Abstract
Background
Previous studies conducted on the association between diabetes and the risk of endometrial cancer have reported controversial results that have raised a variety of questions about the association between diabetes and the incidence of this cancer. Thus, the aim of this systematic review and meta-analysis was to more precisely estimate the effect of diabetes on the risk of endometrial cancer incidence.
Methods
All original articles were searched in international databases, including Medline (PubMed), Web of sciences, Scopus, EMBASE, and CINHAL. Search was done from January 1990 to January 2018 without language limitations. Also, logarithm and standard error logarithm relative risk (RR) were used for meta-analysis.
Results
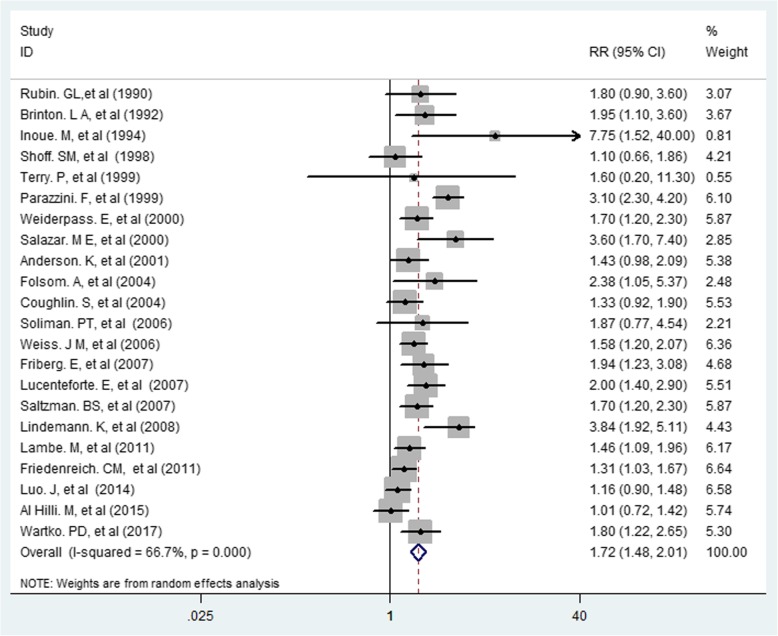
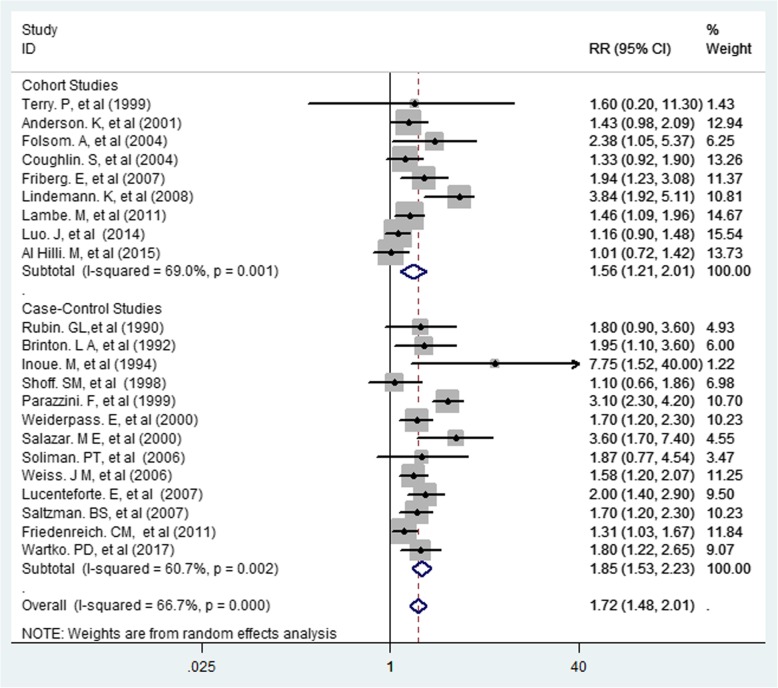
A total of 22 cohort and case-control studies were included in this meta-analysis, of which 14 showed statistically significant associations between diabetes and risk of endometrial cancer. Diabetes was associated with increased risk of endometrial cancer (RR = 1.72, 95% CI 1.48–2.01). The summary of RR for all 9 cohort studies was 1.56 (95% CI 1.21–2.01), and it was 1.85 (95% CI 1.53–2.23) for 13 case control studies. The summary of RR in hospital-based studies was higher than other studies. Thirteen of the primary studies-controlled BMI as a confounding variable, and the combined risk of their results was 1.62 (95% CI 1.34–1.97).
Conclusions
Diabetes seems to increases the risk of endometrial cancer in women, and this finding can be useful in developing endometrial cancer prevention plans for women having diabetes.
Keywords: Diabetes, Endometrial Cancer, Risk, Meta-analysis
Background
A recent study conducted by Lortet-Tieulent, J and colleague show that endometrial cancer is the sixth most commonly occurring cancer in women and the 15th most commonly occurring cancer overall. There were over 380,000 new cases in 2018 [1]. Also, about 142,000 women are diagnosed with endometrial cancer annually worldwide, and about 42,000 women lose their life due to endometrial cancer. The usual curve of endometrial cancer indicates that most cases are diagnosed after menopause, and the highest incidence rate is around the seventh decade of life [2]. The disease is more than 10 times common in North America and Europe than in less developed countries [3]. The incidence and the mortality rate of endometrial cancer increased during 2006 and 2010 [4]. Estrogens, both internal and external, play an important role in increasing endometrial cancer [5]. Several studies have shown that the risk of endometrial cancer increases with older age, early menstruation, late menopause, obesity, family history of endometrial cancer (especially among close relatives), exposure to radiation, infertility (especially due to polycystic ovarian syndrome), and long-term use of estrogens for hormone therapy [4–7]. Estrogens, both internal and external, play an important role in increasing endometrial cancer. Multiple studies have claimed a positive association between diabetes and incidence of endometrial cancer with several biological mechanisms [8]. However, a previous systematic review and meta-analysis was performing by Friberg and colleges [8] but growing several publications afterwards and also considering new variables in adjusted models, we felt to design an updated systematic review and meta-analysis in order to show any posible relationship between diabetes and endometrial cancer.
Methods
This systematic review was performed according to the Meta-Analyses of Observational Studies in Epidemiology (MOOSE) and Strengthening the Reporting of Observationally Studies in Epidemiology (STROBE) guidelines for reviews of analytical observational studies (case-control and cohort) [2, 9, 10].
Search strategy
All original published articles were searched in international databases, including Medline (PubMed), Web of sciences, Scopus, EMBASE, and CINHAL. Search was done from January 1990 to January 2018 without language limitations. The keywords were Diabetes, Diabetes Mellitus (type 1 and 2), Insulin Dependent, IDDM, NIDDM, Noninsulin Dependent, Endometrial Stromal Tumors, Endometrial Neoplasms, and Endometrial. The selected studies were limited to observational studies on humans.
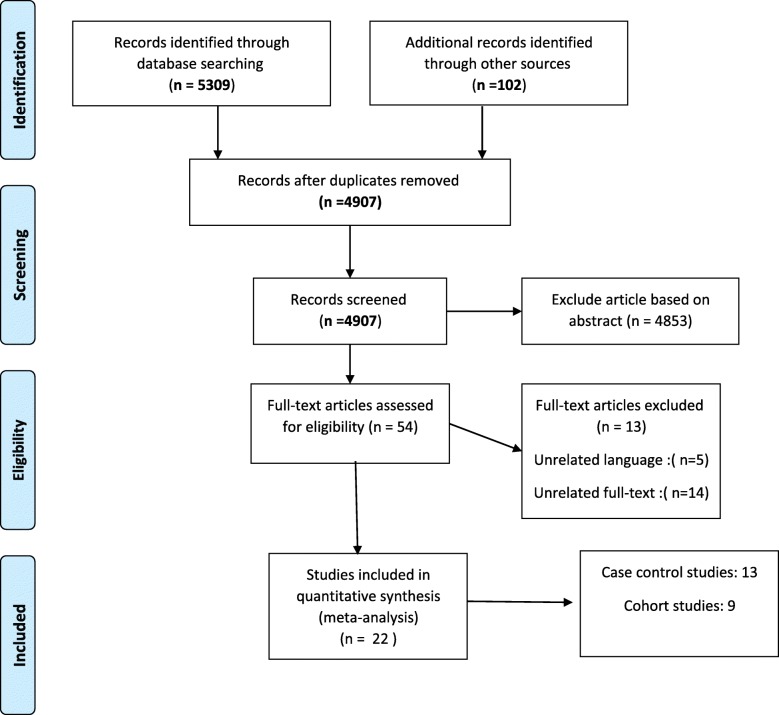
The primary search results were reviewed, and some of the articles were eliminated after reviewing their title and an abstract. Inclusion and exclusion criteria were set by 2 researchers separately (YM, FV) (Fig. 1).
Fig. 1.
Flow Diagram of the Literature Searches and Study Selection
Eligibility criteria
A published study had to meet the following inclusion criteria:
(1) original article, (2) case-control or cohort study, (3) human population, (4) diabetes and patients with diabetes as the main independent variable, and (5) endometrial cancer as the dependent variable. Case reports, reviews, animal studies, and case control or cohort studies with crude estimates about the effect of diabetes on the risk of endometrial cancer were removed from the tabulation. The authors resolved all disputes during the collection, compilation, and analysis of data.
Data extraction
Two researchers evaluated all included articles independently. They assessed the disagreement, if any, and in case an agreement was not reached, a third author (LS) evaluated the study. Two independent matched reviewers extracted the data according to a uniform Excel sheet. Then, a structured checklist was used to extract the following information: (1) author, (2) year of publication, (3) type of study, (4) country, (5) study population, (6) age of women, (7) sample size, (8) type of diabetes, (9) measurement, and (10) adjusted variables of association.
Statistical analysis
In the meta-analysis, 3 measures of association were used: (1) odds ratio (case-control and population-based case-control studies), (2) relative risk (cohort and population cohort studies), and (3) hazard ratio (cohort and population-based cohort studies). As the frequency of endometrial cancer was relatively low, the odds ratio in the case-control and population based case-control studies and the risk ratio in the cohort and population-based cohort studies yielded similar estimates of relative risk (RR) [11].
Logarithm and standard error logarithm relative risk (RR) were used for the meta-analysis. DerSimonian and Laird method was used to compute the pooled estimate of relative risk (RR) with confidence interval (CI 95%) using random models [12]. Because the test for heterogeneity was statistically significant in some analyses, the random effects models were used to estimate RR. In this study, w Cochran’s Q test and I2 statistic were used to evaluate statistical heterogeneity between studies [13]. In addition, a meta-regression and subgroup analysis was performed to assess the source of heterogeneity between studies. Moreover, publication bias was assessed by funnel plot and Egger and Begg’s test [14, 15]. Statistical analysis was performed using STATA 14.0 (Stata Corp, College Station, TX, USA), and statistical significance was set at p < 0.05.
Results
Study characteristics
A total of 22 studies were included in this meta-analysis (Fig. 1), of which 9 were cohort and population cohort studies [4–6, 16–21] (Table 1) and 13 were case-control and population case-control studies [22–34] (Table 2). Also, 12 studies were conducted in the USA [4, 5, 16, 19, 20, 22, 27, 29–32, 34], 4 in Sweden [17, 18, 21, 33], 2 in Italy [25, 26], 1 in Canada [23], 1 in Norway [6], 1 in Mexico [28], and 1 in Japan [24]. The case-control and population case-control studies (n = 13) comprised 22,392 controls and 7698 endometrial cancer cases.
Table 1.
The Main Characteristics of Cohort and Population-based Cohort Studies on Diabetes and Endometrial Cancer Risk
| Authors | Year | Type of study | Country | Study population | Age | Sample size | Type of diabetes | Measurement of association | Controlled variables |
|---|---|---|---|---|---|---|---|---|---|
| Al Hilli. M, et al. [16] | 2015 | Cohort | USA | database for the records of all patients who underwent primary surgical intervention for EC, from January 1, 1999, through December 31, 2008. | All age | 1303 | Diabetes | HR: 1.01; % 95 CI (0.72,1.42) | Age, BMI |
| Friberg. E, et al. [17] | 2007 | Cohort | Sweden |
Exposed group: 1628 women with self-reported DM or DM from national inpatient register Comparison group: 35145 women without self-reported DM or DM from national inpatient register |
50–83 | 36,773 | Diabetes | RR: 1.94; % 95 CI(1.23,3.08) | Age, BMI, total physical activity |
| Anderson. K, et al.[5] | 2001 | Cohort | USA |
Exposed group: 1325 women with self-reported DM Comparison group: 23150 women without self-reported DM |
55–69 | 24,475 | Diabetes | RR: 1.43; % 95 CI(0.98,2.09) | Age, BMI, BMI2, WHR, ovulatory span, gravidity, PMH, menstrual irregularities, hypertension |
| Lindemann. K, et al. [6] | 2008 | Cohort | Norway | Norwegian women during 15.7 years of follow-up. | All age | 36,761 | Diabetes | RR: 3.84 (% 95 CI: 1.92,5.11) | Age |
| Folsom. A, et al. [20] | 2004 | Cohort | USA |
Exposed group: 42 women with self-reported DM and an endometrial cancer diagnosis Comparison group: 373 women with self-reported DM and an endometrial cancer diagnosis |
55–69 | 415 | Diabetes | RR: 2.38 (% 95 CI: 1.05,5.37) | Age, extent of endometrial cancer at diagnosis |
| Luo. J, et al. [4] | 2014 | Cohort | USA | Women’s Health Initiative | 50–79 | 88,107 | Diabetes | HR: 1.16 (% 95 CI: 0.90,1.48) | Age, BMI |
| Terry. P, et al. [21] | 1999 | Cohort | Sweden |
Exposed group: 142 women with self-reported DM Comparison group: 10012 women without self-reported DM |
42–81 | 10,154 | Diabetes | RR: 1.60 (% 95 CI: 0.20,11.30) | Age, physical activity, weight, parity |
| Coughlin. S, et al. [19] | 2004 | Cohort | USA |
Exposed group: 33 women with self-reported DM Comparison group: 448 women without self-reported DM |
> 30 | 481 | Diabetes | RR: 1.33 (% 95 CI: 0.92,1.90) | Age, race, education, BMI, smoking, alcohol, red meat, citrus fruit and juice, vegetables, physical activity, PMH, parity, age at menarche, age at first live birth, menopausal status, OC |
| esLambe. M, et al. [18] | 2011 | Cohort | Sweden | individuals that took part in routine health checkups and primary care patients referred for laboratory testing | All age | 230,737 | Diabetes | HR: 1.46(% 95 CI 1.09,1.96 | Age |
Table 2.
The Main Characteristics of Case-Control and Population Case-Control Studies on Diabetes and Endometrial Cancer Risk
| Authors | Year | Country | Control subjects (selection methods) | Age | Sample size | Type of diabetes | Measurement of association | Controlled variables |
|---|---|---|---|---|---|---|---|---|
| Weiderpass. E, et al. [33] | 2000 | Sweden | Control women were randomly selected from a continuously updated population register that includes all residents. | 50–74 | Case(709) | Diabetes | OR: 1.7 (% 95 CI: 1.2,2.3) | Age, age at menarche, parity, age at last birth, age at menopause, smoking, OC, PMH, BMI |
| Control(3368) | ||||||||
| T(4077) | ||||||||
| Shoff. SM, et al. [30] | 1998 | USA | Community controls were selected randomly from lists | 40–79 | Case(723) | Diabetes | OR: 1.10 (% 95 CI: 0.66,1.86) | Age, BMI, smoking, PMH, parity, education |
| Control(2291) | ||||||||
| T(3014) | ||||||||
| Lucenteforte. E, et al. [25] | 2007 | Italy | Controls women admitted to the same network of hospitals | 18–79 | Case(777) | Diabetes | OR: 2.0 (% 95 CI: 1.4,2.9) | Age, year of interview, study center, education, parity, menopausal status, OC and HRT use |
| Control(1550) | ||||||||
| T(2327) | ||||||||
| Friedenreich. CM, et al. [23] | 2011 | Canada | Controls selected from the Alberta Cancer Registry | 30–79 | Case(515) | Diabetes | OR: 1.31(95% CI: 1.03,1.67) | Age, parity, education, age at menarche, hormone therapy, age at menopause, history of Type 2 diabetes, hormone contraception, oral and non-oral hormone use, history of angina, history of stroke, history of thrombosis, smoking and alcohol consumption |
| Control(962) | ||||||||
| T(1447) | ||||||||
| Saltzman. BS, et al. [29] | 2007 | USA | Controls selected from Women’s Contraceptive and Reproductive Experiences (CARE) breast cancer study | 45–74 | Case(1303) | Diabetes | OR: 1.7(% 95 (CI: 1.2, 2.3) | Country, age, reference year, body mass index, and menopausal hormone use |
| Control(1779) | ||||||||
| T(3082) | ||||||||
| Parazzini. F, et al. [26] | 1999 | Italy | Controls selected from same network of hospitals where cases had been identified. | 28–74 | Case(752) | Diabetes | OR: 3.1 (% 95 CI: 2.3,4.2) | Age, calendar year, education, BMI, parity, OC, PMH, age at menopause, hypertension, smoking |
| Control(2606) | ||||||||
| T(3358) | ||||||||
| Wartko. PD, et al. [32] | 2017 | USA | Control were randomly selected from all other women with delivery records from 1987 to 2013. | All age | Case(593) | Diabetes | OR: 1.80 (% 95 CI: 1.22,2.65) | Race/ethnicity, year of delivery, maternal age at delivery, and body mass index |
| Control(5743) | ||||||||
| T(6336) | ||||||||
| Soliman. PT, et al. [31] | 2006 | USA | Controls patient samples were obtained through a low-risk cancer screening program | All age | Case(117) | Diabetes | OR: 1.87 (%95, CI: 0.77,4.54) | Lower serum adiponectin level, age, BMI, and hypertension |
| Control(238) | ||||||||
| T(355) | ||||||||
| Rubin. GL, et al.[27] | 1990 | USA | population controls, matched for place of residence and age | 20–54 | Case(196) | Diabetes | OR: 1.80 (%95, CI: 0.90,3.60) | Age |
| Control(986) | ||||||||
| T(1182) | ||||||||
| Brinton. L A, et al. [22] | 1992 | USA | Population controls random digit dialing for younger controls and health care financing administration for older controls, older controls were matched on age, race and zip code | 20–74 | Case(405) | Diabetes | OR: 1.95 (%95, CI: 1.10,3.60) | Age, education, number of births, weight, OC, PMH |
| Control(279) | ||||||||
| T(684) | ||||||||
| Inoue. M, et al. [24] | 1994 | Japan | hospital control who underwent hysterectomy due to benign gynecological tumors, matched on year of admittance to hospital and age | 22–79 | Case(143) | Diabetes | OR: 7.75 (%95, CI: 1.52,40.0) | Age, parity, cancer history, hypertension, obesity |
| Control(143) | ||||||||
| T(286) | ||||||||
| Weiss. J M, et al. [34] | 2006 | USA | Population that matched on age | 45–75 | Case(1281) | Diabetes | OR: 1.58 (%95, CI: 1.20,2.07) | Age, PMH, BMI, county, referent year, tumors aggressiveness |
| Control(1779) | ||||||||
| T(3060) | ||||||||
| Salazar. M E, et al.[28] | 2000 | Mexico | Hospital, from primary health center i.e. outpatient, matched on age | NA | Case(85) | Diabetes | OR: 3.60 (%95, CI: 1.70,7.40) | Age, an ovulatory index, smoking, physical activity, menopausal status, hypertension, BMI |
| Control(668) | ||||||||
| T(753) |
The overall and individual results of 22 cohort and case-control studies are shown in Fig. 2. Of the 22 studies, 14 showed statistically significant associations between diabetes and risk of endometrial cancer. Occurrence of diabetes had an association with increased risk of endometrial cancer (RR = 1.72, 95% CI 1.48–2.01) (Figs. 2 and 3). The results demonstrated heterogeneity of the studies (I2 = 66.7%; P < 0.0001). However, no evidence of publication bias was found based on the results of the Egger’s test (Egger’s test: t = 1.90, P = 0.072, 95% CI: − 0.04-0.91).
Fig. 2.
Association between Diabetes and Risk of Endometrial Cancer
Fig. 3.
Association between Diabetes and Risk of Endometrial Cancer by Type of Study
Subgroup analysis
The subgroup analysis was conducted based on the study design, and variables adjustment (Table 3). Individual study results and the overall summary results for 8 cohorts and 7 population-based, 2 hospital-based, and 5 case-control studies investigating the effect of diabetes on the risk of endometrial cancer in women are shown in Table 3. The results indicated that the summary of RR for all the 8 cohort studies combined was 1.52 (95% CI 1.16–2.00), and heterogeneity among these studies was significant (Q = 3.03, I2 = 70.7%; P = 0.001). The summary of RR for all the 7 population-based case–control studies was 1.55 (95% CI 1.37–1.75), however, heterogeneity among these studies was not significant (Q = 6.88, I2 = 0.0%; P = 0.461). In addition, the summary of RR for all the 5 case-control studies was 2.31 (95% CI 1.81–2.96), but heterogeneity was not significant (Q = 6.69, I2 = 22.7%; P = 0.270). Also, the summary of RR was higher in hospital-based studies than in other studies [RR = 4.10 (CI 95% 2.09–8.01), heterogeneity was Q = 4.12, I2 = 0.0%, P = 0.402]. According to the results in Table 3, the summary of RR in hospital-based studies was higher than in other studies. Also, 13 of the primary studies-controlled BMI as a confounding variable, and the combined risk of their results was 1.62 (95% CI 1.34–1.97, test for heterogeneity: Q = 4.14, I2 = 71.0%, P = 0.0001). However, 4 of the primary studies-controlled weight as a confounding variable, and the combined risk of their results was 2.45 (95% CI 1.14–5.26, test for heterogeneity: Q = 2.53, I2 = 21.0%, P = 0.021).
Table 3.
Summary Relative Risk (RR) Estimates [95% confidence intervals (CIs)] for Case–Control and Cohort Studies Conducted on the Association Between Diabetes and Endometrial Cancer Incidence by Study Design, Continent, and Age
| Subgroup | Number of studies | Summery Relative Risk (95% CI) | Between studies | Between subgroups | |||
|---|---|---|---|---|---|---|---|
| I2 | P heterogeneity | Q | Q | P heterogeneity | |||
| Study design | |||||||
| Cohort | 8 | 1.52 (1.16–2.00) | 70.7% | 0.001 | 3.03 | 5.79 | 0.001a |
| Case-Control | 5 | 2.31 (1.81–2.96) | 22.7% | 0.270 | 6.69 | ||
| Population-based | 7 | 1.55 (1.37–1.75) | 0.0% | 0.461 | 6.88 | 4.95 | 0.034b |
| Hospital-based | 2 | 4.10 (2.09–8.01) | 0.0% | 0.402 | 4.12 | ||
| Adjustment | 3 | 1.88 (1.48–2.38) | 81.9% | 0.004 | 5.21 | 8.78 | 0.045 |
| Age | 13 | 1.62 (1.34–1.97) | 71.0% | 0.0001 | 4.14 | ||
| BMI | 3 | 2.45 (1.14–5.26) | 21.0% | 0.021 | 2.53 | ||
| Weight | 4 | 1.89 (1.22–2.94) | 50.6% | 0.108 | 6.08 | ||
| Physical Activity | |||||||
Largely diabetes mellitus
All statistical tests were 2-sided
aTest for heterogeneity between case-control and cohort studies
bTest for heterogeneity between population-based and hospital-based case-control studies
Also, the summary of RR of primary studies, whose results were adjusted based on BMI showed a less value compared to summary of RR of primary studies, whose results were adjusted based on weight control (1.62; 95% CI 1.34–1.97 Vs 2.45; 95% CI 1.14–5.26). Physical activity was adjusted in 4 primary studies, and the summary of RR based on controlling this variable was 1.89 (95% CI 1.22–2.94, test for heterogeneity Q = 6.08, I2 = 50.6%, P = 0.108) (Table 3).
Discussion
The results of this meta-analysis showed that women with diabetes had a 72% increased risk of endometrial cancer compared to those without diabetes as supports the previous meta-analysis conducted by E. Friberg et al. (31) in 2007. Also, other studies have shown that diabetes increased the risk of endometrial cancer, which is in line with the results of the present study [5, 6, 16, 23, 26, 32, 35].
Based on subgroup analysis, the risk of endometrial cancer in case-control studies was higher than in cohort studies, and a higher risk was observed in hospital-based studies compared to population-based studies. [36–38].
Since the results of case-control and hospital-based studies are more prone to be affected by confounders therefore the calculated risk might be over-estimated. [39–41].
In our meta-analysis, heterogeneity was 66.7% for overall risk, which was reduced by subgroup analysis based on type of study, so the heterogeneity for each group for RR in cohort studies, case-control studies, population-based studies, and hospital-based studies were 70.7, 22.7, 0, and 0%, respectively. Furthermore, in this study, it was found that the levels of heterogeneity in physical activity, weight, and BMI had decreased remarkably. It can be concluded from the analysis that the causes of heterogeneity in determining the overall risk of endometrial cancer in women with diabetes in the present meta-analysis were type of study, adjusted co-variables and geographical area (Fig. 2).
Obesity, which is one of the most important factors in diabetes, can cause hormonal imbalances in the body, and this in turn predisposes a person to endometrial cancer [26, 42–44]. One of the risk factors for type 2 diabetes is obesity, which is also a major risk factor for endometrial cancer. Although the precise mechanisms and pathways are uncertain, it could be hypothesized that endometrial carcinogenesis is that exposure of the endometrium to excess estrogen unopposed by progesterone increases the mitogen activity of endometrial cells [45, 46]. In this meta-analysis the summary of RR of primary studies, whose results were adjusted based on BMI showed a less value compared to summary of RR of primary studies, whose results were adjusted based on weight control. In women with obesity the levels of estradiol and estrogen are higher than women with normal weight, [47, 48], and this could be one the possible reason for the increase risk of endometrial cancer because of obesity [48]. However, results of several studies showed that other factors, such as higher insulin levels and growth factors, may also increase the risk of endometrial cancer in women with obesity [49, 50]. Moreover, long-term insulin therapy may also be responsible for increased risk of endometrial cancer in women with diabetes (31).
In this study, the authors performed subgroup analysis based on type of primary studies, geographical area, and adjusted covariate. However, we could not perform subgroup analysis based on type of diabetes (type 1 and type 2) because the early studies did not specify or separate the types of diabetes. Diabetes is a chronic disease, whose diagnosis may not be accurate and specific, in which case it would lead to classification bias (non-differential misclassification). Therefore, the overall results obtained from primary studies should be interpreted with caution.
However most of included case-control and cohort studies in this meta-analysis controlled the variables of obesity and sedentariness but it is of utmost importance to consider the effect of confounding variables (sedentariness, hormonal disorders, and obesity) on determining the relationship between diabetes and risk of endometrial cancer in women. The major strength of this updated meta-analysis in compare to previous one is that more primary studies identified and included [8], therefore distinguished effects of diabetes on risk of developing endometrial cancer based on adjustments to BMI/weight presented with larger sample size (larger effect size).
Limitations
Included primary studies did not mention the duration of diabetes and type of treatment (oral anti hyperglycemic agents and/or insulin). Furthermore, identifying women with diabetes in the primary studies was almost based on their self-reports. Since the primary studies did not consider type of diabetes therefore it was not possible to estimate the possible risk separately in in type 1 and type 2 diabetes.
Conclusions
Diabetes seems to increases the risk of endometrial cancer in women, and this finding can be useful in developing endometrial cancer prevention plans for women having diabetes.
Acknowledgements
Not applicable.
Abbreviations
- BMI
Body weight index
- CI
Confidence interval
- CINAHL
Cumulative index to nursing and allied health literature
- EMBASE
Excerpta medica dataBASE
- HR
Hazard ratio
- IDDM
Insulin-dependent diabetes mellitus
- MOOSE
Meta-Analyses of Observational Studies in Epidemiology
- NIDDM
Non-insulin-dependent diabetes mellitus
- OR
Odds ratio
- RR
Risk ratio or relative risk
- STROBE
Strengthening the Reporting of Observationally Studies in Epidemiology
Authors’ contributions
LS, HRB, and YM conceptualized the idea for this review, formulated the review question, and objectives, assisted with the development of the final search strategy, contributed to the data analysis/ interpretation, and writing the manuscript. ZN, ABSK, SKH and FV contributed to the conceptualization of the final review question, formulation of the review objectives, data analysis/interpretation, and writing the manuscript. EF, SGH, SHT, and ZHK contributed equally to the formulation of the review question/objectives, development of the search strategy, conducting the searches, data extraction, data analysis/interpretation, and writing the manuscript. All authors read and approved the final manuscript.
Funding
This research was funded by Systematic Review Network Center, Iran University of Medical Sciences (Grant Number 97–4–37-13922).
Availability of data and materials
Input data for the analyses are available from the corresponding author on request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lotfolah Saed, Email: Lotfollahsaed@yahoo.com.
Fatemeh Varse, Email: f.varse@yahoo.com.
Hamid Reza Baradaran, Email: baradaran.hr@iums.ac.ir, Email: Hamid.baradaran@abdn.ac.uk.
Yousef Moradi, Phone: 00989183847065, Email: Yousefmoradi211@yahoo.com.
Sorour Khateri, Email: sorur.khateri@yahoo.com.
Emilie Friberg, Email: Emilie.Friberg@ki.se.
Zaher Khazaei, Email: zaherkhazaei@yahoo.com.
Saeedeh Gharahjeh, Email: dr.gharahgeh_1388@yahoo.com.
Shahrzad Tehrani, Email: tehrani2243@gmail.com.
Amir-Babak Sioofy-Khojine, Email: Amirbabak.sioofykhojine@tuni.fi.
Zahra Najmi, Email: zahranaj@yahoo.com.
References
- 1.Lortet-Tieulent J, et al. International patterns and trends in endometrial cancer incidence, 1978–2013. J Natl Cancer Inst. 2017;110(4):354–361. doi: 10.1093/jnci/djx214. [DOI] [PubMed] [Google Scholar]
- 2.Stroup DF, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Jama. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49(1):33–64. doi: 10.3322/canjclin.49.1.33. [DOI] [PubMed] [Google Scholar]
- 4.Luo J, et al. Association between diabetes, diabetes treatment and risk of developing endometrial cancer. Br J Cancer. 2014;111(7):1432. doi: 10.1038/bjc.2014.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson KE, et al. Diabetes and endometrial cancer in the Iowa women’s health study. Cancer Epidemiol Biomarkers Prev. 2001;10(6):611–616. [PubMed] [Google Scholar]
- 6.Lindemann K, et al. Body mass, diabetes and smoking, and endometrial cancer risk: a follow-up study. Br J Cancer. 2008;98(9):1582. doi: 10.1038/sj.bjc.6604313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali A. Risk factors for endometrial cancer. Ceska Gynekol. 2013;78(5):448–459. [PubMed] [Google Scholar]
- 8.Friberg E, et al. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia. 2007;50(7):1365–1374. doi: 10.1007/s00125-007-0681-5. [DOI] [PubMed] [Google Scholar]
- 9.Knottnerus A, Tugwell P. STROBE—a checklist to Strengthen the Reporting of Observational Studies in Epidemiology. 2008;61(4):323. [DOI] [PubMed]
- 10.Von Elm E, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9(1):1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 12.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 13.Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 14.Egger M, Smith GD, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1998;316(7129):470–471. [Google Scholar]
- 15.Egger M, et al. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.AlHilli M, et al. The impact of diabetes and metformin on clinical outcomes is negligible in risk-adjusted endometrial cancer cohorts. Gynecol Oncol. 2015;137:156–157. doi: 10.1016/j.ygyno.2015.01.391. [DOI] [PubMed] [Google Scholar]
- 17.Friberg E, Mantzoros CS, Wolk A. Diabetes and risk of endometrial cancer: a population-based prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2007;16(2):276–280. doi: 10.1158/1055-9965.EPI-06-0751. [DOI] [PubMed] [Google Scholar]
- 18.Lambe M, et al. Impaired glucose metabolism and diabetes and the risk of breast, endometrial, and ovarian cancer. Cancer Causes Control. 2011;22(8):1163–1171. doi: 10.1007/s10552-011-9794-8. [DOI] [PubMed] [Google Scholar]
- 19.Coughlin SS, et al. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159(12):1160–1167. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- 20.Folsom AR, et al. Diabetes as a risk factor for death following endometrial cancer. Gynecol Oncol. 2004;94(3):740–745. doi: 10.1016/j.ygyno.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 21.Terry P, et al. Lifestyle and endometrial cancer risk: a cohort study from the Swedish twin registry. Int J Cancer. 1999;82(1):38–42. doi: 10.1002/(SICI)1097-0215(19990702)82:1<38::AID-IJC8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 22.Brinton LA, et al. Reproductive, menstrual, and medical risk factors for endometrial cancer: results from a case-control study. Am J Obstet Gynecol. 1992;167(5):1317–1325. doi: 10.1016/S0002-9378(11)91709-8. [DOI] [PubMed] [Google Scholar]
- 23.Friedenreich CM, et al. Case–control study of the metabolic syndrome and metabolic risk factors for endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2011;20(11):2384–2395. doi: 10.1158/1055-9965.EPI-11-0715. [DOI] [PubMed] [Google Scholar]
- 24.Inoue M, et al. A case-control study on risk factors for uterine endometrial Cancer in Japan. Cancer Sci. 1994;85(4):346–350. doi: 10.1111/j.1349-7006.1994.tb02365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucenteforte E, et al. Diabetes and endometrial cancer: effect modification by body weight, physical activity and hypertension. Br J Cancer. 2007;97(7):995. doi: 10.1038/sj.bjc.6603933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parazzini F, et al. Diabetes and endometrial cancer: an Italian case-control study. Int J Cancer. 1999;81(4):539–542. doi: 10.1002/(SICI)1097-0215(19990517)81:4<539::AID-IJC6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 27.Rubin GL, et al. Estrogen replacement therapy and the risk of endometrial cancer: remaining controversies. Am J Obstet Gynecol. 1990;162(1):148–154. doi: 10.1016/0002-9378(90)90838-X. [DOI] [PubMed] [Google Scholar]
- 28.Salazar-Martínez E, et al. Case–control study of diabetes, obesity, physical activity and risk of endometrial cancer among Mexican women. Cancer Causes Control. 2000;11(8):707–711. doi: 10.1023/A:1008913619107. [DOI] [PubMed] [Google Scholar]
- 29.Saltzman BS, et al. Diabetes and endometrial cancer: an evaluation of the modifying effects of other known risk factors. 2007;167(5):607–14. [DOI] [PubMed]
- 30.Shoff SM, Newcomb PA. Diabetes, body size, and risk of endometrial cancer. Am J Epidemiol. 1998;148(3):234–240. doi: 10.1093/oxfordjournals.aje.a009630. [DOI] [PubMed] [Google Scholar]
- 31.Soliman PT, et al. Association between adiponectin, insulin resistance, and endometrial cancer. Cancer. 2006;106(11):2376–2381. doi: 10.1002/cncr.21866. [DOI] [PubMed] [Google Scholar]
- 32.Wartko PD, et al. Association of endometrial hyperplasia and cancer with a history of gestational diabetes. Cancer Causes Control. 2017;28(8):819–828. doi: 10.1007/s10552-017-0908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiderpass E, et al. Body size in different periods of life, diabetes mellitus, hypertension, and risk of postmenopausal endometrial cancer (Sweden) Cancer Causes Control. 2000;11(2):185–192. doi: 10.1023/A:1008946825313. [DOI] [PubMed] [Google Scholar]
- 34.Weiss JM, et al. Risk factors for the incidence of endometrial cancer according to the aggressiveness of disease. Am J Epidemiol. 2006;164(1):56–62. doi: 10.1093/aje/kwj152. [DOI] [PubMed] [Google Scholar]
- 35.Tsilidis KK, et al. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. Bmj. 2015;350:g7607. doi: 10.1136/bmj.g7607. [DOI] [PubMed] [Google Scholar]
- 36.Austin MA, et al. The effect of response bias on the odds ratio. Am J Epidemiol. 1981;114(1):137–143. doi: 10.1093/oxfordjournals.aje.a113160. [DOI] [PubMed] [Google Scholar]
- 37.Heid I, et al. On the potential of measurement error to induce differential bias on odds ratio estimates: an example from radon epidemiology. Stat Med. 2002;21(21):3261–3278. doi: 10.1002/sim.1252. [DOI] [PubMed] [Google Scholar]
- 38.Nemes S, et al. Bias in odds ratios by logistic regression modelling and sample size. BMC Med Res Methodol. 2009;9(1):56. doi: 10.1186/1471-2288-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clayton D, Hills M, Pickles A. Statistical models in epidemiology. Oxford: Oxford university press; 1993. [Google Scholar]
- 40.Schulz KF, Grimes DA. Case-control studies: research in reverse. Lancet. 2002;359(9304):431–434. doi: 10.1016/S0140-6736(02)07605-5. [DOI] [PubMed] [Google Scholar]
- 41.Wacholder S, et al. Selection of controls in case-control studies: II. Types of controls. Am J Epidemiol. 1992;135(9):1029–1041. doi: 10.1093/oxfordjournals.aje.a116397. [DOI] [PubMed] [Google Scholar]
- 42.Arima R, et al. Cause-specific mortality in endometrioid endometrial cancer patients with type 2 diabetes using metformin or other types of antidiabetic medication. Gynecol Oncol. 2017;147(3):678–683. doi: 10.1016/j.ygyno.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 43.Arima R, et al. Antidiabetic medication, statins and the risk of endometrioid endometrial cancer in patients with type 2 diabetes. Gynecol Oncol. 2017;146(3):636–641. doi: 10.1016/j.ygyno.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 44.Park Yikyung, Colditz Graham A. Diabetes and adiposity: a heavy load for cancer. The Lancet Diabetes & Endocrinology. 2018;6(2):82–83. doi: 10.1016/S2213-8587(17)30396-0. [DOI] [PubMed] [Google Scholar]
- 45.Nagamani M, Stuart CA. Specific binding and growth-promoting activity of insulin in endometrial cancer cells in culture. Am J Obstet Gynecol. 1998;179(1):6–12. doi: 10.1016/S0002-9378(98)70244-3. [DOI] [PubMed] [Google Scholar]
- 46.Murphy LJ. Growth factors and steroid hormone action in endometrial cancer. J Steroid Biochem Mol Biol. 1994;48(5–6):419–423. doi: 10.1016/0960-0760(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 47.Corocleanu M. Hypothesis for endometrial carcinoma carcinogenesis. Preventive prospects. Clin Exp Obstet Gynecol. 1993;20(4):254–258. [PubMed] [Google Scholar]
- 48.Thiet M-P, Osathanondh R, Yeh J. Localization and timing of appearance of insulin, insulin-like growth factor-I, and their receptors in the human fetal müllerian tract. Am J Obstet Gynecol. 1994;170(1):152–156. doi: 10.1016/S0002-9378(94)70401-5. [DOI] [PubMed] [Google Scholar]
- 49.Ordener C, et al. Epidermal growth factor and insulin induce the proliferation of Guinea pig endometrial stromal cells in serum-free culture, whereas estradiol and progesterone do not. Biol Reprod. 1993;49(5):1032–1044. doi: 10.1095/biolreprod49.5.1032. [DOI] [PubMed] [Google Scholar]
- 50.Friberg E, et al. Coffee drinking and risk of endometrial cancer—a population-based cohort study. Int J Cancer. 2009;125(10):2413–2417. doi: 10.1002/ijc.24543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Input data for the analyses are available from the corresponding author on request.