Abstract
Infectious diseases are a primary driver of bee decline worldwide, but limited understanding of how pathogens are transmitted hampers effective management. Flowers have been implicated as hubs of bee disease transmission, but we know little about how interspecific floral variation affects transmission dynamics. Using bumblebees (Bombus impatiens), a trypanosomatid pathogen (Crithidia bombi) and three plant species varying in floral morphology, we assessed how host infection and plant species affect pathogen deposition on flowers, and plant species and flower parts impact pathogen survival and acquisition at flowers. We found that host infection with Crithidia increased defaecation rates on flowers, and that bees deposited faeces onto bracts of Lobelia siphilitica and Lythrum salicaria more frequently than onto Monarda didyma bracts. Among flower parts, bracts were associated with the lowest pathogen survival but highest resulting infection intensity in bee hosts. Additionally, we found that Crithidia survival across flower parts was reduced with sun exposure. These results suggest that efficiency of pathogen transmission depends on where deposition occurs and the timing and place of acquisition, which varies among plant species and environmental conditions. This information could be used for development of wildflower mixes that maximize forage while minimizing disease spread.
Keywords: Bombus impatiens, Crithidia bombi, pollinator health, disease spread, floral morphology
1. Introduction
Infectious diseases are a global concern for both humans and wildlife, with examples ranging from the shifting ecology of Ebola virus [1] to the rapid and devastating expansion of the chytrid fungus in amphibian populations [2]. Pathogens are one of the primary threats to pollinator health [3]. However, how infectious diseases spread across pollinator communities is poorly understood, limiting effective conservation. Specifically, the mechanisms mediating bee pathogen transmission through shared use of flowers are largely unknown [4,5], despite flowers being linked to pathogen spillover and spread [6]. Increasing dependence on bees for crop pollination heightens the urgency to understand disease transmission dynamics [7].
Effective bee disease transmission requires that pathogens be deposited onto a plant species and flower part where they can survive long enough to be encountered by, acquired and infect new susceptible hosts. Recent findings that transmission rates vary across flower species and floral traits [5,8,9] show that infected foraging bees can transmit disease to susceptible bees that subsequently visit the same flowers [8,9]. Yet, the mechanisms governing how pathogen transmission occurs on flowers, including deposition, survival and acquisition of bee pathogens, are largely unknown. Such information could help us predict which plants are more likely than others to function as disease hubs, which is important, given the increasing role that wildflower plantings play in pollinator protection efforts [10].
Infection can alter behaviour and physiology in ways that facilitate or impede disease spread. For example, infection can induce changes in the social network of ant colonies in ways that suppress pathogen transmission [11]. Conversely, honeybees infected with the faecal–orally transmitted microsporidian Nosema apis often present symptoms of dysentery, which facilitates spread within the colony [12]. Whether infection-induced changes could influence defaecation rates on flowers is unknown. Bumblebees infected with Crithidia bombi, a faecal–orally transmitted trypanostomatid pathogen, are cognitively impaired [13] and less efficient foragers [14,15], spending more time learning floral information and consequently visiting each flower for more time. Either of these mechanisms, physiologically induced defaecation or altered foraging patterns, could result in more faeces deposited on flowers by infected bees. Whether infection affects bee defaecation patterns on flowers represents a serious knowledge gap in bee disease transmission dynamics.
The ways bees interact with flowers vary greatly across floral morphologies and architectures, and depend on traits of the bees themselves, such as body size. Depending on the interaction between a bee and a flower, defaecation patterns and pathogen deposition may be altered [4]. Moreover, bee size is highly variable across and within bee species, and may play an important role in pathogen deposition on flowers [5]. For example, small bodied bees may fit entirely within flowers with long tubular corollas, resulting in higher likelihood of pathogen deposition inside the corolla tube than for larger bees that can only access the nectar at the end of the tubular corolla via their proboscis. Conversely, for flowers with short corollas, bee faeces may be unlikely to be deposited inside the corolla regardless of bee size, but instead may fall onto the bract subtending the flower, or onto other flowers in the inflorescence. These deposition dynamics could have consequences for pathogen survival and transmission, but the role of floral morphology and architecture in mediating host–pathogen dynamics is largely unknown.
Once deposited, horizontally transmitted pathogens depend on environmental conditions to remain infectious before being encountered by a new host. For example, the bee microsporidian N. apis can remain infectious up to 6 years under optimal conditions, but loses infectivity within hours when exposed to ultraviolet (UV) radiation [16]. Similarly, bumblebees develop a stronger infection when inoculated with freshly prepared C. bombi compared to inoculum that has been stored for 45 min [17]. Depending on where pathogens are deposited on a plant, their exposure to UV radiation and phytochemicals may vary (e.g. inside a corolla tube compared to an exposed petal). Moreover, pollen and nectar phytochemicals can have growth-inhibitory effects on C. bombi [18], and floral volatiles can kill certain plant pathogens [19]. Therefore, we predicted that pathogen survival and infectiousness would vary across floral parts within the same plant and across species and environmental conditions, and would be lowest for floral parts more exposed to the sun's UV radiation, such as outside the corolla and on flower bracts.
We evaluated multiple mechanisms hypothesized to contribute to bee disease transmission through shared use of flowers. Specifically, we investigated whether: (i) infection influences faecal deposition on flowers; (ii) the frequency of faeces deposited varies with plant species and flower part (inside the corolla, outside the corolla, flower bract and leaves); (iii) pathogen survival depends on pathogen deposition and environmental conditions (sun exposure) across flower parts; and (iv) pathogen acquisition and subsequent infection of bees vary among different parts of the flower in different plant species. We asked these questions by conducting three experiments. In the first experiment (questions (i) and (ii), we allowed experimentally infected and uninfected bees fed fluorescent diet to forage on three flower species, and determined how many times and where they defaecated on the plants. We predicted that infected bees would defaecate more on flowers than uninfected bees, and that defaecation patterns would depend on how the bees interact with the morphology of each plant species. In the second experiment (question (iii)), we placed pathogen inoculum on three flower parts and determined survival for 3 h across three plant species, either in sun-exposed or shaded conditions. We predicted that the pathogen would survive longer inside the flower corolla and under shaded conditions, owing to reduced exposure to UV radiation. In the third experiment (question (iv)), we allowed uninfected bees to forage on flowers upon which we had placed inoculum on a discrete flower part, and quantified the resulting infection loads one week after exposure. We predicted that resulting infections would be lowest when inoculum was encountered inside the flower corolla, owing to increased presence of phytochemicals in pollen and nectar. This study lies at the intersection of bee foraging ecology and epidemiology, and aims to expand the current understanding of bee disease transmission.
2. Material and methods
(a). Study system
All experiments were conducted using common eastern bumblebee (Bombus impatiens) workers and the trypanosome C. bombi. Native to eastern North America, B. impatiens (Hymenoptera, Apidae) is an abundant generalist bee, frequently used for commercial pollination [20]. The pathogen C. bombi (Kinetoplastea; Trypanosomatida; hereafter Crithidia) is a horizontally transmitted gut pathogen known to reduce bumblebee foraging efficiency and increase mortality under stressful conditions, and is associated with reduced reproduction in wild bumblebee colonies [14,21,22]. All experiments were conducted using Crithidia from wild B. impatiens workers collected in Massachusetts, USA (GPS: 42°22'17.53″ N, 72°35'13.52″ W) and maintained in laboratory bumblebee colonies (Biobest, Leamington, Ontario, Canada); infected colonies were only used as a source of inoculum and not as a source of bees in experimental trials. For the duration of the experiments, we conducted weekly pathogen screenings of five bees from each experimental colony to ensure colonies were Crithidia-free. Crithidia bombi species identity was verified by sequencing the 18S rRNA [23].
This study compared three plant species that are visited by bumblebees in northeastern North America and vary in their floral morphology and architectures: Monarda didyma (Lamiaceae), Lobelia siphilitica (Campanulaceae) and Lythrum salicaria (Lythraceae), hereafter Monarda, Lobelia and Lythrum (figure 1). Monarda and Lobelia are native to eastern North America, whereas Lythrum is a non-native species introduced from Europe that is highly abundant and attractive to pollinators [24].
Figure 1.
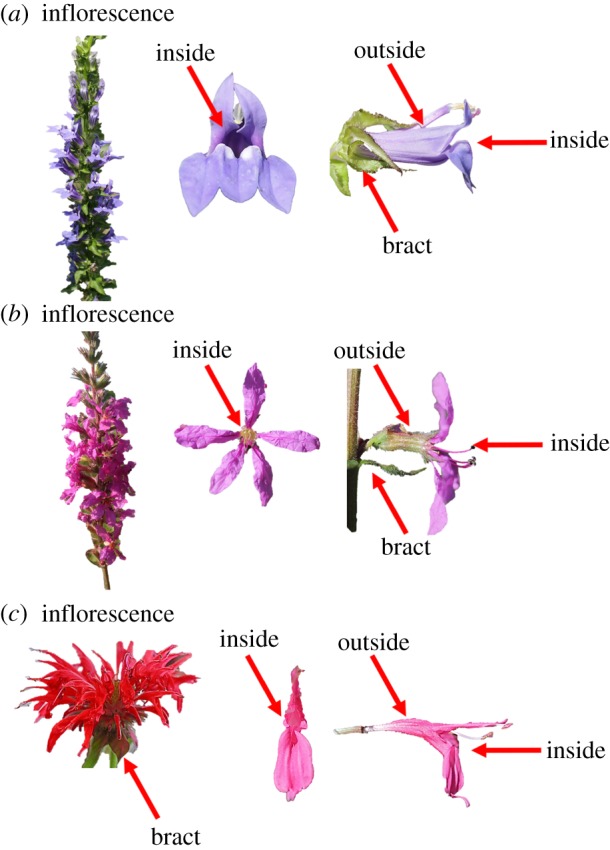
Flower parts where the common eastern bumblebee (Bombus impatiens) defaecated or Crithidia bombi inoculum was placed on (a) Lobelia siphilitica, (b) Lythrum salicaria and (c) Monarda didyma (photo credit: N. Milano). (Online version in colour.)
(b). Experimental protocol
(i). Experiment 1: effect of plant species and infection status on bee defaecation patterns across flower parts
To evaluate the role of infection on bee defaecation across plant species, we infected bees with Crithidia. The Crithidia inoculum used in the trials was prepared fresh daily by dissecting the gut of infected bees maintained in the laboratory and combining with Ringer's solution (Sigma–Aldrich, St Louis, MO, USA) to create a solution with 1200 cells µl−1, which was then mixed with equal amount of 50% sucrose solution to create an inoculum with 25% sucrose and 600 cells µl−1 [25]. We used 25% sucrose in Ringer's solution without Crithidia for a control (sham) inoculum. We selected 18 bees from each of three experimental colonies. Half were infected, while the other half were sham-infected, for a total of 54 bees inoculated on each date (13 days: 10, 12, 16, 19, 21, 26 and 28 July, and 1, 3, 9, 10, 17 and 21 August 2017), by feeding 10 µl of inoculum or sucrose solution using a micropipette. Three similarly sized bees of the same treatment and colony were maintained in microcolony containers with 30% sucrose and pollen provided ad libitum for 7–12 days prior to trial to allow infection to develop [26].
To determine defaecation patterns, bees were given sucrose mixed with fluorescent dye (2.5 g of fluorescent powder (Dayglo Color, Cleveland, OH, USA) dissolved in 500 ml of 30% sucrose) ad libitum 24–48 h prior to field trials. Defaecation trials were conducted during summer 2017 (Monarda 10–19 July, Lythrum 21 July–3 August, Lobelia 9–21 August). The day of the trial, bees were cooled at 4°C and transported in a cooler to the field site in Massachusetts (42°28′45.5″ N, 72°34′46.06″ W). Each trial consisted of a single flight cage (45.7 × 71.0 × 55.6 cm) in which three clipped field-grown inflorescences were placed in tubes with water, held upright by tube racks. The number of flowers per inflorescence was held constant within species. The bottom of each cage was lined with newspaper, which was replaced before each trial to eliminate cross-contamination across trials. Cooling bees prior to trials facilitated foraging. Owing to mortality during the period in which infection was allowed to grow, not all trials included three bees; there was no difference in mortality between infected and uninfected bees ( p = 0.742), nor did the number of bees in a trial affect defaecation patterns ( p = 0.250 and p = 0.200 for presence/absence and number of faecal droplets, respectively). The number of bees and time when each bee was placed in a cage and started foraging were noted. If bees did not forage within 15 min, a flower was raised towards the bees to induce foraging (20% of bees were induced). If presentation of the flower did not induce foraging, that trial was excluded from the experiment. Cages were checked for bee faeces 3 h after foraging began; the cage was brought into a darkened barn and a handheld black light was used to count the number of fluorescent faecal droplets on each plant part (Escolite UV Flashlight Black Light, 51 LED 395 nM). The plant parts were divided into four categories: ‘inside’ the flower (inside the corolla), ‘outside’ the flower (surface of the corolla), on the bract (on the modified leaf subtending the inflorescence) or on a leaf (excluding the bract; figure 1). We also recorded faeces elsewhere in the cage, to determine the proportion of faeces deposited on plants for each plant species. Post-trial, bees were returned to the laboratory and maintained on 30% sucrose until the following day, when they were dissected to confirm infection status. We removed the right forewing and measured marginal cell length as a proxy for bee size [27].
Statistical analyses. Data analyses were conducted using RStudio (R v. 3.5.1) with the lme4 and lsmeans packages [28–30]. We excluded trials for bees that were inoculated but did not develop infection (n = 3) and control trials in which bees developed infection (n = 3), for a resulting sample size of n = 163 trials (Lobelia n = 54, Lythrum n = 61 and Monarda n = 48). To evaluate the factors that predicted defaecation, we constructed a generalized linear mixed model (GLMM) that evaluated faeces on plant (presence/absence) as the response, predicted by bee infection status (infected/uninfected), plant species, average bee size and number of bees in the trial. To determine whether bees were defaecating differently across parts of the plant, we developed a GLMM that included the number of faecal droplets as the response variable and evaluated part (inside of flower, outside of flower, bract or leaf), infection status (infected/uninfected), plant species, average bee size and number of bees in trial as explanatory variables. Both models included observation level (trial), experimental colony and date as random effects, and fit a Poisson distribution, which is suitable for count data [31]. Experimental colony did not explain variance in either model and affected convergence, so was removed from subsequent analyses. No variable in the model produced a variance inflation factor greater than two, indicating low co-linearity [32]. To determine the role of each explanatory variable, we employed a likelihood ratio test to compare the full model to identical models that excluded the variable in question. The significance of interactions was determined by comparing the original model with and without interactions (flower part by either average bee size, plant species or infection status); we removed non-significant interactions. Significant interactions were evaluated using the lstrends function [29].
(ii). Experiment 2: Crithidia survival across plant species and flower parts
Pathogen survival was evaluated across plant species and parts on flowers. We made Crithidia inoculum based on realistic faecal volumes and sugar concentrations; we did not consider other nutrients or compounds that may be in faeces. We used Ringer's solution, a saline solution often used to study insect physiology [33], as we expected, it would be a more realistic proxy for bee faeces than water. We determined realistic faecal volumes by placing 10 worker B. impatiens in individual vials for 2–4 h and measuring faecal volume using microcapillary tubes (Sigma–Aldrich: 20 µl). The largest volume observed was 33 µl, so we used 35 µl of Crithidia inoculum in trials, representing the upper limit of realistic faecal quantity. Given Crithidia's susceptibility to sugar [34], we evaluated the sugar concentration of bee faeces using a refractometer. The values ranged from 0 to 1% sugar, and so, unlike experiments 1 and 3, no sugar was added to inoculum.
Trials were conducted during summer 2017. Inoculum was made fresh each trial day, with at least 3300 Crithidia cells µl−1 of Ringer's solution (mean: 3617, range 3300–3900); this high concentration was chosen for ease of visualization in the hemocytometer. We used the same three plant species from experiment 1, each evaluated in 1 day: Monarda (12 July), Lythrum (21 July) and Lobelia (1 August). Because environmental conditions and inoculum strength varied between days, and flower species did not have co-occurring blooming periods, we are not able to compare viability across plant species. Flowers were bagged in the field 2 days prior to trial to avoid pathogen deposition from foraging bees. On the day of the trial, inflorescences were cut, individually marked and placed in tubes with water. The experiment was conducted in large covered hexagonal tents (71 × 160.5 in.). To evaluate the effect of the sun, one tent had a UV-protected cover, while the other had a mesh cover that allowed UV exposure but prevented wild bees from entering. Monarda was only evaluated in shaded (UV-protected) conditions owing to rainy and overcast weather. Within each tent, we measured the temperature, relative humidity (AcuRite, 01083 Pro Accuracy Indoor Temperature and Humidity Monitor) and UV radiation (Apogee instruments, MU-100).
We placed 35 µl of inoculum on two parts of each inflorescence (inside corolla and bract; the exception was Monarda where we also evaluated outside the corolla). We evaluated pathogen survival for 3 h, taking five inflorescences every 30 min into the laboratory, where the inoculum on each part was pipetted into a hemocytometer to count mobile Crithidia. We did not evaluate the infectivity of Crithidia, using mobility instead as a proxy for survival, in part because infectiousness of Crithidia is highly variable, even within a single day [35]. If the inoculum evaporated, we pipetted 10 µl of distilled water onto the part to collect any Crithidia cells and checked for mobile Crithidia; we were successful in detecting mobile Crithidia in some instances when the inoculum had visibly evaporated. The sample size for the shaded samples were: Lobelia n = 58 parts (29 inflorescences), Lythrum n = 60 (30 inflorescences) and Monarda n = 88 (31 inflorescences). The sample sizes for sun-exposed plants were: Lobelia n = 58 (29 inflorescences) and Lythrum n = 60 (30 inflorescences).
Statistical analyses. We conducted survival analyses using the Cox proportional hazards mixed-models via the coxme package in RStudio [30,36]. The survival analysis evaluated Crithidia survival (count of moving cells per 0.02 µl) by time elapsed when the flower was inspected for each of the three plant species. The model included part on flower and shade treatment as explanatory variables, as well as individual plant as the random effect. To determine the significance of the treatments (flower part and shade), we conducted a likelihood ratio test comparing the full model of each species with a model that included the same random effect structure but excluded either explanatory variable or included an additive relationship instead of an interaction. Differences in survival across flower parts were determined post hoc with Tukey's HSD using the lsmeans function [29].
(iii). Experiment 3: effects of plant species and flower part on pathogen acquisition and subsequent intensity of infection
We evaluated the effect of plant species and flower part on Crithidia transmission by placing pathogen inoculum on flowers, allowing uninfected bees to forage, and subsequently determining infection (presence/absence and intensity) in the bees. Trials were conducted in 2016 on Monarda (30 June–15 July), Lythrum (18 July–9 August) and Lobelia (18–26 August). Experimental bees and inoculum were transported to the field site in a cooler with insulated ice packs. We used bees from four experimental colonies for Monarda, five for Lythrum and six for Lobelia; colonies mostly overlapped for the first two species and had approximately 50% overlap for the second and third species. We accounted for colony origin in the analyses (see Statistical analyses). For each trial, we collected an inflorescence of the target species at the field site and placed it in a tube filled with water. Each trial was randomly assigned to one of three treatments of inoculum placement: inside corolla, outside corolla or bract. For all the treatments, we added four 10 µl drops of inoculum in 25% sucrose solution (see experiment 1 for inoculum preparation) on the inflorescence in the specified treatment part using a micropipette (figure 1); inoculated flowers were marked using a paint pen. Inflorescences were from field-grown plants that were bagged with mesh for at least 2 days prior to trials to prevent Crithidia deposition from wild foraging bees. We placed the prepared inflorescence in a small flight cage and released a single, chilled worker bee into the cage (see experiment 1 for cage details). We allowed the bee to forage and recorded total time spent foraging (i.e. probing flowers, not including time moving between flowers), number of flowers probed and number of drops probed. We also recorded the time of the trial, so that we could calculate elapsed time between inoculum preparation and each trial for use as a covariate. When the bee stopped foraging (usually a clear change in behaviour from probing flowers to flying around the cage), we recaptured it in a vial. Bees were excluded if they did not probe any inoculum drops or foraged for less than 30 s.
Bees were collected and subsequently maintained individually for one week in the laboratory to allow infection to develop. We fed each bee daily 500 µl of 30% sucrose solution and approximately 0.15 g pollen ball (30% sucrose and commercial mixed wildflower pollen (Koppert Biological Systems; Linden Apiaries, Walpole, NH, USA)). We maintained the bees in an incubator set at 27°C in darkness. After 7 days, we dissected each bee and placed the gut in 300 µl of Ringer's solution. The mixture was allowed to incubate for 4 h before Crithidia was quantified using a hemocytometer [25]. We removed the right forewing and measured marginal cell length as a proxy for size [27]. Sample sizes for each species were n = 40 bees for Monarda, n = 67 for Lythrum and n = 89 for Lobelia.
Statistical analyses. Data analyses were conducted using RStudio with packages lme4, DHARMa, RVAidememo and lsmeans [28–30,37,38]. To manage zero-inflated and overdispersed count data, we used manual two-step hurdle models [39]. We first evaluated an ‘incidence’ model (evaluating presence or absence of Crithidia infection), followed by an ‘intensity’ model (Crithidia counts of the infected bees). In the first step, we modelled pathogen incidence using a binomial distribution (logit link), given the binary outcome of whether bees were infected or not. Next, we modelled Crithidia intensity when present (i.e. the non-zero outcomes) with a Poisson distribution (log link). We evaluated overdispersion in the Poisson model using the overdisp.glmer function in the RVAideMemoire package [38]. To ensure our data were well modelled by the specified distributions and to check model assumptions, we used the DHARMa package [37]. Our incidence model was evaluated using a GLMM, with the presence or absence of infection as the response variable, predicted by flower part, plant species, their interaction, bee size, foraging time and time since the inoculum was made (related to its infectiousness). The model included colony and date as random effects, thus accounting for overlap in colonies during trials. The intensity model had the same random effect structure as the incidence model, plus an observation-level random effect to correct for overdispersion [40]. To determine significance, we conducted a likelihood ratio test by comparing the full GLMM to a model that excluded the factor of interest. Significant factors were determined post hoc with Tukey's HSD using the lsmeans function [29].
3. Results
(a). Experiment 1: effect of plant species and infection status on bee defaecation patterns across flower parts
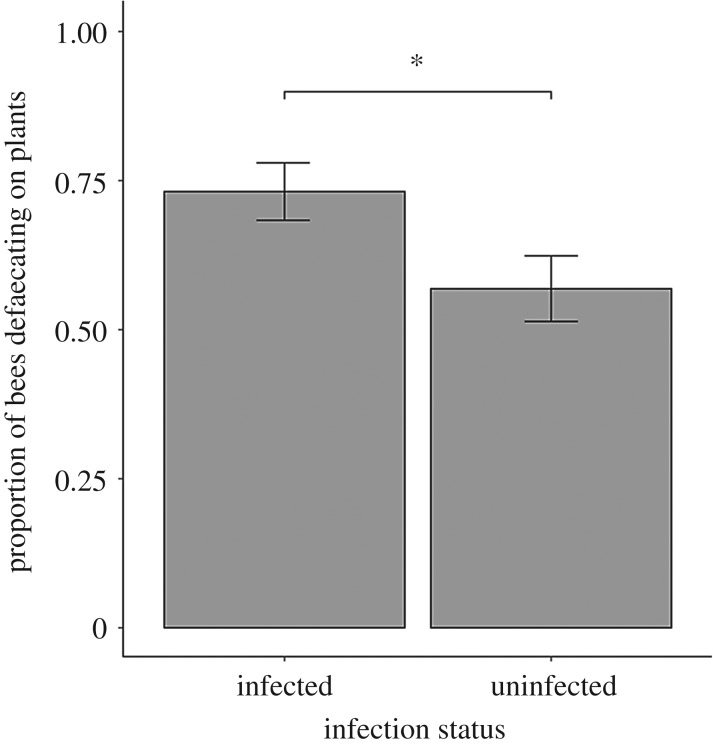
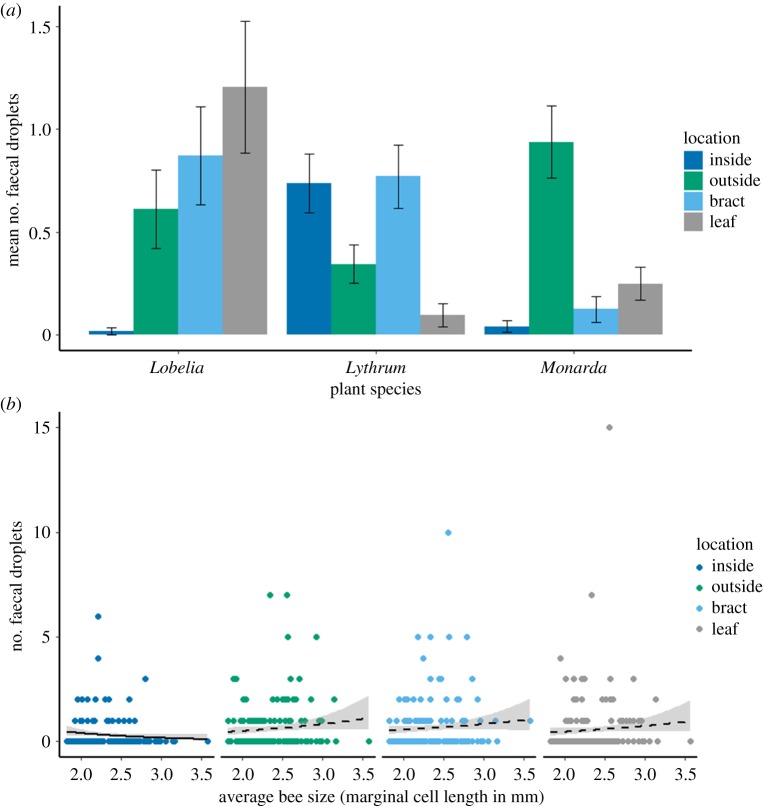
Overall, bees defaecated on plants in 65% of trials. Infected bees were more likely to defaecate on plants than uninfected bees ( p = 0.039; figure 2), although there was no relationship between infection status and the number of faecal droplets observed ( p = 0.306) or where bees defaecated ( p = 0.287). Flower part significantly predicted the number of faecal droplets observed ( p < 0.001). Moreover, we found a strong plant species by part interaction ( p < 0.001; figure 3a; electronic supplementary material, table S1), such that the most deposition occurred on leaves and bracts for Lobelia, on bracts and inside the flower for Lythrum and outside the flower for Monarda. We observed a bee size by flower part interaction for the number of faecal droplets observed ( p = 0.028; figure 3b), whereby fewer droplets were detected inside flowers visited by larger bees (Tukey HSD: z = –2.87, p = 0.004). Plant species and average bee size did not predict the presence or number of faecal droplets observed on flowers ( p = 0.517 and p = 0.991, respectively, for the presence of faeces; p = 0.614 and p = 0.478, respectively, for the number of faecal droplets). Bee size had no relationship with the number of faecal droplets observed on the outside of the flower, on the bract or on leaves (z = 1.55, p = 0.122, z = 1.11, p = 0.268 and z = 1.34 and p = 0.180, respectively). The proportion of total faecal droplets that landed on the plants (compared to elsewhere in the cage) varied across plant species ( p < 0.001), being 0.55, 0.29 and 0.25 for Lobelia, Lythrum and Monarda, respectively.
Figure 2.
Experiment 1: effect of Crithidia infection status on B. impatiens defaecation rate on plants (mean ± s.e). Infected worker bees were more likely to defaecate on plants than uninfected bees. *p < 0.05.
Figure 3.
Experiment 1: (a) effect of plant species and flower part on defaecation by B. impatiens workers. Data are mean ± s.e. (for post hoc comparisons, see the electronic supplementary material, table S1). (b) Effect of B. impatiens size on defaecation among different flower parts. Solid lines indicate significance (p < 0.05), while dashed lines indicate no significant relationship. (Online version in colour.)
(b). Experiment 2: Crithidia survival across plant species and flower parts
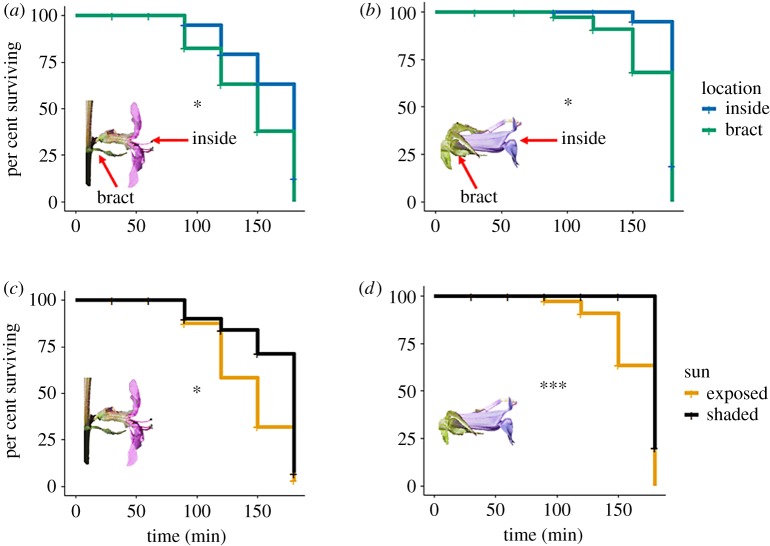
Crithidia became non-motile within 3 h of placement on flowers in 71% of trials. Furthermore, mortality varied by plant species ( p < 0.001), at 90% for Lobelia, 90% for Lythrum and 20% for Monarda. Crithidia survival was influenced by flower part on all plant species ( p = 0.031, p = 0.019 and p = 0.043 for Lobelia, Lythrum and Monarda, respectively; figure 4a,b). For Lobelia and Lythrum, Crithidia survived longer inside the corolla than on the bract (Tukey HSD test: z = 2.09, p = 0.037 and z = 2.29, p = 0.022 for Lobelia and Lythrum, respectively). Post hoc evaluation of Crithidia survival across parts on Monarda flowers did not yield significant pairwise comparisons (electronic supplementary material, table S2), probably owing to low overall mortality in this species. Crithidia survival was also greater in shaded than sunny conditions ( p = 0.009 and p = 0.033 for Lobelia and Lythrum, respectively; figure 4c,d). There was no flower part by sun exposure interaction in either species ( p = 0.892 and p = 0.223, for Lobelia and Lythrum, respectively).
Figure 4.
Experiment 2: Crithidia survival across plant species and flower parts. Survival differed across flower part and exposure to sun in Lythrum (a,c) and Lobelia (b,d). Monarda was only evaluated in shade conditions (see Material and methods); we did not find significant differences among flower parts in Monarda, probably owing to a high overall Crithidia survival (80%). *p < 0.005; ***p < 0.001. (Online version in colour.)
(c). Experiment 3: effects of plant species and flower part on Crithidia acquisition and subsequent intensity of infection
The probability of becoming infected did not depend on plant species, part where inoculum was placed, their interaction or bee size (χ2 < 4.68, p > 0.137 for all). However, part on flower did predict Crithidia intensity for the infected bees ( p = 0.001; figure 5). Specifically, when bees picked up inoculum on the bract of a flower, they developed a more intense Crithidia infection than if they encountered the pathogen on the outside of the flower (Tukey HSD: z = 3.77, p < 0.001). Similarly, bees developed a marginally more intense Crithidia infection when encountered on the bract than the inside of the flower (z = 2.29, p = 0.057). There was no difference in infection intensity between the inside and outside of the flower (z = 1.35, p = 0.370). For infected bees, bee size did not explain Crithidia intensity ( p = 0.363), nor did plant species ( p = 0.602), or plant species by flower part interaction ( p = 0.338).
Figure 5.
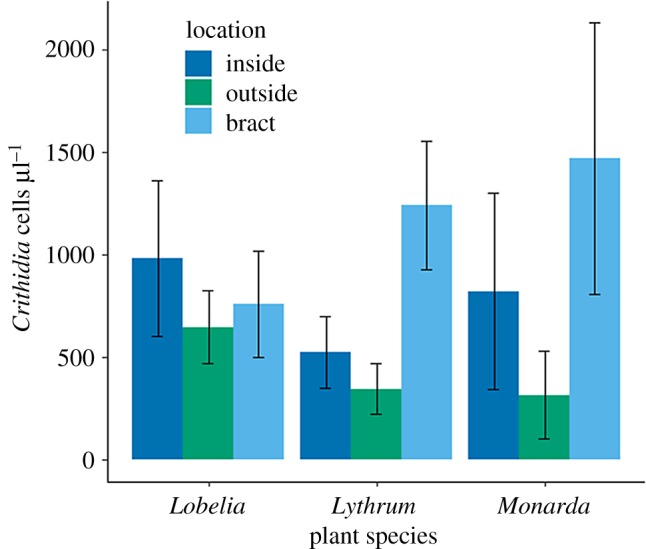
Experiment 3: effects of plant species and flower part on Crithidia acquisition and subsequent intensity of infection (Crithidia cells µl−1) in B. impatiens workers. Data are means ± s.e. (Online version in colour.)
4. Discussion
The intersection of bee foraging ecology and epidemiology is a novel area of research that can give rise to new understanding of pollinator disease spread and evidence-based conservation strategies. Here, we show that foraging bumblebees often defaecate on plants, and do so more when they are infected with Crithidia (figure 2). There is not a universal part on plants where bees are more likely to defaecate. That pattern depends on plant species, which may in turn be related to floral traits, such as shape or size. These deposition dynamics are also influenced by bee traits, with bigger bees defaecating fewer times inside flowers (figure 3b), possibly because they are too large to fit inside the flowers. Similarly, for pathogen survival on flowers, we found differences across flower parts for some species but not for others (figure 4a). Moreover, the flower part where inoculum is encountered influenced the intensity of the resulting infection (figure 5), further highlighting the complexity of bee pathogen transmission dynamics via flowers. Taken together, these data suggest variation in plant–pollinator interaction patterns, from encounter rates to trait matching, are expected to influence pathogen transmission and warrant further research.
Bees defaecated on plants in 65% of trials, and did so significantly more when infected with Crithidia (figure 2). Increased likelihood of defaecation on plants could hasten the spread of multiple diseases, especially because bumblebees are often infected with several faecal–orally transmitted pathogens [16,41]. Whether the increased defaecation is a by-product of dysentery, as in honeybees infected with N. apis [12] or because of increased time spent on each flower by infected bees [15,35], remains unknown.
We found a plant species by part interaction on the number of faecal droplets observed, such that each plant species had a different part where droplets were most likely to be found (figure 3a). Differential handling of the flowers across plant species could have led to this pattern, especially given the diversity of floral morphologies and plant architectures (figure 1). For Monarda (figure 1c), the inside of the small floral tube is only accessible to the bee proboscis, probably explaining why we seldom observed faeces there, compared to the outside of the corolla where the bees crawl to reach subsequent flowers. Similarly, Lobelia (figure 1a) rarely had faeces inside of the flower, despite an entirely different floral morphology. The floral tube of Lobelia is quite large, such that the entire head of the bees can fit inside, but usually the abdomen protrudes, enabling defaecation onto leaves or bracts subtending the flower. However, the smallest bees in the trials fit entirely within the Lobelia flowers, probably contributing to the bee size by part interaction. Lythrum differed in that it often had faeces on the inside of its flowers. This is probably because the tube of Lythrum is extremely short and narrow and surrounded by wide, flat petals (figure 1b), so that bees will crawl over the entire flower after foraging to reach the next flower. These differential deposition dynamics across plant species are the first step towards horizontal transmission, which can result in transferring the pathogen to new colonies via foragers.
Horizontally transmitted pathogens must remain viable to be acquired by a new host. However, the decay rate of many pathogens outside of their host is unknown [42]. Crithidia survived longer on the inside of the corolla than the bract of Lythrum and Lobelia flowers (figure 4a,b). We had predicted that the inside would provide more protection from desiccation, extending survival compared to more exposed parts. However, we did not observe that pattern for Monarda, which aligns with the lower overall Crithidia mortality on this species. Floral chemistry or other unknown mechanisms could mediate the lack of differences across floral parts for this species, as could more humid environmental conditions during the day of trial. In general, we found that within 3 h of being placed on flowers, most Crithidia had died. Incorporating the rate of decay between deposition by infected bees and acquisition by the incoming susceptible foragers could enhance disease spread models [42].
Once pathogens have been deposited on the plant, environmental factors could influence pathogen survival. Crithidia on sun-exposed flowers had shorter survival times than shaded plants (figure 4c,d). This may be because of UV radiation, temperature, and/or increased desiccation, all of which were greater in the sun-exposed conditions. Pulsed UV radiation can decrease Crithidia viability [43]. Otterstatter & Thompson experimentally varied the time and number of Crithidia cells placed on Brassica rapa nectaries encountered by susceptible foraging bumblebees. They found that most foraging bees became infected when exposed to Crithidia that had been placed on the flower for less than 10 min; by 85 min, the probability of infection was under 15% [6]. They determined the half-life of Crithidia to be 77 min, largely mirroring our results. Floral mechanisms that maximize exposure to direct sunlight, such as heliotropism, could reduce bee pathogen survival on flowers and warrant further investigation. Similarly, whether environmental gradients that affect exposure to UV radiation (e.g. along an altitudinal gradient or from the forest canopy to the ground layer) influence bee pathogen transmission dynamics on flowers is entirely unknown and is an important area for future research.
For bees that developed an infection after foraging on inoculated plants (experiment 3), those that encountered inoculum on the bract had more intense Crithidia infections than when they encountered it on the outside of the flower (figure 5). This pattern may be owing to fewer phytochemicals from nectar and pollen encountered on the bract [44]. For Lobelia and Lythrum, bumblebees defaecated many times on the bract (experiment 1), which was also the part associated with the most intense Crithidia infection (experiment 3). However, in this part, Crithidia survived shorter amounts of time, and so the ability to transmit Crithidia will depend on how quickly faeces are encountered by a new host. Lythrum is very frequently visited by bees, especially B. impatiens, in its non-native North American range [24], which could then minimize the impact of short pathogen survival time and facilitate pathogen spread in the community. Conversely, foraging bumblebees seldom defaecated on Monarda bracts, the part that resulted in the greatest infection intensity. These results suggest Lobelia and Lythrum may be more effective disease transmission hubs than Monarda, but transmission will also depend on the frequency of visitation. We hypothesize that floral morphologies which facilitate overlap in where pollinator faeces are deposited and acquired (e.g. flat composites on which bees walk and forage for long periods of time) would result in higher rates of disease transmission compared to morphologies for which deposition and acquisition may be disjointed (e.g. Solanaceous plants that are visited for short period of times and do not have a landing platform).
In the face of increasing dependence on bees for ecosystem services [7], there is a pressing need to understand factors that shape pollinator health. Pathogen-induced stress and spillover from commercial bees via flowers are factors consistently linked to pollinator decline [3,6], yet the mechanisms governing how flowers serve as disease transmission venues have been largely unexplored. Flowers are multifunctional hubs, providing not only nutrition, microbial symbionts [45] and pathogen-suppressing chemical compounds [25,46], but also many of the pathogens themselves [47]. Infection-induced changes in foraging and/or physiology are predicted to affect probability of transmission [35,48], but had yet to be empirically evaluated until now. Understanding how flowers contribute to bee pathogen transmission is a necessary component of promoting pollinator health. Given our results, we recommend assessing floral traits associated with pathogen transmission across a diversity of plant and pollinator species, in an effort to develop wildflower mixes that not only maximize forage but also minimize disease spread.
Supplementary Material
Acknowledgements
We thank Biobest for donating bee colonies, Laura Harrington for support in the methodological design of this experiment and reading a previous draft of the manuscript, Julie Davis and Dana Delaney for field and laboratory assistance, Neal Woodard for field site preparation, Jeff Boettner and Joe Elkinton for providing the field cages, Quinn McFrederick for the Crithidia bombi sequence data and Nelson Milano for flower photographs.
Data accessibility
Data is available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.jc4hf80 [49].
Authors' contributions
L.L.F., L.S.A., R.E.I. and S.H.M. conceived and designed the study; L.L.F., L.S.A., M.B., C.G., A.J., E.K.M., L.A.M., L.E.M. and A.Y.Z. collected field data and conducted experiments; L.L.F. carried out the statistical analyses and drafted the manuscript. All authors edited the manuscript and gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This material is based upon work supported by the National Science Foundation Graduate Research Fellowship under grant no. DGE-1650441 and the National Institute of General Medical Sciences of the National Institutes of Health under award no. R01GM122062, as well as research funds from Garden Club of America (GCA) Board of Associates Centennial Pollinator Fellowship and the Atkinson Center for a Sustainable Future Sustainable Biodiversity Fund. The University of Massachusetts at Amherst Summer Pre-College program provided logistical support.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or National Science Foundation.
References
- 1.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. 2008. Global trends in emerging infectious diseases. Nature 451, 990–993. ( 10.1038/nature06536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lips KR, et al. 2006. Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proc. Natl Acad. Sci. USA 103, 3165–3170. ( 10.1073/pnas.0506889103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goulson D, Nicholls E, Botías C, Rotheray EL. 2015. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347, 1255957 ( 10.1126/science.1255957) [DOI] [PubMed] [Google Scholar]
- 4.McArt SH, Koch H, Irwin RE, Adler LS. 2014. Arranging the bouquet of disease: floral traits and the transmission of plant and animal pathogens. Ecol. Lett. 17, 624–636. ( 10.1111/ele.12257) [DOI] [PubMed] [Google Scholar]
- 5.Adler LS, Michaud KM, Ellner SP, McArt SH, Stevenson PC, Irwin RE. 2018. Disease where you dine: plant species and floral traits associated with pathogen transmission in bumble bees. Ecology 99, 2535–2545. ( 10.1002/ecy.2503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otterstatter MC, Thomson JD. 2008. Does pathogen spillover from commercially reared bumble bees threaten wild pollinators? PLoS ONE 3, e2771 ( 10.1371/journal.pone.0002771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aizen MA, Garibaldi LA, Cunningham SA, Klein AM. 2008. Long-term global trends in crop yield and production reveal no current pollination shortage but increasing pollinator dependency. Curr. Biol. 18, 1572–1575. ( 10.1016/j.cub.2008.08.066) [DOI] [PubMed] [Google Scholar]
- 8.Durrer S, Schmid-Hempel P. 1994. Shared use of flowers leads to horizontal pathogen transmission. Proc. R. Soc. Lond. B 258, 299–302. ( 10.1098/rspb.1994.0176) [DOI] [Google Scholar]
- 9.Graystock P, Goulson D, Hughes WOH. 2015. Parasites in bloom: flowers aid dispersal and transmission of pollinator parasites within and between bee species. Proc. R. Soc. B 282, 20151371 ( 10.1098/rspb.2015.1371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams NM, et al. 2015. Native wildflower plantings support wild bee abundance and diversity in agricultural landscapes across the United States. Ecol. Appl. 25, 2119–2131. ( 10.1890/14-1748.1) [DOI] [PubMed] [Google Scholar]
- 11.Stroeymeyt N, Grasse AV, Crespi A, Mersch DP, Cremer S, Keller L. 2018. Social network plasticity decreases disease transmission in a eusocial insect. Science 362, 941–945. ( 10.1126/science.aat4793) [DOI] [PubMed] [Google Scholar]
- 12.Bailey L, Ball BV. 1991. Honey bee pathology, 2nd edn London, UK: Academic Press. [Google Scholar]
- 13.Gegear RJ, Otterstatter MC, Thomson JD. 2006. Bumble-bee foragers infected by a gut parasite have an impaired ability to utilize floral information. Proc. R. Soc. B 273, 1073–1078. ( 10.1098/rspb.2005.3423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otterstatter MC, Gegear RJ, Colla SR, Thomson JD. 2005. Effects of parasitic mites and protozoa on the flower constancy and foraging rate of bumble bees. Behav. Ecol. Sociobiol. 58, 383–389. ( 10.1007/s00265-005-0945-3) [DOI] [Google Scholar]
- 15.Gegear RJ, Otterstatter MC, Thomson JD. 2005. Does parasitic infection impair the ability of bumblebees to learn flower-handling techniques? Anim. Behav. 70, 209–215. ( 10.1016/j.anbehav.2004.09.025) [DOI] [Google Scholar]
- 16.Schmid-Hempel P. 1998. Parasites in social insects. Princeton, NJ: Princeton University Press. [Google Scholar]
- 17.Schmid-Hempel P, Puhr K, Krüger N, Reber C, Schmid-Hempel R. 1999. Dynamic and genetic consequences of variation in horizontal transmission for a microparasitic infection. Evolution 53, 426–434. ( 10.1111/j.1558-5646.1999.tb03778.x) [DOI] [PubMed] [Google Scholar]
- 18.Palmer-Young EC, Sadd BM, Stevenson PC, Irwin RE, Adler LS. 2016. Bumble bee parasite strains vary in resistance to phytochemicals. Sci. Rep. 6, 37087 ( 10.1038/srep37087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang M, Sanchez-Moreiras AM, Abel C, Sohrabi R, Lee S, Gershenzon J, Tholl D. 2012. The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-β-caryophyllene, is a defense against a bacterial pathogen. New Phytol. 193, 997–1008. ( 10.1111/j.1469-8137.2011.04001.x) [DOI] [PubMed] [Google Scholar]
- 20.Velthuis HHW, van Doorn A.. 2006. A century of advances in bumblebee domestication and the economic and environmental aspects of its commercialization for pollination. Apidologie 37, 421–451. ( 10.1051/apido:2006019) [DOI] [Google Scholar]
- 21.Brown M, Loosli R, Schmid-Hempel P. 2000. Condition-dependent expression of virulence in a trypanosome infecting bumblebees. Oikos 91, 421–427. ( 10.1034/j.1600-0706.2000.910302.x) [DOI] [Google Scholar]
- 22.Goulson D, O'Connor S, Park KJ. 2018. The impacts of predators and parasites on wild bumblebee colonies. Ecol. Entomol. 43, 168–181. ( 10.1111/een.12482) [DOI] [Google Scholar]
- 23.Schmid-Hempel R, Tognazzo M. 2010. Molecular divergence defines two distinct lineages of Crithidia bombi (Trypanosomatidae), parasites of bumblebees. J. Eukaryot. Microbiol. 57, 337–345. ( 10.1111/j.1550-7408.2010.00480.x) [DOI] [PubMed] [Google Scholar]
- 24.Flanagan RJ, Mitchell RJ, Karron JD. 2010. Increased relative abundance of an invasive competitor for pollination, Lythrum salicaria, reduces seed number in Mimulus ringens. Oecologia 164, 445–454. ( 10.1007/s00442-010-1693-2) [DOI] [PubMed] [Google Scholar]
- 25.Richardson LL, Adler LS, Leonard AS, Andicoechea J, Regan KH, Anthony WE, Manson JS, Irwin RE. 2015. Secondary metabolites in floral nectar reduce parasite infections in bumblebees. Proc. R. Soc. B 282, 20142471 ( 10.1098/rspb.2014.2471) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmid-Hempel P, Schmid-Hempel R. 1993. Transmission of a pathogen in Bombus terrestris, with a note on division of labour in social insects. Behav. Ecol. Sociobiol. 33, 319–327. ( 10.1007/BF00172930) [DOI] [Google Scholar]
- 27.Harder LD. 1982. Measurement and estimation of functional proboscis length in bumblebees (Hymenoptera: Apidae). Can. J. Zool. 60, 1073–1079. ( 10.1139/z82-148) [DOI] [Google Scholar]
- 28.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 29.Lenth RV. 2016. Least-squares means: the R package lsmeans. J. Stat. Softw. 69, 1–33. ( 10.18637/jss.v069.i01) [DOI] [Google Scholar]
- 30.R Core Team. 2018. R: a language and environment for statistical computing. Version 3.4.0 Vienna, Austria: R Foundation for Statistical Computing; See https://CRAN.R-project.org. [Google Scholar]
- 31.Ellison G, Gotelli N. 2004. A primer of ecological statistics. Sunderland, MA: Sinauer. [Google Scholar]
- 32.O'Brien RM. 2007. A caution regarding rules of thumb for variance inflation factors. Qual. Quant. 41, 673–690. ( 10.1007/s11135-006-9018-6) [DOI] [Google Scholar]
- 33.Alghamdi A, Dalton L, Phillis A, Rosato E, Mallon EB. 2008. Immune response impairs learning in free-flying bumble-bees. Biol. Lett. 4, 479–481. ( 10.1098/rsbl.2008.0331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cisarovsky G, Schmid-Hempel P. 2014. Combining laboratory and field approaches to investigate the importance of flower nectar in the horizontal transmission of a bumblebee parasite. Entomol. Exp. Appl. 152, 209–215. ( 10.1111/eea.12218) [DOI] [Google Scholar]
- 35.Otterstatter MC, Thomson JD. 2006. Within-host dynamics of an intestinal pathogen of bumble bees. Parasitology 133, 749–761. ( 10.1017/S003118200600120X) [DOI] [PubMed] [Google Scholar]
- 36.Therneau TM. 2018. Package ‘coxme’. Mixed effects cox models. R package version 2.2-10. See https://CRAN.R-project.org/package=coxme.
- 37.Hartig F. 2019. DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.2.4. See https://CRAN.R-project.org/package=DHARMa.
- 38.Hervé M. 2019. RVAideMemoire: testing and plotting procedures for biostatistics. R package version 0.9-73. See https://CRAN.R-project.org/package=RVAideMemoire.
- 39.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Zero-truncated and zero-inflated models for count data. In Mixed effects models and extensions in ecology with R (eds Gail M, Krickeberg K, Samet JM, Tsiatis A, Wong W), pp. 261–293. Berlin, Germany: Springer. [Google Scholar]
- 40.Harrison XA. 2014. Using observation-level random effects to model overdispersion in count data in ecology and evolution. PeerJ 2, e616 ( 10.7717/peerj.616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cordes N, Huang WF, Strange JP, Cameron SA, Griswold TL, Lozier JD, Solter LF. 2012. Interspecific geographic distribution and variation of the pathogens Nosema bombi and Crithidia species in United States bumble bee populations. J. Invertebr. Pathol. 109, 209–216. ( 10.1016/j.jip.2011.11.005) [DOI] [PubMed] [Google Scholar]
- 42.Richardson TO, Gorochowski TE. 2015. Beyond contact-based transmission networks: the role of spatial coincidence. J. R. Soc. Interface 12, 20150705 ( 10.1098/rsif.2015.0705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naughton J, Tiedeken EJ, Garvey M, Stout JC, Rowan NJ. 2017. Pulsed light inactivation of the bumble bee trypanosome parasite Crithidia bombi. J. Apic Res. 56, 144–154. ( 10.1080/00218839.2017.1289668) [DOI] [Google Scholar]
- 44.Palmer-Young EC, Farrell IW, Adler LS, Milano NJ, Egan PA, Junker RR, Irwin RE, Stevenson PC. 2019. Chemistry of floral rewards: intra- and interspecific variability of nectar and pollen secondary metabolites across taxa. Ecol. Monogr. 89, e01335. [DOI] [PubMed] [Google Scholar]
- 45.McFrederick QS, Thomas JM, Neff JL, Vuong HQ, Russell KA, Hale AR, Mueller UG. 2017. Flowers and wild megachilid bees share microbes. Microb. Ecol. 73, 188–200. ( 10.1007/s00248-016-0838-1) [DOI] [PubMed] [Google Scholar]
- 46.Manson JS, Otterstatter MC, Thomson JD. 2010. Consumption of a nectar alkaloid reduces pathogen load in bumble bees. Oecologia 162, 81–89. ( 10.1007/s00442-009-1431-9) [DOI] [PubMed] [Google Scholar]
- 47.Singh R, et al. 2010. RNA viruses in Hymenopteran pollinators: evidence of inter-taxa virus transmission via pollen and potential impact on non-Apis Hymenopteran species. PLoS ONE 5, e14357 ( 10.1371/journal.pone.0014357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koch H, Brown MJF, Stevenson PC. 2017. The role of disease in bee foraging ecology. Curr. Opin. Insect Sci. 21, 60–67. ( 10.1016/j.cois.2017.05.008) [DOI] [PubMed] [Google Scholar]
- 49.Figueroa LL, et al. 2019. Data from: Bee pathogen transmission dynamics: deposition, persistence and acquisition on flowers Dryad Digital Repository. ( 10.5061/dryad.jc4hf80) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Figueroa LL, et al. 2019. Data from: Bee pathogen transmission dynamics: deposition, persistence and acquisition on flowers Dryad Digital Repository. ( 10.5061/dryad.jc4hf80) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data is available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.jc4hf80 [49].