Abstract
The seemingly transparent wings of many insects have recently been found to display unexpected structural coloration. These structural colours (wing interference patterns: WIPs) may be involved in species recognition and mate choice, yet little is known about the evolutionary processes that shape them. Furthermore, to date investigations of WIPs have not fully considered how they are actually perceived by the viewers' colour vision. Here, we use multispectral digital imaging and a model of Drosophila vision to compare WIPs of male and female Drosophila simulans from replicate populations forced to evolve with or without sexual selection for 68 generations. We show that WIPs modelled in Drosophila vision evolve in response to sexual selection and provide evidence that WIPs correlate with male sexual attractiveness. These findings add a new element to the otherwise well-described Drosophila courtship display and confirm that wing colours evolve through sexual selection.
Keywords: sexual selection, evolution, courtship, Drosophila, sensory ecology
1. Introduction
Animal colour patterns are important sources of information that are used in a range of signalling contexts including species recognition [1], intrasexual competition [2] and mate choice [3]. When colour patterns are subject to sexual selection, they covary with sexual fitness components and can be part of multi-modal courtship displays [4]. Wing interference patterns (WIPs) are a newly discovered visual component of many insect wings that are thought to act as visual displays (figure 1). They have been recorded in several Drosophila species [6] and possibly represent previously unrecognized sexual signals in otherwise well-described Drosophila courtship displays—which also involve species-specific movement, song, olfaction and taste [6–9]. WIPs are a form of structural coloration produced by thin-film interference where light striking the wing is refracted and reflected in such a way that the wavelength of the reflected light is dependent on the thickness of the chitinous membrane of the wing [6]. As a result, variation in wing thickness, along with other structural variation including hair placement and venation, determines variation in reflected colour [6].
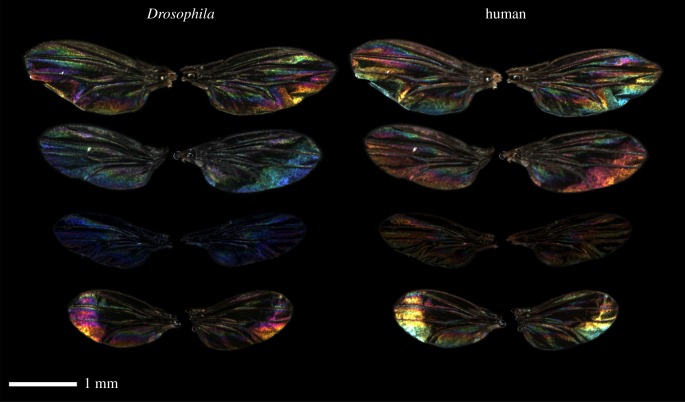
Figure 1.
Examples of Drosophila simulans wing interference patterns (WIPS) photographed for this study using a customized multispectral photography system. Like many insects, Drosophila can see into the ultraviolet range, but not the human ‘red’ range of the spectrum. The images in the left-hand column show ‘false colour’ Drosophila vision (created from cone-catch image stacks using the ‘Make Presentation image’ in the micaToolbox [5]), where the red, green and blue values correspond to normalized Rh6, Rh5 and Rh4 cone-catch quanta, respectively. Images in the right-hand column show the same wings in ‘normal’ human-vision colours. (Online version in colour.)
Recent work shows that WIPs within the human-visible spectrum are heritable and subject to sexual selection via female mate choice in D. melanogaster [8]. Generally, however, little is known about the selective bouts that shape WIPs and how they might respond to any such selection. Furthermore, despite strong evidence that WIPs can be sexual signals [8], all work to date has used uncalibrated digital images where pixel colour values may not correspond linearly with radiance, and this can make colour measurement imprecise [10]. Additionally, no work has yet investigated WIPs explicitly within the spectral sensitivities of the photoreceptors in the Drosophila visual system. This could potentially cloud conclusions about which WIP elements are under selection and how they might evolve.
The Drosophila eye contains five main types of photoreceptor, each expressing a single opsin gene: rhodopsins 1 and 3 through 6 (Rh1 and Rh3 through Rh6) [11]. One of these, Rh1, is thought to be achromatic with broadband spectral sensitivity to both human-visible and UV light, although it could also be used in colour processing [11]. Another two have narrow peak sensitivities in the human-visible spectrum roughly corresponding to green (Rh6) and blue (Rh5) light, and two have narrow peak sensitivities in the UV spectrum at shorter (Rh3) and longer (Rh4) wavelengths [11,12]. These photoreceptors are arranged into bundles of cells called ommatidia, which are often defined as being either ‘pale’ (expressing Rh3 and Rh5) or ‘yellow’ (expressing Rh4 and Rh6) [12–15]. It is currently unclear whether accounting for these attributes of the Drosophila visual system would change conclusions about the precise WIP elements that could act in sexual signalling.
Mating signals and sexual selection has been extensively studied in D. simulans [16–26], but WIPs have not been investigated to assess whether they need to be incorporated into this framework. Female D. simulans are polyandrous, largely determine whether copulation occurs and have a strong preference for certain male genotypes, but do not show clear mate-preference based on male size [16,18,19,21–26]. The attractiveness of male D. simulans is determined by a suite of traits that include dance, smell and song (reviewed in [7,9]), but it can be difficult, and sometimes potentially misleading, to decompose total attractiveness into these contributing characters (e.g. [20]; reviewed in [27]).
Here we investigated the impacts of sexual selection on WIPs in D. simulans using experimental evolution (e.g. [25]). Calibrated digital imaging with Drosophila colour-vision modelling was used to capture WIP colour data. WIP colours can vary within and between wings, but the exact nature of Drosophila colour processing is poorly understood. We therefore measured four visual aspects of WIPs likely to be biologically relevant to Drosophila visual processing. These were mean wing luminance (perceived brightness), luminance contrast (variation in brightness), average colour (hue) and colour contrast (variation in hue: see Material and methods for further details) using male and female wings from experimental populations that had evolved with and without sexual selection (polyandrous and monogamous populations, respectively). We provide the first direct evidence that sexual selection can drive the evolution of WIPs within wavelengths of light visible to the Drosophila visual system and show that variation in WIPs correlates with male sexual attractiveness.
2. Material and methods
(a). Experimental populations
To investigate the ability of sexual selection to drive the evolution of WIPs, we established replicate experimental populations of D. simulans that evolved under either enforced monogamy (1♂:1♀, relaxed sexual selection on males) (n = 4) or under-enforced polyandry (4♂:1♀, elevated sexual selection on males) (n = 4) for 68 non-overlapping generations. This is a standard technique for manipulating the opportunity for sexual selection and allows the action of both pre- and post-copulatory selection [25,28–31].
In each generation, males and females were housed in mating vials at their treatment-specific sex ratio for 6 days (elevated sexual selection: n = 60 per replicate. Relaxed sexual selection: n = 64 per replicate). More mating vials were included in the relaxed treatment to equalize the effective population size (Ne) between treatments [25]. Females were then haphazardly selected to be transferred to treatment- and replicate-specific oviposition vials and housed at a standardized density for 48 h. Virgin adults were collected from oviposition vials after eclosion under light CO2 anaesthesia and separated by sex before being haphazardly assigned to new mating vials for the next generation. Before wings were dissected and photographed all experimental populations were reared for a single generation in mating vials at a standard density (2♂:2♀) to reduce the likelihood of environmental or maternal effects confounding the results [32].
Experimental populations were originally derived from a stock population of D. simulans established from flies originally collected in Australia in 2004 after screening with tetracycline to eliminate Wolbachia infection. Wolbachia infection has been associated with several deleterious effects on fitness in D. simulans [33,34] and can induce cytoplasmic incompatibility in crosses with differences in infection status or strain [35,36]. All flies were housed at a temperature of 25°C under a 12 L : 12 D cycle on an oatmeal-based food media.
We dissected and photographed a total of 480 pairs of wings from 240 individuals all collected after 68 generations of artificial evolution. Thirty-six wings were excluded from analyses due to objects obscuring the wing (e.g. fibres) or wing damage. Final sample sizes were: males evolving with sexual selection n = 55; males without sexual selection n = 58; females with sexual selection n = 57; and females without sexual selection n = 56 (all groups consisted of individuals sampled from each of four replicate populations per treatment).
(b). Wing interference pattern imaging
Wings were photographed in a custom-built assembly using a calibrated Canon camera that had been converted to full-spectrum sensitivity by replacing the sensor's visible-band pass filter with a quartz sheet (conversion by Advanced Camera Systems, Norfolk, UK). The camera was fitted with a Novoflex Noflexar 35 mm lens that transmits in the visible and ultraviolet (UV) range, reverse-mounted on a helicoid to achieve a suitable magnification. Photographs were taken through a Baader UV/IR cut filter that transmits in the human-visible range (400–700 nm) and then through a Baader Venus-U filter that only transmits in the UV (UV, 310–390 nm) range.
WIPs change dramatically as the angle of the wing, light source and viewing angle change under direct (e.g. point source) illumination. We therefore used a custom-built lighting system that provided uniform, diffuse lighting to create standardized illumination and viewing conditions. The lighting assembly used an Iwasaki eyeColor metal halide arc lamp modified to emit UV light by removal of its UV/IR filter. This bulb is designed to match the Commission on Illumination (CIE) standard D65 illuminant, so recreates natural illumination. The bulb was positioned inside a stainless-steel spherical reflector directly below the sample that focused light onto a ring of raw white polytetrafluoroethylene plastic sheet around the lens, simulating a ring-flash. Critically, this light source created standardized and uniformly diffuse illumination that matches natural conditions. The dorsal surfaces of wings were photographed in pairs on a dark, spectrally flat polymethyl methacrylate background that contained a scale bar.
(c). Image processing
Most imaging systems create photographs for viewing on, low dynamic range displays using 8-bits per channel colour spaces. However, such images are also nonlinear, meaning the pixel values do not correspond linearly with radiance, which in turn makes them unsuitable for objective colour measurement [10]. Standard red–green–blue (RGB) systems are also unsuitable for modelling Drosophila vision because they do not capture the UV portion of the spectrum to which Drosophila are sensitive, and previous analyses have included the red portion of the spectrum, which the flies are unable to detect [37]. We therefore processed our whole-wing images using our Multispectral Image Analysis and Calibration Toolbox for ImageJ [38], which enables image calibration, first controlling for lighting conditions and then converting images to animal cone-catch quanta [5].
We used the toolbox to combine the visible and ultraviolet whole-wing images into aligned, normalized multispectral stacks and then used a cone-mapping approach to convert these images to ‘Drosophila vision’ (i.e. Drosophila cone-catch quanta) [5]. Briefly, colour discrimination in Drosophila vision is best explained by a system of opponent colour processing, where neurons receive antagonistic input from two or more photoreceptors and the contrast between these inputs is used to process colour information [11]. To better represent this process, we calculated four ‘opponent channels’ that have been empirically validated to accurately describe Drosophila colour discrimination (Rh5–Rh3, Rh6–Rh4, Rh6–Rh1, and Rh4–Rh1) [11]. These were calculated by dividing the cone-catch quanta values of a focal photoreceptor by the sum of the cone-catch quanta values of that photoreceptor and a second comparator photoreceptor (e.g. Rh5/(Rh3 + Rh5)) [39]. We generated images of these opponent channels from cone-catch data and measured the mean hue (average opponent channel pixel values across the wing) and colour contrast (s.d. in opponent channel pixel values across the entire wing).
Images were normalized (i.e. converted to relative reflectance images that control for lighting conditions) by measuring the background grey in each image, which was in turn calibrated against a Spectralon 99% reflectance standard (Labsphere). Briefly, the cone-mapping process uses the known spectral sensitivities of the camera to estimate the camera's response to a database of thousands of natural reflectance spectra illuminated using the CIE standard D65 illuminant following the von Kries correction. In addition, the Drosophila cone-catch quanta were calculated for the same illuminant using Drosophila spectral sensitivities [11,14]. A polynomial model was then fitted between camera and Drosophila vision. The model reported R2 values greater than 0.993 for all five receptor classes. For more information on the methodology, see [5].
Mean luminance was calculated as the mean Rh1 cone-catch quanta pixel estimates for each wing, and luminance contrast was the s.d. in these estimates. The mean and s.d. in opponent channel pixel values across each wing were then used to analyse wing colour (hue) and colour contrast, respectively, using principal component analysis (see below). Wings of a single colour would therefore have a high average colour, but low colour contrast, while wings containing multiple colours would have a high colour contrast. While all cone-catch quanta were measured for each wing, quanta from pairs of wings were averaged for individual flies. The variance analyses were comparing mean variances across treatments rather than strictly comparing the variances within treatments (see [40] for the importance of this distinction).
(d). Attractiveness assay
After 55 generations of experimental evolution (13 generations before wings were measured—tests were staggered for logistic reasons), including a generation of standardized rearing (all experimental populations were reared for a single generation in mating vials at a standard density (2♂:2♀) to reduce the likelihood of environmental or maternal effects confounding the results [32]), virgin males from each experimental population were collected and housed alone until sexually mature. Attractiveness was then measured using standard protocols [20–22,41] with virgin females from stock populations used as testers (i.e. females that had not been subjected to experimental evolution). In brief, females should mate with attractive males more quickly and we used mating latency (time from pairing until mating: log transformed) as our measure of male attractiveness [20–22,42–45]. A mean latency per population was calculated (since the population was the unit of replication) and then populations were ranked on these means and the rank sum for populations evolving with and without sexual selection was then compared.
(e). Statistical analyses
All statistical analyses were performed in Statview/SuperAnova (attractiveness) or R version 3.1.2 [46], where general linear mixed models (GLMM) were implemented in the package lme4 [47]. Mean wing luminance, luminance contrast, colour (hue) principal components (PCs) and colour contrast PCs were compared between sexes and treatments with GLMMs fitted with sex, treatment and their interaction as fixed effects, and population replicate and sex as random effects with both random intercepts and slopes. Fixed effects were tested for significance using the ANOVA function in the car package [48]. Where a significant sex by treatment interaction was present, Tukey contrasts adjusted for multiple comparisons were obtained from the GLMMs using the lsmeans package [49]. Where no significant interaction between sex and treatment was found, significant GLMM terms explaining differences in WIP traits are reported. Luminance values are presented as Rh1 cone-catch quanta from zero to one.
Principal component analyses (PCA) were conducted on opponent channel data to reduce the dimensionality of the dataset and account for high levels of correlation between the cone-catch values across opponent channels. PCs derived from the PCA were considered significant if their associated eigenvalue was greater than 1.0 [50], and the loading of PCs was considered significant if greater than 0.35 [51]. Statistical testing of the principal component data was conducted in the same manner as for the luminance data. Attractiveness (mating latency) data were highly skewed (electronic supplementary material) and as a result analysed using a non-parametric Mann–Whitney rank sum test.
3. Results
The effect of sexual selection on the brightness of wings (mean luminance measured by the mean cone-catch values of the broadband photoreceptor Rh1) was dependent on sex (i.e. there was a sex-by-treatment interaction: GLMM, , p = 0.017; electronic supplementary material, table S2). The WIPs of males evolving with sexual selection are on average 10.7% brighter than the WIPs of males evolving without sexual selection (LSMeans, t ratio = 3.007, p = 0.016; electronic supplementary material, table S4; figure 2). By contrast, there was no difference in mean luminance between the WIPs of females evolving with or without sexual selection (LSMeans, t ratio = 0.369, p = 0.98). The luminance of female WIPs from both sexual selection treatments was also similar to males evolving with and without sexual selection (electronic supplementary material, tables S1–S4; full statistical models for this and subsequent analyses are presented in the electronic supplementary material).
Figure 2.

Mean luminance of WIPs as measured by average stimulation of the broadband Rh1 photoreceptor in the Drosophila visual system. Boxes represent the interquartile range, black bars are medians, white diamonds are means. SS+ = flies from populations evolving with sexual selection, SS− = flies from populations evolving without sexual selection. Differences in letter annotation denote significance at p < 0.05. (Online version in colour.)
The effect of sexual selection on the brightness contrast (luminance contrast measured by the standard deviation of the cone-catch values of the broadband receptor Rh1) also showed a sex by treatment interaction (GLMM, , p = 0.037; electronic supplementary material, table S6). The WIPs of males evolving with sexual selection have significantly higher luminance contrast than those of males evolving without sexual selection (LSMeans, t ratio = −3.586, p = 0.002; electronic supplementary material, table S8; figure 3), but there was no difference between the WIPs of females evolving with or without sexual selection (LSMeans, t ratio = 1.02, p = 0.74). The brightness contrast of female WIPs from both sexual selection treatments was again similar to males evolving with and without sexual selection (electronic supplementary material, tables S5–S8).
Figure 3.

Luminance contrast of WIPs as measured by the standard deviation of the average stimulation of the broadband Rh1 photoreceptor in the Drosophila visual system. Boxes represent the interquartile range, black bars are medians, white diamonds are means. SS+ = flies from populations evolving with sexual selection, SS− = flies from populations evolving without sexual selection. Differences in letter annotation denote significance at p < 0.01. (Online version in colour.)
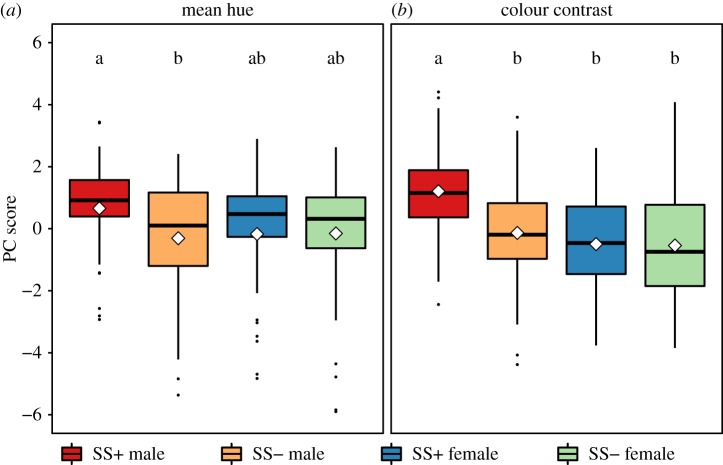
We extracted one significant principal component that explained 92.15% of the variation in the average opponent channel values (i.e. mean hue: electronic supplementary material, table S9), and one significant principal component that explained 78.42% of the variation in colour contrast values (electronic supplementary material, table S13).
The principal component for average hue described variation in the opponency of long versus short wavelength photoreceptors (Rh5 versus Rh3, and Rh6 versus Rh4), and opponency of narrowband photoreceptors in yellow ommatidia versus broadband photoreceptors (Rh6 against Rh1, and Rh4 against Rh1). The Rh4–Rh1 channel was significantly negatively loaded to this principal component while the remaining three channels were significantly positively loaded (electronic supplementary material, table S9). Thus, higher principal component scores indicate higher reflectance of longer wavelength light (measured by Rh5 and Rh6) relative to shorter wavelength UV light (measured by Rh3 and Rh4). Again, we found that the effect of sexual selection on WIPs was different for males and females (GLMM, , p = 0.049; electronic supplementary material, table S10). The WIPs of males evolving with sexual selection differ significantly from those of males evolving without (LSMeans, t ratio = −2.699, p = 0.037; electronic supplementary material, table S12), showing stronger biases towards longer wavelength light (i.e. visible spectrum) relative to shorter wavelength UV light (figure 4; Mean hue). By contrast, the average hues of female WIPs from either evolution treatment were indistinguishable (LSMeans, t ratio = 0.055, p = 0.99; electronic supplementary material, table S12), and did not differ from males from either treatment (electronic supplementary material, tables S9–S12).
Figure 4.
Principal components (PC1) explaining variation in the opponent channels Rh5–Rh3, Rh6–Rh4, Rh6–Rh1 and Rh4–Rh1 for both mean hue (a) and colour contrast (b). SS+ = flies from populations evolving with sexual selection, SS− = flies from populations evolving without sexual selection. Differences in letter annotation denote significance at p < 0.05 for mean hue (a), and significance at p < 0.01 for colour contrast (b). Boxes represent the interquartile range, black bars are medians, white diamonds are means.
The principal component for colour contrast describes variation in the same opponent channels as the component for average hue. All four opponent channels were significantly and positively loaded to this principal component (electronic supplementary material, table S19), and higher principal component scores therefore indicate higher colour contrast levels in all opponent channels (i.e. more variation in the colour axes defined by the opponent channels). Once again, we found that the effect of sexual selection on WIPs was different for males and females (GLMM, , p = 0.003; electronic supplementary material, table S14). The WIPs of males evolving with sexual selection had significantly higher levels of colour contrast than those of males without (LSMeans, t ratio = 5.42, p < 0.001; electronic supplementary material, table S16) (figure 4; colour contrast). However, the colour contrast of female WIPs from both selection regimes were indistinguishable (LSMeans, t ratio =−0.152, p = 0.99), did not differ from the no-sexual selection treatment males (LSMeans, t ratio < −1.067, p > 0.642), but were significantly different from males evolving with sexual selection (LSMeans, t ratio > −5.061, p < 0.003).
These results indicate sexual selection affected wing sexual-signal evolution because in all comparisons males from each treatment differed from one another. As such, males from populations that evolved with sexual selection should be more sexually attractive. To test this, the attractiveness (mating latency, a standard measure of male attractiveness: see Material and methods) of males from experimental populations (when placed with a single virgin tester female) was compared. Ranking population on average attractiveness showed that males from populations evolving with sexual selection (mean latency ± s.e. 66.9 ± 4.0 min; rank sum = 10) were significantly more attractive (better sexual competitors) than males evolving without sexual selection (mean latency ± s.e. 95.0 ± 12.9 min; rank sum = 26) (Mann–Whitney rank sum test: N = 8, Z = −2.31, p = 0.02).
4. Discussion
Here we show that D. simulans wing colour evolved in response to sexual selection when measured using Drosophila visual modelling. Critically, this modelling used the full range of human-visible and ultraviolet wavelengths that Drosophila can perceive. Behavioural experiments then confirmed that these WIPs broadly correlated with male attractiveness. These results also indicate significant additive genetic variation for WIPs and confirm findings of heritable male attractiveness [22]. Importantly, the PCA, which effectively summarize the total dataset into a reduced number of response variables show that males evolving with sexual selection have WIPs that are very different from all other flies. Thus, our findings are consistent with those for D. melanogaster [8] where evidence was found for sexual selection on WIP hue and saturation. Work on sexual coloration in other systems shows that inclusion of the appropriate visual system and colour measurement can sharpen conclusions about colour and sexual selection [52]. Be that as it may, Katayama et al. [8] did not use the explicit model of fly vision we employed here, but there is nonetheless strong congruence in the findings of these two fly studies.
By employing experimental evolution, we have explicitly shown that WIPs evolve via sexual selection as males evolving with mate choice and mate-competition had significantly different wing coloration components than males evolving without sexual selection, and this resulted in males from sexual selection populations being more attractive to females using a standard measure of attractiveness, latency to mate [21,41]. Sexual selection resulted in male wings eliciting a stronger response in longer wavelength light than shorter wavelengths across all four empirically validated opponent channels measured. The wings of males evolving under sexual selection also had higher average luminance (perceived brightness) and luminance contrast than wings from males evolving without sexual selection. Sexual selection therefore seems to favour male wings that have high internal contrast and reflect more light in the human-visible green and blue wavelength regions. However, interpretation of the evolutionary response away from the UV spectrum must be tempered by the low levels of UV light emitted in the controlled environment chambers that housed our populations—this may have constrained evolutionary responses towards the visible spectrum. Despite this, using a standard measure of male attractiveness, males evolving with sexual selection were more attractive to females—they mated faster, and because females determine whether copulation occurs or not [16], mating occurs more rapidly with more attractive males [21,22]. It is important to note that we are not implying that WIP evolution is the sole cause of the differences in attractiveness we documented. For example, we have previously shown that cuticular hydrocarbon profiles, which also confer attractiveness, evolve under sexual selection [25], and we would expect other sexual traits to similarly evolve under our experimental regime (e.g. [53]). However, it is very difficult to experimentally decouple all the other characteristics that generate male attractiveness from WIPs, if only because WIPs are wing structural colours and we would need to remove the wings or run trials in darkness to completely remove their effects. Unfortunately, this would additionally remove male courtship song, which is wing generated, and flies will not mate in the dark. Despite this difficulty, the covariance between attractiveness and WIP evolution is consistent with WIPs being part of the character-set that in sum defines male sexual attractiveness, especially given findings from the closely related D. melanogaster [8].
Higher luminance and colour contrast (i.e. variation of WIP luminance and hues) in males evolving with sexual selection can potentially be explained by trade-offs with other sexually and naturally selected phenotypic optima for wing morphology (e.g. flight performance, or acoustic attractiveness in courtship displays) [54,55]. If selection on wing thickness (which affects WIPs [6,8]) in these other contexts is to some degree orthogonal to selection on WIP coloration from sexual selection, then relaxing sexual selection on WIP coloration could allow these other sources of selection to erode variation in WIP hues that is only relevant in a sexual context.
In contrast to males, female wings have the same mean coloration and colour contrast regardless of the selective regime under which they evolved. This is perhaps unsurprising as sexual selection is typically stronger on males [56,57] and our selection protocol only manipulated the opportunity for sexual selection on them. Furthermore, similar sex-specific responses to sexual selection have been found in other D. simulans studies [25]. While there were some male–female similarities across and within treatments when considering elements of brightness, the PC analysis that summarizes WIPs colour components showed that males evolving with sexual selection were very different from all other flies.
Taken together, our data suggest that sexual selection drives the evolution of a suite of WIP elements in male D. simulans. Specifically, sexual selection favours bright, high-contrast, longwave-shifted male WIPs. This finding is further supported by converting raw colour data into empirically validated opponent channels that reflect the neurological processing of colour discrimination in Drosophila [11]. These data also suggest that differences between treatments and sexes are an evolutionary response to sexual selection (and its relaxation) on males, and that any intersexual genetic correlation underlying WIPs does not appear to be strong enough to prevent detectably independent sexual evolution. Intralocus sexual conflict is a frequent constraint preventing the sexes from reaching sex-specific fitness optima [58,59], but in the D. simulans we study, its effects can be weak [21], which is consistent with the largely sex-specific WIP responses we document here.
5. Conclusion
Here, we provide compelling evidence for the evolution of WIPs through sexual selection. Furthermore, we can be reasonably confident that effects are from female mate choice because males from populations that included sexual selection were more attractive to females and importantly, in the closely related D. melanogaster female choice appears to generate sexual selection on male WIPs [8]. It therefore seems that WIPs are a novel sexual signal that has until very recently been overlooked in sexual selection research, even in well-studied taxa like Drosophila.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank the attendees at the ASAB London Winter Meeting 2017 for comments on these findings, and the Editor and three excellent referees whose comments helped to greatly improve the manuscript.
Data accessibility
All the raw data are included in the electronic supplementary material.
Authors' contributions
The study was conceived by M.D.S., J.T., N.W., J.R. and D.J.H. Experiments were conducted by E.D., R.J., M.F.H. and J.T. Data were analysed by M.F.H., J.T. and D.J.H. The manuscript was written by M.F.H., J.T. and D.J.H. All authors edited and commented on the manuscript.
Competing interests
There are no competing interests.
Funding
This work was funded by The Leverhulme Trust, BBSRC and NERC and by the Foundation for Polish Science, International PhD Projects Program co-financed by the European Regional Development Fund within the project MPD/2009-3/5, ‘Environmental stress, population viability and adaptation’.
References
- 1.Barraclough TG, Harvey PH, Nee S. 1995. Sexual selection and taxonomic diversity in passerine birds. Proc. R. Soc. B 259, 211–215. ( 10.1098/rspb.1995.0031) [DOI] [Google Scholar]
- 2.Siefferman L, Hill GE. 2005. UV-blue structural coloration and competition for nestboxes in male eastern bluebirds. Anim. Behav. 69, 67–72. ( 10.1016/j.anbehav.2003.12.026) [DOI] [Google Scholar]
- 3.Houde AE. 1997. Sex, color, and mate choice in guppies. Princeton, NJ: Princeton University Press. [Google Scholar]
- 4.Candolin U. 2003. The use of multiple cues in mate choice. Biol. Rev. 78, 575–595. ( 10.1017/S1464793103006158) [DOI] [PubMed] [Google Scholar]
- 5.Troscianko J, Stevens M. 2015. Image calibration and analysis toolbox—a free software suite for objectively measuring reflectance, colour and pattern. Methods Ecol. Evol. 6, 1320–1331. ( 10.1111/2041-210X.12439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shevtsova E, Hansson C, Janzen DH, Kjærandsen J. 2011. Stable structural color patterns displayed on transparent insect wings. Proc. Natl Acad. Sci. USA 108, 668–673. ( 10.1073/pnas.1017393108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenspan RJ, Ferveur J-F. 2000. Courtship in Drosophila. Annu. Rev. Genet. 34, 205–232. ( 10.1146/annurev.genet.34.1.205) [DOI] [PubMed] [Google Scholar]
- 8.Katayama N, Abbott JK, Kjærandsen J, Takahashi Y, Svensson EI. 2011. Sexual selection on wing interference patterns in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 111, 15 144–15 148. ( 10.1073/pnas.1407595111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosken DJ, Bretman A, Goodwin SF, Archer CR. 2019. Genes and environment in Drosophila sex. In Genes and behaviour: beyond nature-nurture (eds Hosken DJ, Wedell N, Hunt J). London, UK: Wiley. [Google Scholar]
- 10.Stevens M, Párraga CA, Cuthill IC, Partridge JC, Troscianko TS. 2007. Using digital photography to study animal coloration. Biol. J. Linn. Soc. 90, 211–237. ( 10.1111/j.1095-8312.2007.00725.x) [DOI] [Google Scholar]
- 11.Schnaitmann C, Garbers C, Wachtler T, Tanimoto H. 2013. Color discrimination with broadband photoreceptors. Curr. Biol. 23, 2375–2382. ( 10.1016/j.cub.2013.10.037) [DOI] [PubMed] [Google Scholar]
- 12.Rister J, Desplan C, Vasiliauskas D. 2013. Establishing and maintaining gene expression patterns: insights from sensory receptor patterning. Development 140, 493–503. ( 10.1242/dev.079095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardie RC. 1979. Electrophysiological analysis of fly retina. I: comparative properties of R1–6 and R 7 and 8. J. Comp. Physiol. A 129, 9–33. ( 10.1007/BF00679908) [DOI] [Google Scholar]
- 14.Salcedo E, Huber A, Henrich S, Chadwell LV, Chou W-H, Paulsen R, Britt SG. 1999. Blue- and green-absorbing visual pigments of Drosophila: ectopic expression and physiological characterization of the R8 photoreceptor cell-specific Rh5 and Rh6 rhodopsins. J. Neurosci. 19, 10 716–10 726. ( 10.1523/JNEUROSCI.19-24-10716.1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wernet MF, Çelik A, Mikeladze-Dvali T, Desplan C. 2007. Generation of uniform fly retinas. Curr. Biol. 17, R1002–R1003. ( 10.1016/j.cub.2007.10.006) [DOI] [PubMed] [Google Scholar]
- 16.Spieth HT. 1974. Courtship behavior in Drosophila. Annu. Rev. Entomol. 9, 385–405. ( 10.1146/annurev.en.19.010174.002125) [DOI] [PubMed] [Google Scholar]
- 17.Markow TA, Bustoz D, Pitnick S. 1996. Sexual selection and a secondary sexual character in two Drosophila species. Anim. Behav. 52, 759–766. ( 10.1006/anbe.1996.0220) [DOI] [Google Scholar]
- 18.Ingleby FC, Hosken DJ, Flowers K, Hawkes MF, Lane SM, Rapkin J, Dworkin I, Hunt J. 2013. Genotype-by-environment interactions for cuticular hydrocarbon expression in Drosophila simulans. J. Evol. Biol. 26, 94–107. ( 10.1111/jeb.12030) [DOI] [PubMed] [Google Scholar]
- 19.Ingleby FC, Hunt J, Hosken DJ. 2013. Genotype-by-environment interactions for female mate choice of male cuticular hydrocarbons in Drosophila simulans. PLoS ONE 8, e67623 ( 10.1371/journal.pone.0067623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ingleby FC, Hunt J, Hosken DJ. 2013. Heritability of male attractiveness persists despite evidence for unreliable sexual signals in Drosophila simulans . J. Evol. Biol. 26, 311–324. ( 10.1111/jeb.12045) [DOI] [PubMed] [Google Scholar]
- 21.Taylor ML, Wedell N, Hosken DJ. 2010. Attractive males do not sire superior daughters. Evol. Ecol. 24, 195–205. ( 10.1007/s10682-009-9298-0) [DOI] [Google Scholar]
- 22.Taylor ML, Wedell N, Hosken DJ. 2007. The heritability of attractiveness. Curr. Biol. 17, R959–R960. ( 10.1016/j.cub.2007.09.054) [DOI] [PubMed] [Google Scholar]
- 23.Taylor ML, Wigmore C, Hodgson DJ, Wedell N, Hosken DJ. 2008. Multiple mating increases female fitness in Drosophila simulans. Anim. Behav. 76, 963–970. ( 10.1016/j.anbehav.2008.05.015) [DOI] [Google Scholar]
- 24.Taylor ML, Wedell N, Hosken DJ. 2008. Sexual selection and female fitness in Drosophila simulans. Behav. Ecol. Sociobiol. 65, 721–728. ( 10.1007/s00265-007-0497-9) [DOI] [Google Scholar]
- 25.Sharma MD, Hunt J, Hosken DJ. 2012. Antagonistic responses to natural and sexual selection and the sex-specific evolution of cuticular hydrocarbons in Drosophila simulans. Evolution 66, 665–677. ( 10.1111/j.1558-5646.2011.01468.x) [DOI] [PubMed] [Google Scholar]
- 26.Sharma MD, Tregenza T, Hosken DJ. 2010. Female mate preferences in Drosophila simulans: evolution and costs. J. Evol. Biol. 23, 1672–1679. ( 10.1111/j.1420-9101.2010.02033.x) [DOI] [PubMed] [Google Scholar]
- 27.Prokop ZM, Drobniak SM. 2016. Genetic variation in male attractiveness: it is time to see the forest for the trees. Evolution 70, 913–921. ( 10.1111/evo.12898) [DOI] [PubMed] [Google Scholar]
- 28.Holland B, Rice WR. 1999. Experimental removal of sexual selection reverses intersexual antagonistic coevolution and removes a reproductive load. Proc. Natl Acad. Sci. USA 96, 5083–5088. ( 10.1073/pnas.96.9.5083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosken DJ, Garner TWJ, Ward PI. 2001. Sexual conflict selects for male and female reproductive characters. Curr. Biol. 11, 489–493. ( 10.1016/S0960-9822(01)00146-4) [DOI] [PubMed] [Google Scholar]
- 30.Crudgington HS, Beckerman AP, Brustle L, Green K, Snook RR. 2005. Experimental removal and elevation of sexual selection: does sexual selection generate manipulative males and resistant females? Am. Nat. 165, S72–S87. ( 10.1086/429353) [DOI] [PubMed] [Google Scholar]
- 31.Tilszer M, Antoszczyk K, Sałek N, Zając E, Radwan J. 2006. Evolution under relaxed sexual conflict in the bulb mite. Evolution 60, 1868–1873. ( 10.1111/j.0014-3820.2006.tb00530.x) [DOI] [PubMed] [Google Scholar]
- 32.Magalhães S, Blanchet E, Egas M, Olivieri I. 2011. Environmental effects on the detection of adaptation. J. Evol. Biol. 24, 2653–2662. ( 10.1111/j.1420-9101.2011.02388.x) [DOI] [PubMed] [Google Scholar]
- 33.Snook RR, Cleland SY, Wolfner MF, Karr TL. 2000. Offsetting effects of Wolbachia infection and heat shock on sperm production in Drosophila simulans: analyses of fecundity, fertility and accessory gland proteins. Genetics 155, 167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Champion de Crespigny FE, Wedell N. 2006. Wolbachia infection reduces sperm competitive ability in an insect. Proc. R. Soc. B 273, 1455–1458. ( 10.1098/rspb.2006.3478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Werren JH. 1997. Biology of Wolbachia. Annu. Rev. Entomol. 42, 587–609. ( 10.1146/annurev.ento.42.1.587) [DOI] [PubMed] [Google Scholar]
- 36.Werren JH, Baldo L, Clark ME. 2008. Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Microbiol. 6, 741–751. ( 10.1038/nrmicro1969) [DOI] [PubMed] [Google Scholar]
- 37.Briscoe AD, Chittka L. 2001. The evolution of color vision in insects. Annu. Rev. Entomol. 46, 471–510. ( 10.1146/annurev.ento.46.1.471) [DOI] [PubMed] [Google Scholar]
- 38.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. ( 10.1038/nmeth.2089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelber A, Vorobyev M, Osorio D. 2003. Animal colour vision-behavioural tests and physiological concepts. Biol. Rev. 78, 81–118. ( 10.1017/S1464793102005985) [DOI] [PubMed] [Google Scholar]
- 40.Hosken DJ, Buss DL, Hodgson DJ. 2018. Beware the F test (or how to compare variances). Anim. Behav. 136, 119–126. ( 10.1016/j.anbehav.2017.12.014) [DOI] [Google Scholar]
- 41.Okada K, Blount JD, Sharma MD, Snook RR, Hosken DJ. 2011. Male attractiveness, fertility and susceptibility to oxidative stress are influenced inbreeding in Drosophila simulans. J. Evol. Biol. 24, 363–371. ( 10.1111/j.1420-9101.2010.02170.x) [DOI] [PubMed] [Google Scholar]
- 42.Hosken DJ, Taylor ML, Hoyle K, Higgins S, Wedell N. 2008. Attractive males have greater success in sperm competition. Curr. Biol. 18, R553–R554. ( 10.1016/j.cub.2008.04.028) [DOI] [PubMed] [Google Scholar]
- 43.Kyriacou CP, Hall JC. 1986. Interspecific genetic control of courtship song production and reception in Drosophila. Science 232, 494–497. ( 10.1126/science.3083506) [DOI] [PubMed] [Google Scholar]
- 44.Barth M, Hirsch HVB, Heisenberg M. 1997. Rearing in different light regimes affects courtship behaviour in Drosophila melanogaster. Anim. Behav. 53, 25–38. ( 10.1006/anbe.1996.0275) [DOI] [Google Scholar]
- 45.Ritchie MG, Halsey EJ, Gleason JM. 1999. Drosophila song as a species-specific mating signal and the behavioral importance of Kyriacou & Hall cycles in D. melanogaster song. Anim. Behav. 58, 649–657. ( 10.1006/anbe.1999.1167) [DOI] [PubMed] [Google Scholar]
- 46.R Core Team. 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 47.Bates D, Maechler M, Bolker BM, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 48.Fox J, Weisberg S. 2011. An R companion to applied regression. London, UK: Sage Publications. [Google Scholar]
- 49.Lenth RV. 2016. Least-square means: the R package lsmeans. J. Stat. Softw. 69, 1–33. ( 10.18637/jss.v069.i01) [DOI] [Google Scholar]
- 50.Norman GR, Streiner DL. 2008. Biostatistics: the bare essentials. Shelton, CT: People's Medical Publishing House USA Ltd. [Google Scholar]
- 51.Tabachnick BG, Fidell LS. 2013. Using multivariate statistics. New York, NY: Pearson Education. [Google Scholar]
- 52.Bennett ATD, Cuthill IC, Partridge JC, Lunau K. 1997. Ultraviolet plumage colors predict mate preferences in starlings. Proc. Natl Acad. Sci. USA 94, 8618–8621. ( 10.1073/pnas.94.16.8618) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.House CM, Lewis Z, Hodgson DJ, Wedell N, Sharma MD, Hunt J, Hosken DJ. 2013. Sexual selection and natural selection both influence male genital evolution. PLoS ONE 8, e63807.. ( 10.1371/journal.pone.0063807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Radwan J, Engqvist L, Reinhold K. 2016. A paradox of genetic variance in epigamic traits: beyond ‘good genes’ view of sexual selection . Evol. Biol. 43, 267–275. ( 10.1007/s11692-015-9359-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Radwan J. 2008. Maintenance of genetic variation in sexual ornaments: a review of the mechanisms. Genetica 134, 113–127. ( 10.1007/s10709-007-9203-0) [DOI] [PubMed] [Google Scholar]
- 56.Shuster SM, Wade MJ. 2003. Mating systems and strategies. Princeton, NJ: Princeton University Press. [Google Scholar]
- 57.Hosken DJ, House CM. 2011. Sexual selection. Curr. Biol. 2, R62–R65. ( 10.1016/j.cub.2010.11.053) [DOI] [PubMed] [Google Scholar]
- 58.Rice WR, Chippindale AK. 2001. Intersexual ontogenetic conflict. J. Evol. Biol. 14, 685–693. ( 10.1046/j.1420-9101.2001.00319.x) [DOI] [Google Scholar]
- 59.Harano T, Okada K, Nakayama S, Miyatake T, Hosken DJ. 2010. Intralocus sexual conflict unresolved by sex-limited trait expression. Curr. Biol. 20, 2036–2039. ( 10.1016/j.cub.2010.10.023) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the raw data are included in the electronic supplementary material.