Abstract
Objectives
Acute respiratory distress syndrome (ARDS) is caused by many factors including inhalation of toxicants, acute barotrauma, acid aspiration, and burns. Surfactant function is impaired in ARDS and acute airway injury resulting in high surface tension with alveolar and small airway collapse, edema, hypoxemia, and death. In this study, we explore the mechanisms whereby surfactant becomes dysfunctional in ARDS and bronchiolitis and its repair with a cyclodextrin drug that sequesters cholesterol.
Methods
We used in vitro model systems, a mouse model of ARDS, and samples from patients with acute bronchiolitis. Surface tension was measured by captive bubble surfactometry.
Results
Patient samples showed severe surfactant inhibition even in the absence of elevated cholesterol levels. Surfactant was also impaired in ARDS mice where the cholesterol to phospholipid ratio (W/W%) was increased. Methyl-β-cyclodextrin (MβCD) restored surfactant function to normal in both human and animal samples. Model studies showed that the inhibition of surfactant was due to both elevated cholesterol and an interaction between cholesterol and oxidized phospholipids. MβCD was also shown to have anti-inflammatory effects.
Conclusions
Inhaled cyclodextrins have potential for the treatment of ARDS. They could be delivered in a portable device carried in combat and used following exposure to toxic gases and fumes or shock secondary to hemorrhage and burns.
Keywords: ARDS, pulmonary surfactant, cyclodextrin, oxidation, cholesterol, inflammation, bronchiolitis, mouse lung injury model
INTRODUCTION
Acute respiratory distress syndrome (ARDS) is common in the acute care setting and constitutes a high burden for the health care system and society (78.9/100,000 persons per year in the United States [US]), with mortality ranging between 30% and 50%.1 Four clinical manifestations characterize the disease.1,2 In the initial stage, both tachypnea and dyspnea are noted with relatively normal PaO2. The second stage often begins within 12–72 h of the onset of the initial phase and is characterized by physiological evidence of injury with an influx of granulocytes into the lungs.1,3 At this stage, surfactant becomes dysfunctional, resulting in high surface tension throughout the lung, leading to alveolar collapse, hypoxemia, and onset of respiratory failure.4 Over the next several days, a third stage ensues in which acute respiratory failure necessitates mechanical ventilation. If resolution does not occur, a fourth stage of progressive respiratory failure ensues, leading to pulmonary fibrosis or death.5
Pulmonary surfactant is necessary for normal lung function. It reduces the work required for breathing by decreasing the surface tension within the distal airspaces.6,7 It prevents the collapse of small airways and plays a significant role in host defense.8 Surfactant deficiency is associated with neonatal respiratory distress syndrome, which is rescued by administering exogenous surfactant soon after birth.9 However, studies of patients with ARDS show that endogenous surfactant is not merely deficient but altered by a variety of different inhibitory mediators. In these circumstances, the disease does not respond to exogenous surfactant therapy.10
The pathology of ARDS is complex and involves damage to the alveolar/capillary boundary, ventilation–perfusion mismatch, edema, inflammation, oxidative stress, and surfactant dysfunction.11 Acute respiratory distress syndrome is associated with a wide range of precipitating factors, including inhalation of toxic compounds and gases,11 but also factors as diverse as sepsis, burns, aspiration of gastric contents, barotrauma, and pneumonia.12 Dysfunctional pulmonary surfactant is also associated with reduced patency of small airways in cystic fibrosis.13 Current therapies for ARDS have proven disappointing with continuing high mortality.1 Despite evidence of surfactant deficiency in ARDS, surfactant replacement therapy has been unsuccessful.10
We have previously shown that elevated levels of cholesterol (20%) to be a potent surfactant inhibitor, a condition met in ARDS.4,14 In early ARDS, cholesterol levels are not elevated, yet surfactant is dysfunctional. However, cholesterol is still necessary for surfactant inhibition.15 This finding has led us to investigate other means whereby surfactant is inhibited at normal cholesterol levels.
In the current study, we investigated whether impaired surfactant films can be repaired by exposure to a cholesterol-sequestering agent, methyl-β-cyclodextrin (MβCD), in animal models of acute lung injury, in lung fluids obtained from patients with acute bronchiolitis, and in vitro model studies.
Cyclodextrins are rigid cone-shaped structures with a hydrophilic outer surface and a hydrophobic inner cavity.16 The relatively hydrophobic interior of the toroid-shaped MβCD molecule is able to host various hydrophobic molecules, including cholesterol and pro-inflammatory hydrolysis products resulting from the oxidation of surfactant phospholipids.15,17 The findings reported here provide strong evidence that cyclodextrins can reverse surfactant dysfunction in bronchiolitis and ARDS. Furthermore, cyclodextrins can be easily delivered as an aerosol either by jet nebulizer or via portable dry powder inhalation (DPI) device.
METHODS
Patient Studies
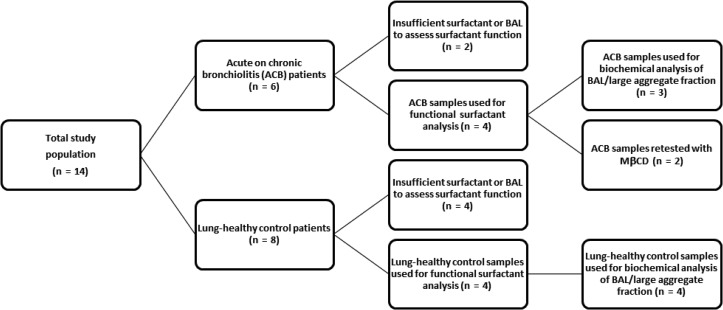
A total of 14 patients were enrolled from the Alberta Children’s Hospital (Calgary, Alberta, Canada) where bronchoalveolar lavage BAL is part of patient care. No changes to the routine clinical management of the patients were required for this research and only BAL surplus to diagnostic needs was used. The BALs were obtained from two groups: the first were eight asymptomatic patients, with a variety of conditions requiring investigative bronchoscopy. These control subjects had no evidence of current respiratory infection and cultures of their BAL were negative. Details of bronchoscopy and BAL procedure have been previously published.18
Bronchoalveolar lavages were also performed on three patients from a family with primary ciliary dyskinesia and one patient with chronic bronchiolitis of unknown cause. These patients had acute on chronic airway inflammation. A flow chart for the two pediatric patient groups is shown in Figure 1. Approval of the research protocol was obtained from the University of Calgary Child Health Scientific Review Committee/Conjoint Health Research Ethics Board, Calgary, Alberta, Canada (id E-17733). Informed consent for the research was obtained from the parents of the children. BAL cell differentials and microbiology were determined by Calgary Laboratory Services. Surplus BAL was centrifuged at 400 g for 10 min at 4°C to separate the supernatant from the cellular pellet and one aliquot was used for biochemical analysis. The remaining aliquot was then centrifuged at 40,000 g for 30 min at 4°C. The resulting pellets, consisting of the large aggregate portion of lung surfactant (Veldhuizen et al 1996), were used for surface tension measurements.
Figure 1.
Flow chart of patient selection. BAL, bronchoalveolar lavage; ACB, acute on chronic bronchiolitis. Not all samples had sufficient sample to conducts all of the tests.
Materials
Bovine lipid extract surfactant (BLES) was donated by BLES Biochemicals Inc. (London, ON, Canada). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). All lipids were stored at −20°C under nitrogen. Surfactant mixtures were stored at 4°C under nitrogen and used within 3 d. Buffers containing HEPES were stored in the dark at 4°C to avoid generating H2O2.
Cell-free BAL fluid was obtained as described above and assessed for protein content using a bicinchoninic acid (BCA) assay (Cat no.: RK-36490-00; Thermo Scientific, Rockford, IL, USA). Sodium dodecyl sulfate (SDS) was added at a final concentration of 2% to minimize interference from lipids. Surfactant was extracted according to the method of Bligh and Dyer.19 Phospholipid concentration was assessed using a phosphate assay (QuantiChrom phosphate assay kit, Cat no.: DIPI-500; BioAssay Systems, Hayward, CA, USA). Cholesterol level (w/w phospholipids) was determined using an enzymatic assay for cholesterol (Amplex Red Cholesterol Assay, Cat no.: A12216; Invitrogen, Eugene, OR, USA).
In Vitro Model Studies
Preparation of the Surfactants
Bovine lipid extract surfactant with a phospholipid concentration of 27 mg/mL was a gift by the manufacturer (BLES Biochemical, London, ON, Canada). Cholesterol was purchased from Sigma Chemicals (St. Louis, MO, USA). A solution of 1:1:1 ratio of methanol, chloroform, and BLES by volume was first vortexed and then centrifuged at 100 g for 5 min. The methanol/water phase was discarded and the BLES in chloroform was retained and none, 5% w/w, or 20% w/w of cholesterol, with respect to phospholipids, was added. Each solution was then dried under N2 and resuspended with buffer (140 mM NaCl, 10 mM HEPES, and 2.5 mM CaCl2; pH 6.9) to obtain an aqueous suspension of BLES and cholesterol at a concentration of 27 mg/mL phospholipids.
In Vitro Oxidation
Reaction mixtures were composed of 27 mg/mL BLES, plus Fenton reagents, hypochlorous acid in working buffer (in mM): 0.150 NaCl, 20 Tris–HCl, and 1.5 CaCl2 at pH 7.4. The samples were incubated at 37°C in a shaking water bath, normally for 24 h. For standard oxidation by the Fenton-like chemistry, BLES at 27 mg/mL was incubated with 0.65 mM FeCl2, 0.65 mM sodium ethylenediamine tetraacetic acid (EDTA), and 30 mM H2O2 in working buffer at a pH of 7.4. Treatment with HOCl/-OCl was carried out at a final concentration of 0.5 mM at pH 7.4 in working buffer. After oxidation or control incubations, the lipid fraction was isolated by the method of Bligh and Dyer19 (organic extraction) and conserved at −20°C until the samples were tested via computer-controlled captive bubble surfactometer (CBS).20 Oxidation of surfactant phospholipids was confirmed by measuring the formation of secondary lipoperoxidation products malondialdehyde (MDA) and 4-hydroxyalkenal (4-HAE) (BIOXYTECH LPO-586; OxisResearch, Burlingame, CA, USA).
Surface Tension Assessment
Surface activity of surfactant was determined with CBS as described in detail by Gunasekara et al21 Interfacial area and surface tension were calculated using the bubble height and diameter. The method is used to determine surface tension after surfactant adsorption and during quasi-static and dynamic compressions of the bubble.
To evaluate the effect of MβCD, powdered MβCD (Sigma-Aldrich, Catalogue-Nr. C4555) was dissolved in buffer to a final concentration of 40 mg/mL and added to the CBS chamber before the addition of a surfactant, whereas 10 mg/mL was used in the animal model. Assessment of surface activity was as described above.
Acid Aspiration Studies of Acute Respiratory Distress Syndrome in Mice
Acute Ex Vivo Study of MβCD in ARDS
Animal Model: To test whether surfactant impairment is seen in ARDS and whether it can be reversed with MβCD, we employed a previously established acid (HCl) injury mouse model of lung inflammation.22 This injury is histologically characterized by an acute inflammatory response with neutrophilic inflammation and release of reactive oxygen species (ROS).
Ten- through twelve-weeks-old C57BLK/6 mice were obtained from Jackson laboratories (Bar Harbor, ME, USA) and housed in a pathogen-free animal care facility at the University of Calgary. The entire protocol (no. AC13-0105) was approved by the University of Calgary Animal Care Committee. The mice were randomly grouped into three groups: acid installation (n = 20), saline instillation (n = 9), and baseline (n = 5). For the induction of acid-mediated lung injury, mice were anesthetized using 3% inhaled isoflurane and acid aspirated as described by Allen et al22 Mice were positioned vertically upright, their tongues were retracted, and their oropharynx carefully instilled with either hydrochloric acid (HCL; pH 0.95–1, 4 μL/g) or normal saline using a long plastic pipette tip. The tongue was retracted carefully until the mice fully aspirated the fluid. After aspiration-associated sounds had ceased, the tongue was released. Then mice were briefly rotated to the left and right lateral decubitus position to equally distribute the fluid among the lung tissue. Subsequently, the mice were allowed to recover from anesthesia and monitored at regular time intervals for a period of 24 h. Animals that appear to be in discomfort were given 0.05 mg/kg buprenorphine subcutaneously every 4–12 h. Animals that appear to be in distress were humanely euthanized.
Following pentobarbital (90 mg/kg) anesthesia, lung mechanics were measured 24 h following acid/saline aspiration using a Flexivent (SCIREQ, Montreal, Canada) system. Tissue elastance (H) and airway resistance (Rn) were recorded.23
Necropsy and Processing of Biologic Samples
After recording pulmonary function, animals were humanely euthanized with intraperitoneal injection (i.p.) of sodium pentobarbital (90 mg/kg). Pulmonary lavage with 0.5 mL of warm 0.9% saline was performed and the BAL fluid immediately centrifuged (400 g × 10 min × 4°C) to remove cellular components. The pellet was resuspended in PBS buffer and differential cell counts determined following centrifugation and staining.
Finally, the supernatant was re-centrifuged using ultra-high-speed centrifugation (40,000 g × 30 min × 4°C) to separate large aggregate (pellet) and small aggregate (supernatant) fractions of pulmonary surfactant. The surfactant was resuspended in 1 μL of PBS buffer and its function measured in a CBS.
Pilot In Vivo Study of MβCD for Potential Toxicity and Efficacy in the Acid Injury Model
Experimental Design and Procedures
A pilot study was conducted to examine the effects of MβCD treatment in the mouse model to determine any potential toxicity of the treatment and to characterize the method of aerosol delivery. These experiments used three groups of five mice followed over an 11-d schedule. Group 1 mice received acid injury using hydrochloric acid as described above followed by aerosolized methyl-β-cyclodextrin (S-1229) powder dissolved in normal saline (10 mg/mL). Group 2 mice were pre-treated with aerosolized acid followed by an aerosol of normal saline (control). A third group received no pre-treatment (naïve) followed by aerosolized methyl-β-cyclodextrin. All mice of a single exposure group were placed in a plexiglass exposure chamber together and treated with either MβCD or saline aerosol for 10 min. This procedure was repeated six times over a 3-d period followed by 3 d of rest.
The S-1229 and saline aerosol were generated using an AeroGen X2 nebulizer head. Particle size was determined by cascade impaction.
On day 10, the mice were weighed and euthanized and BAL with saline performed. After centrifugation of the BAL fluid and cytocentrifugation, slides were stained with Siemens Diff-Quick Stain set (B4132-1A) and differential cell counts performed. The lungs were then inflated to total lung capacity within the thoracic cage with 10% phosphate-buffered formaldehyde. The lungs, heart, liver, kidney, and spleen were also processed for histology and stained with hematoxylin and eosin. All animal protocols were approved by the University of Calgary Animal Care Committee.
RESULTS
Clinical Studies
Surfactant Function in Children with Acute on Chronic Bronchiolitis
Table I shows the demographic information on patients with sufficient BAL fluid for at least one test. There were no significant differences in age or sex between groups, although there were more males than females in both groups. However, two of four acute on chronic bronchiolitis patients had ≥103 CFU/mL of lavage fluid, a level considered indicative of active infection in this patient population. None of the lung-healthy control cases had clinical or laboratory evidence of active airway infection.
Table I.
Demographic Information for Lung-healthy Control and Bronchiolitis Patients
| Subject Details | Lung-Healthy Control (n = 8) | Acute on Chronic Bronchiolitis (n = 4) |
|---|---|---|
| Age (median, range) | 6.10 (1.68–14.68) | 3.84 (2.06–8.68) |
| Sex (male/female) | 6/2 | 3/1 |
| >103 CFUa/mL bacterial/fungal organisms in BAL | 0/8 | 2 (2 not known) |
| Exacerbationb (yes) | 0/8 | 3/4 |
| Bronchoscopic appearances (normal/mild–moderate/severe) | 6/2/0 | 0/2/2 |
Note. No significance observed, Mann–Whitney U-test.
aCFU: Colony-forming units.
bAs determined by the clinician on day of study.
Cellular Analysis of Bronchalveolar Lavage
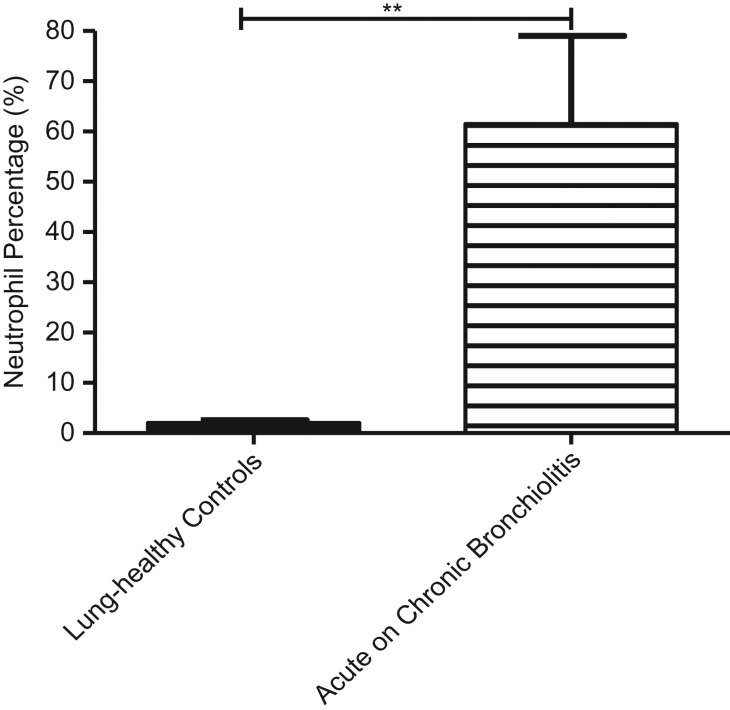
The percentage of polymorphonuclear leukocytes in the BAL was significantly greater in the acute on chronic bronchiolitis patients (p = 0.0011) group compared with lung-healthy control subjects (Fig. 2).
Figure 2.
Mean-(SEM) of % polymorphonuclear leukocytes in the two groups. The proportion of neutrophils in acute on chronic bronchiolitis samples was significantly greater than lung-healthy controls (**p ≤ 0.01).
Biochemical Characterization of BAL Samples
Biochemical analyses of BAL supernatants from the subjects are shown in Table II. Cholesterol levels were not elevated in acute on chronic bronchiolitis patients (4.87 ± 0.48 wt%) compared with lung-healthy controls (4.96 ± 0.70 wt%).
Table II.
Biochemical Analysis of BAL Fluid
| Measured Parameter | Acute on Chronic Bronchiolitis Patients (n = 3) | Lung-Healthy Control Patients (n = 4) |
|---|---|---|
| Total BAL protein (μg/mL) | 247.51 ± 126.00 | 181.97 ± 53.08 |
| Large aggregate phospholipids (wt%) | 86.54 ± 6.61 | 78.30 ± 5.39 |
| Cholesterol: LA phospholipids (wt%) | 4.87 ± 0.48 | 4.96 ± 0.70 |
Note. Data presented as mean ± SEM (no significance between the two tested groups, Mann–Whitney U-test).
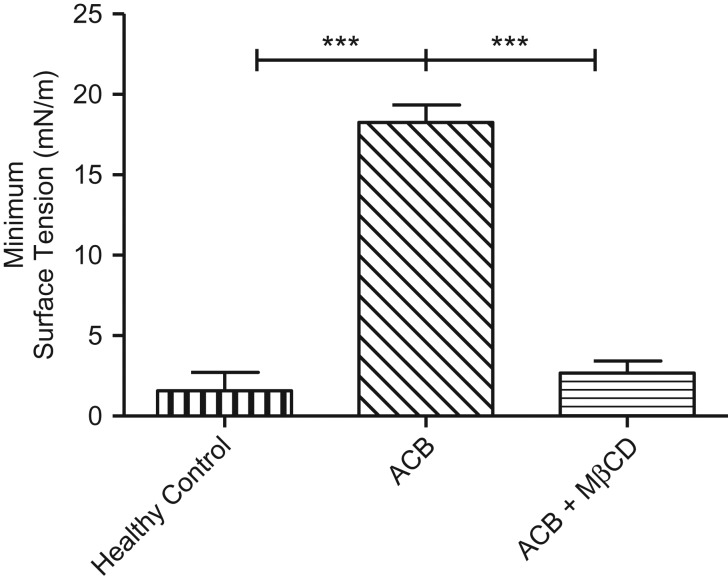
Minimum surface tension during dynamic compression for the two patient groups are shown in Figure 3. Surfactant from healthy lungs revealed a surface tension of almost zero at residual lung capacity. Acute on chronic bronchiolitis patient samples did not achieve low minimum surface tension during CBS compression cycles (γ ≈ 17 mN/m). In summary, we report consistent near-zero surface tensions for lung-healthy control samples and elevated minimum surface tensions (>12 mN/m) for a majority of acute on chronic bronchiolitis cases.
Figure 3.
Mean (SEM) minimum surface tensions following dynamic cycling. Lung-healthy controls achieved low (normal) surface tensions (vertical). Acute on chronic bronchiolitis (ACB) patient samples showed severe impairment in surfactant function (diagonal). ACB samples after methyl-β-cyclodextrin treatment (MβCD 40 mg/mL; horizontal) (***p ≤ 0.001).
To test whether MβCD could recover surfactant function at physiologic cholesterol levels, two of the acute on chronic bronchiolitis samples with sufficient sample were retested in buffer containing MβCD (Fig. 3). During dynamic cycling, minimum surface tension declined from an average of 18.04–3.45 mN/m following treatment with MβCD, suggesting that molecules other than cholesterol, such as pro-inflammatory hydrolysis products of surfactant, play an important role in surfactant impairment in acute bronchiolitis. In summary, after MβCD treatment, both acute on chronic bronchiolitis samples resembled control surfactant in terms of minimum surface tension and film stability.
In Vitro Model Studies
Reactive-Oxygen-Mediated Surfactant Inhibition
In the captive bubble surfactometer, surface tension is manipulated by successive compression–expansion cycles, providing in-depth information about the sample quality and functionality. Surfactant behavior during dynamic cycling was used as the main indicator of surfactant function.
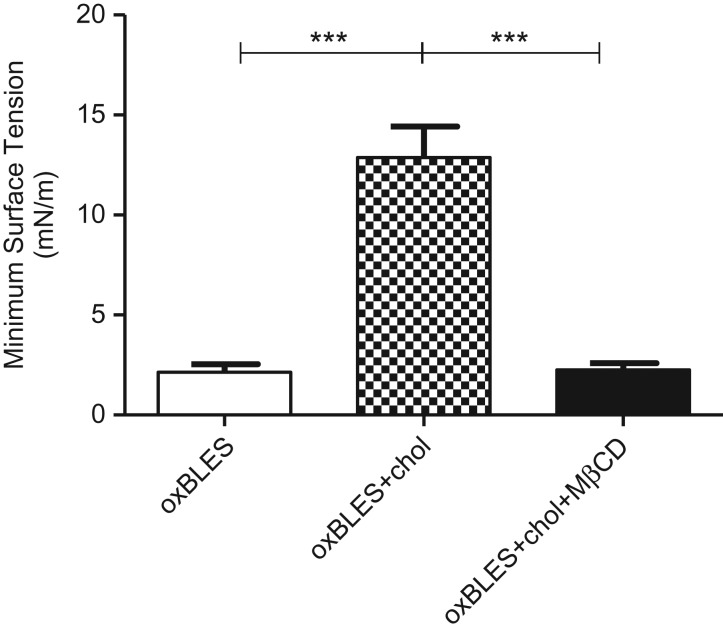
Oxidation (oxBLES) alone had a minimal effect on the function of BLES in the absence of cholesterol. However, this effect was enhanced by the addition of a physiologic amount (10%) of cholesterol (Fig. 4). The role of cholesterol in inducing dysfunction in oxidized surfactant was further confirmed by exposing oxBLES + 10% cholesterol to MβCD, a compound that has been shown to reverse surfactant dysfunction caused by cholesterol by selectively removing cholesterol from the film.15,24 MβCD significantly improved the surface activity of oxBLES + 10% cholesterol in terms of compressibility (data not shown) and minimum surface tension (Fig. 4).
Figure 4.
Minimum surface tensions during dynamic compression–expansion cycles of oxidized bovine lipid extract surfactant (oxBLES, cholesterol free) (white), oxBLES + 10% w/w cholesterol in control CBS buffer (checker), or buffer containing 30 mM MβCD (black).
A prominent target of ROS-induced oxidation is polyunsaturated phospholipid, constituting ≃10% of total phospholipids in surfactant.25 Oxidative inactivation of the surfactant system by oxygen radical introduces significant levels of lipid peroxidation, conjugated dienes, and lipid degradation products (malondialdehyde and 4-hydroxynonenal).26 Further, phospholipid hydrolysis products, free fatty acids (FFA) are generated in considerable quantities (~15–20% w/w) upon ROS exposure and are capable of surfactant inhibition.27 Linoleic acid is increased in ARDS and has been shown to be a major inflammatory mediator.25,28
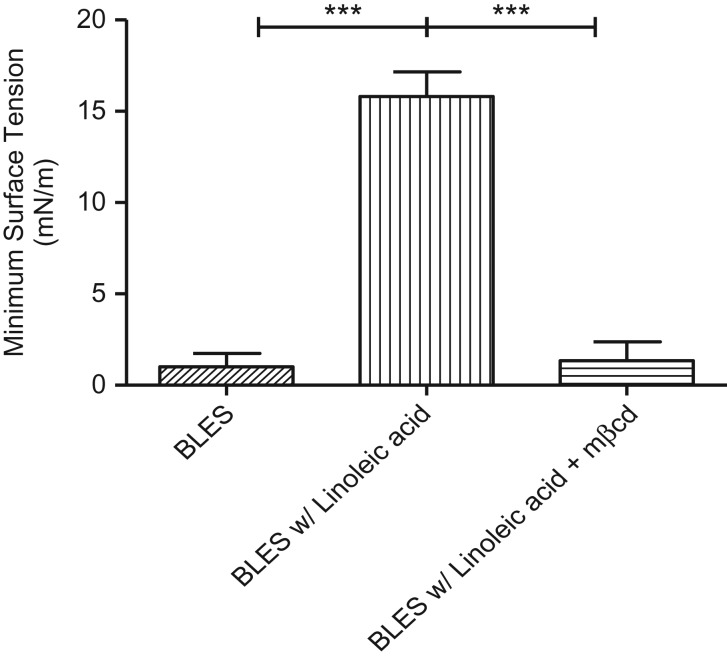
Minimum surface tension during dynamic compression for clinical surfactant with 20% w/w linoleic acid is shown in Figure 5. Linoleic acid impaired surfactant function; samples did not achieve low minimum surface tension following dynamic compression (γ ≈ 16 mN/m) (Fig. 5). Furthermore, FFA-mediated surfactant inhibition was reversed by MβCD, even in the relative absence of cholesterol (Fig. 5). This finding likely reflects the capacity of MβCD to sequester non-steroidal lipids in addition to cholesterol, though with lesser affinity.17 In summary, the effect of oxidation on surfactant function was reversed with MβCD.
Figure 5.
Minimum surface tension during dynamic cycle 20 with BLES containing 27 mg/mL BLES in control CBS buffer (diagonals bar), BLES with 20% w/w free fatty acid (linoleic acid) (vertical bar), and BLES with 20% w/w free fatty acid + 40 mg/mL MβCD (horizontal bar). (**p ≤ 0.01, ***p ≤ 0.001). BLES with FFA or LPC shows marked impairment, which is repaired to normal functionality in the presence of MβCD.
Animal Model Studies
Acute Ex Vivo Study of MβCD in ALI
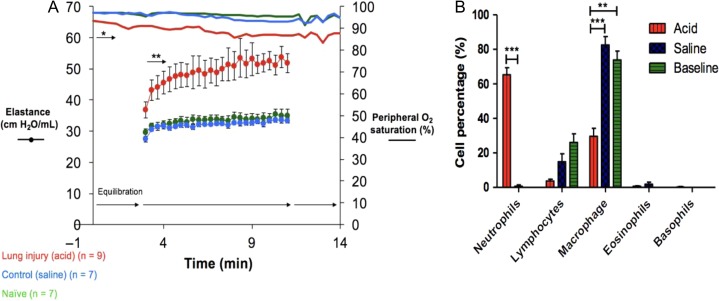
To test the association between surfactant dysfunction and neutrophilic mediated inflammation, we used the acid aspiration model of lung injury in mice. Lung mechanics were compared among the three mice groups during mechanical ventilation (Flexivent) 24 h after acid or saline instillation. The acid-injured mice demonstrated significant increases in elastance (inverse of compliance) compared with baseline and saline-aspirated mice (Fig. 6A). No significant differences in airway resistance were found between groups (data not shown). Neutrophil differentials were significantly increased in acid-exposed animals (p < 0.001) (Fig. 6B). Histologic examination of lung sections in 24 h post-treatment showed acute inflammation in distal small airways and neutrophilic infiltration in the adjacent alveoli and hyaline membranes.
Figure 6.
(A) Mean values (±SE) for elastance (H) and peripheral oxygen saturation are plotted against time at positive end-expiratory pressure (PEEP) level 1 cmH2O in saline-exposed C57BL/6 (n = 6), baseline C57BL/6 (n = 6), acid-exposed C57BL/6 (n = 7) mice. (B) Differential cell count percentage on BAL cytospin films: *p < 0.05; **p < 0.01; ***p < 0.001.
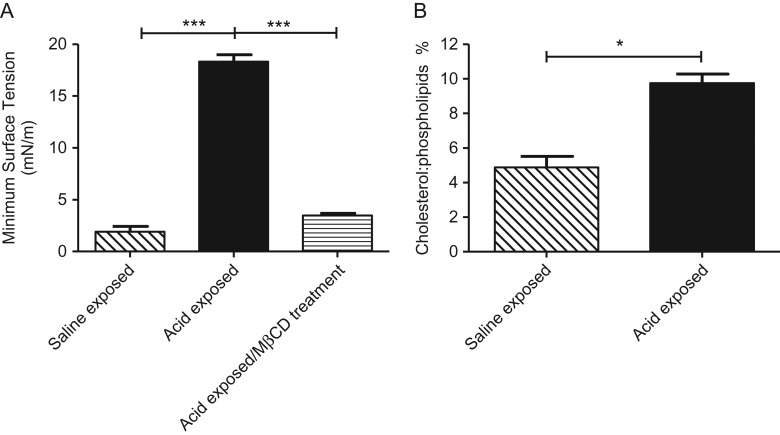
Surfactant obtained from healthy mice (MLES) exposed to aspirated saline displayed normal surface activity. Samples from acid-exposed mice showed marked surfactant dysfunction, which was restored by treatment with MβCD (Fig. 7A).
Figure 7.
(A) Minimum surface tensions during dynamic compression–expansion cycles of mouse lipid extract surfactant (MLES), from saline-exposed, acid-exposed, or CBS buffer containing 30 mM MβCD (horizontal) animals, *p < 0.001. (B) Cholesterol to phospholipid ration (W/W %) for saline-exposed (n = 9 pooled) and acid-exposed (n = 10 pooled) mice large aggregate fraction of the BAL (*p < 0.05).
The cholesterol to phospholipid ratio (w/w %) for saline-treated (n = 9) and acid-treated (n = 10) mouse surfactant fraction of the BAL is shown in Figure 7B. Cholesterol was significantly increased in the acid-aspirated group (p < 0.05) compared with saline-aspirated controls.
Pilot In Vivo Study of MβCD for Potential Toxicity and Efficacy in the Acid Injury Model
With the exception of three animals that were euthanized as per approved animal protocol due to acute respiratory distress immediately following acid aspiration, all animals survived to the 10-d sacrifice. At necropsy, there was patchy evidence of resolving acute lung injury in all acid-exposed mice. However, there were minimal neutrophils within the lesions of the MβCD-treated group compared with the saline-treated group; a finding reflected in the increased neutrophils in the BAL of mice treated with saline (10.6% ± 6.37 SD) compared with those treated with MβCD (1.1% ± 0.36 SD) (p = 0.023). Histological examination of organs removed at necropsy showed no abnormalities, indicative of lack of MβCD toxicity in any group. Specifically, there was no evidence of MβCD toxicity in lungs and kidneys compared with saline-treated animals. The mass median aerodynamic diameter (MMAD) for the MβCD particles as determined with a cascade impactor ranged from 0.9 μm to 1.5 μm.
DISCUSSION
In the studies reported here, we show that surfactant obtained from animals with acute lung injury and patients with acute on chronic bronchiolitis is dysfunctional. Furthermore, we show that dysfunctional surfactant can be restored to normal function in situ with methyl-β-cyclodextrin (MβCD). The animal and human experiments were complemented by in vitro and ex vivo experiments to determine the mechanism of action of surfactant inhibition. We show that the primary mechanism for surfactant inhibition (for two different injurious agents) involves either elevated cholesterol alone or an interaction between normal levels of surfactant cholesterol and oxidized phospholipids. Finally, we show that MβCD not only sequesters cholesterol but can also sequester a pro-inflammatory lipid breakdown product (18-C fatty acid) that causes inflammation in acute lung injury. We focused on linoleic acid as this has been shown to be a major driver of lung inflammation in ARDS.29 MβCD thus has potential for restoring function of damaged surfactant as well as dampening the inflammatory response in acute lung injury.18
A limitation of this study was that the human population we examined for the determination of surfactant dysfunction and its reversal with cyclodextrins may not be representative of an adult human population exposed to environmental airborne toxicants. An ideal study population would have comprised adult military personnel or firefighters exposed to airborne toxicants. Unfortunately, these populations are unlikely to be available for research study as sampling would be invasive and bronchial lavage would not be indicated without prior proof of principle in a human population. We chose this pediatric patient population because the children were already undergoing pulmonary lavage for diagnostic purposes and our study only used surplus lavage material, without compromising patient care. Although not ideal, we believe that this population is relevant because they are susceptible to environmental lung disease due to their young age and genetic predisposition. Most of the children had primary ciliary dyskinesia, a genetic disorder that impairs their ability to clear inhaled particles and bacteria.30
Additional studies are indicated in other high-risk human populations with pre-existing diseases, such as cigarette smokers31 with chronic obstructive pulmonary disease, patients with acute inhalation injuries, immunocompromised patients, and other patient groups undergoing diagnostic pulmonary lavage. These studies can confirm or refute if the findings presented here are generally applicable to acute lung injury.
In this study, we explored the mechanisms whereby surfactant becomes impaired in acute lung injury. Cholesterol is elevated in ARDS patients, rendering surfactant dysfunctional.24,32 A similar finding has been described for children with cystic fibrosis.18 However, we show that for some pulmonary diseases such as acute on chronic bronchiolitis (reported in this study), the surfactant film was dysfunctional despite physiological levels of cholesterol. This finding suggests that surfactant inhibition can be cholesterol-dependent even at physiological levels of cholesterol.
To understand this apparent anomalous finding, we examined the mechanism whereby pulmonary surfactant becomes dysfunctional in the presence of normal cholesterol. We focused on oxidative injury as this plays a central role in the pathogenesis of ARDS and is the primary mechanism of injury caused by a majority of toxicants including, gases and fumes, chemical warfare agents, and exposures related to deployment and experienced by firefighters.31,33–36
The in vitro studies presented here reveal the important role of oxidative stress on surfactant and that an interaction of oxidized phospholipids with cholesterol is an important cause of surfactant film impairment.
It has been reported that the lungs of patients suffering from ARDS have increased levels of ∙NO and O∙2- released by activated alveolar macrophages and neutrophils.37 Because ∙NO and O∙2- rapidly react to form peroxynitrite in the epithelial lining fluid, surfactant function becomes impaired by damage to surfactant-specific proteins and lipid peroxidation. Although it is generally accepted that lipid hydroperoxides are the predominant oxidative products of autoxidation, there is evidence suggesting that epoxide-containing lipids are also produced, where they could also have a deleterious effect on lipid membrane organization and function.38 The presence of lipid epoxides are thought to originate from enzymatic action of monooxygenases. However, it was also shown that lipid epoxides are produced non-enzymatically in the lung, through the process of autoxidation.38
Cyclodextrins are complex sugars with low toxicity, used as vehicles for delivering many drugs and sequestration of toxicants.39 In this study, we used MβCD for its ability to efficiently take up cholesterol. However, we also showed that it can sequester linoleic acid, an important lipid mediator of inflammation in ARDS as well as suppress acute inflammation in our animal model of ALI. Others have shown that cyclodextrins have anti-inflammatory effects in the airways,40 a finding seen in our pilot animal study. Thus, inhaled cyclodextrins may have two important properties for treating acute lung injury; an ability to repair dysfunctional surfactant and an anti-inflammatory effect.
Methyl-β-cyclodextrin can be readily delivered as an aerosol either by jet nebulizer or via a portable dry power inhalation (DPI) device. The particle size measured in this study (MMAD ranging from 0.9 μm to 1.5 μm) are of a size that is predicted to penetrate to the small airways and alveoli. Once a suitable dose has been identified, we will formulate MβCD within a DPI delivery device for further clinical development. A DPI device ultimately allows the most portability and convenience for delivery of MβCD. We anticipate that this small portable device could be deployed in combat (or other circumstances where exposure to toxic gases or smoke is likely to occur) in order to treat personnel after exposure to toxic gases and fumes or shock secondary to hemorrhage and burns. MβCD could be administered as prophylaxis to prevent the onset of ARDS or used to treat established ARDS during transfer or in the ICU. We have obtained US patent (13/629,474) for the treatment of ARDS by cyclodextrins. Before clinical use, we will need to complete further efficacy studies in animals, toxicology, phase I safety, and phase II dose-ranging studies.
CONCLUSIONS
We have shown, using samples from human subjects, animals with acute lung injury and in vitro studies that we can repair/restore normal function to damaged surfactant. We have also shown that the mechanism of the impairment involves elevated cholesterol and/or an interaction between cholesterol and oxidative damage to surfactant. Both types of surfactant dysfunction can be restored/repaired in situ with a cyclodextrin drug (MβCD). Finally, we provide preliminary data showing that MβCD may also have anti-inflammatory properties in situations involving acute lung and airway injury.
Acknowledgments
We thank the CF clinics, doctors, nurses, and Lori Fairchild for assistance with sample collection and Drs. Samuel Schürch and W. Michael Schoel for their pioneer work in surfactant research. We thank Sam Shrestha for his help with manuscript preparation.
Conflicts of Interest
All other authors have no conflict of interest to disclose.
Presentations
Presented as a poster at the 2016 Military Health Systems Research Symposium (abstract number: MHSRS-16-1348).
Funding
This research has been supported by operating grants from the Canadian Institutes for Health Research (CIHR), Alberta Innovates Health Solutions (AIHS-CRIO), Calgary Laboratory Services (CLS), and the Alberta Lung Association (ALA).
REFERENCES
- 1. Zambon M, Vincent JL: Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest 2008; 133(5): 1120–7. [DOI] [PubMed] [Google Scholar]
- 2. Demling RH: The modern version of adult respiratory distress syndrome. Annu Rev Med 1995; 46: 193–202. [DOI] [PubMed] [Google Scholar]
- 3. Li G, Malinchoc M, Cartin-Ceba R, et al. : Eight-year trend of acute respiratory distress syndrome. Am J Respir Crit Care Med 2011; 183(1): 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leonenko Z, Gill S, Baoukina S, et al. : An elevated level of cholesterol impairs self-assembly of pulmonary surfactant into a functional film. Biophys J 2007; 93(2): 674–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ware LB: Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med 2006; 27(212): 337–49. [DOI] [PubMed] [Google Scholar]
- 6. Al-Saiedy M, Tarokh A, Nelson S, et al. : The role of multilayers in preventing the premature buckling of the pulmonary surfactant. Biochim Biophys Acta 2017; 1859(8): 1372–80. [DOI] [PubMed] [Google Scholar]
- 7. Akella A, Deshpande SB: Pulmonary surfactants and their role in pathophysiology of lung disorders. Indian J Exp Biol 2013; 51(1): 5–22. [PubMed] [Google Scholar]
- 8. Sorensen GL, Husby S, Holmskov U: Surfactant protein A and surfactant protein D variation in pulmonary disease. Immunobiology 2007; 212(4): 381–416. [DOI] [PubMed] [Google Scholar]
- 9. Amato M, Petit K, Fiore HH, Doyle CA, Frantz ID, Nielsen HC: Effect of exogenous surfactant on the development of surfactant synthesis in premature rabbit lung. Pediatr Res 2003; 53(4): 671–8. [DOI] [PubMed] [Google Scholar]
- 10. Maruscak A, Lewis JF: Exogenous surfactant therapy for ARDS. Expert Opin Investig Drugs 2006; 15(1): 47–58. [DOI] [PubMed] [Google Scholar]
- 11. Adhikari NKJ, Burns KE, Friedrich JO, Granton JT, Cook DJ, Meade MO: Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. BMJ 2007; 334(7597): 779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leaver SK, Evans TW: Acute respiratory distress syndrome. BMJ 2007; 335: 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Griese M, Essl R, Schmidt R, et al. : Sequential analysis of surfactant, lung function and inflammation in cystic fibrosis patients. Respir Res 2005; 6(1): 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gunasekara L, Schürch S, Schoel WM, et al. : Pulmonary surfactant function is abolished by an elevated proportion of cholesterol. Biochim Biophys Acta 2005; 1737(1): 27–35. [DOI] [PubMed] [Google Scholar]
- 15. Gunasekara LC, Pratt RM, Schoel WM, Gosche S, Prenner EJ, Amrein MW: Methyl-β-cyclodextrin restores the structure and function of pulmonary surfactant films impaired by cholesterol. Biochim Biophys Acta 2010; 1798(5): 986–94. [DOI] [PubMed] [Google Scholar]
- 16. López CA, de Vries AH, Marrink SJ, Swendsen R, Kollman P: Molecular mechanism of cyclodextrin mediated cholesterol extraction. PLoS Comput Biol 2011; 7(3): e1002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brunaldi K, Huang N, Hamilton JA: Fatty acids are rapidly delivered to and extracted from membranes by methyl-beta-cyclodextrin. J Lipid Res 2010; 51(1): 120–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gunasekara L, Al-Saiedy M, Green F, et al. : Pulmonary surfactant dysfunction in pediatric cystic fibrosis: Mechanisms and reversal with a lipid-sequestering drug. J Cyst Fibros 2017; 16(5): 565–72. [DOI] [PubMed] [Google Scholar]
- 19. Bligh E, Dyer W: A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1959; 37(8): 911–7. [DOI] [PubMed] [Google Scholar]
- 20. Manzanares D, Rodriguez-Capote K, Liu S, et al. : Modification of tryptophan and methionine residues is implicated in the oxidative inactivation of surfactant protein B. Biochemistry 2007; 46(18): 5604–15. [DOI] [PubMed] [Google Scholar]
- 21. Gunasekara L, Schoel WM, Schürch S, Amrein MW: A comparative study of mechanisms of surfactant inhibition. Biochim Biophys Acta 2008; 1778(2): 433–44. [DOI] [PubMed] [Google Scholar]
- 22. Allen GB, Cloutier ME, Larrabee YC, Tetenev K, Smiley ST, Bates JHT: Neither fibrin nor plasminogen activator inhibitor-1 deficiency protects lung function in a mouse model of acute lung injury. Am J Physiol Lung Cell Mol Physiol 2009; 296(3): L277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seah AS, Grant KA, Aliyeva M, Allen GB, Bates JHT: Quantifying the roles of tidal volume and PEEP in the pathogenesis of ventilator-induced lung injury. Ann Biomed Eng 2011; 39(5): 1505–16. [DOI] [PubMed] [Google Scholar]
- 24. Vockeroth D, Gunasekara L, Amrein M, Possmayer F, Lewis JF, Veldhuizen RA: Role of cholesterol in the biophysical dysfunction of surfactant in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 2010; 298(1): L117–25. [DOI] [PubMed] [Google Scholar]
- 25. Fessler MB, Summer RS: Surfactant lipids at the host–environment interface. Metabolic sensors, suppressors, and effectors of inflammatory lung disease. Am J Respir Cell Mol Biol 2016; 54(5): 624–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andersson S, Kheiter A, Merritt TA: Oxidative inactivation of surfactants. Lung 1999; 177(3): 179–89. [DOI] [PubMed] [Google Scholar]
- 27. Hite RD, Seeds MC, Jacinto RB, Grier BL, Waite BM, Bass DA: Lysophospholipid and fatty acid inhibition of pulmonary surfactant: non-enzymatic models of phospholipase A2 surfactant hydrolysis. Biochim Biophys Acta 2005; 1720(1–2): 14–21. [DOI] [PubMed] [Google Scholar]
- 28. Bursten SL, Federighi DA, Parsons P, et al. : An increase in serum C18 unsaturated free fatty acids as a predictor of the development of acute respiratory distress syndrome. Crit Care Med 1996; 24(7): 1129–36. [DOI] [PubMed] [Google Scholar]
- 29. Quinlan GJ, Lamb NJ, Evans TW, Gutteridge JMC: Plasma fatty acid changes and increased lipid peroxidation in patients with adult respiratory distress syndrome. Crit Care Med 1996; 24(2): 241–6. [DOI] [PubMed] [Google Scholar]
- 30. Praveen K, Davis EE, Katsanis N: Unique among ciliopathies: primary ciliary dyskinesia, a motile cilia disorder. F1000Prime Rep 2015; 7: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Papaioannou AI, Papiris S, Papadaki G, et al. : Surfactant proteins in smoking-related lung disease. Curr Top Med Chem 2016; 16(14): 1574–81. [DOI] [PubMed] [Google Scholar]
- 32. Manson ME, Corey DA, Bederman I, Burgess JD, Kelley TJ: Regulatory role of β-arrestin-2 in cholesterol processing in cystic fibrosis epithelial cells. J Lipid Res 2012; 53(7): 1268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Falvo MJ, Osinubi OY, Sotolongo AM, Helmer DA: Airborne hazards exposure and respiratory health of Iraq and Afghanistan veterans. Epidemiol Rev 2015; 37(1): 116–30. [DOI] [PubMed] [Google Scholar]
- 34. Filipczak PT, Senft AP, Seagrave J, et al. : NOS-2 inhibition in phosgene-induced acute lung injury. Toxicol Sci 2015; 146(1): 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rose CS: Military service and lung disease. Clin Chest Med 2012; 33(4): 705–14. [DOI] [PubMed] [Google Scholar]
- 36. Gupta RC: The respiratory toxicity of chemical warfare agents In: Handbook of Toxicology of Chemical Warfare Agents, Ed 2, pp 489–518. Hopkinsville, KY, Elsevier, 2009. [Google Scholar]
- 37. Rodríguez-Capote K, Manzanares D, Haines T, Possmayer F: Reactive oxygen species inactivation of surfactant involves structural and functional alterations to surfactant proteins SP-B and SP-C. Biophys J 2006; 90(8): 2808–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sevanian A, Mead JF, Stein RA: Epoxides as products of lipid autoxidation in rat lungs. Lipids 1979; 14(7): 634–43. [DOI] [PubMed] [Google Scholar]
- 39. Kiss T, Fenyvesi F, Bácskay I, et al. : Evaluation of the cytotoxicity of β-cyclodextrin derivatives: evidence for the role of cholesterol extraction. Eur J Pharm Sci 2010; 40(4): 376–80. [DOI] [PubMed] [Google Scholar]
- 40. Cataldo D, Evrard B, Noel A, Foidart J-M: Use of cyclodextrin for treatment and prevention of bronchial inflammatory diseases. Vol. 2, United States Patent. US 7,829,550 B2, 2010:1–8.