Abstract
The growth and virulence of bacteria depends upon a number of factors that are secreted into the environment. These factors can diffuse away from the producing cells, to be either lost or used by cells that do not produce them (cheats). Mechanisms that act to reduce the loss of secreted factors through diffusion are expected to be favoured. One such mechanism may be the production of Fap fibrils, needle-like fibres on the cell surface observed in P. aeruginosa, which can transiently bind several secreted metabolites produced by cells. We test whether Fap fibrils help retain a secreted factor, the iron-scavenging molecule pyoverdine, and hence reduce the potential for exploitation by non-producing, cheating cells. We found that: (i) wild-type cells retain more iron-chelating metabolites than fibril non-producers; (ii) purified Fap fibrils can prevent the loss of the iron-chelators PQS (Pseudomonas quinolone signal) and pyoverdine; and (iii) pyoverdine non-producers have higher fitness in competition with fibril non-producers than with wild-type cells. Our results suggest that by limiting the loss of a costly public good, Fap fibrils may play an important role in stabilizing cooperative production of secreted factors.
Keywords: cooperation, social evolution, Pseudomonas, functional amyloid, pyoverdine
1. Introduction
Bacteria often rely upon the secretion of extracellular public goods to survive and thrive in an environment. These goods are metabolically costly to produce but may be essential for effective communication between cells, scavenging of iron and nutrients, protection from biotic and abiotic stresses and engaging in antagonistic competition [1–4]. Production of these goods can also benefit neighbouring cells. Thus, public goods producers can potentially be exploited by non-producing ‘cheats’ that benefit from the available goods while paying none of the metabolic costs of production [5–7]. Exploitation by cheats can be detrimental to the population; significantly decreasing its density and subsequently increasing its chances of collapse [5,8]. Cheats have been observed to arise de novo under laboratory conditions, and putative social cheats have been identified in a number of natural bacterial populations [9–14].
As well as exploitation by social cheats, cells may lose return on investment in public good production simply through diffusion or advection of costly goods away from the cell in turbulent environments [15–17]. These losses can be minimized through a number of different mechanisms. Cells can avoid exploitation by cheats by ensuring the benefits of public good production accrue to relatives. This can be achieved at its simplest through clonal growth but bacteria can also engage in conflict with, and kill, local unrelated cells [18–20]. Forming a physical barrier can also prevent loss of public goods by slowing diffusion and/or reducing fluid flow rate around the colony. For example, thick layers of biofilm produced by Vibrio cholera can slow the diffusion of digested chitin, preventing its use by protease-deficient mutants [21].
A recently described mechanism that may slow or prevent the loss of secreted goods is the production of needle-like fibrils known collectively as functional amyloids [22]. These fibrils are assembled on the cell surface and are thought to be involved in surface attachment, providing structural rigidity to the extracellular matrix and even aiding in the evasion of host immune responses [23–25]. Recently, it was demonstrated that functional amyloids produced by Pseudomonas aeruginosa, Fap fibrils, are capable of binding several hydrophobic metabolites secreted by the cell such as pyocyanin and the signalling molecule PQS (Pseudomonas quinolone signal) [26,27]. Binding of the metabolites is transient: raising the possibility that serial association and dissociation with Fap fibrils may allow secreted metabolites to be locally ‘bioavailable’ while ensuring they are retained close to producing cells. While the social consequences of metabolite binding by Fap fibrils have yet to be described, if public goods producers are able to maintain preferential access to the costly goods they secrete into the environment, this could have important implications for cooperator–cheat dynamics in bacterial populations [28].
We test whether Fap fibrils help cells retain secreted metabolites involved in iron acquisition and if this can influence cooperator–cheat dynamics. Using strains of Pseudomonas aeruginosa that differ in their ability to produce Fap fibrils, we assess whether production of fibrils influences growth and investment in the iron-scavenging molecule pyoverdine. We also isolate Fap fibrils from cells to determine whether fibrils can bind, and prevent the loss by dilution, of the dedicated siderophore pyoverdine and the signalling molecule PQS, which is also a strong iron-chelator. We subsequently test whether any iron-chelating metabolites retained by Fap fibrils are functionally available to cells. Finally, we determine whether producing Fap fibrils influences the ability of pyoverdine producers to resist invasion by pyoverdine non-producing cheats in an iron-limited environment.
2. Material and methods
(a). Media and bacterial strains
To culture from frozen, we used Kings Broth (KB) medium (20 g Protease Peptone No3 (BD Biosciences), 10 g glycerol, 1.5 g K2HPO4.3H2O and 1.5 g MgSO4.7H20 per litre of dH2O) and for growth in an iron-limited environment we used casamino acid (CAA) medium (5 g CAAs, 1.18 g K2HPO4.3H2O and 0.25 g MgSO4.7H2O per litre of dH2O) supplemented with 20 mM sodium bicarbonate and 100 µg ml−1 of the iron chelator human apo-transferrin. We washed and diluted bacterial cultures using M9 minimal salt media (6.8 g Na2HPO4, 3 g KH2PO4, 0.5 g NaCl and 10 g NH4Cl per litre of dH2O). For purification of Fap fibrils, we cultured strains in colony factor antigen (CFA) medium (10 g hydrolysed casein, 50 mg MgSO4 and 1.5 g yeast extract per litre of dH2O). All reagents were purchased from Sigma unless otherwise stated.
We used strains that differ in their ability to produce Fap fibrils and the siderophore pyoverdine in our experiments (table 1). The PAO1 strain produces wild-type levels of Fap fibrils and pyoverdine. PAO1ΔFap is a mutant with the genes involved in Fap fibril production, FapA-FapF, inactivated by allelic replacement with a gentamicin resistance cassette [26]. We also use a mutant that cannot produce the iron-scavenging siderophore pyoverdine. PAO1ΔpvdD is a clean deletion mutant defective for the pyoverdine synthetase gene pvdD [29]. For isolating Fap fibrils, we use a PAO1 strain harbouring a plasmid containing the Pseudomonas Fap operons under the control of an isopropyl β-D-1-thiogalactopyranoside (IPTG) inducible lacUV5 promoter (PAO1 pFap). Prior to our experiments, we cultured strains overnight for 15 h at 37°C in 6 ml of KB media, at 200 rpm on an orbital shaker.
Table 1.
Summarizing phenotypic differences between strains used in the study.
| strain | Fap fibrils | pyoverdine |
|---|---|---|
| PAO1 | + | + |
| PAO1ΔFap | − | + |
| PAO1 pFap | + | + |
| PAO1ΔPvdD | + | − |
(b). Measuring growth and pyoverdine production
To determine whether producing Fap fibrils influenced the growth of strains, or altered their investment in pyoverdine, we cultured fibril producers and non-producers under iron-limited conditions. We did not quantify the production of pyochelin, the other siderophore produced by P. aeruginosa, as its production is almost completely repressed in the stringently iron-limited conditions used here [30]. We washed overnight cell cultures in M9 media, standardized to an optical density (A600) of 0.2 and diluted 100-fold before inoculating 200 µl of iron-limited CAA media with 2 µl of diluted culture in a 96-well plate. Cell density (absorbance at 600 nm) and pyoverdine production (relative fluorescent units, using an excitation of 400 nm and emission of 460 nm respectively) were measured every 30 min for 24 h using a SpectraMax i3x multi-mode platform (Molecular Devices). We also calculated pyoverdine available per cell at each time point (RFU400–460/A600) and the rate of pyoverdine production per cell per minute (((RFU400–4600(2) − RFU400–460(1))/30min)/A600(2)). Each treatment was replicated six times per strain.
(c). Cells retention of iron-chelating metabolites
To determine whether Fap fibrils influence cells ability to retain iron-chelating metabolites, we measured the iron-chelating activity of fibril producers and non-producers after removing unbound metabolites, using a CAS assay [31]. Overnight cell cultures in M9 media were washed, standardized to an optical density (A600) of 0.2 and diluted 100-fold before inoculating 6 ml of iron-limited CAA media with 60 µl of diluted culture. We incubated vials statically for 48 h at 37°C before vortexing and passing 2 ml of culture through a 0.22 µm syringe filter. Cells held on the membrane were washed to remove unbound iron-chelating metabolites by passing 20 ml of M9 solution through the syringe filter and re-suspended by passing 2 ml of M9 solution through the opposite end of the filter. One hundred microlitres of CAS solution [31] was added to 100 µl of re-suspended cells in a 96-well plate before we incubated the plate in darkness for 30 min. Iron-chelating molecules remove iron from the CAS solution, changing the colour of the solution. We quantified this change by measuring absorbance at A630. We also measured the optical density of the re-suspended cells (A600) and calculated the iron-chelating activity per cell (1 − A630/A600). Each treatment was replicated 22 times.
(d). Binding of iron-chelators to Fap fibrils
We wished to determine if Fap fibrils can bind iron-chelating metabolites, and whether bound metabolites are functionally available to cells. Isolated fibrils were exposed to the iron-chelators PQS and pyoverdine and the iron-chelating activity of the fibrils after repeated washing to remove unbound molecules was quantified. We also assessed the ability of fibrils exposed to iron-chelators to promote the growth of a pyoverdine deficient mutant in iron-limited media.
(i). Isolation of Fap fibrils
We inoculated PAO1 pFAP, an inducible Fap fibril overproducing strain, into KB media and incubated overnight (37°C, 200 rpm) before inoculating 400 ml of CFA media with 4 ml of the overnight culture in a 2 l flask with 40 µg ml−1 of tetracycline. Cultures were incubated at 37°C, 200 rpm to an optical density (A600) of approximately 0.5 before inducing expression of Fap fibrils with 1 mM of IPTG and incubating for a further 8 h. We harvested cells by centrifugation (28 000g, 30 min, 20°C), resuspended cells in 30 ml of buffer (10 mM Tris-HCL, pH 8.0) and homogenized manually before adding 10 ml of enzyme mix (0.4 mg ml−1 RNaseA, 0.4 mg ml−1 DNase I, 4 mg ml−1 lysozyme, 4 mM MgCl2 and 0.4% Triton X-100). This mix was homogenized by three cycles of freeze–thawing using a −80°C freezer and a 37°C water bath, followed by a final incubation of 2 h at 37°C. We then added 5 ml of 20% sodium dodecyl sulfate (SDS), boiled the sample for 20 min and collected insoluble material by centrifugation (28 000g, 30 min, 20°C). The pellet was then resuspended in 27 ml buffer with 3 ml of 20% SDS and boiled for 20 min before insoluble material was collected by centrifugation. We repeated this step twice. To wash away residual SDS, we resuspended the pellet in 25 ml of buffer and centrifuged (28 000g, 30 min, 20°C), repeating this step a further two times before re-suspending the pellet in 3 ml of buffer after the final centrifugation.
To confirm the presence of amyloid structures in our purified protein solution, we used a Thioflavin T fluorescence assay. We suspended 0.5 mg ml−1 of lysozyme and 0.5 mg ml−1 of purified protein solution in 10 mM Tris-HCl (pH 8.0) and 40 µM Thioflavin T. Negative controls consisted of 10 mM Tris-HCl (pH 8.0) and lysozyme, a protein lacking any amyloid structures. Using a Spectramax i3x, we recorded the emission spectra of the samples from 470 to 600 nm after excitation at 450 nm.
(ii). Fap fibril retention of iron-chelating metabolites
We suspended purified Fap fibrils in 400 µl buffer (10 mM HEPES, pH 7.4) to concentrations of 5 and 50 µg ml−1, and included a control with no fibrils (0 µg ml−1). PQS or pyoverdine were added to final concentrations of 200 µM and 100 µM respectively and the mixtures were incubated overnight (22°C, 400 rpm). Each treatment was replicated six times. The mixtures were centrifuged (5 min, 1000g), the supernatant discarded and the retentate resuspended in 1 ml of buffer and vortexed. This step was repeated three times and following the final centrifugation, retentate was resuspended in 400 µl of buffer.
To determine whether fibrils prevented the loss of PQS and pyoverdine by dilution, we quantified the iron chelating activity of the mixes after repeated washing. We added 100 µl of each mixture to 100 µl of CAS solution in a 96-well plate, incubated the plate in darkness for 30 min and measured absorbance at 630 nm.
We also determined whether iron-chelators retained by fibrils were available for use by cells and could stimulate the growth of a pyoverdine non-producer strain, PAO1ΔpvdD, in iron-limited media. Overnight cultures of the pyoverdine non-producer were washed in M9 media, standardized to an OD of 0.2 and diluted 100-fold into 180 µl of iron-limited CAA media in a 96-well plate. We then made up the volume in each well to 200 µl with the different concentrations of fibrils (0, 5 or 50 µg ml−1) exposed to buffer, PQS or pyoverdine. Plates were incubated statically at 37°C and we measured the optical density of cultures (A600) at 0 h and 24 h respectively.
(e). Relative fitness of pyoverdine non-producers
To determine whether producing Fap fibrils influenced the ability of public goods producers to resist invasion by social cheats, we competed fibril producers and fibril non-producers (both pyoverdine producers) against the pyoverdine non-producer PAO1ΔPvdD under iron-limited conditions. After washing overnight cultures in M9 media, standardizing optical density (A600) to 0.2 and diluting 100-fold, we mixed each combination of producer and non-producer at a ratio of 10 : 1 (producers: non-producers). Six millilitres of iron-limited CAA media was inoculated with 60 µl of each mixture and incubated cultures statically at 37°C for 24 h. Each treatment was replicated 20 times. We measured the frequency of pyoverdine producers and non-producers before and after 24 h of incubation by plating onto KB agar (12 g agar per litre of KB medium) and counting the number of colonies of producers and non-producers. We calculated the relative fitness of non-producers using the formula w = (p2(1 − p1))/(p1(1 − p2)) where p1 and p2 are the proportion of pyoverdine non-producers in the population before and after competition occurs respectively. A value of w > 1 indicates that the non-producer has a higher fitness than the pyoverdine producer and w < 1 indicates that the producer has a higher fitness than the non-producer.
(f). Statistical analyses
We carried out all statistical analyses in the R statistical environment (v3.3.3, http://www.R-project.org). Except where stated, we carried out standard analyses (t-test, linear models, etc.) assuming normal errors. All analyses using linear mixed-effect models (LMEM) included day as a random effect, to account for the fact that replicates of the experiments were carried out on different days.
3. Results
(a). Population growth and pyoverdine production
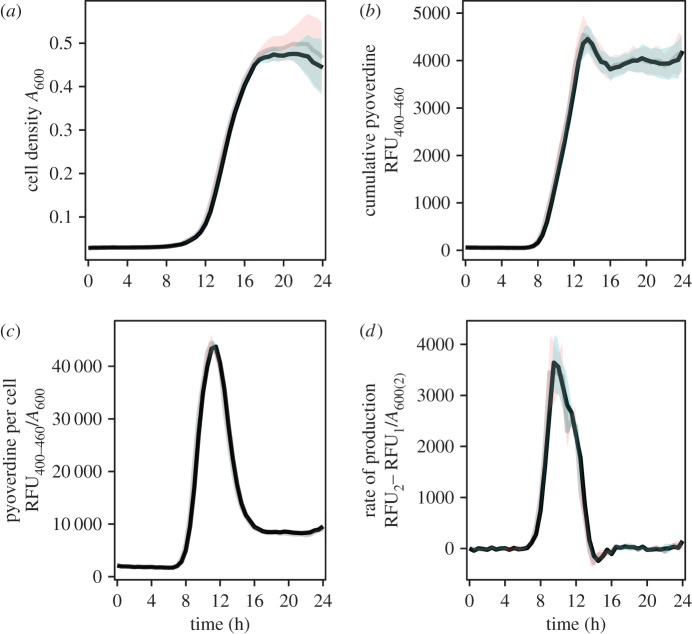
To determine whether the deletion of the Fap operon has consequences for growth or alters investment in pyoverdine, we measured growth and pyoverdine production of fibril producers and non-producers. After 24 h of growth in iron-limited media, we found no significant difference in cell density (t = 0.519, d.f. = 10, p = 0.615), pyoverdine production (t = −0.135, d.f. = 10, p = 0.895) and pyoverdine availability per cell (t = −0.899, d.f. = 10, p = 0.389) (figure 1). These results suggest that not producing fibrils does not significantly alter fibril non-producers investment in pyoverdine production nor detrimentally affect their growth in an iron-limited environment.
Figure 1.
Growth and pyoverdine production of Fap fibril producers (black lines, grey envelope) and non-producers (grey lines, pink envelope) over 24 h in iron-limited media. Mean and standard error envelope are plotted for each. (a) Growth of strains, measured by cell density at A600. (b) Cumulative pyoverdine available, measured by RFU400–460. (c) Pyoverdine available per cell, measured by RFU400–460/A600. (d) Rate of pyoverdine production, measured per cell per 30 min (((RFU400–4600(2) − RFU400–460(1))/30 min)/A600(2)). Cell density, available pyoverdine and pyoverdine available per cell were not significantly different between fibril producers and non-producers.
(b). Retention of iron-chelating metabolites
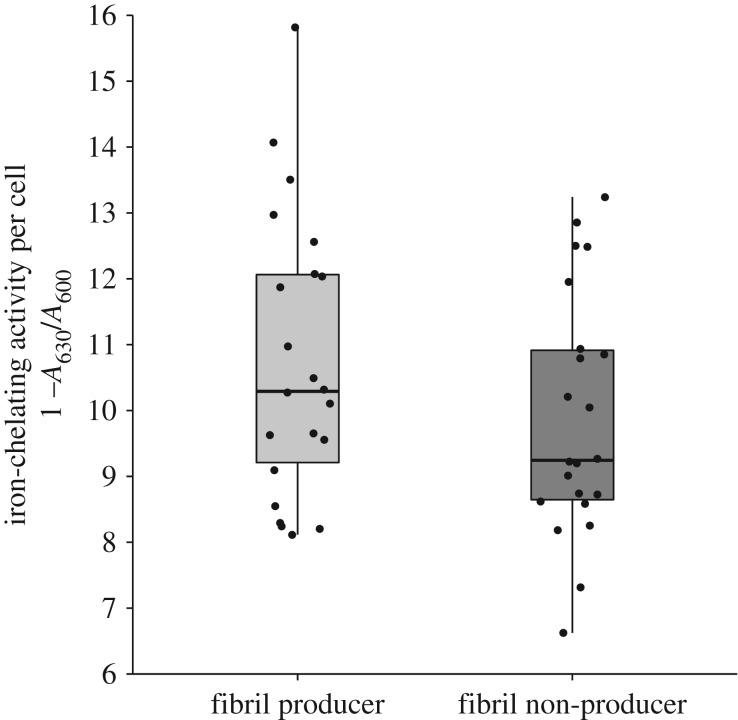
After cells had been washed to remove unattached metabolites, we found that the iron-chelating activity, standardized by cell density, of fibril producers was significantly greater than that of non-producer cells (LMEM, t = 2.256, d.f. = 41, p = 0.0295) (figure 2). This suggests that producing fibrils can promote retention of iron-chelating secreted metabolites under conditions where otherwise they would be removed.
Figure 2.
Retention of iron-chelating metabolites by fibril producers and non-producers. Iron chelating activity was quantified using a CAS assay and standardized by cell density (iron-chelating activity per cell ((1 − A630)/A600)). Boxplots give the median value, boxes show the interquartile range and whiskers extend to the largest or smallest value respectively. Iron-chelating activity of fibril producers was significantly greater than that of non-producers.
We also isolated fibrils and determined whether they could prevent the loss of the known iron-chelators, pyoverdine and PQS, by dilution. A strong fluorescence emission, with a maximum at approximately 490 nm when excited at 450 nm from our Thioflavin T assays confirmed the presence of amyloid structures in our purified protein solution (electronic supplementary material, figure S1). We found that isolated fibrils exposed to pyoverdine or PQS showed increased iron-chelating activity relative to fibrils exposed to buffer, as revealed by a significant interaction effect between fibril concentration and pyoverdine (F3,32 = 12.984, p = 0.00105) or PQS (F3,32 = 82.95, p = 2.12 × 10−10) (figure 3a). Increasing concentrations of fibrils, without the addition of pyoverdine or PQS, did not increase levels of iron-chelation (F1,16 = 0.008, p = 0.931). This suggests that the increase in iron-chelating activity is due to the retention of pyoverdine and PQS by fibrils and not the fibrils themselves.
Figure 3.
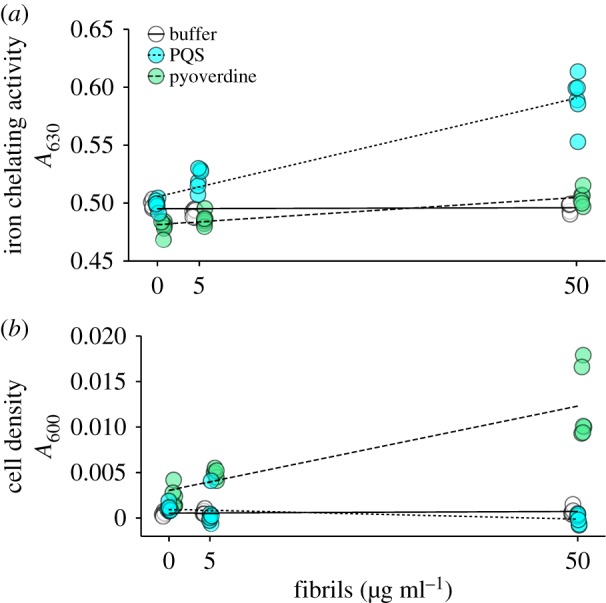
Retention of iron-chelating metabolites by Fap fibrils. (a) Iron-chelating activity, measured as (1 − A630), of 0, 5 and 50 µg ml−1 of untreated purified Fap fibrils (white) or purified Fap fibrils treated with PQS (cyan) or pyoverdine (green). Iron-chelating activity increases with increasing fibril concentration for both pyoverdine and PQS treatments. (b) Growth, measured as A600(24 h) − A600(0 h), of a pyoverdine non-producer (PAO1ΔpvdD) in iron-limited media supplemented with 0, 5 and 50 µg ml−1 of untreated purified Fap fibrils or purified Fap fibrils treated with PQS (cyan) or pyoverdine (green). Growth of a pyoverdine non-producer increases with increasing concentrations of fibrils treated with pyoverdine and decreases with increasing concentrations of fibrils treated with PQS.
We also determined whether pyoverdine or PQS molecules retained by fibrils could be used by pyoverdine non-producers in an iron-limited environment. We found that fibrils exposed to pyoverdine increased the growth of a pyoverdine non-producer strain, PAO1ΔpvdD, relative to fibrils exposed to buffer, demonstrated by a significant interaction effect between fibril concentration and pyoverdine (F3,32 = 50.43, p = 4.68 × 10−8) (figure 3b). Fibrils alone did not increase the growth of cells (F1,16 = 1.281, p = 0.274), suggesting that the growth of the pyoverdine non-producer was promoted by the increasing availability of the siderophore pyoverdine in the iron-limited environment. We found that fibrils exposed to PQS actually repressed the growth of the pyoverdine non-producer relative to fibrils exposed to buffer (F3,32 = −4.983, p = 0.032) (figure 3b). This effect is likely due to the fact that PQS is an iron-chelator but not a dedicated siderophore. Thus, it will bind iron but if cells lack a means of recovering the iron from PQS (by using, for example, a dedicated siderophore such as pyoverdine) it only serves to further iron-limit the surrounding environment.
(c). Competition assays
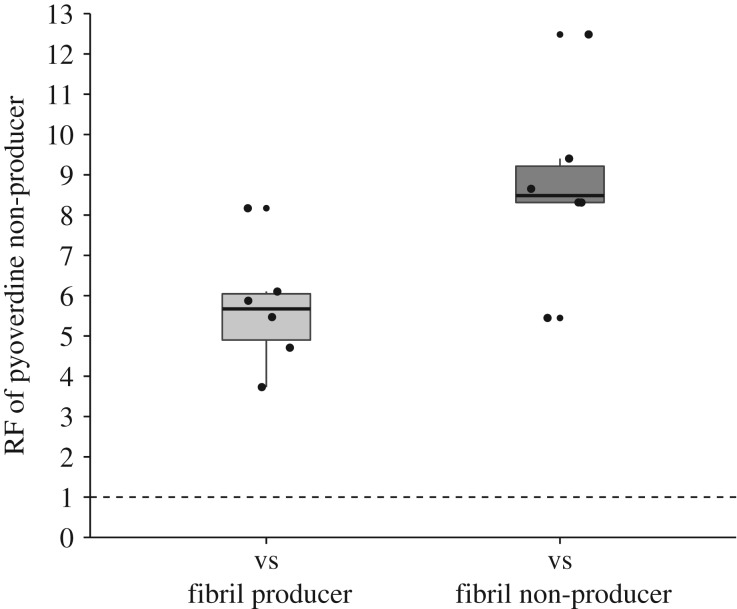
To determine whether producing fibrils can influence the outcome of cooperator–cheat competitive dynamics, we competed fibril producers and non-producers with a pyoverdine non-producing strain (PAO1ΔPvdD). We found that in mixed cultures, pyoverdine non-producers increase in frequency when in competition with both fibril producers (w = 5.67, t = 7.64, d.f. = 5, p = 3.041 × 10−4) and non-producers (w = 8.76, t = 8.39, d.f. = 5, p = 1.959 × 10−4) (figure 4). However, pyoverdine non-producer relative fitness is significantly greater in competition with Fap fibril non-producers than with fibril producers (t = 2.789, d.f. = 10, p = 0.0192). These results suggest that producing fibrils can limit the invasion of a pyoverdine non-producer into a population of pyoverdine producers.
Figure 4.
Relative fitness (RF) of a pyoverdine non-producer (PAO1ΔPvdD) in competition with fibril producers and non-producers in iron-limited media. Boxplots give the median value, boxes show the interquartile range and whiskers extend to either 1.5 times the interquartile range or to the largest or smallest value respectively. Outliers are shown as small circles. Pyoverdine non-producer significantly increase in frequency over 24 h in competition with both fibril producers and non-producers, but pyoverdine non-producer relative fitness is significantly higher when competed against fibril non-producers than against fibril producers.
4. Discussion
Bacterial cells rely upon the secretion of extracellular public goods to acquire resources from the environment. However, cells fail to benefit from these goods if they are lost to the environment through diffusion or are used by social cheats. We present evidence that Fap fibril production in P. aeruginosa limits the loss of secreted public goods involved in iron acquisition, and that this can impact upon competition between public goods producers and cheats over iron. Fibril-producing cells show improved retention of iron-chelating metabolites (figure 2) and isolated Fap fibrils bind both PQS and pyoverdine (figure 3), supporting a role for Fap fibrils in retaining these costly public goods. Secreted public goods such as pyoverdine are often exploited by social cheats: subsequently, cells employ mechanisms that constrain the ability of cheats to benefit from products they produce. We show that fibril production limits the extent to which public goods cheats invade a population of cooperators (figure 4), suggesting that retention of secreted goods by Fap fibrils also has consequences for cooperator–cheat competitive dynamics.
After washing to strip away unbound metabolites from the environment surrounding cells, fibril producers showed improved retention of iron-chelating metabolites (figure 2), and purified fibrils appear to bind both pyoverdine and PQS, preventing their loss by dilution (figure 3a). This supports a role for fibrils in retaining costly iron chelating metabolites. Transient binding of PQS by fibrils occurs due to the hydrophobic nature of both: potentially explaining the strong iron chelating activity of fibrils exposed to PQS despite repeated washing (figure 3a) [27]. Pyoverdine, as a larger amphiphilic molecule, is not expected to bind strongly through hydrophobic interactions [32]. However, it may possess hydrophobic domains that allow a more limited binding to fibrils [33]. Why is binding, and subsequently limited diffusion, of secreted metabolites likely of importance to cells? The majority of cells will experience conditions in their natural environments that remove costly secreted metabolites by diffusion or convection, limiting their utility to producing cells [21,34]. Limiting diffusion of secreted metabolites may allow cells to invest in less of a costly, recyclable public good if it is not constantly being lost to the environment [35]. It may also increase the range of conditions under which producing extracellular metabolites is a viable strategy: for example, limiting diffusion of quorum sensing molecules may expand the range of conditions under which QS controlled behaviours are inducible [16,36].
How does the presence or absence of Fap fibrils influence competition over iron? Our results show that pyoverdine non-producers do significantly better in competition with fibril non-producers than with fibril producers (figure 4). One possible reason for this is that failure to produce fibrils alters investment in pyoverdine production. This is important because non-producers gain a competitive advantage only when a cooperator is paying the cost of producing a public good [37]. Thus, if cells that do not produce fibrils increase per cell pyoverdine production, or increase the duration of pyoverdine production, this may increase the competitive advantage to non-producers. However, we see no significant difference in per cell production of pyoverdine between fibril producers and non-producers (figure 1), suggesting that altered investment in pyoverdine does not explain the success of pyoverdine non-producers in the population.
As fibrils appear to prevent the loss of pyoverdine by dilution (figure 3a), it may be that during competition with social cheats, fibril producers can limit or slow the diffusion of pyoverdine away from producing cells. This would have the effect of partially privatizing pyoverdine: ensuring the benefits of pyoverdine production accrue to producing cells and proximate cooperators, and limiting the competitive advantage of non-producers [38,39]. Another, non-mutually exclusive explanation, is that fibrils can help trap iron close to cells by binding PQS. Our results show that PQS is bound by fibrils but fails to stimulate the growth of a pyoverdine non-producing mutant in an iron-limited environment (figure 3a,b), PQS, while a strong iron chelator, is not a dedicated siderophore as it cannot transfer iron across cell membranes. Thus it has been suggested that PQS may act as an iron trap, binding iron close to cells and making it easier for dedicated siderophores such as pyoverdine to shuttle iron into cells [40]. Previous studies have demonstrated that diffusion of secreted public goods can be limited, through maintaining cell–cell contacts or simply by environmental viscosity [15,18]. Our results suggest that binding of secreted metabolites by Fap fibrils may also limit the diffusion of secreted metabolites and consequently play a role in shaping cooperator–cheat dynamics.
Is the production of functional amyloids an adaptation to reduce or prevent exploitation by social cheats? We have demonstrated the ability of Fap fibrils to partially-privatize secreted metabolites but functional amyloids also play important roles in surface adhesion and biofilm formation and structure in Pseudomonads and many other bacterial species [23–25]. This suggests that functional amyloids may primarily play a role in surface attachment and biofilm formation and maintenance but have been co-opted by Pseudomonas aeruginosa as a means of retaining costly secreted metabolites. Exaptation is not uncommon in bacteria, with many traits co-opted to perform additional, or even novel, functions. For example, PQS is a signalling molecule that also plays an important role in iron acquisition, while bacterial conjugation systems have been co-opted for roles in effector translocation in Type IV secretion systems [40,41].
Cooperative public goods production is common in bacterial populations despite the potential loss of costly public goods through diffusion or advection, or to social cheats [42]. We have demonstrated that Fap fibrils can prevent loss of iron-chelating metabolites by dilution and limit the invasion of social cheats during competition over iron. Interestingly, other secreted metabolites that serve as pubic goods, such as pyochelin, pyocyanin and the QS molecules C4-HSL and C12-HSL are also small, hydrophobic molecules and likely predisposed to binding to Fap fibrils. We have discussed only the benefits of retaining secreted metabolites. However, there may also be costs associated with retention. For example, retaining pyocyanin could increase local oxidative stress. Future work should seek to reveal if Fap fibrils can limit the loss of these secreted metabolites, and any associated costs and benefits of doing so. There is also evidence for intraspecific and interspecific variation in Fap fibril production in pseudomonads [26]. It would also be interesting to see if levels of fibril production correlate with an isolate's ability to retain secreted metabolites in turbulent environments. By helping limit the diffusion of public goods, Fap fibrils may play an important role in explaining the stability of secreted public goods in bacterial populations.
Supplementary Material
Data accessibility
Data used in the manuscript is available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.v8c8h23 [43].
Authors' contributions
J.B.B. conceived of and designed the study, carried out laboratory work, performed the data analysis, and drafted the manuscript. A.S.G. and S.A.W. participated in the design of the study and critically revised the manuscript. All authors gave final approval for publication and agree to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This study was funded by Natural Environment Research Council (1365611) and Royal Society.
References
- 1.West SA, Buckling A. 2003. Cooperation, virulence and siderophore production in bacterial parasites. Proc. R. Soc. Lond. B 270, 37–44. ( 10.1098/rspb.2002.2209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghequire MGK, De Mot R. 2014. Ribosomally encoded antibacterial proteins and peptides from Pseudomonas. FEMS Microbiol. Rev. 38, 523–568. ( 10.1111/1574-6976.12079) [DOI] [PubMed] [Google Scholar]
- 3.Popat R, Cornforth DM, McNally L, Brown SP. 2015. Collective sensing and collective responses in quorum-sensing bacteria. J. R. Soc. Interface 12, 20140882 ( 10.1098/rsif.2014.0882) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S. 2016. Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol. 14, 563–575. ( 10.1038/nrmicro.2016.94) [DOI] [PubMed] [Google Scholar]
- 5.Griffin AS, West SA, Bucking A. 2004. Cooperation and competition in pathogenic bacteria. Nature 430, 1024–1027. ( 10.1038/nature02744) [DOI] [PubMed] [Google Scholar]
- 6.Diggle SP, Griffin AS, Campbell GS, West SA. 2007. Cooperation and conflict in quorum-sensing bacterial populations. Nature 450, 411–414. ( 10.1038/nature06279) [DOI] [PubMed] [Google Scholar]
- 7.Ghoul M, Griffin AS, West SA. 2014. Toward an evolutionary definition of cheating. Evolution 68, 318–331. ( 10.1111/evo.12266) [DOI] [PubMed] [Google Scholar]
- 8.Sanchez A, Gore J. 2013. Feedback between population and evolutionary dynamics determines the fate of social microbial populations. PLoS Biol. 11, p.e1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandoz KM, Mitzimberg SM, Schuster M. 2007. Social cheating in Pseudomonas aeruginosa quorum sensing. Proc. Natl Acad. Sci. USA 104, 15 876–15 881. ( 10.1073/pnas.0705653104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cordero OX, Ventouras LA. 2012. Public good dynamics drive evolution of iron acquisition strategies in natural bacterioplankton populations. Proc. Natl Acad. Sci. USA 109, 20 059–20 064. ( 10.1073/pnas.1213344109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.West SA, Winzer K, Gardner A, Diggle SP. 2012. Quorum sensing and the confusion about diffusion. Trends Microbiol. 20, 586–594. ( 10.1016/j.tim.2012.09.004) [DOI] [PubMed] [Google Scholar]
- 12.Andersen SB, Marvig RL, Molin S, Krogh Johansen H, Griffin AS. 2015. Long-term social dynamics drive loss of function in pathogenic bacteria. Proc. Natl Acad. Sci. USA 112, 10 756–10 761. ( 10.1073/pnas.1508324112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruce JB, Cooper GA, Chabas H, West SA, Griffin AS. 2017. Cheating and resistance to cheating in natural populations of the bacterium Pseudomonas fluorescens. Evolution 71, 2484–2495. ( 10.1111/evo.13328) [DOI] [PubMed] [Google Scholar]
- 14.Butaitė E, Baumgartner M, Wyder S, Kümmerli R. 2017. Siderophore cheating and cheating resistance shape competition for iron in soil and freshwater Pseudomonas communities. Nat. Commun. 8, 414 ( 10.1038/s41467-017-00509-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kümmerli R, Griffin AS, West SA, Buckling A, Harrison F. 2009. Viscous medium promotes cooperation in the pathogenic bacterium Pseudomonas aeruginosa. Proc. R. Soc. Lond. B 276, 3531–3538. ( 10.1098/rspb.2009.0861) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emge P, Moeller J, Jang H, Rusconi R, Yawata Y, Stocker R, Vogel V. 2016. Resilience of bacterial quorum sensing against fluid flow. Sci. Rep. 6, 33115 ( 10.1038/srep33115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mund A, Diggle SP, Harrison F. 2017. The fitness of Pseudomonas aeruginosa quorum sensing signal cheats is influenced by the diffusivity of the environment. MBio 8, e00353-17 ( 10.1128/mbio.00816-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Julou T, Mora T, Guillon L, Croquette V, Schalk IJ, Bensimon D, Desprat N. 2013. Cell–cell contacts confine public goods diffusion inside Pseudomonas aeruginosa clonal microcolonies. Proc. Natl Acad. Sci. USA 110, 12 577–12 582. ( 10.1073/pnas.1301428110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nadell CD, Bucci V, Drescher K, Levin SA, Bassler BL, Xavier JB. 2013. Cutting through the complexity of cell collectives. Proc. R. Soc. B 280, 20122770 ( 10.1098/rspb.2012.2770) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNally L, Bernardy E, Thomas J, Kalziqi A, Pentz J, Brown SP, Hammer BK, Yunker PJ, Ratcliff WC. 2017. Killing by Type VI secretion drives genetic phase separation and correlates with increased cooperation. Nat. Commun. 8, 14371 ( 10.1038/ncomms14371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drescher K, Nadell CD, Stone HA, Wingreen NS, Bassler BL. 2014. Solutions to the public goods dilemma in bacterial biofilms. Curr. Biol. 24, 50–55. ( 10.1016/j.cub.2013.10.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dueholm MS, et al. 2010. Functional amyloid in Pseudomonas. Mol. Microbiol. 77, 1009–1020. [DOI] [PubMed] [Google Scholar]
- 23.Barnhart MM, Chapman MR. 2006. Curli biogenesis and function. Annu. Rev. Microbiol. 60, 131–147. ( 10.1146/annurev.micro.60.080805.142106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng G, Vad BS, Dueholm MS, Christiansen G, Nilsson M, Tolker-Nielsen T, Nielsen PH, Meyer RL, Otzen DE. 2015. Functional bacterial amyloid increases Pseudomonas biofilm hydrophobicity and stiffness. Front. Microbiol. 6, 1099 ( 10.3389/fmicb.2015.01099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taglialegna A, Lasa I, Valle J. 2016. Amyloid structures as biofilm matrix scaffolds. J. Bacteriol. 198, 2579–2588. ( 10.1128/JB.00122-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dueholm MS, et al. 2013. Expression of Fap amyloids in Pseudomonas aeruginosa, P. fluorescens, and P. putida results in aggregation and increased biofilm formation. Microbiologyopen 2, 365–382. ( 10.1002/mbo3.81) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seviour T, et al. 2015. Functional amyloids keep quorum-sensing molecules in check. J. Biol. Chem. 290, 6457–6469. ( 10.1074/jbc.M114.613810) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross-Gillespie A, Gardner A, West SA, Griffin AS. 2007. Frequency dependence and cooperation: theory and a test with bacteria. Am. Nat. 170, 331–342. ( 10.1086/519860) [DOI] [PubMed] [Google Scholar]
- 29.Ghysels B, Dieu TM, Beatson B, Pirnay S, Ochsner J-P, Vasil U, Cornelis M. 2004. FpvB, an alternative type I ferripyoverdine receptor of Pseudomonas aeruginosa. Microbiology 150, 1671–1680. ( 10.1099/mic.0.27035-0) [DOI] [PubMed] [Google Scholar]
- 30.Dumas Z, Ross-Gillespie A, Kümmerli R. 2013. Switching between apparently redundant iron-uptake mechanisms benefits bacteria in changeable environments. Proc. R. Soc. B 280, 20131055 ( 10.1098/rspb.2013.1055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwyn B, Neilands JB. 1987. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160, 47–56. ( 10.1016/0003-2697(87)90612-9) [DOI] [PubMed] [Google Scholar]
- 32.Kümmerli R, Schiessl KT, Waldvogel T, McNeill K, Ackermann M. 2014. Habitat structure and the evolution of diffusible siderophores in bacteria. Ecol. Lett. 17, 1536–1544. ( 10.1111/ele.12371) [DOI] [PubMed] [Google Scholar]
- 33.Visca P, Imperi F, Lamont IL. 2007. Pyoverdine siderophores: from biogenesis to biosignificance. Trends Microbiol. 15, 22–30. ( 10.1016/j.tim.2006.11.004) [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Tay JH. 2002. The essential role of hydrodynamic shear force in the formation of biofilm and granular sludge. Water Res. 36, 1653–1665. ( 10.1016/S0043-1354(01)00379-7) [DOI] [PubMed] [Google Scholar]
- 35.Kümmerli R, Brown SP. 2010. Molecular and regulatory properties of a public good shape the evolution of cooperation. Proc. Natl Acad. Sci. USA 107, 18 921–18 926. ( 10.1073/pnas.1011154107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim MK, Ingremeau F, Zhao A, Bassler BL, Stone HA. 2016. Local and global consequences of flow on bacterial quorum sensing. Nat. Microbiol. 1, 15005 ( 10.1038/nmicrobiol.2015.5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghoul M, West SA, McCorkell FA, Lee ZB, Bruce JB, Griffin AS. 2016. Pyoverdin cheats fail to invade bacterial populations in stationary phase. J. Evol. Biol. 29, 1728–1736. ( 10.1111/jeb.12904) [DOI] [PubMed] [Google Scholar]
- 38.Dobay A, Bagheri HC, Messina A, Kümmerli R, Rankin DJ. 2014. Interaction effects of cell diffusion, cell density and public goods properties on the evolution of cooperation in digital microbes. J. Evol. Biol. 27, 1869–1877. ( 10.1111/jeb.12437) [DOI] [PubMed] [Google Scholar]
- 39.Scholz RL, Greenberg EP. 2015. Sociality in Escherichia coli: enterochelin is a private good at low cell density and can be shared at high cell density. J. Bacteriol. 197, 2122–2128. ( 10.1128/JB.02596-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diggle SP, et al. 2007. The Pseudomonas aeruginosa 4-quinolone signal molecules HHQ and PQS play multifunctional roles in quorum sensing and iron entrapment. Chem. Biol. 14, 87–96. ( 10.1016/j.chembiol.2006.11.014) [DOI] [PubMed] [Google Scholar]
- 41.Guglielmini J, De La Cruz F, Rocha EP. 2012. Evolution of conjugation and type IV secretion systems. Mol. Biol. Evol. 30, 315–331. ( 10.1093/molbev/mss221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hibbing ME, Fuqua C, Parsek MR, Peterson SB. 2010. Bacterial competition: surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 8, 15–25. ( 10.1038/nrmicro2259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruce J, West S, Griffin A. 2019. Data from: Functional amyloids promote retention of public goods in bacteria Dryad Digital Repository. ( 10.5061/dryad.v8c8h23) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Bruce J, West S, Griffin A. 2019. Data from: Functional amyloids promote retention of public goods in bacteria Dryad Digital Repository. ( 10.5061/dryad.v8c8h23) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data used in the manuscript is available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.v8c8h23 [43].