Biocontrol agents are increasingly used to replace chemical pesticides to prevent crop diseases. In the button mushroom field in France, the use of Bacillus velezensis QST713 as a biocontrol agent against the green mold Trichoderma aggressivum has been shown to be efficient. However, the biocontrol mechanisms effective in the Agaricus bisporus/Trichoderma aggressivum/Bacillus velezensis QST713 pathosystem are still unknown. Our paper focuses on the exploration of the bioprotection mechanisms of the biocontrol agent Bacillus velezensis QST713 during culture of the button mushroom (Agaricus bisporus) in a micromodel culture system to study the specific response of strain QST713 in the presence of T. aggressivum and/or A. bisporus.
KEYWORDS: Agaricus bisporus, Bacillus velezensis, biocontrol, biofilm, CLSM, compost, GFP, secondary metabolites, qPCR
ABSTRACT
Bacillus velezensis QST713 is widely used as a biological control agent for crop protection and disease suppression. This strain is used industrially in France for the protection of Agaricus bisporus against Trichoderma aggressivum f. europaeum, which causes green mold disease. The efficacy of this biocontrol process was evaluated in a previous study, yet the mode of its action has not been explored under production conditions. In order to decipher the underlying biocontrol mechanisms for effective biofilm formation by strain QST713 in the compost and for the involvement of antimicrobial compounds, we developed a simplified micromodel for the culture of A. bisporus during its early culture cycle. By using this micromodel system, we studied the transcriptional response of strain QST713 in the presence or absence of A. bisporus and/or T. aggressivum in axenic industrial compost. We report the overexpression of several genes of the biocontrol agent involved in biofilm formation in the compost compared to their expression during growth in broth compost extract either in the exponential growth phase (the epsC, blsA, and tapA genes) or in the stationary growth phase (the tapA gene), while a gene encoding a flagellar protein (hag) was underexpressed. We also report the overexpression of Bacillus velezensis QST713 genes related to surfactin (srfAA) and fengycin (fenA) production in the presence of the fungal pathogen in the compost.
IMPORTANCE Biocontrol agents are increasingly used to replace chemical pesticides to prevent crop diseases. In the button mushroom field in France, the use of Bacillus velezensis QST713 as a biocontrol agent against the green mold Trichoderma aggressivum has been shown to be efficient. However, the biocontrol mechanisms effective in the Agaricus bisporus/Trichoderma aggressivum/Bacillus velezensis QST713 pathosystem are still unknown. Our paper focuses on the exploration of the bioprotection mechanisms of the biocontrol agent Bacillus velezensis QST713 during culture of the button mushroom (Agaricus bisporus) in a micromodel culture system to study the specific response of strain QST713 in the presence of T. aggressivum and/or A. bisporus.
INTRODUCTION
The strain Bacillus velezensis QST713 is a biocontrol agent used in France to protect the white button mushroom (Agaricus bisporus) crop against the fungal pathogen Trichoderma aggressivum, responsible for green mold disease. This fungal pathogen impedes the growth of A. bisporus mycelium and is characterized by the presence of a green color in the infected areas, due to the sporulation of T. aggressivum (1–3). Historically, the QST713 strain was initially commercialized as Bacillus subtilis, yet sequencing of its genome allowed us to recently reclassify it as Bacillus velezensis (4). Hence, we had assessed the impact of this strain on the microbial communities in the culture substrate of A. bisporus and its biocontrol effect on T. aggressivum (3). Many studies have shed light on the involvement of biofilm formation and the production of antimicrobial compounds by Bacillus species in crop bioprotective mechanisms (5–9). In this pathosystem, the mechanisms developed by B. velezensis QST713 during its bioprotective process have not been investigated yet. To determine the involvement of biofilm formation and the synthesis of antimicrobial compounds by B. velezensis QST713 as part of the bioprotective mechanisms during the cultivation of A. bisporus, knowledge of the well-documented regulation network of B. subtilis biofilm formation is first needed. The transition from the planktonic to the biofilm state requires regulatory pathways to set up the expression of genes involved in matrix production or motility at the origin of cellular heterogeneity (10, 11). This cellular heterogeneity is illustrated by the coexistence of different cell phenotypes, such as matrix-producing cells, surfactin-producing cells, flagellated cells, or spore-forming cells. Each cell type is characterized by a different expression profile, with specific genes being regulated by genetic determinants involved in various regulatory pathways for biofilm formation (10, 12, 13). These different regulatory pathways are activated by extracellular signals (the presence of biocides, surfactants, plant exudates, etc.), setting up a phosphorylation cascade relayed by the intracellular kinase phosphatases KinA, KinB, KinC, and KinD. The resulting phosphorylation of the Spo0A transcription factor leads to the activation of B. subtilis biofilm formation (11).
The transcriptional regulator Spo0A controls the expression of about 100 genes, including the genes encoding the transcriptional regulators AbrB, SinI/SinR, and SlrA/SlrR triggering the direct or indirect regulation of genes involved in the synthesis of extracellular matrix components (tasA operon, eps operon) or motility (hag) (11). The tasA operon (tapA-sipW-tasA) is responsible for the synthesis of the TasA structural protein forming amyloid fibers in the matrix. The eps operon (comprising epsA through epsO) is responsible for the production of exopolysaccharides present in the matrix (11, 14, 15). The hag gene encodes a flagellar protein expressed in motile cells (11). Spo0A is involved in the regulation of srf genes, allowing the synthesis of surfactin, a surface-active lipopeptide involved in biofilm formation by induction of polymer synthesis and involved in the regulation of swr genes for the swarming motility system (16). Surfactin can act as a signaling molecule that promotes biofilm formation in other bacilli and also as a broad-spectrum antimicrobial (8, 17). In addition, Spo0A allows the regulation of the sigF gene, involved in sporulation (11–13). DegU, a pleiotropic transcriptional factor, is also involved in the regulation of genes encoding matrix components, including (i) blsA, encoding an amphiphilic hydrophobin (BlsA) forming a hydrophobic protective layer on the interface of the biofilm in contact with air, and (ii) the pgsBCD operon, which allows the synthesis of a polymer, giving a mucosal appearance to the colonies. DegU is also involved in antibiotic production, and it controls the expression of swr genes, involved in swarming motility (6, 11, 12, 16, 18). Moreover, the ComA regulator controls the production of surfactin, as well as the ability to incorporate exogenous DNA from the environment (12, 13, 19).
It has been shown that certain secondary metabolite antimicrobials can be overproduced by the biofilm form of the biocontrol agents (20). We hypothesized that both the biofilm-associated spatial competition and the production of an antifungal by B. velezensis QST713 could be involved in the bioprotective mechanisms against T. aggressivum during the cultivation of A. bisporus. In this work, reverse transcription-quantitative PCR was used to follow the expression of genes related to biofilm formation (epsC, tapA, and blsA), motility (hag and swrA), and secondary metabolite synthesis (e.g., the antifungal surfactin [srfAA ], fengycin [fenA], bacillomycin D [bmyA], the siderophore bacillibactin [dhbA], and two potential antimicrobials [nrpsB, tnrpsC]) in B. velezensis QST713 in broth compost extract for exponential growth phase (EGP) and stationary growth phase (SGP) and in a compost culture with or without the presence of T. aggressivum f. europaeum and A. bisporus. The putative open reading frames of these genes have been identified in silico according to the previously published genomic sequence of B. velezensis QST713 (4). The culture compost of A. bisporus is naturally extremely rich in Bacillus species (3). We therefore chose to carry out this study with autoclaved axenic compost in order to specifically study the cell reprogramming of strain QST713. The spatial organization of the biocontrol agent in the compost was studied by noninvasive confocal laser scanning microscopy (CLSM) with a recombinant B. velezensis strain constitutively producing the green fluorescent protein (GFP).
RESULTS
Growth and biocontrol effect of B. velezensis QST713 in compost micromodels.
The validation of A. bisporus culture micromodels in axenic autoclaved compost was based on the invasion of compost by the mycelium of A. bisporus at the end of the vegetative phase (15 days of cultivation) and on the observation of the biocontrol effect on T. aggressivum by B. velezensis QST713. Three controls were carried out for determination of (i) the normal development of A. bisporus alone in the compost, resulting in the progressive invasion of the compost by white mycelium in pots (Fig. 1, line 1); (ii) the development of the competitor T. aggressivum alone in the compost with massive sporulation at day 6 (Fig. 1, line 2); and (iii) the pathogenic effect of T. aggressivum on A. bisporus, resulting in the absence of A. bisporus mycelium development during culture and colonization by T. aggressivum, which was the most observable at day 6 (Fig. 1, line 3). Moreover, the presence of strain QST713 alone did not affect the natural appearance of the compost (Fig. 1, line 4). Furthermore, when strain QST713 and T. aggressivum were cocultured, we observed the absence of T. aggressivum sporulation in healthy compost, highlighting the inhibition effect of B. velezensis QST713 on T. aggressivum (Fig. 1, line 5). To determine whether the development of A. bisporus was affected by strain QST713, cocultures of A. bisporus and strain QST713 were assessed. We observed the normal growth of A. bisporus in the presence of strain QST713 (Fig. 1, line 6). The last condition studied was the coculture of the tripartite A. bisporus/T. aggressivum/B. velezensis QST713 pathosystem, where we observed the normal growth of the mycelium of A. bisporus and inhibition of the development of T. aggressivum, which thus validated the biocontrol efficiency of strain QST713 (Fig. 1, line 7). B. velezensis QST713 was enumerated to determine its effective development in the compost, and the results are represented in Fig. S1A in the supplemental material. Strain QST713 alone in the compost reached 109 CFU g−1 of compost at day 6 of the cultivation, and its development was not affected by the presence of the fungi (P > 0.05). We demonstrated the biocontrol effect of B. velezensis QST713 on T. aggressivum using A. bisporus culture micromodels in axenic autoclaved compost in early vegetative phase.
FIG 1.
Growth of A. bisporus and T. aggressivum in culture micromodels and effect of B. velezensis QST713 in coculture with one or both partners for 15 days of cultivation. Pictures of the culture pots were taken at days 6 (6d), 10 (10d), and 15 (15d) of cultivation. Line 1, QST713− A. b+ T. a−, A. bisporus (A. b) alone in the compost, resulting in the progressive invasion of the compost by a white mycelium; line 2, QST713− A. b− T. a+, the development of T. aggressivum (T. a) alone in the compost with massive sporulation (mostly at day 6); line 3, QST713− A. b+ T. a+, pathogenic effect of T. aggressivum on A. bisporus in the absence of A. bisporus mycelium development and with the massive sporulation of T. aggressivum (mostly at 6 days); line 4, QST713+ A. b− T. a−, B. velezensis QST713 alone in the compost with natural compost; line 5, QST713+ A. b− T. a+, coculture of B. velezensis QST713 and T. aggressivum, in which the absence of development of T. aggressivum showed the inhibitory effect of strain QST713 on T. aggressivum in natural compost; line 6, QST713+ A. b+ T. a−, coculture of B. velezensis QST713 and A. bisporus showing the normal development of A. bisporus mycelium; line 7, QST713+ A. b+ T. a+, coculture of B. velezensis QST713 with T. aggressivum and A. bisporus showing the normal development of A. bisporus mycelium and the biocontrol effect of B. velezensis QST713 on T. aggressivum.
Biofilm is the preferential mode of life of B. velezensis QST713 in compost micromodels.
To determine the preferential lifestyle of B. velezensis QST713 in the compost, we compared the expression of genes related to biofilm formation by strain QST713 cultivated in broth compost extract (planktonic state) at two different times (exponential growth phase [EGP] and stationary growth phase [SGP]) and in the compost after 6 days of cultivation. The growth curve of B. velezensis QST713 in broth compost extract was determined (Fig. S1B). Strain QST713 reached the stationary growth phase in the broth compost extract after 8 h of cultivation at 25°C (200 rpm) with a maximum optical density at 600 nm (OD600) of 0.74, corresponding to 4 × 107 CFU ml−1. Exponential growth phase occurred from 3 to 6 h. Cells were collected in the mid-exponential growth phase after 5 h of cultivation and in the stationary growth phase after 14 h of cultivation.
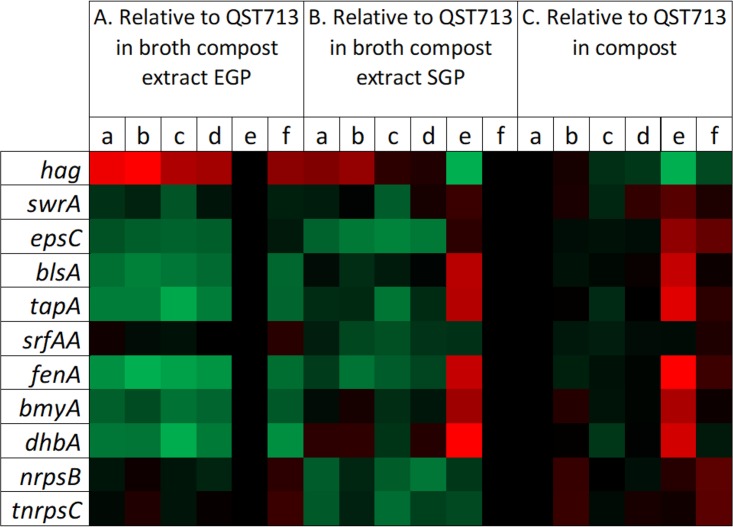
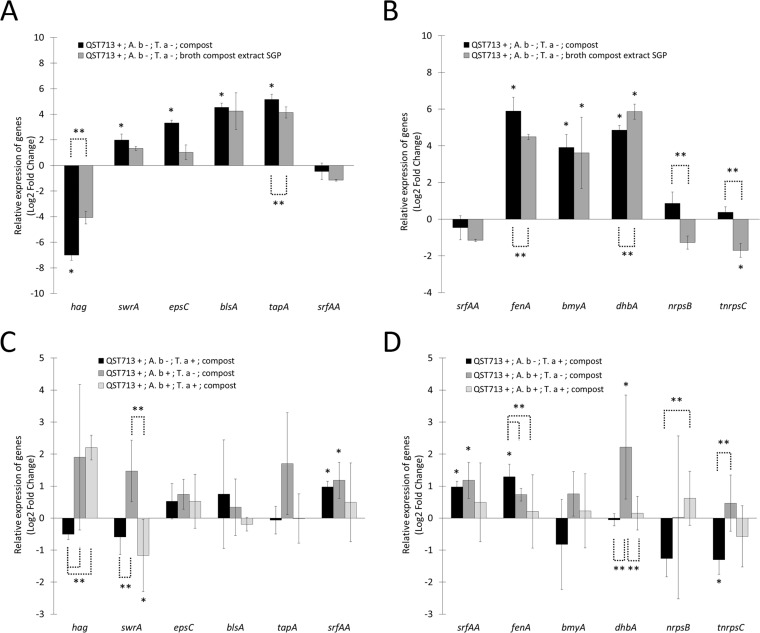
The gene expression profiles of strain QST713 in the compost relative to those when it was grown in the broth compost extract at EGP and SGP are represented as a heat map (Fig. 2A and B, lanes a). Genes related to biofilm formation (epsC, involved in the synthesis of extracellular polysaccharide; blsA, involved in the production of the hydrophobin BlsA; and tapA, involved in the production of amyloid fibers) were upregulated in the compost compared to their expression in EGP (Fig. 3A). On the other hand, hag, a gene involved in flagellin biosynthesis, was strongly downregulated in the compost compared to its expression in both EGP and SGP (Fig. 3A). The gene swrA, involved in swarming motility, was upregulated in the compost compared to its expression in EGP. Altogether, these results strongly suggest that biofilm is the dominant mode of life of strain QST713 in the compost.
FIG 2.
Heat map of the transcription levels of biofilm-, secondary metabolite production-, and motility-related genes of B. velezensis QST713 in the compost and broth compost extract. The transcription levels of motility-related genes (hag and swrA), biofilm-related genes (epsC, blsA, tapA, srfAA), and secondary metabolite synthesis genes (fenA, bmyA, dhbA, nrpsB, tnrpsC) were determined under six different conditions: (i) B. velezensis QST713 alone in the compost (lanes a), (ii) coculture of B. velezensis QST713 and T. aggressivum (lanes b), (iii) coculture of B. velezensis QST713 and A. bisporus (lanes c), (iv) coculture of B. velezensis QST713 with T. aggressivum and A. bisporus (lanes d), (v) exponential growth phase (EGP) of B. velezensis QST713 alone in the broth compost extract (lanes e), and (vi) stationary growth phase (SGP) of B. velezensis QST713 alone in the broth compost extract (lanes f). (A to C) Transcription levels of QST713 genes relative to those during EGP (A) or SGP (B) or for QST713 alone in the compost (C). Green, the values for upregulated genes (maximum value, +7.19 log2 fold change); red, the values for downregulated genes (minimum value, −7.49 log2 fold change). The values for both upregulated and downregulated genes are compared to a control value of 0 (black).
FIG 3.
Compost and interaction effects on the expression of genes related to motility, biofilm formation, and secondary metabolite production in B. velezensis QST713. The transcription levels of motility-related genes (hag and swrA), biofilm-related genes (epsC, blsA, tapA, srfAA), and secondary metabolite synthesis genes (fenA, bmyA, dhbA, nrpsB, tnrpsC) relative to those during the exponential growth phase of strain QST713 alone in the broth compost extract (A and B) and strain QST713 alone in the compost (C and D) are shown. Different culture conditions were studied: strain QST713 alone in the compost (QST713 +; A. b −; T. a −; compost), strain QST713 cocultured with T. aggressivum in the compost (QST713 +; A. b −; T. a +; compost), strain QST713 cocultured with A. bisporus in the compost (QST713 +; A. b +; T. a −; compost), strain QST713 cocultured with T. aggressivum and A. bisporus (QST713 +; A. b +; T. a +; compost), and strain QST713 during stationary growth phase alone in the broth compost extract (QST713 +; A. b −; T. a −; broth compost extract SGP). The gyrA gene was used as an internal reference. Bars represent standard deviations for data from three biological replicates. Results are presented as the log2 fold change, with the fold change being equal to 2−ΔΔCT. *, significant difference compared with the exponential growth phase (A and B) and compared with strain QST713 alone in the compost (C and D) (P < 0.05); **, significant difference between conditions (P < 0.05).
Secondary metabolite synthesis of B. velezensis QST713 in axenic compost micromodels.
The differential expression of genes involved in the production of secondary metabolites by strain QST713 was studied in the planktonic lifestyle (broth compost extract) and compared to that in the compost-associated mode of life (compost) (Fig. 2A and B, lanes a). The gene encoding surfactin, a molecule involved in biofilm formation and associated with antimicrobial activity, was not differentially expressed between the biofilm (compost) and the planktonic (EGP and SGP) lifestyle (Fig. 3B). Three genes encoding secondary metabolites were upregulated in the compost compared to their expression in EGP: fenA, involved in the synthesis of the antifungal fengycin; bmyA, involved in the synthesis of the antifungal bacillomycin D; and dhbA, involved in the synthesis of the siderophore bacillibactin. Only the gene fenA was significantly upregulated in the compost compared to its expression in both EGP and SGP (Fig. 3B). The genes nrpsB and tnrpsC, belonging to two clusters of secondary metabolites with potential antimicrobial activities (4), were both upregulated in the compost compared to their expression in SGP and EGP. These results suggest that fenA, nrpsB, and tnrpsC are overexpressed by the biofilm form in the compost.
The antifungal compounds surfactin and fengycin are overexpressed in the presence of T. aggressivum.
To determine the genes potentially involved in the bioprotection of the A. bisporus culture from T. aggressivum by B. velezensis QST713, the expression of genes involved in biofilm formation and secondary metabolite production was studied in compost micromodels. The four conditions studied were as follows: (i) the culture of B. velezensis QST713 alone in the compost, (ii) the coculture of B. velezensis QST713 and T. aggressivum, (iii) the coculture of B. velezensis QST713 and A. bisporus, and (iv) the coculture of B. velezensis QST713 with T. aggressivum and A. bisporus. The gene expression profiles of strain QST713 in the presence of T. aggressivum, A. bisporus, or both in the compost were compared to those of strain QST713 alone in the compost and are presented in Fig. 2C, lanes a to d. Genes related to biofilm formation did not appear to be significantly up- or downregulated in the presence of T. aggressivum, A. bisporus, or both compared to that by strain QST713 alone in the compost (Fig. 3C). The gene srfAA, involved in the synthesis of surfactin, a molecule involved in the initiation of biofilm formation and with antifungal activity, was upregulated by strain QST713 in the presence of T. aggressivum or A. bisporus compared to its expression by strain QST713 alone in the compost. The fenA gene, encoding an antifungal compound, was upregulated by strain QST713 in the presence of T. aggressivum compared to its expression by strain QST713 alone in the compost (Fig. 3D). The tnrpsC gene, encoding a potential secondary metabolite compound, was downregulated by strain QST713 in the presence of T. aggressivum. The dhbA gene, encoding the siderophore bacillibactin, was upregulated by strain QST713 in the presence of A. bisporus compared to its expression by strain QST713 alone in the compost (Fig. 3D). Competition for iron uptake might occur in the compost between strain QST713 and A. bisporus, an effect that we did not observe in the presence of the three partners. These results suggest that fengycin and surfactin may be involved in the inhibition of T. aggressivum.
Direct visualization of biofilm formation in compost by fluorescent B. velezensis strains.
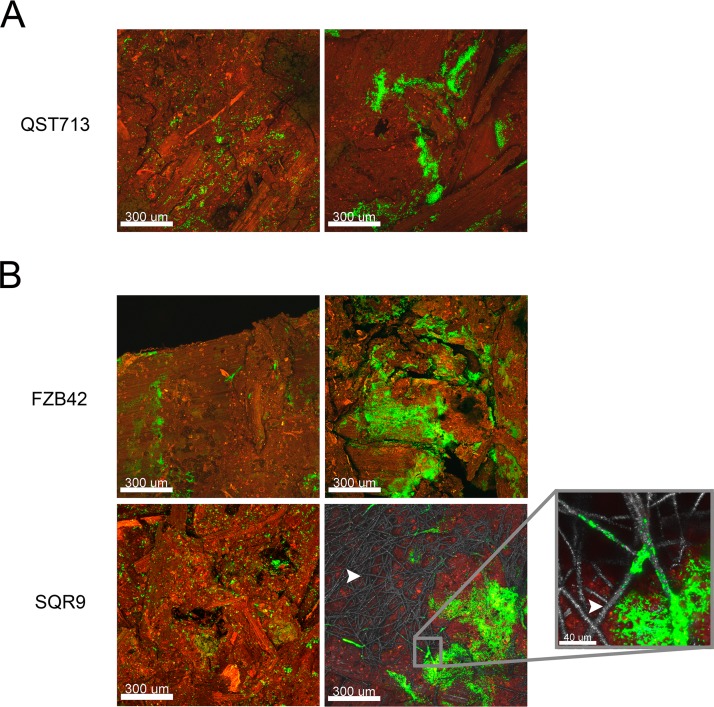
A noninvasive confocal laser scanning microscopy experiment was performed under conditions in which the three partners were present to confirm the effective biofilm formation by strain QST713 in compost micromodels. The plasmid pHAPII (carrying the gene encoding GFP) was electroporated into strain QST713. The transformation of the recalcitrant strain QST713 was complicated and hardly reproducible. Use of the constitutively fluorescent strain QST713/pHAPII allowed visualization of the spatial organization of these bacteria cocultured with A. bisporus and T. aggressivum in the compost at the 6th day of cultivation (Fig. 4A). In the compost, strain QST713 mainly formed microaggregates of cells (Fig. 4A, left) and, sometimes, massive spatially organized biofilms (Fig. 4A, right). We did not observe the systematic colocalization of strain QST713 with A. bisporus mycelium during this experiment.
FIG 4.
Bacillus microcolonies and biofilm on the culture compost of A. bisporus. Confocal laser scanning microscopy observations of B. velezensis QST713 (green) cultivated with A. bisporus and T. aggressivum in the compost (red) (A) and B. velezensis FZB42 (green) or B. velezensis SQR9 (green) cultivated with A. bisporus (gray) and T. aggressivum in the compost (B) are shown. The positive and colocalized spatial interaction between strain SQR9 and the mycelium of A. bisporus is indicated with a white arrowhead.
To determine if other biocontrol strains of B. velezensis form similar biofilm structures in the compost when they are cocultured with T. aggressivum and A. bisporus, two other biocontrol B. velezensis isolates, FZB42-FB01 and SQR9-gfp, were observed in the compost micromodels by CLSM. Similar to strain QST713, both strains showed a heterogeneous colonization of the compost with sparse biofilm structures (Fig. 4B). A specific spatial interaction of strain SQR9 (in green) and the mycelium of A. bisporus (contrasted in gray with reflected light) was observed after 6 days of cocultivation, suggesting an interaction between the two species that could be metabolic cooperation (21).
Antagonism experiments in petri dishes confirmed that the three B. velezensis strains were able to inhibit T. aggressivum (Fig. S2). However, further investigations are needed to determine if the mechanisms of inhibition of T. aggressivum are similar among these three B. velezensis isolates.
DISCUSSION
We previously reported an effective biocontrol effect of B. velezensis QST713 against T. aggressivum during the cultivation of A. bisporus. We showed that this protective effect is significant before the 7th day of cultivation and that the industrial compost is rich in strains assigned to the Bacillus velezensis species (3). We also observed that strain QST713 is capable of forming dense and robust biofilms on inert surfaces and inhibiting the growth of T. aggressivum in vitro (4). In this study, we attempted to explore the mechanisms of bioprotection in a compost micromodel, including both the effective biofilm formation of strain QST713 in the compost and the involvement of antimicrobial compounds. The relative quantification of gene expression related to biofilm formation and antimicrobial production in strain QST713 was assessed by quantitative real-time PCR (qPCR) through the development of a simplified micromodel of A. bisporus cultivation with the addition of the pathogen T. aggressivum. An axenic autoclaved industrial compost was used to remove the native microbiota rich in Bacillus species and to focus specifically on the response of strain QST713 to the presence of T. aggressivum and/or A. bisporus in the compost.
The results showed that genes related to biofilm formation (epsC, tapA, and blsA) were upregulated and the motility gene hag was strongly downregulated in the compost compared to their expression in the exponential growth phase in broth compost extract. The swarming motility gene (swrA) was also active in the compost. The expression profile of strain QST713 in the compost was close to the profile during the stationary growth phase in the broth compost extract, with the exception of that for the hag gene, which was underexpressed, and the tapA gene, which was overexpressed, suggesting effective biofilm formation in the compost. The large variability in gene expression in the compost samples would be related to the heterogeneity of compost colonization observed by CLSM. Moreover, the expression profile of genes encoding secondary metabolites was close to the profile during the stationary growth phase in the broth compost extract, with the exception of that for fenA, which was upregulated, and dhbA, which was downregulated, in the compost, suggesting a higher level of expression of fenA in the compost biofilm mode of life.
We also showed that srfAA and fenA were upregulated in the presence of T. aggressivum, suggesting that surfactin and fengycin would be overproduced in the presence of the pathogen. Previous studies have demonstrated in other Bacillus strains that fengycin synthesis could be regulated by the presence of pathogens (22, 23) and that its toxicity varied depending on the pathogen (23, 24). In addition, surfactin has been shown to be involved in biofilm formation (8), swarming motility and environmental colonization (25, 26), and microbial communication as a signaling molecule that triggers matrix production via the sensor histidine kinase KinC (17, 19) and as an elicitor of plant defense (27) and is also an antifungal compound (28).
In order to evaluate the specificity of the QST713 strain’s behavior, we also studied two biocontrol strains for their ability to colonize the compost and to antagonize T. aggressivum. The strain FZB42 has recently been authorized as a market biocontrol strain for mushroom cultivation in France (French Technical Mushroom Centre, personal communication). However, no public data either on its effect on the yield of A. bisporus or on its biocontrol effect against T. aggressivum are currently available. The strain SQR9 is not currently used in France as a biocontrol agent. Both strains exhibit genes related to biofilm formation and fengycin and surfactin production (4) and can inhibit the growth of T. aggressivum both in vitro and in the compost. The spatial interaction of strain SQR9 and the mycelium of A. bisporus suggests a potential specific interaction between these organisms. Similar observations have been reported for strain SQR9, used to protect maize crops, where the genes implicated in biofilm formation were upregulated in the presence of root exudates (21). This proximity with the mycelium of A. bisporus could also form a protective barrier against the different molecules emitted by T. aggressivum, such as the antifungal compound 3,4-dihydro-8-hydroxy-3-methylisocoumarin (29, 30), as well as proteinase, endochitinase, and β-glucanase, which are cell wall-degrading enzymes that could be involved in T. aggressivum mycoparasitism (31, 32).
Finally, the use of the biocontrol products formulated in the biofilm form with microencapsulated biocontrol agents would permit their introduction in agrosystems. Furthermore, a recent study demonstrated a better persistence of the biocontrol agents at the entrance to the system, as well as a better biocontrol effect on pathogens (33). Hence, additional investigations are needed to formally assign the role of biofilm formation and surfactin and fengycin production in the biocontrol mechanisms against T. aggressivum that occur during the cultivation of A. bisporus.
MATERIALS AND METHODS
Strains and culture conditions.
The strains and plasmid used in this study are described in Table 1. The Bacillus strains used were Bacillus velezensis QST713 (4), Bacillus velezensis SQR9-gfp (34), and Bacillus velezensis FZB42-FB01. Escherichia coli GM48 was used as the host strain for the plasmid pHAPII. Bacillus strains were cultured in 10 ml of Trypticase soy broth (TSB; 1% tryptone, 0.5% yeast extract, 0.5% NaCl; bioMérieux, France) under aerobic conditions (200 rpm) for 24 h at 25°C in an incubator shaker (Infors HT Minitron, Basel, Switzerland). Antibiotics were added as required at the following concentrations: 25 µg ml−1 kanamycin (Kan) and 1 µg ml−1 erythromycin (Em). E. coli GM48 cells were grown in 10 ml of LB medium for 24 h at 37°C under agitation at 200 rpm. When necessary, 25 µg ml−1 of kanamycin was added. Trichoderma aggressivum f. europaeum Ta2 strain Z (1, 2) and Agaricus bisporus (Amycel Delta, white hybrid variety; Amycel, Vendôme, France) were cultured on yeast malt extract agar medium (YMEA; 2 g liter−1 yeast extract, 20 g liter−1 malt extract, 15 g liter−1 agar) supplemented with a mixture of citric acid (250 μg ml−1), streptomycin (100 μg ml−1), and tetracycline (50 μg ml−1) for 15 days at 25°C. All medium components were obtained from Sigma-Aldrich, Saint-Quentin Fallavier, France. Cubes of A. bisporus YMEA cultures were used to produce an initial culture of A. bisporus in autoclaved compost that was incubated in a climatic chamber for 15 days (25°C; air relative humidity, 90%).
TABLE 1.
Strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Description or oligonucleotide sequence (5′–3′) | Source or reference |
|---|---|---|
| Strains | ||
| Escherichia coli GM48 | F− thr-1 araC14 leuB6(Am) fhuA31 lacY1 tsx-78 glnX44(AS) galK2(Oc) galT22 λ− dcm-6 dam-3 thiE1 | Lab strain |
| Bacillus velezensis | ||
| QST713 | Wild type | Serenade (Bayer) |
| QST713-gfp | Carrying plasmid pHAPII, gfp+ Kmr | This study |
| SQR9-gfp | Carrying plasmid pHAPII, gfp+ Kmr | Cao et al. (34) |
| FZB42-FB01 | amyE::(Eryr gfp+) | Fan et al. (40) |
| Trichoderma aggressivum f. europaeum (Ta2) strain Z | Wild type | Mamoun et al. (2) |
| Agaricus bisporus IE-751 (Delta) | Wild type | Amycel |
| Plasmid pHAPII | pUBC19, apha3 containing gfp transcriptionally fused to HAPII promoter, Kanr | Cao et al. (34) |
| Primers for B. velezensis QST713 | ||
| ForblsA | AATTTTTCTCAACTGTCATGGCAAG | This study |
| RevblsA | CGTTGACTGTATCTTTTGTTGTAGC | This study |
| ForbmyA | GAGCTCTGTGACGAATTTTTGAA | This study |
| RevbmyA | CTACTCTTTCATTTGTTTCATCGGA | This study |
| FordhbA | CGTAAATGTGGCAGCTATAGATA | This study |
| RevdhbA | GCAACGTTCGCCAATATTTCAAT | This study |
| ForepsC | TCAGGCTATACGACAGAACAGAT | This study |
| RevepsC | AATCTGCTCCGTATGTACTTCA | This study |
| ForfenA | CAGAAACGGATGTATGTGCTC | This study |
| RevfenA | GCTTCCTCCAGACCAAACG | This study |
| Forhag | ATATCGCGGCTCTTAACACTAG | This study |
| Revhag | ATACGCTGAAGAATGCTGTGA | This study |
| FornrpsB | TTTGGTGGATGTTAGCGGGTGGTA | This study |
| RevnrpsB | TGTAGTTTCTGCTGGACCATAG | This study |
| ForsrfAA | CCTGTCCCGTGTGATCAAAC | This study |
| RevsrfAA | GACTTCCGTATTGCTGACCG | This study |
| ForswrA | GTGGAACAACTAAAAGACAGAACA | This study |
| RevswrA | TCAGATAGTGGTCAACCTCC | This study |
| FortapA | CAAGGTGAGATGGCGGATGA | This study |
| RevtapA | GCTCTTTTCCCTTATTCTCCAG | This study |
| FortnrpsC | ACTGGAGAATACGGTGGGGA | This study |
| RevtnrpsC | CAAGTACAAACATGACATCAAACA | This study |
| ForgyrA | CAGGTGAACAAAGCGAGATTAATT | This study |
| RevgyrA | GTGATCAAGATAATGCTCCAGACA | This study |
Preparation of axenic compost and broth compost extract.
The industrial compost used in this experiment is composed of straw and horse manure supplemented with nitrogen (urea and ammonium sulfate), minerals (gypsum and calcium carbonate), and water (France Champignon; La Tourte, Longué-Jumelles, France). For the preparation of autoclaved axenic compost, 50 g of fresh compost was weighed into individual pots (Microbox full gas; 560 ml; 97- by 80-mm XXL filter lid; Combiness; Dutscher, Brumath, France); autoclaved three times (30 min, 121°C), with the autoclavings spaced by 24 h, at 25°C; and finally, stored at 4°C. For the preparation of broth compost extract, 300 g of fresh compost was boiled in 1 liter of water for 20 min and then centrifuged. The supernatant was collected, supplemented with 5 g of anhydrous glucose (Sigma-Aldrich, Saint-Quentin Fallavier, France), autoclaved for 30 min at 121°C, and then filtered with a Stericup-GP filtration system (pore size, 0.22 μm; Merck-Millipore, Guyancourt, France).
Broth compost extract experiment.
B. velezensis QST713 was cultivated in broth compost extract for 24 h at 25°C and 200 rpm. The optical density at 600 nm (OD600) was measured, serial dilutions were prepared, and the colonies were enumerated to determine the cell concentration (number of CFU milliliter−1) according to the culture age. Samples were collected during the exponential and stationary growth phases to perform RNA extraction. For the broth compost extract samples, 4 ml of the culture was collected in mid-exponential growth phase and 2 ml was collected in late stationary growth phase. Cells were harvested by centrifugation, and 1 ml of LifeGuard solution (Qiagen, Courtaboeuf, France) was added to the pellet. Samples were stored at −80°C (performed in triplicate) until RNA extraction.
Culture compost axenic micromodel experiments.
Cultures in compost were performed and treated with B. velezensis QST713 with or without seeding of A. bisporus and with or without inoculation of T. aggressivum. Hence, four conditions were studied: strain B. velezensis QST713 alone in the compost, strain QST713 with T. aggressivum in the compost, strain QST713 with A. bisporus in the compost, and strain QST713 with both fungi in the compost. For all conditions, individual pots containing 50 g of autoclaved compost were used. At day 0, 5 ml of a B. velezensis QST713 suspension in physiological saline (0.85% NaCl) at 50 CFU ml−1 was added into each pot to reach a final concentration of 2.5 × 102 CFU of strain QST713 per pot. When required, the compost was then seeded at day 0 with 1.6 g of a 15-day-old culture of A. bisporus in autoclaved compost and/or inoculated with 5 ml of T. aggressivum conidiospore solution, made with conidia collected from a 15-day-old YMEA culture, in physiological saline at 200 spores ml−1 to get a final concentration of 1,000 conidiospores per pot. When required, 5 ml of physiological saline was added into the pots to equilibrate the water supply. The compost was then homogenized and compacted at the bottom of the pot. Three replicates per condition were performed, resulting in a total of 12 pots. The pots were then incubated under controlled conditions for 10 days of cultivation in a climatic chamber (25°C; air relative humidity, 90%; Votsh VC 2020). Samples were taken on the 6th day of cultivation. For each compost sample (12 in total), 5 g of compost was collected and crushed with a mortar and pestle in liquid nitrogen for homogenization. Samples were finally stored at −80°C until RNA extraction.
Bacillus enumeration from the compost.
For each pot, compost was collected at days 0, 3, 6, and 10. Enumeration of strain QST713 was performed from 5 g compost in 45 ml sterile physiological saline water (0.85% NaCl) with a sterile filter bag from the Interscience bag system (Interscience, Saint-Nom, France). The mixture was homogenized for 30 min at room temperature and then blended in a commercial laboratory blender according to the manufacturer’s instructions (Seward Laboratory Lab-Blender 400; Seward, Worthing, UK). The filtered liquid was collected and readjusted to 50 ml with sterile physiological saline. Serial dilutions were made from the extracted suspensions in sterile physiological saline (0.85% NaCl), and 0.1 ml of each serial dilution was used to inoculate Trypticase soy agar (TSA; bioMérieux, France) in a 90-mm petri dish. All petri dishes were incubated for 48 h at 25°C.
RNA extraction.
RNA extraction from strain QST713 in the compost was performed with the previously published lysozyme-hexadecyltrimethylammonium bromide (CTAB)-Tri reagent-chloroform method with some modifications (35). Briefly, the CTAB extraction buffer was prepared as described by Wang and Stegemann (36) with some modifications and was composed of 2% CTAB, 2.5% polyvinylpyrrolidone (PVP 40), 2 M sodium chloride (NaCl), 100 mM Tris-HCl (pH 8), 20 mM EDTA, and 2% beta-mercaptoethanol (added just before use) in RNase-free water (Merck-Millipore, Guyancourt, France) to a final volume of 100 ml. All medium components except RNase-free water were obtained from Sigma-Aldrich, Saint-Quentin Fallavier, France. Before the RNA extraction, the broth compost extract culture samples were centrifuged to remove the LifeGuard solution, and 100 mg of each homogenized compost sample was collected. A lysozyme treatment for 30 min at 37°C in 500 µl of lysozyme buffer (30 mM Tris, 10 mM EDTA, 10 mg ml−1 lysozyme [Sigma-Aldrich, Saint-Quentin Fallavier, France], pH 6.2, in RNase-free water) was performed on the 18 samples. After the lysozyme treatment, 500 µl of CTAB buffer supplemented with proteinase K at a 20-mg ml−1 final concentration was added, and the mixture was vortexed for 2 min and incubated in a water bath at 55°C for 10 min. The next steps of the RNA extraction were carried out according to steps 30 to 56 in appendix 5 of the work of Jordon-Thaden et al. (35), followed by DNase treatment in solution with an RNase-free DNase I kit (Norgen Biotec, Proteigene distributor, Saint-Marcel, France) and RNA cleanup kit (Norgen Biotec, Proteigene distributor, Saint-Marcel, France). The RNA concentration was measured with a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Illkirch, France) and a Qubit (version 3.0) fluorometer (Thermo Fisher Scientific, Illkirch, France). The quality and concentration of the RNA were assessed with an Agilent 2100 bioanalyzer (Agilent Technologies, Les Ulis, France) at the @BRIDge platform (http://abridge.inra.fr/). When required, RNA extracts were precipitated with glycogen (molecular biology grade; Thermo Fisher Scientific, Illkirch, France) according to the manufacturer’s protocol to obtain 1 µg of total RNA, required for reverse transcription.
Gene transcription analyses by qPCR.
The reverse transcription of RNA was conducted with an Omniscript reverse transcription kit (Qiagen, Courtaboeuf, France) according to the manufacturer’s protocol with random hexamers (Qiagen, Courtaboeuf, France). Reverse transcripts of the genes involved in biofilm formation (epsC, tapA, blsA), secondary metabolite synthesis (srfAA, fenA, bmyA, dhbA, nrpsB, tnrpsC), and motility (hag, swrA) were quantified by quantitative real-time PCR (qPCR) with Takyon Rox SYBR MasterMix dTTP Blue (Eurogentec, Angers, France) according to the manufacturer’s protocol (10 µl of Takyon master mix, 0.4 µl of each primer [final concentration, 200 nM], 5 µl of cDNA template diluted 20 times, 4.2 µl of water for molecular biology). The qPCR protocol for maximal efficiency was performed: 5 min at 95°C, followed by 40 cycles of denaturation for 15 s at 95°C, annealing for 1 min at 60°C, and elongation for 30 s at 72°C. A final elongation of 5 min at 72°C was performed, followed by a melting curve step. The qPCR efficiency varied from 89% to 100%, depending on the genes to be tested. The gyrA housekeeping gene was used as an internal control (Table 1). The 2−ΔΔCT threshold cycle (CT) method was used to analyze the qPCR data, and results are presented as the log2 fold change (37).
Bacillus velezensis QST713 transformation.
In order to fluorescently contrast strain QST713 in the compost by CLSM, it was transformed with the pHAPII plasmid carrying a gfp gene under the control of a constitutive promoter (34). However, the transformation of strain QST713 is hard to carry out and has not been published yet. We tested several methods (natural transformation, conjugation, electrotransformation, tribos transformation), and electrotransformation was the only successful one; however, it had a very low efficacy. The electrotransformation method used here combines methods described by Zhang et al. (38) and Yi and Kuipers (39), with some modifications. Cells were grown in a hyperosmotic growth medium with glycine to weaken the peptidoglycan. Plasmid pHAPII (carrying the gfp gene) was first extracted from Bacillus velezensis strain SQR9-gfp with a GeneJET plasmid miniprep kit (Thermo Fisher, Illkirch, France) and then transferred to Escherichia coli GM48 (Δdam Δdcm) to obtain an unmethylated pHAPII plasmid. For the transformation of strain QST713, competent cells were prepared according to the following protocol: cells were grown in 50 ml BHIS medium (34 g brain heart infusion [Difco] and 91.1 g sorbitol in 1 liter deionized water, pH 7.2) at 30°C and 200 rpm to an OD600 of 0.85. After 1 h of additional incubation with 1% glycine, the cell culture was transferred into a 50-ml centrifuge tube and cooled on ice for 20 min, and cells were collected (OD600 = 0.95) by centrifugation at 4°C and 8,000 rpm for 5 min with an Eppendorf centrifuge (Eppendorf, Montesson, France). The cells were washed four times in electroporation buffer (prechilled; 10% glycerol, 0.25 M sorbitol, 0.25 M mannitol), and electroporation buffer was added to the pellet at a 1/100 volume of the original culture to obtain electrocompetent cells. Electroporation was performed in 100 µl of electrocompetent cells with 250 ng of the pHAPII plasmid in 2-mm-gap electroporation cuvettes (prechilled) with an Eppendorf Eporator (strength, 2.1 kV cm− 1; Eppendorf, Montesson, France). After a pulse, 1 ml of prewarmed growth medium was added to the electroporation cuvettes and transferred to a 2-ml Eppendorf tube that was then incubated for a recovery time of 5 h at 30°C and 180 rpm. Centrifugation was then performed for 5 min at 8,000 rpm with a Sigma 1k15 centrifuge (Sigma-Aldrich, Saint-Quentin Fallavier, France), and the supernatant was removed. A volume of 100 µl of sterile growth medium was added to resuspend the pellet, and the total resulting volume was plated on LB agar plates (90 mm) containing 25 µg ml−1 of kanamycin. Positive transformants were screened after 48 h of incubation according to their colony fluorescence on a Safe Imager (version 2.0) blue light transilluminator (Thermo Fisher, Illkirch, France).
Confocal laser scanning microscopy of GFP-bacilli in axenic compost.
The condition with Bacillus, Trichoderma, and Agaricus in the compost described above was used to visualize the spatial organization of the biocontrol agent in the compost at day 6 by confocal laser scanning microscopy (CLSM), using a Leica SP8 AOBS inverter microscope (Leica, Microsystems, Germany). The fluorescent bacteria used were Bacillus velezensis QST713 carrying a plasmid encoding GFP (this study) and two relative biocontrol strains: Bacillus velezensis SQR9 carrying a plasmid encoding GFP (34) or Bacillus velezensis FZB42-FB01 tagged with GFP (40). Samples were collected from the compost pots and directly scanned using 10× and 20× HC PL Fluotar dry objective lenses. Samples were scanned at 600 Hz (1,024-by-1,024-pixel images; z step, 4 µm; z range, 480 to 900 µm) with dual excitation wavelengths of 488 nm (argon laser; intensity, 44%) and 561 nm (DPSS 561 laser; intensity, 1.88%). Using ultrasensitive hybrid detectors (HyD Leica Microsystems, Germany), the emitted fluorescences were collected in the range of 497 to 533 nm for the green GFP (bacilli) and in the range of 580 to 740 nm for the red autofluorescence of the compost. Laser reflection was simultaneously used to contrast the mycelium of A. bisporus. Three-dimensional projections from the x-y-z image series were constructed using the Easy 3D function of Imaris software (Bitplane, Switzerland).
Statistical analysis.
The Shapiro-Wilk and Levene tests were applied to determine the normality of the data distribution and the homogeneity of the variances. The data were not normally distributed, and the variances were not homogeneous. Thus, a nonparametric Kruskal-Wallis analysis of variance and the Conover-Iman post hoc test were performed to determine whether the observed differences were significant using XLSTAT-Premium trial software (Microsoft Corporation, USA).
Supplementary Material
ACKNOWLEDGMENTS
C. Pandin was granted a doctoral fellowship by the Ile-de-France Region, DIM ASTREA (project no. ast150075).
We thank the MIMA2 platform (www6.jouy.inra.fr/mima2) for access to the Leica SP8 confocal microscope. We thank G. Condemine for providing the Escherichia coli GM48 strain. We thank J. M. Savoie for providing Trichoderma aggressivum f. europaeum Ta2 strain Z. We thank R. Borriss and R. Zhang for providing the Bacillus velezensis FZB42-FB01 and SQR9-gfp strains, respectively. We thank R. Védie and T. Rousseau for providing the Agaricus bisporus Amycel Delta strain and the industrial compost. Yasmine Dergham is acknowledged for English revision.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00327-19.
REFERENCES
- 1.Largeteau ML, Savoie J-M. 2010. Microbially induced diseases of Agaricus bisporus: biochemical mechanisms and impact on commercial mushroom production. Appl Microbiol Biotechnol 86:63–73. doi: 10.1007/s00253-010-2445-2. [DOI] [PubMed] [Google Scholar]
- 2.Mamoun ML, Savoie JM, Olivier JM. 2000. Interactions between the pathogen Trichoderma harzianum Th2 and Agaricus bisporus in mushroom compost. Mycologia 92:233–240. doi: 10.2307/3761556. [DOI] [Google Scholar]
- 3.Pandin C, Védie R, Rousseau T, Le Coq D, Aymerich S, Briandet R. 2018. Dynamics of compost microbiota during the cultivation of Agaricus bisporus in the presence of Bacillus velezensis QST713 as biocontrol agent against Trichoderma aggressivum. Biol Control 127:39–54. doi: 10.1016/j.biocontrol.2018.08.022. [DOI] [Google Scholar]
- 4.Pandin C, Le Coq D, Deschamps J, Védie R, Rousseau T, Aymerich S, Briandet R. 2018. Complete genome sequence of Bacillus velezensis QST713: a biocontrol agent that protects Agaricus bisporus crops against the green mould disease. J Biotechnol 278:10–19. doi: 10.1016/j.jbiotec.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Khezri M, Ahmadzadeh M, Jouzani GS, Behboudi K, Ahangaran A, Mousivand M, Rahimian H. 2011. Characterization of some biofilm-forming Bacillus subtilis strains and evaluation of their biocontrol potential against Fusarium culmorum. J Plant Pathol 93:373–382. [Google Scholar]
- 6.Xu Z, Zhang R, Wang D, Qiu M, Feng H, Zhang N, Shen Q. 2014. Enhanced control of cucumber wilt disease by Bacillus amyloliquefaciens SQR9 by altering the regulation of its DegU phosphorylation. Appl Environ Microbiol 80:2941–2950. doi: 10.1128/AEM.03943-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeriouh H, de Vicente A, Pérez-García A, Romero D. 2014. Surfactin triggers biofilm formation of Bacillus subtilis in melon phylloplane and contributes to the biocontrol activity. Environ Microbiol 16:2196–2211. doi: 10.1111/1462-2920.12271. [DOI] [PubMed] [Google Scholar]
- 8.Aleti G, Lehner S, Bacher M, Compant S, Nikolic B, Plesko M, Schuhmacher R, Sessitsch A, Brader G. 2016. Surfactin variants mediate species-specific biofilm formation and root colonization in Bacillus. Environ Microbiol 18:2634–2645. doi: 10.1111/1462-2920.13405. [DOI] [PubMed] [Google Scholar]
- 9.Pandin C, Le Coq D, Canette A, Aymerich S, Briandet R. 2017. Should the biofilm mode of life be taken into consideration for microbial biocontrol agents? Microb Biotechnol 10:719–734. doi: 10.1111/1751-7915.12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vlamakis H, Aguilar C, Losick R, Kolter R. 2008. Control of cell fate by the formation of an architecturally complex bacterial community. Genes Dev 22:945–953. doi: 10.1101/gad.1645008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vlamakis H, Chai Y, Beauregard P, Losick R, Kolter R. 2013. Sticking together: building a biofilm the Bacillus subtilis way. Nat Rev Microbiol 11:157–168. doi: 10.1038/nrmicro2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mielich-Süss B, Lopez D. 2015. Molecular mechanisms involved in Bacillus subtilis biofilm formation. Environ Microbiol 17:555–565. doi: 10.1111/1462-2920.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Gestel J, Vlamakis H, Kolter R. 2015. From cell differentiation to cell collectives: Bacillus subtilis uses division of labor to migrate. PLoS Biol 13:e1002141. doi: 10.1371/journal.pbio.1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Branda SS, Chu F, Kearns DB, Losick R, Kolter R. 2006. A major protein component of the Bacillus subtilis biofilm matrix. Mol Microbiol 59:1229–1238. doi: 10.1111/j.1365-2958.2005.05020.x. [DOI] [PubMed] [Google Scholar]
- 15.Romero D, Aguilar C, Losick R, Kolter R. 2010. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc Natl Acad Sci U S A 107:2230–2234. doi: 10.1073/pnas.0910560107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kearns DB, Chu F, Rudner R, Losick R. 2004. Genes governing swarming in Bacillus subtilis and evidence for a phase variation mechanism controlling surface motility. Mol Microbiol 52:357–369. doi: 10.1111/j.1365-2958.2004.03996.x. [DOI] [PubMed] [Google Scholar]
- 17.López D, Vlamakis H, Losick R, Kolter R. 2009. Paracrine signaling in a bacterium. Genes Dev 23:1631–1638. doi: 10.1101/gad.1813709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verhamme DT, Kiley TB, Stanley-Wall NR. 2007. DegU co-ordinates multicellular behaviour exhibited by Bacillus subtilis. Mol Microbiol 65:554–568. doi: 10.1111/j.1365-2958.2007.05810.x. [DOI] [PubMed] [Google Scholar]
- 19.López D, Kolter R. 2010. Extracellular signals that define distinct and coexisting cell fates in Bacillus subtilis. FEMS Microbiol Rev 34:134–149. doi: 10.1111/j.1574-6976.2009.00199.x. [DOI] [PubMed] [Google Scholar]
- 20.Kröber M, Verwaaijen B, Wibberg D, Winkler A, Pühler A, Schlüter A. 2016. Comparative transcriptome analysis of the biocontrol strain Bacillus amyloliquefaciens FZB42 as response to biofilm formation analyzed by RNA sequencing. J Biotechnol 231:212–223. doi: 10.1016/j.jbiotec.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Zhang N, Yang D, Wang D, Miao Y, Shao J, Zhou X, Xu Z, Li Q, Feng H, Li S, Shen Q, Zhang R. 2015. Whole transcriptomic analysis of the plant-beneficial rhizobacterium Bacillus amyloliquefaciens SQR9 during enhanced biofilm formation regulated by maize root exudates. BMC Genomics 16:685. doi: 10.1186/s12864-015-1825-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chowdhury SP, Hartmann A, Gao XW, Borriss R. 2015. Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42—a review. Front Microbiol 6:780. doi: 10.3389/fmicb.2015.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cawoy H, Debois D, Franzil L, De Pauw E, Thonart P, Ongena M. 2015. Lipopeptides as main ingredients for inhibition of fungal phytopathogens by Bacillus subtilis/amyloliquefaciens. Microb Biotechnol 8:281–295. doi: 10.1111/1751-7915.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo Q, Dong W, Li S, Lu X, Wang P, Zhang X, Wang Y, Ma P. 2014. Fengycin produced by Bacillus subtilis NCD-2 plays a major role in biocontrol of cotton seedling damping-off disease. Microbiol Res 169:533–540. doi: 10.1016/j.micres.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Ghelardi E, Salvetti S, Ceragioli M, Gueye SA, Celandroni F, Senesi S. 2012. Contribution of surfactin and SwrA to flagellin expression, swimming, and surface motility in Bacillus subtilis. Appl Environ Microbiol 78:6540–6544. doi: 10.1128/AEM.01341-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bais HP, Fall R, Vivanco JM. 2004. Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol 134:307–319. doi: 10.1104/pp.103.028712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ongena M, Jacques P. 2008. Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16:115–125. doi: 10.1016/j.tim.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Hagberg I, Novitsky L, Hadj-Moussa H, Avis TJ. 2014. Interaction of antimicrobial cyclic lipopeptides from Bacillus subtilis influences their effect on spore germination and membrane permeability in fungal plant pathogens. Fungal Biol 118:855–861. doi: 10.1016/j.funbio.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Dong YH, Wang LH, Xu JL, Zhang HB, Zhang XF, Zhang LH. 2001. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature 411:813–817. doi: 10.1038/35081101. [DOI] [PubMed] [Google Scholar]
- 30.Krupke OA, Castle AJ, Rinker DL. 2003. The North American mushroom competitor, Trichoderma aggressivum f. aggressivum, produces antifungal compounds in mushroom compost that inhibit mycelial growth of the commercial mushroom Agaricus bisporus. Mycol Res 107(Pt 12):1467–1475. doi: 10.1017/S0953756203008621. [DOI] [PubMed] [Google Scholar]
- 31.Guthrie JL, Castle AJ. 2006. Chitinase production during interaction of Trichoderma aggressivum and Agaricus bisporus. Can J Microbiol 52:961–967. doi: 10.1139/w06-054. [DOI] [PubMed] [Google Scholar]
- 32.Abubaker KS, Sjaarda C, Castle AJ. 2013. Regulation of three genes encoding cell-wall-degrading enzymes of Trichoderma aggressivum during interaction with Agaricus bisporus. Can J Microbiol 59:417–424. doi: 10.1139/cjm-2013-0173. [DOI] [PubMed] [Google Scholar]
- 33.Ma X, Wang X, Cheng J, Nie X, Yu X, Zhao Y, Wang W. 2015. Microencapsulation of Bacillus subtilis B99-2 and its biocontrol efficiency against Rhizoctonia solani in tomato. Biol Control 90:34–41. doi: 10.1016/j.biocontrol.2015.05.013. [DOI] [Google Scholar]
- 34.Cao Y, Zhang Z, Ling N, Yuan Y, Zheng X, Shen B, Shen Q. 2011. Bacillus subtilis SQR 9 can control Fusarium wilt in cucumber by colonizing plant roots. Biol Fertil Soils 47:495–506. doi: 10.1007/s00374-011-0556-2. [DOI] [Google Scholar]
- 35.Jordon-Thaden IE, Chanderbali AS, Gitzendanner MA, Soltis DE. 2015. Modified CTAB and TRIzol protocols improve RNA extraction from chemically complex embryophyta. Appl Plant Sci 3:1400105. doi: 10.3732/apps.1400105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Stegemann JP. 2010. Extraction of high quality RNA from polysaccharide matrices using cetlytrimethylammonium bromide. Biomaterials 31:1612–1618. doi: 10.1016/j.biomaterials.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 38.Zhang GQ, Bao P, Zhang Y, Deng AH, Chen N, Wen TY. 2011. Enhancing electro-transformation competency of recalcitrant Bacillus amyloliquefaciens by combining cell-wall weakening and cell-membrane fluidity disturbing. Anal Biochem 409:130–137. doi: 10.1016/j.ab.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 39.Yi Y, Kuipers OP. 2017. Development of an efficient electroporation method for rhizobacterial Bacillus mycoides strains. J Microbiol Methods 133:82–86. doi: 10.1016/j.mimet.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 40.Fan B, Chen XH, Budiharjo A, Bleiss W, Vater J, Borriss R. 2011. Efficient colonization of plant roots by the plant growth promoting bacterium Bacillus amyloliquefaciens FZB42, engineered to express green fluorescent protein. J Biotechnol 151:303–311. doi: 10.1016/j.jbiotec.2010.12.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.