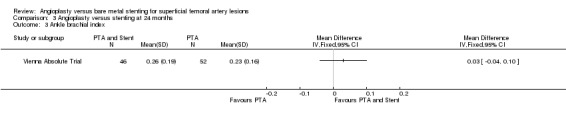
Comparison 3 Angioplasty versus stenting at 24 months, Outcome 3 Ankle brachial index.
Treadmill walking distance
At six months, only one study reports walking distance(Vienna Absolute Trial; 104 participants), which showed a benefit for stenting (Analysis 1.4). However the inclusion of the FAST Trial at 12 months, which was in favour of PTA alone, means that there was no overall difference between the treatment groups (two trials, 240 participants; random effects model; Analysis 2.4). There was significant heterogeneity as a result (Chi² test for heterogeneity P < 0.00001).
1.4. Analysis.

Comparison 1 Angioplasty versus stenting at 6 months, Outcome 4 Treadmill walking distance.
2.4. Analysis.
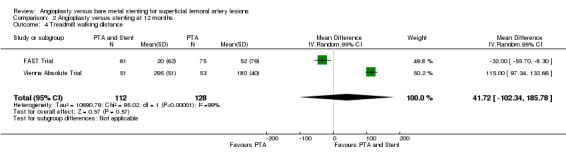
Comparison 2 Angioplasty versus stenting at 12 months, Outcome 4 Treadmill walking distance.
One study (Vienna Absolute Trial; 98 participants) reported treadmill walking distance at 24 months showing no difference between the PTA and PTA plus stenting groups (Analysis 3.3).
Quality of life scores
Quality of life data were only available for meta‐analysis from one RCT (Vienna Absolute Trial). There was no significant difference at six or 12 months in either physical quality of life (P = 0.15 and P = 0.48 respectively) or mental quality of life (P = 0.49 and P = 0.21 respectively) between the two treatment groups (Analysis 1.5; Analysis 2.5). SUPER study reported results from EuroQol 5D questionnaires which could not be included in analysis. The authors of this study found no difference between the treatment groups. RESILIENT reported no difference in quality of life or walking distance QoL scores at any time interval between the angioplasty and stenting groups (P > 0.05 but not reported precisely).
1.5. Analysis.
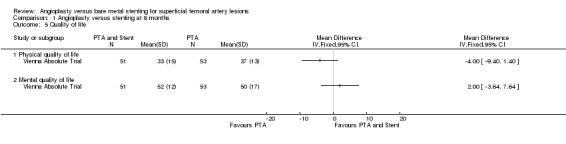
Comparison 1 Angioplasty versus stenting at 6 months, Outcome 5 Quality of life.
2.5. Analysis.
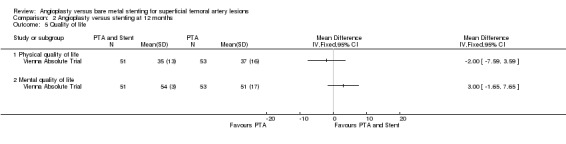
Comparison 2 Angioplasty versus stenting at 12 months, Outcome 5 Quality of life.
Discussion
Summary of main results
The major finding of this analysis was that lesions of the superficial femoral artery treated by percutaneous transluminal angioplasty (PTA) with stent insertion show a small, statistically significant short‐term improvement in primary patency over lesions treated with PTA alone (six trials, 578 participants). This effect is most prominent at six months and diminishes with time. A similar but smaller effect is seen for ankle brachial index (ABI) (one trial, 104 participants), while we observed a more pronounced improvement in treadmill walking distance in participants with PTA with stent insertion at six months (one trial, 104 participants), but not at 12 months (two trials, 240 participants) and 24 months (one trial, 98 participants). Quality of life, however, showed no significant difference between participants treated with PTA alone or PTA with stent insertion at any time interval. Intermittent claudication was the predominant symptom treated.
Overall completeness and applicability of evidence
Heterogeneity between trial outcomes means that while a large number of participants were available, overall some analyses contain small numbers of studies and therefore few participants. These analyses should be interpreted with caution. The Chi² test for heterogeneity for ABI was P < 0.00001 at 12 months, and was P < 0.00001 for treadmill walking distance at 12 months. In each case, the Vienna Absolute Trial reports higher values than the other studies, although the reasons for this are unclear. These results must therefore also be interpreted with caution. The Vienna Absolute Trial found strongly in favour of PTA and stenting and had a relatively large number of participants. This study therefore contributed a large weight towards analyses in which it was included, and is the main reason many forest plots show significance towards PTA and stenting. Nevertheless, the other large RCTs (Cejna 2001; FAST Trial) which showed no difference between groups were included in the primary outcome measure analyses: duplex and angiographic patency, with Vienna Absolute Trial. It is notable that at 12 months the inclusion of data from Becquemin 2003 produces a non‐significant difference between PTA and PTA with stenting for angiographic patency. The finding in favour of stenting for primary duplex patency at 24 months does not include as many studies as at six and 12 months, and is so small that the inclusion of a small amount of data from another study could cause the overall difference to be statistically non‐significant.
Although Grenacher 2004 reports primary duplex patency, the numbers of participants at each time interval is unclear. For this reason we could not include the results in the meta‐analyses. The inclusion of these data would diminish the difference between PTA and PTA with stenting and may have made the 12‐month difference non‐significant. We did not include secondary and primary assisted patency rates as outcome measures due to the differences in reporting between trials.
No trial reported data in a way that allowed results to be separated by Rutherford/Fontaine stages or by chronic (claudicants) and critical ischaemia. This might have been useful, as differences between these groups may be present. No trial was powered in this way, so even if subgroup results were reported their validity would have been questionable.
Quality of the evidence
Six trials used nitinol stents (Dick 2009; FAST Trial; RESILIENT; SUPER study; Vienna Absolute Trial; Zdanowski 1999), while the other trials used stainless steel. There is speculation that nitinol stents may perform differently to stainless steel, although this is not apparent in these data. Indeed, two trials (FAST Trial; Vienna Absolute Trial) used nitinol stents with similar participant numbers and procedural protocols, but showed differing outcomes. The reasons for this are unclear, but one obvious difference between these trials was the antiplatelet therapy protocol used. In the Vienna Absolute Trial clopidogrel is used for long‐term anticoagulation, whereas aspirin is used in both Cejna 2001 and FAST Trial. Additionally, Cejna 2001 excluded participants with ipsilateral iliac artery disease, whereas in the FAST Trial and the Vienna Absolute Trial the authors treated ipsilateral iliac disease with angioplasty and then included the participant in the trial. Medication is thus a major confounding factor between trials, especially the use of clopidogrel which is known to be associated with improved stent patency in coronary artery stents (Becker 2008).
Becquemin 2003 specifically states that 'bailout' stenting was performed in 15% of participants in the PTA‐alone arm, and it is unclear whether these participants were included in the PTA arm for analysis at 12 months. The FAST Trial excluded PTA participants requiring a bailout stent. The fate of such participants in the other trials was unclear. This is another potential source of bias, as in Becquemin 2003 a significant difference may be present if the participants undergoing bailout stenting were subsequently included in the stent arm, or excluded from the trial. RESILIENT provided high participant numbers but cross‐over to stent implantation in the angioplasty arm was high (40%), complicating data analysis.
Trials sponsored by device companies are statistically more likely to produce results in favour of the device (here stents) being tested (Lundh 2012; Djulbegovic 2013). This was true of many of the trials included in this analysis (Becquemin 2003; Cejna 2001; FAST Trial; Grenacher 2004; Grimm 2001; RESILIENT; SUPER study; Vienna Absolute Trial) and therefore may bias outcomes in favour of stents.
Reviewing the acceptability and quality of the evidence, it is therefore difficult to accept that the differences found between the PTA alone and PTA with stenting groups are of clinical relevance.
Potential biases in the review process
Two trials (Vienna Absolute Trial; FAST Trial) quoted medians for ABI which we converted to means for the benefit of the analysis in this review. Given only the median value and interquartile range, we used the reported median value as the mean value and calculated the standard deviation according to guidance described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Trials varied between using participants and limbs as their unit of analysis; however, we converted all data into limbs treated for analysis of the primary outcome patency rates. For the secondary outcomes we deemed the unit of analysis to be the participant.
Agreements and disagreements with other studies or reviews
Meta‐analysis of stenting in femoropopliteal disease has become popular in the past five years. Four others exist (Acin 2012; Kasapis 2009; Mwipatayi 2008; Vardi 2014) as well as this review. Acin 2012 only analysed the nitinol stent trials included in our review. They found a trend in favour of stenting, but the total numbers in that analysis were just under half the total included in this review. Kasapis 2009 found no significant difference between treatment groups, and Vardi 2014 only examined one‐month safety data in detail. An older analysis (Mwipatayi 2008) also found no difference between the treatment groups for primary or secondary patency.
Authors' conclusions
Implications for practice.
The current trial data indicate that there is a small short‐term patency benefit for percutaneous transluminal angioplasty (PTA) with stenting over PTA alone for treating lesions of the superficial femoral artery. However, the magnitude of the benefit is small and inconsistent. Protocols varied between trials, and the benefit may be limited to people with superficial femoral artery (SFA) disease subsequently treated with long‐term clopidogrel. Haemodynamic findings are often used to assess the success of angioplasty and stent insertion. Importantly however, clinical findings, especially quality of life, is not significantly improved by this treatment strategy.
Many of these trials predominantly used claudicants for intervention. Current best evidence shows that exercise therapy is more useful than angioplasty for sustained improvements in quality of life and walking distance as the initial treatment of claudication (Frans 2012; Mazari 2012; Watson 2008). The National Institute for Health and Care Excellence (NICE) therefore recommends supervised exercise therapy as the first‐line treatment (NICE 2012). In this context, measures of angiographic or duplex patency can be misleading, as they do not equate to the most important outcomes for the patient: walking distance and quality of life. Small improvements in short‐term patency using stents are also expensive from a health economics point of view. The current evidence base does not support the use of routine stent insertion following PTA for treatment of lesions of the SFA.
Implications for research.
A trial with quality of life, perceived and actual walking differences, and survival with intact limb would have more clinically useful endpoints than measures of primary patency.
The effect of antiplatelet therapy on PTA and stenting of SFA lesions needs to be properly investigated in a randomised controlled trial. Consideration should also be given to including a non‐intervention control group and two‐year outcomes in the evaluation of new SFA stents. As only subgroups of participants may benefit from stenting, trials should be of sufficient size to allow for heterogeneity in, for example, concurrent lesions (often a finding in clinical practice) and severity of disease, and should offer at least a two‐year follow‐up.
What's new
| Date | Event | Description |
|---|---|---|
| 1 August 2013 | New search has been performed | Searches rerun. Three new studies included, one new study excluded and two ongoing studies identified. |
| 1 August 2013 | New citation required but conclusions have not changed | Seaches rerun. Three new studies included, one new study excluded and two ongoing studies identified. Risk of bias tables completed and review text updated. Conclusions not changed |
History
Protocol first published: Issue 4, 2007 Review first published: Issue 2, 2009
| Date | Event | Description |
|---|---|---|
| 29 May 2008 | Amended | Converted to new review format. |
Appendices
Appendix 1. CENTRAL search strategy
| #1 | MeSH descriptor: [Angioplasty] explode all trees | 4233 |
| #2 | (angioplas* or percutan* or PTA) | 10083 |
| #3 | recanali* or revascular* | 5220 |
| #4 | dilat* | 6100 |
| #5 | balloon | 6019 |
| #6 | MeSH descriptor: [Endovascular Procedures] explode all trees | 5676 |
| #7 | endovascular | 1369 |
| #8 | #1 or #2 or #3 or #4 or #5 or #6 or #7 | 21661 |
| #9 | MeSH descriptor: [Blood Vessel Prosthesis] explode all trees | 443 |
| #10 | MeSH descriptor: [Blood Vessel Prosthesis Implantation] this term only | 489 |
| #11 | MeSH descriptor: [Stents] explode all trees | 3102 |
| #12 | *stent* or graft* or endograft* or endoprosthe* | 50066 |
| #13 | powerlink or talent or excluder or aorfix or zenith or endologix or anaconda or Triascular or Cordis or Endurant or Quantum or Aneurx or Ancure or Advanta or Intracoil or Zilver or Luminex | 372 |
| #14 | #9 or #10 or #11 or #12 or #13 | 50439 |
| #15 | MeSH descriptor: [Arteriosclerosis] this term only | 894 |
| #16 | MeSH descriptor: [Arteriolosclerosis] this term only | 0 |
| #17 | MeSH descriptor: [Arteriosclerosis Obliterans] this term only | 72 |
| #18 | MeSH descriptor: [Atherosclerosis] this term only | 417 |
| #19 | MeSH descriptor: [Arterial Occlusive Diseases] this term only | 770 |
| #20 | MeSH descriptor: [Intermittent Claudication] this term only | 724 |
| #21 | MeSH descriptor: [Peripheral Vascular Diseases] explode all trees | 2191 |
| #22 | MeSH descriptor: [Vascular Diseases] this term only | 393 |
| #23 | MeSH descriptor: [Leg] explode all trees and with qualifiers: [Blood supply ‐ BS] | 1090 |
| #24 | MeSH descriptor: [Femoral Artery] explode all trees | 736 |
| #25 | femoro* | 541 |
| #26 | (atherosclero* or arteriosclero* or PVD or PAOD or PAD) | 17611 |
| #27 | (arter*) near (*occlus* or steno* or obstuct* or lesio* or block* or obliter*) | 4959 |
| #28 | (vascular) near (*occlus* or steno* or obstuct* or lesio* or block* or obliter*) | 1413 |
| #29 | (vein*) near (*occlus* or steno* or obstuct* or lesio* or block* or obliter*) | 744 |
| #30 | (veno*) near (*occlus* or steno* or obstuct* or lesio* or block* or obliter*) | 1000 |
| #31 | (peripher*) near (*occlus* or steno* or obstuct* or lesio* or block* or obliter*) | 1379 |
| #32 | peripheral near/3 dis* | 3350 |
| #33 | arteriopathic | 16 |
| #34 | (claudic* or hinken*) | 1467 |
| #35 | (isch* or CLI) | 17261 |
| #36 | dysvascular* | 25 |
| #37 | leg near/4 (obstruct* or occlus* or steno* or block* or obliter*) | 186 |
| #38 | limb near/4 (obstruct* or occlus* or steno* or block* or obliter*) | 240 |
| #39 | (lower near/3 extrem*) near/4 (obstruct* or occlus* or steno* or block* or obliter*) | 145 |
| #40 | #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 | 40689 |
| #41 | #8 and #14 and #40 in Trials | 1852 |
Data and analyses
Comparison 1. Angioplasty versus stenting at 6 months.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duplex patency | 6 | 578 | Odds Ratio (M‐H, Random, 95% CI) | 2.90 [1.17, 7.18] |
| 2 Angiographic patency | 4 | 329 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.49 [1.49, 4.17] |
| 3 Ankle brachial index | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Treadmill walking distance | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Physical quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Mental quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
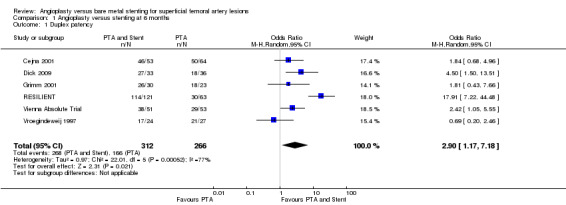
Comparison 1 Angioplasty versus stenting at 6 months, Outcome 1 Duplex patency.
1.2. Analysis.
Comparison 1 Angioplasty versus stenting at 6 months, Outcome 2 Angiographic patency.
1.3. Analysis.
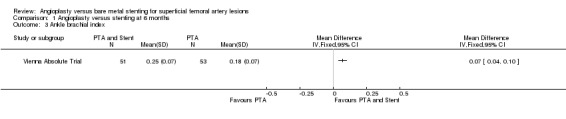
Comparison 1 Angioplasty versus stenting at 6 months, Outcome 3 Ankle brachial index.
Comparison 2. Angioplasty versus stenting at 12 months.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duplex patency | 9 | 858 | Odds Ratio (M‐H, Random, 95% CI) | 1.78 [1.02, 3.10] |
| 2 Angiographic patency | 5 | 384 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.84, 2.00] |
| 3 Ankle brachial index | 4 | 441 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.05, 0.11] |
| 4 Treadmill walking distance | 2 | 240 | Mean Difference (IV, Random, 95% CI) | 41.72 [‐102.34, 185.78] |
| 5 Quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Physical quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Mental quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
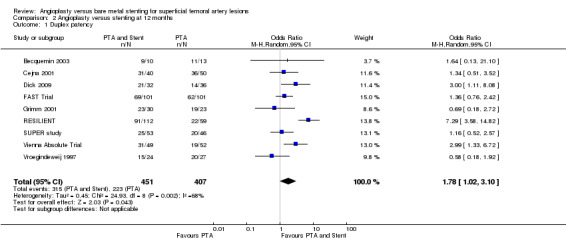
Comparison 2 Angioplasty versus stenting at 12 months, Outcome 1 Duplex patency.
Comparison 3. Angioplasty versus stenting at 24 months.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duplex patency | 3 | 188 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.91, 3.07] |
| 2 Angiographic patency | 2 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.28, 1.77] |
| 3 Ankle brachial index | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Treadmill walking distance | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
3.4. Analysis.
Comparison 3 Angioplasty versus stenting at 24 months, Outcome 4 Treadmill walking distance.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Becquemin 2003.
| Methods | Study design: RCT Method of randomisation: Concealed randomisation using computer software. Blinding: Unblinded, intention‐to‐treat. Exclusions post‐randomisation: 24. Losses to follow‐up: 31 PTA, 32 Stent. Cross‐over to stenting in PTA group: 15. |
|
| Participants | Country: France. Setting: Hospital. No. of participants: 227 (227 limbs). Age (mean): 66 years. Gender: 142 men, 85 women. Inclusion criteria: Inflow vessels free from significant lesion; single SFA lesion 1 cm from origin and 5 cm from proximal projection of knee joint; lesion length 1 cm ‐ 7 cm; at least 1 patent leg artery. Exclusion criteria: Acute ischaemia, previous surgery, haemorrhagic diathesis, hypercoagulation. |
|
| Interventions | PTA (115): over the wire. Stent (112): Stainless steel 'Palmaz'. Low molecular weight heparin for 24 hours, then aspirin or ticlopidine lifelong. |
|
| Outcomes | Pre‐dischage duplex. 12 months: Angiography. Primary patency defined as > 50% stenosis. |
|
| Notes | Salvage stenting used in the PTA arm in 15% of participants. No doses for drugs. Aspirin or ticlopidine. Sponsor: Cordis, Johnson & Johnson, Lafon, Aventis, and Société Française de Chirurgie Vasculaire. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer software used. |
| Allocation concealment (selection bias) | Low risk | Pre‐randomisation carried out using computer software by fax or telephone at least 24 hours before randomisation. During the operation, randomisation was made by telephone call. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Trials of this type cannot be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated but radiological investigations would show stent placement. Becquemin 2003 reports that primary outcome assessment was blinded but it is unclear how this was achieved. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No major attrition. |
| Selective reporting (reporting bias) | Low risk | All participants and outcomes accounted for. |
| Other bias | Unclear risk | Unclear drug prescription and salvage stenting in PTA arm. |
Cejna 2001.
| Methods | Study design: RCT Method of randomisation: Concealed randomisation using randomisation envelopes. Blinding: Unblinded, intention‐to‐treat. Exclusions post‐randomisation: None. Losses to follow‐up: 13 PTA, 20 Stent. Cross‐over to stenting in PTA group: 10. |
|
| Participants | Country: Austria. Setting: Hospital. No. of participants: 141 (154 limbs). Age (mean): 67 years. Gender: 95 men, 59 women. (This number refers to the total number of limbs i.e. 154. The ratio of male/female participants was not quoted in the article) Inclusion criteria: Up to 3 5‐cm lesions in SFA; or proximal popliteal arteries; at least 1 patent runoff vessel. Exclusion criteria: Patients with acute thromboembolism; previous vascular surgery in treated segments; untreated iliac and CFA disease. |
|
| Interventions | PTA (77): over the wire. Stent (77): Stainless steel 'Palmaz'. 100 mg aspirin bolus and 1000 iu heparin intraoperative, IV heparin for 24 hours, 100 mg aspirin lifelong. |
|
| Outcomes | 48 hours, 3, 6 and 12 months: ABI. 6 and 12 months: Angiography. Primary patency defined as greater than 30% stenosis. |
|
| Notes | Many follow‐ups not at prescribed intervals and different numbers of participants for each outcome. Sponsor: Johnson & Johnson Interventional Systems. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Concealed randomisation using randomisation envelopes. |
| Allocation concealment (selection bias) | Low risk | "Participating centres were given a number of closed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible in this type of trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated but radiological investigations would show stent placement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No major attrition. |
| Selective reporting (reporting bias) | Low risk | All participants and outcomes accounted for. |
| Other bias | Low risk | Good medication protocol. |
Dick 2009.
| Methods | Study design: RCT Method of randomisation: concealed, computerised and in sealed envelopes Blinding: Unblinded, intention‐to‐treat Exclusions post randomisation: None. Losses to follow‐up: 3 PTA, 2 Stent. Cross‐over to stenting in PTA group: 7 |
|
| Participants | Country: Austria. Setting: Hospital. No. of participants: 73 (73 limbs). Age (mean): 69 Gender: 50 men, 23 women. Inclusion criteria: clinical, severe intermittent claudication or critical limb ischaemia with rest pain or ulcers. Anatomic, > 50% stenosis or occlusion of the SFA with target lesion between 30 and 200 mm. Exclusion criteria: acute limb ischaemia, previous stenting or bypass surgery of SFA, untreated inflow disease of ipsilateral pelvic arteries and known intolerances to medications/contrast. |
|
| Interventions | 100 mg aspirin clopidogrel 75 mg, statin and antihypertensive PTA: 34 over the wire Stent: 39 nitinol stents |
|
| Outcomes | Restenosis rates ‐ 6‐month Angiography Primary patency defined as > 50% stenosis |
|
| Notes | Salvage stenting used in the PTA arm in 7 participants Sponsor: No statement. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random digits and sealed envelopes. |
| Allocation concealment (selection bias) | Low risk | Computer‐generated random digits and sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible in this type of trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated but radiological investigations would show stent placement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No major attrition. |
| Selective reporting (reporting bias) | Low risk | All outcomes accounted for. |
| Other bias | Unclear risk | No statement on adherence to medication protocol. |
FAST Trial.
| Methods | Study design: RCT Method of randomisation: Concealed randomisation using randomisation envelopes provided by an independent data management organisation. Blinding: Unblinded, intention‐to‐treat. Exclusions post‐randomisation: None. Losses to follow‐up: 6 PTA, 9 stent. Cross‐over to stenting in PTA group: 13. |
|
| Participants | Country: Germany. Setting: Hospital. No. of participants: 244 (244 limbs). Age (mean): 66.5 years. Gender: 168 men, 76 women. Inclusion criteria: SFA lesion at least 1 cm from SFA origin and 1cm ‐ 10 cm long; target lesion diameter at least 70% by visual estimate; all distal vessels patent; at least Rutherford 2 chronic limb ischaemia. Exclusion criteria: Target lesion requiring pretreatment such as debulking; target lesion extending into popliteal artery; previous stent in target SFA; multiple lesions exceeding 10 cm; acute or subacute (≤ 4 weeks) thrombotic occlusion; an untreated ipsilateral iliac artery stenosis; ongoing dialysis and treatment with oral anticoagulation other than antiplatelet therapy. |
|
| Interventions | PTA (121): over the wire. Stent (123): Nitinol. 100 mg aspirin for at least 10 days or 500 mg bolus preoperative. 3000 ‐ 5000 iu heparin intraoperatively |
|
| Outcomes | 1, 6 and 12 month follow‐up: ABI, treadmill test, duplex ultrasound. 12‐month biplane radiographs for participants receiving stent (detection of fractures). Primary patency defined as proximal peak velocity ratio ≥ 2.4 on duplex ultrasound. |
|
| Notes | Participants with ipsilateral iliac artery stenosis underwent angioplasty and were not excluded. Total occlusion rate difference (25% in PTA participants, 37% stent participant) may bias restenosis results (acknowledged). Gender difference in restenosis rates in PTA arm only (acknowledged). Compliance with aspirin/clopidogrel not assessed. Reassessment blinding not stated. Sponsor: C.R. Bard Inc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation envelopes. |
| Allocation concealment (selection bias) | Low risk | 4‐block randomisation envelopes from independent data management company. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible in this type of trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated but radiological investigations would show stent placement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No major attrition. |
| Selective reporting (reporting bias) | Low risk | All outcomes accounted for. |
| Other bias | High risk | No statement of adherence to medication protocol. Medians quoted and participants with ipsilateral iliac artery stenosis underwent angioplasty but were not excluded. |
Grenacher 2004.
| Methods | Study design: RCT Method of randomisation: Concealed randomisation using sealed randomisation envelopes. Blinding: Unblinded, intention‐to‐treat. Exclusions post‐randomisation: None. Losses to follow‐up: 30. Cross‐over to stenting in PTA group: 1. |
|
| Participants | Country: Germany. Setting: Hospital. No. of participants: 116 (124 limbs). Age (mean): 67 years. Gender: 78 men, 38 women. Inclusion criteria: Intermittent claudication or chronic limb ischaemia, lesion < 5 cm, at least 1 patent runoff vessel. Exclusion criteria: Lesions greater than 5 cm in length requiring more than 2 stents; multifocal disease or complete obstruction in the SFA; haemodynamically relevant stenoses in the lower limb previously untreated; occlusion of more than 2 runoff vessels; existing contraindications for vascular surgery or anticoagulation. |
|
| Interventions | PTA (53): over the wire. Stent (71): Stainless steel 'Palmaz'. 100 mg aspirin bolus and 5000 iu heparin intraoperative. Low molecular weight heparin for 48 hours, 100 mg aspirin lifelong. |
|
| Outcomes | 3, 6, 12 and 24 month follow‐up: Angiography and duplex ultrasound. Primary patency defined as greater than 30% stenosis. |
|
| Notes | Numbers of participants undergoing duplex unclear and therefore not included in analysis. Sponsor: No statement. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation envelopes. |
| Allocation concealment (selection bias) | Low risk | "The envelopes were opened after all inclusion and exclusion criteria were examined." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible in this type of trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated but radiological investigations would show stent placement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No major attrition. |
| Selective reporting (reporting bias) | Low risk | All outcomes accounted for. |
| Other bias | Low risk | Good medication and post‐trial protocol. |
Grimm 2001.
| Methods | Study design: RCT Method of randomisation: Concealed randomisation using randomisation envelopes. Blinding: Unblinded, intention‐to‐treat. Exclusions post‐randomisation: None. Losses to follow‐up: 12. Cross‐over to stenting in PTA group: none. |
|
| Participants | Country: Germany. Setting: Hospital. No. of participants: 53 (53 limbs). Age (mean): 69 years. Gender: 32 men, 21 women. Inclusion criteria: SFA lesion at least 1 cm from SFA origin (including proximal popliteal artery); stenosis no longer than 5 cm; target lesion diameter at least 70% by visual estimate; vessel diameter between 4 mm and 8 mm. Exclusion criteria: Lesions > 5 cm in length requiring more than 2 stents; multifocal disease or complete obstruction in the SFA; haemodynamically relevant stenoses in the lower limb previously untreated; occlusion of more than 2 runoff vessels; thrombus within the SFA; existing contraindications for vascular surgery or anticoagulation. |
|
| Interventions | PTA (23): over the wire. Stent (30): Stainless steel 'Palmaz'. 1000 mg aspirin bolus and 5000 iu heparin intraoperative. IV heparin for 24 hours, 100 mg aspirin lifelong. |
|
| Outcomes | 3, 6, 12 and 24 month follow‐up: ABI, treadmill test, duplex ultrasound. 6‐month angiography. Primary patency defined as vessels without 1.5 greater systolic flow than in normal parts of the artery or angiographic reocclusion. |
|
| Notes | Participants with ipsilateral iliac or distal popliteal stenosis underwent angioplasty and were not excluded. No clear mention of distal vessel patency. Primary patency definition for angiography unclear. Aspirin compliance not assessed. Sponsor: Johnson & Johnson Interventional Systems. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation envelopes. |
| Allocation concealment (selection bias) | Low risk | Adequate concealed randomisation using randomisation envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible in this type of trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated but radiological investigations would show stent placement. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No statement on attrition. |
| Selective reporting (reporting bias) | Low risk | All outcomes accounted for. |
| Other bias | Unclear risk | Medication not assessed. Participants with ipsilateral iliac or distal popliteal stenosis underwent angioplasty and were not excluded. |
RESILIENT.
| Methods | Study design: RCT Method of randomisation: Concealed randomisation (computer‐generated) Blinding: Unblinded, intention‐to‐treat. Exclusions post‐randomisation: None. Losses to follow‐up: PTA 13, 22 Stent. Cross‐over to stenting in PTA group: 29 |
|
| Participants | Country: USA (California) and Europe (Austria) ‐ 24 centres. Setting: Hospital. No. of participants: 206 (206 limbs). Age (mean): 67 years. Gender: 143 men, 63 women. Inclusion criteria: symptoms of intermittent claudication (Rutherford categories 1 ‐ 3), candidates for angioplasty or stenting, de novo stenotic, occlusive, or restenotic lesions in SFA Exclusion criteria: critical limb ischaemia (Rutherford categories 4 ‐ 6), sensitivity to contrast media, renal and/or hepatic failure. |
|
| Interventions | 81 mg aspirin (continue for 6 months), 75 mg clopidogrel (12 weeks post‐procedure). Unfractionated heparin intra‐procedure. PTA (72): over the wire. Stent (134): LifeStent nitinol self‐expanding stent. |
|
| Outcomes | Freedom from target lesion revascularisation 6, 12 months. Primary patency, duplex, 6 and 12 months. QoL measures 6, 12 months (Short Form 8 Question Health Survey), walking distance score 6, 12 months (Walking Impairment Questionnaire). |
|
| Notes | Of 72 participants in angioplasty group, 29 underwent secondary bailout stenting procedure (due to inadequate PTA result, flow‐limiting dissection or a residual stenosis). Sponsor: Bard Peripheral Vascular. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | Computer‐generated randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible in this type of trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated but radiological investigations would show stent placement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No major attrition. |
| Selective reporting (reporting bias) | Low risk | All outcomes accounted for. |
| Other bias | Unclear risk | No medication compliance protocol. |
SUPER study.
| Methods | Study design: RCT Method of randomisation: Concealed randomisation (computer‐generated) Blinding: Unblinded, intention‐to‐treat. Exclusions post‐randomisation: None. Losses to follow‐up: Not stated. Cross‐over to stenting in PTA group: 4, cross‐over to PTA in stenting group: 4 |
|
| Participants | Country: UK, 17 centres. Setting: Hospital. No. of participants: 150 (150 limbs). Age (mean): 67 years. Gender: 123 men, 27 women. Inclusion criteria: symptoms of intermittent claudication (Rutherford categories 1 ‐ 5), candidates for angioplasty or stenting, de novo stenotic, occlusive, or restenotic lesions in SFA. Exclusion criteria: critical limb ischaemia (Rutherford 6), sensitivity to contrast media, renal and/or hepatic failure. |
|
| Interventions | PTA 76.
Stent 74. Nitinol. 3000 ‐ 5000 iu heparin intra‐procedure. Unclear antiplatelet as only 'recommended'. |
|
| Outcomes | Primary patency, duplex, baseline to 12 months. QoL measures: EuroQol (EQ)‐5D. |
|
| Notes | No strict antiplatelet protocol. Sponsor: Cordis, Johnson & Johnson Medical Ltd. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | Computer‐generated randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible in this type of trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Radiological investigations would show stent placement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No major attrition. |
| Selective reporting (reporting bias) | Low risk | All outcomes accounted for. |
| Other bias | Unclear risk | No strict antiplatelet protocol. |
Vienna Absolute Trial.
| Methods | Study design: RCT Method of randomisation: Concealed randomisation using computer‐generated randomisation envelopes. Blinding: Unblinded, intention‐to‐treat. Exclusions post‐randomisation: None. Losses to follow‐up: None at 6 months, 3 at 12 months, 6 at 2 years. Cross‐over to stenting in PTA group: 17. |
|
| Participants | Country: Austria. Setting: Hospital. No. of participants: 104 (104 limbs). Age (mean): 66.5 years. Gender: 55 men, 49 women. Inclusion criteria: SFA target lesion > 3 cm long; target lesion diameter at least 50% by visual estimate; at least 1 patent (< 50% stenosed) tibioperoneal runoff vessel; at least Rutherford 3 chronic limb ischaemia. Exclusion criteria: Untreated inflow disease of ipsilateral pelvic arteries (> 50% stenosis or occlusion); previous bypass procedure or stent in target SFA; multiple lesions > 10 cm; acute critical limb ischaemia; an untreated ipsilateral iliac artery stenosis; known intolerance to study medications or contrast agents. |
|
| Interventions | PTA (53): over the wire. Stent (51): Nitinol. 75 mg clopidogrel for at least 2 days or 300 mg bolus preoperative. |
|
| Outcomes | 6 months: Rate of restenosis by angiography. 3, 6 and 12 months: Rate of restenosis (at least 50% luminal diameter) by duplex; occurrence of stent fractures; ABI; treadmill test; QoL (SF‐36 questionnaire, Sabeti 2007). 6 and 12 months: Limb amputation or death. Primary patency defined as > 50% stenosis. 24 months (Schillinger 2007): Rate of restenosis (at least 50% luminal diameter) by duplex; occurrence of stent fractures; ABI; treadmill test; re‐intervention. QoL measures: Short form 36 |
|
| Notes | Full results published over 3 papers: Schillinger 2006; Schillinger 2007; Sabeti 2007. 2‐year follow‐up published for same cohort as Schillinger 2007 (clinical outcomes) and Sabeti 2007 (quality of life). Uses medians for treadmill distance and QoL indicators. Compliance with clopidogrel not assessed. Sponsor: Guidant Endovascular Solutions. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Concealed randomisation using computer‐generated randomisation envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible in this type of trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated but radiological investigations would show stent placement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No major attrition. |
| Selective reporting (reporting bias) | Low risk | All outcomes accounted for. |
| Other bias | Unclear risk | Medians quoted for treadmill distances and QoL; these results may therefore be skewed. |
Vroegindeweij 1997.
| Methods | Study design: RCT Method of randomisation: Concealed randomisation using sealed randomisation envelopes. Blinding: Unblinded, intention‐to‐treat. Exclusions post‐randomisation: None. Losses to follow‐up: None. Cross‐over to stenting in PTA group: 4. |
|
| Participants | Country: The Netherlands. Setting: Hospital. No. of participants: 51 (51 limbs). Age (mean): 64.5 years. Gender: 36 men, 15 women. Inclusion criteria: Lesions confined to femoropopliteal artery. Exclusion criteria: Below‐knee popliteal disease; multisegmental disease; disease > 5 cm in length. |
|
| Interventions | PTA (27): over the wire. Stent (24): stainless steel 'Palmaz'. 5000 iu heparin intraoperative. 3 months warfarin. |
|
| Outcomes | 6 weeks, 3, 6, 9, 12, 18 and 24 month follow‐up: ABI, treadmill test, duplex ultrasound. Primary patency defined as proximal peak velocity ratio > 2.5 on duplex ultrasound. |
|
| Notes | Runoff vessel patency at time of intervention unclear. Participants with coexisting ipsilateral proximal arterial stenosis included. Sponsor: No statement. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Concealed randomisation using sealed randomisation envelopes. |
| Allocation concealment (selection bias) | Low risk | Concealed randomisation using sealed randomisation envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible in this type of trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated but radiological investigations would show stent placement. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No statement on attrition. |
| Selective reporting (reporting bias) | Low risk | All outcomes accounted for. |
| Other bias | Unclear risk | Warfarin used initially then unclear medication protocol. |
Zdanowski 1999.
| Methods | Study design: RCT Method of randomisation: Computerised randomisation. Blinding: Unblinded, intention‐to‐treat. Exclusions post‐randomisation: None. Losses to follow‐up: 1. 4 (2 in each group) subsequently underwent surgery within the trial time period. 7 refused angiography during follow‐up. Cross‐over to stenting in PTA group: none. |
|
| Participants | Country: Sweden. Setting: Hospital. No. of participants: 32 (32 limbs). Age (mean): 71 years. Gender: 14 men, 18 women. Inclusion criteria: Lesions confined to femoropopliteal artery. Exclusion criteria: Unclear. |
|
| Interventions | PTA (27): over the wire. Stent (24): nitinol 'Strecker'. 160 mg aspirin post‐operative. |
|
| Outcomes | 1 year: ABI, angiography. Primary patency defined as > 50% stenosis. |
|
| Notes | Unclear inclusion/exclusion criteria. Poor anticoagulation. Sponsor: No statement. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation. |
| Allocation concealment (selection bias) | Low risk | Computerised randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible in this type of trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated but radiological investigations would show stent placement. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No statement or participant numbers for attrition. |
| Selective reporting (reporting bias) | Unclear risk | Ill‐defined outcomes at trial conception |
| Other bias | Unclear risk | Unclear inclusion and exclusion criteria for participants. |
ABI: ankle brachial index CFA: common femoral artery iu: international units IV: intravenous PTA: percutaneous transluminal angioplasty QoL: quality of life RCT: randomised controlled trial SFA: superficial femoral artery
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahn 1992 | Only a description of the proposal for setting up randomised controlled trial to investigate SFA disease. |
| Brancaccio 2012 | Randomised controlled trial which had the relevant comparisons but not outcomes. |
| Duda 2006 | Randomised controlled trial comparing bare metal stent with drug‐eluting stent; no PTA‐only arm. |
| Saxon 2003 | Randomised controlled trial comparing PTA with PTA plus ePTFE stent graft rather than stent. |
| VascuCoil Trial | Randomised controlled trial designed to enrol 500 participants with stenotic or occluded SFA disease. The trial was stopped early due to slow participant enrolment. Participants (266) were randomised to PTA versus PTA plus stent (Intracoil). Greenberg 2004 reports cost, resource utilisation and in‐hospital complications, outcomes not relevant for this review. |
ePTFE: expanded polytetrafluoroethylene PTA: percutaneous transluminal angioplasty SFA: superficial femoral artery
Characteristics of ongoing studies [ordered by study ID]
NCT01183117.
| Trial name or title | A clinical investigation of SM‐01 stenting versus PTA for the treatment of superficial femoral artery disease |
| Methods | Randomised controlled trial |
| Participants | Men or women 20 years or older. Inclusion criteria: Symptomatic leg ischaemia by Rutherford Classification (category 1, 2, or 3). Lesion length ≥ 40 mm to ≤ 150 mm (must be treatable with no more than 2 SM‐01 stents. Overlap should be about 1 cm if 2 stents are used) Reference vessel diameter (RVD) ≥ 4.0 mm and ≤ 7.0 mm. All lesions are to be located ≥ 3.0 cm proximal to the superior edge of the patella, and ≥ 1.0 cm distal to the SFA/PFA bifurcation. ≥ 50% stenosis or total occlusion. Patent infrapopliteal and popliteal arteries, i.e., single‐vessel runoff or better with at least 1 of 3 vessels patent (< 50% stenosis) to the ankle or foot. |
| Interventions | Device: SM‐01
SM‐01 is a self‐expandable, crush recoverable stent with a diameter larger than that of the arterial lumen. The stent is indicated for use in a vessel with a diameter 1 ‐ 2 mm smaller than the nominal stent diameter. This stent will open to the diameter of the artery and will continue to apply expanding force on the artery. Active comparator: PTA balloon angioplasty. |
| Outcomes | Primary outcome measures: Non‐TVF (Target vessel failure) rate (time frame: 12 months). The primary endpoint will be freedom from TVF defined as any events of clinical driven (confirmed by duplex ultrasound or angiography) TLR/TVR, procedure failure, amputation of the target lesion's leg, procedure‐ or device‐related death, occlusion of target lesion, or > 70% restenosis of target lesion. |
| Starting date | July 2010 |
| Contact information | Takuro Takagi, MD |
| Notes |
Osprey.
| Trial name or title | OSPREY. Occlusive/Stenotic Peripheral artery Revascularization Study |
| Methods | JP arm: randomised trial comparing PTA with stenting |
| Participants | SFA disease Rutherford classification 2,3 or 4 |
| Interventions | PTA versus stenting |
| Outcomes | Bailout stenting, procedure failure, death, amputation |
| Starting date | 2009 |
| Contact information | Hiroyoshi Yokoi, Kokura Memorial Hospital, Kitakyusyu, Japan |
| Notes |
Differences between protocol and review
We assessed clinical outcome as a secondary outcome to allow the assessment of ankle brachial index (ABI) and treadmill walking distance.
We could not stratify data for whether the interventions tested were performed for claudication or for critical limb ischaemia, as RCTs did not report individual data.
We assessed the methodological quality of the included studies using the 'Risk of bias' tool from The Cochrane Collaboration (Higgins 2011).
Contributions of authors
MMC selected trials, assessed the methodological quality of trials, extracted data and analysed results. CPT selected trials, assessed the methodological quality of trials, extracted data and analysed results. MMC and CPT ranked the allocation concealment of the trials and checked the completed manuscript. ADM checked the selection of trials, cross checked data extraction and resolved any disagreements.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The PVD Group editorial base is supported by the Chief Scientist Office.
-
National Institute for Health Research (NIHR), UK.
The PVD Group editorial base is supported by a programme grant from the NIHR.
Declarations of interest
MMC: none known ADM: I act as a consultant to LeMaitre vascular and have presented at their sales meetings. This is compliant with US legislation. LeMaitre vascular makes grafts for bypass surgery. The aortic intervention division of Cook Medical supported my attendance at the Veith meeting in November 2013 by paying for flights, accommodation and meeting fee. I am the examinations director and a member of the executive committee of UEMS Section and Board of Vascular Surgery. They have paid my travel expenses to committee meetings and to run the European Exam in vascular surgery (FEBVS). CPT: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Becquemin 2003 {published data only}
- Becquemin JP, Favre JP, Marzelle J, Nemoz C, Corsin C, Leizorovicz A. Systematic versus selective stent placement after superficial femoral artery balloon angioplasty: a multicenter prospective randomized study. Journal of Vascular Surgery 2003;37(3):487‐94. [DOI] [PubMed] [Google Scholar]
Cejna 2001 {published data only}
- Cejna M, Thurnher S, Illiasch H, Horvath W, Waldenberger P, Hornik K, et al. PTA versus Palmaz stent placement in femoropopliteal artery obstructions: a multicenter prospective randomized study. Journal of Vascular and Interventional Radiology 2001;12(1):23‐31. [DOI] [PubMed] [Google Scholar]
Dick 2009 {published data only}
- Dick P, Wallner H, Sabeti S, Loewe C, Miekusch W, Lammer J, et al. Balloon angioplasty versus stenting with nitinol stents in intermediate length superficial femoral artery lesions. Catheterization and Cardiovascular Interventions 2009;74(7):1090‐5. [DOI] [PubMed] [Google Scholar]
FAST Trial {published data only}
- Bosiers M, Peeters P, Krankenberg H. The Femoral Artery Stenting Trial (FAST): 12‐month outcomes. Abstracts from the 2006 SVS Annual Meeting. June 2006.
- Krankenberg H. FAST (Femoral Artery Stenting Trial): Current evidence in femoral stenting: insights from the FAST trial acute results and 6‐month outcomes. Leipzig Interventional Course; 2006 Jan 26‐28, Leipzig. 2006.
- Krankenberg H. FAST: No advantage to nitinol stents in short lesions. Transcatheter Cardiovascular Therapeutics Conference. 2006.
- Krankenberg H. Randomized comparison of PTA versus femoral artery stenting (FAST): Acute results. Journal of Endovascular Therapy 2005;12(Suppl 1):21‐2. [Google Scholar]
- Krankenberg H, Schluter M, Steinkamp HJ, Burgelin K, Scheinert D, Schulte KL, et al. Nitinol stent implantation versus percutaneous transluminal angioplasty in superficial femoral artery lesions up to 10 cm in length: the femoral artery stenting trial (FAST). Circulation 2007;116(3):285‐92. [DOI] [PubMed] [Google Scholar]
Grenacher 2004 {published data only}
- Grenacher L, Saam T, Geier A, Muller‐Hulsbeck S, Cejna M, Kauffmann GW, et al. PTA versus Palmaz stent placement in femoropopliteal artery stenoses: results of a multicenter prospective randomized study (REFSA) [PTA versus Stent bei Stenosen der A. femoralis und A. poplitea: Ergebnisse einer prospektiv randomisierten Multizenterstudie (REFSA)]. Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 2004;176(9):1302‐10. [DOI] [PubMed] [Google Scholar]
Grimm 2001 {published data only}
- Grimm J, Muller‐Hulsbeck S, Jahnke T, Hilbert C, Brossmann J, Heller M. Randomized study to compare PTA alone versus PTA with Palmaz stent placement for femoropopliteal lesions. Journal of Vascular and Interventional Radiology 2001;12(8):935‐42. [DOI] [PubMed] [Google Scholar]
RESILIENT {published data only}
- Laird JR, Katzen BT, Scheinert D, Lammer J, Carpenter J, Buchbinder M, et al. Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: twelve‐month results from the RESILIENT randomized trial. Circulation. Cardiovascular Interventions 2010;3(3):267‐76. [DOI] [PubMed] [Google Scholar]
- Laird JR, Katzen BT, Scheinert D, Lammer J, Carpenter J, Buchbinder M, et al. Nitinol stent implantation vs. balloon angioplasty for lesions in the superficial femoral and proximal popliteal arteries of patients with claudication: three‐year follow‐up from the RESILIENT randomized trial. Journal of Endovascular Therapy 2012;19(1):1‐9. [DOI] [PubMed] [Google Scholar]
SUPER study {published data only}
- Chalmers N, Walker PT, Belli AM, Thorpe AP, Sidhu PS, Robinson G, et al. Randomized trial of the SMART stent versus balloon angioplasty in long superficial femoral artery lesions: the SUPER study. Cardiovascular and Interventional Radiology 2013;36(2):353‐61. [DOI] [PubMed] [Google Scholar]
Vienna Absolute Trial {published data only}
- Sabeti S, Czerwenka‐Wenkstetten A, Dick P, Schlager O, Amighi J, Mlekusch I, et al. Quality of life after balloon angioplasty versus stent implantation in the superficial femoral artery: findings from a randomized controlled trial. Journal of Endovascular Therapy 2007;14(4):431‐7. [DOI] [PubMed] [Google Scholar]
- Schillinger M, Sabeti S, Dick P, Amighi J, Mlekusch W, Schlager O, et al. Sustained benefit at 2 years of primary femoropopliteal stenting compared with balloon angioplasty with optional stenting. Circulation 2007;115(21):2745‐9. [DOI] [PubMed] [Google Scholar]
- Schillinger M, Sabeti S, Loewe C, Dick P, Amighi J, Mlekusch W, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. New England Journal of Medicine 2006;354(18):1879‐88. [DOI] [PubMed] [Google Scholar]
Vroegindeweij 1997 {published data only}
- Vroegindeweij D, Vos LD, Tielbeek AV, Buth J, vd Bosch HC. Balloon angioplasty combined with primary stenting versus balloon angioplasty alone in femoropopliteal obstructions: A comparative randomized study. Cardiovascular and Interventional Radiology 1997;20(6):420‐5. [DOI] [PubMed] [Google Scholar]
Zdanowski 1999 {published data only}
- Zdanowski Z, Albrechtsson U, Lundin A, Jonung T, Ribbe E, Thorne J, et al. Percutaneous transluminal angioplasty with or without stenting for femoropopliteal occlusions? A randomized controlled study. International Angiology 1999;18(4):251‐5. [PubMed] [Google Scholar]
References to studies excluded from this review
Ahn 1992 {published data only}
- Ahn S, Rutherford RB. A multicenter prospective randomized trial to determine the optimal treatment of patients with claudication and isolated superficial femoral artery occlusive disease: conservative versus endovascular versus surgical therapy. Journal of Vascular Surgery 1992;15(5):889‐91. [DOI] [PubMed] [Google Scholar]
Brancaccio 2012 {published data only}
- Brancaccio G, Lombardi R, Stefanini T, Torri P, Russo D, Gorji N, et al. Comparison of embolic load in femoropopliteal interventions: percutaneous transluminal angioplasty versus stenting. Vascular and Endovascular Surgery 2012;46(3):229‐35. [DOI] [PubMed] [Google Scholar]
Duda 2006 {published data only}
- Duda SH, Bosiers M, Lammer J, Scheinert D, Zeller T, Oliva V, et al. Drug‐eluting and bare nitinol stents for the treatment of atherosclerotic lesions in the superficial femoral artery: long‐term results from the SIROCCO trial. Journal of Endovascular Therapy 2006;13(6):701‐10. [DOI] [PubMed] [Google Scholar]
Saxon 2003 {published data only}
- Saxon RR, Coffman JM, Gooding JM, Natuzzi E, Ponec DJ. Long‐term results of ePTFE stent‐graft versus angioplasty in the femoropopliteal artery: single center experience from a prospective, randomized trial. Journal of Vascular and Interventional Radiology 2003;14(3):303‐11. [DOI] [PubMed] [Google Scholar]
VascuCoil Trial {published data only}
- Anonymous. Intracoil® self‐expanding peripheral stent PO000033. http://www.accessdata.fda.gov/cdrh_docs/pdf/P000033b.pdf 2001:1‐13.
- Greenberg D, Rosenfield K, Garcia LA, Berezin RH, Lavelle T, Fogleman S, et al. In‐hospital costs of self‐expanding nitinol stent implantation versus balloon angioplasty in the femoropopliteal artery (the VascuCoil Trial). Journal of Vascular and Interventional Radiology 2004;15(10):1065‐9. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01183117 {published data only}
- NCT01183117. A clinical investigation of SM‐01 stenting versus PTA for the treatment of superficial femoral artery disease. clinicaltrials.gov/show/NCT01183117 (accessed 10th May 2014).
Osprey {published data only}
- Yokoi H. OSPREY. Occlusive/Stenotic Peripheral artery Revascularization Study. www.leipzig‐interventional‐course.com/index.php?option=com_content&task=view&id=183&Itemid=286 (accessed June 2013).
Additional references
Acin 2012
- Acin F, Haro J, Bleda S, Varela C, Esparza L. Primary nitinol stenting in femoropopliteal occlusive disease: a meta‐analysis of randomized controlled trials. Journal of Endovascular Therapy 2012;19(5):585‐95. [DOI] [PubMed] [Google Scholar]
Becker 2008
- Becker RC, Meade TW, Berger PB, Ezekowitz M, O'Connor CM, Vorchheimer DA, et al. The primary and secondary prevention of coronary artery disease: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition). Chest 2008;133(6 Suppl):776S‐814S. [DOI] [PubMed] [Google Scholar]
Djulbegovic 2013
- Djulbegovic B, Kumar A, Miladinovic B, Reljic T, Galeb S, Mhaskar A, et al. Treatment success in cancer: industry compared to publicly sponsored randomized controlled trials. PloS One 2013;8(3):e58711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fontaine 1954
- Fontaine VR, Kim M, Kienny R. Surgical treatment for peripheral vascular disease [Die chirurgische Behandlung der peripheren Durchblutungsstorungen]. Helvetica Chirurgica Acta 1954;5/6:499‐533. [PubMed] [Google Scholar]
Fowkes 1991
- Fowkes FG, Housley E, Cawood EH, Macintyre CC, Ruckley CV, Prescott RJ. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. International Journal of Epidemiology 1991;20(2):384‐92. [DOI] [PubMed] [Google Scholar]
Frans 2012
- Frans FA, Bipat S, Reekers JA, Legemate DA, Koelemay MJ. Systematic review of exercise training or percutaneous transluminal angioplasty for intermittent claudication. British Journal of Surgery 2012;99(1):16‐28. [DOI] [PubMed] [Google Scholar]
Greenberg 2004
- Greenberg D, Rosenfield K, Garcia LA, Berezin RH, Lavelle T, Fogleman S, et al. In‐hospital costs of self‐expanding nitinol stent implantation versus balloon angioplasty in the femoropopliteal artery (the VascuCoil Trial). Journal of Vascular and Interventional Radiology 2004;15(10):1065‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kasapis 2009
- Kasapis C, Henke PK, Chetcuti SJ, Koenig GC, Rectenwald JE, Krishnamurthy VN, et al. Routine stent implantation vs. percutaneous transluminal angioplasty in femoropopliteal artery disease: a meta‐analysis of randomized controlled trials. European Heart Journal 2009;30(1):44‐55. [DOI] [PubMed] [Google Scholar]
Krankenberg 2007
- Krankenberg H, Schlüter M, Steinkamp HJ, Bürgelin K, Scheinert D, Schulte KL, et al. Nitinol stent implantation versus percutaneous transluminal angioplasty in superficial femoral artery lesions up to 10 cm in length: the femoral artery stenting trial (FAST). Circulation 2007;116(3):285‐92. [DOI] [PubMed] [Google Scholar]
Lundh 2012
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.MR000033.pub2] [DOI] [PubMed] [Google Scholar]
Mazari 2012
- Mazari FA, Khan JA, Carradice D, Samuel N, Abdul Rahman MN, Gulati S, et al. Randomized clinical trial of percutaneous transluminal angioplasty, supervised exercise and combined treatment for intermittent claudication due to femoropopliteal arterial disease. British Journal of Surgery 2012;99(1):39‐48. [DOI] [PubMed] [Google Scholar]
Mwipatayi 2008
- Mwipatayi BP, Hockings A, Hofmann M, Garbowski M, Sieunarine K. Balloon angioplasty compared with stenting for treatment of femoropopliteal occlusive disease: a meta‐analysis. Journal of Vascular Surgery 2008;47(2):461‐9. [PUBMED: 17950563] [DOI] [PubMed] [Google Scholar]
NICE 2012
- National Institute for Health and Clinical Excellence. Lower limb peripheral arterial disease: diagnosis and management. www.nice.org.uk/nicemedia/live/13856/60428/60428.pdf (accessed 16th May 2014).
Norgren 2007
- Norgren L, Hiatt WR, Dormany JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter‐society consensus for the management of peripheral arterial disease (TASC II). European Journal of Vascular and Endovascular Surgery 2007;33(Suppl 1):S1‐75. [DOI] [PubMed] [Google Scholar]
Rutherford 1997
- Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. Journal of Vascular Surgery 1997;26(3):517‐38. [DOI] [PubMed] [Google Scholar]
Sabeti 2007
- Sabeti S, Czerwenka‐Wenkstetten A, Dick P, Schlager O, Amighi J, Mlekusch I, et al. Quality of life after balloon angioplasty versus stent implantation in the superficial femoral artery: findings from a randomized controlled trial. Journal of Endovascular Therapy 2007;14(4):431‐7. [DOI] [PubMed] [Google Scholar]
Schillinger 2006
- Schillinger M, Sabeti S, Loewe C, Dick P, Amighi J, Mlekusch W, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. New England Journal of Medicine 2006;354(18):1879‐88. [DOI] [PubMed] [Google Scholar]
Schillinger 2007
- Schillinger M, Sabeti S, Dick P, Amighi J, Mlekusch W, Schlager O, et al. Sustained benefit at 2 years of primary femoropopliteal stenting compared with balloon angioplasty with optional stenting. Circulation 2007;115(21):2745‐9. [DOI] [PubMed] [Google Scholar]
Selvin 2004
- Selvin E, Erlinger TP. Prevelance of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey 1999‐2000. Circulation 2004;110(6):738‐43. [DOI] [PubMed] [Google Scholar]
Vardi 2014
- Vardi M, Novack V, Pencina MJ, Doros G, Burke DA, Elmariah S, et al. Safety and efficacy metrics for primary nitinol stenting in femoropopliteal occlusive disease: a meta‐analysis and critical examination of current methodologies. Catheterization and Cardiovascular Interventions 2014; Vol. 83, issue 6:975‐83. [DOI: 10.1002/ccd.25179] [DOI] [PubMed]
Watson 2008
- Watson L, Ellis B, Leng GC. Exercise for intermittent claudication. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD000990.pub2] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Twine 2009
- Twine CP, Coulston J, Shandall A, McLain AD. Angioplasty versus stenting for superficial femoral artery lesions. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD006767.pub2] [DOI] [PubMed] [Google Scholar]


