Abstract
Introduction
Prognostic biomarkers are highly needed to properly manage patients with cancer and improve their clinical courses. The relationship between lymphocyte-to-monocyte ratio (LMR) at diagnosis and ovarian cancer prognosis has been extensively studied, but little consensus has been reached regarding its utility as a biomarker of poor outcome. Thus, this study aimed to investigate the potential prognostic value of pretreatment LMR in such patients to shed light on this issue.
Methods
We searched the scientific databases of MEDLINE, Embase, Cochrane Library, and WangFang for relevant studies about the inflammatory prognostic factor LMR in ovarian cancer, based on specific inclusion and exclusion criteria. The following parameters were analyzed among others: LMR values and respective cut-offs, patient’s overall survival (OS) and progression-free survival (PFS), and clinicopathological features.
Results
Eight studies, including 2259 patients, were eligible for inclusion in this meta-analysis. We found that low LMR was associated with both poor OS [Hazard ratio (HR): 1.92; 95% confidence interval (CI): 1.58–2.34; p < 0.001] and PFS (HR: 1.70; 95% CI: 1.54–1.88; p < 0.001). Moreover, our findings revealed that low LMR was correlated with high G2/G3 histological grade (OR: 1.67; 95% CI: 1.26–2.20; p < 0.001) and late III-IV FIGO stage tumors (OR: 3.55; 95% CI: 2.68–4.70; p < 0.001), high serum CA-125 level (OR: 2.18; 95% CI: 1.71–2.77; p < 0.001), and presence of malignant ascites (OR: 1.87; 95% CI: 1.11–3.14; p = 0.02) and lymph node metastases (OR: 1.70; 95% CI: 1.13–2.54; p = 0.01).
Conclusion
Pretreatment LMR is a potential prognostic marker of poor outcome in ovarian cancer patients and may thus be important in clinical care and disease control.
Keywords: Lymphocyte-to-monocyte ratio, Meta-analysis, Ovarian cancer, Prognosis
Introduction
Ovarian cancer is one of the most common gynecological malignant tumors with the highest mortality rate. Over 90% of ovarian cancer is of epithelial origin, and non-epithelial tumors are usually derived from the granulosa or germ cells [1]. These differences in ovarian cancer etiology require different diagnostic approaches and result in distinct treatment regimens.
Despite advances in early diagnosis and targeted drug treatment, as well as improvements in drug cytotoxicity, most patients are still diagnosed at an advanced stage [2, 3]. Furthermore, chemotherapy is frequently not effective in controlling the disease mainly due to the development of primary or secondary resistance to anticancer drugs. In addition, some chemotherapy regimens are also associated with increased relapse and mortality rates among patients with ovarian cancer [4]. Therefore, better understanding of carcinogenic mechanisms is needed. The use of suitable and improved biomarkers could aid in both the diagnosis and prognosis of ovarian cancer.
It is well known that inflammatory and immune responses within the tumor microenvironment play important roles in tumorigenesis and cancer progression [5, 6]. Several inflammatory-related prognostic factors, such as the platelet-to-lymphocyte (PLR), neutrophil-to-lymphocyte (NLR), lymphocyte-to-monocyte (LMR), and C-reactive protein/albumin (CAR) ratios have been recently evaluated for their ability to predict outcomes of patients with various solid cancers [7–9]. In fact, PLR has been continuously reported as a novel inflammation-based prognostic index over the past years.
Similarly, LMR has been associated with a poor prognosis in several cancer types [10–12]. However, its prognostic value in ovarian cancer has not yet been fully elucidated. With this in mind, we decided to carry out this meta-analysis to elucidate the relationship of LMR with the clinicopathology of ovarian cancer and to establish whether it might be useful in predicting patient outcome.
Materials and methods
Search strategy
We searched the MEDLINE, Embase, Cochrane Library, and WanFang databases to identify the relevant articles using the search terms “LMR”, “lymphocyte to monocyte ratio”, “lymphocyte monocyte ratio”, or “lymphocyte-to-monocyte ratio” combined with “ovarian cancer”, “ovarian carcinoma”, “ovarian adenocarcinoma”, “ovarian tumor”, or “ovarian neoplasms”. The literature search was performed up to November 20, 2018.
Inclusion and exclusion criteria
Published articles were selected for study based on the following inclusion criteria: (1) reported association between pretreatment LMR and prognosis in ovarian cancer; (2) patients grouped into “high LMR group” and “low LMR group” according to cut-off values of LMR; and (3) hazard ratios (HRs) with 95% confidence intervals (CIs) calculated for overall survival (OS), progression-free survival (PFS), or cancer-specific survival (CSS). The exclusion criteria were as follows: (1) lack of appropriate data; (2) duplicate publications; and (3) reviews, meta-analysis, letters, and conference abstracts.
Data extraction and quality assessment
Data extraction was conducted independently by two investigators. The following information (on study details and clinopathological features) was collected from the studies: first author, year and country of study, number of patients involved and distribution of age and gender, tumor histological type, grade, stage, and optimal debulking, presence of malignant ascites and lymph node metastases, type of treatment applied (including surgery and chemotherapy dosage and duration), cut-off values of LMR, patient’s survival outcome (assessed by OS and PFS), and duration of follow-up period.
The Newcastle–Ottawa Scale (NOS) [13] was used to assess the methodological quality of the studies. According to this scale, the maximum score is 9; studies with NOS > 6 were considered high-quality studies.
Statistical analysis
We used Review Manager 5.3 (Cochrane Collaboration, Oxford, UK) to pool HRs for OS and PFS and to pool odd ratios (ORs) for clinicopathological parameters. The HRs and 95% CIs were directly obtained from studies that included survival analysis or, when necessary, they were determined from the Kaplan-Meier curve by using Engauge Digitizer 4.1 [14, 15].
The heterogeneity across the eligible studies was assessed by the Cochran’s Q-test and I2 statistic. If I2 ≤ 50% or p > 0.05, indicating low heterogeneity, we used a fixed-effect model with an inverse variance method. Otherwise, we used a random-effect model with the DerSimonian and Laird method, which considers both within-and between-study variations [16]. A subgroup analysis was then performed to examine the potential source of heterogeneity. Sensitivity analysis was undertaken in order to test the robustness of the pooled results by removing each study. When more than eleven studies were included, Begg’s funnel plots and Egger’s linear regression tests were used to evaluate publication bias [17, 18]. In all analyses, a p value < 0.05 was considered statistically significant.
Results
Study selection
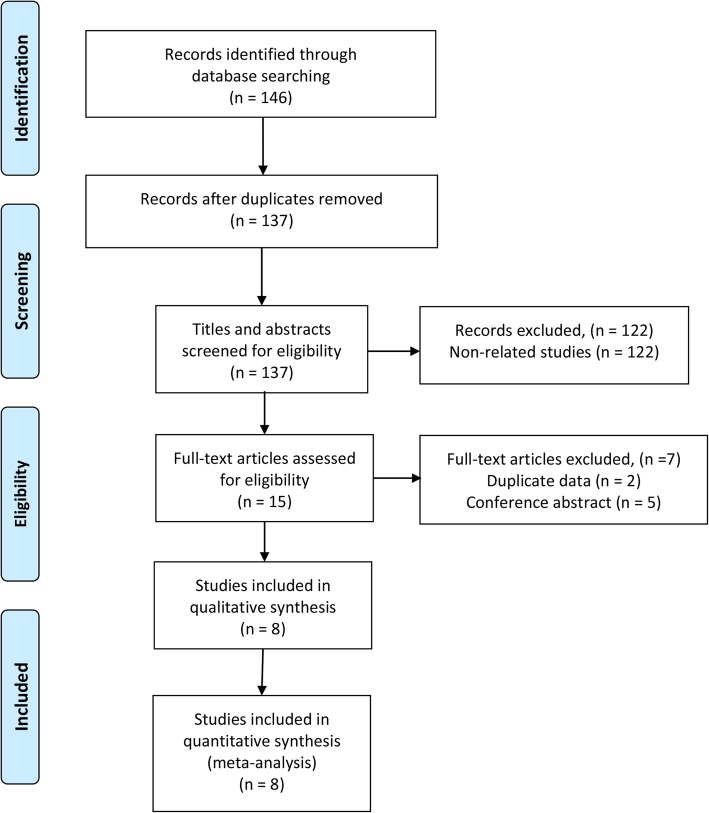
As shown in the flow diagram (Fig. 1), through the electronic search on the relevant databases, we initially retrieved 146 published articles, which were narrowed down to 137 following exclusion of duplicate studies and specific types of articles. After reviewing the title and abstract, 7 articles were excluded according to the exclusion criteria (i.e., lack of appropriate data) and 15 full-text articles were considered for further assessment. From these, 10 articles met the inclusion criteria and thus were used in the quantitative synthesis [19–25].
Fig. 1.
Flow diagram of study retrieval and selection processes
Study characteristics
The main characteristics of the included studies are summarized in Table 1. They were all retrospective studies that were published between 2016 and 2018. There were six and two studies of mixed-stage (I–IV) and advanced-stage (III–IV) diseases, respectively, according to the International Federation of Gynaecology and Obstetrics (FIGO) criteria. All patients underwent surgery and adjuvant chemotherapy. All included studies assessed the prognostic value of LMR in OS, and only six in PFS. The cut-off values of LMR ranged from 1.85 to 4.2, which were determined in seven studies by the receiver operating curve sensitivity and specificity analysis (C-index); in one study, the method used was not reported [22]. Univariate and multivariate analysis were used to evaluate OS in one and seven studies, respectively. For all studies, the NOS scores were ≥ 6 (Table 1).
Table 1.
Characteristics of the studies included in the meta-analysis
| Author | Year | Country | Ethnicity | Follow-up (months) | Treatment | Age (years) | No. of patients | Stage | Cut-off value | Survival analysis | Analysis | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temraz | 2014 | Lebanon | Caucasian | 24 | Mixed | 65 (43–88) | 68 | Mixed | 2.81 | OS/RFS | UV | 8 |
| Lee | 2015 | UK | Caucasian | NA | Surgery | 75 (65–81) | 226 | Early | 1.8 | OS | MV | 7 |
| Zhang | 2015 | China | Asian | 50.8 | Mixed | 65 (30–78) | 124 | Mixed | 4 | OS | MV | 8 |
| Yoshida | 2015 | Japan | Asian | 72 (27.6–111.6) | Mixed | 72 (43–91) | 181 | Mixed | 3.51 | OS | MV | 7 |
| Lucca | 2016 | Austria | Caucasian | NA | Surgery | 68 (61–74) | 310 | Early | 3.3 | OS | MV | 6 |
| D’Andrea | 2017 | Austria | Caucasian | 42.4 (18.3–85.1) | Surgery | 67 (60–73) | 4198 | Mixed | 3.5 | OS/RFS/CSS | MV | 8 |
| Miyake | 2017 | Japan | Asian | 22 (10–64) | Mixed | 72 (61–77) | 117 | Mixed | 3.3 | OS/CSS | UV | 6 |
| Rajwa | 2018 | Poland | Caucasian | 14 (7–40) | Surgery | NA | 144 | Mixed | 2.44 | OS/CSS | MV | 8 |
| Wang | 2018 | China | Asian | NA | Mixed | 63 (20–85) | 270 | Early | 4 | RFS | UV | 7 |
Abbreviations: OS overall survival, RFS recurrence-free survival, CSS cancer-specific survival, MV multivariate, NA not available
Meta-analysis
LMR and overall survival
Eight studies, comprising 2259 patients, investigated the predictive value of LMR in OS, revealing that a low LMR is indicative of a poor prognosis (worse OS) in ovarian cancer patients (HR: 1.92; 95% CI: 1.58–2.34; p < 0.001; Fig. 2). The test for high heterogeneity across the studies was significant (I2 = 70%; p = 0.001).
Fig. 2.
Pooled hazard ratio (HR) of lymphocyte-to-monocyte ratio (LMR) for overall survival (OS) in patients with ovarian cancer
Subgroup analysis was then performed to further explore the prognostic value of LMR (Table 2). In agreement, the results showed that a low ratio significantly predicts a poor OS in patients with both mixed- (HR: 1.91; 95% CI: 1.47–2.47; p < 0.001) and advanced-stage disease (HR: 2.04; 95% CI: 1.26–3.31; p < 0.001). A similar relationship between LMR and OS was also detected in other subgroup analyses (p < 0.05).
Table 2.
Pooled hazard ratios (HRs) for OS according to subgroup analyses
| Subgroup | No. of studies | No. of patients | HR (95% CI) | P value | Heterogeneity | |
|---|---|---|---|---|---|---|
| I2(%) | Ph | |||||
| Overall | 8 | 5368 | 0.63 (0.50–0.80) | < 0.001 | 65.9 | 0.005 |
| Ethnicity | ||||||
| Asian | 2 | 422 | 0.46 (0.25–0.87) | 0.016 | 73.3 | 0.023 |
| Caucasian | 5 | 4946 | 0.80 (0.71–0.89) | < 0.001 | 8.2 | 0.360 |
| Disease stage | ||||||
| Early | 2 | 536 | 0.61 (0.20–1.82) | 0.377 | 0 | 0.705 |
| Mixed | 6 | 4832 | 0.63 (0.49–0.80) | < 0.001 | 75.3 | 0.001 |
| Treatment | ||||||
| Surgery | 4 | 4878 | 0.81 (0.74–0.89) | < 0.001 | 0 | 0.883 |
| Mixed | 4 | 482 | 0.45 (0.27–0.73) | 0.001 | 62.3 | 0.047 |
| Cut-off for LMR | ||||||
| ≥ 3 | 5 | 4930 | 0.56 (0.35–0.88) | 0.011 | 76.6 | 0.002 |
| < 3 | 3 | 438 | 0.65 (0.41–1.04) | 0.075 | 41.7 | 0.180 |
| Analysis method | ||||||
| Univariate | 2 | 185 | 0.46 (0.27–0.79) | 0.005 | 0 | 0.488 |
| Multivariate | 6 | 5183 | 0.67 (0.53–0.86) | 0.001 | 65.9 | 0.005 |
LMR and progression-free survival
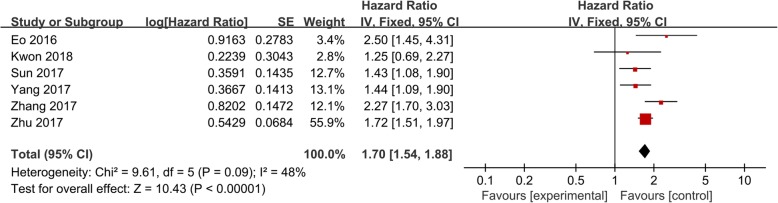
Our findings showed a statistically significant negative relationship between LMR and PFS (Fig. 3), in which low values of LMR were associated with worse PFS (HR: 1.70; 95% CI: 1.54–1.88; p < 0.001). The results of the subgroup analyses based on FIGO stage, sample size, LMR cut-off value, and analysis method were similar to those of OS, meaning that in all cases a low ratio was also predictive of a poor PFS.
Fig. 3.
Pooled hazard ratio (HR) of lymphocyte-to-monocyte (LMR) for progression-free survival (PFS) in patients with ovarian cancer
LMR and clinicopathological parameters
The main results of the relationship between LMR and clinicopathological parameters are summarized in Table 3. A low LMR was associated with an advanced tumor progression, specifically with a high histological grade (G2/G3 vs. low G1 grade; OR: 1.67; 95% CI: 1.26–2.20; p < 0.001) and late FIGO stages (III-IV vs. early I-II stages; OR: 3.55; 95% CI: 2.68–4.70; p < 0.001), as well as with the presence of malignant ascites (OR: 1.87; 95% CI: 1.11–3.14; p = 0.02) and lymph node metastasis (OR: 1.70; 95% CI: 1.13–2.54; p = 0.01). Similarly, a low LMR was related to a high serum CA-125 marker (> median vs. < median; OR: 2.18; 95% CI: 1.71–2.77; p < 0.001). However, no obvious association was found between LMR and patient age (> median vs. < median; OR: 1.18; 95% CI: 0.97–1.44; p = 0.09), histological type (serous vs. others; OR: 1.07; 95% CI: 0.88–1.30; p = 0.51), and evidence of optimal debulking (OR: 1.13; 95% CI: 0.71–1.80; p = 0.62).
Table 3.
Meta-analysis of the association between LMR and clinicopathological features of ovarian cancer
| Characteristics | No. of studies | No. of patients | OR (95% CI) | p | Heterogeneity | |
|---|---|---|---|---|---|---|
| I2 (%) | Ph | |||||
| Age (≥ 60 vs. < 60) | 3 | 626 | 2.07 (1.22–3.50) | 0.007 | 42 | 0.18 |
| Gender (male vs. female) | 4 | 4818 | 1.18 (0.68–2.04) | 0.56 | 70 | 0.02 |
| Smoking status (ever/current vs. never) | 2 | 394 | 0.95 (0.63–1.45) | 0.82 | 0 | 0.80 |
| Differentiation (low vs. moderate/high) | 5 | 4886 | 1.60 (1.10–2.32) | 0.01 | 35 | 0.19 |
| Tumor size (> 3 cm vs. < 3 cm) | 2 | 496 | 1.86 (0.74–4.71) | 0.19 | 71 | 0.06 |
| T stage (III-IV vs. I-II) | 3 | 4390 | 1.13 (1.01–1.28) | 0.04 | 0 | 0.79 |
| Lymph node metastasis (yes vs. no) | 3 | 4390 | 1.22 (1.06–1.39) | 0.005 | 0 | 0.67 |
| Distant metastasis (yes vs. no) | 1 | 124 | 1.46 (0.37–5.73) | 0.59 | – | – |
| Multiplicity (multiple vs. solitary) | 2 | 496 | 1.04 (0.68–1.58) | 0.86 | 0 | 0.49 |
| Concomitant Cis (yes vs. no) | 2 | 4322 | 0.88 (0.78–0.99) | 0.03 | 0 | 0.87 |
Cis carcinoma in situ
Sensitivity analysis
A sensitivity analysis was performed to evaluate the stability/robustness of the meta-analysis results. We found that none of the individual studies substantially altered the combined HRs of all studies, suggesting that the conclusions are relatively reliable.
Discussion
The present study is, to our knowledge, the most comprehensive, up-to-date, and with the largest sample size meta-analysis undertaken to estimate the prognostic value of LMR in ovarian cancer. According to the pooled results confirmed by subgroup analysis, there was a significant association between low LMR and poor survival outcome, specifically poor OS and PFS, in ovarian cancer patients. Therefore, it can be concluded that LMR is an independent prognostic factor in ovarian cancer.
Additionally, in this study, the correlations between LMR and several clinicopathological parameters were evaluated. We found that a low LMR was highly correlated with tumor high G2/G3 histological grades and late III-IV FIGO stages, as well as with high serum CA-125 levels, and presence of malignant ascites and lymph node metastases, in agreement with the poor overall survival outcome associated with this ratio.
However, the potential mechanisms underlying the prognostic ability of LMR have not yet been clarified. It is known that lymphocytes play an important role in cell-mediated antitumor immune responses and in tumor immunological surveillance [26, 27]. Cytotoxic lymphocytes, mainly cytotoxic T cells, are crucial to eliminate residual cancer cells and, as such, are applied in immunotherapy [28, 29]. Monocytes seem to have an impact on tumorigenesis through differentiation into tumor-associated macrophages (TAMs). TAMs are major players in inflammation, being recruited to the tumor site in response to tumor-derived chemotactic factors [30]. Therefore, TAM levels may reflect the tumor burden. Moreover, recent studies reported that an increased local infiltration of TAMs is associated with a poor prognosis in several cancer types [31, 32]. In line with this, LMR may represent the balance between antitumor immune reaction and tumor promotion function. Thus, a low LMR would be associated with a favorable tumor progression, explaining at least in part our results.
Our study presents several limitations. First, all studies included were carried out in Asian countries, implying that more cohort studies from other regions are necessary. Second, our conclusions could have been influenced by the heterogeneity of the results of the studies included in this meta-analysis, as well as by unknown carcinogenesis mechanisms. Third, the cut-off value of LMR was not uniform across the studies analyzed. Finally, only retrospective studies were included, which might have introduced confounding variables; thus, control-test studies are missing. Nevertheless, the present meta-analysis, which is conceptually superior to individual investigations, included sufficient published studies with data from a large number of patients, allowing for adequate evaluation of the prognostic value of LMR in ovarian cancer.
Conclusions
The present study revealed that a low pretreatment (baseline) LMR is associated with a poor OS and PFS in ovarian cancer patients, as well as with severe clinicopathological features including advanced tumor characteristics. Therefore, as LMR is easily accessible, it may be a useful prognostic biomarker in ovarian cancer and thus, relevant in the management of the disease.
Acknowledgments
None.
Authors’ contributions
JG and RFL performed the study, analyzed and interpreted the data, and wrote the manuscript. CS, MQH, YH, and QL also participated in data analysis and writing of the manuscript. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jun Gong, Email: 32218934@qq.com.
Hui Jiang, Email: 279895039@qq.com.
Chang Shu, Email: 81044964@qq.com.
Mei-qin Hu, Email: 344643962@qq.com.
Yan Huang, Email: 61018028@qq.com.
Qin Liu, Email: hszxyylq@126.com.
Rong-feng Li, Phone: +86(714)3062002, Email: rongfengli@yeah.net.
References
- 1.Lim W, Song G. Discovery of prognostic factors for diagnosis and treatment of epithelial-derived ovarian cancer from laying hens. Journal of cancer prevention. 2013;18(3):209–220. doi: 10.15430/JCP.2013.18.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall TR, Dizon DS. Neoadjuvant chemotherapy for advanced epithelial ovarian cancer. Clin Adv Hematol Oncol. 2016;14(4):262–268. [PubMed] [Google Scholar]
- 3.Martin-Camean M, Delgado-Sanchez E, Pinera A, Diestro MD, De Santiago J, Zapardiel I. The role of surgery in advanced epithelial ovarian cancer. Ecancermedicalscience. 2016;10:666. doi: 10.3332/ecancer.2016.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mantia-Smaldone GM, Edwards RP, Vlad AM. Targeted treatment of recurrent platinum-resistant ovarian cancer: current and emerging therapies. Cancer Manag Res. 2011;3:25–38. doi: 10.2147/CMR.S8759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14(12):717–734. doi: 10.1038/nrclinonc.2017.101. [DOI] [PubMed] [Google Scholar]
- 7.Nora I, Shridhar R, Huston J, Meredith K. The accuracy of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as a marker for gastrointestinal malignancies. J Gastrointest Oncol. 2018;9(5):972–978. doi: 10.21037/jgo.2018.08.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phan TT, Ho TT, Nguyen HT, Nguyen HT, Tran TB, Nguyen ST. The prognostic impact of neutrophil to lymphocyte ratio in advanced non-small cell lung cancer patients treated with EGFR TKI. Int J Gen Med. 2018;11:423–430. doi: 10.2147/IJGM.S174605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X, Liu H, He M, Liu M, Zhou G, Gong P, et al. Prognostic value of pretreatment C-reactive protein/albumin ratio in nasopharyngeal carcinoma: a meta-analysis of published literature. Medicine (Baltimore) 2018;97(30):e11574. doi: 10.1097/MD.0000000000011574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mano Y, Yoshizumi T, Yugawa K, Ohira M, Motomura T, Toshima T, et al. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the international liver transplantation society. 2018. Lymphocyte-to-monocyte ratio is a predictor of survival after liver transplantation for hepatocellular carcinoma. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe K, Yasumoto A, Amano Y, Kage H, Goto Y, Yatomi Y, et al. Mean platelet volume and lymphocyte-to-monocyte ratio are associated with shorter progression-free survival in EGFR-mutant lung adenocarcinoma treated by EGFR tyrosine kinase inhibitor. PLoS One. 2018;13(9):e0203625. doi: 10.1371/journal.pone.0203625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimura T, Shibata M, Gonda K, Hayase S, Sakamoto W, Okayama H, et al. Prognostic impact of preoperative lymphocyte-to-monocyte ratio in patients with colorectal cancer with special reference to myeloid-derived suppressor cells. Fukushima J Med Sci. 2018;64(2):64–72. doi: 10.5387/fms.2018-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 14.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815–2834. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 19.Eo WK, Chang HJ, Kwon SH, Koh SB, Kim YO, Ji YI, et al. The lymphocyte-monocyte ratio predicts patient survival and aggressiveness of ovarian Cancer. J Cancer. 2016;7(3):289–296. doi: 10.7150/jca.13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang Y, Li J, Xu F, Hu H. Association between monocyte-to-lymphocyte ratio and prognosis of patients with epithelial ovarian cancer. Chin J Obstet Gynecol Pediatr. 2017;13(5):532–538. [Google Scholar]
- 21.Sun L, Song Y. Effects of lymphocyte and monocyte ratio on prognosis of epithelial ovarian cancer. Chin Clin Oncol. 2017;21(10):909–912. [Google Scholar]
- 22.Zhang W, Ye B, Liang W, Ren Y. Preoperative prognostic nutritional index is a powerful predictor of prognosis in patients with stage. III ovarian cancer Sci Rep. 2017;7(1):9548. doi: 10.1038/s41598-017-10328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon BS, Jeong DH, Byun JM, Lee TH, Choi KU, Song YJ, et al. Prognostic value of preoperative lymphocyte-monocyte ratio in patients with ovarian clear cell carcinoma. J Cancer. 2018;9(7):1127–1134. doi: 10.7150/jca.24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Yuan Z, Qiu H, Zhang R, Ding L, Zhao P. The relationship between preoperative blood lymphocyte-to-monocyte ratio and the prognostic of epithelial ovarian cancer. Prog Obstet Gynecol. 2016;25(9):654–657. [Google Scholar]
- 25.Yang HM, Lou G. The relationship of preoperativelymphocyte-monocyte ratio and the clinicopathological characteristics and prognosis of patients with epithelial ovarian cancer. Zhonghua zhong liu za zhi [Chinese journal of oncology] 2017;39(9):676–680. doi: 10.3760/cma.j.issn.0253-3766.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Chen KJ, Zhou L, Xie HY, Ahmed TE, Feng XW, Zheng SS. Intratumoral regulatory T cells alone or in combination with cytotoxic T cells predict prognosis of hepatocellular carcinoma after resection. Med Oncol. 2012;29(3):1817–1826. doi: 10.1007/s12032-011-0006-x. [DOI] [PubMed] [Google Scholar]
- 27.Zhou J, Ding T, Pan W, Zhu LY, Li L, Zheng L. Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int J Cancer. 2009;125(7):1640–1648. doi: 10.1002/ijc.24556. [DOI] [PubMed] [Google Scholar]
- 28.Zikos TA, Donnenberg AD, Landreneau RJ, Luketich JD, Donnenberg VS. Lung T-cell subset composition at the time of surgical resection is a prognostic indicator in non-small cell lung cancer. Cancer Immunol Immunother. 2011;60(6):819–827. doi: 10.1007/s00262-011-0996-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minami T, Minami T, Shimizu N, Yamamoto Y, De Velasco M, Nozawa M, et al. Identification of programmed death ligand 1-derived peptides capable of inducing Cancer-reactive cytotoxic T lymphocytes from HLA-A24+ patients with renal cell carcinoma. J Immunother. 2015;38(7):285–291. doi: 10.1097/CJI.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 30.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014;6(3):1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang M, Li Z, Ren M, Li S, Zhang L, Zhang X, et al. Stromal infiltration of tumor-associated macrophages conferring poor prognosis of patients with basal-like breast carcinoma. J Cancer. 2018;9(13):2308–2316. doi: 10.7150/jca.25155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang WJ, Wang XH, Gao ST, Chen C, Xu XY, Sun Q, et al. Tumor-associated macrophages correlate with phenomenon of epithelial-mesenchymal transition and contribute to poor prognosis in triple-negative breast cancer patients. J Surg Res. 2018;222:93–101. doi: 10.1016/j.jss.2017.09.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.