Abstract
Background
Patients with primary breast cancer following primary ovarian cancer do not comprise a large clinical entity, and reports of the survival outcomes of this cohort are rare. The purpose of this retrospective population-based research was to investigate the survival outcomes of patients with primary breast cancer after primary ovarian cancer.
Material/Methods
A cohort of patients diagnosed with primary breast cancer following primary ovarian cancer between 1973 and 2014 was drawn from the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) database. Cox proportional hazards survival regression analysis and Kaplan-Meier were applied to calculate overall survival (OS), cancer-specific survival (CSS), and independent predictors of CSS.
Results
A total of 1455 patients with primary breast cancer following primary ovarian cancer were identified. The 5-year and 10-year OS rates for the entire cohort were 81.7% and 67.4%, respectively. The 5-year and 10-year CSS rates were 84.2% and 74.3% for ovarian cancer, and 76.0% and 67.8% for breast cancer, respectively. Multivariate analysis revealed that independent predictors of ovarian cancer CSS include age, cancer stage, diagnosis time, and histological subtype.
Conclusions
Patients diagnosed with breast cancer following ovarian cancer have better survival rates. Patients age, ovarian cancer stage, ovarian cancer histological type, and time of diagnose affect the survival rate.
MeSH Keywords: Ovarian Neoplasms, SEER Program, Triple Negative Breast Neoplasms
Background
Ovarian cancer and breast cancer are the most common malignancies in women. Ovarian cancer is the fifth major cause of cancer mortality among women with 22 440 new cases and 14 080 deaths in the United States (US) during 2017 [1]. In addition, approximately 252 710 new cases and 40 610 deaths due to invasive breast cancer are expected to occur among US women in 2017 [2]. Women with breast cancer have a higher incidence of second primary cancers, particularly ovarian and endometrial cancer [3,4]. Similarly, patients diagnosed with ovarian cancer have an increased incidence of breast cancer. The actuarial risk of developing breast cancer is 7.8% in the 10-year period following ovarian cancer [5]. Most ovarian cancer patients have advanced disease at the time of diagnosis, resulting in poor long-term survival [6], and the 5-year survival (OS) for those with ovarian cancer is only 45.6% [7]. However, improved OS and cancer-specific survival (CSS) have been reported for patients with breast cancer following an ovarian cancer diagnosis [8,9].
Unfortunately, most studies have small sample sizes, and no population-based statistics exist regarding the survival outcomes of women with primary breast cancer following primary ovarian cancer. The main aim of this retrospective population-based analysis was to estimate the survival outcomes and clinical characteristics of patients with primary breast cancer after primary ovarian cancer from the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) database.
Material and Methods
Data source
A cohort of patients diagnosed with primary breast cancer following primary ovarian cancer from 1973 to 2014 was drawn from the National Cancer Institute’s SEER database. The patient data in the present study were derived from 18 population-based cancer registries, as released in November 2016. Patient data are de-identified and made available to the public for research purposes. Because the SEER program is an open-access resource and patients are de-identified, this study was exempt from ethical review and does not contain any personally identifiable information. The SEER program statistical analysis software package (SEER*Stat version 8.3.4) was used to extract the data.
Clinical information
Demographic variables (marital status, year of diagnosis, race, registration area, age at diagnosis, and follow-up of patient status), and clinicopathological variables (tumor grade, American Joint Committee on Cancer (AJCC) stage, histological subtype, survival and cause of death, and associated treatment) were extracted using the “case-listing” option. Primary breast cancer cases and primary ovarian cancer cases were eligible for the study, and cases of metastatic tumors to the breast and ovary from other origins were excluded. Thus, only primary breast cancer and primary ovarian cancer patients were included, and patients with a clinical history of other cancers were excluded. Finally, 1455 patients with breast cancer following ovarian cancer were identified (Figure 1).
Figure 1.
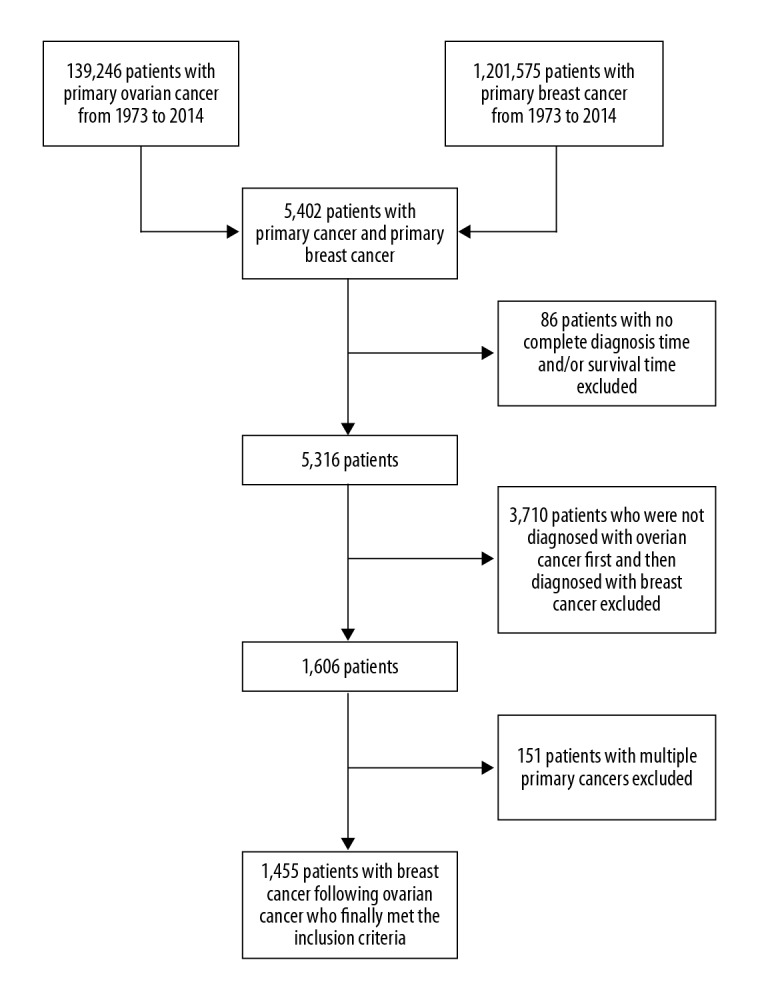
Screening flow chart of patients diagnosed with breast cancer following ovarian cancer.
The ICD-0-3 site/histology validation list and the World Health Organization histological classification were used to identify ovarian cancer and breast cancer. The cancer stage was based on the AJCC staging system. Ovarian cancer staging between 1988 and 2003 was defined by the 3rd edition of the AJCC TNM categories. Ovarian cancer staging between 2004 and 2014 was derived from the 6th edition of AJCC TNM categories. Breast cancer staging between 1988 and 2014 was determined by an adjusted version of the categories of the 6th edition of the AJCC TNM system. In the SEER database, the cause of death is linked to the National Death Index and state mortality records for validation.
Statistical analysis
The diagnosis time interval from ovarian cancer to breast cancer was calculated. The CSS and OS were analyzed as the principal outcomes using the Kaplan-Meier method. The magnitudes of statistical significance were expressed as the hazard ratio (HR) and 95% confidence interval (CI). Univariate and multivariate Cox hazard regression analyses were also applied to determine independent predictors of CSS in patients with breast cancer and ovarian cancer. All statistical analyses were conducted using IBM SPSS 24.0 and Graph Pad Prism 7.0. P<0.05 was considered statistically significant.
Results
Over the past 41 years, 1455 patients were identified who had primary breast cancer following primary ovarian cancer in the SEER database. There were 1.05% of ovarian cancer patients with chemotherapies who developed breast cancers, while 1.03% of patients developed breast cancers who were reported without chemotherapies. The demographic and clinicopathological characteristics are presented in Table 1.
Table 1.
Demographic and clinicopathological characteristics of ovarian cancer and breast cancer patients.
| Characteristic | n (%) | Characteristic | n (%) |
|---|---|---|---|
| Race | Ovarian cancer | ||
| White | 1268 (87.1%) | Age at diagnosis | |
| Black | 94 (6.5%) | <50 | 374 (25.7%) |
| Other/unknown | 93 (6.4%) | 50–64 | 630 (43.3%) |
| Marital status | 65–74 | 304 (20.9) | |
| Married | 842 (57.9%) | ≥75 | 147 (10.1%) |
| Divorced | 114 (7.8%) | Tumor grade | |
| Separated | 23 (1.6%) | Grade I | 161 (11.1%) |
| Single | 238 (16.4%) | Grade II | 248 (17.0%) |
| Widowed | 195 (13.4%) | Grade III | 460 (31.6%) |
| Unknown | 43 (3.0%) | Grade IV | 138 (9.5%) |
| Breast cancer | Unknown | 448 (30.8%) | |
| Age at diagnosis | Histology | ||
| <50 | 163 (11.2%) | Serous | 556 (38.2%) |
| 50–64 | 531 (36.5%) | Mucinous | 162 (11.1%) |
| 65–74 | 400 (27.5%) | Endometrioid | 224 (15.4%) |
| ≥75 | 361 (24.8%) | Clear cell | 88 (6.0%) |
| Tumor grade | Germ cell | 29 (2.0%) | |
| Grade I | 231 (15.9%) | Sex cord/stromal | 45 (3.1%) |
| Grade II | 456 (31.3%) | Others | 351 (24.1%) |
| Grade III | 457 (31.4%) | Stage (AJCC3)# | |
| Grade IV | 20 (1.4%) | I | 214 (14.7%) |
| Unknown | 291 (20.0%) | II | 88 (6.0%) |
| Histology | III | 136 (9.3%) | |
| Infiltrating duct carcinoma | 1029 (70.7%) | IV | 89 (6.1%) |
| Others | 426 (29.3%) | Others | 928 (63.8%) |
| Stage (AJCC6)* | Stage (AJCC6)## | ||
| I | 635 (43.6%) | I | 124 (8.5%) |
| II | 347 (23.8%) | II | 50 (3.4%) |
| III | 116 (8.0%) | III | 133 (9.1%) |
| IV | 64 (4.4%) | IV | 80 (5.5%) |
| Others | 293 (20.1%) | Others | 1068 (73.4%) |
| Year of diagnosis | Year of diagnosis | ||
| 1973–1982 | 68 (4.7%) | 1973–1982 | 257 (17.7%) |
| 1983–1992 | 185 (12.7%) | 1983–1992 | 289 (19.9%) |
| 1993–2002 | 326 (22.4%) | 1993–2002 | 442 (30.4%) |
| 2003–2014 | 876 (60.2%) | 2003–2014 | 467 (32.1%) |
| Surgery | Surgery | ||
| Yes | 1279 (87.9%) | Yes | 1379 (94.8%) |
| Others | 176 (12.1%) | Others | 76 (5.2%) |
| Chemotherapy | Chemotherapy | ||
| Yes | 406 (27.9%) | Yes | 869 (50.7%) |
| No/Unknown | 1049 (72.1%) | No/Unknown | 586 (40.3%) |
| Radiation | Radiation | ||
| Yes | 481 (33.1%) | Yes | 89 (6.1%) |
| No | 972 (66.9%) | No | 1366 (93.9%) |
Breast cancer stage adjusted from AJCC 6th Stage (1988+);
ovarian cancer stage derived from AJCC 3th Stage (1998–2003);
ovarian cancer stage derived from AJCC 6th Stage (2004+).
The median diagnosis time from ovarian cancer to breast cancer was 60 months (range, 1–453 months). The median age at ovarian cancer diagnosis was 58 years (range, 10–94 years).
The majority of the population were white (87.1%), and 69.0% were younger than 65 years. The median age at breast cancer diagnosis was 65 years (range, 22–97 years), and 47.7% were younger than 65 years of age. Of the 1455 patients, 94.8% underwent ovarian cancer-related surgeries, 87.9% underwent breast cancer-related surgeries, 50.7% received ovarian cancer-related chemotherapies, 27.9% received breast cancer-related chemotherapies, 6.1% had ovarian cancer-related radiation therapies, and 33.1% had breast cancer-related radiation therapies, but the detailed surgical approaches, the specific chemotherapy regimens, and the concrete radiation therapies were not known.
The multivariate survival analysis showed that independent predictors of ovarian cancer CSS were age at diagnosis (P<0.001), cancer stage (P<0.001), diagnosis time (P=0.005), and histological types (P=0.001), while age at diagnosis (P<0.001), tumor grade (P=0.044), cancer stage (P<0.001), and diagnosis time (P<0.001) were independent predictors of breast cancer CSS after adjusting for age at diagnosis, marital status, tumor grade, year of diagnosis, stage, chemotherapy, radiation, and histological types. However, chemotherapy was negatively associated with ovarian cancer CSS (P<0.001) and breast cancer CSS (P=0.019) (Table 2). The differences in these adjusted variables were statistically significant in univariate analysis of CSS (Table 3).
Table 2.
Multivariate survival analysis of ovarian cancer and breast cancer patients.
| Variables | Ovarian cancer CSS | Breast cancer CSS | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p-Value | HR | 95%CI | p-Value | |
| Marital status | 0.051 | 0.111 | ||||
| Married | Reference | Reference | ||||
| Divorced | 0.91 | 0.56–1.48 | 1.11 | 0.58–2.09 | ||
| Separated | 1.78 | 0.65–4.85 | 1.85 | 0.73–4.70 | ||
| Single | 0.75 | 0.51–1.11 | 0.69 | 0.45–1.07 | ||
| Widowed | 1.47 | 1.03–2.10 | 1.43 | 0.96–2.14 | ||
| Unknown | 1.45 | 0.72–2.91 | 1.20 | 0.37–3.84 | ||
| Age at diagnosis | <0.001 | <0.001 | ||||
| <50 | Reference | Reference | ||||
| 50–64 | 1.17 | 0.82–1.68 | 0.60 | 0.39–0.93 | ||
| 65–74 | 2.52 | 1.68–3.76 | 0.94 | 0.56–1.51 | ||
| ≥75 | 4.40 | 2.76–7.02 | 2.17 | 1.33–3.52 | ||
| Tumor grade | 0.092 | 0.044 | ||||
| Grade I | Reference | Reference | ||||
| Grade II | 2.21 | 0.97–5.03 | 2.20 | 1.18–4.13 | ||
| Grade III | 2.14 | 0.97–4.71 | 2.63 | 1.43–4.83 | ||
| Grade IV | 2.16 | 0.92–5.07 | 1.98 | 0.61–6.39 | ||
| Unknown | 2.81 | 1.27–6.19 | 2.38 | 1.21–4.68 | ||
| Stage (AJCC)* | <0.001 | <0.001 | ||||
| I | Reference | Reference | ||||
| II | 1.87 | 0.92–3.80 | 2.10 | 1.34–3.32 | ||
| III | 5.10 | 2.85–9.11 | 6.62 | 3.94–11.12 | ||
| IV | 6.88 | 3.78–12.53 | 23.45 | 13.44–40.93 | ||
| Others | 4.09 | 2.20–7.61 | 3.38 | 1.20–5.71 | ||
| Year of diagnosis | 0.005 | <0.001 | ||||
| 1973–1982 | Reference | Reference | ||||
| 1983–1992 | 0.46 | 0.28–0.75 | 0.75 | 0.40–1.41 | ||
| 1993–2002 | 0.47 | 0.28–0.77 | 0.65 | 0.33–1.29 | ||
| 2003–2014 | 0.39 | 0.22–0.67 | 0.27 | 0.13–0.55 | ||
| Histology | 0.001 | |||||
| Serous | Reference | |||||
| Mucinous | 0.46 | 0.25–0.86 | ||||
| Endometrioid | 0.53 | 0.32–0.88 | ||||
| Clear cell | 0.46 | 0.20–1.08 | ||||
| Germ cell | 0.00 | 0.00–6.508E+95 | ||||
| Sex cord/stromal | 0.53 | 0.21–1.34 | ||||
| Others | 1.27 | 0.95–1.69 | ||||
| Histology | 0.498 | |||||
| Infiltrating duct carcinoma | Reference | |||||
| Others | 1.11 | 0.82–1.52 | ||||
| Chemotherapy | ||||||
| Yes | Reference | <0.001 | Reference | 0.019 | ||
| No/unknown | 0.50 | 0.36–0.69 | 0.67 | 0.48–0.94 | ||
| Radiation | 0.284 | |||||
| Yes | Reference | |||||
| No | 1.20 | 0.86–1.68 | ||||
American Joint Committee on Cancer stage,
Table 3.
Univariate survival analysis of ovarian cancer and breast cancer patients.
| Variables | Ovarian cancer CSS | Breast cancer CSS | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p-Value | HR | 95%CI | p-Value | |
| Race | 0.134 | 0.12 | ||||
| White | Reference | Reference | ||||
| Black | 1.44 | 0.89–2.33 | 1.45 | 0.87–2.41 | ||
| Others | 0.72 | 0.43–1.21 | 0.35 | 0.15–0.78 | ||
| Marital status | <0.001 | <0.001 | ||||
| Married | Reference | Reference | ||||
| Divorced | 0.92 | 0.57–1.49 | 0.74 | 0.40–1.36 | ||
| Separated | 1.26 | 0.47–3.41 | 1.82 | 0.75–4.46 | ||
| Single | 0.73 | 0.50–1.06 | 0.67 | 0.45–1.00 | ||
| Widowed | 2.56 | 1.89–3.58 | 1.89 | 1.30–2.75 | ||
| Unknown | 1.15 | 0.58–2.26 | 0.49 | 0.16–1.53 | ||
| Age at diagnosis | <0.001 | <0.001 | ||||
| <50 | Reference | Reference | ||||
| 50–64 | 1.30 | 0.93–1.83 | 0.67 | 0.44–1.01 | ||
| 65–74 | 2.56 | 1.78–3.71 | 0.71 | 0.46–1.11 | ||
| ≥75 | 5.80 | 3.87–8.71 | 1.64 | 1.07–2.52 | ||
| Tumor grade | <0.001 | <0.001 | ||||
| Grade I | Reference | Reference | ||||
| Grade II | 2.76 | 1.22–6.22 | 2.31 | 1.25–4.26 | ||
| Grade III | 5.32 | 2.48–11.44 | 4.17 | 2.33–4.47 | ||
| Grade IV | 4.53 | 1.98–10.34 | 4.44 | 1.45–13.63 | ||
| Unknown | 4.67 | 2.15–10.11 | 6.85 | 3.76–12.49 | ||
| Stage (AJCC)* | <0.001 | <0.001 | ||||
| I | Reference | Reference | ||||
| II | 2.87 | 1.43–5.74 | 2.72 | 1.77–4.17 | ||
| III | 8.45 | 4.89–14.70 | 10.13 | 6.46–15.88 | ||
| IV | 14.09 | 7.97–24.92 | 30.54 | 18.73–49.79 | ||
| Others | 5.90 | 3.49–10.2 | 7.20 | 4.75–10.92 | ||
| Year of diagnosis | 0.047 | <0.001 | ||||
| 1973–1982 | Reference | Reference | ||||
| 1983–1992 | 0.57 | 0.37–0.88 | 0.78 | 0.43–1.32 | ||
| 1993–2002 | 0.62 | 0.43–0.90 | 0.49 | 0.28–0.87 | ||
| 2003–2014 | 0.68 | 0.46–1.00 | 0.18 | 0.10–0.32 | ||
| Histology | <0.001 | |||||
| Serous | Reference | |||||
| Mucinous | 0.30 | 0.17–0.55 | ||||
| Endometrioid | 0.31 | 0.19–0.50 | ||||
| Clear cell | 0.22 | 0.10–0.49 | ||||
| Germ cell | 0.00 | 0.00–3.17E+123 | ||||
| Sex cord/stromal | 0.50 | 0.20–1.21 | ||||
| Others | 1.21 | 0.92–1.58 | ||||
| Histology | 0.001 | |||||
| Infiltrating duct carcinoma | Reference | |||||
| Others | 1.61 | 1.22–2.14 | ||||
| Chemotherapy | <0.001 | <0.001 | ||||
| Yes | Reference | Reference | ||||
| No/unknown | 0.46 | 0.34–0.61 | 0.57 | 0.43–0.75 | ||
| Radiation | 0.709 | <0.001 | ||||
| Yes | Reference | |||||
| No | 0.90 | 0.51–1.57 | 1.96 | 1.44–2.67 | ||
American Joint Committee on Cancer stage.
Of the 1455 patients, 257 patients (26.7%) died of ovarian cancer, 212 patients (23.1%) died of breast cancer, and 272 patients (18.7%) died of other causes. Kaplan-Meier survival analysis showed that a higher survival benefit was observed in patients with breast cancer following ovary cancer than ovary cancer patients without secondary or more malignant tumors (P<0.001). The 5-year and the 10-year OS rates for the entire cohort of 1455 patients were 81.7% and 67.4%, 17.0% and 6.5% for 110 639 ovary cancer patients, respectively (Figure 2). Survival analysis showed that the 5-year and the 10-year CSS rates of ovarian cancer were 84.2% and 74.3% (Figure 3), and those of breast cancer were 76.0% and 67.8% (Figure 4), respectively.
Figure 2.
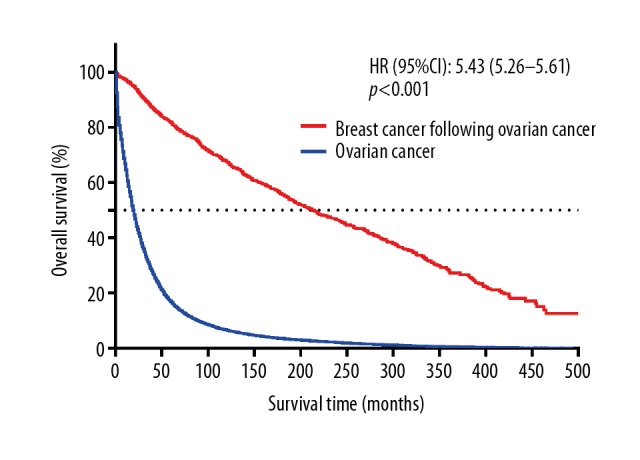
Kaplan-Meier survival curves for overall survival according to the survival state.
Figure 3.
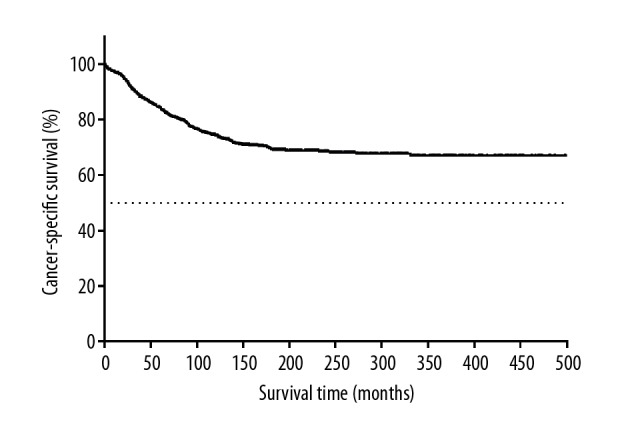
Kaplan-Meier survival curves for cancer-specific survival according to the survival state of patients with ovarian cancer.
Figure 4.
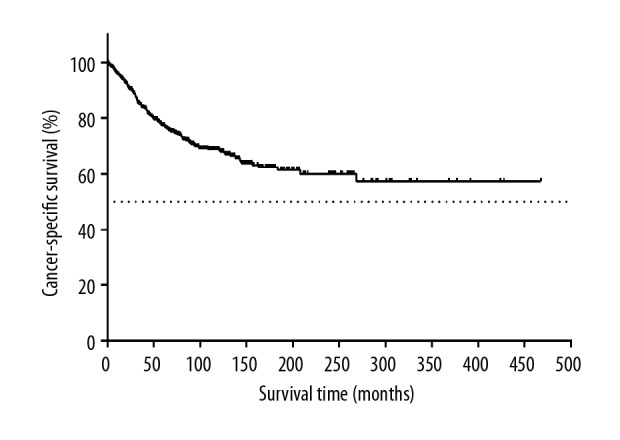
Kaplan-Meier survival curves for cancer-specific survival according to survival state of patients with breast cancer.
The median diagnosis time intervals from ovarian cancer to breast cancer were 63 months (range 1~453 months); the mean interval was 87.91 months. As shown in Figure 5, in the entire group, 50.7% of the patients were diagnosed with breast cancer within 5 years after being diagnosed with ovarian cancer and 74.6% were diagnosed within 10 years. As the time after diagnosis increased, the number of patients diagnosed with breast cancer following ovarian cancer decreased.
Figure 5.
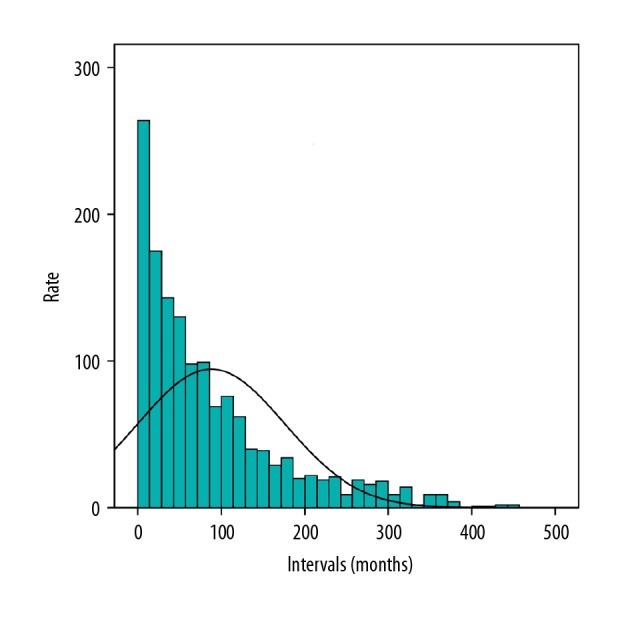
Distribution of time intervals between primary breast cancer and primary ovarian.
Discussion
To the best of our knowledge, few studies have evaluated the survival outcomes of patients with breast cancer after a diagnosis of ovarian cancer. A previous study demonstrated that the 5-year OS rate of ovarian cancer was only 45.6% [7]. However, the survival rates of women with breast cancer following ovarian cancer differ from those of women with ovarian cancer who do not develop breast cancer. A recent study revealed that the 5-year and 10-year OS rates were 58.3% and 50% for BRCA mutation-associated ovarian cancer patients who developed breast cancer but 30.9% and 13.8% for BRCA mutation-associated ovarian cancer patients who did not develop breast cancer [8]. Our study showed that the 5-year and 10-year OS rates for the entire cohort were 81.7% and 67.4%, while the corresponding OS rates of ovarian cancer patients without secondary or more malignant tumors were 17.0% and 6.5%. Our results showed that patients diagnosed with breast cancer following ovarian cancer survived longer than ovarian cancer patients who do not develop breast cancer.
Younger patient age, early cancer stage, and newly diagnosed nonserous ovarian cancer were significantly associated with better CSS of ovarian cancer in our study. Choi et al. [10] also showed that advanced stage, older patient age, poor grade, and serous epithelial ovarian cancer may result in poor survival. Cancer staging is an important factor in determining the survival outcomes and prognosis of women with ovarian cancer. Most patients with advanced-stage cancer have a worse prognosis and die as a result of the disease [11]. Heintz et al. [12] found that the 5-year survival rates were 85%, 70%, 32%, and 18.6% for patients with stage I, IIA, IIIC, and IV disease respectively. Thus, 32.7% of women with ovarian cancer classified as early-stage (stage I, 23.2%; stage II, 9.5%) lived an extended period of time after developing breast cancer.
In addition, the inclusion of fewer serous (38.2%) and younger (<65 years, 69%) ovarian cancer patients may partially contribute to the improved survival. Most studies have reported a low prognostic value for histological type, probably because of bias in analysis due to difficulties in diagnosis and/or small samples. However, in some studies, the serous type of ovarian cancer was an independent prognostic factor and tended to decrease survival [13,14]. Younger patients with epithelial ovarian cancer have been reported to have a better survival rate than older patients [11,15,16]. Moreover, from 1975–2004 to 2005–2011 the 5-year OS rate of ovarian cancer increased from 36.0% to 45.9% and from 74.8% to 90.7% for breast cancer [7]. Our study also found that diagnosis time was associated with better survival, which may be largely attributable to improvements in treatment. However, chemotherapy was negatively linked to ovarian cancer CSS. This may be because there had been more early-stage cases (132 stage I patients account for 38.9% in all kinds of stages) and less serous ovarian cancers cases (97 patients account for 28.6% in histology) in patients without chemotherapy, there had been had less early-stage cases (130 stage I patients account for 20.9% in all kinds of stages) and more serous ovarian cancers cases (297 patients account for 47.7% in histology) in patients with chemotherap.
Several studies have reported that approximately 15% of ovarian cancer patients, irrespective of family history, have BRCA1 or BRCA2 mutations [17], and that BRCA1/2-associated ovarian cancer is related to improved OS and progression-free survival (PFS) regardless of tumor stage, grade, or histological subtype [18–20]. BRCA1 or BRCA2 carrier status can be considered a factor influencing the survival rate. Whether BRCA has any effect on survival in this study remains unknown because of a lack of data regarding BRCA carriers. Although the SEER database lacks information on BRCA carriers, the aforementioned results suggest that younger patient age, early cancer stage, newly diagnosed, and nonserous ovarian cancer can partly explain the good survival of women with breast cancer after ovarian cancer.
Available studies have shown that the median diagnosis time from ovarian cancer to breast cancer was 50.5–108 months [8,9]. McGee et al. [5] reported that the average diagnosis interval from ovarian cancer to breast cancer was 3.5 years. The aforementioned studies were all based on small sample sizes. The present population-based study showed that 50.7% of the patients were diagnosed with breast cancer within 5 years after a diagnosis of ovarian cancer and 74.6% were diagnosed within 10 years. A greater number of ovarian cancer patients were diagnosed at shorter the time interval, but as the time interval progressed, the ovarian cancer incidence gradually decreased. In our study, most breast cancer diagnoses occurred in the first several years after a diagnosis of ovarian cancer. Thus, breast cancer surveillance may need to be advocated during the first few years after a diagnosis of ovarian cancer. However, Gangi et al. [8] reported that 8.9% of BRCA1/2 mutation carriers who have a history of ovarian cancer developed breast cancer, and Vencken et al. [21] showed a 6% 5-year risk of primary breast cancer in 79 women with BRCA-associated ovarian cancer. Domchek et al. [9] reported 5-year and 10-year breast cancer-free survival rates after ovarian cancer diagnosis 97% and 91%, respectively and that all deaths in this cohort were owing to ovarian cancer. These data support the use of less aggressive risk-reduction strategies of breast cancer during the first few years after a diagnosis of ovarian cancer. Peters et al. [22] considered that appropriate breast cancer screening and prevention are necessary for ovarian cancer patients with less favorable advanced-stage disease who achieve sustained remission (>2 years), or with early-stage disease or more favorable advanced disease. In addition, the benefits of early diagnosis of breast cancer need to be balanced the risks of additional testing, including the potential risks/limitations of false-positive test results, overdiagnosis, and patient anxiety. Additionally, decisions need to incorporate the patient’s life expectancy from the ovarian cancer itself [23].
Although multivariate survival analysis revealed that age, histological grade, cancer stage, and diagnosis time were independent predictors of breast cancer CSS, the 5-year CSS rate for breast cancer was only 76.0%, in contrast to 89.4% to 90.3% in other studies [7,24]. Some studies have suggested that OS and the prognosis of patients diagnosed with breast cancer following ovarian cancer was determined by ovarian cancer [8,9]. However, 212 patients (23.1%) died of breast cancer, 257 patients (26.7%) died of ovarian cancer and 272 patients (18.7%) died of other causes in this study; thus, both cancers had serious impacts on overall health. Neither ovarian cancer nor breast cancer obviously can determine the mortality rate of patients with breast cancer following ovarian cancer.
This was the first population-based study examining the survival rate of patients with breast cancer following ovarian cancer. The major advantage of our study was that we reported the survival rates of a large number of patients with ovarian cancer and breast cancer using the well-established SEER cancer registry, which was set up to reflect the general population-based data. In addition, our study contained only patients diagnosed between 1973 and 2014 with the majority diagnosed between 1993 and 2014. Therefore, the management of patients might have been more current and uniform in our study.
The main limitation of our study was that information on the concrete surgical types, detailed chemotherapy, and radiotherapy regimens, and the proportion of BRCA1/2-associated ovarian cancers were not available. As a result, we could not confirm the effects of surgery, chemotherapy, radiotherapy, or BRCA1/2 on the survival of patients with ovarian cancer and breast cancer. Moreover, selection bias may have been introduced due to the retrospective nature of the study.
Conclusions
Our study evaluated the survival of a sub-cohort of patients with breast cancer following ovarian cancer whose demographic and clinicopathological characteristics were obtained from the SEER database of the United States. Patients with breast cancer following ovarian cancer survive longer and have other well-described prognostic factors for improved survival rates including an early cancer stage, nonserous ovarian cancer, a younger patient age, and a new diagnosis.
Footnotes
Source of support: Departmental sources
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Ma J, Goding Sauer A, et al. Breast cancer statistics, 2017, racial disparity in mortality by state. Cancer J Clin. 2017;67:439–48. doi: 10.3322/caac.21412. [DOI] [PubMed] [Google Scholar]
- 3.Bergfeldt K, Rydh B, Granath F, et al. Risk of ovarian cancer in breast-cancer patients with a family history of breast or ovarian cancer: A population-based cohort study. Lancet. 2002;360:891–94. doi: 10.1016/S0140-6736(02)11023-3. [DOI] [PubMed] [Google Scholar]
- 4.Molina-Montes E, Pollan M, Payer T, et al. Risk of second primary cancer among women with breast cancer: A population-based study in Granada (Spain) Gynecol Oncol. 2013;130:340–45. doi: 10.1016/j.ygyno.2013.04.057. [DOI] [PubMed] [Google Scholar]
- 5.McGee J, Giannakeas V, Karlan B, et al. Risk of breast cancer after a diagnosis of ovarian cancer in BRCA mutation carriers: Is preventive mastectomy warranted? Gynecol Oncol. 2017;145:346–51. doi: 10.1016/j.ygyno.2017.02.032. [DOI] [PubMed] [Google Scholar]
- 6.Fishman DA, Bozorgi K. The scientific basis of early detection of epithelial ovarian cancer: The National Ovarian Cancer Early Detection Program (NOCEDP) Cancer Treat Res. 2002;107:3–28. doi: 10.1007/978-1-4757-3587-1_1. [DOI] [PubMed] [Google Scholar]
- 7.Howlader NNA, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2014. National Cancer Institute; Bethesda, MD: Apr, 2017. https://seer.cancer.gov/csr/1975_2014/based on November 2016 SEER data submission, posted to the SEER web site. [Google Scholar]
- 8.Gangi A, Cass I, Paik D, et al. Breast cancer following ovarian cancer in BRCA mutation carriers. JAMA Surg. 2014;149:1306–13. doi: 10.1001/jamasurg.2014.1081. [DOI] [PubMed] [Google Scholar]
- 9.Domchek SM, Jhaveri K, Patil S, et al. Risk of metachronous breast cancer after BRCA mutation-associated ovarian cancer. Cancer. 2013;119:1344–48. doi: 10.1002/cncr.27842. [DOI] [PubMed] [Google Scholar]
- 10.Choi M, Fuller CD, Thomas CR, Jr, Wang SJ. Conditional survival in ovarian cancer: Results from the SEER dataset 1988–2001. Gynecol Oncol. 2008;109:203–9. doi: 10.1016/j.ygyno.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 11.Chan J, Cheung M, Husain A, et al. Patterns and progress in ovarian cancer over 14 years. Obstet Gynecol. 2006;108:521–28. doi: 10.1097/01.AOG.0000231680.58221.a7. [DOI] [PubMed] [Google Scholar]
- 12.Heintz AP, Odicino F, Maisonneuve P, et al. Carcinoma of the ovary. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S161–92. doi: 10.1016/S0020-7292(06)60033-7. [DOI] [PubMed] [Google Scholar]
- 13.Brun JL, Feyler A, Chene G, et al. Long-term results and prognostic factors in patients with epithelial ovarian cancer. Gynecol Oncol. 2000;78:21–27. doi: 10.1006/gyno.2000.5805. [DOI] [PubMed] [Google Scholar]
- 14.Levi F, Franceschi S, La Vecchia C, et al. Epidemiologic pathology of ovarian cancer from the Vaud Cancer Registry, Switzerland. Ann Oncol. 1993;4:289–94. doi: 10.1093/oxfordjournals.annonc.a058484. [DOI] [PubMed] [Google Scholar]
- 15.Chan J, Urban R, Cheung M, et al. Ovarian cancer in younger vs. older women: A population-based analysis. Br J Cancer. 2006;95:1314–20. doi: 10.1038/sj.bjc.6603457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gatta G, Lasota M, Verdecchia A. Survival of European women with gynaecological tumours, during the period 1978–1989. EUROCARE Working Group. Eur J Cancer. 1998;34:2218–25. doi: 10.1016/s0959-8049(98)00326-8. [DOI] [PubMed] [Google Scholar]
- 17.George A, Kaye S, Banerjee S. Delivering widespread BRCA testing and PARP inhibition to patients with ovarian cancer. Nat Rev Clin Oncol. 2017;14:284–96. doi: 10.1038/nrclinonc.2016.191. [DOI] [PubMed] [Google Scholar]
- 18.Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: A report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30:2654–63. doi: 10.1200/JCO.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang D, Khan S, Sun Y, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA. 2011;306:1557–65. doi: 10.1001/jama.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong Q, Peng HL, Zhao X, et al. Effects of BRCA1- and BRCA2-related mutations on ovarian and breast cancer survival: A meta-analysis. Clin Cancer Res. 2015;21:211–20. doi: 10.1158/1078-0432.CCR-14-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vencken P, Kriege M, Hooning M, et al. The risk of primary and contralateral breast cancer after ovarian cancer in BRCA1/BRCA2 mutation carriers: Implications for counseling. Cancer. 2013;119:955–62. doi: 10.1002/cncr.27839. [DOI] [PubMed] [Google Scholar]
- 22.Peters ML, Garber JE, Tung N. Managing hereditary breast cancer risk in women with and without ovarian cancer. Gynecol Oncol. 2017;146:205–14. doi: 10.1016/j.ygyno.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 23.Wahner Hendrickson AE, Bakkum-Gamez JN, Couch J, Bough JC. Management of breast cancer risk in women with ovarian cancer and deleterious BRCA1 or BRCA2 mutations. Ann Surg Oncol. 2017;24:3107–9. doi: 10.1245/s10434-017-5999-8. [DOI] [PubMed] [Google Scholar]
- 24.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. Cancer J Clin. 2014;64:252–71. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]


