Abstract
We previously reported that the cell cycle–related cyclin-dependent kinase 4–retinoblastoma (RB) transcriptional corepressor pathway is essential for stroke-induced cell death both in vitro and in vivo. However, how this signaling pathway induces cell death is unclear. Previously, we found that the cyclin-dependent kinase 4 pathway activates the pro-apoptotic transcriptional co-regulator Cited2 in vitro after DNA damage. In the present study, we report that Cited2 protein expression is also dramatically increased following stroke/ischemic insult. Critically, utilizing conditional knockout mice, we show that Cited2 is required for neuronal cell death, both in culture and in mice after ischemic insult. Importantly, determining the mechanism by which Cited2 levels are regulated, we found that E2F transcription factor (E2F) family members participate in Cited2 regulation. First, E2F1 expression induced Cited2 transcription, and E2F1 deficiency reduced Cited2 expression. Moreover, determining the potential E2F-binding regions on the Cited2 gene regulatory sequence by ChIP analysis, we provide evidence that E2F1/4 proteins bind to this DNA region. A luciferase reporter assay to probe the functional outcomes of this interaction revealed that E2F1 activates and E2F4 inhibits Cited2 transcription. Moreover, we identified the functional binding motif for E2F1 in the Cited2 gene promoter by demonstrating that mutation of this site dramatically reduces E2F1-mediated Cited2 transcription. Finally, E2F1 and E2F4 regulated Cited2 expression in neurons after stroke-related insults. Taken together, these results indicate that the E2F–Cited2 regulatory pathway is critically involved in stroke injury.
Keywords: stroke, ischemia, neuron, hypoxia, E2F transcription factor, brain injury, Cbp/P300-interacting transactivator with Glu/Asp–rich carboxyl-terminal domain 2, cell death, gene promoter, gene regulation
Introduction
Cited2 (cAMP-responsive element-binding protein/p300-interacting transactivator with glutamic acid– and aspartic acid–rich tail 2) is an ubiquitously expressed transcriptional co-activator (1, 2) with essential roles in development such as establishing the left–right body axis (3), heart, gonad (4), lung (5), liver (6), and central nervous system (CNS),3 as well as in cellular transformation and sugar metabolism (5, 7–21). Its importance is highlighted by the observation that Cited2 deficiency in mice is embryonically lethal (7). Although the roles of Cited2 in heart development and cellular transformation have been extensively studied, very little is known about its cellular functions in the CNS. Cited2 is required at least for neurulation, neural tube development (7, 21, 22), and neocortical development (23).
We and others have previously shown that cell cycle–related proteins, i.e. cyclin-dependent kinase 4 (CDK4), E2Fs, and Rb (retinoblastoma protein), are activated in neurons after stroke-related insult and that this signal is important in delayed neuronal death (24–27). In this context, CDK4-mediated phosphorylation of Rb leads to activation of E2F transcription factors, which in turn activate pro-apoptotic genes (28). However, the specific role of each E2F family member is not clear, and reported evidence suggests that distinct E2Fs can differentially associate to Rb family members (Rb, p130, and p107) to activate or repress transcription (29, 30). For example, it appears that E2F1–3 act as transcriptional activators and interact with Rb, whereas E2F4 and E2F5 preferentially bind to p130/p107 to mediate repression of transcription (31). How repression is regulated is not completely clear, although a novel E2F4-containing repressive complex (DREAM complex) has been recently described (32, 33). Whether this complex is relevant in neuronal injury is unclear. Importantly, previous work demonstrates that E2F1 is induced (34) and required for neuronal death after focal ischemia in vivo (35), whereas E2F4 expression appears to be protective after global ischemia in vivo (29). Taken together, these observations point to the importance of CDK4–E2F in neuronal injury following stroke.
In our previously published work (36), we reported that in cultured cortical neurons exposed to DNA damage, Cited2 is a pro-apoptotic signal downstream of the activation of CDK4. In this specific context, Cited2 is up-regulated, leading to its association/activation of peroxisome proliferator-activated receptor γ and the canonical mitochondrial apoptotic pathway. Given the importance of CDK4-induced death in stroke and that Cited2 can potentially act downstream of CDK4, we investigated whether Cited2 may be important in stroke. We also determined whether Cited2 regulation may be mediated by E2F family members. We hypothesized reciprocal effects of E2F1 and E2F4 on Cited2 expression in the context of neuronal stroke-related injury.
Results
Cited2 mediates delayed neuronal death induced by hypoxia/reoxygenation in vitro
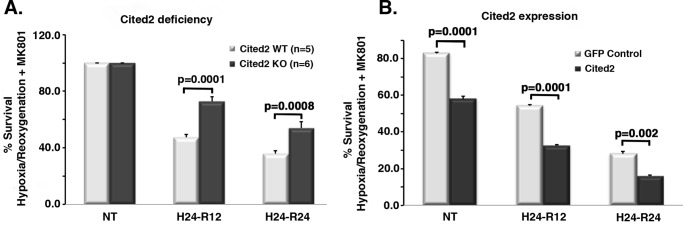
To query the effects of Cited2 on stroke relevant neuronal injury, we first determined the effects of Cited2 deficiency/expression on neuronal survival after hypoxia/reoxygenation in vitro. Our previously published work (28, 29), using in vitro and in vivo studies, demonstrated that the CDK4/E2F/Rb neuronal pro-death pathway is predominantly relevant in the penumbra of the stroke insult, mediating slow neuronal death, versus the most rapid excitotoxic death. Because we previously demonstrated (36) that Cited2 is a downstream target of CDK4 in vitro, we focused our studies on a delayed death model utilizing primary cortical neurons treated with MK801 (a glutamate antagonist) to block excitotoxicity as previously published (28, 29). As shown in Fig. 1A, Cited2 deficiency significantly protects cultured cortical neurons in our delayed model of ischemic death. Conversely, we also expressed Cited2 via adeno-associated virus (AAV) infection of WT cultured neurons. Cited2 ectopic expression alone was sufficient to induce neuronal death when compared with GFP infected controls, and its presence increased death induced by the insult (Fig. 1B). These data indicate the pro-death role of Cited2 in a delayed model of hypoxia/reoxygenation–induced neuronal death, at least in vitro.
Figure 1.
Cited2 deficiency protects against death and Cited2 ectopic expression induces death after hypoxia/reoxygenation in cortical neurons. A, cortical neurons from Cited2 WT and KO littermates (specified n) were exposed to hypoxia/reoxygenation. Neuronal survival was estimated by assessment of nuclei morphology under the microscope. B, cortical neurons were infected by AAV-Cited2: GFP or GFP only–expressing particles and 10 days after that subjected to hypoxia/reoxygenation. Survival was estimated by assessment of nuclei morphology of GFP-positive cells after fixation. Three independent experiments were performed. All stats were done by two-way ANOVA, followed by Tukey's post hoc test. NT, nontreated; H24, 24-h hypoxia; R12 and R24, 12 and 24 h reoxygenation.
Cited2 expression after genotoxic or hypoxic insults correlates with changes in E2F1 and E2F4 protein levels in vitro
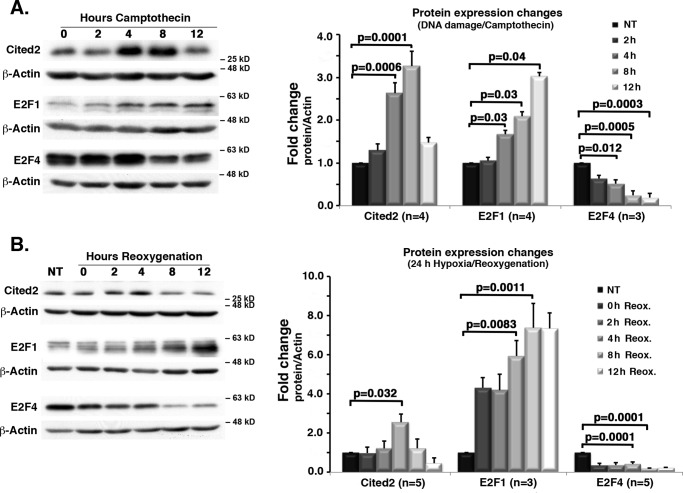
We next determined levels of Cited2 following ischemic insult in vitro. Our previous evidence indicated that Cited2 levels induced by DNA damage are regulated by CDK4 activation, which in turn is known to control E2F function (34, 36–38). Accordingly, we first tested whether E2F family members (in particular E2F1/4) may correlate with any observed changes in Cited2 levels. Also, because the status of Cited2 in more physiological relevant models of ischemia is unknown, we also examined for Cited2 and E2F1/4 levels under conditions of hypoxia/reoxygenation. Because our previous Cited2 data had been obtained using a neuronal DNA damage model of delayed death (36), we decided to first use the same model to explore the correlations between Cited2 and E2F1/4 levels. As shown previously, Cited2 level was dramatically induced upon DNA damage following camptothecin treatment. This increase was accompanied by an increase in E2F1 level and a concomitant decrease in E2F4 level (Fig. 2A). Similar findings were also observed in our models of hypoxia, i.e. induction of Cited2 accompanied by an increase in E2F1 and a decrease in E2F4 levels (Fig. 2B).
Figure 2.
After genotoxic and hypoxic insults, Cited2 and E2F1 levels are up-regulated, whereas E2F4 levels decrease. C57Bl/6 primary cortical neurons were submitted to DNA damage (A) or hypoxia/reoxygenation (B). Protein expression profiles for Cited2, E2F1, and E2F4 were assessed for both models. Representative Western blotting images are shown to the left, and quantification of signals was performed by ImageJ densitometry. The graphs to the right represent average protein changes using specified n independent experiments. Campto, 10 μm camptothecin treatment. The data were analyzed using ANOVA followed by Tukey's test. NT, nontreated; Reox., reoxygenation.
Cited2 neuronal deficiency leads to protection against stroke-induced degeneration in vivo
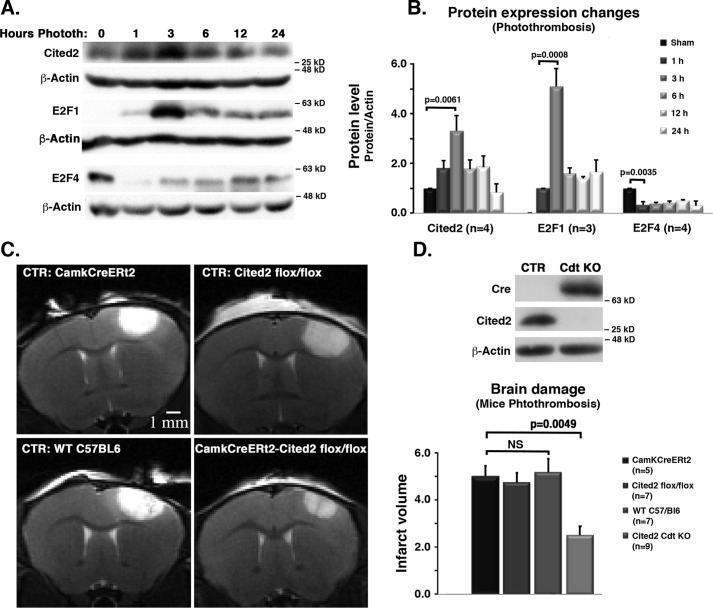
The above evidence suggests but does not demonstrate involvement of an E2F-Cited2 pathway in ischemic death. Accordingly, we next determined whether this pathway is functionally required for damage in an in vivo stroke context utilizing a photothrombosis-induced stroke model in mice. We assessed WT C57Bl/6 mice for changes in protein levels of Cited2, E2F1, and E2F4 in the brain cortex at 1, 3, 6, 12, and 24 h after stroke by Western blotting analyses (Fig. 3, A and B). Cited2 levels significantly increased, peaking at 3 h after stroke insult. Similar to what was observed in vitro, this increase in Cited2 levels correlates with a dramatic elevation in E2F1 expression and reduction of E2F4 levels.
Figure 3.
Comparison of effects of photothrombosis in WT versus Cited2 conditional KO mice. A, mice were subjected to photothrombosis, and tissue samples of the insult brain cortex were processed to assess protein level changes in Cited2, E2F1, and E2F4. Representative images of Western blots are shown. B, quantification by densitometry. The data were analyzed by ANOVA/Tukey's test, and only some of the comparisons are shown. C, transgenic mice carrying a Cited2flox allele together with (conditional KO) or without (CTR) CampKERt2-Cre were treated with tamoxifen to induce transgene expression (Cre activation) followed by photothrombosis and MRI analysis 24 h after insult. Representative images are shown. D, top panel, Western blotting analysis for confirmation of Cited2 deficiency (Cre expression) 24 h after tamoxifen treatment. Bottom panel, quantification of infarct volume 24 h after photothrombosis as assessed by ImageJ densitometry. The data represent the averages of specified n and was analyzed using two-way ANOVA followed by Tukey's post hoc test. Phototh, photothrombosis; CTR, control; Camk, camodulin kinase promoter; Cre, Cre recombinase; ERt2, estrogen receptor; Cdt, conditional; NS, not statistically significant.
We next examined whether Cited2 deficiency in vivo impacts survival. Because germ-line Cited2 deficiency is embryonic lethal, we employed a conditionally inducible transgenic mouse line where experimental animals were homozygous for the Cited2flox allele with (conditional KO) or without (control) a transgenic tamoxifen-responsive CampK-ERt2 gene. C57Bl/6 (WT) and CampK-ERt2-only animals were also used as controls. All mice were treated with tamoxifen as indicated (50 mg/ml, 100 μl/mouse by oral gavage). Western blotting analyses demonstrated that 24 h after the last tamoxifen administration loss of Cited2 protein expression occurred only in animals which expressed Cre, as shown in Fig. 3D (top panel). The mice were then exposed to stroke insult as indicated under “Experimental procedures,” and infarct volume was assessed 24 h later in control (CreNegative/Cited2flox/flox, CrePositive and WT, + tamoxifen) and Cited2-deficient (CrePositive/Cited2flox/flox, + tamoxifen) mice by MRI. Fig. 3C shows representative live MRI images. As quantified in Fig. 3D (bottom panel), neuronal Cited2 loss led to significantly smaller infarct volume when compared with control mice. This is consistent with our hypothesis that Cited2 is a key regulator of neuronal injury after stroke insult.
E2F1 and E2F4 directly regulate Cited2 expression in neurons
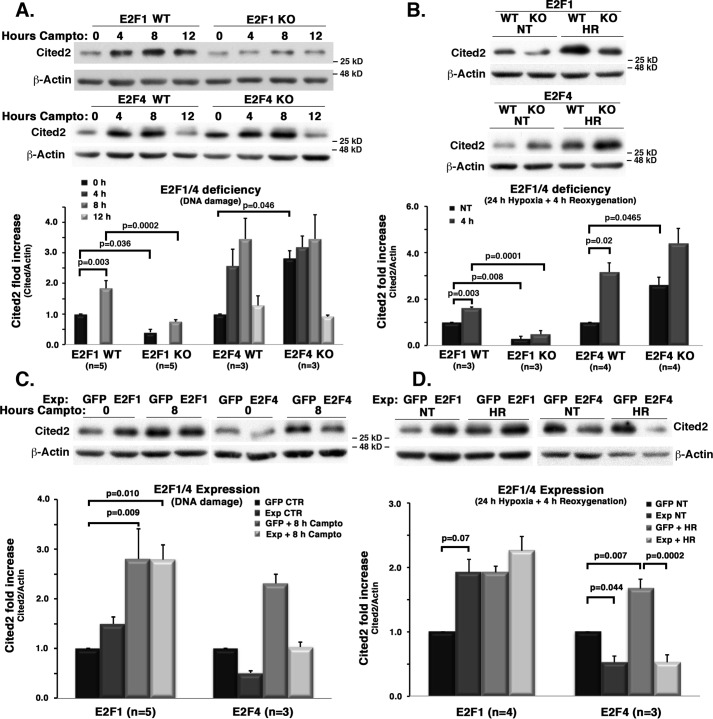
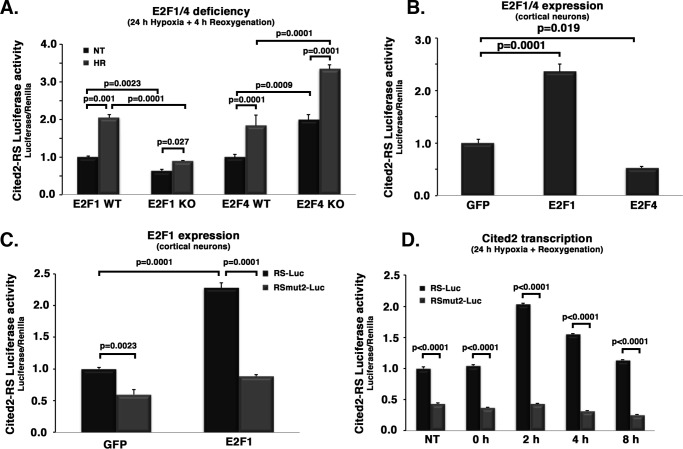
The mechanistic link between E2F changes and levels of Cited2 cannot be inferred from the above experiments. Therefore, we next determined the effects of manipulation of E2F1 or E2F4 on Cited2 levels (Fig. 4). Cited2 protein level, both basally and after DNA damage, was significantly reduced with E2F1 loss (Fig. 4A). Similar results were also observed with E2F1 loss upon hypoxia/reoxygenation (Fig. 4B). In contrast, E2F4 deficiency led to an increase in Cited2 levels basally and upon DNA damage or hypoxia/reoxygenation (Fig. 4, A and B). Conversely, we observed the opposite effects with ectopic E2F1/4 expression (Fig. 4, C and D). E2F1 expression led to an increase in basal levels of Cited2, whereas the up-regulation of Cited2 after DNA damage and hypoxia was similar with or without E2F1 expression (Fig. 4, C and D). This latter observation suggests a saturated nature of the E2F1-mediated Cited2 induction with stress. In contrast, E2F4 expression led to a strong inhibition of Cited2 basally, and also following exposure to DNA damage or hypoxia/reoxygenation (Fig. 4, C and D). These data are consistent with the notion that E2F1 and E2F4 regulate Cited2 levels in an opposite fashion, by which E2F1 increases and E2F4 inhibits Cited2 protein levels.
Figure 4.
E2F1 deficiency leads to inhibition and E2F1 expression leads to induction of Cited2 expression after insult. E2F4 deficiency and expression have the opposite effects. Cortical neurons from WT and KO E2F1 or E2F4 embryos were subjected to DNA damage (A) or hypoxia/reoxygenation (B). Alternatively, WT embryonic cortical neurons were infected with either GFP-expressing control vectors or E2F1 or E2F4-expressing vector to drive their ectopic expression, followed by DNA damage (C) or hypoxia/reoxygenation (D). At specified time points, Cited2 protein levels were assessed by Western blotting and ImageJ densitometry. The top panels show representative images. Campto, 10 μm camptothecin treatment. The data from specified n cultures were averaged and analyzed by two-way ANOVA and Tukey's post hoc test, as shown in bottom panels. HR, hypoxia and reoxygenation; NT, nontreated; Exp, expression.
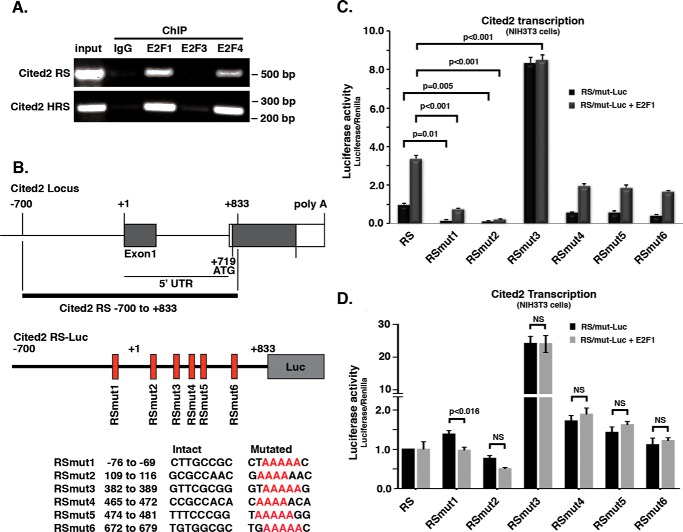
To determine whether E2F transcription factors directly bind the genomic regulatory sequences of Cited2, we first searched for potential binding sites using the ECR browser software (http://ecrbrowser.dcode.org/) (62).4 This would enable us to mutate any specific E2F sites for better assessing their regulatory function on Cited2 expression. To do this, we screened an 8.2-kb genomic region containing most of the intragene, exon 1, and intron 1–2 of the mouse genome. Using the previously reported sequence, BKTSSCGS (39), as the consensus motif to pinpoint potential E2F-binding sites, we identified six sites in the mouse Cited2 genomic region (Fig. 5). We then performed ChIP assay using antibodies to E2F1/3/4, followed by amplification using PCR for the Cited2 genomic regulatory sequence containing mostly intron1–2 (see “Experimental procedures” for details). As shown in Fig. 5A, we looked basally at both murine (primary cortical neurons) and human (HEK293T) cells and found that E2F1 and E2F4, but not E2F3, were able to bind the Cited2 genomic regulating sequences.
Figure 5.
Mutations of mouse Cited2 genomic regulatory sequence modulate E2F1 effects over Cited2 transcription. A, E2F1 and E2F4, but not E2F3, interact with both mouse and human Cited2 genomic regulatory sequences (RS). Representative images of ChIP assay to assess basal interaction of E2Fs is shown. Top panel, using mouse Cited2 RS (C57Bl/6 primary cortical neurons); bottom panel, using human Cited2 RS (HEK 293T). B, top panel, features of the Mus musculus Cited2 locus genomic regulatory sequence (−700 to +833) containing six putative E2F binding sites. Bottom panel, mutated sequences (RSmut) generated and used to produce individual Cited2 Luciferase reporter constructs. C, effect of RS mutations over Cited2 transcription, basally and with E2F1 ectopic expression. D, effect of RS mutations over Cited2 transcription, basally and with E2F4 ectopic expression. The data from C and D present the average values from three and four independent experiments, respectively. Two-way ANOVA and Tukey's post hoc test were used for statistical analysis. Mut, mutant; Luc, luciferase; NS, not statistically significant.
To further confirm the functional nature of this interaction, we generated poly(A) mutations for each of the potential sites individually and then generated luciferase reporter constructs using the original and the mutated versions of the Cited2 regulating sequences (Fig. 5B). Using these constructs, we initially explored the activity of the Cited2-responsive elements under basal conditions and under E2F1 expression in NIH 3T3 cells (Fig. 5C). Our data revealed that mutation of the second potential E2F regulatory site (RS-mut2, residues 109–116) most efficiently eliminated not only the basal expression activity but also the induction of luciferase reporter activity by E2F1 expression. Interestingly, mutation of the third site (RS-mut3) led to a significant increase in Cited2 expression activity, suggesting an inhibitory function at this site. E2F4 expression in NIH 3T3 cells had no significant effect on basal RS reporter activity or on RS-mut2–6 and only a modest repressive effect on RS-mut1. This suggests that at least in mouse fibroblasts, either E2F4 does not a play a major role in Cited2 promoter activity or the basal levels of E2F4 in mouse fibroblast may be sufficient to mediate any E2F4 regulatory effects. Of note, this contrasts the effects of E2F4 observed in cortical neurons (see below).
Both mouse and human Cited2 expression are activated by E2F1 and inhibited by E2F4
Based on these results, we focused on the role of the mut2 regulatory site. We transduced cortical neurons with RS-Luc or the RSmut2-Luc–expressing AAV to test whether levels of E2F1 and E2F4 have any effect on the Cited2 expression profile after ischemic insults. As anticipated, E2F1 deficiency (Fig. 6A) led to a significant decrease in the activity of the WT regulatory sequence (RS-Luc) with or without hypoxia/reoxygenation when compared with controls. In contrast, E2F4 deficiency led to a significant increase in luciferase reporter activity when compared with WT control cells (Fig. 6A). Ectopic expression of E2F1/4 also resulted in an opposite effect on luciferase activity observed utilizing the WT Cited2 regulatory sequence (RS-Luc) (Fig. 6B); that is, E2F1 expression increased, whereas E2F4 decreased luciferase activity. Critically, the effect of E2F1 expression on reporter activity observed with WT Cited2 reporter is abolished in the RS-mut2 reporter construct (RSmut2-Luc; Fig. 6C). These observations support that the second E2F site (murine positions 109–116; Fig. 5B) is functionally relevant for E2F1 regulation over Cited2 expression. Finally, we confirmed that this site is required for the induction of Cited2 reporter activity upon stress response to hypoxic insult. As shown in Fig. 6D, the RS-mut2 sequence displayed significantly less activity basally when compared with WT reporter control; more importantly, induction of Cited2 transcription after insult is abolished for the RS-mut2 sequence (Fig. 6D).
Figure 6.
Mouse Cited2 transcription responds to changes in E2F1 and E2F4 levels. RSmut2 abrogates E2F1-mediated Cited2 up-regulation. Primary cortical neurons were co-transduced with Renilla luciferase as control and firefly luciferase under the control of the specified (native, RS-Luc, or mutated RSmut2-Luc) mouse Cited2 genomic regulatory sequence. Cited2 transcription levels were them measured as Luciferase activity under different condition: A, WT or deficient (KO) neurons form E2F1 and E2F4 transgenic embryos (specified n), after hypoxia/reoxygenation. B, WT neurons after ectopic E2F1 or E2F4 expression. C, WT neurons after 24 h of hypoxia/reoxygenation (specified time points), for both RS and RSmut2-regulatory luciferase. D, WT neurons after 24 h of hypoxia/specified reoxygenation time points, for both RS and RSmut2-regulated luciferase. Except for B (one-way ANOVA/Tukey's), all data were analyzed using two-way ANOVA followed by Tukey's post hoc test. RS, regulatory sequence; Luc, luciferase; mut, mutant.
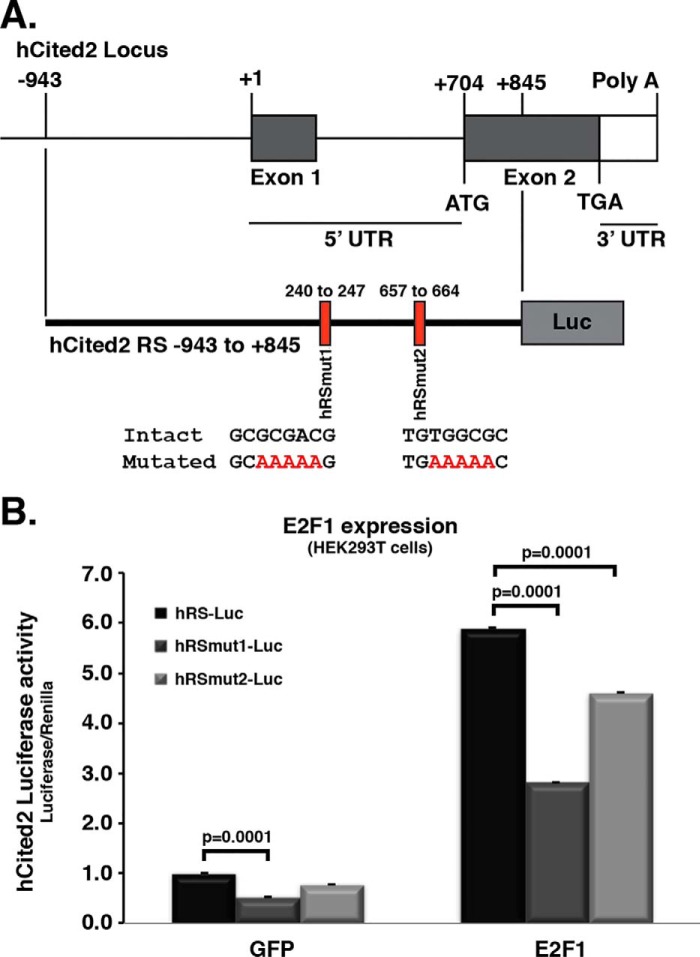
All the above experiments were performed utilizing murine-based genomic sequences. In an effort to test whether the E2Fs/Cited2 regulatory effects are as present in a human context, we first identified two potential E2F sites within the human promoter sequence of Cited2 (Fig. 7A) by methods identical to that used to examine the mouse promoter sites. These sites were then individually mutated as for the murine promoter sequences (hRS-Luc, hRSmut1-Luc, and hRSmut2-Luc). We then assessed their activity basally and in response to E2F1 expression. As shown in Fig. 7B, luciferase activity significantly increased under E2F1 expression when compared with GFP-expressing control. However, this E2F1-induced activity was most significantly decreased when the hRSmut1 site was utilized. These results suggest the importance of select putative E2F1 sites in both murine and human backgrounds.
Figure 7.
Human Cited2 transcription levels also respond to changes in E2F1/4 levels. Mutations of both E2F binding sites significantly decrease the effect of E2F1 levels over Cited2 transcription. A, features of the human Cited2 locus. Two putative E2Fs binding sites are identified, and mutations were introduced to generate two individual reporter constructs (hRSmut1–2). B, HEK293T cells were co-transfected with Renilla luciferase as control and firefly luciferase under the control of the specified (native, hRS-Luc, mutated hRSmut1-Luc, or mutated hRSmut2-Luc) human Cited2 genomic regulatory sequence. Cited2 transcription levels were then measured as luciferase activity after ectopic E2F1 expression. Two-way ANOVA/Tukey's were used to analyze the data. Only some of the Tukey's tests are shown, although all comparisons between GFP and E2F1 groups revealed significant differences. hCited2, human Cited2; RS, regulatory sequence; mut, mutant; Luc, luciferase.
Discussion
The role of cell cycle molecules as pro-apoptotic signals in degenerating neurons has been reported extensively by us and others (27, 28, 40, 41). These signals are required for apoptotic neuronal death induced by insults such as DNA damage (25, 36, 42, 43) and ischemic insult both in vitro and in vivo (28, 44). We and others have reported that during these pathological events, CDK4 is activated very early on by signals like TripBr1 and Cdc25A (45, 46), leading to hyperphosphorylation of Rb and release of E2Fs. Interestingly, we have recently published evidence showing that different E2F family members regulate death in a differentiated manner, i.e. E2F1 induces neuronal death, whereas E2F4 promotes neuronal survival in vivo after global ischemia (29).
Previously, we reported that Cited2 activation is one of the pro-apoptotic signals activated by the CDK4 pathway in vitro after DNA damage (36). However, we did not determine whether E2Fs are the mediators of this regulation or whether Cited2 plays a delayed pro-death role in more physiologically relevant models of injury such as with stroke. The present study explores both questions. Our evidence indicates that Cited2 plays a significant pro-death function in ischemic death both in vitro and in vivo, using a hypoxia/reoxygenation delayed death model in vitro and a photothrombosis model in vivo. This conclusion is further supported by the observation of an increase in Cited2 levels upon ischemic insult in both in vitro and in vivo contexts. In the latter studies, one caveat is that we have not ascertained the specific cell type in which increased Cited2 occurs. This has been difficult to do given the quality of antibodies for immunohistochemistry of endogenous Cited2 levels. However, our results demonstrating increases of Cited2 in isolated neurons and functional effects with neuronal loss of Cited2 support a role of neuronal Cited2 in neuronal delayed death. These studies strongly suggest that the activation/functional involvement of Cited2 in mediating death is not limited to DNA damage but plays a critical role in mediating stroke damage. Our results do not exclude the possibility of Cited2 being induced by any of the pro-death signals involved in excitotoxicity. However, the body of our previous work on the CDK-E2F pathway suggests its importance predominately in delayed death. It should, however, be pointed out that although we did not examine the specific mode of cell death, neuronal loss in our experiments likely represent a spectrum of markers that range from clear and classical apoptosis, with classical markers of apoptosis, to more rapid excitotoxic cell death, with elements that do not reflect programed cell death.
Critically, some reports in the field have suggested a prosurvival role of Cited2. For example, others have reported an up-regulation of Cited2 in areas of brain not affected by stroke (47). However, the bulk of our evidence strongly indicates a pro-death role of Cited2. Most notably, our present data in an in vivo context utilized the conditional model of Cited2 loss specifically in CaMKIIα-positive cells, which demonstrated significant protection in the photothrombosis model of stroke injury. It would be interesting to test in the future whether the difference in infarct volume translates into behavioral differences for these mice. Although these data, along with similar results in vitro, confirm the pro-death role of Cited2 in neurons, it does not rule out more complex functions of Cited2 in other cell types in the CNS in an in vivo context such as with immune cells or astrocytes.
Importantly, our results also provide mechanistic insight into how Cited2 is regulated under conditions of neuronal injury. In this regard, we provide concrete evidence for the importance of E2F-mediated regulation of Cited2 expression. First, we demonstrate that both in vitro (after DNA damage and hypoxia/reoxygenation) and in vivo (photothrombosis induced stroke), Cited2 expression is correlated with a concomitant increase in E2F1 levels. Furthermore, E2F1 deficiency leads to inhibition of Cited2 up-regulation, whereas conversely E2F1 expression alone is sufficient to increase Cited2 expression. We also demonstrate that E2F1 interacts with the Cited2 promoter regulatory sequence in neurons and define the putative functional E2F elements in the Cited2 promoter both in murine and human contexts. With regards to this last point, we demonstrate that E2F1 loss reduces Cited2 promoter activity, whereas its expression increases Cited2 promoter activity. Importantly, we identified functionally relevant E2F sites both in mice and human cell types and showed that mutation of particular sites (RSmut2-murine; RSmut1-human) disrupts the ability of E2F1 to mediate reporter activity. These specific E2F sites were also critical for Cited2 reporter activity observed upon ischemic insult. Taken together, our results define E2F1 as one critical factor in the regulation of Cited2 expression. These results are also consistent with previous reports of the importance of E2F1 in regulation of neuronal injury following stroke (35, 48, 49).
Although E2F1 is likely one mediator of Cited2 expression, the role of E2F members in general is more complex. This is exemplified by (a) our observation that E2F1 levels can remain high, whereas Cited2 levels gradually decrease during hypoxia; and (b) an increasingly defined role of E2F members as part of active repressor complexes (32, 33). Our own present data suggest that some E2F regulatory sites when mutated, at least in a murine context, may actually lead to increase in Cited2 relevant promoter activity. In this regard, E2F4 is one transcriptional family member that likely plays this repressive role in regulating Cited2 expression. First, we have previously shown that unlike E2F1, E2F4 plays a protective role in models of ischemic injury (29). Consistent with this observation, our present data in cortical neurons show that E2F4 affects Cited2 levels in a fashion opposite to that of E2F1. After DNA damage and hypoxia/reoxygenation, its levels decrease at the same time that Cited2 and E2F1 levels increase. This would be consistent with a model whereby E2F4 containing repressive complexes block Cited2 expression, and its loss leads to increased Cited2 levels. Indeed, E2F4 deficiency by itself leads to Cited2 up-regulation. Cited2 levels are further increased by hypoxia/reoxygenation. Finally, E2F4 ectopic expression alone in cortical neurons is sufficient to decrease Cited2 expression and reporter activity both basally and following ischemic insult. The latter observation is further corroborated by our observations that E2F4 also occupies the Cited2 regulatory genomic region basally in neurons, as revealed by ChIP assay. It is important to note, however, that E2F4 regulation of Cited2 promoter activity is not universal in all contexts. Interestingly, E2F4 expression did not appear to significantly affect promoter activity in mouse fibroblasts. The reason for this is unknown but may have to do with the complex makeup of E2F members in differing cell types.
Clinically, stroke is generally treated by vessel recanalization using tissue plasminogen activator (tPA) within 4.5 h of stroke onset (50). Although the reperfusion therapy can decrease infarct size by restoring blood flow to the ischemic region of brain, within minutes of a stroke event, the core of brain tissue exposed to the most dramatic blood flow reduction is fatally injured and subsequently undergoes neuronal cell death. Importantly, our animal stroke model unveiled that Cited2 was dramatically increased after stroke and peaked at 3 h. The increase of Cited2 is within the therapeutic window for vessel recanalization by tPA, indicating that combination of tPA and CDK4 pathway inhibitors for ischemia stroke therapy may ameliorate brain damage more efficiently. Therefore, Cited2 as a downstream effector in CDK4 pathway might be used as a novel potential therapeutic target for drug development.
In summary, our present results define a critical downstream effector pathway of the E2F family of transcriptional factors which regulate the pro-death function of Cited2 in neurons following ischemic stress. Combined with our previous observations demonstrating the importance of the cell cycle–related CDK4-Rb/E2F signal in ischemic death, we provide a plausible model by which E2F mediation regulates the levels of Cited2. It also suggests that the Cited2 is a promising therapeutic target for rescuing delayed neuronal death, by which there would be benefits for a large number of ischemic stroke patients.
Experimental procedures
CamkCreERT2-Cited2flox mice
Cited2flox transgenic mice (51) were crossed with CamkCreERT2 transgenic mice (52), which results in deletion of the floxed Cited2 allele in adult postmitotic cortical neurons by administration of tamoxifen. For maintenance purposes, the mice were kept on their C57Bl/6 background and bred so that they would all be homozygous for the floxed Cited2 allele but either WT (no allele) or heterozygous for the CamkCreERT2 allele.
Tamoxifen administration
Tamoxifen stock (50 mg/ml in 10% ethanol and 90% corn oil) was prepared by mixing tamoxifen (Sigma) in corn oil at 37 °C (protected from light) until completely dissolved. The solution was then administered to mice daily (100 μl/mouse) for 5 consecutive days by oral gavage.
Animal preparation and induction of cortical ischemia
All experiments conformed to the guidelines set forth by the Canadian Council for the Use and Care of Animals in Research and the Canadian Institutes for Health Research. All studies were performed using C57Bl/6, wildtype, or transgenic, 8–10-week-old males. 24 h after the last tamoxifen administration, the animals were anesthetized with 5% isoflurane, maintained under 2.5% of a mixture of air and oxygen (1:1), and then mounted on the stereotaxic apparatus. Body temperature was maintained using a heating pad. Ischemia was induced on the cortex (right hemisphere) using the photothrombotic stroke model previously described (53). Briefly, an incision was made over the skull, the skin flaps were retracted, and the periosteum was removed to allow proper exposure of the skull. 10 μl/g of Rose Bengal dye (10 mg/ml) was injected intraperitoneally 5 min prior to laser illumination. The laser source was placed 3 cm above the skull (Cobolt JiveTM, Solna, Sweden). The skull and underlying tissue were illuminated for 5 min with a laser beam (power, 50 milliwatt; wavelength, 561 nm) at the coordinates relative to bregma: +2.7 mm lateral and +1 mm anterior. The skin was then sutured, and the animals were kept in a heated recovery box until awake and then returned to their home cage. The animals were then either sacrificed for protein analysis (1, 3, 6, 12, and 24 h after insult) or submitted to MRI 24 h after insult.
MRI
In vivo mouse brain MRI was performed at the University of Ottawa preclinical imaging core using a 7-Tesla GE/Agilent MR 901. The mice were anesthetized for the MRI procedure using isoflurane. Changes in respiratory rate were monitored using a small animal monitoring and gating system (Small Animal Instruments, Inc., Stony Brook, NY) to adjust the anesthetic concentration. A 2D fast spin echo sequence pulse was used for the imaging, with the following parameters: 15 prescribed slices; slice thickness, 0.5 mm; spacing, 0 mm; field of view, 2.5 cm; matrix, 256 × 256; echo time, 42 ms; repetition time, 3000 ms; echo train length, 8 echos; bandwidth, 16 kHz; four averages, fat saturation; and imaging time, 6.5 min. Stroke lesions demonstrated marked hyperintensity. Assessment of the lesion size was performed by ImageJ software using the MRI images and the thickness specified above.
Cortical neurons culture
As previously described (36, 54), embryonic cortical neurons were dissected from WT (C57Bl/6 line, Charles River), E2F1 knock-out, E2F4 knock-out, and Cited2 knock-out brains at embryonic days 14 and 15 stages (embryonic days 12.5 and 13.5 for Cited2 embryos). All procedures were performed following the ethical guidelines for investigations of experimental pain in conscious animals as approved by the Institutional Animal Care Committee of the Ottawa University. Briefly, isolated cortices were pooled, trypsinized, dissociated via mechanical trituration, and suspended in plating medium (neurobasal medium with B27, N2, 0.5 mm glutamine, 25 μm glutamic acid, and 0.05 mg/ml penicillin/streptomycin) (all from Invitrogen, San Diego, CA). The cells were counted and then plated on poly-d-lysine–coated 12-well plates at a density of 106 cells/ml. The cultures were maintained in a humidified incubator with 5% CO2 at 37 °C, and after 3 days in vitro (DIV), three-quarters of the plating medium was removed and replaced with maintenance medium (neurobasal medium with B27, N2, 0.5 mm glutamine, and 0.05 mg/ml penicillin/streptomycin). Thereafter, maintenance medium was changed in the same fashion every 3–4 days. Under these conditions, cultures are nearly purely neuronal by day 3 or 4 in vitro (55), a fact that we and other authors (25) attribute to the lack of the nutrient/supplementation needed by dividing cells to survive in culture. For DNA damage experiments, the cells were used after 4 DIV, whereas for hypoxia/reoxygenation experiments, the cells were used after 10 DIV.
DNA damage
As previously described (36), camptothecin, a topoisomerase I inhibitor, was added to cortical neurons at 4 DIV to a final concentration of 10 μm. Nontreated controls were kept alongside treated cells at 37 °C, 5% CO2. At specified times, the cells were processed either for survival or for protein extraction followed by Western blotting.
Hypoxia
For all experiments, immediately before inducing hypoxia and to ensure we model delayed neuronal death, MK801 (SIGMA M108) was added to cultured 10 DIV cortical neurons to a final concentration of 10 μm. Hypoxia was induced by using a humidified environmental chamber (Coy Laboratory Products, Ann Arbor, MI) set at 37 °C, 1% O2, 5% CO2. The cell cultures were subjected to hypoxia for specified times. Control cultures were maintained in a humidified incubator at 37 °C and were not treated with hypoxia.
Cell survival assay
The neuronal cell survival assay was conducted as described previously (36). At indicated time points, the medium was aspirated, and the cells were lysed in 200 μl of lysis buffer (0.1× PBS, pH 7.4, 0.4 mm Na2HPO4, 0.15 mm KH2PO4, 13.5 mm NaCl, 0.25 mm KCl, 0.5% Triton X-100, 2 mm MgCl2, and 0.5 g/100 ml cetyldimethylethylammonium bromide). This solution disrupts cell membranes, whereas the nuclei remain intact and distinguishable under light microscopy. 10 μl of nuclei suspension was loaded onto a hemacytometer, and the number of healthy intact nuclei was assessed per each time point/treatment. Healthy nuclei are defined as nuclei lacking blebbing, disruption of nuclear membrane, or phase bright bodies. These standards in the field have been published extensively by us and others (56–59). The data are presented as the percentage of healthy nuclei relative to nontreated control, averaged for all replicas (n ≥ 3).
Viral construction
Cited2-expressing plasmids were produced by inserting the Cited2-cDNA sequence into the EcoRI–EcoRV sites of the AM/CBA-EGFP-pI-WPRE-bGH vector. Plasmids containing E2F1-cDNA were similarly produced, inserting the appropriate sequence into the AM/CBA-pI-WPRE-bGH vector. Equally, the E2F4 cDNA sequence was subcloned into the SpeI sites of the AM/CBA-pI-WPRE-bGH vector. These plasmids were then used to generate the corresponding recombinant AAVs, as previously described (29).
For luciferase experiments, the Cited2 regulatory sequence was obtained by PCR using DNA from C57Bl/6 mouse brain genomic DNA as template. The sequence (−700 to +833) was amplified using the following primers: 5′-AACCCCTGTCCTTGAAAAGAGTGGAG-3′ and 5′-TGATGCGGGCTCGGGAACTGCCCCAT-3′. Plasmids containing Cited2-luciferase with the WT E2F binding site and the poly(A) mutant construct harboring a mutation that abolishes E2F binding have previously been reported (60). Plasmid containing Cited2 regulatory sequence was produced by subcloning the corresponding sequence into the KpnI–XhoI site of the pGL4.24 vector. The Renilla plasmid (pRL-CMV) was purchased from Promega. We produced the Cited2 regulatory sequence, the luciferase cDNA sequence, and the Renilla cDNA sequence using PCR (primers KpnI-Cited2, 5′-CTAGGTACCAACCCCTGTCCTTGAAAAGAGTGGAG-3′; SalI-lucif, 5′-CATGTCGACTTAGACGTTGATCCTGGCGCTGGCGC-3′; KpnI-Renilla, 5′-CCAGGTACCTCAATATTGGCCATTAGCCATATT-3; and SalI-Renilla 5′-CGGGTCGACTACCACATTTGTAGAGGTTTTACTTG-3′). Plasmids containing Cited2-luciferase or Renilla sequence were then subcloned into the KpnI–SalI sites of the AAV vector to increase efficient delivery into primary neurons.
Male human genomic DNA was obtained from Promega, and the hCited2 regulatory sequence was amplified by PCR (−943/+845, 5′-AGGGCAACAGCAGAAGCAAGTAACTG-3′ and 5′-GCTCTCGAGAGGCGTGCTGGGGCTGCTGCTGCTGG-3′) (2). The sequences containing hCited2 with either the WT or the poly(A) mutant sequence of the E2F binding site were inserted into the KpnI–XhoI site of the luciferase-AAV vector.
Western blotting analysis
For in vitro experiments, neuronal cultures were collected at designated times after insult. For in vivo experiments, the animals were sacrificed at specified times, the brains were dissected out, and small tissue samples were obtained from the cortex of the ischemic (visually clear) area. All samples were homogenized in solubilizing buffer (0.0625 m Tris, pH 7.4, 2.5 mm EDTA, 2.5 mm EGTA, 10% glycerol, 2% SDS, 0.001% bromphenol blue, and 5% β-mercaptoethanol). The total protein lysates were subjected to Western blotting analyses with different primary antibodies: anti-Cited2 (abcam ab108345, 1:2000), anti-E2F4 (abcam SPM179, 1:2000), anti-E2F1 (Santa Cruz Biotechnology sc-251, 1:200), anti-Cre antibody (Novagen 69050, 1:10,000), or anti-β-actin (Sigma A-5316, 1:10,000). The density of bands was estimated using ImageJ software and standardized versus loading signal (β-actin). For each primary signal, the data represent the averages of independent experiments (n ≥ 3).
ChIP assay
Cultured cortical neurons or HEK293T cells were subjected to ChIP assay as previously described (61). The E2Fs antibodies used were as follows: anti-E2F1 (Santa Cruz Biotechnology sc-193), anti-E2F3 (Santa Cruz Biotechnology sc-878), and anti-E2F4 (Santa Cruz Biotechnology sc-866). The PCR primers used to amplify the Cited2 regulatory sequence were the following: mouse sequences, 5′-CGGCTCCGGGGCACTTCCTTTAT-3′ and 5-CCCGCGCCGTAGTGTATGTGCTC-3′ (17443425 to 17443934 on genomic sequence containing most of intron1–2 and beginning of exon2, 540-bp product); human sequences, 5′-GGATGTGCAACAACAGGATG-3′ and 5′-ACCCCCAAGATCCCACTAAC-3′ (139372121 to 139372355 on genomic sequence containing most of intron 1–2, 234-bp product).
Luciferase reporter assays
Luciferase reporter assays were carried out as previous described (60). One day after plating, cortical neurons/NIH3T3/HEK293T cells were infected/transfected with WT or mutant luciferase viruses/plasmids along with AAV-Renilla/Renilla luciferase as an internal control. After hypoxia/reoxygenation, the cells were lysed, and Luciferase assay was performed using a Luciferase system kit (Dual-Luciferase Reporter Assay System, Promega E1910) and following the manufacturer's instructions. Relative luciferase activities were obtained by normalizing the luciferase activity against Renilla luciferase activity. The results were presented as fold increase in reference to control values.
Statistical analysis
All numerical results were expressed as the mean ± S.E. The Statistical analysis was carried out using Prism (version 6.0 for Mac). The means were compared by t test, ANOVA, or two-way ANOVA depending of the nature of the data; followed by Tukey's post hoc test when appropriate.
Data availability
The data that support the findings of present study are available from the corresponding author on reasonable request.
Author contributions
T. H., D. Q., E. H., F. S., R. S. S., and D. S. P. conceptualization; T. H. data curation; T. H., Y. R. G., D. Q., and D. S. P. formal analysis; T. H. funding acquisition; T. H., D. Q., E. W., and D. S. I. investigation; T. H., A. J., S. M. C., W. B., and L. J. methodology; T. H., Y. R. G., and D. S. P. writing-original draft; T. H., Y. R. G., D. Q., and D. S. P. writing-review and editing; Y. R. G. and D. S. P. project administration; F. S., L. J., S. L. D., and R. S. S. resources; D. S. P. supervision.
Acknowledgment
Cited2flox transgenic mice were obtained from Dr. Dunwoodie at the Victor Chang Cardiac Research Institute (Darlinghurst, New South Wales, Australia).
This work was supported by grants from the Canadian Institute of Health Research, the Heart and Stroke Foundation of Ontario, the Canadian Stroke Network, and the Centre for Stroke Recovery. The authors declare that they have no conflicts of interest with the contents of this article.
Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party hosted site.
- CNS
- central nervous system
- CDK
- cyclin-dependent kinase
- AAV
- adeno-associated virus
- tPA
- tissue plasminogen activator
- DIV
- days in vitro
- ANOVA
- analysis of variance.
References
- 1. Bhattacharya S., Michels C. L., Leung M. K., Arany Z. P., Kung A. L., and Livingston D. M. (1999) Functional role of p35srj, a novel p300/CBP binding protein, during transactivation by HIF-1. Genes Dev. 13, 64–75 10.1101/gad.13.1.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leung M. K., Jones T., Michels C. L., Livingston D. M., and Bhattacharya S. (1999) Molecular cloning and chromosomal localization of the human CITED2 gene encoding p35srj/Mrg1. Genomics 61, 307–313 10.1006/geno.1999.5970 [DOI] [PubMed] [Google Scholar]
- 3. Lopes Floro K., Artap S. T., Preis J. I., Fatkin D., Chapman G., Furtado M. B., Harvey R. P., Hamada H., Sparrow D. B., and Dunwoodie S. L. (2011) Loss of Cited2 causes congenital heart disease by perturbing left–right patterning of the body axis. Hum. Mol. Genet. 20, 1097–1110 10.1093/hmg/ddq554 [DOI] [PubMed] [Google Scholar]
- 4. Combes A. N., Spiller C. M., Harley V. R., Sinclair A. H., Dunwoodie S. L., Wilhelm D., and Koopman P. (2010) Gonadal defects in Cited2-mutant mice indicate a role for SF1 in both testis and ovary differentiation. Int. J. Dev. Biol. 54, 683–689 10.1387/ijdb.092920ac [DOI] [PubMed] [Google Scholar]
- 5. Xu B., Qu X., Gu S., Doughman Y. Q., Watanabe M., Dunwoodie S. L., and Yang Y. C. (2008) Cited2 is required for fetal lung maturation. Dev. Biol. 317, 95–105 10.1016/j.ydbio.2008.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qu X., Lam E., Doughman Y. Q., Chen Y., Chou Y. T., Lam M., Turakhia M., Dunwoodie S. L., Watanabe M., Xu B., Duncan S. A., and Yang Y. C. (2007) Cited2, a coactivator of HNF4α, is essential for liver development. EMBO J. 26, 4445–4456 10.1038/sj.emboj.7601883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bamforth S. D., Bragança J., Eloranta J. J., Murdoch J. N., Marques F. I., Kranc K. R., Farza H., Henderson D. J., Hurst H. C., and Bhattacharya S. (2001) Cardiac malformations, adrenal agenesis, neural crest defects and exencephaly in mice lacking Cited2, a new Tfap2 co-activator. Nat. Genet. 29, 469–474 10.1038/ng768 [DOI] [PubMed] [Google Scholar]
- 8. Bamforth S. D., Bragança J., Farthing C. R., Schneider J. E., Broadbent C., Michell A. C., Clarke K., Neubauer S., Norris D., Brown N. A., Anderson R. H., and Bhattacharya S. (2004) Cited2 controls left–right patterning and heart development through a Nodal-Pitx2c pathway. Nat. Genet. 36, 1189–1196 10.1038/ng1446 [DOI] [PubMed] [Google Scholar]
- 9. Bentham J., Michell A. C., Lockstone H., Andrew D., Schneider J. E., Brown N. A., and Bhattacharya S. (2010) Maternal high-fat diet interacts with embryonic Cited2 genotype to reduce Pitx2c expression and enhance penetrance of left–right patterning defects. Hum. Mol. Genet. 19, 3394–3401 10.1093/hmg/ddq251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buaas F. W., Val P., and Swain A. (2009) The transcription co-factor CITED2 functions during sex determination and early gonad development. Hum. Mol. Genet. 18, 2989–3001 10.1093/hmg/ddp237 [DOI] [PubMed] [Google Scholar]
- 11. Du J., and Yang Y. C. (2013) Cited2 in hematopoietic stem cell function. Curr. Opin. Hematol. 20, 301–307 10.1097/MOH.0b013e3283606022 [DOI] [PubMed] [Google Scholar]
- 12. Fonseca D. J., Ojeda D., Lakhal B., Braham R., Eggers S., Turbitt E., White S., Grover S., Warne G., Zacharin M., Nevin Lam A., Landolsi H., Elghezal H., Saâd A., Restrepo C. M., et al. (2012) CITED2 mutations potentially cause idiopathic premature ovarian failure. Transl. Res. 160, 384–388 10.1016/j.trsl.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 13. Haase M., Schott M., Bornstein S. R., Malendowicz L. K., Scherbaum W. A., and Willenberg H. S. (2007) CITED2 is expressed in human adrenocortical cells and regulated by basic fibroblast growth factor. J. Endocrinol. 192, 459–465 10.1677/JOE-06-0083 [DOI] [PubMed] [Google Scholar]
- 14. Jayaraman S., Doucet M., Lau W. M., and Kominsky S. L. (2016) CITED2 modulates breast cancer metastatic ability through effects on IKKα. Mol. Cancer Res. 14, 730–739 10.1158/1541-7786.MCR-16-0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Y. C., Chang P. Y., and Chao C. C. (2015) CITED2 silencing sensitizes cancer cells to cisplatin by inhibiting p53 trans-activation and chromatin relaxation on the ERCC1 DNA repair gene. Nucleic Acids Res. 43, 10760–10781 10.1093/nar/gkv934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sakai M., Matsumoto M., Tujimura T., Yongheng C., Noguchi T., Inagaki K., Inoue H., Hosooka T., Takazawa K., Kido Y., Yasuda K., Hiramatsu R., Matsuki Y., and Kasuga M. (2012) CITED2 links hormonal signaling to PGC-1α acetylation in the regulation of gluconeogenesis. Nat. Med. 18, 612–617 10.1038/nm.2691 [DOI] [PubMed] [Google Scholar]
- 17. Sperling S., Grimm C. H., Dunkel I., Mebus S., Sperling H. P., Ebner A., Galli R., Lehrach H., Fusch C., Berger F., and Hammer S. (2005) Identification and functional analysis of CITED2 mutations in patients with congenital heart defects. Hum. Mutat. 26, 575–582 10.1002/humu.20262 [DOI] [PubMed] [Google Scholar]
- 18. Val P., Martinez-Barbera J. P., and Swain A. (2007) Adrenal development is initiated by Cited2 and Wt1 through modulation of Sf-1 dosage. Development 134, 2349–2358 10.1242/dev.004390 [DOI] [PubMed] [Google Scholar]
- 19. Volcik K. A., Zhu H., Finnell R. H., Shaw G. M., Canfield M., and Lammer E. J. (2004) Evaluation of the Cited2 gene and risk for spina bifida and congenital heart defects. Am. J. Med. Genet. A 126A, 324–325 10.1002/ajmg.a.20578 [DOI] [PubMed] [Google Scholar]
- 20. Weninger W. J., Lopes Floro K., Bennett M. B., Withington S. L., Preis J. I., Barbera J. P., Mohun T. J., and Dunwoodie S. L. (2005) Cited2 is required both for heart morphogenesis and establishment of the left–right axis in mouse development. Development 132, 1337–1348 10.1242/dev.01696 [DOI] [PubMed] [Google Scholar]
- 21. Yin Z., Haynie J., Yang X., Han B., Kiatchoosakun S., Restivo J., Yuan S., Prabhakar N. R., Herrup K., Conlon R. A., Hoit B. D., Watanabe M., and Yang Y. C. (2002) The essential role of Cited2, a negative regulator for HIF-1α, in heart development and neurulation. Proc. Natl. Acad. Sci. U.S.A. 99, 10488–10493 10.1073/pnas.162371799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barbera J. P., Rodriguez T. A., Greene N. D., Weninger W. J., Simeone A., Copp A. J., Beddington R. S., and Dunwoodie S. (2002) Folic acid prevents exencephaly in Cited2 deficient mice. Hum. Mol. Genet. 11, 283–293 10.1093/hmg/11.3.283 [DOI] [PubMed] [Google Scholar]
- 23. Fame R. M., MacDonald J. L., Dunwoodie S. L., Takahashi E., and Macklis J. D. (2016) Cited2 regulates neocortical layer II/III generation and somatosensory callosal projection neuron development and connectivity. J. Neurosci. 36, 6403–6419 10.1523/JNEUROSCI.4067-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Giovanni A., Wirtz-Brugger F., Keramaris E., Slack R., and Park D. S. (1999) Involvement of cell cycle elements, cyclin-dependent kinases, pRb, and E2F x DP, in B-amyloid-induced neuronal death. J. Biol. Chem. 274, 19011–19016 10.1074/jbc.274.27.19011 [DOI] [PubMed] [Google Scholar]
- 25. Kruman I. I., Wersto R. P., Cardozo-Pelaez F., Smilenov L., Chan S. L., Chrest F. J., Emokpae R. Jr, Gorospe M., and Mattson M. P. (2004) Cell cycle activation linked to neuronal cell death initiated by DNA damage. Neuron 41, 549–561 10.1016/S0896-6273(04)00017-0 [DOI] [PubMed] [Google Scholar]
- 26. Park D. S., Morris E. J., Bremner R., Keramaris E., Padmanabhan J., Rosenbaum M., Shelanski M. L., Geller H. M., and Greene L. A. (2000) Involvement of retinoblastoma family members and E2F/DP complexes in the death of neurons evoked by DNA damage. J. Neurosci. 20, 3104–3114 10.1523/JNEUROSCI.20-09-03104.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang F., O'Hare M. J., and Park D. S. (2001) Cyclin-dependent kinases and stroke. Expert Opin. Ther. Targets 5, 557–567 10.1517/14728222.5.5.557 [DOI] [PubMed] [Google Scholar]
- 28. Rashidian J., Iyirhiaro G., Aleyasin H., Rios M., Vincent I., Callaghan S., Bland R. J., Slack R. S., During M. J., and Park D. S. (2005) Multiple cyclin-dependent kinases signals are critical mediators of ischemia/hypoxic neuronal death in vitro and in vivo. Proc. Natl. Acad. Sci. U.S.A. 102, 14080–14085 10.1073/pnas.0500099102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Iyirhiaro G. O., Zhang Y., Estey C., O'Hare M. J., Safarpour F., Parsanejad M., Wang S., Abdel-Messih E., Callaghan S. M., During M. J., Slack R. S., and Park D. S. (2014) Regulation of ischemic neuronal death by E2F4-p130 protein complexes. J. Biol. Chem. 289, 18202–18213 10.1074/jbc.M114.574145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Macaluso M., Montanari M., and Giordano A. (2006) Rb family proteins as modulators of gene expression and new aspects regarding the interaction with chromatin remodeling enzymes. Oncogene 25, 5263–5267 10.1038/sj.onc.1209680 [DOI] [PubMed] [Google Scholar]
- 31. DeGregori J., and Johnson D. G. (2006) Distinct and overlapping roles for E2F family members in transcription, proliferation and apoptosis. Curr. Mol. Med. 6, 739–748 10.2174/156652406778773484,10.2174/1566524010606070739 [DOI] [PubMed] [Google Scholar]
- 32. Tschöp K., Conery A. R., Litovchick L., Decaprio J. A., Settleman J., Harlow E., and Dyson N. (2011) A kinase shRNA screen links LATS2 and the pRB tumor suppressor. Genes Dev. 25, 814–830 10.1101/gad.2000211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tschöp K., and Dyson N. (2011) Identifying players in the functional network around pRB. Cell Cycle 10, 3814–3815 10.4161/cc.10.22.18225 [DOI] [PubMed] [Google Scholar]
- 34. Osuga H., Osuga S., Wang F., Fetni R., Hogan M. J., Slack R. S., Hakim A. M., Ikeda J. E., and Park D. S. (2000) Cyclin-dependent kinases as a therapeutic target for stroke. Proc. Natl. Acad. Sci. U.S.A. 97, 10254–10259 10.1073/pnas.170144197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. MacManus J. P., Jian M., Preston E., Rasquinha I., Webster J., and Zurakowski B. (2003) Absence of the transcription factor E2F1 attenuates brain injury and improves behavior after focal ischemia in mice. J. Cereb. Blood Flow Metab. 23, 1020–1028 10.1097/01.WCB.0000084249.20114.FA [DOI] [PubMed] [Google Scholar]
- 36. Gonzalez Y. R., Zhang Y., Behzadpoor D., Cregan S., Bamforth S., Slack R. S., and Park D. S. (2008) CITED2 signals through peroxisome proliferator-activated receptor-γ to regulate death of cortical neurons after DNA damage. J. Neurosci. 28, 5559–5569 10.1523/JNEUROSCI.1014-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Biswas S. C., Liu D. X., and Greene L. A. (2005) Bim is a direct target of a neuronal E2F-dependent apoptotic pathway. J. Neurosci. 25, 8349–8358 10.1523/JNEUROSCI.1570-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu D. X., and Greene L. A. (2001) Neuronal apoptosis at the G1/S cell cycle checkpoint. Cell Tissue Res. 305, 217–228 10.1007/s004410100396 [DOI] [PubMed] [Google Scholar]
- 39. Rabinovich A., Jin V. X., Rabinovich R., Xu X., and Farnham P. J. (2008) E2F in vivo binding specificity: comparison of consensus versus nonconsensus binding sites. Genome Res. 18, 1763–1777 10.1101/gr.080622.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Herrup K., Neve R., Ackerman S. L., and Copani A. (2004) Divide and die: cell cycle events as triggers of nerve cell death. J. Neurosci. 24, 9232–9239 10.1523/JNEUROSCI.3347-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Timsit S., and Menn B. (2007) Cerebral ischemia, cell cycle elements and Cdk5. Biotechnol. J. 2, 958–966 10.1002/biot.200700072 [DOI] [PubMed] [Google Scholar]
- 42. Park D. S., Morris E. J., Greene L. A., and Geller H. M. (1997) G1/S cell cycle blockers and inhibitors of cyclin-dependent kinases suppress camptothecin-induced neuronal apoptosis. J. Neurosci. 17, 1256–1270 10.1523/JNEUROSCI.17-04-01256.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang Y., Parsanejad M., Huang E., Qu D., Aleyasin H., Rousseaux M. W., Gonzalez Y. R., Cregan S. P., Slack R. S., and Park D. S. (2010) Pim-1 kinase as activator of the cell cycle pathway in neuronal death induced by DNA damage. J. Neurochem. 112, 497–510 10.1111/j.1471-4159.2009.06476.x [DOI] [PubMed] [Google Scholar]
- 44. Rashidian J., Iyirhiaro G. O., and Park D. S. (2007) Cell cycle machinery and stroke. Biochim. Biophys. Acta 1772, 484–493 10.1016/j.bbadis.2006.11.009 [DOI] [PubMed] [Google Scholar]
- 45. Biswas S. C., Zhang Y., Iyirhiaro G., Willett R. T., Rodriguez Gonzalez Y., Cregan S. P., Slack R. S., Park D. S., and Greene L. A. (2010) Sertad1 plays an essential role in developmental and pathological neuron death. J. Neurosci. 30, 3973–3982 10.1523/JNEUROSCI.6421-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Iyirhiaro G. O., Im D. S., Boonying W., Callaghan S. M., During M. J., Slack R. S., and Park D. S. (2017) Cdc25A is a critical mediator of ischemic neuronal death in vitro and in vivo. J. Neurosci. 37, 6729–6740 10.1523/JNEUROSCI.3017-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sun W., Kim K. H., Noh M., Hong S., Huh P. W., Kim Y., and Kim H. (2006) Induction of CITED2 expression in the rat hippocampus following transient global ischemia. Brain Res. 1072, 15–18 10.1016/j.brainres.2005.12.016 [DOI] [PubMed] [Google Scholar]
- 48. Demyanenko S., and Uzdensky A. (2017) Profiling of signaling proteins in penumbra after focal photothrombotic infarct in the rat brain cortex. Mol. Neurobiol. 54, 6839–6856 10.1007/s12035-016-0191-x [DOI] [PubMed] [Google Scholar]
- 49. MacManus J. P., Koch C. J., Jian M., Walker T., and Zurakowski B. (1999) Decreased brain infarct following focal ischemia in mice lacking the transcription factor E2F1. Neuroreport 10, 2711–2714 10.1097/00001756-199909090-00004 [DOI] [PubMed] [Google Scholar]
- 50. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group (1995) Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 333, 1581–1587 10.1056/NEJM199512143332401 [DOI] [PubMed] [Google Scholar]
- 51. Preis J. I., Wise N., Solloway M. J., Harvey R. P., Sparrow D. B., and Dunwoodie S. L. (2006) Generation of conditional Cited2 null alleles. Genesis 44, 579–583 10.1002/dvg.20251 [DOI] [PubMed] [Google Scholar]
- 52. Andrusiak M. G., Vandenbosch R., Dick F. A., Park D. S., and Slack R. S. (2013) LXCXE-independent chromatin remodeling by Rb/E2f mediates neuronal quiescence. Cell Cycle 12, 1416–1423 10.4161/cc.24527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Osman A. M., Porritt M. J., Nilsson M., and Kuhn H. G. (2011) Long-term stimulation of neural progenitor cell migration after cortical ischemia in mice. Stroke 42, 3559–3565 10.1161/STROKEAHA.111.627802 [DOI] [PubMed] [Google Scholar]
- 54. Zhang S., Taghibiglou C., Girling K., Dong Z., Lin S. Z., Lee W., Shyu W. C., and Wang Y. T. (2013) Critical role of increased PTEN nuclear translocation in excitotoxic and ischemic neuronal injuries. J. Neurosci. 33, 7997–8008 10.1523/JNEUROSCI.5661-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Qu D., Rashidian J., Mount M. P., Aleyasin H., Parsanejad M., Lira A., Haque E., Zhang Y., Callaghan S., Daigle M., Rousseaux M. W., Slack R. S., Albert P. R., Vincent I., Woulfe J. M., and Park D. S. (2007) Role of Cdk5-mediated phosphorylation of Prx2 in MPTP toxicity and Parkinson's disease. Neuron 55, 37–52 10.1016/j.neuron.2007.05.033 [DOI] [PubMed] [Google Scholar]
- 56. O'Hare M. J., Hou S. T., Morris E. J., Cregan S. P., Xu Q., Slack R. S., and Park D. S. (2000) Induction and modulation of cerebellar granule neuron death by E2F-1. J. Biol. Chem. 275, 25358–25364 10.1074/jbc.M001725200 [DOI] [PubMed] [Google Scholar]
- 57. Rukenstein A., Rydel R. E., and Greene L. A. (1991) Multiple agents rescue PC12 cells from serum-free cell death by translation- and transcription-independent mechanisms. J. Neurosci. 11, 2552–2563 10.1523/JNEUROSCI.11-08-02552.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Soto A. M., and Sonnenschein C. (1985) The role of estrogens on the proliferation of human breast tumor cells (MCF-7). J. Steroid Biochem. 23, 87–94 10.1016/0022-4731(85)90265-1 [DOI] [PubMed] [Google Scholar]
- 59. Zhang Y., Qu D., Morris E. J., O'Hare M. J., Callaghan S. M., Slack R. S., Geller H. M., and Park D. S. (2006) The Chk1/Cdc25A pathway as activators of the cell cycle in neuronal death induced by camptothecin. J. Neurosci. 26, 8819–8828 10.1523/JNEUROSCI.2593-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Julian L. M., Vandenbosch R., Pakenham C. A., Andrusiak M. G., Nguyen A. P., McClellan K. A., Svoboda D. S., Lagace D. C., Park D. S., Leone G., Blais A., and Slack R. S. (2013) Opposing regulation of Sox2 by cell-cycle effectors E2f3a and E2f3b in neural stem cells. Cell Stem Cell 12, 440–452 10.1016/j.stem.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 61. Liu D. X., Biswas S. C., and Greene L. A. (2004) B-myb and C-myb play required roles in neuronal apoptosis evoked by nerve growth factor deprivation and DNA damage. J. Neurosci. 24, 8720–8725 10.1523/JNEUROSCI.1821-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ovcharenko I., Nobrega M. A., Loots G. G., and Stubbs L. (2004) ECR Browser: A tool for visualizing and accessing data from comparisons of multiple vertebrate genomes. Nucleic Acids Res. 32, W280–W286 10.1093/nar/gkh355 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of present study are available from the corresponding author on reasonable request.