Abstract
The widespread availability and use of modern synthetic therapeutic agents have led to a massive decline in ethnomedical therapies. However, these synthetic agents often possess toxicity leading to various adverse effects. For instance, anti-tubercular treatment (ATT) is toxic, lengthy, and severely impairs host immunity, resulting in posttreatment vulnerability to reinfection and reactivation of tuberculosis (TB). Incomplete ATT enhances the risk for the generation of multidrug- or extensively drug-resistant (MDR or XDR, respectively) variants of Mycobacterium tuberculosis (M. tb), the TB-causing microbe. Therefore, a new therapeutic approach that minimizes these risks is urgently needed to combat this deadly disease and prevent future TB epidemics. Previously, we have shown that the phytochemical bergenin induces T helper 1 (Th1)– and Th17 cell–based protective immune responses and potently inhibits mycobacterial growth in a murine model of M. tb infection, suggesting bergenin as a potential adjunct agent to TB therapy. Here, we combined ATT therapy with bergenin and found that this combination reduces immune impairment and the length of treatment in mice. We observed that co-treatment with the anti-TB drug isoniazid and bergenin produces additive effects and significantly reduces bacterial loads compared with isoniazid treatment alone. The bergenin co-treatment also reduced isoniazid-induced immune impairment; promoted long-lasting, antigen-specific central memory T cell responses; and acted as a self-propelled vaccine. Of note, bergenin treatment significantly reduced the bacterial burden of a multidrug-resistant TB strain. These observations suggest that bergenin is a potent immunomodulatory agent that could be further explored as a potential adjunct to TB therapy.
Keywords: Mycobacterium tuberculosis, immunotherapy, drug resistance, cytokine, T helper cells, infectious disease, adjunct therapy, bergenin, isoniazid, multidrug resistance, natural product, T cells, memory cells, immunity
Introduction
Estimated one third of global population is infected with Mycobacterium tuberculosis (M. tb)3 an intracellular pathogen responsible for TB (1). Amazingly, despite mammoth efforts being made, only vaccine approved against M. tb infection was developed in 1921. Still, the world sees ∼9 million new cases of TB annually, resulting in close to 1.5 million deaths per annum (1–3). The reason for such a huge death toll is the inability of BCG to provide complete protection against pulmonary TB in adults (4–9). Another aspect of tuberculosis is the emergence of drug-resistant tuberculosis because of noncompliance, resulting because of long duration of DOTS therapy that lasts for minimum of 6 months (10, 11), not to mention the hepatic and immune toxicity caused by the administration of these antibiotics, and most often than not this also results in hepatitis (12). Therefore it has become imperative to develop alternate therapies to circumvent above-mentioned challenges.
In approximately 90% of people contacting M. tb infection the host immune response is more than capable of limiting the infection at latent phase; in the rest of the 10%, the disease which progresses to active stage shows significant changes in pro-inflammatory as well as anti-inflammatory cytokines (13, 14). For example, TNFα production peaks after M. tb infection whereas IFN-γ dips after infection (13, 15, 16). Interestingly, IFN-γ production gradually increases in TB patients during the therapy in dose-dependent manner (15, 16). These results along with the studies in mice models with knocked down IFN-γ or IFN-γR having high TB susceptibility (15, 17). These findings point to the fact that IFN-γ signaling is a major signaling pathway in TB infection. Besides, with IFN-γ and TNFα, IL-10 production also varies in untreated versus treated active TB patients. These cytokines display profound effect on downstream activation and transcription factor STAT-4 and T-bet (18, 19). Infected macrophage differentiation to Th1 cells is impeded by dampening of IL-12 production and has already been established, thus making cells more susceptible to M. tb infection (19–21). Apart from these, disease progression is also aided by inhibition of Th1 cell population by IL-4 producing Th2 cells and regulatory T cells (7, 22–26). To summarize there is a fine dynamic balance between subsets of Th cells including Th1, Th2, Th17, and T-reg cells. Ergo, increase in Th1 responses have been shown to help in bacterial clearance by enhancing cell-mediated immune response along with inhibiting unsought humoral immune response. To avoid development and treatment of drug resistant tuberculosis, it is better to implement and develop complete or partial immune therapies including adjunct therapy along with DOTS.
In previous reports from our group, we showed the immunomodulatory effects of bergenin, a phytochemical compound obtained from tender leaves of Shorea robusta. In our study we presented potent cellular immune response generated by selective induction of IFN-γ and IL-17 secreting population of CD4+ and CD8+ T cells. Although the compound showed no direct killing of M. tb, it significantly helped in clearance of bacterial burden by activation of MAPK, ERK1/2, and SAPK/JNK pathways in infected macrophages. In murine models bergenin effectively induced TNFα, NO, and IL-12 producing M. tb–specific T cell proliferatory response to complete soluble antigen of M. tb. Study also showed induction of Th1 and Th17 host-mediated host-protective immune response potent enough to inhibit mycobacterial growth in murine model of M. tb infection. Molecular mechanistic study confirms that bergenin targets the MAP kinase pathway to alter host immune responses (27).
Extending our previous work further, in this study we investigated the effects of bergenin in combination with isoniazid, where the adjunct treatment significantly reduced or cleared M. tb within 45 days post treatment compared with isoniazid alone. To assess the induction of T cell subsets upon addition of bergenin along with isoniazid, we performed adoptive T cell transfer experiments, which demonstrated the M. tb–specific T cell subsets, generated by bergenin treatment.
Taken together, our data suggest that adjunct therapy with bergenin along with conventional antibiotic therapy may reduce various side effects associated with use of antibiotics and can promote clearance of M. tb organisms in patients.
Results
Co-treatment with bergenin and isoniazid enhances host protection against M. tb infection
Previously, it has been shown that isoniazid treatment dramatically reduces M. tb antigen–specific immune responses and induces apoptosis in activated T helper cells and also increases the vulnerability of TB reactivation and reinfection, thus present TB treatment is associated with immune impairment responses (12). It has been well-established that IFN-γ secreting T helper 1 (Th1) cells play a central role in host resistance to M. tb infection whereas Th2 and T-reg cells are associated with pathogenesis (5, 16, 19, 21, 23, 25, 28). However, chemotherapy in tuberculosis dampens the T cell response, rendering the host more vulnerable to other infectious diseases (12). Therefore, here we proposed an adjunct therapy with the inclusion of immunomodulators along with conventional antibiotic treatment regimens. The use of immunomodulators could prevent the loss of antigen-specific T cell responses commonly observed during DOTS treatment resulting in restoration of immune memory required to counter tuberculosis reactivation and reinfection.
Earlier reports from our group highlighted potent immunomodulatory properties of bergenin. In the same report we presented effective reduction of mycobacterial burden in addition to the induction of protective host immune responses (27). We hypothesized that bergenin could be used in adjunct therapy with anti-tuberculosis therapy (ATT) reducing major risks and adverse effects associated with ATT.
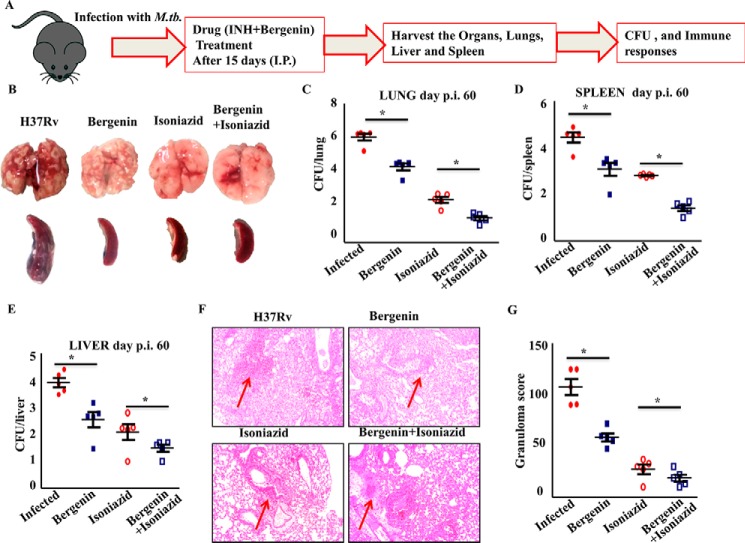
To check our hypothesis we infected groups of C57BL/6 mice with low doses of H37Rv strains of M. tb via aerosol route. The treatment regimen was started 15 days post infection. The treatment regimen consisted of combined therapy with bergenin and isoniazid for one group and other two groups receiving bergenin and isoniazid individually along with untreated mice making up our control group. Organs were harvested from infected animals after 30 and 45 days of treatment to determine the bacterial burden and immune responses (Fig. 1A). The gross picture of the lungs and spleen showed reduced number of granulomatic lesions and necrosis in the mice co-treated with bergenin and isoniazid simultaneously than other groups (Fig. 1B). The administration of bergenin along with isoniazid also resulted in reduced bacterial burden in lungs (Fig. 1C), livers (Fig. 1D) and spleens (Fig. 1E) of the mice. Mice receiving bergenin and isoniazid in combination showed more than 4 log decrease in bacterial burden when compared with the mice receiving bergenin and isoniazid individually. In our study we also observed more than 1 log decrease in bacterial burden in mice receiving bergenin compared with untreated animals. Also, reduced numbers of granulomas in lungs from these mice were observed against the ones harvested from infected controls (Fig. 1, F and G). Previously, we had established that bergenin lacks direct anti-mycobacterial activity and indirectly kills M. tb in infected macrophages by inducing the secretion of NO and TNFα (27). Next, we investigated the effect of bergenin on infection with MDR and XDR strains of M. tb. We observed significant reduction in MDR TB bacterial burden whereas no effect was observed in case of XDR TB in 20 days of treatment (Fig. 2). 20 days' treatment was enough to show the activity of this compound against MDR; however longer treatment may be required against XDR infection. Also, both the MDR and XDR strains induce different and distinct immune response inside the host, which may be one of the reasons why bergenin failed to show any effect on XDR in the time frame tested, as bergenin does not possess strong anti-mycobacterial activity and exerts its effect through immunomodulation. These data suggest that bergenin could be used as an adjunct therapy along with ATT for better results against pulmonary tuberculosis.
Figure 1.
Adjunct therapy with bergenin and isoniazid protects mice against tuberculosis. A, schematic diagram to show the groups of naïve C57BL/6 mice challenged with H37Rv strains of M. tb via the aerosol route with a low-dose inoculum of ∼110 cfu/mouse. After 15 days these mice were treated with bergenin and isoniazid for 45 days. Mice were sacrificed at various time points and lungs were harvested for the estimation of bacterial burden. B, gross picture to show the profile of lungs and spleen of infected and treated mice. C–E, cfu from (C) lung, (D) spleen, and (E) liver homogenates of the different groups of mice. F, lungs from the different groups of mice were harvested, preserved in 4% paraformaldehyde, sectioned, and stained with H&E and AFB. Arrows shows granulomas. G, bar diagram to show the number of granulomas in infected and treated mice. Data are shown for one representative experiment (n = 5 mice/group) of two independent experiments. Error bars indicate S.D. p.i., post infection; *, represents p ≤ 0.05.
Figure 2.
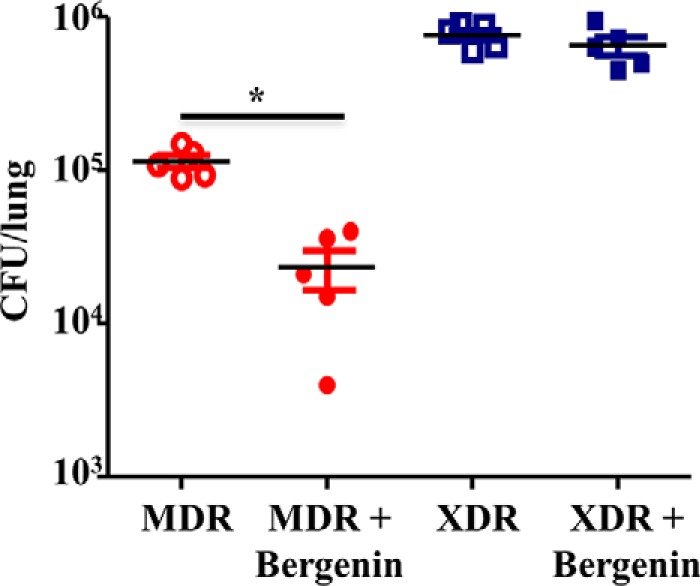
Co-therapy with bergenin and isoniazid protects mice against drug-resistant tuberculosis. Cfu from lung homogenates from the mice infected with MDR and XDR strains of M. tb and with and without treatment. Data are shown for one representative experiment (n = 5 mice/group) of two independent experiments. *, represents p ≤ 0.05.
Combination therapy enhances immunity against M. tb
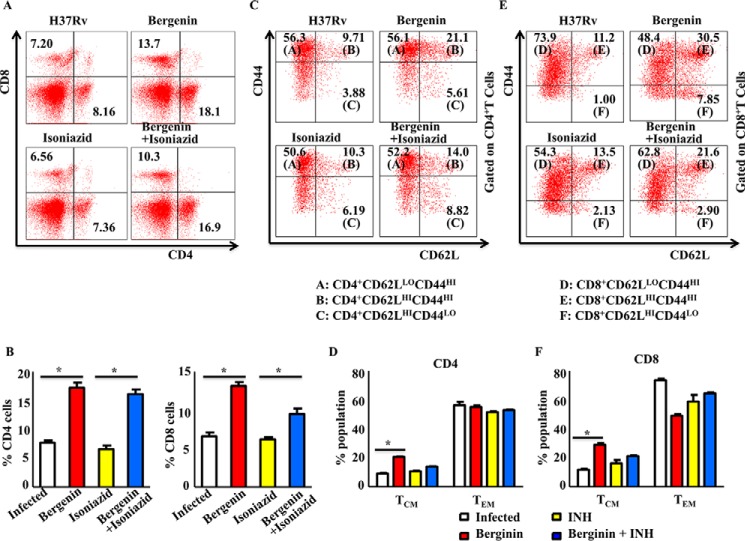
Next, we assessed host protective immune responses induced in the spleens during co-treatment with bergenin and isoniazid when compared with the control groups receiving bergenin or isoniazid treatment. We observed that bergenin treatment enhanced the prevalence of CD4+ and CD8+ T cells in the spleen of the mice than in untreated infected control (Fig. 3, A and B). Furthermore, isoniazid treatment dampens the cellular immune responses as evident from comparatively reduced number of CD4+ and CD8+ T cells. These data are in agreement with previously reported suppression of antigen-specific cytokine responses during isoniazid therapy in murine model of tuberculosis (12). Moreover, co-treatment with bergenin and isoniazid restores the isoniazid-induced suppression of immune responses (Fig. 3, A and B). We further looked at the subsets of memory T cell by analyzing CD62L and CD44 expression. We found that co-treatment enriched the TCM cell pool (CD4+CD62LhiCD44hi) compared with the TEM cell pool (CD4+CD62LloCD44hi) in the spleen (Fig. 3, C and D) of infected mice. Similarly, co-therapy induced CD8+ TCM (CD8+CD62LloCD44hi) cells over CD8+ TEM (CD8+CD62LhiCD44hi) cells (Fig. 3, E and F). These data suggest that bergenin could be used to potentiate the vaccine efficacy of BCG by inducing central memory T cell population, which is the main precursor of memory T cells that can provide long-lasting protection against pathogens. Thus bergenin-treated mice would have fewer chances to get tuberculosis reactivation. After reinfection with the M. tb, these mice would respond faster and reduce the bacteria's ability to expand diseases by selectively inducing M. tb specific T cell responses. Collectively these data suggest that co-treatment with bergenin and isoniazid induces faster and stronger recall responses upon subsequent infection, because of their larger pool of TCM cells in spleen.
Figure 3.
Combined therapy with bergenin and isoniazid induces host protective T cell responses. A and B, FACS data to show the percentage of CD4+ and CD8+ T cells in the spleen of different groups of mice infected with H37Rv strains of M. tb and treated with bergenin and isoniazid. C and D, profiling of memory immune responses (i.e. Tnaive-CD4+CD62LhiCD44lo, TCM-CD4+CD62LhiCD44hi, TEM-CD4+CD62LloCD44hi) of CD4+ T cells isolated from spleen of infected and treated mice. E and F, profiling of memory immune responses (i.e. Tnaive-CD8+CD62LhiCD44lo, TCM-CD8+CD62LhiCD44hi, TEM-CD8+CD62LloCD44hi) of CD8+ T cells isolated from spleen of infected and treated mice. Data shown here are representative of two independent experiments with five mice in each group. Error bars indicate S.D. *, represents p ≤ 0.05.
Adjunct therapy with bergenin induces M. tb–specific, host-protective cytokine responses
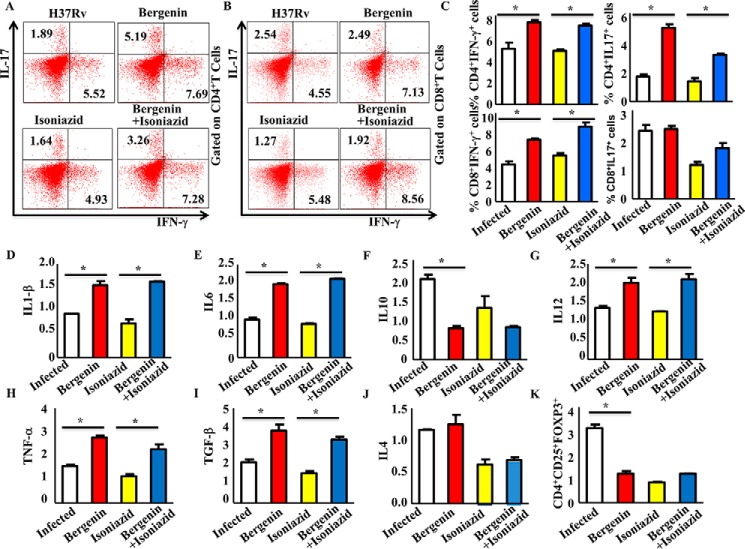
Further extending our study we investigated the effects of co-therapy on cytokine production by T cells. We stained intracellular cytokines for T cells as well as innate cytokines that help in the differentiation of T helper cells during tuberculosis. We observed that antigen-specific T cells from spleen of mice treated with combination therapy produced markedly increased levels of the host-protective cytokines IFN-γ and IL-17 producing CD4+ T cells (Fig. 4, A and C) as well as CD8+ T cells (Fig. 4, B and C). Furthermore, bergenin treatment also induces the level of cytokines IL-1β (Fig. 4D), IL-6 (Fig. 4E), IL-12 (Fig. 4G), TNFα (Fig. 4H), and TGF-β (Fig. 4I) in the spleen of the mice. These cytokines help in the differentiation of different subsets of T helper cells. Reports from the previous study from our lab as well as from others confirm that IL-1β and IL-12 help in the differentiation of IFN-γ producing Th1 cells, whereas, IL-6 and TGF-β help in the differentiation of IL-17 producing Th17 cells (5, 30–34). We did not observe any significant difference for IL-4 producing T cells (Fig. 4J). Moreover, IL-10 (Fig. 4F) and T-reg cell responses were reduced in the spleen of mice treated with combination therapy as compared with infected control groups (Fig. 4K). These findings suggested that bergenin treatment enhances antigen-specific Th1 and Th17 responses and restores the isoniazid-induced immune suppression. These data are in agreement with the previous studies where it has been shown that Th1 and Th17 cells are critical for host resistance to TB whereas Th2 and T-reg cells promote disease by counter-regulating host-protective T cell responses (5, 16, 18, 30, 35).
Figure 4.
Simultaneous treatment with bergenin and isoniazid induces protective Th1 and Th17 responses and reduces diseases progressive T-reg generation in CD4+ T cells. A and C, profiling of IFN-γ and IL-17 cytokines from CD4+ T cells in the spleen of different groups of mice infected with H37Rv strains of M. tb and treated with bergenin and isoniazid. B and C, profiling of IFN-γ and IL-17 cytokines from CD8+ T cells in the spleen of different groups of mice infected with H37Rv strains of M. tb and treated with bergenin and isoniazid. D and I, profiling of cytokines (D) IL-1β, (E) IL-6, (F) IL-10, (G) IL-12, (H) TNFα, and (I) TGF-β cytokines in the spleen of different groups of mice infected with H37Rv strains of M. tb and treated with bergenin and isoniazid. J and K, profiling of (J) IL-4 from CD4+ T cells and (K) CD4+CD25+FOXP3+ cells (T-reg) from CD4+ T cells in the spleen of different groups of mice infected with H37Rv strains of M. tb and treated with bergenin and isoniazid. Data shown here are representative of two independent experiments with five mice in each group. Error bars indicate S.D. *, represents p ≤ 0.05.
T cells from mice infected with M. tb and treated with bergenin confer host-protective immunity against M. tb infection upon adoptive transfer
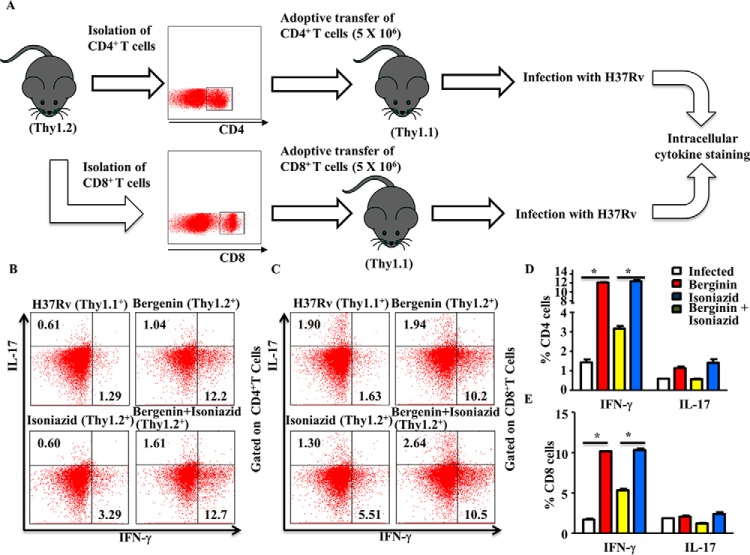
Our findings indicated that combined treatment with bergenin and isoniazid enhanced protective immune responses against M. tb infection. To demonstrate that the protective effects of combination therapy were because of its immune modulatory properties, we next investigated whether adoptive transfer of CD4+ and CD8+ T cells from infected and treated mice can confer protection to M. tb infection to naïve mice. Congenic WT Thy1.2 mice were infected with H37Rv strains of M. tb, treated with bergenin and isoniazid for 45 days, and then rested for 30 days. CD4+ and CD8+ T cells (5 × 106) isolated from these animals were then adoptively transferred into γ-irradiated (sublethal dose of 800 rads/mouse) Thy1.1+ congenic animals. These animals were subsequently challenged with a low dose of M. tb H37Rv by the aerosol route. 25 days after infection, spleens were isolated from the surviving mice in each group for determination of antigen-specific intracellular cytokine responses (Fig. 5A). Results showed that the mice receiving cells from donor animals treated with bergenin and isoniazid maintained increased levels of IFN-γ and IL-17-producing CD4+ T cells (Fig. 5, B and D) and CD8+ T cells (Fig. 5, C and E).
Figure 5.
T cells from mice infected with M. tb and treated with bergenin and isoniazid confer improved protection against TB. A, experimental layout: Thy1.1+ mice were γ-irradiated and rested for 5 days and divided into four groups. The Thy1.1+ control group did not receive any adoptively transferred T cells and served as a negative control. T cells (CD4+ T and CD8+ T cells) were enriched from the lymph nodes of Thy1.2+ animals that had been previously infected with H37Rv strains of M. tb and treated with bergenin and isoniazid for 30 days and then rested for an additional 30 days. These cells were then adoptively transferred into the irradiated recipient mice (5 × 106 cells). 4 days after adoptive transfer all four groups of irradiated Thy1.1+ mice were challenged with M. tb H37Rv through the aerosol route. The mice were euthanized for profiling of intracellular cytokines at 25 days after aerosol challenge. B and D, T cells were then stained for expression of the intracellular cytokines IFN-γ and IL-17 from CD4+ T cells (B and D) and from CD8+ T cells (C and E). The results shown are representative of two independent experiments with six mice in each group. *, represents p ≤ 0.05.
Discussion
Balance between productions of pro- and anti-inflammatory cytokines involved in the differentiation of T helper subsets during M. tb infection decides the extent of host susceptibility (5–7, 28–30), thus there needs to be a dynamic balance between protective cytokines and cytokines associated with diseases progression (6, 7). Previous animal studies have confirmed that mice defective in the cytokines responsible for the differentiation of Th1 cells showed increased susceptibility to M. tb infection by manipulating their response toward Th2 and T-reg phenotype, known to provide a niche for disease progression (6, 7, 18, 35). Others and we have employed various strategies to alter the host protective immune responses but failed to completely eradicate the M. tb from the host (12, 27, 36–39). Therefore, we resorted to ethnobotany to screen various plant-based immunomodulatory as well as antimicrobial compounds for their efficacy to provide protective immunity against tuberculosis. Bergenin is an example of one such plant-based immunomodulatory compound. Several reports have suggested that bergenin possesses various properties, including being anti-hepatotoxic, anti-inflammatory, anti-arrhythmic, and anti-neuroprotective, all major properties sorted to be in a safe M. tb drug (40, 41).
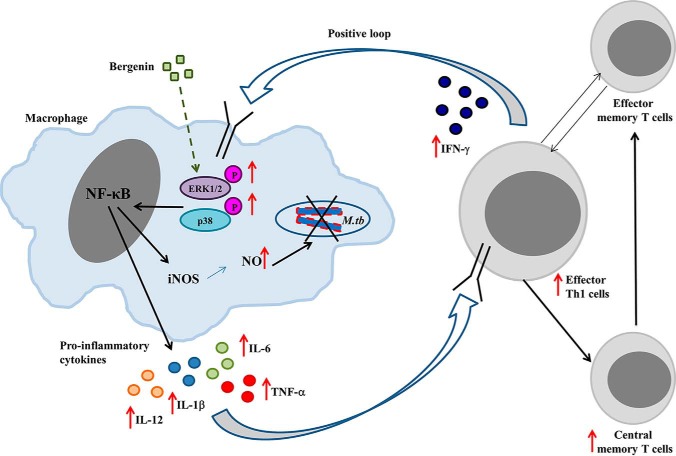
Previously we reported that bergenin induces macrophages to secrete out NO and TNFα, thereby leading to reduced bacterial burden in the infected cells. Our in vivo studies suggested that bergenin not only activates the macrophages and dendritic cells but also induces M. tb–specific T cell proliferation and collectively promotes the killing of bacteria (Fig. 6). Detailed analysis showed that bergenin induces the differentiation of host protective Th1 and Th17 responses, which are believed to be protective T helper subsets against tuberculosis (27). Moreover, our mechanistic studies showed that bergenin induces the activation of MAPK pathway in macrophages infected with M. tb (Fig. 6), which is critically important for the induction of pro-inflammatory cytokines (42, 43). MAPK activation in macrophages provides protective host immunity during M. tb infection and produces various effector molecules that show anti-mycobacterial activity (44). Collectively, we observed that bergenin alone is not able to completely eradicate the bacterial burden from the system, however it induces the host immunity to fight against this deadly disease by activating the MAPK pathway in macrophages (27).
Figure 6.
Proposed mechanism of action of bergenin. Bergenin activates MAPK signaling pathways in the macrophages (27) leading to the induction of NF-κB, which in turn results in the expression of protective pro-inflammatory cytokines and iNOS. The NO thus produced mediates the killing of intracellular mycobacteria and the pro-inflammatory cytokines activate Th cells, which mediates further protection.
We followed up this study and used bergenin in adjunct therapy along with conventional antibiotic therapy against tuberculosis. It is clear from our data that bergenin facilitates the clearance of M. tb by promoting host protective immune responses as well as host protective memory response i.e. central memory T cells (CD4+CD62LhiCD44hi/CD8+CD62LhiCD44hi). Our experiments indicate that the combination therapy may not only enhance faster clearance, but also act as a self-propelled vaccine, which may prevent re-activation and reinfection of TB.
Collectively, we observed that bergenin dramatically reduces the death of effector memory T cells induced by the treatment of isoniazid. Therefore, a combination of ATT could serve a better treatment and have several advantages over current conventional antibiotic therapy, viz. reduce the length of treatment, increase M. tb–specific host protective immune responses, and restore the antibiotic-induced immune suppression. This will open up the possibility for the re-design of TB therapy.
Experimental procedures
Ethics statement
Animal experiments were performed according to the guidelines approved by the Institutional Animal Ethics Committee of the International Centre for Genetic Engineering and Biotechnology (ICGEB; New Delhi, India) (Approval ID: ICGEB/IAEC/08/2016/TH-1) and the Department of Biotechnology guidelines (Government of India). All mice used for experiments were ethically sacrificed by asphyxiation in carbon dioxide according to institutional and Department of Biotechnology, Government of India, regulations.
Mice
C57BL/6 (6–8 weeks of age) mice were provided by ICGEB, New Delhi, India. All animals were maintained in the animal facility of the ICGEB.
Bacteria
M. tb strain H37Rv was a kind gift from the Colorado State University repository. Multi-drug resistant (MDR-Jal2261; resistance for isoniazid, rifampicin and ethambutal) and extensively drug-resistant (XDR-MYC 431) strains of M. tb were kind gift from Dr. Kanury V. S. Rao at ICGEB, New Delhi, India. These organisms were grown in 7H9 (Middlebrooks, DifcoTM) medium supplemented with 10% ADC (albumin, dextrose, and catalase; DifcoTM) and with 0.05% Tween 80 and 0.2% glycerol, and cultures were grown to mid-log phase. Aliquots of the cultures in 20% glycerol were preserved at −80 °C and these cryo-preserved stocks were used for infections.
Antibodies and reagents
We used the following antibodies: anti-CD3 (clone: 145–2C11)-PerCP-Cy5 or -APC, -CD4 (clone: GK1.5, RM4–5)-FITC, -PerCP-Cy5 or -APC, -CD8 (clone: 53–6.7)-FITC, -APC-H7, -PerCP-Cy5 or -APC, -NK1.1 (clone: PK136)-Alexa 700, -PerCP-Cy5 or -PE, -CD44 (clone: IM7)-APC, -CD62L (clone: MEL-14)-PE, -CD25 (clone: 3C7)-PE, -APC, -FOXP3 (clone: MF23, R16–715)-APC, -IFN-γ (clone: XMG1.2)-APC, -IL-4 (clone: 11B11)-PE, -IL-6 (clone: MPS-20F3)-PE, -IL-10 (clone: JES5–16E3)-APC, -IL-12 (clone: C15.6)-PE, -IL-17 (clone: O79–289)-PE, -IL-2 (clone: JES6–5H4)-PerCP or -FITC, -IL-22 (clone: Poly5164)-PE, -TNFα (clone: MP6-XT22)-PE, -CD107A (1DB4)-FITC (all from BD Biosciences), -TGF-β (clone: TW7–16B4)-APC (from BioLegend), and -CD69 (clone: H1.2F3)-PE (from eBioscience).
M. tb infection of mice and estimation of colony forming units (cfu)
Mice were infected with M. tb H37Rv via the aerosol route using a Madison aerosol chamber (University of Wisconsin, Madison, WI) with its nebulizer pre-calibrated to deposit a total of ∼110 cfu to the lungs of each mouse as described previously (5, 28, 29). Briefly, mycobacterial stocks recovered from a −80○C freezer were quickly thawed and were subjected to light ultrasonication to obtain a single cell suspension. 15 ml of the bacterial cell suspension (10 × 106 cells per ml) was placed in the nebulizer of the Madison aerosol chamber pre-calibrated to deliver the desired cfu to the lungs of the mice via aerosol route. At day 1 post infection, three mice were sacrificed and organs were harvested, homogenized in 0.2 μm filtered PBS containing 0.05% Tween 80 and plated onto 7H11 Middlebrooks (Difco) plates containing 10% oleic acid, albumin, dextrose, and catalase (OADC) (Difco). Lung and spleen cell homogenates were plated over 7H11 plates in undiluted, 10-fold, 100-fold dilutions and were incubated at 37 °C. After 21–28 days. M. tb colonies were counted and cfu for lung and spleen were estimated. Mice from various groups were euthanized at the indicated time points in various experiments and their organs were harvested for obtaining cfu counts and/or immune cell subpopulations for immunological studies as described under other subsections.
Drug administration
4 mg of bergenin in 100 μl of PBS was administered intraperitoneally every day during the entire treatment phase. 0.1 g/liter of isoniazid was given to the mice in drinking water and changed every alternate day.
FACS analysis
For intracellular cytokine staining, cells were treated with 50 ng/ml PMA and 500 ng/ml ionomycin in the presence of 10 μg/ml brefeldin A (Sigma-Aldrich or eBioscience) added during the last 6 h of culture. Cells were washed twice with PBS, resuspended in a permeabilization buffer (Cytofix/Cytoperm kit; BD Biosciences), and stained with the following fluorescently conjugated monoclonal antibodies: anti-CD4 (clone GK1.5)-allophycocyanin (APC), anti-IFN-γ (clone XMG1.2)-fluorescein isothiocyanate (FITC), anti-IFN-γ (clone XMG1.2)-APC, anti-IL-4 (clone GK1.5)-phycoerythrin (PE) (eBioscience). Fluorescence intensity was measured by flow cytometry (FACS Canto II; BD Biosciences) and data were analyzed with FlowJo (Treestar).
T cell adoptive transfer
For adoptive transfer experiments, C57BL/6-Thy1.1 mice were γ-irradiated (8 rads/s for 100 s) and rested for a day. CD4+ T cells, isolated from the lymph nodes of C57BL/6-Thy1.2+ mice (infected with H37Rv and treated with bergenin) were then adoptively transferred into the irradiated recipient mice (2–4 × 106 cells/mouse). After 15 days, recipient mice were challenged with H37Rv through the aerosol route.
Histology
Lung tissues were fixed in formalin solution and coated with wax for sectioning. Sections were stained with H&E and Ziel-Nelson Stain (AFB) dyes and slides were analyzed under microscope.
Statistical analysis
All data were derived from at least three independent experiments. Significant differences between the groups were determined by t test. A value of p < 0.05 was accepted as an indication of statistical significance.
Author contributions
S. K., C. S., S. R. K., S. C., and A. B. data curation; S. K., S. R. K., S. C., A. B., and D. C. methodology; C. S., A. B., and V. P. D. writing-original draft; A. K., R. K. N., D. C., and G. D. resources; A. K., A. B., and V. P. D. formal analysis; A. B., G. D., and V. P. D. supervision; A. B., G. D., and V. P. D. writing-review and editing; V. P. D. conceptualization; V. P. D. funding acquisition; V. P. D. validation; V. P. D. investigation.
Acknowledgments
We acknowledge the support of the DBT-supported Tuberculosis Aerosol Challenge Facility and Bio-experimentation Facility (Animal House) at the International Centre for Genetic Engineering and Biotechnology (ICGEB, New Delhi, India) and their staff in accomplishing this work.
This work was supported by the Department of Science and Technology (DST), Government of India and ICGEB, New Delhi, India. This work was also supported by a DST-INSPIRE Faculty Fellowship (to V.P.D. and A.B.) and by an Early Career Research Award (ECRA), Science and Engineering Research Board (SERB), Department of Science and Technology (DST), Government of India (to V.P.D.). The authors declare that they have no conflicts of interest with the contents of this article.
- M. tb
- Mycobacterium tuberculosis
- TB
- tuberculosis
- BCG
- bacille Calmette-Guérin
- DOTS
- Directly Observed Treatment, Short Course
- MAPK
- mitogen-activated protein kinase
- ATT
- anti-tuberculosis therapy
- XDR
- extensively drug-resistant
- MDR
- multidrug-resistant
- ICGEB
- International Centre for Genetic Engineering and Biotechnology
- cfu
- colony forming units.
References
- 1. World Health Organization (2018) Global tuberculosis report. World Health Organization, Geneva [Google Scholar]
- 2. World Health Organization (2015) Tuberculosis vaccine development. World Health Organization, Geneva [Google Scholar]
- 3. World Health Organization (2015) Global tuberculosis report. World Health Organization, Geneva [Google Scholar]
- 4. Douglas Kernodle S. (2010) Decrease in the effectiveness of Bacille Calmette-Guérin vaccine against pulmonary tuberculosis: A consequence of increased immune suppression by microbial antioxidants, not over attenuation. Clin. Infect. Dis. 51, 177–184 10.1086/653533 [DOI] [PubMed] [Google Scholar]
- 5. Chatterjee S., Dwivedi V. P., Singh Y., Siddiqui I., Sharma P., Van Kaer L., Chattopadhyay D., and Das G. (2011) Early secreted antigen ESAT-6 of Mycobacterium tuberculosis promotes protective T helper 17 cell responses in a toll-like receptor-2-dependent manner. PLoS Pathog. 7, e1002378 10.1371/journal.ppat.1002378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhattacharya D., Dwivedi V. P., Kumar S., Reddy M. C., Kaer L. V., Moodley P., and Das G. (2014) Simultaneous inhibition of T helper 2 and T regulatory cell differentiation by small molecules enhances Bacillus Calmette-Guerin vaccine efficacy. J. Biol. Chem. 289, 33404–33411 10.1074/jbc.M114.600452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhattacharya D., Dwivedi V. P., Maiga M., Maiga M., Van Kaer L., Bishai W. R., and Das G. (2014) Small molecule-directed immunotherapy against recurrent infection by Mycobacterium tuberculosis. J. Biol. Chem. 289, 16508–16515 10.1074/jbc.M114.558098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fine P. E. (1989) The BCG story: Lessons from the past and implications for the future. Rev. Infect. Dis. 11, 353–359 [DOI] [PubMed] [Google Scholar]
- 9. Fine P. E. (1995) Variation in protection by BCG: Implications of and for heterologous immunity. Lancet 346, 1339–1345 10.1016/S0140-6736(95)92348-9 [DOI] [PubMed] [Google Scholar]
- 10. Davies J. (1996) Origins and evolution of antibiotic resistance. Microbiologia 12, 9–16 [PubMed] [Google Scholar]
- 11. Byrd T. F., and Davis L. E. (2007) Multidrug-resistant tuberculous meningitis. Curr. Neurol. Neurosci. Rep. 7, 470–475 10.1007/s11910-007-0073-8 [DOI] [PubMed] [Google Scholar]
- 12. Tousif S., Singh D. K., Mukherjee S., Ahmad S., Arya R., Nanda R., Ranganathan A., Bhattacharyya M., Van Kaer L., Kar S. K., and Das G. (2017) Nanoparticle-formulated curcumin prevents posttherapeutic disease reactivation and reinfection with Mycobacterium tuberculosis following isoniazid therapy. Front. Immunol. 8, 739 10.3389/fimmu.2017.00739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Winslow G. M., Cooper A., Reiley W., Chatterjee M., and Woodland D. L. (2008) Early T-cell responses in tuberculosis immunity. Immunol. Rev. 225, 284–299 10.1111/j.1600-065X.2008.00693.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cadena A. M., Flynn J. L., and Fortune S. M. (2016) The importance of first impressions: Early events in Mycobacterium tuberculosis infection influence outcome. mBio 7, e00342–16 10.1128/mBio.00342-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flynn J. L., and Chan J. (2001) Immunology of tuberculosis. Annu. Rev. Immunol. 19, 93–129 10.1146/annurev.immunol.19.1.93 [DOI] [PubMed] [Google Scholar]
- 16. Flynn J. L., Chan J., Triebold K. J., Dalton D. K., Stewart T. A., and Bloom B. R. (1993) An essential role for interferon in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178, 2249–2254 10.1084/jem.178.6.2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sweeney K. A., Dao D. N., Goldberg M. F., Hsu T., Venkataswamy M. M., Henao-Tamayo M., Ordway D., Sellers R. S., Jain P., Chen B., Chen M., Kim J., Lukose R., Chan J., Orme I. M., Porcelli S. A., and Jacobs W. R. Jr. (2011) A recombinant Mycobacterium smegmatis induces potent bactericidal immunity against Mycobacterium tuberculosis. Nat. Med. 17, 1261–1268 10.1038/nm.2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lienhardt C., Azzurri A., Amedei A., Fielding K., Sillah J., Sow O. Y., Bah B., Benagiano M., Diallo A., Manetti R., Manneh K., Gustafson P., Bennett S., D'Elios M. M., McAdam K., and Del Prete G. (2002) Active tuberculosis in Africa is associated with reduced Th1 and increased Th2 activity in vivo. Eur. Immunol. J. 32, 1605–1613 [DOI] [PubMed] [Google Scholar]
- 19. Cooper A. M., Solache A., and Khader S. A. (2007) Interleukin-12 and tuberculosis: An old story revisited. Curr. Opin. Immunol. 19, 441–447 10.1016/j.coi.2007.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hickman S. P., Chan J., and Salgame P. (2002) Mycobacterium tuberculosis induces differential cytokine production from dendritic cells and macrophages with divergent effects on naïve T cell polarization. J. Immunol. 168, 4636–4642 10.4049/jimmunol.168.9.4636 [DOI] [PubMed] [Google Scholar]
- 21. Trinchieri G. (2003) Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3, 133–146 10.1038/nri1001 [DOI] [PubMed] [Google Scholar]
- 22. Kursar M., Koch M., Mittrücker H. W., Nouailles G., Bonhagen K., Kamradt T., and Kaufmann S. H. (2007) Cutting edge: Regulatory T cells prevent efficient clearance of Mycobacterium tuberculosis. J. Immunol. 178, 2661–2665 10.4049/jimmunol.178.5.2661 [DOI] [PubMed] [Google Scholar]
- 23. Scott-Browne J. P., Shafiani S., Tucker-Heard G., Ishida-Tsubota K., Fontenot J. D., Rudensky A. Y., Bevan M. J., and Urdahl K. B. (2007) Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J. Exp. Med. 204, 2159–2169 10.1084/jem.20062105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen W., and Konkel J. E. (2010) TGF-β and “adaptive” Foxp3+ regulatory T cells. J. Mol. Cell. Biol. 2, 30–36 10.1093/jmcb/mjp004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shafiani S., Tucker-Heard G., Kariyone A., Takatsu K., and Urdahl K. B. (2010) Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J. Exp. Med. 207, 1409–1420 10.1084/jem.20091885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yoshimura A., Wakabayashi Y., and Mori T. (2010) Cellular and molecular basis for the regulation of inflammation by TGF-β. J. Biochem. 147, 781–792 10.1093/jb/mvq043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dwivedi V. P., Bhattacharya D., Yadav V., Singh D. K., Kumar S., Singh M., Ojha D., Ranganathan A., Van Kaer L., Chattopadhyay D., and Das G. (2017) The phytochemical bergenin enhances T helper 1 responses and anti-mycobacterial immunity by activating the MAP kinase pathway in macrophages. Front. Cell. Infect. Microbiol. 7, 149 10.3389/fcimb.2017.00149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dwivedi V. P., Bhattacharya D., Chatterjee S., Prasad D. V. R., Chattopadhyay D., Van Kaer L., Bishai W. R., and Das G. (2012) Mycobacterium tuberculosis directs T helper 2 cell differentiation by inducing interleukin-1β production in dendritic cells. J. Biol. Chem. 287, 33656–33663 10.1074/jbc.M112.375154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singh D. K., Dwivedi V. P., Ranganathan A., Bishai W. R., Van Kaer L., and Das G. (2016) Blockade of the Kv1.3 K+ channel enhances BCG vaccine efficacy by expanding central memory T lymphocytes. J. Infect. Dis. 214, 1456–1464 10.1093/infdis/jiw395 [DOI] [PubMed] [Google Scholar]
- 30. Rahman M. A., Sobia P., Dwivedi V. P., Bhawsar A., Singh D. K., Sharma P., Moodley P., Van Kaer L., Bishai W. R., and Das G. (2015) Mycobacterium tuberculosis TlyA negatively regulates Th1 and Th17 differentiation and promotes tuberculosis pathogenesis. J. Biol. Chem. 290, 14407–14417 10.1074/jbc.M115.653600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Santarlasci V., Cosmi L., Maggi L., Liotta F., and Annunziato F. (2013) IL-1 and T helper immune responses. Front. Immunol. 4, 182 10.3389/fimmu.2013.00182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Duhen T., and Campbell D. J. (2014) IL-1β promotes the differentiation of polyfunctional human CCR6+CXCR3+ Th1/17 cells that are specific for pathogenic and commensal microbes. J. Immunol. 193, 120–129 10.4049/jimmunol.1302734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang L., Anderson D. E., Baecher-Allan C., Hastings W. D., Bettelli E., Oukka M., Kuchroo V. K., and Hafler D. A. (2008) IL-21 and TGF-β are required for differentiation of human TH17 cells. Nature 454, 350–352 10.1038/nature07021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Das J., Ren G., Zhang L., Roberts A. I., Zhao X., Bothwell A. L. M., Van Kaer L., Shi Y., and Das G. (2009) Transforming growth factor β is dispensable for the molecular orchestration of Th17 cell differentiation. J. Exp. Med. 206, 2407–2416 10.1084/jem.20082286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rook G. A. (2007) Th2 cytokines in susceptibility to tuberculosis. Curr. Mol. Med. 7, 327–337 [DOI] [PubMed] [Google Scholar]
- 36. Lee H., and Suh J. W. (2016) Anti-tuberculosis lead molecules from natural products targeting Mycobacterium tuberculosis ClpC1. J. Ind. Microbiol. Biotechnol. 43, 205–212 10.1007/s10295-015-1709-3 [DOI] [PubMed] [Google Scholar]
- 37. Jnawali H. N., Jeon D., Jeong M.-C., Lee E., Jin B., Ryoo S., Yoo J., Jung I. D., Lee S. J., Park Y. M., and Kim Y. (2016) Antituberculosis activity of a naturally occurring flavonoid, isorhamnetin. J. Nat. Prod. 79, 961–969 10.1021/acs.jnatprod.5b01033 [DOI] [PubMed] [Google Scholar]
- 38. Gómez-Cansino R., Guzmán-Gutiérrez S. L., Campos-Lara M. G., Espitia-Pinzón C. I., and Reyes-Chilpa R. (2017) Natural compounds from Mexican medicinal plants as potential drug leads for anti-tuberculosis drugs. An. Acad. Bras. Ciênc. 89, 31–43 10.1590/0001-3765201720160298 [DOI] [PubMed] [Google Scholar]
- 39. Stanford J. L., Bahr G. M., Byass P., Corrah T., Dowlati Y., Lucas S., Shaaban M., and Torres P. (1990) A modern approach to the immunotherapy of tuberculosis. Bull. Int. Union Tuberc. Lung Dis. 65, 27–29 [PubMed] [Google Scholar]
- 40. Mukherjee H., Ojha D., Bharitkar Y. P., Ghosh S., Mondal S., Kaity S., Dutta S., Samanta A., Chatterjee T. K., Chakrabarti S., Mondal N. B., and Chattopadhyay D. (2013) Evaluation of the wound healing activity of Shorea robusta, an Indian ethnomedicine, and its isolated constituent(s) in topical formulation. J. Ethnopharmacol. 149, 335–343 10.1016/j.jep.2013.06.045 [DOI] [PubMed] [Google Scholar]
- 41. Patel D. K., Patel K., Kumar R., Gadewar M., and Tahilyani V. (2012) Pharmacological and analytical aspects of bergenin: A concise report. Asian Pacific J. Trop. Dis. 2, 163–167 10.1016/S2222-1808(12)60037-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Whitmarsh A. J., Yang S. H., Su M. S., Sharrocks A. D., and Davis R. J. (1997) Role of p38 and JNK mitogen-activated protein kinases in the activation of ternary complex factors. Mol. Cell. Biol. 17, 2360–2371 10.1128/MCB.17.5.2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zarubin T., and Han J. (2005) Activation and signaling of the p38 MAP kinase pathway. Cell Res. 15, 11–18 10.1038/sj.cr.7290257 [DOI] [PubMed] [Google Scholar]
- 44. Schorey J. S., and Cooper A. M. (2003) Macrophage signalling upon mycobacterial infection: The MAP kinases lead the way. Cell. Microbiol. 5, 133–142 10.1046/j.1462-5822.2003.00263.x [DOI] [PubMed] [Google Scholar]