Abstract
In many solid tumours a desmoplastic reaction takes place, which results in tumour tissue stiffening due to the extensive production of extracellular matrix (ECM) proteins, such as collagen, by stromal cells, mainly fibroblasts (FBs) and cancer-associated fibroblasts (CAFs). In this study, we investigated the effect of collagen stiffness on pancreatic FBs and CAFs, particularly on specific cytoskeleton properties and gene expression involved in tumour invasion. We found that cells become stiffer when they are cultured on stiff substrates and express higher levels of alpha-smooth muscle actin (α-SMA). Also, it was confirmed that on stiff substrates, CAFs are softer than FBs, while on soft substrates they have comparable Young's moduli. Furthermore, the number of spread FBs and CAFs was higher in stiffer substrates, which was also confirmed by Ras-related C3 botulinum toxin substrate 1 (RAC1) mRNA expression, which mediates cell spreading. Although stress fibres in FBs become more oriented on stiff substrates, CAFs have oriented stress fibres regardless of substrate stiffness. Subsequently, we demonstrated that cells' invasion has a differential response to stiffness, which was associated with regulation of Ras homologue family member (RhoA) and Rho-associated, coiled-coil containing protein kinase 1 (ROCK-1) mRNA expression. Overall, our results demonstrate that collagen stiffness modulates FBs and CAFs cytoskeleton remodelling and alters their invasion properties.
Keywords: atomic force microscopy, tumour desmoplasia, biomechanics, collagen, cancer-associated fibroblasts
1. Introduction
In many malignant tumours, including tumours of the pancreas, colon and breast, a desmoplastic reaction is known to take place during progression [1,2]. Tumour desmoplasia is a cancer-specific type of fibrosis characterized by extensive production of extracellular matrix (ECM) proteins, such as collagen type I by tumour stromal cells, mainly fibroblasts (FBs) and cancer-associated fibroblasts (CAFs) [1]. As a result, desmoplasia stiffens the tumour tissue and thus, increases the compressive mechanical forces in the tumour's interior [3–5]. Although it has long been known that this fibrotic response inhibits drug delivery and enhances tumour progression and metastasis, the exact underlying mechanisms are still unclear [6,7].
ECM stiffening and transforming growth factor-β (TGF-β) activation contribute to the conversion of FBs to activated, contractile myofibroblasts, also known as CAFs in the case of the tumour microenvironment [8]. CAFs further stimulate synthesis of ECM proteins reinforcing desmoplasia and tumour stiffening [8,9]. Matrix stiffening, in turn, causes an increase in TGF-β activation [8,10], which further promotes FB conversion [11] suggesting a positive feedback loop. Apart from CAFs, interactions of FBs with cancer cells and the ECM also play a crucial role in tumour progression [12]. Nevertheless, the effect of ECM stiffening has not, so far, been investigated in relation to morphodynamic, cytoskeletal or biophysical alterations of pancreatic stromal cells, which ultimately affect fundamental cellular processes related to cancer cell metastasis such as cell motility, adhesion and invasion into surrounding matrix [13,14].
In the present work, two (2D) and three (3D) dimensional collagen ECM models with varying collagen concentrations and thus, tunable stiffness were developed. In addition, atomic force microscopy (AFM), image processing techniques, in vitro cellular assays, including spreading and invasion assays, and molecular approaches, such as real-time polymerase chain reaction (PCR), were employed in order to investigate the effect of collagen stiffness on pancreatic FBs and CAFs. Finally, we study the effect of stiffness in the presence of TGF-β. Defining the mechanistic interactions between ECM stiffness and FBs/CAFs, can provide the basis for the development of novel treatments that target stromal components to reduce desmoplasia and improve drug delivery and efficacy [9,15–17].
2. Material and methods
2.1. Cell culture
Commercially available pancreatic native human FBs and CAFs (cat. nos. SC00A5 and CAF08, respectively, Neuromics) were cultured in MSC-GRO (VitroPlus III, low serum, complete, cat. no. SC00B1, Neuromics) medium in a 5% CO2-incubator at 37°C.
2.2. Fixation and permeabilization
Cells were first fixed with 4% paraformaldehyde (PFA, cat. no. P6148, Sigma) for 20 min and then a permeabilization buffer containing phosphate-buffered saline (PBS, cat. no. LM-S2041, biosera), 2 mg ml−1 bovine serum albumin (cat. no. A2153, Sigma), and 0.1% Triton X-100 (cat. no. 9002-93-1, Sigma) was used for permeabilizing the cell membranes.
2.3. Cell immunostaining
The α-smooth muscle actin (α-SMA) expression of cells was assessed by staining with anti-α-SMA antibody (cat. no. ab5694, Abcam) and Alexa 647 anti-rabbit antibody (cat. no. ab150079, Abcam) as the secondary antibody. Briefly, cells were fixed with 4% PFA, washed with permeabilization buffer and incubated overnight at 4°C with anti-α-SMA antibody. Samples were then washed three times with the permeabilization buffer and incubated with Alexa 647 anti-rabbit secondary antibody for 1 h at room temperature. Finally, cells were washed again three times with the permeabilization buffer and incubated for 2 min with 4′,6-diamidino-2-phenylindole (DAPI, cat. no. 10236276001 ROCHE, Sigma).
For staining actin stress fibres, the above protocol was followed with a minor change. Cells were incubated with phalloidin (cat. no. 00027, Biotium) for 1 h at room temperature and no secondary antibody was added.
All coverslips were then mounted on a slide and observed under an Olympus BX53 fluorescent microscope equipped with an Olympus XM10 Monochrome CCD camera (1.4 megapixels) and UPLanFLN microscope objective lenses (40×/0.75 and 100×/1.30 oil). Also, appropriate Chroma Technology filters were employed for imaging DAPI, α-SMA and phalloidin, namely the 49000 ET-DAPI (ex. 359 nm, em. 481 nm), the 49006 ET-Cy5 (excitation: 649 nm, emission: 679 nm) and the 49004 ET-Cy3 (ex. 552 nm, em. 579 nm), respectively.
2.4. Stress fibres
The freeware tool FilamentSensor [18] was used to characterize the actin stress fibres from fluorescent images of phalloidin-stained cells. The F-actin filament structure of FBs and CAFs cultured on collagen substrates with different stiffness was reconstructed (different fibre orientations were represented with different colours) using the FilamentSensor tool. Stress fibre orientation was assessed using the order parameter S = cos2θ [19], where θ is the angle formed between each stress fibre of the cell with the long axis of the fitted ellipse. An isotropic cytoskeleton should have S = 0 and a fully aligned (along the major axis of the cell) cytoskeleton should have 1. Therefore, the higher the value of S, the more oriented the fibres become.
2.5. Spreading
FBs and CAFs were cultured on 24-well plates and incubated at 37°C for 30 and 40 min, respectively. The time point chosen for each cell line was the one at which approximately 50% of cells had spread [20]. For assessing cell morphology, cells were fixed in 4% PFA (cat. no. P6148, Sigma) and imaged with an Eclipse TS100 inverted microscope equipped with a digital camera (Olympus XC50 Color CCD camera, 5 megapixel) and a Nikon Ph1 DL 10× 0.25 phase microscope objective lens. We used the optical microscope images from the spread cells so as to measure the cells' circularity and in order to obtain quantified results from the cell spreading assay. The circularity is defined as and it was measured using ImageJ (NIH). ImageJ calculates the circularity in the range of 0–1, where 1 is completely circular and 0 is highly polarized. The cells were divided into three subclasses: rounded (circularity = 0.66–1.0), half rounded (0.33–0.66) and completely spread (0–0.33). For quantifying the percentage of spread cells, at least 300 cells from five randomly selected fields were assessed. Three independent experiments were performed and results represent mean values from all three experiments.
2.6. Collagen I substrates (two-dimensional collagen substrates)
Type I collagen solution from rat tail (cat. no. C3867, Sigma) was mixed with 10% 10× minimal essential medium (MEM, cat. no. 21430020, Gibco) and distilled water so as to get a final collagen concentration of 0.5, 1.0 and 3.0 mg ml−1. The pH was adjusted to 7.4 by adding 1N NaOH. Then, 13 mm glass coverslips (cat. no. L46R13, Agar Scientific) or 35 mm Petri dishes (Thermo Scientific) were coated with 200 µl of the collagen solution and incubated at 37C° for at least 30 min.
2.7. Three-dimensional collagen I gels
In order to form 3D collagen I gels of desired concentration and consequently of different stiffness we used a modification of previously published protocols [21,22]. Briefly, we added the desired amount of a high concentration collagen I solution (cat. no. 354249, Corning) so as to get a final collagen concentration of 0.5, 1.0 or 3.0 mg ml−1, in a solution containing 10% 10× MEM (cat. no. 21430-020, Gibco), 1% human insulin solution (cat. no. I9278, Sigma), and distilled water. Then the pH was adjusted to 7.4 by adding 1N NaOH and the gels were allowed to solidify.
2.8. Atomic force microscopy
2.8.1. Collagen gels imaging
For imaging collagen I gels with AFM, 90 µl of the collagen solution (of the desired concentration), was flushed on 13 mm circular glass cover glasses (cat. no. AGL46R13, Agar Scientific). The samples were then incubated in a cell culture incubator for 30 min. Afterwards the samples were mounted on 15 mm specimen AFM metal discs (cat. no. AGF7003, Agar Scientific) with double-sided adhesive tape and then they were left in room temperature for air drying. For high resolution imaging of collagen gels, AFM images of the samples were obtained in air using a Cypher ES™ Environmental AFM microscope (Asylum Research) in intermittent (also named tapping) mode with AC160TS AFM probes (Olympus). The images were analysed using the ARgyle Light (Asylum Research, ver. 20113.1.4.9) and the WSxM 5.0 dev.2.1 [23] software. All images were acquired at a fixed resolution (512 × 512 data points) with scan rate between 0.5 and 1 Hz.
2.8.2. Mechanical properties characterization of live cells
Cells were cultured in 35 mm Petri dishes coated with collagen of different concentrations for 2 days in their culture medium. Before the AFM experiments, cells were washed twice with PBS, new complete culturing media was added, and were incubated in a cell culture incubator for 30 min. Petri dishes were then directly mounted on AFM sample plates. Young's modulus of cells was acquired by using the PicoPlus AFM system with silicon nitride probes with a round, ball-shape tip (CP-PNPL-BSG-A-5, sQube, 0.08 N m−1 spring constant). The cells Young's modulus was assessed by acquiring 8 × 8 points of force curves in an area of 5 × 5 µm near the centre of the cells (electronic supplementary material, figure S1) and by using the Hertz model (Poisson ratio equal to 0.5) and the freeware software AtomicJ. For the AFM nanoindentation technique a set point of 1 nN normal force at a 2 µm s−1 rate on each of the studied cells was used, while the maximum indentation depth was set to 600 nm. The experiments were performed in less than 40 min per experiment and at least 30 live cells per condition from three independent experiments were studied. Finally, attention was paid so that FBs and CAFs in all experimental conditions were handled in the same way, using the same AFM tips, indenting force, culture media and culture density.
2.8.3. Mechanical properties characterization of collagen substrates and collagen gels
For AFM mechanical properties characterization of the collagen I gels and substrates the PicoPlus AFM system with V-shaped silicon nitride probes (PNP-TR, Nanoword, 0.32 N m−1 spring constant) was used. All AFM experiments were obtained at room temperature under liquid (PBS) conditions. In an area of 5 × 5 µm, 8 × 8 points of force curves were collected and analysed by AtomicJ [24] so as to calculate the sample's Young's modulus using the Hertz model. The spring constant of all tips that were used in the study was assessed with the thermal tune method.
2.9. Assessment of collagen fibres diameters
For assessing collagen fibre diameter, AFM images were analysed using the WSXM software. A 8 × 8 grid was overlaid onto the collected images and fibre diameter was measured for all fibres within the grid-cells [25]. Collagen fibres that were extended in neighbouring grid-cells were measured only once. For each collagen sample, the histogram of fibre diameters was compiled to display the frequency of occurrence of each fibre diameter.
2.10. Cell spheroid formation
For the formation of FB and CAF spheroids the ‘hanging drop’ technique was used as described previously [21,22]. Briefly, after a 2 day incubation period in Petri dishes with pure medium or medium containing TGF-β (5 µg ml−1), FBs and CAFs were collected by trypsinization and put in suspension at a concentration of 2.5 × 104 cells ml−1. Hanging drops containing 500 cells each were formed on the inside of the cover of a culture dish.
2.11. Invasion assay
Formed spheroids were transferred with a glass Pasteur pipette into wells of a 96-well plate containing 0.5, 1.0, and 3.0 mg ml−1 collagen I gel. A Nikon Eclipse optical microscope, equipped with a CCD camera, was used to image the spheroids at time zero (immediately after spheroid transfer) and after 6 h incubation at 37°C. ImageJ software was used to assess spheroids' size (average of the major and minor axis length) at time 0 and 6 h so as to determine cell invasion through surrounding collagen [21] (electronic supplementary material, figure S2).
2.12. Cells culturing in three-dimensional collagen I gels
Cell suspension of 2.5 × 105 cells ml−1 in pure medium or TGF-β-containing medium (5 µg ml−1) were added in collagen gels with different concentrations before being solidified [22]. Cells were cultured at 37°C for 2 days and were then treated with 1 mg ml−1 collagenase D (cat. no. 11088858001, Sigma-Aldrich) for 30 min at 37°C so as to remove collagen and collect cells. Cell suspensions were then centrifuged at 300g for 5 min to remove digested collagen and cell pellets were kept for gene expression analysis.
2.13. RNA isolation and real-time polymerase chain reaction
Trizol (cat. no. 15596026, ThermoFisher Scientific) was used for extracting total RNA from the cells, which was subsequently, purified using RNeasy mini kit (cat. no. 74104, Qiagen) and transcribed to cDNA using Superscript III Reverse Transcriptase (cat. no. 18080093, Thermo-Fisher Scientific). Real-time PCR (CFX96 Real-Time PCR, BioRad) was used for the quantification of gene expression. The ΔΔCt method was used for quantifying the relative gene expression and all the reactions were done in triplicate for at least three independent experiments. All primers used are shown in electronic supplementary material, table S1.
2.14. Statistical analysis
Comparison of means with standard errors was used for the statistical analysis, while Student's t-test with p < 0.05 was regarded as statistically significant and each experimental group was repeated at least three times. Also, the ‘multiple sample comparison’ test was performed using Statgraphics 5.0 (software). After a significant difference was detected between the groups as evidenced from the ANOVA table a ‘multiple range test’ was performed to indicate the differences between groups.
3. Results
3.1. Matrix stiffening increases Young's modulus of fibroblasts and cancer-associated fibroblasts
We first set out to characterize the two human pancreatic cell lines, FBs and CAFs, in terms of cell morphology. Optical microscopy imaging demonstrated that both cell lines are characterized by elongated cells with the characteristic FB-like shape, as seen in electronic supplementary material, figure S3A. Subsequently, we used phalloidin staining and fluorescence microscopy, in order to characterize the actin (F-actin) cytoskeleton in each cell line. The cell cytoskeleton consists of three types of filaments (including actin microfilaments, intermediate filaments and microtubules) with actin filaments being considered the most significant for modulating the mechanical properties of cells [26]. Our results demonstrated that both CAFs and FBs exhibited intense stress fibres (which refers to a qualitative measure of how strong stress fibres are stained with phalloidin), while CAFs also exhibited increased formation of lamellipodia, the sheet-like membrane protrusions involved in cell movement, suggesting that CAFs have a more migratory phenotype (electronic supplementary material, figure S3B), in agreement with previous studies [27].
As tumour stiffening is an important consequence of desmoplasia, we investigated the effect of matrix stiffness on basic cellular characteristics of pancreatic FBs and CAFs, such as cell stiffness, α-SMA expression (the most common FB activation marker), cell spreading and stress fibre formation and orientation. Our approach involved the preparation of collagen substrates of different concentrations which evidently exhibit different stiffness properties [21,28].
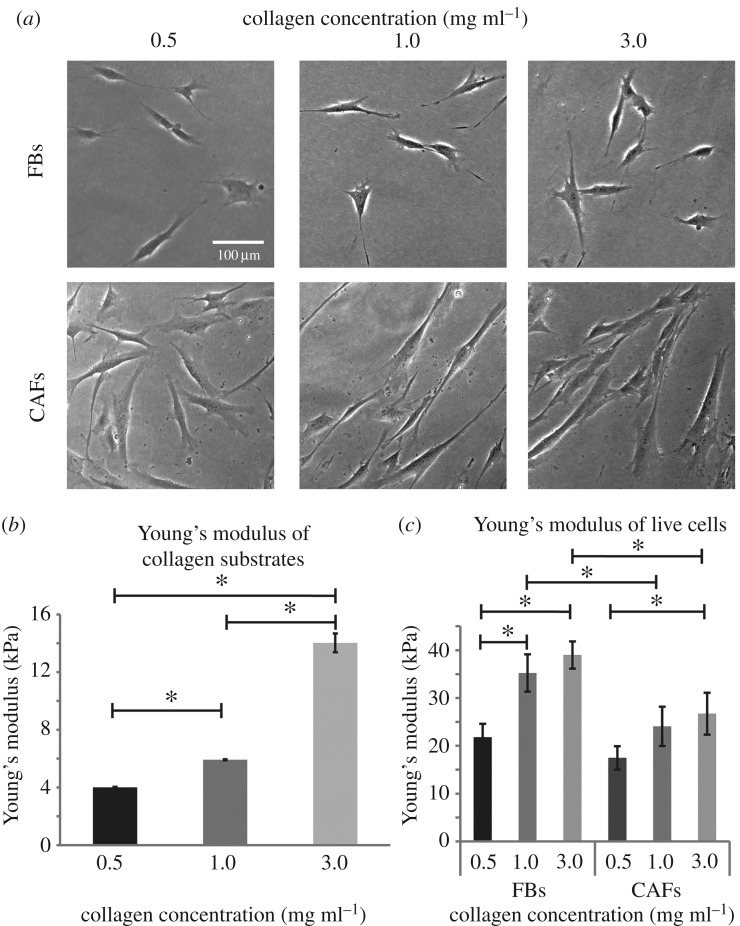
The formed substrates were used for culturing both FBs and CAFs (figure 1a). FBs and CAFs cultured on collagen substrates of different concentration exhibited distinct characteristics with FBs being randomly oriented in all collagen concentrations and CAFs being organized in the same orientation as substrate stiffness increases. Figure 2b and figure S4 (electronic supplementary material) presents the relative Young's modulus of the substrates, measured with AFM, with respect to collagen concentration, confirming the dependence of substrate elastic properties on collagen content [29]. Subsequently, as AFM is gaining ground in cell mechanics [30] and in cancer diagnostics being a novel technique for determining the mechanical properties of various cell types of different metastatic potentials [31–34], we extended our experiments to include AFM force spectroscopy in live cells cultured on different substrates. Figure 1c depicts the Young's modulus of FBs and CAFs as a function of collagen concentration (i.e. substrate stiffness). The Young's modulus increased with substrate stiffness for both cell lines, with changes in the modulus of FBs to be more sensitive to changes in substrate stiffness. As for the Young's modulus of CAFs, a statistically significant increase was found only for the 3.0 mg ml−1 collagen concentration (figure 1c). On the other hand, when cells were treated with Cytochalasin D, a known inhibitor of actin polymerization, their Young's moduli were not affected by the substrate stiffness and the Young's moduli of the different groups did not present statistically significant differences (electronic supplementary material, figure S5). This demonstrates that our measurements correspond to the cells stiffness and it is not an artefact of the AFM measurements.
Figure 1.
Effect of matrix stiffness on cell Young's modulus. (a) Optical microscope imaging of FBs and CAFs cultured on collagen substrates of different concentration, (b) relative substrate Young's modulus and (c) Young's modulus of live cells, measured by AFM. Asterisks indicate a statistically significant difference between compared groups (p < 0.05).
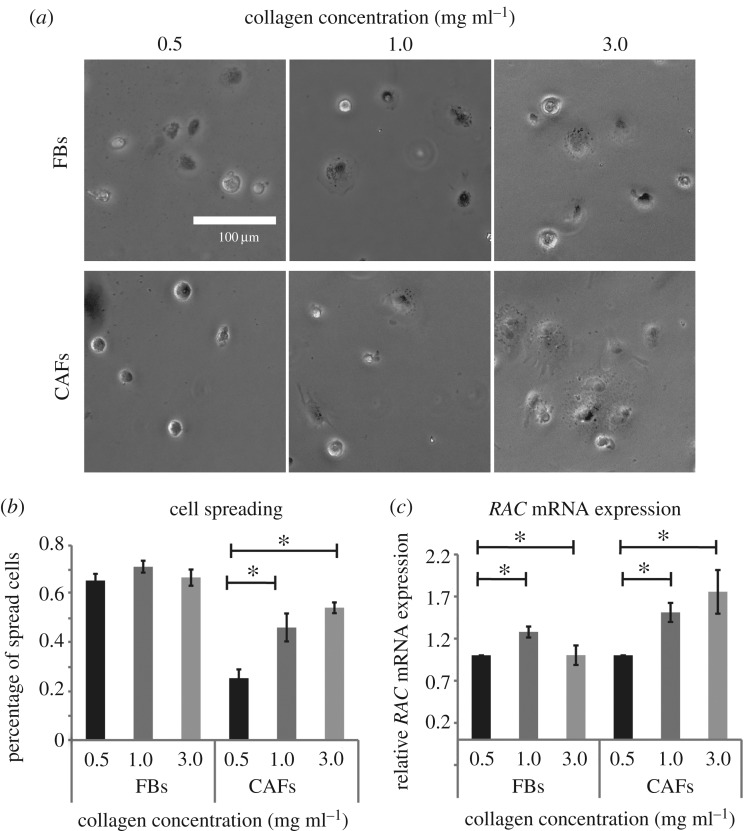
Figure 2.
Substrate stiffness increases cell spreading of CAFs. (a) Representative optical microscope images of cells cultured on collagen substrates of different concentration. (b) Quantitative analysis of cell spreading using the circularity shape factor based on optical microscope images and (c) relative RAC mRNA expression assessed by real-time PCR. Asterisks indicate a statistically significant difference between compared groups (p < 0.05).
Notably, it has been recently demonstrated that CAFs are ‘softer’ compared to FBs and consequently CAFs exhibit a more aggressive phenotype [27]. Thus, our experiments confirmed that for stiffer substrates CAFs have lower values of Young's modules compared to FBs (figure 1c). However, this is not the case for soft substrates. Interestingly, when substrates of 0.5 mg ml−1 collagen were used, FBs and CAFs demonstrated similar Young's modulus values. This result highlights the fact that in desmoplastic tumours, CAFs might acquire a ‘softer’ and more aggressive phenotype.
3.2. Matrix stiffening increases cell spreading
Subsequently, we measured the effect of matrix stiffening on cell spreading, which is related to invasion and metastasis. Our results indicated that increased collagen concentration, and consequently increased stiffness, affects cell spreading of CAFs but not FBs. The cell spreading assay showed that cell spreading was increased in correlation to increased stiffness (figure 2a,b). We also used real-time PCR to assess RAC expression, as RAC is required for actin polymerization and is a known mediator of cell spreading [35]. Real-time PCR, which is more sensitive than optical microscopy observations, showed that stiffness affected RAC mRNA expression both in FBs and CAFs from 0.5 to 1.0 mg ml−1, while in the case of 3.0 mg ml−1 RAC expression was increased only in CAFs (figure 2c).
3.3. Matrix stiffening increases α-smooth muscle actin expression in both fibroblasts and cancer-associated fibroblasts
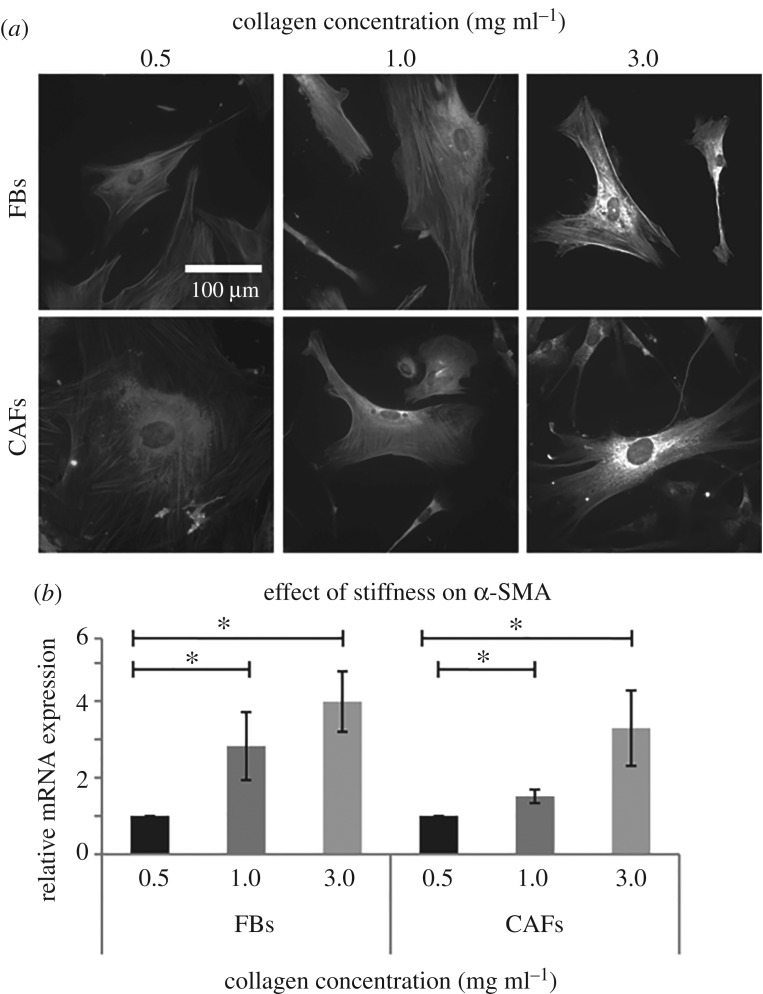
Next, given the fact that α-SMA is the most common marker of activated FBs [36–39], we studied α-SMA expression in FBs and CAFs by means of fluorescence microscopy and real-time PCR. Figure 3a depicts immunofluorescence images of α-SMA protein expression and localization in FBs and CAFs and in correlation with collagen concentration and thus, substrate stiffness. Expression of α-SMA increases with substrate stiffness (electronic supplementary material, figure S6), which is in agreement with the quantitative mRNA expression of α-SMA analysed by real-time PCR (figure 3b).
Figure 3.
FBs and CAFs α-SMA expression. (a) Immunofluorescence images of α-SMA stained cells and (b) relative mRNA α-SMA expression. Asterisks indicate a statistically significant difference between compared groups (p < 0.05).
3.4. Matrix stiffening improves orientation of the less oriented stress fibres of fibroblasts
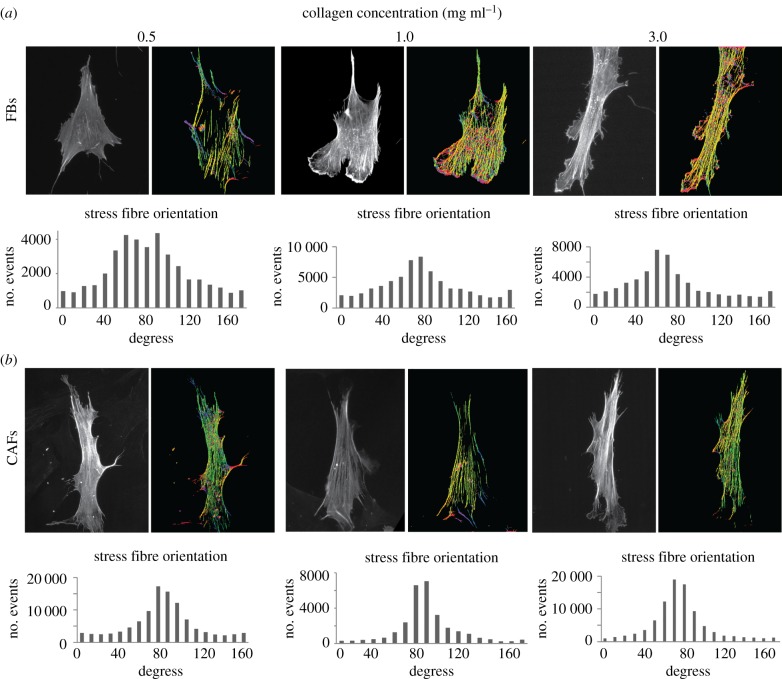
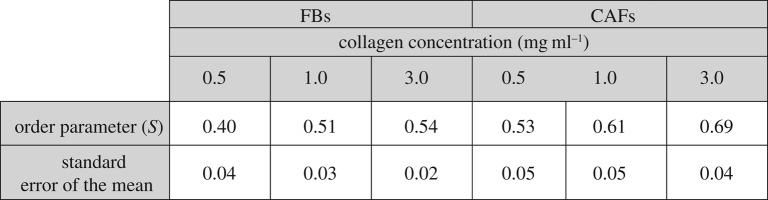
To complete FBs and CAFs cytoskeletal characterization in conditions of increased substrate stiffness, we tested stress fibre orientation by F-actin staining with phaloidin. As F-actin fibre orientation has been associated with increased migration, we used the FilamentSensor tool software to visualize stress fibre orientation [18]. As shown in figure 4, this tool represents the direction of each fibre with a different colour. In the case of soft substrates (0.5 mg ml−1), stress fibres in FBs were randomly organized, while increasing substrate's stiffness, F-actin fibres acquired a more oriented pattern (figure 4a). On the other hand, CAFs independently of substrate stiffness presented well-organized stress fibres with one major orientation, parallel to the major axis of the cell (figure 4b). Also, the stress fibre (F-actin) orientation/alignment was assessed with the order parameter S. Table 1 summarizes the order parameter of FBs and CAFs on different collagen concentration substrates. In general, CAFs present a higher S than FBs, while the order parameter for both cell lines increases with the increase of collagen concentration (and consequently collagen stiffness)
Figure 4.
Effect of substrate stiffness on F-actin stress fibres of FBs and CAFs. (a,b) Phalloidin-stained cells and computationally processed images, presenting stress fibre orientation (each colour corresponds to a different fibre orientation). (Online version in colour.)
Table 1.
Order parameter S of FBs and CAFs on substrates of different collagen concentrations.
 |
3.5. Matrix stiffening inhibits cell spheroid invasion in three-dimensional collagen gels
Given our results from the 2D experiments, we sought to find out how the metastatic potential of FBs and CAFs was affected by ECM stiffness in a 3D set-up that better mimics real ECM and tumour setting [40] as it is evident that the tissue microenvironment regulates cancer cell motility and invasion [41]. Our experimental approach involved the formation of cell spheroids embedded in 3D gels made by purified type I collagen, with tunable stiffness.
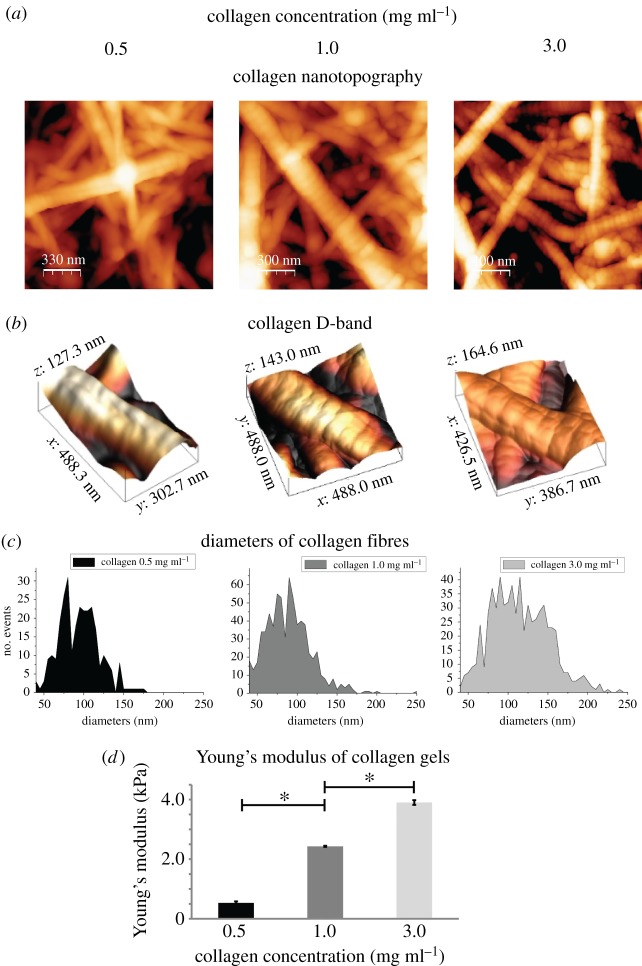
The structure and elastic properties of gels containing 0.5, 1.0 or 3.0 mg ml−1 collagen were analysed using AFM, which does not destroy the gel's microstructure [29,42–45]. Figure 5a depicts the microstructure of the gels consisted of fibres with random orientations, similar to the ECM structure of collagen-rich tumours [46]. Additionally, collagen fibres presented the 67 nm D-band (figure 5b), which is a characteristic of the native forming collagen fibres and it has been proposed to be recognized by cells [47]. Furthermore, AFM microstructural analyses (figure 5a) revealed that changes in collagen concentration increased fibre density and gels with higher concentration consisted of fibres with larger diameters (figure 5c). In order to characterize the stiffness of the gels, we performed AFM analysis under liquid conditions. As expected, stiffness measured by AFM increased with increasing collagen concentration (figure 5d). In particular, we measured an increase in stiffness of about 1.5 and 3.7 times (compared to the 0.5 mg ml−1 condition), when the concentration was increased from 0.5 to 1.0 and 3.0 mg ml−1, respectively.
Figure 5.
AFM characterization of 3D collagen matrices. (a,b) AFM topography images of collagen type I gels with different concentrations, presenting the fibre structure of the gels and the D-band (approximately 67 nm), respectively, (c) diameters of collagen fibres and (d) normalized Young's modulus of the gels relative to the Young's value of the lowest concentration. Asterisks indicate a statistically significant difference between compared groups (p < 0.05). (Online version in colour.)
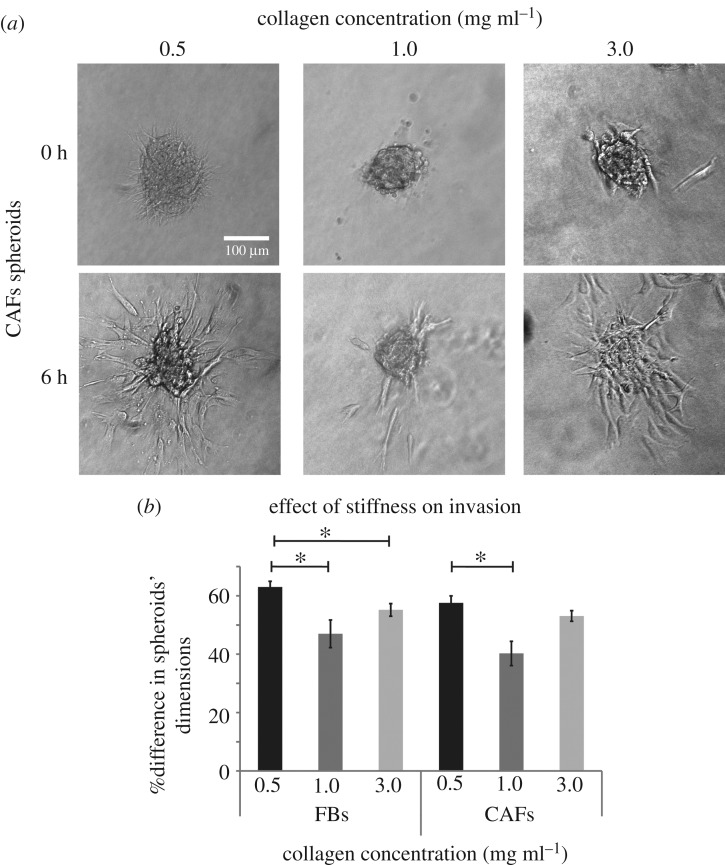
The next step in our study was the formation of FBs and CAFs spheroids using the hanging drop method. Spheroids were embedded in the 3D collagen gels and 6 h later, images were obtained to quantify cell invasion (figure 6a). The analysis revealed that cell invasion was reduced as collagen concentration increased from 0.5 to 1.0 mg ml−1 (figure 6b). FBs, exhibited the same effect when gel concentration was increased from 0.5 to 3.0 mg ml−1 (figure 6b). However, CAFs invasion presented a differential response to stiffness and there was no statistically significant alterations between the 0.5 and 3.0 mg ml−1 collagen concentration (figure 6b).
Figure 6.
Effect of collagen stiffness on FBs and CAFs invasion and expression of RhoA and ROCK in the absence or presence of TGF-β. (a) Representative CAFs spheroids embedded in collagen gels at time 0 and at 6 h post implantation, respectively. (b) Percentage (%) change in FBs and CAFs spheroids' dimensions within 6 h post-implantation. Asterisks indicate a statistically significant difference between compared groups (p < 0.05).
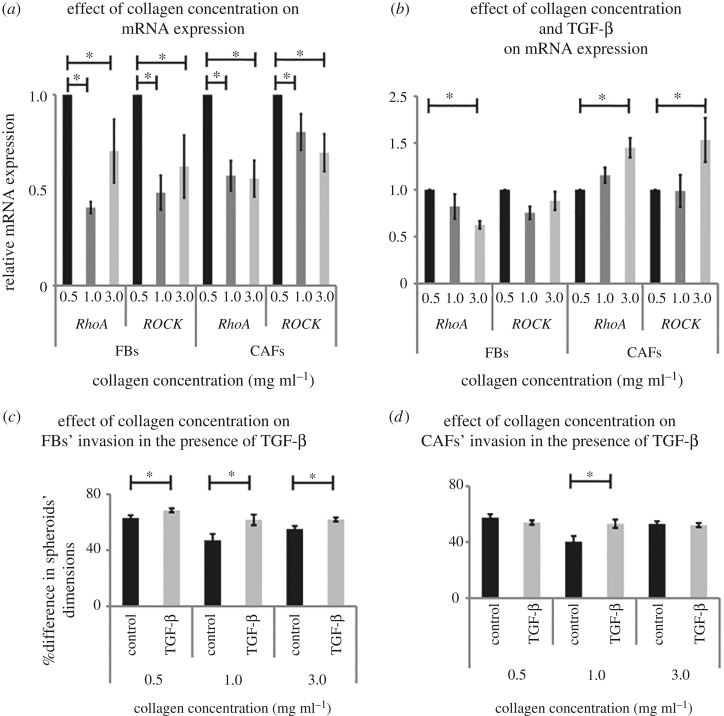
In order to investigate the molecular mechanism involved, we measured the mRNA expression of RhoA and Rho-associated protein kinase-1 (ROCK-1), important members of the Rho GTPase family of proteins that have been previously suggested to be involved in FBs and CAFs ECM remodelling and invasion [27,48,49]. Real-time PCR analyses demonstrated that the mRNA expression of these genes in both cell lines was reduced as the collagen concentration was increased from 0.5 to 1.0 and 3.0 mg ml−1, following the invasion pattern (figure 7a).
Figure 7.
Effect of collagen concentration on FBs and CAFs invasion in the presence of TGF-β. (a) Relative RhoA and ROCK mRNA expression in cells cultured in the 3D collagen gels of different concentrations. (b) Relative RhoA and ROCK mRNA expression in TGF-β-treated FBs and CAFs cultured in the 3D collagen gels. (c,d) Percentage (%) change in cell spheroids' dimensions within 6 h post implantation, respectively. Asterisks indicate a statistically significant difference between compared groups (p < 0.05).
3.6. Transforming growth factor beta increases the invasive potential of both fibroblasts and cancer-associated fibroblasts
Given that TGF-β upregulation is an important component of desmoplasia, we investigated the effect of ECM stiffness on FBs and CAFs in the presence of TGF-β. In that regard, TGF-β treated FBs and CAFs were used for the formation of cell spheroids. The spheroids were embedded in the 3D collagen gels and images were obtained at time 0 and 6 h. In the case of FBs, we found that TGF-β significantly increased FB invasion in all collagen concentrations (figure 7c), whereas TGF-β had negligible effects on the invasive potential of CAFs for 0.5 and 3.0 mg ml−1 collagen, and promoted spheroid invasion only for the 1.0 mg ml−1 condition (figure 7d). Interestingly, even though matrix stiffening reduced cell invasion (figure 6b), addition of TGF-β compensated for the negative effect of stiffness so that spheroid invasion remained the same for both FBs and CAFs (figure 7c,d). Real-time PCR experiments demonstrated that this effect was a consequence of the activation of the RhoA and ROCK pathway, particularly in the case of CAFs (figure 7b). Also, multiple comparisons between the different groups confirmed the activation effect of TGF-β on the RhoA and ROCK pathway (electronic supplementary material, figure S7).
4. Discussion
Pancreatic cancer is the leading cause of cancer-related deaths, with poor survival rates [2]. Therefore, a complete understanding of the mechanisms involved in its pathogenesis is of crucial importance. Pancreatic tumours, such as pancreatic ductal adenocarcinomas, are characterized by desmoplasia, the accumulation of increased amounts of ECM inside the tumour [2]. Although, it has been suggested that ECM stiffening modulates stromal and cancer cell properties, promotes cancer cell metastasis and hinders the effective delivery of drugs [6,7,50], the exact role of matrix stiffness in solid tumours development is not clearly defined yet [51].
As it has been demonstrated that the mechanical properties of a tumour determine cell behaviour, we investigated how collagen stiffness affects pancreatic FBs and CAFs in terms of cytoskeleton and morphodynamic characteristics re-organization as well as in terms of their invasive properties in 2D and 3D collagen models. In our 2D experiments, we demonstrated that the Young's modulus of both FBs and CAFs increased as a function of substrate stiffness (figure 1c). This is in agreement with studies showing that FBs tend to tune their stiffness in order to adapt to that of their environment [52]. Indeed, when cultured on rigid substrates, CAFs proved to be softer than FBs, forming monolayers with oriented cellular patterns (figure 1). AFM studies have demonstrated that cancer cells, which are highly invasive, are also softer than normal cells [32,33], while they also remodel their cytoskeleton so as to facilitate their invasion through surrounding stiff tissues [27,53]. Similarly, we showed here that while CAFs are softer than FBs in stiff substrates and possess a more invasive phenotype reminiscent of that of cancer cells, both cell lines become stiffer in stiffer environments [54,55] when they encounter higher levels of stress. Moreover, the fact that CAFs formed oriented patterns in stiffer environments (figure 1) suggests a coordinated migratory behaviour, which ensures more effective migration than single cells [56]. Furthermore, we showed that α-SMA expression increased in increased substrate stiffness conditions in both cell types (figure 3 and electronic supplementary material, figure S6), confirming the hypothesis that stiffness promotes the activation of FBs into a myofibroblast-like phenotype, which is associated with a higher metastatic potential. Moreover, in accordance with previous studies showing that cells spread better on stiffer substrates [52,57], we found that CAFs, but not FBs, presented better spreading efficiency on stiffer substrates (figure 2) which was corroborated by real-time PCR analysis of RAC mRNA expresssion. The finding, though, that FB spreading was not affected by matrix stiffness may be explained by the fact that alterations in cell stiffness tend to affect cell spreading thus it is plausible to find that CAFs exhibited better spreading properties than FBs, as they were found to be softer. Moreover, CAFs presented oriented stress fibres regardless of substrate stiffness, while FBs had the tendency to remodel F-actin stress fibres in more oriented patterns as substrate stiffness inceased (figure 4 and table 1). This is also in accordance with previous studies showing that actin structure is not organized into stress fibres in soft substrates [52,58]. It should be noted though that such cytoskeleton remodelling is translated into changes in the mechanical properties (reduced stiffness in soft substrates) of the FBs, which can be crucial for the invasive properties of the cells [30,33,54,55,59].
Furthermore, we developed 3D collagen models to study cell invasion in a more physiologically relevant approach [40]. In our 3D experiments, we formed tumour cell spheroids that were embedded in collagen gels of different concentration. AFM nanoscale characterization demonstrated that gels consisted of collagen fibres exhibiting the characteristic D-band periodicity and had random orientation (figure 6). As the collagen concentration increased, the gel density, stiffness and collagen fibre diameter were significantly increased, confirming previous research demonstrating that alterations in collagen concentration are translated into changes in ECM stiffness [28,40]. Concerning fibre diameters, we found a range of 50–300 nm, similar to previous studies performed in high concentrated collagen gels [25] and within the range of fibre diameter of native tissues [28]. Consequently, as the Young's modulus of tissues exhibits great variability and largely depends upon the quantity and organization of collagen [47,60,61] our gels can serve as models to recapitulate tumour ECM stiffening during desmoplasia. Our results revealed that tumour spheroid invasion of FBs and CAFs was hindered when spheroids were cultured in collagen gels of higher concentration (figure 6). Thus, CAFs have most likely developed appropriate mechanisms, such as the development of oriented stress fibres and a ‘softer’ cytoskeleton, to be able to invade through stiff environments. Our findings were further supported by gene expression analysis showing a significant decrease in Rho-GTPases RhoA and ROCK mRNA expression in FBs and CAFs (figure 7a), which have been proposed to mediate cell motility, ECM remodelling by FBs [48,49] and control actin polymerization [26]. Finally, addition of TGF-β reversed the suppressive effects of stiffness on FBs and CAFs invasion (figures 7c and d), confirming the hypothesis that ECM stiffness and TGF-β have synergistic effects in desmoplasia and affect cell invasion.
Overall, our results demonstrated that collagen concentration and consequently ECM stiffening differentially modulates FBs and CAFs cytoskeleton remodelling and invasion with stress fibre orientation being significantly altered in FBs. Finally, the presence of TGF-β reverses the suppressive effect of collagen stiffness on cell invasion.
Supplementary Material
Data accessibility
This article has no additional data.
Authors' contributions
Conceptualization: A.S. and T.S., methodology: A.S. and V.G., investigation: A.S. and M.L., writing, review and editing: A.S., V.G., M.L. and T.S. All authors gave final approval for publication.
Competing interests
The authors declare no conflict of interests.
Funding
This work was supported by a Horizon 2020, Marie Skłodowska-Curie Individual Fellowship (MSCA-IF-2014-658769-MYO-DESMOPLASIA) and the European Research Council (FP7 2007–2013, ERC grant agreement no. 336839-ReEngineeringCancer).
Abbreviations
Alpha-smooth muscle actin (α-SMA)
Atomic force microscopy (AFM)
Cancer-associated fibroblasts (CAFs)
4′,6-Diamidino-2-phenylindole (DAPI)
Extracellular matrix (ECM)
Fibroblasts (FBs)
Minimal essential medium (MEM)
Paraformaldehyde (PFA)
Phosphate-buffered saline (PBS)
Ras-related C3 botulinum toxin substrate 1 (Rac)
Ras homologue family member (RhoA)
Rho-associated, coiled-coil containing protein kinase 1 (ROCK-1)
Scanning electron microscopy (SEM)
Three dimensional (3D)
Transforming growth factor-β (TGF-β)
Two dimensional (2D)
References
- 1.Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell 144, 646–674. ( 10.1016/j.cell.2011.02.013) [DOI] [PubMed] [Google Scholar]
- 2.Rath N, Olson MF. 2016. Regulation of pancreatic cancer aggressiveness by stromal stiffening. Nat. Med. 22, 462 ( 10.1038/nm.4099) [DOI] [PubMed] [Google Scholar]
- 3.Voutouri C, Polydorou C, Papageorgis P, Gkretsi V, Stylianopoulos T. 2016. Hyaluronan-derived swelling of solid tumors, the contribution of collagen and cancer cells, and implications for cancer therapy. Neoplasia 18, 732–741. ( 10.1016/j.neo.2016.10.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voutouri C, Mpekris F, Papageorgis P, Odysseos AD, Stylianopoulos T. 2014. Role of constitutive behavior and tumor–host mechanical interactions in the state of stress and growth of solid tumors. PLoS ONE 9, e0104717 ( 10.1371/journal.pone.0104717) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mpekris F, Angeli S, Pirentis AP, Stylianopoulos T. 2015. Stress-mediated progression of solid tumors: effect of mechanical stress on tissue oxygenation, cancer cell proliferation, and drug delivery. Biomech. Model. Mechanobiol. 14, 1391–1402. ( 10.1007/s10237-015-0682-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox TR, Bird D, Baker AM, Barker HE, Ho MWY, Lang G, Erler JT. 2013. LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res. 73, 1721–1732. ( 10.1158/0008-5472.CAN-12-2233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain RK, Martin JD, Stylianopoulos T. 2014. The role of mechanical forces in tumor growth and therapy. Annu. Rev. Biomed. Eng. 16, 321–346. ( 10.1146/annurev-bioeng-071813-105259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wipff PJ, Hinz B. 2009. Myofibroblasts work best under stress. J. Bodyw. Mov. Ther. 13, 121–127. ( 10.1016/j.jbmt.2008.04.031) [DOI] [PubMed] [Google Scholar]
- 9.Papageorgis P, Stylianopoulos T. 2014. Role of TGFβ in regulation of the tumor microenvironment and drug delivery. Int. J. Oncol. 46, 933–943. ( 10.3892/ijo.2015.2816) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wipff P-J, Rifkin DB, Meister J-J, Hinz B. 2007. Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J. Cell Biol. 179, 1311–1323. ( 10.1083/jcb.200704042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egeblad M, Rasch MG, Weaver VM. 2010. Dynamic interplay between the collagen scaffold and tumor evolution. Curr. Opin. Cell Biol. 22, 697–706. ( 10.1016/j.ceb.2010.08.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalli M, Papageorgis P, Gkretsi V, Stylianopoulos T. 2018. Solid stress facilitates fibroblasts activation to promote pancreatic cancer cell migration. Ann. Biomed. Eng. 46, 657–669. ( 10.1007/s10439-018-1997-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamazaki D, Kurisu S, Takenawa T. 2005. Regulation of cancer cell motility through actin reorganization. Cancer Sci. 96, 379–386. ( 10.1111/j.1349-7006.2005.00062.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cross SE, Jin Y-S, Rao J, Gimzewski JK. 2007. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol. 2, 780–783. ( 10.1038/nnano.2007.388) [DOI] [PubMed] [Google Scholar]
- 15.Papageorgis P, Polydorou C, Mpekris F, Voutouri C, Agathokleous E, Kapnissi-Christodoulou CP, Stylianopoulos T. 2017. Tranilast-induced stress alleviation in solid tumors improves the efficacy of chemo- and nanotherapeutics in a size-independent manner. Sci. Rep. 7, 46140 ( 10.1038/srep46140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polydorou C, Mpekris F, Papageorgis P, Voutouri C, Stylianopoulos T. 2017. Pirfenidone normalizes the tumor microenvironment to improve chemotherapy. Oncotarget 8, 24 506–24 517. ( 10.18632/oncotarget.15534) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mpekris F, Papageorgis P, Polydorou C, Voutouri C, Kalli M, Pirentis AP, Stylianopoulos T. 2017. Sonic-hedgehog pathway inhibition normalizes desmoplastic tumor microenvironment to improve chemo- and nanotherapy. J. Control. Release 261, 105–112. ( 10.1016/j.jconrel.2017.06.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eltzner B, Wollnik C, Gottschlich C, Huckemann S, Rehfeldt F. 2015. The filament sensor for near real-time detection of cytoskeletal fiber structures. PLoS ONE 10, e0126346 ( 10.1371/journal.pone.0126346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zemel A, Rehfeldt F, Brown AE.X., Discher DE, Safran SA. 2010. Optimal matrix rigidity for stress-fibre polarization in stem cells. Nat. Phys. 6, 468 ( 10.1038/nphys1613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tu Y, Wu S, Shi X, Chen K, Wu C. 2003. Migfilin and Mig-2 link focal adhesions to filamin and the actin cytoskeleton and function in cell shape modulation. Cell 113, 37–47. ( 10.1016/S0092-8674(03)00163-6) [DOI] [PubMed] [Google Scholar]
- 21.Gkretsi V, Stylianou A, Louca M, Stylianopoulos T. 2017. Identification of Ras suppressor-1 (RSU-1) as a potential breast cancer metastasis biomarker using a three-dimensional in vitro approach. Oncotarget 8, 27 364–27 379. ( 10.18632/oncotarget.16062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gkretsi V, Stylianou A, Stylianopoulos T. 2017. Vasodilator-stimulated phosphoprotein (VASP) depletion from breast cancer MDA-MB-231 cells inhibits tumor spheroid invasion through downregulation of migfilin, β-catenin and urokinase-plasminogen activator (uPA). Exp. Cell Res. 352, 281–292. ( 10.1016/j.yexcr.2017.02.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horcas I, Fernández R, Gómez-Rodríguez JM, Colchero J, Gómez-Herrero J, Baro AM. 2007. WSXM: a software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 78, 013705 ( 10.1063/1.2432410) [DOI] [PubMed] [Google Scholar]
- 24.Hermanowicz P, Sarna M, Burda K, Gabryś H. 2014. AtomicJ: an open source software for analysis of force curves. Rev. Sci. Instrum. 85, 063703 ( 10.1063/1.4881683) [DOI] [PubMed] [Google Scholar]
- 25.Garvin KA, Vanderburgh J, Hocking DC, Dalecki D. 2013. Controlling collagen fiber microstructure in three-dimensional hydrogels using ultrasound. J. Acoust. Soc. Am. 134, 1491–1502. ( 10.1121/1.4812868) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiou YW, Lin HK, Tang MJ, Lin HH, Yeh ML. 2013. The influence of physical and physiological cues on atomic force microscopy-based cell stiffness assessment. PLoS ONE 8, e77384 ( 10.1371/journal.pone.0077384) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stylianou A, Gkretsi V, Stylianopoulos T. 2018. Transforming growth factor-β modulates pancreatic cancer associated fibroblasts cell shape, stiffness and invasion. Biochim. Biophys. Acta Gen. Subj. 1862, 1537–1546. ( 10.1016/j.bbagen.2018.02.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roeder BA, Kokini K, Sturgis JE, Robinson JP, Voytik-Harbin SL. 2002. Tensile mechanical properties of three-dimensional type I collagen extracellular matrices with varied microstructure. J. Biomech. Eng. 124, 214–222. ( 10.1115/1.1449904) [DOI] [PubMed] [Google Scholar]
- 29.Kontomaris SV, Yova D, Stylianou A, Politopoulos K. 2015. The significance of the percentage differences of Young's modulus in the AFM nanoindentation procedure. Micro Nanosyst. 7, 86–97. ( 10.2174/1876402908666151111234441) [DOI] [Google Scholar]
- 30.Trepat X, Lenormand G, Fredberg JJ. 2008. Universality in cell mechanics. Soft Matter 4, 1750–1759. ( 10.1039/b804866e) [DOI] [Google Scholar]
- 31.Cross SE, Jin Y-S, Rao J, Gimzewski JK. 2009. Applicability of AFM in cancer detection. Nat. Nanotechnol. 4, 72 ( 10.1038/nnano.2009.036) [DOI] [PubMed] [Google Scholar]
- 32.Stylianou A, Stylianopoulos T. 2016. Atomic force microscopy probing of cancer cells and tumor microenvironment components. Bionanoscience 6, 33–46. ( 10.1007/s12668-015-0187-4) [DOI] [Google Scholar]
- 33.Lekka M. 2016. Discrimination between normal and cancerous cells using AFM. Bionanoscience 6, 65–80. ( 10.1007/s12668-016-0191-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plodinec M, et al. 2012. The nanomechanical signature of breast cancer. Nat. Nanotechnol. 7, 757–765. ( 10.1038/nnano.2012.167) [DOI] [PubMed] [Google Scholar]
- 35.Parri M, Chiarugi P. 2010. Rac and Rho GTPases in cancer cell motility control. Cell Commun. Signal. 8, 23 ( 10.1186/1478-811x-8-23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karagiannis GS, Poutahidis T, Erdman SE, Kirsch R, Riddell RH, Diamandis EP. 2012. Cancer-associated fibroblasts drive the progression of metastasis through both paracrine and mechanical pressure on cancer tissue. Mol. Cancer Res. 10, 1403–1418. ( 10.1158/1541-7786.MCR-12-0307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calon A, Tauriello DVF, Batlle E. 2014. TGF-beta in CAF-mediated tumor growth and metastasis. Semin. Cancer Biol. 25, 15–22. ( 10.1016/j.semcancer.2013.12.008) [DOI] [PubMed] [Google Scholar]
- 38.Kalluri R. 2016. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16, 582–598. ( 10.1038/nrc.2016.73) [DOI] [PubMed] [Google Scholar]
- 39.Kalluri R, Zeisberg M. 2006. Fibroblasts in cancer. Nat. Rev. Cancer 6, 392–401. ( 10.1038/nrc1877) [DOI] [PubMed] [Google Scholar]
- 40.Timraz SBH, Rezgui R, Boularaoui SM, Teo JCM. 2015. Stiffness of extracellular matrix components modulates the phenotype of human smooth muscle cells in vitro and allows for the control of properties of engineered tissues. Procedia Eng. 110, 29–36. ( 10.1016/j.proeng.2015.07.006) [DOI] [Google Scholar]
- 41.Brábek J, Mierke CT, Rösel D, Veselý P, Fabry B. 2010. The role of the tissue microenvironment in the regulation of cancer cell motility and invasion. Cell Commun. Signal. 8, 22 ( 10.1186/1478-811x-8-22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stylianou A, Yova D. 2013. Surface nanoscale imaging of collagen thin films by atomic force microscopy. Mater. Sci. Eng. C 33, 2947–2957. ( 10.1016/j.msec.2013.03.029) [DOI] [PubMed] [Google Scholar]
- 43.Stylianou A, Yova D, Alexandratou E. 2014. Investigation of the influence of UV irradiation on collagen thin films by AFM imaging. Mater. Sci. Eng. C 45, 455–468. ( 10.1016/j.msec.2014.09.006) [DOI] [PubMed] [Google Scholar]
- 44.Stylianou A, Kontomaris SV, Yova D. 2014. Assessing collagen nanoscale thin films heterogeneity by AFM multimode imaging and nanoindetation for nanobiomedical applications. Micro Nanosys. 6, 95–102. ( 10.2174/187640290602141127114448#sthash.960XePnt.dpuf) [DOI] [Google Scholar]
- 45.Stylianou A, Yova D, Politopoulos K. 2012. Atomic force microscopy quantitative and qualitative nanoscale characterization of collagen thin films. In Proc. 5th International Conference on Emerging Technologies in Non-Destructive Testing (NDT 21012), Ioannina, Greece, 19–21 September, pp. 415–420.
- 46.Ramanujan S, Pluen A, McKee TD, Brown EB, Boucher Y, Jain RK. 2002. Diffusion and convection in collagen gels: implications for transport in the tumor interstitium. Biophys. J. 83, 1650–1660. ( 10.1016/S0006-3495(02)73933-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fratzl P. 2008. Collagen structure and mechanics. New York, NY: Springer. [Google Scholar]
- 48.Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. 2007. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol. 9, 1392–1400. ( 10.1038/ncb1658) [DOI] [PubMed] [Google Scholar]
- 49.Attieh Y, Vignjevic DM. 2016. The hallmarks of CAFs in cancer invasion. Eur. J. Cell Biol. 95, 493–502. ( 10.1016/j.ejcb.2016.07.004) [DOI] [PubMed] [Google Scholar]
- 50.Laklai H, et al. 2016. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat. Med. 22, 497–505. ( 10.1038/nm.4082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalli M, Stylianopoulos T. 2018. Defining the role of solid stress and matrix stiffness in cancer cell proliferation and metastasis. Front. Oncol. 8, 55 ( 10.3389/fonc.2018.00055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neesse A, Algül H, Tuveson DA, Gress TM. 2015. Stromal biology and therapy in pancreatic cancer: a changing paradigm. Gut 64, 1476–1484. ( 10.1136/gutjnl-2015-309304) [DOI] [PubMed] [Google Scholar]
- 53.Wu TH, Chiou YW, Chiu WT, Tang MJ, Chen CH, Yeh ML. 2014. The F-actin and adherence-dependent mechanical differentiation of normal epithelial cells after TGF-β1-induced EMT (tEMT) using a microplate measurement system. Biomed. Microdevices 16, 465–478. ( 10.1007/s10544-014-9849-1) [DOI] [PubMed] [Google Scholar]
- 54.Prina-Mello A, Jain N, Liu B, Kilpatrick JI, Tutty MA, Bell AP, Jarvis SP, Volkov Y, Movia D. 2018. Culturing substrates influence the morphological, mechanical and biochemical features of lung adenocarcinoma cells cultured in 2D or 3D. Tissue Cell 50, 15–30. ( 10.1016/j.tice.2017.11.003) [DOI] [PubMed] [Google Scholar]
- 55.Xu W, Mezencev R, Kim B, Wang L, McDonald J, Sulchek T. 2012. Cell stiffness is a biomarker of the metastatic potential of ovarian cancer cells. PLoS ONE 7, e46609 ( 10.1371/journal.pone.0046609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mayor R, Etienne-Manneville S. 2016. The front and rear of collective cell migration. Nat. Rev. Mol. Cell Biol. 17, 97–109. ( 10.1038/nrm.2015.14) [DOI] [PubMed] [Google Scholar]
- 57.El-Mohri H, Wu Y, Mohanty S, Ghosh G. 2017. Impact of matrix stiffness on fibroblast function. Mater. Sci. Eng. C 74, 146–151. ( 10.1016/j.msec.2017.02.001) [DOI] [PubMed] [Google Scholar]
- 58.Flanagan LA, Ju YE, Marg B, Osterfield M, Janmey PA. 2002. Neurite branching on deformable substrates. Neuroreport 13, 2411–2415. ( 10.1097/00001756-200212200-00007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lekka M, Pogoda K, Gostek J, Klymenko O, Prauzner-Bechcicki S, Wiltowska-Zuber J, Jaczewska J, Lekki J, Stachura Z. 2012. Cancer cell recognition—mechanical phenotype. Micron 43, 1259–1266. ( 10.1016/j.micron.2012.01.019) [DOI] [PubMed] [Google Scholar]
- 60.van Helvert S, Friedl P.. 2016. Strain stiffening of fibrillar collagen during individual and collective cell migration identified by AFM nanoindentation. ACS Appl. Mater. Interfaces 8, 21 946–21 955. ( 10.1021/acsami.6b01755) [DOI] [PubMed] [Google Scholar]
- 61.Freedman BR, Bade ND, Riggin CN, Zhang S, Haines PG, Ong KL, Janmey PA. 2015. The (dys)functional extracellular matrix. Biochim. Biophys. Acta Mol. Cell Res. 1853, 3153–3164. ( 10.1016/j.bbamcr.2015.04.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.